Abstract
Background:
Botulinum toxin type A (BTX-A) is a potential treatment for cancer pain. This study aimed to analyze the effectiveness and safety of BTX-A in the treatment of pain after cancer treatment.
Patients and Methods:
Systematic searches of PubMed, Cochrane Library, and Embase databases were conducted. Randomized controlled trials evaluating the efficacy and safety of BTX-A compared with either placebo or active treatment in patients with pain after cancer treatment were included. The outcomes included pain intensity, quality of life, and adverse events.
Results:
This systematic review included four studies of which two were included in the meta-analysis. Compared with a placebo, BTX-A injection in patients with pain after cancer treatment had a clinically meaningful reduction in self-reported pain post-treatment [mean difference=−1.79 (95% CI: −2.14–−1.43), P<0.00001, I²=0%].
Conclusion:
This systematic review and meta-analysis demonstrated that BTX-A is safe and effective for pain relief in patients with pain after cancer treatment.
Keywords: botulinum toxin, botulinum toxin type A, cancer, pain
Introduction
Highlights
This study presented a quantitative and qualitative analysis of the use of botulinum toxin type A (BTX-A) in patients with pain after cancer treatment.
This is the first meta-analysis that evaluated the application and therapeutic effect of BTX-A in the treatment of patients with pain after cancer treatment.
Our study proved that BTX-A had a favorable effect and was safe to use in the treatment of patients with pain after cancer treatment.
Cancer patients commonly experience pain. Cancer pain or cancer-related pain can be considered an unpleasant sensory and emotional experience associated with actual or potential tissue damage, which can be caused by tumor growth and related pathophysiological/pathological processes, invasive procedures, treatment toxicities, infection, and physical limitations1. The underlying mechanisms of cancer pain are complex and involve both nociceptive and neuropathic processes2. Nociceptive pain arises from nociceptor stimulation prompted by actual or threatened damage to non-neural tissues and can be further categorized as somatic or visceral based on the level of structures involved3. Pain stemming from an injury or damage to the somatosensory nervous system is classified as neuropathic pain4. Furthermore, cancer pain frequently involves mixed pathophysiology, with both nociceptive and neuropathic components. For instance, a primary nociceptive pain state may in time prompt secondary lesions in the somatosensory nervous system, resulting in the pain acquiring a partial neuropathic nature as well5. Inflammation plays a key role in driving both nociceptive and neuropathic cancer pain through the release of inflammatory mediators like cytokines, chemokines, growth factors, and protons in the tumor microenvironment2,6. Cancer treatment is one of the important causes of cancer pain. According to estimates, discomfort is present at or close to the local radiation or surgery site for cancer in ~25% of patients7,8. Because of fibrosis, scarring, and keloid development, List and Bilir observed postradiation pain in 15–30% of patients with head and neck cancer9. After radiation and surgery, moderate-to-severe local pain may require strong opioid-based systemic analgesics, which, while helpful, frequently have negative side effects and have the potential for significant abuse10,11.
Botulinum toxin is a drug produced by the gram-positive anaerobic bacterium, Clostridium botulinum. Botulinum toxin (BTX or BoNT) was initially discovered by Justinus Kerner, a German physician, and poet12. It is classified into seven neurotoxins(A–G)13. By preventing the release of acetylcholine from the presynaptic terminal, BTX disables the activity of the glands and muscles14,15. Neurotransmitter release is inhibited when the light chain of Botulinum toxin type A (BTX-A) cleaves the intracellular protein known as SNAP-25 (synaptosome-associated protein with a molecular weight of 25 kDa)16. The anticholinergic activity of BTX type A at the neuromuscular junction makes it a broad-spectrum medication. Therapy for involuntary muscle spasms such as spasmodic torticollis, blepharospasm, cervical dystonia, and spasmodic dysphonia makes substantial use of BTX-A17. A crucial neurotransmitter in the peripheral parasympathetic nervous system is acetylcholine. Thus, BTX-A, which can control parasympathetic activation, is used to treat epiphora, sialadenitis, hyperhidrosis, and Frey syndrome18.
In recent years, the use of BTX-A as an antinociceptive agent for cancer pain has been explored. Although the analgesic mechanism of BTX-A has not been fully elucidated, there is evidence that botulinum toxin can inhibit the secretion of pain mediators (substance P, glutamate, and calcitonin gene-related protein) in nerve endings and dorsal root ganglia, directly affecting the anterolateral and trigeminal thalamic systems of pain transmission19–24. GlyT2 was recently found to contribute to the antineoplastic effect of BoNT (BTX-A) in CCI-induced neuropathic pain25. In the study by Daele et al.26, they reported for the first time the application of BTX-A in patients with pain after cancer treatment. The application of BoNT (BTX-A) seems to be more effective, with relatively lower side effects, and a lower risk of drug interactions27. However, it has been reported that low concentration of BTX-A seems to be an effective treatment option for chronic neuropathic pain in the neck and shoulder after neck dissection compared with high concentration of BTX-A28. So how to ensure the safe use and effective analgesic or therapeutic effect of BTX-A is the current hot-point problem in the application of BTX-A in cancer pain treatment. Currently, there is a lack of evidence supporting the effectiveness of BTX-A in patients with pain following cancer therapy. This systematic review and meta-analysis aimed to evaluate the effectiveness and safety of BTX-A in the treatment of pain after cancer treatment. BTX-A was compared to a placebo or an active medication in randomized controlled studies to determine its effectiveness and safety in treating patients with pain after cancer treatment. The outcomes included pain intensity, quality of life, and adverse events.
Material and methods
The study protocol was registered with the Prospective Register of Systematic Reviews (PROSPERO). This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 (Supplemental Digital Content 4, http://links.lww.com/JS9/B342) (Supplemental Digital Content 5, http://links.lww.com/JS9/B343) and AMSTAR (Assessing the methodological quality of systematic reviews) Guidelines (Supplemental Digital Content 6, http://links.lww.com/JS9/B344)29,30. This study differed from the protocol in several key aspects. First, because we finally included studies in which patients with pain caused by cancer treatment were treated with BTX-A, we changed the title to ʽ The Application and therapeutic effect of botulinum toxin type A (BTX-A) in the Treatment of Patients with pain after cancer treatment: A systematic review and Meta-analysisʼ. Second, the intervention was specified as BTX-A rather than the broader ʽapplication of botulinum toxinʼ. The comparator was narrowed to placebo or other active therapy, rather than no botulinum toxin. Third, the primary outcome was modified to pain intensity, and the secondary outcomes were changed to quality of life and adverse events based on the extracted literature data. Fourthly, the strategy for data synthesis relied solely on Review Manager 5.3 as its functions were sufficient for our needs, unlike the original protocol. Finally, we chose the funnel plot to assess reporting bias rather than egger’s test because of the small number of included studies.
Eligibility criteria
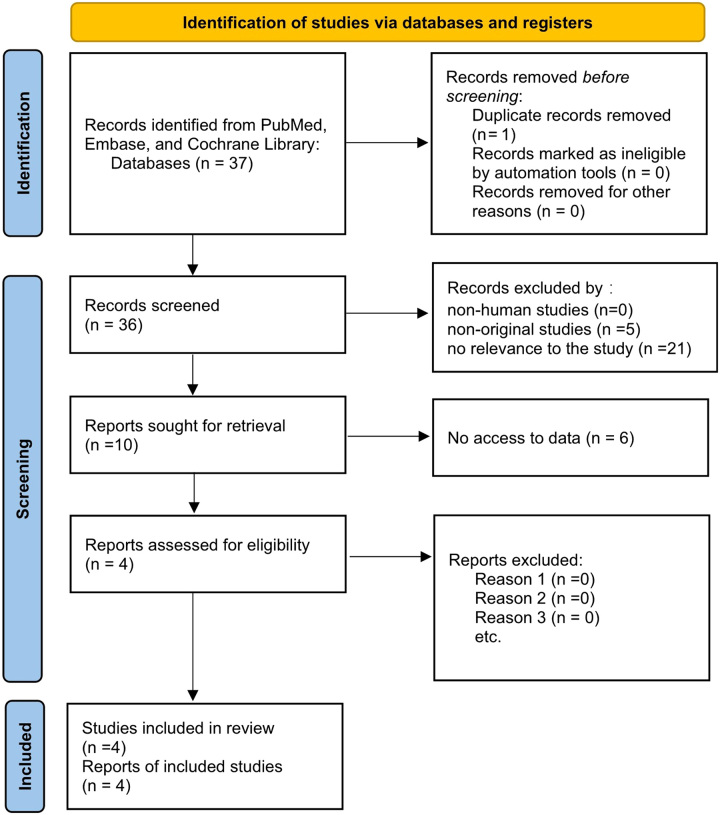
The inclusion criteria for studies were as follows: (1) patients had pain after cancer treatment, (2) the intervention administered was BTX-A, (3) the comparator was either placebo or another active therapy, (4) outcomes included pain intensity, quality of life, and adverse effects, and (5) the study design was a randomized controlled trial. The exclusion criteria were as follows: (1) duplicate records, (2) nonhuman studies, (3) nonoriginal studies (letters, reviews, and editorials), (4) no relevance to the study, and (5) no access to data Figure 1.
Figure 1.
Flow diagram of study selection for the systematic review and meta-analysis.
Data collection and retrieval strategies
We conducted systematic electronic searches for RCTs, regardless of publication status or year of publication. The most recent search was performed on 14 May 2023. The following databases were used to search for relevant studies: PubMed, Embase, and the Cochrane Library. Medical Subject Headings (MeSH) or the keywords ʽCancer painʼ, ʽCancer-Associated Painʼ, and ʽOncological Painʼ were used to search the literature without language restrictions. The specific search strategy is shown in the Supplementary Item 1 (Supplemental Digital Content 1, http://links.lww.com/JS9/B339).
Selection process and data extraction
Following strict respect to previously established inclusion and exclusion criteria, two reviewers independently evaluated all retrieved literature. The first step was to conduct an initial screening by going overall the retrieved studies’ titles and abstracts. After eliminating studies that were duplicates or did not meet the inclusion criteria, the remaining studies were reviewed, and all possibly suitable studies were found by reading the complete text.
With a predesigned table, two authors independently performed data extraction. Any disagreements were resolved by a senior researcher. Demographic and outcome data were also extracted. The demographics of the included studies included the study period, region, duration of pain, follow-up period, sample size, mean age, sex, control arm (placebo and/or active treatment), and outcome measures. The primary outcome was pain intensity. The secondary outcomes were quality of life and adverse events. The baseline value, final value, and change score of each outcome were extracted. When a change score could not be extracted, the final value was used for data analysis.
Data synthesis and statistical analysis
All statistical assessments were conducted using Review Manager (RevMan) version 5.3 (Cochrane Collaboration). SD, 95% CI, and mean difference (MD) or standardized mean difference (SMD) were used to represent continuous data. The standard error, median, range, or 95% CI were imputed if the SD was not supplied. Based on the method described by Wan et al.31, the medians and interquartile ranges of continuous data were converted to means and SDs. A heterogeneity (I²) statistic was used to evaluate the differences in treatment effects among studies. An I² of <40%, 40–60%, and >60% represented low, moderate, and substantial heterogeneity, respectively. When the heterogeneity was low, a fixed-effects model was used. If the heterogeneity was significant, a random-effects model was applied for a more conservative estimation of the differences. Sensitivity analysis was performed using Review Manager (RevMan) version 5.3 (Cochrane Collaboration). Subgroup analysis was performed according to the muscle subgroups at different sites. The funnel plot was used to assess reporting bias. We applied the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system to assess the certainty of the outcomes.
Risk of bias in individual studies
The Cochrane Handbook for Systematic Reviews of Interventions was used to analyze the risk of bias in determining the quality of the included studies. Two reviewers independently analyzed each study. The risk of bias in each study was evaluated across five bias domains: selection bias, performance bias, detection bias, attrition bias, and reporting bias. Each domain was assigned one of the three risk levels: low, high, or unclear. If the reported procedures satisfied the standards for low or high risk of bias in that domain, it was assessed whether they were at low or high risk of bias. The choice of unclear risk of bias was made when there was either a dearth of information or doubt regarding the likelihood of bias32.
Results
Study selection
The electronic and manual systematic searches identified 37 articles. One study was excluded because of duplication before screening. After screening titles and abstracts, 26 articles were excluded. Ten articles were retrieved, of which six were excluded because data could not be obtained. The full texts of four articles were reviewed for eligibility28,33–35. Two studies were included in the qualitative analysis28,34. Consequently, data from two studies were included in the meta-analysis33,35. A flowchart of the study retrieval and selection is illustrated in Figure 1.
Study characteristics
The characteristics of the included studies are shown in Tables 1 and 2. Table 1 described the study period, region, duration of pain, follow-up, basic information of patients (number, mean age, and sex), and the situation of the intervention group and the control group. Table 2 recorded the pain intensity, quality of life, and adverse effects of the patients included in the four articles. All four articles were randomized controlled trials (RCTS) published between 2006 and 2019 and included patients with pain duration of at least 3 months28,33–35. The intervention in each literature was BTX-A, but the control group was different. Unlike other studies, Groef et al.33,34 recorded pain intensity at both sites, and quality of life recorded both aspects of physical functioning and mental functioning.
Table 1.
Characteristics of the included studies.
| Study period | Region | Duration of pain | Follow-up (d) | Patients (n) | Mean age (y) | Male (n) | Intervention (n) | Placebo (n) | Active control (n) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Groef et al.33 2018 | 2017 | Belgium | at least 3 months | 180 | 50 | 55±10 | NRa | BTX-Ab 25 | Saline 25 | |
| Groef et al.34 2020 | 2017 | Belgium | at least 3 months | 180 | 50 | 55±10 | NR | BTX-A 25 | Saline 25 | |
| Li et al.35. 2019 | 2019 | China | NR | 30 | 120 | 51±6.5 | 66 | BTX-A 60 | NR | |
| Wittekindt et al.28 2006 | 2006 | Germany | at least 12 months | 28 | 23 | 60.4±11.4 | 21 | BTX-A 13 | NR | BTX-A 10 |
NR, not reported.
BTX-A, botulinum toxin type A.
Table 2.
Characteristics of the included studies.
| Baseline of pain intensity | Pain intensity | Baseline of quality of life | Quality of life | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome measures | Study group | Control group | Study group | Control group | Study group | Control group | Study group | Control group | Adverse events | |
| Groef et al.33 2018 | VAS a SF-36 b0–100 | The UL cregion 64 ±22 the pectoral region: 56 ±23 | The UL region 64 ±19 the pectoral region: 58±21 | The UL region 9 ±27 the pectoral region 24 ±31 | The UL region 55±28 the pectoral region: 38 ±35 | Physical functioning 62.2±17.6 mental functioning 70.7±17.3 | Physical functioning 43.6 ±20.9 mental functioning: 64.3±18.7 | Physical functioning 68.2±23.4 mental functioning 69.4 ±16.5 | Physical functioning 49.0±19.2 mental functioning 70.4 ±18.7 | No adverse events after the infiltrations occurred |
| Groef et al.34 2020 | NRd | NR | NR | NR | NR | NR | NR | NR | NR | No adverse events after the infiltrations occurred |
| Li et al.35 2019 | NRSe | 4.6±1.1 | 4.2±0.9 | 2.0±0.8 | 3.8±1.2 | NR | NR | NR | NR | No adverse events after the infiltrations occurred |
| Wittekindt et al.28 2006 | VAS EORTC-QLQ-C-30f | 4.3±1.0 | 4.2±1.5 | 3.0±1.9 | 4.3±3.3 | 54.6±15.4 | 56.3±19.2 | 59.4±20.9 | 57.3±28.5 | Serious side effects were not encountered |
VAS, visual analog scale.
SF-36, short form-36.
UL, upper limb.
NR, not reported.
NRS, numerical rating scale.
EORTC-QLQ-C-30, European Organization for Research and Treatment of Cancer Quality of Life Core Questionnaire.
Two articles were expressions of the primary and secondary outcomes of the same study, respectively, and the article expressing the primary outcome was included in the meta-analysis33,34. In the studies included in the meta-analysis, the reported primary outcome measure was a Visual Analog Scale (VAS) on a scale of 0–100 in one study33, and a Numerical Rating Scale (NRS) on a scale of 0–10 in one study35. VAS and NRS were standardized during data consolidation. One article was included in the qualitative analysis because it was a dose study without a control group and the unit could not be compared with the other two studies28. In the study of Li et al.35, pain intensity was measured after postoperative wakefulness and after 3 days of infusion. In the study by Groef et al.33, pain intensity was measured before injection and at 3 and 6 months after injection.
Intervention
In the study by Groef et al.33, patients in the intervention group received intramuscular BTX‐A (Allergan) infiltration in the pectoralis major muscle on the operated side. Patients in the control group received an intramuscular injection of 50 ml saline (Mini-Plasco 20 ml B. Braun NaCl 0.9%). In the study by Li et al.35, the intervention group was treated with BTX-A (Lanzhou Institute of Biological Products) based on the control group 24 h after the operation. In a study by Wittekindt et al.28, patients received BTX-A Dysport formulation (Ipsen, Pharma). BTX-A was reconstituted in saline to a concentration of 10 mouse units (MU)/0.1 ml saline (low-dose group) or 20 MU/0.1 ml saline (high-dose group).
Risk of bias in studies
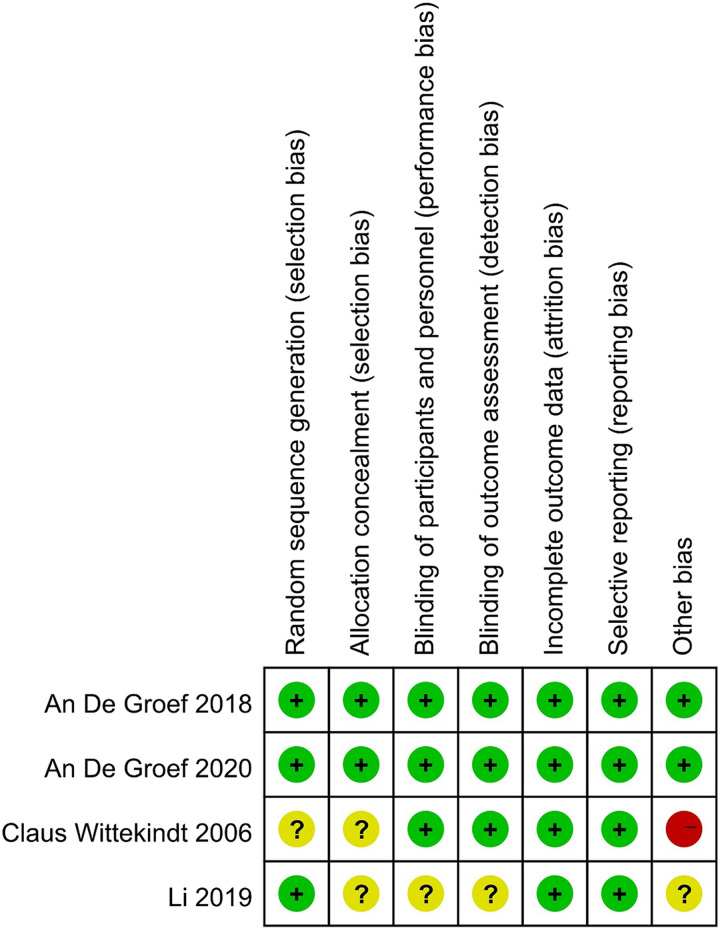
The quality of the articles was evaluated using the Cochrane Collaboration tool to assess the risk of bias. Groef et al.33 and Groef et al.34 had low-risk bias. Li et al.35 did not clearly state that allocation concealment and blinding were subject to unclear selection, performance, and detection biases, and there was insufficient information to determine the existence of other biases. Wittekindt et al.28 had an unclear risk of bias because it did not specify the method of randomization and allocation concealment, and it had a high risk of other bias because it enrolled only 23 patients. Figure 2 summarizes the assessment of the risk of bias for each included study.
Figure 2.
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
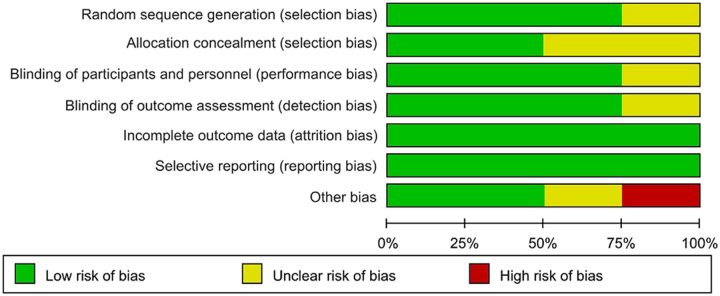
Unclear risks of bias in random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other biases were found in 25, 50, 25, 25, and 25% of the included RCTs, respectively. Twenty-five percent had a high risk of bias for other biases (Fig. 3).
Figure 3.
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
Results of individual studies
A study by Groef et al.33 demonstrated the benefit of a single Botulinum Toxin A infiltration combined with an individual physical therapy program at the upper limb in breast cancer survivors for up to 6 months. There were no differences between the groups in terms of changes in pain intensity from baseline up to 1 and 3 months (primary analysis). Between the groups, there was a substantially distinct change in upper limb pain intensity from baseline to 6 months in favor of the intervention group (P=0.040). The mean difference in change was 16 points on the VAS (0–100) (95% CI:1–31). For pain intensity in the pectoral region, a larger decrease in the intervention group up to 6 months after baseline was also observed. In contrast to the control group, this difference was not statistically significant (mean difference in change 13/100; 95% CI: −4–31). Moreover, both significant results were not clinically relevant, that is, a decrease of at least 20/100 169 on the VAS score. In addition, a hardly significant finding for mental functioning was found in favor of the control group for quality of life from baseline up to 6 months (P=0.049; 95% CI: 0.04–14.68). No other beneficial effects were found, and no adverse events occurred after infiltration.
Groef et al.34 reported the same study in 2020 as in 2018 but with secondary outcomes. This study examined the effect of a single BTX-A infiltration in the pectoralis major muscle, in addition to a routine physical therapy regimen, on shoulder mobility, upper limb strength, shoulder posture and kinematics, and shoulder function in women following breast cancer treatment. No significant differences were found between the groups in the change of the outcome parameters over time. However, improvements in shoulder mobility and function were observed in both groups, indicating the possible beneficial effects of the standard physical therapy program. In terms of therapeutic effect, a single BTX-A infiltrate of the pectoralis major muscle was not recommended outside the standard physiotherapy protocol to improve upper limb injury and dysfunction after breast cancer treatment.
Li et al.35 evaluated 120 patients with low rectal cancer after surgery. There was no significant difference in the pain index scores between the two groups after waking up (P=0.084; t=0.387). Three days after the operation, the pain index of the intervention group was significantly lower than that of the control group (P=0.031; t=10.258). The postoperative hospital stay was significantly shorter in the intervention group than in the control group (P=0.029; t=17.935). Before treatment, there was no significant difference in pelvic floor muscle tension, fast and slow muscle strength, or slow muscle endurance between the two groups (P>0.05). After treatment, the pelvic floor muscle strength of the intervention group was significantly lower than that of the control group (P<0.05). No adverse reactions occurred in the observation group, and the total incidence of postoperative complications was 6.7% in the intervention group and 16.6% in the control group; this difference was statistically significant (P<0.05). The incidence of anastomotic leakage in the intervention group was significantly lower than that in the control group (P<0.05). In conclusion, this study found that botulinum toxin injection into the pelvic floor muscle of postoperative patients with low rectal cancer can not only reduce the pelvic floor muscle strength and prevent the occurrence of anastomotic leakage, but also relieve incision pain, shorten the time of anal exhaust and the length of postoperative hospital stay, without obvious adverse reactions.
Wittekindt et al.28 described a clinical trial. Twenty-three patients with neuropathic pain after neck dissection were included in the trial. All patients underwent extensive conservative treatment for neck and shoulder pain after neck dissection. The patients were divided into low-dose (n=13) (ONA, 80–120 mouse units) and high-dose groups (n=13) (ONA 160–240 mouse units). Pain and quality of life were assessed on days 0 and 28, respectively. Pain assessment was based on a VAS. Quality of life was evaluated by the global quality of life scale (QLQ-C-30) and the multiple-item scale ʽpainʼ (QLQ-H&N35). A lower score denotes better functioning on the functional scale ʽpainʼ in the QLQ-H&N35 (four contributing questions). The mean pain VAS score of all patients was 4.3±1.4 before treatment and showed a nonsignificant decrease to 3.6±2.5 points on day 28 (P=0.15). The self-assessment of pain VAS of patients in the low-dose group decreased considerably (P=0.05) from 4.3±1.0 on day 0 to 3.0±1.9 on day 28. When comparing days 0 and 28 (P=0.86), the high-dose group’s mean VAS scores showed a marginal increase, but the difference was not statistically significant. The low-dose group showed a tendency, but not a significant improvement, in ʽglobal quality of lifeʼ (P=0.15), and a nonsignificant reduction in the functional scale ʽpainʼ (P=0.10). In terms of therapeutic effect, they suggested that local subcutaneous injection of BTX-A may be an effective and well-tolerated treatment modality to reduce chronic neuropathic pain after neck dissection.
Results of syntheses
Primary outcome: pain intensity
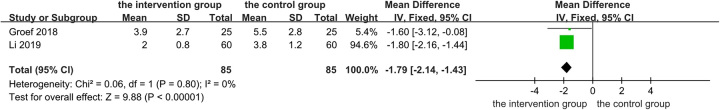
Two articles with a total of 170 patients were included in the meta-analysis of pain intensity. In the study by Groef et al.33, the BTX-A injection group was compared with the placebo group. However, in another study, the intervention group was administered BTX-A 24 h after surgery based on the control group35. Pain intensity scores at the end point of each study were included in the meta-analysis and standardized. As shown in Figure 4, compared with the control group, pain in the patients was significantly relieved after the injection of BTX-A. (MD −1.79, 95% CI: −2.14–−1.43, two RCTs, P<0.00001). An I² of 0% indicated no statistical heterogeneity, so we chose a fixed-effect model. The results were unchanged when the model was changed to a random-effect model. According to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system, the primary outcome was evaluated by the GRADEpro GDT (Guideline Development Tool). The certainty of the evidence for the primary outcome was ʽModerateʼ because of possible biases in the included study. You can find details in the Supplementary Item 3 (Supplemental Digital Content 2, http://links.lww.com/JS9/B340).
Figure 4.
Changes in pain intensity: botulinum toxin type A injection versus no botulinum toxin type A. df, degrees of freedom.
In the study by Wittekindt et al.28, contrary to our common knowledge, patients in the low-dose group improved significantly (P<0.05) in their self-assessment of pain using the VAS. However, in the high-dose group, the mean VAS scores increased slightly, but this change was not statistically significant when comparing data between day 0 and day 2828.
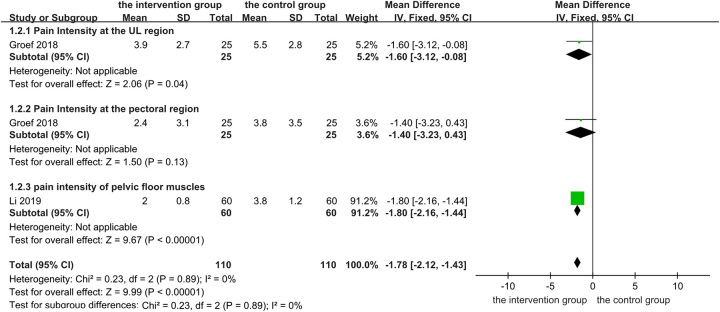
Subgroup analyses by pain intensity in different muscles
In the study by Groef et al.33, the primary outcome was a change in pain intensity in the upper limb, but pain intensity in the pectoral region was also reported; therefore, a subgroup analysis was considered for pain intensity in different muscles (Fig. 5). The results of the subgroup analysis by pain intensity in different muscles were inconclusive because there was only one study in each subgroup. The results showed that BTX-A significantly relieved pain intensity in different muscles (MD−1.78, 95% CI: −2.12–−1.43, P<0.00001). While the subgroup analysis may not provide definitive conclusions in this particular context, it can still contribute valuable insights to the field. From the perspective of systematic review, the subgroup analysis may help readers understand the application and treatment effect of BTX-A in different muscles to a certain extent.
Figure 5.
Changes in pain intensity in different muscles: botulinum toxin type A injection versus no botulinum toxin type A. df, degrees of freedom.
Secondary outcomes: quality of life and adverse events
Quality of life was assessed in only one of the included studies; therefore, no synthesis could be performed33. No adverse events were found in the two studies. In the study by Groef et al.33, a borderline significant outcome for mental functioning was found at 6 months in favor of the control group for quality of life (P=0.049, 95% CI: 0.04–14.68). Additionally, the remark should be made that at baseline the intervention group had higher scores (70.7±17.3 vs. 64.3±18.7).
In the study by Wittekindt et al.28, the low-dose group showed a tendency, but not a significant improvement, in ʽglobal quality of lifeʼ (P=0.15), and a nonsignificant reduction in the functional scale ʽpainʼ (P=0.10).
Reporting biases
Because only two studies were included in the meta-analysis and both were statistically significant, an asymmetric funnel plot was obtained (Supplementary Item 2, Supplemental Digital Content 3, http://links.lww.com/JS9/B341).
Discussion
To our knowledge, this is the first meta-analysis to examine the use of BTX-A and its therapeutic impact on the management of patients with pain after cancer treatment. To a certain extent, this study supports the idea that botulinum toxin can be used to alleviate postcancer treatment pain. Our findings demonstrated the beneficial effect of BTX-A in relieving pain intensity in patients with pain after cancer treatment, enhancing the quality of life to some extent, and causing few adverse events, according to a systematic review and meta-analysis.
Previous studies have demonstrated that local injection of BTX into areas of scar/fibrosis or allodynia may substantially alleviate this type of pain in cancer patients (four prospective and three retrospective trials, as well as multiple case reports)26–28,36–40. In addition, several cases demonstrated the effectiveness of BTX therapy as a low-risk treatment option for cancer pain at the end of life41. Grenda et al.10 summarized data and reports considering BTX use in cancer therapy. This review concluded that there may be noninvasive, very successful therapeutic usage for BTXs in the treatment of many types of neoplasms. One study suggested that botulinum toxin injections are currently one of the most common treatment options for alleviating post-treatment pain in breast cancer patients42. It can achieve pain relief by addressing postoperative muscle spasms and potentially altering pain cascades thus improving nociceptive and neuropathic pain in postbreast cancer surgery patients43,44. Kim et al. reported two cancer patients with intractable pain with psoas muscle invasion who achieved long-term improvement in pain symptoms and movement after botulinum toxin injection into the iliopsoas muscle after ineffective usage of opioids, chemoradiation, anti-inflammatory agents, anesthetic agents, and/or nerve root blocks45. However, a recent review of malignant psoas syndrome that included this study found that three patients reported no improvement in pain symptoms with muscle relaxants, including flunitrazepam, diazepam, and clonazepam, despite concurrent administration of muscle relaxants with agents such as opioids, radiation therapy, chemotherapy, anti-inflammatory agents, SNRIs, anticonvulsants, and anesthetic agents46. Although the evidence from this systematic review and meta-analysis is uncertain because of the small number of studies included, it also shows that BTX-A has a positive therapeutic effect on patients with cancer pain to a certain extent.
This study has several limitations. First, there were insufficient studies included in this review due to the few RCTs of relevant studies. In particular, the evidence for secondary outcomes was even more lacking than for primary outcomes, which made the secondary outcomes more uncertain. Furthermore, subgroup analysis may not provide definitive conclusions in this context. In the future, we will include more newly published studies to continuously improve the reliability of the article. Secondly, there were great differences between the two studies, which led to some clinical or methodological heterogeneity: (1) The characteristics of the patients included in the literature vary significantly. In the study by Groef et al.33, patients had to have undergone relevant surgery, had their radiotherapy stopped at least three months earlier, and had more than 3 months of pain in the pectoral region (that is, maximum pain intensity during the past week during activities >0/100 on the visual analog scale). However, another study selected patients who had just undergone surgery35. (2) In the study by Groef et al.33, the intervention and control groups received physical therapy in addition to BTX-A and saline. However, in another study, the intervention group was treated with BTX-A in addition to the control group’s treatment35. In addition, there are insufficient studies to perform subgroup analyses according to dose. Finally, only subjective outcomes were used in each of the four included studies. Therefore, further studies using well-designed RCTs with objective outcomes are warranted.
This systematic review and meta-analysis illustrated the efficacy and safety of BTX-A in patients with pain after cancer treatment. Despite the limitations of our study, our results have a broader context and potential significance, and it still contributes to the overall body of knowledge in the field. On the basis of our preliminary results, future studies on the use of BTX-A for pain after cancer treatment should include more RCTs followed by high-quality meta-analyses to obtain more definitive results, especially to increase the quantitative evaluation of patient quality of life and adverse effects. Currently, nontoxic BTX constructs are being studied. These novel constructs may exhibit a variety of therapeutic effects and may soon find widespread use. This study might provide support for the future use of BTX-A on a larger scale.
Conclusions
Evidence from four RCTs demonstrated the beneficial effect of BTX-A, which was safe for the treatment of patients with pain after cancer treatment. The BTX-A relieved pain intensity, and there were few adverse effects.
Ethical approval
The study does not involve any ethical.
Consent
The study does not involve any ethical.
Sources of funding
This study received support from the Hunan Provincial Science and Technology Plan Project Funding (2020SK50303), the Hunan Medical Association Medical Research Fund Grant Project (HMA202101004), the Chenzhou Key Project Funding of Science and Technology Plan (ZDYF2020012), the Chenzhou National Sustainable Development Agenda Innovation Demonstration Zone Construction Innovative Province Project (2019sfq04) and the 2023 Annual health appropriate Technology Promotion Project of Hunan Health Commission (202319019440).
Author contribution
S.L.: study design, search, study selection, data collection, data analysis, and drafting of the article; S.P.: search, study selection, data collection, and article revision; F.C.: study selection and data analysis; B.Z.: article revision; Z.Z.: data curation and supervision; Z.Z.: conception, study design, data analysis, drafting of the article, and final approval.
Conflicts of interest disclosure
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Research registration unique identifying number (UIN)
The study protocol was registered with the Prospective Register of Systematic Reviews (PROSPERO), CRD42023430275. This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 and AMSTAR (Assessing the methodological quality of systematic reviews) Guidelines. This study differed from the protocol in terms of outcomes, inclusion and exclusion criteria, and strategy for data synthesis because these aspects were adjusted on the basis of data extracted from the literature.
Guarantor
Zhiming Zhang, MD, PhD. Tel.: +8613875555649. E-mail: otc0735@163.com.
Availability of data
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgements
The authors are very grateful to Doctor Changsheng Huang for his guidance, and the authors also grateful to Yuxiang Xue for his statistical help.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 21 November 2023
Contributor Information
Shuzhai Li, Email: 1610575995@qq.com.
Shixuan Peng, Email: 18773845997@163.com.
Fuchun Chen, Email: hunting588@163.com.
Bin Zeng, Email: zb2119@163.com.
Zhen Zhang, Email: mz0735@163.com.
Zhiming Zhang, Email: otc0735@163.com.
References
- 1. Raja SN, Carr DB, Cohen M, et al. The revised International Association for the study of pain definition of pain: concepts, challenges, and compromises. Pain 2020;161:1976–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falk S, Dickenson AH. Pain and nociception: mechanisms of cancer-induced bone pain. J Clin Oncol 2014;32:1647–1654. [DOI] [PubMed] [Google Scholar]
- 3. Merskey H, Bogduk N. Classification of Chronic Pain, Second Edition. IASP Press; 2012:213–18. [Google Scholar]
- 4. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016;157:1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caraceni A, Shkodra M. Cancer pain assessment and classification. Cancers (Basel) 2019;11:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci 2006;7:797–809. [DOI] [PubMed] [Google Scholar]
- 7. Kanner RM, Foley KM. Patterns of narcotic drug use in a cancer pain clinic. Ann N Y Acad Sci 1981;362:161–172. [DOI] [PubMed] [Google Scholar]
- 8. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–1625. [DOI] [PubMed] [Google Scholar]
- 9. List MA, Bilir SP. Functional outcomes in head and neck cancer. Semin Radiat Oncol 2004;14:178–189. [DOI] [PubMed] [Google Scholar]
- 10. Grenda T, Grenda A, Krawczyk P, et al. Botulinum toxin in cancer therapy-current perspectives and limitations. Appl Microbiol Biotechnol 2022;106:485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mittal SO, Jabbari B. Botulinum neurotoxins and cancer-a review of the literature. Toxins (Basel) 2020;12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erbguth FJ, Naumann M. Historical aspects of botulinum toxin: Justinus Kerner (1786-1862) and the “sausage poison”. Neurology 1999;53:1850–1853. [DOI] [PubMed] [Google Scholar]
- 13. Tighe AP, Schiavo G. Botulinum neurotoxins: mechanism of action. Toxicon 2013;67:87–93. [DOI] [PubMed] [Google Scholar]
- 14. Ozcan C, Ismi O. Botulinum toxin for rhinitis. Curr Allergy Asthma Rep 2016;16:58. [DOI] [PubMed] [Google Scholar]
- 15. Zhang EZ, Tan S, Loh I. Botolinum toxin in rhinitis: literature review and posterior nasal injection in allergic rhinitis. Laryngoscope 2017;127:2447–2454. [DOI] [PubMed] [Google Scholar]
- 16. Aoki KR, Guyer B. Botulinum toxin type A and other botulinum toxin serotypes: a comparative review of biochemical and pharmacological actions. Eur J Neurol 2001;8(Suppl 5):21–29. [DOI] [PubMed] [Google Scholar]
- 17. Davis EC, Barnes MP. Botulinum toxin and spasticity. J Neurol Neurosurg Psychiatry 2000;69:143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laing TA, Laing ME, O’Sullivan ST. Botulinum toxin for treatment of glandular hypersecretory disorders. J Plast Reconstr Aesthet Surg 2008;61:1024–1028. [DOI] [PubMed] [Google Scholar]
- 19. Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology 2005;26:785–793. [DOI] [PubMed] [Google Scholar]
- 20. Aoki KR, Francis J. Updates on the antinociceptive mechanism hypothesis of botulinum toxin A. Parkinsonism Relat Disord 2011;17(Suppl 1):S28–S33. [DOI] [PubMed] [Google Scholar]
- 21. Arezzo JC. Possible mechanisms for the effects of botulinum toxin on pain. Clin J Pain 2002;18(Suppl 6):S125–S132. [DOI] [PubMed] [Google Scholar]
- 22. Cui M, Khanijou S, Rubino J, et al. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain 2004;107:125–133. [DOI] [PubMed] [Google Scholar]
- 23. Intiso D, Basciani M, Santamato A, et al. Botulinum toxin type A for the treatment of neuropathic pain in neuro-rehabilitation. Toxins (Basel) 2015;7:2454–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lang AM. Botulinum toxin type A therapy in chronic pain disorders. Arch Phys Med Rehabil 2003;84(3 Suppl 1):S69–S73. [DOI] [PubMed] [Google Scholar]
- 25. Wang J, Ding Z, Xu W, et al. Botulinum toxin type A counteracts neuropathic pain by countering the increase of GlyT2 expression in the spinal cord of CCI rats. Brain Res 2022;1796:148095. [DOI] [PubMed] [Google Scholar]
- 26. Van Daele DJ, Finnegan EM, Rodnitzky RL, et al. Head and neck muscle spasm after radiotherapy: management with botulinum toxin A injection. Arch Otolaryngol Head Neck Surg 2002;128:956–959. [DOI] [PubMed] [Google Scholar]
- 27. Mittal S, Machado DG, Jabbari B. OnabotulinumtoxinA for treatment of focal cancer pain after surgery and/or radiation. Pain Med 2012;13:1029–1033. [DOI] [PubMed] [Google Scholar]
- 28. Wittekindt C, Liu WC, Preuss SF, et al. Botulinum toxin A for neuropathic pain after neck dissection: a dose-finding study. Laryngoscope 2006;116:1168–1171. [DOI] [PubMed] [Google Scholar]
- 29. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 30. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De Groef A, Devoogdt N, Van Kampen M, et al. Effectiveness of botulinum toxin A for persistent upper limb pain after breast cancer treatment: a double-blinded randomized controlled trial. Arch Phys Med Rehabil 2018;99:1342–1351. [DOI] [PubMed] [Google Scholar]
- 34. De Groef A, Devoogdt N, Van Kampen M, et al. The effectiveness of Botulinum Toxin A for treatment of upper limb impairments and dysfunctions in breast cancer survivors: a randomised controlled trial. Eur J Cancer Care 2020;29:e13175. [DOI] [PubMed] [Google Scholar]
- 35. Li G, Wang G, Song J, et al. Application of botulinum toxin injection to prevent anastomotic fistula in patients with low rectal cancer. Anti-tumor Pharm 2019;9:242–245. [Google Scholar]
- 36. Hartl DM, Cohen M, Juliéron M, et al. Botulinum toxin for radiation-induced facial pain and trismus. Otolaryngol Head Neck Surg 2008;138:459–463. [DOI] [PubMed] [Google Scholar]
- 37. Bach CA, Wagner I, Lachiver X, et al. Botulinum toxin in the treatment of post-radiosurgical neck contracture in head and neck cancer: a novel approach. Eur Ann Otorhinolaryngol Head Neck Dis 2012;129:6–10. [DOI] [PubMed] [Google Scholar]
- 38. Rostami R, Machado D, Richardson D, et al. Focal injection of incobotulinum toxin A improves refractory local cancer pain at the site of radiation/surgery (P3.315). Neurology 2014;82(Suppl 10):P3.315. [Google Scholar]
- 39. Stubblefield MD, Levine A, Custodio CM, et al. The role of botulinum toxin type A in the radiation fibrosis syndrome: a preliminary report. Arch Phys Med Rehabil 2008;89:417–421. [DOI] [PubMed] [Google Scholar]
- 40. Fabregat G, Asensio-Samper JM, Palmisani S, et al. Subcutaneous botulinum toxin for chronic post-thoracotomy pain. Pain Pract 2013;13:231–234. [DOI] [PubMed] [Google Scholar]
- 41. Jabbari B. Botulinum toxin treatment of pain disorders. Botulinum Toxin Treat Pain Disord 2015:213–18. [Google Scholar]
- 42. Murugappan A, Khanna A. Interventional treatment options for post-mastectomy pain. Curr Oncol Rep 2023;25:1175–1179. [DOI] [PubMed] [Google Scholar]
- 43. Zehm A, Kamdar M. Palliative uses of botulinum neurotoxin #324. J Palliat Med 2017;20:300–302. [DOI] [PubMed] [Google Scholar]
- 44. Safarpour Y, Jabbari B. Botulinum toxin treatment of pain syndromes -an evidence based review. Toxicon 2018;147:120–128. [DOI] [PubMed] [Google Scholar]
- 45. Kim SI, Choi Y. Botulinum toxin injection for intractable pain in cancer patients with psoas muscle invasion. J Pain Symptom Manage 2022;63:e441–e444. [DOI] [PubMed] [Google Scholar]
- 46. Suraj D, Zhang A, Appelbaum T, et al. Clinical presentation and management of malignant psoas syndrome: a scoping review of case reports and case series. Cureus 2023;15:e41522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.