Abstract
Bacterial pathogens remain poorly characterized in bats, especially in North America. We describe novel (and in some cases panmictic) hemoplasmas (10.5% positivity) and bartonellae (25.5% positivity) across three colonies of Mexican free-tailed bats (Tadarida brasiliensis), a partially migratory species that can seasonally travel hundreds of kilometers. Molecular analyses identified three novel Candidatus hemoplasma species most similar to another novel Candidatus species in Neotropical molossid bats. We also detected novel hemoplasmas in sympatric cave myotis (Myotis velifer) and pallid bats (Antrozous pallidus), with sequences in the latter 96.5% related to C. Mycoplasma haemohominis. We identified nine Bartonella genogroups, including those in cave myotis with 96.7% similarity to C. Bartonella mayotimonensis. We also detected Bartonella rochalimae in migratory Mexican free-tailed bats, representing the first report of this human pathogen in the Chiroptera. The seasonality and diversity of these bacteria observed here suggest that additional longitudinal, genomic, and immunological studies in bats are warranted.
Keywords: hemoplasmas, Tadarida brasiliensis, migration, One Health
Introduction
Bats have been intensively sampled for viral pathogens, with species in this mammalian order hosting multiple viruses with high virulence in humans (1). However, bats remain understudied for bacterial pathogens, which can be significant for their impacts on both human health and bat morbidity and even mortality (2, 3). Hemotropic mycoplasmas (hemoplasmas) and bartonellae are facultative intracellular bacteria of special interest in bats, given their high prevalence and substantial genetic diversity (4, 5). For example, sampling of Neotropical bat communities has identified many common and co-circulating genotypes of these bacteria (6–8). Surveys in Oceania and Europe have also supported plausible zoonotic transmission of these bacteria from bats to humans, including Candidatus Mycoplasma haemohominis and C. Bartonella mayotimonensis (9, 10). Greater characterization of these bacteria across global bat diversity (over 1,470 species) is therefore warranted to inform infection risks for both bats and humans, although little surveillance has thus far been conducted in North American bats (11, 12).
Although flight enables high mobility of bats, relatively few bat species undertake long-distance migrations (e.g., between maternity and wintering grounds) (13). In North America, Mexican free-tailed bats (Tadarida brasiliensis) display highly variable migratory strategies (14). The southwestern United States contains both non-migratory and migratory populations, with some individuals traveling hundreds to over 1,000 kilometers between wintering grounds in Mexico to northern maternity colonies in Oklahoma, Kansas, and Colorado (14–16). Other colonies across the species range include year-round residents (17, 18). This variation in migratory behavior could shape patterns of infection, including the seasonal dispersal of bacterial pathogens across landscapes to naïve hosts. Hemoplasmas have not yet been detected in this bat species (4, 19), and bartonellae have only been minimally described in the southernmost part of the bat species’ geographic range (i.e., Chile and Argentina) (20).
Here, we conducted an initial characterization of hemoplasmas and bartonellae in Mexican free-tailed bats across multiple populations and seasons. Our goals were to identify novel pathogens in this bat species and to test for differences in prevalence among colonies that differ in migratory strategy and across the bat annual cycle. We also tested whether pathogen lineages were unique to each geography or if migration may facilitate panmixia. Lastly, we used this opportunity to perform a pilot characterization of these pathogens in sympatric bat species.
Material and Methods
Wild bat sampling
We sampled three North American colonies of Mexican free-tailed bats in 2021 and 2022 to compare infections among migratory strategies and provide an initial assessment of pathogen seasonality. We sampled non-migratory individuals in southeastern Louisiana (17), focusing on a colony in Pine Grove of approximately 1,000 bats, in the non-breeding season (October 2021, n=5) and maternity season (July 2022, n=10). We also sampled the partially migratory population of Bracken Cave near San Antonio, Texas, which hosts tens of millions of this bat species in the maternity season and declines to approximately 10,000 bats in winter (16, 21). We sampled the maternity season (August 2021, n=20) and mid-to-late winter (December 2021 and March 2022; n=9 and n=19). We also sampled a fully migratory colony at the Selman Bat Cave near Freedom, Oklahoma, where this maternity roost holds up to 100,000 bats during summer and is empty in winter (16, 22). We sampled bats monthly, from April to September 2022, spanning spring arrival, the maternity season, and fall migration (n=146). In the same Oklahoma site, we also sampled four cave myotis (Myotis velifer), one hoary bat (Lasiurus cinereus), one Townsend’s big-eared bat (Corynorhinus townsendii), and two pallid bats (Antrozous pallidus).
Bats were captured with hand nets and mist nets while emerging from or returning to roosts and placed in individual cloth bags. Bats were identified to species by morphology and identified by sex, reproductive status, and age (23). Blood (<1% body mass) was sampled by lancing the propatagial vein using 27G and 30G needles and collected with heparinized capillaries. Blood was preserved on Whatman FTA cards and held at room temperature until −20°C storage at the University of Oklahoma (OU). Sampling was approved by the Institutional Animal Care and Use Committees of OU (2022–0198) and Southeastern Louisiana University (0064), with permits from the Texas Parks and Wildlife Department (SPR-0521–063), Louisiana Department of Wildlife and Fisheries (WDP-21–101), and Oklahoma Department of Wildlife Conservation (ODWC, 10567389). All bats were released after sampling at the capture site.
Molecular diagnostics
We extracted genomic DNA from blood using QIAamp DNA Investigator Kits (Qiagen). To determine hemoplasma presence, we used PCR targeting the partial 16S and 23S rRNA genes (Table S1; (6, 7, 24, 25), with amplicons purified and sequenced at Psomagen. For DNA samples positive for the 16S or 23S rRNA genes, we also attempted to amplify the partial rpoB gene, using primers newly designed for this study (Table S1). To determine the presence of bartonellae, we used nested PCR targeting the partial gltA gene (Table S1; (26)), with amplicons purified with Zymo kits (DNA Clean & Concentrator-5, Zymoclean Gel DNA Recovery) and sequenced at the North Carolina State University Genomic Sciences Laboratory. We included blank FTA card punches and ultrapure water as extraction and negative controls, respectively, in all PCRs. Hemoplasma PCRs used Candidatus Mycoplasma haemozalophi as a positive control, but we did not include positive controls for Bartonella spp. to reduce cross-contamination risks from nested PCR; instead, amplicons of expected size (~300 bp) were identified during gel electrophoresis. Sequences are available on GenBank through accessions OQ407831–50, OR783320–23, and PQ465198 (Mycoplasma spp. 16S rRNA); OQ359160–75 (Mycoplasma spp. 23S rRNA); OQ554332–38 (Mycoplasma spp. rpoB); and PP317862–72 (Bartonella spp. gltA).
Statistical analysis
We analyzed infection states using generalized linear models (GLMs) or generalized additive models (GAMs) with binary response in R. All GLMs were fit using mean bias reduction methods with the brglm2 package (27), whereas GAMs were fit using restricted maximum likelihood and the mgcv package (28). For each of our two pathogens, we fit the following four GLMs. The first model was fit to all data and compared the odds of infection among Louisiana, Texas, and Oklahoma bats. The second model was limited to the early-to-mid non-breeding season and compared odds of infection between Texas and Louisiana bats. The third model was limited to the late overwintering period and spring arrival to compare odds of infection between Texas and that subset of Oklahoma bats. The fourth model was limited to Texas and included reproductive status (only females were reproductive) and month to test demographic effects and seasonality (i.e., maternity season through late overwintering). Lastly, for our GAMs, we fit a similar model for each pathogen to the Oklahoma data, with a cyclic cubic smooth of month to assess a different aspect of infection seasonality (i.e., spring arrival until onset of fall migration).
Phylogenetic analysis
We used NCBI BLASTn to identify related mycoplasma (16S rRNA, 23S rRNA, rpoB) and bartonellae sequences (gltA), which we aligned with our sequences and reference sequences using MUSCLE. We used MrBayes for phylogenetic analysis, with each gene tree run for 20,000,000 generations using a GTR+I+G model. BLASTn was implemented in Geneious, whereas MUSCLE and MrBayes were implemented using NGPhylogeny.fr (29). We delineated genotypes of hemoplasmas and genogroups of bartonellae based on pairwise similarity among sequences and clustering on their phylogenies, using established criteria for defining novel bacterial lineages (6, 30). For hemoplasmas, we also used multi-loci data to propose novel Candidatus species when the same genotype was identified in at least two samples using 16S rRNA and one other marker (i.e., 16S rRNA and 23S rRNA, 16S rRNA and rpoB) (7, 31).
We conducted two tests to assess if pathogen lineages were unique to each Mexican free-tailed bat colony, which would suggest geographically constrained transmission dynamics. First, we used chi-squared tests with p values generated by a Monte Carlo procedure to quantify associations between geography and pathogen lineage assignments. Next, for any lineages identified in multiple colonies with sufficient sample size, we derived matrices of spatial and phylogenetic distance among sequenced PCR-positive samples and used Mantel tests with the vegan R package to assess isolation by distance (32). These tests used 1,000 randomizations.
Results
Migratory and seasonal effects on bat bacterial infection
We detected hemoplasmas in 21 of 209 Mexican free-tailed bats when targeting the partial 16S or 23S rRNA genes (10.5%, 95% CI: 7.1–15.4%). Sequencing of the 23S rRNA gene showed three other bats (two from Texas and one from Oklahoma) had non-hemotropic mycoplasmas (OQ359169–70, OQ359174) most related to Mycoplasma muris (97.5% sequence identity). We detected bartonellae in 53 of 208 tested bats (25.5%, 95% CI: 20–31.8%). Only six Mexican free-tailed bats were coinfected by bartonellae and any mycoplasmas (2.9%, 95% CI: 1.3–6.1%). Hemoplasmas were detected in all three Mexican free-tailed bat colonies, while bartonellae were only detected in the Texas and Oklahoma colonies. PCR positivity data are fully available in the Pathogen Harmonized Observatory (PHAROS): https://pharos.viralemergence.org/ (33).
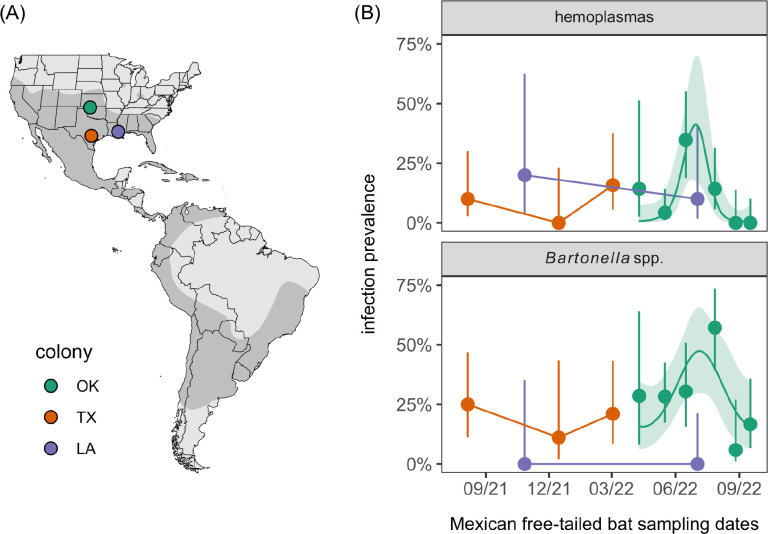
Across all Mexican free-tailed bats (model 1), the odds of infection differed by colony for bartonellae (χ2 = 9.72, p < 0.01) but not hemoplasmas (χ2 = 0.02, p = 0.99; Figure 1). When comparing only the resident and partially migratory populations in the non-breeding season (model 2), Louisiana and Texas bats did not differ in the odds of either infection (hemoplasmas: χ2 = 1.21, p = 0.27; bartonellae: χ2 = 0, p = 1). When comparing only the partially and fully migratory populations in spring (model 3), we did not detect colony differences for hemoplasmas (χ2 = 0, p = 1) or bartonellae (χ2 = 0.11, p = 0.74). When assessing risk factors of infection in the partially migratory Texas colony (model 4), we found no evidence of seasonal or demographic effects for either pathogen (Table S2). However, when assessing these predictors for the fully migratory Oklahoma colony across the full 2022 occupancy period, we identified significant seasonality in infection (Table S3). Prevalence increased from spring arrival and peaked in the maternity season (i.e., June 2022) for both bacteria, declining into fall migration (Figure 1).
Figure 1.
(A) Sampled Mexican free-tailed bat (Tadarida brasiliensis) colonies relative to the host distribution in the Americas. (B) Hemoplasma and Bartonella spp. infection prevalence across months and colonies; segments denote 95% confidence intervals using Wilson’s method. For Oklahoma bats,
Genetic diversity of Mexican free-tailed bat hemoplasmas
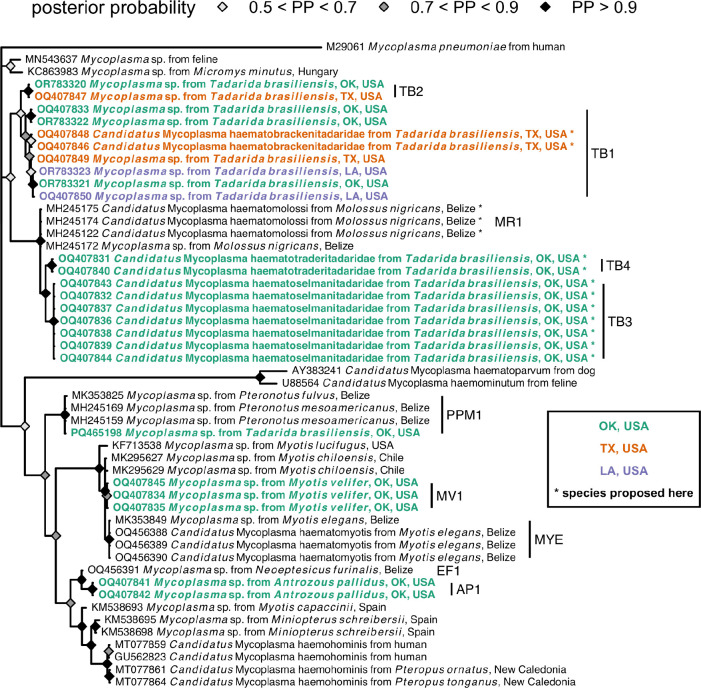
Sequencing of 16S rRNA amplicons revealed four hemoplasma genotypes specific to Mexican free-tailed bats (i.e., TB1–4; Figure 2, Table 1). The TB1 genotype was 97% similar to the MR1 genotype that we earlier isolated from another molossid bat (Molossus nigricans) in Belize (e.g., MH245174) (6), and TB1 was found in all three sampled populations. In contrast, TB2 was only detected in the Texas colony and was ~96–97% similar to hemoplasmas from carnivores and rodents and to the Belize bat MR1 genotype (6, 34, 35). Both TB3 and TB4 were only detected in Oklahoma and were ~99% similar to MR1. We also detected the PPM1 genotype, originally found in Pteronotus mesoamericanus and P. fulvus in Belize (6, 7), in the Oklahoma colony. Amplification of the 23S rRNA gene from two bats (one each from Texas and Oklahoma) also found a genotype we initially detected in cave myotis (i.e., MV1; Figure S1) and the above Mycoplasma muris–like (non-hemotropic) genotype. These seven mycoplasma genotypes were not associated with geography (χ² = 13.07 p = 0.38; Figure S2). When considering the one genotype observed in multiple colonies and in more than one bat per site (TB1), we also found little support for isolation by distance with the 16S rRNA phylogeny (Mantel r = 0.35, p = 0.10).
Figure 2.
Consensus Bayesian phylogeny of the partial 16S rRNA hemoplasma sequences from this study (highlighted in bold and colored by geography; see Table 1 for genotype assignments) and reference sequences from bats and other mammals. Nodes are colored by posterior probability (nodes with less than 50% support are not shown). Hemoplasmas with Candidatus species names proposed here are indicated by asterisks and have paired 23S rRNA or rpoB sequences (see Figures S1 and S3).
Table 1.
Mycoplasma spp. (A) and Bartonella spp. (B) lineages identified from Louisiana, Texas, and Oklahoma bats during this study (2021–2022). Lineages are given with their host species, locations, and mean intra-genotype (mycoplasmas) or intra-genogroup (bartonellae) sequence similarity from the partial 16S rRNA, 23S rRNA, rpoB, or gltA gene sequences identified here.
| Lineage | States | Bat species | Mean intra-lineage similarity (%) | |
|---|---|---|---|---|
| (A) | TB1* | LA, TX, OK | Tadarida brasiliensis | 99.6i, 100iii |
| TB2* | TX, OK | Tadarida brasiliensis | 100i | |
| TB3* | OK | Tadarida brasiliensis | 100i, 100ii | |
| TB4* | OK | Tadarida brasiliensis | 100i, 100ii | |
| PPM1 | OK | Tadarida brasiliensis | NA¶ | |
| MV1* | TX, OK | Myotis velifer, Tadarida brasiliensis | 100i, 100ii | |
| API* | OK | Antrozous pallidus | 100i | |
| M. muris–like† | TX, OK | Tadarida brasiliensis | 99.3ii | |
| (B) | TB1 | TX, OK | Tadarida brasiliensis | 96.8iv |
| TB2* | OK | Tadarida brasiliensis | NA¶ | |
| TB3* | OK | Tadarida brasiliensis | 97.2iv | |
| TB4* | OK, TX | Tadarida brasiliensis, Myotis velifer, Antrozous pallidus | 97.4iv | |
| Bartonella rochalimae | OK | Tadarida brasiliensis | 97.5iv | |
| DR8 | OK | Tadarida brasiliensis | 98.2iv | |
| ML1 | OK | Myotis velifer | NA¶ | |
| C. Bartonella mayotimonensis–like | OK | Myotis velifer | NA¶ | |
| AP1* | OK | Antrozous pallidus | NA¶ | |
| CT1* | OK | Corynorhinus townsendii | NA¶ |
Novel lineages
Non-hemotropic mycoplasma
16S rRNA sequence
23S rRNA sequence
rpoB sequence
gltA sequence
Single sequence
Amplification of paired partial 23S rRNA (Figure S1) and/or rpoB (Figure S3) genes for samples belonging to these 16S rRNA genotypes suggested at least three novel Candidatus hemoplasma species circulate in Mexican free-tailed bats. Based on 100% identity of two rpoB sequences (OQ554335–36) and 100% identity of paired 16S rRNA sequences included in the TB1 genotype, first detected in Bracken Cave (OQ407846, OQ407848), we propose the name C. Mycoplasma haematobrackenitadaridae sp. nov. Similarly, given 99.98% identity among seven 23S rRNA sequences (OQ359161–65, OQ359168, OQ359172) and 100% identity in paired 16S rRNA sequences included in the TB3 genotype from the Selman Bat Cave (OQ407832, OQ407836–39, OQ407843–44), we propose the name C. M. haematoselmanitadaridae sp. nov. Lastly, given 100% identity of two 23S rRNA sequences (OQ359160, OQ359166) and 99.8% identity in paired 16S rRNA sequences from the TB4 genotype (also identified from the Selman Bat Cave; OQ407831, OQ407840), we propose the name C. M. haematotraderitadaridae sp. nov. (Figures 2 and S1), based on the stream running adjacent to the bat cave (Traders Creek) (36).
Given the similarity of Mexican free-tailed bat hemoplasma 16S rRNA sequences to those from molossid bats sampled in Belize (6), we also attempted to amplify the 23S rRNA and rpoB genes from Molossus nigricans sampled in 2017 and 2018 in Belize that previously tested positive for the MR1 and MR2 genotypes (6). We re-extracted DNA from four FTA cards and applied the same additional PCR protocols described earlier. We obtained partial 23S rRNA and rpoB sequences for two (OQ518943–44) and three (OQ554329–31) M. nigricans, respectively. Based on 100% inter-sequence similarity of the rpoB sequences and high () identity of paired 16S rRNA sequences (MH245122, MH245172, MH245174), we propose the name C. Mycoplasma haematomolossi sp. nov. to designate this novel hemoplasma (Figures 2 and S3).
Genetic diversity of Mexican free-tailed bat bartonellae
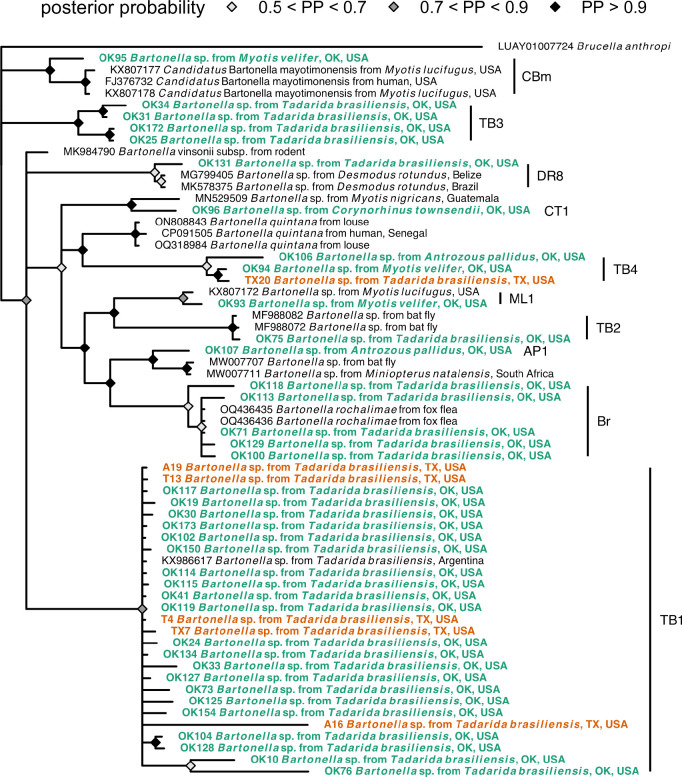
Sequencing of gltA amplicons next revealed at least six Bartonella genogroups circulating in Mexican free-tailed bats (Figure 3, Table 1). The first genogroup was detected in both Texas and Oklahoma, with sequences from all three sampled months across 2021 and 2022 from Bracken Cave but only from May and June in the Selman Bat Cave. The gltA sequences were ≥99.6% similar to those recently detected in Mexican free-tailed bats in Argentina (KX986617) (20), such that we consider these sequences to all form the TB1 genogroup. The TB2–4 genogroups were only found in the Oklahoma colony. TB2 was identical to sequences from streblid bat flies in Mexico (e.g., ≥99.7% identity to MF988072) (37), while TB3 represents a novel genogroup with ~92% similarity to Bartonella vinsonii (e.g., MK984790) (38). Likewise, TB4 was novel and distantly related (~91%) to Bartonella quintana (i.e., Z70014) (39). Oklahoma bats also harbored gltA sequences with 97–100% identity to Bartonella rochalimae in fleas from foxes (OQ436435) (40); these B. rochalimae sequences were only detected in summer months. Lastly, Oklahoma bats had gltA sequences in the same clade as bartonellae originally found in vampire bats (Desmodus rotundus; DR8 genogroup) (26). These six Bartonella genogroups were not associated with geography (χ² = 8.20, p = 0.16; Figure S4), and we also found no support for isolation by distance for the TB1 genotype with our gltA phylogeny (Mantel r = −0.04, p = 0.59).
Figure 3.
Consensus Bayesian phylogeny of the partial gltA Bartonella spp. sequences from this study (highlighted in bold and colored by geography; see Table 1 for genogroup assignments) and reference sequences from bats, other mammals, and ectoparasites. Nodes are colored by posterior probability (nodes with less than 50% support are not shown).
Bacterial infections of sympatric bat species
Opportunistic sampling of other bats in Oklahoma revealed further bacterial diversity (Table 1). Three of the four cave myotis and both pallid bats tested positive for hemoplasmas, whereas three of three cave myotis, both pallid bats, and the single Townsend’s big-eared bat tested positive for bartonellae; the single hoary bat tested negative for both bacterial pathogens.
For hemoplasmas (Figure 2), we identified a single novel hemoplasma genotype in each PCR-positive species (i.e., MV1 and AP1), with 16S rRNA sequences most closely related (i.e., ≥98% similarity) to previously detected genotypes in vesper bats from Chile and Belize (e.g., EF1 and MYE) (6, 19). Notably, 16S rRNA sequences of the AP1 genotype were 96.5% similar to those of Candidatus Mycoplasma haemohominis (i.e., GU562823), whereas those of the MV1 genotype were only ~94% similar. 23S rRNA and rpoB sequences from cave myotis (OQ359173, OQ554337) were entirely novel (<85% similarity to GenBank sequences), with the former 100% similar to select 23S rRNA sequences identified from our Mexican free-tailed bats.
For bartonellae (Figure 3), all three positive cave myotis had their own genogroup. One gltA sequence was ~98% similar to that first identified in little brown bats (Myotis lucifugus) elsewhere in North America (i.e., KX807172), here denoted the ML1 genogroup. Another cave myotis had the TB4 genotype. The final cave myotis gltA sequence clustered within a clade of Candidatus Bartonella mayotimonensis sequences (~96%), isolated from a human endocarditis patient in Iowa, USA (FJ376732) and other little brown bats (KX807177–9) (11, 41). For pallid bats, one sequence also belonged to the TB4 genogroup, whereas the other formed the novel AP1 genogroup most related (~95%) to bartonellae from Natal long-fingered bats (Miniopterus natalensis) and their bat flies in South Africa (e.g., MW007711 and MW007707) (42). Lastly, the single positive Townsend’s big-eared bat hosted a unique genogroup (CT1) only ~94% similar to bartonellae found from Myotis nigricans in Guatemala (e.g., MN529509) (5).
Discussion
Hemoplasmas and bartonellae are emerging as model systems for studying bacterial infections in bats (5, 7), but their infection dynamics and diversity remain poorly characterized, notably in North American systems (11, 12). We here demonstrate novel diversity of hemoplasmas and bartonellae in bats in the south-central United States, including the circulation of lineages of both pathogens with clear infection seasonality in a migratory colony of Mexican free-tailed bats. Such work provides the foundation for further empirical studies to elucidate the transmission dynamics of these bacteria, their pathogenicity in bats, and their possible zoonotic risk.
Our findings suggest relatively common infection with site-specific and panmictic bacterial lineages. Within both bacterial genera, most lineages found in Mexican free-tailed bats were restricted to a single site, indicating spatially constrained transmission. However, we also detected lineages in multiple sites, such as the TB1 hemoplasma genotype in Louisiana, Texas, and Oklahoma; TB1 and MV1 hemoplasma genotypes as well as the Mycoplasma muris–like genotype in Texas and Oklahoma; and TB1 Bartonella genogroup in Texas and Oklahoma (for which sequences were nearly identical to those from Tadarida brasiliensis in Argentina) (20). Such results may be explained by migratory connectivity in Mexican free-tailed bats, for which regional migrations spanning hundreds to over 1,000 kilometers have been well-characterized in North America, including between the Selman Bat Cave and Bracken Cave (14, 15, 43); this suggests the migratory behavior of this species can enhance bacterial dispersal. However, given the presence of these bacterial lineages in migratory and non-migratory populations (i.e., Louisiana) and at the extremes of the bat range (e.g., over 5,000 km between the Selman Bat Cave and the Argentina site for the TB1 Bartonella genogroup), these results also suggest the ancestral spread of these bacteria and limited selection pressure on lineages across the bat range.
Future studies are needed to identify the migratory routes of Mexican free-tailed bats, especially for understanding the origins of possibly zoonotic bacterial lineages and the potential for these bats to disperse infection during spring and fall migrations. Researchers could capitalize on advances in tracking small vertebrates for long periods, such as use of absorbent sutures, to ensure lightweight radiotags stay attached to bats for the duration of migration and winter (44). Such work is also needed to assess if these infections negatively impact bat migration trajectory and success, as observed for blood pathogens in migratory songbirds (45). Longitudinal studies would also inform such analyses, as our data from one full occupancy season in the Oklahoma colony suggest bacterial prevalence peaks in the maternity season. Additional seasonal sampling is needed to assess how infection risk varies across the full migratory cycle, if prevalence tracks bat population size and/or ectoparasite intensity, and whether infections are sufficiently common in autumn to facilitate their dispersal with migration. Further genetic analyses could also inform patterns of bat connectivity and pathogen spread. For example, the TB2 Bartonella genogroup from Oklahoma Mexican free-tailed bats showed 100% identity to bartonellae from bat flies from Morelos, Hidalgo, and Jalisco in central Mexico (37), spanning the likely wintering sites of this bat species (14, 15). Similarly, previous analyses of Trypanasoma cruzi from this same Oklahoma bat population detected lineages similar to those along the Texas–Mexico border, further showing possible southern origins of infection and high pathogen dispersal capacity (46).
Additional molecular and immunological studies are also needed to better characterize these novel bat bacterial pathogens and their health impacts. We identified 16S rRNA and gltA sequences with moderate-to-high similarity to zoonotic pathogens such as C. Mycoplasma haemohominis, C. Bartonella mayotimonensis, and Bartonella rochalimae (9, 41, 47). For the former two pathogens, our bat sequences were ~96% similar to zoonotic lineages, likely indicating divergence from a common ancestor at least tens of million of years ago (7, 48). B. rochalimae has been found in cats and dogs (49), and our detections in Mexican free-tailed bats indicate a broadening of host range into bats. Our sequences showed ~97–100% similarity to those from fox fleas (40), relevant given likely flea transmission (49) and detection of fleas in the Oklahoma bat population where these sequences were found (Dyer, personal communication). Generation of whole genomes for our novel bat pathogens could inform their zoonotic risk, both by better linking them to cryptic human infections (9) and by facilitating machine learning models that predict zoonotic potential from genomic composition, as applied for viruses (50). Other -omics analyses could also elucidate whether these bacterial infections are pathogenic in bats themselves. In addition to assessing impacts of infection on migration outcomes as noted above, approaches such as transcriptomics and proteomics could test if bats have a pronounced immune response to these bacterial infections or appear largely tolerant (51). Such studies could be especially informative when comparing immunity between migratory and non-migratory periods, which could test whether long-distance migration may disrupt immune tolerance in bats.
Lastly, Mexican free-tailed bats and their sympatric bat species provide several important ecosystem services, including but not limited to predating on crop pests and contributing to the tourism economy from bat flight watching (52, 53). Understanding the prevalence, genetic diversity, and pathogenicity of bacterial pathogens in bats can inform One Health approaches that emphasize conservation measures to promote bat, domestic animal, and human health (54).
Supplementary Material
Acknowledgements
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health (P20GM134973), Louisiana Board of Regents Support Fund, Louisiana Biomedical Research Network, and Research Corporation for Science Advancement (RCSA). This work was conducted via Subawards No. 28365 and 29018, part of a USDA Non-Assistance Cooperative Agreement with RCSA Federal Award No. 58-3022-0-005. Additional support was provided by the Edward Mallinckrodt, Jr. Foundation and University of Oklahoma (Data Institute for Societal Challenges, Vice President for Research and Partnerships). Bat Conservation International provided in-kind support. We thank William Caire, Melynda Hickman, and the ODWC for site access; the Selman Living Laboratory for fieldwork support; and Konstantin Chumakov for laboratory support. We thank one anonymous reviewer for helpful feedback.
References
- 1.Letko M, Seifert SN, Olival KJ, Plowright RK, Munster VJ. 2020. Bat-borne virus diversity, spillover and emergence. Nat Rev Microbiol 18:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brook CE, Dobson AP. 2015. Bats as “special”reservoirs for emerging zoonotic pathogens. Trends Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szentivanyi T, McKee C, Jones G, Foster JT. 2023. Trends in Bacterial Pathogens of Bats: Global Distribution and Knowledge Gaps. Transbound Emerg Dis 2023. [Google Scholar]
- 4.Millán J, Di Cataldo S, Volokhov DV, Becker DJ. 2020. Worldwide occurrence of haemoplasmas in wildlife: Insights into the patterns of infection, transmission, pathology and zoonotic potential. Transbound Emerg Dis 10.1111/tbed.13932. [DOI] [PubMed] [Google Scholar]
- 5.McKee CD, Bai Y, Webb CT, Kosoy MY. 2021. Bats are key hosts in the radiation of mammal-associated Bartonella bacteria. Infect Genet Evol 89:104719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker DJ, Speer KA, Brown AM, Fenton MB, Washburne AD, Altizer S, Streicker DG, Plowright RK, Chizhikov VE, Simmons NB, Volokhov DV. 2020. Ecological and evolutionary drivers of haemoplasma infection and bacterial genotype sharing in a Neotropical bat community. Mol Ecol 29:1534–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volokhov DV, Lock LR, Dyer KE, DeAnglis IK, Andrews BR, Simonis MC, Stockmaier S, Carter GG, Downs CJ, Fenton MB, Simmons NB, Becker DJ. 2023. Expanded diversity of novel hemoplasmas in rare and undersampled Neotropical bats. One Health 17:100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai Y, Kosoy M, Recuenco S, Alvarez D, Moran D, Turmelle A, Ellison J, Garcia DL, Estevez A, Lindblade K, Rupprecht C. 2011. Bartonella spp. in Bats, Guatemala. Emerg Infect Dis 17:1269–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Descloux E, Mediannikov O, Gourinat A-C, Colot J, Chauvet M, Mermoud I, Desoutter D, Cazorla C, Klement-Frutos E, Antonini L, Levasseur A, Bossi V, Davoust B, Merlet A, Goujart M-A, Oedin M, Brescia F, Laumond S, Fournier P-E, Raoult D. 2021. Flying Fox Hemolytic Fever, Description of a New Zoonosis Caused by Candidatus Mycoplasma haemohominis. Clin Infect Dis 73:e1445–e1453. [DOI] [PubMed] [Google Scholar]
- 10.Veikkolainen V, Vesterinen EJ, Lilley TM, Pulliainen AT. 2014. Bats as reservoir hosts of human bacterial pathogen, Bartonella mayotimonensis. Emerg Infect Dis 20:960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lilley TM, Wilson CA, Bernard RF, Willcox EV, Vesterinen EJ, Webber QMR, Kurpiers L, Prokkola JM, Ejotre I, Kurta A, Field KA, Reeder DM, Pulliainen AT. 2017. Molecular Detection of Candidatus Bartonella mayotimonensis in North American Bats. Vector Borne Zoonotic Dis 17:243–246. [DOI] [PubMed] [Google Scholar]
- 12.Mascarelli PE, Keel MK, Yabsley M, Last LA, Breitschwerdt EB, Maggi RG. 2014. Hemotropic mycoplasmas in little brown bats (Myotis lucifugus). Parasit Vectors 7:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming TH, Eby P, Kunz TH, Fenton MB. 2003. Ecology of bat migration. Bat ecology 156:164–165. [Google Scholar]
- 14.Russell AL, Medellín RA, McCracken GF. 2005. Genetic variation and migration in the Mexican free-tailed bat (Tadarida brasiliensis mexicana). Mol Ecol 14:2207–2222. [DOI] [PubMed] [Google Scholar]
- 15.Glass BP. 1982. Seasonal Movements of Mexican Freetail Bats Tadarida brasiliensis mexicana Banded in the Great Plains. Southwest Nat 27:127–133. [Google Scholar]
- 16.Wiederholt R, López-Hoffman L, Cline J, Medellín RA, Cryan P, Russell A, McCracken G, Diffendorfer J, Semmens D. 2013. Moving across the border: modeling migratory bat populations. Ecosphere 10.1890/es13-00023.1. [DOI] [Google Scholar]
- 17.LaVal RK. 1973. Observations on the Biology of Tadarida brasiliensis cynocephala in Southeastern Louisiana. Am Midl Nat 89:112–120. [Google Scholar]
- 18.McCracken GF, Gassel MF. 1997. Genetic Structure in Migratory and Nonmigratory Populations of Brazilian Free-Tailed Bats. J Mammal 78:348–357. [Google Scholar]
- 19.Millán J, Cevidanes A, Sacristán I, Alvarado-Rybak M, Sepúlveda G, Ramos-Mella CA, Lisón F. 2019. Detection and Characterization of Hemotropic Mycoplasmas in Bats in Chile. J Wildl Dis 55:977–981. [PubMed] [Google Scholar]
- 20.Cicuttin GL, De Salvo MN, La Rosa I, Dohmen FEG. 2017. Neorickettsia risticii, Rickettsia sp. and Bartonella sp. in Tadarida brasiliensis bats from Buenos Aires, Argentina. Comp Immunol Microbiol Infect Dis 52:1–5. [DOI] [PubMed] [Google Scholar]
- 21.Stepanian PM, Wainwright CE. 2018. Ongoing changes in migration phenology and winter residency at Bracken Bat Cave. Glob Chang Biol 24:3266–3275. [DOI] [PubMed] [Google Scholar]
- 22.Ganow KB, Caire W, Matlack RS. 2015. Use of thermal imaging to estimate the population sizes of Brazilian free-tailed bat, Tadarida brasiliensis, maternity roosts in Oklahoma. swna 60:90–96. [Google Scholar]
- 23.Morgan CN, Ammerman LK, Demere KD, Doty JB, Nakazawa YJ, Mauldin MR. 2019. Field Identification Key and Guide for Bats of the United States of America. Occas Pap Tex Tech Univ Mus 360. [PMC free article] [PubMed] [Google Scholar]
- 24.Volokhov DV, Becker DJ, Bergner LM, Camus MS, Orton RJ, Chizhikov VE, Altizer SM, Streicker DG. 2017. Novel hemotropic mycoplasmas are widespread and genetically diverse in vampire bats. Epidemiol Infect 145:3154–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volokhov DV, Norris T, Rios C, Davidson MK, Messick JB, Gulland FM, Chizhikov VE. 2011. Novel hemotrophic mycoplasma identified in naturally infected California sea lions (Zalophus californianus). Vet Microbiol 149:262–268. [DOI] [PubMed] [Google Scholar]
- 26.Becker DJ, Bergner LM, Bentz AB, Orton RJ, Altizer S, Streicker DG. 2018. Genetic diversity, infection prevalence, and possible transmission routes of Bartonella spp. in vampire bats. PLoS Negl Trop Dis 12:e0006786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosmidis I, Kenne Pagui EC, Sartori N. 2020. Mean and median bias reduction in generalized linear models. Stat Comput 30:43–59. [Google Scholar]
- 28.Wood SN. 2017. Generalized Additive Models: An Introduction with R, Second Edition. CRC Press. [Google Scholar]
- 29.Lemoine F, Correia D, Lefort V, Doppelt-Azeroual O, Mareuil F, Cohen-Boulakia S, Gascuel O. 2019. NGPhylogeny.fr: new generation phylogenetic services for non-specialists. Nucleic Acids Res 47:W260–W265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.La Scola B, Zeaiter Z, Khamis A, Raoult D. 2003. Gene-sequence-based criteria for species definition in bacteriology: the Bartonella paradigm. Trends Microbiol 11:318–321. [DOI] [PubMed] [Google Scholar]
- 31.Volokhov DV, Simonyan V, Davidson MK, Chizhikov VE. 2012. RNA polymerase beta subunit (rpoB) gene and the 16S--23S rRNA intergenic transcribed spacer region (ITS) as complementary molecular markers in addition to the 16S rRNA gene for phylogenetic analysis and identification of the species of the family Mycoplasmataceae. Mol Phylogenet Evol 62:515–528. [DOI] [PubMed] [Google Scholar]
- 32.Legendre P, Legendre L. 2012. Numerical Ecology. 3rd English ed. Development in Environmental Modelling Vol. 24. Elsevier Science BV, Amsterdam: (990 pp.). [Google Scholar]
- 33.Stevens T, Zimmerman R, Albery G, Becker D, Kading R, Keiser C, Khandelwal S, Kramer-Schadt S, Krut-Landau R, McKee C, Montecino-Latorre D, O’Donoghue Z, Olson S, Poisot T, Robertson H, Ryan S, Seifert S, Simons D, Vicente-Santos A, Wood C, Graeden E, Carlson C. 2024. A minimum data standard for wildlife disease studies. EcoEvoRxiv. [Google Scholar]
- 34.Sacristán I, Acuña F, Aguilar E, García S, López MJ, Cevidanes A, Cabello J, Hidalgo-Hermoso E, Johnson WE, Poulin E, Millán J, Napolitano C. 2019. Assessing cross-species transmission of hemoplasmas at the wild-domestic felid interface in Chile using genetic and landscape variables analysis. Sci Rep 9:16816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hornok S, Földvári G, Rigó K, Meli ML, Gönczi E, Répási A, Farkas R, Papp I, Kontschán J, Hofmann-Lehmann R. 2015. Synanthropic rodents and their ectoparasites as carriers of a novel haemoplasma and vector-borne, zoonotic pathogens indoors. Parasit Vectors 8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mc Neely DL, Caire W. The fishes of traders creek in northwestern Oklahoma. http://nanfa.org/education/sll/traderscreek.pdf. Retrieved 4 February 2024.
- 37.Moskaluk AE, Stuckey MJ, Jaffe DA, Kasten RW, Aguilar-Setién A, Olave-Leyva JI, Galvez-Romero G, Obregón-Morales C, Salas-Rojas M, García-Flores MM, Aréchiga-Ceballos N, García-Baltazar A, Chomel BB. 2018. Molecular Detection of Bartonella Species in Blood-Feeding Bat Flies from Mexico. Vector Borne Zoonotic Dis 18:258–265. [DOI] [PubMed] [Google Scholar]
- 38.Beckmann S, Engelbrecht M, Chavez F, Rojas G. 2019. Prevalence of zoonotic Bartonella among prairie rodents in Illinois. J Mammal 101:291–297. [Google Scholar]
- 39.Birtles RJ, Raoult D. 1996. Comparison of partial citrate synthase gene (gltA) sequences for phylogenetic analysis of Bartonella species. Int J Syst Bacteriol 46:891–897. [DOI] [PubMed] [Google Scholar]
- 40.Millán J, Sepúlveda-García P, Di Cataldo S, Canales N, Sallaberry-Pincheira N, Painean J, Cevidanes A, Müller A. 2023. Molecular identification of Bartonella spp. and Rickettsia felis in fox fleas, Chile. Comp Immunol Microbiol Infect Dis 96:101983. [DOI] [PubMed] [Google Scholar]
- 41.Lin EY, Tsigrelis C, Baddour LM, Lepidi H, Rolain JM, Patel R, Raoult D. 2010. Candidatus Bartonella mayotimonensis and endocarditis. Emerg Infect Dis 16:500–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szentiványi T, Markotter W, Dietrich M, Clément L, Ançay L, Brun L, Genzoni E, Kearney T, Seamark E, Estók P, Christe P, Glaizot O. 2020. Host conservation through their parasites: molecular surveillance of vector-borne microorganisms in bats using ectoparasitic bat flies. Parasite 27:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villa BR, Cockrum EL. 1962. Migration in the Guano Bat Tadarida Brasiliensis Mexicana (Saussure). J Mammal 43:43–64. [Google Scholar]
- 44.Castle KT, Weller TJ, Cryan PM, Hein CD, Schirmacher MR. 2015. Using sutures to attach miniature tracking tags to small bats for multimonth movement and behavioral studies. Ecol Evol 5:2980–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emmenegger T, Bensch S, Hahn S, Kishkinev D, Procházka P, Zehtindjiev P, Bauer S. 2021. Effects of blood parasite infections on spatiotemporal migration patterns and activity budgets in a long-distance migratory passerine. Ecol Evol 11:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nichols MD, Lord WD, Haynie ML, Brennan RE, Jackson VL, Monterroso WS. 2019. Trypanosoma cruzi in a Mexican Free-Tailed Bat (Tadarida brasiliensis) in Oklahoma, USA. J Wildl Dis 55:444–448. [DOI] [PubMed] [Google Scholar]
- 47.Eremeeva ME, Gerns HL, Lydy SL, Goo JS, Ryan ET, Mathew SS, Ferraro MJ, Holden JM, Nicholson WL, Dasch GA, Koehler JE. 2007. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med 356:2381–2387. [DOI] [PubMed] [Google Scholar]
- 48.Ochman H, Elwyn S, Moran NA. 1999. Calibrating bacterial evolution. Proc Natl Acad Sci U S A 96:12638–12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pérez-Martínez L, Venzal JM, González-Acuña D, Portillo A, Blanco JR, Oteo JA. 2009. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis 15:1150–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mollentze N, Babayan SA, Streicker DG. 2021. Identifying and prioritizing potential human-infecting viruses from their genome sequences. PLoS Biol 19:e3001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vicente-Santos A, Lock L, Allira M, Dyer K, Dunsmore A, Tu W, Volokhov D, Herrera C, Lei G-S, Relich R, Janech M, Bland A, Simmons N, Neely B, Becker D. 2023. Serum proteomics reveals a tolerant immune phenotype across multiple pathogen taxa in wild vampire bats. Front Immunol 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bagstad KJ, Wiederholt R. 2013. Tourism Values for Mexican Free-Tailed Bat Viewing. Hum Dimensions Wildl 18:307–311. [Google Scholar]
- 53.Cleveland CJ, Betke M, Federico P, Frank JD, Hallam TG, Horn J, López JD Jr, McCracken GF, Medellín RA, Moreno-Valdez A, Sansone CG, Westbrook JK, Kunz TH. 2006. Economic value of the pest control service provided by Brazilian free-tailed bats in south-central Texas. Front Ecol Environ 4:238–243. [Google Scholar]
- 54.Rocha R, Aziz SA, Brook CE, Carvalho WD. 2020. Bat conservation and zoonotic disease risk: a research agenda to prevent misguided persecution in the aftermath of COVID-19. Animal. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.