Abstract
Splenocytes isolated from C57BL/6J female mice 3 to 7 days after inoculation with an attenuated strain of Salmonella typhimurium produced high levels of nitric oxide (39 to 77 μM) and gamma interferon (IFN-γ). Additionally, spleen cell cultures from Salmonella-inoculated mice were markedly suppressed in their ability to generate an in vitro plaque-forming cell (PFC) response to sheep erythrocytes. Depletion of natural killer (NK) cells from the immune splenocyte population markedly reduced nitric oxide production, prevented suppression of PFC responses, and completely abrogated IFN-γ release. Treatment of NK cell-depleted immune cells with IFN-γ restored nitric oxide production to levels comparable to those of intact immune cells and also restored the immunosuppression. These results suggest that NK cells regulate the induction of nitric oxide-mediated immunosuppression following infection with S. typhimurium through the production of IFN-γ.
Natural killer (NK) cells have been shown to be important in the early host defense responses to a number of pathogens (6, 20, 33, 39, 44, 51). NK cells are a major source of gamma interferon (IFN-γ) (12, 52) and have been shown to be critical for T-lymphocyte-independent macrophage (Mφ) activation through production of this cytokine. Previous studies have shown that NK cells play a key role in vivo in protection of mice against challenge with virulent Salmonella typhimurium (41).
The immune response to Salmonella has been of interest because of the desire to develop improved oral vaccines for typhoid fever. Previous studies by our laboratory as well as others have focused on attenuated strains of Salmonella as potential vaccine candidates (reviewed in reference 15). Our studies of the mechanisms of immunity to Salmonella have used a murine model of typhoid fever (14, 22) and an attenuated strain of S. typhimurium (SL3235) blocked in aromatic synthesis (21). We have previously shown that SL3235, while inducing protection against virulent salmonellae, paradoxically induced profound immunosuppression, as evidenced by the inability of splenocytes to generate an antibody response to non-Salmonella antigens and to respond to mitogens with lymphoproliferative responses (3, 4, 13, 31). We found that immunosuppression induced by SL3235 is mediated by the production of nitric oxide by splenic Mφs because nitric oxide production and immunosuppression were blocked by the nitric oxide synthase inhibitor NG-monomethyl-l-arginine, and depletion of macrophages restored immune responses (5, 22). Furthermore, infection of mice with S. typhimurium induces IFN-γ production (36, 38, 40, 49), and our laboratory has shown that IFN-γ is important in the immunosuppression associated with SL3235 inoculation of mice (5, 22).
The present study investigated the role of NK cells in the induction of splenocyte nitric oxide production and immunosuppression following infection with an attenuated strain of S. typhimurium.
MATERIALS AND METHODS
Animals.
Six-week-old female C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, Maine), and mouse chow and water were provided ad libitum. All mice were acclimatized for a minimum of 1 week prior to experimentation.
Bacterial strain and infection model.
An avirulent strain of S. typhimurium, SL3235, was used for all experiments. SL3235 is an aroA mutant which is deficient in aromatic synthesis, and the 50% lethal dose (LD50) of SL3235 is greater than 107 bacteria when administered intraperitoneally (i.p.). Infection was accomplished by injection of 5 × 105 log-phase bacteria i.p., as previously described (31). Control mice were injected i.p. with a comparable volume of endotoxin-free, sterile, isotonic saline (Abbott Laboratories, Chicago, Ill.).
Cell isolation and preparation.
One to seven days after SL3235 inoculation, the mice were sacrificed by cervical dislocation, and the spleens were removed aseptically. Single-cell splenocyte suspensions were prepared as previously described (31).
In vitro depletion of NK cells.
Splenocytes were prepared at a concentration of 107 cells/ml of RPMI 1640 (Gibco) with 1% heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, Utah) and treated for 45 min at 4°C either with antiserum against asialo GM1 (WAKO Chemicals, Richmond, Va.) at a 1:200 dilution or with a 1:3 dilution of supernatants generated by the PK136 hybridoma (American Type Culture Collection, Rockville, Md.). PK136 cells produce a murine monoclonal antibody (immunoglobulin G2a [IgG2a]) against the NK cell antigen NK1.1. In preliminary experiments, a 1:3 dilution of the PK136 supernatants was determined to be optimal for depletion of NK cells. Following the 45-min incubation, the cells were pelleted by centrifugation and resuspended in RPMI 1640 containing 1% FBS and a 1:12 or 1:8 dilution of Low-Tox M rabbit complement. The cell suspension was then incubated for 45 min at 37°C. The cells were pelleted by centrifugation and washed three times in RPMI 1640 containing 1% FBS prior to their use in various experiments.
Nitric oxide production.
Splenocytes were cultured at a concentration of 107 cells/ml (RPMI 1640 containing 5% FBS, 50 U of penicillin-streptomycin per ml [Gibco]) in 96-well tissue culture plates (Costar, Cambridge, Mass.) for 48 h, and nitric oxide production was determined by measuring nitrite, a stable degradation product of nitric oxide according to the method of Ding et al. (11). Briefly, 100 μl of cell supernatant was removed from each well and incubated with an equal volume of Greiss reagent (1% sulfanilamide, 0.1% naphthylethylene diamine dihydrochloride, 2.0% H3PO4 [Sigma Chemical, St. Louis, Mo.]) at room temperature for 10 min. The A550 was determined with a microplate enzyme-linked immunosorbent assay (ELISA) reader. The nitrite concentration was determined by using a standard curve generated with sodium nitrite (Sigma Chemical). In selected experiments, the cells were treated with recombinant murine IFN-γ (Genzyme, Cambridge, Mass.).
IFN-γ ELISA.
Splenocytes were cultured at a concentration of 107 cells/ml (RPMI 1640 containing 5% FBS, 50 U of penicillin-streptomycin per ml [Gibco]) for 24 h, and cell supernatants were collected and stored at −70°C until determination of IFN-γ levels. IFN-γ concentration in the supernatants was determined by sandwich ELISA with a matched pair of monoclonal antibodies and recombinant murine IFN-γ according to the manufacturer’s instructions (PharMingen, San Diego, Calif.). Strepavidin-alkaline phosphatase (Calbiochem, San Diego, Calif.) and p-nitrophenyl phosphate (Sigma) were used for color development. The A405 was determined with a microplate ELISA reader.
Primary in vitro antibody response.
Antibody-producing cells were generated in vitro by the method of Mishell and Dutton (37), with modification, as previously described (3). The plaque-forming cell (PFC) response was determined by the Cunningham modification of the Jerne hemolytic plaque assay (10). Data are expressed as PFC per 107 splenocytes.
Statistical analysis.
All measurements were made by using a minimum of triplicate samples per variable for each experiment. Data are expressed as means ± standard deviations for a representative experiment. Comparisons were analyzed by using Student’s t test. Differences were considered significant when P was ≤0.05.
RESULTS
NK cells mediate nitric oxide production.
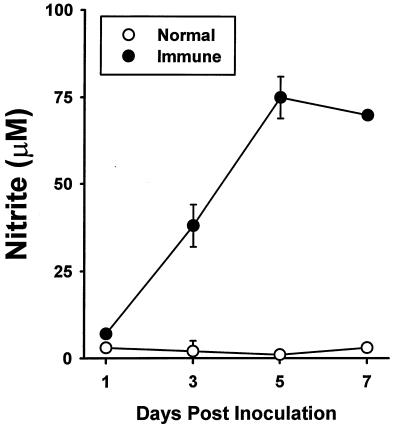
SL3235 inoculation induced splenocyte nitric oxide production in a time-dependent fashion (Fig. 1). By 72 h after in vivo immunization with Salmonella, significant amounts of nitrite were observed, which reached maximal levels by 5 days post-SL3235 inoculation. Nitric oxide levels remained elevated at 7 days postinoculation.
FIG. 1.
Splenocyte nitric oxide production. Splenocytes from saline-inoculated (normal) and SL3235-inoculated (immune) mice were isolated 1 to 7 days after inoculation and cultured at a concentration of 107 cells/ml for 48 h. Nitrite concentrations in cell supernatants were determined. The experiment was done five times. Data are means ± standard deviations of triplicate samples from a representative experiment.
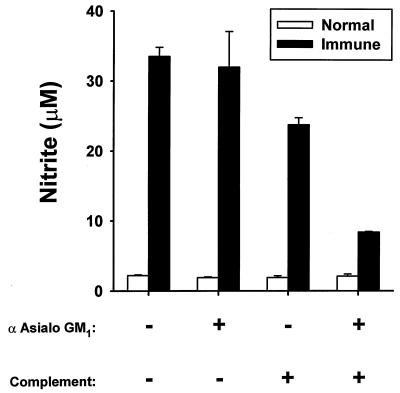
Removal of NK cells from immune splenocytes by treatment with antiserum to asialo GM1 plus complement reduced the high levels of nitric oxide released by these cells by 75% (Fig. 2). Treatment with complement alone resulted in a slight decrease in nitric oxide production by immune splenocytes, whereas treatment with antiserum to asialo GM1 alone had no effect. Nitric oxide production by normal splenocytes was very low (approximately 2 μM) and was unaltered by all treatments.
FIG. 2.
Effect of NK cell depletion on nitric oxide production. Splenocytes from saline-inoculated (normal) and SL3235-inoculated (immune) mice were isolated 7 days postinoculation. The cells were treated with antiserum (α) to asialo GM1 or rabbit complement or were depleted of NK cells by sequential treatment with both agents as described in Materials and Methods. The cells were cultured at a concentration of 107 cells/ml for 48 h, and nitrite concentrations in cell supernatants were determined. The experiment was done six times. Data are means ± standard deviations of triplicate samples from a representative experiment.
NK cell-derived IFN-γ mediates splenocyte nitric oxide production.
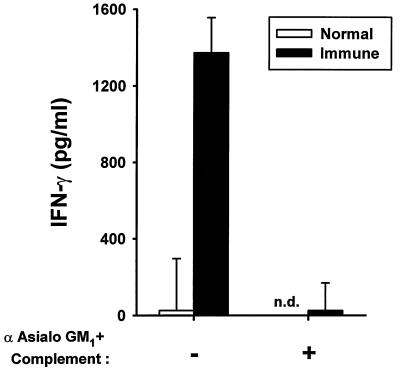
Splenocytes isolated from immune mice 7 days postinoculation and cultured in vitro for 24 h released significant amounts of IFN-γ compared with saline-inoculated controls, in which IFN-γ release was undetectable (Fig. 3). Depletion of NK cells with antiserum to asialo GM1 plus complement completely prevented the production of IFN-γ by immune spleen cells.
FIG. 3.
Effect of NK cell depletion on IFN-γ production. Splenocytes from saline-inoculated (normal) and SL3235-inoculated (immune) mice were isolated 7 days postinoculation. The cells were treated with antiserum to asialo GM1 plus rabbit complement to deplete NK cells. The cells were cultured for 24 h at a concentration of 107 cells/ml, and the IFN-γ concentration in the cell supernatants was determined by ELISA. The experiment was done three times. Data are means ± standard deviations of triplicate samples from a representative experiment. n.d., not done.
The role of NK cells and IFN-γ in the regulation of nitric oxide production by immune spleen cells was investigated further by examining the effect of exogenous IFN-γ on nitric oxide production by NK cell-depleted splenocyte cultures. IFN-γ treatment increased nitric oxide production by NK cell-depleted immune splenocytes in a dose-dependent manner (Table 1). While IFN-γ significantly increased nitric oxide release by both untreated and NK cell-depleted immune cells, the increase was proportionally greater in the NK cell-depleted cultures, particularly at IFN-γ doses up to 10 U/ml. At a dose of 100 U/ml, there was no difference in levels of nitric oxide production by the untreated and NK cell-depleted immune cells. IFN-γ did not induce significant changes in nitric oxide production by normal splenocytes, which was assayed at 2.2 ± 0.1 μM.
TABLE 1.
The effect of IFN-γ on nitrite production following NK cell depletion
| Concn of IFN-γ (U/ml) | Nitrite production by immune splenocytes (μM)a
|
|
|---|---|---|
| Nondepleted | NK cell depleted | |
| 0 | 33.5 ± 1.3b | 8.4 ± 0.1c |
| 1 | 39.2 ± 2.8 | 10.6 ± 0.5c |
| 10 | 45.6 ± 0.6 | 26.8 ± 0.7c |
| 100 | 58.9 ± 5.6 | 52.7 ± 7.0d |
Splenocytes from SL3235-inoculated mice (immune) were isolated 7 days postinoculation and were depleted of NK cells by using asialo GM1 antiserum plus complement, as described in Materials and Methods, or were left intact (nondepleted). The cells (107/ml) were cultured for 48 h in the presence of IFN-γ, when nitrite levels in cell supernatants were determined. Data are means ± standard deviations of triplicate samples from a representative experiment that was done four times.
P ≤ 0.05 compared with nondepleted normal splenocytes. The levels of nitrite production by splenocytes from saline-inoculated mice (normal) were 2.2 ± 0.1 μM for nondepleted cultures and 2.1 ± 0.3 μM for NK cell-depleted cultures.
P ≤ 0.05 compared with the respective nondepleted immune group.
Not significant compared with the respective nondepleted immune group.
Role of NK cells and IFN-γ in Salmonella-induced immunosuppression.
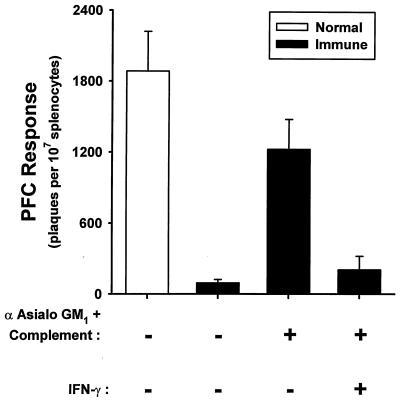
In order to assess the effect of NK cell depletion on immunocompetence, the primary antibody response to sheep erythrocytes was assessed in vitro by using a PFC assay. Consistent with our previously published results (3), SL3235 inoculation resulted in an almost complete suppression of the ability of splenocytes to generate a PFC response (Fig. 4). NK cell depletion by treatment with antiserum to asialo GM1 plus complement reversed the suppression of PFC responses to 67% of normal. Treatment of the NK cell-depleted immune splenocytes with IFN-γ (100 U/ml) restored the suppression (Fig. 4).
FIG. 4.
Effect of NK cell depletion on PFC responses. Splenocytes for saline-inoculated (normal) and SL3235-inoculated (immune) mice were isolated 7 days postinoculation. The cells were treated with asialo GM1 antiserum (α) plus rabbit complement to deplete NK cells, as described in Materials and Methods, or left untreated and cultured for 5 days with sheep erythrocytes. Selected wells were treated with IFN-γ (100 U/ml). PFC responses were assessed as described in Materials and Methods. The experiment was done three times. Data are means ± standard deviations of triplicate samples from a representative experiment.
The effect of NK cell depletion on immunocompetence was probed further by depletion of NK cells by using anti-NK1.1 plus complement (Table 2). Anti-NK1.1 prevented the suppression of PFC responses in a manner similar to that of anti-asialo GM1. Immune cells depleted with anti-NK1.1 and then treated with IFN-γ (100 U/ml) were suppressed to a degree comparable to that of nondepleted immune splenocytes in terms of their ability to generate a PFC response.
TABLE 2.
Effect of depletion of NK1.1+ cells with monoclonal PK136 antibody on PFC responses
| Cell fraction | No. of plaques/107 splenocytesa
|
||
|---|---|---|---|
| Normal splenocytes (no treatment) | Immune splenocytes
|
||
| No treatment | +IFN-γ | ||
| Nondepleted | 1,005 ± 210 | 179 ± 90b | 34 ± 126 |
| NK cell depleted | 847 ± 49 | 994 ± 49c | 533 ± 111d |
Splenocytes from saline-inoculated (normal) or SL3235-inoculated (immune) mice were isolated 7 days postinoculation and left intact (nondepleted) or depleted of NK cells by using anti-NK1.1 antibodies (monoclonal PK136 antibody) plus complement. The cells were cultured for 5 days with no additional treatment or with addition of IFN-γ (100 U/ml), and PFC responses were assessed. Data are means ± standard deviations of triplicate samples from a representative experiment that was done three times.
P ≤ 0.05 compared with the nondepleted normal group.
Not significant compared with the NK cell-depleted normal group.
P < 0.05 compared with the NK cell-depleted immune group receiving no treatment.
DISCUSSION
Consistent with our previously published results, inoculation of mice with SL3235 induced significant quantities of nitric oxide and a profound suppression of the capacity of splenocytes to generate an in vitro immune response to a non-Salmonella antigen, sheep erythrocytes (5, 22). The present study also demonstrated that IFN-γ was constitutively released by immune splenocytes and that NK cells are the likely source of the IFN-γ, because NK cell depletion abrogated its release. Furthermore, NK cells were shown to be critical for maintaining both nitric oxide production and immunosuppression, because NK cell depletion with either antiserum against asialo GM1 or antibodies against NK1.1 ablated nitric oxide production and also restored PFC responses to normal. The marker asialo GM1 is found primarily on NK cells (26, 27); however, other immune cells can express the marker (47, 53). Therefore, we confirmed the finding by using a more specific marker for NK cells, NK1.1 (18). Since our results were consistent, whether NK cells were depleted with anti-asialo GM1 or anti-NK1.1, we conclude that it is NK cells, rather than other cells of the immune system that express the asialo GM1 antigen, which are critical for nitric oxide-mediated immunosuppression induced by Salmonella infection.
Nitric oxide is an important component of Mφ-dependent cytotoxicity and cytostatic activity against tumor cells and microbes (reviewed in references 16 and 35). However, increasing evidence has demonstrated a role for nitric oxide in the induction of immunosuppression (2, 14, 32). Mφs produce nitric oxide via the inducible form of the nitric oxide synthase (iNOS) (35). A number of infectious agents can induce nitric oxide and IFN-γ production and the resultant immunosuppression (1, 5, 8, 19, 25, 45, 46). In vitro, lipopolysaccharide (LPS) in combination with IFN-γ is a potent inducer of iNOS expression by Mφs (11). Our results presented here are consistent with a critical role for IFN-γ in the induction of Mφ nitric oxide production following microbial challenge. An essential role for LPS in the induction of iNOS expression is not evident, because organisms other than gram-negative bacteria (i.e., Leishmania spp. and Mycobacterium bovis BCG) can also induce iNOS expression in Mφs (45, 55).
The importance of IFN-γ in the generation of nitric oxide-mediated immunosuppression was demonstrated by the ability of exogenous IFN-γ to restore nitric oxide production and immunosuppression to NK cell-depleted immune splenocyte cultures. Because NK cells are known to be a major cellular source of IFN-γ, the data suggest that NK cells mediate nitric oxide-induced immunosuppression in Salmonella-inoculated mice via the release of this cytokine. Limited studies have demonstrated a link between NK cells and the induction of nitric oxide production by Mφs (23, 41, 44, 55). The present study supports this conclusion and suggests that NK cell production of IFN-γ is critical. A novel aspect of the present work is the link between NK cells, IFN-γ levels, nitric oxide levels, and immunosuppression. Studies have linked Mφ interleukin 12 (IL-12) release to NK cell activation and nitric oxide production (23, 44). IL-12 is a potent inducer of IFN-γ release by both NK cells and T cells (7). A pathway for the induction of Mφ nitric oxide production by NK cell-derived IFN-γ would be consistent with results from previous studies from our laboratory showing that the administration of anti-IL-12 antibodies to mice prior to Salmonella inoculation completely blocked splenocyte nitric oxide production and immunosuppression (43). Additionally, anti-IFN-γ treatment in vitro blocked nitric oxide production and immunosuppression in immune splenocyte cultures, and IFN-γ treatment of splenocytes from anti-IL-12-treated immune mice, which were not immunosuppressed, restored immunosuppression (43).
Interestingly, some studies have shown that nitric oxide can inhibit NK cell activity (24, 54); however, these studies have focused on pharmacological nitric oxide donors rather than biological phenomena. We have previously shown that NK cell cytotoxic activity is enhanced early after Salmonella infection and returns to basal levels by 7 days postinfection (41). The time course of nitric oxide production presented here (Fig. 1) suggests that nitric oxide may have a role in the down regulation of NK cell activity following Salmonella infection, because elevated nitric oxide production at 3 to 7 days postinfection correlates with the loss of enhanced NK cell cytoxicity previously reported (41). Thus, nitric oxide may provide an important biological feedback mechanism for dampening the proinflammatory cascade induced by Salmonella infection. Conversely, other investigators have suggested that NK cells can produce nitric oxide directly (9, 17). However, this is unlikely to be a major component of the nitric oxide produced by immune splenocytes in the present study, because treatment of NK-depleted cells with IFN-γ restored nitric oxide production to levels comparable to those of nondepleted immune cells. Additionally, previous results have established Mφs as the nitric oxide-producing cells following Salmonella infection (5, 13, 22).
A role for T cells in the induction of nitric oxide-mediated immunosuppression following Salmonella infection cannot be completely ruled out, because specific T-cell subsets have previously been shown to express asialo GM1 (47) and/or NK1.1 (reviewed in reference 50). NK cells may also indirectly stimulate Mφ nitric oxide production via activation of T-cell populations. While we cannot dismiss a role for these specific T-cell subsets, previous observations by our laboratory indicate that immune splenocytes depleted of Thy1.2+ T cells remained immunosuppressive (3), therefore suggesting that immune T cells are not critical to the immunosuppression. The results presented here are consistent with our previous findings demonstrating a critical role for NK cells in the immune response to Salmonella (41) and the work of other investigators using different microbes that induce nitric oxide production through an NK cell-dependent pathway (42, 55). Nonetheless, more detailed analysis of T-cell subsets is warranted to rule out their role in the induction of nitric oxide-mediated immunosuppression following Salmonella infection.
Our previous study demonstrated that a vaccine strain of Salmonella induced NK cell-mediated protection against virulent Salmonella strains (41), while the present work indicates that NK cells are critical to the suppression of the immune response to a non-Salmonella antigen. Therefore, an apparent paradox exists, with NK cells being important to both the immunity to virulent Salmonella challenge and the development of nitric oxide-mediated immunosuppression. Previously we have shown that during the first 3 weeks postimmunization with attenuated Salmonella, T-cell-dependent responses to Salmonella antigen are not evident (28–30), and by 1 month postimmunization, T-cell responses to Salmonella antigen return (29). These observations suggest that NK cells, as part of the innate immune response, are important in the clearance of Salmonella early postimmunization. The mechanism by which NK cells contribute to the clearance of Salmonella from the host is unknown, but nitric oxide derived from iNOS seems to be critical (34). This concept is supported by the findings reported here and other recent studies showing that peroxynitrite, which is formed by the reaction of nitric oxide and superoxide, is an important antimicrobial mechanism against S. typhimurium in mice (48). Therefore, it appears that NK cells mediate both protection against virulent Salmonella strains and immunosuppression of immune responses to non-Salmonella antigens by the same mechanism, namely Mφ-derived nitric oxide.
In conclusion, these findings indicate that NK cell-derived IFN-γ, through the activation of nitric oxide production by Mφs, is a critical factor in inducing immunosuppression in a murine model of Salmonella infection.
ACKNOWLEDGMENTS
These studies were supported by NIH grant AI15613. M. G. Schwacha was supported by a postdoctoral trainee fellowship (NIH grant T32 AI07101) while these studies were being conducted.
REFERENCES
- 1.Abrahamsohn I A, Coffman R L. Cytokine and nitric oxide regulation of the immunosuppression in Trypanosoma cruzi infection. J Immunol. 1992;155:3955–3963. [PubMed] [Google Scholar]
- 2.Albina J E, Abate J A, Henry W L., Jr Nitric oxide production is required for murine resident macrophages to suppress mitogen-stimulated T cell proliferation. J Immunol. 1991;147:144–148. [PubMed] [Google Scholar]
- 3.Al-Ramadi B K, Brodkin M A, Mosser D M, Eisenstein T K. Immunosuppression induced by attenuated Salmonella. Evidence for mediation by macrophage precursors. J Immunol. 1991;146:2737–2746. [PubMed] [Google Scholar]
- 4.Al-Ramadi B K, Brodkin M A, Greene J M, Meissler J J, Jr, Eisenstein T K. Immunosuppression induced by attenuated Salmonella: effect of LPS responsiveness on development of suppression. Microb Pathog. 1992;12:267–278. doi: 10.1016/0882-4010(92)90045-p. [DOI] [PubMed] [Google Scholar]
- 5.Al-Ramadi B K, Meissler J J, Jr, Huang D, Eisenstein T K. Immunosuppression induced by nitric oxide and its inhibition by IL-4. Eur J Immunol. 1992;22:2249–2254. doi: 10.1002/eji.1830220911. [DOI] [PubMed] [Google Scholar]
- 6.Bancroft G J, Schreiber R D, Bosma G C, Bosma M J, Unanue E R. A T cell-independent mechanism of macrophage activation by interferon-γ. J Immunol. 1989;139:1104–1107. [PubMed] [Google Scholar]
- 7.Brunda M J. Interleukin-12. J Leukoc Biol. 1994;55:280–288. doi: 10.1002/jlb.55.2.280. [DOI] [PubMed] [Google Scholar]
- 8.Candolfi E, Hunter C A, Remington J S. Mitogen- and antigen-specific proliferation of T cells in murine toxoplasmosis is inhibited by reactive nitrogen intermediates. Infect Immun. 1994;62:1995–2001. doi: 10.1128/iai.62.5.1995-2001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cifone M G, Festuccia C, Cironi L, Cavallo G, Chessa M A, Pensa V, Tubaro E, Santoni A. Induction of the nitric oxide-synthesizing pathway in fresh and interleukin 2-cultured rat natural killer cells. Cell Immunol. 1994;157:181–194. doi: 10.1006/cimm.1994.1215. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham A, Szenberg A. Further improvements in the plaque technique for detecting single antibody producing cells. Immunology. 1968;14:599–600. [PMC free article] [PubMed] [Google Scholar]
- 11.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 12.Dunn P L, North R J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991;59:2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenstein T K, Dalal N, Killar L, Lee J C, Schafer R. Paradoxes of immunity and immunosuppression in Salmonella infection. Adv Exp Med Biol. 1988;239:353–366. doi: 10.1007/978-1-4757-5421-6_34. [DOI] [PubMed] [Google Scholar]
- 14.Eisenstein T K, Huang D, Meissler J J, Jr, Al-Ramadi B K. Macrophage nitric oxide mediates immunosuppression in infectious inflammation. Immunobiology. 1994;191:493–502. doi: 10.1016/S0171-2985(11)80455-9. [DOI] [PubMed] [Google Scholar]
- 15.Eisenstein T K, Huang D, Schwacha M G. Immunity to Salmonella infection. In: Paradise L, Bendinelli M, Friedman H, editors. Enteric infections and immunity. New York, N.Y: Plenum Press; 1996. pp. 57–78. [Google Scholar]
- 16.Farrell A J, Blake D R. Nitric oxide. Ann Rheumatic Dis. 1996;55:7–20. doi: 10.1136/ard.55.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filep J G, Baron C, Lachance S, Perreault C, Chan J S. Involvement of nitric oxide in target-cell lysis and DNA fragmentation induced by murine natural killer cells. Blood. 1996;87:5136–5143. [PubMed] [Google Scholar]
- 18.Glimcher L, Shen F W, Cantor H. Identification of a cell surface antigen selectively expressed on the natural killer cell. J Exp Med. 1977;145:1–9. doi: 10.1084/jem.145.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregory S H, Wing E J, Hoffman R A, Simmons R L. Reactive nitrogen intermediates suppress the primary immunologic response to Listeria. J Immunol. 1993;150:2901–2909. [PubMed] [Google Scholar]
- 20.Gulay Z, Imir T. Anti-candidial activity of natural killer (NK) and lymphokine activated killer (LAK) lymphocytes in vitro. Immunobiology. 1996;195:220–230. doi: 10.1016/S0171-2985(96)80041-6. [DOI] [PubMed] [Google Scholar]
- 21.Hoiseth L K, Stocker A D. Aromatic dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 22.Huang D, Schwacha M G, Eisenstein T K. Attenuated salmonella vaccine-induced suppression of murine spleen cell responses to mitogen is mediated by macrophage nitric oxide: quantitative aspects. Infect Immun. 1996;64:3786–3792. doi: 10.1128/iai.64.9.3786-3792.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang F P, Feng G J, Lindrop G, Stott D I, Liew F Y. The role of interleukin-12 and nitric oxide in the development of spontaneous autoimmune disease in MRL:MP-1pr:1pr mice. J Exp Med. 1996;183:1447–1459. doi: 10.1084/jem.183.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito M, Watanabe M, Kamiya H, Sakurai M. Inhibition of natural killer cell activity against cytomegalovirus-infected fibroblasts by nitric oxide-releasing agents. Cell Immunol. 1996;174:13–18. doi: 10.1006/cimm.1996.0288. [DOI] [PubMed] [Google Scholar]
- 25.Kamijo R, Gerecitano J, Shapiro D, Green S J, Aguet M, Le J, Vilcek J. Generation of nitric oxide and clearance of interferon-γ after BCG infection are impaired in mice that lack the interferon-γ receptor. J Inflamm. 1996;46:23–31. [PubMed] [Google Scholar]
- 26.Kasai M, Iwamori M, Nagai Y, Okamura K, Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980;10:175–180. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- 27.Kawase I, Urdal D L, Brooks C G, Henney C S. Selective depletion of NK cell activity in vivo and its effect on the growth of NK-sensitive and NK-resistant tumor cell variants. Int J Cancer. 1982;29:567–574. doi: 10.1002/ijc.2910290513. [DOI] [PubMed] [Google Scholar]
- 28.Killar L M, Eisenstein T K. Differences in delayed-type hypersensitivity responses in various mouse strains in the C3H lineage infected with Salmonella typhimurium strain SL3235. J Immunol. 1984;133:1190–1196. [PubMed] [Google Scholar]
- 29.Killar L M, Eisenstein T K. Immunity to Salmonella typhimurium infection in C3H/HeJ and C3H/HeNCr1BR mice: studies with an aromatic-dependent live S. typhimurium strain as a vaccine. Infect Immun. 1985;47:605–612. doi: 10.1128/iai.47.3.605-612.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Killar L M, Eisenstein T K. Delayed-type hypersensitivity and immunity to Salmonella typhimurium. Infect Immun. 1986;52:504–508. doi: 10.1128/iai.52.2.504-508.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J C, Gibson C W, Eisenstein T K. Macrophage-mediated mitogenic suppression induced in mice of the C3H lineage by a vaccine strain of Salmonella typhimurium. Cell Immunol. 1985;91:75–91. doi: 10.1016/0008-8749(85)90033-4. [DOI] [PubMed] [Google Scholar]
- 32.Liew F Y. Regulation of lymphocyte functions by nitric oxide. Curr Opin Immunol. 1995;7:396–399. doi: 10.1016/0952-7915(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 33.Macela A, Stulik J, Hernychova L, Kroca M, Krocova Z, Kovarova H. The immune response against Francisella tularensis live vaccine strain in Lps(n) and Lps(d) mice. FEMS Immunol Med Microbiol. 1996;13:235–238. doi: 10.1111/j.1574-695X.1996.tb00243.x. [DOI] [PubMed] [Google Scholar]
- 34.MacFarlane, A. S., M. G. Schwacha, and T. K. Eisenstein. In vivo blockage of nitric oxide with aminoguanide inhibits immunosuppression induced by attenuated Salmonella, potentiates Salmonella infection, and inhibits macrophage/PMN influx into the spleen. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 35.MacMicking J, Xie Q, Nathan C F. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 36.Matsumura H, Onozuka K, Terada Y, Nakano Y, Nakano M. Effect of murine recombinant interferon-γ in the protection of mice against Salmonella. Int J Immunopharmacol. 1990;12:49–56. doi: 10.1016/0192-0561(90)90067-w. [DOI] [PubMed] [Google Scholar]
- 37.Mishell R I, Dutton R W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967;126:423. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nauciel C, Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992;60:450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orange J S, Wang B, Terhorst C, Biron C A. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramarathinam L, Shaban R A, Niesel D W, Klimpel G R. IFN-γ production by gut-associated lymphoid tissue and spleen following oral S. typhimurium challenge. Microb Pathog. 1991;11:347–352. doi: 10.1016/0882-4010(91)90020-b. [DOI] [PubMed] [Google Scholar]
- 41.Schafer R, Eisenstein T K. Natural killer cells mediate protection induced by a Salmonella aroA mutant. Infect Immun. 1992;60:791–797. doi: 10.1128/iai.60.3.791-797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scharton-Kersten T, Caspar P, Sher A, Denkers E Y. Toxoplasma gondii: evidence for interleukin-12-dependent and -independent pathways of interferon-γ production induced by an attenuated parasite strain. Exp Parasitol. 1996;84:102–114. doi: 10.1006/expr.1996.0096. [DOI] [PubMed] [Google Scholar]
- 43.Schwacha M G, Eisenstein T K. Interleukin-12 is critical for induction of nitric oxide-mediated immunosuppression following vaccination of mice with attenuated Salmonella typhimurium. Infect Immun. 1997;65:4897–4903. doi: 10.1128/iai.65.12.4897-4903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sedegah M, Finkleman F, Hoffman S L. Interleukin 12 induction of interferon-γ-dependent protection against malaria. Proc Natl Acad Sci USA. 1994;91:10700–10702. doi: 10.1073/pnas.91.22.10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stefani M M A, Muller I, Louis J A. Leishmania major-specific CD8+ T cells are inducers and targets of nitric oxide produced by parasitized macrophages. Eur J Immunol. 1994;24:746–752. doi: 10.1002/eji.1830240338. [DOI] [PubMed] [Google Scholar]
- 46.Sternberg J, McGuigan F. Nitric oxide mediates suppression of T cell responses in murine Trypanosoma brucei infection. Eur J Immunol. 1992;22:2741–2744. doi: 10.1002/eji.1830221041. [DOI] [PubMed] [Google Scholar]
- 47.Stitz L, Bainziger J, Pircher H, Hengartner H, Zinkernagel R M. Effect of rabbit anti-asialo GM1 plus complement in vitro on cytotoxic T cell activities. J Immunol. 1986;136:4664–4680. [PubMed] [Google Scholar]
- 48.Umezawa K, Akaike T, Fujii S, Suga M, Setoguchi K, Ozawa A, Maeda H. Induction of nitric oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect Immun. 1997;65:2932–2940. doi: 10.1128/iai.65.7.2932-2940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Cott J L, Staats H F, Pascual D W, Roberts M, Chatfield S N, Yamamoto M, Coste M, Carter P B, Kiyono H, McGhee J R. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J Immunol. 1996;156:1504–1514. [PubMed] [Google Scholar]
- 50.Vicari A P, Zlotnik A. Mouse NK1.1+ T cells: a new family of T cells. Immunol Today. 1996;17:71–76. doi: 10.1016/0167-5699(96)80582-2. [DOI] [PubMed] [Google Scholar]
- 51.Walker W, Roberts C W, Ferguson D J P, Jebbari H, Alexander J. Innate immunity to Toxoplasma gondii is influenced by gender and is associated with differences in interleukin-12 and gamma interferon production. Infect Immun. 1997;65:1119–1121. doi: 10.1128/iai.65.3.1119-1121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wherry J C, Schreiber R D, Unanue E R. Regulation of gamma interferon production by natural killer cells in scid mice. Roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991;59:1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiltrout R H, Santoni A, Peterson E S, Knott D C, Overton W R, Herberman R B, Holden H T. Reactivity of anti-asialo GM1 serum with tumoricidal and non-tumoricidal mouse macrophages. J Leukoc Biol. 1985;37:597–614. doi: 10.1002/jlb.37.5.597. [DOI] [PubMed] [Google Scholar]
- 54.Xiao L, Nilsson C F, Eneroth P H E. Cyclic guanosine 3′,5′-monophosphate mediates 3-morpholinosydnonimine-induced inhibition of human natural killer cells. Biochem Pharmacol. 1995;50:147–153. doi: 10.1016/0006-2952(95)00125-j. [DOI] [PubMed] [Google Scholar]
- 55.Yang J, Kawamura I, Zhu H, Mitsuyama M. Involvement of natural killer cells in nitric oxide production by spleen cells after stimulation with Mycobacterium bovis BCG. Study of the mechanism of the different abilities of viable and killed BCG. J Immunol. 1995;155:5728–5735. [PubMed] [Google Scholar]