Abstract
The enzymatic oxidation of arachidonic acid is proposed to yield trihydroxytetraene species (termed lipoxins) that resolve inflammation via ligand activation of the formyl peptide receptor, FPR2. While cell and murine models activate signaling responses to synthetic lipoxins, primarily 5S,6R,15S-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid (lipoxin A4, LXA4), there are expanding concerns about the biological formation, detection and signaling mechanisms ascribed to LXA4 and related di- and tri-hydroxy ω−6 and ω−3 fatty acids. Herein, the generation and actions of LXA4 and its primary 15-oxo metabolite were assessed in control, LPS-activated and arachidonic acid supplemented RAW 264.7 macrophages. Despite protein expression of all enzymes required for LXA4 synthesis, both LXA4 and its 15-oxo-LXA4 metabolite were undetectable. Moreover, synthetic LXA4 and the membrane permeable 15-oxo-LXA4 methyl ester that is rapidly de-esterified to 15-oxo-LXA4, displayed no ligand activity for the putative LXA4 receptor FPR2, as opposed to the FPR2 ligand WKYMVm. Alternatively, 15-oxo-LXA4, an electrophilic α,β-unsaturated ketone, alkylates nucleophilic amino acids such as cysteine to modulate redox-sensitive transcriptional regulatory protein and enzyme function. 15-oxo-LXA4 activated nuclear factor (erythroid related factor 2)-like 2 (Nrf2)-regulated gene expression of anti-inflammatory and repair genes and inhibited nuclear factor (NF)-κB-regulated pro-inflammatory mediator expression. LXA4 did not impact these macrophage anti-inflammatory and repair responses. In summary, these data show an absence of macrophage LXA4 formation and receptor-mediated signaling actions. Rather, if LXA4 were present in sufficient concentrations, this, and other more abundant mono- and poly-hydroxylated unsaturated fatty acids can be readily oxidized to electrophilic α,β-unsaturated ketone products that modulate the redox-sensitive cysteine proteome via G-protein coupled receptor-independent mechanisms.
Introduction
Inflammatory responses initiate diverse free radical and enzymatic oxidation reactions of unsaturated fatty acids, yielding a broad array of products that can orchestrate either pathogenic or tissue-protective responses (1–3). A subset of unsaturated di- and tri-hydroxy fatty acid lipid mediators, termed lipoxins, resolvins, maresins and protectins, are products of the enzymatic oxygenation of arachidonic acid (AA, 20:4), eicosapentaenoic acid (EPA, 20:5) and docosahexaenoic acid (DHA, 22:6) (4,5). For the AA-derived lipoxins of focus herein [specifically 5S,6R,15S-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid; lipoxin A4, LXA4], trihydroxytetraene formation requires multiple oxygenation reactions catalyzed by cells expressing 5-lipoxgenase (LO), 5-LO-activating protein (FLAP), and 12/15-LO. Two major routes of lipoxin synthesis have been proposed. The first occurs in platelets where leukotriene A4 is a substrate for 12-LO and the second involves the action of two lipoxygenases, 5-LO and 12/15-LO, in leukocytes. Based on previous studies it is most likely that 5-LO and FLAP catalyze the first oxygenation of AA, which is then followed by the second oxygenation by 12/15-LO in the same cell or through transcellular synthesis in a neighboring leukocyte (6–9).
The inactivation of LXA4 bioactivity is proposed to be the further oxidation of the C15 hydroxyl group by a dehydrogenase such as 15-hydroxy prostaglandin dehydrogenase (15-PGDH), yielding an α, β-unsaturated ketone (10). This class of electrophilic metabolites can come from the diet, intermediary metabolism, fatty acid oxidation and in aggregate are conferred with the ability to regulate inflammatory and metabolic homeostasis through the post translational modification of proteins having reactive nucleophilic amino acids, primarily cysteine (11). The electrophilic moiety of 15-oxo-LXA4 can be inactivated by prostaglandin reductase 2 (PTGR2), yielding the non-electrophilic product 13,14-dihydro-15-oxo-LXA4 (Fig. 1) (6,12–14).
Figure 1. Formation and degradation of lipoxin A4.
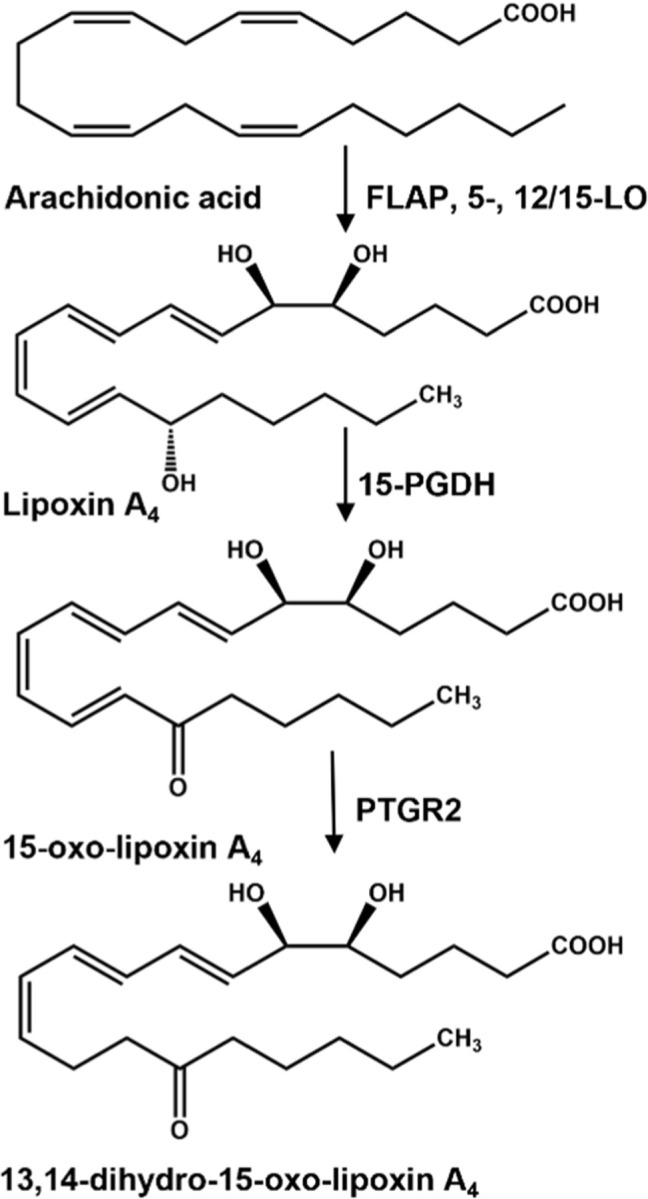
Lipoxin A4 (LXA4) is produced from the oxygenation of free arachidonic acid by a combination of 5-lipoxygenase (5-LO), 5-lipoxygenase activating protein (FLAP) and 12/15-lipoxygenase (12/15-LO). LXA4 can be further oxidized to an alpha, beta-unsaturated carbonyl containing fatty acid, 15-oxo-LXA4, by 15-hydroxyprostaglandin dehydrogenase (15-PGDH).15-oxo-LXA4 is further metabolized by prostaglandin reductase 2 (PTGR2) that reduces the C=C bond at C13-C14 rendering the metabolite, 13, 14-dihydro-15-oxo-LXA4 non-electrophilic.
Experimental support for the anti-inflammatory and tissue repair-related “specialized pro-resolving mediator” (SPM) actions of trihydroxytetraenes stems exclusively from preclinical studies of synthetic LXA4 homologs in biochemical test systems and murine models of inflammatory-related diseases including acute lung injury, asthma, subarachnoid hemorrhage and acute renal failure (8,15–21). Prior to the suggested PTGR2 inactivation of lipoxins, these species are also proposed as specific ligands for the formyl peptide receptor (FPR2), a G-protein coupled receptor (GPCR) that in turn transduces LXA4 inhibition of inflammatory signaling and promotion of tissue repair (8,10,22–25). Multiple investigators have raised concerns about ascribing FPR2 as a transducer of lipoxin signaling, due to contradictory observations coming from a) FPR2 knockdown and FPR2 deficient model systems, b) minimal or no changes in cellular Ca2+ homeostasis and cyclic AMP levels in test systems treated with synthetic lipoxin homologs, c) limited β-arrestin recruitment to membranes of LXA4-activated cells and d) concerns about the stability of LXA4 in commercial products and test systems, all of which are comprehensively reviewed (9,26). Because endogenously-generated lipoxins have not been definitively shown to exert specific signaling responses via FPR2 receptor-dependent mechanisms, nor rise to an in vivo concentration where they would be expected to do so, alternative explanations may account for the anti-inflammatory and adaptive signaling actions of synthetic lipoxin homologs reported for biochemical, cellular and in vivo test systems (6,27).
There are growing numbers of reports contradicting previous data and concepts surrounding the biological generation, endogenous levels, and mechanisms of action of lipoxins and related SPM, initially proposed as endogenous receptor-dependent adaptive signaling mediators. Specifically, multiple labs report undetectable to very low concentrations of SPMs in preclinical and human specimens, even after supplementation with polyunsaturated acids (27–29). In the most rigorous determinations, specimens have been analyzed by mass spectrometry-based methods employing synthetic standards, stable isotope labeled internal standards and the application of internationally-accepted definitions of valid signal to noise responses. These analytical approaches are contrasted by less discriminating approaches to SPM bioanalysis where the identification and quantification of many SPM are based on weak or absent peaks in ion chromatograms and mass spectra that are extracted from sample recordings that are not in concordance with synthetic SPM standard product ion spectra (6,27,30–32).
Multiple reports form the rationale for the present study, including the a) short half-life of most polyunsaturated fatty acid signaling mediators (33–35), b) very low to no detectable tissue, plasma and urine concentrations of trihydroxytetraenes reported in vivo (27,36,37), c) appreciation that diverse oxidoreductases such as 15-PGDH and dehydrogenase reductase 9 (SDR9C4) rapidly oxidize hydroxyl derivatives of unsaturated fatty acids to electrophilic α,β-unsaturated ketones (10,38), and d) knowledge that α,β-unsaturated ketone-containing fatty acid derivatives, such as the LXA4 metabolite 5,6-dihydroxy-15-oxo-7,9,11,13-eicosatetraenoic acid (15-oxo-LXA4), are Michael acceptors and can react with redox-sensing nucleophilic centers of small molecules and proteins to regulate a broad array of electrophile-sensitive transcriptional regulatory and catalytic protein activities, thus impacting downstream signaling (11,39–41). This led to the hypothesis that trihydroxylated unsaturated fatty acids such as LXA4 and other related SPMs, if formed in a sufficient concentration in vivo or as synthetic pharmacological agents, signal via G-protein coupled receptor-independent electrophilic signaling mechanisms. This hypothesis was based on an appreciation that a) there is a broad spectrum of hydroxylated unsaturated fatty acids are generated by metabolism and inflammatory responses (42,43), and b) the cellular oxidation of hydroxy-fatty acids by enzymes such as 15-PGDH, SDR9C4 and many other dehydrogenases, yields electrophilic α,β-unsaturated ketones that display numerous bioactivities in common with SPM (44,45).
Herein we report that RAW 264.7 macrophages are metabolically competent to mediate LXA4 biosynthesis. However, these macrophages did not generate detectable LXA4 or its 15-oxo-LXA4 metabolite, even when supplemented with AA, activated by LPS and analyzed by liquid chromatography high resolution mass spectrometry (LC-HRMS) methods. Treatment of RAW 264.7 macrophages with 15-oxo-LXA4-methyl ester (15-oxo-LXA4-Me), a synthetic membrane-permeable precursor for the electrophilic LXA4 metabolite 15-oxo-LXA4 (46), inhibited NF-ĸB-regulated cytokine expression and promoted nuclear factor (erythroid related factor 2)-like 2 (Nrf2)-dependent adaptive gene expression responses. LXA4 did not impact macrophage Nrf2 target gene expression and NF-ĸB-regulated pro-inflammatory mediator expression. Neither LXA4 nor its 15-oxo-LXA4 metabolite displayed FPR2 ligand activity. It was concluded that multi-target electrophile-mediated signaling may occur through the oxidation of LXA4 to 15-oxo-LXA4, thus accounting for many of the responses to synthetic LXA4 added to ex vivo and in vivo test systems. The present results motivate further investigation as to whether signaling-competent trihydroxytetraenes occur biologically and if so, whether endogenous concentrations of di- and trihydroxy fatty acids or their electrophilic metabolites are present at levels that would transduce signaling responses via electrophilic metabolite-mediated post-translational modification of redox-sensitive target proteins.
Results
RAW 264.7 macrophages express all enzymes needed for the oxidation of AA to LXA4.
The formation of AA hydroperoxide regioisomers needed for the endogenous synthesis of LXA4 are catalyzed by consecutive oxygenation reactions by FLAP and 5-LO, and 12/15-LO (6,12–14). All proteins were detectable in RAW cell lysates over the 24 hr study period (Fig. 2A–C). 15-PGDH, which further oxidizes LXA4 to 15-oxo-LXA4 (44,47), was expressed throughout the 24 hr (Fig. 2D). LPS treatment of RAW macrophages showed the protein expression of FLAP, 5-LO, 12/15-LO and 15-PGDH to be modestly down-regulated (Fig. 2A–D). For subsequent studies, an LPS concentration was selected so that there was a maximum of 20% loss of RAW cell viability over 24 hr, an effect that was mitigated by AA supplementation (Supp. Fig. 1).
Figure 2. Protein expression of lipoxin synthesis enzymes.
Raw 264.7 macrophage express (A) 5-lipoxygenase activating protein (FLAP), (B) 5-lipoxygense (5-LO), (C) 12/15-lipoxygenase (12/15-LO), and (D) 15-hydroxyprostaglandin dehydrogenase (15-PGDH) at basal conditions and upon stimulation with LPS and/or arachidonic acid supplementation over a 24 hr time course.
LXA4 production was not detectable in RAW macrophages under basal, AA and LPS-supplemented conditions.
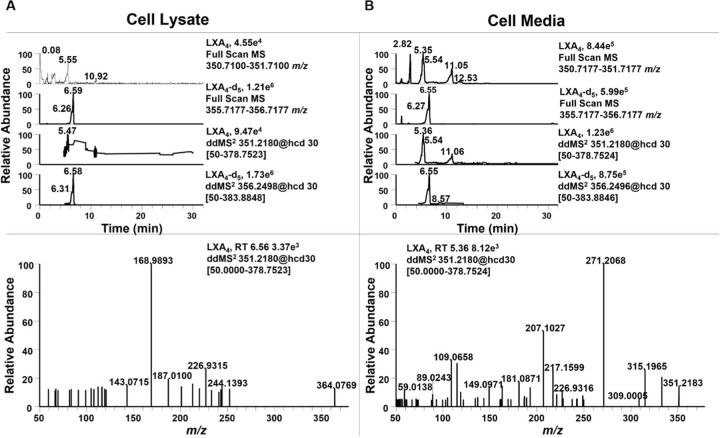
Although all enzymes necessary to produce LXA4 were present, LC-HRMS analysis did not show a peak with the matching retention time, accurate mass and MS2 fragmentation pattern in the cell lysate (Fig. 3A) or cell media (Fig. 3B), even following supplementation with AA and LPS. In the cell supernatant, a peak with the same accurate mass of LXA4 (m/z 351.2179, M-H+) was observed, but at an earlier retention time of 5.36 min, compared to that of the LXA4 standard (Supp. Fig. 2A) and the LXA4-d5 internal standard (m/z 355.2494, M-H+), both at a retention time of 6.55 min (Supp. Fig. 2B). Upon further investigation of the MS2, the isobaric species at 5.36 min was likely a prostaglandin metabolite, as evidenced by the characteristic fragment ion of m/z 271.2068.
Figure 3. Raw 264.7 macrophage do not produce LXA4.
Raw 264.7 macrophage were simultaneously supplemented with 25 µM arachidonic acid and stimulated with 10 ng/mL LPS for 24 hr. (A) Cell lysate and (B) Cell supernatant was extracted using a modified Folch extraction containing the internal standard LXA4-d5 for analysis of oxylipins by LC-HRMS. LXA4 was not observed in cell lysate or supernatant. In cell supernatant an oxylipin with the same accurate m/z of 351.2183, but different retention time (5.36 min vs 6.55 min) was observed. The MS2 for this metabolite contained a diagnostic fragment ion at m/z 271.2068 indicating it was a prostaglandin derivative.
RAW 264.7 macrophages oxidize exogenously-added LXA4 to the electrophilic metabolite 15-oxo-LXA4.
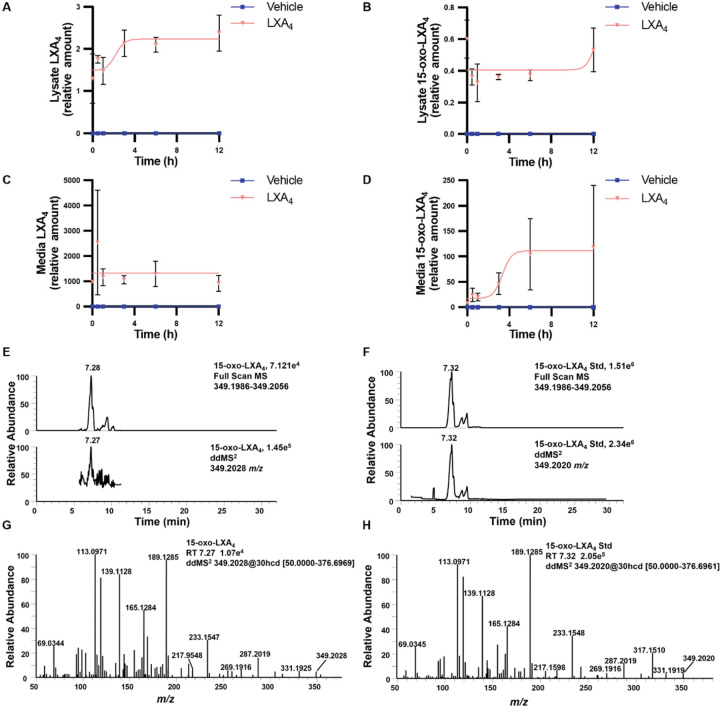
RAW macrophages were treated with 25 µM LXA4 over a 12 hr period to investigate the formation of 15-oxo-LXA4. LXA4 was readily detected in the cell lysate (Fig. 4A), as was 15-oxo-LXA4 (Fig 4B). Cell lysate levels of LXA4 increased early on and remained constant between 3 and 12 hr whereas intracellular levels of 15-oxo-LXA4 remained constant. Inversely, LXA4 levels remained constant in the media (Fig. 4C) and 15-oxo-LXA4 levels increased in the media over the first 6 hr and remained constant between 6 and 12 hr (Fig. 4D). 15-oxo-LXA4 eluted at a retention time of 7.28 min with a m/z of 349.2028 in both full scan and ddMS2 mode (Fig. 4E). To confirm the identity of 15-oxo-LXA4, cells were treated with 15-oxo-LXA4-Me that is rapidly de-esterified by methyl transferases (46). A peak having a retention time 7.32 min was detected for the de-esterified 15-oxo-LXA4-Me product, with a parent m/z of 349.2020 for the M-H+ ion (Fig. 4F). The product ion spectra for 15-oxo-LXA4 and the de-esterified 15-oxo-LXA4-Me standard showed matching diagnostic ions at m/z 331.1919, 287.2019, 233.1548, 189.1285, 165.1284, 139.1128, 113.0971, and 69.0345 (Fig. 4G and 4H).
Figure 4. Raw 264.7 macrophage metabolize exogenous LXA4 to 15-oxo-LXA4.
Raw 264.7 macrophage were treated with 25 µM LXA4 over a 12 hr time course and (A) LXA4 and (B) 15-oxo-LXA4 were measured in cell lysate and (C) LXA4 and (D) 15-oxo-LXA4 were measured in cell media. (E) The endogenously formed 15-oxo-LXA4 was retained at 7.28 min and was detected in both full scan and ddMS2 with a m/z of 349.2028. (F) 15-oxo-LXA4-Me was added to Raw 264.7 cells for 1 hr to create an ionizable standard for comparison of retention time and product ion MS2 and displayed matching m/z at 349.2020 at a retention time of 7.32 min. The product ion spectra of the (G) the endogenous 15-oxo-LXA4 analyte and (H) the 15-oxo-LXA4 standard have corresponding diagnostic fragment ions at m/z 331.1925, 233.1547, 189.1285, 165.1284, 139.1128, and 113.0971.
Signaling actions of LXA4 and 15-oxo-LXA4-Me are FPR2-independent.
The FPR2 receptor is ligand-activated by the formylated peptide fMet-Leu-Phe and related fMet peptides, annexin-1 and amyloid-α and-β. FPR2 is the proposed receptor for LXA4 and other oxygenated unsaturated fatty acids (24,38). Herein, the FPR2 receptor was expressed by RAW macrophages over the course of the study period, with cell protein expression levels not affected by AA supplementation and LPS treatment (Fig. 5A). A sensitive in vitro GTPγS binding assay was performed to investigate the ligand-induced activation of FPR2 (48,49). The assay measures the binding of 35S-GTPγS to Gi, the cognate signaling partner of FPR2, as an indicator of FPR2 activation. The FPR2 peptide agonist, WKYMVm (50,51) was used as a positive control, with FPR2 activation inducing significant 35S-GTPγS binding to Gi. In contrast, LXA4 and its metabolite 15-oxo-LXA4 did not display any FPR2 receptor agonism at any concentration tested (Fig. 5B).
Figure 5. LXA4 and 15-oxo-LXA4-Me do not activate FPR2.
(A) FPR2 expression was measured by western blot in Raw 264.7 macrophage. (B) Increasing concentrations of LXA4, 15-oxo-LXA4 and the FPR2 ligand WKYMVm were incubated with S-GTPγ and binding was determined. (C) Chromatographic overlay of glutathionylated 15-oxo-LXA4 standard and 15-oxo-LXA4 glutathione adduct in Raw cell media at 12 hr after treatment with 15-oxo-LXA4-Me.
To define whether 15-oxo-LXA4 is electrophilic and might signal via FPR2-independent mechanisms, 100 µM glutathione and 10 µM 15-oxo-LXA4-Me were incubated in 50 mM potassium phosphate buffer (pH = 8) for 1 hr at 37 C. Glutathionylated 15-oxo-LXA4 was monitored by LC-MS/MS in positive ion mode using selected reaction monitoring at the m/z transition 658 →308. The GSH adduct was found in cell supernatant of RAW cells treated with 25 µM 15-oxo-LXA4-Me. A representative chromatographic overlay is shown in Fig. 5C.
15-oxo-LXA4-Me inhibits pro-inflammatory signaling.
RAW macrophages were activated with LPS and the activation of NF-ĸB target gene expression was reflected by the increased expression of interleukin (IL)-1β, IL-6, monocyte chemoattractant protein 1 (MCP-1), and inducible nitric oxide synthase (iNOS, Nos2), compared to vehicle controls (Fig. 6A–D). There was upregulation of IL-6 and MCP-1 protein expression measured by ELISA (Fig. 6E and F) and iNOS by western blot analysis (Fig. 6G). The effects of 15-oxo-LXA4-Me on LPS-induced gene expression of pro-inflammatory mediators was variable and primarily occurred at the 12 and 18 hr timepoints. LPS-induced IL-1β mRNA expression was downregulated by 15-oxo-LXA4-Me at all measured times, becoming significant at 12–18 hr (Fig. 6A). IL-6 mRNA expression and cytokine production was inhibited at most time points, with statistically significant decreases at 18–24 hr after LPS plus 15-oxo-LXA4-Me treatment (Fig. 6B and 6E). Ccl2 (MCP-1) mRNA expression and cytokine production were significantly inhibited by 15-oxo-LXA4-Me 12 hr post LPS administration (Fig. 6C and F). 15-oxo-LXA4-Me also significantly inhibited LPS-stimulated Nos2 mRNA expression between 12 and 24 hr (Fig. 6D). Compared with vehicle-treated cells, the maximum loss of viability due to LPS treatment was ~20%, an effect that was mitigated by 15-oxo-LXA4-Me over the 24 hr period. 15-oxo-LXA4-Me supplementation alone did not significantly impact cell viability (Supp. Fig. 1). The signaling actions of LXA4 were compared with 15-oxo-LXA4-Me in LPS treated macrophage at the 12 hr time point. For the cytokines IL-1β and MCP-1 (Fig. 6H and I) and iNOS protein expression (Fig. 6J) LXA4 did not significantly impact the expression of pro-inflammatory signaling mediators when compared to cells treated with 15-oxo-LXA4-Me.
Figure 6. 15-oxo-LXA4-Me inhibits pro-inflammatory pathways.
Treatment of Raw 264.7 macrophage with 15-oxo-LXA4-Me inhibits LPS-mediated upregulation of pro-inflammatory signaling across a 24 hr period. (A) Il1b (B) Il6 (C) Ccl2 (D) Nos2 gene expression, (E) IL-6 protein (F) MCP-1 protein and (G) iNOS protein expression. At 12 hr 15-oxo-LXA4-Me and LXA4 inhibition of LPS-mediated upregulation of inflammatory pathways was compared for (H) IL-1β protein (I) MCP-1 protein and (J) iNOS protein. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
15-oxo-LXA4-Me induces anti-inflammatory signaling.
The activation of Nrf2-regulated gene and protein expression by 15-oxo-LXA4-Me was reflected by the increased expression of its targets including glutamate-cysteine ligase modifier subunit (GCLM), heme oxygenase 1 (HO-1), and NAD(P)H quinone oxidoreductase 1 (NQO1). 15-oxo-LXA4-Me increased both gene and protein expression of the Nrf2 regulated targets (Fig. 7A–F). Gclm and Ho1 mRNA expression peaked at 6 hr (Fig. 7A and C), while the increase in Nqo1 expression at 6 hr was sustained at 12 hr (Fig. 7E). Western blotting of the Nrf2 target proteins further validated the activation of this electrophile-sensitive transcriptional regulatory mechanism by 15-oxo-LXA4-Me at 6 and 12 hr for GCLM and HO-1 (Fig. 7B and D), and at 12 hr for NQO1 (Fig. 7F). In contrast to 15-oxo-LXA4-Me, LXA4 had no significant impact on Nrf2-regulated adaptive signaling responses, both with and without RAW 264.7 cell activation by LPS (Fig. 7G–I).
Figure 7. 15-oxo-LXA4-Me activates the antioxidant response.
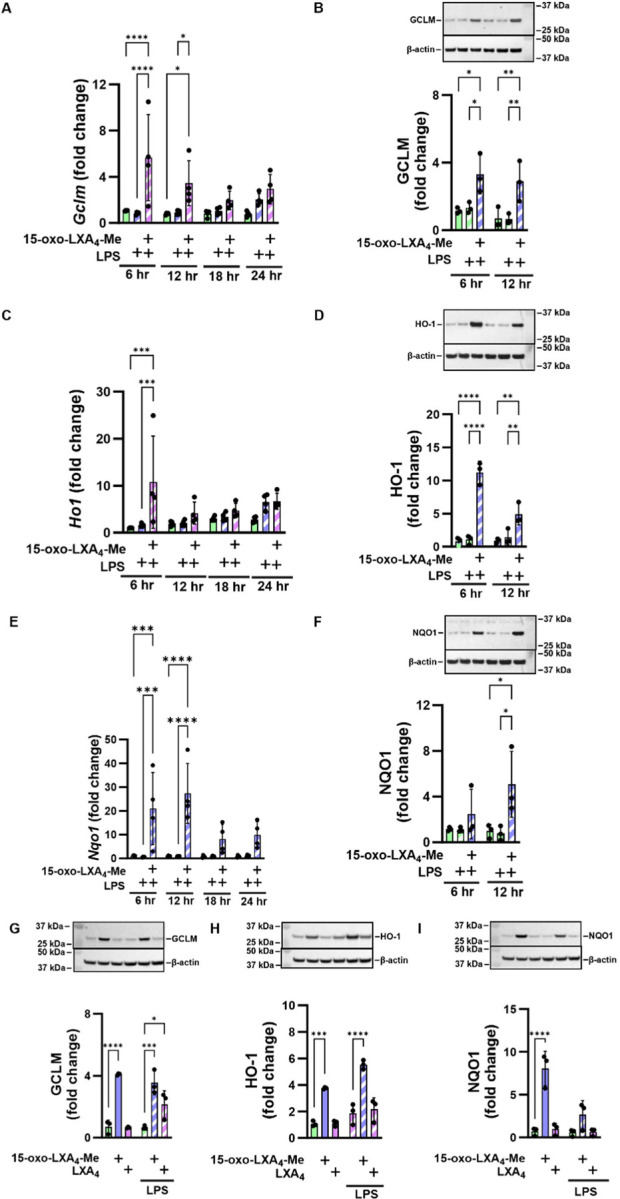
Treatment of Raw 264.7 macrophage with 15-oxo-LXA4-Me results in activation of the antioxidant response after LPS treatment across a 24 hr period. (A) Gclm (B) GCLM protein (C) Ho1 (D) HO-1 protein (E) Nqo1 (F) NQO1 protein. At 12 hr 15-oxo-LXA4-Me and LXA4 activation of the antioxidant response after LPS stimulation was compared for (G) GCLM protein (H) HO-1 protein and (I) NQO1 protein. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Discussion
The formation of di- and tri-hydroxytetraene fatty acids in leukocytes has been studied by various groups in biochemical systems, cell culture and in vivo systems since the 1980s (22,23,52). Although many cells express the enzymes required to synthesize lipoxins, the formation of these trihydroxy species are at very low or undetectable endogenous concentrations and that these concentrations are not definitively linked with the resolution of inflammation (6,53–57). Notably, the initial reports of cellular lipoxin A4 generation required neutrophil priming with the 15-lipoxygenase product 15-hydroperoxy-5,8,11,13-eicosatetraenoic acid (15-HPETE) to accomplish lipoxin biosynthesis (22,58).
Herein, western blot analysis affirmed the expression of 5-, 12/15-LO, and FLAP protein that would be required for lipoxin biosynthesis in RAW 264.7 macrophages (Fig. 2); however, LXA4 generation was undetectable, even with cell activation with LPS and supplementation of AA (Fig. 3). Only upon supplementation of macrophages with synthetic LXA4 was the 15-PGDH metabolite, 15-oxo-LXA4, detected both intra- and extracellularly (Fig. 4). Issues regarding endogenous LXA4 concentrations have gained attention recently, as some investigators are proposing to measure SPM levels to predict clinical and therapeutic outcomes (59–61). Multiple labs, including the present report focusing on 15-oxo-LXA4 as an electrophilic signaling mediator, are unable to replicate the biological detection of sufficient concentrations of trihydroxytetraene and other SPM to concentrations that might induce downstream signaling responses in vitro and in vivo (9,27,36,37). This dilemma is magnified by a) the failure of the primary labs reporting biological SPM generation by LC-MS/MS to apply standard limit-of-detection or limit-of-quantitation criteria to SPM analyses and b) the use of misleading representative LC-MS chromatograms of SPM generation that are not derived from directly-related primary data (37). This has resulted in the triumph of hope over reality in many SPM-related studies, where a flawed characterization of background noise was the criterion for the presence and concentrations of SPM in biological samples. This conundrum motivated the reassessment of past evidence for the presence of SPM in biological matrices and the inferred roles of the SPM LXA4 in the resolution of inflammation.
Herein, FPR2 receptor-independent reactions of 15-oxo-LXA4-Me are evidenced by the engagement of critical constituents of the redox-sensitive cysteine proteome (NF-ĸB and Keap1/Nrf2) and a lack of FPR2 receptor agonism by both LXA4 and 15-oxo-LXA4 (Figs. 5–7). Current dogma holds that trihydroxytetraene oxidation to the 15-oxo metabolite by 15-PGDH inactivates FPR2 ligand activity and downstream signaling; however, this is not the case. Both LXA4 and 15-oxo-LXA4-Me displayed a complete lack of ligand-induced activation of FPR2, as measured by GTPγS binding analysis. This contrasted with the robust activation of FPR2-GTPγS binding induced by WKYMVm, a synthetic peptide mimic of N-formylated bacterial peptides, (Fig. 5B). This does not exclude the possibility that both lipids may act on FPR2 to induce the activation of other FPR2 signaling partners such as β-arrestins, again if produced in sufficient concentration (4,10,38,62,63). The pharmacokinetics (PK) of 15-oxo-LXA4, if present in sufficient concentrations, will distinctly differ from, and might offer advantages over its precursor, LXA4. Following oxidation of LXA4 to 15-oxo-LXA4, there will be an equilibrium between free and nucleophile-adducted 15-oxo-LXA4, as for other small molecule electrophiles. Over time, if LXA4 and 15-oxo-LXA4 generation is sufficient and continues, 15-oxo-LXA4-target protein adducts can accumulate to induce downstream signaling responses from even low rates of LXA4 generation (64). Previous PK interpretations of LXA4 signaling relies on the weak GPCR ligand activity of LXA4, where Michaelis-Menten kinetics will dictate cellular responses. This characteristic could explain how the administration of supra-physiological concentrations of synthetic LXA4, other SPMs or their prodrugs might contribute to the propagation of adaptive anti-inflammatory signaling in vivo.
Diverse electrophilic unsaturated fatty acids are generated by both enzymatic catalysis and as products of the free radical and oxidizing species that are produced during digestion, basal metabolism and inflammatory responses (43,65). These fatty acid species most typically have electron-withdrawing keto, nitro or halogen substituents on or in conjugation with an alkene (42,66,67). At biological concentrations fatty acid electrophiles form Michael addition adducts, primarily with cysteine and to a lesser extent histidine containing peptides and proteins (65,68–70). This cysteinome includes a) cysteines involved in maintaining the 3-dimensional structure of proteins, b) metabolic and transport enzymes, c) signaling proteins such as phosphatases and kinases, d) proteins that regulate cell growth and differentiation and e) transcriptional regulatory factors that modulate the expression of ~1% of the human genome (71–79). RNAseq, in concert with functional enrichment analysis affirms that small molecule electrophiles, such as those derived from fatty acids, can mediate the transcriptional regulation of ~100–250 genes related to metabolism, transport, cell cycle, genome stability, mitochondrion and endoplasmic reticulum organization, proteostasis and inflammation (76,77,79–81). These evolutionarily-conserved transcriptional regulatory mechanisms, that rely on the reactions of a limited number of hyperreactive and functionally-significant nucleophilic amino acids, provides organisms with a capability to respond to increased concentrations of oxidizing and electrophilic species generated during metabolism and inflammation (41,82). In addition to transcriptional regulatory proteins, other proteins that participate in mediating inflammatory and tissue repair responses are also targets of unsaturated fatty acid-derived electrophiles. This includes the nuclear lipid peroxisome proliferator-activated receptor γ (PPARγ), NADPH oxidase-2, xanthine oxidoreductase, cyclooxygenase-2, soluble epoxide hydrolase, diverse protein kinases and phosphatases, matrix metalloproteinase-7 and −9, stimulator of interferon gamma (STING) and calcineurin (83–90).
The generation and metabolism of hydroxyeicosatetraenoic acids (HETEs) is a well-established example of oxidation-induced functional switching of a hydroxy-fatty acid derivative to an α, β-unsaturated ketone product. Following peroxidase reduction of primary unsaturated fatty acid dioxygenation products to hydroxyl moieties (e.g., 5-, 11-, 12- or 15-HETE), 15-PGDH and other cellular dehydrogenases having broad substrate specificities generate α, β-unsaturated ketone products from HETEs. For example, the oxidation of 11-HETE and 15-HETE by 15-PGDH yields 11-oxo-ETE and 15-oxo-ETE, respectively (91–93). Both electrophilic metabolites promote adaptive anti-inflammatory responses by inhibiting NF-ĸB signaling and pro-inflammatory cytokine expression in both murine and human macrophages (91,93). Electrophilic metabolites of hydroxyl moieties in 20 carbon trihydroxypentadienes and 22 carbon trihydroxyhexadienes have been reported both in vitro and in vivo. For example, 8-oxo-resolvin D1 and 17-oxo-resolvin D1 are formed from resolvin D1 and 18-oxo-resolvin E1 from resolvin E1 (94,95). These metabolites, originally viewed to have lost ligand activity for their cognate GPR32, GPR18 and/or ChemR23 receptors, have yet to be characterized in the context of likely downstream signaling actions expected for the corresponding electrophilic α, β-unsaturated ketone-containing metabolites, an exercise motivated by data presented herein (19,94).
In summary, the electrophilic metabolite of LXA4, 15-oxo-LXA4, activated Nrf2-regulated antioxidant and tissue repair responses and inhibited LPS-induced, NF-ĸB-regulated pro-inflammatory cytokine expression in RAW 264.7 macrophages. Many of the SPM properties attributed to LXA4 and other mono-, di- and trihydroxy fatty acids overlap with those reported for electrophilic metabolites. These results indicate that the LXA4 metabolite 15-oxo-LXA4 and other more abundant electrophilic omega-3 fatty acid mono-hydroxy derivatives can exert electrophile-responsive, FPR2 receptor-independent effects after further oxidation. For this property to be of biological significance however, LXA4 must be generated at sufficient concentrations. The extensive literature describing the multi-target signaling actions of lipid electrophiles indicates that many, if not all of the products of non-enzymatic and enzymatically-catalyzed fatty acid oxidation reactions will induce antioxidant and anti-inflammatory responses upon alkylation of critical nucleophilic amino acids of enzymes and transcriptional regulatory proteins (43,71,72). The present data motivates that the concentrations, metabolism, gene expression responses and the temporal differentiation of GPCR-dependent and GPCR-independent responses to unsaturated fatty acid hydroxyl and oxo fatty acid metabolite levels be examined in more detail.
Experimental procedures
Cell cultivation and treatment.
RAW 264.7 (ATCC: TIB-71) murine macrophages were grown in DMEM (Corning: 10-013-CV) with 10% FBS (Gibco: 26140-079) and 1% Penicillin/Streptomycin (P/S, Gibco: 15140-122), and used for all studies. RAW cells were plated at 2x106 cells/well in 6 well plates and cultured for 12 hr in DMEM+10% FBS, next media were replaced with DMEM+1% FBS+1% P/S. Cells were treated with LPS (10 ng/mL, Sigma: L4391, Lot: 067M4036V), AA (25 µM, Cayman: 90010), LXA4 (25 µM, Cayman: 90410), or 15-oxo-LXA4-Me (25 µM) which was synthesized as previously reported (46). Cytotoxicity was determined by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (ThermoFisher: M6494) reduction analysis according to manufacturer’s instructions. Cell lysate and media were collected for endpoint analyses including ELISA, western blot, PCR and liquid chromatography high resolution mass spectrometry (LC-HRMS).
Western blot.
For western blot analysis, cells were scraped into ice cold RIPA buffer (Cell Signaling: 9806) with protease (Roche: 04693132001) and phosphatase (Roche: 4906845001) inhibitors. Samples were further lysed via sonication (10 sec on 5 sec off, repeat 3x, ThermoFisher: FB120, 4°C) and protein was clarified by centrifugation at 21,000 x g for 5 min at 4°C. Protein concentrations were measured using the BCA protein assay (ThermoFisher: 23225) according to manufacturer’s instructions, samples were diluted to 2 mg/mL, and mixed with NuPage sample buffer (ThermoFisher: NP0007) and reducing agent (ThermoFisher: NP0009). Samples were heated at 100°C for 10 min, 20 μL of each sample was loaded onto a polyacrylamide gel (ThermoFisher 4–12%, WG1401BOX and WG1402BOX), 5 μL of dual color standard (Bio-Rad: 1610374) was loaded for molecular weight estimation, and electrophoresis was performed for 1–2 hr at 130 V. Protein was transferred to nitrocellulose membrane (Bio-Rad: 1620115) at 100 V for 1 hr at 4°C. Membranes were washed with TBS-T buffer and blocked with either 5% milk diluted in TBS-T or 1% casein in TBS (ThermoFisher: 37532) for 1 hr at room temperature. Membranes were washed in TBS-T and primary antibodies COX-2 (Cell Signaling: 12282T), FLAP (Invitrogen: PA5-78368), FPR2 (Invitrogen: 720293), GCLM (Invitrogen: PA5-26111), HO-1 (Enzo Life Sciences: ADI-SPA-895-F), iNOS (Cell Signaling: 13120), NQO1 (Abcam: ab80588), 5-LO (Cell signaling: 3289S), 12/15-LO (Invitrogen: MA5-25891), 15-PGDH (Santa Cruz: SC-271418) were added overnight at 4°C. The following day, primary antibodies were washed with TBS-T, and HRP-linked anti-rabbit (Cell Signaling: 7074) or anti-mouse (Cell Signaling: 7076) secondary antibodies were added. Images were taken using ECL substrates (Bio-Rad: 1705061) and a Bio-Rad ChemiDoc imager. Optical density was evaluated in Image Lab software (Bio-Rad). Optical density of β-actin (Sigma: A4700) was used as endogenous control. Representative images of all presented western blots presented for each figure.
RT-PCR.
For PCR analysis, cells were scraped into TRIzol (Invitrogen: 15596026). RNA was isolated, concentration measured, and cDNA prepared as described previously (96). FAM-dyed primers (all purchased from Taqman) were used: Gclm (Mm01324400_m1), Ho1 (Mm00516005_m1), Il1b (Mm00434228_m1), Il6 (Mm99999064_m1), Ccl2 (Mm00441242_m1), Nos2 (Mm00440502_m1), Nqo1 (Mm01253561_m1), and VIC-dyed Actin (Mm00607939_s1) was used as endogenous control.
ELISA.
Media were centrifuged at 500 x g for 5 min at 4ºC and diluted so that absorbance results were in the range of the standard curve. Specific protocols for each cytokine came from the Invitrogen kits: IL1-β (88-7013-88), IL-6 (88-7064-88), and MCP-1 (Ccl2, 88-7391-88).
LC-MS Sample Preparation.
Media were collected and cells washed 3x with PBS, cells were scraped into PBS and immediately frozen at −80ºC. LXA4-d5 (10 µL of 1 µg/mL stock, manufacturer: cat. #10007737) was added to 1 mL of cell lysate or media. To each sample, 4 mL of chloroform:methanol (2:1) was added, vortexed and centrifuged at 2500 rpm for 10 min at 4°C. The organic layer was dried under nitrogen and solvated in 100 µL methanol on the day of analysis.
LC-HRMS Samples were analyzed on a Thermo Fisher Exploris 240 hybrid mass spectrometer coupled to an Vanquish Horizon UHPLC. Samples were applied to a Luna C8 column (2 x100 mm, Phenomenex, cat. 00D-4248-B0) and eluted with a linear gradient using H2O with 0.1% acetic acid as solvent A and acetonitrile with 0.1% acetic acid as solvent B. Samples were loaded at 35% B and the gradient increased to 60% B over 30 min, held at 100% B for 2 min, and equilibrated at 35% B for 3 min at a flow rate of 0.3 mL/min. LXA4 and LXA4 metabolites were measured using negative electrospray ionization under the following MS conditions: source, 2600 V; sheath gas 50, auxiliary gas 10, sweep gas 1, ion transfer temperature 325 °C, and vaporizer temperature 350 °C. Relative levels of LXA4 and 15-oxo-LXA4 were quantified by normalizing to LXA4-d5.
15-oxo-LXA4
GSH adduct standard and LC-MS Analysis A glutathionylated 15-oxo-LXA4 standard was made by incubation of 10 µM 15-oxo-LXA4-Me with 100 µM GSH in 50 mM potassium phosphate buffer (pH = 8) for 1 hr at 37°C (91,97). GSH conjugates were extracted from 1 mL of cell supernatant using Oasis HLB 1 cc solid phase extraction columns (Waters) and applied to a Luna C18 column (2 x 100 mm, Phenomenex) at a flow rate of 0.25 mL/min and eluted with a linear consisting of solvent A (H2O + 0.1% acetic acid) and Solvent B (ACN + 0.1% acetic acid). The gradient started at 20% B at 5 min and increasing to 98% B at 25 min. The gradient was held at 100% B for 2 min and equilibrated at 20% B for 35 min. GSH adducts were analyzed on a 6500+ QTRAP coupled to an Exion LC (Sciex) using multiple reaction monitoring (658→308) and positive ionization with the following MS conditions: CUR 40, CAD med, IS 4500, GS1 70, GS2 65, Temp 550°C, DP 80, EP 7, CE 17, CXP 7.
GTPγS binding assay.
The GTPγS binding assay was conducted using cell membranes overexpressing FPR2 and purified Gi heterotrimer, following our previously reported method (49). Briefly, Sf9 insect cells (ExpressionSystems) were infected with baculovirus expressing FPR2. The cells were collected by centrifugation at 8000 x g for 10 min after 48 hr. For 35S-GTPγS binding analysis, ~200 µg/mL of human FPR2 cell membrane was incubated with 200 nM purified Gi protein for 20 min on ice in buffer containing 20 mM HEPES, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 3 μg/mL BSA, 0.1 μM TCEP, and 5 μM GDP. Next, 25 μL FPR2-Gi mix was transferred to 225 μL reaction buffer containing 20 mM HEPES, pH 7.5, 150 mM NaCl, 5mM MgCl2, 3 μg/mL BSA, 0.1 μM TCEP, 1 μM GDP, 35 pM 35S-GTPγS (Perkin Elmer) and ligands (LXA4 and 15-oxo-LXA4-Me at 2 μM, WKYMVm (Tocris) at 5 μM). After additional 15 min incubation at 25 °C, the reaction was terminated by adding 6 mL of cold wash buffer containing 20 mM HEPES, pH 7.5, 150 mM NaCl and 5mM MgCl2, and filtering through glass fiber prefilters (Millipore Sigma,). After washing 4 times with 6 mL cold wash buffer, the filters were incubated with 5 mL of CytoScint liquid scintillation cocktail (MP Biomedicals) and counted on a Beckman LS6500 scintillation counter.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 9 (GraphPad Software). Bar graph data represent mean values of all results +/- SD, with each individual result represented by black dot. All data presented in the bar graphs were tested via 2-way analysis of variance. Symbols in XY graphs represent mean values of all results +/- SD. Data were tested via nonlinear regression (curve fit), represented by connecting curve. Data represents 3–4 independent experiments. Non-statistically significant results are denoted as ns, meaning p > 0.05, all statistically significant results are denoted by asterisks, with * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001.
Supplementary Material
Acknowledgements
This work was supported by NIH T32GM008424 and F31HL142171 (GJB), R35GM128641 (CZ), R01 HL132550 and P01 HL103455 (BAF), S10OD032141 (SLG), and R33HL157069 (BAF, SLG). We would like to acknowledge Larry Marnett and Valerie O’Donnell for their helpful discussions.
Footnotes
Additional Information
BAF acknowledges an interest in Creegh Pharmaceuticals.
References
- 1.Morales F. S., Koralnik I. J., Gautam S., Samaan S., and Sloane J. A. (2019) Risk factors for lymphopenia in patients with relapsing-remitting multiple sclerosis treated with dimethyl fumarate. Journal of neurology [DOI] [PubMed] [Google Scholar]
- 2.Jaiswal A. K., Sandey M., Suryawanshi A., Cattley R. C., and Mishra A. (2019) Dimethyl fumarate abrogates dust mite-induced allergic asthma by altering dendritic cell function. Immunity, inflammation and disease 7, 201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotas M. E., and Medzhitov R. (2015) Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan C. N. (2017) Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol Aspects Med 58, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan C. N. (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schebb N. H., Kuhn H., Kahnt A. S., Rund K. M., O'Donnell V. B., Flamand N., Peters-Golden M., Jakobsson P. J., Weylandt K. H., Rohwer N., Murphy R. C., Geisslinger G., FitzGerald G. A., Hanson J., Dahlgren C., Alnouri M. W., Offermanns S., and Steinhilber D. (2022) Formation, Signaling and Occurrence of Specialized Pro-Resolving Lipid Mediators-What is the Evidence so far? Front Pharmacol 13, 838782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machado F. S., Johndrow J. E., Esper L., Dias A., Bafica A., Serhan C. N., and Aliberti J. (2006) Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat Med 12, 330–334 [DOI] [PubMed] [Google Scholar]
- 8.Sham H. P., Walker K. H., Abdulnour R. E., Krishnamoorthy N., Douda D. N., Norris P. C., Barkas I., Benito-Figueroa S., Colby J. K., Serhan C. N., and Levy B. D. (2018) 15-epi-Lipoxin A4, Resolvin D2, and Resolvin D3 Induce NF-kappaB Regulators in Bacterial Pneumonia. J Immunol 200, 2757–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahnt A. S., Schebb N. H., and Steinhilber D. (2023) Formation of lipoxins and resolvins in human leukocytes. Prostaglandins Other Lipid Mediat 166, 106726. [DOI] [PubMed] [Google Scholar]
- 10.Clish C. B., Levy B. D., Chiang N., Tai H. H., and Serhan C. N. (2000) Oxidoreductases in lipoxin A4 metabolic inactivation: a novel role for 15-onoprostaglandin 13-reductase/leukotriene B4 12-hydroxydehydrogenase in inflammation. The Journal of biological chemistry 275, 25372–25380 [DOI] [PubMed] [Google Scholar]
- 11.Higdon A. N., Landar A., Barnes S., and Darley-Usmar V. M. (2012) The electrophile responsive proteome: integrating proteomics and lipidomics with cellular function. Antioxidants & redox signaling 17, 1580–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claria J., Lee M. H., and Serhan C. N. (1996) Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Mol Med 2, 583–596 [PMC free article] [PubMed] [Google Scholar]
- 13.Serhan C. N., Hamberg M., and Samuelsson B. (1984) Trihydroxytetraenes: a novel series of compounds formed from arachidonic acid in human leukocytes. Biochem Biophys Res Commun 118, 943–949 [DOI] [PubMed] [Google Scholar]
- 14.Peters-Golden M., and Brock T. G. (2003) 5-lipoxygenase and FLAP. Prostaglandins Leukot Essent Fatty Acids 69, 99–109 [DOI] [PubMed] [Google Scholar]
- 15.Fu C. Y., Chen J., Lu X. Y., Zheng M. Z., Wang L. L., Shen Y. L., and Chen Y. Y. (2019) Dimethyl fumarate attenuates lipopolysaccharide-induced mitochondrial injury by activating Nrf2 pathway in cardiomyocytes. Life sciences 235, 116863. [DOI] [PubMed] [Google Scholar]
- 16.Serhan C. N., Chiang N., Dalli J., and Levy B. D. (2014) Lipid mediators in the resolution of inflammation. Cold Spring Harb Perspect Biol 7, a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy B. D., Lukacs N. W., Berlin A. A., Schmidt B., Guilford W. J., Serhan C. N., and Parkinson J. F. (2007) Lipoxin A4 stable analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism. FASEB J 21, 3877–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieira A. M., Neto E. H., Figueiredo C. C., Barja Fidalgo C., Fierro I. M., and Morandi V. (2014) ATL-1, a synthetic analog of lipoxin, modulates endothelial permeability and interaction with tumor cells through a VEGF-dependent mechanism. Biochem Pharmacol 90, 388–396 [DOI] [PubMed] [Google Scholar]
- 19.Kieran N. E., Doran P. P., Connolly S. B., Greenan M. C., Higgins D. F., Leonard M., Godson C., Taylor C. T., Henger A., Kretzler M., Burne M. J., Rabb H., and Brady H. R. (2003) Modification of the transcriptomic response to renal ischemia/reperfusion injury by lipoxin analog. Kidney Int 64, 480–492 [DOI] [PubMed] [Google Scholar]
- 20.Sasaki A., Koike N., Murakami T., and Suzuki K. (2019) Dimethyl fumarate ameliorates cisplatin-induced renal tubulointerstitial lesions. Journal of toxicologic pathology 32, 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L., Zhang P., Zhang Z., Hu Q., He J., Liu H., Zhao J., Liang Y., He Z., Li X., Sun X., and Guo Z. (2019) LXA4 Ameliorates Cerebrovascular Endothelial Dysfunction by Reducing Acute Inflammation after Subarachnoid Hemorrhage in Rats. Neuroscience [DOI] [PubMed] [Google Scholar]
- 22.Serhan C. N., Hamberg M., and Samuelsson B. (1984) Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci U S A 81, 5335–5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serhan C. N., and Samuelsson B. (1988) Lipoxins: a new series of eicosanoids (biosynthesis, stereochemistry, and biological activities). Adv Exp Med Biol 229, 1–14 [DOI] [PubMed] [Google Scholar]
- 24.Chiang N., Takano T., Arita M., Watanabe S., and Serhan C. N. (2003) A novel rat lipoxin A4 receptor that is conserved in structure and function. Br.J.Pharmacol. 139, 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang N., Serhan C. N., Dahlen S. E., Drazen J. M., Hay D. W. P., Rovati G. E., Shimizu T., Yokomizo T., and Brink C. (2006) The Lipoxin Receptor ALX: Potent Ligand-Specific and Stereoselective Actions in Vivo. Pharmacological Reviews 58, 463–487 [DOI] [PubMed] [Google Scholar]
- 26.Forsman H., and Dahlgren C. (2009) Lipoxin A(4) metabolites/analogues from two commercial sources have no effects on TNF-alpha-mediated priming or activation through the neutrophil formyl peptide receptors. Scand J Immunol 70, 396–402 [DOI] [PubMed] [Google Scholar]
- 27.Skarke C., Alamuddin N., Lawson J. A., Ferguson J. F., Reilly M. P., and FitzGerald G. A. (2015) Bioactive products formed in humans from fish oils. Journal of lipid research [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebert R., Cumbana R., Lehmann C., Kutzner L., Toewe A., Ferreiros N., Parnham M. J., Schebb N. H., Steinhilber D., and Kahnt A. S. (2020) Long-term stimulation of toll-like receptor-2 and −4 upregulates 5-LO and 15-LO-2 expression thereby inducing a lipid mediator shift in human monocyte-derived macrophages. Biochim Biophys Acta Mol Cell Biol Lipids 1865, 158702. [DOI] [PubMed] [Google Scholar]
- 29.Calder P. C. (2020) Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: Concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie 178, 105–123 [DOI] [PubMed] [Google Scholar]
- 30.Sala A., Folco G., and Murphy R. C. (2010) Transcellular biosynthesis of eicosanoids. Pharmacol Rep 62, 503–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folco G., and Robert M. (2006) Eicosanoid Transcellular Biosynthesis: From Cell-Cell Interactions to in Vivo Tissue Responses. Pharmacological reviews 58, 375–388 [DOI] [PubMed] [Google Scholar]
- 32.Pirault J., and Back M. (2018) Lipoxin and Resolvin Receptors Transducing the Resolution of Inflammation in Cardiovascular Disease. Front Pharmacol 9, 1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Offman E., Marenco T., Ferber S., Johnson J., Kling D., Curcio D., and Davidson M. (2013) Steady-state bioavailability of prescription omega-3 on a low-fat diet is significantly improved with a free fatty acid formulation compared with an ethyl ester formulation: the ECLIPSE II study. Vasc Health Risk Manag 9, 563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bygdeman M. (2003) Pharmacokinetics of prostaglandins. Best Practice & Research Clinical Obstetrics & Gynaecology 17, 707–716 [DOI] [PubMed] [Google Scholar]
- 35.Chevalier L., Vachon A., and Plourde M. (2021) Pharmacokinetics of Supplemental Omega-3 Fatty Acids Esterified in Monoglycerides, Ethyl Esters, or Triglycerides in Adults in a Randomized Crossover Trial. The Journal of Nutrition 151, 1111–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schebb N. H., Kühn H., Kahnt A. S., Rund K. M., O’Donnell V. B., Flamand N., Peters-Golden M., Jakobsson P.-J., Weylandt K. H., Rohwer N., Murphy R. C., Geisslinger G., FitzGerald G. A., Hanson J., Dahlgren C., Alnouri M. W., Offermanns S., and Steinhilber D. (2022) Formation, Signaling and Occurrence of Specialized Pro-Resolving Lipid Mediators—What is the Evidence so far? Frontiers in Pharmacology 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valerie B O’Donnell, N. H. S., Milne Ginger L, Murphy Michael P, Thomas Christopher P, Steinhilber Dieter, Wendell Stacy G, Kühn Hartmut, Jakobsson Per-Johan, Blair Ian, Murphy Robert C, Freeman Bruce A Brash Alan R, FitzGerald Garret A. (2021) Failure to apply standard limit-of-detection or limit-of-quantitation criteria to specialized pro-resolving mediator analysis incorrectly characterizes their presence in biological samples. 10.5281/zenodo.5766267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiang N., Serhan C. N., Dahlen S. E., Drazen J. M., Hay D. W., Rovati G. E., Shimizu T., Yokomizo T., and Brink C. (2006) The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev 58, 463–487 [DOI] [PubMed] [Google Scholar]
- 39.Levonen A. L., Hill B. G., Kansanen E., Zhang J., and Darley-Usmar V. M. (2014) Redox regulation of antioxidants, autophagy, and the response to stress: implications for electrophile therapeutics. Free Radic Biol Med 71, 196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y. H., Ko T. P., Guo R. T., Hu S. M., Chuang L. M., and Wang A. H. (2008) Structural basis for catalytic and inhibitory mechanisms of human prostaglandin reductase PTGR2. Structure 16, 1714–1723 [DOI] [PubMed] [Google Scholar]
- 41.Liu X., Long M. J. C., and Aye Y. (2019) Proteomics and Beyond: Cell Decision-Making Shaped by Reactive Electrophiles. Trends Biochem Sci 44, 75–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delmastro-Greenwood M., Freeman B. A., and Wendell S. G. (2014) Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids. Annu Rev Physiol 76, 79–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schopfer F. J., Cipollina C., and Freeman B. A. (2012) Formation and signaling actions of electrophilic lipids. Chem Rev 111, 5997–6021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wendell S. G., Golin-Bisello F., Wenzel S., Sobol R. W., Holguin F., and Freeman B. A. (2015) 15-Hydroxyprostaglandin dehydrogenase generation of electrophilic lipid signaling mediators from hydroxy omega-3 fatty acids. The Journal of biological chemistry 290, 5868–5880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belyaeva O. V., Wirth S. E., Boeglin W. E., Karki S., Goggans K. R., Wendell S. G., Popov K. M., Brash A. R., and Kedishvili N. Y. (2022) Dehydrogenase reductase 9 (SDR9C4) and related homologs recognize a broad spectrum of lipid mediator oxylipins as substrates. The Journal of biological chemistry 298, 101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woodcock S. R., Wendell S. G., Schopfer F. J., and Freeman B. A. (2018) Synthesis of an Electrophilic Keto-Tetraene 15-oxo-Lipoxin A4 Methyl Ester via a MIDA Boronate. Tetrahedron letters 59, 3524–3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Backlund M. G., Mann J. R., Holla V. R., Shi Q., Daikoku T., Dey S. K., and DuBois R. N. (2008) Repression of 15-hydroxyprostaglandin dehydrogenase involves histone deacetylase 2 and snail in colorectal cancer. Cancer research 68, 9331–9337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harrison C., and Traynor J. R. (2003) The [35S]GTPgammaS binding assay: approaches and applications in pharmacology. Life sciences 74, 489–508 [DOI] [PubMed] [Google Scholar]
- 49.Zhuang Y., Liu H., Edward Zhou X., Kumar Verma R., de Waal P. W., Jang W., Xu T. H., Wang L., Meng X., Zhao G., Kang Y., Melcher K., Fan H., Lambert N. A., Eric Xu H., and Zhang C. (2020) Structure of formylpeptide receptor 2-G(i) complex reveals insights into ligand recognition and signaling. Nat Commun 11, 885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christophe T., Karlsson A., Dugave C., Rabiet M. J., Boulay F., and Dahlgren C. (2001) The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/lipoxin A4 receptors and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2. The Journal of biological chemistry 276, 21585–21593 [DOI] [PubMed] [Google Scholar]
- 51.Dahlgren C., Christophe T., Boulay F., Madianos P. N., Rabiet M. J., and Karlsson A. (2000) The synthetic chemoattractant Trp-Lys-Tyr-Met-Val-DMet activates neutrophils preferentially through the lipoxin A(4) receptor. Blood 95, 1810–1818 [PubMed] [Google Scholar]
- 52.Lam B. K., Hirai A., Yoshida S., Tamura Y., and Wong P. Y. (1987) Transformation of 15-hydroperoxyeicosapentaenoic acid to lipoxin A5 and B5, mono- and dihydroxyeicosapentaenoic acids by porcine leukocytes. Biochim Biophys Acta 917, 398–405 [DOI] [PubMed] [Google Scholar]
- 53.Virmani J., Johnson E. N., Klein-Szanto A. J., and Funk C. D. (2001) Role of 'platelet-type' 12-lipoxygenase in skin carcinogenesis. Cancer Lett 162, 161–165 [DOI] [PubMed] [Google Scholar]
- 54.Allen-Redpath K., Aldrovandi M., Lauder S. N., Gketsopoulou A., Tyrrell V. J., Slatter D. A., Andrews R., Watkins W. J., Atkinson G., McNeill E., Gilfedder A., Protty M., Burston J., Johnson S. R. C., Rodrigues P. R. S., Jones D. O., Lee R., Handa A., Channon K., Obaji S., Alvarez-Jarreta J., Kronke G., Ackermann J., Jenkins P. V., Collins P. W., and O'Donnell V. B. (2019) Phospholipid membranes drive abdominal aortic aneurysm development through stimulating coagulation factor activity. Proc Natl Acad Sci U S A 116, 8038–8047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J. L., Liang Y. L., and Wang Y. J. (2019) Knockout of ALOX12 protects against spinal cord injury-mediated nerve injury by inhibition of inflammation and apoptosis. Biochem Biophys Res Commun 516, 991–998 [DOI] [PubMed] [Google Scholar]
- 56.Kristjansson R. P., Benonisdottir S., Davidsson O. B., Oddsson A., Tragante V., Sigurdsson J. K., Stefansdottir L., Jonsson S., Jensson B. O., Arthur J. G., Arnadottir G. A., Sulem G., Halldorsson B. V., Gunnarsson B., Halldorsson G. H., Stefansson O. A., Oskarsson G. R., Deaton A. M., Olafsson I., Eyjolfsson G. I., Sigurdardottir O., Onundarson P. T., Gislason D., Gislason T., Ludviksson B. R., Ludviksdottir D., Olafsdottir T. A., Rafnar T., Masson G., Zink F., Bjornsdottir G., Magnusson O. T., Bjornsdottir U. S., Thorleifsson G., Norddahl G. L., Gudbjartsson D. F., Thorsteinsdottir U., Jonsdottir I., Sulem P., and Stefansson K. (2019) A loss-of-function variant in ALOX15 protects against nasal polyps and chronic rhinosinusitis. Nat Genet 51, 267–276 [DOI] [PubMed] [Google Scholar]
- 57.Marbach-Breitruck E., Rohwer N., Infante-Duarte C., Romero-Suarez S., Labuz D., Machelska H., Kutzner L., Schebb N. H., Rothe M., Reddanna P., Weylandt K. H., Wieler L. H., Heydeck D., and Kuhn H. (2021) Knock-In Mice Expressing a 15-Lipoxygenating Alox5 Mutant Respond Differently to Experimental Inflammation Than Reported Alox5(-/-) Mice. Metabolites 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serhan C. N., Hamberg M., and Samuelsson B. (1984) Trihydroxytetraenes: A novel series of compounds formed from arachidonic acid in human leukocytes. Biochemical and Biophysical Research Communications 118, 943–949 [DOI] [PubMed] [Google Scholar]
- 59.Perretti M., and Dalli J. (2023) Resolution Pharmacology: Focus on Pro-Resolving Annexin A1 and Lipid Mediators for Therapeutic Innovation in Inflammation. Annu Rev Pharmacol Toxicol 63, 449–469 [DOI] [PubMed] [Google Scholar]
- 60.Dalli J., Gomez E. A., and Jouvene C. C. (2022) Utility of the Specialized Pro-Resolving Mediators as Diagnostic and Prognostic Biomarkers in Disease. Biomolecules 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dalli J., Colas R. A., Quintana C., Barragan-Bradford D., Hurwitz S., Levy B. D., Choi A. M., Serhan C. N., and Baron R. M. (2017) Human Sepsis Eicosanoid and Proresolving Lipid Mediator Temporal Profiles: Correlations With Survival and Clinical Outcomes. Crit Care Med 45, 58–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serhan C. N., and Savill J. (2005) Resolution of inflammation: the beginning programs the end. Nat Immunol 6, 1191–1197 [DOI] [PubMed] [Google Scholar]
- 63.Schwab J. M., and Serhan C. N. (2006) Lipoxins and new lipid mediators in the resolution of inflammation. Curr Opin Pharmacol 6, 414–420 [DOI] [PubMed] [Google Scholar]
- 64.Oh J. Y., Giles N., Landar A., and Darley-Usmar V. (2008) Accumulation of 15-deoxy-delta(12,14)-prostaglandin J2 adduct formation with Keap1 over time: effects on potency for intracellular antioxidant defence induction. Biochem J 411, 297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khoo N. K. H., and Schopfer F. J. (2019) Nitrated fatty acids: from diet to disease. Curr Opin Physiol 9, 67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hartman C. L., Duerr M. A., Albert C. J., Neumann W. L., McHowat J., and Ford D. A. (2018) 2-Chlorofatty acids induce Weibel-Palade body mobilization. Journal of lipid research 59, 113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shakya S., Pyles K. D., Albert C. J., Patel R. P., McCommis K. S., and Ford D. A. (2023) Myeloperoxidase-derived hypochlorous acid targets human airway epithelial plasmalogens liberating protein modifying electrophilic 2-chlorofatty aldehydes. Redox Biol 59, 102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baker L. M. S., Baker P. R. S., Golin-Bisello F., Schopfer F. J., Fink M., Woodcock S. R., Branchaud B. P., Radi R., and Freeman B. A. (2007) Nitro-fatty Acid Reaction with Glutathione and Cysteine. Journal of Biological Chemistry 282, 31085–31093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turell L., Vitturi D. A., Coitino E. L., Lebrato L., Moller M. N., Sagasti C., Salvatore S. R., Woodcock S. R., Alvarez B., and Schopfer F. J. (2017) The Chemical Basis of Thiol Addition to Nitro-conjugated Linoleic Acid, a Protective Cell-signaling Lipid. The Journal of biological chemistry 292, 1145–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.LoPachin R. M., Gavin T., Petersen D. R., and Barber D. S. (2009) Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic targets and adduct formation. Chem Res Toxicol 22, 1499–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun R., Fu L., Liu K., Tian C., Yang Y., Tallman K. A., Porter N. A., Liebler D. C., and Yang J. (2017) Chemoproteomics Reveals Chemical Diversity and Dynamics of 4-Oxo-2-nonenal Modifications in Cells. Molecular & cellular proteomics : MCP 16, 1789–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang J., Carroll K. S., and Liebler D. C. (2016) The Expanding Landscape of the Thiol Redox Proteome. Molecular & cellular proteomics : MCP 15, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Codreanu S. G., Ullery J. C., Zhu J., Tallman K. A., Beavers W. N., Porter N. A., Marnett L. J., Zhang B., and Liebler D. C. (2014) Alkylation damage by lipid electrophiles targets functional protein systems. Molecular & cellular proteomics : MCP 13, 849–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang B., Shi Z., Duncan D. T., Prodduturi N., Marnett L. J., and Liebler D. C. (2011) Relating protein adduction to gene expression changes: a systems approach. Molecular bioSystems 7, 2118–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wong H. L., and Liebler D. C. (2008) Mitochondrial protein targets of thiol-reactive electrophiles. Chem Res Toxicol 21, 796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu H., Sun J., Liang W., Zhang J., Rom O., Garcia-Barrio M. T., Li S., Villacorta L., Schopfer F. J., Freeman B. A., Chen Y. E., and Fan Y. (2019) Novel gene regulatory networks identified in response to nitro-conjugated linoleic acid in human endothelial cells. Physiological genomics [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li S., Chang Z., Zhu T., Villacorta L., Li Y., Freeman B. A., Chen Y. E., and Zhang J. (2018) Transcriptomic sequencing reveals diverse adaptive gene expression responses of human vascular smooth muscle cells to nitro-conjugated linoleic acid. Physiological genomics 50, 287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kansanen E., Jyrkkanen H. K., Volger O. L., Leinonen H., Kivela A. M., Hakkinen S. K., Woodcock S. R., Schopfer F. J., Horrevoets A. J., Yla-Herttuala S., Freeman B. A., and Levonen A. L. (2009) Nrf2-dependent and -independent responses to nitro-fatty acids in human endothelial cells: identification of heat shock response as the major pathway activated by nitro-oleic acid. The Journal of biological chemistry 284, 33233–33241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fang M. Y., Huang K. H., Tu W. J., Chen Y. T., Pan P. Y., Hsiao W. C., Ke Y. Y., Tsou L. K., and Zhang M. M. (2021) Chemoproteomic profiling reveals cellular targets of nitro-fatty acids. Redox Biol 46, 102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gould Neal S., Evans P., Martínez-Acedo P., Marino Stefano M., Gladyshev Vadim N., Carroll Kate S., and Ischiropoulos H. (2015) Site-Specific Proteomic Mapping Identifies Selectively Modified Regulatory Cysteine Residues in Functionally Distinct Protein Networks. Chemistry & Biology 22, 965–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuljanin M., Mitchell D. C., Schweppe D. K., Gikandi A. S., Nusinow D. P., Bulloch N. J., Vinogradova E. V., Wilson D. L., Kool E. T., Mancias J. D., Cravatt B. F., and Gygi S. P. (2021) Reimagining high-throughput profiling of reactive cysteines for cell-based screening of large electrophile libraries. Nature Biotechnology 39, 630–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Long M. J., and Aye Y. (2016) The Die Is Cast: Precision Electrophilic Modifications Contribute to Cellular Decision Making. Chem Res Toxicol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saitoh C., Kitada C., Uchida W., Chancellor M. B., de Groat W. C., and Yoshimura N. (2007) The differential contractile responses to capsaicin and anandamide in muscle strips isolated from the rat urinary bladder. Eur J Pharmacol 570, 182–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kelley E. E., Batthyany C. I., Hundley N. J., Woodcock S. R., Bonacci G., Del Rio J. M., Schopfer F. J., Lancaster J. R. Jr., Freeman B. A., and Tarpey M. M. (2008) Nitro-oleic acid, a novel and irreversible inhibitor of xanthine oxidoreductase. The Journal of biological chemistry 283, 36176–36184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trostchansky A., Bonilla L., Thomas C. P., O'Donnell V. B., Marnett L. J., Radi R., and Rubbo H. (2011) Nitroarachidonic acid, a novel peroxidase inhibitor of prostaglandin endoperoxide H synthases 1 and 2. The Journal of biological chemistry 286, 12891–12900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gonzalez-Perilli L., Alvarez M. N., Prolo C., Radi R., Rubbo H., and Trostchansky A. (2013) Nitroarachidonic acid prevents NADPH oxidase assembly and superoxide radical production in activated macrophages. Free Radic Biol Med 58, 126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boike L., Henning N. J., and Nomura D. K. (2022) Advances in covalent drug discovery. Nature Reviews Drug Discovery 21, 881–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bago A., Cayuela M. L., Gil A., Calvo E., Vazquez J., Queiro A., Schopfer F. J., Radi R., Serrador J. M., and Iniguez M. A. (2023) Nitro-oleic acid regulates T cell activation through post-translational modification of calcineurin. Proc Natl Acad Sci U S A 120, e2208924120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hansen A. L., Buchan G. J., Ruhl M., Mukai K., Salvatore S. R., Ogawa E., Andersen S. D., Iversen M. B., Thielke A. L., Gunderstofte C., Motwani M., Moller C. T., Jakobsen A. S., Fitzgerald K. A., Roos J., Lin R. T., Maier T. J., Goldbach-Mansky R., Miner C. A., Qian W., Miner J. J., Rigby R. E., Rehwinkel J., Jakobsen M. R., Arai H., Taguchi T., Schopfer F. J., Olagnier D., and Holm C. K. (2018) Nitro-fatty acids are formed in response to virus infection and are potent inhibitors of STING palmitoylation and signaling. Proceedings of the National Academy of Sciences, U.S.A. 115, E7768–E7775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bonacci G., Schopfer F. J., Batthyany C. I., Rudolph T. K., Rudolph V., Khoo N. K., Kelley E. E., and Freeman B. A. (2011) Electrophilic fatty acids regulate matrix metalloproteinase activity and expression. The Journal of biological chemistry 286, 16074–16081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Snyder N. W., Golin-Bisello F., Gao Y., Blair I. A., Freeman B. A., and Wendell S. G. (2015) 15-Oxoeicosatetraenoic acid is a 15-hydroxyprostaglandin dehydrogenase-derived electrophilic mediator of inflammatory signaling pathways. Chemico-biological interactions 234, 144–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Snyder N. W., Revello S. D., Liu X., Zhang S., and Blair I. A. (2013) Cellular uptake and antiproliferative effects of 11-oxo-eicosatetraenoic acid. Journal of lipid research 54, 3070–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei C., Zhu P., Shah S. J., and Blair I. A. (2009) 15-oxo-Eicosatetraenoic acid, a metabolite of macrophage 15-hydroxyprostaglandin dehydrogenase that inhibits endothelial cell proliferation. Mol Pharmacol 76, 516–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arita M., Oh S. F., Chonan T., Hong S., Elangovan S., Sun Y. P., Uddin J., Petasis N. A., and Serhan C. N. (2006) Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. The Journal of biological chemistry 281, 22847–22854 [DOI] [PubMed] [Google Scholar]
- 95.Sun Y. P., Oh S. F., Uddin J., Yang R., Gotlinger K., Campbell E., Colgan S. P., Petasis N. A., and Serhan C. N. (2007) Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. The Journal of biological chemistry 282, 9323–9334 [DOI] [PubMed] [Google Scholar]
- 96.Koudelka A., Cechova V., Rojas M., Mitash N., Bondonese A., St Croix C., Ross M. A., and Freeman B. A. (2022) Fatty acid nitroalkene reversal of established lung fibrosis. Redox Biol 50, 102226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Woodcock C. C., Huang Y., Woodcock S. R., Salvatore S. R., Singh B., Golin-Bisello F., Davidson N. E., Neumann C. A., Freeman B. A., and Wendell S. G. (2018) Nitro-fatty acid inhibition of triple-negative breast cancer cell viability, migration, invasion, and tumor growth. The Journal of biological chemistry 293, 1120–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.