Summary
Objective:
Neuropsychiatric lupus (NPSLE), a manifestation of the autoimmune disease systemic lupus erythematosus (SLE), is characterized by psychiatric symptoms including anxiety and depression and upregulated autoantibodies. The B6.Nba2 spontaneous mouse model develops SLE, but has not previously been tested for NPSLE.
Methods:
We investigated the NPSLE phenotype in male and female B6.Nba2 mice (n = 12 each) and age- and sex-matched B6 controls (n = 10 each) via behavioral assessments for anxiety, depression, and memory deficits. Serum anti-dsDNA, anti-nRNP, anti-DWEYS peptide reactive IgG autoantibody levels and soluble TWEAK levels were determined by ELISA. Hippocampal regions were stained for activated microglia and neurons.
Results:
Both male and female B6.Nba2 mice showed elevated anti-dsDNA IgG, anti-nRNP IgG and anti-DWEYS reactive antibodies, elevated serum soluble TWEAK levels, and a strong anxiety and depression phenotype (p < 0.05–0.0001). Male B6.Nba2 mice developed this phenotype at a slightly older age than females. Female B6.Nba2 mice displayed reduced numbers of neurons in the hippocampal region compared to female B6 controls (p < 0.05).
Conclusion:
The B6.Nba2 mouse model recapitulates many known NPSLE phenotypes, making it a promising model to investigate the development of NPSLE in the context of SLE.
Keywords: Lupus, neuropsychiatric lupus, mouse model, autoantibody, sex
Introduction
Neuropsychiatric lupus (NPSLE) is a manifestation of systemic lupus erythematosus (SLE) in which the central and peripheral nervous systems are disrupted. NPSLE encompasses a wide array of symptoms including motor deficits, seizures, and cognitive decline; as well as psychiatric conditions such as depression, anxiety and psychosis [1, 2]. In total, the American College of Rheumatology (ACR) has outlined 19 NPSLE symptoms [3]. Up to 80% of SLE patients are also diagnosed with NPSLE at some point during disease progression, increasing both morbidity and mortality of these patients [3–6]. However, as a result of the diverse symptomology of NPSLE the disease is difficult to diagnose and treat [1, 7]. In particular, determining whether NPSLE symptoms are a direct result of SLE, a secondary manifestation of the psychological impact of an SLE diagnosis, and/or a side effect of prescribed medication(s) remains unknown [7]. Thus, more research is needed, using animal models of these diseases, to determine cause and effect relationships.
Similar to many other autoimmune diseases, SLE is predominantly diagnosed in women [8]. Studies have previously suggested a role for both estrogens and testosterone in the disproportional representation of females among lupus patients (reviewed in [9]), and type I interferons have been suggested to act in part to amplify the disease promoting effect of estrogens via a positive regulatory feedback loop [10]. Despite our understanding of sex differences in SLE, very little is known about sex differences in NPSLE. In a single study, a higher rate of seizures has been reported in male NPSLE patients as compared with female NPSLE patients ([11], and reviewed in [12]). Both estrogens and type I interferons have been suggested as promoters of anxiety, depression and cognitive decline in animal models, although recent studies suggested that blockade of the type I interferon receptor had no effect on NPSLE-like disease in at least MRL/lpr mice [13–16].
The presence of elevated levels of anti-dsDNA autoantibodies is commonly observed among SLE patients and is well-known to correlate with disease activity and end-organ damage. Interestingly, a subset of SLE-specific anti-dsDNA autoantibodies was previously found to cross-react with the peptide sequence DWEYSVWLSN (DWEYS), present in the GluN2a and GluN2b (NR2a and NR2b) subunits of the N-methyl-D-aspartate receptor (NMDAR) [17]. Anti-DWEYS reactive antibodies have been associated with increased neuronal death, a potential mechanistic driver of NSPLE symptoms in animal models [17, 18]. Furthermore, the presence of anti-NMDAR specific antibodies in cerebral spinal fluid (CSF) of SLE patients was associated with the development of cognitive deficits or depression in SLE patients, further establishing the pathogenicity of these antibodies [19, 20].
As there is limited patient brain tissue and CSF available for research use, good mouse models are necessary to further our understanding of NPSLE [21]. Female B6.Nba2 lupus-prone mice present with elevated anti-dsDNA IgG autoantibodies, abnormal immune cell activation, splenomegaly and glomerulonephritis [22, 23], however the development of NPSLE-like disease has not been previously investigated. Here, we present data showing elevated levels of anti-DWEYS reactive antibodies coinciding with the levels of anti-dsDNA autoantibodies and the development of anxiety and depression in B6.Nba2 mice. Interestingly, male B6.Nba2 mice also presented with elevated levels of anti-dsDNA autoantibodies along with the development of anxiety and depression, but did so at an older age than their female counterparts. Female, but not male, mice presented with reduced numbers of hippocampal neurons and an increase in microglia area. We conclude that the B6.Nba2 mouse model of SLE represents a new tool in which to study the pathogenesis of NPSLE, sex differences in NPSLE and the effect of anti-NMDAR reactive antibodies in vivo.
Materials and Methods
Mice
Male and female B6 mice (n = 10 each) were obtained from Jackson Laboratory (Stock no. 000664) at 3.5 weeks of age. Male and female B6.Nba2.ABC (B6.Nba2) mice (n = 12 each) were bred at the Biological Research Unit at the Lerner Research Institute. All mice were housed under similar conditions (12hr light/dark cycle; 22°C). Mice were bled for isolation of serum via the tail vein at ages: 8 weeks, 16 weeks + 1 day and 22 weeks + 1 day. Tissue was taken for experiments from mice euthanized at 23 weeks of age. All studies were approved by and performed according to the ARRIVA guidelines and the guidelines of the Institutional Animal Care and Use Committee of Cleveland Clinic.
Behavioral assessments
Habituation and Testing Conditions
Mice were tested during the same lighting and time-of-day conditions. Prior to all tests, mice were given 30 minutes to habituate to the behavioral testing room. All behavioral chambers were cleaned with 70% ethanol before and between mice. Behavioral assessments were conducted in order of least to most invasive in order to limit potential confounding effects of previous behavioral tests (Suppl. Figure 1). Tail vein bleeds were performed on mice at 8, 16 and 22 weeks of age on the day following behavioral testing to limit potentially confounding effects of restraint stress on study results.
Open Field Test
Mice were positioned in the center of a rectangular enclosed arena (50×60 cm) and allowed to explore the arena for 30 minutes. Center of the arena was defined as 8 cm removed from the walls of the arena (34×44 cm). Velocity, distance traveled, and center cumulative duration were recorded using the video tracking system EthoVisionXT (Noldus, Leesburg, VA).
Elevated Plus Maze
Mice were placed in the center of an elevated plus maze (each arm 25 cm) facing the same closed arm for each trial. Mice explored the arena for 10 minutes. EthoVisionXT (Noldus) tracked time spent in open arms, total velocity, and distance traveled. Open arms ratio was defined as open arms duration over sum of closed and open arms duration (excluding the overlapping center area).
Novel Object Placement Test
During the acquisition phase (day 1) two identical Lego™ towers were placed in mirrored quadrants (15 × 22 cm from the wall) in an enclosed arena (50 × 60 cm). Mice were allowed to explore the arena for 10 minutes. EthoVisionXT (Noldus) recorded the time each mouse spent directly touching object 1 and 2. After 24 hours (day 2), one Lego™ tower was placed in a new quadrant (novel object placement), while the second was placed in the same location (original object placement). On day 2 (testing phase), mice were allowed to re-explore the arena for 10 minutes to ensure similar exploratory behavior to the prior day. EthoVisionXT recorded the time each mouse spent directly touching each object. Novel object placement exploration percentage during the testing phase was analyzed as time spent with the object in the novel location divided by the total time spent with both objects. The discrimination index was calculated as (x−y)/(x+y), with x = moved object and y = stable object. Pass rate was defined as 55% or more time spent with the object in the novel location as previously described [24].
Rotarod Test
Mice were positioned on top of a non-accelerating rod (ENV-575, 3 cm diameter, Med Associates, Fairfax, VT) and allowed to balance for 30 s after which the rod rotation would accelerate from 4 rpm to 40 rpm over a 5 min time period. Latency to fall was recorded for three trials separated by 1 hour on each of two separate days. The average of the three trials per day was calculated and displayed for rotarod analysis.
Forced Swim Test
Mice were positioned in a cylindrical tank containing approximately 25 °C water for 6 minutes and videotaped. For analysis of the Forced Swim Test (FST), the first 2 minutes were excluded and the last 4 minutes were analyzed for amount of time mice spent immobile by two independent, blinded observers, and the average time (seconds) was calculated for analysis.
Cued Fear Conditioning
For the cued fear acquisition phase (training), mice were placed in a fear-conditioning chamber (MED-VFC-USB-M, Med Associates) and given 2 minutes to acclimate. After 2 minutes, a 30 s sound cue followed by a 2 s, 0.6 Ma mild electric shock was given. This shock sequence was repeated 1 minute later after which the mice were allowed to recover for 1 min (total 6 minutes in chamber). Twenty-four hours later, mice were placed in a new visual setting (new color walls and floor) and allowed 2 minutes to acclimate. This was followed by the same sequence of sound cues as on day 1, but with no shock (total 6 minutes in chamber). Percent of time frozen was recorded by fear-conditioning chamber software (MED-SYST-VFC-USB, Med Associates) which defines freezing as lack of body movement.
Enzyme-Linked Immunosorbent Assays (ELISA)
DWEYS binding assay
Anti-DWEYS antibody binding assay was performed as previously described [25]. Briefly, a 50 μL solution of 200 μg/mL solution of amidated, acetylated DWEYSVWLSN (DWEYS) (>90 purity; BioMatik Corporation, Cambridge, Ontario) in 1x PBS was absorbed to an Immulon 2HB high binding polystyrene 96-well microtiter plate (ImmunoChemistry Technologies, Bloomington, MN) at 4 °C for 24 hours, followed by a blocking step with 330 μL 3% FBS in 1xPBS for at least 2 hours. After washing the plate, a 50 μL aliquot of 1:25 diluted murine serum in 1xPBS was added and incubated at 37 °C for 2 hours. After aspirating with 1% TWEEN 1xPBS, HRP-conjugated anti-mouse IgG (Southern Biotech, Birmingham, AL) (50 μL, 1:1000 dilution of in 3% FBS 1xPBS) was added and incubated at 37 °C for 1 hour. Plates were developed by adding 50μL of 0.4 g/L TMB substrate for 30 minutes before the reaction was quenched by the addition of 50μL of 0.02% H2O2.
Anti-dsDNA, Anti-nRNP, Anti-Ribosomal P, and Anti-Phospholipid ELISAs
Anti-dsDNA IgG and anti-nRNP IgG were determined using commercially available diagnostic kits per manufacturer’s guidelines (Alpha Diagnostics, San Antonio, TX). Anti-ribosomal P IgG (MyBioSource Inc., San Diego, CA) and anti-phospholipid IgG levels (Antibodies-online Inc., Limerick, PA) were determined using commercially available kits per manufacturer’s guidelines. TWEAK ELISA
Murine tumor necrosis factor-like weak inducer of apoptosis (TWEAK) was measured in a 1:20 serum dilution using a DuoSet kit for murine TWEAK/TNFSF12 (R&D Systems, Minneapolis, MN) and Immulon 2HB high binding polystyrene 96-well microtiter plate (ImmunoChemistry Technologies) according to the manufacturer’s instructions.
Final absorbance for all ELISAs was read at OD 450nm using a Perkin Elmer Wallac 1420 Victor2 microplate reader.
Tissue Processing, Immunofluorescence (IF) and Imaging
At 23 weeks of age, mice were anesthetized with 2.5% isoflurane and 97.5% oxygen anesthesia, and transcardially perfused with PBS followed by 4% paraformaldehyde. Brains were removed, post-fixed overnight, and cryopreserved in 30% sucrose in PBS. Brains were embedded in Optimal Cutting Temperature (OCT) Compound embedding medium (Fisher HealthCare, Waltham, MA) and kept at −80 °C until sliced at 15 μm on a cryostat. Immunofluorescence staining was performed by blocking tissue in 5% goat serum in 0.3% Triton X-100 for 1 h at room temperature, followed by addition of rabbit anti-Iba1 IgG antibodies (019–19741; 1:750 in 0.1% Triton-X/PBS; Wako, Osaka, Japan) and Alexa Fluor 555-conjugated anti-NeuN IgG (MAB377A5; 1:100 in 0.1% Triton-X PBS; Sigma-Aldrich, St. Louis, MO) for incubation overnight at 4°C. The next day, tissue was washed and Alexa Fluor 488-conjugated goat anti-rabbit IgG (A11034; 1:1000 in 0.1% Triton-X/PBS; Invitrogen, Carlsbad, CA) was added and incubated on sections for 1 h, followed by treatment with TruBlack (Cat no. 23007; 1:20; Biotium, Fremont, CA) for 1 minute. Tissue was coverslipped with Fluoromount-G (0100–01; Southern Biotechnology Associates Inc, Birmingham, AL). Tissue was imaged using a BZ-X700 Keyence microscope (Keyence, Itasca, IL) at 2x and 40x. Similar thresholds and acquisition times were used for all images. Area of staining (Iba1) and number of cell bodies (NeuN) for each tissue was quantified within a 200 μm × 200 μm frame using the BZ-X Analyzer program (Keyence). At least two sections were independently stained and analyzed per mouse.
Statistical Analysis
All data were analyzed using GraphPad Prism V8 software (La Jolla, CA). Comparisons between two groups were done using two-tailed unpaired Student’s t-tests with Welch’s correction. Comparisons between all four groups were done using by one-way ANOVA non-parametric tests. Correlation plots were analyzed using linear regression models. For all tests, statistical significance was defined as p < 0.05.
Results
Anti-DWEYS reactive antibodies are elevated in B6.Nba2 mice compared with B6 mice
A subset of anti-dsDNA IgG autoantibodies cross-react with the peptide sequence DWEYS [17], prompting us to evaluate levels of anti-dsDNA IgG and anti-DWEYS reactive antibodies in lupus-prone B6.Nba2 mice. At 8 weeks of age, both anti-DWEYS reactive IgG and anti-dsDNA IgG antibodies were significantly increased in B6.Nba2 females as compared with B6 females (p < 0.0001 and p < 0.01, respectively) (Figure 1A–B). By 16 weeks of age, B6.Nba2 females displayed an even more pronounced increase in anti-DWEYS reactive IgG antibody levels compared to both B6.Nba2 males (p < 0.001) and B6 females (p < 0.0001) as well as in anti-dsDNA IgG autoantibodies (p < 0.001 and p < 0.0001, respectively) (Figure 1A–B). By 16 weeks of age, B6.Nba2 males also displayed significantly higher levels of autoantibodies than their B6 male counterparts (anti-DWEYS IgG: p <0.0001 and anti-dsDNA IgG: p < 0.01) (Figure 1A–B). There was no difference in autoantibody levels between male and female B6 mice at either age. As expected, levels of anti-DWEYS reactive IgG antibodies correlated positively with levels of anti-dsDNA IgG autoantibodies (r2 = 0.3550, p < 0.0001) (Figure 1C).
Figure 1:
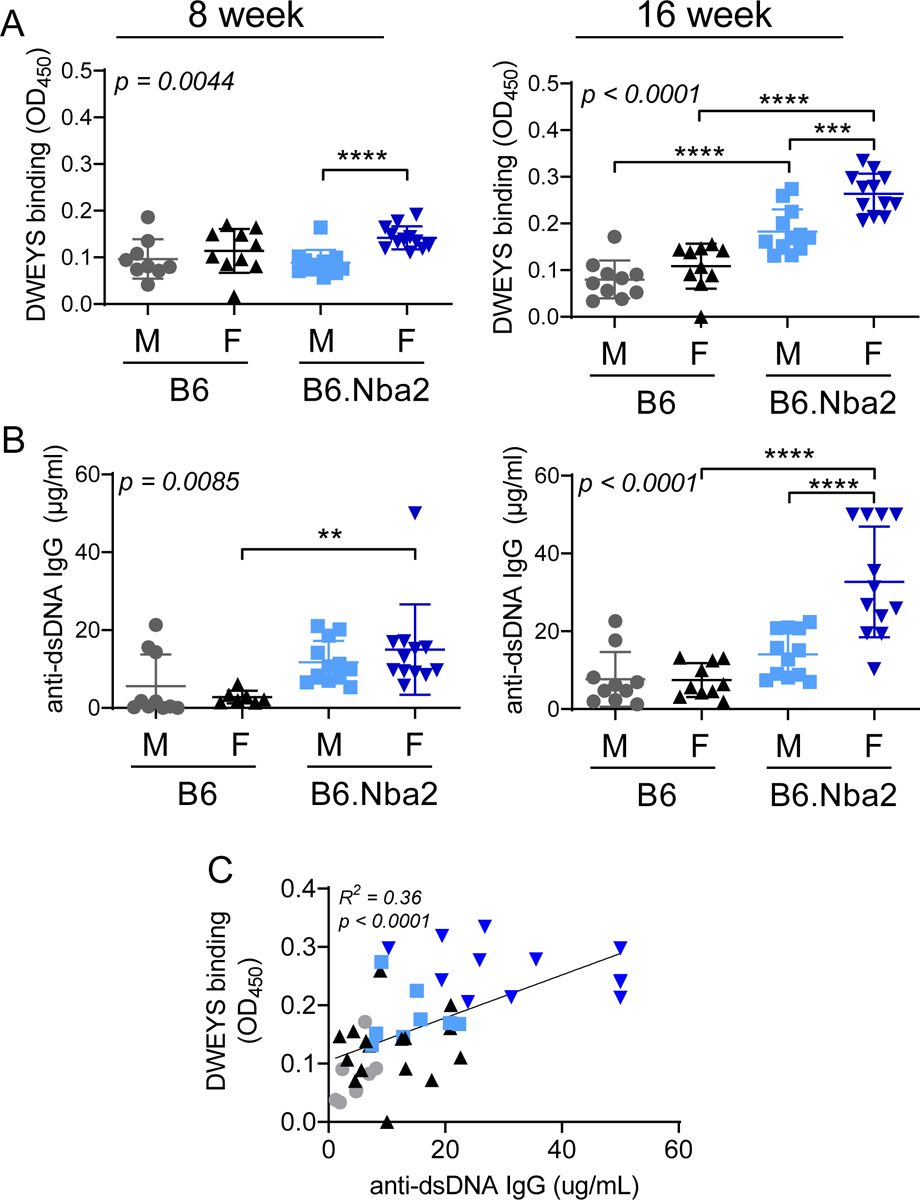
Anti-DWEYS antibody reactivity and anti-dsDNA IgG levels are elevated in B6.Nba2 mice. B6 males (gray circles, n = 10), B6 females (black triangles up, n = 10), B6.Nba2 male (light blue squares, n = 12), B6.Nba2 female (dark blue triangles down, n = 12) mice were bled at 8 and 16 weeks of age and serum was used to determine anti-DWEYS reactivity (A) and anti-dsDNA IgG levels (B) by ELISA. One-way ANOVA result is provided in the upper left corner of each graph. ** p < 0.01; *** p < 0.001; **** p < 0.0001, Student’s unpaired t-test with Welch’s correction. C) Correlation between DWEYS binding and anti-dsDNA IgG concentrations were determined at both. p < 0.0001; Linear regression. Each symbol represents one animal. Horizontal lines indicate mean values and the error bars denote SEM.
Anti-nuclear ribonucleoprotein (anti-nRNP) IgG autoantibodies are elevated in B6.Nba2 mice compared with B6 mice
In SLE patients, serum autoantibodies against phospholipid, ribosomal P, and nuclear ribonucleoprotein (nRNP) have been associated with NPSLE phenotypes [26–28], We tested levels of serum autoantibodies (22 weeks of age) in B6.Nba2 and control B6 mice and found elevated anti-nRNP IgG autoantibody levels (Figure 2A) in both male and female B6.Nba2 mice as compared with age- and sex-matched B6 mice, but no evidence for the presence of anti-ribosomal P IgG or anti-phospholipid IgG autoantibodies in any of the mice (Figure 2B–C). Furthermore, female B6.Nba2 mice displayed elevated levels of anti-nRNP antibodies as compared with male B6.Nba2 mice (p < 0.01) (Figure 2A).
Figure 2:
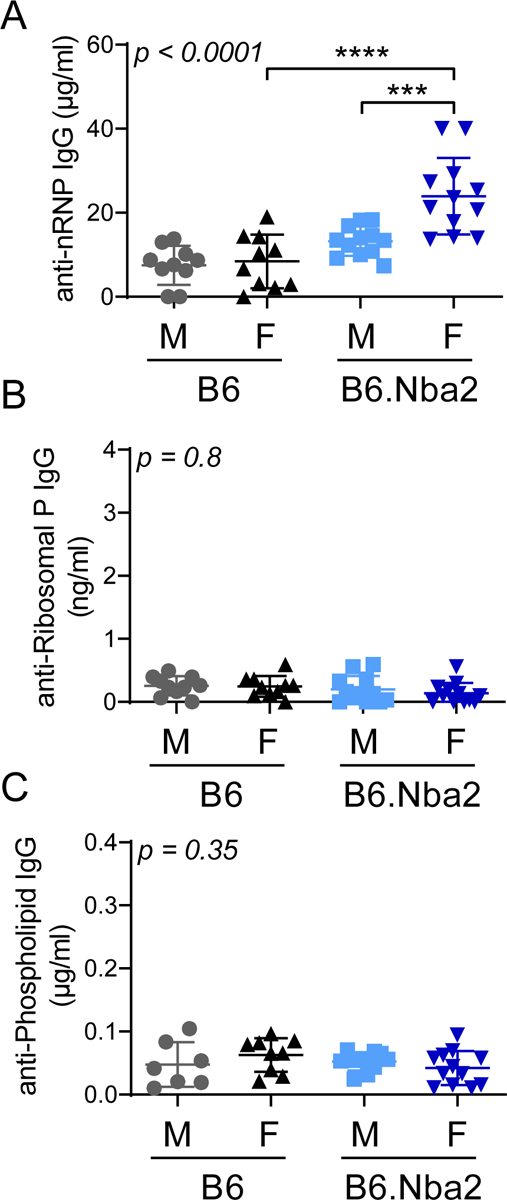
Levels of anti-nRNP IgG autoantibodies are elevated in male and female B6.Nba2 Mice. B6 males (n = 10), B6 females (n = 10), B6.Nba2 male (n = 12), B6.Nba2 female (n = 12) mice were bled at 22 weeks of age and serum was used to determine anti-nRNP IgG (A), anti-ribosomal P IgG (B), and anti-Phospholipid IgG (C) by ELISA. Each symbol represents one animal, mean +/− SEM is indicated by horizontal line and error bars. One-way ANOVA result is provided in the upper left corner of each graph. *** p < 0.001; **** p < 0.0001, Student’s unpaired t-test with Welch’s correction.
Serum soluble TNF-like weak inducer of apoptosis (TWEAK) levels are elevated in B6.Nba2 mice compared with B6 mice
Similar to autoantibodies, elevated levels of soluble TWEAK has been found in the CSF of NPSLE patients [29]. We evaluated levels of serum soluble TWEAK at 16 weeks of age and found elevated levels in B6.Nba2 males and females as compared with B6 males and females (p < 0.01–0.001) (Figure 3A). There was no difference in levels of soluble TWEAK between male and female mice of either strain (Figure 3A). Levels of serum TWEAK correlated positively with levels of anti-dsDNA IgG (p = 0.0022, linear regression) (Figure 3B). Several mice (n = 7) expressed elevated TWEAK without measurable anti-dsDNA IgG levels, while only a single female B6 mouse expressed low levels of anti-dsDNA IgG and no measurable levels of TWEAK.
Figure 3:
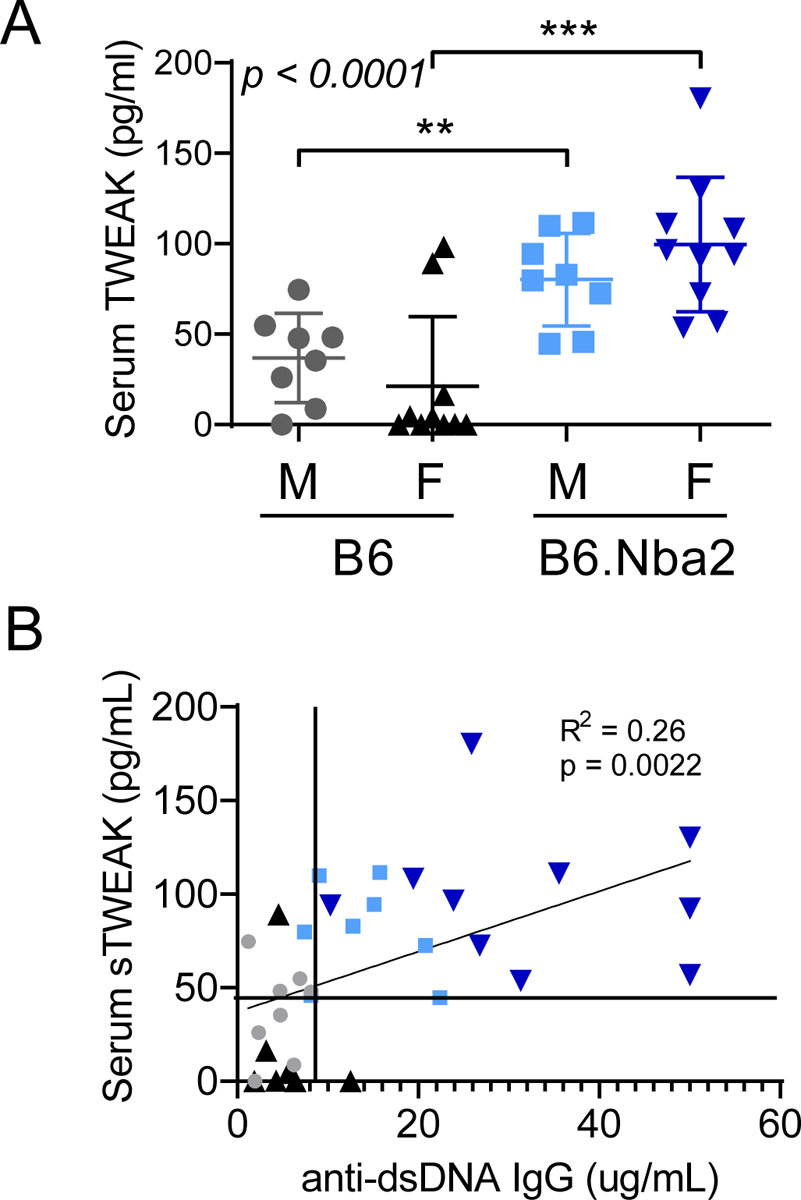
Serum soluble TWEAK levels are elevated in male and female B6.Nba2 Mice. B6 males (n = 10), B6 females (n = 10), B6.Nba2 male (n = 12), B6.Nba2 female (n = 12) mice were bled at 16 weeks of age and serum was used to determine soluble TWEAK (A) by ELISA. One-way ANOVA result is provided in the upper left corner. ** p < 0.01; *** p < 0.001, Student’s unpaired t-test with Welch’s correction. B) Correlation between serum levels of soluble TWEAK and anti-dsDNA autoantibodies in 16 week old mice: p < 0.0022; Linear regression. Each symbol represents one animal, mean +/− SEM is indicated by horizontal line and error bars.
B6.Nba2 mice display a strong and consistent anxiety phenotype
To determine if B6.Nba2 mice exhibited symptoms of NPSLE-like disease, B6 and B6.Nba2 male and female mice were tested using a set of well-established behavioral analyses [30, 31]. Already at 5 weeks of age, female B6.Nba2 mice displayed a slight anxiety phenotype as determined by the mice spending less time in the open field center location than B6 female mice (p < 0.01) (Figure 4A). By 11 weeks of age, this phenotype became even more pronounced (p < 0.0001) (Figure 4A). Interestingly, and in correspondence with the levels of autoantibodies, male B6.Nba2 mice also showed an anxiety phenotype at 11 weeks of age (p < 0.001), but not at 5 weeks of age (Figure 4A). An anxiety phenotype in both sexes was confirmed by similar results from the elevated plus maze testing at 12 weeks of age (Figure 4B). Deficiencies in the open field and elevated plus maze tests were not due to motor deficits in B6.Nba2 mice, as determined by equal performance of B6.Nba2 and B6 male and female mice on the rotarod test (suppl. Figure 2A–B).
Figure 4:
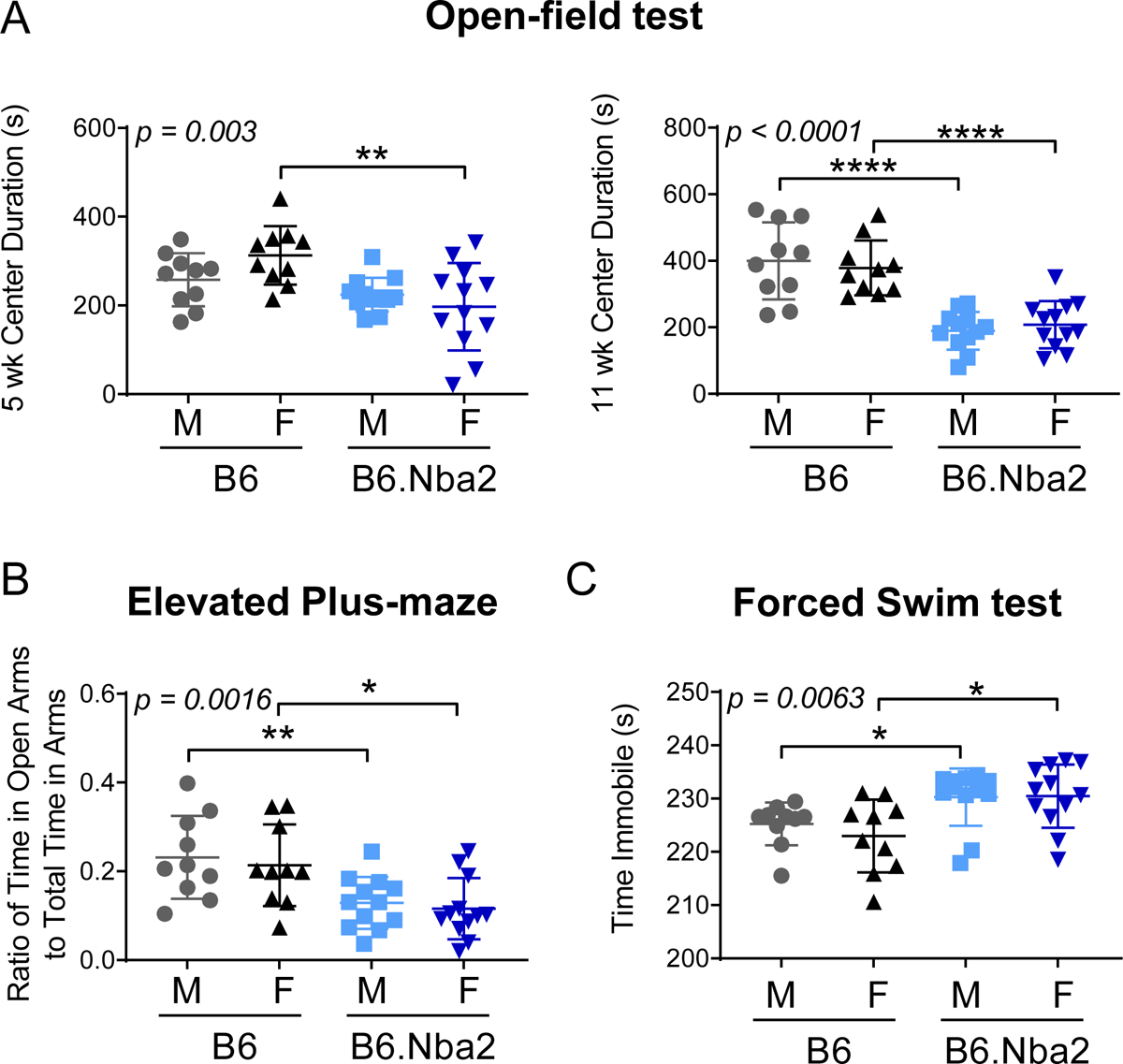
B6.Nba2 mice display an anxiety phenotype not observed in B6 mice. Male and female mice were tested for anxiety phenotype via open field test at 5 and 11 weeks (A), elevated plus maze at 12 weeks (B), and forced swim test at 16 weeks of age (C). Each symbol represents one animal, mean +/− SEM is given by horizontal lines and error bars. One-way ANOVA result is provided in the upper left corner of each graph. * p < 0.05; ** p < 0.01; **** p < 0.0001, Student’s unpaired t-test with Welch’s correction.
B6.Nba2 mice display a mild depression-like phenotype
Depression is a commonly observed symptom among NPSLE patients and mouse models of NPSLE [26, 32]. To determine if B6.Nba2 mice displayed a depression-like phenotype, we tested 16 weeks old male and female B6.Nba2 and B6 control mice for depression-like behavior via the forced swim test. Supporting a mild depression-like phenotype, both male and female B6.Nba2 mice showed longer immobility times than age- and sex-matched B6 mice (p < 0.05) (Figure 4C). Additionally, previous studies on NPSLE have defined the lack of total movement in the open field test as a sign of apathy associated with depression [33]. While our studies showed no significance in total distance traveled or velocity of mice in the open field test at 5 weeks of age, there was a significant reduction in total distance traveled and velocity traveled in the open field test by B6.Nba2 male and female mice at 11 weeks of age compared to B6 mice (p < 0.01), supporting the subsequent development of a depression-like phenotype (suppl. Figure 3).
B6.Nba2 mice display no learning or memory deficits
In NPSLE patients, elevated anti-NR2 IgG autoantibody levels in CSF correlated with the presence of cognitive deficits [34]. To test for hippocampus-dependent spatial memory, we conducted a novel object placement (NOP) test, in which mice were tested for their memory of an object as it was moved to a new location [18, 35]. At 13 weeks of age, we found no statistical significant differences in the percentage of time spent with the replaced object, and no change in the number of mice with a successful NOP ‘pass rate’ of >55% between B6.Nba2 and B6 male and female mice (Figure 5A–C). Thus, at 13 weeks of age B6.Nba2 mice do not suffer from hippocampus-dependent spatial memory deficits despite elevated levels of anti-DWEYS reactive antibodies. Similarly, when evaluating sensiromotor coordination and motor skill learning on the rotarod test at 15 weeks of age, no change in latency to fall between the groups of mice (suppl. Figure 2A–B) and no improvement from day 1 to day 3 for any of the strains (suppl. Figure 2C) were observed. Finally, hippocampus-independent (amygdala-dependent [36]) cued fear conditioning at 22 weeks of age did also not support any cognitive decline, as B6.Nba2 mice exhibited a similar, or even stronger, freezing behavior during conditioning (day 2) than B6 mice (Figure 5D–E).
Figure 5:
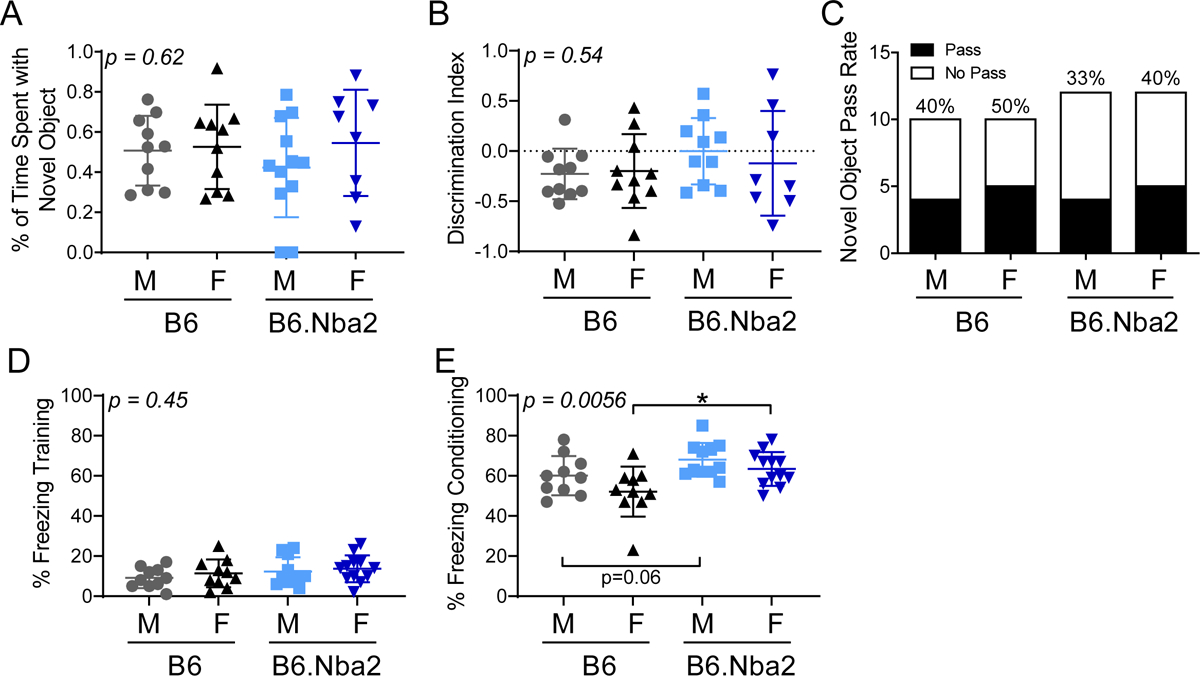
Young B6.Nba2 mice do not display cognitive decline and memory deficits. (A and B) 13 week old B6 females (n = 10), B6 males (n = 10), B6.Nba2 females (n = 12) and B6.Nba2 males (n = 12) were tested for cognitive deficits through the novel object placement test at 13 weeks of age. Neither the percentage of time spent with the novel object (A) nor the percentage of mice passing the test (B) differed among the strains and sexes. (C-D) Cued fear conditioning test was performed on all mice to test for memory deficits at 22 weeks of age. Training (C) and conditioning (D) times are shown for each individual mouse. One-way ANOVA result is provided in the upper left corner of each graph. Each symbol represents one animal. Mean +/− SEM is given by horizontal lines and error bars. * p < 0.05; Student’s unpaired t-test with Welch’s correction.
Decreased neuronal cell counts are observed in female B6.Nba2 hippocampus
Anti-NR2 IgG autoantibodies in NPSLE have been suggested to cause increased neuronal calcium influx similar to NMDAR-induced excitotoxicity, and subsequent neuronal death [37]. In addition, systemic inflammation has been suggested to cause microglial activation, further potentiating neuronal death [38]. We evaluated Iba1 and NeuN expression levels within the CA3 hippocampal region by immunofluorescence to determine microglial activation and neuronal density, respectively, in hippocampal brain tissue (Figure 6A–B). The CA3 (Cornu Ammonis 3) region was selected due to its known expression of NMDAR and association with both spacial memory defects and anxiety [39, 40]. B6.Nba2 females exhibited elevated levels of microglial activation (area Iba1+) as compared with B6 females (p = 0.06), but there was no difference between B6.Nba2 males and B6 males (Figure 6C). Correspondently, we observed decreased neuronal cell counts (NeuN+ cells) in B6.Nba2 females as compared with B6 females (p < 0.05), with no difference between B6.Nba2 and B6 males (Figure 6D). There was also an increase in NeuN+ cells in B6 females as compared with B6 males (p = 0.06), but not between B6.Nba2 males and females (p = 0.9).
Figure 6:
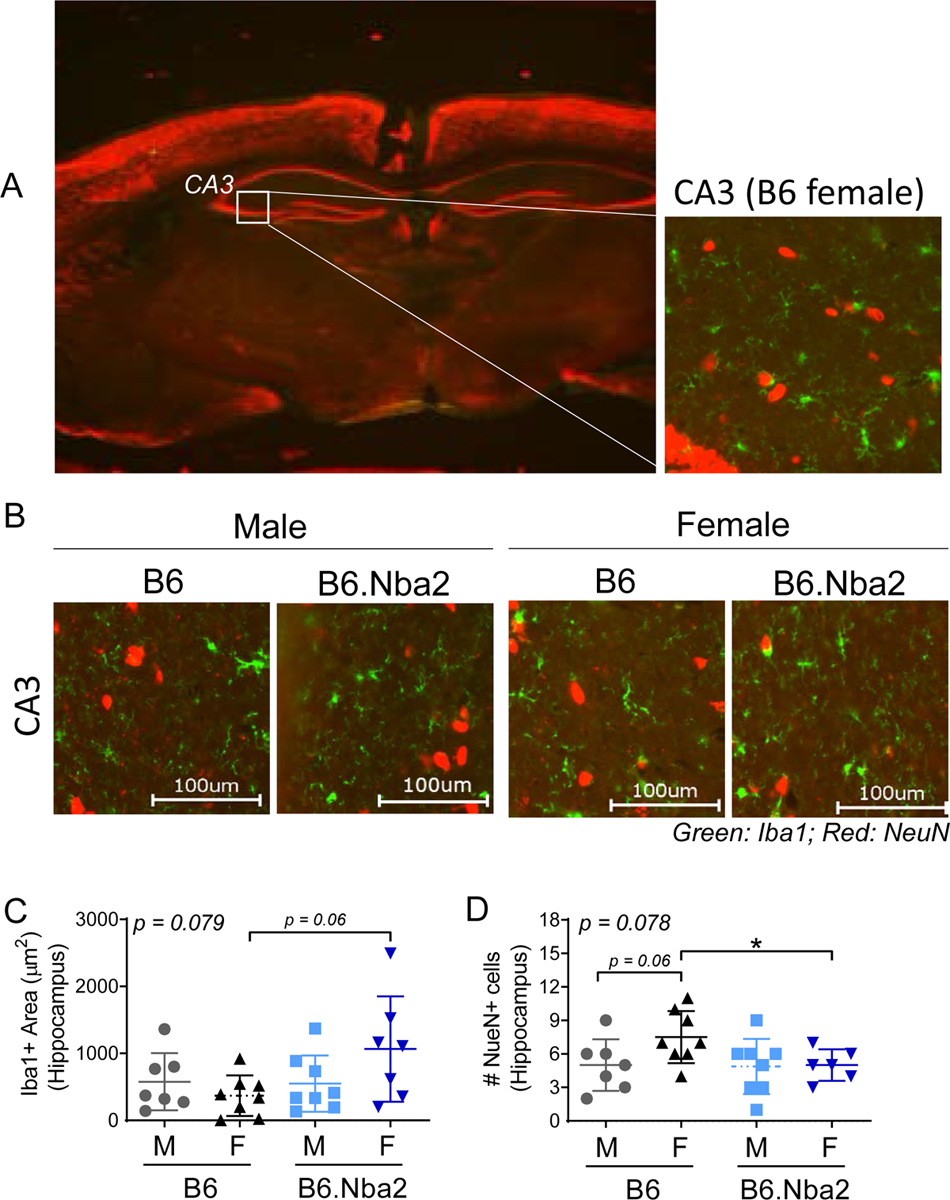
B6.Nba2 females show decreased numbers of hippocampal NeuN+ cells. A) Representative images of the hippocampal region at 2X (left) and 40X (right), stained for Iba1 (green) and NeuN (red), are shown for overall brain tissue orientation. B) Representative images of the CA3 region from male and female, B6 and B6.Nba2 mice (40X). C-D) The total area (μm2) of hippocampal Iba1+ staining was quantified (C), and the total numbers of NeuN+ cells were counted (D) from three separate 200μm × 200μm hippocampal regions from each mouse. Each symbol represents one animal. Mean +/− SEM is given by horizontal lines and error bars. One-way ANOVA result is provided in the upper left corner of each graph. * p < 0.05, Student’s unpaired t-test with Welch’s correction.
Discussion
As a result of the wide array of symptomology, it is difficult to identify the causes of NPSLE development in patients [1, 7]. Mouse models have given some insight into plausible NPSLE biological mechanisms, including the possibility of sphingosine-1-phosphate mediated lymphocyte trafficking [41], dysregulation of microglia [13, 42], or increased autoantibodies paired with breaches in the blood brain barrier (BBB) [20, 24, 34, 43]. However, the extent that each pathway contributes to psychiatric conditions or towards sex-specific differences still remains unknown.
We here introduce the B6.Nba2 lupus-prone strain as a novel model of NPSLE. Presenting with elevated anti-DWEYS reactive antibodies, anxiety and depression, the B6.Nba2 model recapitulates patient studies showing a strong correlation between the presence of anti-DWEYS specific antibodies and the development of NPSLE [17]. Depression, often preceded by anxiety, correlates with the presence of elevated anti-NR2 autoantibody or anti-DWEYS reactive antibody levels [17, 20, 32] and is the most common psychiatric condition observed in patients with NPSLE [44]. In B6.Nba2 mice, correlation analyses showed a strong correlation between levels of anti-DWEYS antibodies and both anxiety and depression, supporting a role for these antibodies in this model of NPSLE (suppl. Figure 4A–C). Depression has also been observed in several other spontaneous mouse models of NPSLE, but only in the MRL/lpr mouse model of NPSLE has the depression phenotype been shown to correlate with elevated levels of anti-NMDAR specific antibodies [32]. Interestingly, while the MRL/lpr model develops a strong depression phenotype, it appears less anxious [45], while an opposite pattern was observed in B6.Nba2 mice.
B6.Nba2 mice present with elevated levels of serum soluble TWEAK, a known marker of inflammation. We did not observe differences in levels of TWEAK between male and female mice, despite previous studies showing that estrogens induce TWEAK production in MRL/lpr mice [46]. Interestingly, TWEAK levels appear to increase prior to elevations in serum anti-dsDNA autoantibody levels, suggesting that inflammation is present and measurable before the induction of autoantibody production. Importantly, TWEAK has been associated with mechanisms driving BBB disruption in MRL/lpr lupus-prone mice [29]. Thus, early increases in TWEAK may predispose B6.Nba2 mice (female > male) to the development of NPSLE-like disease by facilitating BBB disruption and antibody entry into the brain. Future studies evaluating BBB integrity, brain specific antibody levels, and the effect of TWEAK blockade in B6.Nba2 and control animals are needed to confirm such interaction.
Mechanisms mimicking NMDA-receptor excitotoxicity have been suggested to contribute to cognitive decline and mood disorders in NPSLE patients [37]. In particular, the hippocampal region, which controls both memory and emotion, has been hypothesized to be a site for neuronal apoptosis contributing to the development of depression and a site for altered BBB permeability in SLE patients [47, 48]. We observed a pattern of reduced neuronal density in the hippocampal region CA3 along with a depression phenotype in B6.Nba2 females, suggesting a similar association in NPSLE-like disease in B6.Nba2 mice. Whether anti-DWEYS reactive antibodies in B6.Nba2 mice directly account for NMDA-receptor excitotoxicity and subsequent neuronal death similar to that observed in the Balb/c DWEYS-peptide injected model [17], however, remains to be determined. Interestingly, neuronal death has been found to be a predecessor to activation of microglia [38], potentially explaining the pattern of elevated Iba1 expression in female B6.Nba2 mice.
Limitations in our study include the single time point and sole use of the novel object placement test for detection of hippocampus-dependent cognitive decline. We tested B6.Nba2 mice at 13 weeks of age, which may have been too early in the development of cognitive decline for such to be measured. In comparison, MRL/lpr mouse model only started showing cognitive decline at 16 weeks of age [49, 50]. Secondly, the sole use of the novel object placement test might not have adequately captured signs of cognitive decline. In an earlier study conducted by Kowal, et al. no significance was found the in novel object placement test using the Balb/c DWEYS-peptide injected model, despite a cognitive phenotype being detected in T-maze and the Morris water maze tests [18]. It is also possible, that the mice (both B6 controls and B6.Nba2) “forgot” the original placement of the objects in between tests resulting in the mice not being able to identify the replaced object due to the 24 hour interval between habituation and testing in the novel object placement test. Thus, expanded testing of the B6.Nba2 mouse model using a shorter time period (2–4 hours) in between habituation and testing in the novel object placement test, adding additional behavioral tests such as the T-maze and the Morris water maze, and testing mice at older ages correlating with histological observations, are needed to firmly confirm a lack of cognitive phenotype in the B6.Nba2 model.
SLE is predominantly diagnosed in female patients and, as a result, there is less knowledge on the development of NPSLE in males. A single study reported that male SLE patients more often present with seizures than female patients [11], while many studies of female SLE patients have confirmed that anxiety and depression represent the most common psychiatric conditions [45, 51]. Whether this discrepancy represents a true difference in symptoms developing in males and females, respectively, or is a reflection of the low numbers of male patients, or the fact that males are less likely to discuss psychiatric conditions [52], remains unknown. We found that anxiety and depression developed in both male and female B6.Nba2 mice, although the onset was later in males. A similar pattern was observed in the MRL/lpr model, in which males presented with a depression-like phenotype at a much later date than females [50]. A possible explanation surrounds estrogen levels, as ER-alpha knockout and ovariectomized NPSLE-prone mice showed improved memory and exploration times [13]. Neither ER-alpha manipulated nor ovariectomized B6.Nba2 mice have previously been investigated for disease development and thus the role of estrogens remains unknown in this model. In this regard, it is also important to consider the genetic underpinnings of our mouse model, as previously suggested for NZBWF1 and MRL/lpr mice [53, 54]. An altered BBB may allow for earlier changes in neurons and microglial activation, as observed in our model and the possibility for such genetic components selectively impacting female psychiatric phenotypes should not be overlooked. Future studies looking into the differences in presentation of NPSLE-like disease in both male and female mice and how such difference may impact the development of the behavioral phenotype between sexes will be of interest in determining the differential diagnoses of NPSLE between male and female patients.
Finally, the B6.Nba2 mouse model of SLE depends on type I interferons [55, 56]. Whether type I interferons are also driving the NPSLE-like phenotype reported here remains unknown. While previous studies have shown associations between type I interferon levels and anxiety and depression in both animal models and human patients [13–15, 57, 58], recent studies in the MRL/lpr mouse model of NPSLE showed no effect on cognitive behavior upon blockade of the type I interferon receptor [16]. In a preliminary set of analyses, we evaluated levels of type I interferon-inducible genes (Isg15 and Irf7) in whole brain tissue from male and female B6 and B6.Nba2 mice and found no differences in gene expression. Similarly, levels of Ifi202 were found to be similar between the groups, although about 25% of female B6.Nba2 mice showed elevated levels as compared with all other mice (data not shown). Whether this is due to the analyses being done on whole brains rather than isolated cell subsets (neurons, microglia, astrocytes etc.) remains to be determined. Thus, additional studies directly aimed at evaluating the effect of type I interferons are needed to establish their role in B6.Nba2 mice.
In summary, the B6.Nba2 model of SLE is a promising model to conduct future studies pertaining to NPSLE development, particularly within sexes, as a result of a strong anxiety and depression phenotype observed early along with elevated levels of anti-DWEYS reactive antibodies.
Supplementary Material
Funding
Financial support comes from the Department of Defense Lupus Research Program (grant# LR180040 (TJ))
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1.Vivaldo JF, de Amorim JC, Julio PR, de Oliveira RJ, Appenzeller S. Definition of NPSLE: Does the ACR Nomenclature Still Hold? Front Med (Lausanne). 2018;5:138. doi: 10.3389/fmed.2018.00138 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popescu A, Kao AH. Neuropsychiatric systemic lupus erythematosus. Curr Neuropharmacol. 2011;9(3):449–57. doi: 10.2174/157015911796557984 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42(4):599–608. Epub 1999/04/22. doi: . [DOI] [PubMed] [Google Scholar]
- 4.Monahan RC, LJJ, Steup-Beekman GM, Magro-Checa C, Huizinga TWJ, Hoekman J, et al. Neuropsychiatric symptoms in systemic lupus erythematosus: impact on quality of life. Lupus. 2017;26(12):1252–9. doi: 10.1177/0961203317694262 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zirkzee EJ, Huizinga TW, Bollen EL, van Buchem MA, Middelkoop HA, van der Wee NJ, et al. Mortality in neuropsychiatric systemic lupus erythematosus (NPSLE). Lupus. 2014;23(1):31–8. doi: 10.1177/0961203313512540 [doi]. [DOI] [PubMed] [Google Scholar]
- 6.Ahn GY, Kim D, Won S, Song ST, Jeong HJ, Sohn IW, et al. Prevalence, risk factors, and impact on mortality of neuropsychiatric lupus: a prospective, single-center study. Lupus. 2018;27(8):1338–47. doi: 10.1177/0961203318772021 [doi]. [DOI] [PubMed] [Google Scholar]
- 7.Zhang E, Jorgensen TN. Neuropsychiatric SLE: From Immune Mechanisms to Clinical Management [Online First], IntechOpen. Online Book "Lupus". 2018:1–34. [Google Scholar]
- 8.Weckerle CE, Niewold TB. The unexplained female predominance of systemic lupus erythematosus: clues from genetic and cytokine studies. Clin Rev Allergy Immunol. 2011;40(1):42–9. doi: 10.1007/s12016-009-8192-4 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merrheim J, Villegas J, Van Wassenhove J, Khansa R, Berrih-Aknin S, le Panse R, et al. Estrogen, estrogen-like molecules and autoimmune diseases. Autoimmun Rev. 2020;19(3):102468. Epub 2020/01/14. doi: 10.1016/j.autrev.2020.102468 [doi]. [DOI] [PubMed] [Google Scholar]
- 10.Panchanathan R, Shen H, Zhang X, Ho SM, Choubey D. Mutually positive regulatory feedback loop between interferons and estrogen receptor-alpha in mice: implications for sex bias in autoimmunity. PLoS One. 2010;5(5):e10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward MM, Studenski S. Systemic lupus erythematosus in men: a multivariate analysis of gender differences in clinical manifestations. J Rheumatol. 1990;17(2):220–4. [PubMed] [Google Scholar]
- 12.Magro-Checa C, Zirkzee EJ, Huizinga TW, Steup-Beekman GM. Management of Neuropsychiatric Systemic Lupus Erythematosus: Current Approaches and Future Perspectives. Drugs. 2016;76(4):459–83. doi: 10.1007/s40265-015-0534-3 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunningham MA, Wirth JR, Freeman LR, Boger HA, Granholm AC, Gilkeson GS. Estrogen receptor alpha deficiency protects against development of cognitive impairment in murine lupus. J Neuroinflammation. 2014;11:171. doi: 10.1186/s12974-014-0171-x [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19(2):105–23. doi: 10.2165/00023210-200519020-00002 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, et al. Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol Psychiatry. 2007;62(11):1324–33. doi: S0006–3223(07)00522–7 [pii]; 10.1016/j.biopsych.2007.05.026 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang MW, Stock AD, Mike EV, Herlitz L, Kolbeck R, Putterman C. Anti-IFNAR treatment does not reverse neuropsychiatric disease in MRL/lpr lupus mice. Lupus. 2019;28(13):1510–23. doi: 10.1177/0961203319872265 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7(11):1189–93. doi: 10.1038/nm1101-1189 [doi]. [DOI] [PubMed] [Google Scholar]
- 18.Kowal C, DeGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B, et al. Cognition and immunity; antibody impairs memory. Immunity. 2004;21(2):179–88. doi: 10.1016/j.immuni.2004.07.011 [doi]. [DOI] [PubMed] [Google Scholar]
- 19.Omdal R, Brokstad K, Waterloo K, Koldingsnes W, Jonsson R, Mellgren SI. Neuropsychiatric disturbances in SLE are associated with antibodies against NMDA receptors. Eur J Neurol. 2005;12(5):392–8. doi: 10.1111/j.1468-1331.2004.00976.x [doi]. [DOI] [PubMed] [Google Scholar]
- 20.Lapteva L, Nowak M, Yarboro CH, Takada K, Roebuck-Spencer T, Weickert T, et al. Anti-N-methyl-D-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum. 2006;54(8):2505–14. doi: 10.1002/art.22031 [doi]. [DOI] [PubMed] [Google Scholar]
- 21.Stock AD, Gelb S, Pasternak O, Ben-Zvi A, Putterman C. The blood brain barrier and neuropsychiatric lupus: new perspectives in light of advances in understanding the neuroimmune interface. Autoimmun Rev. 2017;16(6):612–9. doi: 10.1016/j.autrev.2017.04.008 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gubbels MR, Jorgensen TN, Metzger TE, Menze K, Steele H, Flannery SA, et al. Effects of MHC and Gender on Lupus-Like Autoimmunity in Nba2 Congenic Mice. The Journal of Immunology. 2005;175(9):6190–6. [DOI] [PubMed] [Google Scholar]
- 23.Jorgensen TN, Alfaro J, Enriquez HL, Jiang C, Loo WM, Atencio S, et al. Development of murine lupus involves the combined genetic contribution of the SLAM and FcgammaR intervals within the Nba2 autoimmune susceptibility locus. J Immunol. 2010;184(2):775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen J, Chen CH, Stock A, Doerner J, Gulinello M, Putterman C. Intracerebroventricular administration of TNF-like weak inducer of apoptosis induces depression-like behavior and cognitive dysfunction in non-autoimmune mice. Brain Behav Immun. 2016;54:27–37. doi: 10.1016/j.bbi.2015.12.017 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putterman C, Diamond B. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J Exp Med. 1998;188(1):29–38. doi: 10.1084/jem.188.1.29 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz N, Stock AD, Putterman C. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat Rev Rheumatol. 2019;15(3):137–52. doi: 10.1038/s41584-018-0156-8 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshio T, Hirata D, Onda K, Nara H, Minota S. Antiribosomal P protein antibodies in cerebrospinal fluid are associated with neuropsychiatric systemic lupus erythematosus. J Rheumatol. 2005;32(1):34–9. [PubMed] [Google Scholar]
- 28.Sato T, Fujii T, Yokoyama T, Fujita Y, Imura Y, Yukawa N, et al. Anti-U1 RNP antibodies in cerebrospinal fluid are associated with central neuropsychiatric manifestations in systemic lupus erythematosus and mixed connective tissue disease. Arthritis Rheum. 2010;62(12):3730–40. doi: 10.1002/art.27700 [doi]. [DOI] [PubMed] [Google Scholar]
- 29.Wen J, Doerner J, Weidenheim K, Xia Y, Stock A, Michaelson JS, et al. TNF-like weak inducer of apoptosis promotes blood brain barrier disruption and increases neuronal cell death in MRL/lpr mice. J Autoimmun. 2015;60:40–50. doi: 10.1016/j.jaut.2015.03.005 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–8. doi: 10.1038/nprot.2007.44 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seibenhener ML, Wooten MC. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. 2015;(96):e52434. doi: 10.3791/52434 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao HX, Campbell SR, Cui MH, Zong P, Hee-Hwang J, Gulinello M, et al. Depression is an early disease manifestation in lupus-prone MRL/lpr mice. J Neuroimmunol. 2009;207(1–2):45–56. doi: S0165–5728(08)00486–4 [pii]; 10.1016/j.jneuroim.2008.11.009 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeltsch-David H, Muller S. Neuropsychiatric systemic lupus erythematosus and cognitive dysfunction: the MRL-lpr mouse strain as a model. Autoimmun Rev. 2014;13(9):963–73. doi: S1568–9972(14)00159–1 [pii]; 10.1016/j.autrev.2014.08.015 [doi]. [DOI] [PubMed] [Google Scholar]
- 34.Hirohata S, Arinuma Y, Yanagida T, Yoshio T. Blood-brain barrier damages and intrathecal synthesis of anti-N-methyl-D-aspartate receptor NR2 antibodies in diffuse psychiatric/neuropsychological syndromes in systemic lupus erythematosus. Arthritis Res Ther. 2014;16(2):R77. doi: 10.1186/ar4518 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evonuk KS, Prabhu SD, Young ME, DeSilva TM. Myocardial ischemia/reperfusion impairs neurogenesis and hippocampal-dependent learning and memory. Brain Behav Immun. 2017;61:266–73. doi:; 10.1016/j.bbi.2016.09.001 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curzon P, Rustay NR, Browman KE. Cued and Contextual Fear Conditioning for Rodents. 2009. doi: NBK5223. [PubMed] [Google Scholar]
- 37.Li V, Wang YT. Molecular mechanisms of NMDA receptor-mediated excitotoxicity: implications for neuroprotective therapeutics for stroke. Neural Regen Res. 2016;11(11):1752–3. doi: 10.4103/1673-5374.194713 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan K, Nestor J, Huerta TS, Certain N, Moody G, Kowal C, et al. Lupus autoantibodies act as positive allosteric modulators at GluN2A-containing NMDA receptors and impair spatial memory. Nat Commun. 2020;11(1):1403. doi: 10.1038/s41467-020-15224-w [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5(5):361–72. Epub 2004/04/22. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- 40.Engin E, Smith KS, Gao Y, Nagy D, Foster RA, Tsvetkov E, et al. Modulation of anxiety and fear via distinct intrahippocampal circuits. Elife. 2016;5:e14120. doi: 10.7554/eLife.14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mike EV, Makinde HM, Der E, Stock A, Gulinello M, Gadhvi GT, et al. Neuropsychiatric Systemic Lupus Erythematosus Is Dependent on Sphingosine-1-Phosphate Signaling. Front Immunol. 2018;9:2189. doi: 10.3389/fimmu.2018.02189 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nomura A, Noto D, Murayama G, Chiba A, Miyake S. Unique primed status of microglia under the systemic autoimmune condition of lupus-prone mice. Arthritis Res Ther. 2019;21(1):303. doi: 10.1186/s13075-019-2067-8 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stock AD, Wen J, Putterman C. Neuropsychiatric Lupus, the Blood Brain Barrier, and the TWEAK/Fn14 Pathway. Front Immunol. 2013;4:484. doi: 10.3389/fimmu.2013.00484 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Figueiredo-Braga M, Cornaby C, Cortez A, Bernardes M, Terroso G, Figueiredo M, et al. Depression and anxiety in systemic lupus erythematosus: The crosstalk between immunological, clinical, and psychosocial factors. Medicine (Baltimore). 2018;97(28):e11376. doi: 10.1097/MD.0000000000011376 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nery FG, Borba EF, Viana VS, Hatch JP, Soares JC, Bonfa E, et al. Prevalence of depressive and anxiety disorders in systemic lupus erythematosus and their association with anti-ribosomal P antibodies. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):695–700. doi: 10.1016/j.pnpbp.2007.11.014 [doi]. [DOI] [PubMed] [Google Scholar]
- 46.Xue L, Liu Z, Hu J, Huang J, Wen J, Liu Z. Estrogen-induced expression of tumor necrosis factor-like weak inducer of apoptosis through ERα accelerates the progression of lupus nephritis. Rheumatology (Oxford). 2016;55(10):1880–8. Epub 2016/06/30. doi: 10.1093/rheumatology/kew248 [doi]. [DOI] [PubMed] [Google Scholar]
- 47.Lee AL, Ogle WO, Sapolsky RM. Stress and depression: possible links to neuron death in the hippocampus. Bipolar Disord. 2002;4(2):117–28. doi: 10.1034/j.1399-5618.2002.01144.x [doi]. [DOI] [PubMed] [Google Scholar]
- 48.Chi JM, Mackay M, Hoang A, Cheng K, Aranow C, Ivanidze J, et al. Alterations in Blood-Brain Barrier Permeability in Patients with Systemic Lupus Erythematosus. AJNR Am J Neuroradiol. 2019;40(3):470–7. doi: 10.3174/ajnr.A5990 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogelweid CM, Wright DC, Johnson JC, Hewett JE, Walker SE. Evaluation of memory, learning ability, and clinical neurologic function in pathogen-free mice with systemic lupus erythematosus. Arthritis Rheum. 1994;37(6):889–97. doi: 10.1002/art.1780370617 [doi]. [DOI] [PubMed] [Google Scholar]
- 50.Gao HX, Sanders E, Tieng AT, Putterman C. Sex and autoantibody titers determine the development of neuropsychiatric manifestations in lupus-prone mice. J Neuroimmunol. 2010;229(1–2):112–22. doi: 10.1016/j.jneuroim.2010.07.020 [doi]. [DOI] [PubMed] [Google Scholar]
- 51.Mok CC, Lau CS, Chan TM, Wong RW. Clinical characteristics and outcome of southern Chinese males with systemic lupus erythematosus. Lupus. 1999;8(3):188–96. doi: 10.1191/096120399678847605 [doi]. [DOI] [PubMed] [Google Scholar]
- 52.Affleck W, Carmichael V, Whitley R. Men's Mental Health: Social Determinants and Implications for Services. Can J Psychiatry. 2018;63(9):581–9. doi: 10.1177/0706743718762388 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kier AB. Clinical neurology and brain histopathology in NZB/NZW F1 lupus mice. J Comp Pathol. 1990;102(2):165–77. doi: 10.1016/s0021-9975(08)80122-3 [doi]. [DOI] [PubMed] [Google Scholar]
- 54.Stock AD, Wen J, Doerner J, Herlitz LC, Gulinello M, Putterman C. Neuropsychiatric systemic lupus erythematosus persists despite attenuation of systemic disease in MRL/lpr mice. J Neuroinflammation. 2015;12:205. doi: 10.1186/s12974-015-0423-4 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jorgensen TN, Roper E, Thurman JM, Marrack P, Kotzin BL. Type I interferon signaling is involved in the spontaneous development of lupus-like disease in B6.Nba2 and (B6.Nba2 x NZW)F(1) mice. Genes Immun. 2007;8(8):653–62. [DOI] [PubMed] [Google Scholar]
- 56.Davison LM, Jorgensen TN. Sialic acid-binding immunoglobulin-type lectin H-positive plasmacytoid dendritic cells drive spontaneous lupus-like disease development in B6.Nba2 mice. Arthritis Rheumatol. 2015;67(4):1012–22. doi: 10.1002/art.38989 [doi]. [DOI] [PubMed] [Google Scholar]
- 57.Fahey B, Hickey B, Kelleher D, O'Dwyer AM, O'Mara SM. The widely-used anti-viral drug interferon-alpha induces depressive- and anxiogenic-like effects in healthy rats. Behav Brain Res. 2007;182(1):80–7. doi: 10.1016/j.bbr.2007.05.005 [doi]. [DOI] [PubMed] [Google Scholar]
- 58.Wang J, Campbell IL, Zhang H. Systemic interferon-alpha regulates interferon-stimulated genes in the central nervous system. Mol Psychiatry. 2008;13(3):293–301. doi: 10.1038/sj.mp.4002013 [doi]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


