Abstract
Background:
Clonal hematopoiesis (CH) has emerged as an independent risk factor for atherosclerotic cardiovascular disease (CVD) with activation of macrophage inflammasomes as a potential underlying mechanism. The NLRP3 inflammasome has a key role in promoting atherosclerosis in mouse models of Tet2 CH, while inhibition of the inflammasome product IL-1β appeared to particularly benefit patients with TET2 CH in CANTOS. TET2 is an epigenetic modifier that decreases promoter methylation. However, the mechanisms underlying macrophage NLRP3 inflammasome activation in TET2 deficiency and potential links with epigenetic modifications are poorly understood.
Methods:
We used cholesterol-loaded TET2 deficient murine and embryonic stem cell derived isogenic human macrophages to evaluate mechanisms of NLRP3 inflammasome activation in vitro and hypercholesterolemic Ldlr−/− mice modeling TET2 CH to assess the role of NLRP3 inflammasome activation in atherosclerosis.
Results:
Tet2 deficiency in murine macrophages acted synergistically with cholesterol loading in cell culture and with hypercholesterolemia in vivo to increase JNK1 phosphorylation and NLRP3 inflammasome activation. The mechanism of JNK activation in TET2 deficiency was increased promoter methylation and decreased expression of the JNK-inactivating dual specificity phosphatase, Dusp10. Active Tet1-deadCas9 targeted editing of Dusp10 promoter methylation abolished cholesterol-induced inflammasome activation in Tet2-deficient macrophages. Increased JNK1 signaling led to NLRP3 deubiquitylation and activation by the deubiquitinase BRCC3. Accelerated atherosclerosis and neutrophil extracellular trap formation (NETosis) in Tet2 CH mice were reversed by holomycin, a BRCC3 deubiquitinase inhibitor, and also by hematopoietic deficiency of Abro1, an essential scaffolding protein in the BRCC3-containing cytosolic complex. Human TET2−/− macrophages displayed increased JNK1 and NLRP3 inflammasome activation, especially following cholesterol loading, with reversal by holomycin treatment, indicating human relevance.
Conclusions:
Hypercholesterolemia and TET2 deficiency converge on a common pathway of NLRP3 inflammasome activation mediated by JNK1 activation and BRCC3-mediated NLRP3 deubiquitylation with potential therapeutic implications for the prevention of CVD in TET2 CH.
Keywords: Atherosclerosis, hypercholesterolemia, clonal hematopoiesis, NLRP3 inflammasome, neutrophil extracellular traps, phosphorylation, deubiquitylation, neutrophils
Subject Codes: TOC category – Basic, TOC subcategory - Atherosclerosis
Introduction:
Cardiovascular disease (CVD) is the leading cause of death worldwide and is increasing in the US again after decades of decline.1 CVD usually arises from atherosclerosis of coronary, cerebral or peripheral arteries.2 Atherosclerosis is initiated by the accumulation of cholesterol-rich lipoproteins in arteries, and their modification and uptake by macrophages, which triggers an inflammatory response. Recent clinical trials employing anti-inflammatory therapies, notably IL-1β antibodies,3 or colchicine,4,5 have shown a reduction in CVD, indicating a central role of the inflammatory response, and specifically involving the macrophage inflammasome.6 However, there was an increased risk of infection and pneumonia in these clinical trials, emphasizing the need for more precise targeting of anti-inflammatory treatments in relevant patient populations for prevention of CVD.
Clonal hematopoiesis (CH) arises from somatic mutations that provide a fitness advantage to hematopoietic stem cells and lead to outgrowth of clones of blood cells. CH commonly involves variants in genes mediating epigenetic modifications (TET2, DNMT3A, ASXL1) or cytokine signaling (JAK2). Recent studies showed that CH increases in frequency with ageing and is present in > 10% of people aged > 70.7 CH has been identified as a major independent risk factor for CVD, with impact comparable to traditional risk factors such as smoking and elevated LDL.7,8 TET2 catalyzes the oxidation of 5-methylcytosine to 5-hydroxymethylcytosine, leading to decreased methylation of DNA and thus TET2 deficiency leads to increased methylation and decreased activity at many gene promoters.9
NLRP3 inflammasome activation is a two-step process with priming events increasing the expression of inflammasome components and activation reflecting assembly of the inflammasome,10 binding of the adaptor ASC and activation of the effector, Caspase-1.11 This leads to cleavage of IL-1β, IL-18 and Gasdermin D (GSDMD), which forms membrane pores,12 and permits the secretion of active IL-1β and IL-18. In a post-hoc analysis of a subset of patients in the CANTOS, subjects with TET2 CH treated with IL-1β antibodies had improved outcomes compared to subjects with other forms of CH or non-CH subjects.13 In murine atherosclerosis studies, Tet2 deficient macrophages exhibited a widespread increase in the expression of inflammatory genes, including Il1b and Nlrp3, indicating increased inflammasome priming,7,14 and the specific NLRP3 inhibitor MCC950 reversed accelerated atherosclerosis in Tet2 CH.14 However, potential mechanisms of NLRP3 inflammasome activation independent of priming effects and the relevance of NLRP3 inflammasomes in human TET2 deficient macrophages have not been explored.
Post-translational modifications of NLRP3 by phosphorylation have been implicated as either increasing,15,16 or decreasing,17 the activation step. In this study, we discovered that a well characterized activating pathway involving JNK1-mediated phosphorylation and deubiquitylation of NLRP3 by BRCC3,16,18 is increased in TET2 deficient macrophages, especially when cholesterol loaded, suggesting potential relevance in the setting of atherosclerosis. We show that the specific BRCC3 inhibitor holomycin,19 or genetic deficiency of Abro1 a scaffolding protein with an essential role in the deubiquitinase activity of BRCC3,18 reduce atherosclerosis in Tet2 CH mice. Studies in human TET2 deficient macrophages indicate the translational relevance of NLRP3 inflammasome activation and the role of the BRCC3 pathway in this process.
Methods:
Upon reasonable request to the corresponding authors, the data, analytic methods, and study materials will be made available to other researchers. All supporting data are available within the article and the Expanded Methods in the Data Supplement.
Mice
All mice except Abro1−/−, Tet2+/−, Tet2+/−Abro1−/− and Tet2−/−Abro1−/− mice were purchased from Jackson Laboratories. Tet2+/−, Tet2+/−Abro1−/− and Tet2−/−Abro1−/− mice were generated in our laboratory by crossbreeding Tet2−/− mice (# 023359) with Abro1−/− mice (kindly provided by Dr. Bin Wang, MD Anderson Cancer Center).20 B6 CD45.1 (# 002014) and Ldlr−/− mice (002207) were bought. Only female mice were used in experiments since they are more prone to developing atherogenesis. All mice used for these studies were on a C57BL/6J background and were housed in a specific pathogen-free facility under standard conditions of temperature (about 23 C) with a 12-h light dark cycle and food available ad lib (humidity was not noted). Cages and water were changed every 14–21 days. All mouse experiments were approved by Institutional Animal Care and Use Committee of Columbia University and were conducted in accordance with the Institutional Animal Care and Use Committee of Columbia University guidelines.
Statistical analysis
All data are presented as means + SEM. The number of mice included in the experiments can be found in the figure legends. The t-test was used to define differences between 2 datasets. For statistical analysis of IL-1β secretion, LPS only or LPS/IFN-γ only conditions are excluded from analysis since IL-1β secretion is very low or sometimes not detectable in LPS only or LPS/IFN-γ only conditions as well as we are interested in conditions where inflammasome is activated by ATP, Nigericin or oxLDL. For statistical analysis of in vitro MAPK phosphorylation such as JNK, ERK and p38 MAPK, No LPS conditions are excluded from analysis since phosphorylation of JNK, ERK and p38 MAPK is very low. The one-way ANOVA coupled with Tukey’s test for multiple comparisons was used define differences between more than 2 datasets if there is only one variable. The two-way ANOVA (with Sidak’s multiple comparison test) was conducted for where two variables (genotype vs treatment or diet or siRNA or virus). Two-way ANOVA results are shown next to dot plot with the exact p-value of genotype, exact p-value of treatment or diet or siRNA used and exact p-value of interactions between genotype and treatment or diet or siRNA used. The criterion for significance was set at ****P<0.0001, ***P<0.001, ** P<0.01, *P<0.05. Statistical analyses were performed using GraphPad Prism 8 software (San Diego, CA).
Results:
Cholesterol loading and Tet2 deficiency promote JNK and NLRP3 inflammasome activation
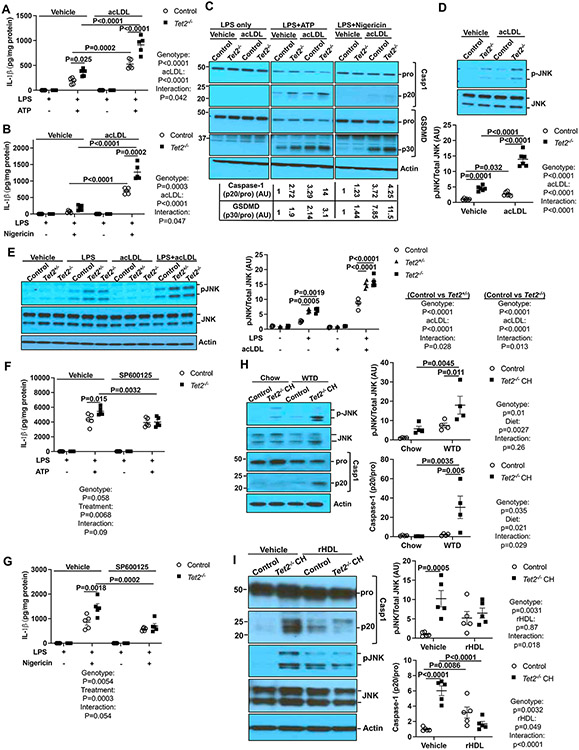
To address the mechanisms of NLRP3 inflammasome activation in Tet2−/− macrophages, wild type (control) and Tet2−/− bone marrow derived macrophages (BMDMs) were primed with 20 ng/ml lipopolysaccharide (LPS) and treated with 2 mM ATP or 10 μg/ml Nigericin to activate the NLRP3 inflammasome.21 To mimic the state of macrophages in atherosclerotic lesions, i.e., as cholesterol-loaded foam cells, we tested the effect of macrophage cholesterol loading on inflammasome activation. Since native LDL does not efficiently load macrophages with cholesterol, BMDMs were incubated with modified LDL (acetyl-LDL, acLDL), a standard approach to inducing foam cell formation.22 AcLDL loading and Tet2 deficiency led to increased IL-1β secretion Caspase-1 and GSDMD cleavage with the largest effects in cholesterol loaded Tet2 deficient macrophages (Fig 1A-C). Two-way ANOVA indicated significant interaction between genotype and acLDL treatment consistent with a synergistic interaction of cholesterol loading and Tet2 deficiency (Figure 1A-B). In contrast, Nlrp3 and Il1b mRNA expression were minimally increased in cholesterol loaded Tet2−/− macrophages (Figure S1A-B), suggesting additional mechanisms promoting NLRP3 activation beyond the priming step. Because post-translational modifications such as phosphorylation and deubiquitylation may be required for full activation of NLRP3,23 we investigated MAPK signaling. AcLDL loading plus Tet2 deficiency resulted in greater JNK phosphorylation than either alone (Figure 1D), while ERK and p38 MAPK phosphorylation were unaffected or only increased by acLDL, respectively (Figure S1C-D). TET2 CH usually involves heterozygous loss of function mutations in the TET2 gene in humans.24 Both Tet2+/−and Tet2−/−BMDMs had elevated JNK phosphorylation in response to LPS treatment and were further increased upon cholesterol loading (Figure 1E). Pre-treatment with SP600125 (a JNK inhibitor) before LPS priming partially reduced IL-1β secretion in Tet2−/− BMDMs bringing it to the same level as controls (Figure 1F-G) while having no effect on Il1b mRNA expression (Figure S1E) suggesting a specific role of increased JNK activity in the promotion of NLRP3 activation in Tet2−/− cells. In a setting mimicking the inflammatory milieu of atherosclerotic lesions, JNK inhibition suppressed IL-1β secretion induced by oxidized LDL (oxLDL) in macrophages activated by LPS/IFN-γ (Figure S1F) while having no effect on Il1b mRNA expression (Figure S1G). These findings indicate that Tet2 deficiency promotes macrophage NLRP3 inflammasome activation via JNK, independent of increases in Il1b mRNA, and that treatment with acLDL or oxLDL enhances these effects.
Figure 1. Elevated JNK activation drives inflammasome activation in Tet2−/− macrophages.
(A-C) Vehicle and acLDL loaded control and Tet2−/− BMDMs were primed with LPS for 3h and treated with ATP or Nigericin for an additional 1h to induce inflammasome activation. (A-B) IL-1β secretion from control and Tet2−/− BMDMs that were treated with ATP (A) or Nigericin (B). (C) Immunoblot of intracellular Caspase-1 and GSDMD cleavage from (A-B). (D) Immunoblot analysis of JNK (Thr183/Tyr185) phosphorylation in vehicle and acLDL loaded control and Tet2−/− BMDMs. (E) Immunoblot of JNK (Thr183/Tyr185) phosphorylation in vehicle and acLDL loaded control, Tet2+/− and Tet2−/− BMDMs upon LPS treatment for 15 min. (F-G) IL-1β from control and Tet2−/− BMDMs that were pretreated with JNK inhibitor SP600125 for 30 min and primed with LPS for 3h and treated with ATP (F) or Nigericin (G) for an additional 1h. (H) Immunoblot analysis of JNK (Thr183/Tyr185) phosphorylation and intracellular Caspase-1 cleavage in Ly6G−CD11b+ monocytes/macrophages isolated from Ldlr−/− mice that were transplanted with bone marrow mixture of WT or 10%Tet2−/−/90%WT and fed with chow or WTD for 4 weeks. (I) Immunoblot analysis of JNK (Thr183/Tyr185) phosphorylation and intracellular Caspase-1 cleavage in Ly6G−CD11b+ monocytes/macrophages isolated from Ldlr−/− mice that were transplanted with bone marrow mixture of WT or 10%Tet2−/−/90%WT and fed with WTD for 2 weeks and infused with vehicle or reconstituted HDL (rHDL, CSL111) once at the end of the first week. ****P<0.0001, ***P<0.001, ** P<0.01, *P<0.05 by two-way ANOVA with Sidak’s multiple comparison test.
To assess in vivo relevance, we prepared control or chimeric Tet2 CH mice by transplanting 90%WT (CD45.1+)/10%Tet2+/+ (CD45.2+) or 90%WT (CD45.1+)/10%Tet2−/− (CD45.2+) bone marrow into Ldlr−/− recipients, then, after a 5-week recovery period, fed the mice chow or high fat/high cholesterol Western type diet (WTD) for 4 weeks. JNK phosphorylation and inflammasome activation (as assessed by Caspase-1 cleavage) were significantly elevated in Ly6G−CD11b+ splenic monocytes/macrophages in Tet2 CH mice fed the WTD but not in Tet2 CH mice fed the chow diet (Figure 1H). Similarly, caspase-1 cleavage and JNK phosphorylation were significantly increased in CD45.2+Ly6G−CD11b+ but not in CD45.1+Ly6G−CD11b+ monocyte/macrophages isolated from Ldlr−/− mice that fed with WTD but not chow diet (Figure S1H). This indicates cell autonomous inflammasome activation in Tet2 deficient macrophages under hypercholesterolemic conditions. In contrast, Il1b mRNA was slightly but significantly increased in both CD45.1+Ly6G−CD11b+ and CD45.2+Ly6G−CD11b+ monocyte/macrophages in WTD-fed Tet2 CH mice (Figure S1I). The increase in Il1b mRNA in both mutant and WT macrophages may be explained by the ability of IL-1β to increase its own gene expression.25-27 To further demonstrate dependence on cellular cholesterol content, we infused mice with a preparation of reconstituted HDL (rHDL, CSL111) that promotes efficient cholesterol efflux from macrophages.28 This resulted in reversal of increased JNK phosphorylation and Caspase-1 cleavage in the Tet2 CH mice (Figure 1I). These results indicate that cholesterol accumulation and TET2 deficiency act synergistically to promote NLRP3 inflammasome activation via JNK activation.
Dusp10 promoter methylation drives JNK activation in Tet2 deficiency
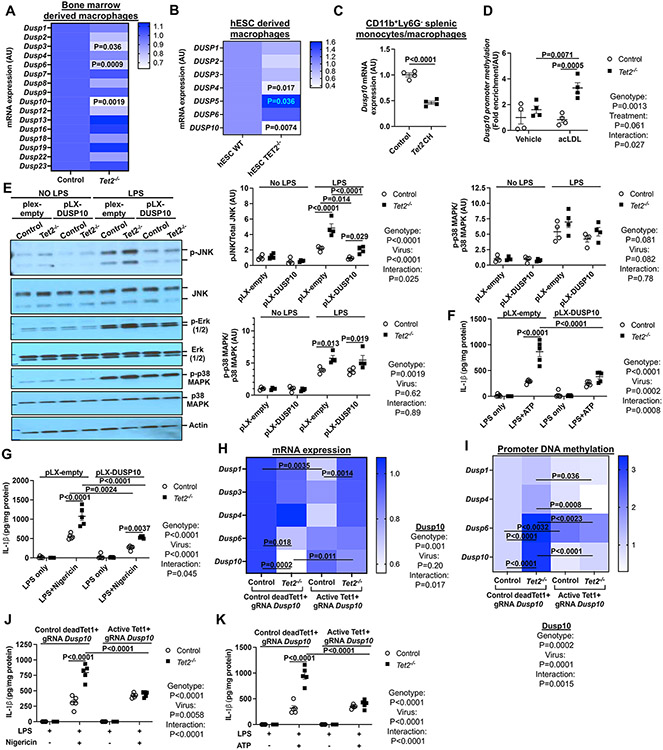
Dual specificity phosphatases (DUSPs) regulate the magnitude and duration of signaling in MAPK pathways and are regulated at the gene expression level.29 Thus, we assessed the effects of Tet2 deficiency on expression of the seventeen DUSPs that have been associated with altered JNK activation.29 Although several Dusps showed moderately reduced expression, only Dusp10 mRNA expression was markedly decreased by TET2 deficiency in both murine Tet2−/− BMDMs and isogenic human TET2−/− embryonic stem cell (hESC)-derived macrophages compared to WT controls (Figure 2A-B). Similarly, Dusp10 expression was decreased in Tet2+/− BMDMs (Figure S2A) and in Ly6G−CD11b+ splenic monocytes/macrophages from WTD fed Tet2 CH Ldlr−/− mice compared to WTD fed Ldlr−/− controls (Figure 2C).
Figure. 2. Dusp10 downregulation due to elevated promoter methylation drives JNK and inflammasome activation in TET2−/− macrophages.
(A) qPCR analysis of dual phosphatases in control and Tet2−/− BMDMs. (B) qPCR analysis of DUSP genes in WT and TET2−/− human embryonic stem cell (hESC)-derived macrophages. (C) Dusp10 mRNA expression in Ly6G−CD11b+ monocytes/macrophages isolated from Ldlr−/− mice that were transplanted with bone marrow mixture of WT or 10%Tet2−/−/90%WT and fed with chow or WTD for 4 weeks. (D) meDIP analysis of Dusp10 promoter methylation in vehicle and acLDL loaded control and Tet2−/− BMDMs. (E-G) Control and Tet2−/− BMDMs were infected with control (pLX-empty) or DUSP10 lentiviruses for 72h. (E) Immunoblot analysis of JNK (Thr183/Tyr185), Erk (1/2) and p38 MAPK phosphorylation from cells primed with LPS for 15 min (F) IL-1β secretion from LPS+ATP. (G) IL-1β secretion from LPS+Nigericin. (H-K) Control and Tet2−/− BMDMs were infected with lentiviruses expressing deadCas9-Tet1 (active dC-T) or deadCas9-a catalytically dead form of Tet1 (control dC-dT) with gRNAs targeting the Dusp10 promoter region for 72 hours. (H) qPCR analysis of Dusp genes. (I) meDIP analysis of Dusp promoter methylation. (J) IL-1β secretion from LPS+Nigericin. (K) IL-1β secretion from LPS+ATP. ****P<0.0001, ***P<0.001, ** P<0.01, *P<0.05 by t-test (A-C) and two-way ANOVA with Sidak’s multiple comparison test (D-K).
Since TET2 demethylates and activates some promoters,30 we next assessed methylation of the Dusp10 promoter using methylated DNA capture (MeDIP) and PCR amplification of CpG rich regions.31 Dusp10 promoter methylation was increased by Tet2 deficiency, but the effect was much more pronounced when macrophages were loaded with cholesterol (Figure 2D), paralleling the synergistic effects of TET2 deficiency and cholesterol loading on JNK phosphorylation and NLRP3 inflammasome activation shown above. Consistently, Dusp10 knockdown significantly increased JNK phosphorylation and IL-1β secretion in control but not Tet2−/− macrophages (Figure S2B-D). Conversely, overexpression of human DUSP10 by lentiviral transduction (Figure S2E) reduced JNK (Figure 2E) but not ERK or p38 MAPK phosphorylation in Tet2−/− macrophages (Figure 2E). In parallel to JNK phosphorylation, DUSP10 overexpression largely reversed the incremental increase in IL-1β secretion in response to LPS+ATP or LPS+Nigericin in Tet2−/− vs control macrophages (Figure 2F-G). DUSP10 overexpression also modestly increased Il1b mRNA expression (Figure S2F). To directly assess the contribution of increased Dusp10 promoter methylation to reduced Dusp10 expression and NLRP3 inflammasome activation, we used an approach,32,33 in which control and Tet2−/− BMDMs were infected with lentiviruses expressing deadCas9-activeTet1 (dC-T) or deadCas9-catalytically inactive Tet1 (dC-dT) and with gRNAs targeting the Dusp10 promoter region. As predicted, the active dC-T but not the control dC-dT combined with Dusp10 gRNAs markedly elevated suppressed Dusp10 mRNA expression in Tet2−/− cells but not in control cells, with minor effects on other Dusps (Figure 2H). While promoter methylation was increased for both Dusp10 and Dusp6, the differential effect of active vs inactive Tet1-dCas9 was much greater for Dusp10 (Figure 2I). Importantly, active Tet1-dCas9 completely reversed the incremental increase in IL-1β secretion observed in Tet2−/− vs control macrophages while having no significant effect in control cells (Figure 2J-K).
While these findings show that decreased expression of Dusp10 due to hypermethylation of its promoter is responsible for increased JNK and inflammasome activation in Tet2−/− macrophages, they do not explain the effects of hypercholesterolemia and cholesterol loading. Since promoter methylation may be increased by DNMTs, we hypothesized that there might be a further increase in promoter methylation in cholesterol loaded Tet2−/− cells due to upregulation of DNMTs. AcLDL loading increased DNMT1 and DNMT3A but not DNTM3B levels specifically in Tet2−/− BMDMs (Figure S3A). DNMT3A and DNMT3B levels were upregulated in Ly6G−CD11b+ splenic monocytes/macrophages from WTD-fed but not chow fed Tet2 CH Ldlr−/− mice (Figure S3B), suggesting increased DNMT3A might contribute to hypermethylation of the Dusp10 promoter in cholesterol loaded Tet2 deficient macrophages. Treatment of the cells with the DNMT inhibitor 5-azacytidine decreased Dusp10 promoter methylation (Figure S3C) and IL-1β secretion (Figure S3D) to a similar level in acLDL loaded control and Tet2−/− macrophages. These findings are consistent with a cholesterol loading induced increase in DNMT3A acting in concert with TET2 deficiency to induce hypermethylation of the Dusp10 promoter, potentially explaining the enhancement of inflammasome activation in Tet2 deficient cells by cholesterol loading.
NLRP3 deubiquitylation promotes inflammasome activation in Tet2−/− macrophages
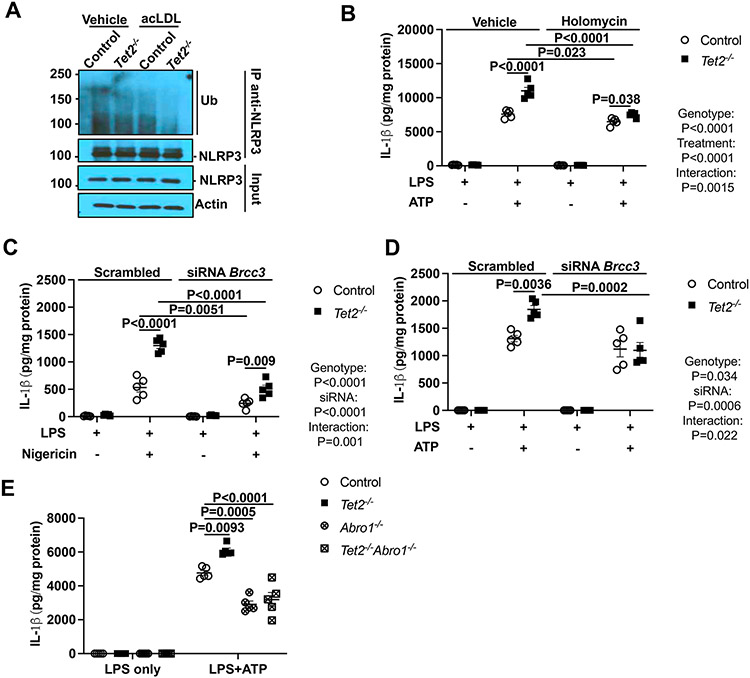
JNK-1 has been reported to activate the NLRP3 inflammasome via specific serine phosphorylation of NLRP3 that leads to NLRP3 deubiquitylation by the cytosolic BRCC3 isopeptidase complex (BRISC) and active NLRP3 inflammasome assembly.16,18 We carried out experiments to assess the role of NLRP3 deubiquitylation by BRCC3 downstream of JNK1 activation,18,34 in cholesterol and TET2 deficiency mediated inflammasome activation. Paralleling the effects on JNK1 activation, acLDL loading+Tet2 deficiency resulted in greater NLRP3 deubiquitylation than either alone (Figure 3A). Pharmacological targeting of NLRP3 deubiquitylation by the specific BRCC3 inhibitor holomycin (HL),19 reduced IL-1β secretion to the same level as controls in Tet2 deficient macrophages (Figure 3B) while having no effect on Il1b mRNA expression (Figure S4A). Similarly, HL treatment suppressed IL-1β secretion induced by oxLDL in macrophages activated by LPS/IFN-γ (Figure S4B) while having no effect on Il1b mRNA expression (Figure S4C). Furthermore, Brcc3 knockdown by siRNA (Figure S4D), largely reversed the incremental increase in IL-1β secretion in Tet2 deficient macrophages to a similar level as controls (Figure 3C-D). Finally, elevated IL-1β secretion induced by LPS+ATP in Tet2−/− macrophages was reversed by deficiency of Abro1, a scaffolding protein that is an essential component of the BRISC,18 (Figure 3E). Similar to Tet2−/− BMDMs, HL decreased IL-1β secretion in response to LPS+ATP or Nigericin in Tet2+/− macrophages (Figure S4E-F). Moreover, elevated IL-1β secretion in response to LPS+ATP or Nigericin in Tet2+/− macrophages was reversed by the deficiency of Abro1 (Figure S4G-H). Finally, Abro1 deficiency decreased IL-1β secretion induced by oxLDL in macrophages activated by LPS/IFN-γ (Figure S4I) while having no effect on Il1b mRNA expression (Figure S4J). Together, these findings suggest that increased NLRP3 deubiquitylation mediated by BRCC3 is a key mechanism underlying NLRP3 mediated inflammasome activation in Tet2−/− macrophages.
Figure 3. BRCC3-mediated NLRP3 deubiquitylation is essential for inflammasome activation in Tet2 deficiency.
(A) Immunoblot of NLRP3 ubiquitylation in cell lysates immunoprecipitated with anti-NLRP3 in vehicle and acLDL loaded control and Tet2−/− BMDMs primed with LPS for 3h (B) IL-1β secretion from control and Tet2−/− BMDMs were primed with LPS for 3h and treated with holomycin (HL) for 30 min and treated with ATP for additional 1h. (C-D) IL-1β secretion from control and Tet2−/− BMDMs transfected with control (Scrambled) or siRNA against Brcc3 for 48h. After 48 hours of transfection, cells were primed with LPS for 3h and and treated with Nigericin (C) or ATP (D) for an additional 1h. (E) IL-1β secretion from control, Tet2−/−, Abro1−/− and Tet2−/−Abro1−/− BMDMs that were primed with LPS for 3h and treated with ATP for an additional 1h. ****P<0.0001, ***P<0.001, ** P<0.01, *P<0.05 by one-way ANOVA coupled with Tukey’s comparison test (E) and two-way ANOVA with Sidak’s multiple comparison test (B-D).
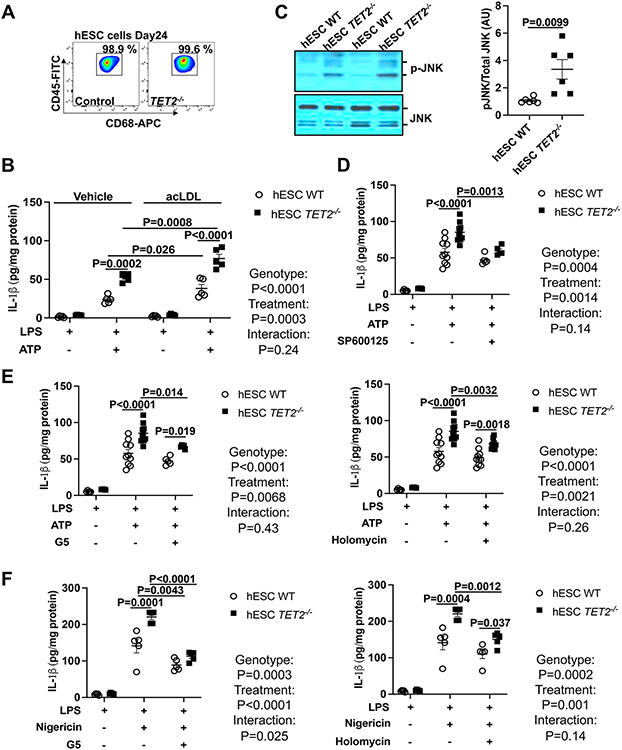
To test the human relevance of our findings, we used a human TET2 deficient embryonic stem cell hESC line (TET2−/−), which was created by CRISPR/Cas9-mediated gene editing and the control isogenic TET2+/+ hESC line.30 We used a macrophage differentiation protocol that we have shown leads to efficient generation of macrophages with minimal differences between WT and TET2−/− cells (Figure 4A).35 TET2 deficiency increased IL-1β secretion in response to LPS+ATP treatment, providing direct evidence for increased NLRP3 inflammasome activation in human cells (Figure 4B). Furthermore, similar to our findings in murine macrophages, IL-1β secretion was further increased in cholesterol-loaded cells (Figure 4B). JNK phosphorylation was elevated in TET2−/− hESC-derived macrophages (Figure 4C) and JNK inhibition decreased IL-1β secretion to levels similar to those in isogenic TET2+/+ hESC-derived macrophages (Figure 4D). Moreover, inhibition of deubiquitinase activity by the isopeptidase inhibitor G5,34 or the more specific BRCC3 inhibitor HL suppressed IL-1β secretion in TET2−/− hESC-derived macrophages (Figure 4E-F). These findings confirm similar findings in human and mouse TET2 deficient macrophages.
Figure 4. JNK1 mediated NLRP3 deubiquitylation drives NLRP3 inflammasome in human embryonic stem cell-derived TET2−/− macrophages.
(A) The verification of WT and TET2−/− human embryonic stem cell (hESC)-derived macrophages via flow cytometry analysis of CD45 and CD68. (B) IL-1β secretion from WT and TET2−/− hESC-derived macrophages loaded with acLDL overnight and primed with LPS for 3h and treated with ATP for additional 1h. (C) Immunoblot of JNK (Thr183/Tyr185) phosphorylation in WT and TET2−/− hESC-derived macrophages. (D) IL-1β secretion from from WT and TET2−/− hESC-derived macrophages with LPS for 3h in the absence or presence of SP600125 (JNK inhibitor) and treated with ATP for an additional 1h. (E-F) IL-1β secretion from WT and TET2−/− hESC-derived macrophages that were primed with LPS for 3h and treated with Holomycin or G5 for 30 min and treated with ATP (E) or Nigericin (F) for an additional 1h. ****P<0.0001, ***P<0.001, ** P<0.01, *P<0.05 by t-test (C) and two-way ANOVA with Sidak’s multiple comparison test (B, D-F).
BRCC3-mediated NLRP3 deubiquitylation accelerates atherosclerosis in Tet2 CH
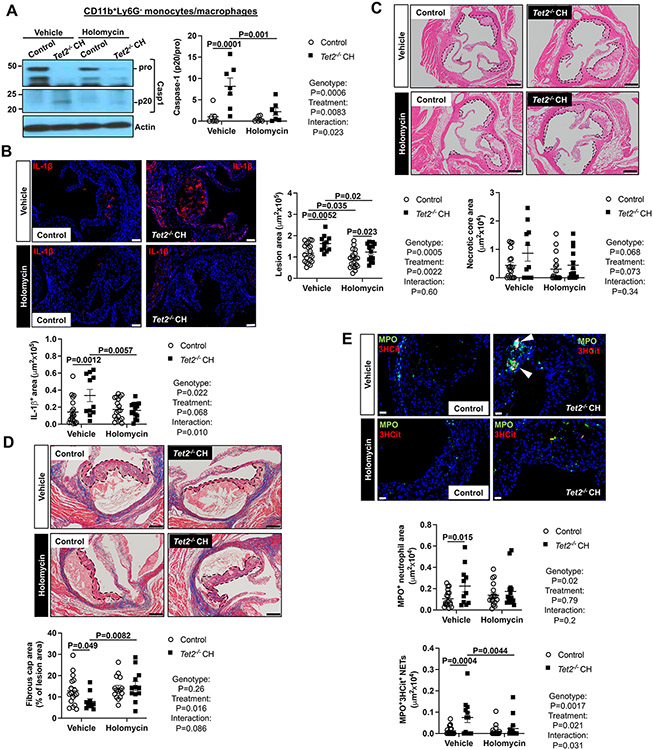
To assess the impact of BRCC3 mediated inflammasome activation on Tet2 CH atherogenesis, we lethally irradiated Ldlr−/− mice and transplanted them with chimeric Tet2−/− bone marrow cells containing 10% CD45.2 Tet2−/− and 90% CD45.1 wild type cells (Tet2 CH). A control group was transplanted with mixed bone marrow from 10% littermate control CD45.2 Tet2+/+ and 90% CD45.1 wild type bone marrow cells. 5-weeks after bone marrow reconstitution and 2-weeks after commencing the WTD, control and Tet2 CH mice were injected intraperitoneally with HL (1 mg/kg) or vehicle control (DMSO/PBS) every second day for 6 weeks. Mice then were sacrificed after a total of 8 weeks of WTD. Tet2 deficient blood cells expanded from about 46 to 71 % during the course of the HL injections similar to previous reports;14 however, HL administration did not affect the expansion of Tet2−/− blood cells (Figure S5A). There was a slight decrease in body weight in control mice (Figure S5B). Splenic weight was higher in Tet2 CH mice without significant change after HL administration (Figure S5C). Hematopoietic Tet2 deficiency or HL treatment did not alter plasma cholesterol (Figure S5D). HL treatment reduced the total white blood cells (WBC), and lymphocyte counts in both groups (Figure S5E-F). HL decreased monocyte counts in the Tet2 CH group while having no effect on neutrophil counts (Figure S5G-H). Importantly, HL administration promoted NLRP3 deubiquitylation in splenic Ly6G−CD11b+ monocyte/macrophages (Figure S5I) and decreased active caspase-1 (p20) in the Tet2 CH group (Figure 5A). In the Tet2 CH group, caspase-1 cleavage was significantly increased in CD45.2+Ly6G−CD11b+ monocyte/macrophages and reversed by HL administration but was not detected in CD45.1+Ly6G−CD11b+ monocyte/macrophages (Figure S5J). The expression of inflammatory genes such as Nlrp3, Il1b and Il6 was upregulated in Ly6G−CD11b+ splenocytes of Tet2 CH group and reduced to control levels by HL administration (Figure S5K-M). Such changes could be secondary to changes in inflammasome activation and IL-1β secretion.25-27,36 Il1b mRNA was elevated in both CD45.1+Ly6G−CD11b+ and CD45.2+Ly6G−CD11b+ monocyte/macrophages in the Tet2 CH group and abrogated by HL treatment (Figure S5N). IL-1β staining was significantly elevated in Tet2 CH lesions and suppressed by HL administration (Figure 5B), consistent with inflammasome inhibition in plaques. These results suggest that NLRP3 deubiquitylation is key factor in NLRP3 inflammasome activation in Tet2 CH mice. HL administration largely reversed the increase in plaque size of Tet2 CH mice (Figure 5C). HL seemed to decrease necrotic core area in Tet2 CH mice, but these trends were not significant (Figure 5C). HL increased fibrous cap thickness in Tet2 CH but not control lesions (Figure 5D). Our recent studies have shown that macrophage inflammasome activation and IL-1β secretion promotes NET formation in atherosclerotic plaques.37 Accordingly, we showed increased NETosis in Tet2 CH mice that was reversed by HL treatment, assessed by both MPO+/3HCit (Figure 5E) or Ly6G +/3HCit staining (Figure S5O). There was no effect of HL on MPO+ (Figure 5E) or Ly6G + (Figure S5O) staining of neutrophils in plaques consistent with activation of neutrophils after entry into plaques.37 Since NETs are formed secondary to NLRP3 activation in myeloid cells,37,38 this suggests a direct connection between cholesterol induced NLRP3 inflammasome activation via BRCC3 in macrophages and plaque NETosis.
Figure 5. Inhibition of NLRP3 deubiquitylation decreases NETosis and atherosclerosis in Tet2 CH mice.
Ldlr−/− mice were transplanted with bone marrow mixture of WT or 10%Tet2−/−/90%WT and fed WTD. After 2 weeks WTD feeding, mice were injected intraperitoneally with holomycin (1 mg/kg) or vehicle control (DMSO/PBS) for 6 weeks. Mice were sacrificed after a total of 8 weeks of WTD. (A) Immunoblot of intracellular Caspase-1 cleavage in Ly6G−CD11b+splenic monocytes/macrophages. (B) Lesions were stained for IL-1β. (C) Atherosclerotic lesion area and necrotic core area in the aortic root. Representative images of hematoxylin and eosin (H&E)–stained sections are shown; atherosclerotic plaques are delineated by dashed lines. Scale bars, 200 μm. (D) Fibrous cap thickness analysis via Masson trichrome staining. (E) Neutrophils were stained in atherosclerotic lesions using MPO and MPO+ percentages of lesion size was quantified. Concomitantly, lesions were stained for 3HCit. To assess NETs, the overlap of MPO and 3HCit was quantified as percentage of the total lesion area. Representative pictures are shown. Scale bars: 200 μm for (B), 75 μm for (C), 100 μm for (D) and 25 μm for (E). Each datapoint represents an individual mouse. ****P<0.0001, ***P<0.001, ** P<0.01, *P<0.05 by two-way ANOVA with Sidak’s multiple comparison test.
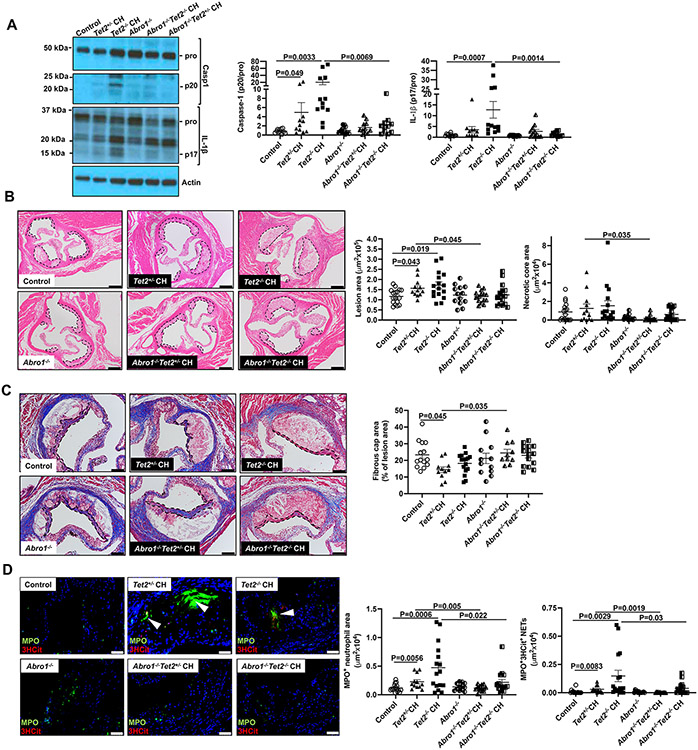
To verify the impact of BRCC3 mediated inflammasome activation on Tet2 CH atherogenesis, we also employed a genetic approach using deficiency of the essential BRCC3 co-factor ABRO1.18 To better simulate human CH, we also employed a group with heterozygous Tet2 deficiency (Tet2+/− mice). Thus, we transplanted WT, 10%Abro1−/−/90%WT, 10%Tet2+/− /90%WT, 10%Tet2−/− /90%WT, 10%Tet2+/−Abro1−/−/90% WT and 10%Tet2−/−Abro1−/−/90% WT BM into Ldlr−/− mice followed by administration of WTD for 8 weeks. Since BRCC3 mutations have also been discovered as potential drivers of clonal hematopoesis,39 we wondered whether Abro1 deficiency could affect the expansion of Tet2 mutant cells. Tet2+/− and Tet2−/− deficient blood cells expanded as expected while Abro1 deficiency modestly decreased the expansion of Tet2+/− blood cells and increased the expansion of Tet2−/− blood cells (Figure S6A). Abro1 deficiency did not alter body weight (Figure S6B). Spleen weight was higher in Tet2−/− CH and reduced by Abro1 deficiency (Figure S6C). Hematopoietic Tet2 or Abro1 deficiency did not change plasma cholesterol or WBC or lymphocyte, monocyte, or neutrophil counts (Figure S6D-H). Abro1 deficiency decreased active caspase-1 (p20) in Ly6G−CD11b+ splenic monocyte/macrophages in the Tet2+/− CH group and decreased both active caspase-1 (p20) and active IL-1β (p17) in Ly6G−CD11b+ splenocytes of Tet2−/− CH group (Figure 6A). Importantly, Abro1 deficiency reduced plaque size in both Tet2+/− and Tet2−/− CH mice (Figure 6B). Abro1 deficiency reduced necrotic core area (Fig. 6B) and improved fibrous cap thickness in Tet2+/− CH lesions (Figure 6C). Abro1 deficiency markedly decreased NETs measured by MPO+/3HCit (Figure 6D) or Ly6G +/3HCit (Figure S6I) co-staining as well as both MPO+ (Figure 6D) or Ly6G+ (Figure S6I) neutrophil content in lesions. Thus, Abro1 deficiency clearly reduced lesion and necrotic core area and increased cap thickness in Tet2+/− CH mice. The changes were similar although less statistically significant effects in the Tet2−/− CH group, possibly reflecting opposite effects on clonal expansion of Tet2 deficient blood cells. Overall, the Abro1 deficiency experiments provide strong confirmation of the changes in atherosclerosis mediated by the BRCC3 inhibitor HL treatment in Tet2 CH mice.
Figure 6. Abro1 deficiency decreases NETosis and atherosclerosis in Tet2 CH mice.
Ldlr−/− mice were transplanted with bone marrow mixture WT, 10%Abro1−/−/90%WT, 10%Tet2+/−/90%WT, 10%Tet2−/−/90%WT, 10%Tet2+/−Abro1−/−/90% WT or 10%Tet2−/−Abro1−/−/90% WT. After 5 weeks of recovery time, they were fed with WTD and then were sacrificed after a total of 8 weeks of WTD. (A) Immunoblot of intracellular Caspase-1 and IL-1β cleavage in Ly6G−CD11b+splenic monocytes/macrophages. (B) Atherosclerotic lesion area and necrotic core area in the aortic root. Representative images of hematoxylin and eosin (H&E)–stained sections are shown; atherosclerotic plaques are delineated by dashed lines. Scale bars, 200 μm. (C) Fibrous cap thickness analysis via Masson trichrome staining. (D) Neutrophils were stained in atherosclerotic lesions using MPO and MPO+ percentages of lesion size was quantified. Concomitantly, lesions were stained for 3HCit. To assess NETs, the overlap of MPO and 3HCit was quantified as percentage of the total lesion area. Representative pictures are shown. Scale bars: 200 μm for (B), 100 μm for (C) and 50 μm for (D). Each datapoint represents an individual mouse. ****P<0.0001, ***P<0.001, ** P<0.01, *P<0.05 by one-way ANOVA coupled with Tukey’s comparison test for 4 groups (Control, Abro1−/−, Tet2+/−, Tet2+/−Abro1−/−) or (Control, Abro1−/−, Tet2−/− and Tet2−/−Abro1−/−).
Discussion:
TET2 CH represents a common somatic genetic variation promoting atherosclerotic CVD especially in the elderly, similarly, affecting men and women and groups of different ancestry in the US.40 Our study provides new insight into the mechanisms of NLRP3 inflammasome activation in TET2 deficient murine and human macrophages, and in Tet2 CH mice, mediated by a JNK1/BRCC3 NLRP3 deubiquitylation pathway that increases NLRP3 inflammasome activation. This mechanism of NLRP3 inflammasome activation has not previously been implicated in accelerated atherosclerosis. Importantly, we were able to link activation of this pathway to epigenetic changes in the Dusp10 promoter resulting from TET2 deficiency. Cholesterol loading of macrophages acted convergently with TET2 deficiency to increase methylation of the Dusp10 promoter and thereby to increase JNK and NLRP3 activation in cell culture and in vivo. Mechanism-based interventions to suppress the BRCC3 pathway of NLRP3 deubiquitylation and activation led to reduced atherosclerotic lesion area with improved features of plaque stabilization and markedly reduced NETosis in Tet2 CH mice. Our studies suggest new therapeutic approaches to the treatment of TET2 CH using BRCC3 inhibitors, and implicate the importance of control of hypercholesterolemia in TET2 CH.
Our study reveals a key role of the JNK1/BRCC3 pathway of NLRP3 activation in accelerated atherosclerosis caused by TET2 CH. The JNK1 pathway of NLRP3 phosphorylation and activation was discovered in a screen for NLRP3 inhibitors,16 and subsequently linked to a BRCC3 K63- deubiquitylation mechanism that decreases NLRP3 serine 195 phosphorylation but does not change NLRP3 abundance.18 This pathway has been shown to promote Cryopyrin mediated periodic syndromes and urate crystal mediated NLRP3 inflammasome activation but has not been previously implicated in atherosclerosis or metabolic diseases.19 Suspecting involvement of the BRCC3 pathway in TET2 deficient macrophages, we performed a screen of JNK related dual specificity phosphatases (Dusps) revealing decreased expression of Dusp10. The role of DUSP10 in inflammasome activation was verified by Dusp10, JNK and Brcc3 knockdown or inhibition and by specific reversal of Dusp10 promoter methylation using active TET1-dCas9. Myeloid Dusp10 deficiency has been shown to increase JNK activation, inflammatory responses and autoimmunity in mice.41 Our findings extend these studies by linking increased Dusp10 promoter methylation to increased JNK1 and NLRP3 inflammasome activation especially in the milieu of hypercholesterolemia and atherosclerosis.
Cholesterol loading and hypercholesterolemia acted convergently with TET2 deficiency in macrophages to increase Dusp10 promoter methylation and NLRP3 inflammasome activation. This was associated with increased expression of DNMT1 and DNMT3A. DNMT3A-mediated Dusp4 promoter methylation was recently shown to sustain ERK activation, enhance macrophage efferocytosis and decrease necrotic core formation in atherosclerotic lesions.42 Interestingly, Tet2 CH was associated with increased atherosclerosis but not with increased necrotic core formation in this or an earlier study.43 Increased DNMTs in cholesterol loaded Tet2 deficient cells could perhaps be compensatory, improving some macrophage functions such as efferocytosis while worsening inflammasome activation. Although global methylome changes in DNMT3A or TET2 deficient cells have been studied,44 the broader impact of TET2 or DNMT3A deficiency in specific metabolic settings such as hypercholesterolemia and atherosclerosis deserve further investigation.
Our study may have therapeutic implications. Despite some recent controversy,40,45,46 CH is increasingly recognized as an independent CVD risk factor.7 However, there are no guidelines or practical approaches to address screening for CH for example in the elderly with multiple risk factors. Our findings that suggest synergistic effects of cholesterol loading and Tet2 deficiency in macrophages and of hypercholesterolemia and Tet2 CH on inflammasome activation imply that TET2 CH subjects could particularly benefit from LDL cholesterol lowering therapies. Moreover, our studies have uncovered a novel way to inhibit NLRP3 in atherosclerosis compared to earlier studies employing the NLRP3 inhibitor MCC950. MCC950 binds the NACHT domain of NLRP3 and has an essential role in NLRP3 activation while not affecting priming.47 Although MCC950 reduced atherosclerosis in Tet2 CH mice,14 clinical development of this drug was halted due to hepatotoxicity.48 Other formulations or related molecules may still have potential in the clinic.48 However, similar to IL-1β inhibition, complete inactivation of NLRP3 may prove to be immunosuppressive. The discovery of the BRCC3 pathway of NLRP3 activation led to the development of specific inhibitors, such as Thiolutin and Holomycin.19 BRCC3 inhibition or Abro1 knockdown reduced inflammasome activation in Cryopyrin disorders but did not completely reverse NLRP3 inflammasome activation.18,19 Similarly in the present study JNK1 inhibitors, holomycin and Abro1 deficiency primarily reduced incremental NLRP3 inflammasome activation mediated by TET2 deficiency without suppressing basal inflammasome activation. This suggests that BRCC3 inhibitors may be less immunosuppressive than direct NLRP3 or IL-1β inhibitors and could provide a new precision medicine approach to reducing CVD risk in TET2 clonal hematopoiesis.
Supplementary Material
CLINICAL PERSPECTIVES.
What is new?
Studies in Tet2 CH mouse models and in human TET2 deficient macrophages reveal a novel pathway of NLRP3 inflammasome activation involving the deubiquitinase BRCC3.
Targeting atherosclerosis risk using BRCC3 inhibitors may represent a new precision medicine approach to reducing CVD risk in individuals with TET2 CH.
Hypercholesterolemia and TET2 deficiency converge on a common pathway of NLRP3 inflammasome activation, which suggests the importance of control of hypercholesterolemia in TET2 CH.
What are the clinical implications?
Targeting the JNK/BRCC3 pathway of NLRP3 inflammasome activation with deubiquitinase inhibitors or LDL lowering drugs may reduce atherosclerotic cardiovascular disease risk in patients with TET2 CH.
Acknowledgments:
Abro1−/− mice were kindly provided by Dr. Bin Wang, MD Anderson Cancer Center.
Funding:
National Institutes of Health grant HL107653 (ART)
National Institutes of Health grant 1RO1HL155431-01 (ART)
National Institutes of Health grant 2RO1HL107653-13A1 (ART)
Netherlands Organization of Scientific Research VIDI 917.15.350 and Aspasia (MW)
Rosalind Franklin Fellowship with EU Co-Fund (MW)
Non-standard Abbreviations and Acronyms
- TET2
Tet methylcytosine dioxygenase 2
- CH
Clonal hematopoiesis
- CVD
Cardiovascular disease
- NETs
Neutrophil extracellular traps
- ASC
Apoptosis-associated speck-like protein containing a C-terminal caspase recruit-ment domain
- IL
Interleukin
- NLRP3
Nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing 3
- GSDMD
Gasdermin D
- BRCC3
BRCA1/BRCA2-containing complex subunit 3
- LPS
Lipopolysaccharide
- IFN
Interferon
- oxLDL
Oxidixed LDL
- acetyl-LDL, acLDL
modified LDL
- WT
Wild type
- WTD
Western-type diet
- rHDL
Reconstituted HDL
- DUSP
Dual specificity phosphatase
- ESC
Embryonic stem cell
- DNMT
DNA methyltransferase
- BRISC
BRCC3 isopeptidase complex
- HL
holomycin
- WBC
white blood cells
- 3HCit
Citrullinated histone
Footnotes
Disclosures: Dr. Tall is a consultant for Amgen, CSL Behring, Astra Zeneca and Foresite Laboratories, and is on the SAB of Staten Biotech, Fortico Biotech and Beren Therapeutics.
References
- 1.Ahmad FB, Anderson RN. The Leading Causes of Death in the US for 2020. JAMA. 2021;325:1829–1830. doi: 10.1001/jama.2021.5469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P. The changing landscape of atherosclerosis. Nature. 2021;592:524–533. doi: 10.1038/s41586-021-03392-8 [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 4.Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu XF, Ireland MA, Lenderink T, et al. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372 [DOI] [PubMed] [Google Scholar]
- 5.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388 [DOI] [PubMed] [Google Scholar]
- 6.Grebe A, Hoss F, Latz E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ Res. 2018;122:1722–1740. doi: 10.1161/CIRCRESAHA.118.311362 [DOI] [PubMed] [Google Scholar]
- 7.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tall AR, Fuster JJ. Clonal hematopoiesis in cardiovascular disease and therapeutic implications. Nat Cardiovasc Res. 2022;1:116–124. doi: 10.1038/s44161-021-00015-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marnell CS, Bick A, Natarajan P. Clonal hematopoiesis of indeterminate potential (CHIP): Linking somatic mutations, hematopoiesis, chronic inflammation and cardiovascular disease. J Mol Cell Cardiol. 2021;161:98–105. doi: 10.1016/j.yjmcc.2021.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreeva L, David L, Rawson S, Shen C, Pasricha T, Pelegrin P, Wu H. NLRP3 cages revealed by full-length mouse NLRP3 structure control pathway activation. Cell. 2021;184:6299–6312 e6222. doi: 10.1016/j.cell.2021.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 12.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- 13.Svensson EC, Madar A, Campbell CD, He Y, Sultan M, Healey ML, Xu H, D'Aco K, Fernandez A, Wache-Mainier C, et al. TET2-Driven Clonal Hematopoiesis and Response to Canakinumab: An Exploratory Analysis of the CANTOS Randomized Clinical Trial. JAMA Cardiol. 2022;7:521–528. doi: 10.1001/jamacardio.2022.0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Meszaros G, He WT, Xu Y, de Fatima Magliarelli H, Mailly L, Mihlan M, Liu Y, Puig Gamez M, Goginashvili A, et al. Protein kinase D at the Golgi controls NLRP3 inflammasome activation. J Exp Med. 2017;214:2671–2693. doi: 10.1084/jem.20162040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, Liu X, Huang YJ, Cai H, Zhan XY, et al. NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Mol Cell. 2017;68:185–197 e186. doi: 10.1016/j.molcel.2017.08.017 [DOI] [PubMed] [Google Scholar]
- 17.Mortimer L, Moreau F, MacDonald JA, Chadee K. NLRP3 inflammasome inhibition is disrupted in a group of auto-inflammatory disease CAPS mutations. Nat Immunol. 2016;17:1176–1186. doi: 10.1038/ni.3538 [DOI] [PubMed] [Google Scholar]
- 18.Ren G, Zhang X, Xiao Y, Zhang W, Wang Y, Ma W, Wang X, Song P, Lai L, Chen H, et al. ABRO1 promotes NLRP3 inflammasome activation through regulation of NLRP3 deubiquitination. EMBO J. 2019;38. doi: 10.15252/embj.2018100376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren GM, Li J, Zhang XC, Wang Y, Xiao Y, Zhang XY, Liu X, Zhang W, Ma WB, Zhang J, et al. Pharmacological targeting of NLRP3 deubiquitination for treatment of NLRP3-associated inflammatory diseases. Sci Immunol. 2021;6. doi: 10.1126/sciimmunol.abe2933 [DOI] [PubMed] [Google Scholar]
- 20.Xu S, Wu X, Wu L, Castillo A, Liu J, Atkinson E, Paul A, Su D, Schlacher K, Komatsu Y, et al. Abro1 maintains genome stability and limits replication stress by protecting replication fork stability. Genes Dev. 2017;31:1469–1482. doi: 10.1101/gad.299172.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basu SK, Goldstein JL, Anderson GW, Brown MS. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976;73:3178–3182. doi: 10.1073/pnas.73.9.3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seok JK, Kang HC, Cho YY, Lee HS, Lee JY. Regulation of the NLRP3 Inflammasome by Post-Translational Modifications and Small Molecules. Front Immunol. 2020;11:618231. doi: 10.3389/fimmu.2020.618231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bick AG, Weinstock JS, Nandakumar SK, Fulco CP, Bao EL, Zekavat SM, Szeto MD, Liao X, Leventhal MJ, Nasser J, et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature. 2020;586:763–768. doi: 10.1038/s41586-020-2819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281:8–27. doi: 10.1111/imr.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinarello CA, Ikejima T, Warner SJ, Orencole SF, Lonnemann G, Cannon JG, Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987;139:1902–1910. [PubMed] [Google Scholar]
- 28.Tardif JC, Gregoire J, L'Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004 [DOI] [PubMed] [Google Scholar]
- 29.Ha J, Kang E, Seo J, Cho S. Phosphorylation Dynamics of JNK Signaling: Effects of Dual-Specificity Phosphatases (DUSPs) on the JNK Pathway. Int J Mol Sci. 2019;20. doi: 10.3390/ijms20246157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verma N, Pan H, Dore LC, Shukla A, Li QV, Pelham-Webb B, Teijeiro V, Gonzalez F, Krivtsov A, Chang CJ, et al. TET proteins safeguard bivalent promoters from de novo methylation in human embryonic stem cells. Nat Genet. 2018;50:83–95. doi: 10.1038/s41588-017-0002-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohn F, Weber M, Schubeler D, Roloff TC. Methylated DNA immunoprecipitation (MeDIP). Methods Mol Biol. 2009;507:55–64. doi: 10.1007/978-1-59745-522-0_5 [DOI] [PubMed] [Google Scholar]
- 32.Marney CB, Anderson ES, Adnan M, Peng KL, Hu Y, Weinhold N, Schmitt AM. p53-intact cancers escape tumor suppression through loss of long noncoding RNA Dino. Cell Rep. 2021;35:109329. doi: 10.1016/j.celrep.2021.109329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu XS, Wu H, Ji X, Stelzer Y, Wu X, Czauderna S, Shu J, Dadon D, Young RA, Jaenisch R. Editing DNA Methylation in the Mammalian Genome. Cell. 2016;167:233–247 e217. doi: 10.1016/j.cell.2016.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013;49:331–338. doi: 10.1016/j.molcel.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 35.Liu W, Yalcinkaya M, Maestre IF, Olszewska M, Ampomah PB, Heimlich JB, Wang R, Vela PS, Xiao T, Bick AG, et al. Blockade of IL-6 signaling alleviates atherosclerosis in Tet2-deficient clonal hematopoiesis. Nat Cardiovasc Res. 2023;2:572–586. doi: 10.1038/s44161-023-00281-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endo-Umeda K, Kim E, Thomas DG, Liu W, Dou H, Yalcinkaya M, Abramowicz S, Xiao T, Antonson P, Gustafsson JA, et al. Myeloid LXR (Liver X Receptor) Deficiency Induces Inflammatory Gene Expression in Foamy Macrophages and Accelerates Atherosclerosis. Arterioscler Thromb Vasc Biol. 2022;42:719–731. doi: 10.1161/ATVBAHA.122.317583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yalcinkaya M, Fotakis P, Liu W, Endo-Umeda K, Dou H, Abramowicz S, Xiao T, Libby P, Wang N, Tall AR, et al. Cholesterol accumulation in macrophages drives NETosis in atherosclerotic plaques via IL-1beta secretion. Cardiovasc Res. 2022. doi: 10.1093/cvr/cvac189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westerterp M, Fotakis P, Ouimet M, Bochem AE, Zhang H, Molusky MM, Wang W, Abramowicz S, la Bastide-van Gemert S, Wang N, et al. Cholesterol Efflux Pathways Suppress Inflammasome Activation, NETosis, and Atherogenesis. Circulation. 2018;138:898–912. doi: 10.1161/CIRCULATIONAHA.117.032636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabre MA, de Almeida JG, Fiorillo E, Mitchell E, Damaskou A, Rak J, Orru V, Marongiu M, Chapman MS, Vijayabaskar MS, et al. The longitudinal dynamics and natural history of clonal haematopoiesis. Nature. 2022;606:335–342. doi: 10.1038/s41586-022-04785-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vlasschaert C, Mack T, Heimlich JB, Niroula A, Uddin MM, Weinstock JS, Sharber B, Silver AJ, Xu Y, Savona MR, et al. A practical approach to curate clonal hematopoiesis of indeterminate potential in human genetic datasets. Blood. 2023. doi: 10.1182/blood.2022018825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Blattman JN, Kennedy NJ, Duong J, Nguyen T, Wang Y, Davis RJ, Greenberg PD, Flavell RA, Dong C. Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature. 2004;430:793–797. doi: 10.1038/nature02764 [DOI] [PubMed] [Google Scholar]
- 42.Ampomah PB, Cai B, Sukka SR, Gerlach BD, Yurdagul A Jr., Wang X, Kuriakose G, Darville LNF, Sun Y, Sidoli S, et al. Macrophages use apoptotic cell-derived methionine and DNMT3A during efferocytosis to promote tissue resolution. Nat Metab. 2022;4:444–457. doi: 10.1038/s42255-022-00551-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wenli Liu MY, Maestre Inés Fernández, Malgorzata Olszewska, Ampomah Patrick B., Heimlich J. Brett, Wang Ranran, Vela Pablo Sanchez, Xiao Tong, Bick Alexander G., Levine Ross, Papapetrou Eirini P., Libby Peter, Tabas Ira, Wang Nan, Tall Alan R.. Blockade of IL-6 signaling alleviates atherosclerosis in Tet2 deficient clonal hematopoiesis. Nat Cardiovasc Res. 2023. doi: In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Su J, Jeong M, Ko M, Huang Y, Park HJ, Guzman A, Lei Y, Huang YH, Rao A, et al. DNMT3A and TET2 compete and cooperate to repress lineage-specific transcription factors in hematopoietic stem cells. Nat Genet. 2016;48:1014–1023. doi: 10.1038/ng.3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kessler MD, Damask A, O'Keeffe S, Banerjee N, Li D, Watanabe K, Marketta A, Van Meter M, Semrau S, Horowitz J, et al. Common and rare variant associations with clonal haematopoiesis phenotypes. Nature. 2022;612:301–309. doi: 10.1038/s41586-022-05448-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bick AG, Pirruccello JP, Griffin GK, Gupta N, Gabriel S, Saleheen D, Libby P, Kathiresan S, Natarajan P. Genetic Interleukin 6 Signaling Deficiency Attenuates Cardiovascular Risk in Clonal Hematopoiesis. Circulation. 2020;141:124–131. doi: 10.1161/CIRCULATIONAHA.119.044362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coll RC, Hill JR, Day CJ, Zamoshnikova A, Boucher D, Massey NL, Chitty JL, Fraser JA, Jennings MP, Robertson AAB, et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol. 2019;15:556–559. doi: 10.1038/s41589-019-0277-7 [DOI] [PubMed] [Google Scholar]
- 48.Corcoran SE, Halai R, Cooper MA. Pharmacological Inhibition of the Nod-Like Receptor Family Pyrin Domain Containing 3 Inflammasome with MCC950. Pharmacol Rev. 2021;73:968–1000. doi: 10.1124/pharmrev.120.000171 [DOI] [PubMed] [Google Scholar]
- 49.Kotini AG, Chang CJ, Chow A, Yuan H, Ho TC, Wang T, Vora S, Solovyov A, Husser C, Olszewska M, et al. Stage-Specific Human Induced Pluripotent Stem Cells Map the Progression of Myeloid Transformation to Transplantable Leukemia. Cell Stem Cell. 2017;20:315–328 e317. doi: 10.1016/j.stem.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.