Abstract
Fluorescent biosensors revolutionized biomedical science by enabling the direct measurement of signaling activities in living cells, yet the current technology is limited in resolution and dimensionality. Here, we introduce highly sensitive chemigenetic kinase activity biosensors that combine the genetically encodable self-labeling protein tag HaloTag7 with bright far-red-emitting synthetic fluorophores. This technology enables five-color biosensor multiplexing, 4D activity imaging, and functional super-resolution imaging via stimulated emission depletion (STED) microscopy.
Introduction
Genetically encoded biosensors are powerful tools to investigate the dynamic biochemical processes underlying physiological functions and pathological derailment1. Despite numerous advances, biosensor technology continues to lag in three major areas. First, sensitive and bright signaling biosensors remain limited to the cyan-to-green spectral region2–5, drastically restricting our capability to monitor multiple biochemical and signaling activities simultaneously. Secondly, there is an urgent need for fluorescent biosensors capable of withstanding the photophysical demands of tissue-scale 4D activity imaging to track biochemical processes in real time within physiologically relevant contexts. Finally, new biosensor designs are needed to make functional super-resolution microscopy6–8 a practical reality for investigating the compartmentalization of signaling networks beyond the diffraction limit and mapping nanoscale biochemical activity architecture9,10.
Results and Discussion
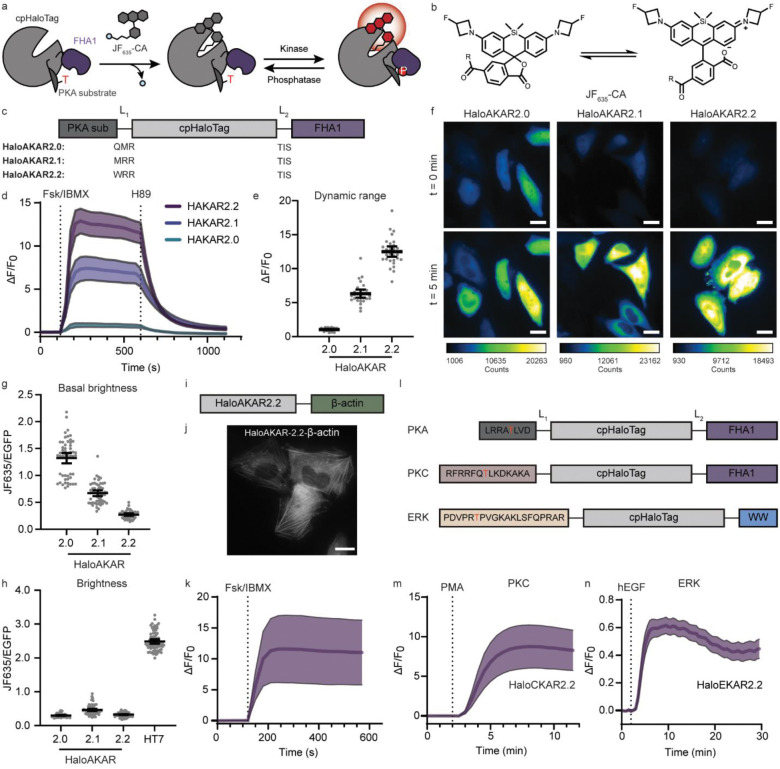
Recognizing that chemigenetic fluorescent biosensors using synthetic fluorophores and self-labeling protein tags11–14 could offer spectral versatility into the far-red, high photostability, and high signal-to-background ratios, we strived to develop chemigenetic far-red kinase activity reporters to address the aforementioned technological gaps. Specifically, we combined a sensing unit consisting of a protein kinase A (PKA)-specific substrate peptide and phosphoamino acid-binding (PAAB) forkhead-associated 1 (FHA1) domain2,15 with a circularly permutated HaloTag reporting unit (Figure 1a, Supplementary Figure S1)12,14, which we labeled with the fluorogenic silicon-rhodamine JF635-chloroalkane (JF635-CA)16. Analogous to recent chemigenetic Ca2+ biosensors12,14, we hypothesized that the kinase-activity-induced conformational change caused by binding of the phosphorylated substrate to FHA1 would influence the open-close equilibrium between the colorless, non-fluorescent spirolactone and the colored, fluorescent zwitterion, and thus the fluorescence intensity of JF635 (Figure 1a–b). We optimized the order of protein domains and the lengths of the interdomain linkers through four rounds of rational engineering. The best candidate, HaloTag-based A kinase activity reporter 1.0 (HaloAKAR1.0-JF635), showed good dynamic range (ΔF/F0 = 129.8%, Supplementary Table S1–2) in living HeLa cells upon maximal PKA stimulation with the adenylate cyclase activator forskolin (Fsk) and the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX). However, the biosensor was rather dim in its basal state (JF635/EGFP = 0.27). We therefore set out to improve the biosensor’s dynamic range and basal brightness simultaneously.
Fig. 1: Design and characterization of HaloAKARs.
a, Schematic of chemigenetic HaloAKAR biosensor based on cpHaloTag labeled with JF635-CA and a phosphorylation-dependent switch that influences JF635 fluorescence intensity. b, Open-close equilibrium of JF635-CA, R = chloroalkane (CA). c, Domain structure of the three selected HaloAKARs, including linker sequences. PKA sub: PKA substrate. d, Average time courses of HeLa cells expressing HaloAKAR2.0, HaloAKAR2.1, or HaloAKAR2.2 labeled with JF635-CA and stimulated with 50 μM Fsk/100 μM IBMX and 20 μM H89 (n = 7, 10, and 13 cells for 2.0, 2.1, and 2.2, respectively). e, Maximum Fsk/IBMX-stimulated response (ΔF/F0) for HaloAKAR-JF635 biosensors. f, Representative pseudo-color widefield images of HeLa cells expressing HaloAKARs labeled with JF635-CA before and after Fsk/IBMX stimulation. Scale bars, 20 μm. g-h, Basal (g) and activated brightness (h) of HaloAKAR-JF635 biosensors. In h, the brightness of HaloTag7-JF635 is given for comparison. i, Domain structure of β-actin-targeted HaloAKAR2.2. j, Representative image of HeLa cells expressing HaloAKAR2.2-β-actin after Fsk/IBMX stimulation. Scale bar, 20 μm. k, Average time course of HeLa cells expressing HaloAKAR2.2-β-actin labeled with JF635-CA upon Fsk/IBMX stimulation (n = 4 cells). l, Domain structure of HaloTag-based PKA, PKC and ERK biosensors (HaloAKAR, HaloCKAR, and HaloEKAR). m-n, Average time courses of HeLa and HEK293T cells expressing HaloCKAR2.2 (m) and HaloEKAR2.2 (n) labeled with JF635-CA stimulated with phorbol 12-myristate 13-acetate (PMA, 100 ng mL−1) or epidermal growth factor (EGF, 100 ng mL−1), respectively (n = 9 and 35 cells). Time courses are representatives of three replicates, and dashed lines indicate addition of drug. Solid lines indicate mean responses; shaded areas correspond to 95% confidence interval. For average measurements, individual data points are shown from three independent repeats along with mean and 95% confidence intervals. Numbers of cells (n) can be found in Supplementary Table S5.
Using a Sort-Seq17,18 approach, we screened >15,000 biosensor variants and identified three new HaloAKAR variants (Figure 1c–h, Supplementary Figure S2, Supplementary Table S3–5). HaloAKAR2.0-JF635 had high basal brightness and good dynamic range (ΔF/F0 = 104.4%, JF635/EGFP = 1.32), although still dimmer than HaloTag7-JF635. HaloAKAR2.1-JF635 showed good basal brightness and high dynamic range (ΔF/F0 = 634%, JF635/EGFP = 0.67), and HaloAKAR2.2-JF635 exhibited low basal brightness but extremely high dynamic range (ΔF/F0 = 1250%, JF635/EGFP = 0.27). All HaloAKARs showed a reversible response upon treatment with the PKA inhibitor H89 and showed no intensity change when the phospho-acceptor Thr on the substrate peptide was mutated to Ala, nor upon stimulation of related ACG kinases (Supplementary Figure S3), indicating that their responses were specific to phosphorylation by PKA.
Purified HaloAKARs rapidly reacted with fluorophore substrate in vitro, albeit at a slower rate than HaloTag7 (Supplementary Figure S4a–d, Supplementary Table S6). Changes in HaloAKARs-JF635 fluorescence emission (ΔF/F0 = 45, 291, and 746% for 2.0, 2.1, and 2.2, respectively) mostly stem from changes in extinction coefficient ε (Δε/ε0 = 42, 165, and 328%), whereas quantum yield φ has no (HaloAKAR2.0: Δφ/φ0 = 2%) or small influence (HaloAKAR2.1 and 2.2: Δφ/φ0 = 48 and 98%). Additionally, we found that the fluorescence lifetime of HaloAKARs-JF635 changed only marginally upon PKA activity (Supplementary Figure S4e–g, Supplementary Table S7). Our data strengthen the model whereby biosensor fluorescence intensity increases are based on a shift in the open-close equilibrium, leading to a larger fraction of fluorophore in the absorbing state and hence an increase in apparent ε. This is further supported by the responses of HaloAKARs labeled with fluorophores whose equilibria are shifted more towards the zwitterionic form compared to JF635 (JF639, JFX646 and JF669), which showed smaller changes upon Fsk/IBMX stimulation than fluorophores with comparable equilibria (JF585, Supplementary Figure S5). The use of JF585 additionally expands the utility of this platform, giving access to high-performance red biosensors.
HaloAKAR2.2-JF635 shows the highest dynamic range reported to date for any kinase biosensor, surpassing ExRai-AKAR2 (480/405 excitation ratio ΔR/R0 = 1,095%)2, which is in the green spectral region, and drastically outperforming the few available NIR Förster resonance energy transfer (FRET) biosensors (< 40%)4,5. This allowed for sensitive measurements of PKA activity at low doses of Fsk (Supplementary Figure S6). Benefiting from their high dynamic ranges, HaloAKARs also enable sensitive PKA activity measurements at different subcellular locations, including the plasma membrane, clathrin-coated pits, the actin or microtubule network, or the outer mitochondrial membrane (Figure 1i–k, Supplementary Figure S7). Further, this chemigenetic biosensor design can be expanded to other kinases by switching the PKA-specific peptide for peptides specific to other kinases, such as protein kinase C (PKC) or protein kinase B (Akt, Figure 1l–m). By further replacing the FHA1 domain with a WW domain, we generated biosensors for extracellular signal-regulated kinase (ERK). Replacing the PAAB domain had a larger impact on the dynamic range than switching out the substrate. Nevertheless, HaloEKARs showed dynamic ranges as high as 60% (HaloEKAR2.2, Figure 1n, Supplementary Figure S8). Taken together, HaloTag-based KARs represent an ideal platform to generate sensitive and specific far-red- and red-emitting biosensors for diverse kinases with promising photophysical properties for advanced bioimaging applications.
These far-red chemigenetic biosensors are ideally suited for multiplexed activity sensing with fluorescent protein-based biosensors in other spectral regions. We first performed three-color imaging using HaloAKAR2.2-JF635, the red Ca2+ biosensor RCaMP1d19, and the green cAMP biosensor GFlamp-120 in MIN6 β-cells. Stimulation with the K+ channel blocker tetraethylammonium chloride led to in-phase oscillations of Ca2+ and PKA activity and inverse oscillations for cAMP (Figure 2a, Supplementary Figure S9), providing single-cell capture of the previously identified oscillatory circuit21 with unprecedented clarity. Further combination with sapphire and blue biosensors allowed for four- and five-color imaging (Supplementary Figures S10–11). For instance, combining HaloAKAR2.1-JF635 with the red cAMP biosensor pinkFlamindo, the yellow cGMP biosensor cGull22, the sapphire PKC biosensor sapphireCKAR3, and the blue Ca2+ biosensor BGeco23 allowed us to follow the concentration and activities of all five analytes upon pharmacological stimulation (Figure 2b). This record-high five-color biosensor multiplexing in the same living cell not only underscores the strengths of this technology, but also sets a new benchmark for future advancements in functional live-cell microscopy.
Figure 2: Application of HaloAKARs in multiplexed biosensor imaging, 4D activity imaging and super-resolution microscopy.
a Three-color multiplexing of cAMP (G-Flamp1, green), Ca2+ (RCaMP, blue) and PKA activity (HaloAKAR2.2, black) in single MIN6 cells stimulated with 20 mM tetraethylammonium chloride (TEA). b, Five-color multiplexing of Ca2+ (B-GECO1, blue), PKC activity (sapphireCKAR (SCKAR), cyan), cGMP (cGull, yellow), cAMP (pinkFlamindo, red), and PKA activity (HaloAKAR2.1, dark-red) in HeLa cells stimulated with 50 μM Fsk/100 μM IBMX (Fsk/IBMX), 100 ng mL−1 PMA, 0.4 μM atrial natriuretic peptide (ANP), and 1 μM ionomycin (Iono). Solid lines indicate mean response; shaded areas correspond to SD (n = 2 cells). c-d, Imaging of 4-d-old HEK293T spheroids stably expressing cytosolic HaloAKAR2.1 (HaloAKAR2.1-NES, magenta) and plasma membrane anchored EGFP (EGFP-CAAX, green) labeled with JF635-CA via lattice light-sheet microscopy (c) and 2P microscopy (d). Representative 3D renderings (c) and overlay images of one z-plane (d) before and after Fsk/IBMX stimulation are shown. Scale bars, 20 μm. E-g, Confocal imaging of isolated pancreatic islets expressing HaloAKAR2.1-T2A-EGFP upon stimulation with 25 mM glucose. Overview (e) and zoomed in images (f) are shown, along with single-cell traces (g) highlighting the three cells in f. h-i, Representative confocal and STED images of HeLa cells expressing HaloAKAR2.1-clathrin-light-chain (CLC) labeled with JF635-CA before and after Fsk/IBMX stimulation. Overview (h) and zoom (i) images are shown. Lines used to quantify the diameter of clathrin-coated pits are indicated in the confocal images (h, white), as well as the line through three neighboring pits (i, blue). Scale bars, 5 μm (h) and 1 μm (i). j, Diameter of clathrin-coated pits. Quantifications are given for HaloAKAR2.1-CLC before and after Fsk/IBMX stimulation, as well as HaloTag7-CLC, for both confocal (C) and STED (S) images. Individual data points are shown along with mean and 95% confidence interval. HaloAKAR2.1, n = 50 pits from 5 cells from 5 replicates; HaloTag7, n = 40 pits from 4 cells from 4 replicates. k-l, Representative normalized line profiles (solid) through a clathrin-coated pit for diameter quantification (k) or three neighboring pits (l) resolved in STED. Confocal (black) and STED (red) curves are shown, as well as fitted curves (dotted) in k. m, Pitwise activity analysis showing the mean F/F0 for pits present after stimulation. Representative images shown under three conditions: untreated (left), 50 μM Fsk (middle), and 50 μM Fsk/100 μM (right). Scale bars, 2.5 μm. n, Zoom of outline region in (m) showing pitwise ratio (top), as well as confocal (middle) and STED (bottom) images after Fsk stimulation. Scale bars, 500 nm. o, Representative normalized line profile through the three clathrin-coated pits, revealing that they are resolved in STED but not in confocal microscopy. p, Density plots of mean ΔF/F0 per clathrin-coated pit. The overall mean is given as a dashed line (no treatment: 900 pits from 5 cells from 5 replicates; Fsk: 934 pits from 7 cells from 7 replicates; Fsk/IBMX: 1377 pits from 9 cells from 9 replicates).
Motivated by the far-red spectral characteristics of HaloAKARs-JF635 and their favorable bleaching kinetics compared with the chemigenetic Ca2+ biosensor HaloCaMP1a-JF635 (Supplementary Figure S4h–j, Supplementary Table S8), we tested their application for 4D activity imaging in more physiologically relevant 3D tissues. First, we imaged 4-d-old HEK293T spheroids (diameter ~60 μm) stably expressing HaloAKAR2.1-NES and EGFP-CAAX via spinning disc confocal and lattice light-sheet microscopy (Figure 2c–d, Supplementary Figures S12–13). PKA responses were visible upon Fsk/IBMX treatment in both modalities, clearly demonstrating the ability of HaloAKAR2.1-JF635 to enable 4D activity imaging. Nonetheless, residual bleaching is still visible and further optimization is required. Secondly, two-photon (2P) microscopy allowed imaging of HaloAKAR2.1-JF635 and EGFP using a single laser line (1031 nm), reducing the excitation light needed (Supplementary Figure S14). Moreover, HaloAKAR2.1 could be labeled with JF585-CA and excited at 1031 nm, leading to a stronger signal, as expected from this fluorophore’s 2P excitation spectrum16. Hence, both red and far-red HaloAKARs allow imaging via 2P microscopy. Lastly, we expressed HaloAKAR2.1 in isolated pancreatic islets via AAV infection. Upon glucose stimulation, confocal microscopy showed asynchronous PKA activity fluctuations in individual β-cells (Figure 2e–g, Supplementary Figure S15 and Supplementary Video S1). While islet Ca2+ imaging has been performed for many years24, the PKA activity imaging in pancreatic islets demonstrated here opens the door for direct interrogation of critical signaling changes in response to important G-protein-coupled receptor ligands, such as glucagon-like peptide 1 receptor agonists25,26. These results demonstrate the power of far-red HaloAKARs for 4D activity imaging in physiologically relevant systems.
The high brightness and photostability of synthetic fluorophores make them ideally suited for live-cell super-resolution microscopy27,28. To test HaloAKAR performance, we used STED microscopy to image PKA activity in HeLa cells expressing clathrin-targeted HaloAKAR2.1 (Figure 2h–l). Analysis of the average diameter of clathrin-coated pits in confocal and STED images revealed a clear resolution improvement both before (FWHMC = 342±4 nm; FWHMS = 241±6 nm) and after Fsk/IBMX treatment (FWHMC = 362±4 nm; FWHMS = 266±7 nm), which was comparable to HaloTag7-labeled clathrin-coated pits (FHWMC = 370±4 nm; FHWMS = 237±7 nm, Supplementary Figure S16). Pit-wise analysis of ΔF/F0 revealed higher mean activity following treatment with Fsk/IBMX (1.9±0.6) versus Fsk alone (1.1±0.6). The broad activity distributions seen with both treatments indicate heterogeneous activity levels on the single-pit level. Most importantly, the enhanced spatial resolution of STED microscopy allowed us to measure PKA activity at individual pits that were not resolvable under confocal microscopy, allowing better capture of signaling heterogeneity (Figure 2m–p, Supplementary Figure S17). HaloAKAR therefore paves the way for future studies of compartmentalized PKA activity at the nanoscale.
In summary, HaloAKARs are a series of chemigenetic PKA activity biosensors with red or far-red spectral properties and exceptionally high dynamic ranges. HaloAKARs expanded biosensor multiplexing, enabled 4D activity imaging, and realized STED-based super-resolution activity imaging. We expect this technology will lead the way in illuminating signaling activity dynamics across scales.
Supplementary Material
Acknowledgements
We thank L. Lavis and J. Grimm (Janelia Research Campus) for providing JF fluorophores. We thank I. Garcia (USC) for assistance with cell culture. We thank C. Alvarez (UCSD) for assistance with cloning. We thank J. Bogomolovas and J. Chen (UCSD) for access and assistance to their fluorescence polarization plate reader. We thank J. Chung and J. Yang (UCSD) for access and assistance to their fluorescence and absorbance spectrometer. We thank Z. Liang (UCSD) for help with processing of sequencing results. We thank H. Farrants (Janelia Research Campus) for discussions on chemigenetic biosensors. We thank A. Linnemann (Indiana University) for providingthe pAAV-Ins-GCaMP plasmid map. We thank Q. Ni (UCSD) for support in material acquisition and tissue culture. We thank P. Guo from the UC San Diego Nikon Imaging Center, as well as J. Santini and M. Erb from the UC San Diego School of Medicine Microscopy Core, for assistance with microscopy. We thank the Translational Imaging Center, A. Shwartz and J. Junge, at USC for their help with STED microscopy. This publication includes data generated at the UC San Diego IGM Genomics Center utilizing an Illumina NovaSeq 6000 that was purchased with funding from a National Institutes of Health SIG grant (#S10 OD026929). The Leica SP8 in the UC San Diego School of Medicine Microscopy Core was funded by NINDS P30NS04710.
Funding
This research was supported by the Swiss National Science Foundation (SNSF) grants P2ELP3_199834 and P500PN_214234 to M.S.F.; the Charles Lee Powell Foundation Powell Fellowship at the University of California, San Diego (UCSD) and the UCSD Interfaces Graduate Training Program National Institutes of Health (NIH)/National Institute of Biomedical Imaging and Bioengineering (NIBIB) T32 Training in Multi-scale Analysis of Biological Structures and Function Training Grant 5T32EB009380-15 to S.A.S.; an EMBO (ALTF 849-2020) and HFSP (LT000404/2021-L) fellowship to F.S.; the National Science Foundation (NSF) Graduate Research Fellowship (DGE-2038238) and American Heart Association Predoctoral Fellowship (24PRE1186687) to A.C.L. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the NSF; the Swiss National Science Foundation (P2BSP3_200177) and the Larry L. Hillblom Foundation (2023-D-012-FEL) to T.V.R.; the U.S. National Institute of Diabetes and Digestive and Kidney Diseases (DK063491 and DK101395) and a grant from Janssen Pharmaceuticals to J.M.O.; NIH R01GM149976, NIH U01AI167892, NIH 5R01NS111039, NIH R21NS125395, NIHU54DK134301, NIHU54 HL165443, and UCSD Startup funds to L.S.; NIH grant R01 CA262815 to J.Z and Y.W.; NIH grants EB029122, R35 GM140929, R01 HL121365, and HD107206 to Y.W.; NIH grants R35 CA197622, R01 DK073368, R01 DE030497, R01 HL162302 and RF1 MH126707 to J.Z.
Footnotes
Competing Interests
We declare that none of the authors have competing financial or non-financial interests.
Code Availability
Custom MATLAB scripts for clathrin-coated pit analysis will be available at https://github.com/jinzhanglab-ucsd. Custom ImageJ macros, R code, and Python code used to analyze imaging data, as well as bash and R scripts used to analyze sequencing data, are available upon reasonable request.
Data Availability
Plasmids encoding pcDNA3.1-HaloAKAR2.0-T2A-EGFP, pcDNA3.1-HaloAKAR2.1-T2A-EGFP, pcDNA3.1-HaloAKAR2.2-T2A-EGFP, pcDNA3.1-HaloAKAR2.1-NES-T2A-EGFP-CAAX, pcDNA3.1-HaloAKAR2.1-CLC, pcDNA3.1-HaloCKAR2.2-T2A-EGFP, pcDNA3.1-HaloAktKAR2.2-T2A-EGFP, pcDNA3.1-HaloEKAR2.2-T2A-EGFP will be deposited on Addgene. The stable cell line generated is available upon request. Source data are provided with this paper. Further data supporting the findings of this study are available upon reasonable request.
References
- 1.Greenwald E. C., Mehta S. & Zhang J. Genetically encoded fluorescent biosensors illuminate the spatiotemporal regulation of signaling networks. Chem. Rev. 118, 11707–11794 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J.-F. et al. An ultrasensitive biosensor for high-resolution kinase activity imaging in awake mice. Nat. Chem. Biol. 17, 39–46 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta S. et al. Single-fluorophore biosensors for sensitive and multiplexed detection of signalling activities. Nat. Cell Biol. 20, 1215–1225 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shcherbakova D. M., Cox Cammer N., Huisman T. M., Verkhusha V. V. & Hodgson L. Direct multiplex imaging and optogenetics of Rho GTPases enabled by near-infrared FRET. Nat. Chem. Biol. 14, 591–600 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliinyk O. S., Shemetov A. A., Pletnev S., Shcherbakova D. M. & Verkhusha V. V. Smallest near-infrared fluorescent protein evolved from cyanobacteriochrome as versatile tag for spectral multiplexing. Nat. Commun. 10, 279 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan R., Wang B. & Xu K. Functional super-resolution microscopy of the cell. Curr. Opin. Chem. Biol. 51, 92–97 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Lu K., Vu C. Q., Matsuda T. & Nagai T. Fluorescent protein-based indicators for functional super-resolution imaging of biomolecular activities in living cells. Int. J. Mol. Sci. 20, 5784 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra K. et al. Genetically encoded photo-switchable molecular sensors for optoacoustic and super-resolution imaging. Nat. Biotechnol. 40, 598–605 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mo G. C. H. et al. Genetically encoded biosensors for visualizing live-cell biochemical activity at super-resolution. Nat. Methods 14, 427–434 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anton S. E. et al. Receptor-associated independent cAMP nanodomains mediate spatiotemporal specificity of GPCR signaling. Cell 185, 1130–1142 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Cook A., Walterspiel F. & Deo C. HaloTag-based reporters for fluorescence imaging and biosensing. ChemBioChem 24, e202300022 (2023). [DOI] [PubMed] [Google Scholar]
- 12.Deo C. et al. The HaloTag as a general scaffold for far-red tunable chemigenetic indicators. Nat. Chem. Biol. 17, 718–723 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Farrants H. et al. A modular chemigenetic calcium indicator enables in vivo functional imaging with near-infrared light. 2023.07.18.549527 Preprint at 10.1101/2023.07.18.549527 (2023). [DOI] [Google Scholar]
- 14.Wang L., Hiblot J., Popp C., Xue L. & Johnsson K. Environmentally sensitive color-shifting fluorophores for bioimaging. Angew. Chem. Int. Ed. 132, 22064–22068 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Depry C., Allen M. D. & Zhang J. Visualization of PKA activity in plasma membrane microdomains. Mol. Biosyst. 7, 52–58 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Grimm J. B. et al. A general method to fine-tune fluorophores for live-cell and in vivo imaging. Nat. Methods 14, 987–994 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L. et al. Integration of FRET and sequencing to engineer kinase biosensors from mammalian cell libraries. Nat. Commun. 12, 5031 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koberstein J. N., Stewart M. L., Mighell T. L., Smith C. B. & Cohen M. S. A Sort-Seq approach to the development of single fluorescent protein biosensors. ACS Chem. Biol. 16, 1709–1720 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akerboom J. et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 6, 1–29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L. et al. A high-performance genetically encoded fluorescent indicator for in vivo cAMP imaging. Nat. Commun. 13, 5363 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tenner B. et al. Spatially compartmentalized phase regulation of a Ca2+-cAMP-PKA oscillatory circuit. eLife 9, e55013 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda S. et al. Generation of a cGMP indicator with an expanded dynamic range by optimization of amino acid linkers between a fluorescent protein and PDE5α. ACS Sens. 2, 46–51 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y. et al. An expanded palette of genetically encoded Ca2+ indicators. Science 333, 1888–1891 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilon P., Chae H.-Y., Rutter G. A. & Ravier M. A. Calcium signaling in pancreatic β-cells in health and in Type 2 diabetes. Cell Calcium 56, 340–361 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Brown E., Heerspink H. J. L., Cuthbertson D. J. & Wilding J. P. H. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. The Lancet 398, 262–276 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Andersen A., Lund A., Knop F. K. & Vilsbøll T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 14, 390–403 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Wang L., Frei M. S., Salim A. & Johnsson K. Small-molecule fluorescent probes for live-cell super-resolution microscopy. J. Am. Chem. Soc. 141, 2770–2781 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Stockhammer A. & Bottanelli F. Appreciating the small things in life: STED microscopy in living cells. J. Phys. Appl. Phys. 54, 033001 (2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Plasmids encoding pcDNA3.1-HaloAKAR2.0-T2A-EGFP, pcDNA3.1-HaloAKAR2.1-T2A-EGFP, pcDNA3.1-HaloAKAR2.2-T2A-EGFP, pcDNA3.1-HaloAKAR2.1-NES-T2A-EGFP-CAAX, pcDNA3.1-HaloAKAR2.1-CLC, pcDNA3.1-HaloCKAR2.2-T2A-EGFP, pcDNA3.1-HaloAktKAR2.2-T2A-EGFP, pcDNA3.1-HaloEKAR2.2-T2A-EGFP will be deposited on Addgene. The stable cell line generated is available upon request. Source data are provided with this paper. Further data supporting the findings of this study are available upon reasonable request.