Abstract
We show that a physiological role of the extensively studied cisternal Golgi rab protein, rab6, is modulation of Golgi apparatus response to stress. Taking exposure of cells to hypotonic media as the best-known example of mammalian Golgi stress response, we found that hypotonic-induced tubule extension from the Golgi apparatus was sensitive to GDP-rab6a expression. Similarly, we found that Golgi tubulation induced by brefeldin A, a known microtubule-dependent process, was inhibited by GDP-restricted rab6a, rab6a′, and rab33b, the most commonly studied cisternal rab proteins. These GDP-rab levels were sufficient to inhibit rab-induced redistribution of Golgi glycosyltransferases into the endoplasmic reticulum (ER), also a microtubule-dependent process, and to depress Golgi membrane association of the GTP-conformer of rab6. Nocodazole-induced Golgi scattering, a microtubule-independent process, also was inhibited by GDP-rab6a expression. In comparison, we found similar GDP-rab expression levels had little inhibitory effect on another microtubule-independent process, constitutive recycling of Golgi resident proteins to the ER. We conclude that Golgi cisternal rabs, and in particular rab6a, are regulators of the Golgi response to stress and presumably the molecular targets of stress-activated signaling pathway(s). Moreover, we conclude that rab6a can regulate select microtubule-independent processes as well as microtubule-dependent processes.
INTRODUCTION
The interrelationship between the Golgi apparatus, the central organelle of the secretory pathway, and the endoplasmic reticulum (ER) is a two-way street. The integral membrane proteins and lipids that comprise the resident molecules of the Golgi apparatus originate in the ER where they are synthesized. The cargo proteins and lipids that are processed in the Golgi apparatus also originate in the ER. Whether resident or cargo, these molecules typically exit the ER in coated vesicles or tubules formed in association with ER exit sites. The known ER coat protein is COPII, a protein complex recruited to ER exit sites by the small GTPase Sar1p. In sum, ER exit site-generated structures are examples of forward or anterograde membrane trafficking. In addition, the Golgi apparatus itself is a source of membrane trafficking directed toward the ER. Membrane trafficking from the Golgi apparatus to the ER provides various examples of backward or retrograde trafficking.
One class of Golgi-to-ER retrograde trafficking is retrieval to ER of proteins that have leaked into the Golgi apparatus during ongoing membrane trafficking and secretion (for reviews, see Storrie, Pepperkok and Nilsson, 2000; Storrie, 2005). The retrieval of these proteins to the ER is dependent on the COPI coat protein complex. COPI is a multi-subunit coat recruited to Golgi membranes. Soluble proteins of the ER containing C-terminal K(H)DEL sequences are retrieved from the Golgi apparatus after binding to the K(H)DEL receptor. Retrieval of leaked ER-membrane proteins containing KKXX, KXKXX, or FFXXRRXX sequences is another example of COPI-dependent Golgi-to-ER retrograde trafficking. In sum, COPI-dependent recycling between the Golgi apparatus and ER occurs in response to defined molecular motifs on the leaked ER proteins. In addition to retrieval of proteins leaked from the ER, COPI-dependent protein recycling has been implicated in intra-Golgi apparatus recycling as part of cisternal maturation, one model of Golgi function (Lanoix et al., 1999, 2001).
A second class of Golgi-to-ER retrograde trafficking is COPI-independent transport of both resident Golgi glycosyltransferases, e.g., GalNAcT2 and GalT, and protein toxins, e.g., Shiga toxin and ricin, to the ER. As recently reviewed (Storrie, 2005), one example is constitutive recycling of Golgi resident proteins to the ER in interphase mammalian cells as revealed by an ER-exit block, e.g., introduction of a mutant Sar1p to block recruitment of the ER-specific coat protein COPII to membranes. In the presence of an ER-exit block, recycling continues and produces an ER accumulation of normally Golgi resident proteins. This COPI-independent process is fairly slow with a half-time of ∼1.5 h; has no known sequence motif specificity, unlike COPI-dependent recycling; and is microtubule independent. In the absence of any other evidence, the process presumably reflects a constitutive or basal recycling rate between the Golgi apparatus and ER. Shiga toxin delivery from the Golgi apparatus to the ER is thought to be by the same process (Girod et al., 1999; Storrie et al., 2000). A second example of COPI-independent Golgi-to-ER retrograde trafficking is rab-induced relocation of Golgi glycosyltransferases to the ER.
As shown originally for the most extensively studied Golgi cisternal rab protein rab6a, overexpression of active rab produces a microtubule-dependent accumulation of GalT in the ER (Martinez et al., 1997). As demonstrated by multiple laboratories, including ours, overexpression of either wild-type or GTP-restricted rab6a, the first identified rab6 isoform (Martinez et al., 1997; White et al., 1999; Echard et al., 2000; Opdam et al., 2000; Shan et al., 2000), or rab33b (Valsdottir et al., 2001) induces the relocation of many medial to trans-Golgi resident proteins to the ER by presumably up-regulating the rate of Golgi-to-ER trafficking. As shown by White et al. (1999), rab6a overexpression leads to the extension of long tubules from the Golgi apparatus that seem to merge with ER at distal sites and hence to transport Shiga toxin into the ER. Rab6a in its active, GTP-conformer has been shown to interact with microtubule-dependent motor proteins/motor protein regulators such as rabkinesin-6 and dynactin (for review, see Storrie, 2005). The closely related rab6a′ isoform does not bind to rabkinesin-6 and has been linked to the regulation of endosomal-to-Golgi apparatus trafficking (Mallard et al., 2002). In sum, COPI-independent Golgi-to-ER trafficking may be constitutive, i.e., as revealed by a Sar1p mutant ER-exit block, or induced as caused by the expression of active rab6 or rab33b. The two processes differ in microtubule dependence with the first being microtubule independent and the second microtubule dependent.
Expression of GDP-rab6a has been reported to have variable effects on COPI-independent Golgi-to-ER toxin delivery, with Shiga toxin transport inhibited (Girod et al., 1999; Chen et al., 2003) and ricin transport unaffected (Chen et al., 2003). At high protein levels, GDP-restricted rab6a and rab33b both inhibit the constitutive, microtubule-independent redistribution of resident Golgi glycosyltransferases to the ER revealed by ER-exit block (Girod et al., 1999; Valsdottir et al., 2001). Golgi redistribution also can be induced by drugs such as brefeldin A (BFA) (Lippincott-Schwartz et al., 1989) or nocodazole (for review, see Burkhardt, 1998) and physiological stress conditions such as hypotonicity (Lee and Linstedt, 1999, 2000). BFA-induced redistribution is COPI independent, and the others are likely to be so also. In the present work, we asked whether a major physiological role of the most extensively cisternal rab protein, rab6a, might be the regulation of Golgi apparatus response to physiological stress. Taking exposure of cells to hypotonic media as the best-known example of mammalian Golgi stress response, we found that hypotonic-induced tubule extension from the Golgi apparatus was sensitive to GDP-rab6a expression. Supporting control experiments confirm the physiological importance of this central finding. We conclude that Golgi cisternal rabs, and in particular rab6a, are regulators of the Golgi response to stress and presumably the molecular targets of a stress activated signaling pathway(s).
MATERIALS AND METHODS
Cell Culture
Wild-type HeLa cells were grown in DMEM supplemented with 10% fetal bovine serum under standard tissue culture conditions. HeLa cells stably expressing tagged Golgi apparatus proteins were maintained in the presence of 0.45 mg/ml G418 sulfate. Cells were grown in a humidified incubator at 37°C and 5% CO2. All cell culture media and sera were obtained from Invitrogen (Carlsbad, CA).
Construction of GDP-rab5 pCMUIV
pCDNA3 plasmids encoding GDP-restricted myc-tagged rab5S34N were provided by Dr. Oddmund Bakke (University of Oslo, Oslo, Norway). The rab5S34N coding sequence was amplified by PCR. The primers used were 5′-GGATCCGGATCCGCCATGGCTAATCGAGGAGCAAC (forward primer) and 5′-GGATCCGGATCCTTAGTTACTACAACACTGACT (reverse primer). The underlined sequences indicate tandem BamHI sites. The PCR fragments were digested with BamHI, purified, and ligated into BamHI-digested pCMUIV, the mammalian expression vector used in all experiments. Flanking primers were used to sequence the insert.
Microinjection of Rab and Sar1p pCMUIV Plasmids
For microinjection, cells were grown for 1–2 d on glass coverslips. Purified pCMUIV plasmids were microinjected into cell nuclei with minor modifications of previous procedures (Storrie et al., 1998; Stroud et al., 2003). DNA concentrations ranged from 2 to 10 ng/μl for rab6a-Q72L (GTP), 20–250 ng/μl for rab6a-T27N (GDP), 20 ng/μl for rab6a′Q72L (GTP), 20–250 ng/μl for rab6a′-T27N (GDP), 10–100 ng/μl for rab33b-Q92L (GTP), 20–250 ng/μl for rab33b-T47N (GDP), 2–10 ng/μl for pSar1T39N (GDP), 2–10 ng/μl for pSar1H79G (GTP), and 200 ng/ml for prab5S34N (GDP). Expression times were 6 h for rab6 or rab5 and 16 h for rab33b unless otherwise specified. The 70-kDa fixable Cascade blue dextran (Molecular Probes, Eugene, OR), at a concentration of 3.33 mg/ml, was used as a coinjection marker. During microinjection cells were maintained at room temperature in CO2-independent medium (Invitrogen), and between 400 and 500 cells were injected per coverslip. Rab6a′ encoding pCMUIV plasmids were a gift from Dr. Joanne Young (European Molecular Biology Laboratory, Heidelberg, Germany). Protein distributions were quantified for an average of 50 cells per condition as described previously (Girod et al., 1999; Stroud et al., 2003). Because all inserts are at the BamHI restriction site of pCMUIV, the relationship of insert to promoter and Kozak sequence is constant. In preliminary experiments, we established that the microinjection of plasmids from increased stock concentrations resulted in increased expression of insert protein as indicated by staining levels with the appropriate antibody (our unpublished data).
Antibodies
Mouse monoclonal antibodies directed against rab33b were gifts from Dr. T. Koda (Hakkaido University, Sapporo, Japan). Rabbit polyclonal IgG antibodies directed against rab6 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse recombinant anti-GTP-rab6 (AA2; Nizak et al., 2003) was a gift from Dr. F. Perez (Institut Curie, Paris, France). Rabbit polyclonal IgG antibodies directed against Sec61 were a gift from Dr. B. Dobberstein (Zentrum für Molekulare Biologie Heidelberg, Heidelberg, Germany). Rabbit polyclonal anti-Sar1p antibodies were a gift from Dr. C. Mansbach (University of Tennessee Medical School, Memphis, TN). Clone 35 mouse antibodies directed against GM130 and mouse antibodies directed against human rab5 (catalogue number 610724) were purchased from BD Biosciences (San Diego, CA). Cy3- or Cy5-conjugated donkey anti-mouse or -rabbit IgG antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). In many experiments, the Golgi markers were stably expressed transfected proteins.
BFA, Hypotonic Shock, and Nocodazole Treatments
HeLa cells were treated with BFA essentially as described by Seemann et al. (2000). To disperse Golgi region proteins, cells cultured on coverslips were incubated with 1 μg/ml BFA for 30 min at 37°C in the presence of cycloheximide (CHX). To investigate the effects of GDP-rab on BFA-induced Golgi apparatus redistribution, cells were microinjected with different concentrations of GDP-rab6a, GDP-rab33b, or mixed GDP-rab plasmids, respectively, and then incubated with BFA in the presence of CHX as described above.
For hypotonic shock, HeLa cells grown on 12-mm glass coverslips were transferred to 35-mm dish containing 1 ml of hypotonic medium (20 mM HEPES, pH 7.2, 60 mM NaCl, 2.5 mM MgOAc, 210 mOsM) and incubated in a 37°C water bath or in a 5% CO2 incubator at 37°C for 15 min. To depolymerize microtubules, GalNAcT2-GFP HeLa cells were incubated with 10 μM nocodazole (Sigma-Aldrich, St. Louis, MO). Microinjection of DNA encoding the GTP-rab6a, GTP-rab6a′, and GTP-rab33b, respectively, was performed in the presence of nocodazole. Cells were then incubated in normal growth medium containing nocodazole in a CO2 incubator at 37°C for an expression period of 6 or 16 h. Preexpression of GDP-rab in HeLa cells for 6 h (for rab6a/a′) or 16 h (for rab33b), and then cells were treated with nocodazole for 1 or 3 h at 37°C.
Immunofluorescence Analysis
Routinely, cells were fixed in 3.5% formaldehyde in phosphate-buffered saline (PBS) for 20 min and permeabilized/blocked with 0.1% saponin/0.2% fish skin gelatin in PBS (SG/phosphate-buffered saline). Cells were incubated for 20 min with primary antibody diluted in SG/phosphate-buffered saline. The cells were washed three times with SG/phosphate-buffered saline and incubated for 20 min with secondary antibody diluted in SG/phosphate-buffered saline. Cells were again washed three times in PBS and mounted on microscope slides in Mowiol. For visualization of rab33b and rab6, cells were permeabilized in 0.1% Triton X-100 rather than saponin.
Microscopy and imaging were performed on a Zeiss Axiovert 200 TV microscope with a 63 or 100×, numerical aperture 1.4, plan-apochromat objective and a CoolSNAP HQ monochrome camera (Roper Scientific, Tuscon, AZ). The images were processed on Macintosh computers by using IPlab 3.9.3 software (Scanalytics, Fairfax, VA) and Adobe Photoshop 7.0 software. Gray scale values were mapped to an optimal intensity range. The incidence of a predominantly ER-like distribution of Golgi proteins was quantified as described previously (Girod et al., 1999; Stroud et al., 2003). The intensity of antibody staining for rab6, its GTP-conformer, and rab5 were quantified on an individual cell basis by using IPLab software. Scatter plots were prepared using KaleidaGraph 3.6 (Synergy Software, Reading, PA).
RESULTS
Variable levels of plasmids encoding GDP-restricted conformers of cisternal rab proteins have been used in previous experiments to probe for the role of cisternal rabs in COPI-independent Golgi retrograde traffic (Girod et al., 1999; Valsdottir et al., 2001; Chen et al., 2003). The premise of such experiments is competition by inactive, GDP-restricted conformer for binding to rab-specific guanine nucleotide exchange factors (rabGEF) at the Golgi membrane (for review, see Feig, 1999). The GDP-restricted conformer of small GTP-binding protein binds more tightly to the GEF than do other rab conformers (Feig, 1999). Hence, binding of inactive rab to the GEF blocks subsequent rab activation and rab effector recruitment. Typically, such experiments are done to achieve variable, high levels of rab protein expression for the lack of a good procedure to calibrate how much competitor protein is needed for efficacy. In the experiments that follow, GalNAcT2 was chosen as the reference Golgi glycosyltransferase because it is distributed across the entire Golgi apparatus (Röttger et al., 1998) and hence serves as a marker for the entire Golgi apparatus. In most experiments, HeLa cells stably expressing GalNAcT2-GFP were used. In all experiments, plasmids were introduced into cells by microinjection to give synchronous initiation of rab protein overexpression under conditions that had no effect on overall cell morphology.
GDP-Rab Conformers Inhibit Rab-induced Redistribution of Golgi Glycosyltransferases to the ER
Overexpression of cisternal rab proteins rab6a, rab6a′, and rab33b induces the redistribution of a Golgi resident glycosyltransferase to the ER. The Golgi glycosyltransferase GalNAcT2, here GFP conjugated, was redistributed to an ER-like pattern of nuclear rim and web-like fluorescence throughout the cytoplasm during a 16-h GTP-rab33b plasmid expression period (Figure 1Aa, asterisks mark plasmid-injected cells). Redistribution occurred in essentially 100% of the plasmid-injected cells. As shown in Figure 1, Bb and Cc, the ER-like distribution of GalNAcT2 was very similar to that of endogenous Sec61p, an ER translocon polypeptide. Extensive overlap of the redistributed GalNAcT2 and Sec61p was observed in a computer overlay (Figure 1, Bb and Cc). Induced redistribution to the ER was completely microtubule dependent. As shown in Figure 1D, the Golgi apparatus in noninjected cells during a 16-h incubation with the microtubule-depolymerizing drug nocodazole scatters into numerous smaller Golgi elements. In the GTP-rab33b–microinjected cells, the same scattered Golgi distribution was seen with no detectable accumulation of GalNAcT2 in the ER. At shorter time periods of expression such as 6 h, the same pattern of results were seen but the incidence of injected cells showing ER redistribution of GalNAcT2 was decidedly lower. Similar microtubule-dependent, induced redistributions were observed with GTP-restricted rab6a and rab6a′ after a 6-h incubation period (our unpublished data), consistent with previous results (Martinez et al., 1997; Young et al., 2005). Our observations confirm the results of Young et al. (2005) that rab6a′ induces microtubule-dependent, Golgi resident protein redistribution to the ER.
Figure 1.
Overexpression of GTP-rab33b induces a microtubule-dependent redistribution of GalNAcT2 to the ER. HeLa cells stably expressing GalNAcT2-GFP were microinjected with pCMUIV plasmids encoding GTP-rab33b (rab33b-Q92L, stock plasmid concentration of 100 ng/ml). After a 16-h expression period, cells were double immunostained for rab33b and Sec61p (ER marker). In GTP-rab33b–overexpressing cells (asterisks), GalNAcT2 redistributed to an ER-like reticulum (Aa) and showed extensive colocalization with Sec61p (Bb–Cc). Noninjected cells serve as an internal control. (D) HeLa cells stably expressing GalN-AcT2-GFP were microinjected with pCMUIV plasmids encoding GTP-rab33b. After a 16-h plasmid expression period in the presence of nocodazole to depolymerize microtubules. GTP-rab33b expressing cells (asterisks) show a similar pattern of scattered Golgi elements as in control cells. Bar, 10 μm.
We next demonstrated quantitatively that GDP-rab expression inhibited rab-induced Golgi redistribution. We first determined the minimal sufficient level of GTP-rab plasmids required to produce general redistribution of Golgi resident proteins such as GalNAcT2 into the ER. In a second titration set of experiments, we determined the level of GDP-rab–encoding plasmids required to inhibit GalNAcT2 relocation in 50% of the microinjected cell population. Because the level of endogenous rab protein is less than the overexpressed level, which includes any endogenously expressed protein, the titered level of GDP-rab plasmids should be more than sufficient to produce strong inhibition of endogenous rab6a or rab33b binding to the respective GEF. Quantitatively, we found that plasmids encoding GTP-restricted rab6a produced general redistribution of GalNAcT2 to the ER in ≥95% of the microinjected cells at a stock DNA concentration of 7.5 ng/μl and a 6-h expression time. For GTP-rab33b encoding plasmids, the stock DNA concentration was 20 ng/μl, and the expression time required was 16 h. The higher plasmid concentration and longer expression time needed were entirely consistent with our previous results (Valsdottir et al., 2001). As shown quantitatively in Figure 2, A and B, coexpression of the GDP-restricted, cisternal rab from varying plasmid stock concentrations together with the homologous GTP-rab plasmid produced a progressive inhibition of induced GalNAcT2 redistribution to the ER. In all experiments, the GTP-rab plasmid stock concentration was fixed at the minimal sufficient dosage necessary to produce redistribution to the ER in almost 100% of the cells, as determined above. When expressed as a molar ratio of GDP-/GTP-plasmid stock concentration, roughly equivalent inhibition was observed for both rab6 isoforms and rab33b, ∼50% inhibition at a molar ratio of ∼7.5:1. Based on the SE bars shown in Figure 2A, there was no statistically significant difference between GDP-rab6a and GDP-rab6a′ in their ability to inhibit GTP-rab induced GalNAcT2 relocation in coexpression experiments. In heterologous competition experiments, GDP-rab6a or GDP-rab6a′ failed to inhibit GTP-rab33b–induced redistribution of GalNAcT2 to the ER (Table 1). Similarly, GDP-rab33b expression had no effect on GTP-rab6a–induced Golgi protein redistribution to the ER. The amount of GTP-rab6a′ encoding plasmids quantitatively required for induced redistribution was not determined, and no other GTP-rab6a′ expression experiments were done. These experiments at face value clearly indicate that GDP-rabs when expressed from stock plasmid concentrations of 50–100 ng/μl should be more than sufficient to inhibit processes regulated by the homologous endogenous cisternal rab protein. This conclusion is greatly strengthened by the outcome of the heterologous competition experiments in which inhibition was not observed with coexpression of the heterologous GDP-restricted rab. A logical conclusion is that synthesis of the GTP-restricted rab is unaffected by coexpression of excess GDP-restricted rab. If so, then it is likely, on the one hand, that the two rab6-isoforms, rab6a and rab6a′, use the same GEF and/or other factors, whereas, on the other hand, the rab6s and rab33b use different GEFs and/or other factors.
Figure 2.
GDP-restricted rab6a, 6a′, or 33b inhibits homologous GTP-restricted rab-induced ER accumulation of Golgi glycosyltrasferases. HeLa cells stably transfected with GalNAcT2-GFP were comicroinjected with a fixed amount of plasmids encoding GTP-rab and variable amounts of plasmids encoding the homologous GDP-rab. After 6-h (for GTP-rab6a expression) or 16-h (for GTP-rab33b expression) incubation at 37°C, cells were fixed, antibody stained for rab33b or rab6, and the localization of GalNAcT2 was determined. (A) Incidence of microinjected cells displaying a predominantly ER distribution for GalNAcT2 was scored relative to the GDP-restrictive rab plasmid concentration as described previously (Girod et al., 1999; Stroud et al., 2003). Cells were microinjected with GTP-rab6a (rab6aQ72L; 7.5 ng/μl) + GDP-rab6a (rab6aT27N; 0–150 ng/μl) (error bars ± SD of three experiments), GTP-rab6a (7.5 ng/μl) + GDP-rab6a′ (rab6a′T27N; 0–150 ng/μl), or GTP-rab33b (rab33bQ79L; 20 ng/μl) +GDP-rab33b (rab33bT47N, 0–200 ng/μl). (B) Data were replotted as molar ratio of GDP/GTP plasmid DNA.
Table 1.
Heterologous competition
| ER distribution of GalNAcT2 (% of cells) rab-GDP concentration (molar ratio of GDP–/GTP–)
|
||||
|---|---|---|---|---|
| Heterologous coexpressed rab proteins | 0 | 50 | 100 | 200 |
| GTP-rab33b + GDP-rab6a | 98 (0) | 97 (2.5) | 98 (5) | 97 (10) |
| GTP-rab33b + GDP-rab6a′ | 97 (0) | 95 (2.5) | 97 (5) | 96 (10) |
| GTP-rab6a + GDP-rab33b | 99 (0) | 99 (6.7) | N.D. | N.D. |
The incidence of microinjected cells displaying a predominantly ER distribution for GalNAcT2 was scored relative to the GDP-restricted rab plasmid concentration (nanograms per microliter). The stock concentration of GTP-rab plasmid was 20 ng/ml for GTP-rab33b, 16-h expression period and 7.5 ng/ml for GTP-rab6a, 6-h expression period. The stock concentrations for the respective heterologous GDP-rab are given in the Table. Molar ratios of GDP/GTP-plasmid stock concentrations are indicated in parentheses. N.D., not done.
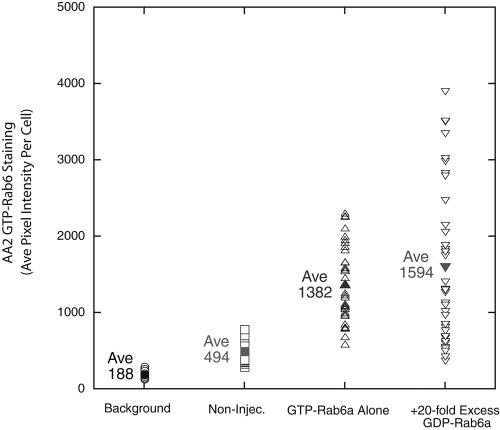
To further test these conclusions, we used recombinant AA2 antibodies specific for the GTP conformer of rab6. Using AA2 reactivity as an assay, we asked whether the production of GTP-restricted rab6a was affected by coexpression of GDP-rab6a from a 20-fold plasmid excess. Quantitatively, there was little-to-no effect of coexpression on the level of GTP-rab6 staining (Figure 3). In fact, if anything, the average GTP-rab6a expression as indicated by AA2 staining levels was ∼15% higher. The wide range in expression per cell is consistent with that observed previously by Young et al. (2005) for microinjection. On average, there was an approximately fourfold increase above background in GTP-rab6 staining with microinjection. Finally, as a positive control to demonstrate the efficacy of GDP-rab concentrations, we tested whether such GDP-rab expression conditions were sufficient to depress the binding of the endogenous GTP-conformer of rab6 to Golgi membranes. To do this, we assessed the Golgi localization of endogenous GTP rab6 conformer in the absence or the presence of overexpressed GDP-rab6a. As shown in Figure 4A, the Golgi apparatus remained intact in cells expressing GDP-rab6a (asterisk). In these cells, in contrast to noninjected cells (arrows), there was little to no detectable GTP-conformer–specific staining of Golgi apparatus (Figure 4B). These data provide direct evidence for the efficacy of GDP-rab6a expression and further support the conclusion that any inhibition of Golgi protein relocalization by overexpression of cisternal GDP-rabs is due to competition with the GTP-conformer.
Figure 3.
Coexpression of GDP-restricted rab6a with GTP-restricted rab6a does not affect the synthesis of GTP-restricted rab6a. HeLa cells stably expressing GalNAcT2-GFP were microinjected with plasmids encoding GTP rab6a (10 ng/μl stock DNA concentration) and GDP-rab6a (200 ng/μl stock DNA concentration). After a 6-h expression period, HeLa cells were stained with AA2, a recombinant antibody specific for the GTP-conformer of rab6, to detect GTP-rab6. AA2 staining intensity in individual cells was determined using IPLab software. Background is average pixel intensity in area between cells; Non-Injec. is average pixel intensity in non-injected control cells.
Figure 4.
Overexpression of GDP-rab6a depresses the binding of GTP-rab6 to Golgi membranes. HeLa cells stably expressing GalN-AcT2-GFP were microinjected with plasmids encoding GDP-rab6a (100 ng/μl stock DNA concentration). Fixable Cascade blue dextran was used as a coinjection marker. After a 6-h expression period, HeLa cells were stained with AA2, a recombinant antibody specific for the GTP-conformer of rab6, to detect GTP-rab6 GalNAcT2-GFP distribution (A) and GTP-rab6 distribution (B). In HeLa cells expressing GDP-rab6a, little to no juxtanuclear, Golgi-specific AA2 staining was detectable. Arrows point to AA2 juxtanuclear Golgi staining in noninjected cells. Asterisks marks injected cells. Bar, 10 μm.
Cisternal Rab Activity Is Required for Stress Response-induced Redistribution of Golgi Resident Proteins
Activated cisternal rab proteins such as rab6a have been implicated in the induction of tubular extensions from the Golgi apparatus to the ER (White et al., 1999). Golgi redistributive responses to a number of drugs or hypotonicity that might be grouped together under the general heading of stress responses also are accompanied by pronounced induction of tubular Golgi extensions. Moreover, in the case of BFA where tubular extensions are a prominent first response to the drug, the formation of extensions has been shown to be dependent on protein prenylation (Ivessa et al., 1997). Rab6 is one example of an important substrate for protein prenylation. Additionally, microtubule-dependent motors or motor factors have been shown to be rab6a effectors (Echard et al., 1998; Matanis et al., 2002; Short et al., 2002). Presumably, these mediate tubule extension. Therefore, we tested the hypothesis that the normal function of cisternal rab proteins in interphase mammalian cells is to regulate known redistributive stress responses of the Golgi apparatus. As a test that the effects observed were specific for Golgi cisternal rab proteins, we overexpressed the endosomal small GTPase rab5.
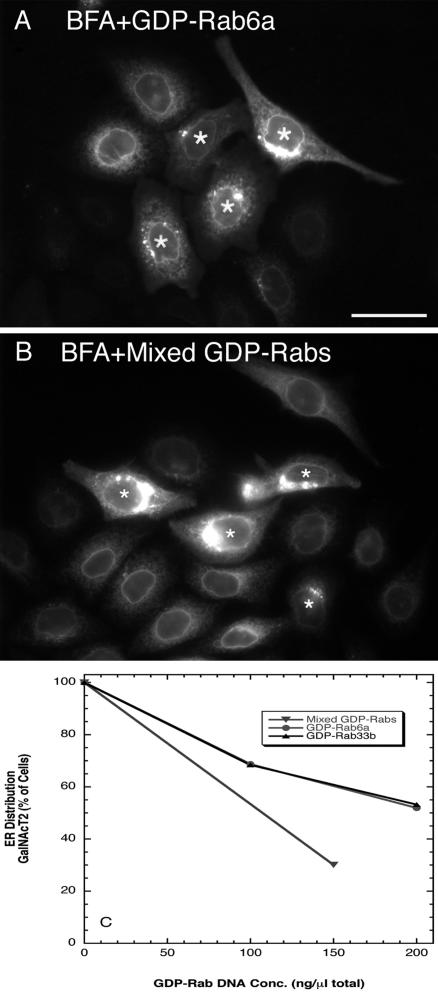
As shown in Figure 5, preexpression of GDP-rab6a or a mix of GDP-rab6a, rab6a′, and rab33b qualitatively inhibited the BFA-induced redistribution of GalNAcT2. As can be seen in the microinjected cells (asterisks), the GalNAcT2 distribution was often much more compact and juxtanuclear (Figure 5A, GDP-rab6a example). In the noninjected cells, the expected, complete ER-like BFA-induced redistribution was found. Similar qualitative results were observed for preexpression of GDP-rab33b followed by the addition of BFA (our unpublished data). Quantitatively, inhibition was observed in ∼50% of the microinjected cell population when cells were preinjected with GDP-rab6a or -rab33b plasmids at stock concentration of 200 ng/μl DNA (Figure 5C). Preexpression of GDP-rab5 at the same plasmid concentration had no inhibitory effect (our unpublished data). When cells were preinjected with a cocktail of GDP-rab6a, -rab6a′, and -rab33b plasmids at a stock concentration of 50 ng/μl each, an even stronger inhibition of Golgi redistribution was observed (Figure 5, B and C). We conclude that BFA induced extension of Golgi tubules and their subsequent merger with ER to redistribute Golgi proteins into the ER is a cisternal rab-regulated process.
Figure 5.
Preexpression of GDP-restricted rab proteins inhibits the BFA-induced ER accumulation of GalNAcT2-GFP. HeLa cells stably expressing GalNAcT2-GFP were microinjected with plasmids encoding either individual rab-GDP proteins or mixed rab-GDP encoding plasmids. After 6-h (for rab6) or 16-h (for mixed rab protein expression) incubation at 37°C, cells were incubated with BFA for 30 min in the presence of cycloheximide, fixed, and stained for rab6 or rab33b by immunofluorescence. The localization of GalNAc-T2 was determined from GFP fluorescence. (A) GDP-rab6a, 200 ng/μl. (B) Mix of GDP rab33b/rab6a/rab6a′ together at 50 ng/μl each. Asterisks over the cell nuclei indicate microinjected cells. Inhibition is indicated by concentrated perinuclear fluorescence. (C) Quantification of fluorescence distributions after BFA treatment. Bar, 20 μm.
To characterize a more physiological situation, we tested the effect of GDP-cisternal rab expression on hypotonic shock-induced Golgi redistribution. Here, we used as the main marker GM130. GM130 is a cis-Golgi protein and is a particularly good marker for Golgi tubulation in response to hypotonic shock (Lee and Linstedt, 2000). As shown in Figure 6A, the compact, juxtanuclear distribution of GM130 was little effected by the expression of GDP-rab6a alone. If anything, the distributions were slightly more compact. With 15-min hypotonic shock at 210 mOsM, there was considerable dispersal of GM130 with the Golgi apparatus in noninjected cells being relatively fragmented with some scattering into the cell periphery; frequently long tubules were observed extending outward from the juxtanuclear Golgi apparatus (Figure 6B, arrows). In the cells expressing GDP-rab6a (Figure 6B, asterisks), the Golgi apparatus maintained a generally compact, juxtanuclear distribution in >90% of the injected cells. GM130-positive tubule extension was blocked. Expression of GDP-rab5 had no effect on hypotonic shock induced Golgi tubule extension (our unpublished data). We conclude an individual cisternal rab protein can modulate a physiological response, i.e., Golgi redistribution in response to hypotonic shock. Moreover, we conclude that a rab protein generally thought to be located across the medial-to-trans-Golgi cisternae exerts a general regulatory effect across the Golgi stack.
Figure 6.
GDP-rab6a inhibits hypotonic-induced redistribution of GM130. HeLa cells were grown to 50% confluence on 12-mm glass coverslips and were injected with GDP-rab6a at a stock concentration of 100 ng/μl and then incubated for 6 h. Coverslips were removed from normal medium and placed in 1 ml of hypotonic (210 mOsM) medium for 15 min. Cells were fixed and processed for double-label immunofluorescence by using a rabbit antibody against rab6 to identify microinjected cells and a monoclonal antibody against GM130. Cells maintained in normal medium are shown in A. In response to hypotonic shock (B), there was considerable dispersal of GM130 with frequently long tubules observed (arrows) in noninjected cells but not in the injected cells (asterisks). Bar, 10 μm.
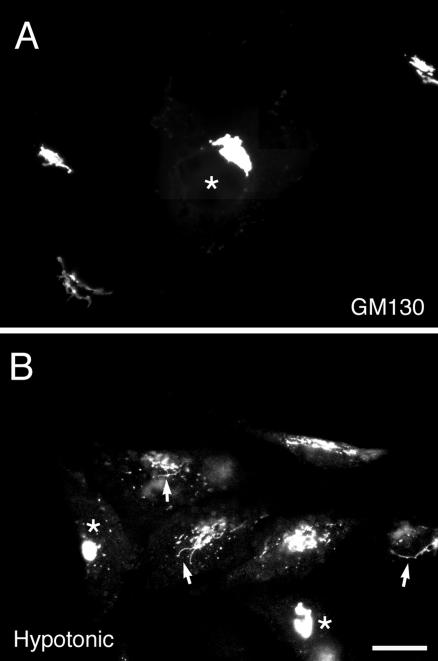
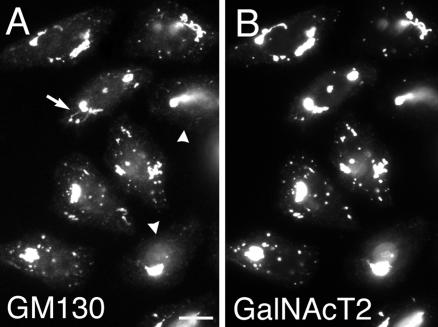
Inhibition by GDP-cisternal rab proteins of induced Golgi redistribution can be explained on the basis of activated rab protein recruitment of microtubule-dependent motors to Golgi membranes. If the total effect of cisternal rab proteins in regulating the distribution of Golgi proteins is due to the recruitment of microtubule-dependent proteins, then expression of GDP-restricted, cisternal rab proteins should have no effect on a microtubule-independent Golgi redistribution process. The response of the Golgi apparatus to drug-induced microtubule depolymerization should be one such example. Treatment of cells with drugs such as nocodazole causes depolymerization of microtubules. This is accompanied by the dispersal over a few hours of the compact, juxtanuclear Golgi apparatus into a series of scattered mini-stacks dispersed throughout the cytoplasm (Cole et al., 1996; Yang and Storrie, 1998; Storrie et al., 1998). As shown in Figure 7, we found surprisingly that preexpression of GDP-rab6a inhibited Golgi scattering. In the noninjected cells, numerous small scattered structures positive for GM130 (Figure 7A) or GalNAcT2 (Figure 7B) were formed 1 h after nocodazole addition, whereas the GDP-rab6a preexpressing cells were essentially devoid of scattered Golgi elements (Figure 7, arrowheads). Such inhibition was observed in >90% of the injected cells. We noted rare GM130-positive tubular extensions in noninjected nocodazole treated cells (Figure 7A, arrows). These projections seemed to be essentially negative for GalNAcT2. The occurrence of such extensions may indicate a role for residual microtubules. At longer nocodazole exposures, e.g., 3 h, there was extensive Golgi scattering in both GDP-rab6a–expressing cells and in control noninjected cells (our unpublished data), indicating that the GDP-rab6a effect was inhibitory rather than a complete block. Again, preexpression of GDP-rab5 was without effect in a Golgi-specific process. We conclude that cisternal rab proteins have an unexpected facilitative role in Golgi scattering in response to microtubule depolymerization.
Figure 7.
Overexpressing GDP-rab6a inhibits Golgi scattering in response to nocodazole. HeLa cells stably expressing GalNAcT2-GFP were microinjected with plasmids encoding GDP-rab6a at a stock concentration of 100 ng/μl. After 6-h incubation at 37°C cells were treated with 10 μM nocodazole at 37°C for 1 h. Then, cells were fixed and antibody stained for rab6 and GM130. The localizations of GM130 (A) and GalNAcT2 (B) are shown. Arrowheads (A) indicate microinjected cells. In the presence of nocodazole, a number of scattered fluorescence structures positive for GalNAcT2 and GM130 were observed in noninjected cells but not in cells injected with rab6a-GDP. Arrow (A), GM130-positive tubular extension. Bar, 10 μm.
In sum, we suggest that a normal physiological role of cisternal rab proteins is to regulate the induced Golgi redistribution in response to stress, with hypotonic shock being an example. Moreover, the data raise the possibility that microtubule-independent processes can play a significant role in rab-regulated Golgi redistribution.
Possible Redundancy of Cisternal Rab Proteins in Regulating Constitutive Recycling of Golgi Resident Proteins to the ER
Having demonstrated an effect of GDP-rab6a expression on a microtubule-independent process, we proceeded to determine whether these levels of individual rab expression affected constitutive recycling of Golgi resident proteins to the ER, a second microtubule-independent process (Storrie et al., 1998). In response to an ER exit block, Golgi resident proteins accumulate with a half-time of ∼1.5 h in the ER (Storrie et al., 1998; Girod et al., 1999; Zaal et al., 1999; Seemann et al., 2000). We have reported previously that constitutive recycling is inhibited in microinjected HeLa cells by preexpression of GDP-rab6a or GDP-rab33b from a plasmid stock concentration of ∼300 or 1000 ng/μl. These are considerably higher than needed to inhibit induced recycling (see above).
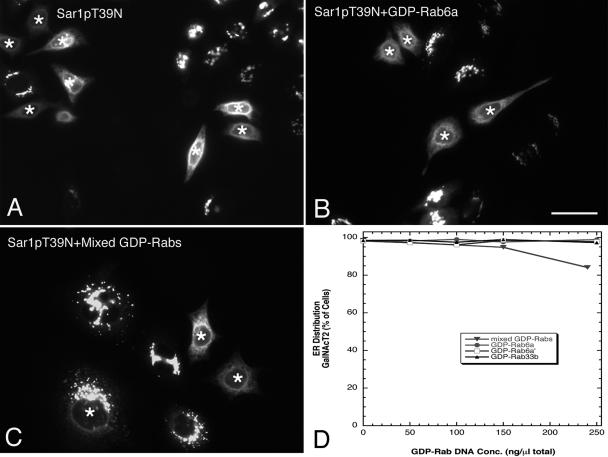
To reveal the constitutive recycling of GalNAcT2, we blocked ER exit by expression of the T39N, GDP-restricted mutant of Sar1p, the small GTPase required for the recruitment of COPII coat protein to ER membranes. COPII is required for vesicles or tubule budding at the ER. The minimal sufficient level of Sar1pT39N plasmids required revealing extensive ER accumulation of Golgi resident proteins after 6-h incubation was 7.5 ng/μl (Figure 8A, asterisks mark injected cells), consistent with previous results (Stroud et al., 2003; Kasap et al., 2004). We found that coexpression of Sar1pT39N with GDP-rab6a (Figure 8B), GDP-rab6a′ (image not shown) or GDP-rab33b (image not shown) individually at stock plasmid concentrations as high as 250 ng/μl had no qualitative effect on ER accumulation of GalNAcT2. When quantified across the microinjected cell population, no inhibition was observed at these concentrations (Figure 8D). In addition, we tested the effect of a cocktail of cisternal GDP-rabs on constitutive recycling. As shown in Figure 8C, the coinjection of plasmids encoding GDP-rab6a, -rab6a′, and -rab33b together with mutant Sar1p plasmids inhibited constitutive recycling in a small portion of the cell population. At stock concentrations of 80 ng/ml each for GDP-rab6a′, -rab6a′, and -rab33b, total plasmid DNA concentration of 240 ng/μl, pronounced inhibition was observed in <20% of the cells (Figure 8D). We did not test higher levels because these would give a total rab plasmid concentration in the range where we observed that GDP-rab6a expression started to inhibit mutant Sar1p protein production as indicated by staining with primary antibodies directed against Sar1p (our unpublished data). This inhibition of Sar1p mutant production may well explain our previously observed inhibition of constitutive recycling with high levels of GDP rab33b plasmids (Valsdottir et al., 2001).
Figure 8.
Effect of GDP-rab expression on constitutive Golgi resident protein cycling. HeLa cells stably expressing GalNAcT2-GFP were comicroinjected either with a fixed concentration of plasmids encoding Sar1pT39N (ER-exit block) and plasmids encoding individual GDP-rab protein at increasing concentrations or with a fixed concentration of Sar1pT39N plasmids and mixed GDP-rab-encoding plasmids at increasing concentrations. After a 6-h (for rab6) or 16-h (for rab33b and mixed rab proteins) expression period, cells were stained for rab6 or rab33b by immunofluorescence, and the localization of GalNAcT2 was determined. Asterisks over the cell nuclei indicate microinjected cells. (A) Sar1pT39N plasmids alone (7.5 ng/μl). (B) Sar1pT39N plasmids (7.5 ng/μl) with 250 ng/μl GDP-rab6a plasmids. (C) Sar1pT39N with mixed GDP-rab (rab6a/rab6a′/rab33b) plasmids together at 80 ng/μl each (240 ng/μl total GDP-rab plasmid stock concentration). (D) Quantitative analysis of the effect of GDP-rab plasmids on the Sar1p-highlighted redistribution of GalNAcT2 to the ER. Bar, 10 μm.
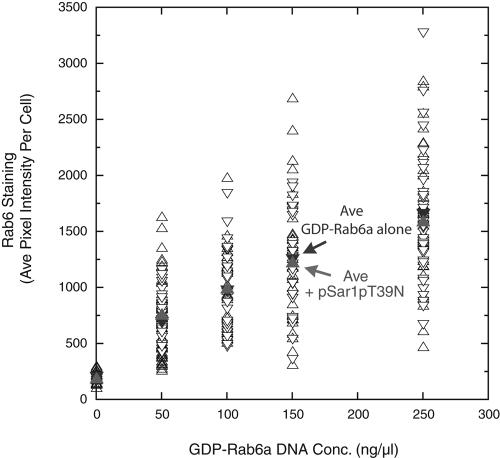
As a final control for the experiments reported here, we asked whether the coexpression of Sar1pT39N with GDP-rab6a–encoding plasmids had an inhibitory effect on rab6 production. As shown in Figure 9 by using a polyclonal antibody, rab6 staining increased curvilinear when cells were microinjected with progressively higher concentrations of GDP-rab6a plasmids. When coexpressed with Sar1pT39N, there was no inhibition of rab6 staining. Hence, the effective level of GDP-rab6a by using a 250 ng/μl plasmid stock coexpressed with Sar1pT39N was unaltered by the coexpresssion; this concentration should produce a >80% reduction in the per cell incidence of GTP-rab6a–induced Golgi redistribution (Figure 2A). In sum, we conclude that expression individually of GDP-restricted cisternal rab proteins has little to no effect on constitutive Golgi resident protein cycling. Only when coexpressed was any inhibition seen at these plasmid levels. We suggest that these rabs may act in a redundant manner to regulate constitutive recycling and accordingly revise our previous conclusions (Girod et al., 1999; Valsdottir et al., 2001) by using higher plasmid concentrations that rab6a or rab33b are individually major regulators of constitutive Golgi recycling. We note here that Young et al. (2005), using specific siRNA knock-downs of rab6a or rab6a′ or the two together, did not observe any inhibition of Sar1p highlighted constitutive Golgi protein recycling unless both rab6a and rab6a′ were knocked down together. Even then, the inhibition was transitory and full redistribution was observed after 7-h Sar1p mutant expression. We used a 6-h endpoint in our experiments and hence were unlikely to observe any transitory inhibition.
Figure 9.
Coexpression of an ER exit block (Sar1pT39N, GDP) with GDP-restricted rab6a has no effect on the synthesis of GDP-restricted rab6a. HeLa cells stably expressing GalNAcT2-GFP were microinjected with mixed plasmid populations encoding either 1) GDP-rab6a alone at various stock DNA concentrations (0–250 ng/ml) or 2) GDP-Sar1p (7.5 ng/ml) and GDP-rab6a (0–250 ng/μl stock DNA concentration). After a 6-h expression period, HeLa cells were stained with polyclonal antibodies directed against rab6. rab6 staining intensity in individual cells was determined using IPLab software.
DISCUSSION
We tested the hypothesis that a major role of Golgi cisternal rab proteins is modulation of the redistributive response of the Golgi apparatus to stress. The best-known physiological regulator of Golgi protein distribution is osmotic stress and its associated cell volume changes (Lee and Linstedt, 1999). Physiologically, cell volume changes are often associated with hormonal stimulation (Lang et al., 1998). In response to hypotonic conditions, mammalian Golgi apparatus proteins redistribute to the ER with an initial stage in this process being the extension of long tubules from the Golgi apparatus resembling those seen after BFA treatment (Lee and Linstedt, 1999; Lee and Linstedt, 2000). Hypotonic Golgi redistribution seems to up-regulate Golgi recycling and is inhibited by the protein kinase A inhibitor H89 as is Golgi scattering in response to microtubule depolymerization (Lee and Linstedt, 2000). We reasoned that cisternal rab proteins such as rab6 that are known to mediate the extension of Golgi tubules to the ER (White et al., 1999) might be the Golgi-associated molecular target of hypotonic stress. Accordingly, we tested whether the expression of inactive, GDP-restricted forms of individual cisternal rab proteins inhibited the Golgi stress response. This was indeed the case. For comparison, we tested whether similar levels of expression of GDP-restricted cisternal rab proteins such as rab6a, rab6a′ and rab33b were efficacious in inhibiting constitutive recycling of Golgi resident proteins to the ER as revealed by an ER-exit block. We found that these levels of expression had little to no effect on constitutive recycling. In control experiments, these levels were sufficient to inhibit homologous GTP-rab induced redistribution of a Golgi glycosyltransferases into the ER and to displace the GTP-conformer of rab6 from Golgi membranes. In sum, we conclude that Golgi cisternal rabs and, in particular, rab6a are individually regulators of the Golgi response to stress and presumably the molecular targets of a stress activated signaling pathway(s). In Table 2, we summarize the apparent properties of constitutive and induced COPI-independent Golgi-to-ER protein trafficking.
Table 2.
Comparative properties of constitutive and induced COPI-independent Golgi recycling to the ER
| Constitutive |
| • Substrates: resident Golgi cisternal enzymes and Golgi matrix proteins |
| • Recycling motif: none known |
| • Kinetics: fairly slow, 1.5-h approximate half-time |
| • Role of microtubules: microtubule independent |
| • Inducers: none known, revealed by an ER-exit block |
| • Cisternal rab protein role: may require low levels of multiple rab proteins |
| • Known physiological stimuli: none known |
| Induced |
| • Substrates: resident Golgi cisternal enzymes and Golgi matrix properties |
| • Recycling motif: none known |
| • Kinetics: can be rapid, GM130 redistribution in response to hypotonic shock |
| • Role of microtubules: microtubule dependent in several cases |
| • Inducers: rab6a, rab6a′, and rab33b |
| • Known physiological stimuli: hypotonic shock |
To the best of our knowledge, we present here the first evidence for the involvement of cisternal rab proteins in the up-regulation of a physiological process, i.e., Golgi stress response. The Golgi cisternal rab proteins: rab6a, rab6a′, and rab33b, have been implicated previously in a variety of COPI coat protein-independent Golgi trafficking events (for review, see Storrie, 2005). Rab6a or rab33b overexpression induces the redistribution of Golgi glycosyltransferases to the ER in a COPI-independent manner (Martinez et al., 1997; Girod et al., 1999; Valsdottir et al., 2001; Chen et al., 2003). This redistribution, at least of rab6a, may be dependent on tubular Golgi extensions (White et al., 1999; see also Nizak et al., 2003). Although rab6a′ was previously thought to be involved exclusively in endosome to trans-Golgi network trafficking (Echard et al., 2000), current evidence indicates that overexpression of rab6a′, as with rab6a, causes resident Golgi protein redistribution to the ER (Young et al., 2005; present work). GTP-rab induced redistribution is in all cases microtubule dependent being inhibited strongly by the microtubule-depolymerizing drug nocodazole (Martinez et al., 1997; Young et al., 2005; present study). Using varying concentrations of plasmids encoding GDP-restricted rab6a or rab33b, we (Girod et al., 1999) and others (Chen et al., 2003) have implicated cisternal rab proteins in constitutive Golgi protein recycling to the ER as revealed by an ER-exit block or by transport of Shiga toxin B subunit to the ER. Toxin B subunit transport presumably uses the same normal COPI-independent Golgi recycling (Girod et al., 1999).
Our present results force a revision of the conclusion that individual cisternal rab proteins are major regulators of constitutive Golgi recycling. We report here that concentrations of GDP-rab6a, -rab6a′, and -rab33b sufficient to inhibit GTP-rab6a, -rab6a′, or -rab33b induced ER redistribution of Golgi proteins in coexpression experiments had individually no effect on constitutive recycling as revealed by a mutant Sar1p ER-exit block. Only when used at much higher concentrations that inhibited the expression of ER-exit block inducer Sar1pT39N was an effect observed. When coexpressed as a cocktail of cisternal rabs, there was a marginal effect on constitutive recycling, suggesting that cisternal rabs may be redundant with respect to constitutive resident Golgi protein recycling; they may coact. Our data with GDP-rab overexpression are entirely consistent with the knock-down experiments of Young et al. (2005) who find only a brief delay in constitutive Golgi recycling with coknock-down of rab6a and rab6a′ and no delay in the single knock-down experiment. We note here that the sequential microinjection experiments reported by Girod et al. (1999) in which plasmid injection is followed by microinjection of mutant Sar1p protein are technically demanding and only limited controls regarding cell viability were provided.
On a technical note, we conclude that rab6a and rab6a′ use the same GEF and/or other factor, wherea rab6s and rab33b use a different GEF/factor. GDP-rab proteins are thought to compete with GTP-rab proteins by binding more tightly to the corresponding GEF and hence preventing exchange of GDP for GTP (for review, see Feig, 1999). In our experiments, both GDP-rab6a and rab6a′ inhibited the phenotypic consequences of coexpressed GTP-rab6a. They did not inhibit synthesis of the GTP-restricted rab. The simplest interpretation is that both compete for the same GEF/factor. In heterologous competition experiments between GDP-rab33b and GTP-rab6a or GDP-rab6a/-rab6a′ and GTP-rab33b, no cross-competition was observed, suggesting that rab6s and rab33b have a different GEF/factor.
Our results indicate that cisternal rab proteins regulate microtubule-independent as well as microtubule-dependent Golgi redistribution. This is an unexpected outcome, considering the evidence that GTP-rab6a, -rab6a′, and rab33b are microtubule-dependent inducers of Golgi glycosyltransferases redistribution to the ER (Martinez et al., 1997; Young et al., 2005; present work). GTP-rab6a interacts with microtubule dependent motor components (Echard et al., 1998; Matanis et al., 2002; Short et al., 2002; Young et al., 2005). Preexpression of GDP-rab6a, -rab6a′, and rab33b inhibited BFA-induced tubule extension, a microtubule-dependent process (Lippincott-Schwartz et al., 1990). In fact, the greater inhibition observed when a cocktail of the GDP-restricted rabs was coexpressed suggests that three rabs coact to promote tubule extension. Similarly, GDP-rab6a inhibited hypotonic shock induced tubule extension. Because the drug H89 that inhibits both BFA- and hypotonic shock-induced Golgi redistribution to the ER also inhibits nocodazole-induced Golgi scattering, we tested whether GDP-rab6a preexpression also would inhibit. Indeed, it did. Nocodazole, a drug that directly inhibits microtubule polymerization, produces Golgi fragmentation into scattered mini-stacks as an indirect result of microtubule depolymerization (for review, see Storrie and Yang, 1998). Hence, Golgi scattering is assumed to be a microtubule-independent process. There must be important microtubule-independent rab6a effectors. Further experiments will be required to identify these and how they act. Golgi scattering has been interpreted as a Golgi assembly process at scattered ER exit sites in which Golgi-to-ER transport is an obligatory step (Cole et al., 1996; Yang and Storrie, 1998; Storrie et al., 1998; Drecktrah and Brown, 1999). Certainly, these results suggest additional and/or other processes than constitutive Golgi recycling to the ER must be involved.
In conclusion, we present evidence that cisternal rab proteins, and in particular, rab6a are modulators of a physiological Golgi redistributive response, i.e., Golgi redistribution in response to hypotonic media. Presumably the activity of cisternal rab proteins is up-regulated in response to a signal cascade. We find little evidence that these proteins individually are regulators of constitutive Golgi recycling to the ER as revealed by an ER-exit block. On this point, our findings force a reinterpretation of previous evidence for such a regulation (Girod et al., 1999). Our data do suggest that they may coact in a redundant manner. To our surprise, cisternal rab proteins seem to regulate the redistribution of Golgi proteins through both microtubule-dependent and independent process as indicated by GDP-rab inhibition of both BFA and nocodazole highlighted Golgi redistribution. Future work will be required to identify microtubule-independent effectors for rab6a.
Acknowledgments
We express our appreciation to colleagues for gifts of plasmids and antibodies as cited in Materials and Methods. Discussion of the research with Drs. T. Lee and A. Linstedt contributed greatly to this work. We gratefully acknowledge the constructive criticisms of wording and organization of this manuscript from our colleagues at University of Arkansas for Medical Sciences, including Drs. G. Baldini, M. Jennings, V. Lupashin, and J. Ware. This work was funded in part by grants from the National Institutes of Health (GM-65233) and the National Science Foundation (MCB-9983332).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-10-0861) on March 9, 2005.
References
- Burkhardt, J. K. (1998). The role of microtubule-based motor proteins in maintaining the structure and function of the Golgi complex. Biochim. Biophys. Acta 1404, 113-126. [DOI] [PubMed] [Google Scholar]
- Chen, A., AbuJarour, R. J., and Draper, R. K. (2003). Evidence that the transport of ricin to the cytoplasm is independent of both rab6A and COPI. J. Cell Sci. 116, 3503-3510. [DOI] [PubMed] [Google Scholar]
- Cole, N. B., Sciaky, N., Marotta, A., Song, J., and Lippincott-Schwartz, J. (1996). Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol. Biol. Cell 7, 631-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drecktrah, D., and Brown, W. J. (1999). Phospholipase A(2) antagonists inhibit nocodazole-induced Golgi ministack formation: evidence of an ER intermediate and constitutive cycling. Mol. Biol. Cell 10, 4021-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echard, A., Jollivet, F., Martinez, O., Lacapere, J. J., Rousselet, A., Janoueix-Lerosey, I., and Goud, B. (1998). Interaction of a Golgi-associated kinesin-like protein with rab6. Science 79, 580-585. [DOI] [PubMed] [Google Scholar]
- Echard, A., Opdam, F. J., Leeuw de, H. J., Jollivet, F., Savelkoul, P., Hendriks, W., Voorberg, J., Goud, B., and Fransen, J. A. (2000). Alternative splicing of the human rab6A gene generates two close but functionally different isoforms. Mol. Biol. Cell 11, 3819-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig, L. A. (1999). Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat. Cell Biol. 1, E25-27. [DOI] [PubMed] [Google Scholar]
- Girod, A., Storrie, B., Simpson, J. C., Johannes, L., Goud, B., Roberts, L. M., Lord. J. M., Nilsson, T., and Pepperkok, R. (1999). Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat. Cell Biol. 1, 423-430. [DOI] [PubMed] [Google Scholar]
- Ivessa, N. E., Gravotta, D., De Lemos-Chiarandini, C., and Kreibich, G. (1997). Functional protein prenylation is required for the brefeldin A-dependent retrograde transport from the Golgi apparatus to the endoplasmic reticulum. J. Biol. Chem. 272, 20828-20834. [DOI] [PubMed] [Google Scholar]
- Kasap, M., Thomas, S., Danaher, E., Holton, V., Jiang, S., and Storrie, B. (2004). Dynamic nucleation of Golgi apparatus assembly from the endoplasmic reticulum in interphase HeLa cells. Traffic 5, 595-605. [DOI] [PubMed] [Google Scholar]
- Lang, F., Busch, G. L., Ritter, M., Volkl, H., Waldegger, S., Gulbins, E., and Haussinger, D. (1998). Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 78, 247-306. [DOI] [PubMed] [Google Scholar]
- Lanoix, J., Ouwendijk, J., Lin, C. C., Stark, A., Love, H. D., Ostermann, J., and Nilsson, T. (1999). GTP hydrolysis by arf-1 mediates sorting and concentration of Golgi resident enzymes into functional COP I vesicles. EMBO J. 18, 4935-4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoix, J., Ouwendijk, J., Stark, A., Szafer, E., Cassel, D., Dejgaard, K., Weiss, M., and Nilsson, T. (2001). Sorting of Golgi resident proteins into different subpopulations of COPI vesicles: a role for ArfGAP1. J. Cell Biol. 155, 1199-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, T. H., and Linstedt, A. D. (1999). Osmotically induced cell volume changes alter anterograde and retrograde transport, Golgi structure, and COPI dissociation. Mol. Biol. Cell 10, 1445-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, T. H., and Linstedt, A. D. (2000). Potential role for protein kinases in regulation of bidirectional endoplasmic reticulum-to-Golgi transport revealed by protein kinase inhibitor H89. Mol. Biol. Cell 11, 2577-25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Schweizer, D. A., Berger, E. G., Hauri, H. P., Yuan, L. C., and Klausner, R. D. (1990). Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. J. Cell Biol. 155, 557-570. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Yuan, L. C., Bonifacino, J. S., and Klausner, R. D. (1989). Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56, 801-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard, F., Tang, B. L., Galli, T., Tenza, D., Saint-Pol, A., Yue, X., Antony, C., Hong, W., Goud, B., and Johannes, L. (2002). Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a rab6 isoform. J. Cell Biol. 156, 653-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez, O., Antony, C., Pehau-Arnaudet, G., Berger, E. G., Salamero, J., and Goud, B. (1997). GTP-bound forms of rab6 induce the redistribution of Golgi proteins into the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 94, 1828-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matanis, T., et al. (2002). Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat. Cell Biol. 4, 986-992. [DOI] [PubMed] [Google Scholar]
- Nizak, C., Monier, S., del Nery, E., Moutel, S., Goud, B., and Perez, F. (2003). Recombinant antibodies to the small GTPase rab6 as conformation sensors. Science 300, 984-987. [DOI] [PubMed] [Google Scholar]
- Opdam, F. J., Echard, A., Croes, H. J., van den Hurk, J. A., van de Vorstenbosch, R. A., Ginsel, L. A., Goud, B., and Fransen, J. A. (2000). The small GTPase rab6B, a novel rab6 subfamily member, is cell-type specifically expressed and localised to the Golgi apparatus. J. Cell Sci. 113, 2725-2735. [DOI] [PubMed] [Google Scholar]
- Röttger, S., White, J., Wandall, H. H., Olivo, J. C., Stark, A., Bennett, E. P., Whitehouse, C., Berger, E. G., Clausen, H., and Nilsson, T. (1998). Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus. J. Cell Sci. 111, 45-60. [DOI] [PubMed] [Google Scholar]
- Seemann, J., Jokitalo, E., Pypaert, M., and Warren, G. (2000). Matrix proteins can generate the higher order architecture of the Golgi apparatus. Nature 407, 1022-1026. [DOI] [PubMed] [Google Scholar]
- Shan, J., Mason, J. M., Yuan, L., Barcia, M., Porti, D., Calabro, A., Budman, D., Vinciguerra, V., and Xu, H. (2000). Rab6c, a new member of the rab gene family, is involved in drug resistance in MCF7/AdrR cells. Gene 257, 67-75. [DOI] [PubMed] [Google Scholar]
- Short, B., Preisinger, C., Schaletzky, J., Kopajtich, R., and Barr, F. A. (2002). The rab6 GTPase regulates recruitment of the dynactin complex to Golgi membranes. Curr. Biol. 12, 1792-1795. [DOI] [PubMed] [Google Scholar]
- Storrie, B. (2005). Maintenance of Golgi apparatus structure in the face of continuous protein recycling to the endoplasmic reticulum: making ends meet. Int. Rev. Cytol. 244, 71-96. [DOI] [PubMed] [Google Scholar]
- Storrie, B., Pepperkok, R., and Nilsson, T. (2000). Breaking the COPI monopoly on Golgi recycling. Trends Cell Biol. 10, 385-391. [DOI] [PubMed] [Google Scholar]
- Storrie, B., White, J., Röttger, S., Stelzer, E. H., Suganuma, T., and Nilsson, T. (1998). Recycling of Golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J. Cell Biol. 143, 1505-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie, B., and Yang, W. (1998). Dynamics of the interphase mammalian Golgi complex as revealed through drugs producing reversible Golgi disassembly. Biochim. Biophys. Acta 1404, 127-137. [DOI] [PubMed] [Google Scholar]
- Stroud, W. J., Jiang, S., Jacks, G., and Storrie, B. (2003). Persistence of Golgi matrix distribution exhibits the same dependence on Sar1p activity as a Golgi glycosyltransferase. Traffic 4, 631-641. [DOI] [PubMed] [Google Scholar]
- Valsdottir, R., Hashimoto, H., Ashman, K., Koda, T., Storrie, B., and Nilsson, T. (2001). Identification of rabaptin-5, rabex-5, and GM130 as putative effectors of rab33b, a regulator of retrograde traffic between the Golgi apparatus and ER. FEBS Lett. 508, 201-209. [DOI] [PubMed] [Google Scholar]
- White, J., Johannes, L., Mallard, F., Girod, A., Grill, S., Reinsch, S., Keller, P., Tzschaschel, B., Echard, A., Goud, B., and Stelzer, E. H. (1999). Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J. Cell Biol. 147, 743-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W., and Storrie, B. (1998). Scattered Golgi elements during microtubule disruption are initially enriched in trans-Golgi proteins. Mol. Biol. Cell 9, 191-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, J., Stauber, T., Vernos, I., Pepperkok, R., and Nilsson, T. (2005). Regulation of microtubule-dependent recycling at the TGN by rab6a and rab6a′. Mol. Biol. Cell 16, 162-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaal, K. J., et al. (1999). Golgi membranes are absorbed into and reemerge from the ER during mitosis. Cell 99, 589-601. [DOI] [PubMed] [Google Scholar]