Abstract
Francisella tularensis is a gram-negative intracellular bacterium that can induce lethal respiratory infection in humans and rodents. However, little is known about the role of innate or adaptive immunity in protection from respiratory tularemia. In the present study, the role of interleukin-12 (IL-12) in inducing protective immunity in the lungs against intranasal infection of mice with the live vaccine strain (LVS) of F. tularensis was investigated. It was found that gamma interferon (IFN-γ) and IL-12 were strictly required for protection, since mice deficient in IFN-γ, IL-12 p35, or IL-12 p40 all succumbed to LVS doses that were sublethal for wild-type mice. Furthermore, exogenous IL-12 treatment 24 h before intranasal infection with a lethal dose of LVS (10,000 CFU) significantly decreased bacterial loads in the lungs, livers, and spleens of wild-type BALB/c and C57BL/6 mice and allowed the animals to survive infection; such protection was not observed in IFN-γ-deficient mice. The resistance induced by IL-12 to LVS infection was still observed in NK cell-deficient beige mice but not in CD8−/− mice. These results demonstrate that exogenous IL-12 delivered intranasally can prevent respiratory tularemia through a mechanism that is at least partially dependent upon the expression of IFN-γ and CD8 T cells.
Tularemia is a vector-borne zoonosis caused by Francisella tularensis, a gram-negative, facultative intracellular bacterium that is ingested by and multiplies within macrophages (7, 9, 10). Disease can be transmitted through bites from infected insects, by handling of infected carcasses, by drinking of contaminated water and, most notably, by inhalation of infectious aerosols. This last route gives rise to the respiratory form of tularemia, the deadliest form of the disease (up to 35% mortality in humans with an infectious dose of 10 to 50 organisms). Because of its extreme infectivity, ease of dissemination, and substantial capacity to cause illness and death, F. tularensis is considered by the Working Group on Civilian Biodefense to be a dangerous potential biological weapon.
Although F. tularensis infection by the inhalation route is the most lethal form of the infection, the majority of research on immune defenses against this organism has focused on systemic infection, including the intradermal (i.d.) and artificial intraperitoneal (i.p.) routes (9). These studies have implicated an important role for gamma interferon (IFN-γ) in protection, although the potential roles of respiratory IFN-γ and interleukin-12 (IL-12) have remained poorly understood (1, 5, 8). Since mucosal surfaces represent the first line of defense against most infectious agents, the present study was designed to investigate strategies for inducing protective immunity directly in the lungs against F. tularensis by using as a model the live vaccine strain (LVS), a type B strain that is highly virulent for mice and induces protective immunity against type A organisms in both mice and humans. Our results demonstrate pivotal roles for both IFN-γ and IL-12 in resistance to respiratory tularemia and show that exogenous IL-12 treatment delivered intranasally (i.n.) can induce protective immunity that is dependent upon CD8 T cells.
MATERIALS AND METHODS
Mice.
BALB/c and C57BL/6 mice, 5 to 8 weeks old, were obtained from the Charles River Laboratories (Raleigh, N.C.) through a contract with the National Cancer Institute (Bethesda, Md.). BALB/c IFN-γ−/− (GKO), BALB/c IL-12 p35−/−, BALB/c IL-12 p40−/−, C57BL/6 beige, and C57BL/6 CD8−/− mice were all obtained from Jackson Laboratories (Bar Harbor, Maine). The mice were maintained and bred at Albany Medical College. All experimental procedures were in compliance with the guidelines of the institutional animal care and use committee.
F. tularensis LVS lung infection.
Mice were anesthetized with ketamine HCl (Fort Dodge Animal Health, Fort Dodge, Iowa) and xylazine (Phoenix Scientific, St. Joseph, Mo.) in phosphate-buffered saline (PBS) and were infected i.n. with 40 μl of 1,000, 3,000, 5,000, or 10,000 CFU of F. tularensis LVS (kindly provided by Karen Elkins, U.S. Food and Drug Administration, Bethesda, Md.) in Ringer's solution. Sham-infected mice received Ringer's solution only. Numbers of CFU inoculated were confirmed at the time of infection by plating on horse blood-cysteine agar plates.
IL-12 treatment.
Mice were inoculated i.n. with murine recombinant IL-12 (kindly provided by Wyeth Vaccines, Pearl River, N.Y.) 24 h before infection. The vehicle for IL-12 delivery was 25 μl of PBS containing 1% normal mouse serum. Results from preliminary experiments indicated that 0.5 μg of IL-12 was optimal for protective efficacy, and no toxicity has been observed with this i.n. dose of IL-12 (15, 21). Control mice received vehicle alone 24 h before infection.
Analysis of bacterial levels in infected mice.
Numbers of LVS bacteria in the lungs, spleens, and livers of mice were determined 3 and 6 days after i.n. infection. The organs were homogenized by using a mortar and pestle, each organ homogenate was diluted in 2 ml of PBS, and serial dilutions of 100 μl of each sample were plated on horse blood-cysteine agar plates to obtain CFU. Statistical comparisons of bacterial levels in different organs in the infected groups were performed by analysis of variance.
RESULTS
Role of IFN-γ in protection from respiratory LVS tularemia.
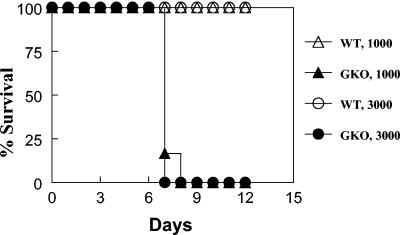
Preliminary results demonstrated a large increase in lung IFN-γ production shortly after i.n. infection with F. tularensis LVS (5 ng of IFN-γ/lung in C57BL/6 mice infected for 3 days with 10,000 CFU of LVS versus no detectable IFN-γ in sham-infected mice). It was previously reported that IFN-γ is required for protection against systemic tularemia, although its importance against respiratory tularemia has been questioned. In fact, Conlan et al. (5) found that treatment of mice with neutralizing anti-IFN-γ antibody significantly altered the course of systemic but not respiratory tularemia; however, it was not clear whether the anti-IFN-γ IgG antibody used in that study was actually able to be transported across the mucosal epithelial barrier and neutralize IFN-γ in the lungs. To readdress this issue, BALB/c GKO mice and wild-type control mice were infected i.n. with sublethal doses of F. tularensis LVS (1,000 and 3,000 CFU), and survival was monitored. It was found that while all wild-type mice survived, as expected, all GKO mice succumbed to the infection within 7 to 8 days (Fig. 1). Thus, IFN-γ is clearly required for survival from respiratory tularemia, in agreement with other recently published results (4).
FIG. 1.
IFN-γ is required for survival from respiratory tularemia. BALB/c GKO mice were infected with 1,000 or 3,000 CFU of F. tularensis LVS, and their survival rates were compared to those of BALB/c wild-type (WT) mice. There were eight mice per group.
Role of IL-12 in protection from respiratory LVS tularemia.
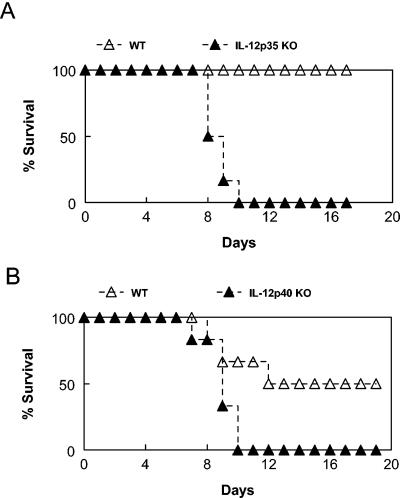
IL-12 is a major inducer of IFN-γ production by NK and T cells (24). Thus, given the importance of IFN-γ in protection and the likelihood that NK and T cells are involved in resistance to the intracellular pathogen F. tularensis, it might be predicted that IL-12 would play a pivotal role in susceptibility to respiratory F. tularensis infection. The actual significance of IL-12 in respiratory tularemia has not yet been investigated, and the role of IL-12 in systemic infection is even unclear, since it has been reported that in vivo clearance of organisms after i.d. infection is dependent upon the IL-12 p40 chain but, surprisingly, not dependent upon the intact molecule (8). The requirement for IL-12 for survival from i.n. F. tularensis LVS infection was tested by using BALB/c IL-12 p35−/− and IL-12 p40−/− mice. It was found that doses of F. tularensis that were sublethal or only mildly lethal for wild-type animals (1,000 and 3,000 CFU) killed all IL-12 p35−/− mice within 9 days (Fig. 2A). In another experiment, the same doses of LVS killed 50% of wild-type animals but 100% of IL-12 p40−/− mice within 10 days (Fig. 2B). Thus, both chains of IL-12 play a critical role in immune protection against respiratory tularemia.
FIG. 2.
IL-12 is required for survival from respiratory tularemia. BALB/c IL-12 p35−/− (KO) mice (A) and BALB/c IL-12 p40−/− (KO) mice (B) were infected with 1,000 CFU of F. tularensis LVS, and their survival rates were compared to those of BALB/c wild-type (WT) mice. There were eight mice per group. Similar results were obtained with an infectious dose of 3,000 CFU.
Ability of exogenous IL-12 treatment to protect against respiratory infection with F. tularensis LVS.
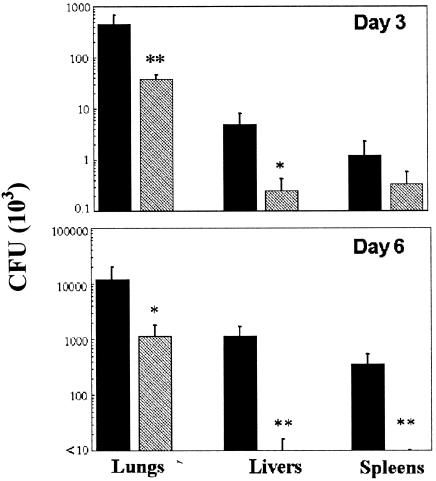
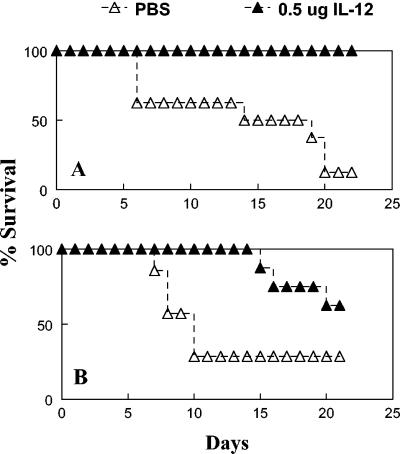
As shown above, IFN-γ and IL-12 are required to survive sublethal i.n. challenges with F. tularensis LVS. To test the ability of exogenous IL-12 treatment to protect against lethal doses of LVS, BALB/c and C57BL/6 mice were inoculated i.n. with 0.5 μg of IL-12 or vehicle 24 h before i.n. challenge with 10,000 CFU of F. tularensis LVS. IL-12 treatment was found to have dramatic protective effects. Analysis of bacterial loads in infected C56BL/6 mice showed that IL-12 treatment reduced the numbers of bacteria in the lungs of the infected animals by approximately 10-fold on days 3 and 6 after i.n. challenge (Fig. 3). In the livers and spleens, IL-12 treatment reduced the bacterial numbers by 10-fold 3 days after infection and by at least 35-fold 6 days after infection. Furthermore, IL-12 treatment allowed all BALB/c mice and more than 60% of C57BL/6 mice to survive subsequent LVS infection (Fig. 4). Even the C57BL/6 mice that ultimately succumbed to infection had a significantly delayed time to death (mean times to death, 20 days in IL-12-treated mice and 10 days in vehicle-treated mice). Similar protection against i.p. LVS challenge was observed in mice treated i.p. with IL-12 (data not shown). The protective capacity of IL-12 was not observed in BALB/c GKO mice, demonstrating the requirement of IFN-γ for IL-12-mediated protection (Fig. 5).
FIG. 3.
Treatment with IL-12 before infection with F. tularensis LVS enhances bacterial clearance from the lungs, livers, and spleens of infected mice. Mice were inoculated i.n. with either PBS vehicle (black bars) or 0.5 μg of IL-12 (gray bars) 24 h before i.n. infection with 10,000 CFU of F. tularensis LVS. CFU (mean ± standard deviations) in the target organs on day 3 (top) and day 6 (bottom) after infection were determined. There were eight mice per group. P values were <0.1 (single asterisk) and <0.05 (double asterisks).
FIG. 4.
Treatment with IL-12 before infection with F. tularensis LVS enhances survival. Mice were inoculated i.n. with either PBS vehicle or 0.5 μg of IL-12 24 h before i.n. infection with 10,000 CFU of F. tularensis LVS. Survival was monitored. BALB/c mice (A) and C57BL/6 mice (B) survived after IL-12 treatment, while all wild-type mice succumbed to the infection. There were seven or eight mice per group.
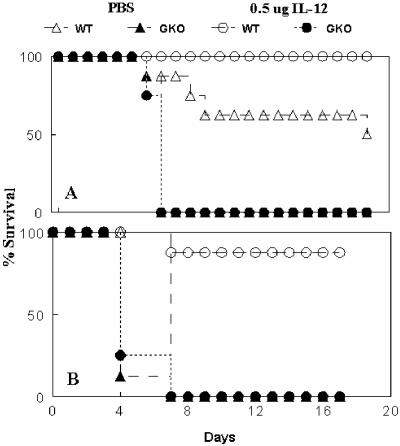
FIG. 5.
Protection by IL-12 requires IFN-γ. Mice were inoculated i.n. with either PBS vehicle or 0.5 μg of IL-12 24 h before i.n. infection with 10,000 CFU of F. tularensis LVS. Survival was monitored. (A and B) Duplicate experiments with 50 and 0% survival, respectively, of vehicle-treated wild-type (WT) mice. After IL-12 treatment, BALB/c WT mice survived the infection, but all BALB/c GKO mice succumbed to the infection. There were eight mice per group.
Roles of NK and CD8 T cells in IL-12-mediated protection.
NK cells represent major target cells for IL-12 activity (24). To examine the potential role of NK cells in protection against respiratory LVS infection, NK cell-deficient C57BL/6 beige mice were infected i.n. with 5,000 and 10,000 CFU of LVS and monitored thereafter for survival. It was found that at both bacterial doses, rates and times to death were nearly identical between C57BL/6 wild-type and beige mice (Fig. 6). In another experiment, wild-type and beige mice were treated with 0.5 μg of IL-12 and subsequently challenged i.n. with 10,000 CFU of LVS. For C57BL/6 wild-type mice, it was again observed that IL-12 pretreatment offered significant protection from fatal disease, compared to the results obtained for animals pretreated with vehicle (Fig. 7). For beige mice treated with IL-12, substantial protection was still observed. Although a larger percentage of beige mice than of wild-type mice succumbed to infection after IL-12 treatment, death occurred at much later time points than in vehicle-treated control animals. We conclude that the beige deficiency has a limited influence on the ability of IL-12 to protect against respiratory tularemia.
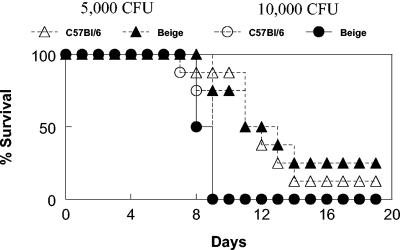
FIG. 6.
The beige mutation does not influence survival from respiratory LVS tularemia. C57BL/6 wild-type mice and C57BL/6 NK cell-deficient beige mice were inoculated i.n. with 5,000 or 10,000 CFU of F. tularensis LVS. Survival was monitored. There were eight mice per group.
FIG. 7.
Protection by IL-12 in beige mice. C57BL/6 wild-type mice and C57BL/6 NK cell-deficient beige mice were inoculated i.n. with either PBS vehicle or 0.5 μg of IL-12 24 h before i.n. infection with 10,000 CFU of F. tularensis LVS. Survival was monitored. There were eight mice per group.
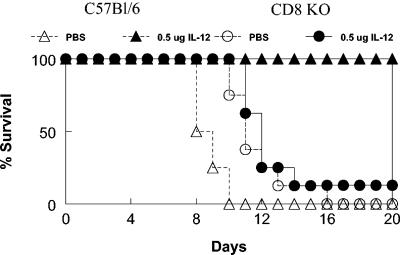
T cells also express the IL-12 receptor and are directly activated by IL-12 (24). Furthermore, it is possible that cytotoxic T cells are critical in lysing LVS-infected cells and therefore in containing the infection. To examine this possibility, the susceptibility of C57BL/6 CD8−/− mice was next tested. It was found that CD8−/− mice were not more susceptible than wild-type mice to high doses of i.n. LVS and even perhaps were slightly more resistant, based upon mean times to death (Fig. 8). However, CD8−/− mice failed to show protection after IL-12 treatment. Thus, while all vehicle-treated wild-type mice were dead by day 10 after i.n. inoculation of 10,000 CFU of LVS and 100% of IL-12-treated wild-type mice survived infection, nearly all CD8−/− mice succumbed by day 13 whether they had been treated with exogenous IL-12 or vehicle only. In addition, times to death were essentially identical between IL-12- and vehicle-treated CD8−/− mice. These results indicate that the activation of CD8 T cells is the primary means for the induction of protection against respiratory LVS F. tularensis by mucosally delivered IL-12.
FIG. 8.
Protection by IL-12 requires CD8 cells. C57BL/6 wild-type mice and CD8−/− (KO) mice were inoculated i.n. with either PBS vehicle or 0.5 μg of IL-12 24 h before i.n. infection with 10,000 CFU of F. tularensis LVS. Survival was monitored. There were eight mice per group.
DISCUSSION
We have found that IFN-γ and IL-12 are necessary for survival from respiratory infection of mice with F. tularensis LVS and that pretreatment with IL-12, a strong inducer of IFN-γ in the lungs, provides greatly increased protection against lethal respiratory tularemia. Protection by IL-12 was not observed in the absence of IFN-γ and was dependent upon CD8 but not NK cells. The results demonstrate the ability of exogenous IL-12 to induce protective immunity against this bacterial threat in the respiratory tract.
It was found that all BALB/c GKO mice quickly succumbed to doses of LVS that were sublethal for wild-type mice. The importance of IFN-γ following i.p. infection of mice with F. tularensis LVS was previously described by others (17). However, Conlan and colleagues (5) concluded that the mechanisms responsible for protection against respiratory and systemic infections might be different, since treatment with a neutralizing anti-IFN-γ antibody potentiated lethality after i.p. but not i.n. LVS challenge. It was not clear from that study whether the injected anti-IFN-γ antibody was actually able to fully neutralize the large amounts of IFN-γ produced in the lungs after infection and, in fact, these same investigators have now found that C57BL/6 GKO mice are more susceptible to LVS infection via the inhalation route than wild-type mice (27). Thus, taken together, the results clearly show that IFN-γ is necessary for optimal protection against both systemic and respiratory F. tularensis infections.
It was also found that IL-12 was necessary for survival from LVS infection in the lungs and that the administration of exogenous IL-12 1 day before infection conferred protective immunity to naive mice. IL-12 is a major inducer of IFN-γ in the lungs (3, 18, 24, 25); as expected, the protective effects of IL-12 against LVS infection were not observed in GKO mice, thus confirming the crucial role of IFN-γ in protective innate immunity against F. tularensis. Although the numbers of bacteria were reduced in the host after IL-12 treatment, cytokine inoculation did not prevent dissemination to the spleen and liver. It should be noted that IL-12 inoculation also did not influence survival during an already established infection (data not shown).
Previous studies with other bacterial infectious agents likewise showed that exogenous IL-12 treatment can protect naive mice against lethal pathogen challenges in the respiratory tract (13, 14, 19) and that IL-12 injection can elicit enhanced resistance against intracellular bacteria that, like F. tularensis, target macrophages (12, 26). However, with regard to F. tularensis LVS, it has been reported that while IL-12 p40 is required for protection against i.d. infection, IL-12 p35 is not needed (8). It was concluded from that study that a cytokine that is dependent upon the p40 chain but not the p35 chain, perhaps IL-23 (20), is critical for protection against this pathogen. Nevertheless, our results clearly indicate a pivotal role for intact IL-12 in resistance against i.n. LVS infection. The reason for the different results obtained in the present study and the earlier investigation (8) could be related to the routes of infection (i.n. versus i.d) and possible differences between skin and lung mucosal immunities. More likely, however, are the possibilities that the immunopathogenesis of F. tularensis depends upon a critical bacterial burden (23) and that the observed requirements for particular immune components, such as IL-12, will differ depending upon the virulence of the pathogen when it is used in a particular system. After i.d. inoculation of F. tularensis, only a subclinical infection occurs unless relatively high doses of bacteria are used (>106 CFU). In this case, redundant immune mechanisms may be able to clear the infection. At the other end of the spectrum, inbred mice are exquisitely sensitive to aerosol delivery of type A strains of F. tularensis (10 or fewer CFU cause 100% lethality); in this case, the immune system may become overwhelmed to such a degree that natural protective immunity cannot be demonstrated, as recently reported (4). With F. tularensis LVS inoculated i.n., as in this study, in which lethality occurred with intermediate doses of 10,000 CFU, the protective roles of IFN-γ and IL-12 may be more apparent. It remains to be determined which experimental system best models the response of humans, who have a fatality rate of 10 to 35% from respiratory tularemia.
The protective effects of IL-12 were primarily mediated by CD8 cells, as determined with mice deficient in NK and CD8 cells, two primary targets of IL-12. While protection was still observed in beige mice, CD8−/− mice demonstrated a complete loss of protection after IL-12 treatment. Naive CD8−/− mice did not show greater susceptibility to F. tularensis infection than wild-type mice, in agreement with previous reports (6, 27). Others (9) have likewise observed that antibody-mediated in vivo depletion of NK cells has little impact on LVS infection, with the caveat that the available antisera may effect only partial NK cell depletion. It should be noted that although beige mice are typically considered to be NK cell deficient, they do also demonstrate decreased T-cell cytolytic activity (22), which could account for the slight loss of IL-12-mediated protection that was observed after infection of these animals. Large numbers of IFN-γ-positive NK cells and concomitant decreases in the numbers of IFN-γ-positive T cells are observed in the lungs of mice within 72 h after i.n. LVS infection (17a). Whether these IFN-γ-positive NK cells are protective or detrimental to the host remains to be determined. Nevertheless, although beige mice have a defect in NK cell cytolytic activity, it is known that they produce normal or even enhanced levels of IFN-γ (2, 11, 16). We also recently began to examine the role of CD4 T cells in infection and, surprisingly, found that mice deficient in CD4 T cells are highly resistant to i.n. infection (S. Olmos and D. W. Metzger, unpublished observations). While the mechanisms responsible for this resistance are presently unknown, such mice would be expected to possess increased numbers of CD8 T cells that could mediate the observed protection, consistent with our above-described findings for CD8−/− mice.
The precise role of IL-12-activated CD8 T cells in mediating protection against respiratory tularemia is presently under investigation. Since F. tularensis is an intracellular pathogen, it is possible that infection is controlled by lysis of infected macrophages. In addition, IL-12 may enhance resistance through a mechanism that is related in some manner to the extreme virulence of this pathogen at mucosal surfaces.
ADDENDUM IN PROOF
Since acceptance of the manuscript, M. A. Pammit, V. N. Budhavarapu, E. K. Raulie, K. E. Klose, J. M. Teale, and B. P. Arulanandam (Antimicrob. Agents Chemother. 48:4513-4519, 2004) have reported limited effectiveness of interleukin-12 (IL-12) treatment in mice infected with very large doses of highly virulent Francisella novicida. However, reduction of the number of bacteria by simultaneous antibiotic treatment did reveal a protective function for IL-12.
Acknowledgments
This work was supported by National Institutes of Health grant PO1 AI056320.
Editor: J. N. Weiser
REFERENCES
- 1.Anthony, L. S., E. Ghadirian, F. P. Nestel, and P. A. Kongshavn. 1989. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb. Pathog. 7:421-428. [DOI] [PubMed] [Google Scholar]
- 2.Appelberg, R., A. G. Castro, S. Gomes, J. Pedrosa, and M. T. Silva. 1995. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect. Immun. 63:3381-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arulanandam, B. P., and D. W. Metzger. 1999. Modulation of mucosal and systemic immunity by intranasal interleukin 12 delivery. Vaccine 17:252-260. [DOI] [PubMed] [Google Scholar]
- 4.Chen, W., R. Kuo Lee, H. Shen, and J. W. Conlan. 2004. Susceptibility of immunodeficient mice to aerosol and systemic infection with virulent strains of Francisella tularensis. Microb. Pathog. 36:311-318. [DOI] [PubMed] [Google Scholar]
- 5.Conlan, J. W., R. Kuo Lee, H. Shen, and A. Webb. 2002. Different host defences are required to protect mice from primary systemic vs pulmonary infection with the facultative intracellular bacterial pathogen, Francisella tularensis LVS. Microb. Pathog. 32:127-134. [DOI] [PubMed] [Google Scholar]
- 6.Conlan, J. W., A. Sjostedt, and R. J. North. 1994. CD4+ and CD8+ T-cell-dependent and -independent host defense mechanisms can operate to control and resolve primary and secondary Francisella tularensis LVS infection in mice. Infect. Immun. 62:5603-5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennis, D. T., T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, M. Layton, S. R. Lillibridge, J. E. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763-2773. [DOI] [PubMed] [Google Scholar]
- 8.Elkins, K. L., A. Cooper, S. M. Colombini, S. C. Cowley, and T. L. Kieffer. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect. Immun. 70:1936-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkins, K. L., S. C. Cowley, and C. M. Bosio. 2003. Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect. 5:135-142. [DOI] [PubMed] [Google Scholar]
- 10.Ellis, J., P. C. Oyston, M. Green, and R. W. Titball. 2002. Tularemia. Clin. Microbiol. Rev. 15:631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Florido, M., R. Appelberg, I. M. Orme, and A. M. Cooper. 1997. Evidence for a reduced chemokine response in the lungs of beige mice infected with Mycobacterium avium. Immunology 90:600-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn, J. L., M. M. Goldstein, K. J. Triebold, J. Sypek, S. Wolf, and B. R. Bloom. 1995. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol. 155:2515-2524. [PubMed] [Google Scholar]
- 13.Greenberger, M. J., S. L. Kunkel, R. M. Strieter, N. W. Lukacs, J. Bramson, J. Gauldie, F. L. Graham, M. Hitt, J. M. Danforth, and T. J. Standiford. 1996. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J. Immunol. 157:3006-3012. [PubMed] [Google Scholar]
- 14.Huang, J., M. D. Wang, S. Lenz, D. Gao, and B. Kaltenboeck. 1999. IL-12 administered during Chlamydia psittaci lung infection in mice confers immediate and long-term protection and reduces macrophage inflammatory protein-2 level and neutrophil infiltration in lung tissue. J. Immunol. 162:2217-2226. [PubMed] [Google Scholar]
- 15.Huber, V. C., B. P. Arulanandam, P. M. Arnaboldi, M. K. Elmore, C. E. Sheehan, B. V. S. Kallakury, and D. W. Metzger. 2003. Delivery of IL-12 intranasally leads to reduced IL-12-mediated toxicity. Int. Immunopharmacol. 3:801-809. [DOI] [PubMed]
- 16.Johnson, L. L., and P. C. Sayles. 1995. Strong cytolytic activity of natural killer cells is neither necessary nor sufficient for preimmune resistance to Toxoplasma gondii infection. Nat. Immunol. 14:209-215. [PubMed] [Google Scholar]
- 17.Leiby, D. A., A. H. Fortier, R. M. Crawford, R. D. Schreiber, and C. A. Nacy. 1992. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect. Immun. 60:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Lopez, M. C., N. S. Duckett, S. D. Baron, and D. W. Metzger. Early activation of NK cells after lung infection with the intracellular bacterium Francisella tularensis LVS. Cell. Immunol., in press. [DOI] [PubMed]
- 18.Lynch, J. M., D. E. Briles, and D. W. Metzger. 2003. Increased protection against pneumococcal disease by mucosal administration of conjugate vaccine plus interleukin-12. Infect. Immun. 71:4780-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metzger, D. W., R. Raeder, V. H. Van Cleave, and M. D. P. Boyle. 1995. Protection of mice from group A streptococcal skin infection by interleukin-12. J. Infect. Dis. 171:1643-1645. [DOI] [PubMed] [Google Scholar]
- 20.Oppmann, B., R. Lesley, B. Blom, J. C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M. Liu, D. Gorman, J. Wagner, S. Zurawski, Y. Liu, J. S. Abrams, K. W. Moore, D. Rennick, R. de Waal-Malefyt, C. Hannum, J. F. Bazan, and R. A. Kastelein. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715-725. [DOI] [PubMed] [Google Scholar]
- 21.Potvin, D. M., D. W. Metzger, W. T. Lee, D. N. Collins, and A. I. Ramsingh. 2003. Exogenous interleukin-12 protects against lethal infection with coxsackievirus B4. J. Virol. 77:8272-8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saxena, R. K., Q. B. Saxena, and W. H. Adler. 1982. Defective T-cell response in beige mutant mice. Nature 295:240-241. [DOI] [PubMed] [Google Scholar]
- 23.Sjostedt, A., R. J. North, and J. W. Conlan. 1996. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology 142:1369-1374. [DOI] [PubMed] [Google Scholar]
- 24.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 25.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251-276. [DOI] [PubMed] [Google Scholar]
- 26.Wagner, R. D., H. Steinberg, J. F. Brown, and C. J. Czuprynski. 1994. Recombinant interleukin-12 enhances resistance of mice to Listeria monocytogenes infection. Microb. Pathog. 17:175-186. [DOI] [PubMed] [Google Scholar]
- 27.Yee, D., T. R. Rhinehart Jones, and K. L. Elkins. 1996. Loss of either CD4(+) or CD8(+) T cells does not affect the magnitude of protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J. Immunol. 157:5042-5048. [PubMed] [Google Scholar]