Abstract
Adoptive immunotherapy using chimeric antigen-receptor (CAR)-engineered T cells can induce robust antitumor responses against hematologic malignancies. However, its efficacy is not durable in the majority of the patients, warranting further improvement of T-cell functions. Cytokine signaling is one of the key cascades regulating T-cell survival and effector functions. In addition to cytokines that use the common γ chain as a receptor subunit, multiple cytokines regulate T-cell functions directly or indirectly. Modulating cytokine signaling in CAR-T cells by genetic engineering is one promising strategy to augment their therapeutic efficacy. These strategies include ectopic expression of cytokines, cytokine receptors, and synthetic molecules that mimic endogenous cytokine signaling. Alternatively, autocrine IL-2 signaling can be augmented through reprogramming of CAR-T cell properties through transcriptional and epigenetic modification. On the other hand, cytokine production by CAR-T cells triggers systemic inflammatory responses, which mainly manifest as adverse events such as cytokine-release syndrome (CRS) and neurotoxicity. In addition to inhibiting direct inflammatory mediators such as IL-6 and IL-1 released from activated macrophages, suppression of T-cell-derived cytokines associated with the priming of macrophages can be accomplished through genetic modification of CAR-T cells. In this review, I will outline recently developed synthetic biology approaches to exploit cytokine signaling to enhance CAR-T cell functions. I will also discuss therapeutic target molecules to prevent or alleviate CAR-T cell-related toxicities.
Keywords: adoptive immunotherapy, cytokine-release syndrome, neurotoxicity
Cytokine signaling for CAR-T cells
Graphical Abstract
Graphical Abstract.

Introduction
Adoptive immunotherapy is a unique therapeutic approach for patients with advanced cancer. The most striking difference of this therapy from other immunotherapy modalities is that it uses living immune cells prepared in vitro as a drug, which theoretically enables persistent and specific antitumor efficacy until target tumor cells are eradicated. Prominent success was achieved by adoptive transfer of chimeric antigen receptor (CAR)-T cells—which express modified versions of T-cell antigen-receptors (TCRs)—to patients with B-cell malignancies and multiple myeloma (1–5).
However, long-term follow-up of the treated patients suggests that a substantial proportion of patients that accomplished an objective response suffer from relapse. For example, recently reported real-world data on one of the CD19-targeting CAR-T cells tisagenlecleucel (tisa-cel) for patients with B-cell lymphoblastic leukemia has shown marked initial response as was seen in clinical trials. Although 85% of the patients infused with tisa-cel accomplished complete remission at Day 28, 37% of the responder patients experienced relapse within 1 year, and the 12-month event-free survival was 50% (6). Relapsed leukemia cells maintained CD19 expression in about 60% of the cases, suggesting that dysfunction or disappearance of the infused CAR-T cells resulted in the regrowth of leukemia cells for these patients. Regarding the use of CAR-T cells against solid tumors, although several clinical trials report a promising efficacy, the majority of the patients cannot attain durable response (7, 8).
Among numerous investigations to fundamentally augment the therapeutic efficacy of CAR-T cells, modulation of cytokine signaling in CAR-T cells is one of the most investigated strategies (Fig. 1). In addition to the therapeutic standpoint, control of serious adverse events that can accompany CAR-T cell therapy such as cytokine-release syndrome (CRS) and neurological toxicity is another urgent issue to be addressed (9). Several cytokines are centrally associated with the pathogenesis of adverse events and are key targets to control or prevent serious side effects.
Figure 1.

Multiple approaches to incorporate cytokine signaling in CAR-T cells. In addition to ectopic expression of soluble or membrane-tethered cytokines, CAR-T cells can be engineered with wild-type or synthetic cytokine receptors. Alternatively, autocrine cytokine signaling (mainly IL-2) can be augmented through genetic modification of CAR-T cells
In this review, I will summarize roles of various cytokines in T-cell functions, the tumor microenvironment and systemic immune responses in the context of antitumor T cells and discuss how these findings can be applied to improve the efficacy and safety of CAR-T cell therapy.
Common γ chain cytokines and antitumor T cells
In addition to the TCR (signal 1) and co-stimulatory (signal 2) signaling, cytokine signaling (signal 3) constitutes an essential part to induce optimal T-cell functions (10). Common γ chain cytokines (IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21)—all of which include the same γ chain subunit in their receptors—are the most established group of molecules that are associated with multiple T-cell attributes. Whereas IL-4 negatively regulates effector T-cell responses, IL-2, IL-7, IL-15, and IL-21 are critically associated with the development, survival, proliferation, and effector functions of T cells (11–13).
Upon cytokine engagement with their cognate receptors, the receptors recruit and activate Janus kinase 1 (JAK1) and JAK3, which then phosphorylate several signal transducer and activator of transcription (STAT) proteins. Different cytokines activate multiple STAT proteins to different extents, which gives rise to their unique and non-redundant functions. Whereas IL-2, IL-7, and IL-15 mainly activate STAT5, the main downstream target of IL-21 is STAT3 (14). Cytokine-mediated STAT5 activation supports the survival of effector T cells partly through transcriptionally upregulating anti-apoptotic BCL2 family proteins (15, 16). STAT5 also regulates the expression of cytolytic molecules such as granzyme B and perforin (17, 18). The role of STAT3 signaling in T cells is more diverse. Whereas STAT3 is required for memory T-cell formation (19, 20), its activation also promotes terminal T-cell differentiation through cooperating with effector-related transcription factors such as BATF and IRF4 (21). Other studies report that STAT3 negatively affects the migration of antitumor T cells in the tumor through downregulating CXCR3 (22, 23).
Despite these complex functions of downstream molecules, IL-2, IL-7, IL-15, and IL-21 overall provide beneficial effects on antitumor and antiviral T-cell immunity. Since these cytokines are not necessarily produced in sufficient amounts in the tumor microenvironment, exogenous cytokine administration was considered as one of the solutions to boost antitumor T-cell immunity (24–26). Early clinical studies explored the antitumor effects of systemically administered cytokines as monotherapy or in the context of adoptive immunotherapy. Although several cytokines such as IL-2 and IL-15 induced objective clinical responses or enhanced the persistence of infused antitumor T cells, they resulted in frequent and sometimes severe toxicities such as capillary leak syndrome followed by multi-organ damage in a dose-dependent manner (27–30). Because of these safety concerns, multiple strategies to locally provide cytokines for CAR-T cells have been studied subsequently (Table 1). In early investigations, Markley and Sadelain (31) expressed individual common γ chain cytokines in CAR-T cells and demonstrated that the ectopic expression of IL-7 or IL-21 prominently enhanced the antitumor activity of CAR-T cells. Adachi et al. (32) showed that co-transduction of CCL19 in addition to IL-7 further enhanced the therapeutic activity of CAR-T cells through recruiting endogenous antitumor immune cells.
Table 1.
Cytokine signaling that enhances CAR-T cell functions
| Cytokine | Strategies to deliver signals | Effects on CAR-T cell properties | References |
|---|---|---|---|
| IL-2 | • Incorporating the receptor within the CAR construct • Orthogonal IL-2/IL-2R |
Enhanced proliferative and effector functions | (35, 37) |
| IL-7 | • Ectopic expression • Expression of the constitutively active receptor |
Long-lived potential; durable effector functions | (31–33) |
| IL-9 | • Orthogonal IL-2/IL-9R | Maintenance of stemness; enhanced effector functions | (38) |
| IL-15 | • Membrane-tethered expression | Prolonged survival | (34) |
| IL-8 | • Ectopic expression of the receptor (CXCR1 and CXCR2) | Enhanced T cell migration into the tumor | (74) |
| IL-10 | • Administration of IL-10:Fc fusion | Restoration of exhausted T cell functions by metabolic reprogramming | (75) |
| IL-12 | • Ectopic expression • Cell-surface expression • Incorporation within the CAR construct |
Direct upregulation of cytotoxicity; modulation of the immunosuppressive tumor microenvironment | (66–69) |
| IL-18 | • Ectopic expression • Expression of GM-CSFR/IL-18R fusion receptor |
Enhanced effector functions; remodeling of the tumor microenvironment | (71–73) |
| IL-23 | • Ectopic expression of the p40 subunit | Increased effector functions; less-exhausted phenotype | (76) |
| IL-33 | • Ectopic expression with high-affinity IL-2 | Modification of the tumor microenvironment | (77) |
| IL-36γ | • Ectopic expression | Enhanced cytotoxic activity; modulation of the tumor microenvironment | (78) |
| IL-37 | • Recombinant cytokine administration | Restoration of exhausted T cell functions | (79) |
In addition to the secretion of soluble cytokines, multiple designs have been invented to induce cytokine-mediated signaling in a constitutive or inducible manner, which include ectopic expression of a constitutively active IL-7 receptor (IL-7R) (33), a membrane-tethered IL-15/IL-15R complex (34) and incorporation of a truncated IL-2R β-chain signaling domain within the CAR construct (Fig. 2) (35). Recent studies have further advanced a state-of-the-art system that transmits desired combinations of cytokine signaling in an inducible manner. Lin et al. (36) designed an artificial cytokine-receptor system, in which extracellular FK506-binding protein 12 (FKBP12) F36V was linked to the transmembrane and intracellular JAK-recruiting domains of the thrombopoietin receptor and desired combinations of cytokine-receptor domains. It can homodimerize and activate cytokine signaling in response to the addition of the FKBP12 ligand AP1903.
Figure 2.
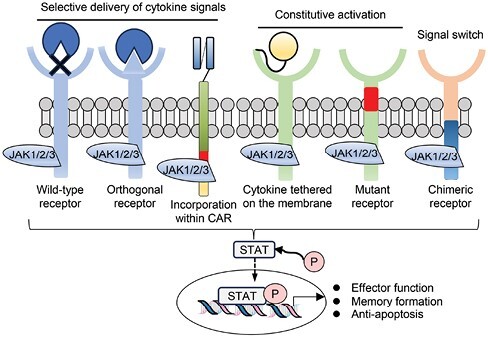
Synthetic biology approaches to provide cytokine signaling. CAR-T cells can be provided with cytokine signaling by administration of orthogonal cytokines, incorporation of a cytokine receptor domain within the CAR structure, or ectopic expression of wild-type, mutated, or synthetic cytokine receptors
Systemic cytokine administration can also deliver cytokine signaling to specific cells by designing synthetic molecules. Zhang et al. (37) developed an orthogonal IL-2 and IL-2R β-chain to avoid the systemic toxicity of IL-2. Ectopic expression of orthogonal IL-2Rβ in CAR-T cells enabled selective induction of IL-2 signaling upon administration of orthogonal IL-2. Although the effect of IL-9 signaling has been less well elucidated compared with other common γ chain cytokines, Kalbasi et al. (38) recently demonstrated that signals through the IL-9R augmented CAR-T cell functions. They tested the orthogonal IL-2R extracellular domain combined with intracellular domains of various cytokine receptors and identified that fusion with the IL-9R most efficiently improved both longevity and cytolytic activity of CAR-T cells.
Enhancing T-cell-intrinsic cytokine secretion
T cells secrete multiple cytokines upon stimulation by antigen. In addition to effector cytokines such as IFN-γ and TNF-α, CD4+ T cells and, to a lesser extent, CD8+ T cells secrete IL-2, which enhances the proliferation and survival of antigen-stimulated CAR-T cells in an autocrine and paracrine manner (39, 40). Antigen-experienced memory T cells progressively differentiate from stem-cell-like memory T cells to central memory T cells and effector memory T cells (41). Whereas IFN-γ and TNF-α secretion is maintained until terminal differentiation, T cells lose the capacity to secrete IL-2 as they differentiate (42, 43). In other words, polyfunctional cytokine secretion is one of the characteristics of immature memory T cells. T cells also undergo extensive alteration of their functional profiles upon exhaustion, which is defined as the impairment of effector functions, including IL-2 secretion, because of chronic and repetitive antigen stimulation (44). Since CAR-T cells go through differentiation along with massive expansion and are rendered exhausted due to persistent encounter with the target tumor cells, they are prone to lose IL-2 production, which further deprives them of survival signals (45).
One of the strategies to maintain cell-intrinsic IL-2 signaling in CAR-T cells is to modify them to acquire resistance to terminal differentiation and/or exhaustion. Both terminal differentiation and exhaustion are associated with global gene expression changes, which are further regulated by epigenetic mechanisms (46–48). Recent studies have extensively explored key epigenetic factors whose modification can enhance the longevity and durable effector responses of CAR-T cells after repetitive antigen exposure (49). We have recently reported that genetic ablation of PRDM1, which encodes the transcriptional repressor Blimp-1, maintained an early memory phenotype and IL-2 production in CAR-T cells after repeated antigen stimulation (50). In addition to its direct repression of IL-2 transcription (51), Blimp-1 promotes terminal differentiation of T cells, which impairs their polyfunctional cytokine secretion (52).
T-cell exhaustion is also controlled by an array of epigenetic factors such as DNMT3A, TOX, the NR4A family, and the BET bromodomain family. Recent work has already demonstrated that genetic or pharmacological inhibition of these proteins positively affects T-cell effector functions through repressing exhaustion-related transcriptional programs of CAR-T cells (45, 53–56). Interestingly, these studies suggest that T-cell differentiation and exhaustion are regulated by distinct transcriptional and epigenetic mechanisms. Blimp-1 ablation did not prevent the upregulation of immunoinhibitory molecules such as PD-1 and their transcriptional regulators in chronically stimulated T cells (50). Simultaneous modification of differentiation- and exhaustion-associated factors may further augment T-cell functions. This concept was recently exemplified by a study showing that dual knockout of PRDM1 and NR4A3 in CAR-T cells enhanced both T-cell longevity and effector functions (57). Further investigation to identify combined modification to optimally improve T-cell stemness and counteract exhaustion would be important to refine this strategy.
Alternatively, cytokine supplementation during in vitro T-cell expansion contributes to generating CAR-T cells with superior antitumor functions. Although IL-2 potently promotes T-cell proliferation, it also accelerates terminal effector differentiation and deprives T cells of long-lived potential (58). Pre-clinical studies demonstrated that T-cell stimulation using other cytokine cocktails such as IL-7 + IL-15 (59) or IL-15 + IL-21 (60) resulted in efficient expansion and acquisition of CAR-T cells with a less differentiated phenotype compared with the standard IL-2 supplementation protocol.
The quality of CAR-T cells can also be modified by different CAR transduction approaches. For example, genomic insertion of a CAR gene using CRISPR/Cas9-mediated homologous recombination or a piggyBac transposon system can efficiently generate CAR-T cells (60–62). These CAR-T cells exhibited immature and non-exhausted phenotypes partly because of attenuated CAR expression levels compared with viral transduction methods, which will potentially result in superior persistence and durable antitumor efficacy.
Non-common γ chain cytokines associated with T-cell properties
In addition to the common γ chain cytokines, a number of studies have shown unique effects of other cytokines on CAR-T cell efficacy. Initial attempts explored the effects of IL-12 on antitumor T cells. IL-12 is a proinflammatory cytokine with antitumor functions. In addition to directly augmenting the cytotoxic activity of T cells (63), IL-12 reduces the immunosuppressive tumor microenvironment through modifying the properties of regulatory T cells and myeloid-lineage cells (64, 65). Consistent with these findings, ectopic expression of IL-12 in CAR-T cells enhanced their proliferation and effector functions in pre-clinical mouse models of solid tumors (66, 67). To deliver IL-12 signaling to intratumoral T cells more locally, subsequent studies have developed multiple synthetic systems, such as cell surface-tethered expression of IL-12 (68) and IL-12 integration into the extracellular CAR domain (69).
IL-18 is another well-known cytokine associated with the induction of inflammatory myeloid cells in conjunction with IL-12 (70). Similar to IL-12, CAR-T cells armored with IL-18 enhanced the antitumor efficacy partly through modulating the tumor microenvironment such as an increase of macrophages with an M1 phenotype and an increase of mature dendritic cells (71). Other studies elucidated the direct effect of IL-18 on effector T-cell functions (72, 73). A list of cytokines that have been reported to affect CAR-T cell functions is shown in Table 1 (66–79). Future clinical trials will elucidate if the effect of these cytokines demonstrated in pre-clinical models really helps to improve the therapeutic efficacy of CAR-T cells.
One of the interesting findings from these studies is that the effect of exogenous administration of cytokines on the overall antitumor response is determined by a complex interplay of multiple immune cells and the metabolic environment, which cannot be predicted precisely by their direct action on T cells evaluated in vitro. For example, IL-10 is a pleiotropic cytokine with both immunosuppressive and immunostimulatory functions. IL-10 dampens the ability of antigen-presenting cells to stimulate T cells (80), whereas it also elevates cytotoxic activity of CD8+ T cells (81). Guo et al. (75) demonstrated that the administration of an IL-10/Fc fusion protein restored mitochondrial respiration in exhausted T cells, which resulted in enhanced effector functions in both endogenous and adoptively transferred antitumor T cells.
Cytokine-release syndrome
Cytokine-release syndrome is the most frequent and serious side effect that accompanies adoptive immunotherapy. Its main symptoms consist of fever, nausea, fatigue, and myalgia (82). In severe cases, patients develop hypotension, hypoxia, and multi-organ dysfunction. Another major toxicity is immune effector cell-associated neurotoxicity syndrome (ICANS), which usually happens at later time points than CRS. It most often manifests as self-limiting delirium, encephalopathy, and aphasia. Prolonged seizures and cerebral edema may happen in life-threatening cases. Although CRS is observed in a variety of treatments, including allogeneic stem cell transplantation and immune-checkpoint inhibitors, its developmental risk is especially high in CAR-T cell therapy and bispecific T-cell engagers (BiTEs), which induce antigen-specific cytotoxicity in endogenous T cells.
The development of severe CRS and ICANS seems to be less frequent in real-world data compared with those in early clinical trials, likely because of improved selection criteria and more established management. In the patients with B-cell leukemia treated by tisa-cel, overall and grade ≥3 CRS was documented in 63% and 21% of the patients, and overall and grade ≥3 ICANS in 21% and 7% of the patients, respectively (6).
Mechanistically, CRS is triggered mainly by the inflammatory cytokine IL-6 derived from activated macrophages, which induces endothelial activation followed by increased vascular permeability (Fig. 3) (83, 84). Macrophage activation is at least partly provoked by GM-CSF secreted by antigen-stimulated CAR-T cells (85). CD40L expressed in CAR-T cells also triggers macrophage activation. As a novel mechanism underlying the development of CRS, Liu et al. (86) demonstrated that granzyme B secreted from CAR-T cells activates caspase 3 within tumor cells, which then cleaves gasdermin E and causes pyroptosis—inflammatory programmed cell death that triggers the activation of adjacent macrophages through secretion of damage-associated molecular patterns (DAMPs).
Figure 3.
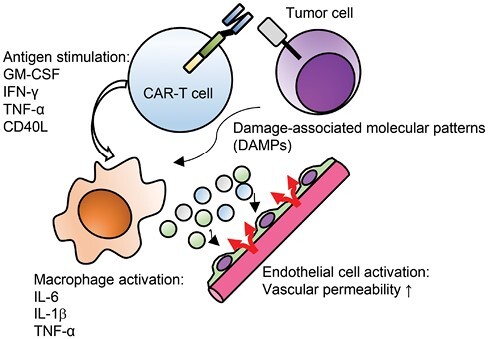
Pathophysiology of CRS. Upon antigen-mediated stimulation, CAR-T cells secrete multiple cytokines that trigger activation of endogenous macrophages, which then secrete inflammatory cytokines including IL-6 and IL-1 and induce systemic inflammatory responses. One of the pathogenetic mechanisms related to CRS is endothelial activation and increased vascular permeability
The anti-IL-6R antibody tocilizumab can effectively sequester soluble IL-6R and then inhibit the activation of IL-6 signaling. ICANS is less frequently responsive to tocilizumab treatment than CRS, suggesting that factors other than IL-6 are associated with its pathogenesis. On the basis of pre-clinical data implicating a role for the IL-1 family—which consists of IL-1α and IL-1β (83, 84)—in neurotoxicity, the IL-1R antagonist anakinra has been investigated for its efficacy and the appropriate timing for administration.
Another strategy is to equip T cells with CRS-preventing machinery. Pharmacologic or genetic inhibition of GM-CSF produced by CAR-T cells has been shown to reduce the risk of CRS and neurotoxicity in a murine CRS model (87). Interestingly, blockade of GM-CSF may also enhance CAR-T cell proliferation. Beneficial effects of GM-CSF blockade were also demonstrated in a different study (88). Chen et al. (89) showed that TNF-α was one the main mediators of endothelial activation, suggesting TNF knockout in CAR-T cells as a potential strategy to alleviate CRS. CAR-T cell-derived IFN-γ is also associated with the activation of macrophages (90). Knockout of IFNG in CAR-T cells mitigated macrophage activation without impairing the antitumor efficacy of CAR-T cells against hematologic malignancies. In fact, recent reports show that the administration of the anti-IFN-γ monoclonal antibody emapalumab was effective to alleviate the symptoms of tocilizumab-refractory CRS (91). These recent findings indicate that the development of CRS is triggered by multiple cytokines and cannot be ascribed to IL-6 alone (Fig. 3).
CAR-NK cells: an alternative option to CAR-T cells
NK cells are innate immune cells with potent cytotoxic activity. Similar to T cells, NK cells can also acquire antigen specificity when engineered with a CAR (92). Recent clinical trials showed that adoptive transfer of CAR-NK cells derived from cord blood cells induced potent therapeutic efficacy against patients with non-Hodgkin’s lymphoma or chronic lymphocytic leukemia: 8 of the 11 treated patients accomplished complete remission and most of them maintained remission beyond 1 year (93). Although NK cells are considered to be short lived compared with T cells, CAR-NK cells were detected in the peripheral blood at least 1 year after infusion. As clearly shown in pre-clinical studies (94), ectopic expression of IL-15 significantly augmented the expansion and persistence of CAR-NK cells.
Contrary to the traditional notion, multiple mouse studies showed that NK cells can induce recall responses to previously encountered antigen (95, 96). These memory-like features were also documented in human NK cells. Priming of NK cells with a combination of IL-12, IL-15, and IL-18 enabled their efficient expansion and potent IFN-γ secretion functions after re-stimulation (97, 98). Phenotypically, they were dim-positive for CD56 and showed increased expression levels of CD94, NKG2A, NKp46, and CD69. NK cells that expanded in the presence of these cytokines exhibited a more durable effector response in vivo compared with those cultured with IL-15 alone (99). In addition to the above cytokines, several other common γ chain cytokines such as IL-2, IL-7, and IL-21 also contribute to enhancing NK cell functions (100–103). Combinatorial incorporation of these cytokines and their signaling may further support the durable therapeutic response of CAR-NK cells.
One of the potential advantages of using NK cells compared with T cells may be that CAR-NK cells are less likely to induce CRS, probably because of their different cytokine-secretion profiles (93). However, the development of CRS is highly dependent on the disease burden and peak expansion of immune cells, making it difficult to perform a fair comparison between independently designed clinical studies. Since NK cells also produce cytokines that are associated with the development of CRS, including IFN-γ and GM-CSF, enhancing their effector functions may result in an increased occurrence of CRS or other side effects with unique manifestations.
Conclusions
A variety of approaches have been invented to boost the therapeutic efficacy of CAR-T cells. Although further investigations are definitely required, especially for CAR-T cells against solid tumors, efficient expansion of cytokine-armored CAR-T cells inevitably increases the risk of CRS and neurotoxicity (104, 105). The therapeutic efficacy of CAR-T cells may be enhanced without increasing toxicities by improving the potential longevity of CAR-T cells instead of their transient effector functions. CAR-T cells generated from naive or stem-cell-like memory T cells induced less CRS, likely because of less-potent effector functions (106). Moreover, infusion of CAR-T cells derived from naive or stem-cell-like memory T cells resulted in more durable antitumor efficacy than standard CAR-T cells because of their increased longevity. These “memory-type” CAR-T cells can also be generated by genetic modification such as the knockout of PRDM1 (50). Another strategy is to design synthetic molecules with dual functions to augment CAR-T cell effector functions and mitigate a cascade related to systemic inflammation. A chimeric cytokine receptor such as GM-CSFR/IL-18R, which captures the CRS-related cytokine GM-CSF secreted by CAR-T cells and activates IL-18 signaling, may be a promising example (73).
For CAR-NK cells, further studies are required to dissect the phenotypic and functional properties of NK cells with enhanced longevity, which may elucidate a differentiation hierarchy among the memory-like NK-cell population as seen in memory T cells. These findings will further accelerate investigations of optimal combinations of cytokine signaling to enhance the durable antitumor responses of CAR-NK cells.
Conflicts of interest statement.
Y. Kagoya received a commercial research grant from Takara Bio.
Funding
This work was supported by the Japan Agency for Medical Research and Development (AMED) under grant numbers JP22bm0704066, JP22ym0126072, and JP22ama221303 (Y.K.); a Japan Society for the Promotion of Science (JSPS) KAKENHI grant number 23H02779; the Takeda Science Foundation; and Multiple myeloma Research Grant from Myeloma Patients and Families.
References
- 1. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–48. 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–44. 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 2020;396:839–52. 10.1016/S0140-6736(20)31366-0 [DOI] [PubMed] [Google Scholar]
- 4. Munshi NC, Anderson LD Jr, Shah N, et al.. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 2021;384:705–16. [DOI] [PubMed] [Google Scholar]
- 5. Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 2021;398:314–24. 10.1016/S0140-6736(21)00933-8 [DOI] [PubMed] [Google Scholar]
- 6. Schultz LM, Baggott C, Prabhu S, et al. Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: a pediatric real-world chimeric antigen receptor consortium report. J Clin Oncol 2022;40:945–55. 10.1200/JCO.20.03585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Del Bufalo F, De Angelis B, Caruana I, et al. ; Precision Medicine Team–IRCCS Ospedale Pediatrico Bambino Gesù. GD2-CART01 for relapsed or refractory high-risk neuroblastoma. N Engl J Med 2023;388:1284–95. 10.1056/NEJMoa2210859 [DOI] [PubMed] [Google Scholar]
- 8. Adusumilli PS, Zauderer MG, Rivière I, et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov 2021;11:2748–63. 10.1158/2159-8290.CD-21-0407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morris EC, Neelapu SS, Giavridis T, et al. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol 2022;22:85–96. 10.1038/s41577-021-00547-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kershaw MH, Westwood JA, Darcy PK.. Gene-engineered T cells for cancer therapy. Nat Rev Cancer 2013;13:525–41. [DOI] [PubMed] [Google Scholar]
- 11. Takeshita T, Asao H, Ohtani K, et al. Cloning of the gamma chain of the human IL-2 receptor. Science 1992;257:379–82. 10.1126/science.1631559 [DOI] [PubMed] [Google Scholar]
- 12. Noguchi M, Yi H, Rosenblatt HM, et al. Interleukin-2 receptor γ chain mutation results in X-linked severe combined immunodeficiency in humans. Cell 1993;73:147–57. 10.1016/0092-8674(93)90167-o [DOI] [PubMed] [Google Scholar]
- 13. Surh CD, Sprent J.. Homeostasis of naive and memory T cells. Immunity 2008;29:848–62. 10.1016/j.immuni.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 14. Asao H, Okuyama C, Kumaki S, et al. Cutting edge: the common γ-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol 2001;167:1–5. 10.4049/jimmunol.167.1.1 [DOI] [PubMed] [Google Scholar]
- 15. Hand TW, Cui W, Jung YW, et al. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc Natl Acad Sci USA 2010;107:16601–6. 10.1073/pnas.1003457107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Opferman JT, Letai A, Beard C, et al. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 2003;426:671–6. 10.1038/nature02067 [DOI] [PubMed] [Google Scholar]
- 17. Verdeil G, Puthier D, Nguyen C, et al. STAT5-mediated signals sustain a TCR-initiated gene expression program toward differentiation of CD8 T cell effectors. J Immunol 2006;176:4834–42. 10.4049/jimmunol.176.8.4834 [DOI] [PubMed] [Google Scholar]
- 18. Pipkin ME, Sacks JA, Cruz-Guilloty F, et al. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 2010;32:79–90. 10.1016/j.immuni.2009.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cui W, Liu Y, Weinstein JS, et al. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity 2011;35:792–805. 10.1016/j.immuni.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siegel AM, Heimall J, Freeman AF, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity 2011;35:806–18. 10.1016/j.immuni.2011.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun Q, Zhao X, Li R, et al. STAT3 regulates CD8+ T cell differentiation and functions in cancer and acute infection. J Exp Med 2023;220:e20220686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yue C, Shen S, Deng J, et al. STAT3 in CD8+ T cells inhibits their tumor accumulation by downregulating CXCR3/CXCL10 Axis. Cancer Immunol Res 2015;3:864–70. 10.1158/2326-6066.CIR-15-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aftabizadeh M, Li Y-J, Zhao Q, et al. Potent antitumor effects of cell-penetrating peptides targeting STAT3 axis. JCI Insight 2021;6:e136176. 10.1172/jci.insight.136176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andorsky DJ, Timmerman JM.. Interleukin-21: biology and application to cancer therapy. Expert Opin Biol Ther 2008;8:1295–307. 10.1517/14712598.8.9.1295 [DOI] [PubMed] [Google Scholar]
- 25. Rosenberg SA, Sportès C, Ahmadzadeh M, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother 2006;29:313–9. 10.1097/01.cji.0000210386.55951.c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 2006;6:595–601. 10.1038/nri1901 [DOI] [PubMed] [Google Scholar]
- 27. Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 1995;13:688–96. 10.1200/JCO.1995.13.3.688 [DOI] [PubMed] [Google Scholar]
- 28. Conlon KC, Lugli E, Welles HC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol 2015;33:74–82. 10.1200/JCO.2014.57.3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 2008;112:2261–71. 10.1182/blood-2007-12-128843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011;17:4550–7. 10.1158/1078-0432.CCR-11-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Markley JC, Sadelain M.. IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell–mediated rejection of systemic lymphoma in immunodeficient mice. Blood 2010;115:3508–19. 10.1182/blood-2009-09-241398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adachi K, Kano Y, Nagai T, et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol 2018;36:346–51. 10.1038/nbt.4086 [DOI] [PubMed] [Google Scholar]
- 33. Shum T, Omer B, Tashiro H, et al. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov 2017;7:1238–47. 10.1158/2159-8290.CD-17-0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hurton LV, Singh H, Najjar AM, et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc Natl Acad Sci USA 2016;113:E7788–97. 10.1073/pnas.1610544113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kagoya Y, Tanaka S, Guo T, et al. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med 2018;24:352–9. 10.1038/nm.4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin RJ, Nager AR, Park S, et al. Design and validation of inducible TurboCARs with tunable induction and combinatorial cytokine signaling. Cancer Immunol Res 2022;10:1069–83. 10.1158/2326-6066.CIR-21-0253 [DOI] [PubMed] [Google Scholar]
- 37. Zhang Q, Hresko ME, Picton LK, et al. A human orthogonal IL-2 and IL-2Rβ system enhances CAR T cell expansion and antitumor activity in a murine model of leukemia. Sci Transl Med 2021;13:eabg6986. 10.1126/scitranslmed.abg6986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kalbasi A, Siurala M, Su LL, et al. Potentiating adoptive cell therapy using synthetic IL-9 receptors. Nature 2022;607:360–5. 10.1038/s41586-022-04801-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Toumi R, Yuzefpolskiy Y, Vegaraju A, et al. Autocrine and paracrine IL-2 signals collaborate to regulate distinct phases of CD8 T cell memory. Cell Rep 2022;39:110632. 10.1016/j.celrep.2022.110632 [DOI] [PubMed] [Google Scholar]
- 40. Kahan SM, Bakshi RK, Ingram JT, et al. Intrinsic IL-2 production by effector CD8 T cells affects IL-2 signaling and promotes fate decisions, stemness, and protection. Sci Immunol 2022;7:eabl6322. 10.1126/sciimmunol.abl6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med 2011;17:1290–7. 10.1038/nm.2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sallusto F, Lenig D, Förster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999;401:708–12. 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- 43. Newell EW, Sigal N, Bendall SC, et al. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity 2012;36:142–52. 10.1016/j.immuni.2012.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wherry EJ. T cell exhaustion. Nat Immunol 2011;12:492–9. 10.1038/ni.2035 [DOI] [PubMed] [Google Scholar]
- 45. Kagoya Y. Dissecting the heterogeneity of exhausted T cells at the molecular level. Int Immunol 2022;34:547–53. 10.1093/intimm/dxac016 [DOI] [PubMed] [Google Scholar]
- 46. Crompton JG, Narayanan M, Cuddapah S, et al. Lineage relationship of CD8 T cell subsets is revealed by progressive changes in the epigenetic landscape. Cell Mol Immunol 2016;13:502–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pauken KE, Sammons MA, Odorizzi PM, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 2016;354:1160–5. 10.1126/science.aaf2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miller BC, Sen DR, Al Abosy R, et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol 2019;20:326–36. 10.1038/s41590-019-0312-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ito Y, Kagoya Y.. Epigenetic engineering for optimal chimeric antigen receptor T cell therapy. Cancer Sci 2022;113:3664–71. 10.1111/cas.15541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yoshikawa T, Wu Z, Inoue S, et al. Genetic ablation of PRDM1 in antitumor T cells enhances therapeutic efficacy of adoptive immunotherapy. Blood 2022;139:2156–72. 10.1182/blood.2021012714 [DOI] [PubMed] [Google Scholar]
- 51. Martins GA, Cimmino L, Liao J, et al. Blimp-1 directly represses Il2 and the Il2 activator Fos, attenuating T cell proliferation and survival. J Exp Med 2008;205:1959–65. 10.1084/jem.20080526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kallies A, Xin A, Belz GT, et al. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity 2009;31:283–95. 10.1016/j.immuni.2009.06.021 [DOI] [PubMed] [Google Scholar]
- 53. Prinzing B, Zebley CC, Petersen CT, et al. Deleting DNMT3A in CAR T cells prevents exhaustion and enhances antitumor activity. Sci Transl Med 2021;13:eabh0272. 10.1126/scitranslmed.abh0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Khan O, Giles JR, McDonald S, et al. TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature 2019;571:211–8. 10.1038/s41586-019-1325-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen J, López-Moyado IF, Seo H, et al. NR4A transcription factors limit CAR T cell function in solid tumours. Nature 2019;567:530–4. 10.1038/s41586-019-0985-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kong W, Dimitri A, Wang W, et al. BET bromodomain protein inhibition reverses chimeric antigen receptor extinction and reinvigorates exhausted T cells in chronic lymphocytic leukemia. J Clin Invest 2021;131:e145459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jung IY, Narayan V, McDonald S, et al. BLIMP1 and NR4A3 transcription factors reciprocally regulate antitumor CAR T cell stemness and exhaustion. Sci Transl Med 2022;14:eabn7336. 10.1126/scitranslmed.abn7336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hinrichs CS, Spolski R, Paulos CM, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood 2008;111:5326–33. 10.1182/blood-2007-09-113050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cieri N, Camisa B, Cocchiarella F, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 2013;121:573–84. 10.1182/blood-2012-05-431718 [DOI] [PubMed] [Google Scholar]
- 60. Batra SA, Rathi P, Guo L, et al. Glypican-3-specific CAR T cells coexpressing IL15 and IL21 have superior expansion and antitumor activity against hepatocellular carcinoma. Cancer Immunol Res 2020;8:309–20. 10.1158/2326-6066.CIR-19-0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Eyquem J, Mansilla-Soto J, Giavridis T, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 2017;543:113–7. 10.1038/nature21405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hamada M, Nishio N, Okuno Y, et al. Integration mapping of piggyBac-mediated CD19 chimeric antigen receptor T cells analyzed by novel tagmentation-assisted PCR. EBioMedicine 2018;34:18–26. 10.1016/j.ebiom.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Curtsinger JM, Lins DC, Mescher MF.. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med 2003;197:1141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cao X, Leonard K, Collins LI, et al. Interleukin 12 stimulates IFN-γ-mediated inhibition of tumor-induced regulatory T-cell proliferation and enhances tumor clearance. Cancer Res 2009;69:8700–9. 10.1158/0008-5472.CAN-09-1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kerkar SP, Goldszmid RS, Muranski P, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest 2011;121:4746–57. 10.1172/JCI58814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pegram HJ, Lee JC, Hayman EG, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 2012;119:4133–41. 10.1182/blood-2011-12-400044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yeku OO, Purdon TJ, Koneru M, et al. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci Rep 2017;7:10541. 10.1038/s41598-017-10940-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jones DS, Nardozzi JD, Sackton KL, et al. Cell surface-tethered IL-12 repolarizes the tumor immune microenvironment to enhance the efficacy of adoptive T cell therapy. Sci Adv 2022;8:eabi8075. 10.1126/sciadv.abi8075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hombach A, Barden M, Hannappel L, et al. IL12 integrated into the CAR exodomain converts CD8+ T cells to poly-functional NK-like cells with superior killing of antigen-loss tumors. Mol Ther 2022;30:593–605. 10.1016/j.ymthe.2021.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schindler H, Lutz MB, Röllinghoff M, et al. The production of IFN-gamma by IL-12/IL-18-activated macrophages requires STAT4 signaling and is inhibited by IL-4. J Immunol 2001;166:3075–82. 10.4049/jimmunol.166.5.3075 [DOI] [PubMed] [Google Scholar]
- 71. Avanzi MP, Yeku O, Li X, et al. Engineered tumor-targeted T cells mediate enhanced anti-tumor efficacy both directly and through activation of the endogenous immune system. Cell Rep 2018;23:2130–41. 10.1016/j.celrep.2018.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hu B, Ren J, Luo Y, et al. Augmentation of antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Rep 2017;20:3025–33. 10.1016/j.celrep.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lange S, Sand LGL, Bell M, et al. A chimeric GM-CSF/IL18 receptor to sustain CAR T-cell function. Cancer Discov 2021;11:1661–71. 10.1158/2159-8290.CD-20-0896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jin L, Tao H, Karachi A, et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat Commun 2019;10:4016. 10.1038/s41467-019-11869-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guo Y, Xie Y-Q, Gao M, et al. Metabolic reprogramming of terminally exhausted CD8+ T cells by IL-10 enhances anti-tumor immunity. Nat Immunol 2021;22:746–56. 10.1038/s41590-021-00940-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ma X, Shou P, Smith C, et al. Interleukin-23 engineering improves CAR T cell function in solid tumors. Nat Biotechnol 2020;38:448–59. 10.1038/s41587-019-0398-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Brog RA, Ferry SL, Schiebout CT, et al. Superkine IL-2 and IL-33 armored CAR T cells reshape the tumor microenvironment and reduce growth of multiple solid tumors. Cancer Immunol Res 2022;10:962–77. 10.1158/2326-6066.CIR-21-0536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li X, Daniyan AF, Lopez AV, et al. Cytokine IL-36γ improves CAR T-cell functionality and induces endogenous antitumor response. Leukemia 2021;35:506–21. 10.1038/s41375-020-0874-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hamilton JAG, Lee MY, Hunter R, et al. Interleukin-37 improves T-cell-mediated immunity and chimeric antigen receptor T-cell therapy in aged backgrounds. Aging Cell 2021;20:e13309. 10.1111/acel.13309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001;19:683–765. 10.1146/annurev.immunol.19.1.683 [DOI] [PubMed] [Google Scholar]
- 81. MacNeil IA, Suda T, Moore KW, et al. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol 1990;145:4167–73. [PubMed] [Google Scholar]
- 82. Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019;25:625–38. 10.1016/j.bbmt.2018.12.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Giavridis T, van der Stegen SJC, Eyquem J, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 2018;24:731–8. 10.1038/s41591-018-0041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med 2018;24:739–48. 10.1038/s41591-018-0036-4 [DOI] [PubMed] [Google Scholar]
- 85. Hamilton JA. GM-CSF in inflammation. J Exp Med 2020;217:e20190945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu Y, Fang Y, Chen X, et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci Immunol 2020;5:eaax7969. 10.1126/sciimmunol.aax7969 [DOI] [PubMed] [Google Scholar]
- 87. Sterner RM, Sakemura R, Cox MJ, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood 2019;133:697–709. 10.1182/blood-2018-10-881722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cox MJ, Manriquez Roman C, Tapper EE, et al. GM-CSF disruption in CART cells modulates T cell activation and enhances CART cell anti-tumor activity. Leukemia 2022;36:1635–45. 10.1038/s41375-022-01572-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chen Y, Li R, Shang S, et al. Therapeutic potential of TNFα and IL1β blockade for CRS/ICANS in CAR-T therapy via ameliorating endothelial activation. Front Immunol 2021;12:623610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bailey SR, Vatsa S, Larson RC, et al. Blockade or deletion of IFNγ reduces macrophage activation without compromising CAR T-cell function in hematologic malignancies. Blood Cancer Discov 2022;3:136–53. 10.1158/2643-3230.BCD-21-0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. McNerney KO, DiNofia AM, Teachey DT, et al. Potential role of IFNγ inhibition in refractory cytokine release syndrome associated with CAR T-cell therapy. Blood Cancer Discov 2022;3:90–4. 10.1158/2643-3230.BCD-21-0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Xie G, Dong H, Liang Y, et al. CAR-NK cells: a promising cellular immunotherapy for cancer. eBioMedicine 2020;59:102975. 10.1016/j.ebiom.2020.102975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liu E, Marin D, Banerjee P, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med 2020;382:545–53. 10.1056/NEJMoa1910607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Liu E, Tong Y, Dotti G, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2018;32:520–31. 10.1038/leu.2017.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sun JC, Beilke JN, Lanier LL.. Adaptive immune features of natural killer cells. Nature 2009;457:557–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. O’Leary JG, Goodarzi M, Drayton DL, et al. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol 2006;7:507–16. 10.1038/ni1332 [DOI] [PubMed] [Google Scholar]
- 97. Romee R, Schneider SE, Leong JW, et al. Cytokine activation induces human memory-like NK cells. Blood 2012;120:4751–60. 10.1182/blood-2012-04-419283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ni J, Miller M, Stojanovic A, et al. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med 2012;209:2351–65. 10.1084/jem.20120944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Romee R, Rosario M, Berrien-Elliott MM, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med 2016;8:357–ra123.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Henney CS, Kuribayashi K, Kern DE, et al. Interleukin-2 augments natural killer cell activity. Nature 1981;291:335–8. 10.1038/291335a0 [DOI] [PubMed] [Google Scholar]
- 101. Cella M, Otero K, Colonna M.. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1β reveals intrinsic functional plasticity. Proc Natl Acad Sci USA 2010;107:10961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shemesh A, Pickering H, Roybal KT, et al. Differential IL-12 signaling induces human natural killer cell activating receptor-mediated ligand-specific expansion. J Exp Med 2022;219:e20212434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Parrish-Novak J, Dillon SR, Nelson A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000;408:57–63. 10.1038/35040504 [DOI] [PubMed] [Google Scholar]
- 104. Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 2017;130:2295–306. 10.1182/blood-2017-06-793141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov 2018;8:958–71. 10.1158/2159-8290.CD-17-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Arcangeli S, Bove C, Mezzanotte C, et al. CAR T cell manufacturing from naive/stem memory T lymphocytes enhances antitumor responses while curtailing cytokine release syndrome. J Clin Invest 2022;132:e150807. 10.1172/JCI150807 [DOI] [PMC free article] [PubMed] [Google Scholar]


