Abstract
Introduction
Needle biopsy is essential for definitive diagnosis of breast malignancy. Significant histologic changes due to tissue damage have been reported in solid tumors. This study investigated the association between time from needle biopsy and inflammation in breast tumors.
Methods
A total of 73 stage I–II invasive breast cancer cases diagnosed by image-guided needle biopsy who had surgery as their first definitive treatment were retrospectively analyzed. Time from biopsy to surgical excision ranged from 8 to 252 days. Histological sections of surgically resected tumors with a visible needle tract were reviewed by histologic evaluation. Data were analyzed by McNemar's test for proportional differences, and the Benjamini-Hochberg procedure was used to assess the association between immune cell prevalence and clinical variables.
Results
Characteristic histology changes, including foreign body giant-cell reaction, synovial-cell metaplasia, desmoplastic repair changes, granulation tissue, fat necrosis, and inflammation, were frequently detected adjacent to the needle tract. Spatial comparison indicated that a higher proportion of cases had neutrophils, eosinophils, and macrophages adjacent to the needle tract than tumors distant from it. The presence of inflammatory cells adjacent to the needle tract was not associated with time from biopsy or subtype. Still, plasma cells were associated with residual carrier material from biopsy markers.
Conclusion
Macrophages and eosinophils are highly abundant and retained adjacent to the needle tract regardless of time from the biopsy.
Keywords: Breast cancer, Needle biopsy, Post-biopsy change
Introduction
Breast cancer is the most commonly diagnosed malignancy worldwide [1, 2]. Historically, symptomatic palpable breast cancers were diagnosed by a one-step intraoperative procedure, in which definitive diagnosis was given in the operating room [3]. Better health education and successful mammography screening programs markedly increased the number of asymptomatic cases with small suspicious lesions [4], which led to the adoption of a two step-procedure where surgery is scheduled only after the malignancy is definitively diagnosed by needle biopsy [3]. Needle biopsy collects a relatively large amount of tissue to ascertain tumors' histologic and molecular subtypes, which help determine the course of treatment. Preoperative diagnosis by needle biopsy for the increased volume of suspicious cases presented a significant advantage over the one-step intraoperative procedure by reducing the number of invasive procedures [3]. However, needle biopsy-related histologic changes have been reported [5, 6].
Sampling tissue using a 9- to 14-gauge biopsy needle and placing a biopsy marker into the resulting cavity lead to responses pertinent to wound healing and foreign body reaction [7]. Thus, inflammation, hemorrhage, fat necrosis, granulation tissue, necrosis, and foreign body giant-cell reaction have been reported as common histologic changes in the area adjacent to the needle tract [6]. The appearance of reactive spindle cell nodules composed of atypical spindle cells and atypical duct-like structures have also been frequently reported in biopsied breast tumors. These cytokeratin-negative cellular structures were networked with blood vessels, macrophages, and lymphocytes, exhibiting moderate nuclear polymorphism with low mitotic count [5]. Aside from such reactive changes, cancer cell displacement (i.e., needle tract seeding) along the needle tract into adjacent tissue due to needle retraction has also been reported [8, 9]. While biopsy-induced inflammation in response to tissue damage is expected, the prevalence of the individual inflammatory cell types and their temporal presence in biopsy wounds of breast tumors are largely unknown. Thus, the primary objective of this study was to evaluate spatial and temporal features of biopsy-associated inflammatory cells in malignant breast tumors.
Materials and Methods
Institutional Review Board approval was obtained at the University of Oklahoma Health Science Center (#6234). A total of 73 surgically resected stage I–II breast carcinomas that had undergone needle biopsy followed by surgery as their first definitive treatment between 2015 and 2020 were retrieved from the pathology archive at the University of Oklahoma Health Science Center. Cases with tissue containing DCIS only, inflammatory breast cancer, other concurrent malignancies, or the absence of a visible needle tract were excluded from the study. Electronic medical records were reviewed to obtain patient demographics (race, age, sex), diagnosis (subtype, Ki67, histology grade, stage), and other clinical factors (comorbidity and BMI). Tumor histologic grade was determined according to the Protocol for the Examination of Specimens From Patients With Invasive Carcinoma of the Breast (ver. 4.4.0.0) [10]. Tumor subtype was classified as luminal A (estrogen receptor [ER]+/progesterone receptor [PR]+/HER2−, Ki67 <15%), luminal B (ER+/PR+/HER2+, Ki67 ≥15%), HER2+ (ER−/PR−/HER2+), and triple negative (ER−/PR−/HER2−) based on immunohistochemical staining positivity (ER, PR, HER2, and Ki67). Patient race/ethnicity was categorized as White, Black, or Hispanic. BMI was calculated based on each patient's height and weight at the time of diagnosis (weight [kg]/height [m]2). Comorbidities were categorized based on the following types of disorders: cardiovascular, endocrine, gynecologic, hematologic, hepatic, hypertension, inflammatory/rheumatic, neurologic, osteoporosis, and psychiatric disorders. Diabetes was determined based on diagnosis code or medications reported in each patients' medical chart. Time after biopsy was defined as days from the first needle biopsy to surgery. Surgically resected, formalin-fixed paraffin-embedded tissue sections (4 μm thickness) were stained with H&E and reviewed by a board-certified pathologist. Inflammatory cells were categorized as acute (neutrophils, eosinophils) or chronic (plasma cells, lymphocytes, and macrophages). The prevalence of each inflammatory cell type per field of view was microscopically counted from multiple fields on low (×4) magnification to arbitrarily score the highest count for each category as 0 (absent), 1 (1–5 cells), 2 (6–19 cells), and 3 (≥20 cells) in an area adjacent to the biopsy needle tract or in tumor distant from it to differentiate biopsy-triggered inflammatory response from background inflammation. High (×40) magnification was used to confirm cytology. We defined residual biopsy cavity, necrotic area, or direct biopsy damage within the tumor tissue as a prior biopsy site. Carcinoma tissues in the area surrounding the aforementioned post-biopsy site within a 5-mm radius were defined as adjacent to the needle tract (AN). Carcinoma tissue at least 1 mm away from the area included in the AN was defined as distant from the needle tract (DN).
Statistical Analysis
Pathology scores for each inflammatory cell were dichotomized as 0 versus 1–3. McNemar's test was used to determine the proportional difference in the prevalence of immune cells between the tumor adjacent to the biopsy needle tract (AN) and the tumor distant from it (DN). Wilcoxon signed-rank sum test was used to compare the differences between median days after biopsy by dichotomized pathology scores. Histograms represent the number of cases with the presence of inflammatory cells AN and DN as a function of time after biopsy. A simple logistic regression model was employed to find the univariate association between the prevalence of inflammatory cells and four independent variables (days after the biopsy, biopsy marker, ER status, HER2 status), and the false discovery rate-adjusted (FDA) p values were calculated using the Benjamini-Hochberg procedure. All statistical analyses were performed using R 4.0.4. Significance was defined as p < 0.05.
Results
Patient Characteristics
A total of 73 stage I–II invasive breast cancer cases whose tumors were diagnosed by image-guided core needle or vacuum-assisted biopsy using 9–14 G needle and surgically resected prior to adjuvant therapy were included. Only cases with a visible needle tract were included in this study (Table 1). The average age of patients at the time of diagnosis was 62.2 years. The cohort was composed of pT1 (76.7%) and pT2 (23.3%); pN− (80.8%), pN+ (15.1%), and pN− unknown (4.1%). Luminal A/B breast tumors were the predominant subtype. Approximately 86.3% (n = 63) of cases had at least one of the listed comorbidities. Hypertension was the most common, followed by endocrine/metabolic disorder, inflammatory/rheumatic disorder, and cardiovascular disorder. Approximately 14% of cases were diabetic. The average time (in days) from biopsy to surgery was 34 days, ranging from 8 to 252 days.
Table 1.
Patients demographic and tumor characteristics
| % | ||
|---|---|---|
| Total, n | 73 | |
| Age, years | 62.2±10.8 (42-83) | |
| BMI | 29.9±6.8 (16-45) | |
| Race, n | ||
| White | 61 | 83.6 |
| African American | 6 | 8.2 |
| Hispanic | 3 | 4.1 |
| Native American | 1 | 1.4 |
| Unknown | 2 | 2.7 |
| Clinical stage, n | ||
| I | 55 | 75.3 |
| II | 18 | 24.7 |
| Lymph node, n | ||
| Negative | 59 | 80.8 |
| Positive | 11 | 15.1 |
| Unknown | 3 | 4.1 |
| Histology grade, n | ||
| 1 | 16 | 21.9 |
| 2 | 32 | 43.8 |
| 3 | 25 | 34.2 |
| Subtype, n | ||
| Luminal A | 45 | 61.6 |
| Luminal B | 10 | 13.7 |
| HER2+ | 1 | 1.4 |
| Triple Negative | 16 | 21.9 |
| Unknown | 1 | 1.4 |
| Time after biopsy, n | ||
| <15 days | 8 | 11.0 |
| 16-30 days | 33 | 45.2 |
| >31 days | 32 | 43.8 |
| Comorbidity, n | ||
| Cardiovascular | 22 | 30.1 |
| Endocrine/metabolic | 26 | 35.6 |
| Gynecologic | 7 | 9.6 |
| Hematologic | 5 | 6.8 |
| Hepatic | 2 | 2.7 |
| Hypertension | 36 | 49.3 |
| Inflammatory/rheumatic | 22 | 30.1 |
| Neurologic | 7 | 9.6 |
| Osteoporosis | 7 | 9.6 |
| Psychiatric | 8 | 11.0 |
| Comorbidity count, n | ||
| 0 | 10 | 13.7 |
| 1 | 22 | 30.1 |
| 2 | 19 | 26.0 |
| ≥3 | 22 | 30.1 |
Histologic Changes Adjacent to the Needle Tract
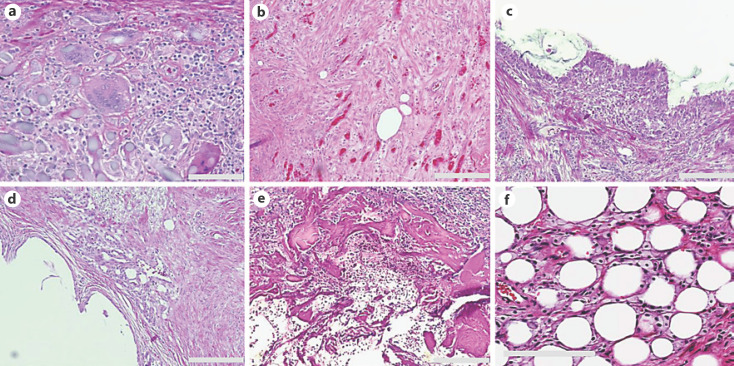
There were marked foreign body giant-cell reactions with macrophage aggregation around the needle tract in 53.4% (n = 39) cases (Fig. 1a). Organized granulation tissue with the presence of fibroblasts and concomitant chronic inflammation was seen in 20.5% (n = 15) of cases (Fig. 1b). Metaplastic synovial mucosa lining the biopsy cavity was present in 12.3% (n = 9) of cases (Fig. 1c). Vascular-rich granulation tissue with extensive angiogenesis AN was present in 20.5% (n = 15) of cases (Fig. 1d). Foreign material, which represents residual carrier material from a biopsy marker, was also present inside the biopsy cavity in 60.2% (n = 44) of cases (Fig. 1e). Fat necrosis (18%) and hemosiderin deposition (2.7%) were also observed (Fig. 1f).
Fig. 1.
H&E images of common histologic changes observed around the needle tract. a Giant-cell reaction with macrophage aggregation. b Granulation tissue with the presence of fibroblasts. c Metaplastic synovial mucosa lining the biopsy cavity. d Vascular-rich granulation tissue. e Residual carrier material from biopsy marker inside the biopsy cavity. f Fat necrosis. Scale bars, 200 μm.
Sustained Inflammation Is Present around the Needle Tract
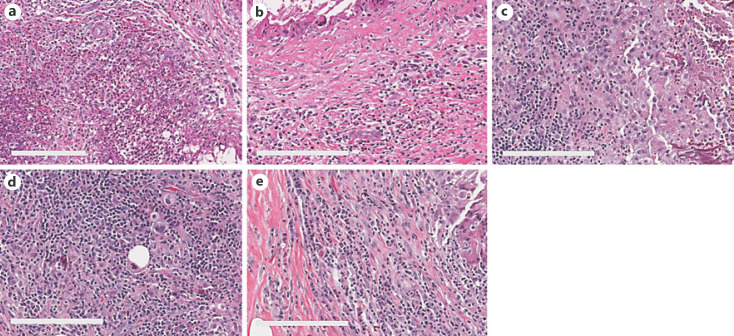
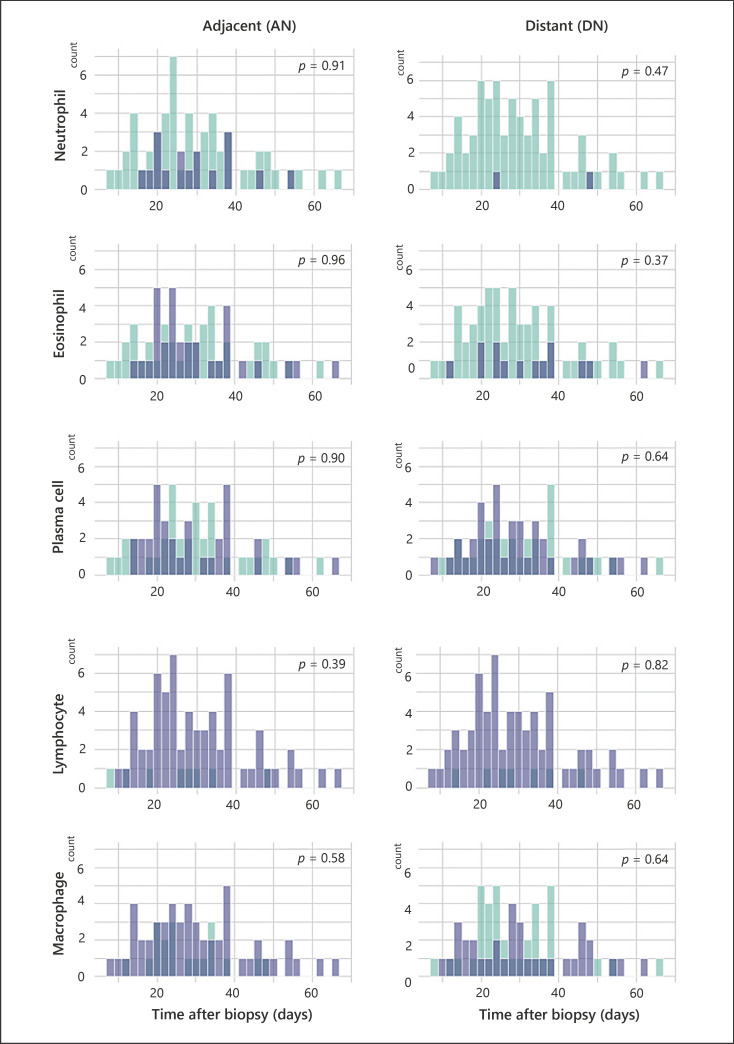
The number and proportion of specimens with individual inflammatory cell types detected AN and DN are shown in Table 2. A portion of specimens had higher levels of neutrophils and eosinophils present within the AN than DN (Fig. 2a, b), which accounted for a proportional difference of 20.5% (p = 0.001) and 24.7% (p = 0.002), respectively (Table 2). Among chronic inflammatory cells, macrophages were more frequently detected at the AN than DN, with a proportional difference of 26.0% (p = 0.001; Fig. 2c). However, no significant proportional difference was detected in other chronic inflammatory cells (plasma cells and lymphocytes), as they were equally abundant at both sites (Fig. 2d, e). Thirteen cases displayed overlapping presence of acute inflammatory cells and macrophages at the AN. To examine the relationship between time after biopsy and inflammation, cases with inflammatory cells present at the AN and DN were plotted as a function of time after biopsy (Fig. 3). Acute inflammatory cells, neutrophils (n = 6), and eosinophils (n = 11) remained present even over a month after biopsy, albeit at a lower frequency. The distribution of lymphocytes and plasma cells by time after biopsy showed considerable similarity between the AN and DN; however, macrophages were more abundant in the AN than DN regardless of time after biopsy (Fig. 3). The Wilcoxon signed-rank test indicated that there was not a statistically significant difference between the presence of inflammatory cells at both the AN and DN by time after biopsy. Additionally, a simple logistic regression model indicated that there was no association between needle sizes and inflammatory cells (data not shown). Further logistic regression analysis showed no association between time after biopsy (as continuous variable) and inflammatory cell presence at the AN, indicating that inflammatory cells were present at the AN regardless of time after biopsy (Table 3). Consistently, additional analysis using a generalized linear model supported the prevalence of eosinophils and macrophages at the AN, even after adjustment for time from biopsy as continuous variable (online suppl. Table. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000524668). Thus, these data collectively suggest prolonged retention of both macrophages and eosinophils at the AN.
Table 2.
Frequency of tumors with inflammatory cells adjacent to or distant from biopsy needle tract
| Inflammatory cell | Site | N | Proportion, % | 95% CI | p value |
|---|---|---|---|---|---|
| Neutrophil | AN | 17 | 23.3 | 14.2–34.7 | |
| DN | 2 | 2.7 | 0.3–9.6 | 0.001* | |
| AN + DN | 0 | 0 | 0–4.9 | ||
| Proportion difference | 20.5 | 9.5–32.6 | |||
|
| |||||
| Eosinophil | AN | 32 | 43.8 | 32.2–56.0 | |
| DN | 14 | 19.2 | 10.9–30.1 | 0.002* | |
| AN + DN | 8 | 11.0 | 4.9–20.5 | ||
| Proportion difference | 24.7 | 9.5–38.3 | |||
|
| |||||
| Macrophage | AN | 54 | 74.0 | 62.4–83.6 | |
| DN | 35 | 48.0 | 36.1–60.0 | 0.001* | |
| AN + DN | 31 | 42.5 | 31.0–54.9 | ||
| Proportion difference | 26.0 | 13.0–39.0 | |||
|
| |||||
| Plasma cell | AN | 35 | 48.0 | 36.1–60.0 | |
| DN | 39 | 53.4 | 41.4–65.2 | 0.607 | |
| AN + DN | 20 | 27.4 | 17.6–39.1 | ||
| Proportion difference | −5.4 | −22.6 to 10.9 | |||
|
| |||||
| Lymphocyte | AN | 65 | 89.0 | 80–95.2 | |
| DN | 65 | 89.0 | 80–95.2 | 1 | |
| AN + DN | 59 | 80.8 | 70–89.1 | ||
| Proportion difference | 0 | −10.9 to 10.9 | |||
AN, adjacent to the needle tract; DN, distant from the needle tract; AN+DN, present in both. Asterisk indicates statistical significance, p < 0.05.
Fig. 2.
H&E images of inflammatory cells adjacent to the biopsy needle tract. a, b Neutrophils and eosinophils were present 31 days after the biopsy. c, d Macrophages and lymphocytes remained present 56 days after the biopsy. e Plasma cell-rich area adjacent to the needle tract 25 days after the biopsy. Scale bars, 50 μm.
Fig. 3.
Histograms represent cases with the presence (blue) or absence (green) of each inflammatory cell at AN or DN in the function of days after the biopsy. p value was calculated by Wilcoxon signed-rank test.
Table 3.
Association between the presence of inflammatory cells and clinical factors
| Inflammatory cell | Odds ratio | 95% CI | p value | FDR-adjusted |
|---|---|---|---|---|
| Neutrophil | ||||
| Time after biopsy | 1 | 0.955–1.043 | 0.992 | 0.994 |
| Carrier material | 4.044 | 1.163–18.96 | 0.043* | 0.462 |
| ER/PR+ | 0.351 | 0.103–1.232 | 0.094 | 0.596 |
| HER2+ | 1.286 | 0.256–5.149 | 0.735 | 0.982 |
| Eosinophil | ||||
| Time after biopsy | 1.005 | 0.968–1.043 | 0.809 | 0.982 |
| Carrier material | 4.135 | 1.516–12.353 | 0.007* | 0.15 |
| ER/PR+ | 0.866 | 0.275–2.773 | 0.804 | 0.982 |
| HER2+ | 2.59 | 0.706–10.769 | 0.161 | 0.692 |
| Plasma cell | ||||
| Time after biopsy | 1.002 | 0.965–1.04 | 0.908 | 0.982 |
| Carrier material | 5.5 | 2.002–16.63 | 0.001 * | 0.043* |
| ER/PR+ | 0.542 | 0.163–1.698 | 0.298 | 0.777 |
| HER2+ | 1.366 | 0.374–5.189 | 0.635 | 0.942 |
| Lymphocyte | ||||
| Time after biopsy | 1.036 | 0.974–1.117 | 0.307 | 0.777 |
| Carrier material | 1.6 | 0.35–7.329 | 0.532 | 0.88 |
| ER/PR+ | 2.65 | 0.491–12.406 | 0.222 | 0.771 |
| HER2+ | 1.078 | 0.216–4.26 | 0.919 | 0.982 |
| Macrophage | ||||
| Time after biopsy | 1.017 | 0.975–1.066 | 0.444 | 0.878 |
| Carrier material | 1.53 | 0.526–4.437 | 0.43 | 0.878 |
| ER/PR+ | 1.571 | 0.43–5.259 | 0.472 | 0.878 |
| HER2+ | 0.928 | 0.235–4.622 | 0.919 | 0.982 |
Days after biopsy (continuous variable), interval between diagnostic biopsy to surgery; Carrier material, residual carrier material from biopsy marker; CI, confidence interval; FDR, false discovery rate. Asterisk indicates statistical significance, p < 0.05.
Biopsy Marker Was Associated with the Presence of Inflammatory Cells
Univariate logistic regression showed that residual carrier material from biopsy marker at the AN was associated with the presence of neutrophils (OR = 4.044; p = 0.043), eosinophils (OR = 4.135; p = 0.007), and plasma cells (OR = 5.5; p = 0.001; Table 3). Statistical significance remained for the association with plasma cells even after FDA adjustment (p = 0.043). However, breast cancer subtypes, either ER/PR− or HER2− status, were not associated with differences in the presence of any types of inflammatory cells (Table 3).
Discussion
Needle biopsy is a standard procedure to extract a small fraction of tissue using a relatively large-gauge needle for breast cancer diagnosis. Disruption of tissue integrity instigates the wound healing cascade, in which inflammation is an essential part of four distinct sequential phases (hemostasis, inflammatory, proliferation, and remodeling) [11, 12]. Typically, the inflammatory phase progresses in a timely and orderly fashion as neutrophils and eosinophils, defined as acute response, are first recruited to damaged tissues [12]. Macrophages follow upon the disappearance of neutrophils and attract fibroblasts by releasing cytokines and chemokines [13, 14]. The wound healing cascade has been investigated in depth using models for incisional and excisional wounds as well as burns on the skin [15]. Yet, the detailed progression of the wound healing cascade in solid tumors has remained unexplored. Inflammation has been reported as a common histologic change at the site where biopsy was performed [6, 16]; we analyzed both spatial and temporal patterns of inflammation using post-biopsy breast tumors. A spatial comparison of the presence of inflammatory cells showed that a high proportion of cases have eosinophils and macrophages AN compared to DN, even after adjustment for time after biopsy. For neutrophils, we were unable to perform statistical analysis due to the absence in the most of cases, presumably attributable to their short lifespan in the tissue. Based on the dogma of timely progression of the inflammatory phase in the wound healing cascade, the inflammatory phase subsides over time as infiltrating immune cells either dislodge or undergo apoptosis [13]. Thus, the prolonged presence of macrophages and eosinophils indicated a delayed healing process at the post-biopsy site within the tumor. We did not find an association between the prolonged presence of macrophages and eosinophils and breast cancer subtypes. Further study to determine a possible driver of such pathologically delayed healing, either host immunity or tumor biology, would add a new dimension to our understanding of the wound healing process within breast tumors.
Preoperative diagnosis unquestionably creates a range of intervals between diagnosis (needle biopsy) and definitive treatment (surgery or therapy). The possible risk of disease progression during the interval between biopsy and definitive treatment as well as the outcome was investigated in the 1950–1960s [17, 18, 19, 20]. Recent studies have addressed histologic changes pertinent to needle biopsy. Histological examination of 352 surgically resected tumors showed tumor displacement (needle tract seeding) in 32% of cases following biopsy with 11- to 14-gauge needles [21]. A systematic review of 15 studies from 1900 to 2008 identified tumor displacement in 22% (150/667) of patients after 11- to 14-gauge needle biopsy [22]. During a biopsy, cancer cells may be displaced in the needle tract, although the local recurrence at the site of the needle tract is low [21]. Additionally, Weber et al. [23] reported an increased level of M2 macrophages in biopsied tumors compared to their matched biopsy sample in oral squamous-cell carcinomas (n = 34) regardless of the time after biopsy (mean = 15 ± 9.6 days). Chen et al. [24] reported an increase in Ki67 in surgically resected breast tumors during the time after biopsy (mean = 4.5 days) compared to matched biopsy samples. These studies suggested possible changes in both the tumor microenvironment and cancer cells following biopsy. Our study found an abundance of macrophages at the post-biopsy site for a more extended period (mean = 34 ± 28.8 days) than former studies. The macrophage phenotype transition from M0→M1→M2 is a physiologic response for tissue repair. While M2ϕ specialized for wound healing and tumor-associated macrophages (TAMs) with the M2 phenotype are distinctly classified, their biologic roles are substantially similar in mediating cellular proliferation and angiogenesis through secretion of cytokines, chemokines, and growth factors [14, 25]. Given the association between M2 TAMs and poorer prognosis [26, 27, 28], it is critical to understand the temporal and spatial information regarding the M2/M1 balance at the post-biopsy site along with possible changes in neighboring cancer cells.
Interestingly, neutrophils, eosinophils, and plasma cells were associated with visible carrier material from the biopsy marker. Biopsy clips, typically composed of a titanium or steel radio-enhancer coated with biocompatible and biodegradable material, are placed into the biopsy cavity to assist surgeons in identifying the location of malignant tumor at the time of surgery [29, 30]. Biodegradable materials such as β-glucan, PGEylated-hydrogel, and bovine collagen that coat a biopsy clip swell within the cavity to prevent movement for as long as 6 months [31]. Thus, a positive association between acute inflammatory and plasma cells with visible carrier material from biopsy markers may represent a foreign body reaction [32]. Unexpectedly, none of the listed comorbid conditions showed an association with altered extent or duration of inflammation following biopsy (data not shown), even comorbid conditions such as diabetes or inflammatory disorders that manifest a pro-inflammatory state that commonly impedes or delays the healing process [33]. Our study focused on the longevity of each immune cell type at the post-biopsy site based on their histologic features but did not address the subset prevalence. Further investigation to identify specific immune cell subsets will advance our understanding of the inflammatory and wound healing process in breast tumors.
Statement of Ethics
This study was reviewed and approved by the Institutional Review Board at the University of Oklahoma Health Science Center (#6234). The retrospective study has been granted an exemption from requiring written informed consent, which approved by the Institutional Review Board at the University of Oklahoma Health Science Center (#6234).
Conflict of Interest Statement
All the authors have no conflict of interest to declare.
Funding Sources
This work was supported by the Oklahoma Center for the Advancement of Science and Technology (HR14-112 to T.T.) and the Oklahoma Center for Adult Stem Cell Research (T.T.).
Author Contributions
Takemi Tanaka and Roy Zhang contributed to study concept, design, and supervised the study. Roy Zhang, Cruz McCarty, and Senna Sous contributed to pathology review and data collection. Macall Leslie, Ezza Tariq, Priya Dondapati, Hiroyasu Kameyama, Natalie Hills, Marielle Wilkerson, Rachel Davis, and Sidra Mesiya contributed to data collection and database development. Misung Yi and Inna Chervoneva performed statistical analysis. Takemi Tanaka, Roy Zhang, Inna Chervoneva, and Hallgeir Rui contributed to data analysis and interpretation. Takemi Tanaka provided funding support. All the authors contributed to manuscript writing and editing.
Data Availability Statement
The data generated in the current study are not publicly available but are available from the corresponding author on reasonable request.
Supplementary Material
Supplementary data
Acknowledgments
Research reported in this publication was supported in part by the National Cancer Institute Cancer Center Support Grant P30CA225520 and the Oklahoma Tobacco Settlement Endowment Trust contract awarded to the University of Oklahoma Stephenson Cancer Center.
Funding Statement
This work was supported by the Oklahoma Center for the Advancement of Science and Technology (HR14-112 to T.T.) and the Oklahoma Center for Adult Stem Cell Research (T.T.).
References
- 1.DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, Jemal A. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2015 Oct;24((10)):1495–14506. doi: 10.1158/1055-9965.EPI-15-0535. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2021 Jan;67((1)):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Lukasiewicz E, Ziemiecka A, Jakubowski W, Vojinovic J, Bogucevska M, Dobruch-Sobczak K. Fine-needle versus core-needle biopsy: which one to choose in preoperative assessment of focal lesions in the breasts? Literature review. J Ultrason. 2017 Dec;17((71)):267–274. doi: 10.15557/JoU.2017.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007 Mar-Apr;57((2)):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 5.Gobbi H, Tse G, Page DL, Olson SJ, Jensen RA, Simpson JF. Reactive spindle cell nodules of the breast after core biopsy or fine-needle aspiration. Am J Clin Pathol. 2000 Feb;113((2)):288–294. doi: 10.1309/RPW4-CXCC-1JHM-0TL7. [DOI] [PubMed] [Google Scholar]
- 6.Layfield LJ, Frazier S, Schanzmeyer E. Histomorphologic features of biopsy sites following excisional and core needle biopsies of the breast. Breast J. 2015 Jul-Aug;21((4)):370–376. doi: 10.1111/tbj.12414. [DOI] [PubMed] [Google Scholar]
- 7.Carmon M, Zilber S, Gekhtman D, Olsha O, Hadar T, Golomb E. Hygroscopic sonographically detectable clips form characteristic breast and lymph node pseudocysts. Mod Pathol. 2018 Jan;31((1)):62–67. doi: 10.1038/modpathol.2017.96. [DOI] [PubMed] [Google Scholar]
- 8.Youngson BJ, Liberman L, Rosen PP. Displacement of carcinomatous epithelium in surgical breast specimens following stereotaxic core biopsy. Am J Clin Pathol. 1995 May;103((5)):598–602. doi: 10.1093/ajcp/103.5.598. [DOI] [PubMed] [Google Scholar]
- 9.Klopfleisch R, Sperling C, Kershaw O, Gruber AD. Does the taking of biopsies affect the metastatic potential of tumours? A systematic review of reports on veterinary and human cases and animal models. Vet J. 2011 Nov;190((2)):e31–42. doi: 10.1016/j.tvjl.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Lester SC, Bose S, Chen YY, Connolly JL, de Baca ME, Fitzgibbons PL, et al. Protocol for the examination of specimens from patients with invasive carcinoma of the breast. Arch Pathol Lab Med. 2009 Oct;133((10)):1515–1538. doi: 10.5858/133.10.1515. [DOI] [PubMed] [Google Scholar]
- 11.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014 Dec 3;6((265)):265sr6. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez AC, Costa TF, Andrade ZA, Medrado AR. Wound healing: a literature review. An Bras Dermatol. 2016 Sep-Oct;91((5)):614–620. doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008 May 15;453((7193)):314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 14.Minutti CM, Knipper JA, Allen JE, Zaiss DM. Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol. 2017 Jan;61:3–11. doi: 10.1016/j.semcdb.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Parnell LKS, Volk SW. The evolution of animal models in wound healing research: 1993–2017. Adv Wound Care. 2019 Dec 1;8((12)):692–702. doi: 10.1089/wound.2019.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porcaro AB, Novella G, Mattevi D, Bizzotto L, Cacciamani G, Luyk ND, et al. Chronic inflammation in prostate biopsy cores is an independent factor that lowers the risk of prostate cancer detection and is inversely associated with the number of positive cores in patients elected to a first biopsy. Curr Urol. 2016 May;9((2)):82–92. doi: 10.1159/000442859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson PP, Pitts HH. Biopsy with delayed radical mastectomy for carcinoma of the breast. Am J Surg. 1959 Aug;98((2)):184–189. doi: 10.1016/0002-9610(59)90062-5. [DOI] [PubMed] [Google Scholar]
- 18.Abramson DJ. 857 breast biopsies as an outpatient procedure: delayed mastectomy in 41 malignant cases. Ann Surg. 1966 Mar;163((3)):478–483. doi: 10.1097/00000658-196603000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertario L, Reduzzi D, Piromalli D, Piva L, Di Pietro S. Outpatient biopsy of breast cancer. Influence on survival. Ann Surg. 1985 Jan;201((1)):64–67. [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher ER, Sass R, Fisher B. Biologic considerations regarding the one and two step procedures in the management of patients with invasive carcinoma of the breast. Surg Gynecol Obstet. 1985 Sep;161((3)):245–249. [PubMed] [Google Scholar]
- 21.Loughran CF, Keeling CR. Seeding of tumour cells following breast biopsy: a literature review. Br J Radiol. 2011 Oct;84((1006)):869–874. doi: 10.1259/bjr/77245199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liebens F, Carly B, Cusumano P, Van Beveren M, Beier B, Fastrez M, et al. Breast cancer seeding associated with core needle biopsies: a systematic review. Maturitas. 2009 Feb 20;62((2)):113–123. doi: 10.1016/j.maturitas.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Weber M, Moebius P, Buttner-Herold M, Amann K, Preidl R, Neukam FW, et al. Macrophage polarisation changes within the time between diagnostic biopsy and tumour resection in oral squamous cell carcinomas: an immunohistochemical study. Br J Cancer. 2015 Jul 28;113((3)):510–519. doi: 10.1038/bjc.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Zhu S, Fei X, Garfield DH, Wu J, Huang O, et al. Surgery time interval and molecular subtype may influence Ki67 change after core needle biopsy in breast cancer patients. BMC Cancer. 2015 Oct 30;15:822. doi: 10.1186/s12885-015-1853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ley K. M1 means kill; M2 means heal. J Immunol. 2017 Oct 1;199((7)):2191–213. doi: 10.4049/jimmunol.1701135. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen M, Corless CL, Kraling BM, Tran C, Atha T, Bischoff J, et al. Vascular expression of E-selectin is increased in estrogen-receptor-negative breast cancer: a role for tumor-cell-secreted interleukin-1 alpha. Am J Pathol. 1997 Apr;150((4)):1307–1314. [PMC free article] [PubMed] [Google Scholar]
- 27.Charpin C, Bergeret D, Garcia S, Andrac L, Martini F, Horschowski N, et al. ELAM selectin expression in breast carcinomas detected by automated and quantitative immunohistochemical assays. Int J Oncol. 1998 May;12((5)):1041–108. doi: 10.3892/ijo.12.5.1041. [DOI] [PubMed] [Google Scholar]
- 28.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004 Jan;4((1)):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 29.Ghate SV, Baker JA, Hawkins AD, Soher BJ. Titanium vs carbon coated ceramic breast tissue marker clips: 3T MR susceptibility artifact and local signal disturbance. Acad Radiol. 2011 Jun;18((6)):770–773. doi: 10.1016/j.acra.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Portnow LH, Thornton CM, Milch HS, Mango VL, Morris EA, Saphier NB. Biopsy marker standardization: what's in a name? AJR Am J Roentgenol. 2019 Apr 11;:1–6. doi: 10.2214/AJR.18.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein RL, Mook JA, Euhus DM, Rao R, Wynn RT, Eastman AB, et al. Evaluation of a hydrogel based breast biopsy marker (HydroMARK(R)) as an alternative to wire and radioactive seed localization for non-palpable breast lesions. J Surg Oncol. 2012 May;105((6)):591–594. doi: 10.1002/jso.22146. [DOI] [PubMed] [Google Scholar]
- 32.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008 Apr;20((2)):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007 Mar;127((3)):514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
The data generated in the current study are not publicly available but are available from the corresponding author on reasonable request.