Abstract
Mycobacterium tuberculosis is a significant threat to global health. Mycobacterium bovis BCG vaccine provides only partial protection, and the skin test reagent used to aid diagnosis of both active and latent tuberculosis, purified protein derivative (PPD), lacks specificity and sensitivity. The use of genetically detoxified Bordetella pertussis adenylate cyclase toxin (CyaA) as a delivery system for two immunodominant proteins of M. tuberculosis that are of greater specificity than PPD, early-secreted antigenic target 6-kDa protein (ESAT-6) and culture filtrate protein 10 (CFP-10), was therefore investigated. CyaA toxoids incorporating these antigens were able to restimulate T cells from more than 91% tuberculosis patients and healthy sensitized donors. Delivery of antigen by CyaA decreased by 10-fold the amount of ESAT-6 and CFP-10 required to restimulate T cells, and in low responders, the overall frequency of gamma interferon-producing cells detected by enzyme-linked immunospot assay was increased (P < 0.01 for both antigens). Delivery of ESAT-6 and CFP-10 by CyaA enabled the detection of both CD4+ and CD8+ T cells: these responses could be blocked by inhibition of major histocompatibility complex class II or class I, respectively. Covalent linkage of antigen to the CyaA vector was required for enhancement to occur, as a mixture of mock CyaA toxoid plus recombinant ESAT-6 did not lead to enhancement. In a simplified whole-blood model to detect tuberculosis infection, the frequency of positive responses to CFP-10 was increased by CyaA delivery, a potentially important attribute that could facilitate the identification of latent infection.
Mycobacterium tuberculosis is a major threat to global health and is responsible for more deaths than any other bacterium. One control strategy is to prevent the progression of latent infection by M. tuberculosis (LTBI) to clinical tuberculosis (TB) by preventive antituberculous drug therapy. However, the tuberculin skin test (TST) used to identify healthy individuals with latent infection suffers operational drawbacks. First, the TST reagent, purified protein derivative (PPD), is cross-reactive, because it contains epitopes found in many mycobacteria. Thus, TST reactivity can arise through sensitization by environmental mycobacteria or from Mycobacterium bovis BCG vaccine. Second, the sensitivity of the TST is reduced by human immunodeficiency virus (HIV) infection (20). Third, the TST requires two visits (administration and reading) and is operator dependent. These limitations impair identification of LTBI and thus wider application of preventive antituberculous drug therapy. Therefore, two major practical goals of immunological studies into TB are the definition of vaccine candidates of greater efficacy than BCG and the development of immunodiagnostic methods of greater sensitivity, specificity, and practicality than TST skin testing.
A major advance in TB research has been the identification of a genomic segment (designated region of deletion 1 [RD1]) that is present in pathogenic members of the M. tuberculosis complex but is absent from all attenuated BCG strains (6, 15, 29). Molecules encoded on this segment clearly could contribute to virulence (33) or stimulate species-specific T-cell responses of protective potential (43). In addition, there has been a great deal of research focused on the potential of RD1-encoded antigens to improve the immunodiagnosis of TB (4, 13). However, protein subunits tend to inefficiently stimulate T-cell responses, and it is notable that the most promising experimental vaccine preparations require powerful adjuvants that are not licensed for use in humans. Along similar operational lines, the best immunodiagnostic methods rely on peptide mixtures and enzyme-linked immunospot assay (ELISPOT) that are arguably too complex for use in medically underserved environments (5). Our research has therefore consistently sought simple methods by which T-cell responses to M. tuberculosis antigens could be enhanced (44, 45).
Bordetella pertussis secretes a calmodulin-activated adenylate cyclase toxin, CyaA, that primarily targets myeloid phagocytic cells expressing the αMβ2 integrin receptor (CD11b/CD18), including professional antigen-presenting cells, such as neutrophils, macrophages, and dendritic cells (16). CyaA is able to deliver its N-terminal catalytic adenylate cyclase domain (400 amino acid residues) into the cytosol of eukaryotic target cells directly through the cytoplasmic membrane (16, 38). Genetically detoxified CyaA can be used as a vehicle to deliver CD4+ and CD8+ T-cell epitopes, inserted within the AC domain of the toxoid, into antigen-presenting cells that can trigger specific T-cell responses (10, 14, 28, 30, 36). As insertion of only peptide fragments (≈20 amino acids) has hitherto been investigated, a primary aim of this study was to determine whether the whole RD1-encoded early-secreted antigenic target 6-kDa protein (ESAT-6) (Rv3875, 95 amino acids) or culture filtrate protein 10 (CFP-10) (Rv3874, 100 amino acids) sequence could be delivered by CyaA. We also wished to determine whether CyaA would affect the dose-response or detection frequency of M. tuberculosis-specific gamma interferon (IFN-γ)-producing cells and if so, what type of response is enhanced and by what mechanism.
MATERIALS AND METHODS
Subjects.
This study was conducted with ethical approval from the Harrow Local Research Ethics Committee (LREC 1646 and 2414). Patients with TB and their healthy contacts were recruited from Northwick Park Hospital, Harrow, United Kingdom (North West London Hospitals National Health Service Trust). Three groups of people with distinct clinical phenotypes were selected. The first group was adults with overt (i.e., culture- or biopsy-positive) TB (21 adults; 14 males, 7 females; average age, 35.1 years). The second group consisted of asymptomatic adults with normal chest radiographs who nevertheless exhibited strongly positive TST reactions (Heaf grade 3 and above) and were thus thought likely to have LTBI (44 adults; 26 males, 18 females; average age, 34.7 years). A third control group consisted of healthy adults with no documented exposure to TB and whose skin test reactions were negative (seven adults; three males, four females; average age, 37.6 years). The first two groups were chosen to maximize the chances of T-cell reactivity to M. tuberculosis-specific antigens and thus allow us to compare the sensitivity of response to recombinant ESAT-6 (rESAT-6) and recombinant CFP-10 (rCFP-10) and CyaA toxoids. All subjects were subsequently advised and, if indicated, treated according to British Thoracic Society guidelines (21).
Cells.
Peripheral blood mononuclear cells (PBMC) were separated from 20 ml of blood by centrifugation over Ficoll-Paque Plus (Pharmacia, Uppsala, Sweden) and suspended in RPMI 1640 supplemented with 2 mM l-glutamine, penicillin (100 U/ml), gentamicin (5 μg/ml), and 10% heat-inactivated fetal bovine serum (Sigma, St. Louis, MO) (R10). CD4+ and CD8+ T cells were depleted using anti-CD4 or anti-CD8 monoclonal antibody (MAb) conjugated to ferrous beads (Dynabeads M-450; Dynal, Oslo, Norway) according to the manufacturer's instructions. These depletions consistently yielded cell populations of 97 to 99% purity. Anti-major histocompatibility complex (MHC) class II blocking antibody (L243; Leinco Technologies), anti-MHC class I blocking antibody (W6/32; Leinco), and isotype control antibody (mouse immunoglobulin G2a; Leinco) were used at 5 μg/ml 30 min after addition of antigens. L243 is known not to inhibit MHC class I-restricted recognition (3, 11), and up to 50 μg/ml of W6/32 does not inhibit MHC class II presentation (19, 35). These antibodies did not inhibit phytohemagglutinin (PHA)-induced IFN-γ production, indicating that they were not cytotoxic under the assay conditions we employed (data not shown). Chloroquine (Sigma) at 20 μM was added to the cultures just before the antigens. A 100 mM concentration of chloroquine has been shown not to affect CD8+ T-cell recognition of cells infected with mycobacteria (27, 39).
Ex vivo ELISPOT for single-cell IFN-γ release.
Polyvinylidene difluoride-backed plates (96-well plates) (MAIPS45; Millipore, Bedford, MA), precoated with 15 μg/ml of anti-IFN-γ MAb 1-D1K (Mabtech, Nacka, Sweden), were blocked with R10 for 2 h. A total of 3 × 105 PBMC were added in 100 μl R10/well. Duplicate wells of CyaA toxoids and recombinant ESAT-6 and CFP10 were used at the optimum concentrations derived from Fig. 1. PPD (Evans Medical, Liverpool, United Kingdom) at 100 U/ml and PHA (ICN Biomedicals, Aurora, OH) at 5 μg/ml were added to positive-control wells. No antigen was added to the negative-control wells. After 14 h incubation at 37°C in 5% CO2, plates were washed with phosphate-buffered saline containing 0.05% Tween 20. Fifty microliters of 1 μg/ml of biotinylated anti-IFN-γ MAb, 7-B6-1-biotin (Mabtech), was added for 2 h. Plates were washed, and streptavidin-alkaline phosphatase toxoid (Mabtech) was added at 1:1,000 dilution. After 1 h and further washing, 50 μl of chromogenic alkaline phosphatase substrate (Bio-Rad, Hercules, CA), diluted 1:25 with deionized water, was added. Ten minutes later, plates were washed and allowed to dry, and spot-forming cells (SFC) were enumerated with a magnifying glass.
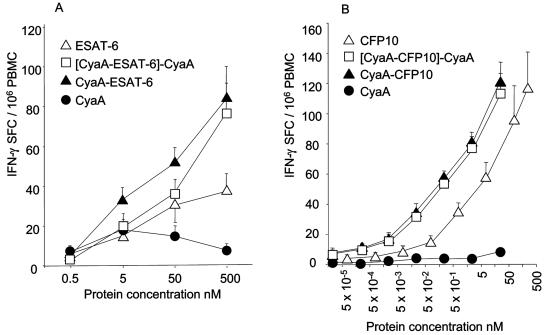
FIG. 1.
The dose of antigen required to restimulate T cells is reduced 10- to 20-fold by CyaA delivery. The numbers of IFN-γ-secreting SFC (IFN-γ SFC) were determined in an overnight ELISPOT in the presence of ESAT-6 or CFP-10 or their CyaA toxoid equivalents or mock CyaA toxoid containing no antigenic insert. The corrected response to antigen containing toxoid (i.e., minus the response to mock toxoid) ([CyaA-ESAT-6]-CyaA) is also shown. A) In nine healthy TST-positive responding donors, the recognition of recombinant ESAT-6 was optimal at 500 nM, whereas similar recognition occurred in the presence of 10-fold-less CyaA-ESAT-6. B) In 10 similar donors who responded to rCFP-10, CyaA delivery also shifted the dose-response curve to the left. Approximately 10 to 20 times less CFP-10 expressed as a CyaA-CFP-10 toxoid elicited the same response. Results are shown as means ± standard errors (error bars).
Recombinant antigen and CyaA toxoid construction.
Recombinant ESAT-6 was prepared as previously described (41). Recombinant CFP-10 was obtained commercially from Lionex (Braunschweig, Germany). N-terminal sequencing confirmed the identity of the cloned antigen. Escherichia coli XL1-Blue (Stratagene) was used throughout this work for recombinant DNA construction and for expression of antigens inserted into CyaA. Bacteria transformed with appropriate plasmids derived from pT7CACT1 (15) were grown at 37°C in Luria-Bertani medium supplemented with 150 μg of ampicillin per ml. The open reading frames of Mycobacterium tuberculosis H37Rv genes esat-6 and cfp-10 were amplified by PCR from the pYUB412 cosmid clone of the RD1 region using the following primers (with restriction sites underlined): Esat6-I, 5′-GATGTGTACACATGACAGAGCAGCAGTGG-3′; Esat6-II, 5′-GATGTGTACACTGAGCGAACATCCCAGTGACG-3′; CFP-10-I, 5′-CATGTGTACACATGGCAGAGATGAAGACC-3′; and CFP-10-II, 5′-CATGTGTACACTGAAGCCCATTTGCGAGGA-3′.
The PCR product was digested by BsrGI at the sites incorporated into the PCR primers, and the purified fragments encoding the antigens were inserted in frame between codons 335 and 336 of CyaA on the pT7CACT-336-BsrGI expression vector (15). The exact sequence of the cloned inserts was verified by DNA sequencing. The control detoxified mock CyaA and the recombinant CyaA proteins carrying the ESAT-6 and CFP-10 antigens were produced in E. coli, purified from inclusion bodies in 8 M urea, 50 mM Tris-Cl, pH 8, 2 mM EDTA and characterized as previously described (30). The resulting proteins were free of any detected adenylate cyclase enzymatic activity.
Whole-blood assay and IFN-γ ELISA.
Venous blood was collected (BD Na heparin Vacutainer; BD Pharmingen catalog no. 368480) and processed within 4 h of sampling. Whole blood was diluted 1:10 in RPMI 1640 (supplemented with glutamine and penicillin/streptomycin). A portion of the diluted blood (180 μl) was plated in 96-well U plates with stimulating antigens in duplicate wells. The final concentrations of the antigens were as follows: 250 nM rESAT-6, 50 nM CyaA-ESAT-6 (CyaA carrying the ESAT-6 antigen), 500 nM rCFP-10, 50 nM CyaA-CFP-10 (CyaA carrying the CFP-10 antigen), 50 nM mock CyaA toxoid, and 5 μg/ml PHA (positive control). Stimulated whole blood was cultured at 37°C in a CO2 incubator. Supernatants from duplicate wells were harvested after 72 h of culture, pooled, and immediately frozen for later IFN-γ measurements by enzyme-linked immunosorbent assay (ELISA). The optimal concentrations of stimulants and the timing of harvesting had been previously determined by dose-response and time course experiments (37).
ELISA reactions were performed in accordance with the instructions of the manufacturer of the antibody. Briefly, 96-well flat-bottomed plates were coated overnight at 4°C with purified mouse anti-human IFN-γ (BD Pharmingen catalog no. 554548). After the wells were blocked and washed, they were plated with supernatants (1:2 dilutions in duplicate) and standards (standard curve dilutions from 15 pg/ml to 10,000 pg/ml [duplicate measurements]) after which the plates were again incubated at 4°C overnight. After the wells were washed, they were incubated with biotinylated mouse anti-human IFN-γ (BD Pharmingen catalog no. 554550) for 1.5 h at room temperature, washed again, and incubated with streptavidin (Sigma) for 30 min. Orthophenylene diamine hydrochloride (OPD) was used as a substrate for detection, and 2 N H2SO4 was used to stop color development. Optical densities were read at 490 nm on a plate reader, and IFN-γ concentrations were calculated from standard curves. Spearman rank correlation coefficients between independent variables were calculated using SPSS-10 software.
RESULTS
The dose of ESAT-6 or CFP-10 required to restimulate M. tuberculosis-specific T cells is reduced 10- to 20-fold by CyaA delivery.
We first determined the optimum stimulatory dose of rESAT-6 and rCFP-10 and their respective CyaA toxoids in vitro. For the CyaA toxoid, the equivalent dose of inserted antigen to the recombinant molecule was used (i.e., the same molar amount of ESAT-6 or CFP-10 protein). The numbers of IFN-γ-secreting SFC were counted in an overnight ELISPOT. The response to CyaA toxoid into which no antigenic stimulus had been inserted (mock toxoid) was also determined. In nine healthy TST-positive donors who responded to rESAT-6, optimal recognition of this molecule was observed at a dose of 500 nM. Tenfold-less ESAT-6 (50 nM) was required when the antigen was presented as CyaA-ESAT-6 (Fig. 1A). However, an increase in the dose of CyaA toxoid beyond 500 nM was associated with a decrease in SFC that we ascertained was due to the high urea content of solutions necessary to solubilize the CyaA toxoid (data not shown). In 10 donors who responded to rCFP-10, CyaA fusion similarly shifted the dose-response curve to the left. Approximately 10 to 20 times less CFP-10 expressed as a CyaA toxoid elicited the same response as native antigen (Fig. 1B). There was low-level reactivity above the background level to mock CyaA toxoid in some donors; thus, we determined that mock CyaA toxoid would be used as a background control in subsequent experiments. Taking this into account, CyaA fusion nevertheless decreased by 10-fold the amount of ESAT-6 and CFP-10 required to restimulate T cells.
The detection of IFN-γ SFC in low-responding subjects is enhanced by CyaA delivery.
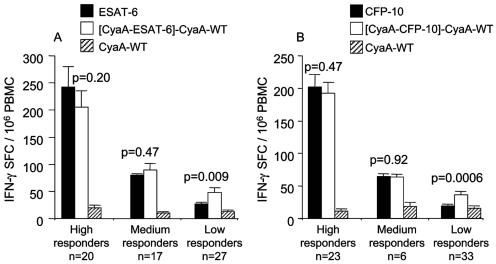
As increasing the dose of toxoid above 500 nM consistently led to toxicity and occasionally toxicity occurred at 500 nM in some donors, we elected to compare equivalent doses for subsequent assays. This choice also reflected our desire to maintain the practicality and potential cost advantage of CyaA fusion. Therefore, for subsequent experiments, 500 nM rESAT-6, 50 nM CyaA-ESAT-6, 250 nM CFP-10, and 25 nM CyaA-CFP-10 were used. We then sought in a larger group of patients and healthy sensitized subjects to clarify whether delivery by CyaA would lead to enhancement in the number of IFN-γ-secreting SFC. There was no difference in the frequency or magnitude of responses to any stimulus between TB patients and healthy sensitized subjects, so results were combined for analysis. Of 68 donors, 63 responded (>10 SFC/106 PBMC) to rESAT-6 (average number of IFN-γ-secreting SFC/106 PBMC was 107.7 ± 16.2) and 64 donors responded to CyaA-ESAT-6 (107.5 ± 12.9); 52 donors responded to rCFP-10 (104.4 ± 14.3) and 62 to CyaA-CFP-10 (94.9 ± 11.3). Thus, no overall difference between the frequencies of IFN-γ-secreting SFC after stimulation of PBMC with the free antigen or toxoids was apparent. However, the proportion of subjects responding to CFP-10 increased from 76.4 to 91.1%. When subjects' responses were stratified by their response to the free antigens (500 nM rESAT-6 or 250 nM CFP-10) into low responders (<50 IFN-γ-secreting SFC/106 PBMC), intermediate responders (>50 but <100 SFC/106 PBMC), or high responders (>100 SFC/106 PBMC), we found clear enhancement in the group of low responders. Thus, the average number of IFN-γ-secreting SFC/106 detected increased from 26.1 ± 2.7 to 48.2 ± 7.3 (n = 27, P = 0.009) for ESAT-6 and from 17.5 ± 2.6 to 36.8 ± 4.3 (n = 34, P = 0.0002) in the case of CFP-10 (Fig. 2). Interestingly, the PBMC of 10 donors who did not respond to rCFP-10 did produce IFN-γ after stimulation with CyaA-CFP-10 (mean number of IFN-γ-secreting SFC/106 PBMC, 37.8 ± 8.2). This indicates that CyaA-CFP-10 more efficiently restimulates CFP-10-specific T cells, thereby increasing the detection frequency of CFP-10 responders.
FIG. 2.
The detection of IFN-γ-secreting SFC (IFN-γ SFC) in low-responding subjects is enhanced by CyaA delivery. Subjects who responded to 500 nM rESAT-6 or 250 nM rCFP-10 were stratified by their magnitude of response into low (<50 IFN-γ-secreting SFC/106 PBMC), intermediate (50 to 100), and high (>100) responders. CyaA delivery significantly increased the detection of IFN-γ-secreting SFC in low-responding subjects. Average CyaA toxoid results are shown corrected for the background response to wild-type CyaA (CyaA-WT) ([CyaA-ESAT-6]-CyaA-WT). Results are shown as means ± standard errors of the means (error bars).
The primary objective of our study was to determine whether the CyaA fusion increased the sensitivity of detection of M. tuberculosis-specific T cells. However, we were also interested to determine whether the well-documented specificity of the RD1-based ex vivo ELISPOT in differentiating BCG-vaccinated individuals from M. tuberculosis-sensitized individuals (13, 26) would be compromised by CyaA fusion. For this purpose, we selected seven BCG vaccinated donors who were thought unlikely to have been exposed to TB and exhibited negative PPD skin test reactions. Of these donors, none reacted to mock CyaA or CFP-10, two showed weak reactivity to ESAT-6 or CyaA-ESAT-6, one reacted to CyaA-CFP-10, and 5 reacted to PPD in vitro (Table 1). These data reassured us that the increment in reactivity we had seen in M. tuberculosis-sensitized donors was due to the presence of T cells specific for M. tuberculosis, rather than Bordetella pertussis CyaA.
TABLE 1.
Effect of CyaA fusion on the specificity of RD1-based detection of sensitization by M. tuberculosisa
| Donor | No. of IFN-γ-secreting SFC/106 PBMC
|
|||||
|---|---|---|---|---|---|---|
| ESAT-6 (500 nM) | CyaA-ESAT-6 (50 nM) | CFP-10 (250 nM) | CyaA-CFP-10 (25 nM) | CyaA (50 nM) | PPD (100 U/ml) | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 100 |
| 3 | 17 | 0 | 2 | 0 | 0 | 0 |
| 4 | 16 | 30 | 3 | 26 | 0 | 98 |
| 5 | 0 | 0 | 0 | 0 | 0 | 65 |
| 6 | 2 | 10 | 0 | 2 | 0 | 93 |
| 7 | 0 | 0 | 0 | 0 | 0 | 97 |
Seven BCG-vaccinated donors who were thought unlikely to have been exposed to TB and exhibited negative PPD skin test reactions were selected. Only weak and occasional ELISPOT responses to ESAT-6, CyaA-ESAT-6, and CyaA-CFP-10 were observed in this group of donors.
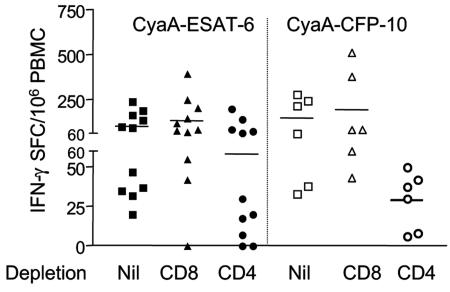
Both CD4+ and CD8+ responses can be detected by CyaA delivery.
In order to define the T-cell subset that recognized the CyaA toxoids, we enriched populations by performing prior immunomagnetic depletion of either CD4+ or CD8+ T cells from PBMC. The remaining cells were set up in the ELISPOT and stimulated overnight with the detoxified CyaA incorporating ESAT-6 or CFP-10. Eleven donors were tested for CyaA-ESAT-6 and six donors for CyaA-CFP-10. Both CD4+ and CD8+ responses were seen to the toxoid antigens, the CD4+ response being dominant (Fig. 3). Compared to the response to corresponding free antigen, responses to the CyaA toxoids increased towards CD4 in three instances and towards CD8 in two instances (data not shown).
FIG. 3.
Both CD4+ and CD8+ responses can be detected by CyaA delivery. Immunomagnetic depletion of either CD4+ or CD8+ T cells from PBMC was performed, and the response of the remaining cells to CyaA toxoids was assayed. The response of CD4+-depleted PBMC was interpreted as CD8+ and vice versa. Both CD4+ and CD8+ responses to the toxoid antigens were seen, with the CD4+ response being dominant. Average responses are indicated by bars. IFN-γ SFC, IFN-γ-secreting SFC.
Increased detection of IFN-γ-secreting SFC requires covalent association of the antigen to CyaA, and antigen presentation is via the MHC.
To determine whether the M. tuberculosis protein has to be covalently linked to CyaA, the effect of mixing ESAT-6 with mock CyaA toxoid was tested. PBMC from four subjects were set up with ESAT-6 (500 nM), CyaA-ESAT-6 (50 nM), or the mixture of rESAT-6 (500 nM) and CyaA (50 nM). The median numbers of IFN-γ-secreting SFC/106 PBMC for these stimulants were 113, 147, and 58, respectively, showing that covalent linkage between the antigen and carrier is required for enhancement to occur. In fact, it appeared that a simple mixture of rESAT-6 and CyaA might have actually decreased the response to rESAT-6.
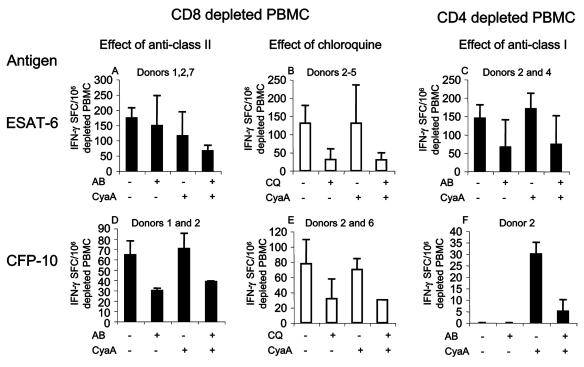
We next determined whether recognition of the CyaA antigen toxoids could be altered by manipulation of MHC class I or II antigen presentation. This was primarily tested by the use of blocking antibodies of well-established specificity (W6/32 and L243) (3, 11, 19, 35). In some experiments we also tested 20 μM chloroquine, a known inhibitor of endosomal processing that therefore decreases presentation via MHC class II (27, 45). CD4- or CD8-depleted cells were set up on the ELISPOT plates from seven donors. Due to limited cell numbers, not all combinations could be tested in every donor. Anti-MHC class I blocking antibody, anti-MHC class II blocking antibody, or isotype control antibody (all at 5 μg/ml) was added to selected wells, and the plates were incubated overnight.
L243 barely inhibited the CD4+ T-cell response to rESAT-6 but inhibited the response to CyaA-ESAT-6 more (15 and 43%, respectively) (Fig. 4A). The effect of preincubation with chloroquine was more marked (77% inhibition in both cases) (Fig. 4B). L243 rather better inhibited the CD4 response to both rCFP-10 and CyaA-CFP-10 (53 and 45%, respectively) (Fig. 4D). The effect of chloroquine was similar (59 and 57%, respectively) (Fig. 4E). W6/32 inhibited CD8+ T-cell recognition of rESAT-6 and CyaA-ESAT-6 by 56 and 57%, respectively (Fig. 4C). No donor in this group had a CD8+ T-cell response to rCFP-10, but in donor 2, W6/32 strongly inhibited the response to CyaA-CFP-10 (Fig. 4F).
FIG. 4.
Effects of inhibition of antigen presentation on the T-cell responses to free and toxoid antigens. CD8+-depleted (i.e., CD4+-enriched) and CD4+-depleted (CD8+-enriched) PBMC were set up in the overnight IFN-γ ELISPOT in the presence or absence of antibodies to MHC class I and II and isotype control (5 μg/ml) or chloroquine (20 μM). Antigens were added as free ESAT-6 (500 nM) or CFP-10 (250 nM) or as CyaA fusions (50 and 25 nM, respectively). There were insufficient cells to test all combinations in all donors; the donors who were tested and responded are indicated. (A and B) Antibody to HLA-DR moderately inhibited the response to both rESAT-6 and CyaA-ESAT-6 (15 and 43%, respectively). The effect of chloroquine was more marked (77% in both cases). (C) Antibody to MHC class I inhibited the CD8+ T-cell recognition of rESAT-6 and CyaA-ESAT-6 by 56 and 57%, respectively. (D and E) Antibody to MHC class II inhibited the response to both rCFP-10 and CyaA-CFP-10 (53 and 45%, respectively). The effect of chloroquine was again more marked (59 and 57%, respectively). (F) No donor had a CD8+ T-cell response to rCFP-10. In donor 2, antibody to MHC class I strongly inhibited the response to CyaA-CFP-10. Isotype control antibody had no effect on antigen recognition. Values are shown as means ± standard deviation (error bars). IFN-γ SFC, IFN-γ-secreting SFC; AB, antibody; CQ, chloroquine; CyaA, antigen presented as CyaA fusion; +, presence of antibody and/or presentation as CyaA toxoid.
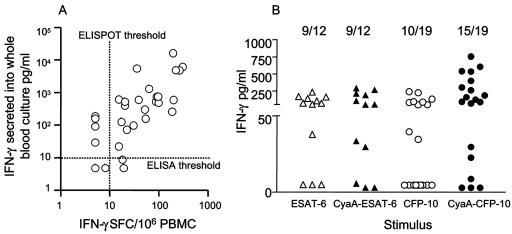
The response to CyaA-CFP-10 is also enhanced in a simple whole-blood IFN-γ production assay.
Detection of IFN-γ secreted into the supernatant of whole-blood cultures requires less blood and is potentially more applicable to epidemiological surveys in resource-poor environments than the ex vivo IFN-γ ELISPOT. However, such whole-blood assays, while retaining specificity, tend to be less sensitive than ELISPOT. We therefore sought to determine whether the enhanced response to CyaA toxoids carrying ESAT-6 or CFP-10 could compensate for this deficiency. Thirty-three patients and healthy sensitized subjects were tested in parallel using the two read-out assays of IFN-γ production. Interestingly, the background reactivity in whole blood to the mock CyaA was greater than we had observed in ELISPOT analysis with 30% subjects having values above 100 pg/ml. This may reflect the presence of Bordetella pertussis-specific memory T cells, undetected by the overnight ELISPOT, that have greater time to differentiate and produce cytokine during the longer whole-blood assay (72 h). However, this potentially confounding factor was compensated for by the greater dynamic range of reactivity to the M. tuberculosis CyaA fusions (0 to 23,993 pg/ml IFN-γ). Therefore, we subtracted the mock CyaA values from the values obtained with the M. tuberculosis CyaA fusions for analysis. The ELISPOT and (mock-CyaA-corrected) whole-blood IFN-γ responses to CyaA-ESAT-6 and CyaA-CFP-10 were positively correlated (r = 0.58 and 0.64, respectively) (P < 0.001 in both cases) (Fig. 5A). Donors were stratified according to their responses to the free antigen into low (<250 pg/ml IFN-γ), intermediate (250 to 1,000 pg/ml IFN-γ), or high (>1,000 pg/ml IFN-γ) responders in the whole-blood assay. Intermediate and high responders had similar responses to free and toxoid antigens. In low responders, the results showed a similar effect of CyaA delivery on antigen recognition as found by the ELISPOT. Thus, the amount of IFN-γ produced in the presence of CyaA-CFP-10 was on average 27.7-fold ± 9.5-fold higher than in the presence of free rCFP-10 (P = 0.021) (Fig. 5B). The response to CyaA-ESAT-6 showed the same general trend (average, 5.6-fold ± 3.2-fold), although this effect did not reach statistical significance.
FIG. 5.
A. Correlation between IFN-γ ELISPOT and whole-blood assay. The mock-CyaA-corrected CyaA-CFP-10-stimulated overnight IFN-γ ELISPOT response was compared to the similarly stimulated 72-h production of IFN-γ in 1/10 diluted whole blood in 31 M. tuberculosis-sensitized donors. Using an ELISPOT cutoff of 10 SFC/106 PBMC and an ELISA cutoff of 10 pg/ml, 81% responses were concordant. The responses were also significantly correlated (Spearman correlation coefficient r = 0.64, P = 0.0002). B. Enhancement of IFN-γ secretion in whole blood stimulated by CyaA carrying M. tuberculosis CFP-10. The M. tuberculosis antigen-specific IFN-γ induced by rESAT-6, rCFP-10, and CyaA toxoids incorporating these antigens in a 72-h whole-blood assay was determined. Donors were then stratified into high (>1,000 pg/ml), medium (250 to 1,000 pg/ml), and low (<250 pg/ml) responders by their response to native antigen. Only the responses of low-responding donors are shown. Enhancement of the M. tuberculosis-specific whole-blood response by CyaA delivery of CFP-10 was significant in subjects classified as low responders to CFP-10 (P = 0.021). The numbers above the scattergram indicate the proportion of responders to each stimulus.
DISCUSSION
We have investigated the use of genetically detoxified Bordetella pertussis adenylate cyclase toxin (CyaA) as a delivery system for two immunodominant proteins of M. tuberculosis, ESAT-6 and CFP-10. We found that CyaA toxoids were able to restimulate T cells from over 91.1% TB patients and healthy sensitized donors. CyaA delivery decreased by 10-fold the amount of ESAT-6 and CFP-10 required to restimulate T cells, and in low responders, the overall frequency of IFN-γ-producing cells detected was increased. Both CD4+ and CD8+ T cells responded to CyaA toxoids: this response could be blocked by inhibition of MHC class II or class I, respectively. In addition, CD4 recognition of toxoids was sensitive to inhibition by chloroquine. In a simplified whole-blood model, the frequency of positive responses to CFP-10 was increased by CyaA delivery, a potentially useful attribute that could help identify LTBI, especially in low responders, such as subjects coinfected with HIV.
A simple mixture of mock CyaA toxoid and rESAT-6 did not increase the response. In addition, CD8+ T-cell responses were sensitive to inhibition by antibody specific for MHC class I (Fig. 4C and F). While we did not investigate in detail the antigen processing of the toxoids, our data are consistent with the interpretation that antigen delivery by CyaA increases the availability of processed M. tuberculosis-derived peptide to nascent MHC class I molecules. It is known that CyaA toxoids become accessible to proteosomic cleavage in the cytoplasm, as CyaA is specifically taken up via CD11b/CD18 (18). In vivo, CyaA has been demonstrated to be delivered efficiently to the cytosol of dendritic cells (17). Thus, CD8 responses are more readily detected when comparing the response to soluble recombinant antigen, because the latter is typically processed in the endosome and thus less accessible to MHC class I. CD8+ T cells potentially contribute to the human protective response against M. tuberculosis (24, 32), but the detection of antigen-specific responses has so far been limited by the need to use peptide pools or recombinant vaccinia viruses that express the antigen of interest (24, 32, 46). Delivery of antigens by CyaA represents a novel method by which the response of CD8+ T cells to whole M. tuberculosis proteins could be assayed.
M. tuberculosis-specific CD4+ T cells also responded to CyaA toxoids; in fact, this was the dominant response (Fig. 3). Enhancement of response to antigens fused to CyaA was especially pronounced in donors who had low responses to free antigen. The CD4+ T-cell response to CyaA toxoids was sensitive to inhibition by chloroquine and to blockade of MHC class II (Fig. 4A, B, D, and E). We have previously documented CD4+ T-cell recognition of CyaA toxoids (28). Furthermore, we have recently demonstrated that CD4+ T cells from cattle infected with M. bovis recognize both the ESAT-6 and CFP-10 CyaA toxoids (42). Like human T cells, bovine T-cell recognition was sensitive to antibody blockade of MHC class II and chloroquine, and it was also sensitive to antibody blockade of CD11b. These data therefore lead us to hypothesize that recombinant antigen is less efficiently taken up by pinocytosis (and thus less available for endosomal processing) than the macromolecular CyaA antigen conjugate that binds specifically to CD11b/CD18 (18) whereupon it is rapidly endocytosed (23). This would explain why 10- to 20-fold-less toxoid antigen (on a molar basis) could restimulate the same response (Fig. 1 and 4) and why some donors with negative responses to recombinant antigen showed a response to the antigen fused to CyaA (Fig. 2, 4D, and 5B).
Although there were differences in the doses favoring the free antigen, in a few donors the recognition of rESAT-6 was actually superior to that of CyaA-ESAT-6 (Fig. 4A). In these circumstances, blockade of the human MHC class II-presenting molecule HLA-DR by L243 antibody poorly inhibited the response to free, but not CyaA delivered, ESAT-6 (Fig. 4A). We note with interest reports that some C-terminal peptides can be recognized without intracellular processing, the proposal being that peptide is accessible to the MHC extracellularly and is cleaved by cell surface exopeptidases (1, 12). Interestingly, both the C- and N-terminal peptides of ESAT-6, which may be similarly accessible to the MHC, are immunodominant in humans (31, 34). The recognition of the N-terminal peptide of ESAT-6 is restricted by HLA-DQ (31), but L243 is specific for HLA-DR (2). Thus, the relative failure of L243 inhibition after the addition of rESAT-6 could be explained by these factors. Extracellular recognition of free ESAT-6 would also tend to offset any beneficial effect of CyaA fusion on intracellular processing, as the C- and N-terminal peptides would not be accessible when ESAT-6 is expressed in this way.
Several years ago, we showed the potential to replace the TST by a test that assays the in vitro production of IFN-γ produced by T cells in response to defined M. tuberculosis antigens (22). This approach has been refined and improved by incorporation of the highly immunogenic RD1-encoded antigens ESAT-6 and CFP-10 (7, 40). Several studies have shown that in vitro responses to RD1 encoded antigens in either whole-blood or ELISPOT format differentiate immune sensitization by BCG from infection by pathogenic mycobacteria (4, 8, 9, 25, 37). The IFN-γ ELISPOT response to multiple peptides of ESAT-6 can be utilized to detect latent or overt tuberculosis infection with a sensitivity of 96% and a specificity of 92% (26). The very high frequency of recognition of the ESAT-6 and CFP-10 antigen toxoids that we observed in M. tuberculosis-sensitized subjects closely accords with these estimates that were made on similar patients and control subjects in our hospital. The more practical approach of using antigen stimulated whole-blood cultures is unfortunately associated with a fall in sensitivity to 72% (8). However, our data suggest that the use of CyaA toxoid as a delivery system may overcome this deficiency. A particular advantage is that smaller amounts of blood are required, making this assay applicable to studies of children. A particular issue that needs to be addressed is whether background reactivity to Bordetella pertussis in whole blood will ultimately compromise specificity. To address both these issues, we plan prospective studies of the utility of CyaA toxoids carrying M. tuberculosis antigens in high-incidence environments. In particular, we are interested in determining whether LTBI in HIV-infected subjects can be detected with greater sensitivity. One limitation is that immunosuppressed patients might have low or undetectable M. tuberculosis-specific T-cell responses. However, we are encouraged that delivery by CyaA best enhanced the detection of IFN-γ in low-responding subjects, an attribute that could be an obvious advantage in the setting of HIV infection.
Acknowledgments
We are grateful to Juraj Ivanyi of King's College London who helped instigate this work and contributed to this work by extremely helpful discussions. We also thank Roland Brosch from Institut Pasteur for the gift of the pYUB412 cosmid clone of the RD1 region and Hana Kubinova and Adam Whelan for important technical assistance.
This work was supported in part by grant 310/01/0934 from the Grant Agency and grant S5020311 to P.S. from the Academy of Sciences of the Czech Republic. R.J.W. is a Wellcome Trust Advanced Fellow in Clinical Science (064261/JW/MW.sf), and E.S. was supported by a Burroughs-Wellcome/Wellcome Trust Infectious Diseases initiative award to M.L. (059141/Z/99/Z). R.J.W., M.S., E.S., P.S., H.M.V., and C.L. have a patent application pending on the diagnostic use of CyaA toxoids in TB.
Editor: J. T. Barbieri
REFERENCES
- 1.Accapezzato, D., R. Nisini, M. Paroli, G. Bruno, F. Bonino, M. Houghton, and V. Barnaba. 1998. Generation of an MHC class II-restricted T cell epitope by extracellular processing of hepatitis delta antigen. J. Immunol. 160:5262-5266. [PubMed] [Google Scholar]
- 2.Agrewala, J., S. Deacock, S. Jurcevic, and R. Wilkinson. 1997. Peptide recognition by T-cell clones of an HLA-DRB1*1501/*0901 heterozygous donor is promiscuous only between parental alleles. Hum. Immunol. 55:34-38. [DOI] [PubMed] [Google Scholar]
- 3.Anichini, A., S. Ress, G. Strassmann, and F. H. Bach. 1985. Inhibition of anti-class I cytotoxicity by anti-class II monoclonal antibodies (MoAb). II. Blocking of anti-class I CTL clones by anti-DR MoAb. Hum. Immunol. 13:139-143. [DOI] [PubMed] [Google Scholar]
- 4.Arend, S. M., P. Andersen, K. E. van Meijgaarden, R. L. Skjot, Y. W. Subronto, J. T. van Dissel, and T. H. Ottenhoff. 2000. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181:1850-1854. [DOI] [PubMed] [Google Scholar]
- 5.Arend, S. M., K. E. van Meijgaarden, K. de Boer, E. C. de Palou, D. van Soolingen, T. H. Ottenhoff, and J. T. van Dissel. 2002. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J. Infect. Dis. 186:1797-1807. [DOI] [PubMed] [Google Scholar]
- 6.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 7.Berthet, F. X., P. B. Rasmussen, I. Rosenkrands, P. Andersen, and B. Gicquel. 1998. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology 144:3195-3203. [DOI] [PubMed] [Google Scholar]
- 8.Brock, I., M. E. Munk, A. Kok-Jensen, and P. Andersen. 2001. Performance of whole blood IFN-gamma test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int. J. Tuberc. Lung Dis. 5:462-467. [PubMed] [Google Scholar]
- 9.Brock, I., K. Weldingh, T. Lillebaek, F. Follmann, and P. Andersen. 2004. Comparison of a new specific blood test and the skin test in tuberculosis contacts. Am. J. Respir. Crit. Care Med. 170:65-69. [DOI] [PubMed] [Google Scholar]
- 10.Dadaglio, G., Z. Moukrim, R. Lo-Man, V. Sheshko, P. Sebo, and C. Leclerc. 2000. Induction of a polarized Th1 response by insertion of multiple copies of a viral T-cell epitope into adenylate cyclase of Bordetella pertussis. Infect. Immun. 68:3867-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darrow, T. L., C. L. Slingluff, Jr., and H. F. Seigler. 1989. The role of HLA class I antigens in recognition of melanoma cells by tumor-specific cytotoxic T lymphocytes. Evidence for shared tumor antigens. J. Immunol. 142:3329-3335. [PubMed] [Google Scholar]
- 12.Diegel, M. L., F. Chen, R. Laus, T. J. Graddis, and D. Vidovic. 2003. Major histocompatibility complex class I-restricted presentation of protein antigens without prior intracellular processing. Scand. J. Immunol. 58:1-8. [DOI] [PubMed] [Google Scholar]
- 13.Ewer, K., J. Deeks, L. Alvarez, G. Bryant, S. Waller, P. Andersen, P. Monk, and A. Lalvani. 2003. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet 361:1168-1173. [DOI] [PubMed] [Google Scholar]
- 14.Fayolle, C., P. Sebo, D. Ladant, A. Ullmann, and C. Leclerc. 1996. In vivo induction of CTL responses by recombinant adenylate cyclase of Bordetella pertussis carrying viral CD8+ T cell epitopes. J. Immunol. 156:4697-4706. [PubMed] [Google Scholar]
- 15.Gordon, S. V., R. Brosch, A. Billault, T. Garnier, K. Eiglmeier, and S. T. Cole. 1999. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol. Microbiol. 32:643-655. [DOI] [PubMed] [Google Scholar]
- 16.Guermonprez, P., C. Fayolle, G. Karimova, A. Ullmann, C. Leclerc, and D. Ladant. 2000. Bordetella pertussis adenylate cyclase toxin: a vehicle to deliver CD8-positive T-cell epitopes into antigen-presenting cells. Methods Enzymol. 326:527-542. [DOI] [PubMed] [Google Scholar]
- 17.Guermonprez, P., C. Fayolle, M. J. Rojas, M. Rescigno, D. Ladant, and C. Leclerc. 2002. In vivo receptor-mediated delivery of a recombinant invasive bacterial toxoid to CD11c+CD8α−CD11bhigh dendritic cells. Eur. J. Immunol. 32:3071-3081. [DOI] [PubMed] [Google Scholar]
- 18.Guermonprez, P., N. Khelef, E. Blouin, P. Rieu, P. Ricciardi-Castagnoli, N. Guiso, D. Ladant, and C. Leclerc. 2001. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the αMβ2 integrin (CD11b/CD18). J. Exp. Med. 193:1035-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson, S., J. R. Richert, W. E. Biddison, A. Satinsky, R. J. Hartzman, and H. F. McFarland. 1984. Measles virus-specific T4+ human cytotoxic T cell clones are restricted by class II HLA antigens. J. Immunol. 133:754-757. [PubMed] [Google Scholar]
- 20.Johnson, J. L., S. Nyole, A. Okwera, C. C. Whalen, P. Nsubuga, V. Pekovic, R. Huebner, R. S. Wallis, P. N. Mugyenyi, R. D. Mugerwa, and J. J. Ellner, for the Uganda-Case Western Reserve University Research Collaboration. 1998. Instability of tuberculin and candida skin test reactivity in HIV-infected Ugandans. Am. J. Respir. Crit. Care Med. 158:1790-1796. [DOI] [PubMed] [Google Scholar]
- 21.Joint Tuberculosis Committee of the British Thoracic Society. 2000. Control and prevention of tuberculosis in the United Kingdom: code of practice 2000. Thorax 55:887-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jurcevic, S., A. Hills, G. Pasvol, R. N. Davidson, J. Ivanyi, and R. J. Wilkinson. 1996. T cell responses to a mixture of Mycobacterium tuberculosis peptides with complementary HLA-DR binding profiles. Clin. Exp. Immunol. 105:416-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khelef, N., P. Gounon, and N. Guiso. 2001. Internalization of Bordetella pertussis adenylate cyclase-haemolysin into endocytic vesicles contributes to macrophage cytotoxicity. Cell. Microbiol. 3:721-730. [DOI] [PubMed] [Google Scholar]
- 24.Lalvani, A., R. Brookes, R. J. Wilkinson, A. S. Malin, A. A. Pathan, P. Andersen, H. M. Dockrell, G. Pasvol, and A. V. S. Hill. 1998. Human cytolytic and interferon-γ secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 95:270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lalvani, A., A. A. Pathan, H. Durkan, K. A. Wilkinson, A. Whelan, J. J. Deeks, W. H. Reece, M. Latif, G. Pasvol, and A. V. Hill. 2001. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet 357:2017-2021. [DOI] [PubMed] [Google Scholar]
- 26.Lalvani, A., A. A. Pathan, H. McShane, R. J. Wilkinson, M. Latif, C. P. Conlon, G. Pasvol, and A. V. Hill. 2001. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am. J. Respir. Crit. Care Med. 163:824-828. [DOI] [PubMed] [Google Scholar]
- 27.Lewinsohn, D. M., M. R. Alderson, A. L. Briden, S. R. Riddell, S. G. Reed, and K. H. Grabstein. 1998. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen-presenting cells. J. Exp. Med. 187:1633-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loucka, J., G. Schlecht, J. Vodolanova, C. Leclerc, and P. Sebo. 2002. Delivery of a MalE CD4+-T-cell epitope into the major histocompatibility complex class II antigen presentation pathway by Bordetella pertussis adenylate cyclase. Infect. Immun. 70:1002-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahairas, G. G., P. J. Sabo, M. J. Hickey, D. C. Singh, and C. K. Stover. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178:1274-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osicka, R., A. Osickova, T. Basar, P. Guermonprez, M. Rojas, C. Leclerc, and P. Sebo. 2000. Delivery of CD8+ T-cell epitopes into major histocompatibility complex class I antigen presentation pathway by Bordetella pertussis adenylate cyclase: delineation of cell invasive structures and permissive insertion sites. Infect. Immun. 68:247-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pathan, A. A., K. A. Wilkinson, P. Klenerman, H. McShane, R. N. Davidson, G. Pasvol, A. V. Hill, and A. Lalvani. 2001. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J. Immunol. 167:5217-5225. [DOI] [PubMed] [Google Scholar]
- 32.Pathan, A. A., K. A. Wilkinson, R. J. Wilkinson, M. Latif, H. McShane, G. Pasvol, A. V. Hill, and A. Lalvani. 2000. Identification of a novel HLA class I restricted epitope in Mycobacterium tuberculosis: high frequencies of circulating peptide-specific IFN-γ secreting CD8+ cytotoxic T lymphocytes in a healthy exposed contact. Eur. J. Immunol. 30:2713-2721. [DOI] [PubMed] [Google Scholar]
- 33.Pym, A. S., P. Brodin, R. Brosch, M. Huerre, and S. T. Cole. 2002. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 46:709-717. [DOI] [PubMed] [Google Scholar]
- 34.Ravn, P., A. Demissie, T. Eguale, H. Wondwosson, D. Lein, H. A. Amoudy, A. S. Mustafa, A. K. Jensen, A. Holm, I. Rosenkrands, F. Oftung, J. Olobo, F. von Reyn, and P. Andersen. 1999. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179:637-645. [DOI] [PubMed] [Google Scholar]
- 35.Santin, A. D., P. L. Hermonat, A. Ravaggi, M. Chiriva-Internati, D. Zhan, S. Pecorelli, G. P. Parham, and M. J. Cannon. 1999. Induction of human papillomavirus-specific CD4+ and CD8+ lymphocytes by E7-pulsed autologous dendritic cells in patients with human papillomavirus type 16- and 18-positive cervical cancer. J. Virol. 73:5402-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saron, M. F., C. Fayolle, P. Sebo, D. Ladant, A. Ullmann, and C. Leclerc. 1997. Anti-viral protection conferred by recombinant adenylate cyclase toxins from Bordetella pertussis carrying a CD8+ T cell epitope from lymphocytic choriomeningitis virus. Proc. Natl. Acad. Sci. USA 94:3314-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scholvinck, E., K. A. Wilkinson, A. O. Whelan, A. R. Martineau, M. Levin, and R. J. Wilkinson. 2004. Gamma interferon-based immunodiagnosis of tuberculosis: comparison between whole-blood and enzyme-linked immunospot methods. J. Clin. Microbiol. 42:829-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sebo, P., C. Fayolle, O. d'Andria, D. Ladant, C. Leclerc, and A. Ullmann. 1995. Cell-invasive activity of epitope-tagged adenylate cyclase of Bordetella pertussis allows in vitro presentation of a foreign epitope to CD8+ cytotoxic T cells. Infect. Immun. 63:3851-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith, S. M., A. S. Malin, P. T. Lukey, S. E. Atkinson, J. Content, K. Huygen, and H. M. Dockrell. 1999. Characterization of human Mycobacterium bovis bacille Calmette-Guérin-reactive CD8+ T cells. Infect. Immun. 67:5223-5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorensen, A. L., S. Nagai, G. Houen, P. Andersen, and A. B. Andersen. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vordermeier, H. M., P. C. Cockle, A. Whelan, S. Rhodes, N. Palmer, D. Bakker, and R. G. Hewinson. 1999. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin. Diagn. Lab. Immunol. 6:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vordermeier, H. M., M. Simsova, K. A. Wilkinson, R. J. Wilkinson, R. G. Hewinson, P. Sebo, and C. Leclerc. 2004. Recognition of mycobacterial antigens delivered by genetically detoxified Bordetella pertussis adenylate cyclase by T cells from cattle with bovine tuberculosis. Infect. Immun. 72:6255-6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinrich Olsen, A., L. A. van Pinxteren, L. Meng Okkels, P. Birk Rasmussen, and P. Andersen. 2001. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Infect. Immun. 69:2773-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilkinson, K. A., J. T. Belisle, M. Mincek, R. J. Wilkinson, and Z. Toossi. 2000. Enhancement of the human T cell response to culture filtrate fractions of Mycobacterium tuberculosis by microspheres. J. Immunol. Methods 235:1-9. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson, K. A., F. Hudecz, H. M. Vordermeier, J. Ivanyi, and R. J. Wilkinson. 1999. Enhancement of the T cell response to a mycobacterial peptide by conjugation to synthetic branched polypeptide. Eur. J. Immunol. 29:2788-2796. [DOI] [PubMed] [Google Scholar]
- 46.Wilkinson, R. J., X. Zhu, K. A. Wilkinson, A. Lalvani, J. Ivanyi, G. Pasvol, and H. M. Vordermeier. 1998. 38000 MW antigen specific MHC class I restricted interferon-γ secreting CD8+ T cells in healthy contacts of tuberculosis. Immunology 95:585-590. [DOI] [PMC free article] [PubMed] [Google Scholar]