Abstract
Rationale
Repeated chemogenetic stimulation is often employed to study circuit function and behavior. Chronic or repeated agonist administration can result in homeostatic changes, but this has not been extensively studied with designer receptors exclusively activated by designer drugs (DREADDs).
Objectives
We sought to evaluate the impact of repeated DREADD activation of dopaminergic (DA) neurons on basal behavior, amphetamine response, and spike firing. We hypothesized that repeated DREADD activation would mimic compensatory effects that we observed with genetic manipulations of DA neurons.
Methods
Excitatory hM3D(Gq) DREADDs were virally expressed in adult TH-Cre and WT mice. In a longitudinal design, clozapine N-oxide (CNO, 1.0 mg/kg) was administered repeatedly. We evaluated basal and CNO- or amphetamine (AMPH)-induced locomotion and stereotypy. DA neuronal activity was assessed using in vivo single-unit recordings.
Results
Acute CNO administration increased locomotion, but basal locomotion decreased after repeated CNO exposure in TH-CrehM3Dq mice relative to littermate controls. Further, after repeated CNO administration, AMPH-induced hyperlocomotion and stereotypy were diminished in TH-CrehM3Dq mice relative to controls. Repeated CNO administration reduced DA neuronal firing in TH-CrehM3Dq mice relative to controls. A two-month CNO washout period rescued the decreases in basal locomotion and AMPH response.
Conclusions
We found that repeated DREADD activation of DA neurons evokes homeostatic changes that should be factored into the interpretation of chronic DREADD applications and their impact on circuit function and behavior. These effects are likely to also be seen in other neuronal systems and underscore the importance of studying neuroadaptive changes with chronic or repeated DREADD activation.
Keywords: Chemogenetics, dopamine, basal ganglia, midbrain, plasticity, amphetamine, locomotion, stereotypic behavior, in vivo electrophysiology, clozapine N-oxide
Introduction
Chemogenetic platforms using designer receptors exclusively activated by designer drugs (DREADDs) (Armbruster et al. 2007) are widely used to evaluate causal links between neuronal activity and behavior. Compared to optogenetics and other conventional pharmacological approaches, DREADD-based platforms can activate or suppress neurons over longer time periods and are minimally invasive, yet specific and repeatable. The most popular DREADD platforms are based on engineered human muscarinic receptors that are rendered insensitive to their natural ligand acetylcholine and respond instead to the pharmacologically inert ligand clozapine N-oxide (CNO). Because of its simplicity and longer-term effects, DREADD-based chemogenetics is frequently used as a method of choice in long-term and longitudinal studies (Burnett and Krashes 2016; Roth 2016; Smith et al. 2016; Sternson and Roth 2014).
As is observed with conventional pharmacological approaches, acute versus chronic DREADD manipulations can result in different, and sometimes even opposite, effects on circuit function and behavior. While acute stimulation or inhibition has more predictable effects, chronic perturbations in neuronal activity may engage compensatory responses. For example, anxiety-like behaviors are seen only with acute but not with chronic stimulation of the dorsal raphe (Urban et al. 2016). Conversely, antidepressant-like effects are observed only with chronic but not with acute stimulation of entorhinal cortex (Yun et al. 2018). As another contrast, acute inhibition of cortical somatostatin interneurons and basal ganglia indirect pathway neurons increases measures of behavioral emotionality and motivation, respectively; whereas chronic inhibition of these neuronal populations leads to behavioral desensitization (Carvalho Poyraz et al. 2016; Soumier and Sibille 2014).
In line with the homeostatic regulations that occur with chronic perturbations of neuronal activity, we previously reported paradoxical decreases in dopaminergic (DA) transmission and amphetamine (AMPH) response in mice with genetically-ablated expression of the glutamate transporter EAAT3 (Zike et al. 2017). Reciprocally, we found that EAAT3 overexpression in DA neurons led to increased DA transmission and AMPH response (Chohan et al. 2022a). Paralleling these observations, chronic DREADD inhibition of DA neurons in postnatal mice paradoxically leads to increases in locomotor activity and stereotypy (Salesse et al. 2020). Conversely, chronic stimulation of DA neurons in an alpha-synuclein-based parkinsonian model produces a decline in motor function (Torre-Muruzabal et al. 2019). Taken together, these findings suggest the recruitment of homeostatic plasticity mechanisms during chronic manipulations of DA neurons.
Given the clinical implications of homeostatic plasticity mechanisms in chronic brain conditions, we sought to examine the impact of repeated DREADD activation of DA neurons on baseline and AMPH-induced behaviors and neuronal activity. Importantly, while psychostimulant-induced neuroadaptations are extensively characterized, to our knowledge, homeostatic adaptations following repeated DREADD activation have not been empirically tested and are assumed on a priori grounds. Based on our EAAT3 findings and prior DREADD studies, we hypothesized that repeated chemogenetic stimulation of DA neurons would evoke homeostatic changes resulting in diminished baseline neuronal activity and decreased AMPH sensitivity. We employed a longitudinal study design to evaluate the evolution of DREADD-related neuroadaptations on baseline and AMPH-induced behaviors. Our findings highlight the importance of considering homeostatic neuroplasticity mechanisms in the interpretation of repeated chemogenetic manipulations and may have ramifications for understanding the neurobiology of chronic brain conditions affecting the DA system.
Methods
Mice
All experimental studies were approved by the New York State Psychiatric Institute Institutional Animal Care and Use Committees (IACUC) in accordance with the NIH’s Guide for the Care and Use of Laboratory Animals (Council 2010). TH-Cre mice (Strain No. 008601, The Jackson Laboratory) (Savitt et al. 2005) were crossed with C57BL/6J wildtype (WT) mice to obtain heterozygous TH-Cre and littermate control WT animals. After ear-tagging and genotyping, alternate animals of each genotype were assigned to experimental groups. Adult, 8-week-old, male and female mice were used for all experiments. In prior work in TH-Cre mice, we did not find sex differences in baseline or AMPH-induced locomotion and stereotypy (Chohan et al. 2020). Data were therefore pooled from both sexes for the primary analysis in the present study. Sexes of mice used in the individual experiments are listed in the ‘Experimental Timeline’ section. All experiments were conducted blind to genotype. Mice were group housed under a 12-hour light/dark cycle in a temperature-controlled environment, with food and water available ad libitum. All experiments were conducted in the light cycle.
Experimental Timeline
Surgeries.
All surgeries took place on postnatal day (P) 60 (termed Day −14 of experiment). Except for the Validation cohort, mice were allowed to recover for 14 days before the commencement of behavioral and electrophysiology experiments.
DREADD Validation.
For Cohort 1/Validation Cohort (Fig. 1; 8 TH-CrehM3D(Gq) (2M/6F) and 10 WThM3D(Gq) (5M/5F)), mice were evaluated for acute 1 mg/kg CNO-induced locomotor response 4 weeks after surgeries. 1 week later, a subset of mice (3 TH-CrehM3D(Gq) (1M/2F) and 3 WThM3D(Gq) (2M/1F)) was evaluated for acute CNO-induced DA neuronal activation.
Fig. 1. Validation of DREADD-based activation of dopaminergic neurons.
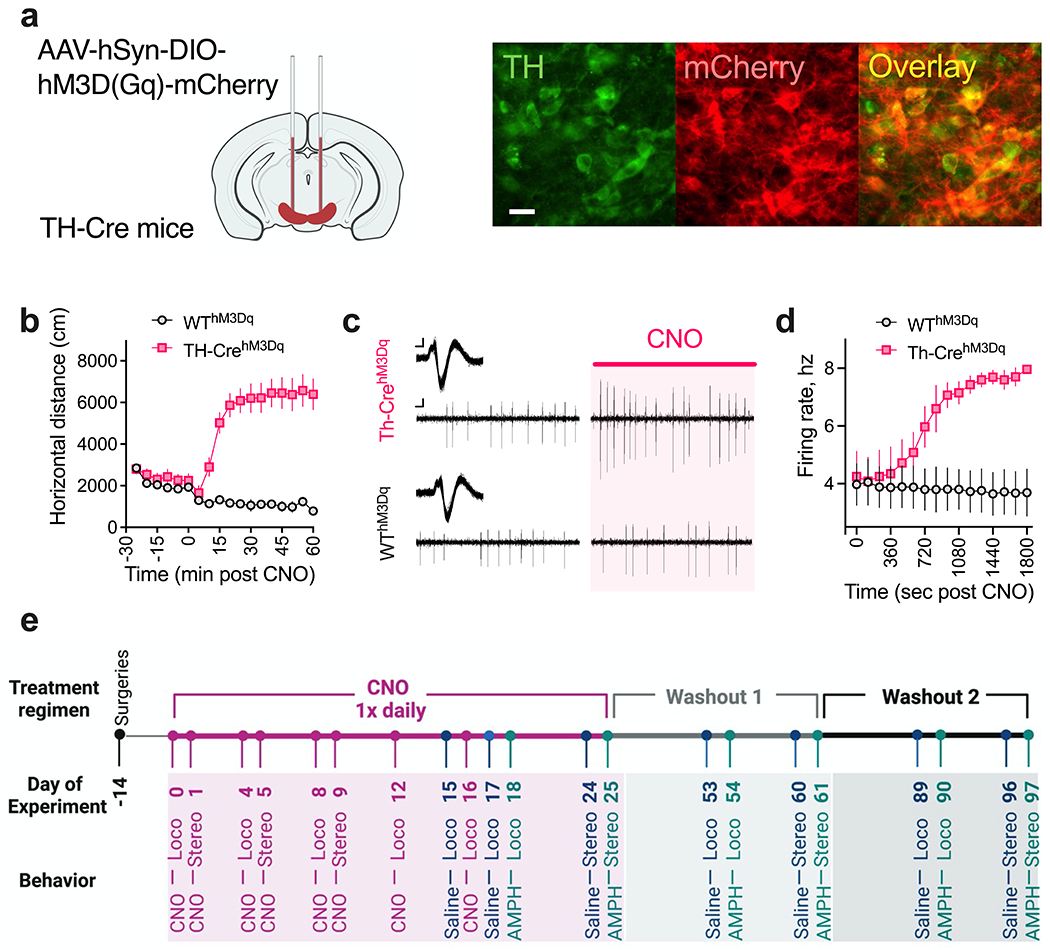
(a) Schematic of AAV-hSyn-DIO-HM3D(Gq)-mCherry injections into the midbrain of TH-Cre and littermate WT control mice. Shown are representative sections from the midbrain region of TH-CrehM3Dq mice. Scale bar: 20 um. (b) Increased open-field locomotion in TH-CrehM3Dq mice following acute 1 mg/kg CNO injection. N = 8 TH-CrehM3Dq and 10 WThM3Dq mice. (c-d) Increased firing rate of DA neurons in TH-CrehM3Dq mice following acute 1 mg/kg CNO administration. (c) Representative extracellularly recorded DA neuronal waveforms (insets) and spike patterns from TH-CrehM3Dq and WThM3Dq mice before (Left) and after ~ 25 mins (Right) of CNO injection. Scale bar: Waveform, 0.05mv/0.5ms; spike patterns, 0.05mv/50ms. (d) Following a baseline recording period of 2 min, mice were administered an IP dose of 1 mg/kg CNO as recording continued for another 30 mins. N = 3 neurons/3 TH-CrehM3Dq and 3 neurons/3 WThM3Dq mice. (e) Experimental timeline for longitudinal behavior. Surgeries were conducted on P60 (Day −14). After a two-week incubation period, mice were administered an IP dose of 1 mg/kg CNO once daily P74-99. CNO was administered during the test sessions on CNO test days (for locomotion: Days 0, 4, 8, 12, 16; for stereotypy: Days 1, 5, 9) and at the conclusion of the session on saline/AMPH test days (for locomotion: Days 15, 17, 18; for stereotypy: Days 24, 25). CNO was administered to mice within their home cages at ~ the same time on non-test days. Mice were tested again for saline and AMPH responses one month (Days 53-54 and 60-61) and two months (Days 89-90 and 96-97) after stopping CNO treatment.
Longitudinal Behavior.
For Cohort 2/Repeated CNO Behavior Cohort (Figs. 2–3; 8 TH-CrehM3D(Gq) (4M/4F) and 10 WThM3D(Gq) (7M/3F)), mice were administered 1 mg/kg CNO 1x daily from P74 (termed Day 0) to P99. During this ‘CNO phase’, mice were evaluated for CNO-induced locomotion every 4 days (i.e., on Days 0, 4, 8, 12, 16), Saline-induced locomotion on Days 15 and Day 17, and AMPH-induced hyperlocomotion on Day 18. CNO was tested repeatedly to examine evolution of CNO-induced response. Saline test days were included to test for any conditioned response to injection experience, and the persistence of locomotor activity patterns from pre-injection period. Mice received CNO injections in their home cages at the conclusion of Saline and AMPH sessions.
Fig. 2. Repeated CNO administration results in diminished baseline and AMPH-induced locomotion in TH-CrehM3D(Gq) mice that is reversed after CNO washout.
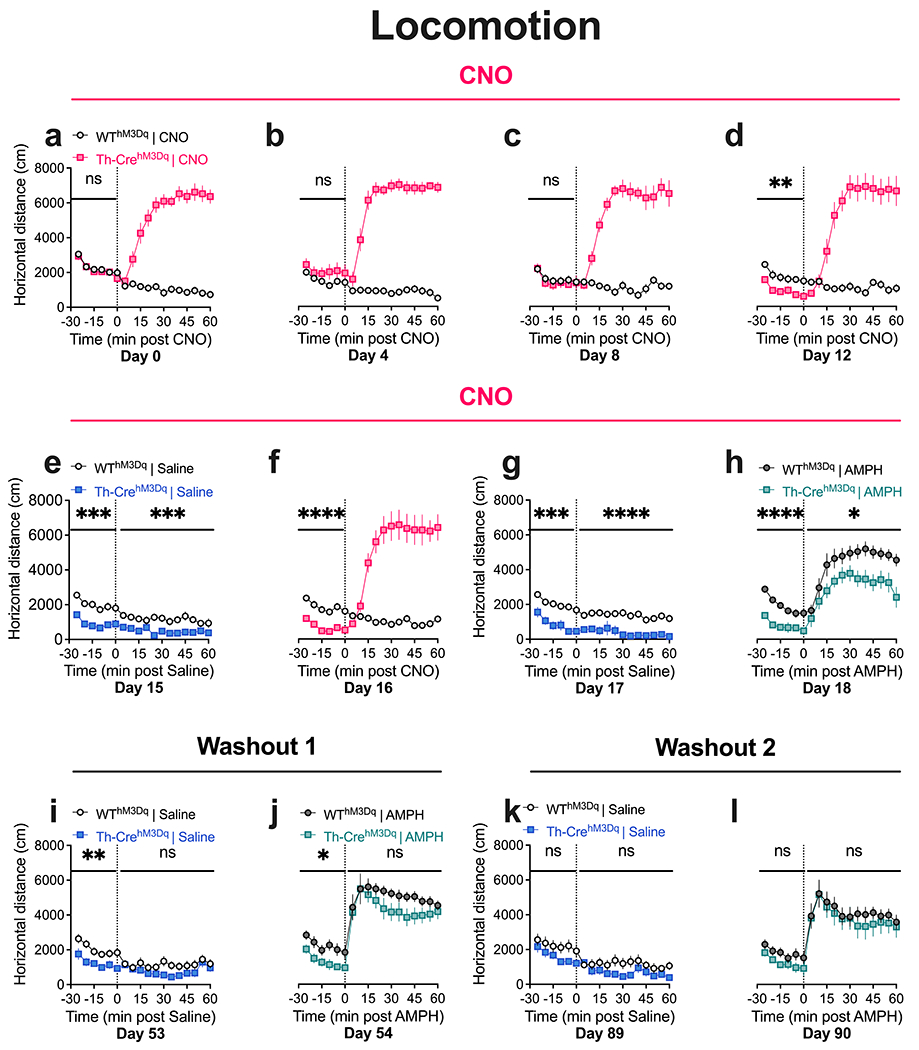
(a) TH-CrehM3Dq mice display increased open-field locomotor activity following acute CNO administration. No between-genotype difference is observed in baseline locomotion on Day 0. (b-d) TH-CrehM3Dq mice show stable increases in locomotion following repeated CNO injections. A decrease in baseline locomotion is observed in TH-CrehM3Dq mice on Day 12. (e-h) The decreases in baseline locomotion in TH-CrehM3Dq mice is again observed on Days 15-18 of the CNO phase. Decreases in locomotor activity is also observed after saline administration (e, g). TH-CrehM3Dq mice show diminished AMPH-induced hyperlocomotion after repeated CNO (h). (i, j) TH-CrehM3Dq mice show diminished baseline locomotion after one month of CNO washout. No between-genotype differences are observed in AMPH-induced hyperlocomotion after one month washout. (k, l) TH-CrehM3Dq mice show comparable baseline and AMPH-induced hyperlocomotor response to WThM3Dq mice after two months of CNO washout. N = 8 TH-CrehM3Dq and 10 WThM3Dq mice. Main effect of genotype, ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05; nsP, not significant. Also see Supplemental Information Fig. SI1 for Area Under Curve (AUC) analysis of this dataset.
Fig. 3. Repeated CNO administration induces decreases in AMPH-induced stereotypy in TH-CrehM3D(Gq) mice that is rescued after CNO washout.
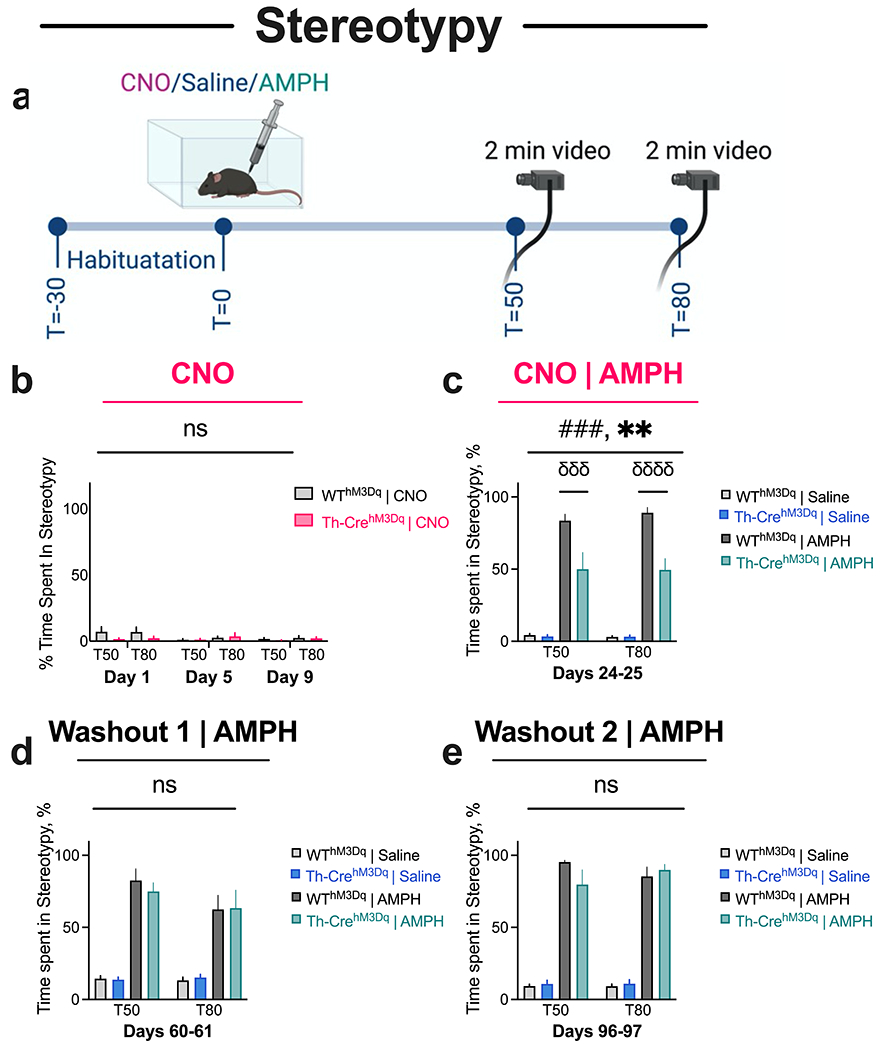
(a) Timeline of Stereotypy experiment. (b) Repeated CNO treatment does not induce stereotypic behavior in TH-CrehM3Dq and WThM3Dq animals. (c) TH-CrehM3Dq mice show diminished AMPH-induced stereotypy compared to WThM3Dq mice in the CNO phase. (d-e) No between-genotype differences are observed in AMPH-induced stereotypy after 1 month (d) and 2 months (e) of CNO washout. N = 8 TH-CrehM3Dq and 10 WThM3Dq mice. Two-way interaction, ###P < 0.001; main effect of genotype, **P < 0.01; post-hoc comparisons, σσσσP < 0.0001, σσσP < 0.001; nsP, not significant.
Mice were additionally evaluated for CNO-induced stereotypic behavior 24 hrs following each of the initial three CNO locomotor test days (i.e., on Day 1, 5, 9). AMPH-induced stereotypic behavior was evaluated 1 week after the AMPH-induced locomotor assay (to allow for AMPH washout) on Days 24-25. For this assay, mice were evaluated for Saline-induced stereotypic response on Day 24 and AMPH-induced response on Day 25. As previously, mice were administered CNO injections in their home cage at the conclusion of the Saline and AMPH sessions.
To evaluate recovery of behavioral response, mice were evaluated for Saline and AMPH-induced locomotion and stereotypic behavior 4 weeks after stopping CNO treatment (i.e., on Days 53-54 and 60-61; termed as ‘Washout 1’). Finally, mice were tested for locomotor and stereotypic behaviors 4 weeks after Washout 1 (i.e., on Days 89-90 and 96-97). Also see Fig. 1e for schematic of this timeline.
In Vivo Single-Unit Recordings.
For Cohort 3/Repeated CNO Electrophysiology Cohort (Fig. 4; 9 TH-CrehM3D(Gq) (4M/5F) and 7 WThM3D(Gq) (4M/3F)), mice were administered 1 mg/kg CNO 1x daily P74-P90 (i.e., Days 0-16). DA neuron recordings were performed on P91/Day17 ~ 24 hrs after the last CNO injection. Note that this cohort was behaviorally naïve. Also see Fig. 4 for schematic of this timeline.
Fig. 4. Repeated CNO administration is associated with decreased spike firing of dopaminergic neurons in TH-CrehM3D(Gq) mice.
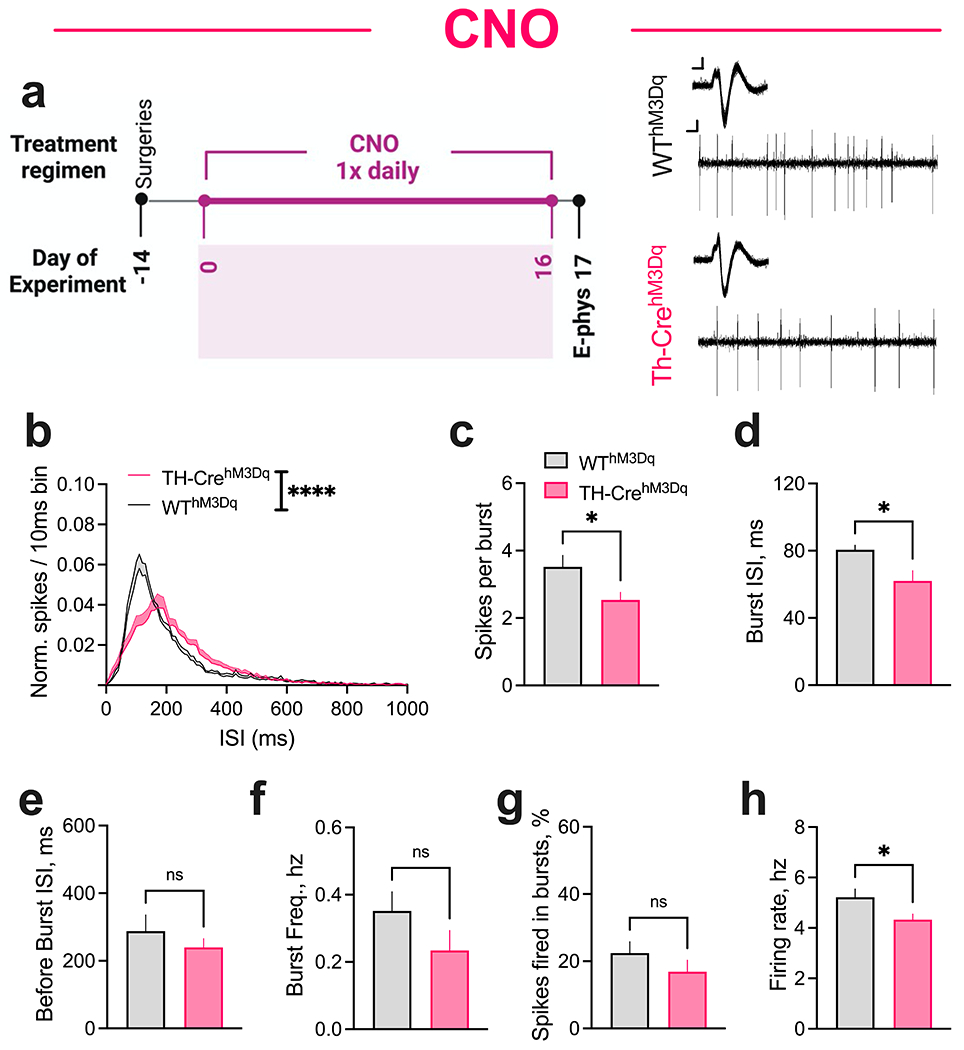
(a) CNO was administered once daily P74-90/Day 0-16. DA neurons were recorded on P91/Day 17 ~24 hours after the final CNO injection. (Left) In vivo recordings experimental timeline. (Right) Representative extracellularly recorded dopaminergic neuron waveforms (inset; overlay of ~100 spikes) and spike patterns from TH-CrehM3Dq and WThM3Dq mice. Scale bar: Waveform, 0.05 mv/0.5 ms; Spike patterns, 0.05 mv/50 ms. (b) Normalized ISI histograms from putative DA neurons showing right-shift of ISI distribution in TH-CrehM3Dq mice relative to WThM3Dq control mice after 16 days of repeated CNO injections. TH-CrehM3Dq mice display significantly decreased (c) spikes per burst and (d) average burst ISI compared to WThM3Dq control mice. No between-genotype differences were observed in (e) average before-burst ISI, (f) burst frequency, and (g) percent spikes fired in bursts. (h) TH-CrehM3Dq mice displayed significantly decreased firing rate compared to WThM3Dq controls. N = 71 neurons/9 TH-CrehM3Dq and 55 neurons/7 WThM3Dq mice. ****P < 0.0001; *P < 0.05; nsP, not significant.
Control cohorts.
For control cohorts Cohort 4/Repeated Vehicle Behavior (Supplemental Information Figs. SI2–3; 7 TH-CrehM3D(Gq) and 7 WThM3D(Gq)), and Cohort 5/Repeated Vehicle Electrophysiology (Supplemental Information Fig. SI4; 6 TH-CrehM3D(Gq) and 5 WThM3D(Gq)), mice were tested with Vehicle using the same timelines as mentioned above. Note that these two control cohorts were independent and behaviorally naïve.
Surgeries
Mice were anesthetized with isoflurane (induction 4-5%, maintenance, 1.5-2% wt/vol) and placed into a stereotactic apparatus (Stoelting). After making a craniotomy, a glass capillary attached to a Nanoject (Drummond) was slowly lowered into the midbrain (AP −3.15, ML +/−0.8, DV −4.0 mm) (Chohan et al. 2022a) and 0.3 ul of undiluted AAV-hSyn-DIO-hM3D(Gq)-mCherry (Addgene, Cat No. 44361-AAV5, titer ≥ 7×10¹² vg/mL) (Krashes et al. 2011) was delivered at a flow rate of 0.1 ul/min. The glass capillary was left in place for 10 min to facilitate diffusion into the brain tissue and to minimize backflow. After 10 min, the capillary was slowly retracted, and the skin was closed with sutures (Henry Schein). Mice were provided two weeks for full recovery and virus expression.
Behavior
Open-field locomotion.
Two weeks after virus expression, mice were placed in an open-field chamber (SmartFrame Open Field System, Kinder Scientific) measuring 40.6 cm long and 40.6 cm wide fitted with 32 infrared photo-beams (16X & 16Y) and connected to the Motor Monitor Software (Kinder Scientific). After an exploratory period of 30 min, mice were removed from the chambers and administered an intraperitoneal (IP) dose of either 1 mg/kg CNO, 0.9% saline, or 3 mg/kg AMPH, depending on the test session (Fig. 1e). Mice were then returned to the chambers, and locomotion was monitored over the next 60 min. Locomotor activity was recorded under bright ambient light conditions.
Stereotypic behavior.
Twenty-four hours after each of the initial three CNO open-field locomotion test days, mice were evaluated for CNO-induced stereotypic behavior. Stereotypic behavior was assessed in shoebox cages (30.5 cm L x 19.7 cm W x 16.5 cm H) that were distinct from the wedge-shaped, Optimouse home cages (34.3 cm L x 29.2 cm W (front) x 15.5 cm H), and the open field chambers (40.6 cm L x 40.6 cm W). Mice were also evaluated for AMPH-induced stereotypic behavior one week after each of the three AMPH open-field tests; for this, mice were administered 0.9% saline (Saline) the first day and 8 mg/kg AMPH the following day. Briefly, following 30 min of acclimation, mice were administered an IP dose of 1 mg/kg CNO, Saline, or 8 mg/kg AMPH, depending on the test session (Figs. 1e, 3a). Mice were then left unperturbed in the shoebox cages for another 90 mins. Mouse behavior was recorded using a video camera (Sony Handycam Flash Memory Camcorder, HDRCX405/B) for 2 min each at 50- and 80-min (termed T50 and T80) post-drug administration. Mice were administered their daily dose of CNO in the home cages at the conclusion of the Saline- and AMPH-induced stereotypic behavior test sessions. Two trained video observers, blind to genotype and treatment, manually recorded the time spent in stationary shuffling, licking and sniffing-like stereotypy, as previously described (Chohan et al. 2020; Chohan et al. 2022a; Zike et al. 2017).
In Vivo Single-Unit Recordings
Anesthetized mice (chloral hydrate, induction 600 mg/kg, maintenance 250 mg/kg, IP) were placed into a stereotactic apparatus (Kopf) and a glass capillary (World Precision Instruments; tip diameter 1 um; impedance 8-10 MΩ) filled with 2M NaCl was slowly lowered into the ventral tegmental area (VTA) (AP −3.15, ML +/−0.8, DV −4.0mm) to detect spontaneously active DA neurons (Chohan et al. 2022a; Gilani et al. 2014). DA neurons were identified by the broad action potential width (>3 msec) and tri-phasic waveform (Grace and Bunney 1984; Ungless et al. 2004). Bursts were defined as per the classical criteria of Grace and Bunney (Grace and Bunney 1984) where an onset of burst is marked by an ISI < 80 msec and its offset is marked by the first spike that is preceded by an ISI > 160 msec. From this starting point, the VTA was sampled in six locations spaced 0.15 mm apart and arranged in a 2 × 3 spaced grid moving in a clockwise direction. Starting locations were counterbalanced across mice and groups. Neuronal activity was amplified and filtered (1000 x gain, 100-10K Hz band pass) and was fed to computer interface with an acquisition and analysis software (NeuroScope V2) for offline analysis. Inter-spike interval (ISI) distributions, burst properties and firing rates for each DA neuron were quantified.
Histology
Mice were perfused with phosphate-buffered saline (PBS), followed by 4% (wt/vol) paraformaldehyde (PFA) (in PBS). The brains were extracted and post-fixed in PFA for 6 hours and then cryoprotected by transferring to ascending concentrations (10, 20, 30%) of sucrose (in PBS) for 24 hours each at 4°C. 40 um thick coronal sections spanning the midbrain region were cut on a cryostat. Sections were collected in PBS. After three 5 min washes in PBS, sections were blocked with 10% normal goat serum and 0.3% Triton X-100 in PBS for 2 hours at room temperature (RT). Sections were then incubated in 10% normal goat serum and 0.3% Triton-X with primary anti-TH (dilution 1:1000, mouse monoclonal, Sigma, T2928) and anti-RFP antibody (dilution 1:500, rabbit polyclonal, Rockland, 600-401-379) in PBS for 20 hours at 4°C. After three 5 min washes in PBS, sections were incubated with Alexa-conjugated secondary antibodies (dilution 1:500, goat anti-mouse, Alexa-Fluor 488, Life Technologies, A-11029; goat anti-rabbit, Alexa-Fluor 594, Abcam, ab150080) for 1 hour at RT. After three more steps of washing in PBS, sections were mounted on a glass slide and cover-slipped. Sections were visualized using a computer assisted morphometry system consisting of a Zeiss Axioplan2 microscope with an internal Z drive, fitted with a Ludl XY motorized stage and 1300 × 1100 pixel digital video camera (manufactured exclusively for Microbrightfield, Inc., VT) and images were captured using Neurolucida software (MBF Biosciences).
Drugs
CNO (Tocris Bioscience) was dissolved in 0.25% DMSO / 0.9% sterile saline (termed ‘Vehicle’). D-amphetamine (AMPH) (Sigma-Aldrich) was dissolved in 0.9% sterile saline (termed ‘Saline’). Mice were administered Saline during the Saline test sessions in the CNO phase. CNO was administrated in the home cage at the conclusion of the Saline/AMPH sessions. Control groups (Supplemental Information Fig. SI2–3) that were repeatedly treated with Vehicle similarly received Saline during the Saline sessions followed by Vehicle in the home cage at the conclusion of the session. All drugs were administered IP at a volume of 10 ml/kg.
Statistical analysis
Data was analyzed with GraphPad Prism (version 9.0.0, GraphPad Software, San Diego, CA, USA). Locomotor activity, expressed as horizontal distance traveled (cm), was collected in 5-min bins and analyzed using 2-way repeated-measures (RM) ANOVA. Area Under the Curve (AUC) was calculated and compared using three-way or two-way RM ANOVA. Stereotypy was analyzed using three-way or two-way RM ANOVA. For in vivo physiology, ISI distributions were compared using lognormal curve fit. All other physiology data was compared using unpaired two-tailed t tests. Multiple comparisons were evaluated by Sidak’s or Tukey’s post hoc tests. All data are reported as the mean ± SEM (standard error of mean). Schematics were created with BioRender.com.
Results
Validation of DREADD-based activation of dopaminergic neurons
We first validated DREADD-based activation of DA neurons. AAVs carrying the excitatory DREADD hM3D(Gq) were bilaterally injected into the midbrain of TH-Cre (TH-CrehM3D(Gq)) and WT (WThM3D(Gq)) mice on postnatal day (P) 60 (Fig. 1a). Four weeks following surgery, mice were acutely administered 1 mg/kg of CNO as locomotor activity was examined. As expected, increased locomotor activity was observed over the hour following CNO (two-way RM ANOVA, time x genotype interaction F (11, 176) = 26.89, P < 0.0001, genotype F (1, 16) = 68.83, P < 0.0001, Fig. 1b). We then used in vivo electrophysiology to functionally validate hM3D(Gq) receptor expression specifically in DA neurons in a sub-set of these animals. In anesthetized mice, acute IP CNO (1 mg/kg) administration increased spike firing frequency (two-way ANOVA, time x genotype interaction F (1919, 7583) = 1.918, P < 0.0001, genotype F (1, 7583) = 6585, P < 0.0001, Fig. 1c–d) for DA neurons in TH-CrehM3D(Gq) mice in comparison to WThM3D(Gq) controls. Having functionally validated hM3D(Gq) receptor activation in DA neurons, we next subjected naïve cohorts of TH-CrehM3D(Gq) and WThM3D(Gq) to a series of behavioral pharmacology experiments to test the impact of repeated DREADD activation (Fig. 1e).
Repeated CNO administration results in diminished baseline and AMPH-induced locomotion in TH-CrehM3D(Gq) mice that is reversed after CNO washout
A two-way RM ANOVA was performed on locomotor data grouped into 5 min bins. We observed robust increases in locomotor activity in response to CNO administration in TH-CrehM3D(Gq) mice on Day 0 (two-way RM ANOVA, time x genotype interaction F (11, 176) = 33.64, P < 0.0001, genotype F (1, 16) = 202.6, P < 0.0001, Fig. 2a). The ability of CNO to increase locomotor activity in TH-CrehM3D(Gq) mice remained strong across CNO test days (Day 4, interaction F = 31.56, P < 0.0001, genotype F = 373.8, P < 0.0001; Day 8, interaction F = 22.90, P < 0.0001, genotype F = 209.9, P < 0.0001; Day 12, interaction F = 40.17, P < 0.0001, genotype F = 61.81, P < 0.0001; Day 16, interaction F = 34.72, P < 0.0001, genotype F = 58.99, P < 0.0001, Figs. 2b–d, f).
Expression of hM3D(Gq) did not impact baseline locomotion, measured over the 30 minute period immediately before IP injection of CNO, in TH-Cre mice on Day 0 (time x genotype interaction F (5, 80) = 1.057, P = 0.3907, genotype F (1, 16) = 0.2731, P = 0.6084, Fig. 2a), supporting previous work showing unchanged baseline locomotion in TH-Cre mice (Chohan et al. 2020). Strikingly, repeated administration of CNO for ~ 2 weeks significantly reduced baseline locomotion in TH-CrehM3D(Gq) mice compared to WThM3D(Gq) control animals (Day 4, interaction F = 0.7618, P = 0.5800, genotype F = 1.969, P = 0.1797; Day 8, interaction F = 0.5849, P = 0.7115, genotype F = 0.1887, P = 0.6698; Day 12, interaction F = 0.3309, P = 0.8929, genotype F = 12.52, P = 0.0027, Figs. 2b–d). Starting on Day 12, a decrease in baseline locomotion in TH-CrehM3D(Gq) mice was observed on every test day for the remainder of the CNO phase (Day 15, interaction F = 0.6144, P = 0.6891, genotype F = 24.95, P = 0.0001; Day 16, interaction F = 0.08453, P = 0.9945, genotype F = 43.93, P < 0.0001; Day 17, interaction F = 1.024, P = 0.4093, genotype F = 25.49, P = 0.0001; Day 18, interaction F = 2.056, P = 0.0797, genotype F = 32.86, P < 0.0001, Figs. 2e–h). Repeated CNO treatment also significantly diminished locomotor hyperactivity in response to AMPH (3 mg/kg) in TH-CrehM3D(Gq) mice compared to WThM3D(Gq) control mice (Day 18, interaction F (11, 176) = 1.491, P = 0.1382, genotype F (1, 16) = 6.459, P = 0.0218, Fig. 2h).
The decrease in baseline locomotion in TH-CrehM3D(Gq) mice was still present following a CNO-free, one month washout period (Day 53, interaction F = 0.7212, P = 0.6094, genotype F = 8.849, P = 0.0089; Day 54, interaction F = 0.8374, P = 0.5271, genotype F = 5.220, P = 0.0363, Figs. 2i–j); however, this effect was not maintained after two months (Day 89, interaction F = 0.8703, P = 0.5052, genotype F = 2.352, P = 0.1460; Day 90, interaction F = 0.9452, P = 0.4570, genotype F = 2.098, P = 0.1680, Figs. 2k–l). No significant genotype difference in AMPH-induced hyperlocomotion was seen following one month or two months of washout (Day 54, interaction F = 1.123, P = 0.3458, genotype F = 1.723, P = 0.2078; Day 90, interaction F = 0.3552, P = 0.9709, genotype F = 0.2449, P = 0.6279, Figs. 2j,l). Area under the curve (AUC) analysis of the pre- and post-drug periods confirmed the time series analyses (Supplemental Information Fig. SI1). Importantly, repeated administration of vehicle did not impact either baseline locomotion or locomotor hyperactivity induced by AMPH in TH-CrehM3D(Gq) animals (Supplemental Information Fig. SI2), indicating that decreases in baseline and AMPH response were not due to transgene effects.
Repeated CNO administration induces decreases in AMPH-induced stereotypy in TH-CrehM3D(Gq) mice that is rescued after CNO washout
We also tracked shuffling, licking, and sniffing-like stereotypies, as previously described (Chohan et al. 2020; Chohan et al. 2022a; Zike et al. 2017), induced by CNO and AMPH by performing stereotypy tests 24 hours following each of the initial three CNO open-field tests and 1 week following each of the three AMPH open-field tests (Figs. 1e, 3a). Repeated administration of CNO over a 10-day period did not produce stereotypic behavior in TH-CrehM3D(Gq) and WThM3D(Gq) mice (Fig. 3b), likely because locomotion was the principal behavior induced at 1 mg/kg dose of CNO (Fig. 2). Three-way ANOVA of stereotypic behavior after repeated CNO showed that there were no main effects of day (F (2, 32) = 2.122, P = 0.1364), genotype (F (1, 16) = 2.156, P = 0.1614), or time (F 1, 16) = 0.9629, P = 0.3411), and no three-way interaction (F (2, 32) = 0.0008, P = 0.9992), day-by-genotype interaction (F (2, 32) = 2.156, P = 0.1323), or time-by-genotype interaction (F (1, 16) = 0.1250, P = 0.7283).
Previous research has shown that stereotypic behavior dominates over locomotion after mice are challenged with a high dose of AMPH (Baldan et al. 2014; Yates et al. 2007). Repeated CNO administration decreased stereotypic behavior produced by administration of 8 mg/kg AMPH in TH-CrehM3D(Gq) mice compared to WThM3D(Gq) control animals (Fig. 3c). There were significant effects of treatment (F (1, 16) = 214.0, P < 0.0001), and genotype (F (1, 16) = 14.57, P = 0.0015), and an interaction between treatment and genotype (F (1, 16) = 16.85, P = 0.0008), based on three-way ANOVA. Sidak’s post hoc comparisons showed that AMPH-treated TH-CrehM3D(Gq) mice displayed lower stereotypy than WThM3D(Gq) controls at both the T50 (P = 0.0001) and T80 (P < 0.0001) time points; whereas there were no differences between the saline-treated TH-CrehM3D(Gq) and WThM3D(Gq) groups (P > 0.99). Importantly, repeated administration of vehicle did not impact AMPH-induced stereotypic behavior in TH-CrehM3D(Gq) animals (Supplemental Information Fig. SI3), indicating that the differences in stereotypic behavior after AMPH administration were mediated by repeated DREADD activation.
The observed decreases in stereotypic behavior in TH-CrehM3D(Gq) mice after repeated CNO administration was not sustained at one month of washout (interaction F = 0.09881, P = 0.7576, genotype F = 0.05807, P = 0.8128, Fig. 3d). Similar results were observed following two months of washout (interaction F = 1.205, P = 0.2909, genotype F = 0.2701, P = 0.6114, Fig. 3e). Finally, three-way ANOVA analysis to determine the effects of phase, treatment and genotype revealed a significant 3-way interaction (F (2, 26) = 4.311, P = 0.0242), phase-by-genotype interaction (F (2, 32) = 4.973, P = 0.0132), phase-by-treatment interaction (F (2, 26) = 5.673, P = 0.0090), and main effects of phase (F (2, 32) = 8.896, P = 0.0008), treatment (F (1, 16) = 434.7, P < 0.0001), and genotype (F (1, 16) = 4.745, P = 0.0447). Tukey’s post hoc comparisons showed that TH-CrehM3D(Gq) mice displayed lower levels of stereotypy in the CNO phase (P = 0.0001) but showed comparable stereotypy to WThM3D(Gq) mice after one month (P > 0.99) and two months (P = 0.99) of washout, indicating that decreases in AMPH-induced stereotypic behavior after repeated CNO were rescued after washout.
Repeated CNO administration is associated with decreased spike firing of dopaminergic neurons in TH-CrehM3D(Gq) mice
We next sought to evaluate whether the altered behavior following repeated CNO administration might be associated with altered spike firing of DA neurons. We used in vivo single-unit recordings to record spontaneous spike firing patterns from the midbrain of anesthetized TH-CrehM3D(Gq) and WThM3D(Gq) mice following 16 days of repeated CNO administration (Fig. 4a), the same regimen that produced diminished behavior in TH-CrehM3D(Gq) mice. DA neurons were identified using the established criteria of long duration (>3 msec), triphasic action potential waveform and tonic irregular firing with intermittent burst patterns (Chohan et al. 2022a; Gilani et al. 2014; Ungless et al. 2004). Bursts were defined using the classic method in which burst onset is marked by two spikes with an ISI ≤ 80 ms and offset is marked by a subsequent ISI ≥ 160 ms (Grace and Bunney 1984; Ungless and Grace 2012).
Strikingly, repeated CNO treatment produced a right-shift in inter-spike interval (ISI) distribution in TH-CrehM3D(Gq) mice relative to WThM3D(Gq) controls (F (1, 1610) = 284.6, P < 0.0001, Fig. 4b). Using a log-normal curve fit of the ISI distribution, the geomean ISI was significantly increased in TH-CrehM3D(Gq) mice compared to WThM3D(Gq) mice (unpaired t test, t = 2.244, P = 0.0416). Repeated CNO treatment had a significant effect on the pattern of activity, reducing the number spikes fired per burst in TH-CrehM3D(Gq) mice (t = 2.498, P = 0.0256, Fig. 4c). Further evaluation of ISIs revealed significantly decreased average burst ISI (t = 2.516, P = 0.0247, Fig. 4d) but unaffected average before-burst ISI (t = 0.9299, P = 0.3682, Fig. 4e) in TH-CrehM3D(Gq) mice. No between-genotype differences were observed in burst frequency (t = 1.395, P = 0.1849, Fig. 4f) or percentage of total spikes fired in bursts (t = 1.110, P = 0.2855, Fig. 4g). Overall, repeated CNO administration decreased firing rate in TH-CrehM3D(Gq) animals (t = 2.339, P = 0.0347, Fig. 4h). Importantly, repeated administration of vehicle for 16 days did not alter ISI distribution or spike firing patterns in TH-CrehM3D(Gq) mice relative to WThM3D(Gq) controls (Supplementary Information Fig. SI4).
Discussion
Using a longitudinal study design, we demonstrate that repeated pharmacogenetic stimulation of DA neurons in the midbrain leads to decreases in basal locomotion as well as AMPH-induced stereotypic behavior. These changes are accompanied by decreases in basal spike firing activity of DA neurons. Specifically, repeated daily DREADD activation induces a right-shift of the inter-spike interval distribution and leads to a reduced number of spikes fired in bursts. Finally, we show that the observed behavioral changes can be rescued after a CNO washout period, indicating that the induced changes are reversible.
There are also some important caveats for our results. First, we were interested in understanding the longitudinal impact of CNO administration and washout on AMPH response, but we cannot exclude the possibility that repeated AMPH administration resulted in sensitization that could contribute to the observed rescue of locomotor and stereotypy response in the washout phase. Future studies could use separate cohorts of mice to evaluate the impact of CNO washout on locomotor and stereotypy response in naïve mice (i.e., not previously exposed to AMPH). Further, because same open field chambers were used for each locomotor session, we cannot fully rule out the role of contextual learning processes in our observed phenotypes. However, while we might expect some level of associative learning to have occurred, the literature on repeated exposure to DA agonists would predict increased, not decreased, activity during the pre-injection period and a conditioned response to saline injections, based on the well-known role of DA in stimulus-reward learning and previous research showing increases in anticipatory (Ma et al. 2010; Mohawk et al. 2013; Sequeira-Cordero and Brenes 2021; Shibata et al. 1994; Shibata et al. 1995; Weiss et al. 1992; Zweifel et al. 2008), and conditioned activity (Alam 1981; Anagnostaras and Robinson 1996; Bevins and Peterson 2004; Borgkvist et al. 2008; Brabant et al. 2003; Browman et al. 1998; Damianopoulos and Carey 1992; Drew and Glick 1988; Gold and Koob 1989; Gold et al. 1988; Hall et al. 2008; Itzhak 1997; Mazurski and Beninger 1987; Michel et al. 2003; Rauhut and Bialecki 2011; Schiff 1982; Tilson and Rech 1973; Tirelli and Terry 1998; Vezina and Leyton 2009), and DA neuron firing (Henry et al. 1989; Lodge and Grace 2008; White and Wang 1984). Importantly, our finding of reduced DA neuron firing in mice that were previously administered CNO in their home cages, suggests that homeostatic adaptions in DA neurons contribute, at least in part, to our observed phenotypes. We also did not evaluate DA neuron firing after CNO washout; although the rescue of basal locomotion would suggest that DA transmission was likely rescued. Finally, we used injection of hM3D(Gq) virus in wildtype animals as a control for the effects of viral injection without Cre-mediated recombination and DREADD expression; however, we did not use a control virus in TH-Cre mice to control for the effects of genotype. This concern is ameliorated somewhat because we focused on change from baseline within animals using a longitudinal design, rather than focusing on cross-sectional comparisons within genotype. Additionally, our previous work showed equivalent baseline and AMPH-induced locomotion in TH-Cre mice compared to wildtype C57BL/6 controls (Chohan et al. 2020).
It is noteworthy that repeated DREADD activation of DA neurons produced changes in behavior and neuronal activity that are in many respects opposite to the changes that are produced with repeated exposure to drugs of abuse. For example, we did not observe sensitization (Robinson and Berridge 1993; Steketee and Kalivas 2011; Stewart and Badiani 1993; Vanderschuren and Kalivas 2000; Vezina 2004; Vezina and Leyton 2009), a well-known property of amphetamine (Pierce and Kalivas 1997; Robinson and Becker 1986; Robinson et al. 1982; Segal and Mandell 1974; Vanderschuren et al. 1999) and cocaine (Guan et al. 1985; Jackson and Nutt 1993; Kalivas and Duffy 1990; Shuster et al. 1977), and which is thought to be mediated, at least in part, by drug-induced adaptations in DA neurons (Carlezon and Nestler 2002; Robinson and Berridge 1993; Wolf 1998). Furthermore, baseline locomotion decreased after repeated DREADD activation, which contrasts with the increased locomotion that is found with repeated exposure to drugs of abuse (Ma et al. 2010; Mohawk et al. 2013; Sequeira-Cordero and Brenes 2021; Shibata et al. 1994; Shibata et al. 1995; Weiss et al. 1992; Zweifel et al. 2008). There was also no conditioned response induced by saline after repeated CNO, which contrasts with the conditioned hyperactivity that is induced with repeated administration of DA enhancer drugs (Alam 1981; Anagnostaras and Robinson 1996; Bevins and Peterson 2004; Borgkvist et al. 2008; Everitt and Wolf 2002; Gold et al. 1988; Hall et al. 2008; Itzhak 1997; Mazurski and Beninger 1987; Michel et al. 2003; Rauhut and Bialecki 2011; Schiff 1982; Tilson and Rech 1973). Repeated CNO did not augment the effects of acute amphetamine, and instead diminished it, ruling out cross-sensitization, which we had also expected to see based upon previous DA agonist experiments (Bonate et al. 1997; Ferrario and Robinson 2007; Holly et al. 2012; Itzhak and Martin 1999; Liu et al. 2007; Schenk et al. 1991; Yang et al. 2003). Importantly, repeated exposure to the DREADD agonist led to decreased DA neuron firing, which contrasts with the increased neuronal activity that is observed with repeated administration of drugs of abuse (Ackerman and White 1990; Borgland et al. 2004; Clark and Overton 1998; Henry et al. 1989; Kalivas and Duffy 1993; Lodge and Grace 2008; Ungless et al. 2001; White and Wang 1984; Zhang et al. 1997). Notably, neuroadaptations underlying DA agonist-induced sensitization response are driven by separate and to some extent independent pre- and postsynaptic mechanisms. Recent work has implicated non-DA neurons in the midbrain and postsynaptic adaptations in striatal medium spiny neurons in the mediation of sensitization response that is associated with repeated exposure to amphetamine and cocaine (Beutler et al. 2011; Fourgeaud et al. 2004; Heusner and Palmiter 2005; Lee et al. 2006; Robinson and Kolb 1997). It thus is plausible that the selective activation of DA neurons with DREADDs leads to neuroadaptations that differ from the homeostatic adaptations that are produced by drugs of abuse.
With regard to our primary findings, the decreased basal locomotion in TH-CrehM3D(Gq) mice is surprising because the acute response to CNO did not change over the course of the experiment, which suggests that motor capacity is intact in these animals. Our data are, however, consistent with recent studies that have reported paradoxical increases in basal locomotion following repeated DREADD inhibition (using twice-daily CNO injections) of DA neurons from P14 to P30 (Salesse et al. 2020), as well as aggravation of diminished motor function following repeated DREADD excitation of the substantia nigra (using once-daily CNO injections over 4 weeks) in an alpha-synuclein-based parkinsonian model (Torre-Muruzabal et al. 2019). These independent reports suggest recruitment of homeostatic mechanisms to offset chronic DREADD-induced changes in DA transmission. Our in vivo single-unit recording data showing decreased DA neuron firing suggest that presynaptic changes mediate at least some of the change in baseline locomotion, but other pre- or post-synaptic mechanisms could also be involved. Future studies examining the impact on presynaptic transmission, DA content, release, and post-synaptic DA receptor function will be important for revealing the molecular mechanisms underlying decreased basal locomotion and spike firing.
While a significant main effect of genotype on AMPH-induced hyperlocomotion was found via two-way RM ANOVA of time-series data in mice that were repeatedly treated with CNO, a lack of treatment-by-genotype interaction via AUC analysis indicates that this was likely driven by decreased basal locomotion. On the contrary, the paradoxical decreases in AMPH-induced stereotypic behavior in TH-CrehM3D(Gq) mice were robust, as evidenced by a significant treatment-by-genotype interaction via three-way ANOVA and full rescue of the diminished behavior after CNO washout, indicating reduced AMPH sensitivity in TH-CrehM3D(Gq) mice. The paradoxical decreases in AMPH-induced behavior in TH-CrehM3D(Gq) mice that were repeatedly treated with CNO parallels what we observed in EAAT3 knockdown and overexpressing mice previously, with a loss or gain of EAAT3 paradoxically leading to decreased or increased AMPH response (Chohan et al. 2022a; Chohan et al. 2022b; Zike et al. 2017), respectively, likely due to homeostatic changes in glutamate transmission after chronic alterations in glutamate exposure. Intriguingly, homeostatic regulations have been posited to underlie the decreased excitatory postsynaptic transcripts in obsessive-compulsive disorder (OCD) (Piantadosi et al. 2019), potentially convergent with recent data pointing to DA’s role in gating of OCD-like grooming behavior (Xue et al. 2022). While we did not evaluate grooming behavior in our mice, our AMPH data are consistent with the suppression of grooming behavior that follows acute optogenetic inhibition of DA neurons (Xue et al. 2022).
Most stimulant drugs increase DA firing and release when administered acutely but lead to blunted DA transmission after repeated exposure (Bloomfield et al. 2016; Martinez et al. 2005; Martinez et al. 2012; Morikawa and Morrisett 2010; Nestler 2005; Subramaniyan and Dani 2015; Wang et al. 2012). Some studies have posited decreased vesicular monoamine transporter binding and structural plasticity as possible mechanisms (Gilman et al. 1998; Little et al. 2003; Narendran et al. 2012), with the latter also induced by chronic chemogenetic manipulations of DA neurons (Bian et al. 2022). Based on our chemogenetic findings, decreased DA neuron spike firing could be an additional mechanism that contributes to the blunted DA transmission that is observed following repeated stimulant use (Ashok et al. 2017; Martinez et al. 2007; Volkow et al. 2014; Volkow et al. 1997; Volkow et al. 2007).
It is intriguing that chemogenetic modulation of DA neurons would impact response to AMPH, which is conventionally believed to increase DA release independent of neuronal firing via reverse transport mechanisms (Freyberg et al. 2016; Jones et al. 1998; Kahlig et al. 2005; Sulzer et al. 1995). Recent in vivo studies point to an additional action potential-dependent mechanism of AMPH effects on behavior (Avelar et al. 2013; Covey et al. 2016; Daberkow et al. 2013; Ramsson et al. 2011). For instance, AMPH increases frequency, amplitude, and duration of phasic DA release (Daberkow et al. 2013) and can improve learning and goal-directed behaviors (Taylor and Jentsch 2001; Wyvell and Berridge 2000; Zhang et al. 2003) that are known to critically rely on phasic DA signaling (Tsai et al. 2009; Zweifel et al. 2009). Further, acute DREADD inhibition or activation of DA neurons decreases or increases AMPH-induced hyperlocomotor response (Runegaard et al. 2019), respectively, revealing a role for DA neuron firing activity in governing AMPH’s motor effects. Importantly, in addition to modulating glutamatergic transmission via EAAT3 (Li et al. 2017; Underhill et al. 2014), AMPH has been shown to increase firing activity of DA neurons via multiple disinhibitory mechanisms, including cannabinoid receptor 1 signaling on GABA neurons (Covey et al. 2016), dopamine transporter-mediated increases in excitability (Ingram et al. 2002), and modulation of noradrenergic signaling (Shi et al. 2000). It is thus plausible that chronic DREADD-induced perturbations in firing result in the recruitment of similar homeostatic mechanisms that are also impacted by exposure to AMPH.
In contrast to our use of repeated stimulation of hM3D(Gq), a recent study using the inhibitory DREADD hM4D(Gi), reported no impact of chronic inhibition (using twice-daily injections of CNO over 16 days) of DA neurons on basal motor activity in adult mice (Salesse et al. 2020). Apart from the different methodologies used, including the use of different Cre lines and dosing regimen, hM3D(Gq) and hM4D(Gi) trigger different intracellular cascades to induce depolarization or hyperpolarization and would thus engage disparate plasticity mechanisms to shape behavior. It would be interesting to evaluate whether repeated DREADD inhibition of DA neurons impacts AMPH-induced hyperlocomotion and stereotypy, as we observed with DREADD stimulation and EAAT3 manipulation. Based on our findings, repeated chemogenetic inhibition of DA neurons might be expected to heighten response to AMPH. This idea would also be consistent with increased AMPH response that is observed following exposure to chronic stress, which also induces blunted DA firing (Antelman et al. 1980; Gomes et al. 2020; Herman et al. 1984; Moore et al. 2001).
Chronic activation of neurons typically induces plastic events and compensatory responses. In the current study, repeated DREADD activation resulted in diminished baseline firing activity of DA neurons. This is not explained by changes in sensitivity or signaling of the DREADD receptors themselves because the diminished baseline firing is observed prior to the daily administration of CNO, and the locomotor responses to each CNO dose are similar across repeated administrations. Other homeostatic mechanisms must be responsible. As one possibility, activation of muscarinic receptors can trigger release of intracellular calcium via activation of the phospholipase C cascade. A rise in NMDA receptor-independent intracellular calcium has been found to be both necessary and sufficient to trigger long-term depression (LTD) in DA neurons (Jones et al. 2000). It is thus plausible that repeated activation of DREADDs results in LTD in DA neurons with the consequences of reduced baseline neuronal firing. Both basal and AMPH responses were restored after a CNO washout period, indicating that the mechanisms responsible for homeostatic adjustment to repeated CNO administration are temporary and dependent upon ongoing DREADD stimulation. Future studies are needed to examine the mechanisms that underlie decreased DA neuron firing following repeated DREADD stimulation, as well as restoration after a period of washout.
Supplementary Material
Acknowledgements
This work was funded by NIH Grant MH114296.
Conflict of interest
JV has served on advisory boards for Roche, Novartis, and SynapDx; has received research funding from NIH, Simon’s Foundation, Roche, Novartis, SynapDx, Forest, Janssen, Yamo, MapLight, Seaside Therapeutics, and Acadia Pharmaceuticals; and has received editorial stipends from Wiley and Springer. The remaining authors declare no competing interests.
References
- Ackerman JM, White FJ (1990) A10 somatodendritic dopamine autoreceptor sensitivity following withdrawal from repeated cocaine treatment Neurosci Lett 117:181–187 doi: 10.1016/0304-3940(90)90141-u [DOI] [PubMed] [Google Scholar]
- Alam MR (1981) Enhancement of motor-accelerating effect induced by repeated administration of methamphetamine in mice: involvement of environmental factors Jpn J Pharmacol 31:897–904 doi: 10.1254/jjp.31.897 [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Robinson TE (1996) Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning Behav Neurosci 110:1397–1414 doi: 10.1037//0735-7044.110.6.1397 [DOI] [PubMed] [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D (1980) Interchangeability of stress and amphetamine in sensitization Science 207:329–331 doi: 10.1126/science.7188649 [DOI] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL (2007) Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand Proc Natl Acad Sci U S A 104:5163–5168 doi: 10.1073/pnas.0700293104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok AH, Mizuno Y, Volkow ND, Howes OD (2017) Association of Stimulant Use With Dopaminergic Alterations in Users of Cocaine, Amphetamine, or Methamphetamine: A Systematic Review and Meta-analysis JAMA Psychiatry 74:511–519 doi: 10.1001/jamapsychiatry.2017.0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avelar AJ, Juliano SA, Garris PA (2013) Amphetamine augments vesicular dopamine release in the dorsal and ventral striatum through different mechanisms J Neurochem 125:373–385 doi: 10.1111/jnc.12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldan LC et al. (2014) Histidine decarboxylase deficiency causes tourette syndrome: parallel findings in humans and mice Neuron 81:77–90 doi: 10.1016/j.neuron.2013.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler LR, Wanat MJ, Quintana A, Sanz E, Bamford NS, Zweifel LS, Palmiter RD (2011) Balanced NMDA receptor activity in dopamine D1 receptor (D1R)- and D2R-expressing medium spiny neurons is required for amphetamine sensitization Proc Natl Acad Sci U S A 108:4206–4211 doi: 10.1073/pnas.1101424108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Peterson JL (2004) Individual differences in rats’ reactivity to novelty and the unconditioned and conditioned locomotor effects of methamphetamine Pharmacol Biochem Behav 79:65–74 doi: 10.1016/j.pbb.2004.06.002 [DOI] [PubMed] [Google Scholar]
- Bian WJ, Brewer CL, Kauer JA, de Lecea L (2022) Adolescent sleep shapes social novelty preference in mice Nat Neurosci 25:912–923 doi: 10.1038/s41593-022-01076-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield MA, Ashok AH, Volkow ND, Howes OD (2016) The effects of Delta(9)-tetrahydrocannabinol on the dopamine system Nature 539:369–377 doi: 10.1038/nature20153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonate PL, Swann A, Silverman PB (1997) Context-dependent cross-sensitization between cocaine and amphetamine Life Sci 60:PL1–7 doi: 10.1016/s0024-3205(96)00591-7 [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Valjent E, Santini E, Herve D, Girault JA, Fisone G (2008) Delayed, context- and dopamine D1 receptor-dependent activation of ERK in morphine-sensitized mice Neuropharmacology 55:230–237 doi: 10.1016/j.neuropharm.2008.05.028 [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A (2004) Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats J Neurosci 24:7482–7490 doi: 10.1523/JNEUROSCI.1312-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabant C, Tambour S, Tirelli E (2003) Quasi-asymptotic development of conditioned hyperactivity induced by intermittent injections of cocaine in C57BL/6J mice Pharmacol Biochem Behav 75:273–280 doi: 10.1016/s0091-3057(03)00083-2 [DOI] [PubMed] [Google Scholar]
- Browman KE, Badiani A, Robinson TE (1998) Modulatory effect of environmental stimuli on the susceptibility to amphetamine sensitization: a dose-effect study in rats J Pharmacol Exp Ther 287:1007–1014 [PubMed] [Google Scholar]
- Burnett CJ, Krashes MJ (2016) Resolving Behavioral Output via Chemogenetic Designer Receptors Exclusively Activated by Designer Drugs J Neurosci 36:9268–9282 doi: 10.1523/JNEUROSCI.1333-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA Jr., Nestler EJ (2002) Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? Trends Neurosci 25:610–615 doi: 10.1016/s0166-2236(02)02289-0 [DOI] [PubMed] [Google Scholar]
- Carvalho Poyraz F et al. (2016) Decreasing Striatopallidal Pathway Function Enhances Motivation by Energizing the Initiation of Goal-Directed Action J Neurosci 36:5988–6001 doi: 10.1523/JNEUROSCI.0444-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chohan MO, Esses S, Haft J, Ahmari S, Veenstra-VanderWeele J (2020) Altered baseline and amphetamine-mediated behavioral profiles in dopamine transporter Cre (DAT-Ires-Cre) mice compared to tyrosine hydroxylase Cre (TH-Cre) mice Psychopharmacology (Berl) doi: 10.1007/s00213-020-05635-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chohan MO et al. (2022a) Developmental impact of glutamate transporter overexpression on dopaminergic neuron activity and stereotypic behavior Mol Psychiatry doi: 10.1038/s41380-021-01424-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chohan MO, Yueh H, Fein H, Kopelman JM, Ahmari SE, Veenstra-VanderWeele J (2022b) Intact amphetamine-induced behavioral sensitization in mice with increased or decreased neuronal glutamate transporter SLC1A1/EAAT3 Neurochem Int 160:105418 doi: 10.1016/j.neuint.2022.105418 [DOI] [PubMed] [Google Scholar]
- Clark D, Overton PG (1998) Alterations in excitatory amino acid-mediated regulation of midbrain dopaminergic neurones induced by chronic psychostimulant administration and stress: relevance to behavioural sensitization and drug addiction Addict Biol 3:109–135 doi: 10.1080/13556219872191 [DOI] [PubMed] [Google Scholar]
- Council NR (2010) Guide for the care and use of laboratory animals. National Academies Press, [Google Scholar]
- Covey DP, Bunner KD, Schuweiler DR, Cheer JF, Garris PA (2016) Amphetamine elevates nucleus accumbens dopamine via an action potential-dependent mechanism that is modulated by endocannabinoids Eur J Neurosci 43:1661–1673 doi: 10.1111/ejn.13248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daberkow DP et al. (2013) Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals J Neurosci 33:452–463 doi: 10.1523/JNEUROSCI.2136-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damianopoulos EN, Carey RJ (1992) Conditioning, habituation and behavioral reorganization factors in chronic cocaine effects Behav Brain Res 49:149–157 doi: 10.1016/s0166-4328(05)80159-7 [DOI] [PubMed] [Google Scholar]
- Drew KL, Glick SD (1988) Characterization of the associative nature of sensitization to amphetamine-induced circling behavior and of the environment dependent placebo-like response Psychopharmacology (Berl) 95:482–487 doi: 10.1007/BF00172959 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME (2002) Psychomotor stimulant addiction: a neural systems perspective J Neurosci 22:3312–3320 doi: 10.1523/JNEUROSCI.22-09-03312.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Robinson TE (2007) Amphetamine pretreatment accelerates the subsequent escalation of cocaine self-administration behavior Eur Neuropsychopharmacol 17:352–357 doi: 10.1016/j.euroneuro.2006.08.005 [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ (2004) A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens J Neurosci 24:6939–6945 doi: 10.1523/JNEUROSCI.0671-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyberg Z et al. (2016) Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain Nat Commun 7:10652 doi: 10.1038/ncomms10652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani AI et al. (2014) Interneuron precursor transplants in adult hippocampus reverse psychosis-relevant features in a mouse model of hippocampal disinhibition Proc Natl Acad Sci U S A 111:7450–7455 doi: 10.1073/pnas.1316488111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S et al. (1998) Decreased striatal monoaminergic terminals in severe chronic alcoholism demonstrated with (+)[11C]dihydrotetrabenazine and positron emission tomography Ann Neurol 44:326–333 doi: 10.1002/ana.410440307 [DOI] [PubMed] [Google Scholar]
- Gold LH, Koob GF (1989) MDMA produces stimulant-like conditioned locomotor activity Psychopharmacology (Berl) 99:352–356 doi: 10.1007/BF00445556 [DOI] [PubMed] [Google Scholar]
- Gold LH, Swerdlow NR, Koob GF (1988) The role of mesolimbic dopamine in conditioned locomotion produced by amphetamine Behav Neurosci 102:544–552 doi: 10.1037//0735-7044.102.4.544 [DOI] [PubMed] [Google Scholar]
- Gomes FV, Zhu X, Grace AA (2020) The pathophysiological impact of stress on the dopamine system is dependent on the state of the critical period of vulnerability Mol Psychiatry 25:3278–3291 doi: 10.1038/s41380-019-0514-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS (1984) The control of firing pattern in nigral dopamine neurons: burst firing J Neurosci 4:2877–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan LC, Robinson TE, Becker JB (1985) Sensitization of rotational behavior produced by a single exposure to cocaine Pharmacol Biochem Behav 22:901–903 doi: 10.1016/0091-3057(85)90545-3 [DOI] [PubMed] [Google Scholar]
- Hall DA, Stanis JJ, Marquez Avila H, Gulley JM (2008) A comparison of amphetamine- and methamphetamine-induced locomotor activity in rats: evidence for qualitative differences in behavior Psychopharmacology (Berl) 195:469–478 doi: 10.1007/s00213-007-0923-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry DJ, Greene MA, White FJ (1989) Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: repeated administration J Pharmacol Exp Ther 251:833–839 [PubMed] [Google Scholar]
- Herman JP, Stinus L, Le Moal M (1984) Repeated stress increases locomotor response to amphetamine Psychopharmacology (Berl) 84:431–435 doi: 10.1007/BF00555227 [DOI] [PubMed] [Google Scholar]
- Heusner CL, Palmiter RD (2005) Expression of mutant NMDA receptors in dopamine D1 receptor-containing cells prevents cocaine sensitization and decreases cocaine preference J Neurosci 25:6651–6657 doi: 10.1523/JNEUROSCI.1474-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly EN, Shimamoto A, Debold JF, Miczek KA (2012) Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats Psychopharmacology (Berl) 224:179–188 doi: 10.1007/s00213-012-2846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram SL, Prasad BM, Amara SG (2002) Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons Nat Neurosci 5:971–978 doi: 10.1038/nn920 [DOI] [PubMed] [Google Scholar]
- Itzhak Y (1997) Modulation of cocaine- and methamphetamine-induced behavioral sensitization by inhibition of brain nitric oxide synthase J Pharmacol Exp Ther 282:521–527 [PubMed] [Google Scholar]
- Itzhak Y, Martin JL (1999) Effects of cocaine, nicotine, dizocipline and alcohol on mice locomotor activity: cocaine-alcohol cross-sensitization involves upregulation of striatal dopamine transporter binding sites Brain Res 818:204–211 doi: 10.1016/s0006-8993(98)01260-8 [DOI] [PubMed] [Google Scholar]
- Jackson HC, Nutt DJ (1993) A single preexposure produces sensitization to the locomotor effects of cocaine in mice Pharmacol Biochem Behav 45:733–735 doi: 10.1016/0091-3057(93)90533-y [DOI] [PubMed] [Google Scholar]
- Jones S, Kornblum JL, Kauer JA (2000) Amphetamine blocks long-term synaptic depression in the ventral tegmental area J Neurosci 20:5575–5580 doi: 10.1523/JNEUROSCI.20-15-05575.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG (1998) Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter J Neurosci 18:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A (2005) Amphetamine induces dopamine efflux through a dopamine transporter channel Proc Natl Acad Sci U S A 102:3495–3500 doi: 10.1073/pnas.0407737102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P (1990) Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens Synapse 5:48–58 doi: 10.1002/syn.890050104 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P (1993) Time course of extracellular dopamine and behavioral sensitization to cocaine. II. Dopamine perikarya J Neurosci 13:276–284 doi: 10.1523/JNEUROSCI.13-01-00276.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ et al. (2011) Rapid, reversible activation of AgRP neurons drives feeding behavior in mice J Clin Invest 121:1424–1428 doi: 10.1172/JCI46229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P (2006) Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens Proc Natl Acad Sci U S A 103:3399–3404 doi: 10.1073/pnas.0511244103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MH, Underhill SM, Reed C, Phillips TJ, Amara SG, Ingram SL (2017) Amphetamine and Methamphetamine Increase NMDAR-GluN2B Synaptic Currents in Midbrain Dopamine Neurons Neuropsychopharmacology 42:1539–1547 doi: 10.1038/npp.2016.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little KY, Krolewski DM, Zhang L, Cassin BJ (2003) Loss of striatal vesicular monoamine transporter protein (VMAT2) in human cocaine users Am J Psychiatry 160:47–55 doi: 10.1176/appi.ajp.160.1.47 [DOI] [PubMed] [Google Scholar]
- Liu Y, Morgan D, Roberts DC (2007) Cross-sensitization of the reinforcing effects of cocaine and amphetamine in rats Psychopharmacology (Berl) 195:369–375 doi: 10.1007/s00213-007-0909-6 [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA (2008) Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization J Neurosci 28:7876–7882 doi: 10.1523/JNEUROSCI.1582-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle CL (2010) Repeated intravenous cocaine experience: development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats Behav Brain Res 212:109–114 doi: 10.1016/j.bbr.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D et al. (2005) Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum Biol Psychiatry 58:779–786 doi: 10.1016/j.biopsych.2005.04.044 [DOI] [PubMed] [Google Scholar]
- Martinez D et al. (2007) Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine Am J Psychiatry 164:622–629 doi: 10.1176/ajp.2007.164.4.622 [DOI] [PubMed] [Google Scholar]
- Martinez D et al. (2012) Deficits in dopamine D(2) receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction Biol Psychiatry 71:192–198 doi: 10.1016/j.biopsych.2011.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurski EJ, Beninger RJ (1987) Environment-specific conditioning and sensitization with (+)-amphetamine Pharmacol Biochem Behav 27:61–65 doi: 10.1016/0091-3057(87)90477-1 [DOI] [PubMed] [Google Scholar]
- Michel A, Tambour S, Tirelli E (2003) The magnitude and the extinction duration of the cocaine-induced conditioned locomotion-activated response are related to the number of cocaine injections paired with the testing context in C57BL/6 J mice Behav Brain Res 145:113–123 doi: 10.1016/s0166-4328(03)00106-2 [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Pezuk P, Menaker M (2013) Methamphetamine and dopamine receptor D1 regulate entrainment of murine circadian oscillators PLoS One 8:e62463 doi: 10.1371/journal.pone.0062463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Rose HJ, Grace AA (2001) Chronic cold stress reduces the spontaneous activity of ventral tegmental dopamine neurons Neuropsychopharmacology 24:410–419 doi: 10.1016/S0893-133X(00)00188-3 [DOI] [PubMed] [Google Scholar]
- Morikawa H, Morrisett RA (2010) Ethanol action on dopaminergic neurons in the ventral tegmental area: interaction with intrinsic ion channels and neurotransmitter inputs Int Rev Neurobiol 91:235–288 doi: 10.1016/S0074-7742(10)91008-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R et al. (2012) In vivo evidence for low striatal vesicular monoamine transporter 2 (VMAT2) availability in cocaine abusers Am J Psychiatry 169:55–63 doi: 10.1176/appi.ajp.2011.11010126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2005) Is there a common molecular pathway for addiction? Nat Neurosci 8:1445–1449 doi: 10.1038/nn1578 [DOI] [PubMed] [Google Scholar]
- Piantadosi SC, Chamberlain BL, Glausier JR, Lewis DA, Ahmari SE (2019) Lower excitatory synaptic gene expression in orbitofrontal cortex and striatum in an initial study of subjects with obsessive compulsive disorder Mol Psychiatry doi: 10.1038/s41380-019-0431-3 [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants Brain Res Brain Res Rev 25:192–216 doi: 10.1016/s0165-0173(97)00021-0 [DOI] [PubMed] [Google Scholar]
- Ramsson ES, Covey DP, Daberkow DP, Litherland MT, Juliano SA, Garris PA (2011) Amphetamine augments action potential-dependent dopaminergic signaling in the striatum in vivo J Neurochem 117:937–948 doi: 10.1111/j.1471-4159.2011.07258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhut AS, Bialecki V (2011) Development and persistence of methamphetamine-conditioned hyperactivity in Swiss-Webster mice Behav Pharmacol 22:228–238 doi: 10.1097/FBP.0b013e328345f741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Becker JB (1986) Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis Brain Res 396:157–198 doi: 10.1016/s0006-8993(86)80193-7 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB, Presty SK (1982) Long-term facilitation of amphetamine-induced rotational behavior and striatal dopamine release produced by a single exposure to amphetamine: sex differences Brain Res 253:231–241 doi: 10.1016/0006-8993(82)90690-4 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction Brain Res Brain Res Rev 18:247–291 doi: 10.1016/0165-0173(93)90013-p [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B (1997) Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine J Neurosci 17:8491–8497 doi: 10.1523/JNEUROSCI.17-21-08491.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL (2016) DREADDs for Neuroscientists Neuron 89:683–694 doi: 10.1016/j.neuron.2016.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runegaard AH, Dencker D, Wortwein G, Gether U (2019) G protein-coupled receptor signaling in VTA dopaminergic neurons bidirectionally regulates the acute locomotor response to amphetamine but does not affect behavioral sensitization Neuropharmacology 161:107663 doi: 10.1016/j.neuropharm.2019.06.002 [DOI] [PubMed] [Google Scholar]
- Salesse C, Charest J, Doucet-Beaupre H, Castonguay AM, Labrecque S, De Koninck P, Levesque M (2020) Opposite Control of Excitatory and Inhibitory Synapse Formation by Slitrk2 and Slitrk5 on Dopamine Neurons Modulates Hyperactivity Behavior Cell Rep 30:2374–2386 e2375 doi: 10.1016/j.celrep.2020.01.084 [DOI] [PubMed] [Google Scholar]
- Savitt JM, Jang SS, Mu W, Dawson VL, Dawson TM (2005) Bcl-x is required for proper development of the mouse substantia nigra J Neurosci 25:6721–6728 doi: 10.1523/JNEUROSCI.0760-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Snow S, Horger BA (1991) Pre-exposure to amphetamine but not nicotine sensitizes rats to the motor activating effect of cocaine Psychopharmacology (Berl) 103:62–66 doi: 10.1007/BF02244075 [DOI] [PubMed] [Google Scholar]
- Schiff SR (1982) Conditioned dopaminergic activity Biol Psychiatry 17:135–154 [PubMed] [Google Scholar]
- Segal DS, Mandell AJ (1974) Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy Pharmacol Biochem Behav 2:249–255 doi: 10.1016/0091-3057(74)90060-4 [DOI] [PubMed] [Google Scholar]
- Sequeira-Cordero A, Brenes JC (2021) Time-dependent changes in striatal monoamine levels and gene expression following single and repeated amphetamine administration in rats Eur J Pharmacol 904:174148 doi: 10.1016/j.ejphar.2021.174148 [DOI] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhang XX, Jones MD, Bunney BS (2000) Dual effects of D-amphetamine on dopamine neurons mediated by dopamine and nondopamine receptors J Neurosci 20:3504–3511 doi: 10.1523/JNEUROSCI.20-09-03504.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Minamoto Y, Ono M, Watanabe S (1994) Aging impairs methamphetamine-induced free-running and anticipatory locomotor activity rhythms in rats Neurosci Lett 172:107–110 doi: 10.1016/0304-3940(94)90673-4 [DOI] [PubMed] [Google Scholar]
- Shibata S, Ono M, Fukuhara N, Watanabe S (1995) Involvement of dopamine, N-methyl-D-aspartate and sigma receptor mechanisms in methamphetamine-induced anticipatory activity rhythm in rats J Pharmacol Exp Ther 274:688–694 [PubMed] [Google Scholar]
- Shuster L, Yu G, Bates A (1977) Sensitization to cocaine stimulation in mice Psychopharmacology (Berl) 52:185–190 doi: 10.1007/BF00439108 [DOI] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW, Mahler SV (2016) DREADDS: Use and application in behavioral neuroscience Behav Neurosci 130:137–155 doi: 10.1037/bne0000135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumier A, Sibille E (2014) Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice Neuropsychopharmacology 39:2252–2262 doi: 10.1038/npp.2014.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW (2011) Drug wanting: behavioral sensitization and relapse to drug-seeking behavior Pharmacol Rev 63:348–365 doi: 10.1124/pr.109.001933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, Roth BL (2014) Chemogenetic tools to interrogate brain functions Annu Rev Neurosci 37:387–407 doi: 10.1146/annurev-neuro-071013-014048 [DOI] [PubMed] [Google Scholar]
- Stewart J, Badiani A (1993) Tolerance and sensitization to the behavioral effects of drugs Behav Pharmacol 4:289–312 [PubMed] [Google Scholar]
- Subramaniyan M, Dani JA (2015) Dopaminergic and cholinergic learning mechanisms in nicotine addiction Ann N Y Acad Sci 1349:46–63 doi: 10.1111/nyas.12871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A (1995) Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport J Neurosci 15:4102–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Jentsch JD (2001) Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, d-amphetamine and 3,4-methylenedioxymethamphetamine (“Ecstasy”) Biol Psychiatry 50:137–143 doi: 10.1016/s0006-3223(01)01106-4 [DOI] [PubMed] [Google Scholar]
- Tilson HA, Rech RH (1973) Conditioned drug effects and absence of tolerance to d-amphetamine induced motor activity Pharmacology Biochemistry and Behavior 1:149–153 doi: 10.1016/0091-3057(73)90091-9 [DOI] [PubMed] [Google Scholar]
- Tirelli E, Terry P (1998) Amphetamine-induced conditioned activity and sensitization: the role of habituation to the test context and the involvement of Pavlovian processes Behav Pharmacol 9:409–419 doi: 10.1097/00008877-199809000-00004 [DOI] [PubMed] [Google Scholar]
- Torre-Muruzabal T, Devoght J, Van den Haute C, Brone B, Van der Perren A, Baekelandt V (2019) Chronic nigral neuromodulation aggravates behavioral deficits and synaptic changes in an alpha-synuclein based rat model for Parkinson’s disease Acta Neuropathol Commun 7:160 doi: 10.1186/s40478-019-0814-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K (2009) Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning Science 324:1080–1084 doi: 10.1126/science.1168878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill SM, Wheeler DS, Li M, Watts SD, Ingram SL, Amara SG (2014) Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons Neuron 83:404–416 doi: 10.1016/j.neuron.2014.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Grace AA (2012) Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons Trends Neurosci 35:422–430 doi: 10.1016/j.tins.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP (2004) Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli Science 303:2040–2042 doi: 10.1126/science.1093360 [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A (2001) Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons Nature 411:583–587 doi: 10.1038/35079077 [DOI] [PubMed] [Google Scholar]
- Urban DJ et al. (2016) Elucidation of The Behavioral Program and Neuronal Network Encoded by Dorsal Raphe Serotonergic Neurons Neuropsychopharmacology 41:1404–1415 doi: 10.1038/npp.2015.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies Psychopharmacology (Berl) 151:99–120 doi: 10.1007/s002130000493 [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Schmidt ED, De Vries TJ, Van Moorsel CA, Tilders FJ, Schoffelmeer AN (1999) A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine, and neurochemical sensitization in rats J Neurosci 19:9579–9586 doi: 10.1523/JNEUROSCI.19-21-09579.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P (2004) Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs Neurosci Biobehav Rev 27:827–839 doi: 10.1016/j.neubiorev.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Vezina P, Leyton M (2009) Conditioned cues and the expression of stimulant sensitization in animals and humans Neuropharmacology 56 Suppl 1:160–168 doi: 10.1016/j.neuropharm.2008.06.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND et al. (2014) Stimulant-induced dopamine increases are markedly blunted in active cocaine abusers Mol Psychiatry 19:1037–1043 doi: 10.1038/mp.2014.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND et al. (1997) Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects Nature 386:830–833 doi: 10.1038/386830a0 [DOI] [PubMed] [Google Scholar]
- Volkow ND et al. (2007) Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement J Neurosci 27:12700–12706 doi: 10.1523/JNEUROSCI.3371-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ et al. (2012) Decreased dopamine activity predicts relapse in methamphetamine abusers Mol Psychiatry 17:918–925 doi: 10.1038/mp.2011.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Hurd YL, Ungerstedt U, Markou A, Plotsky PM, Koob GF (1992) Neurochemical correlates of cocaine and ethanol self-administration Ann N Y Acad Sci 654:220–241 doi: 10.1111/j.1749-6632.1992.tb25970.x [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY (1984) Electrophysiological evidence for A10 dopamine autoreceptor subsensitivity following chronic D-amphetamine treatment Brain Res 309:283–292 doi: 10.1016/0006-8993(84)90594-8 [DOI] [PubMed] [Google Scholar]
- Wolf ME (1998) The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants Prog Neurobiol 54:679–720 doi: 10.1016/s0301-0082(97)00090-7 [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC (2000) Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement J Neurosci 20:8122–8130 doi: 10.1523/JNEUROSCI.20-21-08122.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J et al. (2022) Midbrain dopamine neurons arbiter OCD-like behavior Proc Natl Acad Sci U S A 119:e2207545119 doi: 10.1073/pnas.2207545119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N (2003) Chronic pretreatment with methylphenidate induces cross-sensitization with amphetamine Life Sci 73:2899–2911 doi: 10.1016/s0024-3205(03)00673-8 [DOI] [PubMed] [Google Scholar]
- Yates JW, Meij JT, Sullivan JR, Richtand NM, Yu L (2007) Bimodal effect of amphetamine on motor behaviors in C57BL/6 mice Neurosci Lett 427:66–70 doi: 10.1016/j.neulet.2007.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S et al. (2018) Stimulation of entorhinal cortex-dentate gyrus circuitry is antidepressive Nat Med 24:658–666 doi: 10.1038/s41591-018-0002-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Balmadrid C, Kelley AE (2003) Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat Behav Neurosci 117:202–211 doi: 10.1037/0735-7044.117.2.202 [DOI] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME (1997) Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors J Pharmacol Exp Ther 281:699–706 [PubMed] [Google Scholar]
- Zike ID et al. (2017) OCD candidate gene SLC1A1/EAAT3 impacts basal ganglia-mediated activity and stereotypic behavior Proc Natl Acad Sci U S A 114:5719–5724 doi: 10.1073/pnas.1701736114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Argilli E, Bonci A, Palmiter RD (2008) Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors Neuron 59:486–496 doi: 10.1016/j.neuron.2008.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS et al. (2009) Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior Proc Natl Acad Sci U S A 106:7281–7288 doi: 10.1073/pnas.0813415106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


