Abstract
The Peruvian Andes are the natural habitat of several wild blackberry species that are little known and exploited due to the lack of technological and scientific development to support their agricultural potential. In this context, a study was conducted to understand the physicochemical composition, bioactive compounds, antimicrobial activity, and in vitro multiplication of four wild blackberry (Rubus sp.) species from the northern Peruvian highlands. The results indicate that fruits of R. floribundus presented the highest content of total soluble solids (9.58 ± 1.83°Brix) and titratable acidity (1.88 ± 0.07% citric acid). The fruits of R. weberbaueri recorded the highest total phenolic content (415.06 ± 8.69 mg GAE/100 g Ff). The antioxidant capacity determined by the DPPH assay varied significantly among species, with the highest value found in fruits of R. andicola (50.27 ± 0.11 mg TE/100 g Ff). The fruit extracts of R. weberbaueri and R. andicola showed better antimicrobial activity, with Staphylococcus aureus being the most sensitive bacterium. In the in vitro multiplication phase, the results show that BAP (6-Benzylaminopurine) has a significant effect at a dose of 1.5 mg l−1 on shoot number, leaf number, and shoot length. The results may help in the management of genetic resources.
Keywords: Antimicrobial, Antioxidant, Blackberry, Micropropagation
Subject terms: Biotechnology, Plant sciences
Introduction
Blackberry is one of the most widespread berries of the genus Rubus (Rosaceae), with species (wild and cultivated) widely distributed throughout the world. Today, the popularity of Rubus species has increased due to the high levels of anthocyanins and phenolic compounds in their fruits1–3; bioactive compounds with antioxidant and anticarcinogenic activity4,5.
Numerous wild species, many of which have the potential to be exploited in agriculture, can be found thriving in the high Andean lands of Peru6. These species are widely distributed throughout the territory due to the presence of ecological floors and climates conducive to their development. However, the fruits of the agrobiodiversity of the Rubus genus are little known and exploited due to the lack of knowledge of their physicochemical characteristics and the technological limitations that promote their domestication, valorization, and protection7.
According to a recent study, the genus Rubus contains the most diverse group of berries in the Andes in northern Peru (Amazonas region)8. These wild species can be distinguished by distinctive morphological characteristics, and their fruits can be consumed fresh or used for making liquor, ice cream, yogurt, or jam. They may have promising characteristics given that wild species have been observed to frequently include more phenols than domesticated species9,10. In addition, recent research has shown that a large portion of the fruits of this genus contain compounds that can inhibit or eliminate the development of pathogenic microorganisms11–14. However, it is common that the composition and healthful effects of bioactive substances from wild species are not thoroughly known13,15.
Wild blackberries have significant potential in the global market, but efforts to improve their propagation systems and promote the agricultural development of new Rubus species have been lacking16. Tissue culture is a practical alternative to encourage the cultivation of wild fruits with agricultural potential. This biotechnological tool allows obtaining numerous, healthy, and uniform offspring, while ensuring the transfer of the genetic potential of the parent17,18. However, factors such as species, explant source and growth regulator concentration have been shown to significantly influence in vitro culture19,20.
Therefore, it is important to develop studies that seek to decipher the specific composition of wild blackberries, their health benefits, as well as to optimize the use of growth regulators at each stage of micropropagation. In this context, the objective of the present study was to compare the physicochemical composition, the antioxidant capacity, the antimicrobial activity of four wild blackberry species from the northern Peruvian highlands, and to evaluate the in vitro response during multiplication under the influence of different concentrations of 6-benzylaminopurine (BAP). Thus, the results could provide important information to demonstrate the benefits of consuming this fruit and to assist in the management of genetic resources.
Materials and methods
The study was carried out at the Laboratory of Plant Physiology and Biotechnology of the National University Toribio Rodríguez de Mendoza of Amazonas. First, vigorous plants of four wild Rubus species (R. andicola, R. adenothallus, R. weberbaueri, and R. floribundus) located in the districts of Granada and Molinopampa in northern Peru were selected, at an altitude between 2150 and 3250 m asl (Table 1; Table S1). These species are distinguished by their bushy form, with white, pink, and greenish flowers, and branches with thorns. The plants found grew naturally in acid soils, in Andean cloud forest ecosystems with a very humid climate, with temperatures ranging from 7 to 25 °C and an average annual accumulated rainfall of 1500 mm.
Table 1.
Geographical location of wild species of Rubus.
| Species | Geographical coordinates | Elevation | |
|---|---|---|---|
| Latitude South | Longitude West | ||
| R. weberbaueri | 6° 08′ 59.48″ | 77° 40′ 16.09'' | 3251 |
| R. floribundus | 6° 20′ 15.31″ | 77° 31′ 06.41'' | 2156 |
| R. adenothallus | 6° 06′ 07.01″ | 77° 38′ 28.79'' | 2822 |
| R. andicola | 6° 20′ 23.72″ | 77° 31′ 00.27″ | 2188 |
Ethics statement
The experimental protocol was approved by the Institutional Committee on Research Ethics of the National University Toribio Rodríguez de Mendoza of Amazonas. The plant collection and use were in accordance with all the relevant guidelines. The identification of the species was reported by Tineo et al.8 in the research “Exploring the diversity of andean berries from northern Peru based on molecular analyses”. The samples were deposited in the KUELAP herbarium of the National University Toribio Rodríguez de Mendoza of Amazonas, with registration code KUELAP-254, KUELAP-255, KUELAP-256, and KUELAP-257.
Evaluation of physicochemical characteristics, and antioxidant capacity of fruit
Fruits were harvested when they were completely commercially ripe, undamaged, and firm to the touch (Fig. 1). The longitudinal and transverse diameter was recorded with a digital vernier and the fresh weight with an analytical balance. In addition, firmness was measured with a digital penetrometer equipped with a 4 mm conical tip.
Figure 1.
Wild blackberry fruits of (a) R. andicola, (b) R. floribundum, (c) R. andenothallus, and (d) R. webebaueri. The arrow indicates the maturity stage of fruits collected for analysis.
A pocket PAL-1 digital refractometer (Atago, Japan) was used to measure the total soluble solids content (°Brix). The pH of the pulp extract was determined with a HANNA HI2211 potentiometer (Hanna, USA). Titratable acidity was determined by potentiometric titration using a 0.1 N NaOH solution to pH 8.2 and the results were expressed as % citric acid.
To determine total phenolic compounds, the Folin-Ciocalteu method was used following the methodology of Fascella et al.21, with some modifications. Five g of freeze-dried fruit were combined with 50 mL of methanol/water (80:20, v /v) and then the mixture was centrifuged for 16 h at 400 rpm. Subsequently, a second centrifugation was completed at 5000 rpm for 15 min. Then, 1000 μL of supernatant and 2500 μL of Folin-Ciocalteu 2N were added into amber tubes. After 5 min, 2000 μL of 10% Na2CO3 was added, and the mixture was allowed to stand at room temperature for 2 h in the dark. Finally, the absorbance of the sample was measured at 725 nm on a Genesys 180 UV/Vis spectrophotometer (Thermo Scientific™, Madison, WI, USA). The calibration curve was created with dilutions of gallic acid equivalent (GAE) (0–180 mg L−1), leading to the equation: Y = 150.5x + 5.2; R2 = 0.993. Results were expressed as mg GAE/g Ff (freeze-dried fruit).
Antioxidant activity was determined by the DPPH method following the methodology of Albert et al.22, with some modifications. In an amber flask, 2.4 mg of DPPH (2,2-diphenyl-1- picrylhydrazyl) reagent was dissolved in 100 mL of methanol. The reagent was diluted with methanol to an absorbance of 0.70 ± 0.02 at a wavelength of 515 nm and left without light until use. In tubes containing 3.9 mL of DPPH solution, we added 100 μL of hydromethanolic extract. Subsequently, it was centrifuged for 1 min at 1800 rpm and allowed to stand in the dark for 30 min. The absorbance of the sample was measured at 515 nm on a Genesys 180 UV/Vis spectrophotometer (Thermo Scientific™, Madison, WI, USA). The results were expressed as mg Trolox equivalent (TE)/g Ff. The regression equation was y = 199.29x + 0.3958 (R2 = 0.996). The percentage inhibition activity was calculated as: E % = [(A0 − A1)/A0] × 100, where: A0 = the initial absorbance, and A1 = the absorbance in the presence of the extract.
Evaluation of the antimicrobial activity of blackberry extract
The antimicrobial activity of the hydromethanolic extract (mixture of 2 g of fruit with 2.5 mL of methanol/water (80:20, v/v)) of blackberry was evaluated against a Gram-positive bacterium (Staphylococcus aureus ATCC29213) and a Gram-negative bacterium (Escherichia coli ATCC25922) donated by the Regional Health Directorate—Amazonas, Peru.
To evaluate the antimicrobial activity of blackberry extract, the methodology described by Chen et al.23 was followed, with some modifications. Bacterial strains were inoculated in Mueller–Hinton (MH) broth and incubated for 24 h at 37 °C. Each strain inoculum was suspended in sterile 0.85% saline and the bacterial population was adjusted spectrophotometrically OD600 to the 0.5 McFarland (used to standardize the approximate number of bacteria in a liquid suspension by comparing the turbidity of the suspension with that of the McFarland standard) standards (1.5 × 108 CFU/mL).
The antimicrobial activity of the extracts was evaluated by detection of diameter of inhibition zone (DIZ). Bacterial suspensions (1.5 × 108 CFU/mL) were seeded uniformly on the culture medium and a sterile paper disk (6 mm) impregnated with the extract (8 μL) of blackberries was placed. It was incubated for 24 h at 37 °C. Sterile distilled water (8 μL) was used as a negative control and ampicillin (50 μg/disc) and oxacillin (50 μg/disc) as a positive control for E. coli and S. aureus, respectively.
Evaluation of in vitro multiplication
Actively growing tillers were collected and set up in 4" × 8" polyethylene bags containing a peat and rice husk-based substrate in a 3:1 ratio with a pH of 5.7. Plants were grown in a nursery for two months at a temperature of 20 ± 2 °C and relative humidity > 70%.
For in vitro establishment, sterile explants (2 cm segments with an axillary bud) were grown on MS culture medium24 supplemented with 100 mg myo-inositol, 30 g sucrose, 150 mg ascorbic acid and 6.5 g agar. The pH of the medium was adjusted to 5.8 and autoclaved at 120 °C for 20 min. Cultures were grown for 30 days in a growth room at 20 ± 2 °C and a 16-h photoperiod.
For the multiplication phase, explants adapted to the establishment medium were selected. These were extracted and cut into 1 cm fragments with an axillary bud. The explants were introduced into establishment medium supplemented with different concentrations of 6-benzylaminopurine (1.0, 1.5 and 2.0 mg l−1) and a control treatment. The pH of the medium was adjusted to 5.8 and autoclaved at 120 °C for 20 min. The explants were grown at a temperature of 20 ± 2 °C and a photoperiod of 16 h light with 1.5 k lux light intensity. At 42 days, the number of shoots, number of leaves and shoot height were evaluated. In addition, the percentage of explant loss was determined.
Experimental design and statistical analysis
The trials were conducted under a completely randomized design. A one-way analysis of variance was performed for fruit quality and antimicrobial activity parameters, while a two-way analysis of variance was performed for in vitro multiplication parameters. Each treatment consisted of 10 replicates. Means were compared by Tukey test at 5% probability of error. The analysis was performed with the statistical package InfoStad version 2020p.
Results
Evaluation of physicochemical characteristics, and antioxidant capacity of fruit
Morphometric attributes of wild blackberry fruits showed significant variation among species (Table 2). The fruits of R. andicola were characterized by greater longitudinal (27.27 ± 5.16 mm) and transverse (21.48 ± 2.90 mm) growth. In addition, it is evident that the fruits of the species (R. andicola) had a higher weight (7.56 ± 1.27 g) due to the growth achieved. All the variables contained in Table 2 show that R. floribundus presents the lowest data.
Table 2.
Morphometric fruit quality of four wild blackberry species.
| Species | Longitudinal diameter (mm) | Transverse diameter (mm) | Weight (g) | Firmness (N) |
|---|---|---|---|---|
| R. andicola | 27.27 ± 5.16 a | 21.48 ± 2.90 a | 7.56 ± 1.27 a | 4.29 ± 1.01 bc |
| R. adenothallus | 25.11 ± 1.34 ab | 16.43 ± 1.15 b | 4.12 ± 0.29 b | 5.09 ± 0.78 b |
| R. floribundus | 11.57 ± 0.39 c | 11.43 ± 0.23 c | 0.92 ± 0.11 d | 2.86 ± 0.79 c |
| R. weberbaueri | 20.67 ± 1.60 b | 14.92 ± 0.28 b | 2.38 ± 0.15 c | 6.22 ± 1.05 a |
Means within a column followed by the same letter are not significantly different according to Tukey’s test at P ≤ 0.05.
In contrast to the morphometric characteristics, the fruits of R. floribundus showed a higher content of total soluble solids with 9.58 ± 1.83°Brix. In titratable acidity, measured as percentage of citric acid, it is also observed that R. floribundus stands out over the other species with 1.88 ± 0.07% (Table 3). Regarding the content of total phenols, the fruits of wild blackberries showed significant variation among species (Table 4), with R. weberbaueri presenting the highest concentrations with 415.06 ± 8.69 mg GAE/g Ff. On the other hand, it was observed that the antioxidant capacity (determined by DPPH radical binding activity) of each blackberry species was significantly different, having the highest value in fruits of R. andicola (50.27 ± 0.11 mg TE/g Ff), followed by R. floribundus (49.59 ± 0.40 mg TE/g Ff). Overall, the data obtained showed that wild blackberries had had strong DPPH free radical scavenging effects (> 95% inhibition).
Table 3.
Comparison of total soluble solids (°Brix), pH and titratable acidity contents of fruits of four wild blackberry species.
| Species | Brix | pH | Titratable acidity (% citric acid) |
|---|---|---|---|
| R. andicola | 6.96 ± 0.09 b | 2.87 ± 0.02 d | 1.60 ± 0.11 b |
| R. adenothallus | 6.36 ± 1.09 b | 3.13 ± 0.01 b | 1.30 ± 0.04 c |
| R. floribundus | 9.58 ± 1.83 a | 2.94 ± 0.03 c | 1.88 ± 0.07 a |
| R. weberbaueri | 5.28 ± 0.11 b | 3.32 ± 0.01 a | 1.17 ± 0.04 c |
Means within a column followed by the same letter are not significantly different according to Tukey’s test at P ≤ 0.05.
Table 4.
Total phenol content and antioxidant capacity of fruit extracts from four wild blackberry species.
| Species | Total phenolic content (mg GAE/g Ff) | E% (DPPH Assay) | Antioxidant capacity (mg TE/g Ff) |
|---|---|---|---|
| R. andicola | 119.38 ± 3.30 c | 98.79 ± 0.91 a | 50.27 ± 0.11 a |
| R. adenothallus | 179.53 ± 16.68 b | 96.76 ± 0.43 b | 48.77 ± 0.22 b |
| R. floribundus | 230.15 ± 6.06 b | 98.10 ± 0.25 a | 49.59 ± 0.40 a |
| R. weberbaueri | 415.06 ± 8.69 a | 95.98 ± 0.97 b | 48.19 ± 0.59 b |
Means within a column followed by the same letter are not significantly different according to Tukey’s test at P ≤ 0.05.
Evaluation of antimicrobial activity of blackberry extract.
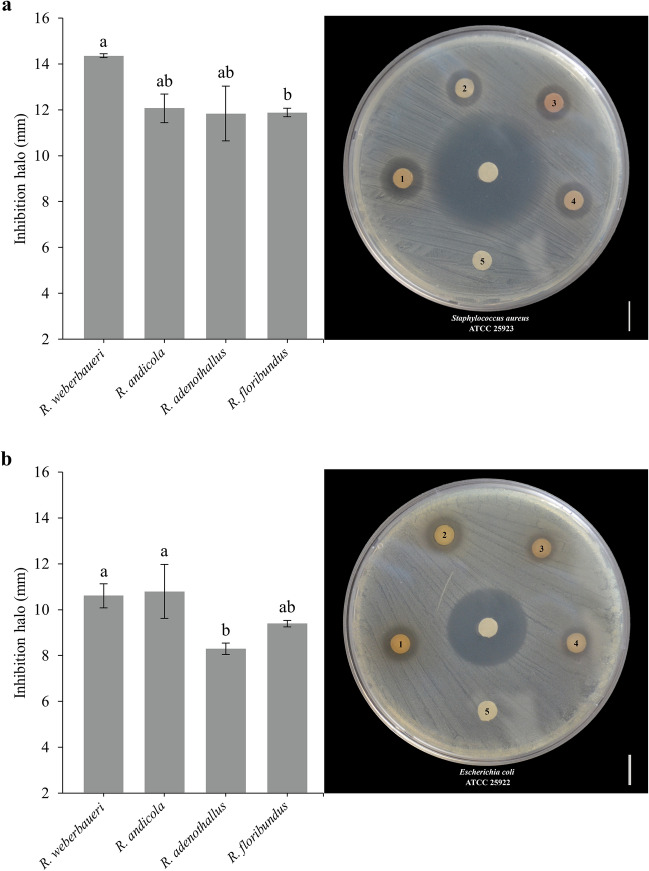
The determinations of DIZ (Fig. 1) demonstrate the high antimicrobial potential of blackberry extracts against Staphylococcus aureus (Fig. 2a) and Escherichia coli (Fig. 2b). R. weberbaueri is particularly notable for showing a wider inhibition halo against S. aureus (Disc 1 = 14 ± 1.26 mm), which places it above the other species that showed an inhibition halo of 11.5–12 mm. On the other hand, it was observed that the extracts of R. weberbaueri and R. andicola were the ones that neutralized E. coli to the greatest extent. With R. andenothallus (Disc 3 = 8 ± 0.23 mm), the minimum inhibition halo was reached.
Figure 2.
Diameter of antibacterial inhibition of wild blackberry extract against (a) Staphylococcus aureus and (b) Escherichia coli bacteria. The discs are labeled 1 for R. webebaueri, 2 for R. andicula, 3 for R. andenothallus, 4 for R. floribundum, and 5 for distilled water. The center disk includes the antibiotic ampicillin and oxacillin.
Evaluation of in vitro multiplication
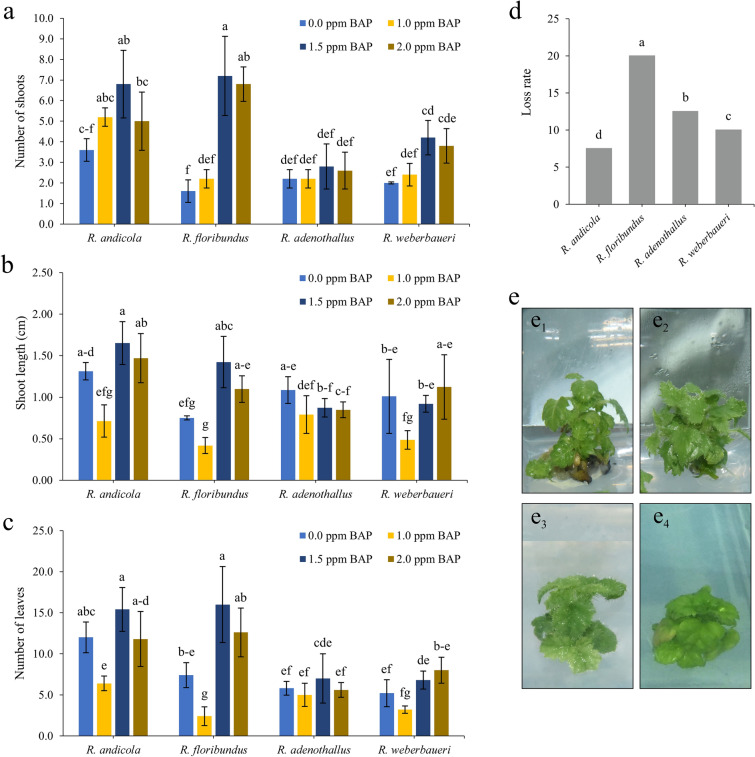
The results show that, except for R. adenothallus, all species developed on average more than 3 shoots per explant when grown on medium supplemented with 1.5 and 2 mg l−1 BAP. Among all species, R. floribundus produced the highest number of shoots (7.2 ± 1.9) when grown under the influence of 1.5 mg l−1 BAP (Fig. 3a–e1). On the other hand, it is observed that when the medium with 1.5 and 2.0 mg l−1 of BAP was used, R. andicola shoots reached a growth of more than 1.2 cm (Fig. 3b). Explants of R. floribundus and R. weberbaueri, which were grown on medium with 1.0 mg l−1 BAP, had significantly lower shoot height (0.42 and 0.49 cm, respectively).
Figure 3.
Effect of different concentrations of 6-benzylaminopurine on in vitro multiplication of four wild blackberry species. Panels are (a) number of shoots, (b) shoot height, (c) number of leaves, (d) loss rate and (e) in vitro plant regeneration. (e1) R. andicola, (e2) R. floribundus, (e3) R. adenothallus, and (e4) R. weberbaueri.
Regarding the number of leaves, R. andicola and R. floribundus developed on average more than 8 leaves when grown on medium supplemented with 1.5 and 2.0 mg l−1 of BAP. These doses improved the average number of leaves obtained by R. adenothallus and R. weberbaueri, but there were no significant differences under any treatment (Fig. 3c). Finally, explant loss varied between 7.5 and 20%, with a minimum in R. andicola and a maximum in R. floribundus (Fig. 3d). In general terms, the four species showed a good survival rate, although marked differences were observed in their development, with R. andicola and R. floribundus being the species with the best response during the multiplication phase (Fig. 3e).
Discussion
Regardless of the species, the pH and acidity values found in the fruits evaluated in this study were as expected, since ripe fruits of Rubus species have pH values ranging from 2.65 to 3.20 and titratable acidity values of 0.29 and 2.3%25. Regarding total soluble solids, Rubus fruits generally register values around 10°Brix26–28. Thus, it can be said that the results indicate that a species contains a % of sugar that is within the range of expected values. It should be noted that although the development of sweetness is crucial, acidity also influences the taste of the fruit, which provides a proper balance of sugar and acid for a pleasant taste29.
Raspberries and blackberries are known to be good sources of phenolic compounds30, which are strongly related to antioxidant capacity31. In addition, there is evidence to support that wild berries have higher levels of phenolic content and antioxidant capacity compared to domesticated and genetically derived crops32. In this study, the total phenolic contents of the species studied ranged from 119.38 to 415.06 mg GAE/g Ff. These results were higher than those obtained by other researchers for fruit extracts of R. fruticosus (31.05 mg GAE/g dw)33 and five blackberry cultivars produced in Brazil (8.23–14.98 mg GAE/g fw)34, and for example were in the range of 226.58–392.9 mg GAE/g reported for root extract of R. hyrcanus35. However, it is important to understand that a variety of factors, including species, climate, soil, ripening phase, location of the fruit on the plant, sun exposure, maturity, post-harvest handling, and different extraction methods, can influence the concentration of these compounds36. All these factors may help explain the differences in phenolic concentrations between the different species examined. On the other hand, the DPPH method used to evaluate the antioxidant capacity of the extracts shows that the antioxidant capacity values of the blackberry species examined ranged from 48.19 to 50.27 mg TE/g Ff. Other researchers obtained lower values in R. coreanus extracts (16.25 mg TE/g dFW)23; however, both investigations demonstrated a high capacity to scavenge DPPH radicals (> 90%). In addition, it was observed that the DPPH radical inhibition efficiency of blackberries in this study was higher than that reported for blackberries of Brazos, Tupy, Arapaho, Choctaw, and Guarani cultivars, which remained in the range of 50–75%37.
Within modern medicine, trends are aimed at overcoming mortality and morbidity problems as a result of the emergence of a resistance of several pathogenic microorganisms to a variety of drugs13,38. In this context, species of the genus Rubus have been reported to be high in flavonoids, tannins, carbohydrates, ascorbic acid, organic acids, and volatile oils39,40. Of these, phenolic compounds exhibit antibacterial mechanisms such as the ability to inhibit nucleic acid synthesis, cytoplasmic membrane functions, biofilm binding and formation, and alter membrane permeability41. These compounds have been shown to be effective against Gram-negative bacteria such as E. coli and Pseudomonas aeruginosa42. Our findings showed that the DIZ values of the blackberry extracts were in the range of 8.29–14.33 mm for the two tested bacteria, especially highlighting the R. weberbaueri and R. andicola extracts. These results are in the range of DIZ values reported for R. coreanus extract against eleven different foodborne pathogens23. In general, it is observed that Gram-negative and Gram-positive bacteria have different sensitivities to antibacterial substances. This may be due to the fact that the outer membrane of the former is composed of hydrophobic lipopolysaccharides, which give them greater resistance43,44.
It has been discovered that berries have a high content of phytochemicals (dependent on genetic and environmental factors) that allows them to combat different microorganisms to a greater or lesser extent45. The microbicidal activity of blackberry extracts has been widely reported on a wide range of large-positive and large-negative bacteria. For example, extracts of R. idaeus, R. moluccanus L., R. fraxinifolius Poir., R. alpestris Blume, R. rosaefolius, and R. chingii, demonstrated high efficacy against Streptococcus typhi, Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus epidermidis, Moraxella catarrhalis, Haemophilus influenzae, Helicobacter pylori, Klebsiella pneumoniae, Enterococcus faecalis, Bacillus subtilis, E. coli, Salmonella enteritidis, Aeromona hydrophila, Pseudomonas aeruginosa, and Pseudomonas fluorescens11,14,46–48. On the other hand, it has been reported that the extracts of these fruits do not affect lactic acid bacteria, used in the fermentation processes of foods48,49. This demonstrates that wild fruits are an important source of potentially healthy phytochemical compounds.
In in vitro multiplication, the use of cytokinins to activate cell division and induce the development of new shoots is frequent50. For example, in the in vitro multiplication of blackberries, the use of BAP has been shown to have significant effects; however, the effect varies depending on the cultivar or genotype to be propagated1,51. In the present study, variation in the in vitro response was not only associated with the source of the explant (species), but also with the concentration of cytokinin (BAP); suggesting an interaction of genotype with the levels of BAP used.
According to the results, it can be mentioned that the variables number and length of shoots were favored by the presence of 1.5 mg l−1 of BAP in the culture medium, but, after this level, they showed a decreasing trend. A similar effect was described by Kefayeti et al.52 and Schiehl et al.53, as they point out that multiplication rates and shoot growth decreased when the culture medium exceeded a certain concentration of BAP. The difference in response to the optimal BAP concentration for in vitro multiplication is likely due to variation between genotypes. In general, determining the appropriate cytokinin concentration decreases the likelihood of toxic effects, while maintaining good multiplication rates and flare-up characteristics54. In addition, the results allow us to generate a baseline for future studies focused on determining the optimization of culture media by genotype or by group of genotypes with the addition of other growth regulators such as gibberellic acid that stimulates shoot elongation and improves leaf formation.
Regarding leaf formation, the results of the present study were superior to the report of Millones6, who when using 0.1 mg l−1 of BAP recorded up to 5.5 leaves per explant in a wild blackberry accession. On the contrary, Cancino-Escalante et al.16 reported that the use of 2 mg l−1 of BAP + 1 mg l−1 of AG3 achieved an average of 14.54 leaves/explant, which is higher than the report of this study. Variations between the above studies may be related to the use of different concentrations of BAP. However, several reports indicate that the use of BAP does not have significant effects on the number of leaves formed55–57, which suggests that they are more closely linked to the response variable of the different genotypes.
The rate of loss of explants reveals that some species are more susceptible than others. These variations, as well as morphometric characteristics, are influenced by genetic factors that determine the sensitivity of plant material, reflecting a variable in vitro growth and morphogenesis response with the genotype58. In the study, explant survival was affected by explant oxidative processes. This process is since explants usually excrete phenolic compounds that manage to oxidize and lead to the generation of radicals that are highly toxic to plant tissues59. Therefore, future studies should consider the addition of antioxidants to the culture medium. BAP generally induces budding, but can affect elongation, leaf production, and rooting, depending on the genotype. In this study, explants grown in BAP medium showed callus formation but not roots, evidencing a possible negative effect of this cytokinin on root development.
Conclusions
The results obtained in our study show that the fruits of wild blackberries from the Peruvian highlands have high antioxidant capacity and antimicrobial activity against E. coli and S. aureus, highlighting their potential to produce functional foods. Evaluations showed that the fruits of R. floribundus presented high content of total soluble solids and titratable acidity. The fruits of R. weberbaueri had the highest total phenolic content. The antioxidant capacity (DPPH assay) of the fruits of the four species varied significantly, but in all cases, they showed the capacity to inhibit more than 95% of the DPPH radicals. The fruit extracts of R. weberbaueri and R. andicola showed higher levels of antimicrobial activity, with S. aureus as the most susceptible bacterium. Results from the in vitro multiplication phase showed that BAP had a significant impact on shoot number, leaf number and shoot length at a dose of 1.5 mg L−1. In addition, it was observed that R. andicola and R. floribundus were the species that showed the most effective response at this stage.
Supplementary Information
Author contributions
Y.K.L.C. and J.B.M.M. Conceptualization; Y.K.L.C., J.B.M.M. and D.C. Methodology; J.J.T.A. and E.H. Data Analysis; E.H. and M.O. Supervision; Y.K.L.C., J.B.M.M., J.J.T.A, and VMNZ. Writing – original draft; J.B.M.M. Writing – review & editing. All authors have read and approved the manuscript for publication.
Funding
The research was funded by SNIP project (312252) / CUI (2252878) “Creación del Servicio de un Laboratorio de Fisiología y Biotecnología Vegetal de la Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas” (“Creation of the Service of a Plant Physiology and Biotechnology Laboratory of the Toribio Rodríguez de Mendoza National University of Amazonas”), executed by the Research Institute for the Sustainable Development of Ceja de Selva.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-54058-0.
References
- 1.Wu J-H, Miller SA, Hall HK, Mooney PA. Factors affecting the efficiency of micropropagation from lateral buds and shoot tips of Rubus. Plant Cell Tissue Organ Cult. PCTOC. 2009;99:17–25. doi: 10.1007/s11240-009-9571-5. [DOI] [Google Scholar]
- 2.Moreno GAL, Espinosa N, Barrero LS, Medina CI. Variabilidad morfológica de variedades nativas de mora (Rubus sp.) en los Andes de Colombia. Rev. Colomb. Cienc. Hortícolas. 2016;10:211–221. doi: 10.17584/rcch.2016v10i2.4755. [DOI] [Google Scholar]
- 3.Raseira MCB, Franzon RC, Feldberg NP, Antunes LEC, Scaranari C. BRS Cainguá, a blackberry fresh-market cultivar. Crop Breed. Appl. Biotechnol. 2020;20:27812014. doi: 10.1590/1984-70332020v20n1c4. [DOI] [Google Scholar]
- 4.Reyes-Carmona J, Yousef GG, Martínez-Peniche RA, Lila MA. Antioxidant capacity of fruit extracts of blackberry (Rubus sp.) produced in different climatic regions. J. Food Sci. 2005;70:s497–s503. doi: 10.1111/j.1365-2621.2005.tb11498.x. [DOI] [Google Scholar]
- 5.Vizzotto M, Raseira MCB, Pereira MC, Fetter M. Teor de compostos fenólicos e atividade antioxidante em diferentes genótipos de amoreira-preta (Rubus sp) Rev Bras Frutic. 2012;34:853–858. doi: 10.1590/S0100-29452012000300027. [DOI] [Google Scholar]
- 6.Millones C. Establecimiento y ensayos preliminares de propagación in vitro de zarzamora silvestre (Rubus Sp) del Centro Poblado San Salvador, región Amazonas. Rev. Científica UNTRM Cienc. Nat. E Ing. 2018;1:31–38. doi: 10.25127/ucni.v3i2.316. [DOI] [Google Scholar]
- 7.Escalante SB, Chuquilín JY, Saldaña E. Identificación botánica y evaluación de los parámetros de calidad de los frutos de zarzamora (Rubus spp), en el distrito de Namora. Cajamarca-Perú. Rev. Caxamarca. 2017;16:51–61. [Google Scholar]
- 8.Tineo D, Bustamante DE, Calderon MS, Huaman E. Exploring the diversity of andean berries from northern Peru based on molecular analyses. Heliyon. 2022;8:e08839. doi: 10.1016/j.heliyon.2022.e08839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caruso MC, et al. Nutraceutical properties of wild berry fruits from Southern Italy. J. Berry Res. 2016;6:321–332. doi: 10.3233/JBR-160140. [DOI] [Google Scholar]
- 10.Mikulic-Petkovsek M, et al. Investigation of anthocyanin profile of four elderberry species and interspecific hybrids. J. Agric. Food Chem. 2014;62:5573–5580. doi: 10.1021/jf5011947. [DOI] [PubMed] [Google Scholar]
- 11.Abu Bakar MF, Ismail NA, Isha A, Mei Ling AL. Phytochemical composition and biological activities of selected wild berries (Rubus moluccanus .L, R. fraxinifolius Poir, and R. alpestris Blume) Evid. Based Complement. Alternat. Med. 2016;2016:e2482930. doi: 10.1155/2016/2482930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grande-Tovar C, Araujo-Pabón L, Flórez-López E, Aranaga-Arias C. Determinación de la actividad antioxidante y antimicrobiana de residuos de mora (Rubus glaucus Benth) Inf. Téc. 2021;85:64–82. doi: 10.23850/22565035.2932. [DOI] [Google Scholar]
- 13.Krzepiłko A, Prażak R, Święciło A. Chemical composition, antioxidant and antimicrobial activity of raspberry, blackberry and raspberry-blackberry hybrid leaf buds. Molecules. 2021;26:327. doi: 10.3390/molecules26020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveira BD, et al. Antioxidant, antimicrobial and anti-quorum sensing activities of Rubus rosaefolius phenolic extract. Ind. Crops Prod. 2016;84:59–66. doi: 10.1016/j.indcrop.2016.01.037. [DOI] [Google Scholar]
- 15.Soto M, Pérez AM, Cerdas M, Vaillant F, Acosta Ó. Physicochemical characteristics and polyphenolic compounds of cultivated blackberries in Costa Rica. J. Berry Res. 2019;9:283–296. doi: 10.3233/JBR-180353. [DOI] [Google Scholar]
- 16.Cancino-Escalante GO, Quevedo García E, Villamizar CE, Díaz Carvajal C. Propagación in vitro de materiales seleccionados de Rubus glaucus Benth (mora de Castilla) en la provincia de Pamplona, región nororiental de Colombia. Rev Colomb Biotecnol. 2015;17:7–15. doi: 10.15446/rev.colomb.biote.v17n2.54262. [DOI] [Google Scholar]
- 17.Pelizza TR, et al. In vitro establishment of blackberry (Rubus sp.) cultivar ‘Xavante’. Ciênc. Rural. 2016;46:1542–1545. doi: 10.1590/0103-8478cr20140988. [DOI] [Google Scholar]
- 18.Borsai O, et al. Evaluation of genetic fidelity of in vitro-propagated blackberry plants using RAPD and SRAP molecular markers. Hortic. Sci. 2020;47:21–27. doi: 10.17221/20/2019-HORTSCI. [DOI] [Google Scholar]
- 19.Villa F, de Araújo AG, Pio LAS, Pasqual M. Multiplicação in vitro da amoreira-preta ‘ÉBANO’ em diferentes concentrações de meio MS e BAP. Ciênc. E Agrotecnologia. 2005;29:582–589. doi: 10.1590/S1413-70542005000300011. [DOI] [Google Scholar]
- 20.Najaf-Abadi AJ, Hamidoghli Y. Micropropagation of thornless trailing blackberry (Rubus sp.) by axillary bud explants. Aust. J. Crop Sci. 2009;3:191–194. [Google Scholar]
- 21.Fascella G, et al. Bioactive compounds and antioxidant activity of four rose hip species from spontaneous Sicilian flora. Food Chem. 2019;289:56–64. doi: 10.1016/j.foodchem.2019.02.127. [DOI] [PubMed] [Google Scholar]
- 22.Albert C, et al. Study of antioxidant activity of garden blackberries (Rubus fruticosus L.) extracts obtained with different extraction solvents. Appl. Sci. 2022;12:4004. doi: 10.3390/app12084004. [DOI] [Google Scholar]
- 23.Chen F, et al. Enzymatic and non-enzymatic bioactive compounds, and antioxidant and antimicrobial activities of the extract from one selected wild berry (Rubus coreanus) as novel natural agent for food preservation. LWT. 2022;171:114133. doi: 10.1016/j.lwt.2022.114133. [DOI] [Google Scholar]
- 24.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 25.Talcott ST. Chemical Components of Berry Fruits. In: Zhao Y, editor. Berry Fruit: Value-Added Products for Health Promotion. CRC Press; 2007. [Google Scholar]
- 26.Acosta-Montoya Ó, et al. Phenolic content and antioxidant capacity of tropical highland blackberry (Rubus adenotrichus Schltdl) during three edible maturity stages. Food Chem. 2010;119:1497–1501. doi: 10.1016/j.foodchem.2009.09.032. [DOI] [Google Scholar]
- 27.de Souza VR, et al. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014;156:362–368. doi: 10.1016/j.foodchem.2014.01.125. [DOI] [PubMed] [Google Scholar]
- 28.Giovanelli G, Limbo S, Buratti S. Effects of new packaging solutions on physico-chemical, nutritional and aromatic characteristics of red raspberries (Rubus idaeus L.) in postharvest storage. Postharvest Biol. Technol. 2014;98:72–81. doi: 10.1016/j.postharvbio.2014.07.002. [DOI] [Google Scholar]
- 29.Mditshwa A, Magwaza LS, Tesfay SZ, Mbili N. Postharvest quality and composition of organically and conventionally produced fruits: A review. Sci. Hortic. 2017;216:148–159. doi: 10.1016/j.scienta.2016.12.033. [DOI] [Google Scholar]
- 30.Schulz M, Chim JF. Nutritional and bioactive value of Rubus berries. Food Biosci. 2019;31:100438. doi: 10.1016/j.fbio.2019.100438. [DOI] [Google Scholar]
- 31.Yang JW, Choi IS. Comparison of the phenolic composition and antioxidant activity of Korean black raspberry, Bokbunja, (Rubus coreanus Miquel) with those of six other berries. CyTA J. Food. 2017;15:110–117. [Google Scholar]
- 32.Burns Kraft TF, et al. Phytochemical composition and metabolic performance-enhancing activity of dietary berries traditionally used by native North Americans. J. Agric. Food Chem. 2008;56:654–660. doi: 10.1021/jf071999d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gil-Martínez L, et al. Phytochemicals determination, and antioxidant, antimicrobial, anti-inflammatory and anticancer activities of blackberry fruits. Foods. 2023;12:1505. doi: 10.3390/foods12071505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Celant VM, Braga GC, Vorpagel JA, Salibe AB. Phenolic composition and antioxidant capacity of aqueous and ethanolic extracts of blackberries. Rev. Bras. Frutic. 2016;38:e-411. doi: 10.1590/0100-29452016411. [DOI] [Google Scholar]
- 35.Yousefbeyk F, et al. Phytochemical analysis, antioxidant, antibacterial, and cytotoxic activities of leaves and roots of Rubus hyrcanus Juz. Eur. Food Res. Technol. 2022;248:141–152. doi: 10.1007/s00217-021-03866-z. [DOI] [Google Scholar]
- 36.Lee J, Dossett M, Finn CE. Rubus fruit phenolic research: The good, the bad, and the confusing. Food Chem. 2012;130:785–796. doi: 10.1016/j.foodchem.2011.08.022. [DOI] [Google Scholar]
- 37.Rigolon TCB, de Barros FAR, Vieira ÉNR, Stringheta PC. Prediction of total phenolics, anthocyanins and antioxidant capacity of blackberry (Rubus sp.), blueberry (Vaccinium sp.) and jaboticaba (Plinia cauliflora (Mart) Kausel) skin using colorimetric parameters. Food Sci. Technol. 2020;40:620–625. doi: 10.1590/fst.34219. [DOI] [Google Scholar]
- 38.McCullough AR, Parekh S, Rathbone J, Del Mar CB, Hoffmann TC. A systematic review of the public’s knowledge and beliefs about antibiotic resistance. J. Antimicrob. Chemother. 2016;71:27–33. doi: 10.1093/jac/dkv310. [DOI] [PubMed] [Google Scholar]
- 39.Sellappan S, Akoh CC, Krewer G. Phenolic compounds and antioxidant capacity of georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002;50:2432–2438. doi: 10.1021/jf011097r. [DOI] [PubMed] [Google Scholar]
- 40.Wada L, Ou B. Antioxidant activity and phenolic content of oregon caneberries. J. Agric. Food Chem. 2002;50:3495–3500. doi: 10.1021/jf011405l. [DOI] [PubMed] [Google Scholar]
- 41.Xie Y, Yang W, Tang F, Chen X, Ren L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015;22:132–149. doi: 10.2174/0929867321666140916113443. [DOI] [PubMed] [Google Scholar]
- 42.Adamczak A, Ożarowski M, Karpiński TM. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2020;9:109. doi: 10.3390/jcm9010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seleshe S, et al. Evaluation of antioxidant and antimicrobial activities of ethanol extracts of three kinds of strawberries. Prev. Nutr. Food Sci. 2017;22:203–210. doi: 10.3746/pnf.2017.22.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puupponen-Pimiä, R. et al. Antimicrobial properties of phenolic compounds from Finnish berries. (1999). [DOI] [PubMed]
- 46.Chen Y, et al. Characterization and functional properties of a pectin/tara gum based edible film with ellagitannins from the unripe fruits of Rubus chingii Hu. Food Chem. 2020;325:126964. doi: 10.1016/j.foodchem.2020.126964. [DOI] [PubMed] [Google Scholar]
- 47.Krauze-Baranowska M, et al. The antimicrobial activity of fruits from some cultivar varieties of Rubus idaeus and Rubus occidentalis. Food Funct. 2014;5:2536–2541. doi: 10.1039/C4FO00129J. [DOI] [PubMed] [Google Scholar]
- 48.Mannino G, et al. Phytochemical profile and antioxidant, antiproliferative, and antimicrobial properties of Rubus idaeus seed powder. Foods. 2022;11:2605. doi: 10.3390/foods11172605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bintsis T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018;4:665–684. doi: 10.3934/microbiol.2018.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aremu AO, et al. Auxin-cytokinin interaction and variations in their metabolic products in the regulation of organogenesis in two Eucomis species. New Biotechnol. 2016;33:883–890. doi: 10.1016/j.nbt.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Fira A, Clapa D, Simu M. Studies regarding the micropropagation of some blackberry cultivars. Bull. UASVM Hortic. 2014;71:29–37. [Google Scholar]
- 52.Kefayeti S, Kafkas E, Ercisli S. Micropropagation of ‘chester thornless’ blackberry cultivar using axillary bud explants. Not. Bot. Horti Agrobot. Cluj-Napoca. 2019;47:162–168. doi: 10.15835/nbha47111280. [DOI] [Google Scholar]
- 53.Schiehl M, de França TO, Biasi LA. Adequação de protocolo para cultivo in vitro de amoreira-preta (Rubus sp) ‘Xingu’. J. Biotechnol. Biodivers. 2020;8:079–087. doi: 10.20873/jbb.uft.cemaf.v8n2.schiehl. [DOI] [Google Scholar]
- 54.Khan N, et al. Optimizing the concentrations of plant growth regulators for in vitro shoot cultures, callus induction and shoot regeneration from calluses of grapes. OENO One. 2015;49:37–45. doi: 10.20870/oeno-one.2015.49.1.95. [DOI] [Google Scholar]
- 55.Bueno PMC, Biasi LA, Tofanelli MBD. Micropropagation protocol for the wild Brazilian greenberry (Rubus erythroclados) Rev. Colomb. Cienc. Hortícolas. 2018;12:405–415. doi: 10.17584/rcch.2018v12i2.7226. [DOI] [Google Scholar]
- 56.Bueno PMC, Biasi LA. Micropropagation of Greenberry (Rubus erythroclados) Acta Hortic. 2015 doi: 10.17660/ActaHortic.2015.1083.48. [DOI] [Google Scholar]
- 57.Schuchovski CS, Biasi LA. Development of an efficient protocol for ‘Brazos’ blackberry in vitro multiplication. Acta Hortic. 2018 doi: 10.17660/ActaHortic.2018.1224.21. [DOI] [Google Scholar]
- 58.Fal MA, Majada JP, Sánchez Tamés R. Physical environment in non-ventilated culture vessels affects in vitro growth and morphogenesis of several cultivars of Dianthus Caryophyllus L. Vitro Cell Dev. Biol. Plant. 2002;38:589–594. doi: 10.1079/IVP2002328. [DOI] [Google Scholar]
- 59.Vega, M. S. Inducción in vitro de variación somaclonal en selecciones de zarzamora (Rubus subgénero Eubatus) productoras en primocañas. (Universidad Michoacana de San Nicolás de Hidalgo, 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.