Abstract
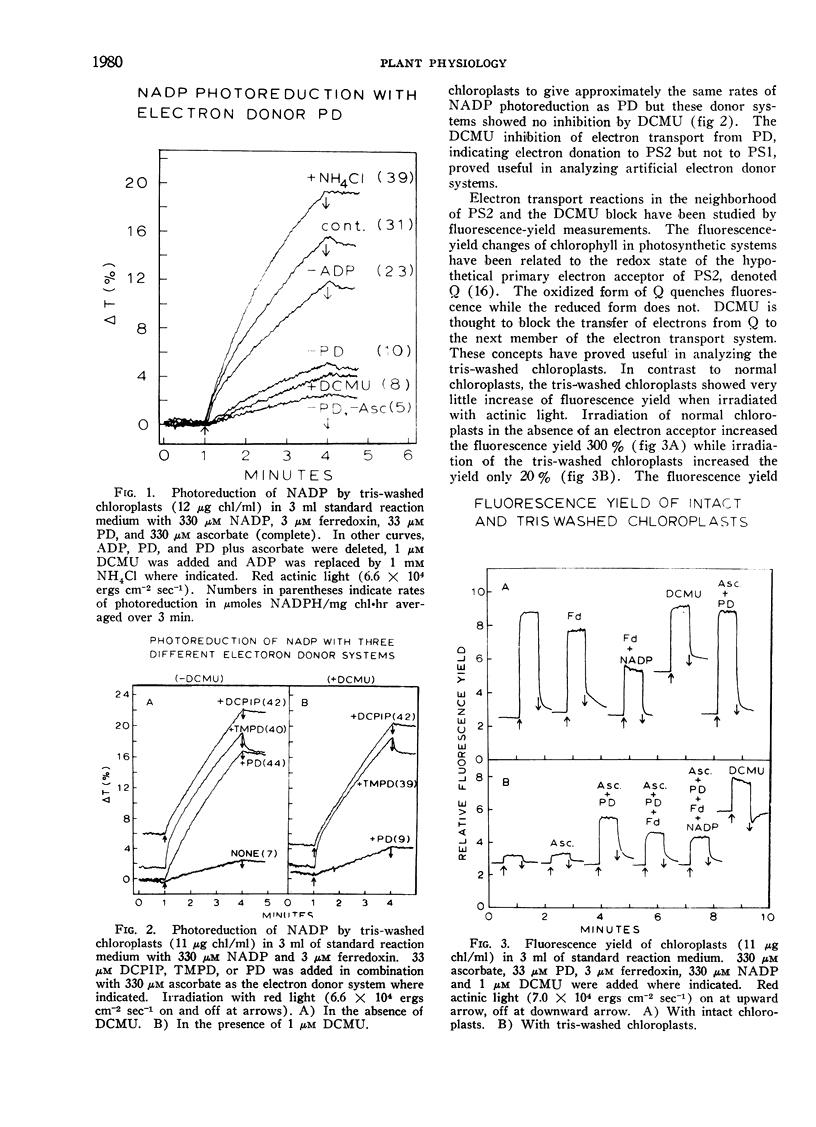
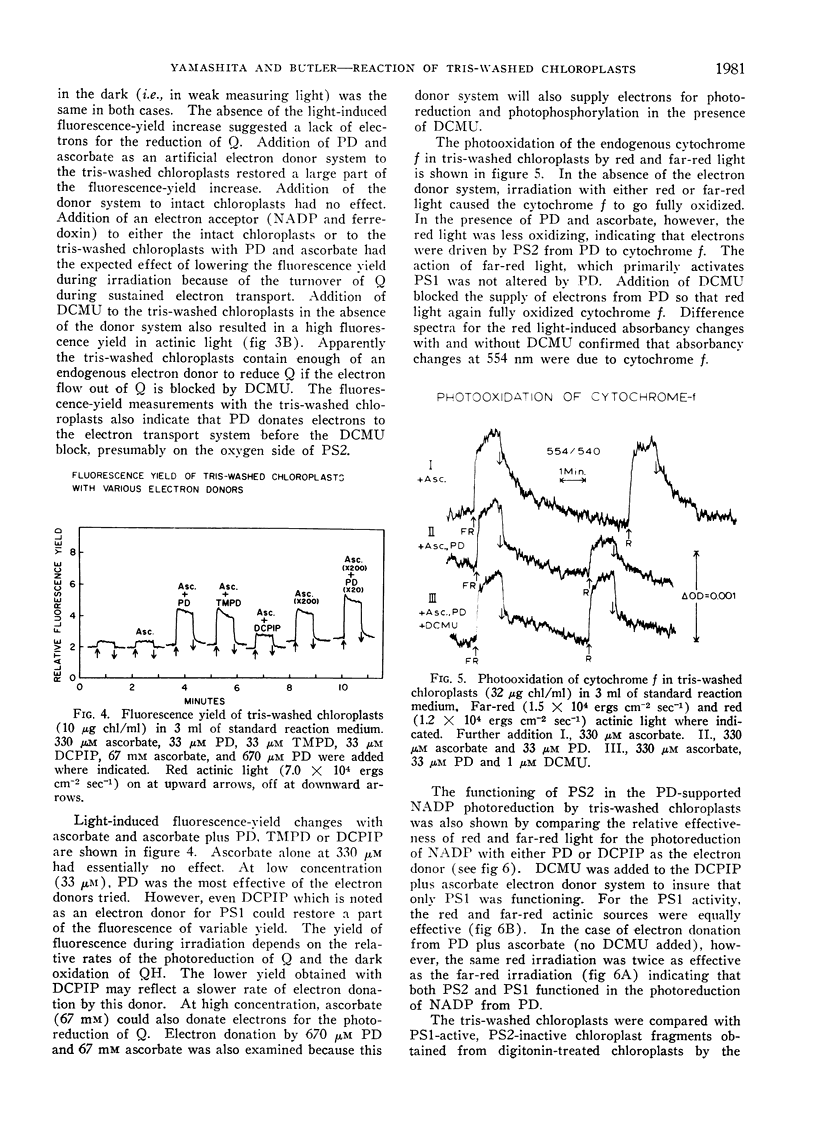
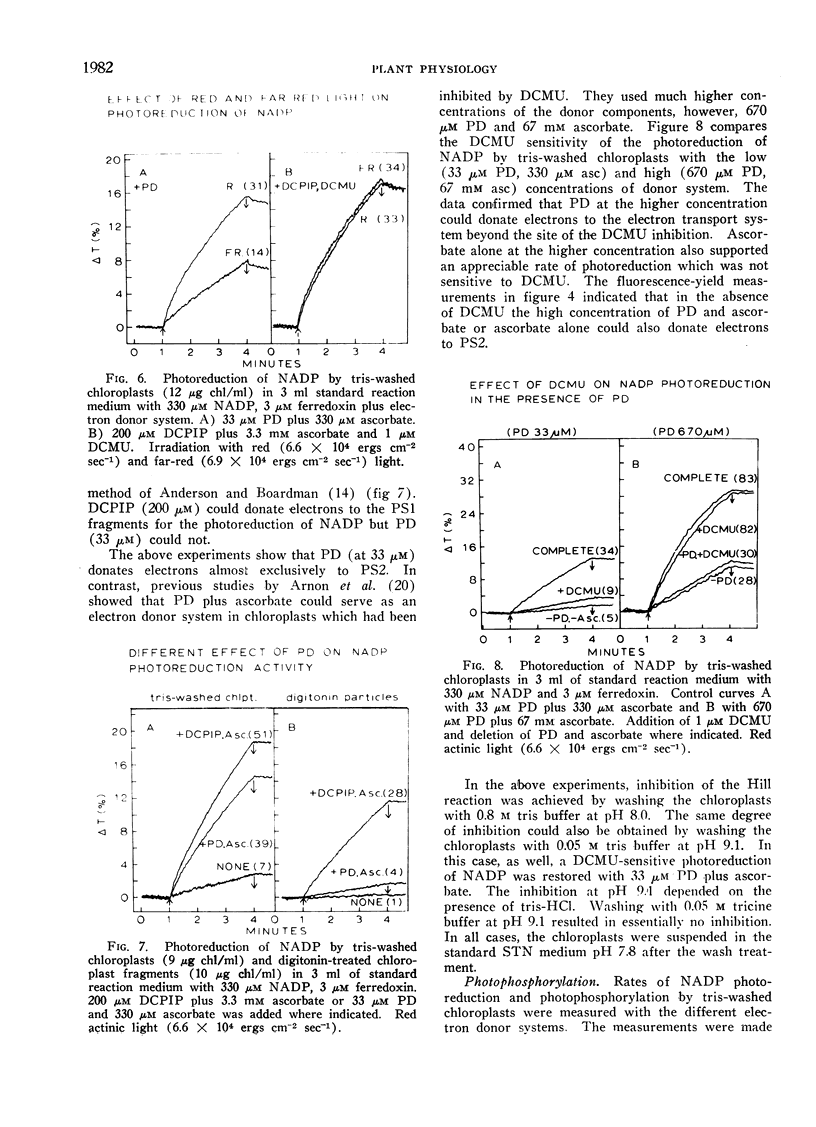
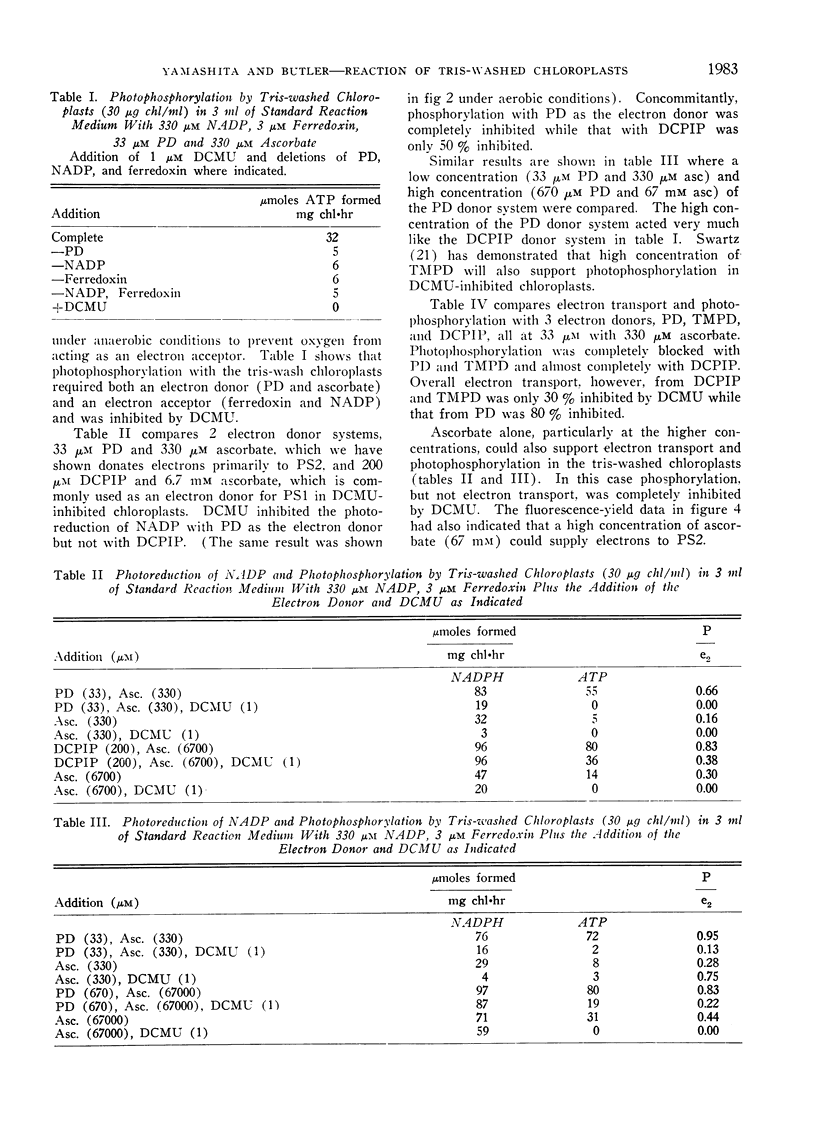
The artificial electron donor compounds p-phenylenediamine (PD), N, N, N′, N′-tetramethyl-p-phenylenediamine (TMPD), and 2,6-dichlorophenol-indophenol (DCPIP) restored the Hill reaction and photophosphorylation in chloroplasts that had been inhibited by washing with 0.8 m tris (hydroxymethyl) aminomethane (tris) buffer, pH 8.0. The tris-wash treatment inhibited the electron transport chain between water and photosystem II and electron donation occurred between the site of inhibition and photosystem II. Photoreduction of nicotinamide adenine dinucleotide phosphate (NADP) supported by 33 μm PD plus 330 μm ascorbate was largely inhibited by 1 μm 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) while that supported by 33 μm TMPD or DCPIP plus ascorbate was relatively insensitive to DCMU. Experiments with the tris-washed chloroplasts indicated that electron donors preferentially donate electrons to photosystem II but in the presence of DCMU the donors (with the exception of PD at low concentrations) could also supply electrons after the DCMU block. The PD-supported photoreduction of NADP showed the relative inefficiency in far-red light characteristic of chloroplast reactions requiring photosystem II. With phosphorylating systems involving electron donors at low concentrations (33 μm donor plus 330 μm ascorbate) photophosphorylation, which occurred with P/e2 ratios approaching unity, was completely inhibited by DCMU but with higher concentrations of the donor systems, photophosphorylation was only partially inhibited.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Boardman N. K. Fractionation of the photochemical systems of photosynthesis. I. Chlorophyll contents and photochemical activities of particles isolated from spinach chloroplasts. Bibl Laeger. 1966 Mar 14;112(3):403–421. doi: 10.1016/0926-6585(66)90244-5. [DOI] [PubMed] [Google Scholar]
- Gromet-Elhanan Z. The relationship of cyclic and non-cyclic electron flow patterns with reduced indophenols to photophosphorylation. Biochim Biophys Acta. 1967 May 9;131(3):526–537. doi: 10.1016/0005-2728(67)90012-6. [DOI] [PubMed] [Google Scholar]
- JAGENDORF A. T., MARGULIES M. Inhibition of spinach chloroplast photosynthetic reactions by p-chlorophenyll, 1-dimethylurea. Arch Biochem Biophys. 1960 Oct;90:184–195. doi: 10.1016/0003-9861(60)90566-x. [DOI] [PubMed] [Google Scholar]
- Katoh S., San Pietro A. Ascorbate-supported NADP photoreduction by heated Euglena chloroplasts. Arch Biochem Biophys. 1967 Oct;122(1):144–152. doi: 10.1016/0003-9861(67)90133-6. [DOI] [PubMed] [Google Scholar]
- LOSADA M., WHATLEY F. R., ARNON D. I. Separation of two light reactions in noncyclic photo-phosphorylation of green plants. Nature. 1961 May 13;190:606–610. doi: 10.1038/190606a0. [DOI] [PubMed] [Google Scholar]
- NIELSEN S. O., LEHNINGER A. L. Phosphorylation coupled to the oxidation of ferrocytochrome c. J Biol Chem. 1955 Aug;215(2):555–570. [PubMed] [Google Scholar]
- VERNON L. P., ZAUGG W. S. Photoreductions by fresh and aged chloropasts: requirement for ascorbate and 2, 6-dichlorophenolindophenol with aged chloroplasts. J Biol Chem. 1960 Sep;235:2728–2733. [PubMed] [Google Scholar]


