Summary
Background
Smoking cessation is challenging, despite making use of established smoking cessation therapies. Preclinical studies and one clinical pilot study suggest the antidiabetic drug glucagon-like peptide-1 (GLP-1) analogue to modulate addictive behaviours and nicotine craving. Previously, we reported the short-term results of a randomised, double-blind, placebo-controlled trial. Herein we report long-term abstinence rates and weight developments after 24 and 52 weeks.
Methods
This single-centre, randomised, double-blind, placebo-controlled, parallel group trial was done at the University Hospital Basel in Switzerland. We randomly assigned (1:1) individuals with at least a moderate nicotine dependence willing to quit smoking to either a 12-week treatment with dulaglutide 1.5 mg or placebo subcutaneously once weekly in addition to standard of care smoking cessation therapy (varenicline 2 mg/day and behavioural counselling). After 12 weeks, dulaglutide or placebo injections were discontinued and the participants were followed up at week 24 and 52. The primary outcome of self-reported and biochemically confirmed point prevalence abstinence rate, and secondary outcome of secondary outcome of weight change were assessed at weeks 24 and 52. All participants who received one dose of the study drug were included in the intention to treat set and participants who received at least 10/12 doses of the study drug formed the per protocol set. The trial was registered at ClinicalTrials.gov, NCT03204396.
Findings
Of the 255 participants who were randomly assigned between June 22, 2017 and December 3, 2020, 63% (80/127) (dulaglutide group) and 65% (83/128) (placebo group) were abstinent after 12 weeks. These abstinence rates declined to 43% (54/127) and 41% (52/128), respectively, after 24 weeks and to 32% (41/127) and 32% (41/128), respectively, after 52 weeks. Post-cessation weight gain was prevented in the dulaglutide group (−1.0 kg, standard deviation [SD] 2.7) as opposed to the placebo group (+1.9 kg, SD 2.4) after 12 weeks. However, at week 24, increases in weight from baseline were observed in both groups (median, interquartile range [IQR]: dulaglutide: +1.5 kg, [−0.4, 4.1], placebo: +3.0 kg, [0.6, 4.6], baseline-adjusted difference in weight change −1.0 kg (97.5% CI [−2.16, 0.16])), and at week 52 the groups showed similar weight gain (median, IQR: dulaglutide: +2.8 kg [−0.4, 4.7], placebo: +3.1 kg [−0.4, 6.0], baseline-adjusted difference in weight change: −0.35 kg (95% CI [−1.72, 1.01])). In the follow-up period (week 12 to week 52) 51 (51%) and 48 (48%) treatment-unrelated adverse events were recorded in the dulaglutide and the placebo group, respectively. No treatment-related serious adverse events or deaths occurred.
Interpretation
Dulaglutide does not improve long-term smoking abstinence, but has potential to counteract weight gain after quitting. However, 3 months of treatment did not have a sustained beneficial effect on weight at 1 year. As post-cessation weight gain is highest in the first year after quitting smoking, future studies should consider a longer treatment duration with a GLP-1 analogue in abstinent individuals.
Funding
Swiss National Science Foundation, the Gottfried and Julia Bangerter-Rhyner Foundation, the Goldschmidt-Jacobson Foundation, the Hemmi-Foundation, the University of Basel, the Swiss Academy of Medical Sciences.
Keywords: Glucagon-like peptide-1 (GLP-1) analogues, Quitting smoking, Long-term abstinence, Post-cessation weight gain
Research in context.
Evidence before this study
We searched for clinical trials on PubMed using the terms “GLP-1” AND “smoking” OR “cigarette” OR “nicotine” (search period May 2016–December 2023). To date, a pilot randomised controlled trial (n = 84) using the glucagon-like peptide-1 (GLP-1) analogue exenatide for 6 weeks as an adjunct to nicotine patches yielded increased short-term smoking abstinence rates and decreased post-cessation weight gain. No studies with longer treatment duration or follow-up have been published and there are no clinical trials on dulaglutide effects on smoking cessation.
Added value of this study
This is the first randomised, placebo-controlled study to assess a treatment with the GLP-1 analogue dulaglutide for 12 weeks concomitant to smoking cessation therapy with a follow-up phase of one year to examine prolonged effects. No effect of dulaglutide on neither short-term (12 weeks) nor long-term (24 and 52 weeks) smoking abstinence rates was detected. Positive effects on short-term post-cessation weight gain wore off after discontinuation of dulaglutide and the participants regained weight similar to the placebo group.
Implications of all the available evidence
Our data show no benefits of dulaglutide on smoking abstinence but emphasise the problem of post-cessation weight gain. A treatment duration of only 12 weeks with dulaglutide as an adjunct to smoking cessation therapy may be too short to have sustained beneficial effects on post-cessation weight gain. A longer treatment duration could yield different conclusions and potentially motivate more individuals who smoke to quit or at least prevent metabolic complications of smoking cessation such as substantial weight gain or diabetes in the long term.
Introduction
Smoking is a leading risk factor for increased morbidity and mortality worldwide.1,2 Smoking cessation is able to mitigate or even reverse the negative consequences of smoking and most individuals who smoke wish to quit and often attempt to quit.3,4 Unfortunately, many do not succeed and relapse rate is high.5, 6, 7 Even with the most effective treatment consisting of pharmacotherapy and behavioural counselling, abstinence rates at one year remain unsatisfactorily low.8,9 Common obstacles of prolonged smoking abstinence are nicotine dependence as well as post-cessation weight gain.10,11 An average weight gain of 4–5 kilograms (kg) within the first year after smoking cessation was reported, although there is great inter-individual variability.12 Useful countermeasures against post-cessation weight gain are not established yet but needed.13 Using the weight loss properties of glucagon-like peptide-1 (GLP-1) analogues might be a new strategy to address this issue, especially as GLP-1 analogues may also play a role in reward regulation and in the pathophysiology of addiction.14, 15, 16 A pilot trial (n = 84) using exenatide for smoking cessation showed promising results with increased abstinence rates after six weeks compared to placebo (46.3% versus 26.8%) and less post-cessation weight gain.17 Previously, we reported the short-term results of a randomised, double-blind, placebo-controlled trial (n = 255) investigating a 12-week treatment with dulaglutide as an adjunct to smoking cessation therapy.18 No effect on abstinence rates after 12 weeks was found (63% in the dulaglutide group versus 65% in the placebo group), however, dulaglutide counteracted post-cessation weight gain, whereas weight increased in the placebo group (baseline-adjusted difference in weight change between groups was −2.9 kg (95% CI [−3.59, −2.3], p < 0.001)). Here we report prespecified outcomes such as long-term smoking abstinence rates as well as changes in body weight and glucose homeostasis at weeks 24 and 52.
Methods
Study design and participants
This is the follow-up of a randomised, double-blind, parallel group, placebo-controlled, single-centre, superiority study conducted at the University Hospital Basel, Switzerland. The study protocol and the results of the main outcomes (abstinence rate and weight change at 12 weeks) have been published previously.18,19 In brief, between June 2017 and July 2021, 255 participants were recruited and randomised to either a 12-week treatment with the GLP-1 analogue dulaglutide or placebo concomitant to a standard of care smoking cessation therapy (partial nicotinic receptor agonist varenicline 2 mg/day plus behavioural counselling). After 12 weeks, dulaglutide or placebo and smoking cessation therapy were discontinued and the participants were followed up at week 24 and 52.
Inclusion criteria entailed daily smoking individuals aged 18–75 years, who were willing to quit and showed at least a moderate nicotine dependence (as defined by a Fagerstroem Score of ≥5 points), and signature of informed consent form.20,21 Pregnancy, severe renal insufficiency and prior therapy with GLP-1 analogues were exclusion criteria.
The study was conducted in accordance to the guidelines on Good Clinical Practice of the International Conference on Harmonization and to the ethical principles of the Declaration of Helsinki and was approved by the Ethics Committee of Northwestern- and Central Switzerland (EKNZ 2017-00286) and the Swiss Agency for Therapeutic Products (Swissmedic 2017DR2066). The trial was registered on ClinicalTrials.gov (NCT03204396).
Randomisation and masking
For details of the randomization, masking, study procedures and standard of care smoking cessation therapy during the treatment phase of 12 weeks we refer to the study protocol and our previous publication of the main results.18,19
Procedures
Participants received dulaglutide or placebo once weekly for a total duration of 12 weeks (subcutaneously either 0.5 ml dulaglutide or 0.5 ml sodium chloride (NaCl 0.9%)) by unblinded study staff at the study site, see Figure S1 in the Supplementary Appendix. Dulaglutide dose was increased from the initial dose of 0.75 mg/0.5 ml at week 1 to 1.5 mg/0.5 ml for the remaining 11 weeks. In parallel, the participants received standard of care smoking cessation therapy. The treatment phase ended after 12 weeks by ceasing dulaglutide or placebo injections and routine treatment with varenicline and behavioural counselling.
The follow-up phase data collection was conducted at the University Hospital Basel at week 24 and week 52 by blinded study staff. Smoking abstinence was assessed by self-report and end-expiratory exhaled carbon monoxide (CO) measurement at week 24 and 52. Furthermore, body weight, glycated haemoglobin (HbA1c) and urine cotinine levels were measured. The participants were asked to report any adverse events for safety assessment. If, for any reasons, participants were not able to come to the study site for assessment, the visit was conducted by phone. Consequently, data on CO measurement, HbA1c or urine cotinine levels were missing for these participants.
Outcomes
We prespecified the assessment of long-term smoking abstinence at week 24 and 52 after a 12-week treatment with dulaglutide or placebo and standard of care smoking cessation therapy. Prespecified outcomes included point prevalence abstinence rates, prolonged abstinence rates and smoking reduction. Point prevalence abstinence was defined as self-reported 7-days nicotine abstinence and biochemically confirmed by an end-expiratory exhaled CO measurement of 10 ppm or less. Additionally, negative urine cotinine levels were required for a stricter definition of abstinence. Prolonged abstinence at week 24 or 52 was given by the condition that the criteria of abstinence were met at previous assessments (week 12 or week 12 and 24, respectively). Smoking reduction was defined by number of cigarettes per day and at least a reduction of 50% of end-expiratory exhaled CO.
Further prespecified outcomes included changes of weight and of haemoglobin A1c (HbA1c, %) levels from baseline to week 24 and 52, switch rates to medically prescribed nicotine substitutes instead of smoking cigarettes and time to quit smoking.
Statistical analysis
Analysis sets
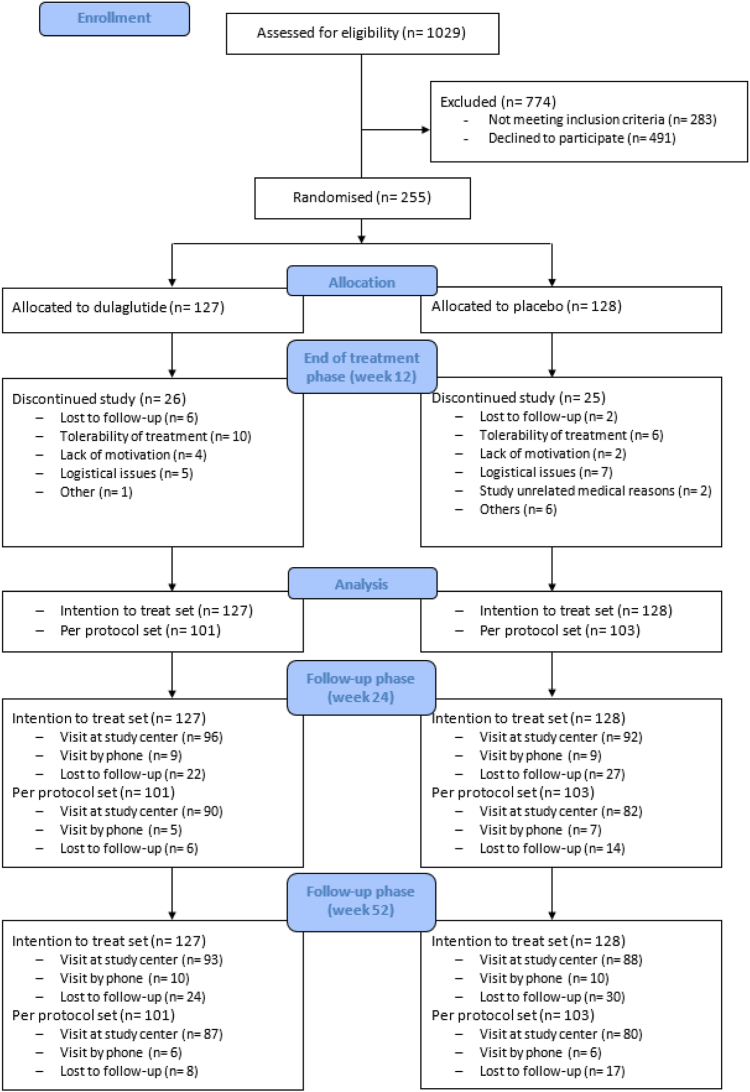
The intention to treat set included all 255 participants. Participants, who received a least 80% of the trial medication during the treatment phase (10/12 doses), formed the per protocol set (n = 204), see Fig. 1.
Fig. 1.
Consort flow diagram. Participant selection and follow-up phase is presented in this diagram. Participants either showed up at the study centre or the visit was done by phone or they were lost to follow-up.
Statistical methods
All analyses were predefined in a statistical analysis plan unless explicitly indicated as post-hoc. Sample size estimation was based on estimated smoking abstinence rates at week 12. For more information see Supplementary Appendix.
Confidence intervals for differences in proportions were constructed using the Wilson score method with continuity correction. Continuous outcomes were analysed using linear regression. HbA1c showed a skewed residual distribution and was therefore analysed using quantile regression for the median.
The outcomes regarding smoking abstinence, weight and HbA1c changes were assumed to measure three different treatment effects of the GLP-1 analogue dulaglutide. Hence, three separate groups of hypotheses were constructed. Confirmatory analyses for each separate group of hypotheses were only performed if a respective short-term treatment effect had been shown at week 12, which was the case for changes in weight and HbA1c levels. For these outcomes, the Bonferroni-Holm procedure was applied to keep the family-wise error rate at 5% within each group and confidence intervals and p-values were adjusted for multiple testing. These analyses were performed on the intention to treat and per protocol set and complete case analysis was carried out additionally. Since no short-term effect has been shown for point prevalence abstinence, prolonged abstinence and smoking reduction, the analyses of the corresponding long-term effects on the intention to treat and per protocol set were purely descriptive. No adjustments between groups of hypotheses were performed nor any adjustments for multiple testing were implemented for the per protocol or supplementary analyses.
Missing data related to smoking abstinence, regardless of the reason, were assumed to be closely linked to active smoking and those participants were hence considered smoking.
Detailed statistical methods, supplemental analyses and further handling of missing data are described in the Supplementary Appendix. All analyses were performed using the statistics program R: Language and environment for statistical computing, Version 4.2.3.22
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. HL, SL and BW had access to all data and had final responsibility for the decision to submit for publication.
Results
The follow-up visits took place between December 2017 and July 2022 and included 186 (72.9%) and 179 (70.2%) of the participants at week 24 and week 52, respectively. Main reasons for missing values were lost to follow-up and study visits by phone, see Fig. 1.
Participants were more female (60.8%) with a mean age of 43.2 years (standard deviation [SD] 13.1). Mean body mass index (BMI) was 27.1 kg/m2 (SD 5.0) and median HbA1c was 5.4% (interquartile range [IQR] 5.1, 5.7), see Table 1. Baseline characteristics of the per protocol set are presented in Table S1 of the Supplementary Appendix.
Table 1.
Baseline characteristics.
| Overall (n = 255) | Dulaglutide (n = 127) | Placebo (n = 128) | |
|---|---|---|---|
| Demographics, clinical parameters, laboratory values | |||
| Gendera | |||
| Female–n (%) | 155 (60.8) | 83 (65.4) | 72 (56.3) |
| Male–n (%) | 100 (39.2) | 44 (34.6) | 56 (43.8) |
| Mean Age (SD)–y | 43.2 (13.1) | 42.7 (13.8) | 43.2 (13.1) |
| Caucasian–n (%) | 247 (96.9) | 126 (99.2) | 121 (94.5) |
| Mean systolic blood pressure (SD) -mmHg | 120.8 (14.2) | 119.5 (13.6) | 122.2 (14.7) |
| Mean diastolic blood pressure (SD)–mmHg | 78.3 (8.4) | 77.6 (8.5) | 78.9 (8.3) |
| Mean heart rate (SD)- bpm | 75.1 (10.3) | 75.3 (10.8) | 75.0 (9.8) |
| Mean weight (SD)–kg | 80.1 (17.9) | 79.0 (17.9) | 81.2 (17.9) |
| Mean BMI (SD)–kg/m2 | 27.1 (5.0) | 27.1 (5.1) | 27.1 (5.0) |
| BMI category | |||
| BMI <25 kg/m2–n (%) | 95 (37.3) | 46 (36.2) | 49 (38.3) |
| BMI 25–29.9 kg/m2–n (%) | 97 (38.0) | 52 (40.9) | 45 (35.2) |
| BMI >30 kg/m2–n (%) | 63 (24.7) | 29 (22.8) | 34 (26.6) |
| Median HbA1c [IQR]–% | 5.4 [5.1, 5.7] | 5.3 [5.1, 5.7] | 5.4 [5.1, 5.6] |
| Median end-expiratory exhaled CO [IQR]–ppm | 19 [13, 27] | 18 [13, 25] | 19 [13, 28] |
| Smoking status | |||
| Median no. of cigarettes per day [IQR] | 20 [15, 20] | 18 [14, 20] | 20 [5, 25] |
| Median no. of total pack years smoked [IQR] | 20 [11, 35] | 19 [10, 35] | 20.5 [13, 36] |
| Mean Fagerstroem (SD)–total sum score | 7.0 (1.5) | 6.9 (1.3) | 7.0 (1.6) |
| Tobacco related diseases, n (%) | |||
| Pulmonal disease | 57 (22.4) | 24 (18.9) | 33 (25.8) |
| Cardiovascular disease | 65 (25.5) | 29 (22.8) | 36 (28.1) |
| Cerebrovascular disease | 10 (3.9) | 7 (5.5) | 3 (2.3) |
| Cancer | 17 (6.7) | 8 (6.3) | 9 (7.0) |
| Gastrointestinal disease | 28 (11.0) | 13 (10.2) | 15 (11.7) |
| Osteoporosis | 10 (3.9) | 5 (3.9) | 5 (3.9) |
| Other comorbidities, n (%) | |||
| Diabetes mellitus | 13 (5.1) | 6 (4.7) | 7 (5.5) |
| Dyslipidemia | 42 (16.5) | 23 (18.1) | 19 (14.8) |
| Psychiatric disease | 71 (27.8) | 36 (28.3) | 35 (27.3) |
| Concomitant medication if > 5%, n (%) | |||
| Any | 178 (69.8) | 88 (69.3) | 90 (70.3) |
| Antihypertensives | 51 (20.0) | 25 (19.7) | 26 (20.3) |
| Contraceptives | 25 (9.8) | 14 (11.0) | 11 (8.6) |
| Acetylsalicylic acid | 19 (7.5) | 8 (6.3) | 11 (8.6) |
| Proton pump inhibitors | 26 (10.2) | 12 (9.4) | 14 (10.9) |
| Lipid-lowering drugs | 29 (11.4) | 17 (13.4) | 12 (9.4) |
| Antipsychotics, neuroleptics | 23 (9.0) | 12 (9.4) | 11 (8.6) |
Percentages may not total 100 because of rounding.
Abbreviations: CO = carbon monoxide, n = numbers, SD = standard deviation, y = years, BMI = body mass index, IQR = interquartile range.
Participants self-identified as female or male.
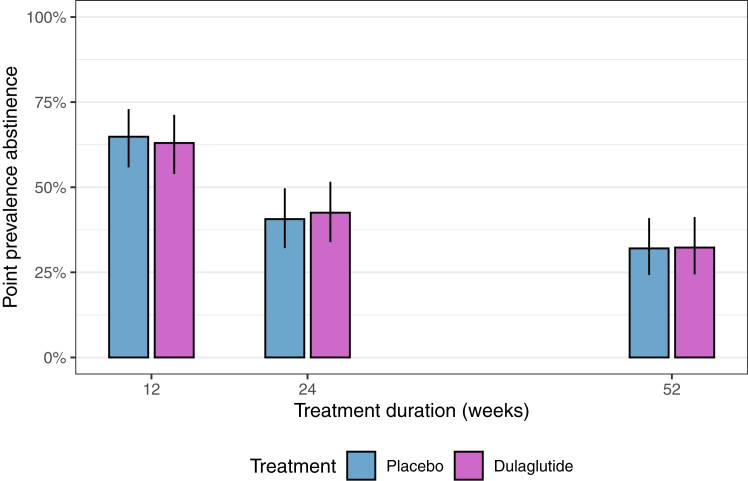
The point prevalence abstinence rates declined from almost two thirds of all participants at week 12 to 43% (54/127) in the dulaglutide and 41% (52/128) in the placebo group at week 24 and to 32% (41/127) and 32% (41/128) at week 52, respectively, see Fig. 2. The proportion of participants with prolonged abstinence at week 24 was 39% (50/127) in the dulaglutide and 40% (51/128) in the placebo group. At week 52, 29% (37/127) and 27% (35/128) of the participants, respectively, were prolonged abstinent. The point prevalence abstinence rates and prolonged abstinence rates in the per protocol set, respectively, were slightly higher, but also similar between groups, see Supplementary Appendix. Similar but slightly lower abstinence rates yielded from a stricter definition of abstinence (including urine cotinine levels), see Supplementary Appendix.
Fig. 2.
Point prevalence abstinence from end of treatment phase to end of follow-up phase according to treatment group (intention to treat set). The y-axis shows the point prevalence abstinence in %, the x-axis represents the weeks 12, 24, 52. Purple and blue colours represent the treatment group (dulaglutide and placebo, respectively).
Smoking reduction, i.e. CO-reduction of more than 50% or by number of cigarettes per day at week 24 and week 52, was similar between the dulaglutide and placebo groups, see Supplementary Appendix. No participant switched to nicotine substitutes instead of smoking cigarettes.
Time to quit and the proportion of participants who quit smoking and maintained prolonged abstinence until the end of the study is depicted in Figure S2 of the Supplementary Appendix. Seventy one participants quit before week 12, only one participant quit between week 12 and 24 and ten participants quit between week 24 and 52. Eighty-four participants, who were abstinent at week 12, did not maintain abstinence until week 52.
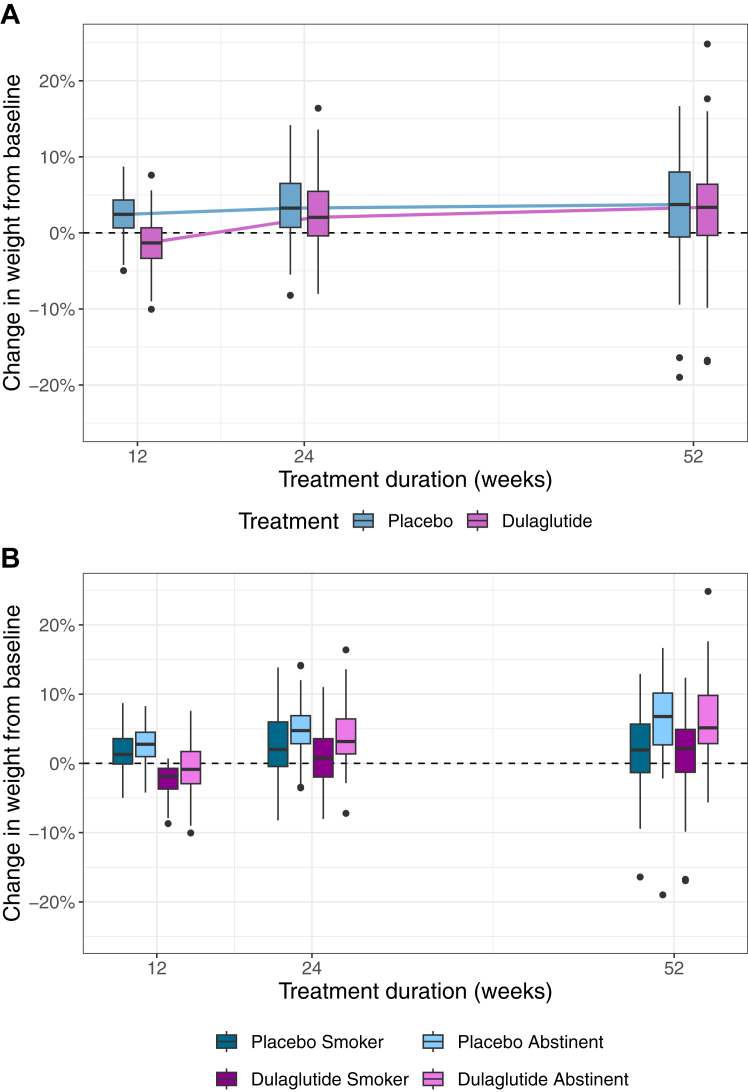
Weight gain since baseline at week 24 was observed in both groups (median, IQR: dulaglutide: +1.5 kg, [−0.4, 4.1], placebo: +3.0 kg, [0.6, 4.6]). At week 52, weight gain since baseline was similar in the dulaglutide group (median, [IQR]: +2.8 kg [−0.4, 4.7]) compared to the placebo group (median, [IQR]: +3.1 kg [−0.4, 6.0]), see Fig. 3A. The baseline-adjusted difference in weight change at week 24 between groups was −1.0 kg (97.5% CI [−2.16, 0.16], p-value = 0.11) and at week 52 it was −0.35 kg (95% CI [−1.72, 1.01], p-value = 0.61). Weight change at week 24 and 52 according to treatment and smoking status is depicted in Fig. 3B and presented in Table S2 and Figure S3 of the Supplementary Appendix. In the complete case and per protocol analysis, a small difference at week 24 was observed, but not at week 52, see Supplementary Appendix. The data provide no support for an association between change in weight with total dose of dulaglutide, which was high overall (median, [IQR]: 15.75 mg [11.25, 16.88]) nor that the effect of dulaglutide depended on baseline HbA1c or baseline BMI, see Tables S3 and S4 of the Supplementary Appendix.
Fig. 3.
Panel A. Relative change in weight (%) from baseline to end of follow-up phase according to treatment group at weeks 12, 24 and 52 (intention to treat set). Panel B. Relative change in weight (%) from baseline to end of follow-up phase according to treatment group and smoking status at weeks 12, 24 and 52 (intention to treat set). Panel A: The y-axis shows the change in weight in %, the x-axis represents the weeks 12, 24, 52. Thick lines indicate the median; boxes indicate the interquartile range (IQR); whiskers include all points within the range of 1.5x the IQR; dots outside the whiskers represent all points outside 1.5x the IQR. Purple and blue colours represent the treatment group (dulaglutide and placebo, respectively). Panel B: The y-axis shows the change in weight in %, the x-axis represents the weeks 12, 24, 52. Box plots are subdivided into 4 groups: (persistent) smokers and quitters on dulaglutide or placebo, respectively. Thick lines indicate the median; boxes indicate the IQR; whiskers include all points within the range of 1.5x the IQR; dots outside the whiskers represent all points outside 1.5x the IQR. Purple and blue colours represent the treatment group (dulaglutide and placebo, respectively), brightness indicates smoking status (bright = abstinent, dark = smoker).
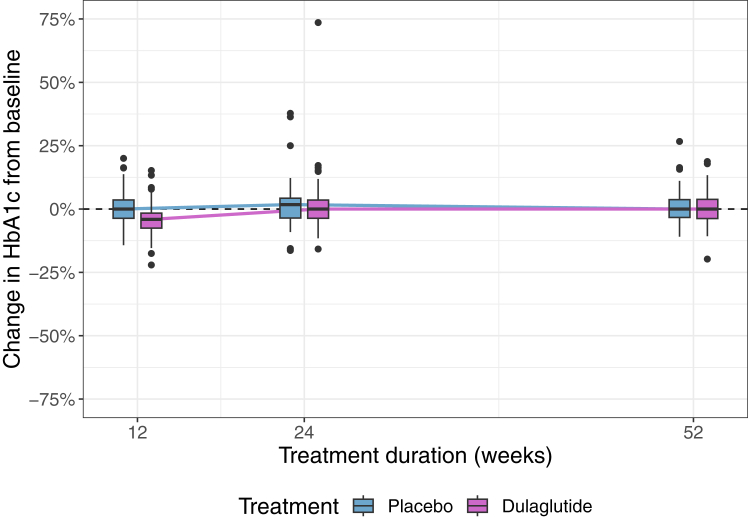
HbA1c did not change substantially from baseline to week 24 nor to week 52, see Fig. 4 and Table S5 of the Supplementary Appendix. The baseline-adjusted median difference in HbA1c between both groups at week 24 was 0.01% (95% CI [−0.12, 0.13], p-value = 0.932) and at week 52 it was −0.04% (97.5% CI [−0.14, 0.06], p-value = 0.83). Results from complete case and per protocol analyses were similar, see Supplementary Appendix. Numbers of participants with new-onset prediabetes or diabetes per group are given in the Supplementary Appendix.
Fig. 4.
Change in HbA1c (%) from baseline to end of follow-up phase according to treatment group at weeks 0, 12, 24 and 52 (intention to treat set). The y-axis shows the glycated haemoglobin (HbA1c) in %, the x-axis represents the weeks 0, 12, 24, 52. Thick lines indicate the median; boxes indicate the interquartile range (IQR); whiskers include all points within the range of 1.5x the IQR; dots outside the whiskers represent all points outside 1.5x the IQR. Purple and blue colours represent the treatment group (dulaglutide and placebo, respectively).
From week 12 to week 52, 99 adverse events (dulaglutide: n = 51, placebo: n = 48) were recorded, see Table S7 of the Supplementary Appendix. Most adverse events were mild or moderate and were judged to be unrelated to the study treatment. Most common adverse events include upper respiratory tract infections and musculoskeletal events. In total, 17 serious adverse events (dulaglutide: n = 11, placebo: n = 6) were recorded and judged to be unrelated to the study treatment, see Table S8 of the Supplementary Appendix.
Discussion
This long-term follow up of a randomised clinical trial found no difference in abstinence rates between the group treated with dulaglutide and the group treated with placebo after 24 and 52 weeks, consistent to the previous findings at week 12. The high point prevalence abstinence rates of almost two thirds in both groups at week 12 dropped continuously to about one third after one year. Furthermore, the positive effect of dulaglutide on post-cessation weight gain and glucose metabolism gradually subsided after discontinuation of dulaglutide.
The decline in abstinence rates observed in the present study highlights the difficulty of achieving long-term abstinence. This is in line with previous results of similar interventions with abstinence rates of around 30% at best after one year since trying to quit smoking.23, 24, 25 As a first requirement for long-term abstinence, short-term abstinence needs to be reached and typically several cessation attempts are necessary.26 The intensive 12-week smoking intervention with pharmacotherapy, behavioural counselling and weekly face-to-face study visits including feedback using CO measurement was successful in achieving higher short-term abstinence rates in this study than in other smoking cessation studies with varenicline and behavioural counselling.27,28 This emphasises the value of a close supervision in the early phase, when the chance of quitting is highest. Once short-term abstinence is achieved, the risk of smoking relapse is particularly high within the first year after the cessation date.7,26 The fact that we observed similar abstinence rates at week 52 to previously reported results of other smoking cessation studies (despite higher abstinence rates at week 12) may be related to the abrupt discontinuation of the smoking cessation intervention after the treatment phase.28
Therefore, a short-term intervention seems not sufficient and the development of new strategies to impede high relapse rates seems inevitable. So far, the continuation of behavioural counselling after smoking cessation failed to lower relapse rates.29 According to a recent meta-analysis on 13 randomised clinical trials, only an extended treatment duration (>12 weeks) with pharmacotherapies (e.g., varenicline) may help prevent smoking relapses.30
In our study, abstinent participants gained more weight after cessation within a year compared to participants who continued to smoke. This was to be expected as post-cessation weight gain is common and occurs mostly within a year after cessation.31 Managing post-cessation weight gain is challenging, which is also highlighted by a similar randomised placebo-controlled clinical trial (n = 84) that investigated the anti-obesity drug lorcaserin during 24 weeks as an adjunct to smoking cessation therapy without showing a substantial difference in post-cessation weight gain among the treatment groups.32 Using dulaglutide as a new strategy to counteract post-cessation weight gain turned out to be promising in the short term.18 Not surprisingly, after discontinuation of dulaglutide, the beneficial effects diminished over time and weight development aligned with that of the placebo group. Weight regain after discontinuation of GLP-1 analogues is a common concern and has been observed in many previous studies.33, 34, 35, 36
In the same line, improvements in cardiometabolic risk factors such as lipid or glucose metabolism also wear off after discontinuation of GLP-1 analogues, consistent with our findings on glucose metabolism.34,37 Post-cessation weight gain is associated with increased risk of type 2 diabetes, especially up to 3 years after quitting.38, 39, 40 In our study, a new onset of prediabetes or diabetes was observed only in a few participants in both groups, possibly due to non-excessive median weight gain. As weight gain, risk of type 2 diabetes and risk of smoking relapse are greatest in the first years after quitting, longer treatment with GLP-1 analogues for the period of highest risk should be evaluated in future studies as a means of controlling the metabolic consequences of smoking cessation. Consistent with previous studies, no adverse events related to dulaglutide occurred after discontinuation.41
This study is subject to the following limitations: First, missing information of approximately 30% on smoking status or metabolic parameters may have influenced the results. However, the proportion of missing information is comparable to other smoking cessation studies.23,24 Second, only two follow-up visits took place, allowing only a rough insight into the time course of smoking abstinence and weight change. Third, using another more potent GLP-1 analogue instead of dulaglutide, might have yielded different results, e.g., exenatide seems to have the best ability to cross the blood–brain barrier, but weight loss capability is limited, whereas semaglutide or dual GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptor agonists have greater potential for weight loss.42, 43, 44 Forth, sample size estimation was based on expected smoking abstinence rates at week 12 and not powered for outcomes such as smoking abstinence, weight change or HbA1c levels at week 24 or 52. Fifth, a monocentric study with a relatively young and predominantly overweight population with a high prevalence of cardio-pulmonary comorbidity and medication limits the generalizability. A key strength of our study was the randomised controlled study design with a follow-up phase of 52 weeks. Our study provides important insights on long-term smoking abstinence and weight development after a smoking cessation intervention with a GLP-1 analogue.
In conclusion, a 12-week treatment with dulaglutide could not impede the decline in long-term abstinence from smoking. Discontinuation of dulaglutide diminished the beneficial metabolic effects on weight and glucose homeostasis observed at week 12. To find an effective countermeasure against post-cessation weight gain, further research should focus on longer treatment duration in individuals who achieve short-term abstinence, ideally with more potent GLP-1 analogues or dual incretin receptor agonists that are available nowadays.
Contributors
HL, SL and BW had accessed and verified the underlying data. HL analysed and interpreted the data, did the literature search and wrote the manuscript. SL contributed to the study design, data collection and to the manuscript. TB and AM were involved in the conceptualisation and contributed to the manuscript. NJ contributed to the study design and collection of data. ML and DRV planned, performed and interpreted the statistical analyses and contributed to the manuscript. TV, KB, MS, CB, COS, SAU, JK, FB and LNL contributed to the data collection. LGH and BS were involved in the study design and contributed to the manuscript. TEE contributed to the methodology. MCC contributed to the methodology, interpreted the data and contributed to the manuscript. BW designed the study, wrote the protocol, collected, analysed and interpreted data, and supervised all steps of the conduct of the study. All authors edited and approved the final manuscript.
Data sharing statement
We may share de-identified, individual participant-level data that underlie the results reported in this article and related documents, including the study protocol and the statistical analysis plan. Data will be available with the publication of our manuscript on receipt of a request detailing the study hypothesis and statistical analysis plan. All requests should be sent to the corresponding author. The steering committee of this study will discuss all requests and decide based on the scientific rigor of the proposal whether data sharing is appropriate. All applicants are asked to sign a data access agreement.
Declaration of interests
All authors declare no competing interests.
Acknowledgements
This study was investigator-initiated. Sources of funding for each author: BW: supported by a grant by the Swiss National Science Foundation (PZ00P3_193206), the Gottfried Julia Bangerter-Rhyner Foundation, the Goldschmidt-Jacobson Foundation, the Hemmi-Foundation and by funds of the University of Basel. SL: research was funded by the Department of Internal Medicine of the University Hospital of Basel, the Gottfried Julia Bangerter-Rhyner Foundation and the Swiss Academy of Medical Sciences. The funders were not involved in designing, conducting nor analysing the study or reporting study results. Other Support: The University Hospital Basel (and the integrated Clinical Trial Unit and Department of Endocrinology, Diabetology and Metabolism) provided the location and infrastructure. We are grateful to our participants for taking part in the trial. We thank the support staff, study and laboratory personnel at the University Hospital Basel, especially Nina Hutter, Joyce Santos de Jesus, Milica Wälchli-Popovic, Cihan Atila, Silke Purschke, Laura Werlen, Klaus Ehrlich, Patrick Simon, Uta Engler, Silvia Caviola, Nathalie Klaus and Nicole Salvisberg and all members of the clinical neuroendocrinology research team for their most helpful support during the study.
Footnotes
Translated abstract For the German translation of the abstract see Supplementary Materials section.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102429.
Appendix A. Supplementary data
References
- 1.Collaborators GBDT Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. 2021;397(10292):2337–2360. doi: 10.1016/S0140-6736(21)01169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaborators GBDRF Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jha P. The hazards of smoking and the benefits of cessation: a critical summation of the epidemiological evidence in high-income countries. Elife. 2020;9:e49979. doi: 10.7554/eLife.49979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babb S., Malarcher A., Schauer G., Asman K., Jamal A. Quitting smoking among adults - United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457–1464. doi: 10.15585/mmwr.mm6552a1. [DOI] [PubMed] [Google Scholar]
- 5.Hughes J.R., Peters E.N., Naud S. Relapse to smoking after 1 year of abstinence: a meta-analysis. Addict Behav. 2008;33(12):1516–1520. doi: 10.1016/j.addbeh.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson J.D., Li L., Chen M., et al. Evaluating the temporal relationships between withdrawal symptoms and smoking relapse. Psychol Addict Behav. 2019;33(2):105–116. doi: 10.1037/adb0000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S.H., Yi Y.H., Lee Y.I., Lee H.Y., Lim K.M. Factors associated with long-term smoking relapse in those who succeeded in smoking cessation using regional smoking cessation programs. Medicine. 2022;101(31) doi: 10.1097/MD.0000000000029595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stead L.F., Koilpillai P., Fanshawe T.R., Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2016;3(3):CD008286. doi: 10.1002/14651858.CD008286.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agboola S.A., Coleman T., McNeill A., Leonardi-Bee J. Abstinence and relapse among smokers who use varenicline in a quit attempt-a pooled analysis of randomized controlled trials. Addiction. 2015;110(7):1182–1193. doi: 10.1111/add.12941. [DOI] [PubMed] [Google Scholar]
- 10.Vangeli E., Stapleton J., Smit E.S., Borland R., West R. Predictors of attempts to stop smoking and their success in adult general population samples: a systematic review. Addiction. 2011;106(12):2110–2121. doi: 10.1111/j.1360-0443.2011.03565.x. [DOI] [PubMed] [Google Scholar]
- 11.Tian J., Venn A., Otahal P., Gall S. The association between quitting smoking and weight gain: a systematic review and meta-analysis of prospective cohort studies. Obes Rev. 2015;16(10):883–901. doi: 10.1111/obr.12304. [DOI] [PubMed] [Google Scholar]
- 12.Aubin H.J., Farley A., Lycett D., Lahmek P., Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ. 2012;345:e4439. doi: 10.1136/bmj.e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartmann-Boyce J., Theodoulou A., Farley A., et al. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst Rev. 2021;10(10):CD006219. doi: 10.1002/14651858.CD006219.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iqbal J., Wu H.X., Hu N., et al. Effect of glucagon-like peptide-1 receptor agonists on body weight in adults with obesity without diabetes mellitus-a systematic review and meta-analysis of randomized control trials. Obes Rev. 2022;23(6) doi: 10.1111/obr.13435. [DOI] [PubMed] [Google Scholar]
- 15.Engel J.A., Jerlhag E. Role of appetite-regulating peptides in the pathophysiology of addiction: implications for pharmacotherapy. CNS Drugs. 2014;28(10):875–886. doi: 10.1007/s40263-014-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klausen M.K., Thomsen M., Wortwein G., Fink-Jensen A. The role of glucagon-like peptide 1 (GLP-1) in addictive disorders. Br J Pharmacol. 2022;179(4):625–641. doi: 10.1111/bph.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yammine L., Green C.E., Kosten T.R., et al. Exenatide adjunct to nicotine patch facilitates smoking cessation and may reduce post-cessation weight gain: a pilot randomized controlled trial. Nicotine Tob Res. 2021;23(10):1682–1690. doi: 10.1093/ntr/ntab066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lengsfeld S., Burkard T., Meienberg A., et al. Effect of dulaglutide in promoting abstinence during smoking cessation: a single-centre, randomized, double-blind, placebo-controlled, parallel group trial. eClinicalMedicine. 2023;57 doi: 10.1016/j.eclinm.2023.101865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lengsfeld S., Burkard T., Meienberg A., et al. Glucagon-like peptide-1 analogues: a new way to quit smoking? (SKIP)-a structured summary of a study protocol for a randomized controlled study. Trials. 2023;24(1):284. doi: 10.1186/s13063-023-07164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heatherton T.F., Kozlowski L.T., Frecker R.C., Fagerstrom K.O. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 21.Fagerstrom K. Determinants of tobacco use and renaming the FTND to the fagerstrom test for cigarette dependence. Nicotine Tob Res. 2012;14(1):75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- 22.R Core Team . 2023. R: a language and environment for statistical computing.https://www.r-project.org/index.html [Google Scholar]
- 23.Windle S.B., Dehghani P., Roy N., et al. Smoking abstinence 1 year after acute coronary syndrome: follow-up from a randomized controlled trial of varenicline in patients admitted to hospital. CMAJ. 2018;190(12):E347–E354. doi: 10.1503/cmaj.170377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo C., Walicka M., Caponnetto P., et al. Efficacy and safety of varenicline for smoking cessation in patients with type 2 diabetes: a randomized clinical trial. JAMA Netw Open. 2022;5(6) doi: 10.1001/jamanetworkopen.2022.17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjornson W., Rand C., Connett J.E., et al. Gender differences in smoking cessation after 3 years in the lung health study. Am J Public Health. 1995;85(2):223–230. doi: 10.2105/ajph.85.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes J.R., Keely J., Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 27.Anthenelli R.M., Benowitz N.L., West R., et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507–2520. doi: 10.1016/S0140-6736(16)30272-0. [DOI] [PubMed] [Google Scholar]
- 28.Jordan C.J., Xi Z.X. Discovery and development of varenicline for smoking cessation. Expert Opin Drug Discov. 2018;13(7):671–683. doi: 10.1080/17460441.2018.1458090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajek P., Stead L.F., West R., Jarvis M., Hartmann-Boyce J., Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev. 2013;8:CD003999. doi: 10.1002/14651858.CD003999.pub4. [DOI] [PubMed] [Google Scholar]
- 30.Murray R.L., Zhang Y.Q., Ross S., et al. Extended duration treatment of tobacco dependence: a systematic review and meta-analysis. Ann Am Thorac Soc. 2022;19(8):1390–1403. doi: 10.1513/AnnalsATS.202110-1140OC. [DOI] [PubMed] [Google Scholar]
- 31.O'Hara P., Connett J.E., Lee W.W., Nides M., Murray R., Wise R. Early and late weight gain following smoking cessation in the Lung Health Study. Am J Epidemiol. 1998;148(9):821–830. doi: 10.1093/oxfordjournals.aje.a009706. [DOI] [PubMed] [Google Scholar]
- 32.Hurt R.T., Croghan I.T., Schroeder D.R., et al. Varenicline and lorcaserin for smoking cessation and weight gain prevention: a randomized clinical trial. Mayo Clin Proc Innov Qual Outcomes. 2022;6(5):465–474. doi: 10.1016/j.mayocpiqo.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubino D., Abrahamsson N., Davies M., et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414–1425. doi: 10.1001/jama.2021.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilding J.P.H., Batterham R.L., Davies M., et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes Metab. 2022;24(8):1553–1564. doi: 10.1111/dom.14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krotter A., Aonso-Diego G., Garcia-Perez A., Garcia-Fernandez G., Secades-Villa R. Post-cessation weight gain among smokers with depression predicts smoking relapse. J Dual Diagn. 2023;19(2–3):62–70. doi: 10.1080/15504263.2023.2192683. [DOI] [PubMed] [Google Scholar]
- 36.Borrelli B., Spring B., Niaura R., Hitsman B., Papandonatos G. Influences of gender and weight gain on short-term relapse to smoking in a cessation trial. J Consult Clin Psychol. 2001;69(3):511–515. doi: 10.1037//0022-006x.69.3.511. [DOI] [PubMed] [Google Scholar]
- 37.Kosiborod M.N., Bhatta M., Davies M., et al. Semaglutide improves cardiometabolic risk factors in adults with overweight or obesity: STEP 1 and 4 exploratory analyses. Diabetes Obes Metab. 2023;25(2):468–478. doi: 10.1111/dom.14890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeh H.C., Duncan B.B., Schmidt M.I., Wang N.Y., Brancati F.L. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2010;152(1):10–17. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu L., Wang X., Dong J.Y., Zhao Y.T., Lou H. Smoking cessation, weight gain, and risk for type 2 diabetes: a prospective study. Int J Public Health. 2022;67 doi: 10.3389/ijph.2022.1604654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Y., Zong G., Liu G., et al. Smoking cessation, weight change, type 2 diabetes, and mortality. N Engl J Med. 2018;379(7):623–632. doi: 10.1056/NEJMoa1803626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filippatos T.D., Panagiotopoulou T.V., Elisaf M.S. Adverse effects of GLP-1 receptor agonists. Rev Diabet Stud. 2014;11(3–4):202–230. doi: 10.1900/RDS.2014.11.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salameh T.S., Rhea E.M., Talbot K., Banks W.A. Brain uptake pharmacokinetics of incretin receptor agonists showing promise as Alzheimer's and Parkinson's disease therapeutics. Biochem Pharmacol. 2020;180 doi: 10.1016/j.bcp.2020.114187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vosoughi K., Atieh J., Khanna L., et al. Association of glucagon-like peptide 1 analogs and agonists administered for obesity with weight loss and adverse events: a systematic review and network meta-analysis. eClinicalMedicine. 2021;42 doi: 10.1016/j.eclinm.2021.101213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jastreboff A.M., Aronne L.J., Ahmad N.N., et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.