Abstract
We previously reported that cholera toxin (CT) was required as a mucosal adjuvant for the induction of peptide-specific cytotoxic T lymphocytes (CTL) following intranasal immunization with CTL epitope peptides (A. Porgador et al., J. Immunol. 158:834–841, 1997). The present study was performed to identify the site and the antigen-presenting cell (APC) population responsible for the presentation of intranasally administered CTL epitope peptide immunogens and to determine whether CT directly affects antigen presentation by these APCs. For these experiments, C57BL/6 mice were intranasally immunized with the ovalbumin H-2Kb-restricted CTL epitope SIINFEKL with or without CT. Cells were then isolated from the cervical lymph nodes (CLN) and the nasal mucosa-associated lymphoid tissue (NALT) and tested for the ability to stimulate the B3Z T-cell hybridoma, which recognizes SIINFEKL in association with H-2Kb. Dendritic cell (DC)-enriched CLN cells from mice immunized with peptide and CT or peptide only could stimulate B3Z cells, while DC-depleted CLN cells from either group were unable to stimulate B3Z cells. NALT cells of mice immunized with peptide and CT, but not with peptide alone, were able to efficiently stimulate B3Z hybridomas. Depletion of N418-positive DC from these NALT cells resulted in significant reduction of B3Z activation. Our results indicate that DC are the APC responsible for the presentation of CTL epitope peptides following intranasal immunization and that CT augments the ability of dendritic cells in the NALT, but not in the draining CLN, to present CLT epitope peptides. This finding suggests that CT acts locally as a mucosal adjuvant and that NALT DC are the predominant APC involved with the induction of immunity after intranasal immunization with peptide immunogens and CT.
Mucosal immunization may lead to the induction of a wide range of antigen-specific immune responses. Immunization via the nasal or gastric route with soluble protein antigens in the absence of mucosal adjuvants may induce antigen-specific immunological tolerance (5, 8, 16, 32). Conversely, immunization via the nasal, gastric, rectal, and vaginal routes with antigen coadministered with cholera toxin (CT) produced by Vibrio cholerae, pertussis toxin (PT) produced by Bordetella pertussis, or labile toxin (LT) produced by enterotoxigenic Escherichia coli commonly induces both systemic and mucosal immune responses, including humoral and cell-mediated immunity (2, 6, 12, 34, 43, 44). These bacterial toxins exhibit mucosal adjuvant activity and all are enzymatically active ADP-ribosylating toxins. These toxins are composed of an A subunit and a pentameric B subunit. The B subunit is responsible for binding to cell surface gangliosides, while the A subunit contains the enzymatically active portion of the toxin. Until recently, mucosal adjuvanticity has been related to the toxic activity of these toxins since coadministration of recombinant CT-B alone (lacking toxic activity) is not able to efficiently enhance the induction of immunity (22, 24); in fact, in some cases, when conjugated to antigens, recombinant CT-B can enhance the development of oral tolerance (1, 49). More recently, however, it has become clear that the requirement of ADP-ribosyltransferase activity for mucosal adjuvanticity by these toxins is not absolute. Thus, it has been shown that CT and LT molecules with isolated mutations in the A subunit resulting in a loss of substantial toxic activity nevertheless maintain some degree of mucosal adjuvanticity with respect to immunoglobulin A (IgA) production and the prevention of oral tolerance (9, 56). It is not clear whether these mutant toxins can augment cytotoxic T-lymphocyte (CTL) responses to peptides administered intranasally (i.n.).
Also unclear are the immunological mechanisms by which the intact (or mutant) toxins affect the induction of IgA responses, the abrogation of oral tolerance, or the induction of CTL. In this regard, CT has been the most extensively studied mucosal adjuvant. CT has been reported to increase the production of proinflammatory cytokines interleukin-1 (IL-1) and IL-6 by mucosal epithelial cells (4) and enhance macrophage production of IL-1 (3). CT also increases the amount of mucosally administered antigen that crosses the mucosal surface and enters the systemic compartment (25). Recent studies indicated that CT increased the expression of the B7-2 costimulatory molecule and the stimulatory capacity of mucosal antigen-presenting cells (APC) (6). In vivo, CT can drive T-cell differentiation into the Th1 or Th2 phenotype, depending on the route of administration and possibly the dose. Oral coadministration of CT and antigens, such as tetanus toxoid, appears to favor Th2-development in the gut, while systemic administration has been shown to skew responses toward the Th1 phenotype (55). While CT is presumed to affect the production of transforming growth factor β by cells in the Peyer’s patch, since CT enhances IgA responses and transforming growth factor β is the only known direct switch factor for IgA B cells, this has never been demonstrated directly. Finally, CT has been shown to have direct effects on B lymphocytes. It inhibits B-cell proliferation in response to anti-IgM and lipopolysaccharide (23, 53) and promotes B-cell differentiation in vitro (26). Thus, it is likely that the adjuvant effects of CT, as well as LT, PT, and the mutant toxins, depend on broad and complex activities on both immune and nonimmune cells.
We have reported that i.n. immunization with a CTL peptide epitope and the mucosal adjuvant CT induced specific CTL and protection against subcutaneous challenge of tumor cells expressing the epitope (34). In this model, however, neither the cells responsible for presenting the CTL epitope peptides nor the mechanism by which CT affects CTL induction is known. The present study addresses these issues. Naive T cells circulate between blood and lymphoid organs and do not penetrate into nonlymphoid tissues. Consequently, immune induction of naive T cells occurs in lymphoid tissues draining the site of antigen exposure. The draining lymphoid tissues for antigens applied i.n. include the nasal-mucosa-associated lymphoid tissue (NALT) as well as the regional lymph nodes (LN) (cervical LN [CLN] and pulmonary LN). A wealth of evidence suggests that the host-derived cells which specialize in presenting antigen to naive T cells, called professional APC, are dendritic cells (DC) of hemopoietic origin (reviewed in references 20, 47, 48, and 39). Consistent with this notion, several studies have documented the exceptional ability of DC to stimulate naive CD4+ and CD8+ T cells in vitro and in vivo (17, 28, 47). In addition, DC have been shown to be recruited into the respiratory tract mucosa during the acute cellular response to local challenge with bacterial (30), viral, and soluble protein antigens (29). Thus, we studied whether DCs are responsible for presenting CTL epitope peptides in the NALT and whether CT affects antigen presentation by these DC. Our results indicate that DC are the APC responsible for the presentation of CTL epitope peptides following i.n. immunization and that CT augments the ability of DC in the NALT, but not in the draining CLN, to present CLT epitope peptides.
MATERIALS AND METHODS
Animals and cell lines.
Female C57BL/6 mice were obtained at 6 to 10 weeks of age. All procedures with animals were carried out in accordance with institutionally approved protocols. B3Z cells (19) were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 2 mM l-glutamine.
Peptides and immunizations.
SIINFEKL is chicken ovalbumin peptide 257-264. Peptides were synthesized by the NIAID Peptide Synthesis Facility by using 9-fluorenylmethoxycarbonyl chemistry and purified to greater than 95% prior to use. For i.n. immunizations, mice were lightly anesthetized with isoflurane and then immunized with 50 μg of peptide with or without 1 μg of mucosal adjuvant CT in a total volume of 16 μl (8 μl/nostril) of phosphate-buffered saline (PBS; Sigma, St. Louis, Mo.).
Collagenase treatment of LN and DC enrichment.
Collagenase-digested LN were prepared as previously described (51). Briefly, LN were dissected and digested with collagenase D (400 Mandl units/ml; Boehringer Mannheim, Indianapolis, Ind.) and DNase I (15 μg/ml; Boehringer Mannheim) for 30 min at 37°C in RPMI 1640–2% FCS. For DC enrichment, released cells (5 × 106 to 10 × 106) were layered over a metrizamide gradient column (Accurate, Westbury, N.Y.; 14.5 g of metrizamide added to 100 ml of RPMI 1640–10% FCS) and centrifuged, and low-density (DC-enriched) and high-density (DC-depleted) cells were collected (27).
Isolation of NALT.
NALT was isolated from mouse nasal tissue as previously described (54), with minor modifications. Briefly, mice were euthanized by cervical dislocation, the skin was stripped from the skull, and incisions were made with scissors through the nasal septum and lateral nasal and facial tissue. Following removal of the remaining tissue of the nasal septum with forceps, the NALT was visualized on floor of the nasal cavity overlying the soft palate. The NALT was removed by gently scraping with a sharp blade and digested with collagenase-DNase, and DC-enriched and -depleted populations were obtained as described above for CLN.
Activation of B3Z T-cell hybridoma.
B3Z is a T-cell hybridoma which recognizes the SIINFEKL peptide in the context of H-2Kb (19) and expresses β-galactosidase (β-Gal) upon activation. Collagenase-digested LN cells from immunized mice were incubated overnight with 5 × 104 or 5 × 105 B3Z cells/well in U-bottom 96-well plates (in triplicates). For β-Gal staining of activated B3Z cells, cells in 96-well plates were fixed in PBS–2% formaldehyde–0.2% glutaraldehyde at 4°C for 7 min, washed in PBS, and incubated with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) substrate (1 mg of X-Gal per ml in PBS–5 mM potassium ferricyanide–5 mM potassium ferrocyanide–2 mM magnesium chloride) at 37°C until positive (blue) cells appeared. Cells from each well were loaded onto a Neubauer counting chamber and were viewed in bright field on a Nikon Labophot microscope. The percentage of activated B3Z cells was calculated by dividing the number of blue cells by the total number of B3Z cells (larger and brighter than residual LN cells) in the field of view. In all cases, a minimum of 500 to 1,000 cells were counted. When SIINFEKL is added directly to cultures of B3Z cells, all cells turn blue because the B3Z cells themselves express major histocompatibility complex type II and present SIINFEKL to neighboring cells.
Flow cytometry.
For CD11c staining, cells were incubated with an antibody that blocks immunoglobulin binding to FcγRII and -III receptors and then with N418 supernatant (anti-CD11c; ATCC HB-224) for 30 min at 4°C, washed with PBS–5% FCS–0.1% sodium azide, and incubated with fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 anti-hamster IgG (Caltag, Burlingame, Calif.) for 30 min at 4°C. The cells were then washed and resuspended in the same medium plus propidium iodide to allow exclusion of dead cells during analysis.
For intracellular IL-2 staining, cells were stained with biotinylated anti-Vα2 (of the transgenic T-cell receptor [TCR]) followed by streptavidin-CyChrome and FITC-anti-CD8α. After being washed with PBS, cells were fixed with PBS containing 4% paraformaldehyde at room temperature for 5 min and then washed with PBS–1% bovine serum albumin (BSA) once. After two additional washes with PBS–0.1% saponin–0.1% BSA–0.01 M HEPES, phycoerythrin-anti-IL-2 was added for 30 min at 4°C; then cells were washed twice with PBS–0.1% saponin–0.1% BSA–0.01 M HEPES. Transgenic T cells (CD8α+ Vα2+) were analyzed for IL-2 staining. Stained cells were analyzed with a FACScan flow cytometer (Becton Dickinson, Mansfield, Mass.).
Depletion of N418-positive cells.
Cells were incubated for 30 min at 4°C with N418 supernatant, washed, and incubated with biotin-conjugated goat F(ab′)2 anti-hamster antibody. Cells were then washed, and antibody-coated cells were removed by M-280 streptavidin-coupled magnetic beads (Dynabeads; Dynal, Oslo, Norway). The beads and cells were mixed at a ratio of 10:1, incubated for 30 min with continuous slow rotation at 4°C, and diluted. Beads and bead-bound cells were removed with a Dynal magnet. Mock-depleted cells were exposed to the same procedure except for the first incubation with monoclonal antibody (MAb) N418.
RESULTS
DC in regional LN present CTL epitope peptides after i.n. immunization.
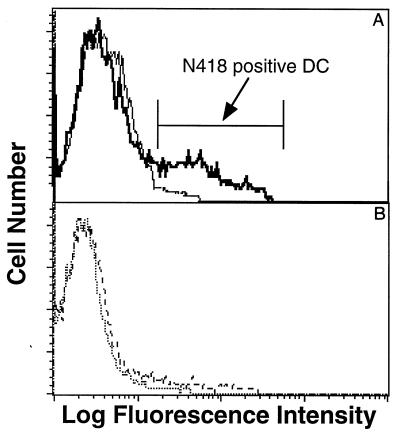
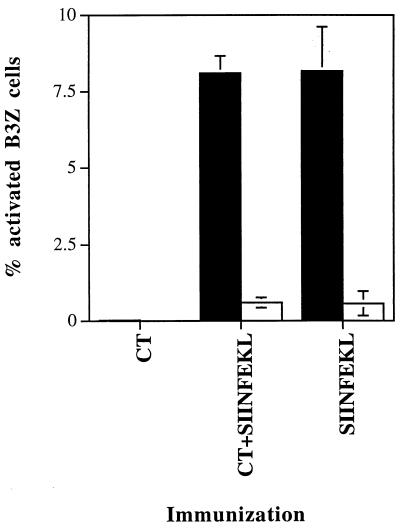
C57BL/6 mice were immunized once i.n. with SIINFEKL peptide either alone or mixed with the mucosal adjuvant CT. CLN cells were harvested 4 h after immunization and separated into DC-enriched and DC-depleted LN cells by centrifugation over metrizamide as described in Materials and Methods. The low-density DC-enriched fraction contained 15 to 30% CD11c+ cells as assayed by staining with MAb N418 (Fig. 1A), while the high-density DC-depleted fraction was practically negative for N418 staining (Fig. 1B). DC-enriched CLN cells either from SIINFEKL- or SIINFEKL-plus-CT-immunized mice activated the B3Z-T cell hybridoma specific for the SIINFEKL-Kb complex (Fig. 2). In contrast, B3Z cells were not activated by the high-density DC-depleted CLN cells or by low-density DC-enriched cells from mice immunized with CT alone (Fig. 2). Similar results were obtained when cells were isolated 24 h after immunization (data not shown). In addition, the non-N418-positive cells of the high-density fractions were made up primarily of B cells, which were also found in similar proportions in the high-density fraction. Therefore, it is unlikely that B cells are responsible for the differential stimulation seen. This conclusion is supported by the fact that DC are known to be the cells responsible for transport of antigens from other epithelial sites (47, 48).
FIG. 1.
N418-positive DC in low- and high-density cells after metrizamide gradient column purification of LN cells. CLN cells from five mice immunized i.n. with SIINFEKL plus CT were pooled and digested as described in Materials and Methods. Suspended cells were layered over a metrizamide gradient column and centrifuged. Low-density (A, thick line) and high density (B, dashed line) cells were incubated with anti-FcγRII/III (clone 2.4G2) to block FcR binding and stained with anti-CD11c MAb N418 followed by FITC-labeled second antibody (goat anti-hamster, mouse and rat adsorbed). Second antibody controls for low-density (A, thin line) and high-density (B, dotted line) cells are also shown. A representative experiment is shown.
FIG. 2.
Antigen presentation by DC-enriched and DC-depleted CLN cells from mice (groups of four) immunized i.n. with CT, SIINFEKL plus CT, or SIINFEKL. CLN were harvested 4 h after immunization, pooled, and digested as described in Materials and Methods. Suspended cells were layered over a metrizamide gradient column and centrifuged. Low-density DC-enriched cells (■) and high-density DC-depleted cells (□) were incubated with B3Z T-hybridoma cells overnight (four APC per B3Z cell). Activation of B3Z cells was determined by β-Gal expression detected by incubation of cells with X-Gal substrate. Activated (blue) B3Z cells were counted in a Neubauer counting chamber. Similar results were obtained in another experiment. The results are expressed as mean percentages of the total number of B3Z cells present ± standard deviations.
CLN-derived DC from mice immunized with SIINFEKL or with SIINFEKL plus CT manifest in vitro similar abilities to present SIINFEKL-Kb.
We previously determined that i.n. immunization with CTL epitope peptide alone did not induce CTL, while i.n. immunization with peptide and CT induced potent CTL activity. Upon first encountering antigen, DC in the regional LN activate naive T cells specific for the antigen. Yet, the results for presentation of SIINFEKL by DC-enriched cells from CLN were comparable between mice immunized with peptide alone or peptide and CT (Fig. 2 to 4). Also, the percentages of CLN DC were similar in the peptide, peptide-plus-CT, and CT-alone groups (data not shown). Thus, it appeared that CT had little effect on the ability of DC to process i.n. peptides or to present antigens in vitro to the B3Z T-cell line. To determine whether this was also true of DC antigen presentation in vivo, we took advantage of the fact that Vβ5.1- and Vβ5.2-expressing CD8+ T cells are dominant in the response against SIINFEKL-Kb (10, 37). Thus, we immunized mice with SIINFEKL, SIINFEKL plus CT, or CT only and similarly boosted them 10 days later. One day after the boost, CLN were harvested and the expression of the activation marker CD69 by Vβ5+ CD8+ T cells was determined by three-color flow cytometry. The percentage of CLN Vβ5+ CD8+ CD69+ T cells from SIINFEKL-immunized mice (average of 19%) was similar to the percentage from naive or CT-immunized mice but twofold less than that for the SIINFEKL-plus-CT-immunized mice (36.4% [data not shown]). Thus, we could determine a measurable effect of CT in mice in that the expression of CD69, a well-established activation marker was enhanced on antigen reactive Vβ5+ CD8+ cells in the presence of CT. Therefore, there was a discrepancy between in vivo and in vitro T-cell activation by DC from SIINFEKL- or SIINFEKL-plus-CT-immunized mice.
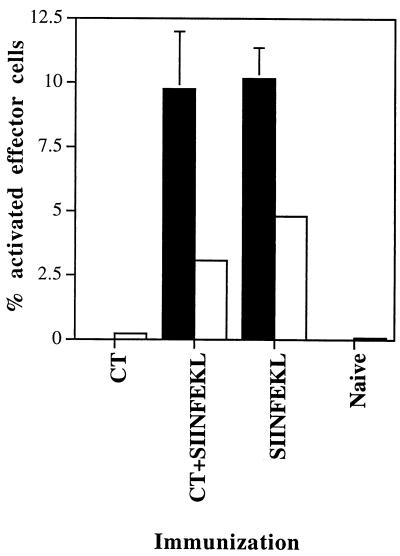
FIG. 4.
Presentation by NALT cells from mice immunized i.n. with CT, SIINFEKL plus CT, or SIINFEKL. Mice (groups of four) were immunized 18 h (A), or mice (groups of four) were immunized 24, 8, and 4 h (three immunizations per mouse; B), prior to harvesting of NALT and CLN. NALT and CLN tissues were pooled separately and digested as described in Materials and Methods. To deplete DC from NALT cells (B), cells were incubated with MAb N418 and then with biotin-conjugated goat F(ab′)2 anti-hamster antibody. N418-positive cells were removed via treatment with streptavidin-coupled Dynabeads M-280 (10 beads per cell). Mock-depleted NALT cells were exposed to the same procedure except for the first incubation with MAb N418. Presenting cells were incubated with B3Z cells overnight, and percent activated B3Z cells was calculated as described for Fig. 1. Results represent means ± standard deviations for a representative experiment. Similar results were obtained in one additional experiment. ■, DC-enriched CLN; □, NALT;  , DC-depleted NALT.
, DC-depleted NALT.
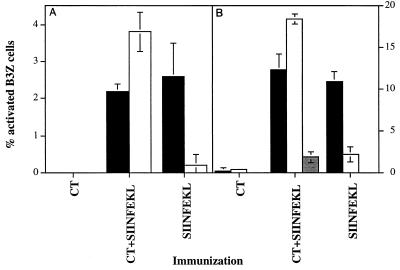
A possible explanation for this discrepancy was that levels of costimulatory molecules expressed by DC loaded with peptide in the presence and absence of CT are different, but the dependence on costimulation differs between the responding cells in vitro and in vivo. Thus, costimulatory molecules may be induced by CT which enables them to activate naive T cells, yet a T-cell hybridoma such as B3Z may be independent of costimulation and thus activated by both DC populations. To further explore this issue, we tested the ability of DC isolated from the CLN of mice immunized with SIINFEKL or SIINFEKL plus CT to activate T cells from mice transgenic for a TCR that recognizes the SIINFEKL-Kb complex. The majority of T cells from these mice are naive and therefore require costimulation for their activation, as would T cells during a primary response in vivo. As shown in Fig. 3, we found that levels of activation of these primary TCR-transgenic T cells, as measured by IL-2 production, were similar upon stimulation by the two populations of DCs. Therefore, it did not appear that the levels of important costimulatory molecules differed between these cells.
FIG. 3.
Comparison of antigen presentation by DC-enriched CLN cells to B3Z T-hybridoma cells and to TCR-transgenic T cells. Mice (groups of four) were immunized i.n. with CT, SIINFEKL plus CT, or SIINFEKL; CLN were harvested 19 h after immunization, pooled, and digested as described in Materials and Methods. DC-enriched cells (low-density cells after passage through a metrizamide column) were incubated either with B3Z cells overnight (■) or with TCR-transgenic T cells for 5 h (□). Activated B3Z cells were evaluated as described for Fig. 1, and the results are expressed as mean percentages of the total number of B3Z cells present ± standard deviations. Transgenic T cells were stained for IL-2 expression (see Methods), and percent IL-2-positive T cells as measured by flow cytometry is shown as percent activated effector T cells.
CT augments the ability of DC from the NALT to present SIINFEKL-Kb.
We then hypothesized that the CLN might not be the site at which CT administered i.n. exerts its adjuvant activity. It seemed possible that the CT administered i.n. might have a more local effect, since it is given at a relatively low dose (1 rather than 50 μg of peptide) and therefore might selectively bind to nasal mucosal cells which are likely to bear high-affinity receptors for CT (11). Therefore, we evaluated the ability of cells isolated from the lymphoid tissues at the site at which CT was administered, the NALT, to present i.n.-administered peptide. For these studies, cells were isolated from the NALT of mice immunized with SIINFEKL, SIINFEKL plus CT, or CT only. In contrast to the results for the CLN, NALT cells isolated from SIINFEKL-plus-CT-immunized mice were able to potently activate B3Z T cells compared to the NALT cells from SIINFEKL-immunized mice (Fig. 4). In addition, we determined that DC present in the NALT population were predominantly responsible for this activation since DC depletion reduced B3Z activation more than ninefold (Fig. 4B).
DISCUSSION
The exact pathway utilized for presentation of peptide immunogens to the immune system after i.n. immunization is not clear. We previously reported that CT was required as a mucosal adjuvant for the induction of peptide-specific CTL after i.n. immunization with CTL epitope peptides. This study was performed to (i) identify the APC responsible for presentation of i.n.-administered CTL epitope peptide immunogens and (ii) determine if the use of CT as a mucosal adjuvant was associated with enhanced presentation of i.n.-administered peptide immunogens.
To address these issues, we compared antigen-specific presentation in draining lymphoid tissues from mice immunized i.n. with SIINFEKL with or without CT. Because cells from the nasal mucosa drain to the CLN (21), CLN cells were first used for this comparison. To test for SIINFEKL-specific APC function, CLN cells were tested for the ability to stimulate the B3Z hybridoma, which recognizes SIINFEKL associated with H-2Kb. DC were found to be the predominant APC in the CLN after i.n. immunization with either SIINFEKL alone or SIINFEKL plus CT (Fig. 2). DC were identified by their low boyant density as well as by their expression of CD11c, a surface-expressed β2 integrin which is expressed by many cell types in humans but primarily by DC in the mouse. Surprisingly, CLN DC isolated from mice immunized with SIINFEKL only (i.e., in the absence of CT) were able to stimulate the B3Z hybridoma as effectively as DC isolated from mice immunized with SIINFEKL plus CT (Fig. 2 to 4). Similar results were obtained when presentation was evaluated by IL-2 production from primary transgenic T cells specific for the SIINFEKL-Kb complex (Fig. 3). These observations suggest that i.n. administration of CT did not affect the uptake and transport of peptide immunogen by DC migrating to the CLN, nor did it appear to affect the levels of costimulatory molecules on the migrating DC, since DC from mice immunized with SIINFEKL alone or SIINFEKL plus CT were equally effective in stimulating naive TCR-transgenic T cells that require costimulation.
We next addressed the question of whether the adjuvant effect of CT was more prominent at the site of administration, in this case, the NALT. Indeed, CT binds monosialoganglioside GM1, which is present on the surface of mucosal epithelial cells, and CT administered by the gastric route predominantly binds gut epithelial cells (21), suggesting that the adjuvant effect of CT, which is applied in minute amounts (1 μg/mouse) compared to the peptide (50 μg/mouse), may be realized at or near the epithelial surface at the site of administration. We therefore compared the SIINFEKL-specific APC function of NALT cells of mice immunized i.n. with SIINFEKL with or without CT. In contrast to the results observed with CLN cells, NALT cells isolated from mice immunized i.n. with SIINFEKL plus CT, but not with SIINFEKL only, were able to efficiently stimulate the B3Z hybridoma (Fig. 4). Depletion of N418-positive DC abrogated the ability of these cells to stimulate the B3Z hybridoma, indicating that DC were the predominant APC in the NALT after i.n. immunization with peptide immunogen and CT (Fig. 4).
Taken together, these data demonstrate that CT enhances the antigen-presenting capacity of DC in the NALT. The mechanism by which it does this is not clear. It is possible that CT acts to enhance antigen transport into the NALT either by affecting nonspecific barrier functions of the nasal mucosal epithelial cells or by enhancing receptor-mediated transport mechanisms (38). This possibility is supported by prior studies of the effects of CT on intestinal mucosa, which demonstrated that CT increases the amount of orally administered antigen that crosses the mucosal surface and enters the systemic compartment (25). Alternatively, CT could act either directly or indirectly to enhance the ability of DC present within the NALT to process antigens or to differentiate and migrate to regions within the NALT where cognate interactions with CD8+ cells could occur. With regard to this possibility, CT has been shown to induce macrophages and mucosal epithelial cells to produce proinflammatory cytokines such as IL-6 and IL-1β (3, 4); the latter has been demonstrated to be important for DC maturation (33) and migration (7, 36, 40) and to enhance the ability of DC to cluster with T cells (14). In addition, CT-mediated induction of proinflammatory cytokine production by DC could directly affect T-cell activation and differentiation (18, 41). Another possibility is that CT induces CD4+ T-cell help for CD8+ T-cell responses simply by acting as a source of CD4+ T-cell epitopes which are lacking on the SIINFEKL peptide (42). Finally, while our studies did not demonstrate the ability of CT to induce costimulatory molecules on DC in the CLN, it is still possible that this effect is operable in vivo in the NALT.
The data presented here may also have implications for responses to peptides administered i.n. without CT. Thus, it has been demonstrated in several experimental system that the induction of antigen-specific T- and B-cell responses including CTL response to soluble antigens requires an adjuvant such as CT (2, 34); conversely, i.n. immunization with peptide epitopes in the absence of adjuvants has been shown to induce antigen-specific tolerance in autoimmune disease and allergy models (15, 31, 35, 45, 52). We showed that DC presented the i.n.-administered peptides, and others have demonstrated that DC can act to tolerize as well as induce T-cell responses (13, 46, 48, 50). Thus, it is logical to speculate that i.n. immunization with peptide immunogens in the absence of CT results in peptide transport, by DC, to the NALT and CLN and that these DC subsequently induce antigen-specific tolerance. Further studies will address this possibility, as well as the ability of CT to alter the expression of major histocompatibility complex class I and costimulatory molecules, such as B7-2. The latter is particularly appropriate given the recent finding that CT can enhance B7-2 but not B7-1 expression on human monocytes (6).
In summary, we showed that DC are the major APC for CD8+ T-cell responses following i.n. peptide immunization and that coadministered CT acts as an adjuvant by enhancing the ability of DC to stimulate T cells at the site of immunization, i.e., the NALT. Additional studies are required to determine the role that costimulatory molecules, CD4 help, and proinflammatory cytokines play in this process.
ACKNOWLEDGMENT
We thank Ron Germain for helpful discussions and comments on the manuscript.
REFERENCES
- 1.Bergerot I, Ploix C, Petersen J, Moulin V, Rask C, Fabien N, Lindblad M, Mayer A, Czerkinsky C, Holmgren J, Thivolet C. A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc Natl Acad Sci USA. 1997;94:4610–4614. doi: 10.1073/pnas.94.9.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowen J C, Nair S K, Reddy R, Rouse B T. Cholera toxin acts as a potent adjuvant for the induction of cytotoxic T-lymphocyte responses with non-replicating antigens. Immunology. 1994;81:338–342. [PMC free article] [PubMed] [Google Scholar]
- 3.Bromander A, Holmgren J, Lycke N. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro. J Immunol. 1991;146:2908–2914. [PubMed] [Google Scholar]
- 4.Bromander A K, Kjerrulf M, Holmgren J, Lycke N. Cholera toxin enhances alloantigen presentation by cultured intestinal epithelial cells. Scand J Immunol. 1993;37:452–458. doi: 10.1111/j.1365-3083.1993.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo V K, Weiner H L. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–180. doi: 10.1038/376177a0. . (Erratum, 21:377.) [DOI] [PubMed] [Google Scholar]
- 6.Cong Y, Weaver C T, Elson C O. The mucosal adjuvanicity of cholera toxin involves enhancement of costimulatory activity by selective up-regulation of B7.2 expression. J Immunol. 1997;159:5301–5308. [PubMed] [Google Scholar]
- 7.Cumberbatch M, Dearman R J, Kimber I. Langerhans cells require signals from both tumour necrosis factor-alpha and interleukin-1 beta for migration. Immunology. 1997;92:388–395. doi: 10.1046/j.1365-2567.1997.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dick A D, Cheng Y F, McKinnon A, Liversidge J, Forrester J V. Nasal administration of retinal antigens suppresses the inflammatory response in experimental allergic uveoretinitis. A preliminary report of intranasal induction of tolerance with retinal antigens. Br J Ophthalmol. 1993;77:171–175. doi: 10.1136/bjo.77.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dillon S R, Jameson S C, Fink P J. Vβ5+ T cell receptors skew toward OVA + H-2Kb recognition, J. Immunol. 1994;152:1790–1801. [PubMed] [Google Scholar]
- 11.Elson C O. Cholera toxin as a mucosal adjuvant. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. New York, N.Y: Academic Press; 1996. pp. 59–65. [Google Scholar]
- 12.Elson C O, Dertzbaugh M T. Mucosal adjuvants. In: Ogra P L, et al., editors. Handbook of mucosal immunology. San Diego, Calif: Academic Press; 1994. pp. 391–402. [Google Scholar]
- 13.Finkelman F D, Lees A, Birnbaum R, Gause W C, Morris S C. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157:1406–1414. [PubMed] [Google Scholar]
- 14.Galkowska H, Wojewodzka U, Olszewski W L. Cytokines and adherence molecules involved in spontaneous dendritic cell-lymphocyte clustering in skin afferent lymph. Scand J Immunol. 1995;42:324–330. doi: 10.1111/j.1365-3083.1995.tb03663.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoyne G F, O’Hehir R E, Wraith D C, Thomas W R, Lamb J R J R. Inhibition of T cell and antibody responses to house dust mite allergen by inhalation of the dominant T cell epitope in naive and sensitized mice. J Exp Med. 1993;178:1783–1788. doi: 10.1084/jem.178.5.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Husby S, Mestecky J, Moldoveanu Z, Holland S, Elson C O. Oral tolerance in humans: T cell but not B cell tolerance after antigen feeding. J Immunol. 1994;152:4663–4670. [PubMed] [Google Scholar]
- 17.Inaba K, Metlay J D, Crowley M T, Steinman R M. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph S B, Miner K T, Croft M. Augmentation of naive, Th1 and Th2 effector CD4 responses by IL-6, IL-1 and TNF. Eur J Immunol. 1998;28:277–289. doi: 10.1002/(SICI)1521-4141(199801)28:01<277::AID-IMMU277>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Karttunen J, Sanderson S, Shastri S. Detection of rare antigen-presenting cells by the LacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc Natl Acad Sci USA. 1992;89:6020–6024. doi: 10.1073/pnas.89.13.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight S C, Stagg A J. Antigen-presenting cell types. Curr Opin Immunol. 1993;5:374–382. doi: 10.1016/0952-7915(93)90056-x. [DOI] [PubMed] [Google Scholar]
- 21.Kuper C F, Koornstra P J, Hameleers D M H, Biewenga J, Spit B J, Duijvestijn A M, van Breda Vriesman P J C, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 22.Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli heat labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992;22:2277–2281. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 23.Lycke N, Bromander A K, Ekman L, Karlsson U, Holmgren J. Cellular basis of immunomodulation by cholera toxin in vitro with possible association to the adjuvant function in vivo. J Immunol. 1989;142:20–27. [PubMed] [Google Scholar]
- 24.Lycke N, Holmgren N. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986;59:301–308. [PMC free article] [PubMed] [Google Scholar]
- 25.Lycke N, Karlsson U, Sjolander A, Magnusson K E. The adjuvant action of cholera toxin is associated with an increased intestinal permeability for luminal antigens. Scand J Immunol. 1991;33:691–698. doi: 10.1111/j.1365-3083.1991.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 26.Lycke N, Strober W. Cholera toxin promotes B cell isotype differentiation. J Immunol. 1989;142:3781–3787. [PubMed] [Google Scholar]
- 27.Macatonia S E, Knight S C, Edwards A J, Griffiths S, Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987;166:1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macatonia, S. E., P. M. Taylor, S. C. Knight, and B. A. Askonas. 1989. Primary stimulation by dendritic cells induces antiviral proliferative and cytotoxic T-cell responses in vitro. J. Exp. Med. 1255–1264. [DOI] [PMC free article] [PubMed]
- 29.McWilliam A S, Napoli S, Marsh A M, Pemper F L, Nelson D J, Pimm C L, Stumbles P A, Wells T N, Holt P G. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med. 1996;184:2429–2432. doi: 10.1084/jem.184.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McWilliam A S, Nelson D, Thomas J A, Holt P G. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med. 1994;179:1331–1336. doi: 10.1084/jem.179.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metzler B, Wraith D C. Inhibition of experimental autoimmune encephalomyelitis by inhalation but not oral administration of the encephalitogenic peptide: influence of MHC binding affinity. Int Immunol. 1993;5:1159–1165. doi: 10.1093/intimm/5.9.1159. [DOI] [PubMed] [Google Scholar]
- 32.Mowat A M. Oral tolerance and regulation of immunity to dietary antigens. In: Ogra P L, et al., editors. Handbook of mucosal immunology. San Diego, Calif: Academic Press; 1994. pp. 185–201. [Google Scholar]
- 33.Ozawa H, Nakagawa S, Tagami H, Aiba S. Interleukin-1 beta and granulocyte-macrophage colony-stimulating factor mediate Langerhans cell maturation differently. J Investig Dermatol. 1996;106:441–445. doi: 10.1111/1523-1747.ep12343589. [DOI] [PubMed] [Google Scholar]
- 34.Porgador A, Staats H F, Faiola B, Gilboa E, Palker T J. Intranasal immunization with CTL epitope peptides from HIV-1 or ovalbumin and the mucosal adjuvant cholera toxin induces peptide-specific CTLs and protection against tumor development in vivo. J Immunol. 1997;158:834–841. [PubMed] [Google Scholar]
- 35.Prakken B J, van der Zee R, Anderton S M, van Kooten P J S, Kuis W, Eden W V. Peptide-induced nasal tolerance for a mycobacterial heat shock protein 60 T cell epitope in rats suppresses both adjuvant arthritis and nonmicrobially induced experimental arthritis. Proc Natl Acad Sci USA. 1997;94:3284–3289. doi: 10.1073/pnas.94.7.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roake J A, Rao A S, Morris P J, Larsen C P, Hankins D F, Austyn J M. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J Exp Med. 1995;181:2237–2247. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandberg J K, Chambers B J, Van Kaer L, Karre K, Ljunggren H G. TAP1-deficient mice select a CD8+ T cell repertoire that displays both diversity and peptide specificity. Eur J Immunol. 1996;26:288–293. doi: 10.1002/eji.1830260203. [DOI] [PubMed] [Google Scholar]
- 38.Sanderson I A, Walker W A. Mucosal barrier. In: Ogra P L, et al., editors. Handbook of mucosal immunology. San Diego, Calif: Academic Press; 1994. pp. 41–51. [Google Scholar]
- 39.Schuler G, Thurner B, Romani N. Dendritic cells: from ignored cells to major players in T-cell-mediated immunity. Int Arch Allergy Immunol. 1997;112:317–322. doi: 10.1159/000237474. [DOI] [PubMed] [Google Scholar]
- 40.Shankar G, Pickard E S, Burnham K. Superantigen-induced Langerhans cell depletion is mediated by epidermal cell-derived IL-1 alpha and TNF alpha. Cell Immunol. 1996;171:240–245. doi: 10.1006/cimm.1996.0199. [DOI] [PubMed] [Google Scholar]
- 41.Shibuya K, Robinson D, Zonin F, Hartley S B, Macatonia S E, Somoza C, Hunter C A, Murphy K M, O’Garra A. IL-1 alpha and TNF-alpha are required for IL-12-induced development of Th1 cells producing high levels of IFN-gamma in BALB/c but not C57BL/6 mice. J Immunol. 1998;160:1708–1716. [PubMed] [Google Scholar]
- 42.Shirai M, Pendleton D, Ahlers J, Takeshita T, Newman M, Berzofsky J A. Helper-cytotoxic T lymphocyte (CTL) determinant linkage required for priming of anti-HIV CD8+ CTL in vivo with peptide vaccine constructs. J Immunol. 1994;152:549–556. [PubMed] [Google Scholar]
- 43.Staats H F, Montgomery S P, Palker T J. Intranasal immunization is superior to vaginal, gastric, or rectal immunization for the induction of systemic and mucosal anti-HIV antibody responses. AIDS Res Hum Retroviruses. 1997;13:945–952. doi: 10.1089/aid.1997.13.945. [DOI] [PubMed] [Google Scholar]
- 44.Staats H F, Nichols W G, Palker T J. Mucosal immunity to HIV-1: systemic and vaginal antibody responses after intranasal immunization with the HIV-1 C4/V3 peptide T1SP10 MN(A) J Immunol. 1996;157:462–472. [PubMed] [Google Scholar]
- 45.Staines N A, Harper N, Ward F J, Malmstrom V, Holmdahl R, Bansal S. Mucosal tolerance and suppression of collagen-induced arthritis (CIA) induced by nasal administration of synthetic peptide 184–198 of bovine type II collagen (CII) expressing a dominant T cell epitope. Clin Exp Immunol. 1996;103:368–375. doi: 10.1111/j.1365-2249.1996.tb08289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk A H. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 47.Steinman R M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 48.Steinman R M, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 49.Sun J B, Rask C, Olsson T, Holmgren J, Czerkinsky C. Treatment of experimental autoimmune encephalomyelitis by feeding myelin basic protein conjugated to cholera toxin B subunit. Proc Natl Acad Sci USA. 1996;93:7196–7201. doi: 10.1073/pnas.93.14.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suss S, Shortman K. A subclass of dendritic cells kills CD4 T cells via fas/fas-ligand-induced apoptosis. J Exp Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swiggard W, Noncas M D, Witmer-Pack M D, Steinman R M. Enrichment of dendritic cells by plastic adherence and EA rosetting. In: Coligan J E, et al., editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1991. pp. 3.7.1–3.7.11. [Google Scholar]
- 52.Tian J, Atkinson M A, Clare-Salzler M, Herschenfeld A, Forsthuber T, Lehmann P V, Kaufman D L. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J Exp Med. 1996;183:1561–1567. doi: 10.1084/jem.183.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woogen S D, Ealding W, Elson C O. Inhibition of murine lymphocyte proliferation by the B subunit of cholera toxin, J. Immunol. 1987;139:3764–3770. [PubMed] [Google Scholar]
- 54.Wu H-Y, Nguyen H H, Russell M W. Nasal lymphoid tissue (NALT) as a mucosal inductive site. Scand J Immunol. 1997;46:506–513. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]
- 55.Xu-Amano J, Kioyno H, Jackson R L, Staats H F, Fujihashi K, Burrows P D, Elson C O, Pillai S, McGhee J R. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med. 1993;178:1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamamoto S, Kiyono H, Yamamoto M, Imaoka K, Fujihashi K, Van Ginkel F W, Noda M, Takeda Y, McGhee J R. A nontoxic mutant of cholera toxin elicits TH2-type responses for enhanced mucosal immunity. Proc Natl Acad Sci USA. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]