Abstract
Background:
The potential neuroprotective effects of regular physical activity on brain structure are unclear, despite links between activity and reduced dementia risk.
Objective:
To investigate the relationships between regular moderate to vigorous physical activity and quantified brain volumes on magnetic resonance neuroimaging.
Methods:
A total of 10,125 healthy participants underwent whole-body MRI scans, with brain sequences including isotropic MP-RAGE. Three deep learning models analyzed axial, sagittal, and coronal views from the scans. Moderate to vigorous physical activity, defined by activities increasing respiration and pulse rate for at least 10 continuous minutes, was modeled with brain volumes via partial correlations. Analyses adjusted for age, sex, and total intracranial volume, and a 5% Benjamini-Hochberg False Discovery Rate addressed multiple comparisons.
Results:
Participant average age was 52.98 ± 13.04 years (range 18–97) and 52.3% were biologically male. Of these, 7,606 (75.1%) reported engaging in moderate or vigorous physical activity approximately 4.05 ± 3.43 days per week. Those with vigorous activity were slightly younger (p < 0.00001), and fewer women compared to men engaged in such activities (p = 3.76e-15). Adjusting for age, sex, body mass index, and multiple comparisons, increased days of moderate to vigorous activity correlated with larger normalized brain volumes in multiple regions including: total gray matter (Partial R = 0.05, p = 1.22e-7), white matter (Partial R = 0.06, p = 9.34e-11), hippocampus (Partial R = 0.05, p = 5.96e-7), and frontal, parietal, and occipital lobes (Partial R = 0.04, p ≤ 1.06e-5).
Conclusions:
Exercise-related physical activity is associated with increased brain volumes, indicating potential neuroprotective effects.
Keywords: Alzheimer’s disease, brain volumes, deep learning, magnetic resonance imaging, physical activity
INTRODUCTION
Physical activity reduces dementia risk by positively impacting brain health. One large study of 78,430 adults in the UK Biobank showed that as little as 3,800 steps per day—less than half of the often recommended daily amount of 10,000—translated to a 25% reduction of incident dementia [1]. A meta-analysis of over 250,000 individuals noted that physical activity was significantly associated with a decreased incidence of all-cause dementia and Alzheimer’s disease (AD), even in follow-ups longer than 20 years [2]. Even light intensity physical activity has been related to a reduced risk of dementia [3]. Increasing physical activity therefore represents a promising non-pharmacological intervention to reduce risk for AD [4].
Multiple mechanisms exist by which physical activity is thought to reduce the rate of age-related cognitive decline and AD. These include the promotion of cardiovascular health, increased brain-derived neurotrophic growth factor, higher insulin sensitivity, and lower neuroinflammation [5]. A key approach to track the influence of physical activity on the brain is through quantitative neuroimaging of structural volumes [6–8].
While the Centers for Disease Control recommends 150 minutes of moderate physical activity a week or 75 minutes of vigorous physical activity a week or equivalent combination [9], considerable difficulties exist with adherence. This challenge was illustrated in one survey where only 23% of U.S. adults aged 18 to 64 years met these guidelines between 2010–2015 [10]. A recent study also did not find substantial improvements in these metrics with only 28% of U.S. adult meeting the recommendations in metropolitan centers—a number that dropped to 16% in rural areas [11]. Thus, understanding the potential brain health benefits of lower thresholds of physical activity can have public health implication as such levels are more likely to be successfully adhered.
We have previously shown that higher physical activity, as quantified by the number of city blocks walked at the equivalent of about a quarter mile a day, is related to larger brain volumes including those targeted by AD pathology such as the hippocampus and precuneus in cognitively normal [12] as well as persons with mild cognitive impairment and AD [13]. Our prior work has also demonstrated that higher kilocalorie expenditure from leisure activities predicts larger brain volumes compared to non-active individuals across normal cognition, mild cognitive impairment, and AD [14]. This work was replicated in the larger UK Biobank cohort of 10,083 participants in which increased physical activity was related to elevated gray matter volume, larger white matter volume as well as higher hippocampal volumes [15].
While these studies suggest that physical activity can strengthen structural brain reserve through mechanisms that can be tracked by larger brain volumes on magnetic resonance imaging (MRI) scans, several gaps in knowledge need to be addressed. First, the intensity of physical activity has not been explored in large datasets. Second, the imaging studies done have focused on midlife to older cohorts and not those encompassing a larger age range of adulthood. Third, prior imaging studies have lacked a cohesive approach to evaluating brain regions that can be influenced by physical activity intensity. Thus, we hypothesized that in a large cohort increased physical activity intensity will relate to larger brain volumes in terms of 1) whole brain parenchyma, gray matter, and white matter; 2) lobar volumes: frontal, temporal, and parietal lobes; and 3) regions at early risk for AD pathology, specifically the hippocampus, posterior cingulate gyrus, and precuneus.
MATERIALS AND METHODS
Participant MR imaging
All analyses were done with IRB approval (Advarra, WPBP-001). Participants were scanned on 1.5T whole body Philips Ingenia Ambition, Siemens Espree and Aera scanners at the following locations: Vancouver, BC, Canada; Redwood City, CA; Los Angeles, CA; Minneapolis, MN; Boca Raton, FL; Dallas, TX. Each participant received a non-contrast whole body MRI scan that has been previously detailed in prior work [16]. Brain sequences included sagittal 3D T1 MPRAGE, axial 2D FLAIR, and time-of-flight MRA.
Evaluation of physical activity (exercise)
Prior to imaging, participants completed intake questionnaires including 1) demographic information (age, sex); 2) available medical history (hypertension, type 2 diabetes mellitus); 3) self-reported physical activity information. For physical activity, participants were asked to report if they engaged in moderate to vigorous aerobic exercise at least 10 minutes a day. Sedentary behavior was defined by a lack of this regular activity. Physical activity intensity was defined with standard CDC definitions [17]. Moderate physical activity intensity was defined as engaging in activities that increased heard rate and respiratory rate that would allow for contemporaneous talking but not singing during the activity. Such activities could include brisk walking, bicycling less than 10 miles per hour, and water aerobics. Vigorous physical activity intensity by contrast does not permit for speaking a few words without pausing to respire. Such activities include jogging, running, swimming laps, and bicycling more than 10 miles per hour. Participants were asked to report the number of days they engaged in such physical activity in a 14-day period. Participants were dichotomized into Exercise and non-Exercise groups if they engaged in 5 days of moderate to vigorous self-reported physical activity in a 14-day period or an average of 2.5 days per week. This threshold was intentionally selected so as it define a level of physical activity that could be attained by more individuals as this threshold is far lower than the 150 minute of physical activity recommended over 5 days per week by the current Centers for Disease Control guidelines [9, 18].
MRI volumetric measurement of regional brain volumes
To generate the respective regional volumetric of the brain from 3D T1-weighted MRI scans, we used FastSurfer network [19]. FastSurfer is a fast and substantially verified deep learning pipeline that can analyze structural MRIs of the human brain in a completely automated fashion. As a result, it produces outputs that are compatible with FreeSurfer and enables scalable big-data analysis as well as time-sensitive clinical applications such as the localization of structures during the process of image acquisition or the extraction of quantitative measures. FastSurfer Convolutional Neural Network (CNN) is composed of three fully convolutional neural networks that operate on coronal, axial, and sagittal 2D slice stacks, as well as a final view aggregation that combines the advantages of 3D patches and 2D slices. Specifically, the three CNNs capture the 2D features of the brain in each plane. After the three networks have processed the 2D slices the final stage of the FastSurfer CNN architecture to produce a 3D segmentation of the brain. Thus, the three CNNs, each of which represents a deep learning model, are used for brain segmentation and the advantage of this approach is that it reduces the time to segment brain volumes to 1 min compared to 60 min using FreeSurfer [20] upon which FastSurfer is based. This time saving feature is quite impactful for rapidly segmenting brain volumes in a large number of scans such as in the cohort evaluated in this work. FastSurfer CNN was trained over 134 participants aged 27–66 and was used to segment 96 distinct regional brain volumes. The FastSurfer CNN was trained on a random sample of available participant scans spanning young adult, midlife, and later life individuals. As the individual scans were randomly sampled, no additional inclusion criteria were applied except that the scans had to be available for the randomly sampled individuals. Scan exclusion criteria were any large structural lesions that could interfere with the computational training process. All MRI brain volumes were conformed to standard slice orientation and resolution (1 mm isotropic) before feeding to the different deep learning networks.
MRI volumetric measurement of intracranial volume (ICV)
To correct for the differences in the head size of the subjects, a separate deep learning model was trained to segment ICV. To estimate the total intracranial volume, we used 60 participants and annotated the intracranial compartment of these individuals manually according to Fig. 3. We used these labeled data to train the nnUNet [21] for intracranial mask generation. As noted above, nnUNet is a self-configuring method for deep learning-based medical image segmentation. It includes preprocessing, network architecture, training, and post-processing for any new task with segmentation quality inspected on renderings of ICV segmentation using the MITK tool [22].
Fig. 3.
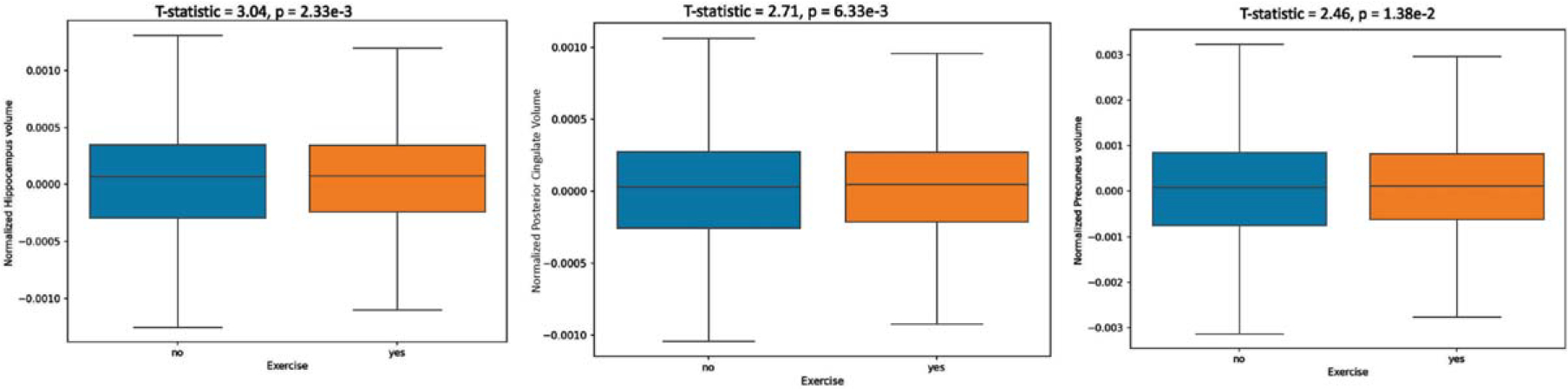
Comparison of brain regions at early risk for Alzheimer’s disease neurodegeneration between exercise and non-exercise groups.
Statistical analyses
All statistical analyses were done in Python using sklearn and the scipy library [23]. Two sample t-tests were done to compare brain volumes between physically active or exercise and non-active or non-exercise groups. Regression analyses were done adjusting for age and sex to understand the relationship between number of days of physical activity and brain regions as defined in the Exercise and Non-Exercise categories. In alignment with our hypothesis, the brain regions evaluated from MR neuroimaging were: 1) total gray matter volume; 2) total white matter volume; Lobar volumes: 1) frontal; 2) temporal; 3) parietal; 4) occipital; and early AD regions: 1) hippocampus; 2) posterior cingulate gyrus; 3) precuneus. Partial correlation coefficients are computed from the residuals of the regression model while adjusting for covariates. Scatterplots relating the total number of days of exercise with selected brain volumes (hippocampus, posterior cingulate, precuneus, and cerebellum) was also done adjusting for age and sex as well with history of hypertension and type 2 diabetes mellitus in a separate set of scatterplots. A subset of analyses evaluated the relationship between moderate and vigorous physical activity intensity separately with gray matter, white matter, AD risk regions, and the cerebellum. All analyses were done on ICV adjusted brain volumes and statistically comparisons accounted for age and sex as covariates. Additionally, Benjamini-Hochberg False Discovery Rate of 5% controlled for multiple comparisons [24–26].
RESULTS
Participant demographics are shown in Table 1.
Table 1.
Participant summary information (n=10,125)
| Variable | Physically Active (n = 7,605) | Physically Non-Active (n = 2,519) | T-statistic, p |
|---|---|---|---|
|
| |||
| Age | 52.5 ± 12.7 (Range: 18–96) | 54.4 ± 13.7 (Range: 18–97) | −6.39, 1.66e-10 |
| Biological Sex (Men/Women) | 54.6% (4,152)/45.4% (3,454) | 45.6% (1,148)/54.5% (1,371) | 7.87, 3.76e-15 |
| Race and Ethnicity | 64.5% (4,906)/0.3% | 46.8% (1,180)/0.4% | 15.97, 9.41e-57 |
| Caucasian/Sub-Saharan African/East | (26)/7.5% (576)/2.4% | (11)/12.5% (316)/3.1% | |
| Asian/West Asian/South Asian/Latin | (187)/6.1% (468)/ 1.3% | (80)/10.7% (271)/3.2% | |
| American/Indigenous/Mixed/Not Sure | (100)/0.07% (6)/15% | (83)/0.2% (6)/18% | |
| Sure | (1144)/2.2% (169) | (455)/4.4% (112) | |
| Body Mass Index | 25.6 ± 4.5 | 27.7 ± 6.3 | −13, 1.94e-38 |
| Hypertension* | 7.6% (579) | 12.3% (312) | −9.02, 2.34e-22 |
| Type 2 Diabetes* | 2.4% (184) | 6.9% (174) | 9.71, 4.41e-22 |
Limited information as the variables only refer to a history of these conditions and does not include information on duration, related medications, or specific metric such as blood pressure for hypertension or hemoglobin A1c for type 2 diabetes mellitus.
Physically active persons from exercise tended to be younger with a higher proportion of Caucasian individuals and lower rates of hypertension and type 2 diabetes.
Figure 1 shows box plots in of whole brain volumes compared between physically active and non-active individuals with the two-sample t-tests results. The physical active group had statistically significant larger gray matter and white matter volumes than the non-active group. There were no statistically significant differences in ventricular volumes.
Fig. 1.
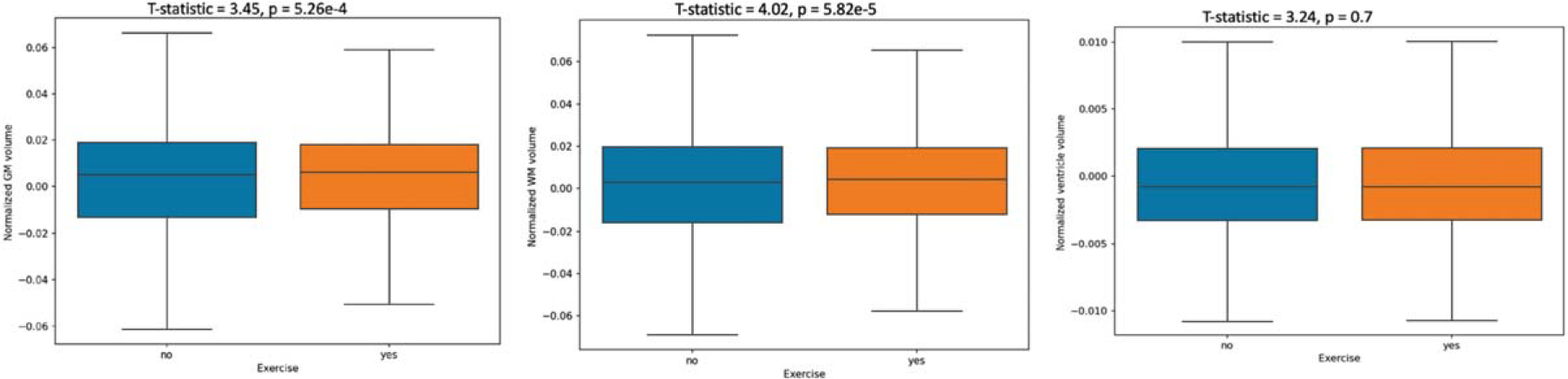
Whole brain volume comparisons between exercise and non-exercise groups. GM, gray matter; WM, white matter.
The box plots in Fig. 2 show similar statistically significant differences across physically active versus non-active persons in brain lobar volumes as well as the cerebellum.
Fig. 2.
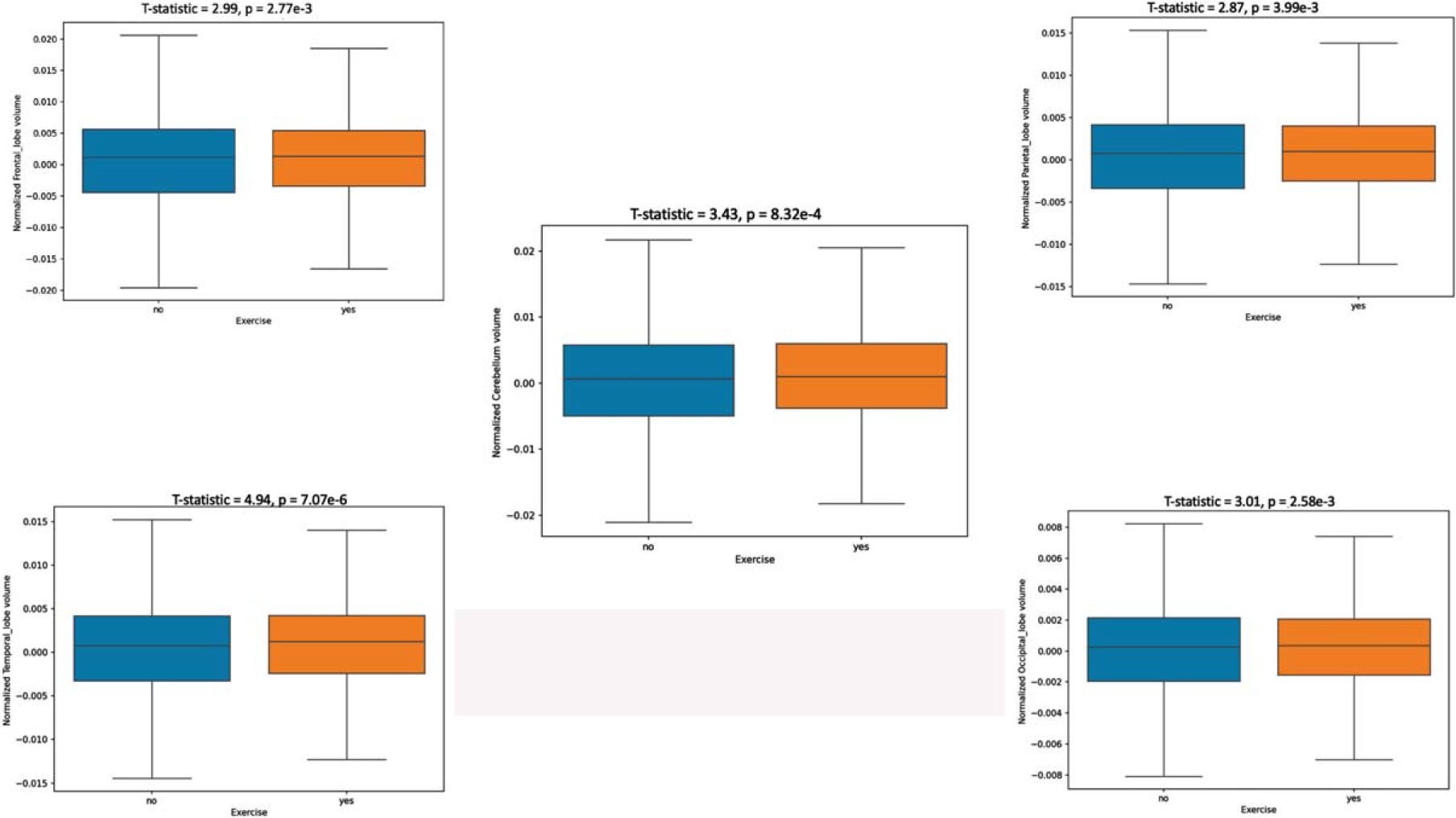
Lobar brain volume comparisons between exercise and non-exercise groups.
Finally, the box plots in Fig. 3 show that in three regions vulnerable to AD neurodegeneration [27], larger volumes are observed in the physically active group for the hippocampus, posterior cingulate gyrus, and precuneus. The effect sizes in these areas were comparable to those noted in other larger brain regions.
Table 2 shows a summary of linear regression of number of days of physical activity and normalized (brain region divided by ICV) brain regions. Displayed are both standardized and unstandardized beta weights, partial correlation coefficients, and p-values adjusted for multiple comparisons with FDR.
Table 2.
Linear regression results relating total number of physical activity days to normalized brain volumes
| Normalized Brain Region | Standardized β Weight | Un-Standardized β Weight | Partial Correlation Coefficient | p |
|---|---|---|---|---|
|
| ||||
| Total Gray Matter | 0.05 | 4.94e-4 | 0.05 | 1.22e-7 |
| Total White Matter | 0.06 | 6.18e-4 | 0.06 | 9.34e-11 |
| Lateral Ventricle | −0.003 | −5.70e-6 | 0.004 | 0.7 |
| Frontal Lobe | 0.04 | 1.21e-4 | 0.04 | 1.06e-5 |
| Temporal Lobe | 0.07 | 1.41e-4 | 0.07 | 8.77e-12 |
| Parietal Lobe | 0.04 | 7.96e-5 | 0.04 | 6.68e-5 |
| Occipital Lobe | 0.04 | 4.61e-5 | 0.04 | 6.96e-6 |
| Cerebellum | 0.06 | 1.63e-4 | 0.06 | 3.72e-9 |
| Hippocampus | 0.05 | 8.4e-6 | 0.05 | 5.96e-7 |
| Posterior Cingulate | 0.04 | 5.41e-6 | 0.04 | 4.47e-5 |
| Precuneus | 0.03 | 1.26e-5 | 0.03 | 1.41e-3 |
In evaluating the influence of exercise intensity and sedentary behavior on brain volumes, we first recharacterize the description of the cohort based upon these physical activity level and intensity as shown in Table 3.
Table 3.
Participant demographics by sedentary status and exercise intensity
| Variable | Sedentary | Moderate | Vigorous | t-stat (p) seden- tary/moderate | t-stat (p) moderate/vigorous | t-stat (p) sedentary/vigorous |
|---|---|---|---|---|---|---|
|
| ||||||
| n | 1963 | 2977 | 5185 | |||
| Age | 54.33 ± 14 | 55.12 ± 13.3 | 51.28 ± 12.1 | −1.98 (0.048) | 13.3 (<0.001) | 9.11 (<0.001) |
| Sex-male (%) | 42.27 | 44.3 | 60.7 | −1.4(0.16) | −14.58< 0.001 | −14.25 <0.001 |
| Sex-female (%) | 57.73 | 55.7 | 39.2 | |||
| Ethnicity (%) | East Asian (14.4) | East Asian (10.3) | East Asian (5.8) | −10.65 <.001 | −4.51 (0.00001) | −15.85 (<0.001) |
| European/ | European/ | European/ | ||||
| Caucasian (45.3) | Caucasian (60.6) | Caucasian (6.5) | ||||
| Indigenous (0.2) | Indigenous (0.1) | Indigenous (0.1) | ||||
| Latin American (3.1) | Latin American (1.2) | Latin American (1.7) | ||||
| Mixed (16.8) | Mixed (15.6) | Mixed (15.5) | ||||
| Not sure (4.6) | Not sure (2.0) | Not sure (2.5) | ||||
| South Asian (11.3) | South Asian (7.3) | South Asian (5.8) | ||||
| Sub-Saharan African (0.5) | Sub-Saharan African (0.4) | Sub-Saharan African (0.3) | ||||
| Western Asian, Arab and Northern African (3.6) | Western Asian, Arab and Northern African (2.3) | Western Asian, Arab and Northern African (2.4) | ||||
| Body mass index | 27.6 ± 6.4 | 26.4±10.5 | 25.6± 5.4 | 3.93 (<0.001) | 4.5 (0.00001) | 11.99 (<0.001) |
| HTN* % (n) | 11.9 (235) | 10.2 (305) | 6.7 (351) | 3.13 (0.002) | 5.88 (<0.001) | 8.78 (<0.001) |
| T2D* % (n) | 7.3 (144) | 4.1 (122) | 1.7 (92) | 5.17 (<0.001) | 5.02 (<0.001) | 10.54 (<0.001) |
Limited information as the variables only refer to a history of these conditions and does not include information on duration, related medications, or specific metric such as blood pressure for hypertension (HTN) or hemoglobin A1c for type 2 diabetes mellitus (T2D).
Figure 4 adds additional information by showing the histogram of sedentary behavior and physical activity intensity across age.
Fig. 4.
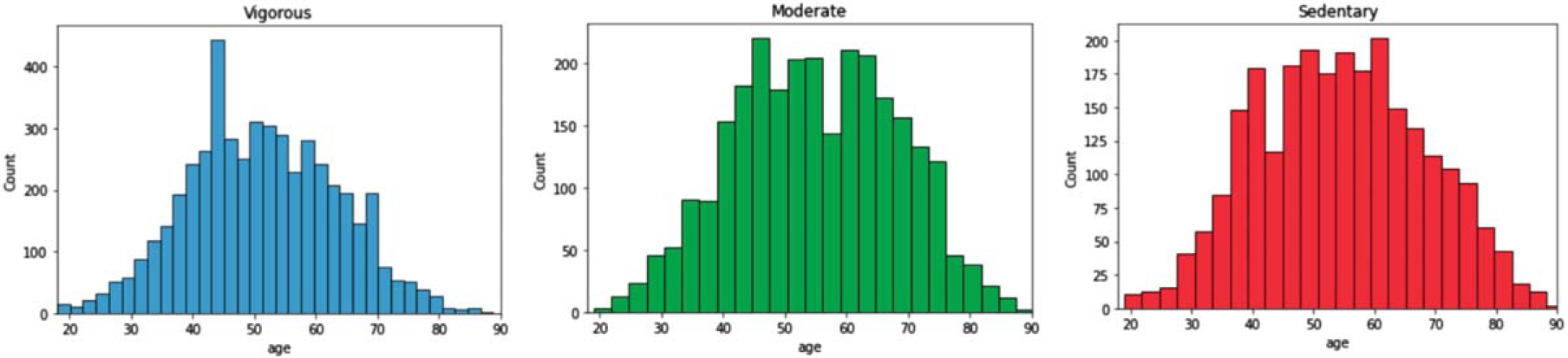
Histograms of physical activity intensity and sedentary status by age.
Scatterplots for selected regions also show that increasing number of exercise dates relates to larger brain volume even when adjusting for age, sex, hypertension, and type 2 diabetes mellitus (Supplementary Figures 1 and 2).
The regression results for the analysis of Moderate and Vigorous Physical activity correlations with brain volumes are displayed in Table 4.
Table 4.
Linear regression results linking physical activity intensity to normalized brain volumes
| Exercise Intensity | Brain Region | Unstandardized β Weight | Partial Correlation Coefficient | P |
|---|---|---|---|---|
|
| ||||
| Moderate | Gray matter | 0.0007 | 0.05 | 0.005 |
| White matter | 0.0007 | 0.05 | 0.004 | |
| Cingulate | 7.95e-06 | 0.03 | 0.03 | |
| Precuneus | 2.45e-05 | 0.04 | 0.02 | |
| Cerebellum | 0.0001 | 0.04 | 0.01 | |
| Hippocampus | 1.36e-05 | 0.05 | 0.004 | |
| Vigorous | ||||
| Gray matter | 0.0003 | 0.02 | 0.10 | |
| White matter | 0.00065726 | 0.04 | 0.002 | |
| Cingulate | 3.18e-06 | 0.01 | 0.311 | |
| Precuneus | 6.33e-06 | 0.01 | 0.471 | |
| Cerebellum | 0.0001 | 0.03 | 0.01 | |
| Hippocampus | 4.58e-06 | 0.01 | 0.21 | |
DISCUSSION
The study aimed to investigate whether moderate to vigorous physical activity may be related to potential neuroprotective effects, specifically improved brain volumes. We found across 10,125 brain MRI scans that a higher number of days of moderate to vigorous physical activity predicted larger normalized brain volumes in multiple regions, including total gray matter volume, total white matter volume, hippocampus, frontal cortex, parietal lobes, and occipital lobe. Our study suggests that engaging in exercise-related physical activity is associated with increased brain volumes from the whole brain to lobar and finally AD risk regional level. We did not find smaller cerebral ventricle size as has been suggested in prior work from the Framingham study [28], although our regression results do suggest that there were non-statistically significant decreasing ventricle size with physical activity. Differences in these results compared to our study may reflect the relative sensitivity of physical activity metrics as well as the cross-sectional design that may not capture ventricle change as sensitively as longitudinal studies [29]. However, another study correlating minutes of physical activity with brain volumes in the Baltimore Longitudinal Study of Aging found similar results to our study, a trending lower cerebral ventricle size that was not statistically significant [30]. Our findings are thus overall consistent with previous research that has found a positive association between physical activity and regional brain volumes [31].
Physical activity has been linked to a reduced risk of dementia, while loss of brain volume as measured by MRI is a marker of neurodegeneration [27]. The benefits of physical activity for improved brain tissue classes and structures has been demonstrated in prior work [32]. One study in 963 late life participants with an average age of 74.1 years showed larger gray and white matter volumes as well as smaller ventricular size related to increased physical activity from walking even when accounting for body mass index [13]. These findings were replicated in a similar sized cohort when considering a variety of leisure activities and related caloric expenditure to larger MRI analyzed brain volumes [14]. Increased physical activity has also been shown to positively impact the hippocampus and precuneus, both regions shown to be improved with frequent physical activity in our study [12]. The UK Biobank also found in 10,083 persons that increased physical activity related to larger total gray and white volumes [15] as well as increased right hippocampal volume. Another study found that wearable technology tracking physical activity related to larger hippocampal and parahippocampal volumes as well as increased functional connectivity between the frontal gyrus, cingulate gyrus, and the inferior occipital lobe [8]. Higher self-reported physical activity in 134 cognitively unimpaired older adults 65 years or older related to larger gray matter volume, improved cerebral glucose metabolism but not with amyloid deposition [33].
The mechanisms by which physical activity can improve brain volumes include neurotrophic growth factors, neuroinflammation, and reduction of amyloid and tau. Exercise has been shown to induce neurogenesis in the hippocampus through increased levels of brain derived neurotrophic growth factor [34, 35]. Physical activity is also thought to improve brain integrity though inflammatory mechanisms. This was illustrated in one study where higher physical activity was related to lower levels of TNF-α, a marker of inflammation, that in turn were related to MRI defined volumes of the inferior parietal lobule [36]. Physical activity was also shown to reduce the burden of neuroinflammation in AD, Parkinson’s disease, amyotrophic lateral sclerosis, and multiple sclerosis [37]. Also, physical activity has been shown to reduce amyloid and tau, which are key pathological features of AD [38, 39].
Physical activity intensity and sedentary behavior varied with declined with age. The fact that sedentary behavior was also reduced with increasing age may suggest a survivor effect whereby such individuals may not live to older age categories. Our finding of moderate physical activity being the intensity level of exercise most correlated to brain regions is reflected by prior work. One randomized exercise intervention noted improved volumes of the hippocampus and prefrontal cortex from moderate intensity exercise over the course of 6 months to 1 year [40]. An earlier randomized clinical trial of moderate intensity exercise intervention versus passive stretching also found increased hippocampal volumes [41]. As little as a single session of moderate intensity exercise induced increased hippocampal activity with functional MRI, higher brain-derived neurotrophic growth factor, and improved long term memory tests; a repeat test with high intensity exercise did not produce these effects [42]. Our findings in context with prior work may suggest that a progressively higher intensity of physical activity does not necessary drive the correlation to larger brain volumes.
Due to the multiple mechanisms through which physical activity can improve brain volumes, there are important implications for this approach for optimizing brain health and improving dementia. Indeed, physical inactivity is noted as in important risk factor for dementia in later life [43]. A structured exercise program is recommended as part of the brain health focus for dementia prevention in a European clinical setting [44]. In the United States, physical activity for dementia prevention is tailored to men and women, with men being recommended an equal to greater region of resistance training to cardiovascular training while for women comparatively more cardiovascular training is recommended [45, 46].
As noted, we intentionally applied a lower threshold of physical activity on brain volumes in our analyses compared to what is formally recommended as these guidelines have poor adherence. This is not only observed in minutes of physical activity per week. For example, the common recommendation of walking 10,000 steps per day. A 2010 study of 2,522 U.S. citizens on average found that on average this cohort only achieved little over half of this recommended amount, at 5,117 steps per day [47]. Even when specific programs attempt to promote additional physical activity of steps walked the results do not fulfill the recommended physical activity metrics. This reality is reflected in a 2016 study where an 8-week employee challenge to improve physical activity with pedometers to track steps walked only found that the 3,820 participants walked an average of 6,886 steps per day—well below the 10,000 recommended standard [48]. Concurrently, the brain health benefits of physical activity are evident at thresholds far below the standard public health recommendations and this may represent an opportunity to improve outcomes even given the lack of adherence to official recommendations [1].
The strengths of our study are its large sample size, quantified high resolution brain volumes, and availability of physical activity frequency as well as intensity. The deep learning approach for volumetric quantification with FastSurfer CNN is also a strength as this approach permitted decreased time to segment brain volumes compared to the usual Freesurfer method. The main weakness of this study is in its cross-sectional design. Self-reporting of physical activity is a study limitation as no specific data are available to verify or support the extend of reported activity. Another limitation is that self-reported physical activity measured in the past two weeks does not reflect a lifetime of exercise/activity levels, a key limitation of this work. Our threshold of physical activity, while intentionally selected to identify attainable levels that may be related to larger brain volumes, does result in an unbalanced sample though this would also be a potential issue with a higher threshold. Also, the correlation we identify between physical activity and brain volumes may not be solely attributable to physical activity alone though our prior work adjusting for covariates did not reveal a confounding influence [12–14]. We lacked a full characterization of such co-variates in this study to additionally confirm earlier observations. We additionally lacked AD biomarker measures to evaluate the influence of physical activity to these important hallmarks of AD pathology.
With respect to neuroimaging methods, while all images were acquired on 1.5T MRI systems we did not use image harmonization software. Thus, our results may potentially be over or underestimated due to potential batch effects [49]. However, batch effects are usually most pronounced when multiple small neuroimaging datasets are combined into one larger sample as opposed to our large dataset aggregated over a relatively small number of sites [50]. Future work should focus on determining the best image harmonization approach for this dataset. Additional directions should also seek to link brain changes in our cohort to generalized and domain specific cognitive changes over time.
Our findings suggest that physical activity may have a positive effect on brain volumes and potential neuroprotective effects that can occur with such changes. Further research is needed to investigate the specific mechanisms underlying the relationship between physical activity and brain health.
Supplementary Material
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This work was supported in part by Providence St. Joseph Health, Seattle, WA [Alzheimer’s Translational Pillar (ATP)]; Saint John’s Health Center Foundation; Pacific Neuroscience Institute and Foundation, including the generous support of the Will and Cary Singleton as well as the McLoughlin family. C.A.R. has received grant support from the NIA 1RF1AG072637-01, NIA R01AG070883.
Footnotes
CONFLICT OF INTEREST
C.A.R. consults to Brainreader ApS, Neurevolution LLC, Apollo Health, Voxelwise Imaging Technology, and the Pacific Neuroscience Foundation. C.A.R. is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review. S.M. consults to Voxelwise Imaging Technology. None of the other co-authors have reported conflicts of interest.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-230740.
DATA AVAILABILITY
The data supporting these findings are available on request to and review by the corresponding author and co-authors. The data are not publicly available due to privacy and ethical restrictions.
REFERENCES
- [1].del Pozo Cruz B, Ahmadi M, Naismith SL, Stamatakis E (2022) Association of daily step count and intensity with incident dementia in 78 430 adults living in the UK. JAMA Neurol 79, 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Iso-Markku P, Kujala UM, Knittle K, Polet J, Vuoksimaa E, Waller K (2022) Physical activity as a protective factor for dementia and Alzheimer’s disease: Systematic review, meta-analysis and quality assessment of cohort and case–control studies. Br J Sports Med 56, 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yoon H, Myung W, Lim SW, Kang HS, Kim S, Won HH, Carroll BJ, Kim DK (2015) Association of the choline acetyltransferase gene with responsiveness to acetylcholinesterase inhibitors in Alzheimer’s disease. Pharmacopsychiatry 48, 111–117. [DOI] [PubMed] [Google Scholar]
- [4].Alty J, Farrow M, Lawler K (2020) Exercise and dementia prevention. Pract Neurol 20, 234–240. [DOI] [PubMed] [Google Scholar]
- [5].Kennedy G, Hardman RJ, Macpherson H, Scholey AB, Pipingas A (2016) How does exercise reduce the rate of age-associated cognitive decline? A review of potential mechanisms. J Alzheimers Dis 55, 1–18. [DOI] [PubMed] [Google Scholar]
- [6].Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA (2007) An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A 104, 5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Melo Neves L, Ritti-Dias R, Juday V, Marquesini R, Mendes Gerage A, Cândido Laurentino G, Hoffmann Nunes R, Stubbs B, Ugrinowitsch C (2023) Objective physical activity accumulation and brain volume in older adults: An MRI and whole-brain volume study. J Gerontol A Biol Sci Med Sci 78, 902–910. [DOI] [PubMed] [Google Scholar]
- [8].Domingos C, Picó-Pérez M, Magalhães R, Moreira M, Sousa N, Pêgo JM, Santos NC (2021) Free-living physical activity measured with a wearable device is associated with larger hippocampus volume and greater functional connectivity in healthy older adults: An observational, cross-sectional study in Northern Portugal. Front Aging Neurosci 13, 729060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD (2018) The physical activity guidelines for Americans. JAMA 320, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Blackwell DL, Clarke TC (2018) State variation in meeting the 2008 Federal Guidelines for both aerobic and muscle-strengthening activities through leisure-time physical activity among adults aged 18–64: United States, 2010–2015. Natl Health Stat Report, pp. 1–22. [PubMed] [Google Scholar]
- [11].Abildso CG, Daily SM, Umstattd Meyer MR, Perry CK, Eyler A (2023) Prevalence of meeting aerobic, muscle-strengthening, and combined physical activity guidelines during leisure time among adults, by rural-urban classification and region — United States, 2020. MMWR Morb Mortal Wkly Rep 72, 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Gach HM, Thompson PM, Ho AJ, Kuller LH (2010) Physical activity predicts gray matter volume in late adulthood: The Cardiovascular Health Study. Neurology 75, 1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Boyle CP, Raji CA, Erickson KI, Lopez OL, Becker JT, Gach HM, Longstreth WT Jr, Teverovskiy L, Kuller LH, Carmichael OT, Thompson PM (2015) Physical activity, body mass index, and brain atrophy in Alzheimer’s disease. Neurobiol Aging 36 Suppl 1, S194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Raji CA, Merrill DA, Eyre H, Mallam S, Torosyan N, Erickson KI, Lopez OL, Becker JT, Carmichael OT, Gach HM, Thompson PM, Longstreth WT, Kuller LH (2016) Longitudinal relationships between caloric expenditure and gray matter in the Cardiovascular Health Study. J Alzheimers Dis 52, 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brown BM, De Frutos Lucas J, Porter T, Frost N, Vacher M, Peiffer JJ, Laws SM (2022) Non-modifiable factors as moderators of the relationship between physical activity and brain volume: A cross-sectional UK Biobank Study. J Alzheimers Dis 88, 1091–1101. [DOI] [PubMed] [Google Scholar]
- [16].Petralia G, Koh D-M, Attariwala R, Busch JJ, Eeles R, Karow D, Lo GG, Messiou C, Sala E, Vargas HA, Zugni F, Padhani AR (2021) Oncologically Relevant Findings Reporting and Data System (ONCO-RADS): Guidelines for the acquisition, interpretation, and reporting of whole-body MRI for cancer screening. Radiology 299, 494–507. [DOI] [PubMed] [Google Scholar]
- [17].Centers for Disease Control and Prevention, Measuring Physical Activity Intensity | Physical Activity. https://www.cdc.gov/physicalactivity/basics/measuring/index.html. Accessed on October 20, 2022.
- [18].Centers for Disease Control and Prevention, Move More; Sit Less, https://www.cdc.gov/physicalactivity/basics/adults/index.htm. Accessed on March 23, 2023.
- [19].Henschel L, Conjeti S, Estrada S, Diers K, Fischl B, Reuter M (2020) FastSurfer – A fast and accurate deep learning based neuroimaging pipeline. Neuroimage 219, 117012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fischl B (2012) FreeSurfer. Neuroimage 62, 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Isensee F, Jaeger PF, Kohl SAA, Petersen J, Maier-Hein KH (2021) nnU-Net: A self-configuring method for deep learning-based biomedical image segmentation. Nat Methods 18, 203–211. [DOI] [PubMed] [Google Scholar]
- [22].Nolden M, Zelzer S, Seitel A, Wald D, Müller M, Franz AM, Maleike D, Fangerau M, Baumhauer M, Maier-Hein L, Maier-Hein KH, Meinzer H-P, Wolf I (2013) The Medical Imaging Interaction Toolkit: Challenges and advances: 10 years of open-source development. Int J Comput Assist Radiol Surg 8, 607–620. [DOI] [PubMed] [Google Scholar]
- [23].Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, Vanderplas J, Passos A, Cournapeau D, Brucher M, Perrot M, Duchesnay É (2011) Scikit-learn: Machine learning in Python. J Mach Learn Res 12, 2825–2830. [Google Scholar]
- [24].Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol 57, 289–300. [Google Scholar]
- [25].Genovese CR, Lazar NA, Nichols TE (2002) Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15, 870–878. [DOI] [PubMed] [Google Scholar]
- [26].Bennett CM, Wolford GL, Miller MB (2009) The principled control of false positives in neuroimaging. Soc Cogn Affect Neurosci 4, 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Spartano NL, Davis-Plourde KL, Himali JJ, Andersson C, Pase MP, Maillard P, DeCarli C, Murabito JM, Beiser AS, Vasan RS, Seshadri S (2019) Association of accelerometer-measured light-intensity physical activity with brain volume: The Framingham Heart Study. JAMA Netw Open 2, e192745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Carmichael OT, Kuller LH, Lopez OL, Thompson PM, Dutton RA, Lu A, Lee JY, Aizenstein HJ, Meltzer CC, Liu Y, Toga AW, Becker JT (2007) Cerebral ventricular changes associated with transitions between normal cognitive function, mild cognitive impairment, and dementia. Alzheimer Dis Relat Disord 21, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wanigatunga AA, Wang H, An Y, Simonsick EM, Tian Q, Davatzikos C, Urbanek JK, Zipunnikov V, Spira AP, Ferrucci L, Resnick SM, Schrack JA (2021) Association between brain volumes and patterns of physical activity in community-dwelling older adults. J Gerontol A Biol Sci Med Sci 76, 1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hofman A, Rodriguez-Ayllon M, Vernooij MW, Croll PH, Luik AI, Neumann A, Niessen WJ, Ikram MA, Voortman T, Muetzel RL (2023) Physical activity levels and brain structure in middle-aged and older adults: A bidirectional longitudinal population-based study. Neurobiol Aging 121, 28–37. [DOI] [PubMed] [Google Scholar]
- [32].Gregory S, Parker B, Thompson P (2012) Physical activity, cognitive function, and brain health: What is the role of exercise training in the prevention of dementia? Brain Sci 2, 684–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Felisatti F, Gonneaud J, Palix C, Garnier-Crussard A, Mézenge F, Brigitte L, Chocat A, Quillard A, Ferrand-Devouge E, De La Sayette V, Vivien D, Chételat G, Poisnel G, on behalf of the Medit-Ageing Research Group (2022) Role of cardiovascular risk factors on the association between physical activity and brain integrity markers in older adults. Neurology 98, e2023–e2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Adlard PA, Perreau VM, Pop V, Cotman CW (2005) Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci 25, 4217–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu PZ, Nusslock R (2018) Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci 12, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Braskie MN, Boyle CP, Rajagopalan P, Gutman BA, Toga AW, Raji CA, Tracy RP, Kuller LH, Becker JT, Lopez OL, Thompson PM (2014) Physical activity, inflammation, and volume of the aging brain. Neuroscience 273, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Seo D-Y, Heo J-W, Ko JR, Kwak H-B (2019) Exercise and neuroinflammation in health and disease. Int Neurourol J 23, S82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Raja D, Ravichandran S, Chandrasekaran B, Kadavigere R, Babu MGR, Almeshari M, Alyahyawi AR, Alzamil Y, Abanomy A, Sukumar S (2022) Association between physical activity levels and brain volumes in adults visiting radio-imaging center of tertiary care hospital. Int J Environ Res Public Health 19, 17079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Liu Y, Yan T, Chu JM-T, Chen Y, Dunnett S, Ho Y-S, Wong GT-C, Chang RC-C (2019) The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab Invest 99, 943–957. [DOI] [PubMed] [Google Scholar]
- [40].Erickson KI, Leckie RL, Weinstein AM (2014) Physical activity, fitness, and gray matter volume. Neurobiol Aging 35, S20–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad SciUSA 108, 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Marin Bosch B, Bringard A, Logrieco MG, Lauer E, Imobersteg N, Thomas A, Ferretti G, Schwartz S, Igloi K (2021) A single session of moderate intensity exercise influences memory, endocannabinoids and brain derived neurotrophic factor levels in men. Sci Rep 11, 14371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Frisoni GB, Altomare D, Ribaldi F, Villain N, Brayne C, Mukadam N, Abramowicz M, Barkhof F, Berthier M, Bieler-Aeschlimann M, Blennow K, Brioschi Guevara A, Carrera E, Chételat G, Csajka C, Demonet J-F, Dodich A, Garibotto V, Georges J, Hurst S, Jessen F, Kivipelto M, Llewellyn DJ, McWhirter L, Milne R, Minguillón C, Miniussi C, Molinuevo JL, Nilsson PM, Noyce A, Ranson JM, Grau-Rivera O, Schott JM, Solomon A, Stephen R, Van Der Flier W, Van Duijn C, Vellas B, Visser LNC, Cummings JL, Scheltens P, Ritchie C, Dubois B (2023) Dementia prevention in memory clinics: Recommendations from the European task force for brain health services. Lancet Reg Health Eur 26, 100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Niotis K, Akiyoshi K, Carlton C, Isaacson R (2022) Dementia prevention in clinical practice. Semin Neurol 42, 525–548. [DOI] [PubMed] [Google Scholar]
- [46].Saif N, Hristov H, Akiyoshi K, Niotis K, Ariza IE, Malviya N, Lee P, Melendez J, Sadek G, Hackett K, Rahman A, Meléndez-Cabrero J, Greer CE, Mosconi L, Krikorian R, Isaacson RS (2022) Sex-driven differences in the effectiveness of individualized clinical management of Alzheimer’s disease risk. J Prev Alzheimers Dis 9, 731–742. [DOI] [PubMed] [Google Scholar]
- [47].Bassett DR, Wyatt HR, Thompson H, Peters JC, Hill JO (2010) Pedometer-measured physical activity and health behaviors in U.S. adults. Med Sci Sports Exerc 42, 1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Berko J, Goetzel RZ, Roemer EC, Kent K, Marchibroda J (2016) Results from the Bipartisan Policy Center’s CEO Council Physical Activity Challenge to American Business. J Occup Environ Med 58, 1239–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pizarro R, Assemlal H-E, De Nigris D, Elliott C, Antel S, Arnold D, Shmuel A (2019) Using deep learning algorithms to automatically identify the brain MRI contrast: Implications for managing large databases. Neuroinformatics 17, 115–130. [DOI] [PubMed] [Google Scholar]
- [50].Hu F, Chen AA, Horng H, Bashyam V, Davatzikos C, Alexander-Bloch A, Li M, Shou H, Satterthwaite TD, Yu M, Shinohara RT (2023) Image harmonization: A review of statistical and deep learning methods for removing batch effects and evaluation metrics for effective harmonization. Neuroimage 274, 120125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting these findings are available on request to and review by the corresponding author and co-authors. The data are not publicly available due to privacy and ethical restrictions.


