Abstract
Over 124 methicillin-susceptible Staphylococcus aureus 0/74 fluoroquinolone-susceptible versus 5/50 fluoroquinolone-resistant isolates were hypermutable. Hypermutable isolates combined mutations in gyrA, parC, and/or parE genes. One strain had a large deletion of the mutator mutS and mutL genes. No relevant mutation in mutS and mutL genes was found in the other isolates.
Fluoroquinolones (FQ) are widely used in staphylococcal infections. However, emergence of resistance to these antimicrobials has limited their use (1, 10). Mechanisms of FQ resistance in Staphylococcus aureus have been extensively studied and are mostly related to mutations in the drug target, DNA topoisomerase IV and DNA gyrase (10, 20). Resistance mutations occur within the quinolone resistance-determining regions (QRDR) located in the A and C subunits of DNA gyrase and topoisomerase encoded by the gyrA and parC genes, respectively. In S. aureus, DNA topoisomerase IV is the primary target of FQ, and first-step resistance to most of these antimicrobials has been associated with mutations in parC (10, 20). Single mutations in parC appear to be sufficient to increase MICs of ciprofloxacin up to levels that exceed the laboratory breakpoints for susceptibility (10). Additional mutations in gyrA and, less commonly, mutations in parE and gyrB genes, encoding the B subunits of the DNA topoisomerase IV and DNA gyrase, respectively, generate an increased level of ciprofloxacin MIC and full resistance to the drug (10). Moreover, mutations resulting in overexpression of efflux pumps may contribute to fluoroquinolone resistance (10).
In this context where mutation is the major mechanism reported to confer FQ resistance in S. aureus, mutation frequency of clinical strains is important to consider. Recently, an increased mutation frequency has been reported to the development of high-level resistance to FQ in Escherichia coli (12). A small proportion of strains displaying high mutation frequencies (mutator strains) has been reported in populations of commensal or pathogenic bacteria (8, 14, 17). These variants display mutation frequencies increased up to 10,000 times compared to their wild-type counterparts (13) and could contribute to the emergence of antibiotic resistance (5, 9). Hypermutability has been related in most cases to defects in the methyl-directed mismatch repair system (MMR), particularly in the mutS or mutL genes (11). This system repairs mismatches occurring during DNA replication, and an alteration of any of its major components, MutS and MutL, leads to the rapid accumulation of mutations in the newly synthesized strand.
We investigated the possible relationship between hypermutability and quinolone resistance in S. aureus clinical isolates.
Bacterial isolates and antibiotic susceptibility testing.
One-hundred twenty-four methicillin-susceptible S. aureus isolates, including 74 FQ-susceptible and 50 FQ-resistant isolates, were studied. These strains were obtained from various clinical samples including respiratory secretions, pus from soft tissue, and bone infections between 2000 and 2003 in 124 different patients in various wards. S. aureus UCN22 was isolated in a patient with chronic prostatitis who was treated with ofloxacin. S. aureus UCN46, UCN47, UCN48, and UCN49 were isolated from wound infections. No information on previous antibiotic treatments was available for the corresponding patients. Strains isolated in patients suffering from cystic fibrosis were excluded from the study since it has been shown that hypermutable S. aureus are frequently isolated in this particular pathology. MRSA were also excluded since isolates are often epidemiologically closely related (17). Susceptibility to ciprofloxacin and ofloxacin was screened for by the disk-diffusion method and MICs of these antimicrobials were determined by the agar dilution method, as recommended previously (7). S. aureus ATCC 29213 was used as a control strain.
Determination of mutation frequencies.
For each strain, approximately 108 to 109 cells were spread on Trypticase-soy agar plates (Bio-Rad, Marne-La-Coquette, France) supplemented with rifampin (100 μg/ml) or streptomycin (50 μg/ml) (Sigma-Aldrich, Saint Louis, Mo.). Plates were incubated 48 h and 72 h at 37°C for rifampin and streptomycin, respectively. The number of colonies was counted and mutation frequencies were calculated. The experiment was repeated in triplicate and results are indicated as means. Except for 5 FQ-resistant isolates, all isolates yielded mutants on rifampin and streptomycin agar plates with frequencies around 10−7 (between 5 × 10−8 and 1.7 × 10−7 for rifampin and between 10−8 and 10−7 for streptomycin) which correspond to previous reports for S. aureus (2). Five FQ-resistant isolates displayed mutation frequencies increased by 4 to 200 times for rifampin and by 20 to 200 times for streptomycin as compared to the mean for the other isolates (frequencies ranging from 3.9 × 10−7 to 1 × 10−5 for rifampin and 3 × 10−6 to 3 × 10−5 for streptomycin). A statistically significant difference between the FQ-resistant and -susceptible groups was found by the Fisher's exact test (P = 0.009). pulsed-field gel electrophoresis analysis showed that hypermutable isolates were unrelated (data not shown). These findings showed a significant link between hypermutability and FQ resistance. However, the number of mutator strains among the FQ-resistant isolates remained low.
Fluoroquinolone resistance.
As shown in Table 1, the MICs of ciprofloxacin and ofloxacin for four hypermutable strains ranged from 64 μg/ml to 128 μg/ml and 16 μg/ml to 32 μg/ml, respectively, except for the S. aureus UCN46 isolate that was moderately resistant with MICs of ciprofloxacin and ofloxacin equal to 16 μg/ml and 2 μg/ml, respectively. PCRs were performed as described in (21) with the primers listed in Table 2 to amplify the QRDR or conserved regions of the gyrA, gyrB, parC, and parE genes of the 5 hypermutable strains. Sequencing of the fragments obtained showed that, consistent with their high level of fluoroquinolone resistance, four strains, S. aureus UCN22, UCN47, UCN48, and UCN49, combined the classical mutations of Serine 84 and Serine 80 in the deduced amino acid sequences of GyrA and ParC QRDR, respectively. UCN46 had only a S80Y substitution in ParC and no mutation in the QRDR of GyrA, which is consistent with the moderate level of resistance displayed by the strain.
TABLE 1.
MICs of fluoroquinolones for hypermutable strains and mutations detected in the quinolone targets
| S. aureus strain | MIC (μg/ml)
|
Amino acid (codon) substitution in:
|
||||
|---|---|---|---|---|---|---|
| Ofloxacin | Ciprofoxacin | GyrA | GyrB | ParC | ParE | |
| UCN22 | 32 | 128 | S84L (TCA→TTA) | None | S80Y (TCC→TAC) | None |
| UCN46 | 2 | 16 | None | Nonea | S80Y (TCC→TAC) | H474Y (TAC→CAC) |
| UCN47 | 32 | 128 | S84V (TCA→GTA) | None | S80F (TCC→TTC) | A434V (GTA→GCA) |
| UCN48 | 32 | 128 | S84V (TCA→GTA) | None | S80F (TCC→TTC) | None |
| UCN49 | 16 | 64 | S84V (TCA→GTA) | None | S80F (TCC→TTC) | None |
| ATCC 29213 | 0.25 | 0.25 | ||||
Silent mutations in gyrB.
TABLE 2.
Primers used in this study
| Gene | Primer name | Primer sequence (5′ to 3′)a | Positionb | Product size (bp) | Reference |
|---|---|---|---|---|---|
| gyrA | gyrAF | +AATGAACAAGGTATGACACC | 2311 | 223 | 21 |
| gyrAR | −TACGCGCTTCAGTATAACGC | 2533 | |||
| gyrB | gyrBF | +CAGCGTTAGATGTAGCAAGC | 1400 | 249 | 21 |
| gyrBR | −CCAATTCCTGTACCAAATGC | 1650 | |||
| parC | parCF | +ACTTGAAGATGTTTTAGGTGAT | 2402 | 559 | 21 |
| parCR | −TTAGGAAATCTTGATGGCAA | 2961 | |||
| parE | parEF | +CGATTAAAGCACAACAAGCAAG | 1520 | 375 | 21 |
| parER | −CATCAGTCATAATAATTACAC | 1874 | |||
| mutS | SU1 | +AATAATTAATAATTAAAAAGC | −83 | 489 | 17 |
| SL1 | −CAGGTTGATTCATAACAAAAC | +406 | |||
| SU2 | +AACTGTGATGGAGCAAGGTGG | +332 | 585 | 17 | |
| SL2 | −GTGCTTCAATTTGTTCTTTAC | +916 | |||
| SU3 | +ACACCAATGGGAGCACGCCGC | +844 | 567 | 17 | |
| SL3 | −TTCAAAATTTTGCAAGTTGGC | +1410 | |||
| SU4 | +AAGCTTTAATAAAGTGTTTGG | +1347 | 570 | 17 | |
| SL4 | −TCAAATATA GGTAACACTGCC | +1916 | |||
| SU5 | +GTTGCCATAATTAGTATAATG | +1843 | 830 | 17 | |
| SL5 | −TTATTTGCTAATGAGGTTTGG | +2672 | |||
| Sinv | +ACTGTGATGTTTCTACTGGCG | +422 | This study | ||
| mutL | LU1 | +TAATATGACACCAATTGAGGC | −73 | 495 | This study |
| LL1 | −CCTTTTTTCGCTTTTGCAGGC | +422 | |||
| LU2 | +TTGCACGGATAATGCTAATGG | +339 | 514 | This study | |
| LL2 | −ATAACAAATCGGGAACCTACC | +852 | |||
| LU3 | +GATACATTAAAAACTTTATGC | +767 | 512 | This study | |
| LL3 | −GTAGTCTTCATCTTTTTCACG | +1278 | |||
| LU4 | +AACTGATGATGAATCTTCTGG | +1224 | 490 | This study | |
| LL4 | −TACAATATAATCATGACCACC | +1713 | |||
| LU5 | +AAATGAGCTTCAACAAGTAGG | +1653 | 518 | This study | |
| LL5 | −TCTATATGATTTTGCTTCAGA | +2170 |
+, sense primer; −, antisense primer.
Base relative to ATG.
No mutation was found in the deduced amino acids sequence of the conserved region of the gyrB gene, although S. aureus UCN46 displayed silent mutations in this structure. Possibly, these alterations which did not confer any advantage to the strain in terms of resistance could be the consequence of the hypermutable status of the strain. UCN22 and UCN47 displayed an additional mutation in parE. To the best of our knowledge, these two mutations in the parE gene have not been reported previously. Their role in FQ-resistance remains speculative.
As already reported before in S. aureus (21) and E. coli (12), the number of altered structures correlated with the phenotype of the strain, as the highest MICs are reached by the four strains displaying mutations in both FQ targets, DNA gyrase and DNA topoisomerase IV. Hypermutability could thus not only favor the emergence of resistant mutants but also favor the acquisition of high-level resistance to FQ by facilitating the occurrence of combined mutations.
Mutations in mutSL genes.
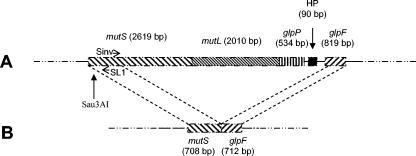
Deduced amino acid sequences of mutSL genes in the 6 S. aureus genomic complete sequences available at the National Institutes of Health website (www.ncbi.nlm.nih.gov) were submitted to multiple alignments. We found that the MutS and MutL sequences were highly conserved, differing by only one substitution at the C-terminal end of MutS or few substitutions in the central part of MutL. On the basis of these alignments, we designed 10 pairs of deoxynucleotide primers that allowed amplification of overlapping fragments in mutS and mutL genes, listed in Table 2. The mutS gene of the S. aureus UCN22 mutator strain could only be partially amplified with a single pair of primers (U1S and L1S), whereas no amplification was obtained with the mutL primers. The mutS and mutL genes were further studied by inverse PCR. A new Sinv primer, pairing to the DNA of S. aureus UCN22 just downstream SL1 primer and a Sau3AI restriction site, was designed (Table 2 and Fig. 1). Total DNA of the strain was submitted to digestion with the restriction enzyme Sau3AI. The fragments obtained were self-ligated and a SL1/Sinv PCR was performed, resulting in a single 1.5-kb PCR product. Sequencing showed that S. aureus UCN22 displayed a large mutS and mutL deletion, as shown in Fig. 1. These results confirmed the role of alterations of the mutS and mutL genes of S. aureus in hypermutability (15).
FIG. 1.
Schematic representation of the mutSL deletion in S. aureus UCN22. A. Representation of the mutSL genes and three downstream open reading frames in the S. aureus chromosome. Positions of the Sau3AI restriction site and of the primers used for the inverse PCR are shown. B. Representation of the mutSL region of S. aureus UCN22 as determined by inverse PCR. A total of 5,060 bp were deleted between the mutS and the glpF genes.
The mutS and mutL genes of four hypermutable isolates were amplified as described previously (17). Differences in the deduced amino acid sequence of the MutL proteins were found in three of the four remaining strains but only in variable regions of the protein and were therefore probably devoid of significance. In S. aureus UCN46, two substitutions (Q430H and L434F) of conserved amino acids located in the very middle of the MutL protein were detected. However, these amino acids are not known to have a particular importance for the functions of the protein (3, 4). Thus, the role of these mutations in hypermutability remains speculative. Similarly to the silent gyrB mutations detected in this very strain, these MutL mutations may only be a consequence of the hypermutable phenotype. Alterations of other mutator genes might be the cause of hypermutability in these four isolates. Other putative mutator genes have already been identified in the Bacillus subtilis chromosome (18), and some have homologues in the S. aureus chromosome.
It is generally considered that mutator strains constitute a real risk factor for the emergence of antibiotic resistance in many bacterial species (6). However, there are conflicting data on the role of hypermutability in acquiring antibiotic resistance in S. aureus (16, 19). So far, in clinical settings, the risk was established only for macrolide resistance by ribosomal mutation in staphylococci isolated from cystic fibrosis patients (17).
We showed that similar observations could be done for other staphylococcal infections. Interestingly, S. aureus UCN22 was isolated in the context of a chronic prostatitis where bacteria may have adaptational requirements and are exposed to prolonged therapy.
Acknowledgments
This work was supported by grants from Conseil Régional de Basse-Normandie and the association “Vaincre la Mucoviscidose.”
REFERENCES
- 1.Acar, J. F., and F. W. Goldstein. 1997. Trends in bacterial resistance to fluoroquinolones. Clin. Infect. Dis. 1(Suppl. 24):S67-S73. [DOI] [PubMed] [Google Scholar]
- 2.Aubry-Damon, H., C. J. Soussy, and P. Courvalin. 1998. Characterization of mutations in the rpoB gene that confer rifampin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 42:2590-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ban, C., M. Junop, and W. Yang. 1999. Transformation of MutL by ATP binding and hydrolysis: a switch in DNA mismatch repair. Cell 97:85-97. [DOI] [PubMed] [Google Scholar]
- 4.Ban, C., and W. Yang. 1998. Crystal structure and ATPase activity of MutL: implications for DNA repair and mutagenesis. Cell 95:541-552. [DOI] [PubMed] [Google Scholar]
- 5.Blazquez, J. 2003. Hypermutation as a factor contributing to the acquisition of antimicrobial resistance. Clin. Infect. Dis. 37:1201-1209. [DOI] [PubMed] [Google Scholar]
- 6.Chopra, I., A. J. O′Neill, and K. Miller. 2003. The role of mutators in the emergence of antibiotic-resistant bacteria. Drug Resist. Update 6:137-145. [DOI] [PubMed] [Google Scholar]
- 7.Comité de l'Antibiogramme de la Société Française de Microbiologie. Updated 2. April 2004. www.sfm.asso.fr.
- 8.Denamur, E., S. Bonacorsi, A. Giraud, P. Duriez, F. Hilali, C. Amorin, E. Bingen, A. Andremont, B. Picard, F. Taddei, and I. Matic. 2002. High frequency of mutator strains among human uropathogenic Escherichia coli isolates. J. Bacteriol. 184:605-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giraud, A., I. Matic, M. Radman, M. Fons, and F. Taddei. 2002. Mutator bacteria as a risk factor in treatment of infectious diseases. Antimicrob. Agents Chemother. 46:863-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hooper, D. C. 2002. Fluoroquinolone resistance among gram-positive cocci. Lancet Infect. Dis. 2:530-538. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh, P. 2001. Molecular mechanisms of DNA mismatch repair. Mutat. Res. 486:71-87. [DOI] [PubMed] [Google Scholar]
- 12.Komp Lindgren, P., A. Karlsson, and D. Hughes. 2003. Mutation rate and evolution of fluoroquinolone resistance in Escherichia coli isolates from patients with urinary tract infections. Antimicrob. Agents Chemother. 47:3222-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller, J. H. 1996. Spontaneous mutators in bacteria: insights into pathways of mutagenesis and repair. Annu. Rev. Microbiol. 50:625-643. [DOI] [PubMed] [Google Scholar]
- 14.Oliver, A., R. Canton, P. Campo, F. Baquero, and J. Blazquez. 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288:1251-1254. [DOI] [PubMed] [Google Scholar]
- 15.O′Neill, A. J., and I. Chopra. 2002. Insertional inactivation of mutS in Staphylococcus aureus reveals potential for elevated mutation frequencies, although the prevalence of mutators in clinical isolates is low. J. Antimicrob. Chemother. 50:161-169. [DOI] [PubMed] [Google Scholar]
- 16.O'Neill, A. J., and I. Chopra. 2003. Lack of evidence for involvement of hypermutability in emergence of vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1484-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prunier, A. L., B. Malbruny, M. Laurans, J. Brouard, J. F. Duhamel, and R. Leclercq. 2003. High rate of macrolide resistance in Staphylococcus aureus strains from patients with cystic fibrosis reveals high proportions of hypermutable strains. J. Infect. Dis. 187:1709-1716. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki, M., Y. Yonemura, and Y. Kurusu. 2000. Genetic analysis of Bacillus subtilis mutator genes. J. Gen. Appl. Microbiol. 46:183-187. [DOI] [PubMed] [Google Scholar]
- 19.Schaaff, F., A. Reipert, and G. Bierbaum. 2002. An elevated mutation frequency favors development of vancomycin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 46:3540-3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitz, F. J., P. G. Higgins, S. Mayer, A. C. Fluit, and A. Dalhoff. 2002. Activity of quinolones against gram-positive cocci: mechanisms of drug action and bacterial resistance. Eur. J. Clin. Microbiol. Infect. Dis. 21:647-659. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz, F. J., B. Hofmann, B. Hansen, S. Scheuring, M. Luckefahr, M. Klootwijk, J. Verhoef, A. Fluit, H. P. Heinz, K. Kohrer, and M. E. Jones. 1998. Relationship between ciprofloxacin, ofloxacin, levofloxacin, sparfloxacin and moxifloxacin (BAY 12-8039) MICs and mutations in grlA, grlB, gyrA and gyrB in 116 unrelated clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 41:481-484. [DOI] [PubMed] [Google Scholar]