Abstract
The histone variant H2AX is rapidly phosphorylated at the sites of DNA double-strand breaks (DSBs). This phosphorylated H2AX (γ-H2AX) is involved in the retention of repair and signaling factor complexes at sites of DNA damage. The dependency of this phosphorylation on the various PI3K-related protein kinases (in mammals, ataxia telangiectasia mutated and Rad3-related [ATR], ataxia telangiectasia mutated [ATM], and DNA-PKCs) has been a subject of debate; it has been suggested that ATM is required for the induction of foci at DSBs, whereas ATR is involved in the recognition of stalled replication forks. In this study, using Arabidopsis as a model system, we investigated the ATR and ATM dependency of the formation of γ-H2AX foci in M-phase cells exposed to ionizing radiation (IR). We find that although the majority of these foci are ATM-dependent, ∼10% of IR-induced γ-H2AX foci require, instead, functional ATR. This indicates that even in the absence of DNA replication, a distinct subset of IR-induced damage is recognized by ATR. In addition, we find that in plants, γ-H2AX foci are induced at only one-third the rate observed in yeasts and mammals. This result may partly account for the relatively high radioresistance of plants versus yeast and mammals.
INTRODUCTION
The induction of DNA double-strand breaks (DSBs) in eukaryotes triggers a number of protective responses including the upregulation of repair pathways, initiation of cell cycle arrest, and, in some organisms, the induction of programmed cell death. DSBs in actively dividing cells are particularly dangerous. Repair to form translocations and deletions can lead to loss of heterozygosity, which in turn leads to carcinogenesis in mammals or lethality in haploid yeast. For this reason all living things possess the ability to detect the presence of DSBs and relay this information to the cell cycle.
Two important protein kinases involved in sensing and signaling DNA damage in eukaryotes are ataxia telangiectasia mutated (ATM) and ataxia telangiectasia mutated and rad3-related (ATR; Abraham, 2001; Sancar et al., 2004). In mammals, ATM is critical for responses to DSBs and signals downstream cell cycle checkpoint regulators including p53 and Chk2 to coordinate apoptotic responses and/or cell cycle arrest (Fernandez-Capetillo et al., 2002). In addition to checkpoint regulation, ATM responds to DSBs by interacting with proteins intimately involved in DNA repair such as the Mre11-Rad50-Nbs1 (M-R-N) complex (Gatei et al., 2000; van den Bosch et al., 2003) and RAD51 (Chen et al., 1999). In comparison, ATR, in a complex with the ATR interacting protein (ATRIP), is thought to respond primarily to agents that block replication, recognizing stalled replication forks and then signaling to Chk1 and p53 to induce cell cycle arrest, replication restart, and apoptosis (Abraham, 2001). In striking contrast to ATM, ATR is an essential gene in mammals; defects in the murine homolog cause early embryonic lethality and loss of ATR in conditional knock-out embryonic stem cells rapidly induces death (Brown and Baltimore, 2000; de Klein et al., 2000). This is thought to reflect a requirement for ATR in the normal replicative cycle (Brown and Baltimore, 2003; Dart et al., 2004).
One of the earliest known responses to DSB induction is the phosphorylation of thousands of molecules of the histone variant H2AX at the break site (Rogakou et al., 1998). Phosphorylated H2AX, known as γ-H2AX, forms “foci” at DSBs induced by ionizing radiation (IR; Rogakou et al., 1999), meiosis (Mahadevaiah et al., 2001), replication (Ward and Chen, 2001), and V(D)J recombination (Chen et al., 2000). Recently, H2AX has been identified as one of the targets of mammalian ATM and ATR phosphorylation. Probably reflecting their somewhat specialized roles in DNA damage recognition and signaling, it was determined that γ-H2AX forms in an ATM-dependent manner in response to DSB-inducing agents such as X-rays, etoposide, and bleomycin, but not UV light or methyl methanesulfonate, agents that primarily induce DNA dimers and alkyl adducts, respectively (Burma et al., 2001). In contrast, it was found that H2AX is phosphorylated in an ATR-dependent manner in situations where DNA synthesis is blocked. In response to hydroxyurea or UV light, ATR-mediated γ-H2AX foci were found in the majority of S-phase cells, whereas very few nonreplicating G1 cells had γ-H2AX foci after the same treatment (Ward and Chen, 2001). This, taken together with the facts that ATR-mediated foci colocalize with proliferating cell nuclear antigen (PCNA; Ward et al., 2004) and that RPA-bound single-stranded DNA (ssDNA) is a good substrate for ATR/ATRIP binding (Zou and Elledge, 2003) suggests that the ATR/ATRIP complex binds ssDNA and phosphorylates H2AX at stalled replication forks.
Recently, plants defective in homologues of both ATM and ATR were found to have a number of features in common with the mammalian mutants. Loss of ATM in Arabidopsis thaliana (Arabidopsis) causes meiotic defects, hypersensitivity to DSB-inducing agents, and an inability to properly induce IR-mediated transcription of several DNA repair genes (Garcia et al., 2003). Similarly, defects in the mammalian homolog cause IR sensitivity, prevent activation of ATM interacting proteins (Shiloh, 2003), and affect meiosis (Barlow et al., 1998). Additional hallmarks of mammalian ATM deficiency include severe growth defects and neurodegenerative disease (Shiloh, 2003). In contrast, plant ATM mutants do not exhibit obvious developmental defects in the absence of DNA damaging agents. Arabidopsis ATR knockout mutants are viable, fertile, and phenotypically normal in the absence of exogenous DNA damaging agents. Like human conditional ATR knockout lines, mutant atr plants are hypersensitive to replication antagonists, including UV light, aphidicolin, and hydroxyurea, and are defective in G2 checkpoints induced by these agents (Culligan et al., 2004). Thus, many of ATR's and ATM's functions are conserved in plants and mammals.
In this article, using both immunoblotting and immunocytochemistry we demonstrate that ATM and ATR-dependent γ-H2AX induction is conserved in plants and that IR-dependent γ-H2AX foci are formed at less than one-third the rate of mammalian γ-H2AX foci, suggesting a lower rate of DSB induction in plants. Significantly, because we limit our examination to M-phase cells, we show that ATR contributes to IR-induced γ-H2AX focus formation in the absence of DNA replication, demonstrating a clear role for ATR in response to IR distinct from its role during S phase. Furthermore, using mutants defective in either or both of the Arabidopsis ATM and ATR homologues, we quantify the contributions of each to IR-induced γ-H2AX formation. These results suggest that ATM and ATR are recognizing two distinct classes of IR-induced DNA damage products.
MATERIALS AND METHODS
Plant Lines and Human Cells
Wild-type, atr-3, and atm-1 plants are in the Ws ecotype, and atm-2 is in the Col ecotype (Garcia et al., 2003; Culligan et al., 2004). atm,atr double mutant plants were constructed using the atm-1 and atr-3 alleles. The atm,atr double mutant is completely sterile; to isolate double mutants, we PCR-genotyped progeny of an atr-3 homozygous atm-1 heterozygous plant, which is fertile and produces seeds that segregate in the expected 1:2:1 manner for atm in a homozygous atr background. Plants for protein extraction were grown on Premier Prosoil mix (Premier Horticulture, Dorval, Canada), whereas plants for root tip excision were grown on nutritive MS agar; all plants were grown at 21°C as described (Friesner and Britt, 2003). Human HCT116 colorectal cells, graciously donated by Dr. Ken Kaplan (UC Davis), were grown and maintained as described (Green and Kaplan, 2003).
Ionizing Radiation
Plants for protein extraction were sown on soil as described (Friesner and Britt, 2003). Approximately 3-wk-old single mutant plants (before bolting), or 4–6-wk-old double mutant plants with buds removed, were irradiated at doses indicated in the figures, with a 137Cs source (7.5 Gy min-1; Institute of Toxicology and Environmental Health, University of California, Davis). Plants were harvested at times indicated, placed into liquid nitrogen, and then stored at -80°C until protein extraction. Seeds grown for root tip excision were sterilized and plated onto nutritive agar as described (Friesner and Britt, 2003). Five- to 8-d-old seedlings were irradiated at doses indicated and immersed in fixative at times indicated, and root tips were excised (see Root Tip Excision, below). Plants involved in repair time course studies were returned to growth chambers and harvested at times indicated. Flasks of HCT116 cells were exposed to 100 Gy of IR or mock-irradiated, trypsinharvested 30 min later, lysed, and subject to acid extraction as described (Karlsson and Stenerlow, 2004). After removal from incubation, cells were kept at room temperature before extraction.
Antibody Production
Antiplant γ-H2AX antibody was prepared by AnaSpec (San Jose, CA) against the synthetic peptide CVGKNKGDIGSA(p)SQGEF (the cysteine was added during preparation). These amino acids are identical in both the putative Arabidopsis H2AX proteins H2AXa and H2AXb. The peptide was conjugated to keyhole limpet hemocyanin and injected into two rabbits. Immune serum was first passed over a column containing immobilized CVGKNKGDIGSA(p)SQGEF to retain phospho-specific antibodies, eluted, and passed over a second column containing immobilized CVGKNKGDIGSASQGEF to remove antibodies to unphosphorylated H2AX. The flow-through from this column was used in experiments in this study. ELISA analysis was performed by AnaSpec to demonstrate the specificity of the antibody to phosphorylated H2AX peptide.
Acid Extraction of Proteins
To enrich samples for histones, acid extraction of plant tissue was performed essentially as described (Jackson et al., 2004), with the following modifications; Sodium fluoride (Acros, Morris Plains, NJ) and sodium ortho-vanadate (Acros) were added to concentrations of 30 and 100 mM, respectively, to inhibit protein phosphatases. Protein samples were quantified using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Richmond, CA), using bovine serum albumin (BSA) to create a standard curve. Human HCT116 cells were acid extracted as described (Karlsson and Stenerlow, 2004) with benzamide and aprotinin omitted. Protein concentration was estimated by comparing serial dilutions of samples on a gel stained with Coomassie-blue. Five percent 2-mercaptoethanol, 5–10% 1.5 M Tris-HCl, pH 8.8 (to neutralize samples, if needed), and 5× sample loading buffer (10% SDS, 0.5% bromophenol blue, 313 mM Tris-HCl, pH 6.8, 50% glycerol) were then added to each tube, and samples were boiled for 5 min. Samples were stored at -20°C until used for immunoblotting. Before loading, samples were reboiled for 3 min.
Immunoblotting
For plant protein blots, ∼20 μg of protein samples was loaded into each well of a 4–20% Tris-glycine-SDS gradient precast polyacrylamide gel (iGel, Gradipore, Hawthorne, NY) before electrophoresis. Gels were transferred to PVDF membranes (Immobilon-P, Millipore, Bedford, MA) overnight at 95 mA, 4°C with either CAPS/methanol buffer (10 mM CAPS, 10% methanol, pH 11) or nonmethanol buffer (48 mM Tris base, 39 mM glycine, to pH 9.3 with NaOH). Blots were incubated in 3% skim milk in 1× Tris-buffered saline (TBS)-T (0.05% final concentration Tween-20) either overnight at 4°C, or at room temperature for 3–5 h, on a rotating platform. Blots were then incubated overnight at 4°C in rabbit antiplant γ-H2AX primary antibody diluted 1:5000 in 3% skim-milk/1× TBS-T. Blots were briefly rinsed three times with ddH20, washed three times for 5 min each in large volumes of ddH20, and then once in 1× TBS-T for 15 min at room temperature on a rotating platform. Blots were then incubated with anti-rabbit immunoglobulin horseradish peroxidase–linked secondary antibody (Amersham Biosciences NA934V, Piscataway, NJ) at a dilution of 1:10,000 in 3% skim milk/1× TBS-T for 1–2 h at room temperature on a rotating platform. Blots were washed as before and exposed for various times, typically 45 s to 5 min, to x-ray film (Hyperfilm ECL, Amersham Biosciences; or CL-Xposure film, Pierce, Rockford, IL) after incubation with enhanced chemiluminescence reagents (ECL+) as described (RPN 2132, Amersham Biosciences). After film exposures, blots were stained for 5 min in Ponceau S (P-3504, Sigma, St. Louis, MO) solution, and destained with ddH20 to visualize proteins and estimate protein loading. For Western blots containing human cell, protein samples were separated on a 12.5% polyacrylamide gel and transferred to nitrocellulose membranes for 4 h at 400 mA and 4°C using 20% methanol transfer buffer. Blots were incubated in 2% nonfat dry milk in 1× TBS-T for 1 h at room temperature. Blots were incubated for 2 h at room temperature in either polyclonal anti-human γ-H2AX primary antibody (Upstate Biotechnology, Lake Placid, NY) diluted 1:1000 or polyclonal antiplant γ-H2AX primary antibody diluted 1:4000 in phosphate-buffered saline (PBS)-gelatin. Blots were washed and incubated for 1 h at room temperature in anti-rabbit secondary antibody as described above. Blots were exposed to x-ray film as described above, except the SuperSignal West Pico detection system by Pierce was used as directed. Film exposures and Ponceau S–stained blots were scanned to retain digital images.
Root Tip Excision and Slide Preparation
Root tips were excised as described (Liu et al., 1993), with the following modifications: root tips were fixed for 45 min in freshly prepared 4% formaldehyde solution in 1× PME and then washed with 1× PME five times for 5 min each. Tips were digested for 30 min in a 1% cellulase solution (Onuzuka RS cellulase, Research Products International, Mount Prospect, IL) prepared in 1× PME and washed five times for 5 min with 1× PME as before. Root tips were squashed gently onto gelatin-coated slides as described (Liu et al., 1993); slides were allowed to air dry and placed at -80°C until antibody incubation. Before immunostaining, slides were removed from -80°C, warmed at room temperature for 15–25 min, and rehydrated in 1× PME. Slides were incubated with 0.5% Triton X-100 (Sigma) in 1× PME for 10–15 min, rinsed with 1× PME, incubated three times for 5 min in 1× PME, and then immersed in -20°C methanol for 10 min. Slides were immediately placed in 1× PBS for 10 min and then washed three times for 5 min with 1× PBS. All washes were performed at room temperature.
Immunostaining
Slides were incubated with our rabbit antiplant γ-H2AX and commercially available mouse monoclonal antialpha tubulin (Sigma, clone DM1A) antibodies. Antibodies were diluted 1:400–1:800 for anti-γ-H2AX, and 1:600–1:800 for anti-α-tubulin in dilution solution (3% BSA, 0.05% Tween-20, 0.02% NaN3 in 1× PBS, stored at 4°C). Fifty microliters of diluted primary antibodies were applied to each slide for 3 h at room temperature or overnight at 4°C. Slides were washed three times in 1× PBS for 5 min each and incubated for 2–3 h at room temperature in 50 μl antibody dilution solution consisting of FITC-conjugated donkey anti-rabbit (Rockland Immunochemicals, Gilbertsville, PA, 1:800 or 1:1000) or Alexa 488–conjugated goat anti-rabbit (Molecular Probes, Eugene, OR, 1:800 or 1:1000) and Texas red-X–conjugated goat anti-mouse (Molecular Probes, 1:800 or 1:1000) secondary antibodies. Slides were washed as before. Finally, slides were mounted in DAPI-containing mounting medium to visualize plant chromosomes (100 mM Tris-HCl, pH 9.2, 50% glycerol, 2 μg/ml DAPI, 1 μg/ml phenylene diamine, stored at -80°C); coverslips were applied and sealed with clear nail polish, and slides were placed at 4°C overnight before examining. Nuclei were visualized using a Nikon Eclipse E600 epi-fluorescence microscope (Melville, NY) equipped with a mercury lamp. Images were viewed using ImagePro Plus software (Media Cybernetics, Silver Spring, MD) and captured using a Hamamatsu digital camera (C4742–95, Bridgewater, NJ), equipped with a UniBlitz shutter driver (model D122). Images were captured using a 100× oil lens, and exported as 24-bit RGB composite images, and 8-bit grayscale individual images. A scale bar was drawn in Adobe Photoshop (San Jose, CA) after capturing an image of a micrometer (AO, 0.1 mm/10 μm divisions) at 100× magnification. A 5-μm scale bar was inserted into unmanipulated images and then resized as part of the image.
RESULTS
Putative Arabidopsis H2AX Protein Homologues
The presence of H2AX sequence homologues in widely divergent organisms including Homo sapiens, Saccharomyces cerevisiae, and Arabidopsis (Redon et al., 2002) suggests that these proteins are conserved throughout eukaryotes. Our search of the Arabidopsis genome database, using the human H2AX amino acid sequence as a query, revealed two putative H2AX protein homologues that we term H2AXa and H2AXb. The genes encoding the proteins are located ∼17 Mb apart on opposite sides of the centromere on Chromosome I. Both predicted proteins are 143 amino acids in length and differ in only two positions: H2AXa (GenBank locus At1g08880) codes for threonine at position 3, whereas H2AXb (GenBank locus At1g54690) codes for serine, and at position 44, H2AXa codes for serine, whereas H2AXb codes for alanine. Both possess the canonical C-terminal SQ motif shared by all H2AX protein homologues that is the site of DNA DSB-induced phosphorylation. Histones are highly conserved proteins in eukaryotes, and 13 putative Arabidopsis H2A homologues have been identified in the plant chromatin database (http://www.chromdb.org/). Of these sequences, H2AXa and H2AXb share more identity throughout their coding regions than either shares with any other H2A homolog, suggesting that they may represent a recent gene duplication event (Arabidopsis Genome, 2000; Mitchell-Olds and Clauss, 2002). Expressed sequence tags (ESTs) for both genes are present in GenBank, indicating that both are transcribed, but functional data will be needed to demonstrate whether the two genes have distinct or overlapping roles.
In addition to these two putative H2AX homologues, we found a third Arabidopsis mRNA sequence in GenBank (locus tag BT002503) more closely related to the human H2AX sequence than the previous two. The amino acid sequence corresponding to the translated mRNA was so similar to the human sequence that when used in a database search, the most significant matches were mammalian, rather than plant sequences. Furthermore, BLAST searches could not map the mRNA sequence to any Arabidopsis chromosomal locus, suggesting it was encoded by a different organism. The sequence is probably an incorrectly labeled database contaminant, presumably of mammalian origin.
A Plant-specific γ-H2AX Antibody Recognizes an IR-induced Antigen in Plant and Human Cells
Previous research had demonstrated that an antibody raised against the C-terminus of human γ-H2AX could detect H2AX phosphorylation in Xenopus laevis, Drosophila melanogaster, and S. cerevisiae, suggesting it might similarly cross-react with plant γ-H2AX (Rogakou et al., 1999). We obtained both monoclonal and polyclonal antibodies raised against the C-terminus of human γ-H2AX (Upstate Biotechnology) and performed immunoblotting of irradiated and unirradiated plants. We found that the human antibodies detected a faint 16-kDa protein in plant extracts, approximately the predicted size for plant γ-H2AX, but the level of cross-reactivity was low and in some blots the protein was observed in both irradiated and unirradiated samples (unpublished data). This lack of specificity for irradiated plant tissues suggested that either plant H2AX is always phosphorylated to some extent, or that, regardless of the high degree of similarity between the plant and human proteins, some critical difference must allow the antibody to also recognize unphosphorylated plant H2AX and/or some other protein of similar size.
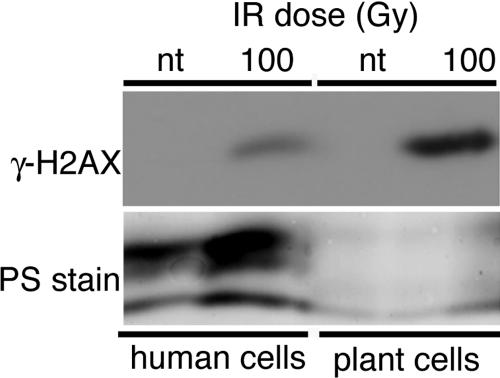
Because of the low cross-reactivity of the human γ-H2AX antibody for irradiated plant extracts, we generated an antibody to the putative plant homologues. A synthetic peptide corresponding to the phosphorylated C-terminal 14 amino acids of Arabidopsis γ-H2AX (identical in H2AXa and H2AXb) was synthesized and a polyclonal antibody was raised in rabbits. The plant γ-H2AX antibody recognized an ∼16-kDa protein in extracts made from irradiated wild-type plants. The antibody also recognized a minimal background band that comigrated with γ-H2AX in unirradiated plants; this may represent a very low steady state level of phosphorylated H2AX, recognition of the nonphosphorylated protein by the polyclonal antibodies, or recognition of some unrelated protein. Within 2 min after treatment with 50 Gy, a dose requiring approximately 7 min of IR exposure, we observed a strong induction of γ-H2AX (unpublished data) that reached maximal amounts within 20 min (Figure 1A). Our plant γ-H2AX antibody detected an IR-induced 16-kDa protein in human cells as well, although detection of this signal required a larger application of protein to the blot (Figure 2). In contrast, a commercially available human antibody, although recognizing both the plant and animal IR-induced protein, was able to detect the human protein with much greater sensitivity (unpublished data). The fact that both the human and plant antibodies detect the same IR-induced signal in both plant and animal cells indicates that our antibody is indeed detecting γ-H2AX. The identity of the higher molecular weight protein at ∼28 kDa is not known (Figure 1) but it typically appears in all samples, regardless of IR exposure. We believe these bands may represent background cross-reacting protein; however, we do observe some variability in the presence and prominence of this high-molecular-weight protein between immunoblots.
Figure 1.
γ-H2AX protein induction in wild-type plants. (A) γ-H2AX induction was assessed over a time course of 10–40 min by immunoblotting. Plants were irradiated with 50 Gy of IR and harvested at the times indicated, which included the time required to deliver the radiation (∼7 min). (B) γ-H2AX induction assessed after increasing doses of IR. Plants were treated with IR as indicated and harvested 15 min after removal from the gamma source. Immunoblots were stained with Ponceau S (PS) and the major protein bands at the top of the blot were compared with qualitatively control for protein loading. Figures are representative blots from two experiments. nt, not treated with IR.
Figure 2.
Protein recognition by the antiplant γ-H2AX antibody in irradiated human cells. Human HCT116 colorectal cells were exposed to 100 Gy of IR and harvested 30 min after removal from the gamma source. A membrane containing equal amounts of irradiated and mock-irradiated protein samples was probed with the plant γ-H2AX antibody. Plant samples were included in the experiment for comparison. PS stain, Ponceau S stain as in Figure 1. nt, not treated with IR.
Induction of Plant γ-H2AX Is IR Dose Dependent
Protein samples from wild-type Arabidopsis plants exposed to increasing doses of IR were subjected to immunoblotting and probed with the plant γ-H2AX antibody. We observed a strong induction of γ-H2AX after IR exposure and protein levels increased with dose (Figure 1B). These data support the prediction that by increasing IR dose, H2AX protein phosphorylation will increase in response to higher levels of DNA damage and suggests that plant γ-H2AX may serve as an indicator of DSB induction in the plant genome.
IR-induced γ-H2AX Foci in M-phase Nuclei
Even correcting for its small genome size, Arabidopsis is a relatively radiation-resistant organism. For this reason we are interested in determining whether the rate of DSB formation by IR is reduced in plants. In mammalian cells, the appearance of γ-H2AX foci clearly correlates with DSB induction, and its loss, with DSB repair (DSBR) (Rothkamm and Löbrich, 2003). Given the obvious value of this assay for DSB induction and repair, identifying an analogous plant process would contribute significantly to our ability to elucidate similar and/or unique features of plant DSBR.
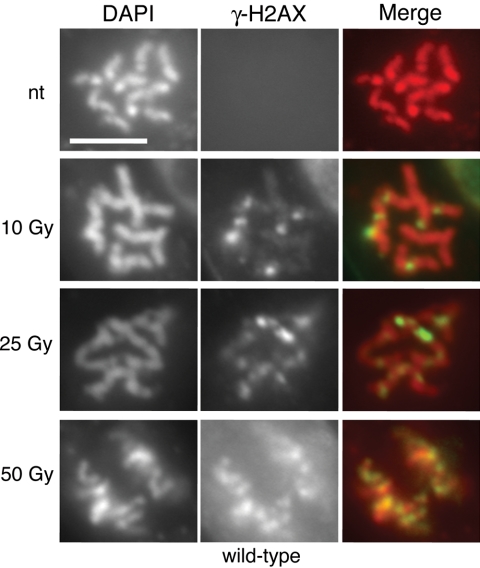
To determine whether plant γ-H2AX foci could also be visualized in response to IR-induced damage, we performed in situ immunostaining experiments using Arabidopsis root tip nuclei. However, most of the DNA in plant cells is endoreduplicated to varying degrees, confounding our ability to accurately determine the number of foci per Gy per Gbp (gigabase pair). Thus, we chose to look at M-phase cells, which in the primary root tip meristem are strictly diploid (D'Amato, 1964). We did not observe any γ-H2AX foci in unirradiated wild-type M-phase cells, but discrete, chromosomally located γ-H2AX foci appeared very quickly after exposure to IR. The intense, localized γ-H2AX staining on chromosomes made it possible to count individual foci (Figure 3). Induction of foci by gamma was approximately linear with dose (Table 1, Figure 4). If we assume the diploid replicated (4N) Arabidopsis genome of an M-phase cell is ∼500 Mbp, this corresponds to ∼1.1–2 foci/Gy-Gbp.
Figure 3.
γ-H2AX focus formation in wild-type plants. Immunofluorescence of root tip nuclei demonstrates IR-dependent γ-H2AX focus induction. Wild-type plants were irradiated with increasing doses of IR and then paraformaldehyde-fixed 5 min after removal from the gamma source. DNA is visualized with DAPI, and merged images overlay γ-H2AX foci onto chromosomes. Scale bar, 5 μm. nt, not treated with IR.
Table 1.
γ-H2AX focus formation in wild-type plants
| Dose
|
|||||
|---|---|---|---|---|---|
| 0 Gy | 2.5 Gy | 5 Gy | 10 Gy | 25 Gy | |
| Average no. of foci/cell | |||||
| Trial 1 | 0 ± 0 (6) | 2.75 ± 0.28 (16) | 3.91 ± 0.23 (28) | 6.4 ± 0.24 (24) | 14.3 ± 1.14 (6) |
| Trial 2 | 0 ± 0 (13) | 2.38 ± 0.12 (45) | 4.53 ± 0.33 (18) | 6.2 ± 0.22 (20) | 12.7 ± 1.20 (3) |
| Overall | 0 ± 0 (19) | 2.48 ± 0.12 (61) | 4.16 ± 0.19 (46) | 6.3 ± 0.16 (44) | 14.1 ± 0.89 (9) |
Values are ± SE with the number of cells in parentheses.
Figure 4.
Approximately linear induction of γ-H2AX foci as a function of IR dose. Error bars, SEM of the values obtained from two experiments. Except for the 25-Gy data point, SEM bars are smaller than the data point symbols.
γ-H2AX Induction and Disappearance in Repair-proficient Plants
It has been demonstrated that each γ-H2AX focus is equivalent to one DSB (Rogakou et al., 1999), and disappearance of γ-H2AX is a reliable indicator of DSBR in mammalian cells (Rothkamm and Löbrich, 2003). To determine the rate at which plant γ-H2AX disappears after induction, we irradiated wild-type plants and harvested tissue after allowing variable time for DNA repair. Similar to our previous experiments, immunoblotting revealed a large initial induction of γ-H2AX in plants harvested shortly after irradiation (Figure 5). Within 2 h, the majority of γ-H2AX had disappeared, and by 48 h postirradiation, γ-H2AX staining had decreased to unirradiated levels, suggesting that most DSBs are repaired quite rapidly in wild-type plants, and only a small number of IR-induced lesions persist. The different rates of γ-H2AX loss we observe may represent the well-characterized phenomenon of differential rates of DSBR (Glasunov et al., 1989; Kodym and Horth, 1995; Iliakis et al., 2004); although the majority of DSBs are quickly repaired, a small subset, perhaps requiring more time and/or different repair complexes for resolution, persist (Nazarov et al., 2003). An alternative, but not exclusive possibility, is that some of the persisting fraction represents indirectly induced DSBs triggered by replication fork collapse in S-phase cells, or γ-H2AX focus formation at non-DSB lesions (Limoli et al., 2002; Ward et al., 2004).
Figure 5.
γ-H2AX induction and disappearance in wild-type plants. Plants received 100 Gy of IR and then were returned to growth chambers and harvested at the times indicated. The immunoblot is representative of four experiments. PS stain, Ponceau S stain as in Figure 1. nt, not treated with IR.
AtATR and AtATM Have Complementary Roles in IR-induced γ-H2AX Formation
To determine if either AtATM or AtATR are responsible for the phosphorylation of histone H2AX in response to ionizing radiation, we examined γ-H2AX induction in atm and atr mutant plants. In this study, we used two alleles of ATM, atm-1 and atm-2 (Garcia et al., 2003), and one of ATR, atr-3 (Culligan et al., 2004). All three lines were generated by T-DNA insertional mutagenesis and each contains a T-DNA insertion in the 3′ region of the gene. The mutation in atm-1 prevents ATM protein expression, and atm-2 is phenotypically indistinguishable from atm-1, suggesting both are null alleles. Both are sensitive to IR and defective in meiosis (Garcia et al., 2003). The insertion in atr-3 lies in the highly conserved C-terminal kinase domain and RT-PCR analysis confirms the ATR transcript is absent, suggesting it is also a null allele (Culligan et al., 2004).
We observed IR-dependent γ-H2AX induction in wild-type, as well as ATM- and ATR-deficient plants via immunoblotting (Figure 6). To support these qualitative data we quantified IR-induced γ-H2AX foci in atm and atr root tips. While γ-H2AX foci were readily apparent in atr nuclei (Figure 7A), the number was severely reduced in atm nuclei (Figure 7B). Typically, one or two foci were seen in atm nuclei, and ∼25% had no foci at all. In comparison to wild-type plants, which have ∼14 foci per cell at this dose (25 Gy), the average number of γ-H2AX foci was 10.5 ± 0.31 in atr plants and 1.2 ± 0.17 in atm plants (Table 2). Because the foci averages for wild-type and atr plants were close in value, and because at this dose, some foci overlap, we also examined atr plants after 10 Gy of IR. Similar to the data at 25 Gy, foci induction in atr plants was slightly, but significantly, reduced after 10 Gy in comparison to wild-type plants (Figure 8). The level of focus formation in atm plants was some-what puzzling given the immunoblot data that suggested loss of AtATM caused only a slight reduction in γ-H2AX induction in comparison to wild-type plants (Figure 6). It may be that the differences we observe in the two methods of examining γ-H2AX induction are due merely to the qualitative nature of our immunoblots. Alternatively, it is possible that the fairly robust γ-H2AX induction observed in atm mutants in Figure 6 is due to the use of cycling cells in immunoblotting experiments while we limit our examination to M-phase cells during immunofluorescent microscopy. Some proportion of cycling cells will be undergoing replication at the time of IR exposure and it is well established in mammalian cells that γ-H2AX induction is primarily dependent on ATR, rather then ATM, during S phase (Ward and Chen, 2001). It is possible that some of the γ-H2AX observed in atm immunoblots is due to ATR-dependent H2AX phosphorylation at sites of IR-induced replicational stress.
Figure 6.
γ-H2AX induction in atm and atr mutants. Immunoblot of wild-type, atm, and atr plants, in the presence or absence of IR. Plants were irradiated with 100 Gy and harvested 15 min after removal from the gamma source. PS, Ponceau S stain as in Figure 1.
Figure 7.
γ-H2AX induction in atm and atr mutants. (A) IR-dependent γ-H2AX focus formation detected by immunofluorescence in atr mutant plants. (a1 and a2) Two focal planes of one unirradiated cell; (b) one focal plane of another unirradiated cell. (c1 and c2) Two focal planes of one cell; (d1 and d2) two focal planes of a different cell treated with 25 Gy. (B) IR-dependent γ-H2AX focus formation detected by immunofluorescence in atm mutant plants. (a and b1/b2) Two unirradiated atm nuclei, the second with two focal planes shown. (c–e) A single focal plane from three atm nuclei exposed to 25 Gy. Scale bar, 5 μm. (C) γ-H2AX foci in ATM-deficient cells going through anaphase (a) or late metaphase (b). Mitotic microtubules are marked with mouse anti-α-tubulin antibodies and visualized in conjunction with γ-H2AX foci and plant chromosomes. nt, not treated with IR.
Table 2.
AtATR and AtATM both contribute to γ-H2AX focus formation
| Dose
|
|||
|---|---|---|---|
| Plant line | 0 Gy | 10 Gy | 25 Gy |
| Wild-type | |||
| Trial 1 | 0 ± 0 (6) | 6.4 ± 0.24 (24) | 14.8 ± 1.14 (6) |
| Trial 2 | 0 ± 0 (13) | 6.2 ± 0.22 (20) | 12.7 ± 1.20 (3) |
| Overall | 0 ± 0 (19) | 6.3 ± 0.16 (44) | 14.1 ± 0.89 (9) |
| atr | |||
| Trial 1 | 0 ± 0 (14) | 5.3 ± 0.29 (17) | 10.3 ± 0.80 (6) |
| Trial 2 | 0 ± 0 (14) | 5.5 ± 0.29 (23) | 10.5 ± 0.31 (14) |
| Overall | 0 ± 0 (28) | 5.4 ± 0.21 (30) | 10.5 ± 0.89 (20) |
| atm | |||
| Trial 1 | 0 ± 0 (24) | 1.4 ± 0.26 (12) | |
| Trial 2 | 0 ± 0 (23) | 1.1 ± 0.22 (19) | |
| Overall | 0 ± 0 (47) | n.d. | 1.2 ± 0.17 (31) |
| atm,atr | |||
| Trial 1 | 0 ± 0 (3) | ||
| Trial 2 | 0 ± 0 (5) | ||
| Trial 3 | 0 ± 0 (7) | ||
| Overall | n.d. | n.d. | 0 ± 0 (15) |
Values are the average number of foci/cell ± SE, with the number of cells in parentheses. Data are from Table 1. n.d., not determined.
Figure 8.
γ-H2AX induction in wild-type and atr plants. Comparison of γ-H2AX focus induction averages from wild-type and atr plants exposed to 10 or 25 Gy. Error bars, SEM of the values obtained from two experiments.
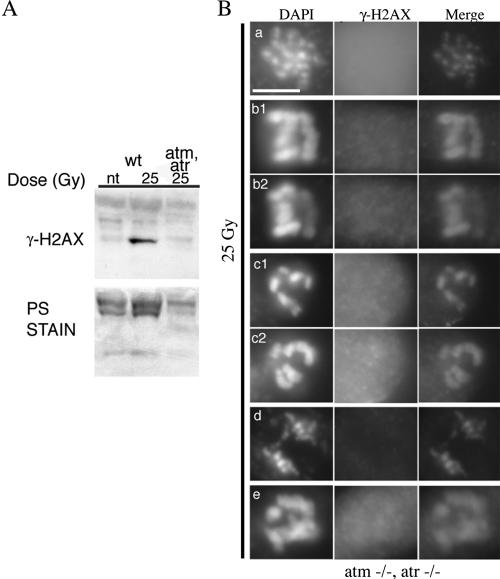
These data suggest that both AtATR and AtATM are involved in the IR-induced phosphorylation of H2AX and that AtATM is directly or indirectly responsible for the majority (∼90%) of focus formation in M-phase cells. The low residual number of foci in atm plants suggests that, in the absence of ATM, another kinase can phosphorylate H2AX in response to IR; one obvious candidate is ATR. To determine if the ATR homolog was responsible for the few remaining IR-induced foci in atm plants, we examined γ-H2AX induction in an atm,atr double mutant. In contrast to wild-type, atm, and atr single mutant plants (Figure 6), no γ-H2AX induction was observed in immunoblots of atm,atr double mutants after IR exposure (Figure 9A). To confirm these data, atm,atr seedlings were irradiated with 25 Gy, and root tip nuclei examined for the presence of γ-H2AX foci. As expected based on the immunoblotting data, no γ-H2AX foci were observed in atm,atr nuclei (Figure 9B, Table 2). These data suggest that although formation of most γ-H2AX foci is dependent on AtATM, AtATR is capable of phosphorylating a limited subset of IR-induced lesions.
Figure 9.
γ-H2AX induction in atm,atr double mutant plants. (A) Immunoblot of wild-type and atm,atr plants. Plants were treated with 25 Gy and harvested 15 min after removal from the gamma source. The immunoblot is representative of three experiments. PS stain as in Figure 1. (B) IR-dependent γ-H2AX focus formation detected by immunofluorescence in atm,atr double mutant plants. (a) One focal plane of a single nucleus; (b1/b2 and c1/c2) two focal planes each of two separate nuclei; (d and e) one focal plane of two separate nuclei. All nuclei are from plants irradiated with 25 Gy. Scale bar, 5 μm. n.t., not treated with IR.
It is known that ATR-dependent γ-H2AX foci form in response to replicational stress (Ward et al., 2004) raising the obvious question of whether the foci observed in atm plants were from cells exposed to IR while in S phase. Plants were harvested between 5 and 20 min after irradiation and all cells examined were in prometaphase or later, well past nuclear envelope breakdown, a process requiring more than 20 min to complete (Wolniak and Larsen, 1992; Hush et al., 1996; Vos et al., 2000). Thus, it is clear that the ATR-dependent foci seen in atm mutants are induced during mitosis rather than in the preceding S phase. We used an antibody against tubulin to visualize the mitotic spindle and determine the phase of all cells examined; in one experiment, 24 of 32 atm cells were in anaphase or metaphase with the remainder in prometaphase (Figure 7C). In the absence of both AtATM and AtATR, γ-H2AX induction in response to IR is completely abrogated, suggesting both play a role in response to IR-induced DNA damage. Given that the average number of foci is reduced in each mutant, these data might be further interpreted to suggest that each kinase recognizes a discrete subset of IR induced lesions in M-phase cells.
DISCUSSION
Data suggest that plants are more resistant than mammals to the induction of DNA lesions. One possible explanation is that in plants, fewer DNA lesions are formed in response to IR, resulting in a lesser insult to the genome. Alternatively, but not exclusively, it is possible that plants possess a greater capacity for DNA repair and/or damage tolerance than do mammals. Thus far it has been difficult to address these questions in plants due to the lack of a method for measuring DSB induction and repair that is both uncomplicated and robust. In this study we describe the development of such a technique and use it to demonstrate that comparable doses of IR appear to induce fewer DSBs in Arabidopsis than in mammalian cells. Additionally, using mutants defective in the plant homologues of ATM and ATR, we demonstrate the contribution of each to the phosphorylation of histone H2AX in response to IR. Because we limit our focus to M-phase nuclei, these data demonstrate a role for ATR in γ-H2AX induction distinct from its conventional role during S phase.
IR Induces Fewer γ-H2AX Foci in Plants than in Mammals
γ-H2AX formation is rapid in mammals, with the first molecules observed within 20 s of irradiation. γ-H2AX protein levels continue to rise with peak formation between ∼10 and 30 min after irradiation (Rogakou et al., 1998). We found that in plants, γ-H2AX formation was observable soon after irradiation, peaking at some point between 20 and 40 min after exposure. As observed in mammals and fungi, the production of γ-H2AX increases with increasing damage to the DNA. In contrast, however, the number of foci generated per Gy per Gbp is significantly lower in Arabidopsis than mammals or fungi. We find that ∼1.25–2 γ-H2AX foci/Gy-Gbp are induced in M-phase root tip nuclei. In comparison, 6 foci/Gy-Gbp, shown to be equivalent to 6 DSBs/Gy-Gbp, are induced in human cells (Rothkamm and Löbrich, 2003), and 5.4 DSBs/Gy-Gbp are induced in yeast (Prise et al., 1998). This suggests that IR actually induces less damage per base pair in the plant genome or that plants fail to form foci at a majority of IR-induced damage sites.
Studies performed on γ-H2AX induction in mammalian cells have convincingly demonstrated that each focus is approximately equivalent to one DSB (Sedelnikova et al., 2002; Rothkamm and Löbrich, 2003), with focus formation and loss reflecting DSB induction and repair. Supporting the notion that we too are observing focus formation primarily at DSBs, other investigators, directly assaying DSB formation in plant cells via pulse-field gel electrophoresis (PFGE), have found a relatively low rate of induction of breaks/Gy-Gbp. Yokota et al. performed PFGE (Rydberg et al., 1994; Lobrich et al., 1995; Whitaker et al., 1995) in tobacco BY-2 cells and Chinese hamster ovary (CHO) cells to directly quantitate and compare IR-dependent DSB induction. They determined DSB induction in tobacco cells was only one-third the rate of CHO cells (2.0 ± 0.1 vs. 6.6 ± 0.2 DSBs/Gy-Gbp; Yokota et al., 2005), and the consistency of their CHO DSB induction data with previously published data suggests their methodology is reliable (Table 3; Cedervall et al., 1995). This suggests that plants are, like yeast and mammals, forming γ-H2AX foci at DSBs and that the lower rate of focus formation observed in plants is simply due to a lower rate of damage induction by IR.
Table 3.
Lower DSB induction in plants
| Organism | Average no. of foci or DSBs per Gy-Gbp | Reference |
|---|---|---|
| Arabidopsis | 1.25–2a | This study |
| Tobacco | ||
| BY-2 cells | 2.0b | Yokota et al. (2005) |
| Yeast (S. cerevisiae) | 5.4b | Prise et al. (1998) |
| CHO-K1 cells | 6.6,b 5.5b | Yokota et al. (2005),b Cedervall et al. (1995)b |
| Human fibroblasts | 6.0,a 6.5b | Rothkamm and Lobrich (2003) |
Foci or
DSBs
What is the basis for lower IR-dependent DSB induction in plants? One possibility is that endogenous plant materials can prevent strand breakage by physically intercepting DNA-damaging molecules. As obligate photosynthesizers, plants frequently must cope with oxidative stress because the photosynthetic process forms reactive oxygen species (ROS) that can interact with and damage intracellular components including nucleic acids (Noctor and Foyer, 1998). The same ROS are also induced by IR-mediated ionization of water molecules that, if formed within radical diffusion distance of DNA, can create lesions including DSBs (Friedberg, 1985). To cope with photo-oxidative damage, plants possess a large repertoire of antioxidants (reviewed in Larson, 1988), compounds that effectively eliminate ROS produced via photosynthesis as well as those from nonphotochemical sources. Thus, it is possible that the lower γ-H2AX induction we observe in plants is due to the action of antioxidants that scavenge IR-induced ROS before they encounter DNA.
The finding that IR induces fewer DSBs in plants is consistent with their higher relative radioresistance. Because fewer lesions are induced per Gy, it is reasonable that a higher dose is needed to effect damage comparable to that inflicted upon mammalian and yeast cells. But is the elevated radioresistance observed in plants directly equivalent to the decrease in DSB induction? One way this question can be addressed is to compare the number of DSBs required to effect 50% lethality (LD50) in yeast and mammals, or in the case of Arabidopsis, to completely arrest growth. The LD50 is ∼1.5 Gy for diploid human fibroblasts (Cole et al., 1988) and ∼400 Gy, or 250-fold higher, for diploid yeast (Resnick, 1969; Resnick and Martin, 1976). However, because the yeast genome is ∼250-fold smaller than that of humans, the LD50 values correspond to induction of ∼54 and 52 DSBs respectively making them comparable on a per DSB basis. In comparison, 400 Gy is required simply to arrest growth in wild-type Arabidopsis plants (J. Friesner, unpublished results; Hefner et al., 2003), yet the plant's genome is only 25-fold smaller than the human genome. Therefore, even after accounting for the lower rate of DSB induction, it requires between 125 and 200 DSBs to completely arrest growth in Arabidopsis.
Arabidopsis ATM and ATR Both Contribute to γ-H2AX Focus Formation
In addition to ATM and ATR, a related mammalian protein, the DNA-dependent protein kinase (DNA-PK), has been implicated in the γ-H2AX induction response to DSBs (Park et al., 2003; Stiff et al., 2004). Although it is clear that ATM, ATR, and DNA-PK are important for responding to DNA damage and effecting DSBR and/or cell cycle regulation, there are a number of contradictory reports in the current literature about the involvement of each in DSB-dependent γ-H2AX induction. In an early report, a 95% reduction in γ-H2AX focus formation was observed in atm-deficient mouse fibroblasts, suggesting that ATM was primarily responsible for IR-induced focus formation. On the other hand, more recent studies report normal (Kuhne et al., 2004; Stiff et al., 2004), or at most, only mild reduction (Karlsson and Stenerlow, 2004) in focus formation in primary fibroblasts derived from atm patients, making it unclear to what extent ATM is involved in IR-dependent γ-H2AX induction. Complicating matters further, in studies that examined γ-H2AX formation in the DNA-PK-deficient cell line M059J, one reported atypical focus induction (Paull et al., 2000), whereas two concluded induction was normal (Karlsson and Stenerlow, 2004; Stiff et al., 2004). The difficulty in interpreting these data are compounded by the fact that M059J cells are also partially defective in ATM (Chan et al., 1998; Gately et al., 1998).
In contrast to ATM and DNA-PK, ATR is believed to primarily respond to DNA damage that results from replication blockage (Abraham, 2001; Furuta et al., 2003; Ward et al., 2004), although there is evidence that ATR can compensate for the absence of ATM in certain situations (Siliciano et al., 1997; Cliby et al., 1998). Interestingly, chromosomes in ATR-deficient mouse blastocyst mitotic spreads are extensively fragmented (Brown and Baltimore, 2000), and “fragile sites,” regions in the genome that are especially susceptible to genomic instability after replication stress and appear cytologically as gaps or breaks on metaphase chromosomes, are increased 10–20-fold in ATR-deficient cells (Casper et al., 2002). Although these data may suggest that ATR protects against DSB formation via a role in M phase, it appears more likely that these aberrations are actually due to errors that occurred in the preceding S phase and simply become apparent in M phase (Brown and Baltimore, 2000; Casper et al., 2002; Cimprich, 2003). Thus, the combined literature places the functions of ATR primarily in situations of replicational distress.
The mammalian studies that seek to determine the relative contribution(s) of PIKK family members to H2AX phosphorylation are hampered by the inability to delete ATR, an essential gene in mammals, thus precluding combining ATR null alleles with ATM, and/or DNA-PK deficiencies. Homologues of DNA-PK have not been found in any nonvertebrates, suggesting that AtATM and/or AtATR are responsible for phosphorylating H2AX in Arabidopsis. The lack of DNA-PK, and the availability of viable ATM and ATR Arabidopsis null mutants allowed us to definitively determine the kinases involved in, and their relative contributions to, IR-induced γ-H2AX formation in M-phase cells.
We find that γ-H2AX focus formation in atm M-phase nuclei is reduced to <10% the level in wild-type nuclei, suggesting that AtATM is primarily responsible for IR-induced γ-H2AX formation in M-phase cells (Table 2). We also find that atr single mutants have slightly, but significantly, reduced focus formation in irradiated M-phase cells, suggesting that ATR is responsible for a subset of foci independent of those induced by ATM (Figure 8, Table 2). Combining ATM and ATR deficiencies results in complete abolishment of γ-H2AX induction, in M-phase nuclei and cycling cells, demonstrating that these two proteins are complementary, and completely responsible for IR-dependent γ-H2AX induction, at least over the time course we examined (Figure 9, Table 2). Because we restrict our analysis to cells that have already undergone nuclear breakdown, it is clear that the foci we are seeing were induced in M phase. Thus, ATR clearly plays a role in damage recognition outside of (as well as within) the context of normal replicative DNA synthesis.
What is the lesion that ATR is recognizing? One possibility is that a subset of IR-induced lesions, or their repair intermediates, are characterized by the presence of extensive ssDNA, which, when complexed with replication protein A (RPA), is an attractive substrate for ATR/ATRIP complex binding (Zou and Elledge, 2003). Alternatively, it was recently shown that ATR is necessary for the S-phase DNA cross-link checkpoint in human cells, demonstrating that this lesion, in S-phase cells at least, is recognized preferentially by ATR, and not ATM (Pichierri and Roselli, 2004). Although repair of interstrand cross-links (ICLs) is poorly understood, they are believed to be minor products of IR-induced DNA damage (Friedberg et al., 1995). However, current research suggests that cells must first enter S phase to convert ICLs into DSBs (Niedernhofer et al., 2004; Rothfuss and Grompe, 2004), thus, it remains to be determined what lesion(s) are recognized by AtATR in M-phase cells.
In this article, using immunological detection of plant γ-H2AX, we demonstrate that phosphorylation of H2AX occurs rapidly after IR, as in fungi and mammals, and that the rate of γ-H2AX focus formation in plants is about threefold lower, on a per Gy, per Gbp basis, than in mammalian and yeast cells. We also examined γ-H2AX focus formation in atm and atr plants and determined that AtATM is responsible for the majority (∼90%) of IR-induced foci in M-phase cells. Focus formation is completely abolished in atm,atr double mutants, confirming that AtATR is responsible for the residual foci induced in atm plants. Significantly, because we limit our examination to M-phase cells, we show that ATR contributes to IR-dependent γ-H2AX focus formation in non–S-phase cells, suggesting a novel role for ATR in response to IR distinct from its role during replication.
The development of the plant-specific γ-H2AX antibody should be useful for future studies of plant DNA metabolism. Using the γ-H2AX antibody, we have also observed the presence of γ-H2AX in unirradiated floral bud tissue, and in irradiated interphase atm-deficient cells (J. Friesner, unpublished observations), suggesting that the conservation of γ-H2AX function will extend to cells undergoing meiotic recombination, as well DNA replication.
Acknowledgments
Dr. Bo Liu provided expertise, reagents, and equipment required for cytological studies. Dr. Kevin Culligan provided the atr and atr,atm segregating lines. We are extremely grateful to Dr. Steve Jacobsen and members of his lab for sharing histone preparation protocols and technical advice and to Dr. Y. Yokota for sharing in press data (Yokota et al., 2005). We thank Dr. Eli Hefner for ordering the γ-H2AX peptide and antibody. We also thank Drs. ScottMerlino, Julin Maloof, Dan Kliebenstein, and Steve Theg for helpful discussion; Dr. Julie Lee for technical advice regarding sample preparation and microscopy; and Christy Caldwell and Dr. Ken Kaplan for providing human cells and assistance with immunoblotting. This work was made possible by an National Institute of Environmental Health Sciences fellowship provided to J. Friesner (grant 5-32-ES07059). Support was also provided by United States–Israel Binational Agricultural Research Fund (US-3223-01C) and the United States Department of Agriculture (04-35301-14740).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-10-0890) on March 16, 2005.
References
- Abraham, R. (2001). Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15, 2177-2196. [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome, I. (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796-815. [DOI] [PubMed] [Google Scholar]
- Barlow, C., Liyanage, M., Moens, P. B., Tarsounas, M., Nagashima, K., Brown, K., Rottinghaus, S., S.P., J., Tagle, D., Ried, T., and Wynshaw-Boris, A. (1998). Atm deficiency results in severe meiotic disruption as early as leptonema of prophase I. Development 125, 40007-40017. [DOI] [PubMed] [Google Scholar]
- Brown, E., and Baltimore, D. (2003). Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 17, 615-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, E.J., and Baltimore, D. (2000). ATR disruption lead to chromosome fragmentation and early embryonic lethality. Genes Dev. 15, 397-402. [PMC free article] [PubMed] [Google Scholar]
- Burma, S., Chen, B. P., Murphy, M., Kurimasa, A., and Chen, D. J. (2001). ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276, 42462-42467. [DOI] [PubMed] [Google Scholar]
- Casper, A. M., Nghiem, P., Arlt, M. F., and Glover, T. W. (2002). ATR regulates fragile site stability. Cell 111, 779-789. [DOI] [PubMed] [Google Scholar]
- Cedervall, B., Wong, R., Albright, N., Dynlacht, J., Lambin, P., and Dewey, W. C. (1995). Methods for the quantification of DNA double-strand breaks determined from the distribution of DNA fragment sizes measured by pulsed-field gel electrophoresis. Rad. Res. 143, 8-16. [PubMed] [Google Scholar]
- Chan, D. W., Gately, D. P., Urban, S., Galloway, A. M., Lees-Miller, S. P., Yen, T., and Allalunis-Turner, J. (1998). Lack of correlation between ATM protein expression and tumour cell radiosensitivity. Int. J. Rad. Biol. 74, 217-224. [DOI] [PubMed] [Google Scholar]
- Chen, G. et al. (1999). Radiation-induced assembly of Rad51 and Rad52 recombination complex requires ATM and c-Abl. J. Biol. Chem. 274, 12748-12752. [DOI] [PubMed] [Google Scholar]
- Chen, H. T. et al. (2000). Response to RAG-mediated V(D)J cleavage by NBS1 and gamma-H2AX. Science 290, 1962-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimprich, K. A. (2003). Fragile sites: breaking up over a slowdown. Curr. Biol. 13, R231-R233. [DOI] [PubMed] [Google Scholar]
- Cliby, W., Roberts, C., Cimprich, K., Stringer, C., Lamb, J., Schreiber, S., and Friend, S. (1998). Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J. 17, 159-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, J., Arlett, C. F., Green, M. H., Harcourt, S. A., Priestley, A., Henderson, L., Cole, H., James, S. E., and Richmond, F. (1988). Comparative human cellular radiosensitivity: II. The survival following gamma-irradiation of unstimulated (G0) T-lymphocytes, T-lymphocyte lines, lymphoblastoid cell lines and fibroblasts from normal donors, afrom ataxia-telangiectasia patients and from ataxia-telangiectasia heterozygotes. Int. J. Radiat. Biol. 54, 929-943. [DOI] [PubMed] [Google Scholar]
- Culligan, K. M., Tissier, A., and Britt, A. B. (2004). ATR regulates a G2-phase cell-cycle checkpoint in Arabidopsis thaliana. Plant Cell 16, 1091-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amato, F. (1964). Endopolyploidy as a factor in plant tissue development. Caryologia 17, 41-52. [Google Scholar]
- Dart, D., Adams, K., Akerman, I., and Lakin, N. (2004). Recruitment of the cell-cycle checkpoint kinase ATR to chromatin during S-phase. J. Biol. Chem. 279, 16433-16440. [DOI] [PubMed] [Google Scholar]
- de Klein, A., Muijtjens, M., van, O., s.R., Verhoeven, Y., Smit, B., Carr, A., Lehmann, A., and Hoeijmakers, J. (2000). Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr. Biol. 20, 188-189. [DOI] [PubMed] [Google Scholar]
- Fernandez-Capetillo, O. et al. (2002). DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat. Cell Biol. 4, 993-997. [DOI] [PubMed] [Google Scholar]
- Friedberg, E. C. (1985). DNA repair. San Francisco: W. H. Freeman and Co.
- Friedberg, E. C., Walker, G. C., and Siede, W. (1995). DNA repair and mutagenesis. Washington, DC: ASM Press.
- Friesner, J. D., and Britt, A. B. (2003). Ku80- and DNA ligase IV-deficient plants are sensitive to ionizing radiation and defective in T-DNA integration. Plant J. 34, 427-440. [DOI] [PubMed] [Google Scholar]
- Furuta, T. et al. (2003). Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J. Biol. Chem. 278, 20303-20312. [DOI] [PubMed] [Google Scholar]
- Garcia, V., Bruchet, H., Camescasse, D., Granier, F., Bouchez, D. L., and Tissier, A. (2003). AtATM is essential for meiosis and the somatic response to DNA damage in plants. Plant Cell 15, 119-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatei, M., Young, D., Cerosaletti, K. M., Desai-Mehta, A., Spring, K., Kozlov, S., Lavin, M. F., Gatti, R. A., Concannon, P., and Khanna, K. (2000). ATM-dependent phosphorylation of nibrin in response to radiation exposure. Nat. Gen. 25, 115-119. [DOI] [PubMed] [Google Scholar]
- Gately, D. P., Hittle, J. C., Chan, G.K.T., and Yen, T. J. (1998). Characterization of ATM expression, localization, and associated DNA-dependent protein kinase activity. Mol. Biol. Cell 9, 2361-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasunov, A. V., Glaser, V. M., and Kapultsevich, Y. G. (1989). Two pathways of DNA double-strand break repair in G1 cells of Saccharomyces cerevisiae. Yeast 5, 131-140. [DOI] [PubMed] [Google Scholar]
- Green, R. A., and Kaplan, K. B. (2003). Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC. J. Cell Biol. 163, 949-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner, E. A., Preuss, S. B., and Britt, A. B. (2003). Arabidopsis mutants sensitive to gamma radiation include the homolog of the human repair gene ERCC1. J. Exp. Bot. 54, 669-680. [DOI] [PubMed] [Google Scholar]
- Hush, J., Wu, L., John, P.C.L., Hepler, L. H., and Hepler, P. K. (1996). Plant mitosis promoting factor disassembles the microtubule preprophase band and accelerates prophase progression in Tradescantia. Cell Biol. Int. 20, 275-287. [DOI] [PubMed] [Google Scholar]
- Iliakis, G., Wang, H., Perrault, A. R., Boecker, W., Rosidi, B., Windhofer, F., Wu, W., Guan, J., Terzoudi, G., and Pantelias, G. (2004). Mechanisms of DNA double strand break repair and chromosome aberration formation. Cytogenet. Genome Res. 104, 14-20. [DOI] [PubMed] [Google Scholar]
- Jackson, J. P., Johnson, L., Jasencakova, Z., Zhang, X., PerezBurgos, L., Singh, P. B., Cheng, X., Schubert, I., Jenuwein, T., and Jacobsen, S. E. (2004). Dimethylation of histone H3 lysine 9 is a critical mark for DNA methylation and gene silencing in Arabidopsis thaliana. Chromosoma 112, 308-315. [DOI] [PubMed] [Google Scholar]
- Karlsson, K., and Stenerlow, B. (2004). Focus formation of DNA repair proteins in normal and repair-deficient cells irradiated with high-LET ions. Rad. Res. 161, 517-527. [DOI] [PubMed] [Google Scholar]
- Kodym, R., and Horth, E. (1995). Determination of radiation-induced DNA strand breaks in individual cells by non-radioactive labelling of 3′ OH ends. Int. J. Rad. Biol. 68, 133-139. [DOI] [PubMed] [Google Scholar]
- Kuhne, M., Riball, E., Rief, N., Rothkamm, K., Jeggo, P. A., and Lobrich, M. (2004). A double-strand break defect in ATM-deficient cells contributes to radiosensitivity. Cancer Res. 64, 500-508. [DOI] [PubMed] [Google Scholar]
- Larson, R. A. (1988). The antioxidants of higher plants. Phytochemistry 27, 969-978. [Google Scholar]
- Limoli, C. L., Giedzinski, E., Bonner, W. M., and Cleaver, J. E. (2002). UV-induced replication arrest in the xeroderma pigmentosum variant leads to DNA double-strand breaks, gamma-H2AX formation, and Mre11 relocalization. Proc. Natl. Acad. Sci. USA 99, 233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, B., Joshi, H., and Palevitz, B. (1993). A gamma tubulin related protein associated with the microtubule arrays o higher plants in a cell cycle dependent manner. J. Cell Sci. 104, 1217-1228. [DOI] [PubMed] [Google Scholar]
- Lobrich, M., Rydberg, B., and Cooper, P. K. (1995). Repair of x-ray-induced DNA double-strand breaks in specific Not I restriction fragments in human fibroblasts: joining of correct and incorrect ends. Proc. Natl. Acad. Sci. USA 92, 12050-12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah, S. K., Turner, J.M.A., Baudat, F., Rogakou, E. P., de Boer, P., Blanco-Rodriguez, J., Jasin, M., Keeney, S., Bonner, W. M., and Burgoyne, P. S. (2001). Recombinational DNA double-strand breaks in mice precede synapsis. Nat. Gen. 27, 271-276. [DOI] [PubMed] [Google Scholar]
- Mitchell-Olds, T., and Clauss, M. J. (2002). Plant evolutionary genomics. Curr. Opin. Plant Biol. 5, 74-79. [DOI] [PubMed] [Google Scholar]
- Nazarov, I. B. et al. (2003). Dephosphorylation of histone gamma-H2AX during repair of DNA double-strand breaks in mammalian cells and its inhibition by calyculin A. Rad. Res. 160, 309-317. [DOI] [PubMed] [Google Scholar]
- Niedernhofer, L. J., Odijk, H., Budzowska, M., van Drunen, E., Maas, A., Theil, A. F., de Wit, J., Jaspers, N.G.J., Beverloo, H. B., Hoeijmakers, J.H.J., and Kanaar, R. (2004). The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol. Cell. Biol. 24, 5776-5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor, G., and Foyer, C. H. (1998). Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Physiol. Mol. Biol. 49, 249-279. [DOI] [PubMed] [Google Scholar]
- Park, E.-J., Chan, D. W., Park, J.-H., Oettinger, M. A., and Kwon, J. (2003). DNA-PK is activated by nucleosomes and phosphorylates H2AX within the nucleosomes in an acetylation-dependent manner. Nucleic Acids Res. 31, 6819-6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull, T. T., Rogakou, E. P., Yamazaki, V., Kirchgessner, C. U., Gellert, M., and Bonner, W. M. (2000). A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10, 886-895. [DOI] [PubMed] [Google Scholar]
- Pichierri, P., and Roselli, F. (2004). The DNA crosslink-induced S-phase checkpoint depends on ATR-CHK1 and ATR-NBS1-FANCD2 pathways. EMBO J. 23, 1178-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prise, K.M. et al. (1998). A review of dsb induction data for varying quality radiations. Int. J. Rad. Biol. 74, 173-184. [DOI] [PubMed] [Google Scholar]
- Redon, C., Pilch, D., Rogakou, E., Sedelnikova, O., Newrock, K., and Bonner, W. (2002). Histone H2A variants H2AX and H2AZ. Curr. Opin. Gen. Dev. 12, 162-169. [DOI] [PubMed] [Google Scholar]
- Resnick, M. A. (1969). Genetic control of radiation sensitivity in Saccharomyces cerevisiae. Genetics 62, 519-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick, M. A., and Martin, P. (1976). The repair of double-strand breaks in the nuclear DNA of Saccharomyces cerevisiae and its genetic control. Mol. Gen. Genet. 143, 119-129. [DOI] [PubMed] [Google Scholar]
- Rogakou, E., Pich, D., Orr, A., Ivanova, V., and Bonner, W. (1998). DNA double stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858-5868. [DOI] [PubMed] [Google Scholar]
- Rogakou, E. P., Boon, C., Redon, C., and Bonner, W. M. (1999). Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 146, 905-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfuss, A., and Grompe, M. (2004). Repair kinetics of genomic interstrand DNA cross-links: Evidence for DNA double-strand break-dependent activation of the Fanconi anemia/BRCA pathway. Mol. Cell. Biol. 24, 123-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkamm, K., and Löbrich, M. (2003). Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc. Natl. Acad. Sci. USA 100, 5057-5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydberg, B., Lobrich, M., and Cooper, P. K. (1994). DNA double-strand breaks induced by high-energy neon and iron ions in human fibroblasts. I. Pulsed-field gel electrophoresis method. Rad. Res. 139, 133-141. [PubMed] [Google Scholar]
- Sancar, A., Lindsey-Boltz, L. A., Unsal-Kaccmaz, K., and Linn, S. (2004). Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 73, 39-85. [DOI] [PubMed] [Google Scholar]
- Sedelnikova, O. A., Rogakou, E. P., Panyutin, I. G., and Bonner, W. M. (2002). Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Rad. Res. 158, 486-492. [DOI] [PubMed] [Google Scholar]
- Shiloh, Y. (2003). ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3, 155-168. [DOI] [PubMed] [Google Scholar]
- Siliciano, J. D., Canman, C. E., Taya, Y., Sakaguchi, K., Appella, E., and Kastan, M. B. (1997). DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 11, 3471-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiff, T., O'Driscoll, M., Rief, N., Iwabuchi, K., Lobrich, M., and Jeggo, P. A. (2004). ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 64, 2390-2396. [DOI] [PubMed] [Google Scholar]
- van den Bosch, M., Bree, R., and Lowndes, N. F. (2003). The MRN complex: coordinating and mediating the response to broken chromosomes. EMBO Rep. 4, 844-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos, J. W., Safadi, F., Reddy, A.S.N., and Hepler, P. K. (2000). The kinesin-like calmodulin binding protein is differentially involved in cell division. Plant Cell 12, 979-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, I., Minn, K., and Chen, J. (2004). UV-induced ATR activation requires replicational stress. J. Biol. Chem. 279, 9677-9680. [DOI] [PubMed] [Google Scholar]
- Ward, I. M., and Chen, J. (2001). Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 276, 47759-47762. [DOI] [PubMed] [Google Scholar]
- Whitaker, S. J., Ung, Y. C., and McMillan, T. J. (1995). DNA double-strand break induction and rejoining as determinants of human tumour cell radiosensitivity: A pulsed-field gel electrophoresis study. Int. J. Rad. Biol. 67, 7-18. [DOI] [PubMed] [Google Scholar]
- Wolniak, S. M., and Larsen, P. M. (1992). Changes in the metaphase transit times and the pattern of sister chromatid separation in stamen hair cells of tradescantia after treatment with protein phosphatase inhibitors. J. Cell Sci. 102, 691-715. [DOI] [PubMed] [Google Scholar]
- Yokota, Y., Shikazono, N., Tanaka, A., Hase, Y., Funayama, S., Wada, S., and Inoue, M. (2005).Comparative radiation tolerance based on the induction of DNA double-strand breaks in tobacco BY-2 cells and CHO-K1 cells irradiated with gamma-rays. Rad. Res. (in press). [DOI] [PubMed]
- Zou, L., and Elledge, S. J. (2003). Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300, 1542-1548. [DOI] [PubMed] [Google Scholar]