Abstract
The bacterial plant pathogen Xanthomonas axonopodis pv. vesicatoria, also known as Xanthomonas campestris pv. vesicatoria group A, is the causal agent of bacterial spot in pepper and tomato. In order to test different models that may explain the coevolution of avrBs2 with its host plants, we sequenced avrBs2 and six chromosomal loci (total of 5.5 kb per strain) from a global sample of 55 X. axonopodis pv. vesicatoria strains collected from diseased peppers. We found an extreme lack of genetic variation among all X. axonopodis pv. vesicatoria genomic loci (average nucleotide diversity, π = 9.1 × 10−5), including avrBs2. This lack of diversity is consistent with X. axonopodis pv. vesicatoria having undergone a recent population bottleneck and/or selective sweep followed by population expansion. Coalescent analysis determined that approximately 1.4 × 104 to 7.16 × 104 bacterial generations have passed since the most recent common ancestor (MRCA) of the current X. axonopodis pv. vesicatoria population. Assuming a range of 50 to 500 bacterial generations per year, only 28 to 1,432 years have passed since the MRCA. This time frame coincides with human intervention with the pathogen's host plants, from domestication to modern agricultural practices. Examination of 19 mutated (loss-of-function) avrBs2 alleles detected nine classes of mutations. All mutations affected protein coding, while no synonymous changes were found. The nature of at least one of the avrBs2 mutations suggests that it may be possible to observe one stage of an evolutionary arms race as X. axonopodis pv. vesicatoria responds to selection pressure to alter avrBs2 to escape host plant resistance.
Plant resistance (R) genes and the pathogen elicitor genes they detect play a crucial role in the ability of a plant to successfully defend itself against pathogen attack (10). The genes encoding the pathogen elicitors that are detected by plant R genes historically have been termed “avirulence” (avr) genes. The ability of a plant R gene to recognize a pathogen elicitor is very specific; hence, the interaction is known as the “gene-for-gene” interaction. Plant R gene recognition of a pathogen elicitor sets up a rapid and robust defense response in the plant that severely limits the ability of a pathogen to multiply within plant tissues (10, 19). While our understanding of the molecular biology of gene-for-gene interactions has greatly increased within the last few years (1, 5, 16, 29, 47), we still know very little about the coevolutionary forces that shape these systems. Most of the progress made in our understanding of the evolution and maintenance of genes involved in this interaction has focused on the plant side of the story, i.e., on R genes (9, 49, 50, 52, 64, 72). Although a few studies have examined the variation within alleles of bacterial avr genes (27, 38, 67), these studies used small, nonrandom samples of pathogens and thus were not designed to examine the evolutionary history of avr genes or the pathogens that carry them.
Two evolutionary strategies are often invoked to explain gene-for-gene coevolution, balancing selection or an “arms race.” These two strategies are expected to leave distinct patterns of genetic diversity at the target locus (8). An “arms race” model of coevolution predicts that selective sweeps of adaptation between host (R gene) and pathogen (avr gene) should reduce both the number of alleles and the genetic diversity at these loci. In contrast, under balancing selection, multiple alleles at R gene and avr gene loci are maintained over long stretches of evolutionary time, which results in the maintenance of high genetic diversity linked to these loci. Within bacteria, selection for a single gene will result in selection for the entire bacterial chromosome harboring that gene (a phenomenon known as genetic hitchhiking), due to the fact that the bacterial chromosome comprises a single linkage group. Hence, strong selection for a single bacterial gene (a selective sweep) may cause an entire chromosome to become predominant in the population, leading to a populationwide reduction in genetic variation (62). The extent of reduced genetic variation within the bacterial chromosome depends on the amount of subsequent recombination (i.e., horizontal gene transfer) in the bacterial population; a high recombination rate will keep the linkage block around the selected gene small and lead to more chromosomal diversity away from the selected gene. For these reasons, in order to accurately assess the evolutionary pressure acting on a bacterial gene, it is necessary to understand the pattern of genetic variation (and the pattern of linkage disequilibrium) across the entire bacterial chromosome.
We chose to focus on the bacterium Xanthomonas axonopodis pv. vesicatoria and two of its avr genes: avrBs2 and avrBs1. X. axonopodis pv. vesicatoria is the causal agent of bacterial spot disease in peppers and tomatoes and can cause severe economic losses in both of these crops, especially in warm and humid regions like the southeastern United States. One of these avr genes, avrBs2, is found within the chromosomes of many species of Xanthomonas (37) and has been found in all X. axonopodis pv. vesicatoria isolates examined. This is unlike many other avr genes, which are often carried on plasmids and are not found across a large number of strains or species. avrBs2 is an important gene in the pathogenesis of Xanthomonas, and the pathogen incurs a significant fitness cost upon the removal of avrBs2 (37, 70, 77). For this reason, the R gene that recognizes avrBs2, named Bs2, has been introduced extensively into cultivated pepper within the past decade. Indeed, by the mid 1990s, many cultivated pepper varieties in the United States carried at least one copy of Bs2 (39), creating intensive selective pressure on the pathogen to respond with changes within avrBs2 (27, 39). Unlike avrBs2, avrBs1 is carried on a large endogenous plasmid (66) and is not found within all strains. avrBs1 has been shown to confer a fitness benefit on X. axonopodis pv. vesicatoria in the field, but this benefit is much smaller than, and has an epistatic interaction with, the benefit contributed by avrBs2 (77).
In this study, we attempted to understand the evolutionary forces that have acted on avrBs2, avrBs1, and X. axonopodis pv. vesicatoria in general. To our knowledge, this is the first attempt to directly examine historic and recent responses of avr genes to selection pressures within the context of the evolution of the pathogen. Without knowledge of the evolutionary processes shaping avr genes in bacterial populations, we cannot fully understand the dynamics underlying gene-for-gene interactions. In addition, understanding the genomic context and underlying population structure in which avr genes exist is valuable, as genes do not act independently but are influenced by the genomic makeup of the species. Ultimately, this knowledge will help us to understand an organism's potential as a pathogen (59).
We examined the genetic variation in two X. axonopodis pv. vesicatoria avr genes and within the X. axonopodis pv. vesicatoria chromosome in 55 strains of this pathogen, representing a worldwide sample of pathogens collected from diseased peppers. We found that the global X. axonopodis pv. vesicatoria population is extremely clonal, with very little genetic variation throughout the chromosome, including avrBs2 and the plasmidborne avrBs1. The paucity of genetic variation is consistent with recent evolution or population expansion of the species. We calculate that the most recent common ancestor of the current X. axonopodis pv. vesicatoria population occurred between 28 and 1,432 years ago, which overlaps well with human manipulation of the bacterium's host plants: pepper and tomatoes.
MATERIALS AND METHODS
Bacterial strains, culturing, and DNA extraction.
Table 1 describes the 55 Xanthomonas strains used in this study. An initial set of 36 bacterial strains representing a sample of worldwide Xanthomonas bacteria collected from diseased cultivated peppers (Capsicum annuum) came from the collections of D. Ritchie and J. Jones (University of Florida, Gainesville). This collection was gathered from 1969 through 2001, and all 36 strains contain functional avrBs2 (avrBs2+) alleles. Functionality is defined as the ability to elicit a defense response in plants containing the matching pepper resistance gene, Bs2. To avoid overlooking sources of variation within avrBs2 alleles, we examined a second set of 19 Xanthomonas strains (collected by D. Ritchie) that were known to contain alleles of avrBs2 that evade Bs2 gene detection. These 19 strains with altered avrBs2 genes were arbitrarily collected from diseased cultivated pepper plants (both resistant and susceptible) in the southeastern United States from 1995 to 2001.
TABLE 1.
Strains, polymorphisms, and phenotypes
| Strain | Origin | Yr | avrBs2a | avrBs1a,b | avrBs2 polymorphsc | vieAd | Unique poly.e | Colony morph.f | Pheno. var.g |
|---|---|---|---|---|---|---|---|---|---|
| Xav43 | NC | 1989 | + | Yes (+) | T | 1 | |||
| Xav75-4 | USA | 1975 | + | Yes (+) | 870-871 = TC > CA | T | 1 | ||
| Xav133 | FL | 1993 | + | Yes (+) | T | 1 | |||
| Xav190 | Nicaragua | 1990 | + | Yes (+) | T | 1 | |||
| Xav196 | Nicaragua | 1990 | + | Yes (+) | T | 1 | |||
| Xav326 | GA | 1997 | + | Yes (+) | T | 1 | |||
| Xav880 | Mexico | 1992 | + | Yes (+) | 870-871 = TC > CA | T | 1 | ||
| Xav950 | Mexico | 1992 | + | Yes (+) | 870-871 = TC > CA | T | 1 | ||
| Xav996 | Mexico | 1992 | + | Yes (+) | 870-871 = TC > CA | T | 1 | ||
| Xav1928 | Taiwan | 1989 | + | Yes (+) | T | 1 | |||
| Xav19 | NC | 1987 | + | Yes (+) | C | 1 | |||
| Xav206 | FL | 1995 | − | Yes (+) | See Table 3 | T | 1 | ||
| Xav294 | NC | 1995 | − | Yes (+) | See Table 3 | T | 1 | ||
| Xav374 | Barbados | 1990 | + | Yes (−) | 870-871 = TC > CA | T | 1 | ||
| Xav488 | Costa Rica | 1991 | + | Yes (−) | C | 1 | |||
| Xav1924 | Spain | 2001 | + | Yes (−) | C | 1 | |||
| Xav76-4 | USA | 1976 | + | No | T | 1 | |||
| Xav82-8 | FL | 1982 | + | No | T | 2 | |||
| Xav69-1 | USA | 1969 | + | No | C | 1 | |||
| Xav71-21 | USA | 1971 | + | No | C | 1 | |||
| Xav77-3 | USA | 1973 | + | No | C | 1 | |||
| Xav79 | NC | 1991 | + | No | C | 1 | |||
| Xav96 | VA | 1992 | + | No | C | 1 | |||
| Xav135 | NC | 1993 | + | No | C | 1 | A | ||
| Xav178 | OH | 1994 | + | No | C | 1 | A | ||
| Xav235 | Guadaloupe | 1990 | + | No | 126 = A > G 804 = A > G | C | Frag. 2 | 1 | |
| Xav290 | Guadaloupe | 1990 | + | No | C | 1 | |||
| Xav333 | GA | 1998 | + | No | C | 1 | |||
| Xav458 | Costa Rica | 1991 | + | No | C | IGS | 1 | ||
| Xav583 | Hawaii | 1991 | + | No | C | 1 | |||
| Xav600 | Bahamas | 1991 | + | No | C | 1 | |||
| Xav630 | Bahamas | 1991 | + | No | C | 1 | |||
| Xav699 | Puerto Rico | 1991 | + | No | C | 1 | |||
| Xav851 | Hungary | 1992 | + | No | C | 1 | |||
| Xav852 | Hungary | 1992 | + | No | C | 1 | F | ||
| Xav1103 | Senegal | 1979 | + | No | C | 1 | F | ||
| Xav1925 | Spain | 1999 | + | No | C | 1 | |||
| Xav1929 | Taiwan | 1989 | + | No | C | 2 | |||
| Xav182 | NC | 1994 | − | No | See Table 3 | C | 1 | ||
| Xav314 | KY | 1996 | − | No | See Table 3 | C | 1 | ||
| Xav329 | FL | 1997 | − | No | See Table 3 | C | 1 | ||
| Xav361 | FL | 1998 | − | No | See Table 3 | C | 1 | ||
| Xav376 | NC | 1995 | − | No | See Table 3 | C | 1 | A | |
| Xav390 | NC | 1995 | − | No | See Table 3 | C | 2 | ||
| Xav416 | NC | 1998 | − | No | See Table 3 | C | 1 | ||
| Xav76-4 | USA | 1976 | + | No | T | 1 | |||
| Xav82-8 | FL | 1982 | + | No | T | 2 | |||
| Xav69-1 | USA | 1969 | + | No | C | 1 | |||
| Xav71-21 | USA | 1971 | + | No | C | 1 | |||
| Xav77-3 | USA | 1973 | + | No | C | 1 | |||
| Xav79 | NC | 1991 | + | No | C | 1 | |||
| Xav96 | VA | 1992 | + | No | C | 1 | |||
| Xav135 | NC | 1993 | + | No | C | 1 | A | ||
| Xav178 | OH | 1994 | + | No | C | 1 | A | ||
| Xav235 | Guadaloupe | 1990 | + | No | 126 = A > G 804 = A > G | C | Frag. 2 | 1 | |
| Xav290 | Guadaloupe | 1990 | + | No | C | 1 | |||
| Xav333 | GA, USA | 1998 | + | No | C | 1 | |||
| Xav458 | Costa Rica | 1991 | + | No | C | IGS | 1 | ||
| Xav583 | Hawaii | 1991 | + | No | C | 1 | |||
| Xav600 | Bahamas | 1991 | + | No | C | 1 | |||
| Xav630 | Bahamas | 1991 | + | No | C | 1 | |||
| Xav699 | Puerto Rico | 1991 | + | No | C | 1 | |||
| Xav851 | Hungary | 1992 | + | No | C | 1 | |||
| Xav852 | Hungary | 1992 | + | No | C | 1 | F | ||
| Xav1103 | Senegal | 1979 | + | No | C | 1 | F | ||
| Xav1925 | Spain | 1999 | + | No | C | 1 | |||
| Xav1929 | Taiwan | 1989 | + | No | C | 2 | |||
| Xav182 | NC | 1994 | − | No | See Table 3 | C | 1 | ||
| Xav314 | KY | 1996 | − | No | See Table 3 | C | 1 | ||
| Xav329 | FL | 1997 | − | No | See Table 3 | C | 1 | ||
| Xav361 | FL | 1998 | − | No | See Table 3 | C | 1 | ||
| Xav376 | NC | 1995 | − | No | See Table 3 | C | 1 | A | |
| Xav390 | NC | 1995 | − | No | See Table 3 | C | 2 | ||
| Xav416 | NC | 1998 | − | No | See Table 3 | C | 1 | ||
| Xav437 | FL | 1998 | − | No | See Table 3 | C | 1 | ||
| Xav445 | FL | 1998 | − | No | See Table 3 | C | 2 | ||
| Xav454 | FL | 1998 | − | No | See Table 3 | C | 1 | ||
| Xav467 | FL | 1999 | − | No | See Table 3 | C | 1 | ||
| Xav487 | FL | 1999 | − | No | See Table 3 | C | 2 | ||
| Xav516 | FL | 1999 | − | No | See Table 3 | C | 1 | ||
| Xav536 | FL | 2000 | − | No | See Table 3 | C | 1 | ||
| Xav544 | KY | 2000 | − | No | See Table 3 | C | 1 | ||
| Xav554 | FL | 2000 | − | No | See Table 3 | C | 2 | ||
| Xav571 | NC | 2001 | − | No | See Table 3 | C | 2 |
Functionality of avrBs1 and avrBs2, as defined by the ability to elicit a hypersensitive response in resistant plants (+) or lack of ability (−).
Presence (yes) or absence (no) of an avrBs1 gene in a strain, as determined by PCR.
Nucleotide position numbers for avrBs2 are based on GenBank AF114720.
C or T polymorphism found in the X. axonopodis pv. vesicatoria vieA homologue (genomic fragment 5).
Strains which contain a unique polymorphism (poly.) within the genomic fragment (Frag.) or locus listed.
Colony morphology: 1, colonies are smaller and dark yellow; 2, colonies are larger, pale yellow, and more mucoid.
Phenotypic variation: A, strain has amylolytic activity; F, strain is flocculent in liquid culture.
Unless otherwise stated, two different media were used interchangeably to culture Xanthomonas bacteria: nutrient broth and nutrient yeast glycerol (5 g peptone, 3 g yeast extract, and 20 g glycerol per liter). For plates, 15 g agar per liter was added. For long-term storage, bacterial stocks were kept at −80°C in 15% glycerol. Total genomic and plasmid DNA was extracted using the cetyltrimethylammonium bromide method of Ausubel et al. (4).
Xanthomonas strain typing.
To confirm the species (or groups) of the 55 Xanthomonas strains, we analyzed the restriction patterns of PCR-amplified HrpB, after the method of Obradovic et al. (53). Distinct restriction digest patterns enable differentiation among all four Xanthomonas species or groups. To assess general colony morphology, strains were grown on nutrient yeast glycerol plates for 3 days at 30°C. To test for amylolytic ability, strains were grown on nutrient broth plates with 1% soluble potato starch for 3 days at 30°C, after which a clearly defined halo surrounded colonies with amylolytic ability.
Plasmid profiles.
Endogenous plasmids were analyzed using a method developed by D. Dahlbeck (personal communication). Cells were scraped from a fresh plate and resuspended in 1 ml of 10 mM potassium phosphate buffer to an optical density of approximately 0.25 at 600 nm. The cells were then pelleted, resuspended in 25 μl of buffer, and lysed with the addition of 175 μl lysis buffer (125 mM NaOH, 30 mM NaCl, 50 mM Tris, pH 8.0, 5 mM EDTA, 3% sodium dodecyl sulfate [sodium dodecyl sulfate added after NaOH]). After addition of lysis buffer, the cells were vortexed vigorously for 1 second and then incubated at 65°C for 5 min. DNA was extracted by adding 400 μl of phenol-chloroform-isoamyl alcohol (25:24:1) and then vortexing the emulsion vigorously for 30 seconds. The emulsion was broken by centrifugation at 14,000 rpm for 5 min. Plasmid preps are stable and reusable for several weeks. An aliquot (10 to 15 μl) of the upper phase was mixed with 3 μl of loading dye (0.2% bromophenol blue, 0.2% xylene cyanol FF, 40% glycerol) and loaded onto a 0.7% gel. The gels were run at 80 V for 2.5 h in 1× TAE buffer (0.04 M Tris acetate, 0.001 M EDTA, pH 8.0) and stained with ethidium bromide for visualization. Plasmid preps and gel electrophoresis were performed at least twice for each strain; plasmid sizing was calculated once per strain. Plasmids sizes up to approximately 300 kb can be resolved using this method.
Plasmid sizing.
Two supercoiled DNA ladders were used to determine the sizes of endogenous plasmids: BAC-Tracker from Epicentre (8 to 165 kb) and Invitrogen (2.067 to 16.21 kb). Plasmid sizing was performed as described by Canteros et al. (15). Briefly, the distance traveled by each band in the ladders was measured and log10 transformed. A linear regression equation for each gel was calculated by considering the log10 of the migration distance as the independent variable and the log10 of the ladder band size (kb) as the dependent variable. The approximate sizes of the endogenous X. axonopodis pv. vesicatoria plasmids were calculated using the regression equations for each gel, based on the log10-transformed migration distance for each plasmid band in each strain. The two ladders were treated independently, resulting in two regression equations per gel. Only the 28- to 165-kb bands were used to calculate the regression equations for the larger size standard (BAC-Tracker; Epicentre). Regression equations based on the smaller size standard (2.067 to 16.21 kb; Invitrogen) were applied only to plasmids of approximately 18 kb or smaller. The reported plasmid profiles are conservative; very faint bands appearing in a strain were not counted toward the strain's plasmid profile. Also, very subtle differences in the distance traveled by bands of nearly identical sizes were assumed to be gel variations and not reflective of different-size plasmids.
PCR conditions and sequencing.
Table 2 lists all PCR primers and conditions used in this study; PCR primers also doubled as sequencing primers. Four sets of primers were used to perform PCR and to sequence a 2.2-kb fragment containing the entire avrBs2 coding region plus roughly 150 bp of 5′ flanking DNA. avrBs2 primers were designed based on the avrBs2 sequence submitted to (GenBank accession no. AF114720). We tested for the presence of avrBs1 in all Xanthomonas strains by a PCR-based assay. We first attempted to amplify the full-length avrBs1 gene, open reading frame 2 (ORF 2) (58). All strains for which no full-length avrBs1 was amplified were tested with four additional sets of internal avrBs1 primers to determine if polymorphism at the initial primers prevented amplification. No internal PCR product was ever generated from strains that were negative for full-length avrBs1. All strains that tested positive for avrBs1 by PCR were tested on pepper plants carrying the matching resistance gene (Bs1) to determine whether the avr gene was functional. All sequencing reactions used Big Dye (Applied Biosystems) version 2.0 or version 3.0 and were run on an ABI 3700 sequencing machine. All PCR products were sequenced in both directions and aligned using the program Sequencher 4.0.
TABLE 2.
PCR conditions and primers used in this study
| Gene target | Primer name | Primer sequence (5′-3′) | Anneal tempa | Extension time (s) |
|---|---|---|---|---|
| avrBs2 | avrBs2-28U | GGCAACGCGTCCAAACAAC | ||
| avrBs2-922L | GCACGAGCGACTTTTGATGA | |||
| avrBs2-639U | CACAACAAGGTCGCATCATC | |||
| avrBs2-1431L | TCAAAGCCGCCCGTGTAGT | 50 | 50 | |
| avrBs2-1334U | CTGGACTGCAAGGAAAACA | |||
| avrBs2-1949L | GATCGGTCAACAGGCTTTC | |||
| avrBs2-1611U | GAAGCAATGAGCAGGGCG | |||
| avrBs2-2324L | GAAGCCGTGATTGGAAGGT | |||
| Full-length avrBs1 | avrBs1-642U | TGAGCTCCTATGACGGACTTGTGCTCG | 58 | 90 |
| avrBs1-2050L | TGCATGCGTGGCGGATACTTCTTCTCT | |||
| Internal avrBs1 | avrBs1-913 U | AACTGTGGGATGCTAAAGCTA | ||
| avrBs1-617U | GCATAAATCGCAAGTACATT | 58 | 30 | |
| avrBs1-1329L | CTTCTTCTCTTACGCTTCTCC | |||
| avrBs1-1643L | TAGCTTTAGCATCCCACAGTT | |||
| X. axonopodis pv. vesicatoria | 1-U3 | TCGCAACTACGCCACTGT | 58 | 30 |
| genomic fragment 1 | 1-L616 | GCTTCCCCTGCCTCAATG | ||
| X. axonopodis pv. vesicatoria | 2-U19 | CTCTCGCACGGCACGGT | 61 | 30 |
| genomic fragment 2 | 2-L520 | CATTCCACGCCCACACCA | ||
| X. axonopodis pv. vesicatoria | 3-U142 | TTTGCCGTGCCCTCGTCC | 55 | 30 |
| genomic fragment 3 | 3-L668 | CGGTCGGGATGCTGTAAG | ||
| X. axonopodis pv. vesicatoria | 4-U22 | TGCCGACCTCACCGACAG | 58 | 30 |
| genomic fragment 4 | 4-L581 | CCTTCTGCTTGATGGTGCC | ||
| X. axonopodis pv. vesicatoria | 5-U86n | CCTTGAACCTCTCGCCC | 58 | 30 |
| genomic fragment 5 | 5-L600n | CCGCATCGCTGTGGAAC | ||
| IGS | rrn16s | GAAGTCGTAACAAGG | 55 | 40 |
| rrn23s | CAAGGCATCCACCGT | |||
| HrpB | HrpB1 | GTCGTCGTTACGGCAAGGTGGTCG | 61 | 45 |
| HrpB2 | TCGCCCAGCGTCATCAGGCCATC |
All PCRs had an initial denaturation time of 3 min at 95°C, followed by 35 cycles of 30 s at 95°C, 30 s at the specified annealing temperature (in °C), and 72°C at the specified extension time, with a final 5-min extension at 72°C.
Shotgun sequencing and determination of the six chromosomal Xanthomonas loci.
For all 55 strains, we PCR amplified and sequenced six chromosomal loci. Five random genomic fragments were derived by shotgun sequencing of total genomic DNA from strain Xav19 (64). Genomic DNA was digested with MboI, and fragments of 0.5 to 1 kb were gel purified, blunted with T4 DNA polymerase, and then blunt ligated into the positive selection cloning vector pZero2.0 (Invitrogen). Inserts were sequenced using universal M13 primers, and the sequences were subjected to a BLAST search to determine homology to any known proteins. Genomic fragments with homology to known or suspected pathogenicity genes were eliminated. Five fragments of sufficient length were then randomly chosen from the remaining pool; these fragments ranged from 500 to 625 bp. The sixth sequenced locus was the intergenic spacer (IGS) between the 16S and 23S rRNA genes. There are two copies of the IGS within Xanthomonas species; each copy is 493 bp long and contains two tRNA genes (alanine and isoleucine) (20). The tRNA genes are 75 bp each, leaving 70% of the IGS noncoding. The five random genomic fragments amplified, plus the IGS region, totaled 5.5 kb of sequence for each strain.
Coalescent analysis.
We tested the null model of a constant (effective) population size, panmictic mating, and neutral mutations within X. axonopodis pv. vesicatoria by Monte Carlo simulation (30), using the test statistic Tajima's D (71). Tajima's D was calculated in the program DNAsp 3.0. Ten thousand simulations were run with a fixed number of segregating sites (31, 76), using the code available from R. Hudson (http://home.uchicago.edu/∼rhudson1/).
We used two methods to calculate the time back to the MRCA of the current global population of X. axonopodis pv. vesicatoria. One method assumed that the X. axonopodis pv. vesicatoria population underwent a recent bottleneck and/or selective sweep. Under this assumption, the genealogies of samples drawn from a global distribution are expected to have a star-like phylogeny, with the MRCA being the vertex of the star, and the neutral mutations acquired within strains since the MRCA are expected to have a Poisson distribution (61). To generate a point estimate for the MRCA under this model, we used equation 1,
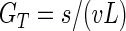 |
(1) |
where GT is the total number of bacterial generations (the sum of all the lineages) in the star phylogeny from the present-day population back to the most recent common ancestor, s is the number of segregating sites (the number of polymorphisms) in the sample, v is the neutral mutation rate per base pair per generation, and L is the length (bp) of DNA sequenced. To obtain G, the number of bacterial generations from the MRCA to a single current strain, we divided GT by n, the number of lineages (strains) in the sample (n = 55). (This equation ignores the possibility of multiple hits at a single site.) To determine the total upper (GTU) and total lower (GTL) confidence intervals for the MRCA, we used the Poisson distribution, where the mean (vGL) is the number of mutations expected to occur in a given number of bacterial generations (61). To find the GTU, we used equation 2 to find the number of bacterial generations at which the sum of the probability of seeing s or fewer mutations equaled 0.025.
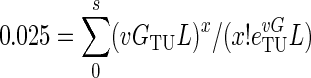 |
(2) |
To calculate GTL, we used equation 3 to find the number of bacterial generations at which the sum of the probability of seeing at least s mutations equaled 0.025.
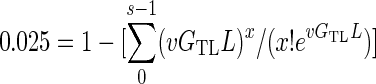 |
(3) |
Both GTU and GTL were divided by n to determine the confidence intervals (GU and GL) for the time since the MRCA to a single current strain.
The second method for calculating the MRCA assumes that the population has evolved neutrally over time since the MRCA (with a constant population size) and does not assume any specific phylogenetic structure. Simply,
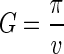 |
(4) |
where π is the nucleotide diversity (the number of nucleotide differences per base pair between any two randomly chosen sequences). We calculated π by using equation 5 (45),
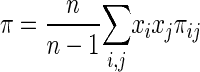 |
(5) |
in which xi and xj are the frequencies of the ith and jth alleles in the sample and πij is the number of nucleotide differences between the ith and jth sequences. The total number of nucleotide differences between sequences was determined using the program Megalign (DNASTAR, Madison, WI); sequences were aligned using the ClustalW algorithm. The ratios of nonsynonymous (amino acid-altering) to synonymous (silent) base pair changes (Ka/Ks ratios) were determined using the program DNAsp 3.0.
For tests of population structure within X. axonopodis pv. vesicatoria (n = 55), DNA sequences from the seven chromosomal loci (avrBs2, X. axonopodis pv. vesicatoria fragments 1, 2, 3, 4, and 5, and the IGS) were concatenated to form one long hypothetical DNA sequence of 5,524 bp for each strain. This was necessitated by the near absence of polymorphism. The protein-altering polymorphisms found in the 19 mutant avrBs2 strains (Table 3) do not represent random, neutral mutations accumulated over the entire time since the MRCA. Instead, these mutations are the result of recent intense agricultural selection against functional avrBs2 and are inappropriate to use in the coalescence analysis of X. axonopodis pv. vesicatoria strains. Therefore, when creating the concatenated DNA sequences for the 19 strains harboring avrBs2 mutations, we replaced the nonsynonymous mutations within the altered avrBs2 alleles with the avrBs2+ consensus sequence. (No sequence differences were found in the other six genomic loci for the 19 avrBs2 mutant strains compared to the consensus sequences for the other 36 avrBs2+ strains.) This yielded an s value of 7 for the number of segregating sites within the X. axonopodis pv. vesicatoria population (Table 1).
TABLE 3.
Mutations in avrBs2 detected in 19 strains of X. axonopodis pv. vesicatoria known to evade Bs2 gene detection
| Strain | Yr | Location collected | Profilea | State | Mutation | Site and commentsb |
|---|---|---|---|---|---|---|
| Xav294 | 1995 | SHRS; research plotc | A | NC | 5-bp deletion | One CGCGC repeat deleted (bp 1522-1526) |
| Xav314 | 1996 | Research plot | N | KY | 5-bp deletion | One CGCGC repeat deleted (bp 1522-1526) |
| Xav182 | 1994 | SHRS; research plot | P | NC | 5-bp insertion | One CGCGC repeat inserted (bp 1522-1526) |
| Xav206 | 1995 | Pine Island | D | FL | 5-bp insertion | One CGCGC repeat inserted (bp 1522-1526) |
| Xav416 | 1998 | Goldsboro | P | NC | 5-bp insertion | One CGCGC repeat inserted (bp 1522-1526) |
| Xav437 | 1998 | Pompano Beach | Z | FL | 5-bp insertion | One CGCGC repeat inserted (bp 1522-1526) |
| Xav454 | 1998 | Southeastern Florida no. 1 | P | FL | 5-bp insertion | One CGCGC repeat inserted (bp 1522-1526) |
| Xav544 | 2000 | Research plot | BB | KY | 5-bp insertion | One CGCGC repeat inserted (bp 1522-1526) |
| Xav329 | 1997 | Clewiston | I | FL | 12-bp insertion | Insert after bp 1305; repeats preceding 12 bp |
| Xav536 | 2000 | South Florida no. 2 | I | FL | 12-bp insertion; 5-bp deletion | Insert after bp 1305; repeats preceding 12 bp 761-765 |
| Xav390 | 1995 | SHRS; research plot | P | NC | 97-bp deletion | bp 218-314 |
| Xav516 | 2000 | South Florida no. 1 | AA | FL | CC → AA | bp 1165-1166 change Asp, Pro → Glu, Thr |
| Xav445 | 1998 | Southeastern Florida no. 1 | I | FL | G → A | bp 1100 change Glu → Lys |
| Xav571 | 2001 | Goldsboro | I | NC | G → A | bp 1100 change Glu → Lys |
| Xav361 | 1998 | Ft. Pierce | I | FL | G → C | bp 1386 change Arg → Pro |
| Xav467 | 1999 | Southeastern Florida no. 2 | I | FL | G → C | bp 1386 change Arg → Pro |
| Xav487 | 1999 | Ft. Pierce | I | FL | G → C | bp 1386 change Arg → Pro |
| Xav554 | 2000 | South Florida no. 3 | I | FL | G → C | bp 1386 change Arg → Pro |
| Xav376 | 1995 | SHRS; research plot | S | NC | IS insertion | Start bp 113 |
Pattern of plasmid profiles; each uppercase letter(s) represents a unique plasmid profile.
Base pair positions are based on GenBank accession no. AF114720.
Strains isolated from a “research plot” evolved from wild-type strains collected from the southeastern United States and released into the plots. SHRS, Sandhills Research Station.
Nucleotide sequence accession numbers.
The sequences of genomic fragments 1 to 5 and the IGS consensus sequence for the 55 Xav strains have been deposited in GenBank under accession numbers AY613941 to AY613945 and AY613946, respectively.
RESULTS
Strain classification.
Although the species designation Xanthomonas campestris pv. vesicatoria is still used to describe bacteria that cause bacterial spot disease in peppers and tomatoes, it has been shown that the bacteria classified within this species can be classified into four distinct groups (termed groups A, B, C, and D), constituting at least three separate species (33, 65, 74). Based on the HrpB digestion patterns (53), our initial sample of 36 Xanthomonas pathogens consisted purely of group A strains (Table 1). In addition, our second set of 19 Xanthomonas strains known to evade Bs2 gene detection (i.e., carrying mutated avrBs2 alleles) were also all group A strains. Suggested names for the A group species are Xanthomonas axonopodis pv. vesicatoria (74) and Xanthomonas campestris pv. vesicatoria (60). In this paper, we use the nomenclature of Vauterin et al. (74) and refer to the A group species as Xanthomonas axonopodis pv. vesicatoria.
Strains were also tested for their ability to degrade starch (amylolytic ability). Generally, group A strains are expected to be amylolytically deficient, although exceptions have been noted (12, 33). Comparison of amylolytic ability and species designation revealed that three strains were amylolytic strains (5.5%) (Table 1). Amylolytic group A strains have been previously identified in North and Central America and are considered a subgroup (A1) within the A group (33). Some other variation was observed among the X. axonopodis pv. vesicatoria strains during routine culturing, both for colony morphology and clumping (flocculence) in liquid culture (Table 1). Disease and defense response phenotypes of the X. axonopodis pv. vesicatoria strains were indistinguishable from each other when used to infect susceptible and resistant pepper plants, respectively.
Identification of X. axonopodis pv. vesicatoria chromosomal fragments.
Completion of the genomic sequences of the related pathogens Xanthomonas axonopodis pv. citri strain 306 (NC_003919) and Xanthomonas campestris pv. campestris strain ATCC 33913 (NC_003902) after the start of this project allowed us to further identify the five random chromosomal genes we analyzed for genetic variation. For all BLASTn search results, a sequence from Xanthomonas axonopodis pv. citri had the highest similarity to X. axonopodis pv. vesicatoria genomic sequences, with a sequence from Xanthomonas campestris pv. campestris the next closest match. (Percent nucleotide identities for X. campestris pv. campestris sequences were generally 1 to 15% less similar than X. axonopodis pv. citri sequences.) The first 300 bp of X. axonopodis pv. vesicatoria genomic fragment 1 (AY613941) has no homology to any sequence; the remaining 309 bp has 94% identity to a stretch of DNA from X. axonopodis pv. citri containing a tRNA glycine (Xac3299) gene and the first 116 bp of estA, a lipase/esterase gene (Xac3300). The first 55 bp of X. axonopodis pv. vesicatoria fragment 2 (AY613942) are 94% identical to the end of a two-component system regulatory protein (Xac3992), while the last 235 bp are 92% identical to the beginning of a conserved hypothetical protein (Xac3993). The 173-bp intervening region between these two genes is less well conserved between X. axonopodis pv. vesicatoria and X. axonopodis pv. citri (57% identical). X. axonopodis pv. vesicatoria fragment 3 (AY613943) is a partial sequence of another conserved hypothetical protein (Xac1554) with 91% identity over the fragment. X. axonopodis pv. vesicatoria fragment 4 (AY613944) is a partial sequence of fhuE (Xac3370), an outer membrane receptor for the uptake of ferric iron, with 91% identity over the fragment. Finally, X. axonopodis pv. vesicatoria fragment 5 (AY613945) is a partial sequence of the response regulator vieA (Xac2868) with 95% identity over the fragment. Assuming that the gene order in X. axonopodis pv. vesicatoria is highly colinear with the X. axonopodis pv. citri genome, we were able to build an approximate map of where the X. axonopodis pv. vesicatoria genomic fragment sequences lie within the chromosome (Fig. 1). Colinearity between X. axonopodis pv. vesicatoria and X. axonopodis pv. citri is likely, given that 85% of the genes shared between the more distantly related X. axonopodis pv. citri and X. campestris pv. campestris genomes are colinear (20).
FIG. 1.
Schematic representation of the positions of the seven genomic loci from Xanthomonas axonopodis pv. vesicatoria used in this study, based on the gene order of the closely related species Xanthomonas axonopodis pv. citri strain 306 and Xanthomonas campestris pv. campestris strain ATCC 33913. Both copies of the X. axonopodis pv. vesicatoria IGS were amplified using a single set of primers.
Genetic variation within X. axonopodis pv. vesicatoria alleles of avrBs2.
Sequence analysis of avrBs2+ alleles from the initial set of 36 X. axonopodis pv. vesicatoria strains revealed extremely low levels of genetic variation. Thirty alleles were 100% identical to each other (as well as to the GenBank sequence AF114720). The remaining six alleles contained three polymorphic sites (Table 1). The first polymorphic site was shared by five X. axonopodis pv. vesicatoria strains from North and Central America and the Caribbean: Xav880 (Mexico), Xav950 (Mexico), Xav996 (Mexico), Xav374 (Barbados), and Xav75-4 (United States). This shared polymorphism consists of two adjacent base pair changes at positions 870 and 871 in the coding sequence. These two changes always occur together and affect the second and third base pairs in a single amino acid (amino acid 231), changing TTC (phenylalanine) to TCA (serine). We consider it likely that these two base pair differences are the results of a single mutational event, and hence, consider them as a single polymorphic site in our coalescent analysis below. The remaining two polymorphic sites are unique mutations found only in strain Xav235 from Guadaloupe. One of these mutations is an A-to-G transition 53 base pairs before the start codon. This mutation does not occur within the putative plant-inducible promoter that lies upstream of the coding sequence (70), and its effect on gene transcription, if any, is unknown. The second mutation within Xav235 occurs at base pair 626 in the coding sequence, changing AAG (lysine) to AGG (arginine).
Examination of the second set of 19 X. axonopodis pv. vesicatoria strains known to contain mutated (nonfunctional) avrBs2 alleles detected nine different mutations, all of which cause amino acid alterations to the avrBs2 protein (Table 3). Three of the mutations are base pair replacement changes, one strain contains the insertion of a large transposon (in GenBank as AF077016; IS1646), and the remaining five mutations represent smaller insertions or deletions. The most common mutational class, found in eight strains, is the insertion or deletion of a 5-bp CGCGC repeat; functional avrBs2+ alleles contain three copies of this repeat toward the 3′ end of the protein coding region. The next most common mutation, found in four strains, is a G-to-C transversion at position 1386. Two strains have a G-to-A transition at position 1100, and the remaining five mutations are found only once.
Genetic variation within the X. axonopodis pv. vesicatoria chromosome.
The five chromosomal DNA fragments from all 55 X. axonopodis pv. vesicatoria strains (strains carrying both avrBs2+ and mutant avrBs2 alleles) contain almost no polymorphism (Table 1). We found only two polymorphic sites within a total of 154,000 sequenced base pairs. One of these sites is unique, again in strain Xav235, and is a C-to-A transversion in genomic fragment 2 (within the conserved hypothetical protein). The second polymorphic site is a C-to-T transition occurring in fragment 5 (putative response regulator). This “T” polymorphism is found in 15 strains (27%), all of which contain an avrBs2+ allele. Interestingly, all five strains containing the 2-base-pair (870 and 871) polymorphism in avrBs2+ occur within these 15 “T” strains. The 19 X. axonopodis pv. vesicatoria strains carrying mutant avrBs2 alleles are indistinguishable from the 36 strains carrying avrBs2+ based on genetic diversity of the background chromosome.
Diversity within the IGS rRNA operon (AY613946) was also nearly absent among the 55 X. axonopodis pv. vesicatoria strains. Only a single X. axonopodis pv. vesicatoria strain, Xav458 (Costa Rica; avrBs2+), contains any polymorphisms. Within Xav458, there are two heterozygous sites (the two IGS copies in the chromosome contain a different base at each position). One site in Xav458 is heterozygous for T/C, while all other X. axonopodis pv. vesicatoria strains are homozygous for T. The second site is heterozygous for C/A, while all other X. axonopodis pv. vesicatoria strains are homozygous for C. Both of these heterozygous sites occur in a noncoding region of the IGS, after the two tRNA genes and before the 23S rRNA gene. The calculated nucleotide diversity per base pair between any two strains in X. axonopodis pv. vesicatoria is very low (π = 9.1 × 10−5), based on the 5,524-bp concatenation of all seven genomic loci.
Analysis of avrBs1.
PCR revealed the presence of avrBs1 (ORF 2) in 16 of the 55 X. axonopodis pv. vesicatoria strains (Table 1). Fifteen of the avrBs1 alleles are 100% identical to each other. The remaining strain, Xav374, contains a 1.2-kb transposable insertion sequence (IS) element insertion but otherwise is identical to the other 15 avrBs1 sequences. The IS element present in Xav374 is 98% identical to IS476, a previously described active insertion element in X. axonopodis pv. vesicatoria (36). All 16 avrBs1 alleles differ from the published avrBs1 sequence (58) (M32142) at bp 1390. The published sequence contains a G at this position, while all our sequences contain a C. This changes the published amino acid 226 (ORF 2) from TTG (leucine) to TTC (phenylalanine). A significant correlation exists between the presence of avrBs1 and the “T” polymorphism within X. axonopodis pv. vesicatoria fragment 5 (Table 1); 13 out of the 15 strains which carry a “T” at this position also contain avrBs1, while only 3 out of the 40 strains which carry a “C” contain avrBs1 (Fisher's exact test; P < 0.0001). Three strains carrying avrBs1 fail to elicit a defense response in plants carrying the corresponding Bs1 R gene. One of the three strains is Xav374 (IS insertion), but the other two strains, Xav1924 and Xav488, contain no sequence differences between them and the other functional alleles over the entire coding region.
Plasmid profiles in X. axonopodis pv. vesicatoria.
Table 4 describes the plasmid profiles found in the 55 X. axonopodis pv. vesicatoria strains; only two strains do not have any plasmids. Of the remaining 53 strains with plasmids, the number of plasmids per strain ranges from one to eight, while the majority of strains (55%) have four plasmids. A total of 20 different plasmids (based on size) were identified in the 55 strains. The most frequently observed plasmids were 35 kb (80%), 25 kb (66%), and 155 kb (66%) (Fig. 2). Most of the strains (75%) harbor one of the two largest plasmids, either 190 kb or 155 kb. While some plasmids were found to be very prevalent in the strains, the overall plasmid profiles of each strain were highly diverse. Of the 53 strains with plasmids, there were 28 different plasmid profiles. The majority of these profiles (19 out of 28, or 68%) were detected only once, and 89% of the profiles occurred no more than twice. Only three plasmid profiles (designated A, I, and P) (Table 4) were found more than twice among the 55 strains. Three out of the four A profile strains were collected before 1990. Both the I profile (found in 10 strains) and P profile (found in eight strains) were predominately found in the sample of 19 mutant avrBs2 strains collected more recently in the southeastern United States. Within this sample, 12 out of the 19 strains (63%) contain either I or P profiles.
TABLE 4.
Distribution of plasmids within X. axonopodis pv. vesicatoria
| Strain | Origin | Yr | avrBs2a | avrBs1a,b | Profilec | Approx sizes of plasmids found (kb) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xav43 | NC | 1989 | + | Yes (+) | A | 155 | 35 | 25 | 1.8 | ||||||||||||||||
| Xav75-4 | USA | 1975 | + | Yes (+) | A | 155 | 35 | 25 | 1.8 | ||||||||||||||||
| Xav133 | FL | 1993 | + | Yes (+) | B | 190 | 110 | 25 | 2.6 | 1.8 | |||||||||||||||
| Xav190 | Nicaragua | 1990 | + | Yes (+) | C | 155 | 25 | 1.8 | |||||||||||||||||
| Xav196 | Nicaragua | 1990 | + | Yes (+) | C | 155 | 25 | 1.8 | |||||||||||||||||
| Xav326 | GA | 1997 | + | Yes (+) | D | 155 | 110 | 25 | 1.8 | ||||||||||||||||
| Xav880 | Mexico | 1992 | + | Yes (+) | E | 190 | 35 | 30 | 1.8 | ||||||||||||||||
| Xav950 | Mexico | 1992 | + | Yes (+) | E | 190 | 35 | 30 | 1.8 | ||||||||||||||||
| Xav996 | Mexico | 1992 | + | Yes (+) | F | 190 | 35 | 30 | 14 | 1.8 | |||||||||||||||
| Xav1928 | Taiwan | 1989 | + | Yes (+) | A | 155 | 35 | 25 | 1.8 | ||||||||||||||||
| Xav19 | NC | 1987 | + | Yes (+) | G | 190 | 65 | 35 | |||||||||||||||||
| Xav206 | FL | 1995 | − | Yes (+) | D | 155 | 110 | 25 | 1.8 | ||||||||||||||||
| Xav294 | NC | 1995 | − | Yes (+) | A | 155 | 35 | 25 | 1.8 | ||||||||||||||||
| Xav374 | Barbados | 1990 | + | Yes (−) | H | 155 | 110 | 35 | 25 | 22 | 2.6 | ||||||||||||||
| Xav488 | Costa Rica | 1991 | + | Yes (−) | I | 155 | 65 | 35 | 25 | ||||||||||||||||
| Xav1924 | Spain | 2001 | + | Yes (−) | J | 155 | 35 | ||||||||||||||||||
| Xav76-4 | USA | 1976 | + | No | K | 65 | 35 | 25 | 1.8 | ||||||||||||||||
| Xav82-8 | FL | 1982 | + | No | L | 75 | 35 | 25 | 1.8 | ||||||||||||||||
| Xav69-1 | USA | 1969 | + | No | M | 110 | 25 | 17 | |||||||||||||||||
| Xav71-21 | USA | 1971 | + | No | N | 35 | |||||||||||||||||||
| Xav77-3 | USA | 1973 | + | No | O | 25 | |||||||||||||||||||
| Xav79 | NC | 1991 | + | No | P | 155 | 75 | 35 | 25 | ||||||||||||||||
| Xav96 | VA | 1992 | + | No | Q | 155 | 65 | 35 | 2.6 | ||||||||||||||||
| Xav135 | NC | 1993 | + | No | R | 155 | 35 | 13 | 7 | 5 | 2.6 | ||||||||||||||
| Xav178 | OH | 1994 | + | No | S | 155 | 65 | 35 | |||||||||||||||||
| Xav235 | Guadaloupe | 1990 | + | No | T | 190 | 35 | 25 | 2.6 | 1.8 | |||||||||||||||
| Xav290 | Guadaloupe | 1990 | + | No | U | 155 | 75 | 35 | 25 | 1.8 | |||||||||||||||
| Xav333 | GA | 1998 | + | No | V | 65 | 35 | ||||||||||||||||||
| Xav458 | Costa Rica | 1991 | + | No | W | 155 | 130 | 50 | 25 | 14 | 12 | 10 | 1.8 | ||||||||||||
| Xav583 | Hawaii | 1991 | + | No | X | 35 | 30 | 17 | 14 | ||||||||||||||||
| Xav600 | Bahamas | 1991 | + | No | P | 155 | 75 | 35 | 25 | ||||||||||||||||
| Xav630 | Bahamas | 1991 | + | No | P | 155 | 75 | 35 | 25 | ||||||||||||||||
| Xav699 | Puerto Rico | 1991 | + | No | P | 155 | 75 | 35 | 25 | ||||||||||||||||
| Xav851 | Hungary | 1992 | + | No | |||||||||||||||||||||
| Xav852 | Hungary | 1992 | + | No | J | 155 | 35 | ||||||||||||||||||
| Xav1103 | Senegal | 1979 | + | No | Y | 110 | 35 | ||||||||||||||||||
| Xav1925 | Spain | 1999 | + | No | |||||||||||||||||||||
| Xav1929 | Taiwan | 1989 | + | No | I | 155 | 65 | 35 | 25 | ||||||||||||||||
| Xav182 | NC | 1994 | − | No | P | 155 | 75 | 35 | 25 | ||||||||||||||||
| Xav314 | KY | 1996 | − | No | N | 35 | |||||||||||||||||||
| Xav329 | FL | 1997 | − | No | I | 155 | 65 | 35 | 25 | ||||||||||||||||
| Xav361 | FL | 1998 | − | No | I | 155 | 65 | 35 | 25 | ||||||||||||||||
| Xav376 | NC | 1995 | − | No | S | 155 | 65 | 35 | |||||||||||||||||
| Xav390 | NC | 1995 | − | No | P | 155 | 75 | 35 | 25 | ||||||||||||||||
| Xav416 | NC | 1998 | − | No | P | 155 | 75 | 35 | 25 | ||||||||||||||||
| Xav437 | FL | 1998 | − | No | Z | 65 | 35 | 25 | |||||||||||||||||
| Xav445 | FL | 1998 | − | No | I | 155 | 65 | 35 | 25 | ||||||||||||||||
| Xav190 | Nicaragua | 1990 | + | Yes (+) | C | 155 | 25 | 1.8 | |||||||||||||||||
| Xav196 | Nicaragua | 1990 | + | Yes (+) | C | 155 | 25 | 1.8 | |||||||||||||||||
| Xav326 | GA | 1997 | + | Yes (+) | D | 155 | 110 | 25 | 1.8 | ||||||||||||||||
| Xav880 | Mexico | 1992 | + | Yes (+) | E | 190 | 35 | 30 | 1.8 | ||||||||||||||||
| Xav950 | Mexico | 1992 | + | Yes (+) | E | 190 | 35 | 30 | 1.8 | ||||||||||||||||
| Xav996 | Mexico | 1992 | + | Yes (+) | F | 190 | 35 | 30 | 14 | 1.8 | |||||||||||||||
| Xav1928 | Taiwan | 1989 | + | Yes (+) | A | 155 | 35 | 25 | 1.8 | ||||||||||||||||
| Xav19 | NC | 1987 | + | Yes (+) | G | 190 | 65 | 35 | |||||||||||||||||
| Xav206 | FL | 1995 | − | Yes (+) | D | 155 | 110 | 25 | 1.8 | ||||||||||||||||
| Xav294 | NC | 1995 | − | Yes (+) | A | 155 | 35 | 25 | 1.8 | ||||||||||||||||
| Xav374 | Barbados | 1990 | + | Yes (−) | H | 155 | 110 | 35 | 25 | 22 | 2.6 | ||||||||||||||
| Xav488 | Costa Rica | 1991 | + | Yes (−) | I | 155 | 65 | 35 | 25 | ||||||||||||||||
| Xav1924 | Spain | 2001 | + | Yes (−) | J | 155 | 35 | ||||||||||||||||||
| Xav76-4 | USA | 1976 | + | No | K | 65 | 35 | 25 | 1.8 | ||||||||||||||||
| Xav82-8 | FL | 1982 | + | No | L | 75 | 35 | 25 | 1.8 | ||||||||||||||||
| Xav69-1 | USA | 1969 | + | No | M | 110 | 25 | 17 | |||||||||||||||||
| Xav71-21 | USA | 1971 | + | No | N | 35 | |||||||||||||||||||
| Xav77-3 | USA | 1973 | + | No | O | 25 | |||||||||||||||||||
| Xav79 | NC | 1991 | + | No | P | 155 | 75 | 35 | 25 | ||||||||||||||||
| Xav96 | VA | 1992 | + | No | Q | 155 | 65 | 35 | 2.6 | ||||||||||||||||
| Xav135 | NC | 1993 | + | No | R | 155 | 35 | 13 | 7 | 5 | 2.6 | ||||||||||||||
| Xav178 | OH | 1994 | + | No | S | 155 | 65 | 35 | |||||||||||||||||
| Xav235 | Guadaloupe | 1990 | + | No | T | 190 | 35 | 25 | 2.6 | 1.8 | |||||||||||||||
| Xav290 | Guadaloupe | 1990 | + | No | U | 155 | 75 | 35 | 25 | 1.8 | |||||||||||||||
| Xav333 | GA, USA | 1998 | + | No | V | 65 | 35 | ||||||||||||||||||
| Xav458 | Costa Rica | 1991 | + | No | W | 155 | 130 | 50 | 25 | 14 | 12 | 10 | 1.8 | ||||||||||||
| Xav583 | Hawaii | 1991 | + | No | X | 35 | 30 | 17 | 14 | ||||||||||||||||
| Xav600 | Bahamas | 1991 | + | No | P | 155 | 75 | 35 | 25 | ||||||||||||||||
| Xav630 | Bahamas | 1991 | + | No | P | 155 | 75 | 35 | 25 | ||||||||||||||||
| Xav699 | Puerto Rico | 1991 | + | No | P | 155 | 75 | 35 | 25 | ||||||||||||||||
| Xav851 | Hungary | 1992 | + | No | |||||||||||||||||||||
| Xav852 | Hungary | 1992 | + | No | J | 155 | 35 | ||||||||||||||||||
| Xav1103 | Senegal | 1979 | + | No | Y | 110 | 35 | ||||||||||||||||||
| Xav1925 | Spain | 1999 | + | No | |||||||||||||||||||||
| Xav1929 | Taiwan | 1989 | + | No | I | 155 | 65 | 35 | 25 | ||||||||||||||||
| Xav182 | NC | 1994 | − | No | P | 155 | 75 | 35 | 25 | ||||||||||||||||
| Xav314 | KY | 1996 | − | No | N | 35 | |||||||||||||||||||
| Xav329 | FL | 1997 | − | No | I | 155 | 65 | 35 | 25 | ||||||||||||||||
| Xav361 | FL | 1998 | − | No | I | 155 | 65 | 35 | 25 | ||||||||||||||||
| Xav376 | NC | 1995 | − | No | S | 155 | 65 | 35 | |||||||||||||||||
| Xav390 | NC | 1995 | − | No | P | 155 | 75 | 35 | 25 | ||||||||||||||||
| Xav416 | NC | 1998 | − | No | P | 155 | 75 | 35 | 25 | ||||||||||||||||
| Xav437 | FL | 1998 | − | No | Z | 65 | 35 | 25 | |||||||||||||||||
| Xav445 | FL | 1998 | − | No | I | 155 | 65 | 35 | 25 | ||||||||||||||||
| Xav454 | FL | 1998 | − | No | P | 155 | 75 | 35 | 25 | ||||||||||||||||
| Xav467 | FL | 1999 | − | No | I | 155 | 65 | 35 | 25 | ||||||||||||||||
| Xav487 | FL | 1999 | − | No | I | 155 | 65 | 35 | 25 | ||||||||||||||||
| Xav516 | FL | 1999 | − | No | AA | 155 | 65 | 35 | 30 | 22 | |||||||||||||||
| Xav536 | FL | 2000 | − | No | I | 155 | 65 | 35 | 25 | ||||||||||||||||
| Xav544 | KY | 2000 | − | No | BB | 110 | |||||||||||||||||||
| Xav554 | FL | 2000 | − | No | I | 155 | 65 | 35 | 25 | ||||||||||||||||
| Xav571 | NC | 2001 | − | No | I | 155 | 65 | 35 | 25 | ||||||||||||||||
| Sumd | 6 | 36 | 1 | 7 | 10 | 18 | 1 | 44 | 5 | 36 | 2 | 2 | 3 | 1 | 1 | 1 | 1 | 1 | 5 | 17 | |||||
| %e | 11 | 66 | 2 | 13 | 18 | 33 | 2 | 80 | 9 | 66 | 4 | 6 | 2 | 2 | 2 | 2 | 2 | 2 | 9 | 31 | |||||
Functionality of avrBs1 and avrBs2, as defined by the ability to elicit a hypersensitive response in resistant plants (+) lack of ability (−).
Presence (yes) or absence (no) of an avrBs1 gene in a strain.
Pattern of plasmid profiles; each uppercase letter(s) represents a unique plasmid profile.
Total number of times a plasmid of a given size was found in one of the 55 X. axonopodis pv. vesicatoria strains.
Percentage of strains carrying a plasmid of a given size [percent = (sum/55) × 100].
FIG. 2.
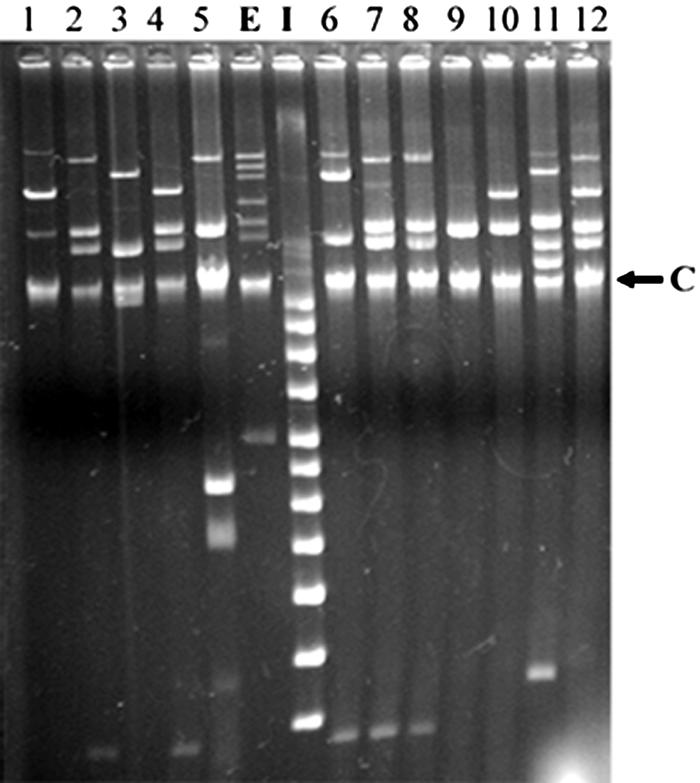
Plasmid profiles of X. axonopodis pv. vesicatoria strains. A representative gel of plasmid DNAs from 12 X. axonopodis pv. vesicatoria strains, showing the diversity of the plasmid profiles. Lanes E and I are the two supercoiled DNA ladders. E (BAC-Tracker; Epicentre) ladder: 165, 120, 95, 55, 38, 28, and 8 kb. I (supecoiled DNA ladder; Invitrogen) ladder: 16, 14, 12, 10, 8, 7, 6, 5, 4, 3, and 2 kb. The arrow points to nonplasmid/chromosomal DNA. In lanes 1 to 12 are X. axonopodis pv. vesicatoria strains: Xav19, -43, -69-1, -76-4, -135, -206, -290, -294, -314, -333, -374, and -571.
The avrBs1 gene has been shown to reside on a large (approximately 190-kb), transmissible plasmid with X. axonopodis pv. vesicatoria strains (66). We found that all strains carrying a copy of the avrBs1 gene contained a large plasmid, either 155 or 190 kb. However plasmids of these sizes are not confined solely to strains carrying avrBs1; indeed, 75% of all X. axonopodis pv. vesicatoria strains harbor one of these two plasmids. In addition, there was not a single plasmid size that correlated with a particular phenotype (i.e., colony morphology, amylolytic ability, or flocculence). The only significant correlation between a particular plasmid size and a polymorphism was between strains harboring the “T” polymorphism within X. axonopodis pv. vesicatoria fragment 5 and the presence of the 1.8-kb plasmid (Fisher's exact test; P < 0.0001). Interestingly, the only strain to contain variation within the IGS region, Xav458, also has a significantly different plasmid profile: Xav458 contains the highest number of plasmids detected within a strain, and four of its eight plasmids were found in no other X. axonopodis pv. vesicatoria strain.
Coalescent analysis of Xanthomonas species.
The extremely low level of genetic variation found in X. axonopodis pv. vesicatoria implies that the current X. axonopodis pv. vesicatoria population can trace its origin to a very recent common ancestor. This could have resulted from a recent selective sweep or a population bottleneck followed by recent expansion. These two explanations are both consistent with the results of Tajima's D test on the concatenated sequences for the 55 X. axonopodis pv. vesicatoria strains, which yielded a negative value of D (D = −1.267; neutral expectation, D = 0). However, we cannot reject the null hypothesis of neutral evolution and constant population size due to the marginal significance of the P value (P = 0.090) from the Monte Carlo simulations on the data.
Because we could not formally reject the null hypothesis, we used two calculations to determine the time back to the MRCA of X. axonopodis pv. vesicatoria. One equation (equation 1) assumes a recent population bottleneck and/or selective sweep, and hence, expects the strains to have a star-like phylogeny (61). The other equation (equation 4) assumes a neutral model of evolution with a constant population size but no specific phylogenetic relationship among strains. For these equations, L is 5,524 bp, n is 55 strains, π is 9.1 × 10−5, and s is 7. To estimate ν, we assumed a neutral evolutionary mutation rate of 0.0034 per genome per generation, based on the empirical calculations for DNA-based microbes of Drake et al. (22, 23). We estimated the genome size of X. axonopodis pv. vesicatoria (5,126 kb) by taking the average of the published genome sequences of the two close Xanthomonas relatives, X. axonopodis pv. citri (5,176 kb) and X. campestris pv. campestris (5,076 kb) (20). The neutral mutation rate for Xanthomonas was thus calculated as follows: v = 0.0034/5,126,000 bp = 6.63 × 10−10 mutations per base pair per generation. This estimate of the Xanthomonas neutral mutation rate is consistent with the empirical calculation of the neutral mutation rate (1.44 × 10−10) for Escherichia coli by Lenski et al. (43). With these parameters, equation 1 (which assumes a recent bottleneck/selective sweep) yields an estimated G of 3.48 × 104 bacterial generations back to the MRCA, with confidence intervals as follows: GU = 7.16 × 104 and GL = 1.40 × 104 (equations 2 and 3). Equation 4 (which assumes neutral evolution) yields a higher estimate: G = 1.37 × 105 generations back to the MRCA.
To translate the number of generations into years, it is necessary to know the number of bacterial generations that occur per year, which is very difficult to estimate. To determine an estimate of the number of bacterial generations per year, we first calculated the expected number of bacterial generations per day in infected plant tissue. We then estimated the length (in days) of the pepper growing season per year; this yields a maximum estimate of bacterial generations per year. Specifically, we determined r, the intrinsic growth rate of X. axonopodis pv. vesicatoria, by using the equation nt = n0ert. The parameters nt and n0 were determined using data (39, 77) on the ability of X. axonopodis pv. vesicatoria to grow in pepper plants under optimal greenhouse conditions. In an average growth curve experiment, n0 is 5 × 102 cells, and after a t of 5 days, nt is 5 × 107, yielding an r of 2.3026. The doubling time of a species is equal to (ln 2)/r; therefore, the doubling time of X. axonopodis pv. vesicatoria in a growth curve infection under optimal conditions with unlimited susceptible plant tissue is 7.22 h, or 3.32 generations per day. Although X. axonopodis pv. vesicatoria may overwinter in dead plant tissue or seeds, most bacterial reproduction is expected to occur in infections of host plants because X. axonopodis pv. vesicatoria does not survive long in the soil and is not a good epiphyte (34, 55). The pepper-tomato growing season lasts up to 4 months, but susceptible plant tissue will be depleted from the field rather quickly during a severe epidemic (maximum bacterial replication). An experimental X. axonopodis pv. vesicatoria epidemic of cultivated pepper fields found that approximately 6 to 8 weeks after inoculation, very little susceptible pepper plant tissue remains uninfected (40). In warmer climates, three crops may be planted within a single year. This leads us to estimate the number of bacterial generations per year to be no more than 500 generations (3.32 generations per day × 50 days of susceptible tissue per crop × 3 crops per year). Indeed, this is probably a gross overestimate, since such a high, constant reproductive rate is unrealistic under natural conditions in the field. Hence, we arbitrarily set a much more conservative lower limit of 50 bacterial generations per year. Assuming, then, a range of 50 to 500 bacterial generations per year, the time back to the MRCA of species X. axonopodis pv. vesicatoria, using equation 1, is estimated to be 28 (i.e., GL/500) to 1,432 (i.e., GU/50) years ago. Using the point estimate from equation 2, we obtain a slightly longer time back to the MRCA, namely, 274 to 2,740 years ago.
DISCUSSION
Lack of genetic diversity within the X. axonopodis pv. vesicatoria (group A) species.
Within the last 10 years, it has become clear that the bacterial pathogen known as “Xanthomonas campestris pv. vesicatoria” is actually made up of four distinct groups (A, B, C, and D) representing at least three distinct species (33, 65, 74). The vast majority of bacterial spot disease on pepper and tomato is caused by groups A and B; group C has a limited distribution, and group D is very rare (35). Based on reactions to antibodies, enzymatic tests, DNA-DNA hybridization studies, and rRNA sequencing, the groups A (Xanthomonas axonopodis pv. vesicatoria) and B (Xanthomonas vesicatoria) have been shown to be distinct bacterial species (33). The distributions of these two species among host plants are not uniform: group A bacteria infect peppers and tomatoes equally, while group B predominately infects tomatoes (13). Given that all the strains used in this study were collected from infected peppers, it is not surprising that all of them are group A (X. axonopodis pv. vesicatoria).
Within our 55 X. axonopodis pv. vesicatoria strains, we found extremely low levels of variation in both the avr genes and the bacterial chromosomes. The most variable strain (Xav235) contains only three base pair changes over a total 5.5 kb of sequenced genomic DNA. The lack of variation in X. axonopodis pv. vesicatoria is unusual compared to other pathogenic xanthomonads. For example, four published studies of carefully defined Xanthomonas species or pathovars all found very deep levels of diversity among bacteria collected over a wide geographic area, with the greatest diversity often found in areas of host plant origin: X. campestris pv. mangiferaeindicae (mango bacterial black spot) (26), X. campestris pv. campestris and X. campestris pv. armoraciae (crucifer black rot) (2), X. axonopodis pv. manihotis (cassava bacterial blight) (57), and X. oryzae pv. oryzae (rice bacterial blight) (44). Similarly, a recent study of many pathovars of the closely related pathogen Pseudomonas syringae found deep diversity among the core genomes, suggesting that this pathogen has been an endemic plant pathogen over a long span of evolutionary time (59). Finally, studies of human bacterial pathogens have found that many species contain a high level of genetic diversity (25, 28, 51, 63).
Two possible biases may have led to a skewed view of the genetic variation in this species. First, our samples are restricted to strains found on peppers, whereas X. axonopodis pv. vesicatoria is also commonly found infecting tomatoes. However, a study of diagnostic PCRs on a worldwide sample of X. axonopodis pv. vesicatoria strains (X. campestris pv. vesicatoria group A) collected from both peppers and tomatoes showed that the tomato strains are neither more variable than nor differentiated from strains collected from peppers (46). The second possible bias comes from the fact that all our samples came from diseased plant tissue. It is known from the medical literature on human bacterial pathogens that epidemics are often caused by a single clone or group of closely related clones (51, 63), suggesting that diseased hosts may not carry all the variation present in a bacterial population. We suspect that such a subsampling issue is not relevant for X. axonopodis pv. vesicatoria because these bacteria do not survive long outside of plant tissue or in the soil (34, 55). In addition, the studies of genetic diversity among other Xanthomonas species cited above all found high levels of genetic diversity when collecting was done solely from diseased plant tissue. Finally, the strains we analyzed were collected over a 33-year period, making it very unlikely that our samples are the result of the spread of a single epidemic. Nonetheless, it is important to note that all the X. axonopodis pv. vesicatoria strains examined in this study came from cultivated peppers. We cannot rule out the possibility that the genetic profile of X. axonopodis pv. vesicatoria (group A) strains associated with wild, uncultivated pepper and tomato species may be different from the genetic profile seen in X. axonopodis pv. vesicatoria strains that infect cultivated plants. Neither can we rule out the possibility that the genetic variation may be different for those regions of the world from which we did not have samples. Therefore, the results of this study are limited to the populations of X. axonopodis pv. vesicatoria infecting cultivated peppers and tomatoes in the regions of the world that were sampled.
Although we found very little genetic variation in the chromosome of X. axonopodis pv. vesicatoria, several studies have shown phenotypic variation among pepper and tomato leaf spot pathogens for traits such as carbon utilization, pectolytic and amylolytic activity, serology, and fatty acid profiles (12, 13, 33, 65). We also found some phenotypic diversity among our X. axonopodis pv. vesicatoria strains (Table 1). One possible explanation for this dichotomy between genetic and phenotypic variation is the introduction of novel DNA sequences into the chromosome by large mobile elements, such as bacteriophages and transposons. These mobile DNA elements are known to be important sources of novel genetic variation in bacterial evolution and often contain genes important to bacterial fitness and virulence (7, 54). The analysis presented here is not extensive enough to determine if variations in transposon or phage integrations have occurred within our sample of X. axonopodis pv. vesicatoria chromosomes, possibly contributing to the phenotypic differences observed. However, the work of Louws et al. (46) suggests that large-scale genomic differences among X. axonopodis pv. vesicatoria strains are unlikely.
Plasmid profiles and variation.
A second possible source of phenotypic variation within X. axonopodis pv. vesicatoria strains may be encoding by the many different endogenous plasmids this species harbors. Bacterial plasmids are known to encode a wide variety of ecologically important traits, such as antibiotic resistance (21), degradation of xenobiotic organic compounds (73), resistance to heavy metals (13, 73), pathogenic virulence factors (32, 75), and various other phenotypic traits, including pigmentation and mucoidy (18, 75). Our results are consistent with the report of Canteros et al. (15), which found a large diversity of plasmids (up to 300 kb) among Xanthomonas pathogens collected from peppers and tomatoes (both groups A and B). Within our sample, 69% of the X. axonopodis pv. vesicatoria strains contain four or more plasmids, and the strains harbor at least 20 different plasmids; this level of plasmid diversity is seen consistently over time within our collection as well. Because we only categorized the plasmids by size, it is likely that several different plasmids were classified within the same size category, and therefore, our estimate of plasmid diversity within X. axonopodis pv. vesicatoria is conservative. X. axonopodis pv. vesicatoria appears to contain a higher number of plasmids than is usually observed within soil- or plant-associated bacteria. Although studies of plasmid diversity among a global sample of a particular bacterium are not common, examinations of plasmids within populations of various Pseudomonas syringae pathovars, Xanthomonas species, and Bacillus species have generally found that the bacteria carry zero, one, or two plasmids per strain (3, 6, 17, 42, 68, 69, 78). Still, the relatively high number of plasmids observed within X. axonopodis pv. vesicatoria is not unprecedented. A study of plasmids within a global sample of the closely related pathogen Xanthomonas axonopodis pv. citri found a high degree of plasmid diversity and that strains harboring three or four plasmids were common (56). In addition, Escherichia coli has been shown to carry on average four plasmids per strain (14). The presence of so many different plasmids within X. axonopodis pv. vesicatoria offers the species a potentially large source of genetic variation.
We were unable to detect a significant correlation between a single plasmid size and most of the phenotypic and/or genetic variation observed within the 55 X. axonopodis pv. vesicatoria strains in our sample, including the presence or absence of avrBs1. avrBs1 has been shown to be associated with a large plasmid (pXvCu) that also carries genes for copper resistance and streptomycin resistance (66). While all of the X. axonopodis pv. vesicatoria strains in our sample that contain avrBs1 also contain a large plasmid (155 kb to 190 kb), many strains negative for the avrBs1 gene also contain large plasmids. This result is typical of studies that try to find a correlation between plasmids categorized by size and a particular phenotypic trait. For example, one recent study of plasmids conferring copper and streptomycin resistance in the plant pathogenic bacterium Pseudomonas syringae pv. syringae found that a ubiquitous, 62-kb plasmid often contained genes for copper resistance, but the same-size plasmid in copper-sensitive strains did not contain these genes (17). Conversely, it has been shown that plasmid-encoded traits, such as antibiotic resistance or metal tolerance, are often located on different-size plasmids within a single species (17, 69). The heterogenic nature of plasmids is not surprising given the ability of plasmids to readily exchange genetic information, due to their ability to self-mobilize into other bacterial strains and species, as well their possession of other mobile genetic elements, such as transposons. Indeed, an examination of recombination within several conjugative plasmids in E. coli found extensive recombination among plasmid genes, with rates much higher than that detected for chromosomal genes (14).
Variation within avr gene alleles.
The most straightforward way to interpret avr-driven evolutionary dynamics is to detect a difference between the evolutionary history of an avr gene sequence and those of other chromosomal genes. However, we detected no such differences between the X. axonopodis pv. vesicatoria chromosome and avrBs2 sequences. Instead, we found very low levels of genetic variation throughout the entire genome. The 16 alleles of avrBs1 identified in X. axonopodis pv. vesicatoria are also nearly uniform, although the gene is variable in its presence or absence among strains. Interestingly, the avrBs1 alleles identified in our X. axonopodis pv. vesicatoria strains are 100% identical to the avrBs1 allele found in the chromosome of X. campestris pv. campestris (20). This perfect identity is at odds with the rest of the X. axonopodis pv. vesicatoria and X. campestris pv. campestris genomic sequences, which are generally between 80 and 90% identical at the nucleotide level, and with the avrBs2 genes from X. axonopodis pv. vesicatoria and X. campestris pv. campestris (Xcc0052), which are 78% identical at the nucleotide level and differ in the presence of an additional four amino acids in X. campestris pv. campestris. (In contrast, the avrBs2 genes from X. axonopodis pv. vesicatoria and X. axonopodis pv. citri [Xac0076] are 96% identical at the nucleotide level). The perfect identity between the X. campestris pv. campestris avrBs1 gene and the avrBs1 alleles found in our X. axonopodis pv. vesicatoria strains suggests that X. campestris pv. campestris may have acquired the avrBs1 gene recently via horizontal gene transfer. This speculation is supported by the fact that the X. campestris pv. campestris avrBs1 gene is surrounded by four different transposases, two on either side.
Recent evolution of avrBs2 within X. axonopodis pv. vesicatoria.
The R gene Bs2 has been introduced extensively into cultivated pepper within the past decade. Indeed, by the mid-1990s, many cultivated pepper varieties in the United States carried at least one copy of this R gene (39). Pepper resistance by Bs2 has proven effective in controlling bacterial spot; however, new strains of X. axonopodis pv. vesicatoria that evade Bs2 detection were quickly found in pepper fields (39, 41). Indeed, before the mid-1990s, only X. axonopodis pv. vesicatoria strains carrying wild-type avrBs2 (avrBs2+) had been detected. Although X. axonopodis pv. vesicatoria strains harboring mutated alleles of avrBs2 are now common in the southeastern United States, the prevalence of these strains within the population has not yet been reported. Therefore, we calculated the frequency of mutated avrBs2 alleles in X. axonopodis pv. vesicatoria strains collected from pepper and tomato fields in the southeastern United States between the years 1986 and 2000 (Fig. 3). Examination of Fig. 3 shows that bacterial populations were nearly fixed for wild-type avrBs2 until 1997, when mutant avrBs2 alleles were detected at significant levels (10%) for the first time. By the year 2000, the amount of mutant avrBs2 in bacterial populations had reached around 50%.
FIG. 3.
Frequencies of avrBs2 mutant alleles in X. axonopodis pv. vesicatoria populations. X. axonopodis pv. vesicatoria strains were isolated by D. Ritchie from cultivated pepper and tomato plants in the southeastern United States. Use of resistant (Bs2) pepper plants became widespread in the mid-1990s.
Previous work has shown that the loss of avrBs2 in X. axonopodis pv. vesicatoria leads to reduced fitness of the bacteria, i.e., in planta growth reduction (37), reduced disease transmission, delayed symptom development, and reduced epiphytic survival (77). Despite these fitness costs, both field and greenhouse tests have shown that strains containing mutated avrBs2 alleles are still pathogenic and can cause significant disease and crop loss (27, 39, 41, 77). Nevertheless, the fact that 50% of X. axonopodis pv. vesicatoria strains in the field still carry the avrBs2+ allele suggests that avrBs2+ may be selectively favored in some situations. Total loss of avrBs2 function does not appear to be a good evolutionary pathway for the bacteria, at least when pathogens still have the chance of infecting a nonresistant host.
Analysis of avrBs2 alleles from strains that evade R gene detection revealed evidence of recent selection for mutations that affect the encoded protein, including large transposon insertions, frameshifting insertions/deletions, and single amino acid changes (Table 3) (27). A very similar pattern of nucleotide change was observed among nonfunctional alleles of the avr genes avrD from Pseudomonas syringae pv. glycinea and avrPphE from Pseudomonas syringae pv. phaseolicola (38, 67). For these two avr genes, most mutations within the nonfunctional alleles were not synonymous but were single base pair changes affecting critical amino acids. We were not surprised to find that the most common mutation within our sample of 19 mutant avrBs2 alleles in Xanthomonas is a change in the repeat structure of the 5-bp CGCGC repeat. This area of the gene would be expected to have a higher mutation rate, due to the possibility of polymerase slippage during replication (24). Aside from the mutations in avrBs2 listed in Table 3, the 19 X. axonopodis pv. vesicatoria strains carrying mutant avrBs2 alleles were identical (they contained no synonymous changes) to the consensus sequence from the 36 avrBs2+ alleles. We conclude from these data that the 19 X. axonopodis pv. vesicatoria strains carrying mutant avrBs2 alleles in our sample were recently derived from the local X. axonopodis pv. vesicatoria population, which harbored the ubiquitous avrBs2+ allele. Further, the six additional sequenced chromosomal loci from the 19 X. axonopodis pv. vesicatoria strains carrying mutated avrBs2 were 100% identical to the consensus sequences of the 36 X. axonopodis pv. vesicatoria strains carrying avrBs2+, indicating that the release of resistant pepper varieties in the United States has not resulted in an altered pattern of X. axonopodis pv. vesicatoria chromosomal variation.
An examination of the plasmid profiles within the strains carrying mutated avrBs2 alleles supports the idea that a few avrBs2 mutations arose recently within a few strains. All strains sharing a particular mutation (excluding loss or gain of the 5-bp CGCGC repeat) also share the same plasmid profile (Table 3). This result seems uncharacteristic for a species with such high plasmid diversity. Instead, it seems more likely that selection has spread a few founding isolates, carrying mutated avrBs2, throughout the greater X. axonopodis pv. vesicatoria population. The only class of mutation not to show a conserved plasmid profile is the expansion/contraction of the 5-bp CGCGC repeat (although one plasmid profile is found in three out of the six strains carrying a 5-bp insertion). Again, because the frequency of this particular mutation is expected to be higher than single base pair changes or other genomic insertions/deletions, it is not surprising that changes within the CGCGC repeat structure appear to have arisen independently in several different X. axonopodis pv. vesicatoria strains.
Under an arms race model, the pathogen is expected to accumulate discrete sequence changes that maintain gene function (i.e., full virulence) but evade R gene detection. We did not find this pattern for the majority of our mutant alleles. Instead, five mutations, found in 58% of the mutant strains, are predicted to completely alter a significant proportion of the avrBs2 protein sequence, which presumably has a detrimental effect on protein function. Without a functioning Avr protein, it is unlikely that a host plant could mount an evolutionary response that would lead to avr gene recognition, as would be expected under an arms race model. However, one of the mutations we found in our set of 19 mutant avrBs2 alleles has been shown to be consistent with an evolutionary arms race. In particular, Gassmann et al. (27) showed that the G-to-C transversion at 1386 retains virulence (as measured by in planta bacterial growth) on resistant plants yet also completely evades Bs2 gene detection. This particular mutation was found in 21% of our mutant strains, the second most common mutation in our study. Another mutation identified by Gassmann et al., but one we did not find in our study, is moderately consistent with an arms race model. This mutation is a C-to-A transversion at 1407 and results in partial bacterial virulence and partial plant defense induction in resistant plants. It is unclear how the three untested, in-frame, nonsynonymous mutations uncovered in our study (the 12-bp insertion at 1305, the CC-to-AA transversion at 1165 to 1166, and the G-to-A transversion at 1100) would affect avrBs2 function/virulence. In addition, it is important to remember that secondary mutations may arise in another gene which compensate for a mutation in avrBs2, restoring full virulence to the pathogen. Further study of a possible arms race between avrBs2 and Bs2 would be best served by focusing on the avrBs2 mutations that have little or no effect on pathogen fitness. This, however, would require a more thorough understanding of the impact of a particular avrBs2 mutation on bacterial fitness, such as field analysis of transmission and lesion development. It would also be necessary to determine if secondary mutations within other genes are able to suppress any fitness costs associated with avrBs2 mutations.
Evolutionary interpretation of low genetic variation in X. axonopodis pv. vesicatoria.
There are two possible explanations for the reduced level of genetic variation we observed in X. axonopodis pv. vesicatoria. Either the population experienced a bottleneck in the effective population size in the recent past, followed by population expansion to its current level, or some gene(s) on the chromosome was subjected to a selective sweep with insufficient recombination to reduce genetic hitchhiking. The extents of recombination among different bacterial populations are highly variable (24a, 28, 28a, 62a, 62b). Because of the lack of genetic diversity within our X. axonopodis pv. vesicatoria strains, we were unable to detect any recombination among the genomic sequences, although a recent report by Sarkar and Guttmann (59) found very low levels of recombination among many pathovars of the plant pathogen Pseudomonas syringae.
A recent selective sweep would be consistent with the predictions of an arms race between avr and R genes. However, it is impossible to know which gene(s) was the target of selection during the sweep, since the reduction in genetic diversity extends throughout the X. axonopodis pv. vesicatoria chromosome (71). A negative Tajima's D, such as we saw (Tajima's D = −1.267), indicates that there is an overabundance of rare (singleton) alleles in the population of sequences, supporting the occurrence of a population bottleneck or selective sweep. Note, however, that the null hypothesis of neutral evolution and constant population size could not be rejected due to the marginally significant P value (P = 0.09). This lack of power may stem from the near absence of genetic variation among the X. axonopodis pv. vesicatoria sequences (π = 9.1 × 10−5). In other words, this test relies on the presence of genetic variation; if all sequences are 100% identical, then Tajima's D will equal zero, even though a complete lack of genetic variation would indicate nonneutral evolution within a population.
The lack of genetic variation in X. axonopodis pv. vesicatoria implies that the present-day population can trace its ancestry back to a relatively recent common ancestor. Although we cannot formally reject the null hypothesis that the current X. axonopodis pv. vesicatoria population has evolved neutrally, with a constant population size, from its MRCA, we believe that the pattern of extremely low genetic diversity in the X. axonopodis pv. vesicatoria strains supports the hypothesis that this species has undergone a recent population bottleneck and/or selective sweep. For this reason, we believe equations 1 to 3 are a better calculation of the number of bacterial generations that have passed since the MRCA of the current X. axonopodis pv. vesicatoria population. Using these equations, we estimate that the modern population of X. axonopodis pv. vesicatoria coalesces to an MRCA roughly 14,000 to 71,600 bacterial generations ago. Assuming a conservative estimate of 50 bacterial generations per year, this yields an upper limit of approximately 1,400 years back to the MRCA (or around AD 500 to 600). Increasing the number of bacterial generations to 500 per year yields a dramatically lower limit of only 28 years back to the MRCA.
It is interesting that this range of coalescent dates coincides well with human intervention with the two host plants, pepper (Capsicum spp.) and tomatoes (Lycopersicon spp.). The domestication of pepper in the New World is thought to have been completed between 1500 and 900 BC (48). While the domestication of the tomato is not as well known, it is thought to have occurred much later, perhaps as late as AD 700. Further human manipulation of these two species came in the guise of Western conquerors, who brought peppers and tomatoes back from the New World in the AD 1500s; cultivation of these two crops, especially peppers, quickly spread across the globe (11). It is possible that X. axonopodis pv. vesicatoria underwent a population bottleneck or selective sweep, or both, due to its association with pepper and/or tomato during their domestication or later dissemination throughout the world. Alternatively, large-scale modern agricultural changes within the 20th century (including the development of global distribution of hybrid seed produced in a few areas of world) may have selected for and/or disseminated a single dominant clone of X. axonopodis pv. vesicatoria throughout the world. Although we cannot know for certain the historical event(s) which led to the reduced genetic variation within X. axonopodis pv. vesicatoria strains that infect cultivated plants, it is evident from the work presented here that the strains come from a highly clonal population. Additionally, it is highly probable that the population can trace its lineage back to a very recent common ancestor. To gain a more thorough understanding of this pathogen's evolutionary history, it will be necessary to identify Xanthomonas pathogens from wild peppers and tomatoes and compare the pattern of genetic diversity seen in these strains to the pattern of genetic diversity observed among strains from cultivated plants. If human intervention had a role to play in the evolution of the current X. axonopodis pv. vesicatoria population found on cultivated plants, then it is possible that this particular Xanthomonas species may not be found on wild plants. Alternatively, if a single clone of X. axonopodis pv. vesicatoria was selected during human domestication/manipulation of the host plants, it is possible that wild plants would harbor an array of genetically diverse X. axonopodis pv. vesicatoria strains. Finally, it would be informative to examine the evolutionary relationship between X. axonopodis pv. vesicatoria (group A) strains and strains from the group B species, which is the other predominant Xanthomonas pepper and tomato pathogen. Examination of these two species might reveal if one species recently arose from the other or if they have distinct evolutionary histories.
REFERENCES
- 1.Abramovitch, R. B., Y.-J. Kim, S. Chen, M. B. Dickman, and G. B. Martin. 2003. Pseudomonas type III effector AvrPtoB induces plant disease susceptibility by inhibition of host programmed cell death. EMBO J. 22:60-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez, A. M., A. A. Benedict, C. Y. Mizumoto, J. E. Hunter, and D. W. Gabriel. 1994. Serological, pathological, and genetic diversity among strains of Xanthomonas campestris infecting crucifers. Phytopathology 84:1449-1457. [Google Scholar]
- 3.Amuthan, G., and A. Mahadevan. 1994. Plasmid and pathogenicity in Xanthomonas oryzae pathovar oryzae, the bacterial-blight pathogen of Oryza sativa. J. Appl. Bacteriol. 76:529-538. [Google Scholar]
- 4.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Deidamn, J. A. Smith, D. Struhl, L. M. Albright, D. M. Coen, and A. Varki (ed.). 1994. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 5.Axtell, M. J., and B. Staskawicz. 2003. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 112:369-377. [DOI] [PubMed] [Google Scholar]
- 6.Bender, C. L., and D. A. Cooksey. 1986. Indigenous plasmids in Pseudomonas syringae pv tomato: conjugative transfer and role in copper resistance. J. Bacteriol. 165:534-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beres, S. B., G. L. Sylva, K. D. Barbian, B. F. Lei, J. S. Hoff, N. D. Mammarella, M. Y. Liu, J. C. Smoot, S. F. Porcella, L. D. Parkins, D. S. Campbell, T. M. Smith, J. K. McCormick, D. Y. M. Leung, P. M. Schlievert, and J. M. Musser. 2002. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc. Natl. Acad. Sci. USA 99:10078-10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergelson, J., M. Kreitman, E. A. Stahl, and D. C. Tian. 2001. Evolutionary dynamics of plant R-genes. Science. 292:2281-2285. [DOI] [PubMed] [Google Scholar]
- 9.Bittner-Eddy, P. D., I. R. Crute, E. B. Holub, and J. L. Beynon. 2000. RPP13 is a simple locus in Arabidopsis thaliana for alleles that specify downy mildew resistance to different avirulence determinants in Peronospora parasitica. Plant J. 21:177-188. [DOI] [PubMed] [Google Scholar]
- 10.Bonas, U., and T. Lahaye. 2002. Plant disease resistance triggered by pathogen-derived molecules: refined models of specific recognition. Curr. Opin. Microbiol. 5:44-50. [DOI] [PubMed] [Google Scholar]
- 11.Bosland, P. W. 1999. Chiles: a gift from a fiery God. Hortscience 34:809-811. [Google Scholar]
- 12.Bouzar, H., J. B. Jones, G. V. Minsavage, R. E. Stall, and J. W. Scott. 1994. Proteins unique to phenotypically distinct groups of Xanthomonas campestris pv. vesicatoria revealed by silver staining. Phytopathology 84:39-44. [Google Scholar]
- 13.Bouzar, H., J. B. Jones, R. E. Stall, N. C. Hodge, G. V. Minsavage, A. A. Benedict, and A. M. Alvarez. 1994. Physiological, chemical, serological, and pathogenic analyses of a worldwide collection of Xanthomonas campestris pv. vesicatoria strains. Phytopathology 84:663-671. [Google Scholar]
- 14.Boyd, E. F., C. W. Hill, S. M. Rich, and D. L. Hartl. 1996. Mosaic structure of plasmids from natural populations of Escherichia coli. Genetics 143:1091-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canteros, B. I., G. V. Minsavage, J. B. Jones, and R. E. Stall. 1995. Diversity of plasmids in Xanthomonas campestris pv. vesicatoria. Phytopathology 85:1482-1486. [Google Scholar]
- 16.Casper-Lindley, C., D. Dahlbeck, E. T. Clark, and B. J. Staskawicz. 2002. Direct biochemical evidence for type III secretion-dependent translocation of the AvrBs2 effector protein into plant cells. Proc. Natl. Acad. Sci. USA 99:8336-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cazorla, F. M., E. Arrebola, A. Sesma, A. Perez-Garcia, J. C. Codina, J. Murillo, and A. de Vicente. 2002. Copper resistance in Pseudomonas syringae strains isolated from mango is encoded mainly by plasmids. Phytopathology 92:909-916. [DOI] [PubMed] [Google Scholar]
- 18.Chakrabarty, P. K., A. Mahadevan, S. Raj, M. K. Meshram, and D. W. Gabriel. 1995. Plasmid-borne determinants of pigmentation, exopolysaccharide production, and virulence in Xanthomonas campestris pv. malvacearum. Can. J. Microbiol. 41:740-745. [Google Scholar]
- 19.Dangl, J. L., and J. D. G. Jones. 2001. Plant pathogens and integrated defence responses to infection. Nature 411:826-833. [DOI] [PubMed] [Google Scholar]
- 20.da Silva, A. C. R., J. A. Ferro, F. C. Reinach, C. S. Farah, L. R. Furlan, R. B. Quaggio, C. B. Monteiro-Vitorello, M. A. Van Sluys, N. F. Almeida, L. M. C. Alves, A. M. do Amaral, M. C. Bertolini, L. E. A. Camargo, G. Camarotte, F. Cannavan, J. Cardozo, F. Chambergo, L. P. Clapina, R. M. B. Cicarelli, L. L. Coutinho, J. R. Cursino-Santos, H. El-Dorry, J. B. Faria, A. J. S. Ferreira, R. C. C. Ferreira, M. I. T. Ferro, E. F. Formighieri, M. C. Franco, C. C. Greggio, A. Gruber, A. M. Katsuyama, L. T. Kishi, R. P. Leite, E. G. M. Lemos, M. V. F. Lemos, E. C. Locali, M. A. Machado, A. Madeira, N. M. Martinez-Rossi, E. C. Martins, J. Meidanis, C. F. M. Menck, C. Y. Miyaki, D. H. Moon, L. M. Moreira, M. T. M. Novo, V. K. Okura, M. C. Oliveira, V. R. Oliveira, H. A. Pereira, A. Rossi, J. A. D. Sena, C. Silva, R. F. de Souza, L. A. F. Spinola, M. A. Takita, R. E. Tamura, E. C. Teixeira, R. I. D. Tezza, M. T. dos Santos, D. Truffi, S. M. Tsai, F. F. White, J. C. Setubal, and J. P. Kitajima. 2002. Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417:459-463. [DOI] [PubMed] [Google Scholar]
- 21.Davies, J. 1994. Inactivation of antibiotics and the dissemination of resistance genes. Science 264:375-382. [DOI] [PubMed] [Google Scholar]
- 22.Drake, J. W. 1991. A constant rate of spontaneous mutation in DNA-based microbes. Proc. Natl. Acad. Sci. USA 88:7160-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drake, J. W., B. Charlesworth, D. Charlesworth, and J. F. Crow. 1998. Rates of spontaneous mutation. Genetics 148:1667-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellegren, H. 2000. Microsatellite mutations in the germline: implications for evolutionary inference. Trends Genet. 16:551-558. [DOI] [PubMed] [Google Scholar]
- 24a.Feil, E. J., and B. G. Spratt. 2001. Recombination and the population structures of bacterial pathogens. Annu. Rev. Microbiol. 55:561-590. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald, J. R., and J. M. Musser. 2001. Evolutionary genomics of pathogenic bacteria. Trends Microbiol. 9:547-553. [DOI] [PubMed] [Google Scholar]
- 26.Gagnevin, L., J. E. Leach, and O. Pruvost. 1997. Genomic variability of the Xanthomonas pathovar mangiferaeindicae, agent of mango bacterial black spot. Appl. Environ. Microbiol. 63:246-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gassmann, W., D. Dahlbeck, O. Chesnokova, G. V. Minsavage, J. B. Jones, and B. J. Staskawicz. 2000. Molecular evolution of virulence in natural field strains of Xanthomonas campestris pv. vesicatoria. J. Bacteriol. 182:7053-7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Go, M. F., V. Kapur, D. Y. Graham, and J. M. Musser. 1996. Population genetic analysis of Helicobacter pylori by multilocus enzyme electrophoresis: extensive allelic diversity and recombinational population structure. J. Bacteriol. 178:3934-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Guttman, D. S. 1997. Recombination and clonality in natural populations of Escherichia coli. Trends Ecol. Evol. 12:16-22. [DOI] [PubMed] [Google Scholar]
- 29.Hauck, P., R. Thilmony, and S. Y. He. 2003. A Pseudomonas syringae type III effector suppresses cell wall-based extracellular defense in susceptible Arabidopsis plants. Proc. Natl. Acad. Sci. USA 100:8577-8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudson, R. R. 1990. Gene genealogies and the coalescent process. Oxford Surv. Evol. Biol. 7:1-44. [Google Scholar]
- 31.Hudson, R. R. (ed.). 1993. The how and why of generating gene genealogies. Sinauer Associates, Sunderland, Mass.
- 32.Jackson, R. W., E. Athanassopoulos, G. Tsiamis, J. W. Mansfield, A. Sesma, D. L. Arnold, M. J. Gibbon, J. Murillo, J. D. Taylor, and A. Vivian. 1999. Identification of a pathogenicity island, which contains genes for virulence and avirulence, on a large native plasmid in the bean pathogen Pseudomonas syringae pathovar phaseolicola. Proc. Natl. Acad. Sci. USA 96:10875-10880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones, J. B., H. Bouzar, R. E. Stall, E. C. Almira, P. D. Roberts, B. W. Bowen, J. Sudberry, P. M. Strickler, and J. Chun. 2000. Systematic analysis of xanthomonads (Xanthomonas spp.) associated with pepper and tomato lesions. Int. J. Syst. Evol. Microbiol. 50:1211-1219. [DOI] [PubMed] [Google Scholar]
- 34.Jones, J. B., K. L. Pohronezny, R. E. Stall, and J. P. Jones. 1986. Survival of Xanthomonas campestris pv. besicatoria in Florida on tomato crop residue, weeds, seeds, and volunteer tomato plants. Phytopathology 76:430-434. [Google Scholar]
- 35.Jones, J. B., R. E. Stall, and H. Bouzar. 1998. Diversity among xanthomonads pathogenic on pepper and tomato. Annu. Rev. Phytopathol. 36:41-58. [DOI] [PubMed] [Google Scholar]
- 36.Kearney, B., and B. J. Staskawicz. 1990. Characterization of IS476 and its role in bacterial spot disease of tomato and pepper. J. Bacteriol. 172:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kearney, B., and B. J. Staskawicz. 1990. Widespread distribution and fitness contribution of Xanthomonas campestris avirulence gene Avrbs2. Nature 346:385-386. [DOI] [PubMed] [Google Scholar]
- 38.Keith, L. W., C. Boyd, N. T. Keen, and J. E. Partridge. 1997. Comparison of avrD alleles from Pseudomonas syringae pv. glycinea. Mol. Plant-Microbe Interact. 10:416-422. [DOI] [PubMed] [Google Scholar]
- 39.Kousik, C. S., and D. F. Ritchie. 1996. Disease potential of pepper bacterial spot pathogen races that overcome the Bs2 gene for resistance. Phytopathology 86:1336-1343. [Google Scholar]
- 40.Kousik, C. S., and D. F. Ritchie. 1996. Race shift in Xanthomonas campestris pv. vesicatoria within a season in field-grown pepper. Phytopathology 86:952-958. [Google Scholar]
- 41.Kousik, C. S., and D. F. Ritchie. 1998. Response of bell pepper cultivars to bacterial spot pathogen races that individually overcome major resistance genes. Plant Dis. 82:181-186. [DOI] [PubMed] [Google Scholar]
- 42.Lazo, G. R., and D. W. Gabriel. 1987. Conservation of plasmid DNA sequences and pathovar identification of strains of Xanthomonas campestris. Phytopathology 77:448-453. [Google Scholar]
- 43.Lenski, R. E., C. L. Winkworth, and M. A. Riley. 2003. Rates of DNA sequence evolution in experimental populations of Escherichia coli during 20,000 generations. J. Mol. Evol. 56:498-508. [DOI] [PubMed] [Google Scholar]
- 44.Leung, H., C. V. Cruz, and J. Leach (ed.). 2002. Population genetics of Xanthomonas oryzae: applications to disease control. APS Press, St. Paul, Minn.
- 45.Li, W.-H. 1997. Molecular evolution. Sinauer Associates, Sunderland, Mass.
- 46.Louws, F. J., D. W. Fulbright, C. T. Stephens, and F. J. Debruijn. 1995. Differentiation of genomic structure by REP-PCR fingerprinting to rapidly classify Xanthomonas campestris pv. vesicatoria. Phytopathology 85:528-536. [Google Scholar]
- 47.Mackey, D., Y. Belkhadir, J. M. Alonso, J. R. Ecker, and J. L. Dangl. 2003. Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112:379-389. [DOI] [PubMed] [Google Scholar]
- 48.Macneish, R. S. 1964. Ancient Mesoamerican civilization—long archeological sequence from Tehuacan Mexico may give new data about rise of this civilization. Science 143:531-537. [DOI] [PubMed] [Google Scholar]
- 49.Mauricio, R., E. A. Stahl, T. Korves, D. Tian, M. Kreitman, and J. Bergelson. 2003. Natural selection for polymorphism in the disease resistance gene Rps2 of Arabidopsis thaliana. Genetics 163:735-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDowell, J. M., M. Dhandaydham, T. A. Long, M. G. M. Aarts, S. Goff, E. B. Holub, and J. L. Dangl. 1998. Intragenic recombination and diversifying selection contribute to the evolution of downy mildew resistance at the RPP8 locus of arabidopsis. Plant Cell 10:1861-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musser, J. M. 1996. Molecular population genetic analysis of emerged bacterial pathogens: selected insights. Emerg. Infect. Dis. 2:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noel, L., T. L. Moores, E. A. van der Biezen, M. Parniske, M. J. Daniels, J. E. Parker, and J. D. G. Jones. 1999. Pronounced intraspecific haplotype divergence at the RPP5 complex disease resistance locus of Arabidopsis. Plant Cell 11:2099-2111. [PMC free article] [PubMed] [Google Scholar]
- 53.Obradovic, A., A. Mavridis, K. Rudolph, J. D. Janse, M. Arsenijevic, J. B. Jones, G. V. Minsavage, and J. F. Wang. 2004. Characterization and PCR-based typing of Xanthomonas campestris pv. vesicatoria from peppers and tomatoes in Serbia. Eur. J. Plant Pathol. 110:285-292. [Google Scholar]
- 54.Ochman, H., J. G. Lawrence, and E. A. Groisman. 2000. Lateral gene transfer and the nature of bacterial innovation. Nature 405:299-304. [DOI] [PubMed] [Google Scholar]
- 55.Ogarro, L. W., H. Gibbs, and A. Newton. 1997. Mutation in the avrBs1 avirulence gene of Xanthomonas campestris pv. vesicatoria influences survival of the bacterium in soil and detached leaf tissue. Phytopathology 87:960-966. [DOI] [PubMed] [Google Scholar]
- 56.Pruvost, O., J. S. Hartung, E. L. Civerolo, C. Dubois, and X. Perrier. 1992. Plasmid DNA fingerprints distinguish pathotypes of Xanthomonas campestris pv. citri, the causal agent of citrus bacterial canker disease. Phytopathology 82:485-490. [Google Scholar]
- 57.Restrepo, S., T. L. Valle, M. C. Duque, and V. Verdier. 1999. Assessing genetic variability among Brazilian strains of Xanthomonas axonopodis pv. manihotis through restriction fragment length polymorphism and amplified fragment length polymorphism analyses. Can. J. Microbiol. 45:754-763. [Google Scholar]
- 58.Ronald, P., and B. Staskawicz. 1988. The avirulence gene avrBs1 from Xanthomonas campestris pv. vesicatoria encodes a 50-kD protein. Mol. Plant-Microbe Interact. 1:191-198. [PubMed] [Google Scholar]
- 59.Sarkar, S. F., and D. S. Guttman. 2004. Evolution of the core genome of Pseudomonas syringae, a highly clonal, endemic plant pathogen. Appl. Environ. Microbiol. 70:1999-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schaad, N. W., A. K. Vidaver, G. H. Lacy, K. Rudolph, and J. B. Jones. 2000. Evaluation of proposed amended names of several pseudomonads and xanthomonads and recommendations. Phytopathology 90:208-213. [DOI] [PubMed] [Google Scholar]
- 61.Slatkin, M., and R. R. Hudson. 1991. Pairwise comparisons of mitochondrial-DNA sequences in stable and exponentially growing populations. Genetics 129:555-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith, J. M., and J. Haigh. 1974. Hitch-hiking effect of a favorable gene. Genet. Res. 23:23-35. [PubMed] [Google Scholar]
- 62a.Smith, J. M., N. H. Smith, M. O’Rourke, and B. G. Spratt. 1993. How clonal are bacteria? Proc. Natl. Acad. Sci. USA 90:4384-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62b.Smith, J. M., E. J. Feil, and N. H. Smith. 2000. Population structure and evolutionary dynamics of pathogenic bacteria. Bioessays 22:1115-1122. [DOI] [PubMed] [Google Scholar]
- 63.Spratt, B. G., and M. C. J. Maiden. 1999. Bacterial population genetics, evolution and epidemiology. Phil. Trans. R. Soc. Lond. B 354:701-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stahl, E. A., G. Dwyer, R. Mauricio, M. Kreitman, and J. Bergelson. 1999. Dynamics of disease resistance polymorphism at the Rpm1 locus of Arabidopsis. Nature 400:667-671. [DOI] [PubMed] [Google Scholar]
- 65.Stall, R. E., C. Beaulieu, D. Egel, N. C. Hodge, R. P. Leite, G. V. Minsavage, H. Bouzar, J. B. Jones, A. M. Alvarez, and A. A. Benedict. 1994. Two genetically diverse groups of strains are included in Xanthomonas campestris pv. vesicatoria. Int. J. Syst. Bacteriol. 44:47-53. [Google Scholar]
- 66.Stall, R. E., D. C. Loschke, and J. B. Jones. 1986. Linkage of copper resistance and avirulence loci on a self-transmissible plasmid in Xanthomonas campestris pv. vesicatoria. Phytopathology 76:240-243. [Google Scholar]
- 67.Stevens, C., M. A. Bennett, E. Athanassopoulos, G. Tsiamis, J. D. Taylor, and J. W. Mansfield. 1998. Sequence variations in alleles of the avirulence gene avrPphE.R2 from Pseudomonas syringae pv. phaseolicola lead to loss of recognition of the AvrPphE protein within bean cells and a gain in cultivar-specific virulence. Mol. Microbiol. 29:165-177. [DOI] [PubMed] [Google Scholar]
- 68.Sundin, G. W., and C. L. Bender. 1993. Ecological and genetic analysis of copper and streptomycin resistance in Pseudomonas syringae pv. syringae. Appl. Environ. Microbiol. 59:1018-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sundin, G. W., D. H. Demezas, and C. L. Bender. 1994. Genetic and plasmid diversity within natural populations of Pseudomonas syringae with various exposures to copper and streptomycin bactericides. Appl. Environ. Microbiol. 60:4421-4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Swords, K. M. M., D. Dahlbeck, B. Kearney, M. Roy, and B. J. Staskawicz. 1996. Spontaneous and induced mutations in a single open reading frame alter both virulence and avirulence in Xanthomonas campestris pv. vesicatoria avrBs2. J. Bacteriol. 178:4661-4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tajima, F. 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tian, D. C., H. Araki, E. Stahl, J. Bergelson, and M. Kreitman. 2002. Signature of balancing selection in Arabidopsis. Proc. Natl. Acad. Sci. USA 99:11525-11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Top, E. M., D. Springael, and N. Boon. 2002. Catabolic mobile genetic elements and their potential use in bioaugmentation of polluted soils and waters. FEMS Microbiol. Ecol. 42:199-208. [DOI] [PubMed] [Google Scholar]
- 74.Vauterin, L., B. Hoste, K. Kersters, and J. Swings. 1995. Reclassification of Xanthomonas. Int. J. Syst. Bacteriol. 45:472-489. [Google Scholar]
- 75.Vivian, A., J. Murillo, and R. W. Jackson. 2001. The roles of plasmids in phytopathogenic bacteria: mobile arsenals? Microbiology 147:763-780. [DOI] [PubMed] [Google Scholar]
- 76.Wall, J. D., and R. R. Hudson. 2001. Coalescent simulations and statistical tests of neutrality. Mol. Biol. Evol. 18:1134-1135. [DOI] [PubMed] [Google Scholar]
- 77.Wichmann, G., and J. Bergelson. 2004. Effector genes of Xanthomonas axonopodis pv. vesicatoria promote transmission and enhance other fitness traits in the field. Genetics 166:693-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zawadzki, P., M. A. Riley, and F. M. Cohan. 1996. Homology among nearly all plasmids infecting three Bacillus species. J. Bacteriol. 178:191-198. [DOI] [PMC free article] [PubMed] [Google Scholar]




