Abstract
Objective:
Back pain and radiculopathy caused by disc herniation are major health issues worldwide. While macrophages are key players in disc herniation induced inflammation, their roles and origin in disease progression remain unclear. We aim to study the roles of monocytes and derivatives in a mouse model of disc herniation.
Methods:
Using a CCR2-CreER; R26R-EGFP (Ai6) transgenic mouse strain, we fate-mapped C-C chemokine receptor type 2 (CCR2) expressing monocytes and derivatives at disc herniation sites, and employed a CCR2RFP/RFP mouse strain and a CCR2-specific antagonist to study the effects of CCR2+ monocytes on local inflammatory responses, pain level, and disc degeneration by immunostaining, flow cytometry, and histology.
Results:
CCR2+ monocytes (GFP+) increased at the sites of disc hernia over postoperative day (POD) 4, 6, and 9 in CCR2-CreER; Ai6 mice. F4/80+ cells increased, and meanwhile CD11b+ cells trended downward. Co-localization analysis revealed that both GFP+CD11b+ and GFP+F4/80+ constituted the majority of CD11b+ and F4/80+ cells at disc hernia sites. Fluorescence activated cell sorter purified GFP+ cells exhibited higher cytokine expressions than GFP− cells. Inhibition of CCR2 signaling reduced infiltration of monocytes and macrophages, alleviated pain, maintained disc height, and reduced osteoclast activity in adjacent cortical bone for up to 1 month.
Conclusion:
Our findings suggest that circulating CCR2+ monocytes play important roles in initiating and promoting the local inflammatory responses, pain sensitization, and degenerative changes after disc herniation, and thus may serve as therapeutic targets for disc herniation induced back and leg pain.
Keywords: Monocytes, macrophages, disc herniation, CCR2, inflammation, radiculopathy
Graphical abstract

Introduction
Inflammation plays a critical role in the pathogenesis of intervertebral disc degeneration, a leading cause of back and leg pain (1,2). Inflammatory mediators in degenerated discs have been shown to correlate with the severity of degeneration (3–5). Surgical specimens of degenerative disc tissues, including herniated discs, contain high levels of inflammatory cytokines, such as IL-1β, TNF-α, IL-6, IL-8, IL-17, IFN-γ, monocyte chemoattractant protein-1 (MCP-1), and IL-4. These inflammatory mediators may be produced by degenerated disc cells and macrophages (4,6,7), which attracts and activates additional macrophages and lymphocytes to the disc hernia site, exacerbating the inflammatory response.
However, the origins and functions of macrophages in the pathogenesis of disc herniation and associated pain remain unclear. For example, it remains unclear whether these macrophages are bone marrow-derived or resident macrophages. Using green fluorescent protein (GFP) bone marrow chimeric mice, a study showed CD86+F4/80+ macrophages increased in GFP+ populations on days 3, 7, and 14, while GFP− macrophages rose on day 3, and then declined on days 7 and 14 after disc herniation (8). Therefore, infiltrated macrophages are likely derived from circulating monocytes (9). Monocytes belong to the mononuclear phagocyte system, which migrates to replace and replenish tissue macrophages (10–12), essential for host defense and tissue remodeling (13). C-C chemokine receptor 2 (CCR2) is a monocyte specific receptor that plays a pivotal role in monocyte migration and recruitment (14) and responds to chemotaxis through its main ligand MCP-1 (15). MCP-1/CCR2 pathways are critical in mediating monocyte mobilization from bone marrow and migration into target tissues, such as in mediating osteoarthritis pain (16,17). In a disc herniation rabbit model, intra-discal injection of CCR2 antagonist during surgery reduced mRNA levels of MCP-1, CCL5, IL-8 in discs after 3 weeks (18). Zhang et al reported that inhibition of CCR2 monocyte influx reduced pain behaviors in an inflammatory dorsal root ganglion (DRG) mouse model of back pain (19). However, the precise roles of CCR2 monocytes in the progression of disc herniation remains unclear. We hypothesize that monocytes infiltrate disc hernia sites and differentiate into a heterogeneous population of macrophages, and that disruption of this monocytic infiltration by blocking CCR2 signaling can attenuate local inflammation and hyperalgesia following acute disc herniation.
To test this hypothesis, we used genetic and pharmacological approaches in a mouse model of disc herniation to monitor and block the infiltration of monocytes. Specifically, we adopted an innovative transgenic tamoxifen-induced CCR2-CreER; R26R-EGFP (Ai6) mouse strain to trace the influx of CCR2+ monocytes and monocyte-derived macrophages at disc hernia, and investigated the impact of CCR2 signaling on pain sensitivity, local inflammation, and disc degeneration. Our results indicated that CCR2+ monocytes are essential in initiating and sustaining inflammation at disc hernia sites, influencing pain sensitivity and disc degeneration. Blocking monocyte infiltration could offer a promising therapy for acute disc herniation associated pain.
Materials and Methods
Animals
BALB/c (#000651), C57BL/6J (#000664), and CCR2RFP/RFP mice (#017586) were purchased from the Jackson Laboratories. CCR2RFP/+ mice were generated by breeding C57BL/6J mice with CCR2RFP/RFP mice. For fate-mapping, CCR2-CreER; R26R-EGFP (Ai6) mice were used (20). Animal care and drug administration see Supplemental Information for details.
Mouse model of disc herniation
All experimental procedures were approved by the Institutional Animal Care and Use Committee and conformed to the National Institutes of Health Guide for the care and use of laboratory animals. Needle annular puncture-induced acute disc herniation was performed per our established protocol (7). Local inflammatory tissues (creamy granular tissue at injury sites with a gross size of ~1–2 mm) corresponding to L3–L4, L4–L5 and L5–L6 three discs were harvested under a surgical microscope (Fig. S1). For immunostaining, tissues were fixed in 2% p-formaldehyde-lysine-periodate (PLP) and embedded for cryo-sectioning as previously described (7). Lumbar spines (n = 4 – 5 per group) were fixed in 4% paraformaldehyde and processed for histology.
Details for animal surgery, drug administration, histology and immunofluorescence staining, Tartrate-resistant acid phosphatase (TRAP) staining, RNA extraction, qRT-PCR, and GAG and collagen measurements were included in the Supplemental Information.
Mechanical hyperalgesia
Paw withdrawal threshold (PWT) was measured on both hind paws every other day post-surgery for up to 2 weeks and every week for up to 1 month using an electronic Von Frey Aesthesiometer (IITC Life science, CA) per our established protocol (21,22).
Micro-Computed tomography (micro-CT)
Micro-CT and calculation of percentage disc height (PDH) were performed per our established protocol (23) (Fig. S2).
Flow cytometry and fluorescence activated cell sorting (FACS)
To analyze the infiltrating immune cells at disc injury sites, a single-cell suspension of inflammatory tissues was generated by gentle dissection and enzyme digestion (33 μg/mL Liberase TM and 50 μg/ml DNase I) at 37 °C for 30 minutes (n = 4 animals per group, samples were pooled from three levels L3-L6 of infiltrated tissues from one mouse. Both male and female mice were used). Cells were filtered through a 70 μm cell strainer and stained with antibodies for 1 hour at 4°C and analyzed on an Attune Nxt analyzer (DeNovo Software). FACS sorting of GFP+ bone marrow cells, GFP+ infiltrate cells, and GFP− infiltrate cells was performed on a BD Influx cell sorter. GFP+ cells were gated on live singlet GFP+ cells. Details were included in the supplement information.
Statistical analyses
Statistical analysis was performed using GraphPad Prism version 9.0. All animals were included in the analyses. Data were shown as mean ± 95% CI. Differences between groups were analyzed by one-way ANOVA followed by Holm-Šídák’s multiple comparisons test or t test. For comparisons among groups across two fixed-effect factors (genotype and treatment), two-way ANOVA was applied. For behavior assays, multiple t tests were used to determine significance at each time point. A p value < 0.05 was considered statistically significant. The investigators were blinded to grouping during cell counting.
Results
Using CCR2-CreER; Ai6 mice to trace the infiltrated monocytes and derivatives in disc herniation.
We recently established a CCR2-CreER: Ai6 mouse strain after crossing with a GFP-reporter line (Ai6) and constitutive expression of GFP upon tamoxifen injection, allowing longitudinal tracking of CCR2+ monocytes and derivatives (20). Animals were i.p. administered with tamoxifen for 3 days prior to surgery (Fig. 1A). Immunofluorescence staining of infiltration tissue at disc hernia showed abundant and increasing percentages of GFP+ cells on POD 4 (29.0%), POD 6 (45.6%), and POD 9 (47.9%) (Fig. 1B, 1C), while few GFP+ cells was detected in intact discs or inside herniated discs (Fig. S4A, S4B). While proportion of CD11b+ cells showed a downward trending from 31% on POD 4 to 18% on POD 9 without a statistical significance (p=0.052) and CD68+ cells remained stable over time (Fig. 1B, 1C), F4/80+ cells increased from 9% on POD 4 to 18% on POD 9 (p=0.0047). Co-localization analysis showed that majority of CD11b+ and CD68+ (60–80%) cells were GFP+ (white arrows), suggesting dominance of monocytes-derived macrophages on POD 6 (Fig. 1D, 1E). There were a few GFP+CD11b− or GFP+CD68− cells (Fig. 1D green arrows), indicating possible transformation of infiltrated monocytes to non-macrophages.
Figure 1.

Detection of infiltrating CCR2 monocytes and their derivatives at disc herniation sites in CCR2-CreER; Ai6 mice. (A) Experimental design. (B) Representative fluorescence images of GFP+, CD11b+, F4/80+, CD68+ cells on POD4, POD6, and POD9. (C) Quantification showed a stable total DAPI stained cells and an increasing GFP+ cells over time; a decreasing trend of CD11b+ and significant increase of F4/80+ from POD 4 to POD 9, and relatively stable CD68+ cells. (D) Representative fluorescence images of GFP+CD11b+, GFP+F4/80+, and GFP+CD68+ cells on POD 6 after disc herniation. Arrows indicate co-localized cells. (E) Quantification of co-localized staining suggested the majority of CD11b+, F4/80+, and CD68+ cells were derived from GFP+ monocytes. Cells were counted in 10–15 sections per animal. n = 4 for POD 4 and POD 9, n = 3 for POD 6. Data combining samples from both genders was analyzed. Statistical analysis was performed one-way ANOVA followed by Holm-Šídák’s multiple comparisons test. *p<0.05; **p<0.01; Scale bar = 50 μm.
Local infiltrated monocytes/macrophages displayed both proinflammatory and anti-inflammatory phenotypes
Macrophages are traditionally classified as pro-inflammatory (M1) and anti-inflammatory (M2) macrophages (24,25). As shown in Fig. 2A, over total cells, MMR+ (M2 marker) cells remained stable (~33–44%) across three time points (n = 4), while iNOS+ (M1 marker) ratio were very low on POD 4 and POD 6 (less than 1%) and showed an increase to 9% on POD 9 (p=0.0077 vs POD 6; p=0.0070 vs POD 4). The expression of cytokines IL-1β, TNF-α, and IL-33 in the infiltrates were detected as early as POD 4 and gradually increased on POD 9 (Fig. 2B). There was an upward trend of TNF-α+ cells (6% POD 4 to 10% POD 9, p=0.0548), and a significant increase of IL-33+ cells from 2% on POD 4, 10% on POD 6 to 25% on POD 9 (p=0.01 vs POD 4), but no change in IL-1β+ cells (3% POD 4 to 7% POD 9, p=0.2642) (Fig. 2B & 2C). Most IL-1β+ and TNF-a+ and some IL-33+ signals were co-localized with GFP signal (Fig. S5, white arrows).
Figure 2.
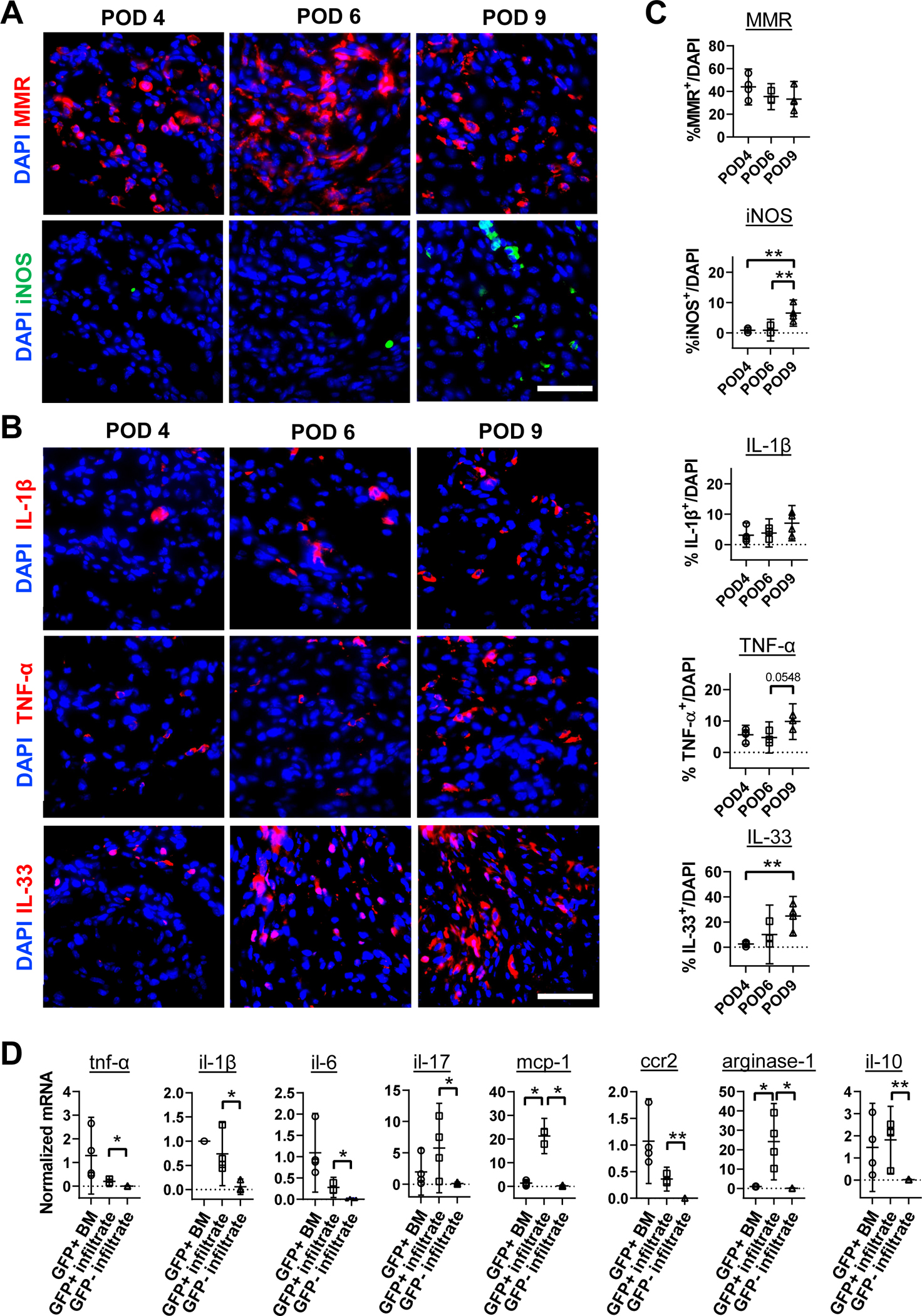
Detection of macrophage subsets and cytokines at disc herniation sites in CCR2-CreER; Ai6 mice. (A) Representative fluorescence images of iNOS+ and MMR+ cells on POD 4, 6 and 9. (B) Representative fluorescence images of cytokines IL-1β, TNF-α and IL-33 at disc hernia sites on POD 4, 6 and 9. (C) Quantification showed a comparable number of MMR+ cells from POD 4 to POD 9 while iNOS+ cells increased on POD 9; and all three cytokines increased from POD 4 to POD 9 and peaked on POD 9. (D) RT-PCR analysis shows that GFP+ monocytes/macrophages (GFP+ infiltrate) at disc hernia sites expressed significantly higher mRNA levels of tnf-α, il1b, il6, il33, il17, ccr2, mcp-1, arginase-1 and il10 compared to that of GFP− cells (GFP− infiltrate) from disc hernia sites on POD 6. GFP+ and GFP− cells were sorted by FACS. Of note, mRNA levels of mcp-1 and arginase-1 in GFP+ cells at hernia sites are higher than that of GFP+ bone marrow cells. For immunofluorescence images, cells were counted in 10–15 sections per animal. n = 4 for POD 4, n = 3 for POD 6, n = 3–4 for POD 9; For RT-qPCR, n = 4. Data combining samples from both genders was analyzed. For C, statistical analysis was performed by one-way ANOVA followed by Holm-Šídák’s multiple comparisons test. For D, significance was determined by paired t-test between GFP+ bone marrow and GFP+ infiltrate or between GFP+ infiltrate and GFP− infiltrate. *p<0.05; **p<0.01; Scale bar = 50 μm.
GFP+ and GFP− cells were sorted from local infiltrates on POD 6. The mRNA levels of pro-inflammatory mediators (TNF-α, IL-6, IL-1β, IL-17, MCP-1, and CCR2) and anti-inflammatory mediators (IL-10 and arginase-1) were significantly higher in GFP+ infiltrates than GFP− cells (Fig. 2D), with GFP+ cells from bone marrow (BM) as controls. Notably, mRNA levels of mcp-1 and arginase-1 were more than 20-fold higher in GFP+ infiltrates than GFP+ bone marrow cells, with no significant difference between the two groups for other cytokines. These findings suggest that GFP+ monocytes/macrophages were the dominant cells involved in inflammatory responses to acute disc herniation.
Loss of CCR2 reduced monocyte infiltration and disc herniation induced mechanical hyperalgesia
To investigate the involvement of CCR2 signaling in the disease progression, various outcomes were measured in CCR2RFP+ and CCR2RFP/RFP mice (Fig. 3A). Loss of CCR2 reduced RFP+ (by >50%, p=0.0317) and F4/80+CD11b+ cells (by >50%, p=0.0317), but increased neutrophils by 2-fold (p=0.0079, n = 4 – 5) in infiltrated tissues of CCR2RFP/RFP mice compared to CCR2RFP+ mice on POD 6 (Fig. 3B, Fig. S6). Immunostaining showed the total (DAPI) and RFP+ cells decreased in CCR2RFP/RFP mice compared to CCR2RFP+ mice by ~26% (p=0.022, n = 3) and ~54% (p=0.0054, n = 3), respectively. On POD 6, various macrophage markers also significantly declined in CCR2RFP/RFP mice compared to their CCR2RFP+ counterparts by more than 50% for CD11b (p=0.0055), F4/80 (p=0.0092), and CD68 (p=0.0069), while Ly6G+ neutrophils increased from 3±4% in CCR2RFP+ mice to 21±6% in CCR2RFP/RFP mice (p=0.0005, n=3) (Fig. 3C & 3D).
Figure 3.

Loss of CCR2 attenuated infiltration of monocytes and derivatives at disc hernia sites on POD 6 and disc herniation induced mechanical hyperalgesia for up to 1 month. (A) Experiment workflow. (B) Quantification of flow cytometry analysis showed a significant decrease of RFP+ cells and macrophages at disc hernia sites in CCR2RFP/RFP vs CCR2RFP+ mice, while neutrophils increased. (C) Representative fluorescence images of RFP+ monocytes and derivatives between CCR2RFP+ and CCR2RFP/RFP mice on POD 6. Scale bar = 50 μm. (D) In CCR2RFP/RFP, quantification showed decreased total infiltrated cells, RFP+, CD11b+, F4/80+, CD68+, and increased neutrophils at disc hernia sites. (E) CCR2RFP/RFP mice showed significantly lower mechanical hyperalgesia after disc herniation on the ipsilateral hind legs for up to 1 month compared to CCR2RFP+ mice, while no significant difference was observed on the mechanical sensitivity of contralateral hind legs. Data combining samples from both genders was analyzed. Statistical analyses were performed by unpaired t-test. n = 4–5 animals per group for (B) and n = 3 per group for (D). *p<0.05; **p<0.01; ***p<0.001 by unpaired t-test. Data shown are mean ± 95% CI. For (E), Statistical analysis at various time point were performed by multiple t-test at each time point. **p<0.01; ***p<0.001; n =12–14 animals per group including both male and female.
We studied the impact of CCR2 deficiency on pain sensitivity in animals for 1-month post-surgery. For left ipsilateral hind paw, CCR2RFP+ mice showed a significant decreased PWT (severe level of pain and increased mechanical sensitivity), whereas the PWT of CCR2RFP/RFP mice remained comparable to baselines. The difference between CCR2RFP+ and CCR2RFP/RFP mice lasted for up to one month (p<0.05, n = 12 – 14) (Fig. 3E, left panel). The contralateral PWT of both CCR2RFP+ and CCR2RFP/RFP mice declined on POD 2 (p=0.0367) due to surgery and recovered by POD 6 without significant differences later on (Fig. 3E, right panel). Both male and female mice showed similar PWT values and changes, suggesting that mechanical sensitivity is independent of sex in this animal model (Fig. S7A–D). There was a transient loss of body weights (>10%) during two days after surgery, which gradually recovered in both sexes and genetic phenotypes (Fig. S7E, S7F). Consistently, the expression of pain mediators (CGRP and substance P) in dorsal root ganglia (DRG) were much higher in CCRRFP+ than CCRRFP/RFP on POD 6 (Fig. S8). These findings underscore the significant contribution of CCR2 monocytes to the development of inflammation and pain sensitivity. Absence of functional CCR2 in monocytes led to a significant reduction in monocyte recruitment and subsequent macrophage numbers at the local hernia sites, which may impact the pain sensation.
Loss of CCR2 attenuated disc herniation induced ossification and loss of disc height
Vertebral osteophyte formation has been reported in rabbits with disc herniation (26). Micro-CT showed no significant differences in cortical bone volume and thickness (BV/TV ratio) and normalized disc height between CCR2RFP+ and CCR2RFP/RFP sham mice (Fig. 4A & 4B). On POD 30, CCR2RFP+ mice exhibited a rough surface on vertebrae adjacent to the hernia site (CCR2RFP+ Injury, red arrows) with woven bone deposition on the original cortical bone surface, while the CCR2RFP/RFP animals showed much less abnormal bone formation on the ipsilateral side (CCR2RFP/RFP Injury, red arrows) (Fig. 4A). Contralateral bone surfaces of both genotypes remained unchanged. Quantification revealed increased osteophyte formation on ipsilateral side of cortical bone (BV/TV, p=0.0094 injury vs sham, n = 5) and decreased disc height (p=0.0056 injury vs sham, n = 5) in CCR2RFP+ mice (Fig. 4B). Absence of CCR2 reduced osteophyte formation (BV/TV, CCR2RFP+ 0.48 ± 0.07 vs CCR2RFP/RFP0.40 ± 0.05, p=0.0361) and restored disc height (CCR2RFP+ 0.79 ± 0.12 vs CCR2RFP/RFP 1.04 ± 0.09, p=0.0014) for herniated discs compared to CCR2RFP+ animals (Fig. 4B) on POD 30. Similar trends were observed for disc heights (Fig. S9B, S9C, and S10) and osteophyte formation (Fig. S9A, S9C) on POD 16.
Figure 4.

CCR2RFP/RFP mice restored vertebral cortical bone thickness and disc height loss induced by disc herniation for up to 1 month. (A) Representative μCT scan of lumbar motion segments (upper) and cross section of cortical bone (lower) one month after surgery. Red arrows indicate the increased cortical bone roughness. Scale bar in (A) =1 mm. (B) Quantification of cortical bone volume fraction (BV/TV) of lumbar vertebrae and normalized disc height. Dotted line indicates the normalized baseline at 1. (C) Picrosirius red/alcian blue staining of sagittal sections in CCR2RFP+ and CCR2RFP/RFP mice one month after disc herniation surgery. EP, endplate; NP, nucleus pulposus; AF, annulus fibrosis; # indicates ectopic bone formation in endplates. Yellow arrows indicate disrupted AF structure by needle puncture. Scale bar in (C) = 100 μm. (D) Histology scoring of disc structure showed no differences between CCR2RFP+ and CCR2RFP/RFP mice. Annular puncture induced significant disc degeneration in both CCR2RFP+ and CCR2RFP/RFP groups compared to sham discs but no significant difference between two genotypes on POD 30. (E) Biochemical assays showed declined GAG and collagen contents in injured discs of CCR2RFP+ and CCR2RFP/RFP groups compared to their non-injured baseline controls, with no significant difference in injured discs between groups after 30 days injury. n = 3–5 mice per group. *p<0.05; ** p<0.01; *** p<0.001; ****p<0.0001 by two-way ANOVA with Šídák’s multiple comparisons test.
Histology illustrated typical degenerative changes in injured discs (e.g. loss of NP tissue, disrupted AF/NP boundaries, and clefts and fissures in AF tissues) in both CCR2RFP+ and CCR2RFP/RFP mice compared to non-injured sham controls (Fig. 4D), while no distinct differences were observed in injured discs between two groups on POD 16 (Fig. S9D) and POD 30 (Fig. 4D). Histological scoring (27–29) confirmed the degenerative changes in discs after injury (Fig. 4E) (p<0.0001 injury vs sham) in both CCR2RFP+ and CCR2RFP/RFP mice. Loss of CCR2 did not restore disc degeneration on POD 16 (Fig. S9D) and POD 30 (Fig. 4E) based on histology scoring.
Injured discs exhibited lower GAG (by 20–27%, p=0.0302 CCR2RFP+ injury vs Sham; p=0.0026 CCR2RFP/RFP injury vs Sham) and collagen contents (by 30–40%, p=0.0154 CCR2RFP+ injury vs Sham; p<0.0001 CCR2RFP/RFP injury vs Sham) compared to intact discs on POD 30 (Fig 4F). On POD 6, a similar trend was observed for GAG (Fig. S11A), while collagen content did not change among groups (Fig. S11B).
Blocking CCR2 with antagonists reduced monocytic infiltrates after disc herniation
To demonstrate potential of targeting CCR2+ monocytes for translation, we investigated the effects of a small molecule CCR2-specific antagonist PF-4136309 in BALB/c mice. Compared to vehicle controls, three consecutive injections of PF-4136309 (20 mg/kg/day, on POD −1, 0 and 1) reduced total (DAPI counts, p=0.0263), CD11b+ (p=0.008), F4/80+ (p=0.0135), and CD68+ cells (p=0.0149) at disc hernia sites on POD 6 (Fig. 5A, 5B). The antagonist treatment reduced iNOS+ by 80% and MMR+ cells by 78% (Fig. 5B), reduced TNF-α+ cells by 80% (p=0.0441) did not change IL-1β+ and MCP-1+ counts compared to vehicle controls (Fig. 5C & 5D). Thus, CCR2 antagonist PF-4136309 is a potent inhibitor of monocytic infiltration in our mouse model of disc herniation.
Figure 5.

CCR2 antagonist PF-4136309 treatment reduced total macrophages and cytokines at disc hernia sites on POD 6 of BALB/C mice. Three doses of PF-4136309 (20 mg/kg/day or vehicle) was applied to adult BALB/c mice from −1 to 1 day post-injury, and the infiltrated tissues were harvested on POD 6. (A) Representative fluorescence images of CD11b+, F4/80+, CD68+, iNOS+, and MMR+ cells were compared at 6 days’ post-injury. Arrow indicates co-localized iNOS+ and MMR+ cells. (B) Quantification showed PF-4136309 significantly decreased the number of total cells and monocytes/macrophages at disc hernia sites. (C) Representative fluorescence images of proinflammatory cytokines in vehicle and antagonist treated groups. (D) Quantification showed decreased TNF-α and unchanged IL-1β and MCP-1 expressing cells in the antagonist PF-416309 groups compared to vehicle controls. Cells were counted on 15 ROIs per section and 3–4 sections per animal (n = 3 per group). Data combining samples from both genders was analyzed. Each data point represents mean of one animal. Significance was determined by unpaired t-test. *p<0.05; *** p<0.001. Scale bar = 50 μm.
Blocking CCR2 with antagonists alleviated the narrowing of disc height, ossification of adjacent cortical bone and acute mechanical hyperalgesia
We also studied the effect of antagonist PF-4136309 on pain behavior in CCR2RFP+ animals (Fig. 6A). Early administration (POD −1, 0, and 1) of CCR2 antagonist in CCR2RFP+ mice restored the ipsilateral PWT to near baseline levels at day 6–8 and maintained for up to 16 days (Fig 6B, upper), while vehicle treated mice showed a marked reduction in PWT starting POD 2 on ipsilateral paws. There was no significant difference between two groups in contralateral PWT (remained stable) (Fig. 6B, lower) and body weights (Fig. S12). PF-4136309 protected vertebral cortical bone from inflammation induced osteophyte formation (Fig. 6C, red arrows) with reduced BV/TV ratio (p=0.0097, Fig. 6D left) and partially restored disc height loss on POD 16 (p=0.0195, Fig. 6D right, Fig. S13A). Similar to CCR2RFP/RFP mice, PF-4136309 did not rescue the structural disruption of annular puncture in histology (Fig. S13B) and disc scores (Fig. S13C).
Figure 6.

CCR2 antagonist PF-4136309 treatment restored deformation of vertebral cortical bone and loss of disc height induced by disc herniation up to POD 16. (A) Experimental workflow. (B) Antagonist treatment (POD −1 – POD 1) significantly attenuated disc herniation induced mechanical hyperalgesia. n = 5 per group. (C) Representative μCT images of lumbar motion segments (upper) and transaxial section of cortical bone (lower) on POD 16 showed less cortical bone thickness and cortical bone volume fraction (BV/TV) in antagonist group compared to the vehicle control. Red arrows indicate the thickened cortical bone. Scale bar in (C) = 1 mm. (D) Quantification of BV/TV of lumbar vertebrae and normalized disc height change. Dashed lines indicate baseline disc heights at 1 on y-axis. (E) Representative histological images and TRAP staining of osteoclasts showed abnormal vertebral cortical bone ossification with increased osteoclasts in vertebrae adjacent to herniated discs of vehicle control and PF-4136309 treated animals. Dotted lines delineate anterior cortical bone boundaries. Arrow indicates TRAP+ osteoclast. Pound sign indicates ectopic bone formation. BM, bone marrow; Scale bar in (E) =100 μm. (F) Quantification showed PF-4136309 markedly inhibited the increased cortical bone thickness and tended to reduce TRAP+ osteoclast in cortical bone compared to the vehicle control. For (B), comparison was performed by multiple t-test at each time point. For (D), comparison was performed by unpaired t-test, n=3–5 mice per group. For (F), each data represents one cortical bone with 14–15 cortical bones from 5 mice per group. Data combining samples from both genders was analyzed. *p<0.05; **p<0.01: *** p<0.001.
Pharmacological inhibition of CCR2 decreased osteoclast activity in disc herniation
Disc herniation induced abnormal bone formation and increased osteoclast activity in the cortical bone adjacent to the injured discs (Fig. S14). The CCR2 antagonist PF-4136309 reduced the cortical bone thickness by ~51% (p=0.0002) and TRAP+ cells (black arrows) in cortical bone (dotted line) by >65% (p=0.0009), compared to vehicle controls on POD 16 (Fig. 6E, Fig. 6F). Inhibiting CCR2 signaling appears to reduce the pathological osteophyte formation and osteoclast activity in the cortical bone near herniated discs.
Administration of CCR2 antagonist at later time points reduced monocytes infiltration but not pre-infiltrated macrophages
Next, we administered antagonists into animals immediately after surgery (POD 0–2) or a few days after the surgery (POD 2–4) to study their effects when the inflammatory response had already been initiated (Fig. 7A). Contrary to reduced total cellularity at disc hernia sites on POD 6 when administer antagonist during POD −1 to 1 (Fig. 5A), administration during POD 0–2 and POD 2–4 did not change total cells, but decreased RFP+ monocytes (p=0.0002 for POD 0–2; p=0.0013 for POD 2–4), reduced CD11b+ cells (p<0.0001) (Fig. 7B, Fig. 7C). Administration of antagonist on POD 2–4 decreased numbers of CD68+ cells (p=0.0133) but not for administration during POD 0–2 (Fig. 7C). Disc scores remained comparable among groups on POD 9 (Fig. S15).
Figure 7.

CCR2 antagonist PF-4136309 treatment at later time points reduced infiltration of monocytes/macrophages at disc hernia sites of CCR2RFP+ mice. (A) Experimental design. Three doses of PF-4136309 (20 mg/kg/day or vehicle controls, i.p.) was administered to mice from POD 0–2 or POD 2–4 post-injury, and the infiltrated tissues were harvested on POD 9. (B) Representative fluorescence images of RFP+, CD11b+, F4/80+, and CD68+ cells were compared on POD 9 among three groups. (C) Quantification showed PF-4136309 significantly decreased the number of RFP+ and CD11b+ cells at disc hernia sites, compared to vehicle control with no difference observed between POD 0–2 and POD 2–4 groups, while POD 2–4 group showed a dramatic drop in CD68+ macrophages compared to the vehicle control. Cells were counted on 15 ROIs per section and 3–4 sections per animal (n=3–4 per group). Each data point represents mean of one animal. Data combining samples from both genders was analyzed. Comparisons were performed by one-way ANOVA test with Holm-Šídák’s multiple comparisons test. *p<0.05; **p<0.01: *** p<0.001; **** p<0.0001. Scale bar =50 μm.
Discussion
Despite the significance of disc herniation and associated pain, no disease-modifying treatment is currently available due to incomplete understanding of disease mechanisms. Macrophages play a critical role in the progression of disc herniation and associated pain (6,7,30,31). Our recent study showed that transient depletion of macrophages altered the local inflammatory response at the site of disc herniation using a macrophage Fas-induced apoptosis (MaFIA) transgenic mouse strain (32), also suggesting the importance of monocytes/macrophages in disc herniation. However, the functions of monocyte-derived macrophages have not been distinguished from resident macrophages.
When tissue damage or infection occurs, monocytes are promptly recruited to the affected tissue, where they can differentiate into tissue macrophages (33). Monocytes can differentiate into either pro-inflammatory or anti-inflammatory subsets depending on microenvironmental cues. Here we used an innovative mouse strain to fate-map CCR2+ monocytes at disc hernia sites (16). GFP+ cells increased from POD 4 to POD 9, suggesting infiltration of CCR2 monocytes is a continuous process (Fig 1B, 1C), and majority of local infiltrated macrophage were derived from CCR2+ monocytes (Fig. 1D, 1E). The downward trend of CD11b+ cells and the rise of F4/80+ at the disc hernia suggest a phenotypic change of CCR2+ monocytes. It remains unclear if this change is due to gene regulation within the same population of monocytes or the infiltration of new subsets. Unexpectedly, iNOS+ cells increased from POD 4 to POD 9 as well as TNF-α+, and IL-33+ cells, whereas IL-1β+ and MMR+ cells remained unchanged over time (Fig. 2A–2C). The increase of IL-33+ cells suggested possible metabolic reprogramming at disc hernia (34). Contrary to literature reported various macrophage markers, such as CD68, CCR7, and CD163 in human disc cells (30,35), we did not observe GFP+ signals in disc cells (Fig. S4) of CCR2-CreER; Ai6 mouse model. The discrepancy may be due to conventional methods such as immunostaining and flow cytometry limited in providing comprehensive information and lack of reliable markers to distinguish macrophage origins.
Existing research has shown CCR2 and its ligands play critical role in recruiting inflammatory monocytes into targeted tissues (36). GFP+ cells at local infiltrates showed higher mRNA levels of both pro-inflammatory and anti-inflammatory cytokines compared to GFP− cells (Fig. 2D) in CCR2-CreER; Ai6 mice, confirming presence of monocyte infiltration at disc hernia sites and the heterogeneity of macrophages derived from monocytes. Administering the CCR2 antagonist after disc herniation reduced RFP+ (monocyte-derived macrophages), CD11b+, and CD68+ macrophages (Fig. 7B & 7C), suggesting blocking CCR2 prevented infiltration of monocytes and monocyte-derived macrophages at disc hernia. CCR2RFP+ mice displayed higher ipsilateral sensitivity (more pain) toward mechanical stimuli, whereas CCR2RFP/RFP mice demonstrated significantly less pain sensitivity (Fig. 3E). Similarly, administration of CCR2-specific antagonist PF-4136309 virtually restored the decreased ipsilateral PWT induced by disc herniation in CCR2RFP+ mice (Fig. 6B). Our study corroborates with prior findings that inhibition of CCR2 reduced pain behaviors in an inflammatory DRG mouse model of back pain (19). The alleviated mechanical pain hypersensitivity in CCR2RFP/RFP mice may associate with reduced monocyte infiltration and cytokine expression (Fig. 3B–D) and reduction of neurotransmitters in DRGs (Fig. S8). Besides the local inflammation, MCP-1/CCR2 signaling facilitates excitatory effects at both DRG and spinal cord levels (37). In a mouse model of osteoarthritis, Miller et al showed macrophage infiltration in the DRGs at 8 weeks, but not in CCR2 null mice (17). Since loss of CCR2 signaling also impairs microglial activity (38,39), reduced pain in the CCR2RFP/RFP and antagonists treated groups may be associate with altered microglial activities. CCR2 antagonist in BALB/c mice with Th2 immune responses confirmed similar reduction of total cellularity, macrophages, and cytokines at disc hernia sites (Fig. 5). In contrast to a prior study that showed gender differences in mechanical allodynia in a rat annular puncture model (40), we did not observe a noticeable gender difference in mechanical hyperalgesia (Fig. S7A–D). The discrepancy may be caused by different surgical protocols to induce disc herniation (midline anteriorly and left and right anterolaterally in literature vs. lateral puncture + spinal nerve exposure in our study) and rodents species (rat vs. mouse).
Blocking CCR2 monocyte infiltration reduced the loss of disc height induced by annular puncture (Fig. 4B, Fig. 6C). Abnormal ectopic bone formation in adjacent vertebral cortical bone was reduced in both CCR2RFP/RFP (Fig. 4A, 4B) and antagonist treated animals (Fig. 6C, 6D), due to reduced osteoclasts differentiation and activity (Fig. 6E, 6F), possibly associated with reduced monocyte/macrophage infiltration. This corroborates with our prior study showing transient depletion of macrophages alleviated vertebral bone anomaly after disc herniation(32). It was reasonable to observe similar levels of GAG and collagen as well as structural changes of discs between the CCR2RFP+ and CCR2RFP/RFP mice (Fig. 4C–4E), since annular puncturing surgery caused severe disruption of AF and loss of NP tissue and might be difficult to rescue in a short period. Increased neutrophils at local injury sites in CCR2RFP/RFP mice (Fig. 3B–D), possibly by a compensation feedback loop, may also contribute to the inflammation and degenerative process (41). When administering the antagonist after surgery on POD 0–2 and POD 2–4, despite significant reduction in RFP+ monocyte-derived macrophages, the total cells remained unchanged (Fig. 7). These findings suggest that the timing of CCR2 inhibition is critical to achieve desired therapeutic effects.
Our study renders a few limitations. In addition to CCR2 monocytes, other chemokine receptors, like CCR1-MCP-3 axis may play important roles in recruitment of effector immune cells. Chou et al. injected 8 μL of antagonists of either CCR1 or CCR2 intradiscally after an annular puncture in rabbits and found decreased mRNA expression of CCL2, CCL3, CCL5, and IL-8 after three weeks of surgery (18). Administering antagonists targeting both CCR1 and CCR2 may elicit better effects in modulating the inflammation and disc degeneration. Additionally, a longer follow-up period (>1 month) may provide more information regarding the impacts of CCR2 signaling on chronic pain. Detailed mechanism on how CCR2 triggers and maintains pain behavior requires further investigation.
In summary, we utilized CCR2 transgenic mouse models for fate mapping and a specific CCR2 antagonist to investigate the role of CCR2 monocytes/macrophages in disc herniation induced inflammation and pain sensitization. Our findings demonstrate that CCR2+ monocytes are crucial for initiating local inflammation and pain sensitization following disc herniation. Additionally, disc herniation-induced inflammation causes abdominal cortical bone changes external to the disc. This suggests that targeting CCR2+ monocytes could be a promising therapeutic strategy for alleviating radicular pain caused by disc herniation.
Supplementary Material
Acknowledgements
This work is supported by research grants from NIH NIAMS R01AR064792, R01AR078888, R21AR072334, R21AR078547, R21AR082052, North American Spine Society, and Commonwealth Health Research Board (CHRB) 207–10-18. We thank the flow cytometry core and histology core for assisting in sample processing.
Abbreviation
- AF
annulus fibrosus
- BM
bone marrow
- BV/TV
bone volume ratio
- CCR2
C-C chemokine receptor type 2
- DMMB
dimethylmethylene blue
- DMBA
dimethyllaminobenzal-dehyde
- DMSO
dimethyl sulfoxide
- DRG
dorsal root ganglion
- EDTA
ethylenediaminetetraacetic acid
- EP
endplate
- GAG
glycosaminoglycan
- GFP
green fluorescence protein
- iNOS
inducible nitric oxide synthase
- i.p.
intraperitoneal
- MaFIA
macrophage Fas-induced apoptosis
- MCP-1
monocyte chemoattractant protein-1
- MMR
macrophage mannose receptor
- micro-CT or μCT
micro-Computed tomography
- NP
nucleus pulposus
- PDH
percentage disc height
- POD
post-operative day
- PLP
formaldehyde-lysine-periodate
- PFA
paraformaldehyde
- PWT
Paw withdrawal threshold
- RFP
red fluorescence protein
- RT-PCR
real-time quantitative reverse transcription polymerase chain reaction
- TRAP
Tartrate-resistant acid phosphatase
Footnotes
Competing Interests
The authors have no competing interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
Data availability statement
Data supporting the findings of this study are available upon request.
Bibliography
- 1.Johnson ZI, Schoepflin ZR, Choi H, Shapiro IM, Risbud MV. Disc in flames: Roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur Cell Mater. 2015. Sep 21;30:104–16; discussion 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Allen C, Arora M. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016. Oct 8;388(10053):1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shamji MF, Setton LA, Jarvis W, So S, Chen J, Jing L, et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010. Jul;62(7):1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunha C, Silva AJ, Pereira P, Vaz R, Gonçalves RM, Barbosa MA. The inflammatory response in the regression of lumbar disc herniation. Arthritis Res Ther. 2018. Nov 6;20(1):251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawaguchi S, Yamashita T, Yokogushi K, Murakami T, Ohwada O, Sato N. Immunophenotypic analysis of the inflammatory infiltrates in herniated intervertebral discs. Spine. 2001. Jun 1;26(11):1209–1214. [DOI] [PubMed] [Google Scholar]
- 6.Xiao L, Ding M, Zhang Y, Chordia M, Pan D, Shimer A, et al. A novel modality for functional imaging in acute intervertebral disk herniation via tracking leukocyte infiltration. Mol Imaging Biol. 2017. Oct;19(5):703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin L, Xiao L, Ding M, Pan A, Balian G, Sung S-SJ, et al. Heterogeneous macrophages contribute to the pathology of disc herniation induced radiculopathy. Spine J. 2022. Apr;22(4):677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawakubo A, Uchida K, Miyagi M, Nakawaki M, Satoh M, Sekiguchi H, et al. Investigation of resident and recruited macrophages following disc injury in mice. J Orthop Res. 2020. Aug;38(8):1703–1709. [DOI] [PubMed] [Google Scholar]
- 9.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003. Jul;19(1):71–82. [DOI] [PubMed] [Google Scholar]
- 10.Hettinger J, Richards DM, Hansson J, Barra MM, Joschko A-C, Krijgsveld J, et al. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013. Aug;14(8):821–830. [DOI] [PubMed] [Google Scholar]
- 11.Hume DA. Differentiation and heterogeneity in the mononuclear phagocyte system. Mucosal Immunol. 2008. Nov;1(6):432–441. [DOI] [PubMed] [Google Scholar]
- 12.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968. Sep 1;128(3):415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kratofil RM, Kubes P, Deniset JF. Monocyte conversion during inflammation and injury. Arterioscler Thromb Vasc Biol. 2017. Jan;37(1):35–42. [DOI] [PubMed] [Google Scholar]
- 14.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006. Mar;7(3):311–317. [DOI] [PubMed] [Google Scholar]
- 15.Zhao BN, Campbell JJ, Salanga CL, Ertl LS, Wang Y, Yau S, et al. CCR2-Mediated Uptake of Constitutively Produced CCL2: A Mechanism for Regulating Chemokine Levels in the Blood. J Immunol. 2019. Dec 15;203(12):3157–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H-R, Chen C-W, Mandhani N, Short-Miller JC, Smucker MR, Sun Y-Y, et al. Monocytic Infiltrates Contribute to Autistic-like Behaviors in a Two-Hit Model of Neurodevelopmental Defects. J Neurosci. 2020. Dec 2;40(49):9386–9400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci USA. 2012. Dec 11;109(50):20602–20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou P-H, Chee A, Shi P, Lin C-L, Zhao Y, Zhang L, et al. Small molecule antagonist of C-C chemokine receptor 1 (CCR1) reduces disc inflammation in the rabbit model. Spine J. 2020. Dec;20(12):2025–2036. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Xie W, Zhang J, Shanahan H, Tonello R, Lee SH, et al. Key role of CCR2-expressing macrophages in a mouse model of low back pain and radiculopathy. Brain Behav Immun. 2021. Jan;91:556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H-R, Sun Y-Y, Chen C-W, Kuo Y-M, Kuan IS, Tiger Li Z-R, et al. Fate mapping via CCR2-CreER mice reveals monocyte-to-microglia transition in development and neonatal stroke. Sci Adv. 2020. Aug 26;6(35):eabb2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin L, Ding M, Oklopcic A, Aghdasi B, Xiao L, Li Z, et al. Nanoparticle fullerol alleviates radiculopathy via NLRP3 inflammasome and neuropeptides. Nanomedicine. 2017. Aug;13(6):2049–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao L, Ding M, Fernandez A, Zhao P, Jin L, Li X. Curcumin alleviates lumbar radiculopathy by reducing neuroinflammation, oxidative stress and nociceptive factors. Eur Cell Mater. 2017. May 9;33:279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao L, Hong K, Roberson C, Ding M, Fernandez A, Shen F, et al. Hydroxylated Fullerene: A Stellar Nanomedicine to Treat Lumbar Radiculopathy via Antagonizing TNF-α-Induced Ion Channel Activation, Calcium Signaling, and Neuropeptide Production. ACS Biomater Sci Eng. 2018. Jan 8;4(1):266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills CD, Harris RA, Ley K. Macrophage polarization: decisions that affect health. J Clin Cell Immunol. 2015. Oct 30;6(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS−) vs. Alternatively Activated Macrophages. Front Immunol. 2019. May 24;10:1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipson SJ, Muir H. Vertebral osteophyte formation in experimental disc degeneration. Morphologic and proteoglycan changes over time. Arthritis Rheum. 1980. Mar;23(3):319–324. [DOI] [PubMed] [Google Scholar]
- 27.Xiao L, Majumdar R, Dai J, Li Y, Xie L, Shen FH, et al. Molecular detection and assessment of intervertebral disc degeneration via a collagen hybridizing peptide. ACS Biomater Sci Eng. 2019. Apr 8;5(4):1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai J, Xing Y, Xiao L, Li J, Cao R, He Y, et al. Microfluidic Disc-on-a-Chip Device for Mouse Intervertebral Disc-Pitching a Next-Generation Research Platform To Study Disc Degeneration. ACS Biomater Sci Eng. 2019. Apr 8;5(4):2041–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam V, Chan WCW, Leung VYL, Cheah KSE, Cheung KMC, Sakai D, et al. Histological and reference system for the analysis of mouse intervertebral disc. J Orthop Res. 2018. Jan;36(1):233–243. [DOI] [PubMed] [Google Scholar]
- 30.Nakazawa KR, Walter BA, Laudier DM, Krishnamoorthy D, Mosley GE, Spiller KL, et al. Accumulation and localization of macrophage phenotypes with human intervertebral disc degeneration. Spine J. 2018. Feb;18(2):343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamagishi A, Nakajima H, Kokubo Y, Yamamoto Y, Matsumine A. Polarization of infiltrating macrophages in the outer annulus fibrosus layer associated with the process of intervertebral disc degeneration and neural ingrowth in the human cervical spine. Spine J. 2022. May;22(5):877–886. [DOI] [PubMed] [Google Scholar]
- 32.Xiao L, Matharoo J, Chi J, Ma J, Chen M, Manley B, et al. Transient depletion of macrophages alters local inflammatory response at the site of disc herniation in a transgenic mouse model. Osteoarthr Cartil. 2023. Feb 7; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guilliams M, Mildner A, Yona S. Developmental and functional heterogeneity of monocytes. Immunity. 2018. Oct 16;49(4):595–613. [DOI] [PubMed] [Google Scholar]
- 34.Faas M, Ipseiz N, Ackermann J, Culemann S, Grüneboom A, Schröder F, et al. IL-33-induced metabolic reprogramming controls the differentiation of alternatively activated macrophages and the resolution of inflammation. Immunity. 2021. Nov 9;54(11):2531–2546.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nerlich AG, Weiler C, Zipperer J, Narozny M, Boos N. Immunolocalization of phagocytic cells in normal and degenerated intervertebral discs. Spine. 2002. Nov 15;27(22):2484–2490. [DOI] [PubMed] [Google Scholar]
- 36.Mysore V, Tahir S, Furuhashi K, Arora J, Rosetti F, Cullere X, et al. Monocytes transition to macrophages within the inflamed vasculature via monocyte CCR2 and endothelial TNFR2. J Exp Med. 2022. May 2;219(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White FA, Miller RJ. Insights into the regulation of chemokine receptors by molecular signaling pathways: functional roles in neuropathic pain. Brain Behav Immun. 2010. Aug;24(6):859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007. Nov 7;27(45):12396–12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen H-R, Chen C-W, Kuo Y-M, Chen B, Kuan IS, Huang H, et al. Monocytes promote acute neuroinflammation and become pathological microglia in neonatal hypoxic-ischemic brain injury. Theranostics. 2022. Jan 1;12(2):512–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosley GE, Wang M, Nasser P, Lai A, Charen DA, Zhang B, et al. Males and females exhibit distinct relationships between intervertebral disc degeneration and pain in a rat model. Sci Rep. 2020. Sep 15;10(1):15120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parisien M, Lima LV, Dagostino C, El-Hachem N, Drury GL, Grant AV, et al. Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci Transl Med. 2022. May 11;14(644):eabj9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available upon request.


