Abstract
Diabetic retinopathy (DR) is a severe microvascular complication of diabetes and is one of the primary causes of blindness in the working-age population in Europe and the United States. At present, no cure is available for DR, but early detection and timely intervention can prevent the rapid progression of the disease. Several treatments for DR are known, primarily ophthalmic treatment based on glycemia, blood pressure, and lipid control, which includes laser photocoagulation, glucocorticoids, vitrectomy, and antivascular endothelial growth factor (anti-VEGF) medications. Despite the clinical efficacy of the aforementioned therapies, none of them can entirely shorten the clinical course of DR or reverse retinopathy. MicroRNAs (miRNAs) are vital regulators of gene expression and participate in cell growth, differentiation, development, and apoptosis. MicroRNAs have been shown to play a significant role in DR, particularly in the molecular mechanisms of inflammation, oxidative stress, and neurodegeneration. The aim of this review is to systematically summarize the signaling pathways and molecular mechanisms of miRNAs involved in the occurrence and development of DR, mainly from the pathogenesis of oxidative stress, inflammation, and neovascularization. Meanwhile, this article also discusses the research progress and application of miRNA-specific therapies for DR.
1. Introduction
Diabetic retinopathy (DR) is a severe microvascular complication of diabetes and is one of the leading causes of blindness in the working-age population. Globally, DR accounts for 1.4% of cases in adults with moderate or severe visual impairment [1, 2]. The prevalence of diabetes has been rapidly increasing worldwide, with 8.8% of adults affected in 2015 and projected to rise to 10.4% by 2040 [3]. As the prevalence of diabetes increases and people with diabetes live longer, the incidence of DR is also expected to rise, as it is a complication of diabetes. Currently, there is no permanent cure for DR, but the disease can be prevented or managed. Early detection and timely intervention can prevent the majority of cases resulting in visual impairment [4].
Hyperglycemia can cause various changes in the retina, such as increased retinal vascular permeability, loss of vascular endothelial cells and pericytes, thickening of the vascular basement membrane, abnormalities of retinal neurons and glia, tissue ischemia, and the release of various vasoactive substances, ultimately leading to neovascularization [5, 6]. The classical pathways involved in the pathogenesis of DR include activation through the polyol and hexosamine pathways, accumulation of advanced glycation end products (AGEs), and activation of protein kinase C (PKC) [7–9]. Activation of these biochemical pathways can lead to mitochondrial dysfunction, increased oxidative stress, dysfunction of proinflammatory mediators, activation of the renin-angiotensin system, and upregulation of vascular endothelial growth factor (VEGF) expression, ultimately resulting in increased vascular permeability, vascular dysfunction, and neovascularization [10–13].
Patients with DR are usually unable to detect symptoms in the early stages of the disease [14]. As the disease progresses and affects the macular region, patients may begin to experience varying degrees of vision loss [15]. Clinically, DR is classified into nonproliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) based on its developmental stage and severity [16]. NPDR is the early stage of DR and presents with progressive development. During this period, the blood-retinal barrier (BRB) undergoes significant changes, including increased retinal vascular permeability, thickening of the basement membrane, and selective loss of peripheral cells [17, 18]. The primary physical signs of NPDR range from mild to severe, with small retinal bleeding spots, microangiomas, venous beading, and retinal microvascular abnormalities [19, 20]. DR is considered to have progressed to PDR when one or more of the following three changes occur: neovascularization, vitreous hemorrhage, or preretinal hemorrhage [21–23]. The key to distinguishing NPDR from PDR is the presence or absence of new blood vessels. PDR is characterized by the possibility of neovascularization of the iris (NVI), neovascularization of the disk (NVD), and neovascularization elsewhere (NVE) [19]. The formation of neovascularization is usually accompanied by a surge in inflammatory factors and myofibroblasts [19]. Due to the unstable microenvironment of neovascularization, new blood vessels are fragile, and vascular contents are prone to exudation, leading to vitreous/preretinal hemorrhage and retinal detachment [24, 25].
Several treatments are known for DR, primarily ophthalmic treatments based on glycemia, blood pressure, and lipid control, including laser photocoagulation [26], intravitreal steroid therapy [27, 28], glucocorticoids, vitrectomy [29], and anti-VEGF medications [30]. Laser photocoagulation is the preferred treatment for diabetic macular edema that is not centrally involved [31, 32]. The mainstay of treatment for PDR is total retinal photocoagulation surgery (PRP) [33, 34]. In addition, vitrectomy is the primary surgical intervention for persistent vitreous hemorrhage and retinal detachment that occurs in the later stages of DR [35], while anti-VEGF medications are effective in treating centrally involved diabetic macular edema with the visual loss [36]. Although these therapies are clinically effective, none of them can completely reduce the clinical course of DR or reverse the retinal lesions [8]. Therefore, there is an urgent need for new treatment discoveries. Many researchers have explored new treatment options for DR. New aspects of therapeutic research currently underway include mediators of the angiopoietin signaling axis, immunosuppressants, nonsteroidal anti-inflammatory drugs (NSAIDs), inhibitors of oxidative stress, and inhibitors of viral viscosity (VVIs) [8, 37]. Moreover, diet is also closely related to DR, and diets including fruits and vegetables can have a protective effect on DR [38].
Nutrients and nutritional supplements have also been found to play important roles in DR; for example, vitamins can affect protein glycosylation, oxidative stress, and retinal blood vessels [39]. This also provides a new direction for the treatment of DR in the future. Furthermore, some preclinical studies suggest that DR gene therapy has great potential in the future to provide patients with a better treatment experience through personalized therapy, enabling patients to decrease the frequency of ocular injections or laser treatments, which can better meet patients' needs in terms of effectiveness and sustainability [40].
Several related studies have reported that genetics and other factors may influence DR progression, which may result in dysregulation of related epigenetic mechanisms such as DNA methylation, posttranscriptional modification of histones in chromatin, and the formation of noncoding RNAs, leading to changes in the expression of various genes involved in the DR process, particularly noncoding RNAs [41–44]. miRNAs have been extensively studied as a novel class of small noncoding RNA. A large body of work has shown that aberrantly expressed miRNAs play key roles in the pathogenesis of microvascular complications, such as oxidative stress, apoptosis, inflammation, and angiogenesis, and are believed to play a critical role in the pathogenesis of DR via the aforementioned pathways [45–47]. miRNAs were also found to control insulin synthesis and regulate cytokines to control processes such as immune response, indirectly affecting the occurrence and development of DR [48].
2. Structural and Biological Processes of MicroRNAs
MicroRNAs (miRNAs) are a class of small noncoding RNAs, approximately twenty-four nucleotides in length, found in animals, plants, and viruses. They are key posttranscriptional regulators of gene expression. MicroRNAs are involved in the growth, differentiation, development, and apoptosis of the cell body, and a single miRNA can bind to hundreds of different mRNAs and take part in different biological processes by pairing with different bases of targeted mRNA [49].
The biological process of miRNA starts in the nucleus and ends in the cytoplasm. miRNA transcription is initiated by RNA polymerase II, followed by capping, splicing, and polyadenylation, and, finally, the formation of primary miRNA (pri-miRNA) [50]. In the nucleus, the microprocessor complex consisting of the DROSHA ribonuclease and DiGeorge critical region 8 (Dgcr8) double-stranded RNA-binding protein processes pri-miRNA to form hairpin-structured miRNA precursors (pre-miRNA) [51]. The pre-miRNA is formed by shearing and modification of the stem loop structure, which results in a miRNA: the miRNA double strand [52, 53]. With the help of the human ribonuclease Dicer and double-stranded RNA-binding proteins (dsRBPs), the human immunodeficiency virus transactivating response RNA-binding protein (TRBP) or protein activator of PKR (PACT), one of the double strands (guide strand), is selected and combines with Argonaute family proteins to form an RNA-induced silencing complex [54–58]. During which, the other strand (passenger strand) is degraded [59], and the guide strand, which is retained in the miRISC, eventually forms a mature single-stranded miRNA [60–62]. The RNA-induced silencing complex induces the degradation of the target mRNA or suppresses its translation through complementary pairing with the 3′ UTR of its target mRNA molecule. It is important to note that a single miRNA can target hundreds of mRNAs, and a single mRNA can be suppressed by multiple miRNAs [63].
Recent studies have shown that in addition to the above canonical miRNA biogenesis, some miRNA precursors can be processed to produce the abundant two miRNA strands, where passenger strand is not always degraded, and it can be loaded into the Ago 2 protein and contribute to the regulation of mRNA translation [64–66]. For example, Schober et al. [67] found that the precursor miRNA pre-miR-126 produces two mature miRNA chains, miR-126-3p and miR-126-5p. The guide strand miR-126-3p mediates the formation of angiogenesis and anti-inflammatory factor. The passenger chain miR-126-5p maintains endothelial integrity by targeting delta-like 1 homolog (Dlk1), a negative regulator of endothelial cell proliferation. Villain et al. [68] also reported the role of miR-126-5p in endothelial cell survival during the establishment of the retinal vasculature through the regulation of SetD5 and Sema3A. Yang et al. [64] found that transgenic mice expressing miR-17 precursor can produce considerable levels of guide strand miR-17-5p and passenger strand miR-17-3p, while both miRNA-17-3p and miRNA-17-5p can inhibit the expression of TIMP metalloproteinase inhibitor 3 (TIMP3), and the effect of the combination of them is much greater than that of single miRNA. These effects can greatly increase the invasive ability of tumor cells. It has also been found that the guide strand miR-9-5p [69] can promote the proliferation, migration, and invasion of hepatocellular carcinoma cells by targeting ESR1, while the passenger strand miR-9-3p [70] has been proved to inhibit the proliferation and apoptosis of glioma cells through forkhead box G1 (FOXG1).
3. MicroRNAs as Biomarkers of DR
Being a class of small noncoding RNAs, approximately 24 nucleotides in length, miRNAs can migrate outside of cells and enter the humoral circulation by binding with other molecules, a process referred to as circulating miRNAs [71]. However, the mechanism by which circulating miRNAs are sorted into exocrine/small extracellular vesicles (sEV) or retained in cells is still largely unknown [72, 73]. Studies have suggested that the sequence or structural features of miRNAs may influence binding affinity and secretion [74]. Koppers-Lalic et al. proposed a miRNA sorting mechanism based on the 3′ terminal structure [75]. They found that 3′-end adenylated miRNA isoforms were mainly enriched in cells, while their 3′-end uridylated miRNA isoforms were overrepresented in exosomes. Further studies have also shown that miRNAs have sorting sequences that determine their sEV secretion or cell retention, which may be related to the existence of sEV export (EXOmotifs) versus cellular retention (CELLmotifs) sequences [73]. This selective release makes it possible to view circulating miRNAs as biomarkers for various diseases.
Studies have shown that approximately 10% of circulating miRNAs enter circulation through exosomes, while the majority of other circulating miRNAs form complexes with RNA binding proteins such as Ago2, NPM1 (nuclear phosphoprotein 1), and high-density lipoprotein (HDL) [76, 77]. Circulating miRNAs have been detected in various bodily fluids, including blood, tear fluid, and vitreous fluid. These miRNAs can be stably present in bodily fluids and serve as biomarkers for several diseases such as cancer, DR, diabetes, and prostate cancer [71, 78–82]. Several studies have demonstrated that miRNAs have high sensitivity and specificity for the diagnosis of DR and can be used as an effective noninvasive biomarker, and the diagnostic effectiveness can be further improved by using a combination of multiple miRNAs [80, 83]. For instance, it has been reported that miR-9–5p and miR-17–3p are downregulated while miR-210 is upregulated in the serum of patients with DR [84, 85]. In the vitreous humor, miR-125a-5p, miR-125b-5p, and miR-204–5p are downregulated, while miR-21–5p, miR-660–5p, miR-142–3p, miR-19a-3p, miR-142–5p, and miR-15a-5p are upregulated [86]. In aqueous humor, miR-200b-3p, miR-199a-3p, and miR-365–3p are downregulated [87]. The following is a summary of the research progress of microRNA as a biomarker of DR in recent years (Table 1).
Table 1.
Summary of microRNAs as biomarkers of DR.
| miRNAs | Changes in DR | Tested tissues | Targets | Cite |
|---|---|---|---|---|
| miR-21 | ↑ | Endothelial cells | Endothelial cell dysfunction | [88] |
| miR-216a | ↓ | Rat retina, human retinal microvascular endothelial cells (HRMECS) | Suppression of nitric oxide synthase 2 (NOS2)/the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) | [89] |
| miR-29b-3p | ↑ | HRMECS | Blocking SIRT1 | [90] |
| miR-203a-3p | ↓ | Rat retina, OIR | VEGFA, hypoxia-inducible factor-1α (HIF-1α) | [91] |
| miR-183 | ↓ | Rat retina | PI3K/Akt/VEGF, upregulating BTG1 | [92] |
| miR-200b | ↓ | Diabetic mouse retina, RMECs | VEGF | [93] |
| miR-150 | ↓ | Endothelial cells | TNF-α, NF-κB | [94] |
| miR-204 | ↑ | Endothelial cells | SIRT1, Bcl-2 | [95] |
| miR-3197, miR-2116-5p | ↑ | Retinal endothelial cells (RECs) | Notch homolog 2 (NOTCH2) | [96] |
| miR-93 | ↓ | Serum | Transforming growth factor-β (TGFβ), VEGFA | [97] |
| miR-335-3p | ↓ | Plasma | Endothelial growth factor (EGFR) | [98] |
| miR-205-5p | ↓ | HRMEC | Malat1, VEGF | [99] |
| miR-425-5p | ↑ | Serum | — | [100] |
| miR-320a | ↓ | Plasma | PTEN | [101] |
| miR-122 | ↑ | Serum | VEGF | [102] |
| miR-26a-5p | ↓ | Plasma | PTEN | [103] |
| miR-431-5p | ↑ | Serum extracellular vesicles | — | [104] |
| miR-29c-3p | ↓ | Plasma | VEGF | [105] |
| miR-6a-56p, miR-20a-5p, miR-20b, miR-3a-20p | ↓ | Retina and serum | VEGFA | [106] |
| miR-3-320p, miR-495b | ↑ | Plasma and serum | — | [107] |
| miR-1281 | ↑ | Plasma and serum | VEGFA | [108] |
| miR-4448, miR-338-3p, miR-485-5p, miR-9-5p | ↓ | Serum | SIRT, forkhead box O (FOXO) 1, FOXO3 | [84] |
| miR-374a | ↑ | Serum | — | [109] |
| miR-214-3p, miR-218-5p, miR145-5P | ↓ | Tear exosomes | — | [110] |
| miR-4328, miR-4422, miR-548z, miR-628-5p | ↓ | Serum | — | [111] |
| miR-21-3p, miR-30b-5p | ↑ | Plasma and serum | — | [112] |
| miR-17-3p, miR-20b | ↓ | Serum | — | [113] |
4. MicroRNAs Participate in DR Development by Different Mechanisms
MicroRNAs have been reported to play a regulatory role in various biological processes, including angiogenesis, oxidative stress, immune response, and inflammation. Dysregulation of these processes can lead to the development of DR and other complications, such as choroidal neovascularization [80, 114–116]. Although several studies have investigated the involvement of miRNAs in DR development, their specific mechanisms and target molecules remain largely unknown. Therefore, it is imperative to investigate the underlying signaling pathways and molecular mechanisms through which miRNAs are involved in DR development and progression.
4.1. Oxidative Stress Pathways
4.1.1. HIF-1α Signaling Pathway
Hypoxia-inducible factor-1α (HIF-1α) is a crucial transcription factor in the cellular response to hypoxia. It is highly responsive to changes in oxygen concentration in the cellular environment and plays an important role in responding to decreased oxygen levels or hypoxia [117]. Abnormalities in tissue metabolism can arise due to changes in morphology, function, and insufficient oxygen delivery. Under hypoxic conditions, the expression of HIF-1α is increased, resulting in the production of reactive oxygen species (ROS). Excessive ROS accumulation can cause mitochondrial damage, apoptosis, inflammation, lipid peroxidation, and structural and functional changes in the retina [6, 118]. Additionally, oxidative stress, which results from excessive ROS production and inhibition of ROS scavenging by antioxidant defense systems, has been reported to be a pathological consequence of several diseases, including diabetes and its complications [119, 120].
Liu et al. [121] reported that miR-135b-5p expression was elevated in retinal tissue and retinal vascular endothelial cells from DR mice, suggesting its involvement in DR pathogenesis. The authors also investigated the effect of miR-135b-5p on HIF-1α expression in DR mouse retinas. Their data showed that miR-135b-5p inhibition suppressed HIF-1α expression in DR mouse retinal tissues, leading to a reduction in pathological retinal tissue damage, apoptosis, the formation of the lumen, and the activity and migration of retinal DR vascular endothelial cells. The study further revealed that the downregulation of miR-135b-5p promoted the expression of Von Hippel-Lindau (VHL) protein in DR retinal tissue and retinal vascular endothelial cells. The results suggested that the positive regulation of VHL in DR mice in vivo and in vitro was opposite to the negative regulation of miR-135b-5p. In addition, the inhibition of miR-135b-5p or upregulation of VHL played a protective role in DR by suppressing HIF-1α expression.
Similarly, Han et al. [91] found that upregulation of miR-203a-3p could inhibit pathological angiogenesis of the retina in PDR by targeting VEGFA and HIF-1α. In a murine model of PDR, the authors observed reduced levels of microRNA-203–3p (miR-203–3p). In contrast, upregulation of miR-203a-3p expression decreased the levels of VEGFA and HIF-1α in the retinal tissues of the mouse model of PDR. The dual luciferase reporter gene assay showed that miR-203a-3p is specifically bound to the 3′ UTR of VEGFA and HIF-1α. The study also found that upregulation of miR-203a-3p expression inhibited HG-induced proliferation, migration, and tube formation in human retinal microvascular endothelial cells (HRMECS).
Guan et al. [122] identified miR-18a-5p as a possible therapeutic target for the treatment of ocular neovascular diseases. The authors found that miR-18a-5p targets fibroblast growth factor 1 (FGF1) and HIF-1α. Overexpression of miR-18a-5p in HRMECS led to a significant reduction in the expression levels of FGF1 and HIF-1α, thereby inhibiting pathologic neovascularization.
Chen et al. [123] found that miR-320a can reduce the damage to Müller cells in DR. The mechanism may be that lncRNA MALAT1 affects the retinal angiogenesis in DR by regulating the miR-320a/HIF-1α axis. The results showed that MALAT1 and HIF-1α were highly expressed in Müller cells induced by HG, while miR-320a was lowly expressed, and MALAT1 could bind competitively with HIF-1α. The overexpression of miR-320a downregulates HIF-1α and inhibits the invasion, angiogenesis, and vascular permeability of mouse retinal microvascular endothelial cells (MRMECs). We summarize the miRNAs in HIF-1α signaling pathways in DR and present them in Figure 1.
Figure 1.
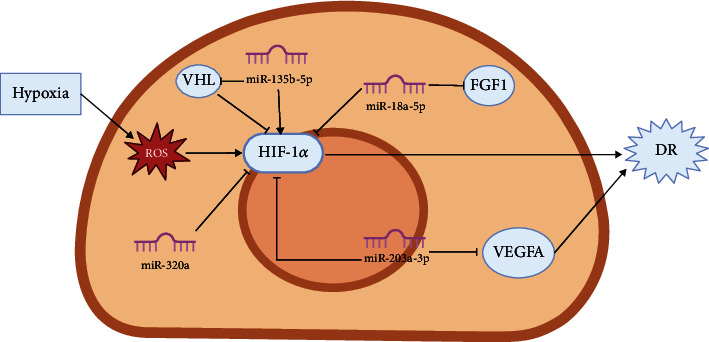
Diagrammatic representation of HIF-1α/microRNA mechanism in DR.
4.1.2. Nrf2 Signaling Pathway
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a crucial participant in preserving mitochondrial homeostasis and structural integrity and acts as a major regulator of cellular redox homeostasis. It plays a significant role in antioxidant stress responses and drug detoxification [124–126]. Activation of Nrf2 and downstream target genes (e.g., superoxide dismutase, glutathione reductase, and glutathione peroxidase) [127] is critical for defending against oxidative stress. An increase in oxidative stress is considered a key metabolic abnormality associated with DR development. Such an increase leads to excessive production of reactive oxygen species (ROS), which damages the inner mitochondrial membrane and results in high levels of superoxide, causing further damage to membrane proteins and disrupting antioxidant defense systems [128, 129]. As mitochondria are the primary sites of ROS generation, they are more susceptible to ROS damage. Damage to mitochondria activates apoptotic mechanisms, leading to accelerated apoptosis of retinal cells and promoting the development of DR [130–132]. Some related studies suggest that Nrf2 may have an important role in maintaining mitochondrial homeostasis in the diabetic retina, thus enabling it to withstand oxidative stress and the development of DR [133–135].
Jadeja et al. [136] conducted correlation expression analysis and demonstrated that miR-144–3p and miR-144–5p target the 3′ UTR of Nrf2 in retinal pigment epithelial cells. They evaluated the expression of miR-144–3p and miR-144–5p in the retina of mice and observed a significant increase in the expression of miR-144–3p in retinal pigment epithelium cells during oxidative stress, while miR-144–5p expression was significantly elevated during the course of oxidative stress. Inhibition of miR-144–3p and miR-144–5p resulted in a significant increase in the expression of Nrf2 and its downstream antioxidant signaling in retinal pigment epithelial cells. The study concluded that miR-144–3p and miR-144–5p have a protective effect on the retina, reducing oxidative stress, and may have important implications for the prevention and treatment of DR.
Luo et al. [137] found decreased expression of Nrf2 and increased expression of miR-93 in blood samples from DR patients and in human retinal pigment epithelial (RPE) cells treated with HG. They demonstrated that overexpression of miR-93 inhibited cellular proliferation and promoted apoptosis, while Nrf2 overexpression abrogated the proapoptotic effect of miR-93, promoted cellular proliferation, and inhibited inflammation. They also found that Nrf2 is a target gene of miR-93 and that miR-93 directly downregulates Nrf2 expression.
Rasoulinejad et al. [138] found that the expression of Nrf2 and miR-146a-5p was significantly reduced in the eye tissues of diabetic rats. They observed a positive correlation between miR-146a-5p and Nrf2 expression and suggested that miR-146a-5p can regulate the expression of Nrf2 as well as inflammation and oxidative stress in diabetic rat eye tissues.
Wang et al. [139] found that overexpression of miR-489–3p impairs the role of transmembrane phosphatase with tensin homology pseudogene 1 (TPTEP1) and that Nrf2 targeted by miR-489–3p is downregulated in human retinal vascular endothelial cells treated with high glucose. They also observed that Nrf2 knockout enhances the impact of miR-489–3p and antagonizes the impact of TPTEP1.
Tang et al. [140] found that miR-138-5p can promote the expression of Sirt1 and Nrf2 in the nucleus to alleviate HG damage. Astragaloside IV can increase the expression of miR-138-5p, thereby increasing Sirt1/Nrf2 activity, enhancing cell antioxidant capacity, alleviating cell apoptosis, and inhibiting the progression of DR. Figure 2 shows the relevant miRNAs in Nrf2 signaling pathways in the progress in DR.
Figure 2.
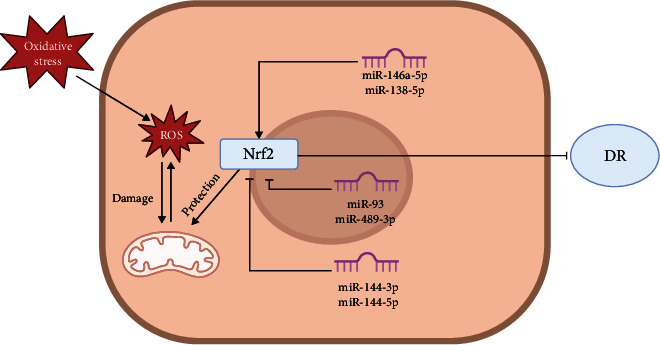
Diagrammatic representation of Nrf2/microRNA mechanism in DR.
4.1.3. SIRT1 Signaling Pathway
Sirtuin 1 (SIRT1), a highly conserved NAD-dependent deacetylase, is known for its antioxidant and anti-inflammatory properties [141–143]. SIRT1 affects various biological processes, including cellular senescence, apoptosis, lipid metabolism, oxidative stress, and inflammation, by deacetylating histone and nonhistone proteins [144–147]. Due to its ability to reduce apoptosis, inflammation, oxidative stress, and mitochondrial damage, SIRT1 is considered a potential target for the treatment of DR [148–150].
Ji et al. [151] found that miR-34a is upregulated, while SIRT1 expression is decreased in DR rats and retinal tissue cells induced by HG, suggesting that miR-34a and SIRT1 share some common regulation and have a role in DR injury. Overexpression of miR-34a leads to the inhibition of retinal endothelial cell proliferation, while downregulation of SIRT1 can achieve the same effect. These findings suggest that the miR-34a/SIRT1 axis could be a target for DR therapy.
Zeng et al. [90] found that miR-29b-3p was upregulated, and SIRT1 protein was downregulated in DR rats, and subsequent studies showed that in microvascular endothelial cells of the retina, miR-29b-3p was upregulated, while SIRT1 was downregulated. This resulted in an increase in Bax/Bcl-2 expression, which can be reversed by downregulating miR-29b-3p. Therefore, the miR-29b-3p/SIRT1 axis is a potential therapeutic target for DR.
Yang et al. [152] investigated the miR-128–3p and SIRT1 axis and found that in HG-treated retinal microvascular endothelial cells, HG significantly downregulated SIRT1 expression and upregulated miR-128–3p expression, suggesting that SIRT1, a downstream gene of miR-128–3p, has a negative regulatory role, which in turn has an effect on diabetic lesions.
Xiao and Liu [153] found that the expression of miR-217 was upregulated in HG-induced cells, and SIRT1 was discovered to be a direct target of miR-217 by a dual luciferase reporter gene assay. They also discovered that supplementation with miR-217 inhibitors reduced HG-induced cellular injury. This suggests that miR-217 inhibitors would target the SIRT1 gene to reverse the impairment, making miR-217 an important target for DR processing.
Pan et al. [154] found that SIRT1 expression was downregulated in DR- and HG-treated retinal microvascular endothelial cells and that overexpression of SIRT1 reversed proliferation in well-proliferating cells. Although the subsequent studies indicated that SIRT1 positively regulates the expression of miR-20a, miR-20a negatively regulates the activity of the yes-associated protein (YAP)/HIF-1a/VEGFA axis in RMEC. This study also revealed that SIRT1 upregulation could inhibit DR disease progression via the induction of other growth factors by miR-20a.
Shan et al. [155] reported increased expression of miR-195 and BAX and decreased expression of BCL-2 and SIRT1 in retinal cells induced by HG. They also demonstrated a significant targeting relationship between miR-195 and SIRT1, and the knockdown of miR-195 led to increased expression of BCL-2 and SIRT1 and a decrease in apoptosis. These findings suggest that miR-195 is an important driver of DR progression by reducing cellular growth and accelerating apoptosis through the inhibition of SIRT1.
Wang et al. [156] identified a targeting relationship between miR-93–5p and SIRT1 using luciferase reporter gene assay. They found that miR-93–5p is upregulated and SIRT1 is downregulated in rats with DR. Furthermore, expression of SIRT1 was upregulated by the addition of miR-93–5p inhibitors, which led to a reduction in VEGF expression levels and proinflammatory cytokines. These results suggest that miR-93–5p may prevent and treat DR by targeting SIRT1 expression and reducing the inflammatory response to DR.
Wang et al. [157] also found that miR-30b was upregulated and SIRT1 was downregulated in the retina of diabetic mice, indicating a regulatory relationship between miR-30b and SIRT1. Inhibiting miR-30b led to an increase in SIRT1 expression, thereby reducing angiogenesis in retinal microvascular endothelial cells. Additionally, inhibiting miR-30b prevented the progression of PDR in mice by promoting SIRT1 expression.
Shi et al. [158] found that the expression of circKMT2E in the retina of diabetic mice was more than twice as high as that of miR-204–5p and that circKMT2E plays a role in DR pathogenesis by acting as a sponge for miR-204–5p. They speculated that miR-204–5p can bind to SIRT1 and interact with its target protein to regulate various functions of cells, including the inflammatory response, proliferation, and apoptosis. We have collated the current research on miRNAs in SIRT1 signaling pathways in DR (Figure 3).
Figure 3.
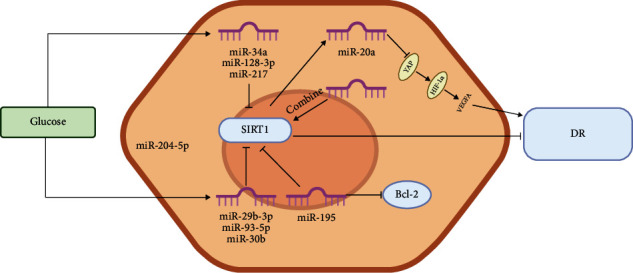
Diagrammatic representation of SIRT1/microRNA mechanism in DR.
4.1.4. AKT Signaling Pathway
The serine/threonine kinase AKT, also known as protein kinase B (PKB), is a proto-oncogene that has three isoforms, AKT1 (PKBα), AKT2 (PKBβ), and AKT3 (Pub), which have markedly different or even opposing functions in cancer and physiology [159–161]. AKT is a key component of the PI3K/AKT signaling pathway, which is activated by the second messenger PI3K and negatively regulated by PTEN. AKT primarily mediates disease development by promoting cell survival and inhibiting apoptosis [162]. Several studies have also demonstrated that the ability of AKT to inhibit apoptosis is dependent on glucose metabolism. Activation of AKT does not inhibit cell death but instead renders cells more susceptible to metabolic stress [163–165]. Since the development of DR is closely linked to the diabetic process, studies have shown that the signaling pathways related to AKT are closely related to oxidative stress and proinflammatory responses in DR [166–168].
Lu et al. [169] found increased expression of miR-21 and AKT-related genes and decreased expression of phosphatase and tensin homolog (PTEN) in DR rats, suggesting a role for miR-21 in DR. They reported that miR-21 can activate signaling pathways related to AKT, and furthermore, miR-21 inhibitors can cause cells to overexpress PTEN, inhibit apoptosis, and promote angiogenesis. However, miR-21 represents an important target for DR therapy.
In order to study its role in the BTG1-mediated AKT pathway, Zhang et al. [92] used rats to establish a DR model with miR-183 mimetic and inhibitors. They found that miR-183 expression was upregulated and BTG1 was decreased in the retinal cells of DR rats. Recent studies have shown that BTG1 is the target gene of miR-183, and inhibition of miR-183 expression could positively regulate BTG1, thereby inhibiting vascular endothelial cell growth in DR rats in vivo through signaling pathways related to AKT. Thus, miR-183 silencing is a target for DR therapy.
Wang et al. [170] found that the expression of miR-199a-3p was decreased but that VEGF expression was increased in HG-induced cells. The miR-199a-3p inhibitor may also cause cellular growth and promote angiogenesis. Upregulation of miR-199a-3p has an opposing effect. In this study, it is reported that miR-199a-3p can inhibit signaling pathways related to AKT, resulting in reduced angiogenesis in DR, which may act through VEGF factors.
Liu et al. [171] noted that Müller glia are the sole source of aberrant expression of miR-9–3p, which activates phosphorylation of VEGFR2. The specific pathway could be accomplished with the binding of miR-9–3p to sphingosine-1-phosphate receptor S1P1 through the S1P1/AKT/VEGFR2 signaling pathway, opening up new opportunities for potential therapeutic targets for DR and elucidation of mechanisms of disease progression.
Zha et al. [172] found that high glucose inhibited the expression of METTL3 and miR-25-3p in RPE cells. After upregulating miR-25-3p, the pathological condition of DR improved. In addition, this study suggests that PTEN may be negatively regulated by miR-25-3p, and overexpression of METTL3 increases p-AKT levels by targeting the miR-25-3p/PTEN axis. The upregulation of PTEN consistently hindered the protective effect of METTL3. In summary, METTL3 enhanced the vitality of HG cells by targeting the miR-25-3p/PTEN/AKT axis. Figure 4 shows the miRNAs in AKT signaling pathways in the progress of DR.
Figure 4.
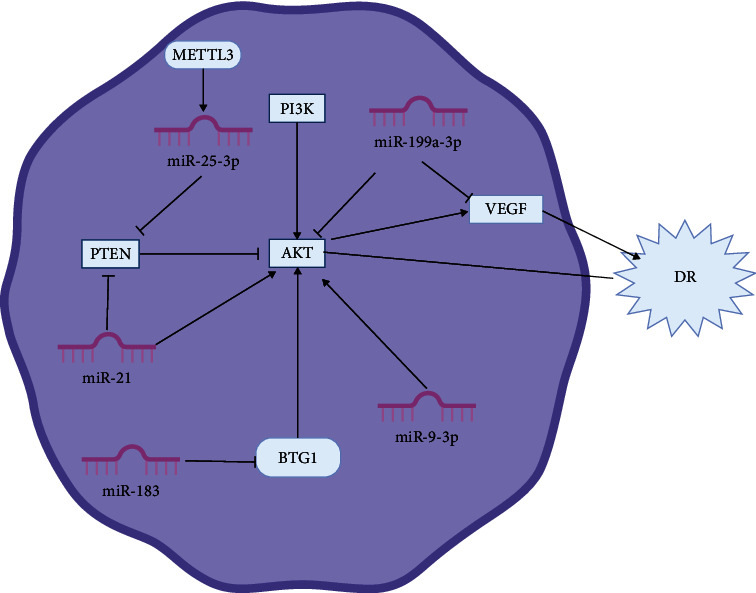
Diagrammatic representation of AKT/microRNA mechanism in DR.
4.2. Inflammation-Related Signaling Pathways
4.2.1. NF-κB Signaling Pathway
Nuclear factor-κB (NF-κB) is a family of core dimer transcription factors that coordinate inflammatory responses and can be activated by various stimuli and complex signaling pathway networks [173, 174]. NF-κB is a highly important intracellular nuclear transcription factor and is found in nearly all animal cells. The NF-κB signaling pathway is a typical proinflammatory pathway involved in a variety of biological processes, such as inflammatory and immune responses, cell proliferation, and apoptosis [175, 176]. In related studies, NF-κB has been shown to be associated with apoptosis and oxidative stress in peripapillary retinal cells from DR patients [177–179].
He et al. [180] found that miR-30c-5p inhibited the expression of the PLCG1 target gene, thereby suppressing the activation of the PKC/NF-κB pathway and reducing the inflammatory response in DR. PLCG1 is a member of the PLC family, and its downstream signaling factor, PKC, activates the NF-κB pathway, resulting in the induction of inflammation [181]. Upregulation of miR-30c-5p improved retinal vascular inflammation in DR, as verified by in vivo experiments.
Shi et al. [182] discovered that DR rats exhibit a significant reduction in miR-26a-5p expression levels compared to normal rats. The injection of miR-26a-5p analogs resulted in the attenuation of retinal nerve layer thickness and ganglion cell count reductions in DR mice. These findings suggest that miR-26a-5p plays a role in DR prevention. Additionally, the study demonstrated that miR-26a-5p could decrease the expression of NF-κB in DR rats by negatively regulating PTEN expression and reducing inflammation-induced damage.
For example, Xu et al. [183] found that miR-18b expression was significantly reduced in DR rat retina, and inhibition of miR-18b expression was shown to promote human retinal microvascular endothelial cell proliferation in high-glucose culture. The authors found that miR-18b dampened the inflammatory response by activating MAP3K1 and inhibiting the phosphorylation of NF-κB, which in turn slowed the progression of DR.
Rasoulinejad et al. [138] observed increased expression of NF-κB and decreased expression of miR-146a-5p in DR rat eye tissues. This study suggested that miR-146a-5p might be involved in the preventative therapeutic mechanism of DR. It was also established that miR-146a could inhibit NF-κB-mediated activation of inflammation through the suppression of the expression of its target genes, such as IRAK1 and TRAF6. The progression of DR could also be prevented by increasing miR-146a expression to enhance endothelial function.
Li et al. [184] found that miR-874 can target the degradation of p65. This study suggests that miR-874 regulates NF-κB signaling pathway by targeting p65 for degradation to further improve DR. Compared with normal rats, DR rats showed a decrease in miR-874 expression, an increase in VEGF and Ang II protein expression, a decrease in capillary pericytes, an increase in vascular endothelial cells, and an increase in the p65 expression in the retina. These changes were better in diabetic rats injected with miR-874 mimetics but worsened in diabetic rats injected with miR-874 inhibitors. We summarize the current studies of NF-κB signaling pathways in DR and categorize the relevant miRNAs in Figure 5.
Figure 5.
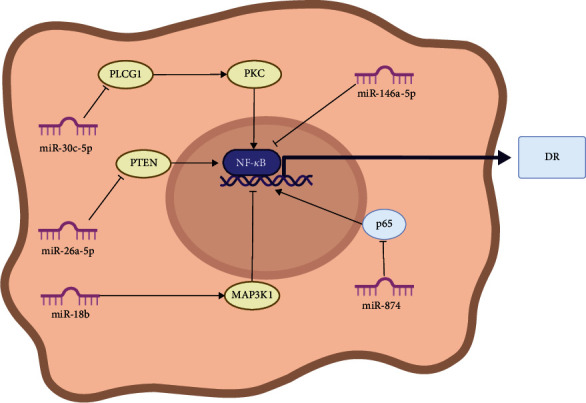
Diagrammatic representation of NF-κB/microRNA mechanism in DR.
4.2.2. MAPK Signaling Pathway
The mitogen-activated protein kinase (MAPK) signaling pathway is a ubiquitous signaling cascade in eukaryotic cells that regulates various physiological functions, including cell proliferation, differentiation, apoptosis, and stress response, and its dysregulation has been linked to cancer development [185, 186]. The MAPKs are a family of nuclear-expressed kinases that phosphorylate downstream targets to regulate gene expression. Major pathways include the c-Jun amino-terminal kinase (JNK), p38MAPK, and extracellular signal-regulated kinase (ERK), which includes extracellular signaling-regulated protein-1/2/3/4/5/7/8, amino-terminal c-Jun 1/2/3, and p38-MAPK (α, β, δ, and γ) [187, 188]. In related studies, various extracellular stimuli, such as oxidative stress and inflammation, have been shown to activate the MAPK signaling pathway [189, 190].
Zhang et al. [191] investigated the role of microRNA-141–3p (miR-141–3p) in docking protein 5- (DOK5-) mediated mitogen-activated protein kinase (MAPK) signaling pathway and found that miR-141–3p can activate the MAPK pathway by inhibiting the DOK5 gene. Upregulation of miR-141–3p was found to inhibit the proliferation of retinal vascular epithelial cells, as well as angiogenesis, and promote apoptosis of RGCs.
Cheng et al. [192] observed reduced levels of miR-181b in diabetic mice and showed that overexpression of miR-181b-improved endothelial function inhibited the generation of intravascular reactive oxygen species (ROS) and suppressed vascular inflammation in diabetic mice. The authors also found that miR-181b may be located downstream of the AMP-activated protein kinase (AMPK) signaling pathway, and activation of AMPK was able to upregulate miR-181b levels in endothelial cells. Activation of the AMPK/miR-181b axis was shown to ameliorate impaired endothelial cell function in a high-glucose environment.
4.2.3. TLR4 Signaling Pathway
Toll-like receptor 4 (TLR4) is a member of the toll-like receptor (TLR) family of receptors and is involved in the immune response. Excessive activation of TLR4 initiates the production of various inflammatory factors that are associated with the development of numerous diseases, such as sepsis, endothelial dysfunction, atherosclerosis, diabetes, rheumatoid arthritis, cardiovascular disease, and metabolic syndrome [193–198]. In DR, the progression of the disease is associated with TLR4 signaling pathway activation, and inhibiting the expression of TLR4 signaling pathways may slow down the DR process [199–201].
For instance, Liu et al. [202] found that interferon alpha 2 (IFNA2) was reduced in diabetic rats, and TLR4 signaling pathway activation was associated with the progression of DR lesions in rats. In this study, they showed that IFNA2 is a target gene of miR-499–3p, which downregulates IFNA2. In DR rats, the upregulation of miR-499–3p expression led to a decrease in IFNA2, which led to the activation of TLR4 signaling pathways. This study also found that downregulation of miR-499–3p attenuated DR lesions in the retina.
Li et al. [203] found that miR-486–3p can be induced within mesenchymal stem cells of bone marrow to generate exosomes, and TLR4 is a target of miR-486–3p, suggesting that there is a link between miR-486–3p and DR disease pathology. In this study, they showed that positive regulation of miR-486–3p can downregulate TLR4, thus inhibiting oxidative stress, inflammation, and apoptosis via the TLR4/nuclear transcription factor-κB (NF-κB) axis, thereby inhibiting DR lesion progression.
Cao et al. [204] found that the secretion of bone marrow mesenchymal stem cell miR-146a reduced TNF-α and the levels of IL-1 and IL-6, suggesting that miR-146a can reduce the inflammatory response in DR mice. Further research has found that miR-146a can reduce the activity of TLR4. In addition, overexpression of TLR4 reverses the effects of miR-146a on the proliferation, apoptosis, and inflammation of microglia. Prompt miR-146a can be adjusted by adjusting toll-like receptor 4 (TLR4)/myeloid differentiation factor 88(MyD88)/nuclear transcription factor-κB (NF-κB), which in turn regulates the inflammatory response pathway of DR. This serves as experimental evidence for the prevention and treatment of DR. Figure 6 shows the relevant miRNAs in the MAPK signaling pathways and TLR4 signaling pathways in the progress of DR.
Figure 6.
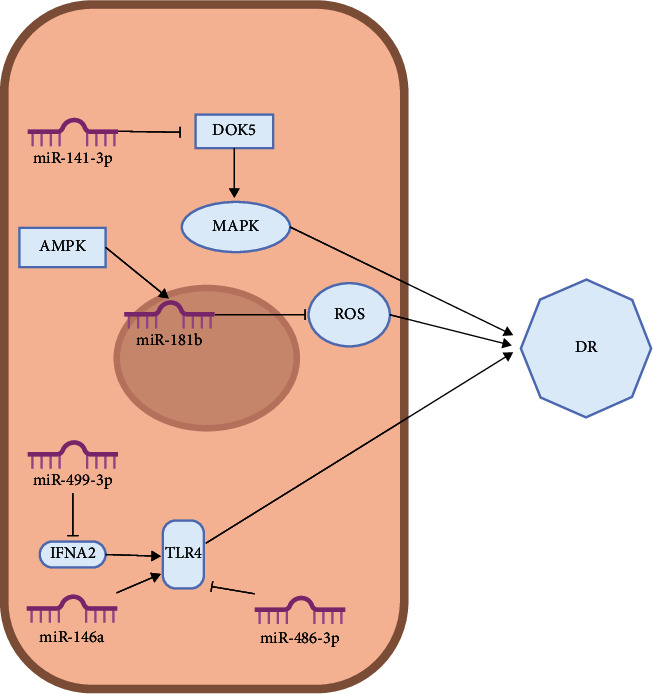
Diagrammatic representation of MAPK/TLR4/microRNA mechanism in DR.
4.3. Antineovascular Signaling Pathways
Vascular endothelial growth factor (VEGF) is a prominent factor involved in the pathogenesis of DR. Dysregulated production and release of VEGF lead to vascular endothelial cell proliferation and migration, resulting in neovascularization and increased vascular permeability [205]. VEGF comprises several isoforms, including VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and placental growth factor (PGF) [206]. Among these isoforms, VEGF-A plays a crucial role in regulating angiogenesis [207]. Although anti-VEGF pharmacotherapy has been extensively used in the clinical management of DR, its efficacy remains limited, and adverse side effects are possible. In this review, we summarize the roles of VEGF-related miRNAs in DR with the aim of providing new insights into antineovascular therapy in DR (Table 2).
Table 2.
Summary of miRNA treatment of DR via anti-VEGF.
| miRNA | Expression in DR | Mechanism of action | Regulation of VEGF by miRNA | Pathogenic function | Reference |
|---|---|---|---|---|---|
| miR-377–3p | Downregulation in serum exosomes | Lack of it increases VEGF expression | Negative regulation in serum exosomes | Inhibit the development of DR | [208] |
| miR-15b | Downregulation in serum | miR-15b regulates the expression of VEGF by targeting the 3′ untranslated regions to inhibit its transcription | Negative regulation in serum | Inhibit the development of DR | [115] |
| miR-409–5p | Upregulation in retinal tissues, in mRMECs, and in vitreous fluid | Its overexpression increases the expression and secretion of VEGF | Positive regulation in retinal tissues, in mRMECs, and in vitreous fluid | Promote the development of DR | [209] |
| miR-203a-3p | Downregulation in HRMECS | Reduce the levels of VEGFA and HIF-α, PCNA, and MMPs in cells | Negative regulation in HRMECS | Inhibit the development of DR | [91] |
| miR-29b-3p | Downregulation in RMECS | miR-29b-3p negatively regulated the expression of angiogenic factors in RMECs | Negative regulation in RMECS | Inhibit the development of DR | [210] |
| miR-20a | Downregulation in retinal tissues | Upregulation of SIRT1 inhibits the development of DR via miR-20a-induced downregulation of YAP/HIF1α/VEGFA | Negative regulation in retinal tissues | Inhibit the development of DR | [154] |
| miR-152 | Downregulation in hRECs and HRMECS | Its overexpression can inhibit VEGF signaling | Negative regulation in hRECs and HRMECS | Inhibit the development of DR | [211] |
| miR-200b-3p | Downregulation in RMECs | AP1 may exert some promotive effects on the development of DR through its regulation of the MALAT1/miR-200b-3p/VEGFA axis, highlighting that YAP1 silencing may be instrumental for the therapeutic targeting of DR | Negative regulation in RMECs | Inhibit the development of DR | [93] |
| miR-141–3p | Downregulation in retinal neovascularization and retinal ganglion cells (RGCs) | Impede the activation of the DOK5-mediated MAPK signaling pathway | Negative regulation in retinal neovascularization and retinal ganglion cells (RGCs) | Inhibit the development of DR | [191] |
| miR-26a | Downregulation in retinal tissues | Reduce the levels of VEGF, IL-1β, and NF-κB | Negative regulation in retinal tissues | miR-26a can protect against retinal neuronal impairment in diabetic mice by downregulating PTEN | [182] |
| miR-145 | Downregulation in HRMECS | Reduce the levels of VEGF, IL-1β, and NF-κB | Negative regulation in HRMECS | Inhibition of miR-145 abolished the beneficial role of TUG1 knockdown in HG-treated HRMECS. Inhibit the development of DR | [212] |
| miR-23a | Downregulation in blood and tear | Lack of it increases VEGF expression | Negative regulation in blood and tear | May regulate microvascular growth at the retina via VEGF and contribute to DR progression | [213] |
| miR-9–3p | Upregulation in Müller glia cells | Mechanistically, exosomal miRNA-9–3p was transferred to retinal endothelial cells and bound to the sphingosine-1-phosphate receptor S1P1 coding sequence, which subsequently activated VEGFR2 phosphorylation and internalization in the presence or absence of exogenous VEGF-A | Positive regulation in Müller glia cells | S1P1 was identified as the key component of miR-9–3p to regulate abnormal angiogenesis | [171] |
| miR-1281 | Upregulation in serum | miR-1281 positively regulates VEGFA protein expression through activation of VEGFA gene transcription | Positive regulation in serum | Promote the expression of VEGFA | [108] |
| miR-223–3p | Upregulation in a zebrafish model | Overexpression increases VEGF levels | Positive regulation in a zebrafish model | miR-223–3p negatively regulates eukaryotic translation initiation factor 4E family member 3 (EIF4E3) and insulin-like growth factor 1 receptor (IGF1R) | [214] |
| miR-181d-5p | Downregulation in HRMECS | miR-181d-5p directly targeted and negatively regulated VEGFA | Negative regulation in HRMECS | miR-181d-5p inhibition augmented cell proliferation, migration, and angiogenesis of HRMECS caused by HG | [215] |
| miR-139-5p | Upregulation in RMECs | Targeted inhibition PTEN | Positive regulation in RMECs | Promotes cell migration, tube formation, and VEGF protein level | [216] |
5. MicroRNA Therapy
MicroRNAs are key regulators of cellular signaling cascades, and changes in their expression play a critical role in the alteration of protein expression in many diseases [217]. There are two main strategies for miRNA therapy: inhibition of disease-driving miRNA expression to reduce or block its expression and promotion of the expression of pathologic suppressors of disease [218].
MicroRNAs that drive pathology are often upregulated during the disease process and must be suppressed and returned to normal levels. Five approaches are typically used to inhibit miRNA expression: (i) anti-miRNA oligonucleotides, which are chemically modified single-stranded oligonucleotides complementary to the target miRNA that prevent its interaction with the target gene by binding to the mature miRNA of interest [219, 220]; (ii) miRNA sponges, which are competitive inhibitors with multiple miRNA target binding sites to block the interaction between miRNA and their target mRNA [221]; (iii) small molecule inhibitors, designed using bioinformatics tools or identified by experimental screening of pharmacologically active compounds [222, 223], which function through protein interactions involved in miRNA biogenesis or through inhibition of miRNA-target interactions via binding to specific miRNA secondary structures [221]; (iv) masking of miRNAs, a novel method developed by Xiao et al. consisting of single-stranded 2′-O-methyl-modified antisense oligonucleotides with a high affinity to the predicted miRNA binding site in the 3′ UTR of the target mRNA, rendering the miRNA incapable of binding to the corresponding binding site and thereby inhibiting its target gene (mRNA) [224]; and (v) nucleic acid immobilization using locked nucleic acids (LNA), an oligonucleotide analog with a ring structure formed by the 2′-O atom and the 4′-C atom of ribose linked by a methylene bridge. Locked nucleic acids (LNA) can form a complementary pairing with RNA, single-stranded DNA, and double-stranded DNA with higher affinity [225, 226].
MicroRNAs that act as disease suppressors are often lost during disease development. Promoting the expression of these suppressors can help suppress disease development. MicroRNA mimics are an effective tool for this purpose. These are chemically engineered small double-stranded RNA molecules that mimic endogenous mature miRNA molecules, complementing the alternative processing of the relevant miRNA molecules [227].
6. Summary and Discussion
The most effective approach to managing DR is early detection, prevention, and treatment. As many patients are asymptomatic during the early stages of the disease, early detection is crucial [228]. Ophthalmological examinations, such as pupillary dilation and retinal assessment, are currently the available screening modalities [229]. However, early detection and diagnosis of DR still present a challenge worldwide, particularly in middle- and low-income countries. The cost of ophthalmic screening devices and the insufficient number of ophthalmologists hinder the early screening process. Nevertheless, miRNAs can circulate in the blood, providing a novel means of early screening for DR. The identification of a miRNA or group of miRNAs as molecular biological markers for the onset of DR, and the detection of the levels of such markers in the blood, may lead to the early diagnosis of DR and even classification of DR progression by magnitude of grade change, making it a more specific treatment. This has far-reaching implications for DR prevention and treatment in the future.
In recent years, an increasing number of miRNA research reports have been published, and miRNAs have become the focus of attention as both biomarkers and therapeutics. miRNAs can be found stably in serum and other bodily fluids and have been used as biomarkers in numerous diseases due to their endogenous nature, stability, and noninvasive biopsies, such as diabetes, diabetic nephropathy, DR, ophthalmic diseases, cardiovascular diseases, and various cancers [230–236]. However, it should be noted that miRNAs still have many limitations as a biomarker of DR, such as lack of specificity, sample source limitations, and other methodological limitations. miRNAs may have overlapping expression in different diseases, and the variability between different populations and the variability between ethnic groups can limit its guiding characteristics. The abundance of miRNAs in the body fluids of DR patients is low, and most of them can only be detected in blood samples or aqueous humor, which to some extent limits the source of samples. At the same time, the lack of highly efficient clinical collection methods, the immature miRNA isolation and purification techniques, and the lack of standardized procedures ultimately limit its application in clinical practice. Nevertheless, miRNAs still have great potential in the detection of DR, and future research may address these limitations.
miRNAs may also serve as therapeutic agents for various cancers, and their ability to target multiple molecules provides them with a unique advantage over approaches that target single genes [237, 238]. Several miRNA therapies have demonstrated substantial preclinical efficacy for various diseases. For example, miR-10b-5p is in preclinical development and is believed to be useful in the treatment of diabetes and related disorders of gut motility [239]. Preclinical studies have also shown that inhibitors of miR-103/107 improve insulin sensitivity in obese mice [240]. MicroRNA therapeutics have shown great therapeutic promise in clinical settings. However, it still faces many challenges, such as the selection of effective drug delivery routes, controlling the in vivo stability of relevant designer drugs, efficient targeting of specific tissues and cells, determining drug delivery mode and dose, controlling vector-miRNA interaction, and reducing immune responses and side effects.
MicroRNAs have unlimited therapeutic potential and may offer novel therapeutic options for DR. However, it is undeniable that much work needs to be done before miRNA therapeutics can be translated into the clinic. Further research is required to refine miRNA therapeutics.
Acknowledgments
This search was supported by grants from the Guoyiqiangyou Foundation of Shanghai Hongkou (2022-2024) (HKGYQYXM-2022-13).
Data Availability
Data availability is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Xu T., Wang B., Liu H., et al. Prevalence and causes of vision loss in China from 1990 to 2019: findings from the Global Burden of Disease Study 2019. The Lancet Public Health . 2020;5(12):e682–e691. doi: 10.1016/S2468-2667(20)30254-1. [DOI] [PubMed] [Google Scholar]
- 2.Cheung N., Mitchell P., Wong T. Y. Diabetic retinopathy. Lancet . 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 3.Ogurtsova K., da Rocha Fernandes J. D., Huang Y., et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Research and Clinical Practice . 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2019 Blindness and Vision Impairment Collaborators. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the Global Burden of Disease Study. The Lancet Global Health . 2021;9(2):e144–e160. doi: 10.1016/S2214-109X(20)30489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lechner J., O'Leary O. E., Stitt A. W. The pathology associated with diabetic retinopathy. Vision Research . 2017;139:7–14. doi: 10.1016/j.visres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Kang Q., Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biology . 2020;37, article 101799 doi: 10.1016/j.redox.2020.101799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oshitari T. Neurovascular impairment and therapeutic strategies in diabetic retinopathy. International Journal of Environmental Research and Public Health . 2021;19(1):p. 439. doi: 10.3390/ijerph19010439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitehead M., Wickremasinghe S., Osborne A., Van Wijngaarden P., Martin K. R. Diabetic retinopathy: a complex pathophysiology requiring novel therapeutic strategies. Expert Opinion on Biological Therapy . 2018;18(12):1257–1270. doi: 10.1080/14712598.2018.1545836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammes H. P. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia . 2018;61(1):29–38. doi: 10.1007/s00125-017-4435-8. [DOI] [PubMed] [Google Scholar]
- 10.Mahajan N., Arora P., Sandhir R. Perturbed biochemical pathways and associated oxidative stress lead to vascular dysfunctions in diabetic retinopathy. Oxidative Medicine and Cellular Longevity . 2019;2019:16. doi: 10.1155/2019/8458472.8458472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phipps J. A., Jobling A. I., Greferath U., Fletcher E. L., Vessey K. A. Alternative pathways in the development of diabetic retinopathy: the renin-angiotensin and kallikrein-kinin systems. Clinical & Experimental Optometry . 2012;95(3):282–289. doi: 10.1111/j.1444-0938.2012.00747.x. [DOI] [PubMed] [Google Scholar]
- 12.Morgan M. J., Liu Z. G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Research . 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nawaz I. M., Rezzola S., Cancarini A., et al. Human vitreous in proliferative diabetic retinopathy: characterization and translational implications. Progress in Retinal and Eye Research . 2019;72, article 100756 doi: 10.1016/j.preteyeres.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Oh K., Kang H. M., Leem D., Lee H., Seo K. Y., Yoon S. Early detection of diabetic retinopathy based on deep learning and ultra-wide-field fundus images. Scientific Reports . 2021;11(1):p. 1897. doi: 10.1038/s41598-021-81539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin K. Y., Hsih W. H., Lin Y. B., Wen C. Y., Chang T. J. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. Journal of Diabetes Investigation . 2021;12(8):1322–1325. doi: 10.1111/jdi.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkinson C. P., Ferris F. L., 3rd, Klein R. E., et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology . 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 17.Das A., Stroud S., Mehta A., Rangasamy S. New treatments for diabetic retinopathy. Diabetes, Obesity & Metabolism . 2015;17(3):219–230. doi: 10.1111/dom.12384. [DOI] [PubMed] [Google Scholar]
- 18.Stitt A. W., Curtis T. M., Chen M., et al. The progress in understanding and treatment of diabetic retinopathy. Progress in Retinal and Eye Research . 2016;51:156–186. doi: 10.1016/j.preteyeres.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Kollias A. N., Ulbig M. W. Diabetic retinopathy: early diagnosis and effective treatment. Deutsches Arzteblatt International . 2010;107(5):75–84. doi: 10.3238/arztebl.2010.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodríguez M. L., Pérez S., Mena-Mollá S., Desco M. C., Ortega Á. L. Oxidative stress and microvascular alterations in diabetic retinopathy: future therapies. Oxidative Medicine and Cellular Longevity . 2019;2019:18. doi: 10.1155/2019/4940825.4940825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno A., Lozano M., Salinas P. Diabetic retinopathy. Nutricion Hospitalaria . 2013;28(Supplement 2):53–56. doi: 10.3305/nh.2013.28.sup2.6714. [DOI] [PubMed] [Google Scholar]
- 22.Santiago A. R., Boia R., Aires I. D., Ambrósio A. F., Fernandes R. Sweet stress: coping with vascular dysfunction in diabetic retinopathy. Frontiers in Physiology . 2018;9:p. 820. doi: 10.3389/fphys.2018.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viswanath K., McGavin D. D. Diabetic retinopathy: clinical findings and management. Community Eye Health . 2003;16(46):21–24. [PMC free article] [PubMed] [Google Scholar]
- 24.Mohamed Q., Gillies M. C., Wong T. Y. Management of diabetic retinopathy. JAMA . 2007;298(8):902–916. doi: 10.1001/jama.298.8.902. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhary S., Zaveri J., Becker N. Proliferative diabetic retinopathy (PDR) Disease-a-Month . 2021;67(5, article 101140) doi: 10.1016/j.disamonth.2021.101140. [DOI] [PubMed] [Google Scholar]
- 26.Moutray T., Evans J. R., Lois N., Armstrong D. J., Peto T., Azuara-Blanco A. Different lasers and techniques for proliferative diabetic retinopathy. The Cochrane Database of Systematic Reviews . 2018;3(3, article CD012314) doi: 10.1002/14651858.CD012314.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rittiphairoj T., Mir T. A., Li T., Virgili G. Intravitreal steroids for macular edema in diabetes. The Cochrane Database of Systematic Reviews . 2020;11(11, article CD005656) doi: 10.1002/14651858.CD005656.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyer D. S., Yoon Y. H., Belfort R., Jr., et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology . 2014;121(10):1904–1914. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 29.Schreur V., Brouwers J., Van Huet R. A. C., et al. Long-term outcomes of vitrectomy for proliferative diabetic retinopathy. Acta Ophthalmologica . 2021;99(1):83–89. doi: 10.1111/aos.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liberski S., Wichrowska M., Kocięcki J. Aflibercept versus faricimab in the treatment of neovascular age-related macular degeneration and diabetic macular edema: a review. International Journal of Molecular Sciences . 2022;23(16):p. 9424. doi: 10.3390/ijms23169424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Photocoagulation for diabetic macular edema. Photocoagulation for diabetic Macular Edema. Archives of Ophthalmology . 1985;103(12):1796–1806. doi: 10.1001/archopht.1985.01050120030015. [DOI] [PubMed] [Google Scholar]
- 32.Everett L. A., Paulus Y. M. Laser therapy in the treatment of diabetic retinopathy and diabetic macular edema. Current Diabetes Reports . 2021;21(9):p. 35. doi: 10.1007/s11892-021-01403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torp T. L., Kawasaki R., Wong T. Y., Peto T., Grauslund J. Temporal changes in retinal vascular parameters associated with successful panretinal photocoagulation in proliferative diabetic retinopathy: a prospective clinical interventional study. Acta Ophthalmologica . 2018;96(4):405–410. doi: 10.1111/aos.13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Early photocoagulation for diabetic retinopathy. Early Photocoagulation for diabetic retinopathy. Ophthalmology . 1991;98(5) 5 Supplement:766–785. doi: 10.1016/S0161-6420(13)38011-7. [DOI] [PubMed] [Google Scholar]
- 35.Stewart M. W., Browning D. J., Landers M. B. Current management of diabetic tractional retinal detachments. Indian Journal of Ophthalmology . 2018;66(12):1751–1762. doi: 10.4103/ijo.IJO_1217_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wells J. A., Glassman A. R., Ayala A. R., et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology . 2016;123(6):1351–1359. doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolinger M. T., Antonetti D. A. Moving past anti-VEGF: novel therapies for treating diabetic retinopathy. International Journal of Molecular Sciences . 2016;17(9):p. 1498. doi: 10.3390/ijms17091498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah J., Cheong Z. Y., Tan B., Wong D., Liu X., Chua J. Dietary intake and diabetic retinopathy: a systematic review of the literature. Nutrients . 2022;14(23):p. 5021. doi: 10.3390/nu14235021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milluzzo A., Barchitta M., Maugeri A., et al. Do nutrients and nutraceuticals play a role in diabetic retinopathy? A systematic review. Nutrients . 2022;14(20):p. 4430. doi: 10.3390/nu14204430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le N. T., Kroeger Z. A., Lin W. V., Khanani A. M., Weng C. Y. Novel treatments for diabetic macular edema and proliferative diabetic retinopathy. Current Diabetes Reports . 2021;21(10):p. 43. doi: 10.1007/s11892-021-01412-5. [DOI] [PubMed] [Google Scholar]
- 41.He Y., Dan Y., Gao X., Huang L., Lv H., Chen J. DNMT1-mediated lncRNA MEG3 methylation accelerates endothelial-mesenchymal transition in diabetic retinopathy through the PI3K/Akt/mTOR signaling pathway. American Journal of Physiology. Endocrinology and Metabolism . 2021;320(3):E598–E608. doi: 10.1152/ajpendo.00089.2020. [DOI] [PubMed] [Google Scholar]
- 42.Duraisamy A. J., Mishra M., Kowluru A., Kowluru R. A. Epigenetics and regulation of oxidative stress in diabetic retinopathy. Investigative Ophthalmology & Visual Science . 2018;59(12):4831–4840. doi: 10.1167/iovs.18-24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho H., Sobrin L. Genetics of diabetic retinopathy. Current Diabetes Reports . 2014;14(8):p. 515. doi: 10.1007/s11892-014-0515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Becker K., Klein H., Simon E., et al. In-depth transcriptomic analysis of human retina reveals molecular mechanisms underlying diabetic retinopathy. Scientific Reports . 2021;11(1, article 10494) doi: 10.1038/s41598-021-88698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X., Yu Z. W., Wang Y., Fu Y. H., Gao X. Y. MicroRNAs: potential targets in diabetic retinopathy. Hormone and Metabolic Research . 2020;52(3):142–148. doi: 10.1055/a-1107-2943. [DOI] [PubMed] [Google Scholar]
- 46.Gui F., You Z., Fu S., Wu H., Zhang Y. Endothelial dysfunction in diabetic retinopathy. Frontiers in Endocrinology . 2020;11:p. 591. doi: 10.3389/fendo.2020.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao X., Ling F., Zhang G. W., Yu N., Yang J., Xin X. Y. The correlation between MicroRNAs and diabetic retinopathy. Frontiers in Immunology . 2022;13, article 941982 doi: 10.3389/fimmu.2022.941982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milluzzo A., Maugeri A., Barchitta M., Sciacca L., Agodi A. Epigenetic mechanisms in type 2 diabetes retinopathy: a systematic review. International Journal of Molecular Sciences . 2021;22(19, article 10502) doi: 10.3390/ijms221910502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhaskaran M., Mohan M. MicroRNAs: history, biogenesis, and their evolving role in animal development and disease. Veterinary Pathology . 2014;51(4):759–774. doi: 10.1177/0300985813502820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee Y., Ahn C., Han J., et al. The nuclear RNase III Drosha initiates microRNA processing. Nature . 2003;425(6956):415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 51.Bartel D. P. Metazoan microRNAs. Cell . 2018;173(1):20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carthew R. W., Sontheimer E. J. Origins and mechanisms of miRNAs and siRNAs. Cell . 2009;136(4):642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siomi H., Siomi M. C. Posttranscriptional regulation of microRNA biogenesis in animals. Molecular Cell . 2010;38(3):323–332. doi: 10.1016/j.molcel.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 54.Starega-Roslan J., Koscianska E., Kozlowski P., Krzyzosiak W. J. The role of the precursor structure in the biogenesis of microRNA. Cellular and Molecular Life Sciences: CMLS . 2011;68(17):2859–2871. doi: 10.1007/s00018-011-0726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chendrimada T. P., Gregory R. I., Kumaraswamy E., et al. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature . 2005;436(7051):740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson R. C., Tambe A., Kidwell M. A., Noland C. L., Schneider C. P., Doudna J. A. Dicer-TRBP complex formation ensures accurate mammalian microRNA biogenesis. Molecular Cell . 2015;57(3):397–407. doi: 10.1016/j.molcel.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Treiber T., Treiber N., Plessmann U., et al. A compendium of RNA-binding proteins that regulate microRNA biogenesis. Molecular Cell . 2017;66(2):270–284.e13. doi: 10.1016/j.molcel.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 58.Noland C. L., Ma E., Doudna J. A. siRNA repositioning for guide strand selection by human Dicer complexes. Molecular Cell . 2011;43(1):110–121. doi: 10.1016/j.molcel.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Treiber T., Treiber N., Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nature Reviews Molecular Cell Biology . 2019;20(1):5–20. doi: 10.1038/s41580-018-0059-1. [DOI] [PubMed] [Google Scholar]
- 60.Bartel D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell . 2004;116(2):281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 61.Gulyaeva L. F., Kushlinskiy N. E. Regulatory mechanisms of microRNA expression. Journal of Translational Medicine . 2016;14(1):p. 143. doi: 10.1186/s12967-016-0893-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ho P. T. B., Clark I. M., Le L. T. T. MicroRNA-based diagnosis and therapy. International Journal of Molecular Sciences . 2022;23(13):p. 7167. doi: 10.3390/ijms23137167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Correia de Sousa M., Gjorgjieva M., Dolicka D., Sobolewski C., Foti M. Deciphering miRNAs’ action through miRNA editing. International Journal of Molecular Sciences . 2019;20(24):p. 6249. doi: 10.3390/ijms20246249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang X., Du W. W., Li H., et al. Both mature miR-17-5p and passenger strand miR-17-3p target TIMP3 and induce prostate tumor growth and invasion. Nucleic Acids Research . 2013;41(21):9688–9704. doi: 10.1093/nar/gkt680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ro S., Park C., Young D., Sanders K. M., Yan W. Tissue-dependent paired expression of miRNAs. Nucleic Acids Research . 2007;35(17):5944–5953. doi: 10.1093/nar/gkm641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okamura K., Phillips M. D., Tyler D. M., Duan H., Chou Y. T., Lai E. C. The regulatory activity of microRNA∗ species has substantial influence on microRNA and 3′ UTR evolution. Nature Structural & Molecular Biology . 2008;15(4):354–363. doi: 10.1038/nsmb.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schober A., Nazari-Jahantigh M., Wei Y., et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nature Medicine . 2014;20(4):368–376. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Villain G., Poissonnier L., Noueihed B., et al. miR-126-5p promotes retinal endothelial cell survival through SetD5 regulation in neurons. Development . 2018;145(1, article dev156232) doi: 10.1242/dev.156232. [DOI] [PubMed] [Google Scholar]
- 69.Wang L., Cui M., Cheng D., et al. miR-9-5p facilitates hepatocellular carcinoma cell proliferation, migration and invasion by targeting ESR1. Molecular and Cellular Biochemistry . 2021;476(2):575–583. doi: 10.1007/s11010-020-03927-z. [DOI] [PubMed] [Google Scholar]
- 70.Zhen J., Zhang H., Dong H., Tong X. miR-9-3p inhibits glioma cell proliferation and apoptosis by directly targeting FOXG1. Oncology Letters . 2020;20(2):2007–2015. doi: 10.3892/ol.2020.11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arroyo J. D., Chevillet J. R., Kroh E. M., et al. Argonaute 2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proceedings of the National Academy of Sciences of the United States of America . 2011;108(12):5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pozniak T., Shcharbin D., Bryszewska M. Circulating microRNAs in medicine. International Journal of Molecular Sciences . 2022;23(7):p. 3996. doi: 10.3390/ijms23073996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia-Martin R., Wang G., Brandão B. B., et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature . 2022;601(7893):446–451. doi: 10.1038/s41586-021-04234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cui J., Shu J. Circulating microRNA trafficking and regulation: computational principles and practice. Briefings in Bioinformatics . 2020;21(4):1313–1326. doi: 10.1093/bib/bbz079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koppers-Lalic D., Hackenberg M., Bijnsdorp I. V., et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Reports . 2014;8(6):1649–1658. doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 76.Théry C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nature Reviews Immunology . 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 77.Weber J. A., Baxter D. H., Zhang S., et al. The microRNA spectrum in 12 body fluids. Clinical Chemistry . 2010;56(11):1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Osipov I. D., Zaporozhchenko I. A., Bondar A. A., et al. Cell-free miRNA-141 and miRNA-205 as prostate cancer biomarkers. Advances in Experimental Medicine and Biology . 2016;924:9–12. doi: 10.1007/978-3-319-42044-8_2. [DOI] [PubMed] [Google Scholar]
- 79.Mitchell P. S., Parkin R. K., Kroh E. M., et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proceedings of the National Academy of Sciences of the United States of America . 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smit-McBride Z., Morse L. S. MicroRNA and diabetic retinopathy-biomarkers and novel therapeutics. Annals of Translational Medicine . 2021;9(15):p. 1280. doi: 10.21037/atm-20-5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zampetaki A., Kiechl S., Drozdov I., et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circulation Research . 2010;107(6):810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 82.Karolina D. S., Armugam A., Tavintharan S., et al. MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes mellitus. PLoS One . 2011;6(8, article e22839) doi: 10.1371/journal.pone.0022839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma L., Wen Y., Li Z., Wu N., Wang Q. Circulating microRNAs as potential diagnostic biomarkers for diabetic retinopathy: a meta-analysis. Frontiers in Endocrinology . 2022;13, article 929924 doi: 10.3389/fendo.2022.929924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Z., Dong Y., He C., et al. RNA-Seq revealed novel non-proliferative retinopathy specific circulating miRNAs in T2DM patients. Frontiers in Genetics . 2019;10:p. 531. doi: 10.3389/fgene.2019.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yin C., Lin X., Sun Y., Ji X. Dysregulation of miR-210 is involved in the development of diabetic retinopathy and serves a regulatory role in retinal vascular endothelial cell proliferation. European Journal of Medical Research . 2020;25(1):p. 20. doi: 10.1186/s40001-020-00416-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kot A., Kaczmarek R. Exosomal miRNA profiling in vitreous humor in proliferative diabetic retinopathy. Cell . 2023;12(1):p. 123. doi: 10.3390/cells12010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grieco G. E., Sebastiani G., Eandi C. M., et al. MicroRNA expression in the aqueous humor of patients with diabetic macular edema. International Journal of Molecular Sciences . 2020;21(19):p. 7328. doi: 10.3390/ijms21197328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roy D., Modi A., Khokhar M., et al. MicroRNA 21 emerging role in diabetic complications: a critical update. Current Diabetes Reviews . 2021;17(2):122–135. doi: 10.2174/1573399816666200503035035. [DOI] [PubMed] [Google Scholar]
- 89.Liu Y., Xiao J., Zhao Y., et al. MicroRNA-216a protects against human retinal microvascular endothelial cell injury in diabetic retinopathy by suppressing the NOS2/JAK/STAT axis. Experimental and Molecular Pathology . 2020;115, article 104445 doi: 10.1016/j.yexmp.2020.104445. [DOI] [PubMed] [Google Scholar]
- 90.Zeng Y., Cui Z., Liu J., Chen J., Tang S. MicroRNA-29b-3p promotes human retinal microvascular endothelial cell apoptosis via blocking SIRT1 in diabetic retinopathy. Frontiers in Physiology . 2020;10:p. 1621. doi: 10.3389/fphys.2019.01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han N., Xu H., Yu N., Wu Y., Yu L. miR-203a-3p inhibits retinal angiogenesis and alleviates proliferative diabetic retinopathy in oxygen-induced retinopathy (OIR) rat model via targeting VEGFA and HIF-1α. Clinical and Experimental Pharmacology & Physiology . 2020;47(1):85–94. doi: 10.1111/1440-1681.13163. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Z. Z., Qin X. H., Zhang J. MicroRNA-183 inhibition exerts suppressive effects on diabetic retinopathy by inactivating BTG1-mediated PI3K/Akt/VEGF signaling pathway. American Journal of Physiology. Endocrinology and Metabolism . 2019;316(6):E1050–E1060. doi: 10.1152/ajpendo.00444.2018. [DOI] [PubMed] [Google Scholar]
- 93.Han N., Tian W., Yu N., Yu L. YAP1 is required for the angiogenesis in retinal microvascular endothelial cells via the inhibition of MALAT1-mediated miR-200b-3p in high glucose-induced diabetic retinopathy. Journal of Cellular Physiology . 2020;235(2):1309–1320. doi: 10.1002/jcp.29047. [DOI] [PubMed] [Google Scholar]
- 94.Ko G. Y., Yu F., Bayless K. J., Ko M. L. MicroRNA-150 (miR-150) and diabetic retinopathy: is miR-150 only a biomarker or does it contribute to disease progression? International Journal of Molecular Sciences . 2022;23(20, article 12099) doi: 10.3390/ijms232012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bereimipour A., Najafi H., Mirsane E. S., Moradi S., Satarian L. Roles of miR-204 in retinal development and maintenance. Experimental Cell Research . 2021;406(1, article 112737) doi: 10.1016/j.yexcr.2021.112737. [DOI] [PubMed] [Google Scholar]
- 96.Ji H., Yi Q., Chen L., et al. Circulating miR-3197 and miR-2116-5p as novel biomarkers for diabetic retinopathy. Clinica Chimica Acta . 2020;501:147–153. doi: 10.1016/j.cca.2019.10.036. [DOI] [PubMed] [Google Scholar]
- 97.Saleh A. A., El-Hefnawy S. M., Kasemy Z. A., Alhagaa A. A., Nooh M. Z., Arafat E. S. Mi-RNA-93 and mi-RNA-152 in the diagnosis of type 2 diabetes and diabetic retinopathy. British Journal of Biomedical Science . 2022;79, article 10192 doi: 10.3389/bjbs.2021.10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xia Z., Yang X., Zheng Y., Yi G., Wu S. Plasma levels and diagnostic significance of miR-335-3p and EGFR in diabetic retinopathy. Clinical Laboratory . 2022;68(4) doi: 10.7754/Clin.Lab.2021.210447. [DOI] [PubMed] [Google Scholar]
- 99.Tan A., Li T., Ruan L., et al. Knockdown of Malat1 alleviates high-glucose-induced angiogenesis through regulating miR-205-5p/VEGF-A axis. Experimental Eye Research . 2021;207, article 108585 doi: 10.1016/j.exer.2021.108585. [DOI] [PubMed] [Google Scholar]
- 100.Liu X., Zhou Y., Liu Y., Wang Q., Pan L. MicroRNA-425-5p is involved in the development of diabetic retinopathy and regulates the proliferation and migration of retinal microvascular endothelial cells. Ophthalmic Research . 2022;65(1):60–67. doi: 10.1159/000516906. [DOI] [PubMed] [Google Scholar]
- 101.Prado M. S. G., de Jesus M. L., de Goes T. C., Mendonça L. S. O., Kaneto C. M. Downregulation of circulating miR-320a and target gene prediction in patients with diabetic retinopathy. BMC Research Notes . 2020;13(1):p. 155. doi: 10.1186/s13104-020-05001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pastukh N., Meerson A., Kalish D., Jabaly H., Blum A. Serum miR-122 levels correlate with diabetic retinopathy. Clinical and Experimental Medicine . 2019;19(2):255–260. doi: 10.1007/s10238-019-00546-x. [DOI] [PubMed] [Google Scholar]
- 103.Shi R., Chen L., Wang W., et al. Plasma miR-26a-5p is a biomarker for retinal neurodegeneration of early diabetic retinopathy. Eye . 2021;35(6):1587–1599. doi: 10.1038/s41433-021-01393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu B., Xiao M., Yang F., et al. MicroRNA-431-5p encapsulated in serum extracellular vesicles as a biomarker for proliferative diabetic retinopathy. The International Journal of Biochemistry & Cell Biology . 2021;135, article 105975 doi: 10.1016/j.biocel.2021.105975. [DOI] [PubMed] [Google Scholar]
- 105.Torus B., Korkmaz H., Ozturk K. H., et al. Downregulation of plasma microRNA-29c-3p expression may be a new risk factor for diabetic retinopathy. Minerva Endocrinology . 2023;48(1):42–50. doi: 10.23736/S2724-6507.20.03278-2. [DOI] [PubMed] [Google Scholar]
- 106.Platania C. B. M., Maisto R., Trotta M. C., et al. Retinal and circulating miRNA expression patterns in diabetic retinopathy: an in silico and in vivo approach. British Journal of Pharmacology . 2019;176(13):2179–2194. doi: 10.1111/bph.14665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Santovito D., Toto L., De Nardis V., et al. Plasma microRNA signature associated with retinopathy in patients with type 2 diabetes. Scientific Reports . 2021;11(1):p. 4136. doi: 10.1038/s41598-021-83047-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Greco M., Chiefari E., Accattato F., et al. MicroRNA-1281 as a novel circulating biomarker in patients with diabetic retinopathy. Frontiers in Endocrinology . 2020;11:p. 528. doi: 10.3389/fendo.2020.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Z., Zhang X., Wang Y., Xiao D. Dysregulation of miR-374a is involved in the progression of diabetic retinopathy and regulates the proliferation and migration of retinal microvascular endothelial cells. Clinical & Experimental Optometry . 2022;105(3):287–292. doi: 10.1080/08164622.2021.1913043. [DOI] [PubMed] [Google Scholar]
- 110.Hu L., Zhang T., Ma H., et al. Discovering the secret of diseases by incorporated tear exosomes analysis via rapid-isolation system: iTEARS. ACS Nano . 2022;16(8):11720–11732. doi: 10.1021/acsnano.2c02531. [DOI] [PubMed] [Google Scholar]
- 111.Liu W., Luo Z., Zhang L., et al. hsa-mir-(4328, 4422, 548z and -628-5p) in diabetic retinopathy: diagnosis, prediction and linking a new therapeutic target. Acta Diabetologica . 2023;60(7):929–942. doi: 10.1007/s00592-023-02077-0. [DOI] [PubMed] [Google Scholar]
- 112.Mazzeo A., Lopatina T., Gai C., Trento M., Porta M., Beltramo E. Functional analysis of miR-21-3p, miR-30b-5p and miR-150-5p shuttled by extracellular vesicles from diabetic subjects reveals their association with diabetic retinopathy. Experimental Eye Research . 2019;184:56–63. doi: 10.1016/j.exer.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 113.Shaker O. G., Abdelaleem O. O., Mahmoud R. H., et al. Diagnostic and prognostic role of serum miR-20b, miR-17-3p, HOTAIR, and MALAT1 in diabetic retinopathy. IUBMB Life . 2019;71(3):310–320. doi: 10.1002/iub.1970. [DOI] [PubMed] [Google Scholar]
- 114.Ye E. A., Steinle J. J. Regulatory role of microRNA on inflammatory responses of diabetic retinopathy. Neural Regeneration Research . 2017;12(4):580–581. doi: 10.4103/1673-5374.205095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang Y., Liu Y., Li Y., et al. MicroRNA-15b targets VEGF and inhibits angiogenesis in proliferative diabetic retinopathy. The Journal of Clinical Endocrinology and Metabolism . 2020;105(11):3404–3415. doi: 10.1210/clinem/dgaa538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shan K., Liu C., Liu B. H., et al. Circular noncoding RNA HIPK3 mediates retinal vascular dysfunction in diabetes mellitus. Circulation . 2017;136(17):1629–1642. doi: 10.1161/CIRCULATIONAHA.117.029004. [DOI] [PubMed] [Google Scholar]
- 117.Li H. S., Zhou Y. N., Li L., et al. HIF-1α protects against oxidative stress by directly targeting mitochondria. Redox Biology . 2019;25, article 101109 doi: 10.1016/j.redox.2019.101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang S., Ji L. Y., Li L., Li J. M. Oxidative stress, autophagy and pyroptosis in the neovascularization of oxygen-induced retinopathy in mice. Molecular Medicine Reports . 2019;19(2):927–934. doi: 10.3892/mmr.2018.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pizzino G., Irrera N., Cucinotta M., et al. Oxidative stress: harms and benefits for human health. Oxidative Medicine and Cellular Longevity . 2017;2017:13. doi: 10.1155/2017/8416763.8416763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Newsholme P., Cruzat V. F., Keane K. N., Carlessi R., de Bittencourt P. I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochemical Journal . 2016;473(24):4527–4550. doi: 10.1042/BCJ20160503C. [DOI] [PubMed] [Google Scholar]
- 121.Liu L., Xu H., Zhao H., Sui D. MicroRNA-135b-5p promotes endothelial cell proliferation and angiogenesis in diabetic retinopathy mice by inhibiting Von Hipp-el-Lindau and elevating hypoxia inducible factor α expression. Journal of Drug Targeting . 2021;29(3):300–309. doi: 10.1080/1061186X.2020.1833017. [DOI] [PubMed] [Google Scholar]
- 122.Guan J. T., Li X. X., Peng D. W., et al. MicroRNA-18a-5p administration suppresses retinal neovascularization by targeting FGF1 and HIF1A. Frontiers in Pharmacology . 2020;11:p. 276. doi: 10.3389/fphar.2020.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen Z., Yang J., Gao Y., et al. LncRNA MALAT1 aggravates the retinal angiogenesis via miR-320a/HIF-1α axis in diabetic retinopathy. Experimental Eye Research . 2022;218, article 108984 doi: 10.1016/j.exer.2022.108984. [DOI] [PubMed] [Google Scholar]
- 124.Dinkova-Kostova A. T., Abramov A. Y. The emerging role of Nrf2 in mitochondrial function. Free Radical Biology & Medicine . 2015;88(Part B):179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hayes J. D., Dinkova-Kostova A. T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends in Biochemical Sciences . 2014;39(4):199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 126.He F., Ru X., Wen T. NRF2, a transcription factor for stress response and beyond. International Journal of Molecular Sciences . 2020;21(13):p. 4777. doi: 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kensler T. W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annual Review of Pharmacology and Toxicology . 2007;47(1):89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 128.Kowluru R. A., Kowluru A., Mishra M., Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Progress in Retinal and Eye Research . 2015;48:40–61. doi: 10.1016/j.preteyeres.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brownlee M. The pathobiology of diabetic complications. Diabetes . 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 130.Mizutani M., Kern T. S., Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. The Journal of Clinical Investigation . 1996;97(12):2883–2890. doi: 10.1172/JCI118746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kowluru R. A., Abbas S. N. Diabetes-induced mitochondrial dysfunction in the retina. Investigative Ophthalmology & Visual Science . 2003;44(12):5327–5334. doi: 10.1167/iovs.03-0353. [DOI] [PubMed] [Google Scholar]
- 132.Madsen-Bouterse S. A., Kowluru R. A. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Reviews in Endocrine & Metabolic Disorders . 2008;9(4):315–327. doi: 10.1007/s11154-008-9090-4. [DOI] [PubMed] [Google Scholar]
- 133.Cheng X., Siow R. C., Mann G. E. Impaired redox signaling and antioxidant gene expression in endothelial cells in diabetes: a role for mitochondria and the nuclear factor-E2-related factor 2-Kelch-like ECH-associated protein 1 defense pathway. Antioxidants & Redox Signaling . 2011;14(3):469–487. doi: 10.1089/ars.2010.3283. [DOI] [PubMed] [Google Scholar]
- 134.Gao L., Mann G. E. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovascular Research . 2009;82(1):9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 135.Itoh K., Ye P., Matsumiya T., Tanji K., Ozaki T. Emerging functional cross-talk between the Keap1-Nrf2 system and mitochondria. Journal of Clinical Biochemistry and Nutrition . 2015;56(2):91–97. doi: 10.3164/jcbn.14-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jadeja R. N., Jones M. A., Abdelrahman A. A., et al. Inhibiting microRNA-144 potentiates Nrf2-dependent antioxidant signaling in RPE and protects against oxidative stress-induced outer retinal degeneration. Redox Biology . 2020;28, article 101336 doi: 10.1016/j.redox.2019.101336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Luo R., Jin H., Li L., Hu Y. X., Xiao F. Long noncoding RNA _MEG3_ inhibits apoptosis of retinal pigment epithelium cells induced by high glucose via the miR-93/Nrf2 axis. The American Journal of Pathology . 2020;190(9):1813–1822. doi: 10.1016/j.ajpath.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 138.Rasoulinejad S. A., Akbari A., Nasiri K. Interaction of miR-146a-5p with oxidative stress and inflammation in complications of type 2 diabetes mellitus in male rats: anti-oxidant and anti-inflammatory protection strategies in type 2 diabetic retinopathy. Iranian Journal of Basic Medical Sciences . 2021;24(8):1078–1086. doi: 10.22038/IJBMS.2021.56958.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang X., Zhou X., Wang F., et al. Long noncoding RNA TPTEP1 suppresses diabetic retinopathy by reducing oxidative stress and targeting the miR-489-3p/NRF2 axis. Acta Biochimica Polonica . 2023;70(1):45–50. doi: 10.18388/abp.2020_6034. [DOI] [PubMed] [Google Scholar]
- 140.Tang X., Li X., Zhang D., Han W. Astragaloside-IV alleviates high glucose-induced ferroptosis in retinal pigment epithelial cells by disrupting the expression of miR-138-5p/Sirt1/Nrf2. Bioengineered . 2022;13(4):8240–8254. doi: 10.1080/21655979.2022.2049471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rada P., Pardo V., Mobasher M. A., et al. SIRT1 controls acetaminophen hepatotoxicity by modulating inflammation and oxidative stress. Antioxidants & Redox Signaling . 2018;28(13):1187–1208. doi: 10.1089/ars.2017.7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang Y., Liu Y., Wang Y., et al. Regulation of SIRT1 and its roles in inflammation. Frontiers in Immunology . 2022;13, article 831168 doi: 10.3389/fimmu.2022.831168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tang B. L. Sirt1 and the mitochondria. Molecules and Cells . 2016;39(2):87–95. doi: 10.14348/molcells.2016.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang W., Feng Y., Guo Q., et al. SIRT1 modulates cell cycle progression by regulating CHK2 acetylation–phosphorylation. Cell Death & Differentiation . 2020;27(2):482–496. doi: 10.1038/s41418-019-0369-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Begum M. K., Konja D., Singh S., Chlopicki S., Wang Y. Endothelial SIRT1 as a target for the prevention of arterial aging: promises and challenges. Journal of Cardiovascular Pharmacology . 2021;78(6S):S63–S77. doi: 10.1097/FJC.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 146.Majeed Y., Halabi N., Madani A. Y., et al. SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways. Scientific Reports . 2021;11(1):p. 8177. doi: 10.1038/s41598-021-87759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.He F., Li Q., Sheng B., Yang H., Jiang W. SIRT1 inhibits apoptosis by promoting autophagic flux in human nucleus pulposus cells in the key stage of degeneration via ERK signal pathway. BioMed Research International . 2021;2021:10. doi: 10.1155/2021/8818713.8818713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Karbasforooshan H., Karimi G. The role of SIRT1 in diabetic cardiomyopathy. Biomedicine & Pharmacotherapy . 2017;90:386–392. doi: 10.1016/j.biopha.2017.03.056. [DOI] [PubMed] [Google Scholar]
- 149.Karbasforooshan H., Karimi G. The role of SIRT1 in diabetic retinopathy. Biomedicine & Pharmacotherapy . 2018;97:190–194. doi: 10.1016/j.biopha.2017.10.075. [DOI] [PubMed] [Google Scholar]
- 150.Qi F., Jiang X., Tong T., Chang H., Li R. X. miR-204 inhibits inflammation and cell apoptosis in retinopathy rats with diabetic retinopathy by regulating Bcl-2 and SIRT1 expressions. European Review for Medical and Pharmacological Sciences . 2020;24(12):6486–6493. doi: 10.26355/eurrev_202006_21631. [DOI] [PubMed] [Google Scholar]
- 151.Ji Q., Han J., Wang L., et al. MicroRNA-34a promotes apoptosis of retinal vascular endothelial cells by targeting SIRT1 in rats with diabetic retinopathy. Cell Cycle . 2020;19(21):2886–2896. doi: 10.1080/15384101.2020.1827509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang J., Yang F. J., Wang Y. G., Su G. F., Miao X. LncRNA MIR497HG inhibits proliferation and migration of retinal endothelial cells under high-level glucose treatment via miRNA-128-3p/SIRT1 axis. European Review for Medical and Pharmacological Sciences . 2020;24(11):5871–5877. doi: 10.26355/eurrev_202006_21479. [DOI] [PubMed] [Google Scholar]
- 153.Xiao H., Liu Z. Effects of microRNA-217 on high glucose-induced inflammation and apoptosis of human retinal pigment epithelial cells (ARPE-19) and its underlying mechanism. Molecular Medicine Reports . 2019;20(6):5125–5133. doi: 10.3892/mmr.2019.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pan Q., Gao Z., Zhu C., Peng Z., Song M., Li L. Overexpression of histone deacetylase SIRT1 exerts an antiangiogenic role in diabetic retinopathy via miR-20a elevation and YAP/HIF1α/VEGFA depletion. American Journal of Physiology Endocrinology and Metabolism . 2020;319(5):E932–E943. doi: 10.1152/ajpendo.00051.2020. [DOI] [PubMed] [Google Scholar]
- 155.Shan L., Zhang H., Han Y., Kuang R. Expression and mechanism of microRNA 195 in diabetic retinopathy. Endocrine Journal . 2022;69(5):529–537. doi: 10.1507/endocrj.EJ21-0231. [DOI] [PubMed] [Google Scholar]
- 156.Wang H., Su X., Zhang Q. Q., et al. MicroRNA-93-5p participates in type 2 diabetic retinopathy through targeting Sirt1. International Ophthalmology . 2021;41(11):3837–3848. doi: 10.1007/s10792-021-01953-4. [DOI] [PubMed] [Google Scholar]
- 157.Wang P., Li C., Deng Y., et al. Effect of plasma-derived extracellular vesicles on angiogenesis and the ensuing proliferative diabetic retinopathy through a miR-30b-dependent mechanism. Diabetology & Metabolic Syndrome . 2022;14(1):p. 188. doi: 10.1186/s13098-022-00937-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shi J., Li L. circKMT2E protect retina from early diabetic retinopathy through SIRT1 signaling pathway via sponging miR-204-5p. Computational and Mathematical Methods in Medicine . 2022;2022:12. doi: 10.1155/2022/7188193.7188193 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 159.Jones P. F., Jakubowicz T., Pitossi F. J., Maurer F., Hemmings B. A. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proceedings of the National Academy of Sciences of the United States of America . 1991;88(10):4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jones P. F., Jakubowicz T., Hemmings B. A. Molecular cloning of a second form of rac protein kinase. Cell Regulation . 1991;2(12):1001–1009. doi: 10.1091/mbc.2.12.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Konishi H., Kuroda S., Tanaka M., et al. Molecular cloning and characterization of a new member of the RAC protein kinase family: association of the Pleckstrin homology domain of 3 types of RAC protein kinase with protein kinase C subspecies and βγ subunits of G proteins. Biochemical and Biophysical Research Communications . 1995;216(2):526–534. doi: 10.1006/bbrc.1995.2654. [DOI] [PubMed] [Google Scholar]
- 162.Manning B. D., Cantley L. C. AKT/PKB signaling: navigating downstream. Cell . 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Los M., Maddika S., Erb B., Schulze-Osthoff K. Switching Akt: from survival signaling to deadly response. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology . 2009;31(5):492–495. doi: 10.1002/bies.200900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nogueira V., Park Y., Chen C. C., et al. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell . 2008;14(6):458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Elstrom R. L., Bauer D. E., Buzzai M., et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Research . 2004;64(11):3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 166.Yang X., Huo F., Liu B., et al. Crocin inhibits oxidative stress and pro-inflammatory response of microglial cells associated with diabetic retinopathy through the activation of PI3K/Akt signaling pathway. Journal of Molecular Neuroscience: MN . 2017;61(4):581–589. doi: 10.1007/s12031-017-0899-8. [DOI] [PubMed] [Google Scholar]
- 167.Shi J., Lv H., Tang C., Li Y., Huang J., Zhang H. Mangiferin inhibits cell migration and angiogenesis via PI3K/AKT/mTOR signaling in high glucose- and hypoxia-induced RRCECs. Molecular Medicine Reports . 2021;23(6):p. 473. doi: 10.3892/mmr.2021.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Huang H., He J., Johnson D., et al. Deletion of placental growth factor prevents diabetic retinopathy and is associated with Akt activation and HIF1α-VEGF pathway inhibition. Diabetes . 2015;64(1):200–212. doi: 10.2337/db14-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lu J. M., Zhang Z. Z., Ma X., Fang S. F., Qin X. H. Repression of microRNA-21 inhibits retinal vascular endothelial cell growth and angiogenesis via _PTEN_ dependent-PI3K/Akt/VEGF signaling pathway in diabetic retinopathy. Experimental Eye Research . 2020;190, article 107886 doi: 10.1016/j.exer.2019.107886. [DOI] [PubMed] [Google Scholar]
- 170.Wang L., Liu W. X., Huang X. G. MicroRNA-199a-3p inhibits angiogenesis by targeting the VEGF/PI3K/AKT signalling pathway in an _in vitro_ model of diabetic retinopathy. Experimental and Molecular Pathology . 2020;116, article 104488 doi: 10.1016/j.yexmp.2020.104488. [DOI] [PubMed] [Google Scholar]
- 171.Liu Y., Yang Q., Fu H., et al. Müller glia-derived exosomal miR-9-3p promotes angiogenesis by restricting sphingosine-1-phosphate receptor S1P1 in diabetic retinopathy. Molecular Therapy-Nucleic Acids . 2022;27:491–504. doi: 10.1016/j.omtn.2021.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zha X., Xi X., Fan X., Ma M., Zhang Y., Yang Y. Overexpression of METTL3 attenuates high-glucose induced RPE cell pyroptosis by regulating miR-25-3p/PTEN/Akt signaling cascade through DGCR8. Aging . 2020;12(9):8137–8150. doi: 10.18632/aging.103130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang Q., Lenardo M. J., Baltimore D. 30 years of NF-κB: a blossoming of relevance to human pathobiology. Cell . 2017;168(1-2):37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hoesel B., Schmid J. A. The complexity of NF-κB signaling in inflammation and cancer. Molecular Cancer . 2013;12(1):p. 86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.DiDonato J. A., Mercurio F., Karin M. NF-κB and the link between inflammation and cancer. Immunological Reviews . 2012;246(1):379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 176.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspectives in Biology . 2009;1(6, article a001651) doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Romeo G., Liu W. H., Asnaghi V., Kern T. S., Lorenzi M. Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes . 2002;51(7):2241–2248. doi: 10.2337/diabetes.51.7.2241. [DOI] [PubMed] [Google Scholar]
- 178.Al-Kharashi A. S. Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi Journal of Ophthalmology . 2018;32(4):318–323. doi: 10.1016/j.sjopt.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ejaz S., Chekarova I., Ejaz A., Sohail A., Lim C. W. Importance of pericytes and mechanisms of pericyte loss during diabetic retinopathy. Diabetes, Obesity & Metabolism . 2008;10(1):53–63. doi: 10.1111/j.1463-1326.2007.00795.x. [DOI] [PubMed] [Google Scholar]
- 180.He Y., Zhang Z., Yao T., et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells relieves diabetic retinopathy through a microRNA-30c-5p-dependent mechanism. Diabetes Research and Clinical Practice . 2022;190, article 109861 doi: 10.1016/j.diabres.2022.109861. [DOI] [PubMed] [Google Scholar]
- 181.Shi H., Berger E. A. Characterization of site-specific phosphorylation of NF-κB p65 in retinal cells in response to high glucose and cytokine polarization. Mediators of Inflammation . 2018;2018:15. doi: 10.1155/2018/3020675.3020675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shi R., Liu D. D., Cao Y., Xue Y. S. microRNA-26a-5p prevents retinal neuronal cell death in diabetic mice by targeting PTEN. Current Eye Research . 2022;47(3):409–417. doi: 10.1080/02713683.2021.1975760. [DOI] [PubMed] [Google Scholar]
- 183.Xu Z., Tian N., Li S., et al. Extracellular vesicles secreted from mesenchymal stem cells exert anti-apoptotic and anti-inflammatory effects via transmitting microRNA-18b in rats with diabetic retinopathy. International Immunopharmacology . 2021;101(Part B, article 108234) doi: 10.1016/j.intimp.2021.108234. [DOI] [PubMed] [Google Scholar]
- 184.Li R., Yuan H., Zhao T., et al. miR-874 ameliorates retinopathy in diabetic rats by NF-κB signaling pathway. Advances in Clinical and Experimental Medicine: Official Organ Wroclaw Medical University . 2021;30(4):421–430. doi: 10.21203/rs.2.14609/v4. [DOI] [PubMed] [Google Scholar]
- 185.Khavari T. A., Rinn J. Ras/Erk MAPK signaling in epidermal homeostasis and neoplasia. Cell Cycle . 2007;6(23):2928–2931. doi: 10.4161/cc.6.23.4998. [DOI] [PubMed] [Google Scholar]
- 186.Rubinfeld H., Seger R. The ERK cascade: a prototype of MAPK signaling. Molecular Biotechnology . 2005;31(2):151–174. doi: 10.1385/MB:31:2:151. [DOI] [PubMed] [Google Scholar]
- 187.Kim E. K., Choi E. J. Compromised MAPK signaling in human diseases: an update. Archives of Toxicology . 2015;89(6):867–882. doi: 10.1007/s00204-015-1472-2. [DOI] [PubMed] [Google Scholar]
- 188.Kyriakis J. M., Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiological Reviews . 2012;92(2):689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 189.Arthur J. S., Ley S. C. Mitogen-activated protein kinases in innate immunity. Nature Reviews Immunology . 2013;13(9):679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 190.Sui X., Kong N., Ye L., et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Letters . 2014;344(2):174–179. doi: 10.1016/j.canlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 191.Zhang L. Q., Cui H., Yu Y. B., Shi H. Q., Zhou Y., Liu M. J. MicroRNA-141-3p inhibits retinal neovascularization and retinal ganglion cell apoptosis in glaucoma mice through the inactivation of docking protein 5-dependent mitogen-activated protein kinase signaling pathway. Journal of Cellular Physiology . 2019;234(6):8873–8887. doi: 10.1002/jcp.27549. [DOI] [PubMed] [Google Scholar]
- 192.Cheng C. K., Shang W., Liu J., et al. Activation of AMPK/miR-181b axis alleviates endothelial dysfunction and vascular inflammation in diabetic mice. Antioxidants . 2022;11(6):p. 1137. doi: 10.3390/antiox11061137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rogero M. M., Calder P. C. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients . 2018;10(4):p. 432. doi: 10.3390/nu10040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hernanz R., Martínez-Revelles S., Palacios R., et al. Toll-like receptor 4 contributes to vascular remodelling and endothelial dysfunction in angiotensin II-induced hypertension. British Journal of Pharmacology . 2015;172(12):3159–3176. doi: 10.1111/bph.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Thon P., Rump K., Knorr A., et al. The distinctive activation of toll-like receptor 4 in human samples with sepsis. Cell . 2022;11(19):p. 3020. doi: 10.3390/cells11193020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Alonso-Pérez A., Franco-Trepat E., Guillán-Fresco M., et al. Role of toll-like receptor 4 on osteoblast metabolism and function. Frontiers in Physiology . 2018;9:p. 504. doi: 10.3389/fphys.2018.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yang Z., Liu W., Wan X., Liu R., Zhang Y. Association of toll-like receptor 4 Rs7873784 G/C polymorphism with rheumatoid arthritis risk in a Chinese population. Immunological Investigations . 2022;51(3):660–669. doi: 10.1080/08820139.2020.1864397. [DOI] [PubMed] [Google Scholar]
- 198.Lu P., Sodhi C. P., Yamaguchi Y., et al. Intestinal epithelial toll-like receptor 4 prevents metabolic syndrome by regulating interactions between microbes and intestinal epithelial cells in mice. Mucosal Immunology . 2018;11(3):727–740. doi: 10.1038/mi.2017.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bayan N., Yazdanpanah N., Rezaei N. Role of toll-like receptor 4 in diabetic retinopathy. Pharmacological Research . 2022;175, article 105960 doi: 10.1016/j.phrs.2021.105960. [DOI] [PubMed] [Google Scholar]
- 200.Wang H., Shi H., Zhang J., et al. Toll-like receptor 4 in bone marrow-derived cells contributes to the progression of diabetic retinopathy. Mediators of Inflammation . 2014;2014:7. doi: 10.1155/2014/858763.858763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fu H., Liu H. Deletion of toll-like receptor 4 ameliorates diabetic retinopathy in mice. Archives of Physiology and Biochemistry . 2023;129(2):519–525. doi: 10.1080/13813455.2020.1841795. [DOI] [PubMed] [Google Scholar]
- 202.Liu X., Zhang Y., Liang H., Zhang Y., Xu Y. MicroRNA-499-3p inhibits proliferation and promotes apoptosis of retinal cells in diabetic retinopathy through activation of the TLR4 signaling pathway by targeting IFNA2. Gene . 2020;741, article 144539 doi: 10.1016/j.gene.2020.144539. [DOI] [PubMed] [Google Scholar]
- 203.Li W., Jin L., Cui Y., Nie A., Xie N., Liang G. Bone marrow mesenchymal stem cells-induced exosomal microRNA-486-3p protects against diabetic retinopathy through TLR4/NF-κB axis repression. Journal of Endocrinological Investigation . 2021;44(6):1193–1207. doi: 10.1007/s40618-020-01405-3. [DOI] [PubMed] [Google Scholar]
- 204.Gu C., Zhang H., Zhao S., He D., Gao Y. Mesenchymal stem cell exosomal miR-146a mediates the regulation of the TLR4/MyD88/NF-κB signaling pathway in inflammation due to diabetic retinopathy. Computational and Mathematical Methods in Medicine . 2022;2022:10. doi: 10.1155/2022/3864863.3864863 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 205.Aiello L. P., Wong J. S. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney International Supplement . 2000;77:S113–S119. doi: 10.1046/j.1523-1755.2000.07718.x. [DOI] [PubMed] [Google Scholar]
- 206.Ferrara N., Adamis A. P. Ten years of anti-vascular endothelial growth factor therapy. Nature Reviews Drug Discovery . 2016;15(6):385–403. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 207.Senger D. R., Van de Water L., Brown L. F., et al. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Reviews . 1993;12(3-4):303–324. doi: 10.1007/BF00665960. [DOI] [PubMed] [Google Scholar]
- 208.Jiang L., Cao H., Deng T., et al. Serum exosomal miR-377-3p inhibits retinal pigment epithelium proliferation and offers a biomarker for diabetic macular edema. The Journal of International Medical Research . 2021;49(4, article 3000605211002975) doi: 10.1177/03000605211002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang Y., Lin W., Ju J. MicroRNA-409-5p promotes retinal neovascularization in diabetic retinopathy. Cell Cycle . 2020;19(11):1314–1325. doi: 10.1080/15384101.2020.1749484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tang W., Guo J., Gu R., et al. MicroRNA-29b-3p inhibits cell proliferation and angiogenesis by targeting VEGFA and PDGFB in retinal microvascular endothelial cells. Molecular Vision . 2020;26:64–75. [PMC free article] [PubMed] [Google Scholar]
- 211.Fu X., Ou B. miR-152/LIN28B axis modulates high-glucose-induced angiogenesis in human retinal endothelial cells via VEGF signaling. Journal of Cellular Biochemistry . 2020;121(2):954–962. doi: 10.1002/jcb.28978. [DOI] [PubMed] [Google Scholar]
- 212.Shi Q., Tang J., Wang M., Xu L., Shi L. Knockdown of long non-coding RNA TUG1 suppresses migration and tube formation in high glucose-stimulated human retinal microvascular endothelial cells by sponging miRNA-145. Molecular Biotechnology . 2022;64(2):171–177. doi: 10.1007/s12033-021-00398-5. [DOI] [PubMed] [Google Scholar]
- 213.Sun L., Liu X., Zuo Z. Regulatory role of miRNA-23a in diabetic retinopathy. Experimental and Therapeutic Medicine . 2021;22(6):p. 1477. doi: 10.3892/etm.2021.10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Da’as S. I., Ahmed I., Hasan W. H., et al. The link between glycemic control measures and eye microvascular complications in a clinical cohort of type 2 diabetes with microRNA-223-3p signature. Journal of Translational Medicine . 2023;21(1):p. 171. doi: 10.1186/s12967-023-03893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang F., Yu C. In vitro protective effect of miR-181d-5p in high glucose-induced human retinal microvascular endothelial cells by targeting the angiogenic factor VEGFA. European Review for Medical and Pharmacological Sciences . 2022;26(17):6199–6207. doi: 10.26355/eurrev_202209_29637. [DOI] [PubMed] [Google Scholar]
- 216.Zhang Z., Song C., Wang T., Sun L., Qin L., Ju J. miR-139-5p promotes neovascularization in diabetic retinopathy by regulating the phosphatase and tensin homolog. Archives of Pharmacal Research . 2021;44(2):205–218. doi: 10.1007/s12272-021-01308-8. [DOI] [PubMed] [Google Scholar]
- 217.Subramanian S., Steer C. J. Special issue: microRNA regulation in health and disease. Genes . 2019;10(6):p. 457. doi: 10.3390/genes10060457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shah M. Y., Ferrajoli A., Sood A. K., Lopez-Berestein G., Calin G. A. microRNA therapeutics in cancer -- an emerging concept. eBioMedicine . 2016;12:34–42. doi: 10.1016/j.ebiom.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Garzon R., Marcucci G., Croce C. M. Targeting microRNAs in cancer: rationale, strategies and challenges. Nature Reviews Drug Discovery . 2010;9(10):775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Garzon R., Heaphy C. E., Havelange V., et al. MicroRNA 29b functions in acute myeloid leukemia. Blood . 2009;114(26):5331–5341. doi: 10.1182/blood-2009-03-211938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ebert M. S., Neilson J. R., Sharp P. A. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nature Methods . 2007;4(9):721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Disney M. D., Winkelsas A. M., Velagapudi S. P., Southern M., Fallahi M., Childs-Disney J. L. Inforna 2.0: a platform for the sequence-based design of small molecules targeting structured RNAs. ACS Chemical Biology . 2016;11(6):1720–1728. doi: 10.1021/acschembio.6b00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Suresh B. M., Li W., Zhang P., et al. A general fragment-based approach to identify and optimize bioactive ligands targeting RNA. Proceedings of the National Academy of Sciences of the United States of America . 2020;117(52):33197–33203. doi: 10.1073/pnas.2012217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Choi W. Y., Giraldez A. J., Schier A. F. Target protectors reveal dampening and balancing of Nodal agonist and antagonist by miR-430. Science . 2007;318(5848):271–274. doi: 10.1126/science.1147535. [DOI] [PubMed] [Google Scholar]
- 225.Vester B., Wengel J. LNA (locked nucleic acid): high-affinity targeting of complementary RNA and DNA. Biochemistry . 2004;43(42):13233–13241. doi: 10.1021/bi0485732. [DOI] [PubMed] [Google Scholar]
- 226.Elmén J., Lindow M., Schütz S., et al. LNA-mediated microRNA silencing in non-human primates. Nature . 2008;452(7189):896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 227.Li C., Feng Y., Coukos G., Zhang L. Therapeutic microRNA strategies in human cancer. The AAPS Journal . 2009;11(4):747–757. doi: 10.1208/s12248-009-9145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Safi H., Safi S., Hafezi-Moghadam A., Ahmadieh H. Early detection of diabetic retinopathy. Survey of Ophthalmology . 2018;63(5):601–608. doi: 10.1016/j.survophthal.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 229.Ellis M. P., Lent-Schochet D., Lo T., Yiu G. Emerging concepts in the treatment of diabetic retinopathy. Current Diabetes Reports . 2019;19(11):p. 137. doi: 10.1007/s11892-019-1276-5. [DOI] [PubMed] [Google Scholar]
- 230.Sekar D. miRNA 21: a novel biomarker in the treatment of bladder cancer. Biomarkers in Medicine . 2020;14(12):1065–1067. doi: 10.2217/bmm-2020-0319. [DOI] [PubMed] [Google Scholar]
- 231.Fabris L., Ceder Y., Chinnaiyan A. M., et al. The potential of microRNAs as prostate cancer biomarkers. European Urology . 2016;70(2):312–322. doi: 10.1016/j.eururo.2015.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.McGuire A., Brown J. A., Kerin M. J. Metastatic breast cancer: the potential of miRNA for diagnosis and treatment monitoring. Cancer Metastasis Reviews . 2015;34(1):145–155. doi: 10.1007/s10555-015-9551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Shahouzehi B., Eghbalian M., Fallah H., Aminizadeh S., Masoumi-Ardakani Y. Serum microRNA-33 levels in pre-diabetic and diabetic patients. Molecular Biology Reports . 2021;48(5):4121–4128. doi: 10.1007/s11033-021-06425-7. [DOI] [PubMed] [Google Scholar]
- 234.Ishii H., Kaneko S., Yanai K., et al. MicroRNA expression profiling in diabetic kidney disease. Translational Research: the Journal of Laboratory and Clinical Medicine . 2021;237:31–52. doi: 10.1016/j.trsl.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 235.Zhao Y., Shen A., Guo F., et al. Urinary exosomal miRNA-4534 as a novel diagnostic biomarker for diabetic kidney disease. Frontiers in Endocrinology . 2020;11:p. 590. doi: 10.3389/fendo.2020.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yang X., Yu X., Zhou R. H., Liu C. Serum miRNA-27b-3p is a biomarker of diabetic retinopathy. Journal of Biological Regulators and Homeostatic Agents . 2020;34(4):1431–1435. doi: 10.23812/20-191-L. [DOI] [PubMed] [Google Scholar]
- 237.Lal A., Navarro F., Maher C. A., et al. miR-24 inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to "seedless" 3′UTR microRNA recognition elements. Molecular Cell . 2009;35(5):610–625. doi: 10.1016/j.molcel.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.O'Day E., Lal A. MicroRNAs and their target gene networks in breast cancer. Breast Cancer Research: BCR . 2010;12(2):p. 201. doi: 10.1186/bcr2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Singh R., Ha S. E., Wei L., et al. miR-10b-5p rescues diabetes and gastrointestinal dysmotility. Gastroenterology . 2021;160(5):1662–1678.e18. doi: 10.1053/j.gastro.2020.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Trajkovski M., Hausser J., Soutschek J., et al. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature . 2011;474(7353):649–653. doi: 10.1038/nature10112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.


