Abstract
Context
Lipodystrophy syndromes are a heterogeneous group of rare genetic or acquired disorders characterized by generalized or partial loss of adipose tissue. LMNA-related lipodystrophy syndromes are classified based on the severity and distribution of adipose tissue loss.
Objective
We aimed to annotate all clinical and metabolic features of patients with lipodystrophy syndromes carrying pathogenic LMNA variants and assess potential genotype-phenotype relationships.
Methods
We retrospectively reviewed and analyzed all our cases (n = 115) and all published cases (n = 379) curated from 94 studies in the literature.
Results
The study included 494 patients. The most common variants in our study, R482Q and R482W, were associated with similar metabolic characteristics and complications though those with the R482W variant were younger (aged 33 [24] years vs 44 [25] years; P < .001), had an earlier diabetes diagnosis (aged 27 [18] vs 40 [17] years; P < .001) and had lower body mass index levels (24 [5] vs 25 [4]; P = .037). Dyslipidemia was the earliest biochemical evidence described in 83% of all patients at a median age of 26 (10) years, while diabetes was reported in 61% of cases. Among 39 patients with an episode of acute pancreatitis, the median age at acute pancreatitis diagnosis was 20 (17) years. Patients who were reported to have diabetes had 3.2 times, while those with hypertriglyceridemia had 12.0 times, the odds of having pancreatitis compared to those who did not.
Conclusion
This study reports the largest number of patients with LMNA-related lipodystrophy syndromes to date. Our report helps to quantify the prevalence of the known and rare complications associated with different phenotypes and serves as a comprehensive catalog of all known cases.
Keywords: LMNA, laminopathies, adipose tissue, lipodystrophy
Lipodystrophy syndromes are a heterogeneous group of rare genetic or acquired disorders characterized by generalized or partial loss of adipose tissue (1). While earlier reports estimated a prevalence between 1 in 10 million and 1 in 1 million, recent studies have predicted that the clinical prevalence of inherited lipodystrophy syndromes is as high as 1 in 20 000 individuals, while the molecular prevalences of the specific subtypes is even more common, up to 1 in 7000 (2, 3). Pathogenic variants in various genes, including AGPAT1, BSCL2, CAV1, PPARG, AKT2, and LMNA have been associated with the recognizable phenotypes of inherited lipodystrophy syndromes (3).
LMNA-related lipodystrophy syndromes are classified based on the severity and distribution of adipose tissue loss (2). The most common form is familial partial lipodystrophy type 2 (FPLD2), also known as the Dunnigan-type lipodystrophy (1, 4, 5). However, patients carrying different pathogenic LMNA variants may also present as having atypical FPLD2, multisystem FPLD syndrome, non-Dunnigan FPLD, atypical Werner syndrome, atypical progeroid syndrome, mandibuloacral dysplasia, or generalized lipodystrophy syndromes with or without progeroid features (1, 3, 4, 6-8). The LMNA (lamin A/C) gene, which encodes the nuclear lamina proteins lamin A and its isoform lamin C, has been associated with pivotal roles in the maintenance of the nuclear organization and nuclear matrix homeostasis (9, 10). Due to lamins' ubiquitous and variable expression throughout the body, laminopathies can have far-reaching consequences, affecting not just adipose tissue but also skeletal muscle, the cardiovascular system, kidneys, and peripheral nerves (10, 11). The clinical manifestations of LMNA variants can vary widely, even among members of the same family who carry the same variant (12). Patients with FPLD2 show ectopic fat accumulation in the absence of peripheral adipose depots (13). As lipid spills over to ectopic sites driven by adipose tissue loss, a state of low-grade systemic inflammation (14) develops along with several hormonal and metabolic complications, such as hypertriglyceridemia and insulin resistance that can progress to diabetes. This can lead to several debilitating organ involvements, including pancreatitis, cardiomyopathy, early atherosclerosis, nephropathy, retinopathy, hepatic steatosis, and fibrosis, among others (1, 15).
Diverse phenotypical and clinical features of atypical adipose distribution, progeroid features, and multisystemic involvements pose a challenge to clinicians in the diagnosis, follow-up, and treatment of these rare disorders. Hence, several reviews and meta-analyses have focused on the FPLD2 phenotypes (4) and the overall systemic involvements of the LMNA gene (16, 17), while others reviewed the LMNA-related cardiomyopathy diseases or muscular dystrophies (18, 19). Nevertheless, due to the rarity and heterogeneity of this syndrome, additional data compiling all available information on the genotype-phenotype relationships of LMNA-related lipodystrophy syndromes is a missing gap in the literature (1, 3, 20, 21).
In this study, we analyzed all 106 available reports (6-8, 12, 13, 20-120) on cases with various LMNA-related lipodystrophy phenotypes, omitting the distinct and well-defined form of Hutchinson-Gilford progeroid syndrome (100, 121, 122). We also included follow-up data for our 90 previously published cases (13, 20, 21, 92, 110-118), in addition to an unreported 25 patients, to provide a comprehensive and detailed analysis of the genotype-phenotype correlations of patients with lipodystrophy who carry distinct pathogenic LMNA variants.
Materials and Methods
Case Series
We retrospectively collected the clinical data of all patients with lipodystrophy carrying pathogenic LMNA variants from the divisions of metabolism and endocrinology of 4 medical centers: the University of Michigan (USA), the University of São Paulo (Brazil), Dokuz Eylul University (Turkey), and Ege University (Turkey).
Online Search Strategy
We further selected cases for inclusion in the study using web search tools including PubMed, OMIM, and Google Scholar using the following search terms: “LMNA,” “lipodystrophy,” and “partial lipodystrophy” (1997 to present, last accessed June 14, 2023). The electronic search strategy is presented in Fig. 1. Studies eligible for our analysis had to include information on the pathogenic LMNA variant and provide at least one of the following clinical characteristics per individual: body mass index (BMI) and the presence of insulin resistance, diabetes, dyslipidemia, hypertriglyceridemia, hypertension, hepatic steatosis, pancreatitis, nephropathy, or retinopathy.
Figure 1.
Electronic search strategy.
In this study, lipodystrophy phenotypes were classified as FPLD2 and other overlapping phenotypes. FPLD2 was classified as typical and atypical FPLD2 (4). Typical FPLD2 was defined by loss of adipose tissue from the upper and lower extremities and trunk, with an excess of adipose tissue in the face, neck, and supraclavicular region. Atypical FPLD2 was defined when adipose tissue loss was less severe, with lipoatrophy being more evident in the legs (4). Other overlapping phenotypes were considered (i) in the presence of atypical progeroid features, defined when the individual's appearance appeared older than their chronological age, which may have been accompanied by premature gray hair, beaked nose, or pinched facies, and (ii) when skeletal defects were observed, including those with mandibuloacral dysplasia, which is a heterogeneous phenotype, primarily characterized by mandibular and clavicular hypoplasia (8).
Exclusion criteria were articles not written in English, animal studies, and review articles not containing the required detailed LMNA variant-phenotype information. Patients with Hutchinson-Gilford progeroid syndrome were not included in the analysis. To avoid duplications, previous reports on our patients, on whom we collected the follow-up data for this analysis, were also excluded from the literature review. Two reviewers (O.B. and M.C.F.F.) independently screened titles and abstracts to be included in the study.
The following data on the patients' clinical and laboratory characteristics were recorded: sex, age at diagnosis, BMI, pathogenic LMNA variant, and presence and age of onset of additional complications/features including insulin resistance, diabetes, dyslipidemia, hypertriglyceridemia, hypertension, hepatic steatosis, pancreatitis, nephropathy, and retinopathy. Details of circulating lipid concentrations (triglycerides [TGs], total cholesterol, high-density lipoprotein [HDL], low-density lipoprotein [LDL]), glycated hemoglobin A1c (HbA1c), leptin, adiponectin, and fibroblast growth factor-21 (FGF-21) concentrations were also noted. Additional data on previously unreported patients regarding complaints of pain, and body composition demonstrating the pattern of adipose tissue loss have also been included. Fat and lean body mass were estimated using dual x-ray absorptiometry. The ratio of percentage fat mass of the trunk to percentage fat mass of the legs (FMR) was also presented.
Diabetes was diagnosed when HbA1c was greater than or equal to 6.5% or fasting plasma glucose was greater than or equal to 126 mg/dL (123), or with a report of antidiabetic agent use. Dyslipidemia was diagnosed with a total cholesterol level of 200 mg/dL or greater or TGs of 150 mg/dL or greater, or HDL of less than 50 mg/dL for women and less than 40 mg/dL for men, or LDL level of 130 mg/dL or greater, or treatment with lipid-lowering drugs (124). Hypertension was defined when blood pressure was 140/90 mm Hg or greater or the patient was using antihypertensive medications (124). Nephropathy was diagnosed by the presence of diabetic kidney disease with albuminuria of more than 30 mg/g and/or reduced glomerular filtration rate or in the presence of focal segmental glomerulosclerosis or its clinical features (125). Abnormal cardiac function was defined with the presence of valvular defects, conduction abnormalities, or anatomical defects identified via echocardiography or electrocardiogram.
Ethical Approval
This study was approved by the ethical committee of the University of Michigan (institutional review board [IRB] Med approval No. LD-LYNC HUM#00127427) and the research ethics committee of the Clinical Hospital of Ribeirao Preto (IRB Med approval No. CAAE 56714421.3.0000.5440) and was performed according to the principles of the Declaration of Helsinki.
Statistical Analysis
Results were analyzed using SPSS v.24 for Windows. The data are summarized as medians (interquartile range) unless otherwise stated. The numbers (N) of the patients affected (X), and the total number of patients (Y) whose data were available for analyses were given as (N, X/Y). The Mann-Whitney U test was used for comparison between groups. Categorical variables were analyzed by χ2 or Fisher exact test. A P value of less than .05 was considered statistically significant.
Results
Characteristics of 25 Previously Unreported Patients
Detailed clinical and biochemical characteristics of all patients are presented in Table 1. Twenty-five (19 women, 76%) patients from 6 unrelated families were followed up with a median duration of 15 (15) years (range, 1-32 years) were evaluated. Six of these patients were male. The median age at diagnosis was 34 (21) years (range, 5-52 years). The patients carrying the R644C variant demonstrated an atypical FPLD2 phenotype, while the rest of the patients demonstrated the classic FPLD2 phenotype. Three (12%) patients had complaints of gastroesophageal reflux, 1 (4%) had bowel problems, 1 (4%) had back pain, 1 (4%) had muscle pain, and 2 (8%) had joint pain. Seven patients harbored R644C (same family), while 18 carried the R482W (5 different families) variant. Patients with a BMI level of 25 (range, 20–31) had an FMR of 1.4 (1) (range, 1.3-2.0), all of whom had an FMR above the 95% CI of reference median values (126). Seventeen patients (68%) had diabetes diagnosed at a median age of 36 (19) years with a median HbA1c of 8.8% (4), while 2 patients had prediabetes. Five (20%) patients had retinopathy, 5 (20%) had nephropathy, and 12 (48%) patients had nonalcoholic fatty liver disease. None of the patients had myopathy. Twenty-three (92%) patients had dyslipidemia, of whom 22 also had hypertriglyceridemia. Median TG, cholesterol, HDL, and LDL concentrations were 264 (153), 198 (80), 38 (9), and 105 (70) mg/dL, respectively. Three (13%) patients had at least 1 pancreatitis attack, diagnosed at ages 32 (R644C), 32 (R482W), and 48 (R482W) years. The patients carrying R644C (n = 7) and R482W (n = 18) had similar ages at diagnosis, BMI values, and concentrations of cholesterol, HDL, TGs, LDL, and HbA1c.
Table 1.
Clinical characteristics of our previously unreported patients
| Pt | Variant | M/F | Age, y | BMI | FMR | Diabetes | Diabetes age, y | Dys lipidemia | Pancreatitis | Hepato steatosis | HbA1c, % | LDL, mg/dL | HDL, mg/dL | TGs, mg/dL | Cholesterol, mg/dL | Retinopathy | Nephro pathy | Cardiac abnormality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.1 | R644C | F | 33 | 26 | 1.8 | Y | 32 | Y | Ya | 1 | 11.7 | na | 60 | 675 | 336 | Y | Y | N |
| 1.2 | R644C | F | 44 | 31 | 1.8 | Y | 44 | Y | N | 1 | 5.6 | 90 | 37 | 105 | 148 | Y | Y | Yf |
| 1.3 | R644C | F | 39 | 24 | 1.4 | Y | 46 | Y | N | 1 | 9.4 | 116 | 35 | 275 | 207 | N | N | N |
| 1.4 | R644C | M | 32 | na | na | N | — | Y | N | na | 6.0 | 148 | 46 | 76 | 209 | N | N | N |
| 1.5 | R644C | M | 44 | na | 1.4 | Y | 44 | Y | N | na | 5.4 | 168 | 43 | 88 | 229 | N | N | N |
| 1.6 | R644C | M | 45 | 29 | na | Y | 45 | Y | N | na | na | 141 | 35 | 376 | 251 | N | N | N |
| 1.7 | R644C | M | na | na | na | N | — | N | N | na | na | 100 | 46 | 33 | 153 | N | N | N |
| 2.1 | R482W | F | 52 | 23 | 2.0 | Y | 56 | Y | N | na | 6.2 | 157 | 42 | 239 | 248 | N | N | N |
| 2.2 | R482W | F | 14 | 21 | 1.4 | N | — | Y | N | na | 5.1 | 46 | 45 | 354 | 162 | N | N | N |
| 3.1 | R482W | M | 49 | 22 | na | Y | 35 | Y | Yb | 1d | na | 49 | 38 | 65 | 100 | Y | Y | N |
| 3.2 | R482W | F | 23 | 20 | 1.3 | Y | 23 | Y | N | 1 | 10.4 | 137 | 28 | 334 | 232 | na | na | N |
| 3.3 | R482W | F | 40 | 25 | 1.8 | Y | 47 | Y | N | 1e | 5.8 | 71 | 38 | 314 | 171 | N | N | N |
| 3.4 | R482W | F | 45 | 27 | 1.4 | Y | 46 | Y | N | 1 | 9.4 | 137 | 45 | 278 | 238 | N | Y | Yg |
| 3.5 | R482W | F | 5 | 25 | 1.7 | N | — | Y | N | N | 5.5 | 77 | 38 | 341 | 183 | N | N | N |
| 3.6 | R482W | F | 42 | 21 | 1.1 | Y | 43 | Y | N | 1e | 7.8 | 93 | 23 | 224 | 161 | N | Y | N |
| 3.7 | R482W | M | 28 | 31 | na | Y | 28 | Y | N | 1 | 5.1 | 29 | 1487 | 275 | N | N | N | |
| 3.8 | R482W | F | 20 | na | 1.4 | N | — | Y | N | 1 | 4.5 | 63 | 47 | 183 | 147 | N | na | N |
| 4.1 | R482W | F | 20 | 27 | 1.6 | Y | 20 | Y | N | 1 | 9.7 | 106 | 40 | 264 | 198 | N | Y | N |
| 4.2 | R482W | F | 12 | 27 | 1.7 | Y | 21 | Y | N | 1 | 5.8 | 41 | 35 | 276 | 131 | na | na | N |
| 4.3 | R482W | F | 31 | na | 1.5 | N | — | Y | N | na | 5.1 | 89 | 32 | 230 | 167 | na | na | N |
| 4.4 | R482W | F | 25 | 22 | 1.8 | N | — | Y | N | N | 5.8 | 150 | 37 | 296 | 246 | na | na | N |
| 5.1 | R482W | F | 29 | 31 | 1.7 | Y | 30 | Y | Yc | 1 | 8.1 | 146 | 33 | 282 | 236 | Y | N | N |
| 5.2 | R482W | F | 39 | 25 | 1.5 | Y | 26 | Y | N | 1 | 9.7 | 105 | 37 | 159 | 174 | N | Y | N |
| 5.3 | R482W | F | 35 | 24 | 1.7 | Y | 36 | Y | N | 1 | 9.7 | 60 | 42 | 200 | 142 | Y | Y | Yh |
| 6.1 | R482W | F | 46 | 25 | na | na | na | Y | N | N | 5.6 | 122 | 37 | 247 | 209 | na | na | N |
Abbreviations: BMI, body mass index; F, female; HbA1c, glycated hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; M, male; N, not present; na, not available; PT, patient codes; TGs, triglycerides; Y, yes, present;
Age at diagnosis, aat age 32 years; bat age 32 years; cat age 48 years; dportal vein thrombosis; ehepatitis C cirrhosis; fmild dilatation of ascending aorta; gearly coronary artery disease, hfirst-degree atrioventricular block.
Follow-Up Characteristics of 90 Previously Reported Patients
Detailed clinical and biochemical characteristics of all our patients have been previously reported (13, 20, 21, 92, 110-118). Follow-up data for these patients are presented in Tables 2 and 3. Ninety (67 women, 74%) patients from 42 different families with a median duration follow-up of 12 (15) years (range, 1-32 years) were included. The median age at the cross-sectional evaluation point was 41 (33) years (range, 1-77 years). Ninety-eight percent (n = 89) of patients presented with FPLD2 phenotypes, while one had generalized lipodystrophy without progeroid features (n = 1, T10I). Sixteen patients had gastroesophageal reflux, 11 had bowel problems, and 13 had myopathy. R482W was the most common variant (n = 37, 41%) in our cohort, while the other variants identified were as follows: R482Q (n = 36, 40%), R482L (n = 3, 3%), R349W (n = 2, 2%), c.1488 + 5G > C, intron 8 (n = 1, 1%), D300N (n = 1, 1%), G535Q (n = 1, 1%), R349W (n = 1, 1%), R541P (n = 1, 1%), R582C (n = 1, 1%), R582H (n = 1, 1%), R582L (n = 1, 1%), R584H (n = 1, 1%), R60G (n = 1, 1%), R62G (n = 1, 1%), and T10I (n = 1, 1%). The median BMI level was 26 (8) (range, 16-41). Sixty-four (72%) patients had diabetes with a median HbA1c value of 7.2% (3%). Abnormal cardiac examination, nephropathy, and retinopathy were diagnosed in 29 (32%), 29 (32%), and 7 patients (7%), respectively. Seventy patients (83%) had dyslipidemia, 93% (n = 65) of whom had increased TG concentrations. The median levels of cholesterol, TGs, HDL, and LDL were 184 (69), 255 (317), 38 (16), and 100 (47) mg/dL, respectively. Thirteen patients (14%) carrying R482Q (n = 4), R482W (n = 4), D300N (n = 1), c.1488 + 5G > C (n = 1), R584H (n = 1), R62G (n = 1), and T10I (n = 1) variants had at least one episode of pancreatitis.
Table 2.
| Pt | Variant | M/F | Age, y | BMI | Diabetes | Diabetes age, y | Dys lipidemia | Pancreatitis | Hepato steatosis | HbA1c, % | LDL, mg/dL | HDL, mg/dL | TGs, mg/dL | Cholesterol, mg/dL | Retinopathy | Nephro pathy | Cardiac abnormality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UM1 | R482Q | F | 41 | 33 | N | — | Y | N | N | 7.3 | 84 | 41 | 255 | 176 | N | N | na |
| UM2 | R482Q | F | 42 | 31 | N | — | Y | N | N | 9.0 | 144 | 49 | 249 | 243 | N | N | na |
| UM3 | R482Q | F | 33 | 23 | Y | 33 | Y | N | Y | 5.8 | 79 | 69 | 253 | 198 | N | N | N |
| UM4 | R482Q | F | 47 | na | Y | 47 | Y | N | Y | 6.8 | 48 | 45 | 364 | 166 | N | N | N |
| UM5 | R482Q | F | 62 | 34 | Y | 60 | Y | N | Y | 6.6 | 46 | 45 | 280 | 147 | N | N | Yk |
| UM6 | R482L | F | 28 | 29 | Y | 20 | N | N | Y | 5.2 | 110 | 45 | 137 | 183 | Y | Y | Yl |
| UM7 | R482Q | F | 38 | 27 | Y | 47 | Y | N | Y | 5.4 | 82 | 38 | 662 | 218 | N | N | N |
| UM8 | R482Q | M | 16 | 31 | N | — | N | N | N | 5.0 | 89 | 58 | 104 | 168 | N | N | na |
| UM9 | R482Q | M | 15 | 23 | N | — | N | N | N | 6.6 | 73 | 48 | 120 | 145 | N | N | na |
| UM10 | R482L | F | 20 | 30 | N | — | Y | N | N | 11.1 | 56 | 37 | 262 | 145 | N | N | na |
| UM11 | c.1488 + 5G > C | F | 18 | 22 | Y | 12 | Y | Ya | Y | 10.8 | 67 | 39 | 868 | 191 | N | Y | Ym |
| UM12 | R482W | F | 20 | 26 | Y | 19 | Y | Yb | N | 11.9 | 152 | 32 | 359 | 256 | N | N | N |
| UM13 | R482W | F | 55 | 20 | Y | 20 | Y | Yc | N | 5.2 | 153 | 34 | 355 | 187 | Y | N | Yn |
| UM14 | R482W | M | 44 | 38 | Y | na | Y | N | Y | 7.7 | 125 | 28 | 326 | 196 | N | N | na |
| UM15 | R482Q | F | 23 | 23 | N | — | N | N | N | 7.2 | 84 | 52 | 40 | 148 | N | N | N |
| UM16 | R482Q | F | 63 | 38 | Y | 64 | Y | N | Y | na | 127 | 35 | 469 | 262 | Y | N | Yn |
| UM17 | R582H | F | 47 | 26 | Y | 47 | Y | N | Y | 11.9 | 47 | 30 | 398 | 157 | N | N | na |
| UM18 | R62G | F | 55 | 29 | Y | 38 | Y | Yd | Y | 7.2 | 77 | 19 | 150 | 125 | N | Y | |
| UM19 | R482Q | M | 58 | 27 | Y | 45 | Y | N | Y | 8.7 | 63 | 30 | 333 | 160 | N | N | N |
| UM20 | R482Q | F | 58 | 27 | Y | 53 | Y | N | Y | 9.7 | 89 | 26 | 249 | 142 | N | Y | Yn |
| UM21 | R482Q | M | 48 | 39 | Y | 46 | Y | Ye | Y | na | na | 32 | 1454 | 347 | N | Y | Yl |
| UM22 | R482Q | F | 44 | 23 | Y | 23 | N | N | Y | 5.2 | 120 | 24 | 150 | 174 | N | N | Yn |
| UM23 | R482Q | M | 76 | na | N | — | na | N | N | 5.2 | 57 | 29 | 118 | 124 | N | N | Yn |
| UM24 | R482Q | M | 19 | na | N | — | N | N | N | na | 108 | 49 | 85 | 174 | N | N | na |
| UM25 | R482Q | F | 32 | 32 | N | — | Y | Yf | Y | 5.6 | 121 | 43 | 308 | 226 | N | N | N |
| UM26 | R482W | F | 45 | 41 | Y | 25 | Y | N | Y | 5.6 | 95 | 51 | 244 | 195 | N | N | N |
| UM27 | R482W | F | 19 | 28 | N | — | Y | N | N | 7.3 | 73 | 36 | na | 143 | N | N | na |
| UM28 | R582L | F | 44 | 28 | N | — | N | N | Y | na | 116 | 42 | 116 | 181 | N | N | N |
| UM29 | R60G | F | 26 | 19 | Y | 26 | Y | N | Y | 6.7 | 84 | 35 | 402 | 159 | N | Y | N |
| UM30 | D300N | F | 34 | na | Y | 44 | Y | Yg | Y | 5.3 | 93 | 38 | 126 | 156 | N | N | Yl |
| UM31 | R482Q | F | 43 | 37 | Y | 26 | Y | Y | Y | na | na | 26 | 3461 | 236 | N | Y | N |
| UM32 | R482Q | F | 29 | 18 | N | — | Y | N | Y | 8.5 | 226 | 39 | 72 | 262 | N | N | na |
| UM33 | R482Q | F | 21 | na | Y | 30 | Y | Yh | Y | na | na | na | 484 | 248 | N | N | N |
| UM34 | R482Q | F | 30 | 22 | Y | 42 | Y | N | Y | 6.8 | 112 | 31 | 3602 | 376 | Y | N | na |
| UM35 | R482Q | F | 59 | na | Y | 36 | na | N | N | 7.6 | 111 | 41 | 372 | 226 | N | N | N |
| UM36 | R482L | F | 40 | 20 | Y | 55 | N | N | Y | 8.4 | 81 | 58 | 105 | 160 | N | N | Yk |
| UM37 | R482W | F | 60 | 26 | Y | 31 | Y | N | Y | 6.9 | 84 | 30 | 346 | 182 | N | N | N |
| UM38 | R482W | M | 42 | 38 | Y | 43 | Y | N | Y | 6.4 | 117 | 38 | 195 | 193 | N | N | Yl |
| UM39 | R482W | M | 43 | 29 | Y | 50 | Y | N | N | 5.9 | 29 | 36 | 363 | 137 | N | N | Yl |
| UM40 | R453W | F | 54 | 22 | N | — | Y | N | N | 13.3 | 113 | 52 | 196 | 204 | N | N | N |
| UM41 | R541P | F | 41 | 25 | Y | 57 | Y | N | Y | 5.8 | 130 | 45 | 192 | 213 | N | Y | Yn |
| UM42 | R584H | F | 18 | 30 | Y | 25 | Y | Yi | Y | na | 91 | 39 | 434 | 237 | N | N | Yn |
| UM43 | R482Q | F | 37 | 23 | Y | 29 | Y | N | Y | 8.2 | 145 | 36 | 211 | 223 | na | N | Yn |
| UM44 | R349W | F | 37 | 26 | Y | 20 | Y | N | Y | 6.6 | 174 | 44 | 236 | 271 | N | Y | Yn |
| UM45 | R349W | M | 26 | 17 | Y | 20 | N | N | Y | 11.9 | 107 | 37 | 89 | 162 | N | N | Yo |
| UM46 | R582C | F | 9 | 24 | N | — | N | N | Y | 5.5 | 93 | 39 | 113 | 155 | N | N | N |
| UM47 | T10I | F | 4 | 16 | Y | 6 | Y | Yj | Y | 8.0 | 150 | 17 | 4727 | 670 | N | Y | Yn |
| UM48 | G535Q | F | 48 | 23 | Y | 47 | Y | N | Y | 9.3 | na | 47 | 912 | 303 | N | Y | Yl |
| UM49 | R482Q | F | 70 | na | Y | 79 | na | N | N | 7.5 | na | na | na | na | N | N | na |
| UM50 | R482Q | M | 77 | 26 | Y | 77 | na | N | Y | 7.5 | na | na | na | na | Y | Y | na |
| UM51 | R482Q | M | 46 | 26 | na | na | na | N | na | na | na | na | na | na | na | na | |
| UM52 | R482Q | M | 71 | 26 | Y | na | na | N | na | 7.5 | na | na | na | na | Y | Y | na |
| UM53 | R482Q | F | 47 | 22 | N | — | Y | N | Y | 5.3 | 127 | 41 | 212 | 284 | N | N | N |
Abbreviations: BMI, body mass index; F, female; HbA1c, glycated hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; M, male; N, not present; na, not available; PT, patient codes; TGs, triglycerides; Y, yes, present.
a At age 18 years; bat age 19 years; cat age 45 years; dat age 46 years; eat age 10 years; fat age 43 years; gat age 26 years; hat age 38 years; iat age 36 years; jat age 5 years; kdiastolic dysfunction; lventricular hypertrophy; mantrioventricular block; nvalvular problems; oearly coronary artery disease.
Table 3.
| Pt | Variant | M/F | Age, y | BMI | Diabetes | Diabetes age, y | Dys lipidemia | Pancreatitis | Hepato steatosis | HbA1c, % | LDL, mg/dL | HDL, mg/dL | TGs, mg/dL | Cholesterol, mg/dL | Retinopathy | Nephro pathy | Cardiac abnormality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TR1 | R482Q | F | 69 | 26 | Y | 28 | Y | N | Y | 8.4 | 52 | 36 | 196 | 127 | N | Y | Ya |
| TR2 | R482Q | F | 58 | 25 | Y | 38 | Y | N | Y | 7.8 | 130 | 44 | 221 | 210 | N | N | N |
| TR3 | R482Q | F | 50 | 26 | Y | 42 | Y | N | Y | 6.3 | 158 | 35 | 1358 | 229 | N | Y | N |
| TR4 | R482Q | F | 57 | 25 | Y | 45 | Y | N | Y | 6.0 | 84 | 44 | 285 | 185 | N | N | N |
| TR5 | R482Q | F | 55 | 20 | Y | 40 | Y | N | Y | 8.0 | 131 | 46 | 197 | 217 | N | N | N |
| TR6 | R482Q | F | 43 | 26 | N | — | Y | N | Y | na | 140 | 49 | 148 | 229 | N | N | N |
| TR7 | R482Q | M | 58 | 29 | Y | 46 | Y | N | Y | 9.5 | 171 | 36 | 483 | 252 | N | N | N |
| TR8 | R482Q | F | 32 | 19 | N | — | N | N | N | 5.4 | 144 | 42 | 129 | 212 | N | N | N |
| TR9 | R482W | M | 55 | na | N | — | Y | N | Y | 6.0 | 54 | 27 | 553 | 164 | N | N | N |
| TR10 | R482W | F | 38 | 20 | Y | 22 | Y | Y | Y | 9.8 | 126 | 33 | 3216 | 367 | N | Y | N |
| TR11 | R482W | M | 65 | 25 | Y | 40 | N | N | 1 | 5.5 | 179 | 47 | 138 | 254 | N | N | N |
| TR12 | R482W | F | 7 | 16 | N | N | N | na | na | 49 | 51 | 74 | 115 | N | N | N | |
| TR13 | R482W | F | 32 | 22 | Y | 22 | Y | N | Y | 10.2 | 61 | 21 | 799 | 175 | N | Y | N |
| TR14 | R482W | M | 11 | 20 | N | — | N | N | N | na | 51 | 49 | 97 | 119 | N | N | N |
| TR15 | R482W | M | 50 | 26 | Y | 42 | Y | N | Y | 6.9 | 97 | 39 | 232 | 182 | N | N | N |
| TR16 | R482W | M | 47 | 30 | Y | 45 | Y | N | Y | 5.3 | 125 | 49 | 162 | 181 | N | Y | N |
| TR17 | R482W | F | 39 | 20 | Y | 24 | Y | N | Y | 9.0 | 105 | 27 | 342 | 176 | N | Y | Yb |
| TR18 | R482W | F | 46 | 22 | Y | 26 | Y | N | Y | 8.6 | 129 | 21 | 544 | 238 | N | Y | Yc |
| TR19 | R482W | F | 31 | 21 | N | — | Y | N | Y | 4.3 | 61 | 28 | 485 | 107 | N | N | N |
| TR20 | R482W | F | 34 | 20 | Y | 27 | Y | N | Y | 5.3 | 125 | 29 | 485 | 166 | N | Y | N |
| TR21 | R482W | F | 58 | 25 | Y | 51 | Y | N | Y | 8.0 | na | 30 | 1080 | 317 | N | Y | N |
| TR22 | R482W | F | 53 | 26 | Y | 40 | Y | N | Y | 11.4 | 87 | 47 | 293 | 193 | N | N | N |
| TR23 | R482W | F | 41 | 24 | N | — | Y | N | Y | 5.4 | 110 | 30 | 208 | 182 | N | N | N |
| TR24 | R482W | F | 41 | 25 | N | — | Y | N | Y | 5.0 | 97 | 36 | 268 | 187 | N | N | N |
| TR25 | R482W | F | 17 | 21 | Y | 12 | Y | Y | Y | 12.2 | 126 | 21 | 2993 | 419 | N | Y | N |
| TR26 | R482W | M | 37 | 25 | N | — | N | N | Y | 5.7 | 92 | 44 | 128 | 162 | N | N | N |
| TR27 | R482W | F | 11 | 16 | N | — | N | N | na | 5.6 | 82 | 59 | 66 | 154 | N | Y | N |
| TR28 | R482W | F | 18 | 23 | Y | 17 | N | N | Y | 5.2 | 68 | 57 | na | 147 | N | Y | N |
| TR29 | R482W | F | 29 | 26 | Y | 23 | Y | N | Y | 5.4 | 110 | 32 | 450 | 190 | N | N | N |
| TR30 | R482W | F | 23 | 28 | Y | 18 | Y | N | Y | 6.4 | 81 | 27 | 134 | 132 | N | N | N |
| TR31 | R482W | M | 54 | 29 | Y | 49 | Y | N | N | 6.2 | 112 | 38 | 195 | 184 | N | N | N |
| TR32 | R482W | F | 41 | 22 | Y | 21 | Y | N | Y | 11 | 104 | 22 | 803 | 226 | Y | Y | Yd |
| TR33 | R482W | F | 70 | na | Y | 30 | Y | N | Y | na | na | na | na | na | N | N | Ye |
| TR34 | R482W | M | 75 | 27 | Y | 45 | Y | N | Y | 8.7 | 104 | 21 | 422 | 176 | N | Y | Yf |
| TR35 | R482W | M | 57 | 28 | Y | 46 | Y | N | Y | 7.1 | 118 | 47 | 136 | 171 | N | Y | Yg |
| TR36 | R482W | F | 42 | 24 | Y | 25 | Y | N | Y | na | na | na | na | na | N | Y | Yh |
| TR37 | R482W | F | 40 | na | Y | 28 | Y | N | Y | na | na | na | na | na | N | N | N |
Abbreviations: BMI, body mass index; CoD, cause of death; F, female; HbA1c, glycated hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; M, male; MI, myocardial infarction; N, not present; na, not available; PT, patient codes; TGs, triglycerides; Y, yes, present.
a Coronary artery disease, catheter; bcardiomyopathy; ccoronary artery disease, catheter, MI; dcoronary artery disease, catheter, MI, died at age 41; eearly coronary disease, died at age 70 years, CoD: MI; fearly coronary disease, died at age 75 years, CoD: stroke; gearly coronary disease, died at age 58 years, CoD: coronary artery disease; hearly coronary disease, died at age 42 years, CoD: heart failure after MI.
R482W and R482Q Correlations of Our In-House Patients
Comparison of the clinical features of the 2 most common variants, R482W (n = 55) and R482Q (n = 36), in our in-house data demonstrated that patients carrying R482W were younger (age 31 [30] years vs 46 [26] years; P < .001), were of the same sex (females %, 75% vs 72%; P = .812), and had similar BMI levels (25 [6] vs 26 [8]; P = .08) in comparison to those harboring the R482Q variant.
The median levels of HbA1c, cholesterol, TGs, and LDL were similar between the 2 groups (all values listed as R482W first followed by R482Q) (HbA1c [6.3 (4) vs 7.2 (2) %, P = .785], cholesterol [182 (61) mg/dL vs 215 (75) mg/dL; P = .121], TGs [280 (183) mg/dL vs 251 (296) mg/dL; P = .543], and LDL [101 (55) mg/dL vs 111 (55) mg/dL; P = .334], and HDL [36 (15) vs 41 (11) mg/dL; P = .061]). Other than the ages at diagnosis of diabetes (28 [21] vs 44 [16] years; P = .002) and hepatic steatosis (34 [19] vs 45 [23] years; P = .012), which were earlier in patients carrying R482W variants, all accompanying clinical features that have been reported, including the history of pancreatitis attack, the presence of diabetes, fatty liver disease, dyslipidemia, hypertriglyceridemia, nephropathy, retinopathy, hypertension, abnormal cardiac examination, and the respective ages of diagnosis of the aforementioned complications, were similar between the patients carrying R482W and R482Q.
Genotype-Phenotype Correlations of 494 Patients
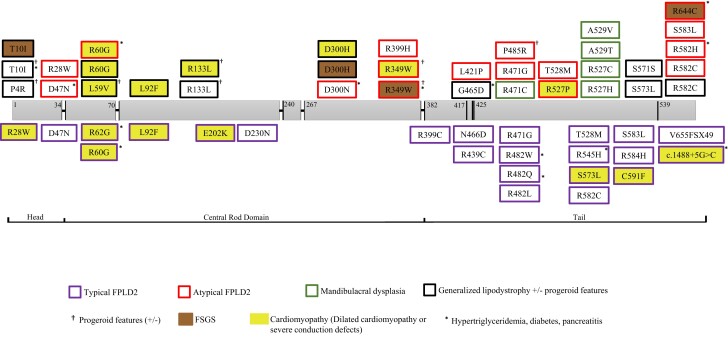
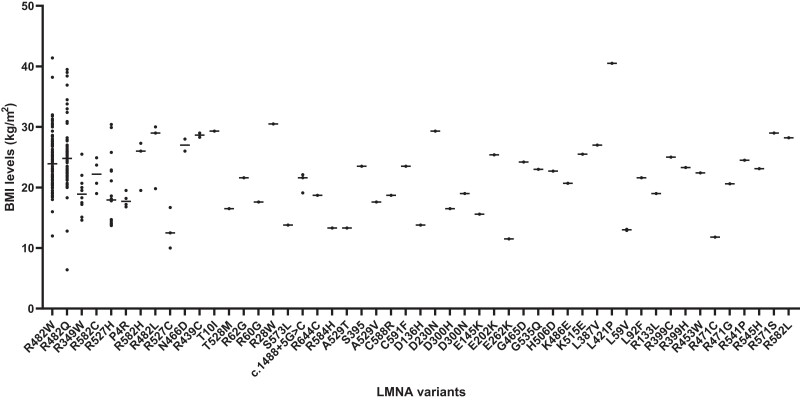
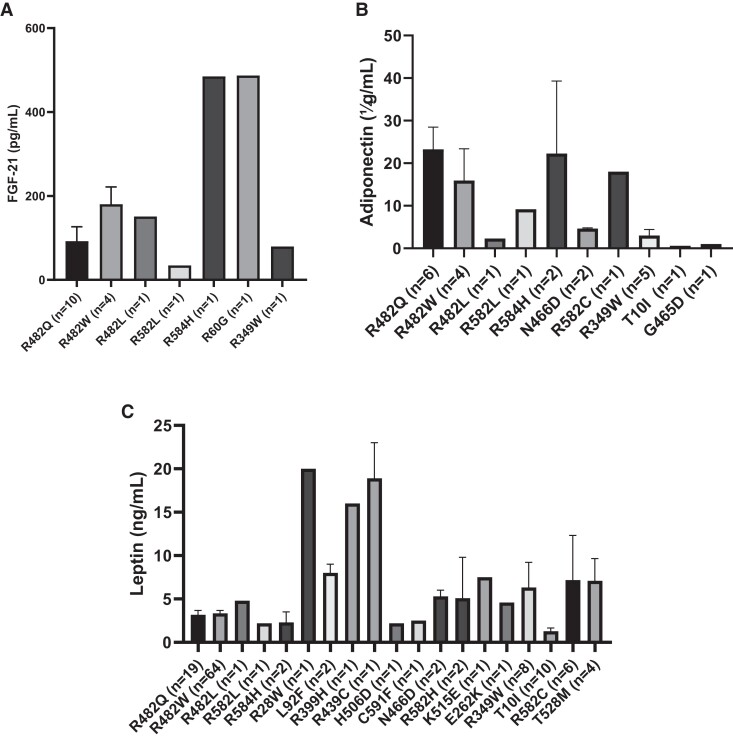
Combined analyses of our patients (n = 115) from 4 centers and all other cases (n = 379) curated from 95 studies in the literature were performed. Detailed clinical characteristics of all patients are presented in Supplementary Table S1 (128). The study included a total of 494 patients (females/males: 366/126) with a mean age of 33 (±17) years (median: 33; range, 1-77 years). The range of pathogenic variants was as follows: R482W (n = 190), R482Q (n = 93), R349W (n = 24), R527H (n = 20), T10I (n = 17), R62G (n = 9), S583L (n = 9), R60G (n = 8), R644C (n = 9), R582H (n = 9), T528M (n = 8), P4R (n = 7), T655FSX49 (n = 6), R582C (n = 5), L306V (n = 5), R28W (n = 4), R584H (n = 4), D47N (n = 4), N466D (n = 3), R471G (n = 3), R482L (n = 3), R527C (n = 3), c.1488 + 5G > C (n = 3), R133L (n = 3), A529V (n = 2), C591F (n = 2), D300N (n = 2), L59V (n = 2), L92F (n = 2), R439C (n = 2), R527P (n = 2), R571S (n = 2), S573L (n = 2), A529T (n = 1), C588R (n = 1), D136H (n = 1), D192V (n = 1), D230N (n = 1), D300H (n = 1), E145K (n = 1), E202K (n = 1), E262K (n = 1), G465D (n = 1), G535Q (n = 1), H506D (n = 1), K486E (n = 1), K486T (n = 1), K515E (n = 1), L387V (n = 1), L421P (n = 1), R399C (n = 1), R399H (n = 1), R453W (n = 1), R471C (n = 1), R541P (n = 1), R545H (n = 1), R582L (n = 1), P485R (n = 1), S395 (n = 1), and V440M (n = 1). Clinical characteristics of the common patients carrying the most common variants are presented in Table 4. Most patients were heterozygous for the changes (n = 464), with a minority presenting with either homozygous (n = 25) or compound heterozygous (n = 5) variants. Domain topology of the variants included in this analysis and associated phenotypes are presented in Fig. 2. BMI values of all variants included in this analysis are shown in Fig. 3. Circulating concentrations of leptin, adiponectin, and FGF-21 are presented in Fig. 4A–C.
Table 4.
Clinical characteristics of the patients harboring the most common variants. The results are presented as median (interquartile ranges) or as number of patients (percentages)
| All variants | R482W | R482Q | R349W | R527H | T10I | R62G | S583L | R582H | T528M | R60G | R644C | P4R | R582C | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 494 | n = 190 (39%) | n = 93 (19%) | n = 24 (5%) | n = 20 (4%) | n = 17 (3%) | n = 9 (2%) | n = 9 (2%) | n = 9 (2%) | n = 8 (2%) | n = 8 (2%) | n = 8 (2%) | n = 7 (1%) | n = 5 (1%) | |
| Common presenting phenotype | FPLD2 | FPLD2 | aFPLD | MAD | GLDS | FPLD2 | FPLD2 | aFPLD | aFPLD2 | FPLD2 | aFPLD2 | APS | aFPLD | |
| Sex (female %) | 366/492 (74%) | 134/190 (71%) | 82/93 (88%) | 15/24 (63%) | 13/20 (65%) | 8/17 (44%) | 5/9 (55%) | 6/9 (66%) | 6/9 (86%) | 7/8 (88%) | 5/8 (63%) | 4/8 (50%) | 7/7 (100%) | 4/5 (80%) |
| Age at diagnosis, y | 33 (25) n = 471 | 31 (26) n = 185 | 44 (25) n = 93 | 30 (20) n = 24 | 15 (21) n = 20 | 14 (6) n = 17 | 38.5 (12) n = 8 | 40 (17) n = 9 | 33 (15) n = 7 | 44 (23) n = 8 | 26 (13) n = 8 | 39 (12) n = 7 | 25 (17) n = 7 | 22 (43) n = 5 |
| BMI | 23 (6) n = 347 | 24 (5) n = 134 | 25 (4) n = 78 | 19(3) n = 12 | 18 (9) n = 16 | 15 (2) n = 15 | NA n = 1 | 27 (7) n = 8 | 26 (4) n = 3 | 24 (7) n = 7 | NA n = 1 | 26 (6) n = 5 | 18(2) n = 4 | 22 (5) n = 4 |
| Presence of additional features | ||||||||||||||
| Diabetes mellitus | 241/395 (61%) | 96/153 (63%) | 42/64 (66%) | 11/20 (55%) | 1/7 (14%) | 13/17 (72%) | 6/9 (67%) | 4/9 (44%) | 4/7 (57%) | 4/8 (50%) | 4/8 (50%) | 6/8 (75%) | 1/7 (14%) | 3/7 (43%) |
| Age at diagnosis, y | 30 (22) n = 176 | 27 (19) n = 61 | 40 (17) n = 31 | 22.5(12.3) n = 8 | NA | 12 (6) n = 11 | 38 (24) n = 5 | NA | 32 (23) n = 4 | 44 n = 3 | 42(18) n = 4 | 44 (15) n = 6 | NA | 23 n = 3 |
| Hypertriglyceridemia | 300/392 (77%) | 125/157 (80%) | 56/73 (76%) | 15/20 (75%) | 4/15 (27%) | 17/17 (100%) | 3/3 (100%) | 7/8 (88%) | 3/3 (100%) | 8/8 (100%) | 2/2 (100%) | 6/8 (75%) | 2/6 (33%) | 3/4 (75%) |
| Dyslipidemia | 355/428 (83%) | 142/165 (86%) | 66/82 (80%) | 17/24 (75%) | 7/15 (47%) | 17/17 (100%) | 5/5 (100%) | 7/7 (100%) | 5/5 (100%) | 8/8 (100%) | 2/2 (100%) | 7/8 (88%) | 2/7 (29%) | 4/5 (80%) |
| Age at diagnosis, y | 26 (20) n = 111 | 28 (22) n = 48 | 33 (16) n = 20 | 20 (17) n = 8 | NA n = 1 | 13.5 (6) n = 12 | 28 n = 2 | NA | 33 n = 3 | 32 n = 2 | NA | NA n = 1 | 25.5 n = 2 | 12 n = 3 |
| Pancreatitisp | 39/494 (8%) | 11/190 (6%) | 6/92 (6.5%) | 1/24 (4%) | — | 5/17 (23.5%) | 2/9 (22%) | — | 1/7 (14%) | — | 1/8 (13%) | 1/8 (13%) | — | — |
| Age at diagnosis, y | 20 (18) n = 23 | 26 (25) n = 4 | 24 (24) n = 6 | NA n = 1 | — | 6 n = 3 | NA n = 1 | — | NA | — | NA | NA n = 1 | — | — |
| Hepatic steatosis | 149/172 (87%) | 32/38 (84%) | 28/38 (73%) | 6/6 (100%) | NA | 16/16 (100%) | 1/1 (100%) | NA | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | NA | 1/1 (100%) | 1/1 (100%) |
| Age at diagnosis, y | 32 (25) n = 101 | 35 (21) n = 41 | 40 (21) n = 33 | 24.5 (11) n = 4 | NA | 15 n = 3 | NA | NA | NA n = 1 | NA n = 1 | NA n = 1 | NA | NA n = 1 | NA n = 1 |
Abbreviations: aFPLD, atypical form of familial partial lipodystrophy; APS, atypical progeroid syndrome; BMI, body mass index; FPLD2, typical form of familial partial lipodystrophy, type 2; GLDS, generalized lipodystrophy syndrome; MAD, mandibuloacral dysplasia; NA, not applicable.
Figure 2.
Domain topology of the variants in LMNA and the association of the most common clinical phenotypes. FPLD2, familial partial lipodystrophy type 2; FSGS, focal segmental glomerulosclerosis.
Figure 3.
Body mass index (BMI) values of the patients harboring different LMNA variants.
Figure 4.
Concentrations of leptin, adiponectin, and fibroblast growth factor-21 (FGF-21).
R482W vs R482Q Variants
Comparison of available data of the clinical features of the 2 most common variants, R482W and R482Q, demonstrated that patients carrying R482W were predominantly female (134/190 vs 82/93, respectively; P < .001) and were younger (n = 185, 31 [26] years vs n = 93, 44 [23] years, respectively; P < .001) and had lower BMI levels (n = 134, 24 [5] vs n = 78, 25 [4], respectively; P = .037) in comparison to those harboring the R482Q variant at the cross-sectional evaluation point.
The median levels of HbA1c, cholesterol, TGs, LDL, and HDL were similar between the 2 groups (all values listed as R482W first followed by R482Q) (HbA1c [n = 56, 6.8 (4) vs n = 37, 7.2 (3); P = .997], cholesterol [n = 92, 193 (70) mg/dL vs n = 65, 198 (67) mg/dL; P = .907], TGs [n = 107, 293 (290) mg/dL vs n = 66, 248 (259) mg/dL; P = .141], LDL [n = 88, 105 (54) mg/dL vs n = 57, 111 (51) mg/dL; P = .606], and HDL [n = 93, 37 (18) vs n = 63, 41 (13); P = .065 mg/dL]). Other than age at diagnosis of diabetes (n = 61, 27 [18] vs n = 31, 40 [17] years; P < .001) and hepatic steatosis (n = 41, 35 [21] vs 40 [21] years; P = .029), which were earlier in patients carrying R482W variants, all accompanying clinical features that have been reported, including the history of pancreatitis attack, the presence of diabetes, fatty liver disease, dyslipidemia, hypertriglyceridemia, nephropathy, retinopathy, hypertension, abnormal cardiac examination, and the respective ages of diagnosis of the aforementioned complications, were similar between the patients carrying R482W and R482Q.
Sexual Dimorphism
Among all patients, a higher percentage of female patients were reported to have diabetes (79% vs 21%; P = .012), hypertriglyceridemia (78% vs 22%, P = .019), and pancreatitis (90% vs 10%; P = .020). The distribution of dyslipidemia, hypertension, pancreatitis, hepatic steatosis, nephropathy, and abnormal cardiac examination were similar between the female and male patients. The median ages at lipodystrophy diagnosis, dyslipidemia, pancreatitis, and hepatic steatosis were similar between female and male patients. The levels of BMI, the concentrations of cholesterol, TGs, HDL, LDL, and percentage of HbA1c were also similar between female and male patients.
Dyslipidemia
Dyslipidemia was the earliest biochemical evidence described in 354 (83%) of the 427 patients (277 females, 78%), diagnosed at a median age of 26 (20) years (range, 7-68 years). The median age and BMI of patients with dyslipidemia at the cross-sectional evaluation point was 35 (24) years (range, 1-75 years) and 24 (6) (range, 11-41). The 4 most frequent variants were R482W (n = 142), R482Q (N = 66), T10I (n = 17), and R349W (n = 19).
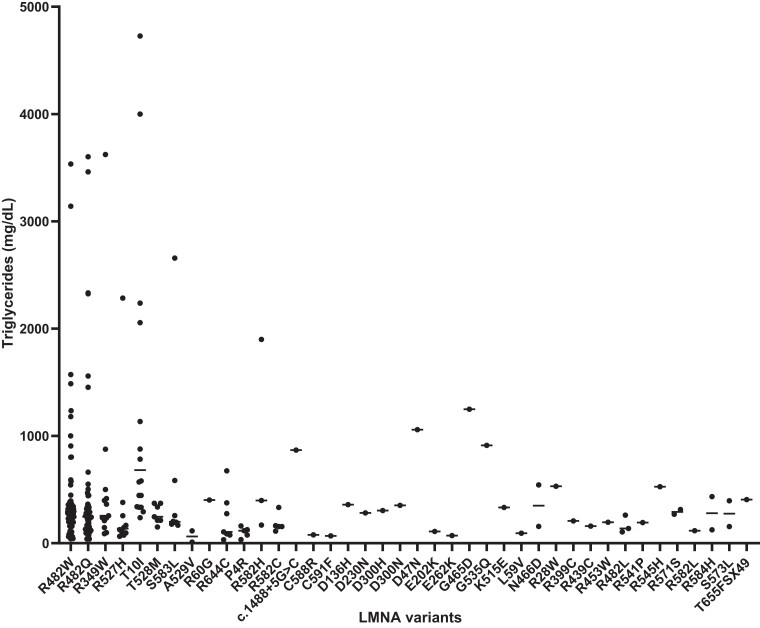
Hypertriglyceridemia was the most frequent cause of dyslipidemia (n = 291, 91%). TG concentrations corresponding to all the variants included in this analysis are presented in Fig. 5. Of all patients whose levels of lipids had been reported numerically, increased concentrations of TGs, HDL, cholesterol, LDL were identified in 77%, 77%, 55%, and 71% of all patients, respectively.
Figure 5.
Triglyceride concentrations and the association of all variants included in these analyses.
The concomitant diagnosis of dyslipidemia and hepatic steatosis was reported in 89% (n = 109/123), with diabetes present in 70% (n = 212/303), abnormal cardiac examination present in 22% (n = 77/354), pancreatitis present in 10% (n = 36/354), nephropathy present in 8% (n = 29/354), and retinopathy present in 3% (n = 11/354) of these cases. Those with dyslipidemia had 8.4 times the odds (95% CI, 1.1-62.3) ([37/317]/[1/72]) of developing acute pancreatitis compared to those without dyslipidemia, while those with hypertriglyceridemia had 12.0 times the odds (95% CI, 1.6-89.0) ([35/265]/[1/91]) compared to those without. Circulating TGs were negatively correlated with age at diabetes onset (Spearman rho [rs] = −0.312; P = .001) and HDL levels ([rs] = −0.557; P < .001), and positively correlated with HbA1c ([rs] = 0.310; P < .001) and cholesterol levels ([rs] = 0.402; P < .001). There were no other statistically significant correlations between TG levels, age at lipodystrophy diagnosis, BMI values, and LDL concentrations. The patients with hypertriglyceridemia had higher BMI levels (23 [6] vs 22 [7]; P = .003) and lower HbA1c concentrations (8 [3] vs 6 [1]%; P = .004) in comparison to those without.
Diabetes
Diabetes had been reported in 241 (61%) of 395 patients (191 females, 79%) at a median age onset of 30 (21) years (range, 6-79 years). Patients with diabetes had a median age of 39 (24) years (range, 4-77 years) with a BMI level of 23 (6) (range, 12–41) at the cross-sectional evaluation point. The median age at onset of dyslipidemia was significantly earlier than that of diabetes (n = 108, 26 [21] vs n = 179, 30 [22] years; P = .020). The most common variants identified among patients with diabetes were R482W (n = 96), R482Q (n = 42), T10I (n = 13), R349W (n = 11), R644C (n = 7), T655fsX49 (n = 6), R582H (n = 6), R62G (n = 5), T528M (n = 4), S583L (n = 4), and R60G (n = 4).
The concomitant presence of diabetes with dyslipidemia was reported in 95% (n = 213/224), hypertriglyceridemia in 91% (n = 181/198), hepatic steatosis in 93% (n = 92/99), nephropathy in 27% (n = 64/241), abnormal cardiac examination in 34% (n = 83/241), pancreatitis in 13% (n = 32/241), and retinopathy in 2% (n = 12/241) of all cases. The median ages of diagnosis of dyslipidemia, pancreatitis, and hepatic steatosis were 27.5 (19), 23 (20), and 34 (25) years, which had been reported in 70, 20, and 73 patients, respectively. Patients with diabetes were diagnosed at older ages (39 [24] vs 25 [23] years; P < .001) with higher BMI levels (23 [6] vs 22 [7]; P = .046) in comparison to those without diabetes. Patients with diabetes had significantly higher levels of HbA1c (7.6 [3] vs 5.5 [1]; P < .001), TGs (350 [421] vs 209 [204] mg/dL; P < .001), cholesterol (195 [73] vs 178 [56] mg/dL; P = .008), and lower concentrations of HDL (36 [13] vs 41 [10] mg/dL; P < .001) in comparison to those without diabetes, while the levels of LDL were similar between the 2 groups. Patients who were reported to have diabetes had 3.2 times the odds (95% CI, 1.4-7.5) ([32/209]/[7/147]) of having pancreatitis compared to those who did not have diabetes.
Pancreatitis
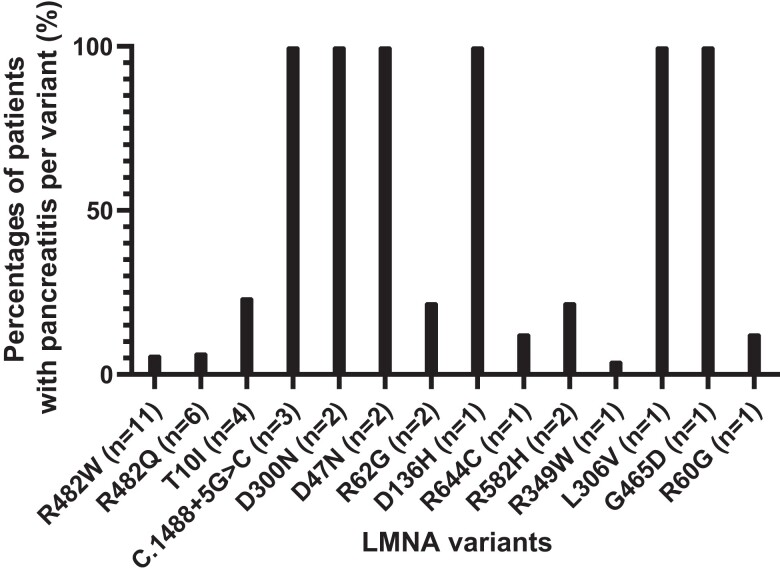
Among 39 patients (female: 35, 90%) with an episode of acute pancreatitis history, the median age at acute pancreatitis diagnosis was 20 (17) years (range, 5-48 years). The distribution of all the patients with a history of pancreatitis included in this analysis is shown in Fig. 6. Patients had a median age of 29 (18) years (range, 4-55 years) and BMI of 24 (9) (range, 11–39) at the cross-sectional evaluation point. Patients with a history of acute pancreatitis harbored R482W (n = 11), R482Q (n = 6), T10I (n = 4), c.1488 + 5G > C (n = 3), D47N (n = 2), D300N (n = 2), R582H (n = 2), R62G (n = 2), D136H (n = 1), G465D (n = 1), L306V (n = 1), R349W (n = 1), R584H (n = 1), R60G (n = 1), and R644C (n = 1) variants (see Table 1). Ten (26%) patients were reported to have multiple pancreatitis ( > 1) episodes. Evaluations of patients with a history of acute pancreatitis revealed that dyslipidemia was present in 97% (n = 37/39), hypertriglyceridemia in 90% (n = 35/39), hepatic steatosis in 90% (n = 23/25), diabetes in 83% (n = 32/39), abnormal cardiac examination in 46% (n = 11/39), nephropathy in 28% (n = 11/39), and retinopathy in 10% (n = 4/39) of all patients.
Figure 6.
The distribution of all patients with a history of acute pancreatitis. The percentages shown demonstrate the occurrence of pancreatitis for each variant.
Other Clinical Characteristics and Mortality
Evidence of hypertension was reported in 21% (n = 105) of patients. The median age of diagnosis of hypertension was 24 (17) years (range, 10-71 years). Among patients who had been evaluated with liver ultrasound or biopsy, 87% (n = 149/172) were found to have hepatic steatosis. The median age at diagnosis was 33 (25) years (n = 105; range, 6-65 years). Abnormal cardiac examination was reported in 26% (n = 130) of all patients. Early coronary heart disease (n = 30), valvular abnormalities (n = 30), ventricular hypertrophy (right or left) (n = 27), conduction (n = 24), and dilated cardiomyopathy (n = 23) were among the most common cardiac problems. Whereas 32% (n = 41) of cases with cardiac defects were found to have variants in exon 8, the majority of cases (68%) had defects affecting regions outside exon 8 (P < .001). Defects outside exon 8 were most frequently found in exon 1 (38%; n = 34/52), exon 6 (25%; n = 22/25), and exon 11 (11%; n = 10/24). Evidence of nephropathy and retinopathy were reported in 15% (n = 76) and 3% (n = 15) of all patients. While mild proteinuria as a complication of diabetes was the most common evidence of nephropathy reported in most studies, there were also reports on cases with chronic renal disease (n = 5) and focal segmental glomerulosclerosis (n = 9).
Skeletal abnormalities, including clavicular hypoplasia, scoliosis, acro-osteolysis, mandibular hypoplasia, short stature, joint contractures, micrognathia, evident shoulders, thin clavicles, vertebral sclerosis, and dental crowding were mostly reported among patients carrying the R527H variant. Other variants presenting with skeletal abnormalities were as follows: T10I (eg, joint contractures, spinal deformity, mandibular hypoplasia), R60G (eg, contractures), K486E (eg, mandibular hypoplasia), R527C (eg, short stature, joint contractures), R571S (eg, short stature, clinodactyly, joint contractures), A529V (eg, short statures, dysmorphic extremities, mandibular hypoplasia, acro-osteolysis), and A529T (eg, short stature, clavicle aplasia).
Based on our analysis, personalized treatment strategies mostly focusing on treating the complications of lipodystrophies, including diabetes, pancreatitis, hypertriglyceridemia, hypertension, and cardiac failure, were followed. Twenty-five percent (n = 122) of all patients were reported to be treated with combination treatments such as insulin, metformin, fibrates, statins, angiotensin-converting enzyme inhibitors, diuretics, and pioglitazone. Treatment with metreleptin was also well tolerated and resulted in improvements of the complications in several cohorts (21, 23, 42, 104, 107, 112, 117)
Cardiovascular disease was the leading cause of mortality occurring at a median age of 43 (20) years (n = 16; range, 7-75 years). The following causes of death were reported: paroxysmal arrhythmia (n = 1), myocardial infarction (n = 5), heart failure (n = 4), graft failure from heart transplant (n = 1), hemorrhagic stroke (n = 2), bacterial endocarditis (n = 1), sepsis due to foot infection (n = 1), cerebral hemorrhage after hemodialysis (n = 1), and other (n = 1).
Discussion
This study reports the distribution of various clinical and metabolic characteristics, including dyslipidemia, diabetes, pancreatitis, hypertension, cardiac pathology, nephropathy, and hepatic steatosis, in the largest number of patients with LMNA-related lipodystrophy syndromes to date, using clinical cases reported in more than 100 studies in addition to our cohort of 115 patients, some of whom we had been following for more than 20 years. The results confirm that dyslipidemia precedes the onset of diabetes. As each rare variant may present with different clinical features, this study demonstrates that the most common variants, R482W and R482Q, do not exhibit distinct metabolic characteristics.
Consistent with earlier publications, we observed disparities among clinical phenotypes, with certain variants exhibiting a milder phenotype and others having a more aggressive clinical course (4, 27). Several authors have tried to derive specific phenotypic associations with respect to lamin domain topology. As an example, the pathogenic variants affecting only the structure of exon 11 (protein domains 566-656), which is not transcribed in lamin C, as opposed to exon 8, had initially been held accountable for the milder phenotypes; however, the patients with homozygosity in exon 11 or variants, such as R582H, have blurred this hypothesis (27, 61, 65, 84, 102). Further, those pathogenic variants located in the amino-terminal (5′-end of the gene) to the nuclear localization signal (NLS, 417-422) (exon 7; protein domains 386-466) of lamin A had been initially classified as class 1 laminopathies, while those carboxy-terminal (3′-end of the gene) to the NLS were clustered as class 2 laminopathies (129). However, the presumption that the class 1 laminopathies would affect the cardiac, skeletal muscle, and neurological system, whereas class 2 would cause partial lipodystrophy or progeria syndromes, has been proven otherwise through analyses of the R28W, D230N, R399C, and R471G variants (31, 35, 40, 41). Our study showed that patients with the T10I variant, who typically presented with generalized lipodystrophy, exhibited the most severe complications. All patients with T10I were diagnosed with dyslipidemia, with the greatest circulating TGs compared to other variants. Patients carrying the R582H variant, which is related to mandibuloacral dysplasia, and the P4R variant, which is associated with atypical progeroid syndromes, showed a milder phenotype than those carrying the R482Q, R482W, R349W, and R62G variants, which most commonly present with the FPLD2 phenotype. The most common variants in our study, R482Q and R482W, were associated with similar metabolic characteristics and complications, despite the fact that those with the R482W variant were younger and had statistically lower BMI levels at the time they were evaluated. Given that we had more cases of the R482W variant, it is possible that the efforts to expand family studies to investigate younger family members may have skewed the data collected. To state that R482W is representative of a more severe clinical phenotype would require age-adjusted studies looking at hard end points such as mortality, and organ-specific morbidity (diabetes complications, liver failure, cardiovascular disease, etc). The classification of these patients with respect to different variants may serve as a map for those interested in the field to better understand the associations and develop individualized approaches for all the distinctions in these overlapping phenotypes.
Our study showed that the median ages of diagnosis of lipodystrophy and several metabolic complications were comparable between females and males with LMNA-related lipodystrophy syndromes. This finding should be interpreted carefully, as it does not necessarily contradict that females with FPLD2 are diagnosed at an earlier age than males, since our study included many types of lipodystrophy syndromes including those with progeroid forms (4). Although this finding does not cover the whole lifespan, similar ages at onset of complications for both sexes support the notion that age correlates with phenotype (2).
Dyslipidemia and diabetes, the 2 most common metabolic abnormalities in patients with lipodystrophy syndromes, were seen frequently among all LMNA variants (3). While previous studies have reported a prevalence of dyslipidemia ranging from 59% to 100% in patients with FPLD2 (4, 37, 130, 131), our study showed that 83% of all patients had dyslipidemia, which was also comparable in the most common variants, R482W and R482Q, which were 86% and 80%, respectively. It was still interesting to note the totality of the distribution width in each variant, which likely represents contributions not only from the LMNA variants themselves, but also from additional genetic, epigenetic, and environmental factors.
Consistent with previous studies indicating that the lipoprotein abnormalities initially contribute to the development of diabetes, we also found that the age at dyslipidemia onset was earlier than that of diabetes (15, 29, 132). Hypertriglyceridemia, which we have previously demonstrated to have a positive correlation with liver lipid content and a negative association with leg fat mass (13), was the most common lipid abnormality among patients with dyslipidemia carrying different variants. While the consensus in 2018 by Grundy et al (133) recommends adding therapeutic modalities at specified cutoff levels for each lipid subfraction, it is important to note that normal mean levels of different lipid subfractions have been reported to be much lower, depending strictly on a variety of factors, including age, race, ethnicity, and secular trends. It is also worth mentioning that Aberra et al (134) have reported that TG concentrations even in the reference ranges may be associated with increased cardiovascular risks. Similarly, despite the recommendations on initiating TG-lowering treatment at levels greater than or equal to 500 mg/dL, mild-to-moderate hypertriglyceridemia (>177 mg/dL) has also been shown to be associated with high risk of acute pancreatitis in a prospective cohort study by Pedersen et al (135). The strong association of high cholesterol, LDL, and TGs, and low HDL levels with the development of atherosclerosis and cardiovascular disease, the presence of cardiac complications, and the risk of acute pancreatitis in lipodystrophy syndromes highlight the importance of strict lipid control at the earliest possible point in the clinical care of patients afflicted with these diseases (124).
Diabetes, the second most prevalent characteristic in our study, has been reported to range in prevalence from 28% to 77% among patients with FPLD2 (4, 130, 131, 136). According to our findings, the prevalence of diabetes was 61% in all patients with LMNA-related lipodystrophy syndromes, as well as among those harboring the most common R482W variant. It is interesting to note the discrepancies in cause-and-effect relationships among patients of similar ages who have different metabolic derangements. Although there was a positive correlation between TGs and HbA1c levels, those with diabetes had even higher TG concentrations. This result may be anticipated, since both are independent risk factors for the development of the other (137). However, along with the earlier onset of dyslipidemia, this distinction may also suggest that hypertriglyceridemia is a risk factor for the development of diabetes, and it gets worse once diabetes has been established (132).
Severe atherosclerotic cardiovascular disease and acute pancreatitis have been reported to be the 2 most common causes of mortality and morbidity in patients with lipodystrophy syndromes (138, 139). Comparable to a previous study (140) reporting the overall prevalence of pancreatitis as 4% (140) among a cohort of 74 patients predominantly carrying R482W variants (n = 51, 69%), we found that the overall prevalence of pancreatitis was 8%, while the patients with R482W and R482Q variants had a lower prevalence of 6%. The difference is presumably due to the pathogenic variants associated with the generalized type, which we also included in our study for comparative purposes, which are associated with more severe complications. Although the age of onset of acute pancreatitis was found to be younger in comparison to dyslipidemia, it is well known that dyslipidemia precedes the onset of acute pancreatitis in lipodystrophy syndromes. This contradictory finding has presumably arisen due to the symptomatic nature of acute pancreatitis, and possible data skewness of higher number of patients with dyslipidemia. However, one patient had normal lipid levels despite pancreatitis, highlighting that acute pancreatitis may in some instances precede dyslipidemia, and since some biological lipid abnormalities may arise without biochemical evidence of dyslipidemia (135). Further, pancreatic acinar cell–specific LMNA-deletion in mice has shown that these animals develop histological abnormalities comparable to chronic pancreatitis due to changes in p53 signaling, cell cycle, and basal transcription factors, which collectively lead to cell-autonomous endoplasmic reticulum stress with loss of acinar cells of the pancreas (141). In line with previous reports (13, 55, 140) demonstrating an increased risk of pancreatitis with hypertriglyceridemia in FPLD2, we also found that the risk of acute pancreatitis increased in the presence of diabetes and dyslipidemia. The patients with hypertriglyceridemia had 12.0 times the odds of having acute pancreatitis in comparison to those who did not, while those with diabetes had 3.2 times the odds.
Cardiovascular events were the most prevalent cause associated with mortality. We have previously shown that 78% of all patients with lipodystrophy may have evidence of a severe cardiac pathology (110). In line with the literature, this study showed that cardiac defects due to variants in LMNA, which is associated with poor prognosis and higher rates of sudden cardiac death, were more common in patients carrying variants outside exon 8 (18). As several prediction models underpin the changes in the protein structure of lamins with respect to the subdomains and hotspot mutations, the exact underlying mechanisms still need further research (18, 142).
Although the abnormal cardiac observations reported among the patients carrying the R482W variant outnumbered the cases seen among all other variants, its prevalence was similar to that of R482Q, the second most frequent variant. Hence, this higher number could just indicate a possible reporting bias, since R482W is the most frequent variant overall. Although the prevalence of cardiovascular abnormalities in our study was not particularly high, significant valvular, conduction, or morphological abnormalities were found in most patients who were able to undergo a comprehensive cardiac examination. These findings support the need for routine cardiac monitoring, in accordance with our previous recommendations (110).
Despite serious metabolic complications, most patients with LMNA variants had similarly low or normal BMI values in comparison to the healthy population, though the distribution did include some individuals with BMI levels as high as in the 40s. We have previously demonstrated this particular discordance between BMI and metabolic dysfunction, which in our view may be related to the distinct distribution of adiposity observed in lipodystrophy syndromes (127). Loss of lower-extremity adipose tissue, which is very sensitive to insulin, contributes to excess free fatty acids in circulation, which may then lead to the deterioration of cardiovascular and metabolic health (14, 143, 144). Further, the loss of brown adipose tissue characteristics is inversely correlated with BMI (145). Brown adipose tissue is predominantly found in the neck and upper body, resonating the distribution pattern of preserved depots in FPLD2, and thus may contribute to metabolic dysfunction. We believe that the limited storage capacity conferred by reduced overall adipose tissue and the altered characteristics of the residual adipose depots are deserving of additional studies.
It is also important to note that the adipose tissue deficiency and perturbations in circulating factors coming out the adipose tissue per se cause systemic metabolic effects. These are well-established observations given the overlapping clinical phenotypes in all forms of lipodystrophy, classical studies of fat transplantation in rodent models, and studies of leptin therapy in patients as well as animal models (21, 115, 146). While it is known that leptin levels are quite low in patients with the LMNA-related lipodystrophy from previous reports and the data compiled here, the fact that leptin therapy is not universally effective in this subgroup suggests that molecular mechanisms leading to the adipocyte loss in some depots and alterations in the preserved depots may be additional important contributors to overall pathophysiology (13, 30, 49, 112, 113, 147, 148). Other circulating factors that have been evaluated here are adiponectin and FGF-21. Interestingly, adiponectin levels were quite varied according to genotype and were not universally low as previously implicated in singular reports (104, 149-151). On the other hand, FGF21 levels were not low and surprisingly elevated in some genotypes, suggesting a potential compensatory role for this hormone (152, 153).
Understanding how specific LMNA gene variants alter or impair lamin A/C function is critical to investigate mechanisms of lipodystrophy syndromes and to reveal therapeutic targets. Regarding mechanisms leading to the diverse pathologies observed in different laminopathy syndromes, several theories exist. Specific cell types may be more sensitive to perturbed lamin A/C function, which may explain why cells like adipocytes, cardiomyocytes, and myocytes are predominantly affected by laminopathies. The nuclear lamina maintains the shape of the nuclear membrane, is associated with chromatin, and interacts with other intracellular intermediate filament and transmembrane nuclear proteins to coordinate movement and shape change in response to external and internal stimuli. Due to the association of lamin A/C with chromatin, lamin A/C plays a key role in nuclear location and accessibility of transcribed genes, some of which may exert control over the differentiation and maintenance of different cell types. Lamin A/C also maintains shape and stability for the nucleus while undergoing cell division and the overall cell structure under plastic stressors, like mechanical stress. Our laboratories are currently investigating the mechanisms by which adipocyte loss occurs in different LMNA-related lipodystrophy phenotypes. We have demonstrated that LMNA is essential to the maintenance of mature adipocytes by creating an adipocyte-specific LMNA knockout mouse with Cre recombinase activity driven by the adiponectin promoter (154). These mice recapitulate many of the features observed in FPLD2 clinically, including adipose tissue loss coincident with puberty, dyslipidemia, hepatic steatosis, and insulin resistance. Ongoing work in the laboratory includes development of tamoxifen-inducible conditional LMNA knockouts, which allows us to examine the earliest molecular events after lamin A/C deletion that lead to adipocyte loss. We are also actively working on the creation of a topographic map of lamin-associated domains in chromatin, which may reveal key genes involved in adipocyte maintenance. In addition, 6 different human variants have been recreated in mice through knockin-mutant murine models. Finally, we are examining adipose tissue biopsies of affected teenagers before and after adipose loss at single-cell nuclei resolution to correlate the changes we observe in the conditional knockout models. We believe that these approaches will allow us to determine the precise etiology of adipose loss in patients and rodent models and venture into precision therapeutics.
Study Limitations
Our study was limited by the retrospective nature of our data and the potential reporting bias of the previous 100 published reports. Our analyses could include only those cases with detailed clinical characteristics. We did not include patient cohorts that lacked the genotype-phenotype characteristics per individual. Although the presence of clinical features related to disease was noted, the ages at which certain clinical features were diagnosed were not included in most of the studies either. For this report, we did not focus on clinical characteristics such as pancreatic exocrine function or morphology, smooth muscle function, neurocognitive characteristics, mood, pain perception, and other mental diseases or reproductive function. However, we are keenly aware that such characteristics need to be investigated in future studies either because there is a link to lamin A/C function specifically at a tissue level or abnormalities have been reported in a limited fashion in case series. Finally, it is important to note the differences between different countries’ access to health care when interpreting the results. Even regarding our own cohort, the parameters such as the ages at diagnosis or onset of certain complications completely depend on the information collected from the patients. Hence, we believe that although it is important to describe the comparisons, strong statements on genotype-phenotype correlations should be avoided.
Conclusion
This study identifies genotype-phenotype relationships in the largest cohort of LMNA-related lipodystrophy syndrome patients to date. This study will serve as a comprehensive directory for obtaining a deeper understanding of the clinical characteristics and underlying mechanisms of this rare disorder. Our report helps to quantify prevalence of rare complications associated with different LMNA variants. Dyslipidemia was the most common and earliest metabolic dysfunction, affecting 83% of all patients, leading to the earliest symptomatic and significant clinical manifestation, acute pancreatitis, which was diagnosed at a median age of 20 years. The patients with hypertriglyceridemia had 12.0 times, while those with diabetes had 3.2 times, the odds of having acute pancreatitis in comparison to those who did not. Even though patients carrying different variants had varying features, most of the clinical and metabolic features between the patients harboring the most common variants, R482W and R482Q, were indistinguishable for the variables we interrogated for this analysis.
Acknowledgments
We thank the patients and their families, caregivers, and physicians involved in the care of these patients.
Abbreviations
- BMI
body mass index
- FGF-21
fibroblast growth factor-21
- FMR
ratio of percentage fat mass of trunk to percentage fat mass of legs
- FPLD2
familial partial lipodystrophy type 2
- HbA1c
glycated hemoglobin A1c
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- LMNA
lamin A/C gene
- MI
myocardial infarction
- TGs
triglycerides
Contributor Information
Ozge Besci, Division of Metabolism, Endocrinology & Diabetes, University of Michigan, Ann Arbor, MI 48109, USA; Division of Pediatric Endocrinology, Dokuz Eylul University, Izmir 35340, Turkey.
Maria Christina Foss de Freitas, Division of Metabolism, Endocrinology & Diabetes, University of Michigan, Ann Arbor, MI 48109, USA.
Natália Rossin Guidorizzi, Division of Internal Medicine, University of São Paulo, Ribeirão Preto, São Paulo 05508, Brazil.
Merve Celik Guler, Division of Metabolism, Endocrinology & Diabetes, University of Michigan, Ann Arbor, MI 48109, USA; Division of Internal Medicine, Dokuz Eylul University, Izmir 35340, Turkey.
Donatella Gilio, Division of Metabolism, Endocrinology & Diabetes, University of Michigan, Ann Arbor, MI 48109, USA; Department of Clinical and Translational Sciences, University of Pisa, Pisa 56126, Italy.
Jessica N Maung, Department of Molecular & Integrative Physiology, University of Michigan, Ann Arbor, MI 48105, USA.
Rebecca L Schill, Department of Molecular & Integrative Physiology, University of Michigan, Ann Arbor, MI 48105, USA.
Keegan S Hoose, Department of Molecular & Integrative Physiology, University of Michigan, Ann Arbor, MI 48105, USA.
Bonje N Obua, Department of Molecular & Integrative Physiology, University of Michigan, Ann Arbor, MI 48105, USA.
Anabela D Gomes, Division of Metabolism, Endocrinology & Diabetes, University of Michigan, Ann Arbor, MI 48109, USA.
Ilgın Yıldırım Şimşir, Division of Endocrinology and Metabolism, Department of Internal Medicine, Ege University, Izmir 35100, Turkey.
Korcan Demir, Division of Pediatric Endocrinology, Dokuz Eylul University, Izmir 35340, Turkey.
Baris Akinci, DEPARK, Dokuz Eylul University & Izmir Biomedicine and Genome Center, Izmir, Turkey; Izmir Biomedicine and Genome Center, Izmir 35340, Turkey.
Ormond A MacDougald, Division of Metabolism, Endocrinology & Diabetes, University of Michigan, Ann Arbor, MI 48109, USA; Department of Molecular & Integrative Physiology, University of Michigan, Ann Arbor, MI 48105, USA.
Elif A Oral, Division of Metabolism, Endocrinology & Diabetes, University of Michigan, Ann Arbor, MI 48109, USA.
Funding
This work was supported by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases grant number 2R56DK125513-04).
Author Contributions
E.A.O. conceived the idea for this work and funded team members. She also designed the studies in which cases are collected and has collected cases and their data. She assisted with the initial draft and reviewed all drafts including the final one. E.A.O. assumes full responsibility for the integrity of the data. O.B. collected data, performed statistical analyses, interpreted the data, and wrote the initial draft. M.C.F.F., N.R.G., M.C.G., D.G., A.D.G., I.Y.S., and B.A. participated in patient care and data collection. J.N.M., R.L.S., K.S.H., B.O., K.D., B.A., and O.A.M. edited and revised the manuscript critically. All authors read and approved the final manuscript.
Disclosures
E.A.O. has intellectual property related to the use of metreleptin and related compounds in patients with all forms of lipodystrophy. She has provided consulting services to Ionis and Akcea in the past 3 years. She is actively providing consulting services to Amryt Pharmaceuticals, Regeneron Pharmaceuticals Inc, and Third Rock Ventures. She has received either grant or clinical trial support from Amryt Pharmaceuticals and Ionis related to the lipodystrophy space. She has previously received writing support or educational grants from Amryt Pharmaceuticals and Regeneron Pharmaceuticals, Inc, as well as Akcea Therapeutics. Her current clinical trial portfolio also includes support from Rhythm Pharmaceuticals, GI Dynamics, Novo Nordisk, Fractyl (current), and Gemphire Therapeutics (in the last 3 years). She is on the scientific advisory board of Rejuvenate Bio and has ongoing grant support. O.A.M. has received grant support from Regeneron Pharmaceuticals, Inc, CombiGene AB, and Rejuvenate Bio. J.N.M. and R.S. have received grant support from Regeneron Pharmaceuticals Inc, Rejuvenate Bio, and CombiGene. B.A. receives consulting fees from Amryt Pharmaceuticals and Regeneron Pharmaceuticals, Inc. The remaining authors have nothing to disclose with respect to this manuscript.
Data Availability
Original data generated and analyzed during this study are included in the data repositories listed in “References.”
References
- 1. Brown RJ, Araujo-Vilar D, Cheung PT, et al. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab. 2016;101(12):4500‐4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gonzaga-Jauregui C, Ge W, Staples J, et al. Clinical and molecular prevalence of lipodystrophy in an unascertained large clinical care cohort. Diabetes. 2019;69(2):249‐258. [DOI] [PubMed] [Google Scholar]
- 3. Garg A. Clinical review#: lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96(11):3313‐3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernandez-Pombo A, Diaz-Lopez EJ, Castro AI, et al. Clinical spectrum of LMNA-associated type 2 familial partial lipodystrophy: a systematic review. Cells. 2023;12(5):725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pellegrini C, Columbaro M, Schena E, et al. Altered adipocyte differentiation and unbalanced autophagy in type 2 familial partial lipodystrophy: an in vitro and in vivo study of adipose tissue browning. Exp Mol Med. 2019;51(8):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garg A, Subramanyam L, Agarwal AK, et al. Atypical progeroid syndrome due to heterozygous missense LMNA mutations. J Clin Endocrinol Metab. 2009;94(12):4971‐4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garg A, Speckman RA, Bowcock AM. Multisystem dystrophy syndrome due to novel missense mutations in the amino-terminal head and alpha-helical rod domains of the lamin A/C gene. Am J Med. 2002;112(7):549‐555. [DOI] [PubMed] [Google Scholar]
- 8. Garg A, Cogulu O, Ozkinay F, Onay H, Agarwal AK. A novel homozygous Ala529Val LMNA mutation in turkish patients with mandibuloacral dysplasia. J Clin Endocrinol Metab. 2005;90(9):5259‐5264. [DOI] [PubMed] [Google Scholar]
- 9. Kim HJ, Lee PCW, Hong JH. Overview of cellular homeostasis-associated nuclear envelope lamins and associated input signals. Front Cell Dev Biol. 2023;11:1173514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lazarte J, Hegele RA. Lamin A/C missense variants: from discovery to functional validation. NPJ Genom Med. 2021;6(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burke B, Stewart CL. The nuclear lamins: flexibility in function. Nat Rev Mol Cell Biol. 2013;14(1):13‐24. [DOI] [PubMed] [Google Scholar]
- 12. Montenegro RM, Jr., Costa-Riquetto AD, Fernandes VO, et al. Homozygous and heterozygous nuclear lamin A p.R582C mutation: different lipodystrophic phenotypes in the same kindred. Front Endocrinol (Lausanne). 2018;9:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ajluni N, Meral R, Neidert AH, et al. Spectrum of disease associated with partial lipodystrophy: lessons from a trial cohort. Clin Endocrinol (Oxf). 2017;86(5):698‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tchkonia T, Thomou T, Zhu Y, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17(5):644‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bidault G, Garcia M, Vantyghem MC, et al. Lipodystrophy-linked LMNA p.R482W mutation induces clinical early atherosclerosis and in vitro endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2013;33(9):2162‐2171. [DOI] [PubMed] [Google Scholar]
- 16. Carboni N, Politano L, Floris M, et al. Overlapping syndromes in laminopathies: a meta-analysis of the reported literature. Acta Myol. 2013;32(1):7‐17. doi. Published 2013/07/16. [PMC free article] [PubMed] [Google Scholar]
- 17. Scharner J, Gnocchi Viola F, Ellis Juliet A, Zammit Peter S. Genotype–phenotype correlations in laminopathies: how does fate translate? Biochem Soc Trans. 2010;38(1):257‐262. [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Dobreva G. Epigenetics in LMNA-related cardiomyopathy. Cells. 2023;12(5):783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valenti AC, Albini A, Imberti JF, et al. Clinical profile, arrhythmias, and adverse cardiac outcomes in emery-dreifuss muscular dystrophies: a systematic review of the literature. Biology (Basel). 2022;11(4):530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akinci B, Onay H, Demir T, et al. Clinical presentations, metabolic abnormalities and end-organ complications in patients with familial partial lipodystrophy. Metab Clin Exp. 2017;72:109‐119. [DOI] [PubMed] [Google Scholar]
- 21. Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570‐578. [DOI] [PubMed] [Google Scholar]
- 22. Peters JM, Barnes R, Bennett L, Gitomer WM, Bowcock AM, Garg A. Localization of the gene for familial partial lipodystrophy (Dunnigan variety) to chromosome 1q21–22. Nat Genet. 1998;18(3):292‐295. [DOI] [PubMed] [Google Scholar]
- 23. Speckman RA, Garg A, Du F, et al. Mutational and haplotype analyses of families with familial partial lipodystrophy (Dunnigan variety) reveal recurrent missense mutations in the globular C-terminal domain of lamin A/C. Am J Hum Genet. 2000;66(4):1192‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garg A, Peshock RM, Fleckenstein JL. Adipose tissue distribution pattern in patients with familial partial lipodystrophy (Dunnigan variety)1. J Clin Endocrinol Metab. 1999;84(1):170‐174. [DOI] [PubMed] [Google Scholar]
- 25. Hegele RA, Cao H, Anderson CM, Hramiak IM. Heterogeneity of nuclear lamin a mutations in dunnigan-type familial partial lipodystrophy*. J Clin Endocrinol Metab. 2000;85(9):3431‐3435. [DOI] [PubMed] [Google Scholar]
- 26. Vigouroux C, Magré J, Vantyghem MC, et al. Lamin A/C gene: sex-determined expression of mutations in Dunnigan-type familial partial lipodystrophy and absence of coding mutations in congenital and acquired generalized lipoatrophy. Diabetes. 2000;49(11):1958‐1962. [DOI] [PubMed] [Google Scholar]
- 27. Garg A, Vinaitheerthan M, Weatherall PT, Bowcock AM. Phenotypic heterogeneity in patients with familial partial lipodystrophy (Dunnigan variety) related to the site of missense mutations in lamin A/C gene1. J Clin Endocrinol Metab. 2001;86(1):59‐65. [DOI] [PubMed] [Google Scholar]
- 28. Caux F, Dubosclard E, Lascols O, et al. A new clinical condition linked to a novel mutation in lamins A and C with generalized lipoatrophy, insulin-resistant diabetes, disseminated leukomelanodermic papules, liver steatosis, and cardiomyopathy. J Clin Endocrinol Metab. 2003;88(3):1006‐1013. [DOI] [PubMed] [Google Scholar]
- 29. Schmidt HH-J, Genschel J, Baier P, et al. Dyslipemia in familial partial lipodystrophy caused by an R482W mutation in the LMNA gene. J Clin Endocrinol Metab. 2001;86(5):2289‐2295. [DOI] [PubMed] [Google Scholar]
- 30. Vantyghem MC, Faivre-Defrance F, Marcelli-Tourvieille S, et al. Familial partial lipodystrophy due to the LMNA R482W mutation with multinodular goitre, extrapyramidal syndrome and primary hyperaldosteronism. Clin Endocrinol. 2007;67(2):247‐249. [DOI] [PubMed] [Google Scholar]
- 31. Lanktree M, Cao H, Rabkin SW, Hanna A, Hegele RA. Novel LMNA mutations seen in patients with familial partial lipodystrophy subtype 2 (FPLD2; MIM 151660). Clin Genet. 2007;71(2):183‐186. [DOI] [PubMed] [Google Scholar]
- 32. van Tintelen JP, Hofstra RM, Katerberg H, et al. High yield of LMNA mutations in patients with dilated cardiomyopathy and/or conduction disease referred to cardiogenetics outpatient clinics. Am Heart J. 2007;154(6):1130‐1139. [DOI] [PubMed] [Google Scholar]
- 33. Hegele RA, Al-Attar SA, Rutt BK. Obstructive sleep apnea in 2 women with familial partial lipodystrophy due to a heterozygous LMNA R482Q mutation. CMAJ. 2007;177(7):743‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Araújo-Vilar D, Loidi L, Domínguez F, Cabezas-Cerrato J. Phenotypic gender differences in subjects with familial partial lipodystrophy (Dunnigan variety) due to a nuclear lamin A/C R482W mutation. Horm Metab Res. 2003;35(1):29‐35. [DOI] [PubMed] [Google Scholar]
- 35. Vantyghem MC, Pigny P, Maurage CA, et al. Patients with familial partial lipodystrophy of the dunnigan type due to a LMNA R482W mutation show muscular and cardiac abnormalities. J Clin Endocrinol Metab. 2004;89(11):5337‐5346. [DOI] [PubMed] [Google Scholar]
- 36. Savage DB, Soos MA, Powlson A, et al. Familial partial lipodystrophy associated with compound heterozygosity for novel mutations in the LMNA gene. Diabetologia. 2004;47(4):753‐756. [DOI] [PubMed] [Google Scholar]
- 37. Lüdtke A, Genschel J, Brabant G, et al. Hepatic steatosis in dunnigan-type familial partial lipodystrophy. Am J Gastroenterol. 2005;100(10):2218‐2224. https://journals.lww.com/ajg/Fulltext/2005/10000/Hepatic_Steatosis_in_Dunnigan_Type_Familial.14.aspx. [DOI] [PubMed] [Google Scholar]
- 38. van der Kooi AJ, Bonne G, Eymard B, et al. Lamin A/C mutations with lipodystrophy, cardiac abnormalities, and muscular dystrophy. Neurology. 2002;59(4):620‐623. [DOI] [PubMed] [Google Scholar]
- 39. Al-Attar SA, Pollex RL, Robinson JF, et al. Semi-automated segmentation and quantification of adipose tissue in calf and thigh by MRI: a preliminary study in patients with monogenic metabolic syndrome. BMC Med Imaging. 2006;6(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muschke P, Kölsch U, Jakubiczka S, Wieland I, Brune T, Wieacker P. The heterozygous LMNA mutation p.R471G causes a variable phenotype with features of two types of familial partial lipodystrophy. Am J Med Genet A. 2007;143A(23):2810‐2814. [DOI] [PubMed] [Google Scholar]
- 41. Decaudain A, Vantyghem M-C, Guerci B, et al. New metabolic phenotypes in laminopathies: LMNA mutations in patients with severe metabolic syndrome. J Clin Endocrinol Metab. 2007;92(12):4835‐4844. [DOI] [PubMed] [Google Scholar]
- 42. Park JY, Javor ED, Cochran EK, DePaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with Dunnigan-type familial partial lipodystrophy. Metab Clin Exp. 2007;56(4):508‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Araújo-Vilar D, Lado-Abeal J, Palos-Paz F, et al. A novel phenotypic expression associated with a new mutation in LMNA gene, characterized by partial lipodystrophy, insulin resistance, aortic stenosis and hypertrophic cardiomyopathy. Clin Endocrinol (Oxf). 2008;69(1):61‐68. [DOI] [PubMed] [Google Scholar]
- 44. Owen KR, Donohoe M, Ellard S, Hattersley AT. Response to treatment with rosiglitazone in familial partial lipodystrophy due to a mutation in the LMNA gene. Diabet Med. 2003;20(10):823‐827. [DOI] [PubMed] [Google Scholar]
- 45. Vantyghem MC, Vincent-Desplanques D, Defrance-Faivre F, et al. Fertility and obstetrical complications in women with LMNA-related familial partial lipodystrophy. J Clin Endocrinol Metab. 2008;93(6):2223‐2229. [DOI] [PubMed] [Google Scholar]
- 46. Gambineri A, Semple RK, Forlani G, et al. Monogenic polycystic ovary syndrome due to a mutation in the lamin A/C gene is sensitive to thiazolidinediones but not to metformin. Eur J Endocrinol. 2008;159(3):347‐353. [DOI] [PubMed] [Google Scholar]
- 47. Joy TR, Hegele RA. Prevalence of reproductive abnormalities among women with familial partial lipodystrophy. Endocr Pract. 2008;14(9):1126‐1132. [DOI] [PubMed] [Google Scholar]
- 48. Imachi H, Murao K, Ohtsuka S, et al. A case of Dunnigan-type familial partial lipodystrophy (FPLD) due to lamin A/C (LMNA) mutations complicated by end-stage renal disease. Endocrine. 2009;35(1):18‐21. [DOI] [PubMed] [Google Scholar]
- 49. Keller J, Subramanyam L, Simha V, Gustofson R, Minjarez D, Garg A. Lipodystrophy: an unusual diagnosis in a case of oligomenorrhea and hirsutism. Obstet Gynecol. 2009;114(2):427‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Klupa T, Szopa M, Skupien J, et al. LMNA Gene mutation search in Polish patients: new features of the heterozygous Arg482Gln mutation phenotype. Endocrine. 2009;36(3):518‐523. [DOI] [PubMed] [Google Scholar]
- 51. Subramanyam L, Simha V, Garg A. Overlapping syndrome with familial partial lipodystrophy, Dunnigan variety and cardiomyopathy due to amino-terminal heterozygous missense lamin A/C mutations. Clin Genet. 2010;78(1):66‐73. 10.1111/j.1399-0004.2009.01350.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mory PB, Crispim F, Freire MBS, et al. Phenotypic diversity in patients with lipodystrophy associated with LMNA mutations. Eur J Endocrinol. 2012;167(3):423‐431. [DOI] [PubMed] [Google Scholar]
- 53. Valerio CM, Zajdenverg L, de Oliveira JEP, Mory PB, Moyses R, Godoy-Matos AF. Body composition study by dual-energy x-ray absorptiometry in familial partial lipodystrophy: finding new tools for an objective evaluation. Diabetol Metab Syndr. 2012;4(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Luedtke A, Boschmann M, Colpe C, et al. Thiazolidinedione response in familial lipodystrophy patients with LMNA mutations: a case series. Horm Metab Res. 2012;44(04):306‐311. [DOI] [PubMed] [Google Scholar]
- 55. Nabrdalik K, Strózik A, Minkina-Pędras M, et al. Dunnigan-type familial partial lipodystrophy associated with the heterozygous R482W mutation in LMNA gene - case study of three women from one family. Endokrynol Pol. 2013;64(4):306‐311. [DOI] [PubMed] [Google Scholar]
- 56. Thong KM, Xu Y, Cook J, et al. Cosegregation of focal segmental glomerulosclerosis in a family with familial partial lipodystrophy due to a mutation in LMNA. Nephron Clin Pract. 2013;124(1–2):31‐37. [DOI] [PubMed] [Google Scholar]
- 57. Demir T, Onay H, Savage DB, et al. Familial partial lipodystrophy linked to a novel peroxisome proliferator activator receptor -γ (PPARG) mutation, H449L: a comparison of people with this mutation and those with classic codon 482 lamin A/C (LMNA) mutations. Diabet Med. 2016;33(10):1445‐1450. [DOI] [PubMed] [Google Scholar]
- 58. Kutbay NO, Yurekli BS, Onay H, et al. A case of familial partial lipodystrophy caused by a novel lamin A/C (LMNA) mutation in exon 1 (D47N). Eur J Intern Med. 2016;29:37‐39. [DOI] [PubMed] [Google Scholar]
- 59. Krawiec P, Mełges B, Pac-Kożuchowska E, Mroczkowska-Juchkiewicz A, Czerska K. Fitting the pieces of the puzzle together: a case report of the Dunnigan-type of familial partial lipodystrophy in the adolescent girl. BMC Pediatr. 2016;16:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vouillarmet J, Laville M. A case of familial partial lipodystrophy: from clinical phenotype to genetics. Can J Diabetes. 2016;40(5):376‐378. [DOI] [PubMed] [Google Scholar]
- 61. Chan D, McIntyre AD, Hegele RA, Don-Wauchope AC. Familial partial lipodystrophy presenting as metabolic syndrome. J Clin Lipidol. 2016;10(6):1488‐1491. [DOI] [PubMed] [Google Scholar]
- 62. Rankin J, Auer-Grumbach M, Bagg W, et al. Extreme phenotypic diversity and nonpenetrance in families with the LMNA gene mutation R644C. Am J Med Genet A. 2008;146A(12):1530‐1542. [DOI] [PubMed] [Google Scholar]
- 63. Araújo-Vilar D, Fernández-Pombo A, Victoria B, et al. Variable expressivity and allelic heterogeneity in type 2 familial partial lipodystrophy: the p.(Thr528Met) LMNA variant. J Clin Med. 2021;10(7):1497.https://www.mdpi.com/2077-0383/10/7/1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Saha B, Lessel D, Hisama FM, et al. A novel LMNA mutation causes altered nuclear morphology and symptoms of familial partial lipodystrophy (Dunnigan variety) with progeroid features. Mol Syndromol. 2010;1(3):127‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Soyaltin UE, Simsir IY, Akinci B, et al. Homozygous LMNA p.R582H pathogenic variant reveals increasing effect on the severity of fat loss in lipodystrophy. Clin Diabetes Endocrinol. 2020;6(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Grundfest-Broniatowski S, Yan J, Kroh M, Kilim H, Stephenson A. Successful treatment of an unusual case of FPLD2: the role of roux-en-Y gastric bypass—case report and literature review. J Gastrointest Surg. 2017;21(4):739‐743. [DOI] [PubMed] [Google Scholar]
- 67. Fountas A, Giotaki Z, Dounousi E, et al. Familial partial lipodystrophy and proteinuric renal disease due to a missense c.1045C>T LMNA mutation. Endocrinol Diabetes Metab Case Rep. 2017;2017:17-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vu NP, Tran HT, Vu NB, et al . Severe loss of adipose tissue in a Vietnamese lipodystrophy patient caused by LMNA p.G465D mutation: a first clinical characterization and two-year follow-up. J Pediatr Endocrinol Metab. 2022;35(9):1206‐1210. [DOI] [PubMed] [Google Scholar]
- 69. Drac H, Madej-Pilarczyk A, Gospodarczyk-Szot K, Gaweł M, Kwieciński H, Hausmanowa-Petrusewicz I. Familial partial lipodystrophy associated with the heterozygous LMNA mutation 1445G>A (Arg482Gln) in a Polish family. Neurol Neurochir Pol. 2010;44(3):291‐296. [DOI] [PubMed] [Google Scholar]
- 70. Belo SP, Magalhães ÂC, Freitas P, Carvalho DM. Familial partial lipodystrophy, Dunnigan variety - challenges for patient care during pregnancy: a case report. BMC Res Notes. 2015;8(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Collet-Gaudillat C, Billon-Bancel A, Beressi JP. Long-term improvement of metabolic control with pioglitazone in a woman with diabetes mellitus related to Dunnigan syndrome: a case report. Diabetes Metab. 2009;35(2):151‐154. [DOI] [PubMed] [Google Scholar]
- 72. Laudes M, Oberhauser F, Walgenbach K, et al. Comparison of phenotypes in male and female individuals of a new family with Dunnigan type of familial partial lipodystrophy due to a lamin A/C R482W mutation. Horm Metab Res. 2009;41(5):414‐417. [DOI] [PubMed] [Google Scholar]
- 73. Chirico V, Ferraù V, Loddo I, et al. LMNA Gene mutation as a model of cardiometabolic dysfunction: from genetic analysis to treatment response. Diabetes Metab. 2014;40(3):224‐228. [DOI] [PubMed] [Google Scholar]
- 74. Panikkath R, Panikkath D, Sanchez-Iglesias S, Araujo-Vilar D, Lado-Abeal J. An uncommon association of familial partial lipodystrophy, dilated cardiomyopathy, and conduction system disease. J Investig Med High Impact Case Rep. 2016;4(3):2324709616658495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lewandowski KC, Lewiński A, Dąbrowska K, Jakubowski L, Gach A. Familial partial lipodystrophy as differential diagnosis of polycystic ovary syndrome. Endokrynol Pol. 2015;66(6):550‐554. [DOI] [PubMed] [Google Scholar]
- 76. Turkyilmaz A, Geçkinli BB, Alavanda C, Ates EA, Arman A. Novel clinical features and pleiotropic effect in three unrelated patients with LMNA variant. Clin Dysmorphol. 2021;30(1):10‐16. [DOI] [PubMed] [Google Scholar]
- 77. Mory PB, Crispim F, Kasamatsu T, Gabbay MA, Dib SA, Moisés RS. Atypical generalized lipoatrophy and severe insulin resistance due to a heterozygous LMNA p.T10I mutation. Arq Bras Endocrinol Metabol. 2008;52(8):1252‐1256. [DOI] [PubMed] [Google Scholar]
- 78. Toni L, Dušátková P, Novotná D, Zemková D, Průhová Š, Lebl J. Short stature in a boy with atypical progeria syndrome due to LMNA c.433G>A [p.(Glu145Lys)]: apparent growth hormone deficiency but poor response to growth hormone therapy. J Pediatr Endocrinol Metab. 2019;32(7):775‐779. [DOI] [PubMed] [Google Scholar]
- 79. Carboni N, Porcu M, Mura M, et al. Evolution of the phenotype in a family with an LMNA gene mutation presenting with isolated cardiac involvement. Muscle Nerve. 2010;41(1):85‐91. [DOI] [PubMed] [Google Scholar]
- 80. Patni N, Xing C, Agarwal AK, Garg A. Juvenile-onset generalized lipodystrophy due to a novel heterozygous missense LMNA mutation affecting lamin C. Am J Med Genet A. 2017;173(9):2517‐2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Simha V, Agarwal AK, Oral EA, Fryns JP, Garg A. Genetic and phenotypic heterogeneity in patients with mandibuloacral dysplasia-associated lipodystrophy. J Clin Endocrinol Metab. 2003;88(6):2821‐2824. [DOI] [PubMed] [Google Scholar]
- 82. Lombardi F, Gullotta F, Columbaro M, et al. Compound heterozygosity for mutations in LMNA in a patient with a myopathic and lipodystrophic mandibuloacral dysplasia type A phenotype. J Clin Endocrinol Metab. 2007;92(11):4467‐4471. [DOI] [PubMed] [Google Scholar]
- 83. Garavelli L, D’Apice MR, Rivieri F, et al. Mandibuloacral dysplasia type A in childhood. Am J Med Genet A. 2009;149a(10):2258‐2264. [DOI] [PubMed] [Google Scholar]
- 84. Kosho T, Takahashi J, Momose T, et al. Mandibuloacral dysplasia and a novel LMNA mutation in a woman with severe progressive skeletal changes. Am J Med Genet A. 2007;143a(21):2598‐2603. [DOI] [PubMed] [Google Scholar]
- 85. Luo DQ, Wang XZ, Meng Y, et al. Mandibuloacral dysplasia type A-associated progeria caused by homozygous LMNA mutation in a family from southern China. BMC Pediatr. 2014;14(1):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shen JJ, Brown CA, Lupski JR, Potocki L. Mandibuloacral dysplasia caused by homozygosity for the R527H mutation in lamin A/C. J Med Genet. 2003;40(11):854‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jéru I, Nabil A, El-Makkawy G, Lascols O, Vigouroux C, Abdalla E. Two decades after mandibuloacral dysplasia discovery: additional cases and comprehensive view of disease characteristics. Genes (Basel). 2021;12(10):1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Agarwal AK, Kazachkova I, Ten S, Garg A. Severe mandibuloacral dysplasia-associated lipodystrophy and progeria in a young girl with a novel homozygous Arg527Cys LMNA mutation. J Clin Endocrinol Metab. 2008;93(12):4617‐4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zirn B, Kress W, Grimm T, et al. Association of homozygous LMNA mutation R471C with new phenotype: mandibuloacral dysplasia, progeria, and rigid spine muscular dystrophy. Am J Med Genet A. 2008;146a(8):1049‐1054. [DOI] [PubMed] [Google Scholar]
- 90. Jacob KN, Baptista F, dos Santos HG, Oshima J, Agarwal AK, Garg A. Phenotypic heterogeneity in body fat distribution in patients with atypical Werner's Syndrome due to heterozygous Arg133Leu lamin A/C mutation. J Clin Endocrinol Metab. 2005;90(12):6699‐6706. [DOI] [PubMed] [Google Scholar]
- 91. Van Esch H, Agarwal AK, Debeer P, Fryns JP, Garg A. A homozygous mutation in the lamin A/C gene associated with a novel syndrome of arthropathy, tendinous calcinosis, and progeroid features. J Clin Endocrinol Metab. 2006;91(2):517‐521. [DOI] [PubMed] [Google Scholar]
- 92. de Andrade NXS, Adiyaman SC, Yuksel BD, et al. UNUSUAL PRESENTATIONS OF LMNA-ASSOCIATED LIPODYSTROPHY WITH COMPLEX PHENOTYPES AND GENERALIZED FAT LOSS: WHEN THE GENETIC DIAGNOSIS UNCOVERS NOVEL FEATURES. AACE Clin Case Rep. 2020;6(2):e79‐e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Schultz B, Miller DD, Maguiness S. Diffuse, mottled hyperpigmentation and mutations in LMNA gene in a 5-year-old boy, his mother, and his grandmother: atypical progeroid syndrome. Pediatr Dermatol. 2019;36(6):913‐917. [DOI] [PubMed] [Google Scholar]
- 94. Al-Haggar M, Madej-Pilarczyk A, Kozlowski L, et al. A novel homozygous p.Arg527Leu LMNA mutation in two unrelated Egyptian families causes overlapping mandibuloacral dysplasia and progeria syndrome. Eur J Hum Genet. 2012;20(11):1134‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Guo H, Luo N, Hao F, Bai Y. P.Pro4Arg mutation in LMNA gene: a new atypical progeria phenotype without metabolism abnormalities. Gene. 2014;546(1):35‐39. [DOI] [PubMed] [Google Scholar]
- 96. Guo X, Ling C, Liu Y, Zhang X, Zhang S. A case of novel lamin A/C mutation manifesting as atypical progeroid syndrome and cardiomyopathy. Can J Cardiol. 2016;32(9):1166.e1129‐1131. [DOI] [PubMed] [Google Scholar]
- 97. He G, Yan Z, Sun L, et al. Diabetes mellitus coexisted with progeria: a case report of atypical werner syndrome with novel LMNA mutations and literature review. Endocr J. 2019;66(11):961‐969. [DOI] [PubMed] [Google Scholar]
- 98. Yukina M, Nuralieva N, Sorkina E, et al. Atypical progeroid syndrome (p.E262K LMNA mutation): a rare cause of short stature and osteoporosis. Endocrinol Diabetes Metab Case Rep. 2021;2021:20-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mahdi L, Kahn A, Dhamija R, Vargas HE. Hepatic steatosis resulting from LMNA-associated familial lipodystrophy. ACG Case Rep J. 2020;7(4):e00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hussain I, Jin RR, Baum HBA, et al. Multisystem progeroid syndrome with lipodystrophy, cardiomyopathy, and nephropathy due to an LMNA p.R349W variant. J Endocr Soc. 2020;4(10):bvaa104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Magno S, Ceccarini G, Pelosini C, et al. Atypical progeroid syndrome and partial lipodystrophy due to LMNA gene p.R349W mutation. J Endocr Soc. 2020;4(10):bvaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Xiao C, Liu J, Yang C, et al. The clinical characteristics and potential molecular mechanism of LMNA mutation-related lipodystrophy. Adv Biol (Weinh). 2023;7(9):e2200301. [DOI] [PubMed] [Google Scholar]
- 103. Jang S, Ahn YH, Ko JM, Ko JS, Lim S, Kang HG. Case report: focal segmental glomerulosclerosis in a pediatric atypical progeroid syndrome. Front Pediatr. 2022;10:1032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sasako T, Kadowaki H, Fujiwara T, et al. Severe aortic stenosis during leptin replacement therapy in a patient with generalized lipodystrophy-associated progeroid syndrome due to an LMNA variant: A case report. J Diabetes Investig. 2022;13(9):1636‐1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Doanh LH, Phuong HT, Phuong NTT, Thu LTH, Thuong NV. Mandibuloacral dysplasia in a young Vietnamese girl caused by homozygous missense variant c.1579C>T in the LMNA gene with progeria and severe skin lesions. JAAD Case Rep. 2021;16:5‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cecchetti C, D’Apice MR, Morini E, et al. Case report: an atypical form of familial partial lipodystrophy type 2 due to mutation in the rod domain of lamin A/C. Front Endocrinol (Lausanne). 2021;12:675096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fukaishi T, Minami I, Masuda S, et al. A case of generalized lipodystrophy-associated progeroid syndrome treated by leptin replacement with short and long-term monitoring of the metabolic and endocrine profiles. Endocr J. 2020;67(2):211‐218. [DOI] [PubMed] [Google Scholar]
- 108. Akamnonu C, Ueda M, Shah A. Rare diagnosis of familial partial lipodystrophy in a patient with life-threatening pancreatitis due to hypertriglyceridemia. AACE Clin Case Rep. 2022;8(1):11‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yanhua X, Suxian Z. Cerebral haemorrhage in a young patient with atypical werner syndrome due to mutations in LMNA. Front Endocrinol (Lausanne). 2018;9:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Eldin AJ, Akinci B, da Rocha AM, et al. Cardiac phenotype in familial partial lipodystrophy. Clin Endocrinol (Oxf). 2021;94(6):1043‐1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Saydam O, Ozgen Saydam B, Adiyaman SC, et al. Risk factors for diabetic foot ulcers in metreleptin naïve patients with lipodystrophy. Clin Diabetes Endocrinol. 2021;7(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Akinci B, Subauste A, Ajluni N, et al. Metreleptin therapy for nonalcoholic steatohepatitis: open-label therapy interventions in two different clinical settings. Med. 2021;2(7):814‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Oral EA, Garg A, Tami J, et al. Assessment of efficacy and safety of volanesorsen for treatment of metabolic complications in patients with familial partial lipodystrophy: results of the BROADEN study: volanesorsen in FPLD; the BROADEN study. J Clin Lipidol. 2022;16(6):833‐849. [DOI] [PubMed] [Google Scholar]
- 114. Meral R, Ryan BJ, Malandrino N, et al. “Fat shadows” from DXA for the qualitative assessment of lipodystrophy: when a picture is worth a thousand numbers. Diabetes Care. 2018;41(10):2255‐2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Brown RJ, Oral EA, Cochran E, et al. Long-term effectiveness and safety of metreleptin in the treatment of patients with generalized lipodystrophy. Endocrine. 2018;60(3):479‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Akinci B, Unlu SM, Celik A, et al. Renal complications of lipodystrophy: a closer look at the natural history of kidney disease. Clin Endocrinol (Oxf). 2018;89(1):65‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ajluni N, Dar M, Xu J, Neidert AH, Oral EA. Efficacy and safety of metreleptin in patients with partial lipodystrophy: lessons from an expanded access program. J Diabetes Metab. 2016;7(3):659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Foss-Freitas MC, Akinci B, Neidert A, et al. Selective targeting of angiopoietin-like 3 (ANGPTL3) with vupanorsen for the treatment of patients with familial partial lipodystrophy (FPLD): results of a proof-of-concept study. Lipids Health Dis. 2021;20(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Morel CF, Thomas MA, Cao H, et al. A LMNA splicing mutation in two sisters with severe Dunnigan-type familial partial lipodystrophy type 2. J Clin Endocrinol Metab. 2006;91(7):2689‐2695. [DOI] [PubMed] [Google Scholar]
- 120. Le Dour C, Schneebeli S, Bakiri F, et al. A homozygous mutation of prelamin-A preventing its farnesylation and maturation leads to a severe lipodystrophic phenotype: new insights into the pathogenicity of nonfarnesylated prelamin-A. J Clin Endocrinol Metab. 2011;96(5):E856‐E862. [DOI] [PubMed] [Google Scholar]
- 121. Lamis A, Siddiqui SW, Ashok T, Patni N, Fatima M, Aneef AN. Hutchinson-Gilford progeria syndrome: a literature review. Cureus. 2022;14(8):e28629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Eriksson M, Brown WT, Gordon LB, et al. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature. 2003;423(6937):293‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. ElSayed NA, Aleppo G, Aroda VR, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes—2023. Diabetes Care. 2022;46(Supplement_1):S19‐S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2023 update: a report from the American heart association. Circulation. 2023;147(8):e93‐e621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Committee ADAPP . 11. Chronic kidney disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(Supplement_1):S175‐S184. [DOI] [PubMed] [Google Scholar]
- 126. Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4(9):e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Koo E, Foss-Freitas MC, Meral R, et al. The metabolic equivalent BMI in patients with familial partial lipodystrophy (FPLD) compared with those with severe obesity. Obesity (Silver Spring). 2021;29(2):274‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Besci O, Foss de Freitas MC, Guidorizzi GR, et al. Data from: Deciphering the Clinical Presentations in LMNA-Related Lipodystrophy: Report of 115 cases and a Systematic Review. Figshare. 2023. https://figshare.com/articles/dataset/supplementaary_file/23682441.
- 129. Hegele R. LMNA Mutation position predicts organ system involvement in laminopathies. Clin Genet. 2005;68(1):31‐34. [DOI] [PubMed] [Google Scholar]
- 130. Treiber G, Flaus Furmaniuk A, Guilleux A, et al. A recurrent familial partial lipodystrophy due to a monoallelic or biallelic LMNA founder variant highlights the multifaceted cardiac manifestations of metabolic laminopathies. Eur J Endocrinol. 2021;185(4):453‐462. [DOI] [PubMed] [Google Scholar]
- 131. Vasandani C, Li X, Sekizkardes H, Brown RJ, Garg A. Phenotypic differences among familial partial lipodystrophy due to LMNA or PPARG variants. J Endocr Soc. 2022;6(12):bvac155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Haque WA, Oral EA, Dietz K, Bowcock AM, Agarwal AK, Garg A. Risk factors for diabetes in familial partial lipodystrophy, Dunnigan variety. Diabetes Care. 2003;26(5):1350‐1355. [DOI] [PubMed] [Google Scholar]
- 133. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;139(25):e1082‐e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Aberra T, Peterson ED, Pagidipati NJ, et al. The association between triglycerides and incident cardiovascular disease: what is “optimal”? J Clin Lipidol. 2020;14(4):438‐447.e433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Pedersen SB, Langsted A, Nordestgaard BG. Nonfasting mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis. JAMA Intern Med. 2016;176(12):1834‐1842. [DOI] [PubMed] [Google Scholar]
- 136. Treiber G, Guilleux A, Huynh K, et al. Lipoatrophic diabetes in familial partial lipodystrophy type 2: from insulin resistance to diabetes. Diabetes Metab. 2023;49(2):101409. [DOI] [PubMed] [Google Scholar]
- 137. Al Quran TM, Bataineh ZA, Al-Mistarehi AH, et al. Prevalence and pattern of dyslipidemia and its associated factors among patients with type 2 diabetes mellitus in Jordan: a cross-sectional study. Int J Gen Med. 2022;15:7669‐7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Haque WA, Vuitch F, Garg A. Post-mortem findings in familial partial lipodystrophy, Dunnigan variety. Diabet Med. 2002;19(12):1022‐1025. [DOI] [PubMed] [Google Scholar]
- 139. Garg A. Gender differences in the prevalence of metabolic complications in familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab. 2000;85(5):1776‐1782. [DOI] [PubMed] [Google Scholar]
- 140. Lazarte J, Wang J, McIntyre AD, Hegele RA. Prevalence of severe hypertriglyceridemia and pancreatitis in familial partial lipodystrophy type 2. J Clin Lipidol. 2021;15(5):653‐657. [DOI] [PubMed] [Google Scholar]
- 141. Elenbaas JS, Bragazzi Cunha J, Azuero-Dajud R, et al. Lamin A/C maintains exocrine pancreas homeostasis by regulating stability of RB and activity of E2F. Gastroenterology. 2018;154(6):1625‐1629.e1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lin EW, Brady GF, Kwan R, Nesvizhskii AI, Omary MB. Genotype-phenotype analysis of LMNA-related diseases predicts phenotype-selective alterations in lamin phosphorylation. FASEB J. 2020;34(7):9051‐9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue—link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11(2):90‐100. [DOI] [PubMed] [Google Scholar]
- 144. Malandrino N, Reynolds JC, Brychta RJ, et al. Visceral fat does not contribute to metabolic disease in lipodystrophy. Obes Sci Pract. 2019;5(1):75‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Colombo C, Cutson JJ, Yamauchi T, et al. Transplantation of adipose tissue lacking leptin is unable to reverse the metabolic abnormalities associated with lipoatrophy. Diabetes. 2002;51(9):2727‐2733. [DOI] [PubMed] [Google Scholar]
- 147. Hussain I, Patni N, Ueda M, et al. A novel generalized lipodystrophy-associated progeroid syndrome due to recurrent heterozygous LMNA p.T10I mutation. J Clin Endocrinol Metab. 2018;103(3):1005‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Sekizkardes H, Cochran E, Malandrino N, Garg A, Brown RJ. Efficacy of metreleptin treatment in familial partial lipodystrophy due to PPARG vs LMNA pathogenic variants. J Clin Endocrinol Metab. 2019;104(8):3068‐3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87(5):2395. [DOI] [PubMed] [Google Scholar]
- 150. Garg A. Adipose tissue dysfunction in obesity and lipodystrophy. Clin Cornerstone. 2006;8:S7‐S13. [DOI] [PubMed] [Google Scholar]
- 151. Treiber G, Gonthier MP, Guilleux A, et al. Familial partial lipodystrophy type 2 and obesity, two adipose tissue pathologies with different inflammatory profiles. Diabetol Metab Syndr. 2023;15(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Miehle K, Ebert T, Kralisch S, et al. Serum concentrations of fibroblast growth factor 21 are elevated in patients with congenital or acquired lipodystrophy. Cytokine. 2016;83:239‐244. [DOI] [PubMed] [Google Scholar]
- 153. Mann JP, Savage DB. What lipodystrophies teach us about the metabolic syndrome. J Clin Invest. 2019;129(10):4009‐4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Corsa CAS, Walsh CM, Bagchi DP, et al. Adipocyte-specific deletion of lamin A/C largely models human familial partial lipodystrophy type 2. Diabetes. 2021;70(9):1970‐1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in the data repositories listed in “References.”