Abstract
Background:
Fecal immunochemical test (FIT) is an effective colorectal cancer (CRC) screening modality. Little is known about prevalence, reasons, and testing after unsatisfactory FIT, or a FIT that cannot be processed by the laboratory due to inadequate stool specimen or incomplete labeling.
Methods:
Our retrospective cohort study examined unsatisfactory FIT among average-risk individuals aged 50–74 years in a large, integrated, safety-net health system who completed an index FIT from 2010–2019. We determined prevalence of unsatisfactory FIT and categorized reasons hierarchically. We used multivariable logistic regression models to identify factors associated with: a) unsatisfactory FIT; and b) subsequent testing within 15 months of the unsatisfactory FIT.
Results:
Of 56,980 individuals completing an index FIT, 10.2% had an unsatisfactory FIT. Reasons included inadequate specimen (51%), incomplete labeling (27%), old specimen (13%), and broken/leaking container (8%). Unsatisfactory FIT was associated with being male (OR :1.10, CI:1.03–1.16), Black (OR:1.46, CI:1.33–1.61), Spanish speaking (OR:1.12, CI:1.01–1.24), on Medicaid (OR:1.42, CI:1.28–1.58), and received FIT by mail (OR:2.66, CI:2.35–3.01). Among those with an unsatisfactory FIT, fewer than half (41%) completed a subsequent test within 15 months (median: 4.4 months). Adults aged 50–54 years (OR: 1.16, CI:1.01–1.39) and those who received FIT by mail (OR:1.92, CI:1.49–2.09) were more likely to complete a subsequent test.
Conclusion:
One in ten returned a FIT that could not be processed – mostly due to patient-related reasons. Fewer than half completed a subsequent test after unsatisfactory FIT.
Impact:
Screening programs should address these breakdowns such as specimen collection and labeling to improve real-world effectiveness.
Keywords: colorectal cancer screening, fecal immunochemical test, quality improvement
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the US with more than 50,000 deaths annually (1). CRC screening can detect and remove precancerous and cancerous lesions and has therefore contributed to the substantial decline in CRC incidence and mortality rates over the past decades (2). However, despite the known benefits, CRC screening is underused. Only 70.9% of eligible adults in the US were up-to-date on CRC screening in 2021(3).
Stool-based tests – initially fecal occult blood tests (FOBT) and later fecal immunochemical tests (FIT) – are recommended as a screening option by the US Preventive Services Task Force as one of several CRC screening modalities and are the most commonly used modalities worldwide (4,5). Randomized clinical trials and large cohort studies have shown that stool-based tests reduce CRC mortality (6,7). Further, stool-based tests are convenient, non-invasive, and inexpensive, and have consequently resulted in higher participation, compared to other screening modalities, especially in underserved populations (8–14). CRC screening programs that include stool-based tests increase screening participation, particularly in large population-based settings where access to endoscopy may be limited (8,15–18). Stool-based tests are particularly important during and after the COVID-19 pandemic, where they were shown to be an effective strategy to improve screening participation and reduce disparities (3,19).
Effectiveness of stool-based tests depends on high participation, satisfactory test completion, follow-up of abnormal results, and repeat testing annually. The US Multi-Society Task Force (MSTF) recommended that the proportion of returned FIT that cannot be processed should be less than 5% (4). Prior studies have focused largely on the first part of the process (increasing screening participation (20)), or the later steps (improving timely follow-up of abnormal FIT (21,22), and ensuring repeat testing (4,23)). However, few large-scale studies have evaluated the multiple steps and potential failures in the middle of the FIT process,(24,25) including: inadequate stool specimen, incomplete labeling, delayed transfer to laboratories for processing (collectively, “unsatisfactory FIT”), and subsequent testing after unsatisfactory FIT. To address this gap, we: 1) characterized prevalence and reasons for unsatisfactory FIT in a large integrated, safety-net health system, 2) identified patient- and system-level factors associated with unsatisfactory FIT, and 3) assessed prevalence of and factors associated with subsequent testing within 15 months of the unsatisfactory FIT.
Materials and Methods
We conducted a population-based, retrospective cohort study using electronic health record (EHR) data from Parkland Health (Parkland) after obtaining ethical approval from the Institutional Review Board at University of Texas Southwestern Medical Center in accordance with the U.S. Common Rule. Parkland is a regional, integrated healthcare delivery system, and the sole safety-net hospital in Dallas County, Texas, and includes the tertiary-care Parkland Hospital, specialty clinics (including gastroenterology), and 14 (at the time of study) community-oriented primary care clinics throughout Dallas County. Parkland is responsible for providing comprehensive primary, specialty, and acute care to more than one million low-income, uninsured Dallas County residents. Healthcare services are provided for free or at minimal cost, according to income levels, through Parkland Financial Assistance.
During the study period, Parkland offered both opportunistic and organized CRC screening programs (Supplementary Fig S1). Opportunistic screening included FIT ordered during primary care visits accompanied by verbal instructions at the discretion of clinic or laboratory staff. A patient label may have been affixed to the FIT by staff, though this process and instructions were not standardized across clinics during the study period. Hemoccult ICT 3-card tests were used from 2010 to 2017 and were later replaced by one sample Polymedco OC-Auto FIT CHEK starting in 2018. Organized screening with mailed outreach was implemented starting 2013 and in parallel to opportunistic screening. Specifically, from March 2013 to July 2016, more than 2,000 patients were mailed a Polymedco FIT as part of a pragmatic trial to increase CRC screening process completion (26). Based on success of the pragmatic trial, mailed outreach was expanded to mail ~15,000 Polymedco FIT kits from September 2018 to August 2019 to patients not up-to-date with CRC screening. The mailed FIT kit included: one sample Polymedco test, bilingual written and info-graphical instructions, and pre-paid return envelopes, followed by telephone calls from bilingual staff to remind patients to complete and return the test, and to complete another test if the initial FIT was unsatisfactory (27).
Completed FIT results were reported as normal, abnormal, or unsatisfactory. For Hemoccult 3-card test kit, results (positive or negative) were reported if at least 1 card was deemed satisfactory for testing. Reasons for unsatisfactory FIT were included in the EHR report for both Hemoccult and Polymedco kit. We categorized reasons for unsatisfactory FIT based on the free text entered into laboratory system’s EHR by the lab technician. This would include entries such as “no sample on card”, “too much stool”, “back of card”, “improperly collected.”, “sample too old”, “leaked”, and so on. The complete list of free text entries are tabulated in Supplementary Table S1. These free text entries were hierarchically categorized into reasons for unsatisfactory FIT as: broken/leaking container, incomplete labeling (incomplete patient information or no completion date), inadequate specimen, old specimen, and other.
Study Population
The study population comprised of patients between the ages of 50–74 years who completed at least one FIT between January 2010 and December 2019, and had a primary care visit at Parkland in the year prior to the test. For participants completing more than one FIT during the study period, we used the first FIT only (“index” test), regardless of result. The EHR in Parkland started in 2007. We included receipt and results of FIT before study period (2007 to 2009) as baseline characteristics. We excluded individuals who had colonoscopy in the past 10 years or sigmoidoscopy in the past 5 years at the time of index FIT, as they were considered screen up-to-date. We also excluded individuals with a prior abnormal stool test, those with a prior diagnosis of CRC, inflammatory bowel disease, and prior colectomy or gastrointestinal surgery.
Factors Associated with Unsatisfactory FIT
We examined associations of unsatisfactory FIT with these pre-specified patient-level factors: age, sex, race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Hispanic, others), preferred language (English, Spanish, others), insurance (commercial, Medicare, Medicaid, Parkland Financial Assistance, others), Charlson comorbidity score (categorized as 0, 1 and ≥2) based on diagnoses in the 12 months prior to FIT, and body mass index (<25, 25–30, and ≥30 kg/m2). We also assessed the associations of unsatisfactory FIT with several system and process-of-care factors, including type of FIT (3-card Hemoccult test vs Polymedco test), completion of FIT in the past 3 years, prior unsatisfactory FIT, number primary care visits within 2 years, and receiving FIT via opportunistic screening vs. mailed outreach.
Subsequent Testing after Unsatisfactory FIT
Because unsatisfactory FIT should be followed with another CRC screening test, we examined prevalence of and factors associated with receipt of any subsequent test within 15 months of the unsatisfactory FIT (e.g., another FIT, colonoscopy, flexible sigmoidoscopy, CT colonography, or barium enema). We chose 15-month cutoff to evaluate longer-term participants’ behavior after index FIT.
Statistical Analyses
We estimated the proportion of adults with unsatisfactory FIT among eligible participants with an index FIT between 2010 to 2019, and then contrasted patient, system, and process of care differences for those with a satisfactory vs. unsatisfactory FIT using chi-square and t-tests.
To examine subsequent testing after unsatisfactory FIT, we restricted analyses to patients with at least 15-months of follow-up time after the unsatisfactory FIT, and reported the proportion with subsequent tests and the median time to subsequent testing within 15 months. It is possible that subsequent tests in the 15-month window could be a repeat annual FIT and not a re-test after an unsatisfactory FIT. Therefore, we conducted sensitivity analyses to estimate prevalence of and factors associated with subsequent tests within 9 months of an unsatisfactory FIT.
We used logistic regression to identify factors associated unsatisfactory FIT, as well as factors associated with completing any subsequent test within 9 or 15 months of an unsatisfactory FIT. Variables significant at p<0.20 in univariable analysis were included in the multivariable model (p<.05 to be statistically significant in the multivariable model). All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC).
Data Availability
A subset of these data is available from the PROSPR II consortium after appropriate approvals and agreements are completed. Additional details are provided at: https://healthcaredelivery.cancer.gov/prospr/datashare/
Results
A study flow diagram is shown in Figure 1. Characteristics of the 56,980 patients who completed an index FIT are displayed in Table 1. Patients were predominantly younger (40% aged 50–54 years), female, racial/ethnic minorities, preferred speaking English, uninsured or received medical assistance, without comorbidities, and overweight or obese. They were engaged in primary care with a median of three visits in the prior two years, though few had a prior FIT (8.6%) or received a FIT by mail (9.4%). We also assessed patients’ engagement in primary care after index FIT and found that about 88% patients had a subsequent PCP visit after a satisfactory FIT, while approximately 83% patients had a PCP visit following an unsatisfactory FIT.
Figure 1.
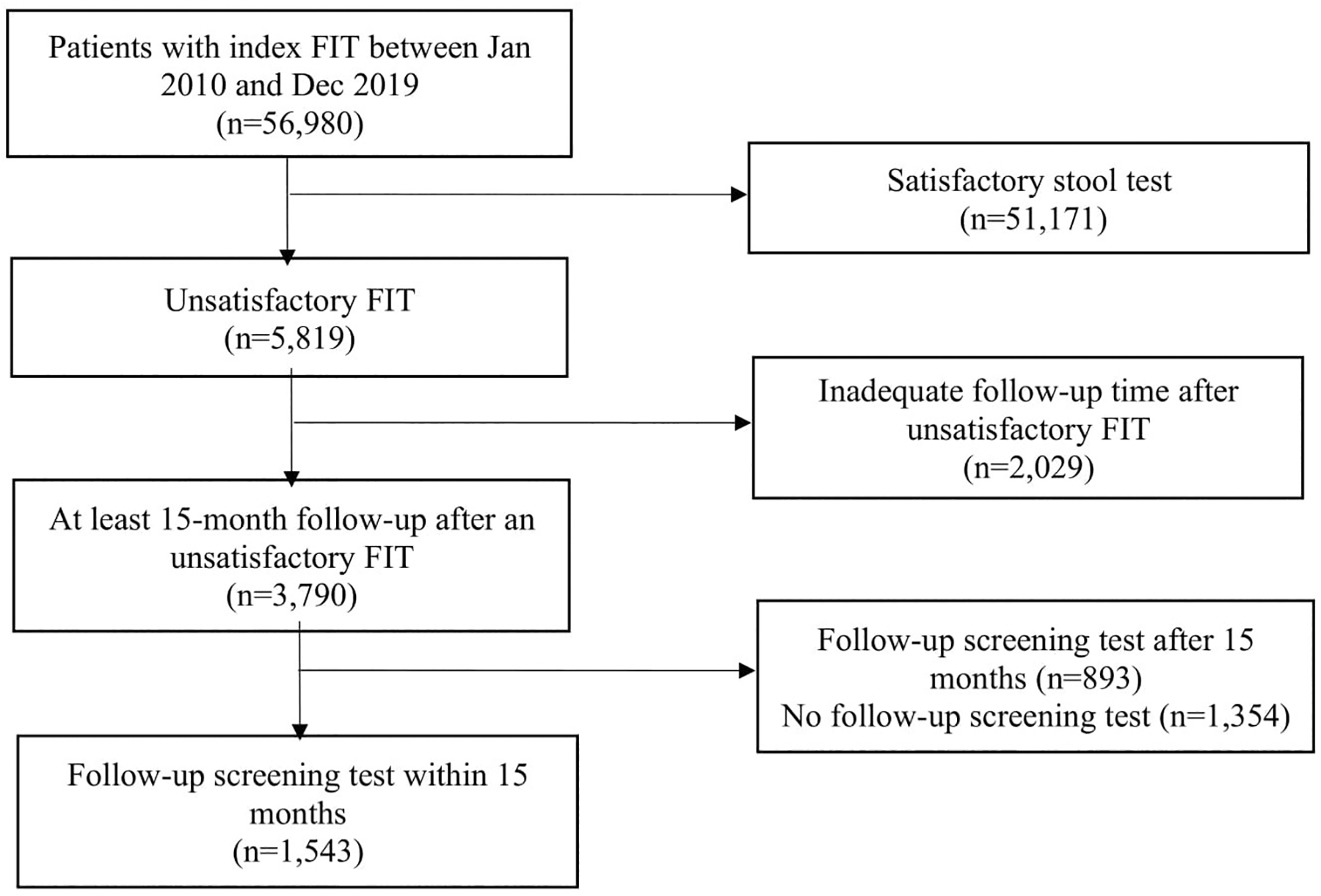
Study cohort assembly flowchart.
The entire analytical sample of 56,980 patients was used to calculate prevalence and assess predictors of unsatisfactory FIT. Of these, 3,790 patients had at least 15-month follow-up after the unsatisfactory FIT and were used to determine prevalence of subsequent test after the unsatisfactory FIT.
Table 1.
Characteristics of participants with index fecal immunochemical tests between 2010–2019*
| Unsatisfactory FIT n=5,819 n (%) | Satisfactory FIT n=51,161 n (%) | Total FIT n=56,980 n (%) | p-value | |
|---|---|---|---|---|
| Age (years) | <.01 | |||
| 50–54 | 2,341 (40.2%) | 20,282 (39.6%) | 22,623 (39.7%) | |
| 55–59 | 1,557 (26.8%) | 12,368 (24.2%) | 13,925 (24.4%) | |
| 60–65 | 1,192 (20.5%) | 10,791 (21.1%) | 11,983 (21.0%) | |
| 65+ | 729 (12.5%) | 7,720 (15.1%) | 8,449 (14.8%) | |
| Sex | <.01 | |||
| Male | 2,384 (41.0%) | 19,685 (38.5%) | 22,069 (38.7%) | |
| Female | 3,435 (59.0%) | 31,476 (61.5%) | 34,911 (61.3%) | |
| Race/ethnicity | <.01 | |||
| Non-Hispanic White | 620 (10.7%) | 6,504 (12.7%) | 7,124 (12.5%) | |
| Non-Hispanic Black | 2,222 (38.2%) | 16,012 (31.3%) | 18,234 (32.0%) | |
| Hispanic | 2,706 (46.5%) | 25,064 (49.0%) | 27,770 (48.7%) | |
| Other | 271 (4.7%) | 3,581 (7.0%) | 3,852 (6.8%) | |
| Preferred language | <.01 | |||
| English | 3,281 (56.4%) | 27,166 (53.1%) | 30,447 (53.4%) | |
| Spanish | 2,295 (39.4%) | 20,952 (41.0%) | 23,247 (40.8%) | |
| Others | 243 (4.2%) | 3,043 (5.9%) | 3,286 (5.8%) | |
| Insurance type | <.01 | |||
| Commercial | 833 (14.7%) | 7,726 (15.3%) | 8,559 (15.3%) | |
| Medicaid | 774 (13.7%) | 4,959 (9.8%) | 5,733 (10.2%) | |
| Medicare | 668 (11.8%) | 6,038 (12.0%) | 6,706 (12.0%) | |
| Charity | 3,039 (53.6%) | 28,672 (56.9%) | 31,711 (56.5%) | |
| Other | 350 (6.2%) | 3,027 (6.0%) | 3,377 (6.0%) | |
| Comorbidity score | <.01 | |||
| 0 | 3,063 (52.7%) | 28,458 (55.6%) | 31,521 (55.3%) | |
| 1 | 1,724 (29.6%) | 14,241 (27.8%) | 15,965 (28.0%) | |
| ≥2 | 1,032 (17.7%) | 8,462 (16.6%) | 9,494 (16.7%) | |
| Body mass index (kg/m2) | 0.15 | |||
| <25 | 1,068 (18.4%) | 9,252 (18.1%) | 10,320 (18.1%) | |
| 25–30 | 1,915 (32.9%) | 16,306 (31.9%) | 18,221 (32.0%) | |
| ≥30 | 2,836 (48.7%) | 25,603 (50.0%) | 28,439 (49.9%) | |
| Type of index FIT | <.01 | |||
| Hemoccult 3-card test | 4,403 (75.7%) | 43,225 (84.5%) | 47,628 (83.6%) | |
| Polymedco tube test | 1,416 (24.3%) | 7,936 (15.5%) | 9,352 (16.4) | |
| Prior FIT within 3 years | 0.65 | |||
| No | 5,318 (91.4%) | 46,840 (91.5%) | 52,158 (91.5%) | |
| Yes | 503 (8.6%) | 4,331 (8.5%) | 4,834 (8.5%) | |
| Prior unsatisfactory FIT** | <.01 | |||
| No | 5,767 (99.1%) | 50,993 (99.7%) | 56,760 (99.6%) | |
| Yes | 54 (0.9%) | 178 (0.3%) | 232 (0.4%) | |
| Number of PCP visits (2 years) | <.01 | |||
| Median (IQR) | 3 (2–5) | 3 (1–5) | 3 (1–5) | |
| Exposed to mailed FIT outreach | <.01 | |||
| No | 5,275 (90.6%) | 49,651 (97.0%) | 54,926 (96.4%) | |
| Yes | 546 (9.4%) | 1,520 (3.0%) | 2,066 (3.6%) |
FIT, fecal immunochemical test; IQR, interquartile range,
Values at the time of index FIT completion
Values 3 years before index FIT completion
Overall, 5,819 (10.2%) of FIT were unsatisfactory. Prevalence of unsatisfactory FIT ranged from 8.2% to 12.7% during the study period (Supplementary Fig S2). Half of unsatisfactory FIT (51%) were due to an inadequate specimen, one-quarter (27%) were due to incomplete labeling, 13% of the specimens were too old, and 8% had a broken or leaking container (Figure 2a). Among those exposed to mailed FIT outreach program, inadequate specimen accounted for 41%, incomplete label for 37%, and broken or leaking container for 16% of all unsatisfactory FIT (Figure 2b). When compared by type of FIT, unsatisfactory Polymedco tests were most commonly due to incomplete label or broken container, while unsatisfactory Hemoccult tests were due to insufficient specimen or old specimen (Supplementary Figure S3).
Figure 2.
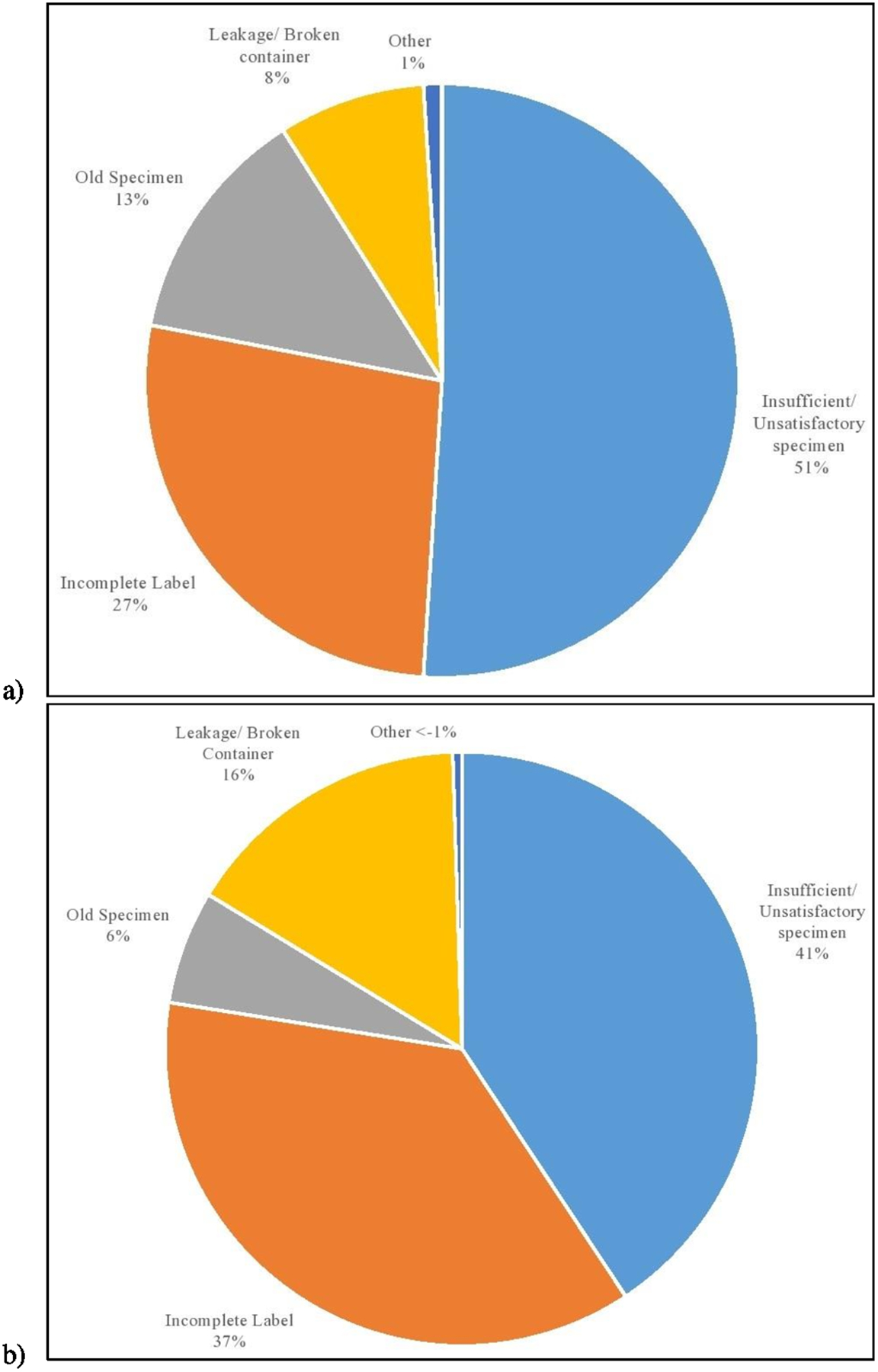
Reasons for an unsatisfactory fecal immunochemical test. a) For all participants, b) For those in the mailed FIT outreach program
Insufficient sample was the most common reason for unsatisfactory FIT among the entire analytical sample as well as among those were exposed to the mailed FIT outreach program.
Relative to patients with a satisfactory FIT, patients with an unsatisfactory FIT were more likely to be men (OR 0.91, 95% CI 0.86–0.97), younger (55–59 years, OR 1.09, 95% CI 1.01–1.16), non-Hispanic Black (OR 1.46, 95% CI 1.33–1.61), prefer speaking Spanish (OR 1.12, 95% CI 1.01–1.24), covered by Medicaid (OR 1.42, 95% CI 1.28–1.58), and diagnosed with a comorbidity (OR 1.14, 95% CI 1.07–1.21) (Table 2). Unsatisfactory FIT was associated with having a Polymedco test (OR 1.32, 95% CI 1.22–1.43), prior unsatisfactory FIT (OR 2.74, 95% CI 2.00–3.74), prior primary care encounters (OR 1.03, 95% CI 1.02–1.04), and receiving a FIT via mailed outreach (OR 2.66, 95% CI 2.35–3.01). In sensitivity analyses, stratifying the multivariable model by type of test, the results remained similar (Supplementary Table S2).
Table 2.
Factors associated with unsatisfactory FIT among patients completing FIT in 2010–2019 (N=56,980)
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p- value * | OR | 95% CI | p-value** | |
| Age (years) | ||||||
| 50–54 | Ref | Ref | ||||
| 55–59 | 1.09 | (1.02, 1.17) | <.01 | 1.09 | (1.01,1.16) | <.01 |
| 60–64 | 0.96 | (0.90, 1.03) | 0.88 | 0.94 | (0.87, 1.02) | 0.26 |
| 65+ | 0.82 | (0.75, 0.89) | <.01 | 0.87 | (0.78, 0.96) | 0.01 |
| Sex | ||||||
| Male | Ref | Ref | ||||
| Female | 0.90 | (0.85, 0.95) | <.01 | 0.91 | (0.86, 0.97) | <.01 |
| Race/ethnicity | ||||||
| Non-Hispanic White | Ref | Ref | ||||
| Non-Hispanic Black | 1.45 | (1.32,1.60) | <.01 | 1.46 | (1.33, 1.61) | <.01 |
| Hispanic | 1.13 | (1.03, 1.24) | 0.02 | 1.07 | (0.95, 1.21) | 0.81 |
| Other | 0.79 | (0.68, 0.92) | <.01 | 0.87 | (0.73, 0.99) | <.01 |
| Preferred language | ||||||
| English | Ref | Ref | ||||
| Spanish | 0.91 | (0.86, 0.96) | <.01 | 1.12 | (1.01, 1.24) | 0.03 |
| Others | 0.66 | (0.58, 0.76) | <.01 | 0.95 | (0.81, 1.11) | 0.20 |
| Insurance type | ||||||
| Commercial | Ref | Ref | ||||
| Medicaid | 1.45 | (1.30, 1.61) | <.01 | 1.42 | (1.28, 1.58) | <.01 |
| Medicare | 1.03 | (0.92, 1.14) | 0.08 | 1.19 | (0.99, 1.30) | 0.68 |
| Charity | 0.98 | (0.91, 1.07) | 0.05 | 1.13 | (0.98, 1.20) | 0.11 |
| Other | 1.08 | (0.94, 1.23) | 0.70 | 1.16 | (1.00, 1.32) | 0.79 |
| BMI (kg/m2) | ||||||
| <25 | Ref | |||||
| 2 −30 | 1.02 | (0.94, 1.10) | 0.21 | 1.07 | (0.98, 1.16) | 0.08 |
| ≥30 | 0.96 | (0.89, 1.03) | 0.08 | 0.97 | (0.89, 1.05) | 0.12 |
| Comorbidity score | ||||||
| 0 | Ref | Ref | ||||
| 1 | 1.12 | (0.98, 1.20) | 0.09 | 1.14 | (1.07, 1.21) | 0.01 |
| ≥2 | 1.13 | (0.89, 1.22) | 0.07 | 1.08 | (1.00, 1.17) | 0.72 |
| Type of index FIT | ||||||
| Hemoccult 3-card test | Ref | Ref | ||||
| Polymedco tube test | 1.75 | (1.64, 1.87) | <.01 | 1.32 | (1.22, 1.43) | <.01 |
| Prior FIT within 3 years | ||||||
| No | Ref | |||||
| Yes | 1.02 | (0.93, 1.13) | 0.63 | |||
| Prior unsatisfactory FIT | ||||||
| No | Ref | Ref | ||||
| Yes | 2.68 | (1.98, 3.64) | <.01 | 2.74 | (2.00, 3.74) | <.01 |
| Prior primary care encounters | 1.03 | (1.02,1.04) | <.01 | 1.03 | (1.02, 1.04) | <.01 |
| Exposed to mailed FIT outreach | ||||||
| No | Ref | Ref | ||||
| Yes | 3.38 | (3.06, 3.75) | <.01 | 2.66 | (2.35, 3.01) | <.01 |
CI, confidence interval; FIT, fecal immunochemical test; OR, odds ratio
p-value <0.2 considered significant to be included for multivariable model
p-value<.05 considered statistically significant
Of the 5,819 patients with unsatisfactory FIT, 3,790 (65%) had at least 15-months of follow-up after the unsatisfactory FIT. Of these 3,790 patients, fewer than half (n=1,543, 40.7%) had a subsequent test within 15 months of their unsatisfactory FIT. Among those with at least 15-months follow-up time, 1,445 patients (38.1%) had a subsequent FIT with a median time to subsequent test of 4.2 months (Interquartile range; IQR 1.2–8.2 months). Only about2.5% (95 patients) had a subsequent colonoscopy (median time to test, 6.8 months; IQR 3.1–10.7 months). One patient (0.03%) had flexible sigmoidoscopy within 4.3 months, while 0.05% (2 patients) had barium enema within a median time of 3.4 months (IQR: 1.2–5.6 months). (Supplementary Table S3).
Multivariable logistic regression (Table 3) revealed that older adults (60–64 years, OR 0.86, 95% CI 0.72–0.99) and those on Medicaid (OR 0.67, 95% CI 0.51–0.88) were less likely to have a subsequent CRC screening test within 15 months of an unsatisfactory FIT. By contrast, people who completed the Polymedco tube test (OR 1.77, 95% CI 1.42–2.20), had more primary care encounters (OR 1.05, 95% CI 1.02–1.07), or were exposed to the mailed FIT outreach program (OR 1.92, 95% CI 1.49–2.09) were more likely to have a subsequent CRC screening test within 15 months of an unsatisfactory FIT.
Table 3.
Factors associated with subsequent testing within 15 months among patients with unsatisfactory fecal immunochemical test (N=3790)
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value * | OR | 95% CI | p-value** | |
| Age (years) | ||||||
| 50–54 | Ref | Ref | ||||
| 55–59 | 1.07 | (0.91, 1.27) | 0.32 | 1.08 | (0.91,1.28) | 0.20 |
| 60–64 | 0.89 | (0.74, 1.06) | 0.03 | 0.86 | (0.72, 0.99) | 0.01 |
| 65+ | 1.11 | (0.91, 1.35) | 0.18 | 1.08 | (0.86, 1.35) | 0.34 |
| Sex | ||||||
| Male | Ref | |||||
| Female | 1.08 | (0.95, 1.24) | 0.25 | |||
| Race/ethnicity | ||||||
| Non-Hispanic White | Ref | Ref | ||||
| Non-Hispanic Black | 0.79 | (0.64, 0.99) | <.01 | 0.82 | (0.65, 1.03) | 0.08 |
| Hispanic | 1.07 | (0.87, 1.33) | 0.07 | 0.95 | (0.71, 1.26) | 0.86 |
| Other | 1.03 | (0.73, 1.44) | 0.58 | 0.97 | (0.64, 1.48) | 0.77 |
| Language | ||||||
| English | Ref | Ref | ||||
| Spanish | 1.31 | (1.15, 1.50) | 0.09 | 1.18 | (0.92, 1.50) | 0.76 |
| Others | 1.26 | (0.94, 1.69) | 0.52 | 1.26 | (0.86, 1.85) | 0.46 |
| Insurance type | ||||||
| Commercial | Ref | Ref | ||||
| Medicaid | 0.64 | (0.49, 0.83) | <.01 | 0.67 | (0.51, 0.88) | 0.02 |
| Medicare | 0.76 | (0.59, 0.97) | 0.23 | 0.79 | (0.60, 1.02) | 0.60 |
| Charity | 0.85 | (0.69, 1.03) | 0.88 | 0.81 | (0.66, 1.00) | 0.80 |
| Other | 1.01 | (0.73, 1.40) | 0.12 | 0.89 | (0.63, 1.25) | 0.53 |
| BMI (kg/m2) | ||||||
| < 25 | Ref | |||||
| ≥25 - < 30 | 1.36 | (1.12, 1.64) | 0.03 | 1.14 | (0.93, 1.39) | 0.14 |
| ≥ 30 | 1.35 | (1.12, 1.64) | 0.03 | 1.14 | (0.94, 1.39) | 0.24 |
| Comorbidity score | ||||||
| 0 | Ref | Ref | ||||
| 1 | 1.14 | (0.98, 1.32) | 0.02 | 1.10 | (0.95, 1.29) | 0.06 |
| ≥2 | 0.92 | (0.77, 1.10) | 0.09 | 0.91 | (0.75, 1.09) | 0.11 |
| Type of index FIT | ||||||
| Hemoccult 3-card test | Ref | Ref | ||||
| Polymedco test | 1.65 | (1.35, 2.01) | <.01 | 1.77 | (1.42, 2.20) | <.01 |
| Prior FIT within 3 years | ||||||
| No | Ref | |||||
| Yes | 1.69 | (1.38, 2.06) | <.01 | |||
| Prior unsatisfactory FIT | ||||||
| No | Ref | Ref | ||||
| Yes | 1.59 | (0.91, 2.78) | 0.10 | 1.39 | (0.78, 2.48) | 0.27 |
| Prior primary care encounters | 1.05 | (1.03,1.08) | <.01 | 1.05 | (1.02, 1.07) | <.01 |
| Exposed to mailed FIT outreach | ||||||
| No | Ref | Ref | ||||
| Yes | 1.46 | (0.82, 2.62) | 0.19 | 1.92 | (1.49, 2.09) | 0.03 |
CI, confidence interval; FIT, fecal immunochemical test; OR, odds ratio
p-value <0.2 considered significant to be included for multivariable model
p-value<.05 considered statistically significant
In sensitivity analyses, prevalence of a subsequent test within 9 months of the unsatisfactory FIT (32%) was lower than prevalence of a subsequent test within 15 months (41%). However, multivariable analyses revealed that factors associated with subsequent testing within 9 months were similar to those with subsequent test within 15 months (Supplementary Table S4).
Discussion
In this population-based cohort of 56,980 patients who completed a FIT over a 10-year period in an integrated safety-net health system, 10% of FIT were unsatisfactory. Half of the inadequate tests were due to specimen problems, one-quarter due to sample labeling, and 13% mailed in too late after specimen collection. Our findings also highlight another important breakdown in the screening process, as less than half of patients with unsatisfactory FIT completed a subsequent test within 15 months. While many studies have sought to optimize stool-based testing, few had focused on ensuring return of satisfactory stool samples (24,25,28). Our study provides real-world data to inform quality assurance for CRC screening programs.
We identified several groups of patients more likely to have unsatisfactory stool tests: younger adults (those aged 50–54 years), male, non-Hispanic Black, Spanish-speaking, and Medicaid beneficiaries. These findings are consistent with those of prior research and are likely associated with multiple barriers to care, such as lower socioeconomic status, health literacy, and language barriers (29). Patients who received FIT in mailed FIT outreach programs had approximately 3-fold higher odds of unsatisfactory tests. This may be because patients who received a FIT by mail received only written instructions (in both English and Spanish) on specimen collection, whereas clinic-based distribution of FIT likely included more detailed or direct oral instructions from clinic or laboratory staff, with reminders to avoid common missteps (e.g. “please write your name and the date on the tube”) (25).
In this study, fewer than half (41%) of patients with unsatisfactory FIT completed a subsequent test within 15 months. Just over a third (38%) had another stool-based test, while only 3% received a colonoscopy. Patients who received FIT via mailed outreach had about 2-fold increase in completing a subsequent test, perhaps because navigators in the mailed outreach program called patients who returned an unsatisfactory FIT whereas providers who distributed FIT in the office were not notified of an uncollected or unsatisfactory test. Frequently, the primary care provider would only become aware of this during the next clinical encounter 6–12 months later when a FIT would have to be reordered.
Based on our findings and best practices from the literature, Table 4 outlines strategies to address common reasons for unsatisfactory FIT that can be used to further improve effectiveness of CRC screening programs. First, more robust patient education strategies are needed to address these common breakdowns. Low literacy, wordless, visual instructions can improve patient handling of stool samples (28,30). System solutions can also mitigate common breakdowns. Some of the most successful mailed FIT programs in the U.S. affix a patient name or unique test order bar code onto the FIT or add a patient reminder sticker on the test to write your name and the completion date to minimize the common “no patient name” or “no date” errors (25). Laboratory policy changes can allow using sample ordering, mailing, or receiving date as the collection date, if the date is missing and the test was returned within two weeks of being ordered. While sensitivity of FIT decreases with longer sample return time (31), changes in laboratory policies can also allow processing of samples beyond the two week return window and report abnormal results as true positives for certain test kits (14). Other strategies may include automated test kit distribution for unsatisfactory stool tests, administrative registries that identify incomplete tests in addition to any abnormal results, and patient navigation to reduce gaps and disparities in CRC screening (23,32,33).
Table 4.
Strategies to address common reasons for unsatisfactory FIT tests
| Reason for unsatisfactory FIT | Examples | Potential intervention strategies |
|---|---|---|
| Inadequate specimen |
|
|
| Incomplete label |
|
|
| Old specimen |
|
|
| Broken container |
|
|
| Failed to complete subsequent test |
|
|
CRC, colorectal cancer; FIT, fecal immunochemical test; URL, uniform resource locator
This refers to differences such as DD/MM/YYYY, MM/DD/YYYY, or YYYY/MM/DD for non-US people
Based on expert opinion, the US MSTF suggests the proportion of returning FIT that cannot be processed by the laboratory should be less than 5% (4), whereas the Irish National Screening Service has a threshold of unacceptable tests less than 3%, with ≥ 95% repeat test kits dispatched to clients within 10 working days following receipt of unacceptable stool tests (34). Our study, reflecting a decade of experience in a large, US safety net health system, found a higher proportion of unsatisfactory FIT in real-world practice than the optimal rate expressed in guidelines. In a large Veterans Affairs health system in Los Angeles, the FIT rejection rate went down from 29% to 7% after local quality improvement efforts (24). The STOP-CRC trial showed around 41% of FIT were unsatisfactory, which improved to 25% after quality improvement initiatives (25). A large integrated safety-net health system in San Francisco reported nearly 20% of mishandled FIT samples (28). Elsewhere, pre-printed patient labels and manual review of FIT with no date or returned more than 14 days from collection resulted in only 2% of FIT not processed in a large health system in North California(35). Taken together, this and prior studies provide real-world evidence on the prevalence and reasons for breakdowns in the FIT-based screening process that can inform quality standards and population health improvement initiatives.
Strengths of the study include a decade worth of longitudinal EHR data on unsatisfactory FIT among a large, racially and ethnically diverse, population-based cohort in an integrated safety-net health system. The laboratory in our health system documented the reasons for unsatisfactory FIT whereas many do not. While the specific absolute rates of different breakdowns in the process might be different in other settings, the most common reasons for unsatisfactory results should be generalizable and can inform quality improvement strategies. However, several limitations are worth noting. This study was performed in a safety-net health system that may not be generalizable to other practices and populations. We were limited to using the reasons for an unsatisfactory FIT as reported by the laboratory results system, and therefore lacked details about failures in the screening process (patient, provider, or system-level). For instance, if the unsatisfactory FIT was due to an incomplete label, we could not delineate if the name, date, or both were missing or unreadable. Additionally, we could not ascertain whether patients received subsequent screening outside of the safety-net health system, although this was unlikely because the vast majority of patients were uninsured or underinsured, so had very few care options outside of Parkland. Finally, because we used 15-month cutoff for subsequent testing after unsatisfactory FIT, it is possible that a small portion of subsequent tests were not related to the unsatisfactory FIT but rather annual, repeat FIT. The median time to subsequent testing was 4.4 months (Interquartile range: 1.2–8.3 months), and sensitivity analyses using a 9-month cutoff found similar associations.
In summary, one in ten of the nearly 57,000 FIT completed for CRC screening could not be processed by a laboratory, mostly due to errors in completing the test. That most unsatisfactory stool tests did not have a subsequent screening test highlights the need for systems to have a better, more comprehensive approach to flagging and following up unsatisfactory FIT. Unsatisfactory stool tests can reduce effectiveness of this screening modality, especially without timely and appropriate repeat testing. With the growing use of stool-based CRC screening in the US and around the world, our results may inform design of patient education and system improvement strategies to improve screening delivery in real-world settings.
Supplementary Material
Acknowledgements
Research reported in this article was supported by the NIH under award numbers U54 CA163308 and UM1 CA222035 (to C.S. Skinner, E.A. Halm), T32 DK007745 (to E. Burstein), and the Cancer Prevention and Research Institute of Texas under award number PP160075 (to A.G. Singal).
Role of sponsor:
The study sponsors have no role in the study design, collection, analysis, and interpretation of data.
Abbreviations:
- CRC
Colorectal cancer
- FOBT
Fecal occult blood test
- FIT
fecal immunochemical test
- CT
Computed Tomography
Footnotes
Competing interests:
CCM reports consulting for Freenome. BBG is a member of the National Colorectal Cancer Round Table Steering Committee, sponsored by the American Cancer Society and Centers for Disease and Infection Control; she receives no compensation for this position except for travel expenses to attend meetings. TRL reports research funding from Freenome. The remaining authors have no conflicts to disclose.
References
- 1.Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;(December 2022):233–54. [DOI] [PubMed] [Google Scholar]
- 2.Welch HG, Robertson DJ. Colorectal Cancer on the Decline — Why Screening Can’t Explain It All. N Engl J Med. 2016;374(17):1605–7. [DOI] [PubMed] [Google Scholar]
- 3.Liu PH, Singal AG, Murphy CC. Stool-Based Tests Mitigate Impacts of COVID-19 on Colorectal Cancer Screening. Clin Gastroenterol Hepatol. 2023;(January). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology [Internet]. 2017;153(1):307–23. Available from: 10.1053/j.gastro.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 5.Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, García FAR, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA - J Am Med Assoc. 2016;315(23):2564–75. [DOI] [PubMed] [Google Scholar]
- 6.Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA - J Am Med Assoc. 2021;325(19):1978–97. [DOI] [PubMed] [Google Scholar]
- 7.Chiu HM, Chen SLS, Yen AMF, Chiu SYH, Fann JCY, Lee YC, et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the One Million Taiwanese Screening Program. Cancer. 2015;121(18):3221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robertson DJ, Lee JK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, et al. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology [Internet]. 2017;152(5):1217–1237.e3. Available from: 10.1053/j.gastro.2016.08.053 [DOI] [PubMed] [Google Scholar]
- 9.DeBourcy AC, Lichtenberger S, Felton S, Butterfield KT, Ahnen DJ, Denberg TD. Community-based preferences for stool cards versus colonoscopy in colorectal cancer screening. J Gen Intern Med. 2008;23(2):169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas Á, et al. Colonoscopy versus Fecal Immunochemical Testing in Colorectal-Cancer Screening. N Engl J Med. 2012;366(8):697–706. [DOI] [PubMed] [Google Scholar]
- 11.Singal AG, Gupta S, Tiro JA, Skinner CS, McCallister K, Sanders JM, et al. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: A randomized controlled trial in a safety net health system. Cancer [Internet]. 2016;122:456–63. Available from: https://www.ptonline.com/articles/how-to-get-better-mfi-results [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coronado GD, Leo MC, Ramsey K, Coury J, Petrik AF, Patzel M, et al. Mailed fecal testing and patient navigation versus usual care to improve rates of colorectal cancer screening and follow-up colonoscopy in rural Medicaid enrollees: a cluster-randomized controlled trial. Implement Sci Commun [Internet]. 2022;3(1):1–15. Available from: 10.1186/s43058-022-00285-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Somsouk M, Rachocki C, Mannalithara A, Garcia D, Laleau V, Grimes B, et al. Effectiveness and cost of organized outreach for colorectal cancer screening: A randomized, controlled trial. J Natl Cancer Inst. 2021;112(3):305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta S, Coronado GD, Argenbright K, Brenner AT, Castañeda SF, Dominitz JA, et al. Mailed fecal immunochemical test outreach for colorectal cancer screening: Summary of a Centers for Disease Control and Prevention–sponsored Summit. CA Cancer J Clin. 2020;70(4):283–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson DJ, Selby K. Fecal Immunochemical Test: The World’s Colorectal Cancer Screening Test. Gastrointest Endosc Clin N Am [Internet]. 2020;30(3):511–26. Available from: 10.1016/j.giec.2020.02.011 [DOI] [PubMed] [Google Scholar]
- 16.Jager M, Demb J, Asghar A, Selby K, Mello EM, Heskett KM, et al. Mailed Outreach Is Superior to Usual Care Alone for Colorectal Cancer Screening in the USA: A Systematic Review and Meta-analysis. Dig Dis Sci. 2019;64(9):2489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dougherty MK, Brenner AT, Crockett SD, Gupta S, Wheeler SB, Coker-Schwimmer M, et al. Evaluation of Interventions Intended to Increase Colorectal Cancer Screening Rates in the United States: A Systematic Review and Meta-analysis. JAMA Intern Med. 2018;178(12):1645–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Issaka RB, Akinsoto NO, Strait E, Chaudhari V, Flum DR, Inadomi JM. Effectiveness of a mailed fecal immunochemical test outreach: a Medicare Advantage pilot study. Therap Adv Gastroenterol. 2020;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Star J, Bandi P, Siegel RL, Han X, Minihan A, Smith RA, et al. Cancer Screening in the United States During the Second Year of the COVID-19 Pandemic. J Clin Oncol [Internet]. 2023; Available from: 10.1200/JCO.22.02170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladabaum U, Dominitz JA, Kahi C, Schoen RE. Strategies for Colorectal Cancer Screening. Gastroenterology [Internet]. 2020;158(2):418–32. Available from: 10.1053/j.gastro.2019.06.043 [DOI] [PubMed] [Google Scholar]
- 21.Selby K, Baumgartner C, Levin TR, Doubeni CA, Zauber AG, Schottinger J, et al. Interventions to improve follow-up of positive results on fecal blood tests: A systematic review. Ann Intern Med. 2017;167(8):565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corley DA, Jensen CD, Quinn VP, Doubeni CA, Zauber AG, Lee JK, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal Cancer and Cancer stage at Diagnosis. JAMA - J Am Med Assoc. 2017;317(16):1631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy CC, Halm EA, Skinner CS, Balasubramanian BA, Singal AG. Challenges and approaches to measuring repeat fecal immunochemical test for colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2020;29(8):1557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng C, Ganz DA, Chang ET, Huynh A, De Peralta S. Reducing Rejected Fecal Immunochemical Tests Received in the Laboratory for Colorectal Cancer Screening. J Healthc Qual. 2019;41(2):75–82. [DOI] [PubMed] [Google Scholar]
- 25.Coury J, Schneider JL, Rivelli JS, Petrik AF, Seibel E, D’Agostini B, et al. Applying the Plan-Do-Study-Act (PDSA) approach to a large pragmatic study involving safety net clinics. BMC Health Serv Res. 2017;17(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singal AG, Gupta S, Skinner CS, Ahn C, Santini NO, Agrawal D, et al. Effect of colonoscopy outreach vs fecal immunochemical test outreach on colorectal cancer screening completion a randomized clinical trial. JAMA - J Am Med Assoc. 2017;318(9):806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy CC, Halm EA, Zaki T, Johnson C, Yekkaluri S, Quirk L, et al. Colorectal Cancer Screening and Yield in a Mailed Outreach Program in a Safety-Net Healthcare System. Dig Dis Sci [Internet]. 2022;67(9):4403–9. Available from: 10.1007/s10620-021-07313-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang A, Rachocki C, Shapiro JA, Issaka RB, Somsouk M. Low Literacy Level Instructions and Reminder Calls Improve Patient Handling of Fecal Immunochemical Test Samples. Clin Gastroenterol Hepatol [Internet]. 2019;17(9):1822–8. Available from: 10.1016/j.cgh.2018.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carethers JM, Doubeni CA. Causes of Socioeconomic Disparities in Colorectal Cancer and Intervention Framework and Strategies. Gastroenterology [Internet]. 2020;158(2):354–67. Available from: 10.1053/j.gastro.2019.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coronado GD, Sanchez J, Petrik A, Kapka T, Devoe J, Green B. Advantages of wordless instructions on how to complete a fecal immunochemical test: Lessons from patient advisory council members of a federally qualified health center. J Cancer Educ. 2014;29(1):86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Roon AH, Hol L, van Vuuren AJ, Francke J, Ouwendijk M, Heijens A, et al. Are Fecal Immunochemical Test Characteristics Influenced by Sample Return Time? A Population-Based Colorectal Cancer Screening Trial. Am J Gastroenterol. 2012;107(1):99–107. [DOI] [PubMed] [Google Scholar]
- 32.Cusumano VT, Myint A, Corona E, Yang L, Bocek J, Lopez AG, et al. Patient Navigation After Positive Fecal Immunochemical Test Results Increases Diagnostic Colonoscopy and Highlights Multilevel Barriers to Follow-Up. Dig Dis Sci [Internet]. 2021;66(11):3760–8. Available from: 10.1007/s10620-021-06866-x [DOI] [PubMed] [Google Scholar]
- 33.Zapka JM, Edwards HM, Chollette V, Taplin SH. Follow-up to abnormal cancer screening tests: Considering the multilevel context of care. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Cancer Screening Service. Guidelines for quality assurance in colorectal cancer screening. 2nd ed. Dublin: [Internet]. 2017. [cited 2022 May 20]. Available from: https://www.screeningservice.ie/publications/BS-Guidelines-for-Quality-Assurance-in-Colorectal-Screening.pdf [Google Scholar]
- 35.Selby K, Jensen CD, Levin TR, Lee JK, Schottinger JE, Zhao WK, et al. Program Components and Results From an Organized Colorectal Cancer Screening Program Using Annual Fecal Immunochemical Testing. Clin Gastroenterol Hepatol. 2022;20(1):145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A subset of these data is available from the PROSPR II consortium after appropriate approvals and agreements are completed. Additional details are provided at: https://healthcaredelivery.cancer.gov/prospr/datashare/


