Abstract
Weight loss achieved through a combination of healthy eating patterns that encompass the principles of the Mediterranean diet and regular physical activity is the most evidence-based treatment for nonalcoholic fatty liver disease. Although other types of diets have demonstrated efficacy in liver fat reduction, the Mediterranean diet confers additional cardiometabolic benefits. Macronutrient composition, food choices, and timing of eating can be tailored to individual preferences, culture, and financial circumstances; however, recommended healthy eating patterns are characterized by minimally processed or unprocessed foods (vegetables, legumes, nuts and seeds, fruits, whole grains, and unprocessed meats and fish) that are low in sugar, refined carbohydrates, and saturated fat and high in fiber, polyphenols, vitamins, minerals, and healthy fats. Physical activity can independently improve steatosis, prevent fibrosis and cirrhosis, and reduce mortality.
Nonalcoholic fatty liver disease (NAFLD) is a multifactorial condition characterized by the accumulation of fat in the liver. It is estimated to affect up to 32% of the global population and is associated with an increased risk of all-cause mortality. Notably, although people with NAFLD have an increased risk of liver-related complications, their leading causes of death are cardiovascular disease (CVD) and extrahepatic malignancies (1).
Closely linked to obesity, insulin resistance, dyslipidemia, and type 2 diabetes, NAFLD is considered the hepatic manifestation of the metabolic syndrome. Although NAFLD pathogenesis can be viewed simplistically as an imbalance between lipid accumulation and removal in the liver, the mechanisms that drive the development and progression of NAFLD are not fully understood, and there is significant interindividual variation in the dominant drivers of NAFLD pathogenesis (2,3). Genetic risk factors have been identified; however, these interact strongly with environmental factors (e.g., obesity, dietary factors, and physical activity) to greatly amplify the genetic risk (4). NAFLD is a systemic disease that involves cross-talk between multiple organs (i.e., adipose, pancreas, gut, and immune cells), with increasing evidence of a role for gut microbiota in NAFLD pathogenesis (5). In short, NAFLD is a complex phenotype that arises from dynamic interactions among diet, lifestyle, and genetic factors; multiple organs; and the intestinal microbiome (6).
Weight reduction through caloric restriction is the most evidence-based way to improve NAFLD across the disease spectrum (7,8) and therefore is the first-line treatment recommended in multiple clinical practice guidelines (9–12). Comprehensive lifestyle change should involve simultaneous implementation of dietary changes to reduce energy intake, lifestyle and behavioral training, and an increase in physical activity. Changes should also include the avoidance of smoking, which has been associated with NAFLD, fibrosis progression, and hepatocellular carcinoma (HCC) (13).
Alongside a discussion about the benefits of lifestyle change for reducing NAFLD severity and overall cardiometabolic risk, patients should be advised that there are currently no licensed medications for NAFLD treatment. Of course, dietary changes and long-term weight reduction may not be achievable or might not work for patients with advanced fibrosis, and concomitant pharmacological treatment may be needed once it becomes available.
Given the complexity of NAFLD pathogenesis, there are many pharmaceutical agents against many different targets either currently in trials or in development. These include glucagon-like peptide 1 (GLP-1) receptor agonists that are currently approved for the treatment of type 2 diabetes and weight loss (discussed further below). Although these agents appear promising for patients with nonalcoholic steatohepatitis (NASH) without cirrhosis, phase 3 clinical trials with liver end points are still ongoing, and future treatments may involve combination therapy.
Weight Loss: The Most Evidence-Based Available Treatment
It has been repeatedly demonstrated in clinical trials that weight reduction achieved by caloric restriction, either with or without increased physical activity, leads to improvements in multiple NAFLD biomarkers, including liver enzymes, liver fat, NASH, and fibrosis (14,15). There is a dose-dependent association between the amount of weight loss and the extent of improvement in biomarkers of liver damage (16). A seminal interventional trial with histological end points suggested that body weight reduction of ≥5% is required to reduce liver fat, 7–10% to improve liver inflammation, and ≥10% to improve fibrosis/scarring (7). A more recent meta-analysis concluded that a clinically meaningful absolute improvement in steatosis (∼5%) can be achieved with an initial weight loss of about 5 kg (16). This is encouraging because many people may experience difficulty in achieving and maintaining a 7–10% weight loss. Nonetheless, larger weight losses are associated with greater improvements in markers of NAFLD (7,16) as well as type 2 diabetes remission (17).
Importantly, NAFLD can also develop in individuals with a BMI within ethnicity-specific ranges of normal weight. NAFLD patients of normal weight have a greater waist circumference than normal-weight control subjects (18), suggesting that increased visceral fat may be an independent risk factor for NAFLD in individuals of normal weight (19). In fact, normal-weight NAFLD patients can achieve remission of steatosis with a body weight reduction of 3–5% (20) and will benefit from increasing physical exercise as recommended by both the European Society for Clinical Nutrition and Metabolism guidelines (9) and the recent American Gastroenterological Association clinical practice update on normal-weight NAFLD (21). Sugar-sweetened beverages in particular are associated with increased visceral and liver fat and should be avoided (21).
One Goal, Many Paths: Multiple Dietary Approaches to Treating NAFLD
There are multiple dietary approaches to choose from to lose weight. Patients should be cautioned that marketing for diet books and commercial weight loss programs can be hyperbolic, with many suggesting that theirs is the single best approach. However, multiple randomized controlled trials (RCTs) and subsequent meta-analyses have shown no differences in efficacy for weight loss among different macronutrient-focused approaches (e.g., low-carbohydrate or low-fat diets) or named diet programs (22). The sections below describe common diets and the evidence for their effectiveness in treating NAFLD.
Mediterranean Eating Pattern
One of the most studied healthy eating patterns is the traditional Mediterranean-style diet, characterized by a high intake of olive oil and healthy fatty acids (monounsaturated fat and omega-3 fatty acids), vegetables, fruits, nuts and seeds, legumes, whole grains and fiber, and fish and seafood and a low intake of red and processed meats. In addition to being high in vitamins and minerals, the Mediterranean diet is high in polyphenols, which are bioactive compounds that act as antioxidants found in high amounts in plant foods and olive oil. The Mediterranean diet is also typically characterized by a reduced intake of sugars and refined carbohydrates, meaning that this is also often a reduced-carbohydrate eating pattern.
The Mediterranean diet repeatedly has been shown to provide hepatic and extrahepatic, especially cardiovascular (23–25), health benefits, even without weight loss (26). Observational prospective studies show an inverse association of NAFLD with the Mediterranean diet (27,28), and clinical trials also support its beneficial effect on liver fat. Therefore, this diet and overlapping dietary patterns are recommended by all relevant clinical practice guidelines (9–12,29).
The two largest and longest RCTs (18 months each) have provided the best evidence for the valuable role of two modified Mediterranean diets. The first showed the superiority of a Mediterranean diet that was also low in carbohydrate over a low-fat diet in decreasing intrahepatic fat (30). The second showed that a Mediterranean diet enriched with polyphenols and more strictly restricted in red and processed meats led to a twofold liver fat loss compared with an isocaloric regular Mediterranean diet, despite similar weight reduction (31). Both diets were better than standard nutritional counseling. Similarly, in a 24-month RCT of two energy-restricted dietary strategies, the Mediterranean diet group showed a more significant decrease in body weight (−7.6 vs. −4.8%), ALT, liver stiffness, and liver fat at 24 months compared with a group following the standard low-fat (30% of calories) American Heart Association diet. These findings may indicate that the Mediterranean diet is easier to maintain in the long term (32). However, importantly, no liver histology was obtained in either of the RCTs; thus, the effect of the Mediterranean diet on NASH and fibrosis is unknown (33).
Low-Carbohydrate Versus Low-Fat Diets
As recently summarized in a scientific statement from the National Lipid Association, although in the short-term (≤6 months) use of hypoenergetic low-carbohydrate diets may result in greater weight loss than hypoenergetic low-fat diets, after 1–2 years of follow-up, there are no differences in weight loss, lipid levels, A1C, or blood pressure (34). Although there are fewer trials with liver end points, hypoenergetic, low-carbohydrate diets and low-fat diets appear to be similarly effective in reducing liver fat and related biomarkers (35). For example, in a recent 6-month intervention of low-carbohydrate and low-fat calorie-unrestricted diets, greater reductions in body weight and A1C were observed at 6 months with the low-carbohydrate diet, but there was no difference seen between groups in the secondary end point of a 2-point improvement in NAFLD activity score (36). Moreover, differences between groups in body weight and glycemic control were not sustained at the 9-month follow-up.
Very-low-carbohydrate ketogenic diets (VLCKDs) are characterized by extreme carbohydrate restriction to <20–50 g/day (10–25% of total calories) and high fat and protein content (33). These are highly restrictive diets and should be followed under a health professional’s supervision and in a time-limited manner, followed by a gradual increase of calories, carbohydrates, and food variety. To date, no long-term RCTs allow conclusions regarding the effect of VLCKDs on NAFLD and NASH or their safety or efficacy in reducing fibrosis. Only small, short-term trials among NAFLD patients have found a higher or comparable reduction in hepatic fat content after following a VLCKD diet compared with standard caloric restriction (33). For healthful dietary changes that are to be maintained in the long term, as expanded on below, patients should understand the importance of carbohydrate and fat quality, indeed of overall food quality, rather than just food or macronutrient quantity.
Time-Restricted Eating
Time-restricted eating (TRE), also called intermittent fasting, is a new diet strategy in which calories are consumed in a defined time window (37) considered optional for patients with obesity and metabolic syndrome, including NAFLD. There is currently very little evidence for a beneficial effect of TRE beyond that of caloric restriction on hepatic fat in NAFLD patients.
To date, all trials have been relatively short, not all have been RCTs, and there are few long-term follow-up data (38). In an 8-week RCT, alternate-day calorie restriction (fasting day: 30% of calorie requirement, nonfasting day ad libitum) compared with no intervention resulted in a greater reduction of steatosis and fibrosis, as measured by ultrasound and elastography (39). However, in comparison with a hypoenergetic VLCKD, 12 weeks of TRE using the 5:2 diet (500 kcal/day for women and 600 kcal/day for men on two nonconsecutive days per week and normal eating on the other 5 days) was found equally effective in reducing weight and steatosis (40). A recent long-term RCT involving 88 NAFLD patients confirmed these findings; 12-month calorie-restricted diets either in the form of TRE (eating only between 8:00 a.m. and 4:00 p.m.) or with habitual meal timing led to similar reductions in liver fat and liver stiffness (41). Interestingly, a recent 12-week RCT involving 80 adults with obesity and NAFLD compared alternate day fasting (“fast day”: 600 kcal; “feast day”: ad libitum intake) with or without moderate-intensity aerobic exercise for five 60-minute sessions per week) versus exercise alone. The results underscore the additive benefit of diet plus exercise (discussed further below); liver fat was reduced by 5.5% in the diet-and-exercise group compared with 1.3% in the exercise-only group and 2.3% in the diet-only group (42).
Dietary Quality, Not Quantity: Nutrient Composition Is Key
Adherence to healthier eating patterns has been associated with lower risk of all-cause, CVD-specific, and cancer-related mortality in U.S. adults with NAFLD (43). Importantly, healthier patterns are defined by dietary quality, not quantity; and, for all-cause mortality, the effects are observed to be linear, suggesting that any improvements in dietary quality may help. In addition, benefits have been observed across both sexes, all racial/ethnic groups, and various age-groups (43).
Several prospective studies have provided evidence for the independent, beneficial association of healthy dietary composition with NAFLD regression (27,28,44,45). Notably, the Mediterranean diet’s emphases on reducing sugars and refined carbohydrates, saturated fat, red and processed meats, and ultra-processed foods are all relevant to NAFLD pathogenesis and even HCC incidence (46). Saturated fat has been shown to have a negative effect on liver fat accumulation in several clinical trials (35). Therefore, reducing the intake of saturated fat is important. Foods rich in saturated fat include palm oil, which is found mostly in processed foods; butter and high-fat dairy products; high-fat meats such as internal organs and processed meats such as sausages; and cakes, cookies, ice cream, and other sweets. Several studies have shown a harmful association between NAFLD and high intake of red meats (e.g., beef, lamb, and pork) and processed meats (e.g., hamburgers, salami, sausages, and processed schnitzel) (47–49).
“Added sugar” refers to sucrose (table sugar) or other refined sugars (e.g., fructose and high-fructose corn syrup), which are incorporated into foods, fruit drinks, and other beverages (50). Sugar-sweetened beverages are the leading source of added sugar worldwide. High intake of added sugars, especially fructose, plays a major role in NAFLD development. Evidence from many studies shows an association between added sugars, especially in the form of sugar-sweetened beverages, and NAFLD (51). Importantly, reduced sugar consumption leads to an improvement in liver fat within weeks (52).
Conversely, coffee consumption has been shown, albeit only in observational studies, to be related to reduced risk of NAFLD and especially fibrosis, and there is also evidence for a relationship between coffee consumption and reduced risk for HCC (53,54). The American Association for the Study of Liver Diseases NAFLD guidance (12) recommends ≥3 cups of coffee (caffeinated or decaffeinated) daily because of its association with less advanced liver disease.
Clinicians should advise that foods that are beneficial or harmful for NAFLD are also related to the risk of liver cancer. High intakes of red meat, saturated fat, cholesterol, and refined sugars are associated with an increased liver cancer risk (55,56). Consumption of omega-3 polyunsaturated fat–rich fish is inversely associated with liver cancer, as is vegetable intake (55). Altogether, the Mediterranean diet or similar healthy eating patterns are associated with lower risks for liver cancer (56,57).
Physical Activity and Exercise: Which Regimen Promotes Liver Health?
The importance of incorporating physical activity and exercise with dietary changes in the treatment of NAFLD should be emphasized to all patients (58). Whereas low physical activity is associated with a greater risk for NAFLD progression (59), several RCTs have demonstrated that exercise alone reduces liver fat in patients with NAFLD (15). Furthermore, several recent studies have demonstrated that increased physical activity is associated with reduced risk for liver fibrosis, cirrhosis, and all-cause mortality (60–63). Exercise independent of weight loss has hepatic and cardiometabolic benefit and should be routinely recommended and tailored to patients’ preferences and physical abilities (10). Although patients should be cautioned that exercise alone will not induce the same amount of body weight loss as dietary changes, they should also be told that even small changes in waist circumference are indicative of a reduction in visceral fat.
When starting from a very inactive baseline, patients should be encouraged to set achievable goals for increasing total activity toward the recommended minimum of at least 150 minutes/week (30 minutes/day on 5 days/week) of moderate activity (10,59). Moderate activity is defined as anything that will raise a person’s heart rate and break a sweat but still permit talking. Current guidelines recommend that a combination of aerobic (i.e., “cardio,” such as brisk walking, cycling, and swimming) and resistance (i.e., “strength,” such as weight-lifting) training be used (10,12,59). However, depending on preferences, culture, and financial constraints, this may not be achievable for all patients. Patients should be encouraged that dose-dependent beneficial effects have been observed for both volume and intensity of physical activity, with no minimal threshold for benefit on mortality (43). This suggests that even small amounts of physical activity may benefit patients, and an initial goal for some may be just getting to 30 minutes of uninterrupted walking. Nonetheless, physical activity goals and targets should evolve as patients become more fit, because the data suggest further risk reduction with increasing total amounts of activity and intensity (43,60).
A Little Help From Friends: Bariatric Surgery and Medical Support for Weight Loss
In the context of obesity, focusing on health outcomes rather than weight loss is a core tenant of patient-centered care. Lifestyle changes should be the foundation of care for all patients with NAFLD, because all individuals, regardless of their weight, benefit from healthy eating and regular physical activity. Nonetheless, depending on an individual patient’s history, extent of adiposity, and number of comorbidities, medical nutrition therapy should not stand in isolation, and pharmacological and surgical interventions should be considered and tailored to improve individuals’ health-related or weight-related outcomes (64).
Bariatric surgery induces large amounts (25–30%) of weight loss very effectively and improves steatosis and NASH for many patients. In a meta-analysis of 37 studies and 3,751 bariatric surgery patients, 45–56% of participants experienced resolution in histological features of inflammation, ballooning, and steatosis and 25% for fibrosis (65). Bariatric surgery also rapidly improves glycemic control and induces type 2 diabetes remission for the majority of patients (>80%). However, bariatric surgery is invasive, with potential risks of complications and serious adverse effects, and clinicians considering making a referral for bariatric surgery should explain both the risks and benefits to patients.
Until recently, successful long-term pharmacological treatment of obesity to induce weight loss comparable to bariatric surgery safely and within patients’ tolerability for side effects seemed unattainable. More than 25 antiobesity drugs have been withdrawn from markets worldwide because of serious adverse effects (66). At present, six agents are approved by the U.S. Food and Drug Administration for adults with obesity, including orlistat, phentermine, phentermine-topiramate, bupropion, and the more recently approved GLP-1 receptor agonists liraglutide and semaglutide. These, as well as dual agonists such as tirzepatide, are discussed in detail elsewhere in this article collection (67).
Although the excitement around the use of GLP-1 receptor agonists for weight loss and type 2 diabetes remission appears warranted, patients should be cautioned that trials in patients with NAFLD are still ongoing. Furthermore, there is a heterogenous response to the currently approved agents in terms of percentage of weight loss (e.g., in the RCT of semaglutide, 14% of patients did not achieve a weight loss ≥5% weight loss) (68). Additionally, there will be rapid weight regain if these agents are discontinued (69). As with bariatric surgery, access to and costs of these drugs can also be a challenge, depending on the health care system and insurance plan.
How To: Practical Guidance for Facilitating Lifestyle Modification in Patients With NAFLD
Obesity and its cardiometabolic complications, including NAFLD, are complex, chronic diseases; therefore, dietary and lifestyle changes must be adopted for life to prevent the progression of NAFLD and its common comorbidities—namely, CVD and type 2 diabetes. Behavioral therapy is crucial for successful lifestyle change and can be at least partially facilitated through digital devices such as smartphones and smartwatches, which enable monitoring of activity and food intake, and digital health coaching. Greater weight loss at 1 year has been shown to be associated with the number of behavioral sessions attended and the number of minutes of physical activity per week (22).
Health care providers clearly have a key role to play as educators. Awareness of disease severity and effectiveness of dietary measures to treat it are important elements contributing to successful lifestyle modification. In an international survey of adults with NAFLD, lack of awareness about their fibrosis stage was significantly associated with poor adherence to lifestyle adjustments, suggesting that improving patient-provider communication about liver fibrosis stage, its associated risks, and how to mitigate it, is greatly needed (70). Improved nutrition-related behavior in NAFLD patients has been shown to be related to patients’ awareness that lifestyle treatment is effective for disease regression and their degree of self-efficacy (71).
Behavioral-focused approaches teach healthy eating skills, enhance confidence in the benefits of adopting a healthy eating pattern, engage family members in treatment to gain support and avoid conflicts, discuss potential barriers (e.g., life stressors and the so-called “obesogenic environment”), and help participants find shared solutions to overcome barriers (72). Setting SMART (specific, measurable, achievable, relevant, and timely) weight loss, dietary, and physical activity goals can help to improve patients’ motivation, self-efficacy, and maintenance.
Importantly, the exact type of diet in terms of macronutrient composition, food choices, and timing of eating can and should be tailored to patients’ preferences, culture, and economic ability. With the support of a multidisciplinary team, patients can choose a diet with health benefits that they will be able to follow in the long term (33).
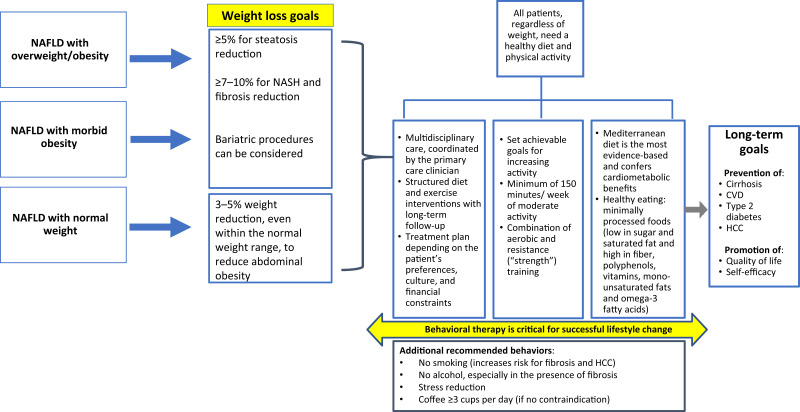
Peer support networks, whether involving family, friends, or fellow participants in a commercial weight loss program, are beneficial for many and increasingly available online (73). A Web-based intervention compared with a standard group-based intervention was shown to have comparable long-term effectiveness in diabetes prevention among NAFLD patients followed up to 60 months, with similar results on weight change (74). Indeed, the use of digital devices for health interventions is increasing, and smartphone apps can be used for self-monitoring, participating in educational and counseling sessions, and communicating with clinicians, other health care professionals, and peer support networks. Efficacy trials of such digital apps are beginning in patients with NAFLD (75). A lifestyle modification checklist for health care professionals is provided in Table 1, and a lifestyle management algorithm for NAFLD is depicted in Figure 1.
Table 1.
Lifestyle Checklist for Health Care Practitioners
| To-Do | Description |
|---|---|
|
Ask open-ended questions to assess patients’ willingness and readiness to discuss the topic and consider treatment. |
|
Ask about patients, history of weight reduction attempts. Agree together in an open discussion on weight reduction goals that are achievable in a reasonable time and can be maintained with a reasonable effort. For most patients, a 10% weight reduction is hard to achieve and maintain in the long term. Be sure to tell the patients that even smaller weight reductions accompanied by a healthier diet and increased physical activity can be helpful and suggest medications or bariatric interventions, when appropriate. |
|
Ask patients about their dietary intake, which many times contains sugar-sweetened beverages and high-sugar/high-fat foods or ultra-processed foods. Recommend a healthy eating pattern. Many of normal-weight patients with NAFLD have abdominal obesity and thus will also benefit from a small weight reduction and increased physical activity. |
|
Ultra-processed foods and drinks can be recognized by a long list of ingredients, many of which are not ingredients typically used in home kitchens (e.g., high-fructose corn syrup, hydrogenated oils, food coloring agents, and other additives). Explain to patients that these foods are usually high in calories, salt, sugars, and fat and low in nutritional value and that they are designed to be hyper-palatable and thus promote overeating and obesity. Provide patients with examples such as sausages; sugar-sweetened beverages, including fruit juice; sweet and savory snacks; and store-bought frozen meals. |
|
Different types of fat have differential effects on the liver. Therefore, the recommendation for fat reduction needs to be specifically focused on saturated fat. The Mediterranean diet is not a low-fat diet; typically, 35–40% of total energy is derived from healthy fats in this eating pattern. Make sure patients understand these differences and know how to translate them to guide their food intake (e.g., reducing their intake of red meat and internal organs, high-fat dairy products, bakery goods, and ultra-processed foods and increasing intake of fish, olive oil, nuts, avocados, and tahini). |
|
Vegetables can be eaten freely; recommend cooking them with olive oil. There is no evidence of the harmful effect of fruits in their natural form if they are eaten in a reasonable amount (usually 1–3 medium-sized portions/day). Recommend reducing intake of fruit juice, which is high in rapidly absorbed sugars, including fructose. |
|
Note that cultural or financial barriers may prevent adherence to the Mediterranean diet. Some of the foods in the Mediterranean diet tend to be expensive (e.g., fish, nuts, olive oil, and fresh fruit). Emphasize that even partial adherence to the principles of the Mediterranean diet or using overlapping diets (such as combining elements of the Mediterranean diet with moderately low-carbohydrate diets or intermittent fasting) can be beneficial if it facilitates the reduction of added sugars and saturated fat and promotes intake of minimally processed foods and home cooking. |
|
Successful lifestyle change requires skills training; patients should have access to counseling and follow-up. Behavior change techniques include setting goals, actively self-monitoring (e.g., keeping a food diary and using a wearable activity monitor), and addressing barriers related to diet and physical activity. Explain to patients that these modifications are a lifelong treatment. |
|
The role of clinicians is to enhance self-efficacy and motivation by providing positive feedback. Show appreciation for any positive change patients make in their lifestyle or for any increase in physical activity. Do not set goals that the patients are unable to reach or maintain because failing to do so will harm their sense of self-efficacy. Negotiate all goals together and start with the easiest ones to achieve. |
|
Generally, 150 minutes/week of moderate aerobic activity is advised, plus resistance training, but the exercise routine should be tailored to patients’ ability. Explain that exercise is crucial for general health, weight reduction and maintenance, prevention of sarcopenia, and improvement of quality of life. |
Figure 1.
Lifestyle management algorithm for NAFLD.
Conclusion
Weight reduction achieved by adopting a healthy eating pattern and increasing physical activity is the most beneficial treatment for NAFLD. Improving dietary quality and increasing physical activity reduces risks of both NAFLD progression and CVD incidence. The benefits are additive, but clinically meaningful improvements are observed in males and females across all age and racial/ethnic groups, even with small amounts of weight loss and small increases in physical activity.
Although many hypoenergetic diets can induce liver fat and induce weight reduction, the Mediterranean diet has additional cardiometabolic benefits relevant to CVD risk reduction, which is the primary cause of death in most patients with NAFLD. Lifestyle changes should be tailored to patients’ preferences, culture, and financial circumstances, with the goal of lifelong adherence.
In an obesogenic environment, changes to lifestyle are difficult to make, but even small improvements make a difference. Health care providers play a crucial role in facilitating their patients’ long-term success.
Article Information
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
The authors contributed equally to all aspects of writing and preparing this article for publication. Both authors are guarantors of this work and take responsibility for the accuracy of the data presented and the integrity of the content.
References
- 1. Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2022;7:851–861 [DOI] [PubMed] [Google Scholar]
- 2. Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020;158:1999–2014.e1991 [DOI] [PubMed] [Google Scholar]
- 3. Schattenberg JM, Allen AM, Jarvis H, et al. A multistakeholder approach to innovations in NAFLD care. Commun Med (Lond) 2023;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J Hepatol 2018;68:268–279 [DOI] [PubMed] [Google Scholar]
- 5. Tilg H, Adolph TE, Dudek M, Knolle P. Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nat Metab 2021;3:1596–1607 [DOI] [PubMed] [Google Scholar]
- 6. Moore JB. From sugar to liver fat and public health: systems biology driven studies in understanding non-alcoholic fatty liver disease pathogenesis. Proc Nutr Soc 2019;78:290–304 [DOI] [PubMed] [Google Scholar]
- 7. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149:367–78.e5; quiz e14–e15 [DOI] [PubMed] [Google Scholar]
- 8. Glass LM, Dickson RC, Anderson JC, et al. Total body weight loss of ≥ 10 % is associated with improved hepatic fibrosis in patients with nonalcoholic steatohepatitis. Dig Dis Sci 2015;60:1024–1030 [DOI] [PubMed] [Google Scholar]
- 9. Plauth M, Bernal W, Dasarathy S, et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr 2019;38:485–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Francque SM, Marchesini G, Kautz A, et al. Non-alcoholic fatty liver disease: a patient guideline. JHEP Rep 2021;3:100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cusi K, Isaacs S, Barb D, et al. American Association of Clinical Endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract 2022;28:528–562 [DOI] [PubMed] [Google Scholar]
- 12. Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023;77:1797–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marti-Aguado D, Clemente-Sanchez A, Bataller R. Cigarette smoking and liver diseases. J Hepatol 2022;77:191–205 [DOI] [PubMed] [Google Scholar]
- 14. Kenneally S, Sier JH, Moore JB. Efficacy of dietary and physical activity intervention in non-alcoholic fatty liver disease: a systematic review. BMJ Open Gastroenterol 2017;4:e000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fernández T, Viñuela M, Vidal C, Barrera F. Lifestyle changes in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. PLoS One 2022;17:e0263931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koutoukidis DA, Koshiaris C, Henry JA, et al. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism 2021;115:154455. [DOI] [PubMed] [Google Scholar]
- 17. Taylor R, Al-Mrabeh A, Zhyzhneuskaya S, et al. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for β cell recovery. Cell Metab 2018;28:547–556.e3 [DOI] [PubMed] [Google Scholar]
- 18. Sookoian S, Pirola CJ. Systematic review with meta-analysis: risk factors for non-alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment Pharmacol Ther 2017;46:85–95 [DOI] [PubMed] [Google Scholar]
- 19. Lee S, Kim KW, Lee J, et al. Visceral adiposity as a risk factor for lean non-alcoholic fatty liver disease in potential living liver donors. J Gastroenterol Hepatol 2021;36:3212–3218 [DOI] [PubMed] [Google Scholar]
- 20. Wong VW, Wong GL, Chan RS, et al. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol 2018;69:1349–1356 [DOI] [PubMed] [Google Scholar]
- 21. Long MT, Noureddin M, Lim JK. AGA clinical practice update: diagnosis and management of nonalcoholic fatty liver disease in lean individuals: expert review. Gastroenterology 2022;163:764–774.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bray GA, Frühbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet 2016;387:1947–1956 [DOI] [PubMed] [Google Scholar]
- 23. Hassani Zadeh S, Mansoori A, Hosseinzadeh M. Relationship between dietary patterns and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Gastroenterol Hepatol 2021;36:1470–1478 [DOI] [PubMed] [Google Scholar]
- 24. Gepner Y, Shelef I, Komy O, et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J Hepatol 2019;71:379–388 [DOI] [PubMed] [Google Scholar]
- 25. Tsaban G, Yaskolka Meir A, Rinott E, et al. The effect of green Mediterranean diet on cardiometabolic risk; a randomised controlled trial. Heart 2021;107:1054–1061 [DOI] [PubMed] [Google Scholar]
- 26. Properzi C, O’Sullivan TA, Sherriff JL, et al. Ad libitum Mediterranean and low-fat diets both significantly reduce hepatic steatosis: a randomized controlled trial. Hepatology 2018;68:1741–1754 [DOI] [PubMed] [Google Scholar]
- 27. Maskarinec G, Lim U, Jacobs S, et al. Diet quality in midadulthood predicts visceral adiposity and liver fatness in older ages: the Multiethnic Cohort Study. Obesity (Silver Spring) 2017;25:1442–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma J, Hennein R, Liu C, et al. Improved diet quality associates with reduction in liver fat, particularly in individuals with high genetic risk scores for nonalcoholic fatty liver disease. Gastroenterology 2018;155:107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) . EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–1402 [DOI] [PubMed] [Google Scholar]
- 30. Gepner Y, Shelef I, Schwarzfuchs D, et al. Effect of distinct lifestyle interventions on mobilization of fat storage pools: CENTRAL magnetic resonance imaging randomized controlled trial. Circulation 2018;137:1143–1157 [DOI] [PubMed] [Google Scholar]
- 31. Yaskolka Meir A, Rinott E, Tsaban G, et al. Effect of green-Mediterranean diet on intrahepatic fat: the DIRECT PLUS randomised controlled trial. Gut 2021;70:2085–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marin-Alejandre BA, Cantero I, Perez-Diaz-Del-Campo N, et al. Effects of two personalized dietary strategies during a 2-year intervention in subjects with nonalcoholic fatty liver disease: a randomized trial. Liver Int 2021;41:1532–1544 [DOI] [PubMed] [Google Scholar]
- 33. Zelber-Sagi S, Grinshpan LS, Ivancovsky-Wajcman D, Goldenshluger A, Gepner Y. One size does not fit all: practical, personal tailoring of the diet to NAFLD patients. Liver Int 2022;42:1731–1750 [DOI] [PubMed] [Google Scholar]
- 34. Kirkpatrick CF, Bolick JP, Kris-Etherton PM, et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol 2019;13:689–711.e1 [DOI] [PubMed] [Google Scholar]
- 35. Yki-Järvinen H, Luukkonen PK, Hodson L, Moore JB. Dietary carbohydrates and fats in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2021;18:770–786 [DOI] [PubMed] [Google Scholar]
- 36. Hansen CD, Gram-Kampmann EM, Hansen JK, et al. Effect of calorie-unrestricted low-carbohydrate, high-fat diet versus high-carbohydrate, low-fat diet on type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial. Ann Intern Med 2023;176:10–21 [DOI] [PubMed] [Google Scholar]
- 37. Rynders CA, Thomas EA, Zaman A, Pan Z, Catenacci VA, Melanson EL. Effectiveness of intermittent fasting and time-restricted feeding compared to continuous energy restriction for weight loss. Nutrients 2019;11:2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lange M, Nadkarni D, Martin L, Newberry C, Kumar S, Kushner T. Intermittent fasting improves hepatic end points in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Hepatol Commun 2023;7:e0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johari MI, Yusoff K, Haron J, et al. A randomised controlled trial on the effectiveness and adherence of modified alternate-day calorie restriction in improving activity of non-alcoholic fatty liver disease. Sci Rep 2019;9:11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Holmer M, Lindqvist C, Petersson S, et al. Treatment of NAFLD with intermittent calorie restriction or low-carb high-fat diet: a randomised controlled trial. JHEP Rep 2021;3:100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wei X, Lin B, Huang Y, et al. Effects of time-restricted eating on nonalcoholic fatty liver disease: the TREATY-FLD randomized clinical trial. JAMA Netw Open 2023;6:e233513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ezpeleta M, Gabel K, Cienfuegos S, et al. Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: a randomized controlled trial. Cell Metab 2023;35:56–70.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vilar-Gomez E, Vuppalanchi R, Gawrieh S, Pike F, Samala N, Chalasani N. Significant dose-response association of physical activity and diet quality with mortality in adults with suspected NAFLD in a population study. Am J Gastroenterol 2023;118:1576–1591 [DOI] [PubMed] [Google Scholar]
- 44. Alferink LJM, Erler NS, de Knegt RJ, et al. Adherence to a plant-based, high-fibre dietary pattern is related to regression of non-alcoholic fatty liver disease in an elderly population. Eur J Epidemiol 2020;35:1069–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maskarinec G, Namatame LA, Kang M, et al. Differences in the association of diet quality with body fat distribution between men and women. Eur J Clin Nutr 2020;74:1434–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hadefi A, Arvanitakis M, Trépo E, Zelber-Sagi S. Dietary strategies in non-alcoholic fatty liver disease patients: from evidence to daily clinical practice, a systematic review. United European Gastroenterol J 2023;11:663–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Etemadi A, Sinha R, Ward MH, et al. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: population based cohort study. BMJ 2017;357:j1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zelber-Sagi S, Ivancovsky-Wajcman D, Fliss Isakov N, et al. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J Hepatol 2018;68:1239–1246 [DOI] [PubMed] [Google Scholar]
- 49. Noureddin M, Zelber-Sagi S, Wilkens LR, et al. Diet associations with nonalcoholic fatty liver disease in an ethnically diverse population: the Multiethnic Cohort. Hepatology 2020;71:1940–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moore JB, Fielding BA. Demystifying dietary sugars. In Nutrition Guide for Physicians and Related Healthcare Professions. Wilson T, Temple NJ, Bray GA, Eds. Cham, Switzerland, Springer International Publishing, 2022, p. 319–328 [Google Scholar]
- 51. Jensen T, Abdelmalek MF, Sullivan S, et al. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol 2018;68:1063–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schwimmer JB, Ugalde-Nicalo P, Welsh JA, et al. Effect of a low free sugar diet vs usual diet on nonalcoholic fatty liver disease in adolescent boys: a randomized clinical trial. JAMA 2019;321:256–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hayat U, Siddiqui AA, Okut H, Afroz S, Tasleem S, Haris A. The effect of coffee consumption on the non-alcoholic fatty liver disease and liver fibrosis: a meta-analysis of 11 epidemiological studies. Ann Hepatol 2021;20:100254. [DOI] [PubMed] [Google Scholar]
- 54. Niezen S, Mehta M, Jiang ZG, Tapper EB. Coffee consumption is associated with lower liver stiffness: a nationally representative study. Clin Gastroenterol Hepatol 2022;20:2032–2040.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zelber-Sagi S, Noureddin M, Shibolet O. Lifestyle and hepatocellular carcinoma: what is the evidence and prevention recommendations? Cancers (Basel) 2021;14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ma Y, Yang W, Simon TG, et al. Dietary patterns and risk of hepatocellular carcinoma among U.S. men and women. Hepatology 2019;70:577–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Turati F, Trichopoulos D, Polesel J, et al. Mediterranean diet and hepatocellular carcinoma. J Hepatol 2014;60:606–611 [DOI] [PubMed] [Google Scholar]
- 58. Younossi ZM, Zelber-Sagi S, Henry L, Gerber LH. Lifestyle interventions in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2023;20:708–722 [DOI] [PubMed] [Google Scholar]
- 59. Stine JG, Long MT, Corey KE, et al. American College of Sports Medicine (ACSM) International Multidisciplinary Roundtable report on physical activity and nonalcoholic fatty liver disease. Hepatol Commun 2023;7:e0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim D, Murag S, Cholankeril G, et al. Physical activity, measured objectively, is associated with lower mortality in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2021;19:1240–1247.e5 [DOI] [PubMed] [Google Scholar]
- 61. Kim D, Konyn P, Cholankeril G, Ahmed A. Physical activity is associated with nonalcoholic fatty liver disease and significant fibrosis measured by FibroScan. Clin Gastroenterol Hepatol 2022;20:e1438–e1455 [DOI] [PubMed] [Google Scholar]
- 62. Chun HS, Lee M, Lee HA, et al. Association of physical activity with risk of liver fibrosis, sarcopenia, and cardiovascular disease in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2023;21:358–369.e12 [DOI] [PubMed] [Google Scholar]
- 63. Henry A, Paik JM, Austin P, et al. Vigorous physical activity provides protection against all-cause deaths among adults patients with nonalcoholic fatty liver disease (NAFLD). Aliment Pharmacol Ther 2023;57:709–722 [DOI] [PubMed] [Google Scholar]
- 64. Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ 2020;192:E875–E891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhou H, Luo P, Li P, et al. Bariatric surgery improves nonalcoholic fatty liver disease: systematic review and meta-analysis. Obes Surg 2022;32:1872–1883 [DOI] [PubMed] [Google Scholar]
- 66. Müller TD, Blüher M, Tschöp MH, DiMarchi RD. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov 2022;21:201–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Genua I, Cusi K. Pharmacological approach to NAFLD: current and future therapies. Diabetes Spectr 2024;37:48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wilding JPH, Batterham RL, Calanna S, et al.; STEP 1 Study Group . Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021;384:989–1002 [DOI] [PubMed] [Google Scholar]
- 69. Wilding JPH, Batterham RL, Davies M, et al.; STEP 1 Study Group . Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes Metab 2022;24:1553–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Carrieri P, Mourad A, Marcellin F, et al. Knowledge of liver fibrosis stage among adults with NAFLD/NASH improves adherence to lifestyle changes. Liver Int 2022;42:984–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zelber-Sagi S, Bord S, Dror-Lavi G, et al. Role of illness perception and self-efficacy in lifestyle modification among non-alcoholic fatty liver disease patients. World J Gastroenterol 2017;23:1881–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Haigh L, Bremner S, Houghton D, et al. Barriers and facilitators to Mediterranean diet adoption by patients with nonalcoholic fatty liver disease in Northern Europe. Clin Gastroenterol Hepatol 2019;17:1364–1371.e3 [DOI] [PubMed] [Google Scholar]
- 73. Ufholz K. Peer support groups for weight loss. Curr Cardiovasc Risk Rep 2020;14:19 [Google Scholar]
- 74. Petroni ML, Brodosi L, Armandi A, Marchignoli F, Bugianesi E, Marchesini G. Lifestyle intervention in NAFLD: long-term diabetes incidence in subjects treated by Web- and group-based programs. Nutrients 2023;15:792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sato M, Akamatsu M, Shima T, et al. Impact of a novel digital therapeutics system on nonalcoholic steatohepatitis: the NASH App clinical trial. Am J Gastroenterol 2023;118:1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]