Abstract
The unique properties of oligonucleotide (and small interfering RNA)-modified gold nanoparticle conjugates make them promising intracellular gene regulation agents. We found that gold nanoparticles stably functionalized with covalently attached oligonucleotides activate immune-related genes and pathways in human peripheral blood mononuclear cells, but not an immortalized, lineage-restricted cell line. These findings have strong implications for the application of oligonucleotide-modified gold nanoparticle conjugates in translational research and in the development of therapeutics and gene delivery systems.
Keywords: gold nanoparticle–oligonucleotide complexes, gene delivery, gene expression, signaling pathways, immune activation
INTRODUCTION
New nanotechnologies that deliver oligonucleotides or small interfering RNA into cells to regulate gene transcript and protein abundance are emerging. By conjugating antisense constructs to the surface of gold nanoparticles, for example, polynucleotide stability, binding properties and cellular uptake can be enhanced, and ostensibly gene expression can be suppressed.1 This extent of regulation mediated by a nanoparticulate system could provide an effective strategy to decipher gene pathways and function, and improve the efficacy of gene therapy. Nevertheless, targeted delivery of nucleic acids into immune cells by engineered nanoparticles may trigger effector functions and elicit untoward molecular and biological effects.2–6
Most cells have natural sensors of pathogen-associated molecular patterns, other pathogen-associated molecules, cellular damage and environmental irritants. Pattern recognition receptors (PRRs) of the innate immune system, including the transmembrane Toll-like receptors (TLRs) and C-type lectin receptors as well as the cytoplasmic RIG-I-like receptors and NOD-like receptors, recognize these structures and initiate signaling pathways that lead to the production of type-1 interferons, proinflammatory cytokines and chemokines.7 These inducible gene products regulate the sensitivity and intensity of the innate immune response to infection and injury. Though oligonucleotide-modified gold nanoparticle conjugates are purportedly nontoxic for lineage-specific cell types, their effects on signaling pathways that respond to activating stimuli in primary immune cells, which are differentially activated depending upon the cell type involved, has not been studied. Here, we show that human peripheral blood mononuclear cells (PBMCs) exposed to gold nanoparticles stably functionalized with covalently attached oligonucleotides lead to widespread transcriptional activation of innate immune responses.
RESULTS AND DISCUSSION
Because pathogen-associated molecular patterns or other non-microbial structures trigger signaling pathways and effector mechanisms of innate immunity, we determined whether gold nanoparticle–oligonucleotide complexes impact gene expression with a functional genomics approach. A phosphorothioate-modified non-targeting antisense DNA sequence complementary to enhanced green fluorescent protein (EGFP) that inhibits green fluorescent protein expression was conjugated to the gold nanoparticle (13 ± 1 nm) surface with a mono-thiol group.1 In experiments meant to visualize their uptake in the cell, the gold nanoparticle–oligonucleotide complexes were functionalized with a 5′ Cy5.5-label; for experiments aimed at determining the effect on cell signaling and gene expression, they were not labeled with the fluorophore. The size and morphology of the gold nanoparticle–oligonucleotide complexes were well characterized as described.8 (Supplementary Figures S1 and S2). Real-time quantitative PCR (RT-qPCR) analysis of the 16S ribosomal RNA gene and limulus ameobocyte lysate analysis of bacterial endotoxin ruled out the possibility for contamination of the gold nanoparticle–oligonucleotide complex preparations by microbial products.9,10
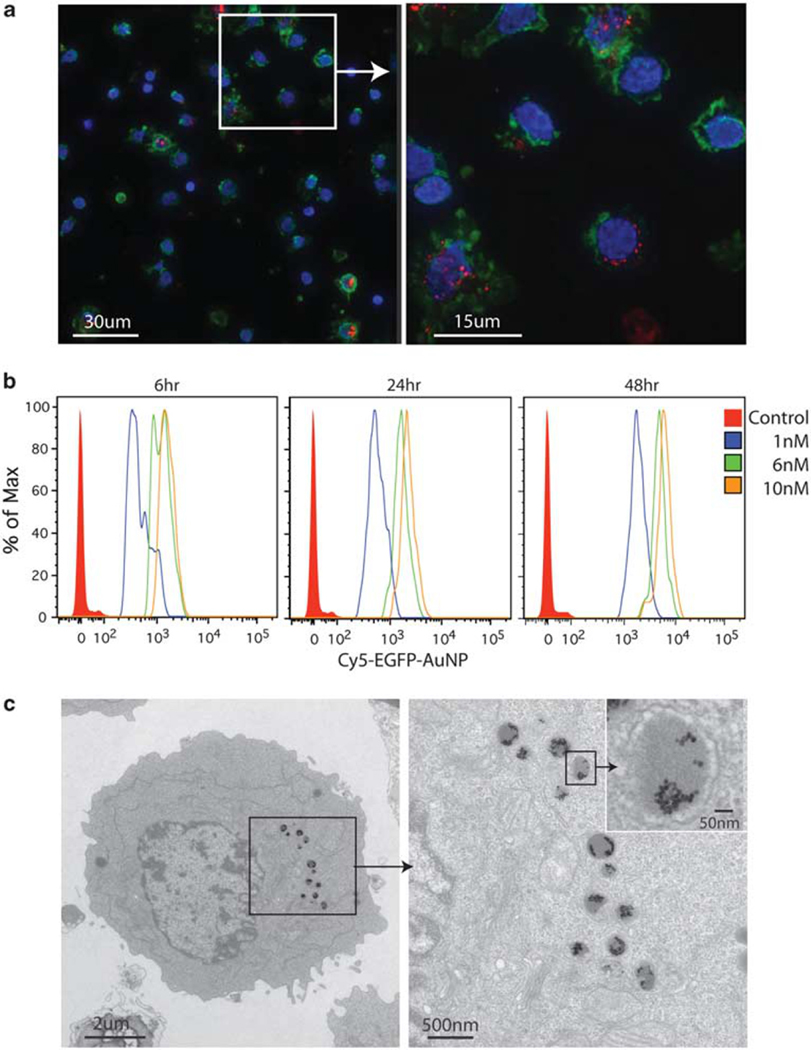
The gold nanoparticle–oligonucleotide complexes or a commercially available cationic lipid transfectant (Oligofectamine, Invitrogen, Carlsbad, CA, USA) with an equivalent amount of antisense EGFP oligonucleotide were introduced onto PBMCs or 293T cells. There were no differences in cell morphology in relation to the treated cells or cell viability under the conditions of our experiments as assessed by Trypan blue staining. As shown in Figure 1, there was efficient internalization of gold nanoparticle–oligonucleotide complexes in almost all PBMCs, regardless of cell type (Figure 1a). The amount of gold nanoparticle–oligonucleotide complex uptake in the cytoplasm increased in a time (and dose)-dependent manner (Figure 1b), but did not further enter the nucleus.
Figure 1.
Gold nanoparticle–oligonucleotide complexes are localized within membrane-bound organelles with electron-dense cores. (a) PBMCs were treated with a 10 nm concentration of gold nanoparticle–oligonucleotide complexes with a 5′ Cy5.5-label for 24h. Representative cells from independent healthy blood donors are shown (Cy5.5 fluorescence is red, actin is green and DNA is blue). A deconvoluted magnified image (×100) with stitched panels (left) and a single panel (right). Images were acquired with constant microscope settings. The uptake of the gold nanoparticle–oligonucleotide complexes is not specific to a particular cell type. Almost every mononuclear cell or granulocyte incorporated the gold nanoparticle–oligonucleotide complexes in its cytoplasm. (b) PBMCs were treated with 1, 6 and 10 nm concentrations of gold nanoparticle–oligonucleotide complexes with a 5′ Cy5.5-label for 6, 24 or 48h. Uptake was measured by flow cytometry. The data represent five independent experiments. (c) A representative primary human PBMC treated with the gold nanoparticle–oligonucleotide complexes without a 5′ Cy5.5-label (10 nm concentration) for 24h is shown (× 2900, bar=2 μm (left); and × 11000, bar=500 nm, with ×12000 inset magnification (right)). The distribution of discrete, highly electron-dense particles (alone or in aggregate) is confined exclusively to cytoplasmic membrane-bound organelles.
To understand where the gold nanoparticle–oligonucleotide complexes are localized in the cytoplasmic compartment, we imaged transverse sections of individual cells treated with a 10 nm concentration of the same gold nanoparticle–oligonucleotide complexes with the use of transmission electron microscopy. For a given cell, discrete gold nanoparticles (singly or in aggregate) were located mainly in ~0.5-μm-diameter membrane-bound organelles with electron-dense cores (Figure 1c), and not free in the cytosol or in the nucleus. Electron microscopic morphological studies of trafficking of vesicles in the endolysosomal system suggest that this cell structure is formed by direct fusion of late endosomes and lysosomes to create a hybrid organelle for the digestion of endocytosed macromolecules.11–14 To regulate gene transcript and protein abundance, the covalently attached antisense polynucleotides or the engineered gold nanoparticle constructs per se must transverse this membrane-bound cellular compartment to penetrate into the cytosol.15
mRNA levels were quantified in PBMCs under five conditions and 293T cells under four conditions for ~47 000 gene transcripts with the Affymetrix U133 Plus 2.0 expression array (Affymetrix, Santa Clara, CA, USA; Supplementary Table S1). To match other studies of the HeLa cell response to these gold nanoparticle–oligonucleotide complexes, we performed genome-wide expression profiling of PBMCs and 293T cells treated with gold nanoparticle–oligonucleotide complexes.12,16 Three of the five PBMC conditions (24 and 48 h after treatment with the gold nanoparticle–oligonucleotide complexes and the negative control, respectively) were assayed independently a second and a third time (technical and biological replicates, respectively) to control for experimental variability (15 microarrays hybridized in total). A different batch of these same gold nanoparticle–oligonucleotide complexes was used in the generation of the four 293T cell samples and the three biologically replicate PBMC samples, and the microarrays for these samples with calibration controls included were processed separately.
The gene expression profiles of the replicate PBMC samples were highly correlated (r2>0.92), indicating high experimental reproducibility. This was true for the 293T cell samples irrespective of the treatment condition (r2>0.98), consistent with the finding that gold nanoparticle–oligonucleotide complexes have little effect on gene expression in immortalized lineage-restricted cell types, such as 293T cells and HeLa cells.12,16 In contrast, the correlation between PBMCs and 293T cell samples was low (r2<0.46), indicating that primary human cells in the blood and 293T cells have very distinct transcription profiles. Unsupervised hierarchical clustering of the transcription profiles confirmed and refined these observations.
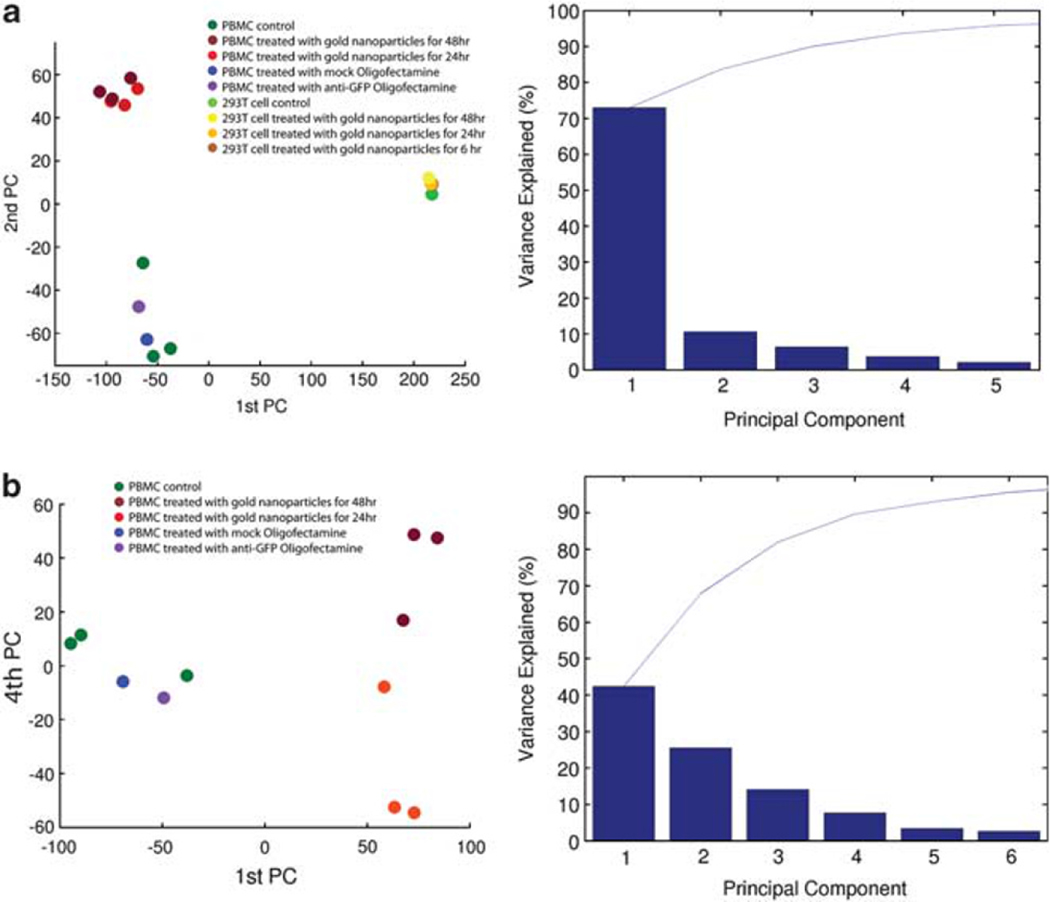
We quantified the contributions of cell type and treatment with gold nanoparticle–oligonucleotide complexes to the overall variance in the microarray data using principal components analysis. We first performed this analysis with the entire data set. The projection of the transcription profiles onto the plane spanned by the leading principal components showed that 72% of the variance in gene expression across all samples was due to the difference between the PBMC and 293T cell types (Figure 2a). The second largest source of variance (11%) was the difference between treatment of PBMCs with gold nanoparticle–oligonucleotide complexes and the EGFP oligonucleotide cationic lipid transfectant control or no treatment (Figure 2a). This difference had little effect on gene expression in 293T cells.
Figure 2.
Principal component analysis of the gene expression profiles and the distribution of the percent explained variance across the first five principal components. (a) Projection of all transcription profiles onto the plane spanned by the first two principal components, that is, the two orthogonal dimensions along which the data exhibit the largest variance (the two eigenvectors whose eigen values have the largest variances). The first principal component explains 72% of the variance in the data and corresponds to a separation of the sample set into PBMCs (left) and 293T cells (right), that is, most of the variance in gene expression across all arrays was caused by the difference in cell types. The second principal component explains 11% of the overall variance and represents the difference between treatment with gold nanoparticle–oligonucleotide complexes and oligofectamine or no treatment of PBMCs. The 293T cell samples were not separated along the second principal component axis. Accordingly, their gene expression hardly varied in response to treatment. (b) The transcription profiles are shown in the space spanned by the first and fourth principal components for the PBMC samples only, explaining 42% and 8% of the variance, respectively. Along the first principal component, the samples treated with gold nanoparticle–oligonucleotide complexes were widely separated from the other samples, that is, most of the changes in PBMC gene expression occurred between these two sample groups. The fourth principal component represented the variance introduced by the two different durations (24 h versus 48h) of treatment with gold nanoparticle–oligonucleotide complexes.
To further examine the contribution of treatment to changes in the gene expression profile, we restricted the analysis to PBMCs only (Figures 2b). For these samples, the largest source of gene expression variance (42%) was the treatment with gold nanoparticle–oligonucleotide complexes versus the EGFP oligonucleotide cationic lipid transfectant control or no treatments. The second and third principal components (26% and 14% of variance, respectively) represent the differences between medium-only-negative PBMC controls and the other controls, as well as nonbiological differences between groups of samples. This batch effect explains ~20% of the variance in the PBMC data. After adjusting for batch effects or other types of systematic bias in the microarray data using principal component analysis, we found that the source of nonbiological variation did not correlate with the differences in gene expression between PBMCs treated with gold nanoparticle–oligonucleotide complexes and PBMCs not treated or treated otherwise. Thus, any technical bias in the microarray data did not convolve true biological differences. The fourth principal component (8% of variance) partitioned the four samples treated with gold nanoparticle–oligonucleotide complexes according to the length of treatment (24 h versus 48 h).
We interrogated the gold nanoparticle–oligonucleotide complex regulatory effects in PBMCs by exploring the functional composition of significantly differentially expressed genes. At a significance level of 0.01 (multiple testing-corrected P-value <0.01), 2919 microarray probe sets detected a change in gene expression between the treated and the untreated (both the EGFP oligonucleotide cationic lipid transfectant control or no treatment) PBMCs (Supplementary Figure S3 and Supplementary Table S2). Thus, the non-targeting EGFP oligonucleotide by itself was not responsible for the cell response. We used RT-qPCR to confirm the microarray expression changes for 12 genes—evaluating the TLR signaling, T-cell activation, endocytosis and apoptosis pathways. Genes were selected based on P-values <0.05 and fold change >2 in PBMCs. RT-qPCR analysis of the representative genes experimentally confirmed the microarray data (Supplementary Table S3). This indicates that the gold nanoparticle–oligonucleotide complexes per se can have a significant impact on gene expression in PBMCs.
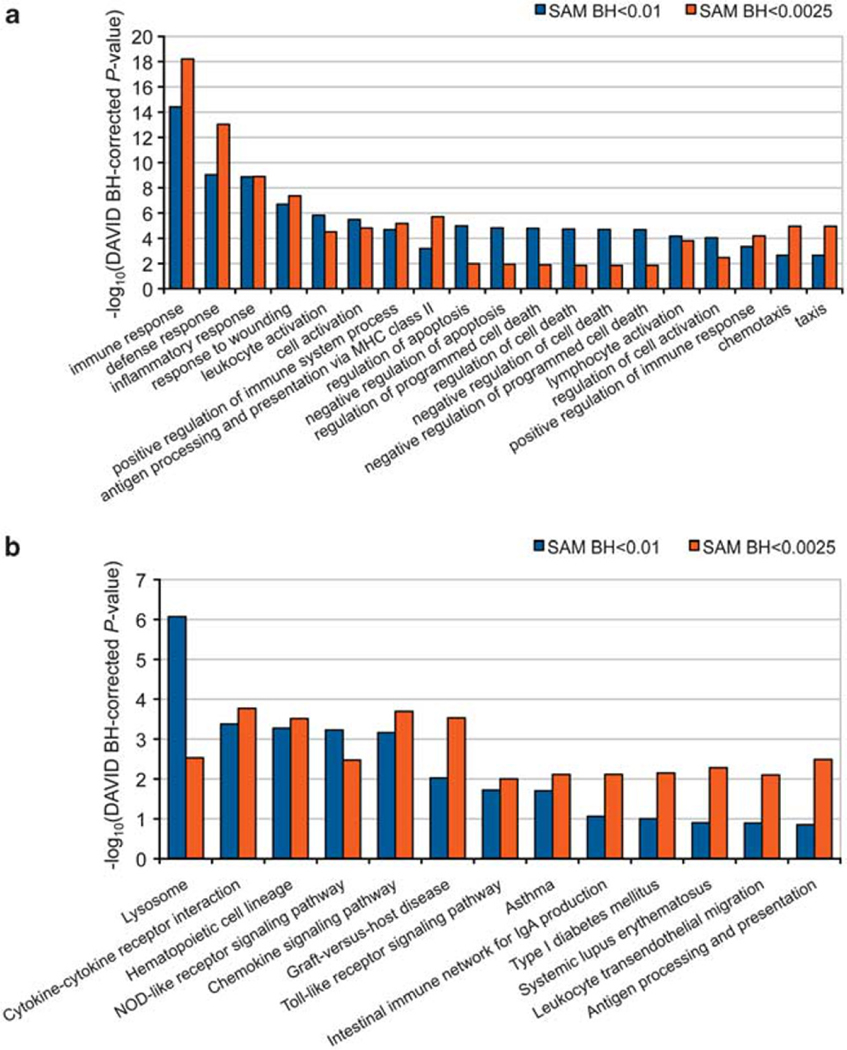
To discover dynamic changes of cellular processes, we determined the categories of functionally related genes, using gene ontology (GO) term and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis, which contained a significant number of genes with altered expression levels in response to treatment with gold nanoparticle–oligonucleotide complexes. A GO analysis revealed an overrepresentation of differentially expressed genes linked to an innate immune response (Figure 3a and Supplementary Table S4). The same result could be produced at a significance level of 0.0025 (multiple testing-corrected P-value <0.0025) for 1341 probe sets, indicating that the functional composition of the differentially expressed genes was invariant with respect to arbitrary changes of the significance level, and therefore robust. A similar pattern of enrichment was observed for the KEGG pathways that reflected the identified GO terms involved in innate immunity (Figure 3b and Supplementary Table S5; multiple testing-corrected P-value <0.001; Figure 3 and Supplementary Figure S4). Most consistently overrepresented were the immune response, inflammatory response, response to wounding and defense response categories (multiple testing-corrected P-value <10−5).
Figure 3.
The most overrepresented GO terms and KEGG pathways in PBMCs treated with gold nanoparticle–oligonucleotide complexes. Shown are the – log10-transformed multiple testing-corrected P-values of the (a) 19 most overrepresented GO term and (b) the 13 most overrepresented KEGG pathways for two significance thresholds of differential gene expression (multiple testing-corrected SAM P-value <0.01 (blue) versus P-value <0.0025 (orange)). DAVID, Database for Annotation, Visualization and Integrated Discovery; MHC, major histocompatibility complex.
We did not identify any significant changes in the gene expression profile for 293T cells treated with gold nanoparticle–oligonucleotide complexes for at least 24 h after correction for multiple testing (Supplementary Table S6). Nevertheless, we performed GO and KEGG pathway analyses for 293T cells using the genes that exhibited significant differential expression before multiple test correction to determine whether there was any overlap with respect to overrepresented gene categories (Supplementary Tables S7 and S8). We found no overlap between 293T cells and PBMCs for the raw P-value thresholds examined (P-value <0.05: 2349 probe sets, P-value <0.0125: 628 probe sets; Supplementary Figure S5). Consequently, the extent of functional gene category sharing across the gold nanoparticle–oligonucleotide complex-treated PBMC and 293T cell types is insignificant.
Although innate immune signaling receptors evolved to recognize specific microbial products, it appears that these same receptors can detect a broad range of other activating stimuli. The changes in the signaling pathways activated in response to the gold nanoparticle–oligonucleotide complexes were highly complex, suggesting that multiple PRRs were responsible for recognizing these exogenous molecules. Because the oligonucleotide-modified gold nanoparticle conjugates activate signaling cascades that lead to the induction of genes involved in cytokine signaling and immune regulation, it is not surprising that there is a direct effect on the physiological state of PBMCs and not cell lines. The engineered nanoparticles presumably interact with PRRs outside the cell and in endosomes and lysosomes to produce these signaling events in innate immune cells, such as dendritic cells, macrophages and neutrophils, among others, through mechanisms that are currently not clear. Activation of PRRs by these non-microbial-activating signals induces the synthesis and secretion of regulatory molecules known to mediate cell–cell communication that can strongly amplify the innate immune response to cellular stress.
Our results highlight the need to study the potential harmful interactions and system effects caused by engineered nanoparticle structures with a relevant biological system and the appropriate discovery tools that can identify perturbations of genes important for cell signaling and innate immunity. Our data furthermore question the mechanism by which gold nanoparticles stably functionalized with covalently attached oligonucleotides could regulate gene transcript and protein abundance.1 In light of the cell-type-specific differences in cell signaling and effector responses, our data imply that assessments of the toxic potential for engineered nanoparticles in immortalized lineage-restricted cell lines may not predict their phenotypic effects in relevant biological systems and should be interpreted with caution.
MATERIALS AND METHODS
Cell culture and DNA transfection
PBMCs derived from healthy donors were isolated on Ficoll-Paque gradients (MP Biomedicals, Santa Ana, CA, USA). 293T cells were obtained from the American Type Culture Collection. Cells were grown in 5% CO2 at 37 °C in RPMI1640 medium or Dulbecco’s modied Eagle’s medium (Invitrogen) with 10% fetal bovine serum supplemented with 100Uml−1 penicillin, 100 ìgml−1 streptomycin and 200 μM l-glutamine. Cells were seeded at 50% confluent in 24-well plates, grown for 24 h, washed with 1× phosphate-buffered saline (PBS) and fresh media was added. Antisense EGFP oligonucleotide was delivered onto the freshly washed cells using cationic lipid transfection (Oligofectamine, Invitrogen) according to the manufacturer’s direction. Gold nanoparticle–oligonucleotide complexes were filtered (0.20 μm) and added onto the freshly washed cells. After 6, 24 or 48 h, the cells were washed in 1× PBS and collected for analysis. The gold nanoparticle–oligonucleotide complexes had no shift in color from red to purple associated with their aggregation, and therefore remained stable under the cell culture conditions we used. Cell viability was assessed by the Trypan blue exclusion method (Sigma-Aldrich, St Louis, MO, USA).
Limulus amoebocyte lysate assay analysis
Because nanoparticles often interfere with limulus amoebocyte lysate assays owing to their intrinsic physicochemical properties, endotoxin contamination was assessed with the Lonza gel-clot limulus amoebocyte lysate assay format (sensitivity of 0.03 EU ml−1) using certified reagents and supplies according to the manufacturer’s instructions (Lonza BioWitthaker, Walkersville, MD, USA). A United States Pharmacopeia (USP)-certified Escherichia coli 055:B5 endotoxin standard spiked into endotoxin-free water was used to generate a standard curve and allow calculation of the input amount of endotoxin. A gold nanoparticle–oligonucleotide complex control and inhibition/enhancement control (USP-certified endotoxin standard at the standard curve midpoint concentration added to the gold nanoparticle–oligonucleotide complexes) were used to screen for potential false-positives or false-negatives.9,10 Controls were used within 30min of their preparation. Replicate samples were tested at four different dilutions in triplicate. The parameters for the gel-clot limulus amoebocyte lysate assay met the validity criteria for evaluation of endotoxin contamination as described.9
Bacterial culture
Gold nanoparticle–oligonucleotide complexes were inoculated into sterile Luria–Bertani broth without antibiotics and grown in a shaking incubator (225 r.p.m.) at 37 °C. After overnight culture, the tubes were screened for visible bacteria growth and then centrifuged (6000 g, 15min, at 4 °C) to concentrate any bacteria for RT-qPCR analysis for bacterial 16S ribosomal DNA.
Bacterial 16S ribosomal DNA
We used RT-qPCR to determine the presence of bacterial 16S ribosomal DNA in the gold nanoparticle–oligonucleotide complex preparations directly and from the concentrated overnight cultures. Samples were amplified with Taqman Universal PCR master mix reagent (Applied Biosystems, Foster City, CA, USA) as per manufacture’s protocol. Primer sequences for the bacterial 16S ribosomal gene were forward (8F: 5′-AGTTTGATCCTGGCTCAG-3′), reverse (515R: 5′-GWATTACCGCGGCKGCTG-3′) and probe (338P: 5′-FAM-GCTGCCTCCCGTAGGAGT-BHQ1-3′). After Taq polymerase activation at 94 °C for 10 min, the cycling was performed with 45 cycles of denaturation at 94 °C for 15 s and primer–probe annealing at 60 °C for 1min with the ABI 7900 Sequence Detection System (Applied Biosystems). Data were analyzed with the system’s SDS software (v2.1).17 Serial dilutions of a standard and negative controls were included to generate a standard curve and allow calculation of the input amount of bacterial 16S ribosomal DNA. Reactions were performed in triplicate and results for the reaction plate were confirmed by gel electrophoresis.
Cell imaging studies
Cells were grown in a 24-well tissue culture plate. The gold nanoparticle–oligonucleotide complexes were then added to the culture plate wells. After 6, 24, or 48 h of incubation, the cells were washed with PBS, immobilized onto chambered glass coverslip treated with Cell Tak (BD Bioscience, Bedford, MA, USA), and then fixed with 3.7% formaldehyde (Polysciences, Warrington, PA, USA) in 0.1 m PIPES buffer (pH 6.8). Cells were stained with fluorescein isothiocyanate-labeled anti-actin mouse monoclonal antibody (Cytoskeleton Inc., Denver, CO, USA) to access the cytosol and Hoechst dye to access nuclear morphology, washed and mounted on glass slides for imaging. Images were collected with a digital camera (CoolSnap HQ; Photometrics, Tucson, AZ, USA) using a DeltaVision Deconvolution Microscope equipped with a ×100 1.4 NA objective lens (Applied Precision, Issaquah, WA, USA).
Electron microscopy
Cells treated with the gold nanoparticle–oligonucleotide complexes were washed, fixed with 2% paraformaldehyde/2.5% glutaraldehyde in cacaodylate buffer (0.1 m), and then embedded in 2% agar. After additional fixation, cells dehydrated through a graded ethanol series, and infiltrated and embedded in LX112 resin at 60 °C for 24 h. Ultrathin sections (60nm) were cut from the embedded blocks, mounted onto a 200-mesh copper grid, and then stained with uranyl acetate and lead citrate by standard methods. Stained grids were examined using a FEI Tecnai Spirit G2 120 kV transmission electron microscope and imaged with a FEI Eagle 4k CCD camera (FEI, Hillsboro, OR, USA). The gold nanoparticle–oligonucleotide complex preparations were added directly onto a 3-mm diameter copper grid coated with carbon film. The particle size distribution was obtained from the transmission electron microscope images.
Ultraviolet–visible absorption spectrum
The ultraviolet–visible absorption spectrum of the gold nanoparticle–oligonucleotide complexes was recorded on a SpectraMax M2 spectrometer (Molecular Devices, Sunnyvale, CA, USA) with a spectral resolution of 1 nm.18
Microarray studies
Total RNA was extracted from cell cultures using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA). RNA quantity (concentration greater than 0.5 μg μl−1) and purity (OD260/OD280 ratio above 1.8) were verified in the extracted samples. The microarray workflow was monitored at the cDNA production, in vitro transcription, complementary RNA fragmentation and labeling, and target hybridization stages. Internal hybridization controls were included. Biotinylated complementary RNA probes prepared according to standard protocols were hybridized to Affymetrix Human HG-U133 Plus 2.0 arrays (~52 000 probe sets). The arrays were scanned with the Affymetrix GeneChip Scanner 3000 (Affymetrix). Microarray data probe-level analysis and filtering were performed using the xps v1.6.2 BioConductor package implementations of the FARMS19 and I/NI methods.20 Differential gene expression was ascertained using the siggenes v1.18.0 and multitest v2.2.0 BioConductor package implementations of the SAM and Benjamini–Hochberg multiple testing correction methods.21,22 The Matlab r2007a platform was used for correlation, hierarchical clustering and principal component analyses. The Database for Annotation, Visualization and Integrated Discovery (DAVID) tool v6.7 (http://david.abcc.ncifcrf.gov/) was used for in silico discovery of GO term and KEGG biochemical pathways.23,24 Non-informative probe sets (I/NI P-value >0.6) did not enter subsequent analyses.
RT-qPCR gene expression analysis
RT-qPCR analysis for selected genes (Supplementary Table S1) was performed using Taqman RNA assays (Applied Biosystems), according to standard protocols. Total RNA was reverse transcribed with random hexamers using a Superscript III First strand cDNA synthesis kit (Invitrogen). Messenger RNA expression was quantified by real-time PCR with an ABI 7900HT Real-Time PCR machine (Applied Biosystems). Primer sequences were designed and optimized by ABI Assays-on-Demand for human IL1B (Hs01555410_m1), JUN (Hs99999141_s1), TLR1 (Hs00413978_m1), NFKBIA (Hs00153283_m1), IL2RA (Hs00907779_m1), EHD1 (Hs00199030_m1), TLR4 (Hs00152939_m1), CLTA (Hs00747351_mH), NFKB2 (Hs00174517_m1), CTLA4 (Hs03044418_m1), FYN (Hs00176628_m1), MAP2K7 (Hs00178198_m1), GAPDH (Hs99999905_m1) and β-actin (Hs99999903_m1). Reactions were performed in triplicate and analyzed with the ABI SDS software (v2.1). Results for the duplicate samples were averaged. Values were then normalized by the amount of β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in each sample by means of the 2–ΔΔCT method.
Statistical analysis
Statistical analyses compared results from gold nanoparticle–oligonucleotide complex-treated and -untreated controls in primary immune cells microarray, flow cytometry and RT-qPCR analyses by using each analysis software and GraphPad Prism 4.1. (GraphPad Prism, San Diego, CA, USA).
Supplementary Material
ACKNOWLEDGEMENTS
The National Institutes of Health, the National Science Foundation, the United Kingdom Medical Research Council and the International Institute for Nanotechnology within Northwestern University supported this study. Data were presented at the 2009 meeting of the International Institute for Nanotechnology, Nanoscale Science and Engineering Center for Integrated Nanopatterning and Detection Technologies, Northwestern University. EK is the recipient of a Baxter Award from the Institute for BioNanotechnology in Medicine. RS is a Research Councils UK Academic Fellow. All patient samples were collected with Northwestern University Institutional Review Board (IRB) consent. We thank David Giljohann and Chad Mirkin for generously providing the gold nanoparticles stably functionalized with covalently attached oligonucleotides; Edward Campbell and Kelly Fahrbach for assistance with fluorescent microscopy; Robert Goldman and Lennell Raynolds for assistance with transmission electron microscopy; Carlos Nahas and Maurice O’Gorman for assistance with FACS analysis; and Jaejung Kim for assistance with the human microarrays. The microarray data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo) and are accessible through GSE 20677 Series accession numbers GSM518597 to GSM518609 and GSM535541 to GSM535543.
Footnotes
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AK, Han MS, Mirkin CA. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science 2006; 312: 1027–1030. [DOI] [PubMed] [Google Scholar]
- 2.Fox BA, Drury M, Hu HM, Cao Z, Huntzicker EG, Qie W et al. Lipofection indirectly increases expression of endogenous major histocompatibility complex class I molecules on tumor cells. Cancer Gene Ther 1998; 5: 307–312. [PubMed] [Google Scholar]
- 3.Hornung V, Latz E. Intracellular DNA recognition. Nat Rev Immunol 2010; 10: 123–130. [DOI] [PubMed] [Google Scholar]
- 4.Marano F, Hussain S, Rodrigues-Lima F, Baeza-Squiban A, Boland S. Nanoparticles: molecular targets and cell signalling. Arch Toxicol 2010. [DOI] [PubMed] [Google Scholar]
- 5.Stacey KJ, Ross IL, Hume DA. Electroporation and DNA-dependent cell death in murine macrophages. Immunol Cell Biol 1993; 71 (Part 2): 75–85. [DOI] [PubMed] [Google Scholar]
- 6.Brown DM, Dickson C, Duncan P, Al-Attili F, Stone V. Interaction between nanoparticles and cytokine proteins: impact on protein and particle functionality. Nanotechnology 2010; 21: 215104. [DOI] [PubMed] [Google Scholar]
- 7.Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002; 20: 197–216. [DOI] [PubMed] [Google Scholar]
- 8.Hazarika P, Giorgi T, Reibner M, Ceyhan B, Niemeyer CM. Synthesis and characterization of deoxyribonucleic acid-conjugated gold nanoparticles. Methods Mol Biol 2004; 283: 295–304. [DOI] [PubMed] [Google Scholar]
- 9.Dobrovolskaia MA, Germolec DR, Weaver JL. Evaluation of nanoparticle immunotoxicity. Nat Nanotechnol 2009; 4: 411–414. [DOI] [PubMed] [Google Scholar]
- 10.Vallhov H, Qin J, Johansson SM, Ahlborg N, Muhammed MA, Scheynius A et al. The importance of an endotoxin-free environment during the production of nanoparticles used in medical applications. Nano Lett 2006; 6: 1682–1686. [DOI] [PubMed] [Google Scholar]
- 11.Bright NA, Reaves BJ, Mullock BM, Luzio JP. Dense core lysosomes can fuse with late endosomes and are re-formed from the resultant hybrid organelles. J Cell Sci 1997; 110 (Part 17): 2027–2040. [DOI] [PubMed] [Google Scholar]
- 12.Khan JA, Pillai B, Das TK, Singh Y, Maiti S. Molecular effects of uptake of gold nanoparticles in HeLa cells. Chembiochem 2007; 8: 1237–1240. [DOI] [PubMed] [Google Scholar]
- 13.Mandal D, Maran A, Yaszemski MJ, Bolander ME, Sarkar G. Cellular uptake of gold nanoparticles directly cross-linked with carrier peptides by osteosarcoma cells. J Mater Sci Mater Med 2009; 20: 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nativo P, Prior IA, Brust M. Uptake and intracellular fate of surface-modified gold nanoparticles. ACS Nano 2008; 2: 1639–1644. [DOI] [PubMed] [Google Scholar]
- 15.Dominska M, Dykxhoorn DM. Breaking down the barriers: siRNA delivery and endosome escape. J Cell Sci 2010; 123 (Part 8): 1183–1189. [DOI] [PubMed] [Google Scholar]
- 16.Massich MD, Giljohann DA, Seferos DS, Ludlow LE, Horvath CM, Mirkin CA. Regulating immune response using polyvalent nucleic acid-gold nanoparticle conjugates. Mol Pharm 2009; 6: 1934–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis 2009; 199: 1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haiss W, Thanh NT, Aveyard J, Fernig DG. Determination of size and concentration of gold nanoparticles from UV-vis spectra. Anal Chem 2007; 79: 4215–4221. [DOI] [PubMed] [Google Scholar]
- 19.Hochreiter S, Clevert DA, Obermayer K. A new summarization method for Affymetrix probe level data. Bioinformatics 2006; 22: 943–949. [DOI] [PubMed] [Google Scholar]
- 20.Talloen W, Clevert DA, Hochreiter S, Amaratunga D, Bijnens L, Kass S et al. I/NI-calls for the exclusion of non-informative genes: a highly effective filtering tool for microarray data. Bioinformatics 2007; 23: 2897–2902. [DOI] [PubMed] [Google Scholar]
- 21.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 2001; 98: 5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoav Benjamini YH. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B (Methodol) 1995; 57: 289–300. [Google Scholar]
- 23.Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 2003; 4 (5): P3. [PubMed] [Google Scholar]
- 24.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4: 44–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.