Abstract
Fission yeast capping protein SpCP is a heterodimer of two subunits (Acp1p and Acp2p) that binds actin filament barbed ends. Neither acp1 nor acp2 is required for viability, but cells lacking either or both subunits have cytokinesis defects under stressful conditions, including elevated temperature, osmotic stress, or in combination with numerous mild mutations in genes important for cytokinesis. Defects arise as the contractile ring constricts and disassembles, resulting in delays in cell separation. Genetic and biochemical interactions show that the cytokinesis formin Cdc12p competes with capping protein for actin filament barbed ends in cells. Deletion of acp2 partly suppresses cytokinesis defects in temperature-sensitive cdc12-112 cells and mild overexpression of capping protein kills cdc12-112 cells. Biochemically, profilin has opposite effects on filaments capped with Cdc12p and capping protein. Profilin depolymerizes actin filaments capped by capping protein but allows filaments capped by Cdc12p to grow at their barbed ends. Once associated with a barbed end, either Cdc12p or capping protein prevents the other from influencing polymerization at that end. Given that capping protein arrives at the division site 20 min later than Cdc12p, capping protein may slowly replace Cdc12p on filament barbed ends in preparation for filament disassembly during ring constriction.
INTRODUCTION
Actin filaments in cytokinetic contractile rings have the remarkable ability to transmit the force that pinches daughter cells in two in spite of the fact that they turn over rapidly (Carter, 1967; Schroeder, 1970; Pelham and Chang, 2002) and depolymerize naturally as the ring constricts (Schroeder, 1972; Wu et al., 2003). Actin binding proteins, including formins, profilin, and ADF/cofilins, are required for cytokinesis, but the mere dependence of cytokinesis on these proteins provides few insights about how they contribute to actin filament dynamics in the contractile ring.
Until recently, nothing motivated curiosity about a role for capping protein in cytokinesis. Capping proteins bind actin filament barbed ends, blocking subunit association and dissociation (Isenberg et al., 1980; Cooper and Schafer, 2000). No one had observed capping protein in a contractile ring, and both budding yeast (Amatruda et al., 1990, 1992) and fission yeast (Nakano et al., 2001) survive deletion of capping protein genes without defects in cytokinesis. Capping protein is essential for viability of Drosophila (Hopmann et al., 1996) and Dictyostelium (Hug et al., 1995), but a link to cytokinesis was not explored. On the other hand, capping protein-yellow fluorescent protein (YFP) concentrates in the cleavage furrow of some fission yeast cells just before constriction of the contractile ring (Wu et al., 2003), capping protein purifies with midbodies from Chinese hamster ovary (CHO) cells (Skop et al., 2004), and capping protein transiently accumulates at the midbody of asymmetrically dividing cells of Caenorhabditis elegans embryos (Waddle et al., 1994).
These observations prompted us to reconsider the participation of capping protein in cytokinesis. Our experiments on fission yeast revealed a network of genetic and biochemical interactions among capping protein, the actin monomer binding protein profilin, and the cytokinesis formin Cdc12p that contribute to cytokinesis. Because both capping protein and formin interact with actin filament barbed ends, our results focus attention of the role of actin filament barbed ends during cytokinesis.
Formins are required for cytokinesis in many cell types, including flies, yeast, and worms (Evangelista et al., 2003). Formin formin homology (FH)2 domains nucleate actin filaments and interact with barbed ends (Pruyne et al., 2002; Kovar et al., 2003; Zigmond et al., 2003; Harris et al., 2004; Kovar and Pollard, 2004). Some formins allow elongation of barbed ends (Zigmond et al., 2003; Harris et al., 2004; Kovar and Pollard, 2004; Moseley et al., 2004), but elongation of a barbed end associated with fission yeast Cdc12p requires gating by profilin (Kovar et al., 2003; Kovar and Pollard, 2004). Fission yeast has genes for three formins that are important for specific processes: For3p (interphase actin cables), Fus1p (mating), and Cdc12p (cytokinesis) (Chang et al., 1997; Petersen et al., 1998b; Feierbach and Chang, 2001; Nakano et al., 2002).
Capping protein is a heterodimer of structurally related α- and β-subunits with COOH-terminal “tentacles” (Yamashita et al., 2003) that attach to the barbed end of an actin filament (Wear et al., 2003). Mutational analysis established that the main physiological function of capping protein in budding yeast is capping the barbed ends of filaments nucleated by Arp2/3 complex in actin patches (Kim et al., 2004). Depletion of capping protein compromises the actin-based lamellae of Dictyostelium (Hug et al., 1995) and Drosophila S2 cells (Kiger et al., 2003; Rogers et al., 2003), structures that also depend on Arp2/3 complex.
Fission yeast require the actin monomer binding protein profilin for viability and contractile ring assembly (Balasubramanian et al., 1994). Viability depends on the ability of profilin to bind both actin and poly-l-proline (Lu and Pollard, 2001). Actin-profilin elongates actin filament barbed ends but not pointed ends (Pollard and Cooper, 1984), so the combination of capping protein and profilin inhibits elongation at both ends. On the other hand, profilin binding to the proline-rich FH1 domain of the cytokinesis formin Cdc12p (Chang et al., 1997) allows filaments nucleated by Cdc12p to grow at their barbed ends while remaining attached to the formin (Kovar et al., 2003; Kovar and Pollard, 2004).
Here, we show that fission yeast capping protein SpCP is required for cytokinesis under a variety of stressful conditions, including high temperature (36°C), hyperosmotic media or mild mutations in numerous genes that participate in cell division. Capping protein competes with formin Cdc12p for actin filament barbed ends, both in biochemical experiments and in fission yeast cells, because SpCP and Cdc12p are genetically antagonistic. Without capping protein, excess actin filaments accumulate in patches, and actin cables are severely compromised.
MATERIALS AND METHODS
Strains, Growth Conditions, and Cellular Methods
Supplemental Table 5 lists the Schizosaccharomyces pombe strains used in this study (available online). We used standard growth media (YE5S complete medium and EMM5S minimal medium) and methods for genetics, electroporation, and molecular biology (http://www.bio.uva.nl/pombe/handbook; Sambrook et al., 1989; Wu et al., 2001). We used 100 μM latrunculin-A (Molecular Probes, Eugene, OR) to depolymerize actin (Wu et al., 2001). We stained nuclei and septa with Hoechst (Sigma-Aldrich, St. Louis, MO) or with 4,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich) and calcofluor (Sigma-Aldrich), and filamentous actin with rhodamine-phalloidin (Fluka Chemical, Ronkonkoma, NY) (Kovar et al., 2003). For each experimental condition, we counted at least 400 total cells from two separate cultures.
acp1 (Nakano et al., 2001), acp2 (accession AL391713), and twf1 (accession AL034490) genes were tagged and/or replaced at their chromosomal loci by PCR-based gene targeting by using kanMX6 as the selectable marker with pairs of primers that were 80–100 base pairs in length (Bahler et al., 1998). A 3× GFP kanMX6 module, used as a PCR template for C-terminal integration of 3× GFP, was made by amplifying a budding yeast 3× GFP module (Lee et al., 2003) with primers that introduced 5′ PacI and 3′ Asc1 restriction sites. The resulting fragment was cloned into pFA6a-kanMX6 (Bahler et al., 1998), creating plasmid pFA6a-3× GFP-kanMX6. acp2, twf1, and cdc12 were tagged at their C termini with green fluorescent protein (GFP), YFP, cyan fluorescent protein (CFP), and/or 3× GFP by using forward primers that ended just upstream of the stop codon, and reverse primers that ended 1 base pair downstream of the stop codon. All tagged proteins were functional by several criteria: colony growth, generation time, morphology at different temperatures, and crosses with mutations known to give synthetic phenotypes (i.e., acp2-GFP × cdc3-6). High-level expression P3nmt1 and medium-level P41nmt1 thiamine-inducible promoters were integrated in front of acp1 and acp2 by using forward primers that end just upstream of the start codon and reverse primers that ended with the start codon. Expression under the nmt1 promoter was regulated in EMM5S medium with the presence or absence of 10.0 μg/ml thiamine (Sigma-Aldrich).
Plasmids
Plasmids pREP42-GFP-cdc4 (Balasubramanian et al., 1997), pSGP573-41×-YFP-cdc8 (Wu et al., 2003) and pET21-cdc12(882-1390) (Kovar and Pollard, 2004) have been described previously. To make p573-81×-YFP-act1 and pREP81-cdc12(882-1375), the entire act1 coding region or appropriate cdc12 region was amplified from an S. pombe cDNA library and cloned into pSGP573-81× (Pasion and Forsburg, 1999) (GFP was subsequently replaced by YFP) or pREP81 (Maundrell, 1990), respectively. A bacteria expression vector for fission yeast capping protein, SpCP, was made by a published strategy (Soeno et al., 1998). The entire coding regions (including start and stop) of both acp1 and acp2 were amplified from an S. pombe cDNA library, with primers that introduced 5′ NdeI and 3′ BamHI restriction sites, and cloned into plasmid pET3a (Novagen, Madison, WI). pET3a-acp2 was cut with restriction enzymes BglII and ClaI, and the resulting fragment was cloned into the EcoRV site of pET3a-acp1, creating plasmid pET3a-acp1acp2. Inserts of all new plasmids were sequenced to confirm fidelity of PCR amplification.
Microscopy of Cells
For most experiments, cells were observed by differential interference contrast (DIC) and fluorescence microscopy by using an Olympus IX-71 inverted microscope equipped with a 60× 1.4 numerical aperture Plan-apo objective and appropriate filter sets (DIC, DAPI, CFP, fluorescein isothiocyanate, and YFP) and a Hamamatsu Orca-ER cooled charge-coupled device (CCD) camera (Bridgewater, NJ). Images in Figure 2E, Figure 5F, and Supplemental Figure 2C were taken with an Ultraview spinning disk confocal microscope (PerkinElmer Life and Analytical Sciences, Boston, MA). Time-lapse microscopy, kymograph preparation, and data analysis have been described previously (Wu et al., 2003). The amount of filamentous actin in patches was determined by measuring the integrated fluorescence intensity of pixels at the ends of rhodamine-phalloidin–stained interphase cells (no contractile ring present) by using ImageJ software (http://rsb.info.nih.gov/ij/). Twenty randomly chosen wild-type and acp1Δ acp2Δ cells from at least four different fields were measured.
Figure 2.
Late cytokinesis defects in the absence of capping protein. (A and B) DIC and fluorescence micrographs of wild-type (MLP198) and acp2Δ (KV301) cells expressing integrated myosin regulatory light chain-GFP (Rlc1p-GFP) from its native promoter and stained with Hoechst (bisbenzimide) to visualize nuclei and septa. Cells were grown exponentially in liquid minimal media at 36°C (A) or liquid minimal media with 1 M ethylene glycol at 25°C (B). (C and D) Effect of capping protein deletion on the time course of cytokinesis. Wild-type and acp2Δ cells expressing Rlc1p-GFP were initially grown exponentially in liquid YE5S complete media at 25°C or YE5S complete media with 1 M ethylene glycol at 25°C, and then washed and grown in minimal media plus 25% gelatin at 25°C with or without 1 M ethylene glycol. Fluorescence micrographs were recorded every minute. (C) Kymographs showing assembly, constriction, and disassembly of the Rlc1p-GFP contractile ring. Kymographs were constructed in ImageJ with a 5-μm slit across the midplane of the cell and aligned at the time of Rlc1p-GFP appearance at the division site (time = 0 min). (D) Plot of cytokinesis events versus time for (○) wild-type and (•) acp2Δ cells in minimal media or (□) wild-type and (▪) acp2Δ cells in minimal media with 1 M ethylene glycol. The arrival of SpCP at the division site is marked (Wu et al., 2003). At least 10 cells were analyzed for each condition. (E) Fluorescence micrographs of actin filaments stained with rhodamine-phalloidin in fixed wild-type (FY436) and acp1Δacp2Δ (KV149) cells grown exponentially in liquid minimal media at 36°C. (F) Colocalization of Acp2p-CFP with contractile ring actin filaments. Fixed cells expressing integrated Acp2p-CFP from its native promoter (KV184) were stained with DAPI for DNA and rhodamine-phalloidin for actin filaments and photographed with CFP, tetramethylrhodamine B isothiocyanate, and DAPI filters.
Figure 5.
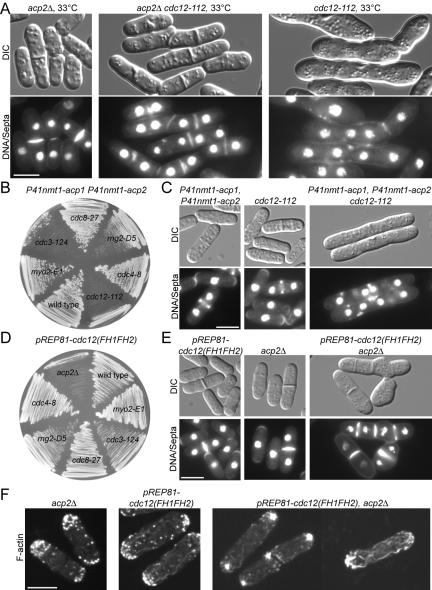
Antagonism of capping protein and the formin Cdc12p in cells. (A) A capping protein null mutant mildly suppresses a temperature sensitive cdc12 mutant. DIC and fluorescence micrographs of acp2Δ (KV21), cdc12-112 (MBY310), and acp2Δ cdc12-112 (KV81) cells grown at 33°C in YE5S complete liquid media for 10 h and stained with Hoechst to visualize DNA and septa. (B and C) Mild overexpression of SpCP kills cells with ts mutations of formin cdc12-112 and profilin cdc3-124 at a semipermissive temperature. (B) SpCP was mildly overexpressed (P41nmt1-acp1 P41nmt1-acp2) in various genetic backgrounds. Cells were streaked on minimal media without thiamine at 30°C for 72 h. (C) DIC and fluorescence micrographs of cells grown in liquid minimal media without thiamine for 20 h at 28°C and stained with Hoechst. (D–F) Low level overexpression of Cdc12(FH1FH2)p is lethal specifically in SpCP null cells. (D) The formin Cdc12(FH1FH2)p was weakly overexpressed from a plasmid [pREP81-cdc12(FH1FH2)] in various genetic backgrounds. Cells were streaked on minimal media without thiamine and grown at 30°C for 72 h. (E and F) DIC and fluorescence micrographs. Cells were grown in liquid minimal media without thiamine for 20 h at 28°C and then either stained with Hoechst (E) or fixed and stained with rhodamine-phalloidin (F) to visualize actin filaments. Bars, 5 μm.
Protein Purification
Recombinant fission yeast capping protein SpCP (Acp1p and Acp2p) was purified from bacteria. The pET3a-acp1acp2 construct was transformed into Escherichia coli strain BL21-DE3 pLysS (Stratagene, La Jolla, CA) and grown overnight at 37°C. After subculturing into fresh media, cells were grown at 37°C for ∼2 h and then induced for 6 h with the addition of 0.5 mM isopropyl β-d-thiogalactopyranoside. Cells harvested by centrifugation were frozen, resuspended in extraction buffer (50 mM Tris, pH 8.0, 1 mM EDTA, 1 mM dithiothreitol [DTT], and 50 mM NaCl) supplemented with a complete protease inhibitor tablet (Roche Diagnostics, Indianapolis, IN), and sonicated. The sonicate was clarified at 30,000 and 50,000 × g for 25 min each. A 45–65% ammonium sulfate cut was taken, and the pellet was resuspended in (15 ml) and dialyzed against 2 liters (for 2 h) of DE52 buffer (10 mM Tris, pH 8.0, 100 mM NaCl, 1 mM EDTA, 1 mM DTT, and 0.01% NaN3). The dialyzed protein was separated on a 30-ml bed volume DE52 (Whatman, Clifton, NJ) column eluted with a 400-ml 100–400 mM NaCl gradient. SpCP-containing fractions were dialyzed overnight against 2 liters of HA buffer (500 mM KCl, 10 mM KH2PO4, 1 mM DTT, and 0.01% NaN3) and separated on a 15-ml Hydroxyapatite (Bio-Rad, Hercules, CA) column eluted with a 400-ml 10–250 mM KH2PO4 gradient. SpCP-containing fractions were dialyzed overnight against 2 liters of SQ(7.5) buffer (10 mM Tris, pH 7.5, 100 mM NaCl, 1 mM DTT, and 0.01% NaN3) and separated on a 1-ml Source 15Q (Amersham Biosciences, Piscataway, NJ) column eluted with a 100-ml 100–300 mM NaCl gradient. SpCP-containing fractions were dialyzed overnight against 2 liters of SQ(6.0) buffer [10 mM 2-(N-morpholino)ethanesulfonic acid, pH 6.0, 50 mM NaCl, 1 mM DTT, and 0.01% NaCl] and separated on a 1-ml Source 15Q (Amersham Biosciences) column eluted with a 100-ml 50–300 mM NaCl gradient. Pure SpCP was dialyzed against CP buffer (10 mM Tris, pH 7.5, 40 mM KCl, 0.5 mM DTT, 50% glycerol, and 0.01% NaN3), flash frozen in liquid nitrogen, and stored at –80°C.
Mouse capping protein MmCP (Palmgren et al., 2001), Cdc12(FH1FH2)p residues 882-1390 (Kovar and Pollard, 2004), and S. pombe profilin (Lu and Pollard, 2001) were purified from bacteria. Ca-ATP actin was purified from rabbit skeletal muscle (Kovar et al., 2003). Gel filtered actin was labeled on Cys-374 with pyrenyliodoacetamide or Oregon green 488 iodoacetamide (Molecular Probes) (Kovar et al., 2003). Just before each experiment, Ca-ATP actin was converted to Mg-ATP actin by adding 0.2 volume of 5 mM EGTA and 0.5 mM MgCl2 for 5 min at 25°C.
Protein concentrations were calculated with the following extinction coefficients: actin, A290 = 26,600 M–1 cm–1 (Houk and Ue, 1974); profilin, A280 = 1.63 OD mg–1 ml–1 (Lu and Pollard, 2001); MmCP, A280 = 76.3 mM–1 cm–1 (Palmgren et al., 2001); Cdc12(882-1390)p, A280 = 49,860 M–1 cm–1 (Kovar and Pollard, 2004); and SpCP, A280 = 72,000 M–1 cm–1 (estimated based on amino acid composition by ProtParam; http://us.expasy.org/tools/).
Fluorescence Spectroscopy
Actin assembly was measured from the fluorescence of a trace of pyrene-actin (excitation at 365 nm and emission at 407 nm) with a PTI Alphascan spectroflourimeter (Photon Technology International, Monmouth Junction, NJ) (Higgs et al., 1999; Kovar et al., 2003). Final protein concentrations are indicated in the figure legends.
For spontaneous assembly assays (Higgs et al., 1999; Kovar et al., 2003), separate drops of a 20 μM mixture of pyrene-labeled and unlabeled Mg-ATP-actin, other proteins, and 10× KMEI (500 mM KCl, 10 mM MgCl2, and 100 mM imidazole, pH 7.0) were placed on the side of a plastic Eppendorf tube, and the reaction was started by mixing with Mg-buffer G (2 mM Tris, pH 8.0, 0.2 mM ATP, 0.1 mM MgCl2, and 0.5 mM DTT).
For assembly from F-actin seeds, 6 μM unlabeled preassembled actin filaments was incubated in a drop for 5 min with a range of concentrations of SpCP or Cdc12(FH1FH2)p. To start the reaction, a separate drop with 15 μM (5% pyrene-labeled) Mg-ATP-actin and 75 μM profilin was washed into the F-actin seeds with F buffer (G buffer supplemented with 1× KMEI). In some cases, indicated in the Figure 6 legend, the seeds were incubated with either Cdc12p or capping protein (CP) for 5 min and then either CP or Cdc12p was added at the start of the reaction.
Figure 6.
Biochemical competition between SpCP and formin Cdc12p for actin filament barbed ends. The conditions were as follows: 10 mM imidazole, pH 7.0, 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 0.5 mM DTT, 0.2 mM ATP, 90 μM CaCl2, and 0.25% glycerol at 25°C. Polymer concentrations were measured by the fluorescence of pyrene-labeled actin. (A and B) Critical concentration for actin assembly. (A) Dependence of actin polymer concentration on total actin concentration with fits by linear regression. Actin (5% pyrene labeled) was assembled for 16 h. Conditions with critical concentrations: (▵) actin alone, Cc = 0.15 μM; (○) 100 nM SpCP, Cc = 0.9 μM; (•) 100 nM SpCP, 2.5 μM profilin, Cc = 1.95 μM; (□) 50 nM Cdc12(FH1FH2)p, Cc = 0.85 μM; (▪) 50 nM Cdc12(FH1FH2)p, 2.5 μM profilin, Cc = 0.1 μM. (B) Dependence of steady-state polymerization on profilin, SpCP, and Cdc12p. Actin filaments (5 μM, 10% pyrene labeled) were diluted to 1.5 μM in a range of profilin concentrations and incubated for 16 h. Conditions: (▵) actin alone, (○) 100 nM SpCP or (□) 50 nM Cdc12(FH1FH2)p. (C and D) Effects of profilin, SpCP, and Cdc12p on the time course of spontaneous assembly of 4 μM Mg-ATP actin (5% pyrene labeled). (C) Conditions: (thick curve) actin alone; (▵) 5 μM profilin; (○) 25 nM SpCP; (•) 25 nM SpCP, 5 μM profilin; (□) 10 nM Cdc12(FH1FH2)p; (▪) 10 nM Cdc12(FH1FH2)p, 5 μM profilin. (D) Conditions: (thick curve) actin alone; (□) 100 nM SpCP, 2.5 μM profilin; (∘ to ○) 100 nM SpCP, 2.5 μM profilin with a range of indicated Cdc12(FH1FH2)p concentrations (nM). (E–H) Effect of SpCP, mouse capping protein MmCP, and Cdc12(FH1FH2)p on elongation of the barbed ends of preassembled actin filaments. (E) Time course of elongation of barbed ends of 400 nM actin filaments upon addition of 1 μM actin (5% pyrene labeled). Conditions: (thick curve) actin alone; (○) 125 nM SpCP, 2.5 μM profilin; (□) 5 nM Cdc12(FH1FH2)p; (▪) 5 nM Cdc12(FH1FH2)p, 2.5 μM profilin; (⋄) 2.5 μM profilin with 125 nM SpCP and 5 nM Cdc12(FH1FH2)p added simultaneously. (♦) 1 μM actin with 5 nM Cdc12(FH1FH2)p and 2.5 μM profilin without preassembled filaments. (F) Effect of Cdc12(FH1FH2)p concentration on the initial rate of barbed end elongation of 400 nM actin filaments by 1 μM actin with 125 nM SpCP and 2.5 μM profilin. (G and H) Elongation of actin filaments preincubated with SpCP/MmCP or Cdc12p. (G) Time course of elongation of barbed ends of 400 nM actin filaments upon addition of 1 μM actin (5% pyrene labeled). Conditions: (thick curve) filaments were preincubated with buffer alone followed by actin and 2.5 μM profilin, (○) filaments were preincubated with 125 nM SpCP followed by actin with 5 nM Cdc12(FH1FH2)p and 2.5 μM profilin, (⋄) filaments were preincubated with 5 nM Cdc12(FH1FH2)p followed by actin with 125 nM SpCP and 2.5 μM profilin, (□) filaments were preincubated with buffer alone followed by actin with 5 nM Cdc12(FH1FH2)p, 125 nM SpCP and 2.5 μM profilin. (H) Time course of elongation of barbed ends of 400 nM actin filaments upon addition of 1 μM actin (5% pyrene labeled). Conditions: (thick curve) filaments were preincubated with buffer alone followed by actin and 2.5 μM profilin, (○) filaments were preincubated with 10 nM MmCP followed by actin with 5 nM Cdc12p and 2.5 μM profilin, (⋄) filaments were preincubated with 5 nM Cdc12(FH1FH2)p followed by actin with 10 nM MmCP and 2.5 μM profilin, (□) filaments were preincubated with buffer alone followed by actin with 5 nM Cdc12(FH1FH2)p, 10 nM MmCP, and 2.5 μM profilin.
The critical concentration for actin assembly was determined two ways (Kovar et al., 2003). First, a range of concentrations of Mg-ATP actin (5% pyrene labeled) was polymerized alone or with the indicated concentrations of either SpCP, Cdc12(882-1390)p, and/or profilin in F buffer for 16 h in the dark at 25°C. Second, a 5 μM stock of 10% pyrene F-actin was diluted to 1.0 μM with F buffer, in the presence of a range of concentrations of SpCP, Cdc12(882-1390)p, and/or profilin (Caldwell et al., 1989), and incubated for 16 h in the dark at 25°C.
Calculation of the Concentration of Apparent Ends, Initial Polymerization Rates, and Depolymerization Rates
The concentration of apparent ends was calculated from elongation rates by using the equation [Endsapp] = elongation rate/(k+[actin monomers]), where k+ = 11.6 μM–1 s–1 at pH 7.0 (Higgs et al., 1999), where 25% (1 μM) of the actin had polymerized. Barbed end polymerization rates from preassembled F-actin seeds were measured from the slope of a linear fit of the first 300 s. The affinity of capping proteins for actin filament barbed ends was determined by fitting a plot of the dependence of the initial assembly rate on the concentration of capping protein with the equation Vi = Vif + (Vib – Vif) ((Kd + [ends] + [CP] – square root((Kd + [ends] + [CP])2 – 4[ends][CP])/2[ends])), where Vi is the observed elongation rate, Vif is the elongation rate when barbed ends are free, Vib is the elongation rate when barbed ends are capped, [ends] is barbed end concentration, and [CP] is capping protein concentration (Huang et al., 2003). The rate of depolymerization was calculated by fitting the data from 300 to 1000 s with a single exponential curve. Depolymerization rates were expressed as a percent normalized to the rate of actin alone.
Total Internal Reflection Fluorescence (TIRF) Microscopy
Images of Oregon green-labeled fluorescent actin filaments excited by total internal reflection were collected at 15-s intervals with a cooled CCD camera (Orca-ER) as described previously (Kovar et al., 2003; Kovar and Pollard, 2004). Initially, a mixture of unlabeled Mg-ATP actin and 20% Mg-ATP Oregon green actin was mixed with 2× TIRF buffer (1×: 10 mM imdazole, pH 7.0, 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 50 mM DTT, 0.2 mM ATP, 50 μM CaCl2, 15 mM glucose, 20 μg/ml catalase, 100 μg/ml glucose oxidase, and 0.5% [4000 centipoise] methylcellulose) and water and transferred to a flow cell for imaging. Subsequently, a second sample with 40% labeled actin in the absence or presence of the indicated concentrations of capping protein replaced the initial sample during continuous imaging.
RESULTS
Biochemical Comparison of Fission Yeast and Mouse Capping Proteins
SpCP is a heterodimer of α-subunits (Acp1p; Nakano et al., 2001) and β-subunits (Acp2p) (Figure 1A) with sequences similar to budding yeast capping protein (Amatruda et al., 1990, 1992). Like mouse capping protein MmCP, purified recombinant fission yeast capping protein SpCP increased the critical concentration for actin assembly (Figure 1B), but this required 10-fold more SpCP than MmCP (Figure 1C). Direct observation of actin filament elongation in real time by evanescent wave fluorescence microscopy (Figure 1D) confirmed that MmCP (Figure 1E) and SpCP (Figure 1F) stopped subunit addition at barbed ends but not pointed ends. Inhibition of elongation (Figure 1G) suggested barbed end affinities of 16 nM for SpCP and 0.8 nM for MmCP (Figure 1H). Similarly, 20-fold more SpCP than MmCP was required to inhibit the depolymerization of actin filament barbed ends (Figure 1, I and J). MmCP stimulated spontaneous assembly of Mg-actin monomers (Cooper and Pollard, 1985; Caldwell et al., 1989; Kovar et al., 2003), but SpCP inhibited spontaneous assembly (Figure 1K). Inhibition of nucleation (the apparent concentration of ends produced) was biphasic, with maximal inhibition at 100 nM SpCP and less inhibition at higher concentrations (Figure 1L). Inhibition of end-to-end annealing of actin filaments required 10-fold more SpCP than MmCP (Figure 1M).
Figure 1.
Biochemical comparison of purified SpCP and MmCP. The conditions were as follows: 10 mM imidazole, pH 7.0, 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 0.5 mM DTT, 0.2 mM ATP, 90 μM CaCl2, and 0.25% glycerol at 25°C. (A) A Coomassie Blue-stained gel showing purification of SpCP. Lane 1, bacteria extract; lane 2, 45–65% ammonium sulfate cut; lane 3, DE52 column; lane 4, Hydroxyapatite column; lane 5, Source 15Q column, pH 7.5; and lane 6, Source 15Q column, pH 6.0. Molecular weights are indicated on the left. (B and C) Critical concentration for assembly of rabbit skeletal muscle actin. (B) Dependence of actin polymer concentration on total actin concentration in the (•) absence or presence of either (□) 15 nM MmCP (○) 150 nM SpCP. Actin (5% pyrene labeled) was assembled for 16 h. The polymer concentration was measured from the pyrene fluorescence, plotted versus actin concentration, and fit by linear regression. The Cc values were 0.1 μM for actin alone, 0.95 μM with 15 nM MmCP, and 0.90 M with 150 μ nM SpCP. (C) Dependence of the polymer concentration of 1 μM actin on the concentration of (□) MmCP or (○) SpCP. Actin filaments (5 μM) (10% pyrene labeled) were diluted to 1 μM in the presence of a range of CP concentrations. After 16 h, the pyrene fluorescence was measured, and the actin polymer concentration was plotted versus the log of CP concentration. (D–F) Direct visualization of the effect of capping protein on growing actin filaments by time-lapse evanescent wave fluorescence microscopy. Mg-ATP actin (1.0 μM) with 0.25 or 0.65 μM Oregon green (OG) 488-labeled Mg-ATP actin. Black triangles indicate time when the second solution replaced the initial solution. Left, kymograph of the length (y-axis) of a representative filament versus time (x-axis; 1200 s). Right, lifetime plots for eight filaments of the growth of barbed and pointed ends versus time. An initial solution that contained 1.0 μM actin with 0.25 μM OG-actin was followed by a second solution that contained 1.0 μM actin with 0.65 μM OG-actin (D) alone, or with either (E) 10 nM MmCP or (F) 250 nM SpCP. (G and H) Barbed end addition of monomer to preassembled actin filaments in the presence of capping protein. (G) Time course of the assembly of 1 μM actin (5% pyrene labeled) saturated with 5 μM profilin upon addition to 400 nM actin filaments (thick curve) alone, or in the presence of either (•) 2.5, (▪) 5 and (□) 10 nM MmCP or (○) 10, (□) 35 and (□) 100 nM SpCP. (H) Dependence of the initial rate of barbed end assembly on the concentration of (□) MmCP or (○) SpCP. Curve fits of the plotted data (see Materials and Methods) revealed dissociation equilibrium constants of 0.8 nM for MmCP and 16 nM for SpCP. (I and J) Time course of the depolymerization of 5 μM actin filaments (70% pyrene labeled) after dilution to 0.1 μM in the (thick curve) absence or presence of (□) 1 nM MmCP, (▪) 5 nM MmCP, (○) 10 nM SpCP, and (•) 250 nM SpCP. (J) Dependence of the rate of depolymerization on the concentration of (□) MmCP or (○) SpCP. The data from 300 to 1000 s of each curve was fit with single exponentials, and the depolymerization rates were expressed as a fraction of the rate of actin alone. (K and L) Time course of the spontaneous assembly of 4 μM Mg-ATP actin (5% pyrene labeled) in the absence (thick curve) or presence of either a range of concentrations of MmCP (•) 5 nM, (▪) 25 nM, and (□) 100 nM, or a range of concentrations of SpCP, (□) 5 nM, (○) 25 nM, and (□) 100 nM. (L) Biphasic dependence of the concentration of apparent ends (nanomolar) on the concentration of SpCP calculated from the rate of polymerization at the time where 25% (1 μM) of the actin was polymerized. (M) Effects of capping protein on actin filament annealing. Merged micrographs of red and green fluorescence are shown. Equal concentrations (0.25 μM) of red (rhodamine-phalloidin)- and green (Alexa green-phalloidin)–labeled actin filaments were sheared through a 26-gauge needle in the absence or presence of the indicated concentrations of CP and allowed to anneal for 60 min before dilution and absorption to poly-l-lysine–coated coverslips. Bar, 5 μm.
Background Information on Fission Yeast Capping Protein
This section summarizes the basic cellular properties of fission yeast capping protein SpCP, which are documented by supplemental materials (available online), because SpCP is similar to orthologues in other cells. GFP-tagged SpCP concentrates with actin filaments in patches at the ends of interphase cells (Supplemental Figure 1, A–G). These patches form, move, and disappear on a time scale of seconds (Supplemental Figure 1, E and F). Localization of Acp2p-GFP to patches depends on Acp1p (Supplemental Figure 1A) and actin filaments (Supplemental Figure 1B). Acp2p-CFP patches overlap with some but not all patches of PCH protein Cdc15p-YFP (Supplemental Figure 1D). As in budding yeast (Goode et al., 1998; Palmgren et al., 2001), SpCP is required for twinfilin (Twf1p-GFP) to concentrate in actin patches (Supplemental Figure 1G), but deletion of twinfilin (twf1Δ) has no effect on cellular morphology or localization of Acp2p-GFP to patches (Supplemental Figure 1G) under any condition that we tested. We confirmed that cells lacking Acp1p are viable with normal morphology in complete media at 25°C (Nakano et al., 2001) and found the same to be true for cells lacking Acp2p or both Acp1p and Acp2p (Supplemental Figure 2 and Supplemental Table 1).
During cell division, patches marked by Acp2p-GFP accumulate lateral to the cleavage site (Supplemental Figure 1, A–C and F), and Acp2p-CFP concentrates along with actin filaments in ∼2% of contractile rings (Figure 2F). The contractile ring signal bleaches rapidly and is obscured by the bright fluorescence of patches clustered near the cleavage furrow, so 2% may be an underestimate. Because cells lacking Acp1p are viable without cytokinesis defects (Nakano et al., 2001), SpCP seemed unlikely to have a role in cytokinesis. Nonetheless, the following sections document evidence that SpCP does function in cytokinesis in a role that is antagonistic to the formin Cdc12p.
Cytokinesis Depends on Capping Protein under a Variety of Stressful Conditions
Cells lacking one or both capping protein genes have conditional defects in cytokinesis that are evident at elevated temperatures or in 1 M ethylene glycol at 25°C. At 36°C in minimal EMM5S media ∼20% of acp1Δ, acp2Δ, and acp1Δ acp2Δ cells have cytokinesis defects (Figure 2A, Supplemental Figure 2, A and B, and Supplemental Table 1) with disorganized contractile rings (Figure 2, A and E and Supplemental Figure 2B) and improper deposition of septal material (Figure 2A and Supplemental Figure 2A). At 25°C in 1 M ethylene glycol, ∼15% of capping protein null cells have cytokinesis defects in minimal EMM5S media (Figure 2B and Supplemental Table 1).
Contractile rings form in acp2Δ cells with the same time course as wild-type cells, but delays emerge late in the process as the contractile ring disassembles and daughter cells separate (Figure 2, C and D). We followed these events by time-lapse microscopy of cells expressing integrated myosin regulatory light chain-GFP (Rlc1p-GFP) (Wu et al., 2003). In the presence of 1 M ethylene glycol in minimal media at 25°C, these defects are more severe and extend to include a delay in contractile ring constriction. With the exception of cell separation, wild-type cells in both the absence and presence of 1 M ethylene glycol faithfully follow the time course of cytokinesis with remarkably little variation (Figure 2D; Wu et al., 2003). On the other hand, the timing of all late cytokinesis events was highly variable in acp2Δ cells as reflected in larger standard deviations (Figure 2D).
Excess capping protein also caused cytokinesis defects. Cells overexpressing both α- and β-subunits, but neither alone, grew abnormally long (Figure 3A and Supplemental Table 1). Nuclei divided normally but contractile rings failed to form (Figure 3C), and septa were incomplete (Figure 3B). Cells accumulated up to eight nuclei before lysing. Because actin patches and actin cables looked normal (Figure 3C), SpCP overexpression specifically affected contractile ring assembly. The formin Cdc12p concentrated at the division site but failed to form a contractile ring between separated nuclei in cells overexpressing SpCP (Figure 3D). Capping protein activity (measured by the effect on the low shear viscosity of pure actin filaments; Isenberg et al., 1980) was 10 times higher in extracts from strains overexpressing SpCP than control extracts.
Figure 3.
Overexpression of capping protein causes a specific lethal defect in cytokinesis. (A–D) Wild-type, capping protein α-subunit overexpressing (P3nmt1-acp1; KV37), capping protein β-subunit overexpressing (P3nmt1-acp2; KV40), and both α- and β-subunit overexpressing (P3nmt1-acp1 P3nmt1-acp2; KV56) cells were grown in liquid minimal media with 0.2 μM thiamine at 25°C. Overexpression was induced by washing the cells in media without thiamine and allowing them to grow 18–24 h more at 25°C. (A) Overexpression of both capping protein subunits is required for a defect in cytokinesis. DIC and fluorescent micrographs of wild-type and capping protein overexpressing cells (24 h) stained with Hoechst to visualize DNA and septa. (B and C) Cells overexpressing capping protein cannot form contractile rings. (B) DIC and fluorescent micrographs of cells stained with Hoechst that are overexpressing capping protein (18 h) and expressing either GFP-myosin essential light chain (pGFP-Cdc4p; KV295) or YFP-tropomyosin (pYFP-Cdc8p; KV296) from plasmids. (C) Fluorescent micrographs of cells fixed and stained with DAPI (DNA) and rhodamine-phalloidin (actin filaments) that were overexpressing only the capping protein α subunit or both capping protein subunits for 20 h. (D) DIC and fluorescent micrographs of cells expressing Cdc12-3XGFP and overexpressing capping protein for 0 or 18 h. Nuclei and septa were stained with Hoechst. Bars, 5 μm.
Genetic crosses revealed synthetic interactions between the capping protein null mutation acp2Δ and mutations in other genes required for cytokinesis. We tested the ability of the double mutants to form colonies on complete YE5S media agar plates at a range of temperatures (Supplemental Table 2) where acp2Δ single mutant cells formed normal colonies. We stained selected single and double mutants with Hoechst or DAPI and calcofluor to visualize and count nuclei and septa (Figure 4 and Supplemental Table 3).
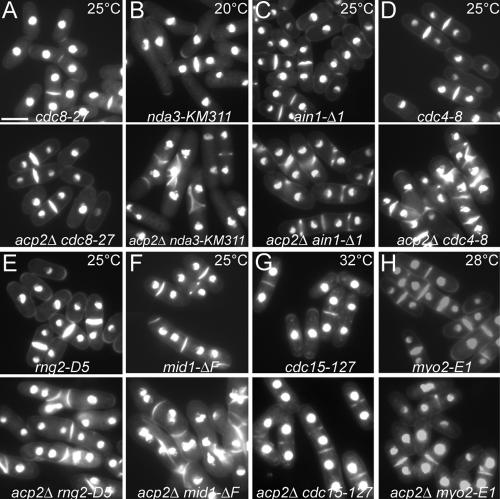
Figure 4.
Genetic interactions of capping protein null mutants with other mutations. Fluorescent micrographs of single and double mutant cells grown at the indicated temperature in complete YE5S liquid media and stained with Hoechst to visualize DNA and septa. (A) An example of no genetic interaction. Capping protein and tropomyosin: acp2Δ cdc8-27 (KV79). (B–G) Synthetic interactions where double mutant were sicker than either single mutant. (B) Capping protein and β-tubulin: acp2Δ nda-KM311 (KV134). (C) Capping protein and α-actinin: acp2Δ ain1-Δ1 (KV67). (D) Capping protein and myosin essential light chain: acp2Δ cdc4-8 (KV85). (E) Capping protein and IQGAP: acp2Δ rng2-D5 (KV132). (F) Capping protein and anillin-like: acp2Δ mid1-ΔF (KV136). (G) Capping protein and PCH protein: acp2Δ cdc15-127 (KV83). (H) Suppression where the double mutant of capping protein null and type II myosin ts (acp2Δ myo2-E1, KV105) was healthier than the myo2-E1 single mutant. Bar, 5 μm.
Double mutants that formed colonies less well than the single non-acp2Δ mutant parent had more severe defects in septal organization and contained more multinucleated cells. Whereas 1% of tubulin cold-sensitive nda3-KM311 cells had abnormal septa, 14% of nda3-KM311 acp2Δ double mutant cells had abnormal septa at 20°C (Figure 4B). Compared with α-actinin null ain1-Δ1 cells at 25°C, 26% more ain1-Δ1 acp2Δ double mutant cells had abnormal septa (Figure 4C). Compared with myosin essential light chain temperature-sensitive (ts) cdc4-8 cells at 25°C, 47% more cdc4-8 acp2Δ double mutant cells had abnormal septa (Figure 4D). Compared with IQGAP ts rng2-D5 cells at 25°C, 41% more rng2-D5 acp2Δ double mutant cells had abnormal septa (Figure 4E). Compared with anillin-like mid1-ΔF null cells at 25°C, 49% more mid1-ΔF acp2Δ double mutant cells had abnormal septa (Figure 4F). Compared with PCH protein cdc15-127 ts cells at 32°C, 16% more cdc15-127 acp2Δ double mutant cells had abnormal septa (Figure 4G).
On the other hand, some double mutants were indistinguishable morphologically from the non-acp2Δ parent mutant. For example, tropomyosin ts cdc8-27 and cdc8-27 acp2Δ double mutant cells both formed normal septa and had similar numbers of binucleate cells at 25°C (Figure 4A).
Cellular and Biochemical Competition between SpCP and Formin Cdc12p
Genetic crosses revealed suppressor interactions between the capping protein null mutation acp2Δ and temperaturesensitive mutations in genes for two proteins required for cytokinesis: the formin cdc12 and type-II myosin myo2. Compared with myo2-E1 ts cells at 28°C, myo2-E1 acp2Δ double mutant cells had 16% fewer abnormal septa and 26% less were multinucleate (Figure 4H). We studied the formin interaction in detail.
No genetic interaction was found between acp2Δ and for3Δ, a formin required for actin cables (our unpublished data). However, deleting acp2 partly suppresses the severe defects of ts cdc12-112 cells grown at 33°C in complete YE5S liquid media (Figure 5A and Supplemental Table 4). Compared with formin cdc12-112 ts cells at 33°C, 25% fewer cdc12-112 acp2Δ double mutant cells had abnormal septa and 11% fewer were multinucleate (Figure 5A). In 87% of cdc12-112 cells the septa failed to span the entire cell width, whereas only 25% of cdc12-112 acp2Δ double mutant cells had this “partial” septa defect.
On the other hand, mild overexpression of SpCP (α- and β-subunits expressed from the P41nmt1 promoter) was lethal in formin ts cdc12-112 and profilin ts cdc3-124 cells (Figure 5B), but not wild-type, tropomyosin ts cdc8-27, IQ-GAP ts rng2-D5, myosin essential light chain ts cdc4-8, or type-II myosin ts myo2-E1 cells at semipermissive temperature (30°C) (Figure 5, B and C, and Supplemental Table 4). Mild overexpression of SpCP was not lethal in cells with deletion of for3, the formin required for interphase actin cables (our unpublished data), suggesting a specific antagonistic relationship between SpCP and the cytokinesis formin Cdc12p. P41nmt1-acp1 P41nmt1acp2 cdc12-112 cells continued cycles of nuclear division but could not assemble contractile rings, resulting in partial septa (Figure 5C), like wild-type cells strongly overexpressing SpCP (Figure 3).
Mild overexpression of Cdc12(FH1FH2)p from the P81nmt1 promoter on a plasmid was lethal in acp2Δ cells, but not in a variety of other ts genetic backgrounds (Figure 5, D–F, and Supplemental Table 4). These pREP81-cdc12(FH1FH2) acp2Δ cells had aberrant thick actin cables in aster-like accumulations (Figure 5F) and suffered a variety of defects in morphology and cytokinesis (Figure 5E).
Biochemical experiments showed competition between SpCP and formin Cdc12p for actin filament barbed ends (Figure 6). We used an active fragment of Cdc12p consisting of the FH1 and 2 domains, Cdc12(FH1FH2)p (Kovar et al., 2003; Kovar and Pollard, 2004). We consider first the individual proteins.
At steady state, both SpCP and Cdc12(FH1FH2)p cap actin filament barbed ends and increase the critical concentration for assembly to the critical concentration of the pointed end (Figure 6, A and B). Profilin has opposite effects on these capped filaments, depolymerizing filaments capped by SpCP (Figure 6, A and B) (like other capping proteins; Cooper and Schafer, 2000) but overcoming barbed end capping by Cdc12(FH1FH2)p (Figure 6, A and B; Kovar et al., 2003). Both effects depend on the concentration of profilin. Similarly, profilin allowed Cdc12(FH1FH2)p capped filaments (Kovar et al., 2003), but not SpCP capped filaments, to elongate their barbed ends (Figure 6E). Both SpCP and Cdc12(FH1FH2)p stimulate spontaneous assembly of Mg-actin monomers by nucleating filaments that grow at their pointed ends. Profilin has opposite effects on these reactions, inhibiting spontaneous assembly in the presence of SpCP but accelerating spontaneous assembly by Cdc12(FH1FH2)p (Figure 6C).
Three different experiments showed that SpCP and Cdc12(FH1FH2)p exclude each other from actin filament barbed ends. The effects of the two proteins are additive in the absence of profilin, because both cap tightly. Profilin allows barbed ends capped by Cdc12(FH1FH2)p but not SpCP to elongate. With both proteins, the outcome depends entirely on which protein binds the barbed end first.
When SpCP, profilin and Cdc12(FH1FH2)p are added to Mg-actin monomers simultaneously, Cdc12(FH1FH2)p overcomes the inhibition of spontaneous assembly of Mg-actin by SpCP and profilin, strongly stimulating polymerization in a concentration dependent manner, even at concentrations 10-fold lower than SpCP (Figure 6D).
Both SpCP and Cdc12(FH1FH2)p inhibit actin filament elongation, but if the two proteins are added to filaments simultaneously in the presence of profilin, substoichiometric concentrations of Cdc12(FH1FH2)p overcome capping by SpCP (Figure 6E). The rate of elongation depends on the concentration of Cdc12(FH1FH2)p (Figure 6F).
Preincubation of filaments with SpCP or MmCP prevents elongation of the barbed ends by actin monomers, Cdc12(FH1FH2)p, and profilin. Conversely, preincubation of filaments with Cdc12(FH1FH2)p allows actin monomers and profilin to elongate the barbed ends even with excess SpCP or MmCP (Figure 6, G and H). This is explained by persistent association of Cdc12(FH1FH2)p (Kovar and Pollard, 2004) and the capping proteins with barbed ends, such that neither a formin nor a capping protein can displace the other from a barbed end.
Other Functions of Fission Yeast Capping Protein
Actin Filament Cables and Patches. Cells lacking either capping protein subunit had fewer actin cables but ∼35% more actin filaments in actin patches judging from rhodamine-phalloidin fluorescence (Supplemental Figure 2C). The actin cable deficiency resulted in mislocalization of the type V myosin Myo52p (Supplemental Figure 2D). In wild-type cells, Myo52p-GFP localized to patches primarily at the ends during interphase and the middle during division. Spots of Myo52p-GFP were dispersed throughout acp2Δ cells, similar to cells lacking for3 (Feierbach and Chang, 2001; Nakano et al., 2002), the formin required for actin filament cables (Supplemental Figure 2D; Feierbach and Chang, 2001).
Sexual Cycle. Like actin and fimbrin (Petersen et al., 1998a; Wu et al., 2001), Acp2p-GFP concentrates in patches at the tips of mating cells, in patches in the cortex of developing spores and in patches at the tips of germinating spores (Supplemental Figure 3A). Crosses of two wild-type strains yielded four spores in 99% of the asci after 48 h (Supplemental Figure 3B). However, crosses of two acp1Δ strains, two acp2Δ strains, and two fim1-1Δ strains yielded four spores in only 58, 61, and 7% of asci, respectively (Supplemental Figure 3B). Few additional spores formed with longer incubation.
DISCUSSION
Capping protein SpCP and formin Cdc12p bind to actin filament barbed ends. In the absence of profilin, both simply prevent subunit association and dissociation. The presence of profilin reveals an important difference: ends capped by Cdc12p elongate, but ends capped by SpCP do not. Because both SpCP and Cdc12p dissociate slowly from barbed ends, whichever protein is bound will dominate the behavior of the associated end persistently. Thus, excess capping protein cannot prevent barbed ends capped by Cdc12p from elongating in the presence of profilin. Interactions of profilin, SpCP, and Cdc12p contribute to contractile ring function as detailed below.
Competition between Capping Protein and Formin in Dividing Cells
Fission yeast capping protein contributes to the fidelity of cytokinesis, but it is not absolutely required for assembly or constriction of the contractile ring. Under nonstress conditions (25°C), most cells lacking capping protein (acp1Δ, acp2Δ, and acp1Δ acp2Δ) completed cytokinesis normally, although the fraction of cells with two nuclei was ∼5% higher than wild-type cells (Supplemental Table 1). Under stressful conditions (36°C or 1 M ethylene glycol), ∼20% of cells lacking capping protein had aberrant septa resulting from disorganized contractile rings. Consistent with the arrival of capping protein at the division site well after the contractile ring forms (Wu et al., 2003), defects arise only as the contractile ring constricts and disassembles.
Biochemical and genetic evidence support the hypothesis that fission yeast cytokinesis is influenced by profilin-mediated competition between capping protein and the formin Cdc12p for actin filament barbed ends at the division site. Fission yeast depend on Cdc12p and profilin to assemble contractile ring actin filaments de novo (Balasubramanian et al., 1994; Chang et al., 1997; Pelham and Chang, 2002). SpCP competes with Cdc12p in vivo, because they are genetically antagonistic (Figure 5): the absence of capping protein partly suppresses the septation defect of temperature sensitive cdc12-112 cells (acp2Δ cdc12-112) at a semipermissive temperature; and mild overexpression of SpCP kills cdc12-112 cells as does mild overexpression of Cdc12(FH1FH2)p in capping protein null cells (acp2Δ). Furthermore, temperature-sensitive mutations in the gene for fission yeast profilin (cdc3) are synthetically lethal with both capping protein null acp2Δ and cdc12-112 temperature-sensitive mutations (Chang et al., 1997). On the contrary, mutations in Drosophila profilin (chickadee) suppress bristle abnormalities in capping protein (cpb) mutants (Hopmann and Miller, 2003).
Profilin is key to the competition between SpCP and formin Cdc12p. Mg-ATP-actin bound to profilin elongates free barbed ends but not pointed ends (Pollard and Cooper, 1984; Kang et al., 1999; Blanchoin and Pollard, 2002). Therefore, profilin-Mg-ATP-actin elongates neither end of filaments capped by SpCP. Instead, subunits dissociate from pointed ends and result in depolymerization in proportion to the concentration of profilin (Pantaloni and Carlier, 1993). On the other hand, by interacting with both an actin monomer and the FH1 domain of Cdc12p (Chang et al., 1997), profilin allows barbed ends to elongate at near full-speed while continuously associated with Cdc12(FH1FH2)p (Kovar et al., 2003; Kovar and Pollard, 2004). Once Cdc12(FH1HF2)p is bound, this profilin-gated, barbed end elongation persists even with saturating concentrations of SpCP (Figure 6). Other formins such as budding yeast Bni1p (Pruyne et al., 2002; Sagot et al., 2002) and mDiaI (Li and Higgs, 2003; Harris et al., 2004) are less dependent on profilin to gate barbed end elongation. However, Bni1p absolutely requires profilin to assemble actin filaments in cells (Evangelista et al., 2002; Sagot et al., 2002), and Bni1p and mDia1 compete with capping protein for barbed ends (Zigmond et al., 2003; Harris et al., 2004; Moseley et al., 2004; Romero et al., 2004).
The evidence suggests that Cdc12p and SpCP interact sequentially with actin filament barbed ends at two distinct stages of contractile ring function. Cdc12p (Cdc12p-3XGFP) arrives early at the division site and cooperates with profilin to trigger the polymerization of the actin filaments required to assemble a compact contractile ring. SpCP arrives 20 min later (Wu et al., 2003) and may slowly replace Cdc12p on some of the barbed ends in preparation for disassembly (of pointed ends) during constriction of the ring (Wu et al., 2003). Regulation of the competition between formins and capping protein during cytokinesis is far from understood. Profilin regulates Cdc12p, Rho family GTPases overcome autoinhibition of some formins (Wallar and Alberts, 2003), and polyphosphoinositides inhibit capping proteins in vitro (Cooper and Schafer, 2000), but none of these simple binary interactions begins to explain the biology.
An alternative mechanism also should be considered. Because the contractile ring assembles de novo (Marks and Hyams, 1985; Pelham and Chang, 2002), the loss of capping protein may compromise the contractile ring indirectly, as postulated for interphase cables. However, some evidence is consistent with direct participation: 1) a small fraction of Acp2p-CFP concentrates in the contractile ring; 2) capping protein null mutations interact synthetically with mutations in some (i.e., anillin-like mid1 and IQGAP rng2) but not all (i.e., myosin-II myp2 and regulatory light chain rlc1) genes contributing to cytokinesis; 3) capping protein deletion (acp2Δ) mildly suppresses mutations in both the myosin-II (myo2) and formin (cdc12) required for cytokinesis; and 4) the timing of the arrival of Rlc1p-GFP to the division site, ring assembly, and initiation of constriction seem normal in acp2Δ cells (only late cytokinetic events are aberrant). Additionally, capping protein null mutations do not interact with mutations in tropomyosin cdc8 and the formin for3, which are required for actin cables, although cables are affected in capping protein null cells.
Participation of capping protein in cytokinesis is less clear in other eukaryotes. Capping protein purifies with midbodies from CHO cells (Skop et al., 2004) and accumulates transiently in the midbodies of C. elegans embryos (Waddle et al., 1994). On the other hand, depletion of capping protein from either Dictyostelium (Hug et al., 1995) and cultured Drosophila cells (Kiger et al., 2003; Rogers et al., 2003) compromises the leading lamellae with little impact on cytokinesis, although the ploidy of Dictyostelium increased slightly. Deletion of the Drosophila capping protein β-subunit is lethal (Hopmann et al., 1996), but the mechanism was not investigated. Budding yeast accomplish cytokinesis without capping protein (Amatruda et al., 1990, 1992), perhaps because only limited constriction of the contractile ring is required (Watts et al., 1987; Bi et al., 1998; Tolliday et al., 2003).
Biochemical Properties of SpCP
In each biochemical assay, SpCP was ∼10- to 20-fold less active than mouse capping protein. The affinity of SpCP for muscle actin barbed ends seems similar to plant and budding yeast capping proteins (Amatruda and Cooper, 1992; Huang et al., 2003; Kim et al., 2004) but lower than vertebrate capping protein (Wear et al., 2003). SpCP may bind differently to fission yeast actin, although budding yeast capping protein has the same affinity for muscle actin and yeast actin barbed ends (Kim et al., 2004). Most capping proteins stimulate spontaneous assembly of actin monomers by nucleating filaments (capturing actin monomers and dimers) that grow slowly at their pointed ends (Cooper and Pollard, 1985; Caldwell et al., 1989; Kovar et al., 2003), but concentrations of SpCP up to 100 nM inhibit spontaneous assembly of actin. Higher concentrations of SpCP stimulate spontaneous assembly like other capping proteins (Figure 1L). The biphasic dependence of actin assembly on the concentration of SpCP indicates that SpCP has a much higher affinity for filament barbed ends than for monomers and/or dimers. Nucleation of actin filaments by capping proteins may not be relevant in cells (Hug et al., 1995), because these filaments cannot grow at either end in the presence of profilin, and dimers bound by capping protein may not be accessible to formin.
Capping Protein Functions during Interphase
Although fission yeast lacking either or both capping protein subunits had normal morphology at 25°C, interphase actin patches stained more intensely with rhodamine-phalloidin, and interphase actin cables were diminished compared with wild-type cells. Actin filaments are also more abundant in patches of budding yeast lacking capping protein (Li et al., 1995; Kim et al., 2004). Kim et al. (2004) proposed a reasonable explanation that may apply to fission yeast: filaments nucleated in patches by Arp2/3 complex grow unchecked without capping such that each nucleation event produces a longer actin filament until most monomers are consumed. Budding yeast actin patches contain a network of branched actin filaments (Young et al., 2004), similar to the leading edge of motile cells (Pollard and Borisy, 2003). Surprisingly, actin filaments in latrunculin treated patches isolated from budding yeast cells lacking capping protein are not longer than filaments from wild-type patches (Young et al., 2004).
The reason for compromised actin cables in capping protein null cells is less clear. Cable assembly and stability depend on formins, For3p in the case of fission yeast (Feierbach and Chang, 2001; Nakano et al., 2002), which remain associated with growing barbed ends (Higashida et al., 2004; Kovar et al., 2004; Romero et al., 2004) and prevent capping protein from inhibiting barbed end growth (Zigmond et al., 2003; Harris et al., 2004; Moseley et al., 2004; Romero et al., 2004; this report). The persistent association of formins with barbed ends protects them from capping protein and accounts for the absence of capping protein in cables.
Because neither Acp2p-GFP, GFP-Acp2p, nor budding yeast capping protein (Amatruda and Cooper, 1992) concentrates in cables, the loss of capping protein may compromise cables indirectly. Unregulated incorporation of actin into filaments in patches may deplete the cytoplasmic pool of actin monomers, compromising cable growth. This indirect mechanism requires that nucleation by formins be more sensitive to the actin monomer concentration than nucleation by Arp2/3 complex. Consistent with this idea, yeast formins are inefficient nucleators at actin concentrations below 1 μM in vitro (Pruyne et al., 2002; Kovar et al., 2003), whereas Arp2/3 complex efficiently nucleates assembly until all monomers are consumed (Higgs et al., 1999).
Acknowledgments
We thank Fred Chang (Columbia University, New York, NY) for helpful suggestions and strains; Greg Law (Perkin Elmer-Cetus Life Sciences) for help with the spinning disk confocal microscope; Matt Lord for Rlc1p-GFP strains; and members of our laboratory for discussions, reagents, and technical expertise. This work was supported by National Institutes of Health research grants GM-26338 and GM-26132 (to T.D.P.), a National Institutes of Health postdoctoral fellowship (to D.R.K.), and an Anna Fuller Fund postdoctoral fellowship (to J.-Q.W.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–09–0781) on March 2, 2005.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Amatruda, J. F., Cannon, J. F., Tatchell, K., Hug, C., and Cooper, J. A. (1990). Disruption of the actin cytoskeleton in yeast capping protein mutants. Nature 344, 352–354. [DOI] [PubMed] [Google Scholar]
- Amatruda, J. F., and Cooper, J. A. (1992). Purification, characterization, and immunofluorescence localization of Saccharomyces cerevisiae capping protein. J. Cell Biol. 117, 1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatruda, J. F., Gattermeir, D. J., Karpova, T. S., and Cooper, J. A. (1992). Effects of null mutations and overexpression of capping protein on morphogenesis, actin distribution and polarized secretion in yeast. J. Cell Biol. 119, 1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler, J., Wu, J. Q., Longtine, M. S., Shah, N. G., McKenzie, A., 3rd, Steever, A. B., Wach, A., Philippsen, P., and Pringle, J. R. (1998). Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943–951. [DOI] [PubMed] [Google Scholar]
- Balasubramanian, M. K., Hirani, B. R., Burke, J. D., and Gould, K. L. (1994). The Schizosaccharomyces pombe cdc3+ gene encodes a profilin essential for cytokinesis. J. Cell Biol. 125, 1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian, M. K., McCollum, D., and Gould, K. L. (1997). Cytokinesis in fission yeast Schizosaccharomyces pombe. Methods Enzymol. 283, 494–506. [DOI] [PubMed] [Google Scholar]
- Bi, E., Maddox, P., Lew, D. J., Salmon, E. D., McMillan, J. N., Yeh, E., and Pringle, J. R. (1998). Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J. Cell Biol. 142, 1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchoin, L., and Pollard, T. D. (2002). Hydrolysis of ATP by polymerized actin depends on the bound divalent cation but not profilin. Biochemistry 41, 597–602. [DOI] [PubMed] [Google Scholar]
- Caldwell, J. E., Heiss, S. G., Mermall, V., and Cooper, J. A. (1989). Effects of CapZ, an actin capping protein of muscle, on the polymerization of actin. Biochemistry 28, 8506–8514. [DOI] [PubMed] [Google Scholar]
- Carter, S. B. (1967). Effects of cytochalasins on mammalian cells. Nature 213, 261–264. [DOI] [PubMed] [Google Scholar]
- Chang, F., Drubin, D., and Nurse, P. (1997). cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J. Cell Biol. 137, 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, J. A., and Pollard, T. D. (1985). Effect of capping protein on the kinetics of actin polymerization. Biochemistry 24, 793–799. [DOI] [PubMed] [Google Scholar]
- Cooper, J. A., and Schafer, D. A. (2000). Control of actin assembly and disassembly at filament ends. Curr. Opin. Cell Biol. 12, 97–103. [DOI] [PubMed] [Google Scholar]
- Evangelista, M., Pruyne, D., Amberg, D. C., Boone, C., and Bretscher, A. (2002). Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat. Cell Biol. 4, 260–269. [DOI] [PubMed] [Google Scholar]
- Evangelista, M., Zigmond, S., and Boone, C. (2003). Formins: signaling effectors for assembly and polarization of actin filaments. J. Cell Sci. 116, 2603–2611. [DOI] [PubMed] [Google Scholar]
- Feierbach, B., and Chang, F. (2001). Roles of the fission yeast formin for3p in cell polarity, actin cable formation and symmetric cell division. Curr. Biol. 11, 1656–1665. [DOI] [PubMed] [Google Scholar]
- Goode, B. L., Drubin, D. G., and Lappalainen, P. (1998). Regulation of the cortical actin cytoskeleton in budding yeast by twinfilin, a ubiquitous actin monomer-sequestering protein. J. Cell Biol. 142, 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, E. S., Li, F., and Higgs, H. N. (2004). The mouse formin, FRLalpha, slows actin filament barbed end elongation, competes with capping protein, accelerates polymerization from monomers, and severs filaments. J. Biol. Chem. [DOI] [PubMed]
- Higashida, C., Miyoshi, T., Fujita, A., Oceguera-Yanez, F., Monypenny, J., Andou, Y., Narumiya, S., and Watanabe, N. (2004). Actin polymerization-driven molecular movement of mDia1 in living cells. Science 303, 2007–2010. [DOI] [PubMed] [Google Scholar]
- Higgs, H. N., Blanchoin, L., and Pollard, T. D. (1999). Influence of the C terminus of Wiskott-Aldrich syndrome protein (WASp) and the Arp2/3 complex on actin polymerization. Biochemistry 38, 15212–15222. [DOI] [PubMed] [Google Scholar]
- Hopmann, R., Cooper, J. A., and Miller, K. G. (1996). Actin organization, bristle morphology, and viability are affected by actin capping protein mutations in Drosophila. J. Cell Biol. 133, 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopmann, R., and Miller, K. G. (2003). A balance of capping protein and profilin functions is required to regulate actin polymerization in Drosophila bristle. Mol. Biol. Cell 14, 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk, T. W., Jr., and Ue, K. (1974). The measurement of actin concentration in solution: a comparison of methods. Anal. Biochem. 62, 66–74. [DOI] [PubMed] [Google Scholar]
- Huang, S., Blanchoin, L., Kovar, D. R., and Staiger, C. J. (2003). Arabidopsis capping protein (AtCP) is a heterodimer that regulates assembly at the barbed ends of actin filaments. J. Biol. Chem. 278, 44832–44842. [DOI] [PubMed] [Google Scholar]
- Hug, C., Jay, P. Y., Reddy, I., McNally, J. G., Bridgman, P. C., Elson, E. L., and Cooper, J. A. (1995). Capping protein levels influence actin assembly and cell motility in Dictyostelium. Cell 81, 591–600. [DOI] [PubMed] [Google Scholar]
- Isenberg, G., Aebi, U., and Pollard, T. D. (1980). An actin-binding protein from Acanthamoeba regulates actin filament polymerization and interactions. Nature 288, 455–459. [DOI] [PubMed] [Google Scholar]
- Kang, F., Purich, D. L., and Southwick, F. S. (1999). Profilin promotes barbedend actin filament assembly without lowering the critical concentration. J. Biol. Chem. 274, 36963–36972. [DOI] [PubMed] [Google Scholar]
- Kiger, A., Baum, B., Jones, S., Jones, M., Coulson, A., Echeverri, C., and Perrimon, N. (2003). A functional genomic analysis of cell morphology using RNA interference. J. Biol. 2, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K., Yamashita, A., Wear, M. A., Maeda, Y., and Cooper, J. A. (2004). Capping protein binding to actin in yeast: biochemical mechanism and physiological relevance. J. Cell Biol. 164, 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar, D. R., Kuhn, J. R., Tichy, A. L., and Pollard, T. D. (2003). The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J. Cell Biol. 161, 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar, D. R., and Pollard, T. D. (2004). Insertional assembly of actin filament barbed ends in association with formins produces piconewton forces. Proc. Natl. Acad. Sci. USA 101, 14725–14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, W. L., Oberle, J. R., and Cooper, J. A. (2003). The role of the lissencephaly protein Pac1 during nuclear migration in budding yeast. J. Cell Biol. 160, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F., and Higgs, H. N. (2003). The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr. Biol. 13, 1335–1340. [DOI] [PubMed] [Google Scholar]
- Li, R., Zheng, Y., and Drubin, D. G. (1995). Regulation of cortical actin cytoskeleton assembly during polarized cell growth in budding yeast. J. Cell Biol. 128, 599–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, J., and Pollard, T. D. (2001). Profilin binding to poly-L-proline and actin monomers along with ability to catalyze actin nucleotide exchange is required for viability of fission yeast. Mol. Biol. Cell 12, 1161–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks, J., and Hyams, J. S. (1985). Localization of F-actin through the cell division cycle of Schizosaccharomyces pombe. Eur. J. Cell Biol. 39, 27–32. [Google Scholar]
- Maundrell, K. (1990). nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J. Biol. Chem. 265, 10857–10864. [PubMed] [Google Scholar]
- Moseley, J. B., Sagot, I., Manning, A. L., Xu, Y., Eck, M. J., Pellman, D., and Goode, B. L. (2004). A conserved mechanism for Bni1- and mDia1-induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol. Biol. Cell 15, 896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano, K., Imai, J., Arai, R., Toh, E. A., Matsui, Y., and Mabuchi, I. (2002). The small GTPase Rho3 and the diaphanous/formin For3 function in polarized cell growth in fission yeast. J. Cell Sci. 115, 4629–4639. [DOI] [PubMed] [Google Scholar]
- Nakano, K., Satoh, K., Morimatsu, A., Ohnuma, M., and Mabuchi, I. (2001). Interactions among a fimbrin, a capping protein, and an actin-depolymerizing factor in organization of the fission yeast actin cytoskeleton. Mol. Biol. Cell 12, 3515–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren, S., Ojala, P. J., Wear, M. A., Cooper, J. A., and Lappalainen, P. (2001). Interactions with PIP2, ADP-actin monomers, and capping protein regulate the activity and localization of yeast twinfilin. J. Cell Biol. 155, 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaloni, D., and Carlier, M. F. (1993). How profilin promotes actin filament assembly in the presence of thymosin beta 4. Cell 75, 1007–1014. [DOI] [PubMed] [Google Scholar]
- Pasion, S. G., and Forsburg, S. L. (1999). Nuclear localization of Schizosaccharomyces pombe Mcm2/Cdc19p requires MCM complex assembly. Mol. Biol. Cell 10, 4043–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham, R. J., and Chang, F. (2002). Actin dynamics in the contractile ring during cytokinesis in fission yeast. Nature 419, 82–86. [DOI] [PubMed] [Google Scholar]
- Petersen, J., Nielsen, O., Egel, R., and Hagan, I. M. (1998a). F-actin distribution and function during sexual differentiation in Schizosaccharomyces pombe. J. Cell Sci. 111, 867–876. [DOI] [PubMed] [Google Scholar]
- Petersen, J., Nielsen, O., Egel, R., and Hagan, I. M. (1998b). FH3, a domain found in formins, targets the fission yeast formin Fus1 to the projection tip during conjugation. J. Cell Biol. 141, 1217–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard, T. D., and Borisy, G. G. (2003). Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465. [DOI] [PubMed] [Google Scholar]
- Pollard, T. D., and Cooper, J. A. (1984). Quantitative analysis of the effect of Acanthamoeba profilin on actin filament nucleation and elongation. Biochemistry 23, 6631–6641. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., Evangelista, M., Yang, C., Bi, E., Zigmond, S., Bretscher, A., and Boone, C. (2002). Role of formins in actin assembly: nucleation and barbedend association. Science 297, 612–615. [DOI] [PubMed] [Google Scholar]
- Rogers, S. L., Wiedemann, U., Stuurman, N., and Vale, R. D. (2003). Molecular requirements for actin-based lamella formation in Drosophila S2 cells. J. Cell Biol. 162, 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, S., Le Clainche, C., Didry, D., Egile, C., Pantaloni, D., and Carlier, M. F. (2004). Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell 119, 419–429. [DOI] [PubMed] [Google Scholar]
- Sagot, I., Rodal, A. A., Moseley, J., Goode, B. L., and Pellman, D. (2002). An actin nucleation mechanism mediated by Bni1 and profilin. Nat. Cell Biol. 4, 626–631. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Schroeder, T. E. (1970). The contractile ring. I. Fine structure of dividing mammalian (HeLa) cells and the effects of cytochalasin B. Z Zellforsch Mikrosk Anat. 109, 431–449. [PubMed] [Google Scholar]
- Schroeder, T. E. (1972). The contractile ring. II. Determining its brief existence, volumetric changes, and vital role in cleaving Arbacia eggs. J. Cell Biol. 53, 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skop, A. R., Liu, H., Yates, I. J., Meyer, B. J., and Heald, R. (2004). Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science 305, 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeno, Y., Abe, H., Kimura, S., Maruyama, K., and Obinata, T. (1998). Generation of functional beta-actinin (CapZ) in an E. coli expression system. J. Muscle Res. Cell Motil. 19, 639–646. [DOI] [PubMed] [Google Scholar]
- Tolliday, N., Pitcher, M., and Li, R. (2003). Direct evidence for a critical role of myosin II in budding yeast cytokinesis and the evolvability of new cytokinetic mechanisms in the absence of myosin II. Mol. Biol. Cell 14, 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddle, J. A., Cooper, J. A., and Waterston, R. H. (1994). Transient localized accumulation of actin in Caenorhabditis elegans blastomeres with oriented asymmetric divisions. Development 120, 2317–2328. [DOI] [PubMed] [Google Scholar]
- Wallar, B. J., and Alberts, A. S. (2003). The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol. 13, 435–446. [DOI] [PubMed] [Google Scholar]
- Watts, F. Z., Shiels, G., and Orr, E. (1987). The yeast MYO1 gene encoding a myosin-like protein required for cell division. EMBO J. 6, 3499–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wear, M. A., Yamashita, A., Kim, K., Maeda, Y., and Cooper, J. A. (2003). How capping protein binds the barbed end of the actin filament. Curr. Biol. 13, 1531–1537. [DOI] [PubMed] [Google Scholar]
- Wu, J. Q., Bahler, J., and Pringle, J. R. (2001). Roles of a fimbrin and an alpha-actinin-like protein in fission yeast cell polarization and cytokinesis. Mol. Biol. Cell 12, 1061–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. Q., Kuhn, J. R., Kovar, D. R., and Pollard, T. D. (2003). Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell 5, 723–734. [DOI] [PubMed] [Google Scholar]
- Yamashita, A., Maeda, K., and Maeda, Y. (2003). Crystal structure of CapZ: structural basis for actin filament barbed end capping. EMBO J. 22, 1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, M. E., Cooper, J. A., and Bridgman, P. C. (2004). Yeast actin patches are networks of branched actin filaments. J. Cell Biol. 166, 629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond, S. H., Evangelista, M., Boone, C., Yang, C., Dar, A. C., Sicheri, F., Forkey, J., and Pring, M. (2003). Formin leaky cap allows elongation in the presence of tight capping proteins. Curr. Biol. 13, 1820–1823. [DOI] [PubMed] [Google Scholar]