Abstract
Carbohydrates have been thought to stimulate immune responses independently of T cells; however, zwitterionic polysaccharides (ZPSs) from the capsules of some bacteria elicit potent CD4+-T-cell responses in vivo and in vitro. We demonstrated that HLA-DR on professional antigen-presenting cells (APCs) is required for ZPS-induced T-cell proliferation in vitro (15). Recently, it was shown that ZPSs are processed to low-molecular-weight carbohydrates by a nitric oxide-mediated mechanism in endosomes and locate in the major histocompatibility complex class II pathway (5, 15). The effect of the ZPS-mediated expression of HLA-DR and costimulatory molecules on the APC and T-cell engagement and subsequent T-cell activation has not been elucidated. Herein, we report that ZPS-mediated induction of HLA-DR-surface expression and T-cell proliferation are maximally enhanced after incubation of APCs for 8 h with ZPS. Treatment of APCs with bafilomycin A inhibits the up-regulation of ZPS-mediated HLA-DR surface expression and leads to inhibition of T-cell proliferation. Monoclonal antibodies (MAbs) to the costimulatory molecules B7-2 and CD40L specifically block ZPS-mediated T-cell activation, while a MAb to B7-1 does not. Surface expression of B7-2 and B7-1 but not of CD40 is maximally enhanced at 8 to 16 h of treatment of APCs with ZPS. The results demonstrate that the cellular immune response to ZPS depends on the translocation of HLA-DR to the cell surface and requires costimulation via B7-2 and CD40 on activated APCs. The implication is that activation of ZPS-specific T cells requires an orchestrated arrangement of both presenting and costimulatory molecules to form an immunological synapse.
Most pathogenic extracellular bacteria produce large-molecular-mass surface polysaccharides, usually in the form of a capsule that coats the bacterial cell surface. The classification of polysaccharides as T-cell-independent type 2 (TI-2) antigens is based on the lack of T-cell-dependent responses to these typically negatively charged or uncharged molecules (12, 13). In view of their immunogenic characteristics, bacterial zwitterionic polysaccharides (ZPSs) isolated from strains of Bacteroides fragilis, Staphylococcus aureus, and Streptococcus pneumoniae type 1 represent an unusual group of bacterial carbohydrates. ZPSs have unique immunological properties: molecules as small as 17 kDa elicit a potent CD4+-T-cell response in vitro and in vivo (16). Structure-function studies have shown that the proliferative response of T cells depends on free amino (positively charged) and carboxyl or phosphate groups (negatively charged) on each repeating unit (31, 32). The mediation of proliferation of human T cells in vitro is characterized by a requirement for direct interaction of T cells with HLA-DR-bearing antigen-presenting cells (APCs). ZPSs colocalize with HLA-DR in compartments of the endocytic pathway and on the cell surface (15). They are processed to low-molecular-weight carbohydrates by a nitric-oxide-dependent mechanism in endosomes and bind to major histocompatibility complex class II (MHC-II) inside APCs (5). Nuclear magnetic resonance studies of ZPS from B. fragilis and S. pneumoniae type 1 suggest possible sites for binding to the α-helices of HLA-DR (17, 33, 34).
Contact of T cells with processed antigen presented by MHC molecules results in T-cell activation through the dynamic interaction of multiple membrane molecules generating the necessary intracellular signals. Through the mediation of the MHC molecules, the T-cell receptor (TCR) and the CD3 molecule initiate the activation signal 1. However, this interaction by itself is not sufficient to fully activate naïve T cells. A subsequent antigen-nonspecific costimulatory signal (signal 2) is required. Interaction of CD28 on T cells with its ligands B7-1 and B7-2 on APCs comprises a major costimulatory pathway controlling T-cell response to a variety of antigens, promoting cell cycle progression, and increasing interleukin-2 production (3, 28). Once a naïve T cell is activated through signal 1 and signal 2, it expresses a number of proteins that contribute to sustaining or modifying the costimulatory signals. For example, binding of CD40-CD40 ligand (CD154) transmits bidirectional activation signals to the T cell and the APC (10, 35). These best-characterized costimulatory molecules can be either constitutively expressed or induced on activated APCs.
The activation of APCs by TI-2 polysaccharides and the role of costimulatory molecules during the humoral immune response to these antigens have been reported in various experimental systems. Heparan sulfate and hyaluronic acid induce a phenotypic and functional maturation of dendritic cells (DCs) (18, 27). The immune response to alpha (1→ 3) dextran was dependent on the CD28-B7-1 pathway. In contrast, immune responses to capsular polysaccharides of some S. pneumoniae serotypes, to NP-Ficoll, and to the capsular polysaccharide of Cryptococcus neoformans depended upon the CD40-CD40L pathway (1, 8, 14, 25). In an animal model for abscess formation, inhibition of the CD28-B7-2 pathway prevented abscess formation following challenge with live B. fragilis plus adjuvant (30). However, the role of costimulation in the cell-mediated immune response to purified ZPS in vitro has not been investigated. In this study, we show that ZPSs induce APC activation and expression of HLA-DR. T-cell proliferation resulting from ZPS activation of APCs depends upon the costimulatory molecules B7-2 and CD40. In vitro, ZPS-mediated cellular immune response requires the B7-2 and CD40 molecules in addition to the retrograde transport of the HLA-DR molecule to the cell surface.
MATERIALS AND METHODS
Antigens.
S. pneumoniae type 1 capsular polysaccharide (Sp1) was obtained from the American Type Culture Collection (Manassas, Va.) and treated with 2 M NaOH for 1 h at 80°C to remove C substance (a contaminating cell wall polysaccharide). After purification by gel filtration chromatography with Sephracyl S-400 HR (Amersham Pharmacia Biotech, Piscataway, N.J.), the Sp1 was concentrated by ultrafiltration and lyophilization and stored at a concentration of 1 mg/ml in 0.15 M phosphate-buffered saline. The polysaccharide antigen was purified aseptically with sterile water. The instruments and devices used in the antigen purification process were deproteinated by treatment with sulfuric and chromic acid and depyrogenated by heat inactivation for 4 h at 240°C or by treatment with 1 to 2 M sodium hydroxide buffer. The antigens were analyzed for protein by the bicinchoninic acid method (Pierce, Rockford, Ill.) and by UV absorbance at 280 nm, for nucleic acid by UV absorbance at 260 nm, and for endotoxin (lipopolysaccharide [LPS]) by the limulus amebocyte lysate test (Charles River Endosafe, Charleston, S.C.). In the limulus test, the antigens were evaluated alone and in the presence of LPS; LPS alone served as a positive control. In addition, the polysaccharide antigen was subjected to high-resolution (500 MHz) proton nuclear magnetic resonance spectroscopy (16). Sp1 was found to contain no detectable protein and no detectable nucleic acid. Endotoxin was not detectable in the above preparations according to the limulus test with a sensitivity of <8 pg of LPS/mg of Sp1 (<0.4 pg of LPS/ml of culture medium containing 50 μg of Sp1) which corresponds to <0.028 endotoxin units [EU/mg of Sp1 (<0.0012 EU/ml of culture medium containing 50 μg of Sp1). Tetanus toxoid (TT) was a generous gift from Chiron Behring, Marburg, Germany, and was further purified by gel filtration chromatography with Sephracyl S-400 HR (Amersham Pharmacia Biotech) and ultrafiltration. It was found to contain no detectable nucleic acid and <8 pg of LPS/mg of TT containing 293 limit of flocculation (LF) units (0.054 pg of LPS/ml of culture medium containing 2 LF TT) which corresponds to <0.028 EU/mg of TT (<0.00019 EU/ml of culture medium containing 2 LF TT). S. aureus enterotoxin A (SEA) and LPS from Escherichia coli O111:B4 were purchased from Sigma Chemical (St. Louis, Mo.).
APCs and cell culture.
Mononuclear cells from healthy donors were isolated by centrifugation in Ficoll-Hypaque gradients (7). CD19+ B cells and CD14+ monocytes were negatively selected by immunomagnetic separation (Dynal, Inc., Lake Success, N.Y.). Immature DCs were generated from CD14+ monocytes by the addition of interleukin-4 (1,500 U/ml of culture medium) and granulocyte-macrophage colony-stimulating factor (500 U/ml of culture medium) every 48 h for 5 days in RPMI-1640 supplemented with l-glutamine, sodium pyruvate, penicillin-streptomycin, nonessential amino acids, 2-mercaptoethanol, and 10% fetal bovine serum (FBS; Life Technologies, Gaithersburg, Md.) (2, 29). The purity of the B-cell, monocyte, and DC populations was confirmed by flow cytometry (≥95%). For the surface expression studies, mononuclear cells, monocytes, B cells, and immature DCs were incubated for different time periods ranging from 1 to 48 h with Sp1, TT, SEA, or in medium alone. The concentrations of antigens used was shown to be optimal to induce 80% of maximal T-cell proliferation in preliminary experiments. In the case of treatment of APCs with bafilomycin A1 (BFA), APCs were treated for 30 min with BFA before antigens were added.
T-cell proliferation assays.
T cells were isolated by centrifugation in Ficoll-Hypaque gradients and purified with nylon wool and immunomagnetic beads (7). The purity of the CD3+ and CD4+CD8− cell populations was confirmed by flow cytometry (≥95%). T cells (5 × 104 per well) were incubated with γ-irradiated mononuclear cells (APCs; (105/well) in triplicate (at 37°C in 5% CO2) with antigens, antibodies, or medium alone for different intervals in a 96-well plate in RPMI-1640 supplemented with l-glutamine, sodium pyruvate, penicillin-streptomycin, nonessential amino acids, 2-mercaptoethanol, and 10% FBS (Life Technologies). APCs were irradiated with 2,500 rad. The number of APCs was shown to be optimal in preliminary experiments, as were the concentrations of antigens (80% of maximal T-cell proliferation) and antibodies (maximal blocking effect) and the incubation period (4 to 9 days). In proliferation assays in the presence of blocking antibodies, the following purified monoclonal antibodies (MAbs) without azide and endotoxin were used: B7-1 (also known as CD80; L307.4), B7-2 (CD86; IT2.2), CD40L (CD154; TRAP-1), and isotype controls from BD PharMingen (San Diego, Calif.). The antibodies were added 30 min before the antigens. In proliferation assays studying the influence of the antigen incubation time or of BFA treatment of APCs on T-cell proliferation, APCs were washed 10 times after a defined time interval before T cells were added. T-cell proliferation was quantitated by [3H]thymidine incorporation (1 μCi/well) for 6 h. Assays were performed at three or more independent time points. The results were expressed as counts per minute (cpm), stimulation index, or percentage of inhibition of the stimulation index of the positive control.
Flow cytometry.
After washing, cells were incubated with MAbs at the concentration recommended by the manufacturer at 4°C for 30 min in phosphate-buffered saline with 10% FBS. Subsequently, they were fixed—after gating for viable cells by forward and side scatter characteristics—and analyzed by FACScan (Becton Dickinson) using CELLQuest software (Becton Dickinson). The following purified fluorescein isothiocyanate- or phycoerythrin-conjugated MAbs directed to various cell surface antigens were used: MAbs against human CD14 (M5E2), CD19 (Leu-12), CD40 (5C3), B7-1 (also CD80; L307.4), B7-2 (also CD86; IT2.2), and HLA-DR (L243 and TÜ36), all of which plus the isotype control antibodies were obtained from BD PharMingen. The results were expressed as mean fluorescence intensity (MFI) of fluorescence-labeled surface cells in case the whole APC population of nonstimulated cells was 100% positive for the surface marker or as percentage of fluorescence-labeled APCs from the whole APC population in case the nontreated cells were not 100% positive for the surface marker.
Statistical analysis.
Results for the various groups in T-cell proliferation assays were compared by a Student's t test.
RESULTS
ZPSs increase surface expression of HLA-DR in APCs.
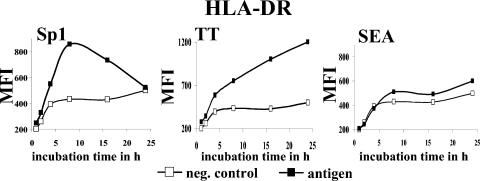
Fluorescent staining of the surface marker HLA-DR showed that Sp1 induced a twofold increase of HLA-DR surface expression on monocytes, which peaked at 8 h of incubation (Fig. 1). Induced HLA-DR expression was eliminated at 24 h of incubation. Incubation of B cells with Sp1 showed increased HLA-DR surface expression with similar kinetics (data not shown). HLA-DR surface expression was upregulated after 48 h of incubation of immature monocyte-derived DCs with Sp1 (Table 1). The increased surface expression of HLA-DR on ZPS-treated APCs correlated with specific mRNA transcription. Monocytes incubated with Sp1 for 8 h exhibited a 6.4-fold increase of HLA-DR mRNA transcription (data not shown). We compared the kinetics of ZPS-mediated HLA-DR surface expression with two control antigens. TT is a conventional protein antigen that exhibits a T-cell response after being processed in the endocytic pathway and presented on the cell surface by MHC-II molecules. Processing and presentation by APCs require cellular activation through transport of MHC-II-peptide complexes from lysosomes to the cell surface. In contrast, the superantigen SEA does not require processing for induction of an immune response, and therefore APCs do not need to be activated. SEA binds simultaneously to the α-chain of the MHC-II molecule and β-chain of the TCR. Incubation of APCs with TT (optimal dose) resulted in a nearly linear increase in HLA-DR over a 24-h period. Treating APCs with SEA resulted in no significant increase in HLA-DR (Fig. 1).
FIG. 1.
ZPS increases surface expression of HLA-DR. Monocytes (4 × 106 cells/ml in a 24-well plate) were incubated in culture medium with Sp1 (50 μg/ml), TT (2 LF/ml), or SEA (10 ng/ml) or in medium alone for different intervals ranging from 1 to 24 h. Surface expression of HLA-DR was then assessed by flow cytometry as described in Materials and Methods. The result shown is representative of three independent experiments with blood from different donors.
TABLE 1.
ZPS activates immature monocyte-derived DCsa
| Antigen | HLA-DR expression in:
|
|
|---|---|---|
| Untreated cells | Cells treated with Sp1 | |
| HLA-DR (MFI) | 393 | 623 |
| B7-1 (%) | 15.1 | 30.7 |
| B7-2 (%) | 32.8 | 68.0 |
Immature DCs were generated as described in Materials and Methods, Sp1 (50 μg/ml) was added to the culture medium, and the cultures were incubated for 48 h. Cells were harvested and prepared for flow cytometric analysis as described in Materials and Methods. This result is representative of three independent experiments with blood from different donors.
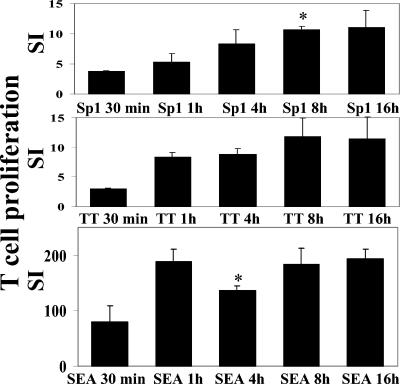
Duration of APC treatment with ZPS influences T-cell proliferation.
Kinetics studies examining the influence of the duration of incubation with antigen at 37°C on T-cell proliferation by Sp1 showed, as with control TT antigen, maximal proliferation after an incubation period of 8 h. In contrast, T-cell proliferation induced by SEA reached maximal levels after incubation for 30 min (Fig. 2).
FIG. 2.
ZPS-mediated T-cell proliferation depends on incubation time of APCs with ZPS. Freshly isolated and irradiated mononuclear cells were incubated for 30 min and 1, 4, 8, and 16 h at 37°C with Sp1 (50 μg/ml), TT (2 LF/ml), SEA (10 ng/ml), or medium alone before incubation with autologous CD3+ T cells. This T-cell proliferation assay is representative of three independent experiments with blood from different donors. SI, stimulation index. *, P < 0.05.
ZPS-mediated T-cell proliferation requires upregulation of HLA-DR surface expression.
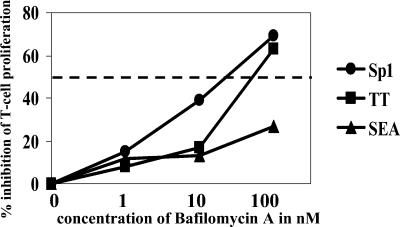
To address whether enhanced HLA-DR transcription and HLA-DR transport to the cell surface with subsequent surface expression are required for ZPS-mediated T-cell proliferation, APCs were treated with BFA. BFA prevents the acidification in endolysosomal compartments through specific inhibition of the vacuolar-type H+-ATPase. As a result, loading of antigens on MHC-II molecules and transport of these molecules to the cell surface are decreased. Gamma-irradiated mononuclear cells were treated with BFA at different concentrations, with or without antigens, or in medium alone. The density of HLA-DR molecules on the cell surface of APCs treated with the highest dose of BFA and stimulated with Sp1, TT, and SEA was comparable to the HLA-DR surface expression of nonstimulated APCs (Table 2). After extensive washing, T cells were added, and T-cell proliferation was evaluated. BFA reduced T-cell proliferation in a dose-dependent manner by over 50% when APCs were stimulated with Sp1 and TT but not with SEA (Fig. 3). Therefore, decreased T-cell proliferation was associated with decreased HLA-DR translocation to the cell surface.
TABLE 2.
BFA treatment reduces HLA-DR surface expression of stimulated APCsa
| MFI of HLA-DR-positive APCs treated with:
| ||||||
|---|---|---|---|---|---|---|
| Medium | Sp1 | Sp1+BFA | TT | TT+BFA | SEA | SEA+BFA |
| 236 | 924 | 237 | 970 | 355 | 570 | 200 |
γ-Irradiated mononuclear cells were treated with Sp1 (50 μg/ml), TT (2Lf/ml), and SEA (10 ng/ml) for 8 h at 37°C in the absence or presence of 100 nM BFA. Surface expression of HLA-DR was then assessed by flow cytometry as described in Materials and Methods. This result is representative of three independent experiments.
FIG. 3.
BFA inhibits ZPS-mediated T-cell proliferation. T-cell proliferation assays with CD3+ T cells as responders were performed with APCs treated for 8 h with Sp1 (50 μg/ml), TT (2 LF/ml), and SEA (10 ng/ml), or in medium alone in the presence of BFA at increasing concentrations or the absence of BFA, as described in Materials and Methods. This T-cell proliferation assay is representative of three independent experiments.
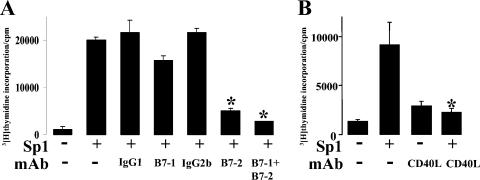
ZPS-induced T-cell proliferation requires costimulation by B7-2/CD28 and CD40/CD40L.
In vitro, T-cell proliferation assays were performed in the presence of blocking antibodies to B7-1, B7-2, and CD40L and appropriate isotype-matched control antibodies. T-cell proliferation mediated by Sp1 was significantly inhibited by specific antibodies to B7-2 compared with Sp1-mediated T-cell proliferation in the presence of the isotype-matched control. A specific antibody to B7-1 did not influence the Sp1-mediated T-cell proliferation in vitro (Fig. 4, panel A). The addition of an antibody to CD40L decreased T-cell proliferation significantly compared with the T-cell response in the absence of the blocking antibody (Fig. 4, panel B).
FIG. 4.
Antibodies to B7-2 and CD40L block ZPS-induced T-cell proliferation. In these T-cell proliferation assays in the absence or presence of blocking antibodies to B7-1 and B7-2 (A) and to CD40L (B) (5 μg/ml) and their isotype controls, mononuclear cells served as APCs and autologous CD3+ cells as responders. Sp1 was added at a concentration of 50 μg/ml. This T-cell proliferation assay is representative of three independent experiments with blood from different donors. *, P < 0.05.
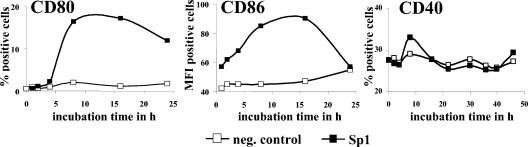
ZPS increases surface expression of B7-1 and B7-2, but not of CD40.
Fluorescent staining of APCs for the costimulation surface markers B7-1, B7-2, and CD40 showed that treatment with Sp1 increased the expression of B7-1 and B7-2 on monocyte-derived DCs (Table 1), on monocytes (Fig. 5), and on B cells (data not shown). Upregulation was maximally increased at 8 to 16 h of incubation of monocytes with Sp1. After a 24-h incubation with ZPS, B7-2 surface expression was eliminated, while B7-1 expression was still increased at a low level. The MFI of B7-1 of Sp1-treated monocytes was maximally 21 (data not shown). CD40 surface expression was induced neither on B cells (Fig. 5) nor on monocytes (data not shown) when incubated with Sp1 alone or with Sp1 in the presence of T cells. About 30% of the nontreated or Sp1-treated B cells expressed CD40 with a maximum MFI of 30.
FIG. 5.
ZPS increases surface expression of B7-1 and B7-2, but not of CD40. For the determination of surface expression of B7-1, B7-2, and CD40, monocytes (4 × 106 cells/ml in a 24-well plate) were incubated in culture medium with Sp1 (50 μg/ml) or in medium alone for different intervals ranging from 1 to 24 h. To determine the surface expression of CD40 on CD19+ B cells, mononuclear cells were incubated with Sp1 (50 μg/ml) or in medium alone for different time intervals ranging from 1 to 48 h. Surface expression of B7-1, B7-2, or CD40 was then assessed by flow cytometry as described in Materials and Methods. This analysis is representative of three independent experiments.
DISCUSSION
Previous reports have shown that ZPSs can stimulate CD4+-T-cell activation in vitro and that T-cell activation by these polysaccharides, which have both positive and negative charges, is specific to the charge motif of the ZPS tested and is not due to contaminating peptides (4, 31). We showed that T-cell activation by ZPS requires direct interaction of CD4+ T cells and APCs expressing HLA-DR (15). Recent studies demonstrate depolymerization of ZPS in endosomes and localization of ZPS in the MHC-II endocytic pathway (5, 15). These observations imply presentation to T cells through MHC-II and a fundamental shift in the MHC-II presentation paradigm. To investigate the detailed mechanisms of APC activation that result in APC and T-cell engagement with immune synapse formation and subsequent T-cell stimulation, we hypothesized (i) that activation of APCs resulting in transcription, translation, retrograde transport, and upregulation of surface expression of HLA-DR is required for ZPS-mediated T-cell stimulation in vitro and (ii) that T-cell activation relies on costimulatory interactions between APCs and T cells via the B7-CD28 pathway and on specific interaction between CD40 and CD40L. We have shown previously that monocytes, DCs, and B cells were all able to serve as APCs for ZPS-mediated T-cell activation. Electron and fluorescence microscopy studies have revealed colocalization of ZPS with HLA-DR in compartments of the endocytic pathway (5, 15). Kinetics studies investigating the influence of the duration of APC treatment with ZPS show that incubation times comparable to those for TT (conventional antigen) are required for the induction of T-cell activation, thus supporting the implication that time for intracellular processing and transport of the MHC-II and ZPS complex to the cell surface might be a prerequisite for T-cell activation. We demonstrate that all professional APCs become activated by ZPS, as is evident through the upregulation of mRNA transcription and surface expression of HLA-DR, which indicate transport of newly synthesized HLA-DR molecules to the cell surface. Kinetics studies of monocytes and B cells demonstrated that they are maximally activated after 8 h. Expression of HLA-DR on the APC following ZPS incubation was eliminated at 24 h. Previously, antibody-mediated blockade of HLA-DR was shown to inhibit T-cell proliferation (15). Here we demonstrate that, associated with a BFA-induced quantitative decrease in expression of HLA-DR molecules on the APC, there is a decrease in ZPS-mediated T-cell proliferation. These results might be explained by a failure of APC activation and subsequent decreased transport of HLA-DR to the cell surface. However, if ZPS stimulated T cells via a superantigen-like mechanism, the quantity of HLA-DR on the cell surface should be sufficient (as demonstrated with SEA). The inhibition of ZPS-induced T-cell proliferation by BFA may also reflect a decreased transport of HLA-DR/ZPS complex to the cell surface because an acidic pH in lysosomes and exchange of self-peptide with the polysaccharide are also inhibited by BFA.
Recently published studies have demonstrated differential requirements for B7-1 and B7-2 in mediating T-cell-dependent host responses to a bacterial polysaccharide linked to an immunogenic protein or to a lipopolysaccharide. The antibody response to a group C meningococcal capsular conjugate vaccine is inhibited by antibodies to B7-2 but not to B7-1 (20). In another report, the immunoglobulin G antibody response to a type III group B Streptococcus glycoconjugate vaccine depended on either B7-1 or B7-2 (11). B7-1 is crucial for LPS-induced T-cell proliferation (21). The finding that specific antibody to B7-2 and not to B7-1 prevented T-cell proliferation stimulated by ZPS is intriguing. The result indicates that B7-1 is not required for ZPS-induced T-cell proliferation. Treatment of APCs induced at 8 to 16 h a maximum increase of B7-1 surface expression at a low level on about 20% of monocytes. The blocking antibody to B7-1 might not exhibit an effect on T-cell proliferation because surface expression of B7-1 is not high enough and of no biological relevance. Surface expression of B7-2 was maximally increased at 8 to 16 h of treatment on all APCs. After a 24-h incubation with ZPS, B7-2 surface expression was eliminated. In an earlier report, we demonstrated that abscess formation induced by B. fragilis was prevented by a specific antibody to B7-2 and not to B7-1. Furthermore, blockade of the B7-CD28 axis by administration of the CTLA4Ig fusion protein at the time of challenge with B. fragilis prevented abscess formation, whereas administration delayed by 24 or 48 h resulted in a loss of protective efficacy (30). These results corroborate our finding that activation of APCs, including the induction of HLA-DR and costimulatory molecules, is initiated shortly following treatment with ZPS.
Thymus-dependent antigens (e.g., proteins) require participation of CD4+ T cells to generate an immune response. The immune response to thymus-dependent antigens critically depends on the interaction between CD40L, a molecule that is transiently expressed on the surface of activated CD4+ T lymphocytes, and CD40 expressed on surface APCs, predominantly on B cells (23). As for ZPS, neutralization of CD40L resulted in an inhibition of ZPS-mediated T-cell proliferation. CD40 surface expression was not induced on B cells or monocytes by stimulation with ZPS. About 30% of nontreated and ZPS-treated B cells expressed CD40 on their surface. The results demonstrate that upregulation of CD40 surface expression is not a prerequisite for ZPS-induced T-cell proliferation. However, binding of CD40-CD40L is required to sustain the bidirectional signals necessary for the initiation of T-cell proliferation mediated by ZPS.
T cells are normally activated through direct contact with an APC. When T cells and APCs come in contact, they must overcome the electrostatic repulsion caused by their negative surface electric charges (24, 26). The attractive force necessary to overcome this barrier is largely mediated by the action of adhesion molecules. ZPSs rapidly bind to the surface of APCs (15). The high density of charge on ZPS molecules facilitates electrostatic interactions between APCs and T cells and may help to bypass the electrostatic repulsion, resulting in the uncommon kinetics of surface expression of HLA-DR and costimulatory molecules. During the initiation of T-cell activation, a number of key receptor-ligand complexes accumulate in discrete geometric patterns at the APC-T-cell interface (6, 9, 19, 22, 36). This complex has been referred to as the “supramolecular activation cluster” (22) or the “immunological synapse” (9) and has been suggested to potentiate TCR signaling (6). Our data demonstrate that ZPSs promote CD4+-T-cell proliferation via the first signal, provided by retrogradely transported HLA-DR, and the second signal, provided by the costimulatory molecules B7-2 and CD40, on activated APCs in a unique pattern.
Acknowledgments
We thank Helena Frank and April Arrasate for their excellent technical assistance, Thomas Berger and Paula Bryant for helpful technical advice, and Jaylyn Olivo for editorial services.
This work was supported in part by the Deutsche Forschungsgemeinschaft (KA 1398/2 to W.M.K.), by the Bundesministerium für Bildung und Forschung (01KI9953 to W.M.K.), by the Maria-Pesch Foundation (364552 to W.M.K.), and by the National Institute of Allergy and Infectious Diseases (AI 34073 and AI 39576 to D.L.K.).
Editor: J. N. Weiser
REFERENCES
- 1.Almeida, G. M., R. M. Andrade, and C. A. Bento. 2001. The capsular polysaccharides of Cryptococcus neoformans activate normal CD4(+) T cells in a dominant Th2 pattern. J. Immunol. 167:5845-5851. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 3.Bluestone, J. A. 1995. New perspectives of CD28-B7-mediated T cell costimulation. Immunity 2:555-559. [DOI] [PubMed] [Google Scholar]
- 4.Brubaker, J. O., Q. Li, A. O. Tzianabos, D. L. Kasper, and R. W. Finberg. 1999. Mitogenic activity of purified capsular polysaccharide A from Bacteroides fragilis: differential stimulatory effect on mouse and rat lymphocytes in vitro. J. Immunol. 162:2235-2242. [PubMed] [Google Scholar]
- 5.Cobb, B. A., Q. Wang, A. O. Tzianabos, and D. L. Kasper. 2004. Polysaccharide processing and presentation by the MHCII pathway. Cell 117:677-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dustin, M. L., and A. S. Shaw. 1999. Costimulation: building an immunological synapse. Science 283:649-650. [DOI] [PubMed] [Google Scholar]
- 7.Finberg, R. W., W. White, and W. A. Nicholson. 1992. Decay-accelerating factor expression on either effector or target cells inhibits cytotoxicity by human natural killer cells. J. Immunol. 149:2055-2060. [PubMed] [Google Scholar]
- 8.Garcia de Vinuesa, C., I. C. MacLennan, M. Holman, and G. G. Klaus. 1999. Anti-CD40 antibody enhances responses to polysaccharide without mimicking T cell help. Eur. J. Immunol. 29:3216-3224. [DOI] [PubMed] [Google Scholar]
- 9.Grakoui, A., S. K. Bromley, C. Sumen, M. M. Davis, A. S. Shaw, P. M. Allen, and M. L. Dustin. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science 285:221-227. [DOI] [PubMed] [Google Scholar]
- 10.Grewal, I. S., and R. A. Flavell. 1998. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16:111-135. [DOI] [PubMed] [Google Scholar]
- 11.Guttormsen, H. K., A. H. Sharpe, A. K. Chandraker, A. K. Brigtsen, M. H. Sayegh, and D. L. Kasper. 1999. Cognate stimulatory B-cell-T-cell interactions are critical for T-cell help recruited by glycoconjugate vaccines. Infect. Immun. 67:6375-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harding, C. V., R. W. Roof, P. M. Allen, and E. R. Unanue. 1991. Effects of pH and polysaccharides on peptide binding to class II major histocompatibility complex molecules. Proc. Natl. Acad. Sci. USA 88:2740-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishioka, G. Y., A. G. Lamont, D. Thomson, N. Bulbow, F. C. A. Gaeta, A. Sette, and H. M. Grey. 1992. MHC Interaction and T cell recognition of carbohydrates and glycopeptides. J. Immunol. 148:2446-2451. [PubMed] [Google Scholar]
- 14.Jeurissen, A., M. Wuyts, A. Kasran, S. Ramdien-Murli, L. Boon, J. L. Ceuppens, and X. Bossuyt. 2002. Essential role for CD40 ligand interactions in T lymphocyte-mediated modulation of the murine immune response to pneumococcal capsular polysaccharides. J. Immunol. 168:2773-2781. [DOI] [PubMed] [Google Scholar]
- 15.Kalka-Moll, W. M., A. O. Tzianabos, P. W. Bryant, M. Niemeyer, H. L. Ploegh, and D. L. Kasper. 2002. Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions. J. Immunol. 169:6149-6153. [DOI] [PubMed] [Google Scholar]
- 16.Kalka-Moll, W. M., A. O. Tzianabos, Y. Wang, V. J. Carey, R. W. Finberg, A. B. Onderdonk, and D. L. Kasper. 2000. Effect of molecular size on the ability of zwitterionic polysaccharides to stimulate cellular immunity. J. Immunol. 164:719-724. [DOI] [PubMed] [Google Scholar]
- 17.Kalka-Moll, W. M., Y. Wang, L. E. Comstock, S. E. Gonzalez, A. O. Tzianabos, and D. L. Kasper. 2001. Immunochemical and biological characterization of three capsular polysaccharides from a single Bacteroides fragilis strain. Infect. Immun. 69:2339-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kodaira, Y., S. K. Nair, L. E. Wrenshall, E. Gilboa, and J. L. Platt. 2000. Phenotypic and functional maturation of dendritic cells mediated by heparan sulfate. J. Immunol. 165:1599-1604. [DOI] [PubMed] [Google Scholar]
- 19.Krummel, M. F., M. D. Sjaastad, C. Wulfing, and M. M. Davis. 2000. Differential clustering of CD4 and CD3zeta during T cell recognition. Science 289:1349-1352. [DOI] [PubMed] [Google Scholar]
- 20.Mackinnon, F. G., Y. Ho, M. S. Blake, F. Michon, A. Chandraker, M. H. Sayegh, and L. M. Wetzler. 1999. The role of B/T costimulatory signals in the immunopotentiating activity of neisserial porin. J. Infect. Dis. 180:755-761. [DOI] [PubMed] [Google Scholar]
- 21.Mattern, T., H. D. Flad, L. Brade, E. T. Rietschel, and A. J. Ulmer. 1998. Stimulation of human T lymphocytes by LPS is MHC unrestricted, but strongly dependent on B7 interactions. J. Immunol. 160:3412-3418. [PubMed] [Google Scholar]
- 22.Monks, C. R., B. A. Freiberg, H. Kupfer, N. Sciaky, and A. Kupfer. 1998. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395:82-86. [DOI] [PubMed] [Google Scholar]
- 23.Noelle, R. J., M. Roy, D. M. Shepherd, I. Stamenkovic, J. A. Ledbetter, and A. Aruffo. 1992. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc. Natl. Acad. Sci. USA 89:6550-6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw, A. S., and M. L. Dustin. 1997. Making the T cell receptor go the distance: a topological view of T cell activation. Immunity 6:361-369. [DOI] [PubMed] [Google Scholar]
- 25.Specht, C., R. Junker, A. Kruger, A. Rademaekers, H. Redlich, and E. Kolsch. 1999. Involvement of CD28 cosignalling in the T cell-mediated suppression of the IgG antibody response against the TI-2 antigen alpha(1→3) dextran. Immunobiology 201:49-63. [DOI] [PubMed] [Google Scholar]
- 26.Springer, T. A., M. L. Dustin, T. K. Kishimoto, and S. D. Marlin. 1987. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu. Rev. Immunol. 5:223-252. [DOI] [PubMed] [Google Scholar]
- 27.Termeer, C., F. Benedix, J. Sleeman, C. Fieber, U. Voith, T. Ahrens, K. Miyake, M. Freudenberg, C. Galanos, and J. C. Simon. 2002. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J. Exp Med. 195:99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson, C. B. 1995. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation. Cell 81:979-982. [DOI] [PubMed] [Google Scholar]
- 29.Thurner, B., C. Roder, D. Dieckmann, M. Heuer, M. Kruse, A. Glaser, P. Keikavoussi, E. Kampgen, A. Bender, and G. Schuler. 1999. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J. Immunol. Methods 223:1-15. [DOI] [PubMed] [Google Scholar]
- 30.Tzianabos, A. O., A. Chandraker, W. Kalka-Moll, F. Stingele, V. M. Dong, R. W. Finberg, R. Peach, and M. H. Sayegh. 2000. Bacterial pathogens induce abscess formation by CD4+ T-cell activation via the CD28-B7-2 costimulatory pathway. Infect. Immun. 68:6650-6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzianabos, A. O., R. W. Finberg, Y. Wang, M. Chan, A. B. Onderdonk, H. J. Jennings, and D. L. Kasper. 2000. T cells activated by zwitterionic molecules prevent abscesses induced by pathogenic bacteria. J. Biol. Chem. 275:6733-6740. [DOI] [PubMed] [Google Scholar]
- 32.Tzianabos, A. O., J. Y. Wang, and J. C. Lee. 2001. Structural rationale for the modulation of abscess formation by Staphylococcus aureus capsular polysaccharides. Proc. Natl. Acad. Sci. USA 98:9365-9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, J. Y., and M. H. Roehrl. 2002. Glycosaminoglycans are a potential cause of rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 99:14362-14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, Y., W. M. Kalka-Moll, M. H. Roehrl, and D. L. Kasper. 2000. Structural basis of the abscess-modulating polysaccharide A2 from Bacteroides fragilis. Proc. Natl. Acad. Sci. USA 97:13478-13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitmire, J. K., R. A. Flavell, I. S. Grewal, C. P. Larsen, T. C. Pearson, and R. Ahmed. 1999. CD40-CD40 ligand costimulation is required for generating antiviral CD4 T cell responses but is dispensable for CD8 T cell responses. J. Immunol. 163:3194-3201. [PubMed] [Google Scholar]
- 36.Wulfing, C., A. Bauch, G. R. Crabtree, and M. M. Davis. 2000. The vav exchange factor is an essential regulator in actin-dependent receptor translocation to the lymphocyte-antigen-presenting cell interface. Proc. Natl. Acad. Sci. USA 97:10150-10155. [DOI] [PMC free article] [PubMed] [Google Scholar]