Abstract
The structure and location of Toxoplasma gondii apicoplasts were examined in intermediate and definitive hosts and shown to vary in a stage-specific manner. Immunocytochemistry and electron microscopy studies were used to identify changes in the morphology of apicoplasts and in their enoyl reductase (ENR) content during asexual and sexual development. Apicoplasts in tachyzoites were small, multimembraned organelles anterior to nuclei that divided and segregated with the nuclei during endodyogeny. In nonproliferating bradyzoites within mature tissue cysts (1 to 24 months), apicoplasts had high levels of ENR. During coccidian development, asexual multiplication (endopolygeny), resulting in simultaneous formation of up to 30 daughters (merozoites), involved an initial growth phase associated with repeated nuclear divisions during which apicoplasts appeared as single, elongated, branched structures with increased levels of ENR. At initiation of merozoite formation, enlarged apicoplasts divided simultaneously, with constrictions, into portions that segregated to developing daughters. In sexual stages, apicoplast division did not occur during microgametogony, and apicoplasts were absent from the microgametes that were formed. In contrast, during macrogametogony, the apicoplast appeared as a large, branched, perinuclear structure that had very high levels of ENR in the absence of nuclear division. Marked increases in the size of apicoplasts and levels of ENR may be related to requirements of the macrogametocytes to synthesize and store all components necessary for oocyst formation and subsequent extracellular sporulation. Thus, it is shown that apicoplasts are present and contain ENR in all T. gondii life cycle stages except microgametes, which will result in maternal inheritance of the organelle.
Toxoplasma gondii is a member of the Apicomplexa, a diverse group containing numerous parasites of major medical and veterinary importance. T. gondii is a highly prevalent parasite that infects and then persists throughout life in the brains of approximately 30% of humans; an estimated two billion people are thought to be infected worldwide (7). It is a unique parasite, even among the cyst-forming Coccidia, in its broad host range, with all warm-blooded animals capable of acting as intermediate hosts, although only members of the cat family can act as the definitive host. This wide distribution is facilitated by a complex life cycle that allows transmission via the ingestion of either oocysts resulting from coccidian development in the cat or tissue cysts present in chronically infected intermediate hosts (4). Development in the intermediate host involves an initial acute phase with rapid tachyzoite proliferation, followed by the development of tissue cysts containing slowly dividing or resting bradyzoites. Extensive in vivo studies have shown that tachyzoites and bradyzoites divide by endodyogeny or repeated endodyogeny, a process involving the formation of two daughters within the mother cell. In contrast, the coccidian development in the cat involves both asexual and sexual (microgametogony and macrogametogony) processes. However, the process of asexual multiplication is significantly different from that seen in the intermediate host. Thus, in enterocytes, the parasite undergoes a number of nuclear divisions prior to initiation of daughter formation within the cytoplasm. Daughter formation occurs in a similar manner to endodyogeny, giving rise to the simultaneous formation of up to 30 daughters. The process has been termed endopolygeny to differentiate it from the “classical” schizogony undergone by other apicomplexans such as Plasmodium sp. or Eimeria sp. in which, after the multiple nuclear divisions, daughter formation is initiated adjacent to the plasmalemma, and the daughters form by budding into the parasitophorous vacuole. To date it has been impossible to create an in vitro model system of any aspect of the coccidian development of T. gondii.
In common with other apicomplexan parasites, T. gondii harbors a residual plastid derived from an ancient secondary endosymbiotic acquisition of an alga (37). The discovery of a vestigial plastid organelle, with a 35-kb genome, prompted reexamination of the organellar components of T. gondii. Early ultrastructural studies of tachyzoites described a multimembranous organelle of unknown function in close association with the single Golgi body located just anterior to the nucleus (33, 35). It was subsequently shown to be the location of the plastid genome in T. gondii, and the term apicoplast was proposed for this distinctive organelle (20, 24). However, large multiple multimembraned structures have also been observed in other coccidians during both asexual and sexual development, but whether they represent apicoplasts is unknown (8, 9, 12, 13). The function of the apicoplast is the subject of intense investigation as a potential target for therapeutic intervention (10, 19, 25, 27, 31, 32, 36, 38, 39). It has been shown in recent in vitro studies of tachyzoites that the apicoplast is essential for parasite viability and that tachyzoites lacking an apicoplast are able to invade, but not multiply, in new host cells (17). A number of nuclear-encoded proteins have been identified that appear to localize to the apicoplast and are involved in its biological function (19, 25, 27, 30, 32, 38, 39). These proteins have been characterized by a bipartite targeting sequence consisting of a von-Heijne secretory sequence and a chloroplast-like transit sequence, both of which are required for entry into the multimembraned organelle (25, 31, 36, 39). The presence of this bipartite apicoplast targeting sequence suggested that enoyl reductase (ENR) is localized to the apicoplast (25, 27, 30, 31), as in plants (29), and therefore that antibodies to this molecule could be a suitable marker for identifying the apicoplast.
To date, all studies on the developmental role of the apicoplast have been limited to the in vitro examination of certain asexual stages: the tachyzoites of T. gondii and the erythrocyte-associated merozoites in Plasmodium sp. (34, 36). To the best of our knowledge, the role of the T. gondii apicoplast during in vivo development has not been examined. In addition, although an apicoplast-like structure has been described in the macrogamete (13), there has been no confirmation that the structure is a functional apicoplast or that it has a specific role during macrogamete development. In the present study, immunocytochemistry, with an antibody against ENR, has been used to identify the apicoplast and correlated with parallel electron microscopic examination during in vivo development in both the intermediate host (tachyzoite or bradyzoite development) and the definitive host (coccidian development). In addition, the changes in shape and size of the apicoplast and the level of expression of ENR were semiquantitatively assessed during asexual division (endodyogeny and endopolygeny) and sexual development (microgametogony and macrogametogony) of T. gondii.
MATERIALS AND METHODS
Parasites
Parasites of the RRA, NTE, and M3 strains were used to infect mice and cats as described previously (10). In addition, mice were infected by intraperitoneal injection of the RH strain. Tissues were removed at various intervals postinfection and processed for histological examination (10).
Recombinant protein.
A construct of T. gondii ENR (TgENR) containing residues 103 to 417 (and lacking the putative signal and transit peptides) was designed for in vivo cleavage by the tobacco etch virus (TEV) protease. Amplified DNA encoding residues 103 to 417 of TgENR was ligated into a modified version of the pMALc2x vector (pMALcHT) in which the linker region was altered to contain nucleotides encoding a TEV protease cleavage site followed by a six-histidine tag (27). The resulting ligation product, pSTP8, was transformed into BL21 Star (DE3) cells (Invitrogen). These cells were cotransformed with the pRIL plasmid from BL21-CodonPlus (DE3) cells (Stratagene) and a plasmid (pKM586) encoding the TEV protease (26, 31). Cells were grown, harvested, and lysed as described (27, 31). Cell lysate was clarified by centrifugation and applied to a 5-ml HiTrap Chelating HP column (Pharmacia). Column fractions containing cut TgENR were desalted with a HiPrep 26/10 desalting column (Pharmacia) and loaded on a 5-ml HiTrap Q Fast Flow column (Pharmacia). Fractions containing pure TgENR were then flash frozen for later use in antibody production.
Antibodies.
Antibodies to recombinant ENR (27, 31) were produced in mice. Briefly, mice were injected subcutaneously with 50 μg of ENR emulsified in Freund's complete adjuvant and boosted 14 days later with 50 μg of ENR emulsified in Freund's incomplete adjuvant. In addition, the following rabbit antibodies were used for double labeling experiments: antibodies to the isoenzymes enolase 1 and 2 (14) (kindly supplied by S. Tomavo), the tachyzoite-specific marker SAG1 (15) (kindly supplied by E. Petersen), the bradyzoite-specific marker BAG1 (2) (kindly supplied by U. Gross), and NTPase (1) (kindly supplied by K. Joiner), plus antibodies to plant and bacterial FtsZ (22, 28) (kindly provided by W. Margolin and K. Osteryoung).
Western blot analysis of recombinant TgENR protein.
Recombinant ENR was produced in Escherichia coli and purified (S. P. Muench et al., unpublished observations), and recombinant TgENR protein (Muench et al., unpublished observations) was added to sodium dodecyl sulfate (SDS) sample buffer (50 mM Tris Cl, 100 mM dithiothreitol, 2% SDS, and 10% glycerol [pH 6.8]) and immediately boiled for 5 min. SDS gel electrophoresis and electroblotting onto a polyvinylidene fluoride membrane were performed according to standard protocols. The membrane was blocked overnight at 4°C with 5% fat-free powdered milk in phosphate-buffered saline (PBS-M) and cut into strips that each contained approximately 0.4 μg of recombinant TgENR protein. After three washes in PBS containing 0.1% Tween 20, strips were incubated for 1 h with anti-TgENR mouse serum or preimmune mouse serum diluted 1:4,000 in PBS-M. The strips were then washed three times in PBS containing 0.1% Tween 20 and incubated for 1 h with goat anti-mouse immunoglobulin G (IgG) peroxidase conjugate antibody (Sigma) at a ratio of 1:12,000 in PBS-M. Peroxidase activity was detected by enhanced chemiluminescence (Amersham Biosciences).
Immunocytochemistry.
Sections of cat small intestine containing the coccidian stages and mouse lung and brain containing the tachyzoite and bradyzoite stages of T. gondii were obtained as described previously (10, 14). In immunofluorescence experiments, sections were pretreated by being incubated in a stainless steel pressure cooker (Prestige Ltd., Birkenhead, United Kingdom) in sodium citrate buffer, pH 6, at full pressure for 2 min prior to staining with mouse anti-ENR either alone or in combination with rabbit anti-ENO2, anti-NTPase, anti-SAG1, or anti-BAG1. After sections were washed, they were stained with goat anti-mouse Ig conjugated to fluorescein isothiocyanate (FITC) or goat anti-rabbit Ig conjugated to Texas red and goat anti-mouse Ig conjugated to FITC. The cell nuclei were counterstained with DAPI (4′,6′-diamidino-2-phenylindole), and the sections were examined with a fluorescence microscope equipped with Openlab software (10).
Electron microscopy.
Tissue for routine electron microscopy was processed as described previously (10, 11). In summary, tissue was fixed in glutaraldehyde, postfixed in osmium tetroxide, dehydrated in ethanol, and treated with propylene oxide prior to embedding in Spurr's epoxy resin. Thin sections were stained with uranyl acetate and lead citrate and examined with a Jeol 1200EX electron microscope.
RESULTS
Western blotting.
The mouse polyclonal antibody to recombinant ENR recognizes a single protein of 33 kDa in gels prepared from tachyzoites. This is consistent with the predicted molecular mass of native ENR (Fig. 1).
FIG. 1.

Western blot of T. gondii recombinant ENR showing the negative result with the preimmune serum while the anti-ENR serum recognizes a 33-kDa protein, the predicted molecular mass of ENR.
Exo-enteric development.
When tachyzoites located in the lung and brain of acutely infected mice were examined by immunostaining with anti-ENR, a small positive-staining structure, the apicoplast, was observed just anterior to the nucleus (Fig. 2c). During tachyzoite development the apicoplast appeared elongated (Fig. 2d), and in certain cases, a central constriction appears to begin to separate the apicoplast, although there are not yet two entities (Fig. 2e). This also correlates with the shape and location of the multimembraned vacuole, which is now identified as the apicoplast, within intracellular tachyzoites observed by electron microscopy (Fig. 3a and b).
FIG. 2.
Immunofluorescent images through the brains of mice infected with T. gondii at 15 days (a to e and g), 21 days (h), and 15 months (f, i, and j) postinfection. Sections were stained with anti-SAG1/anti-BAG1 (a), anti-SAG1/anti-ENR (b to e), or anti-BAG1/anti-ENR (f to i) and visualized with secondary antibodies conjugated to Texas red (red) and FITC (green), respectively, and the nuclei were counterstained with DAPI (blue). Bars, 5 (a, b, and f) and 1 μm (c, d e, g, h, and i). (a) Small lesion in which parasites undergoing stage conversion can be identified by the presence of both SAG1+ tachyzoites (T; red) and early cysts (Cy) containing BAG1+ bradyzoites (green). (b) Similar lesion to that shown in panel a in which the tachyzoites (T) can be identified as positive for SAG1 (red) while the early tissue cysts (Cy) are unstained. It was observed that theapicoplasts in both the tachyzoites and bradyzoites were positively stained with anti-ENR (green). (c to e) Details of SAG1+ tachyzoites (red) showing the apicoplast stained with anti-ENR (green). Note the range in shape from spherical in panel c to an elongated structure in panel d and finally division into two in panel e. (f) A mature tissue cyst (15 months postinfection) showing numerous bradyzoites with BAG1+ cytoplasm (red) and ENR+ apicoplasts anterior to the nuclei (arrows). (g) An early small tissue cyst (15 days postinfection) with a few BAG1+ bradyzoites (red). Note that the bradyzoites possess an elongated ENR+ apicoplast (green) just anterior to the nucleus. (h) Small tissue cyst containing BAG1+ bradyzoites (red). Note that certain bradyzoites appear to contain possibly two ENR-positive (green) apicoplasts (arrowheads). (i) Detail from panel f showing the presence of enlarged ENR+ apicoplasts (green) within the mature bradyzoites. (j) Section stained with hematoxylin and eosin showing the morphology of a tissue cyst in mouse brain. N, nucleus.
FIG. 3.
Ultrastructural appearances of the apicoplast during tachyzoite (a and b) and bradyzoite (c to f) development. (a) Electron micrograph of a section through intracellular tachyzoite showing an enlarged mitochondrion (Mi) and an elongated structure (arrowhead) anterior to the nucleus (N). Bar, 500 nm. (b) Enlargement of the enclosed area shown in panel a showing the nucleus, the Golgi body (G), and the multimembranous apicoplast (arrow). Note that the membranes are less distinct on the face adjacent to the Golgi body (arrowheads). Bar, 100 nm. (c) Detail of the anterior cytoplasm of a bradyzoite illustrating an elongated multimembraned apicoplast (arrows). Bar, 100 nm. (d) Enlargement of a serial section of the upper apicoplast shown in panel f in which four membranes can be readily identified along the lateral surfaces (arrowheads). Bar, 100 nm. (e) Low-power electron micrograph of a section through a bradyzoite showing the posteriorly located nucleus (N), numerous polysaccharide granules (PG), micronemes (M), and rhoptry (R). CW, tissue cyst wall. Bar, 500 nm. (f) Enlargement of the enclosed area shown in panel e showing the Golgi body (G) anterior of which are dense granules (DG) and profiles of two apicoplasts (A). Bar, 100 nm.
It was not possible to identify the apicoplast by using DAPI staining in tissue sections. It is probable that the additional processing involved in producing the tissue sections reduces the sensitivity of the DAPI staining.
To directly compare the apicoplast during tachyzoite and bradyzoite development, brain lesions at 15 days postinfection were examined. In these lesions, stage conversion was taking place, and it was possible to identify both tachyzoites and bradyzoites within early tissue cysts by immunocytochemistry and electron microscopy. The presence of both tachyzoites and bradyzoites was confirmed by double labeling with antibodies to the stage-specific antigens SAG1 (tachyzoite specific) and BAG1 (bradyzoite specific) (Fig. 2a). Examination of both tachyzoite and bradyzoite apicoplasts by using antibodies to ENR allowed direct comparison of the organelle in identically treated parasites (Fig. 2b). The apicoplast of the tachyzoite was as described above (Fig. 2c to e). In the early tissue cysts, the BAG1-positive bradyzoites contained elongated ENR-positive apicoplasts (Fig. 2g), and in certain cases there appeared to be two structures (Fig. 2h). However, as observed by electron microscopy, many bradyzoites in early tissue cysts were undergoing repeated cycles of endodyogeny (11), and the changes in the bradyzoite apicoplast could relate to cell division.
To examine the situation within tissue cysts in chronically infected mice (1 to 24 months postinfection) when little or no bradyzoite division is occurring (11), the tissue cysts in the brain (Fig. 2j) were double labeled with anti-ENR and anti-BAG1. It was observed that all the BAG1-positive bradyzoites continued to display an elongated ENR-positive structure located just anterior to the nucleus in tissues obtained from throughout the 24-month study period (Fig. 2g and i).
When examined by electron microscopy, the characteristic multimembraned apicoplast could be identified in bradyzoites (Fig. 3c, d, e, and f). The large elongated and possibly multiple apicoplasts in the nondividing bradyzoite contrasts with the small subspherical structure found in the nondividing tachyzoite. In this situation, the changes in the apicoplast cannot be related to parasite division since no proliferation is occurring.
Coccidian development.
Coccidian development was examined in sections of the small intestine of a cat infected with T. gondii. It was possible to identify various coccidian stages involving asexual development (endopolygeny) and sexual development (microgametogony and macrogametogony) by using light microscopy (Fig. 4a, d, and i, and 5a and c). For immunocytochemistry, sections were labeled with anti-ENR or double labeled with anti-ENR and either anti-ENO2 or anti-NTPase and counterstained with DAPI. These staining combinations assisted in the identification of the various developmental stages, with the ENO2 strongly labeling the active nuclei of the trophozoites and early- to mid-stage schizonts and also the cytoplasm of the macrogametocyte and with the anti-NTPase localizing to the parasitophorous vacuole and the dense granules within mature merozoites (10, 14). During the early stages of intracellular development a single small low-intensity staining structure was observed, but this increased in size as the trophozoite enlarged (Fig. 4b). In the case of asexual development, as the parasite grew it underwent a number of nuclear divisions (Fig. 4a and d). During this process, the apicoplast developed into an elongated, branched tubular structure and displayed what appears to be a significantly increased ENR signal (Fig. 4b, c, e, and f). In control sections stained with preimmune serum, no ENR signal was observed (Fig. 4g). In the later stages of asexual development, the apicoplast exhibited multiple constrictions (Fig. 4e) and divided into a number of portions (Fig. 4 h). It appeared that these apicoplasts became associated with the nuclei at the initiation of daughter formation, and a single apicoplast segregated within each developing daughter and appeared as a small spherical structure with relatively low signal in the fully formed merozoites (Fig. 4j). In the extracellular merozoite in the gut lumen, the apicoplast was located adjacent to the nucleus and between it and the NTPase-positive dense granules (Fig. 4k and l).
FIG. 4.
Sections illustrating the coccidian (asexual and sexual) development of T. gondii in the villus of the small intestine of the cat. Panels a, d, and i show 1-μm plastic sections stained with Azure A from material processed for electron microscopy. Panels b, c, e, f, h, j, k, and l are sections that have been immunolabeled with anti-ENR in combination with other primary antibodies visualized with FITC (green) and Texas red (red), respectively. Panel g shows a control section stained with preimmune sera. The nuclei stained with DAPI (blue). Bars, 5 μm. (a) Part of a villus showing the morphological appearances of the developing parasites within the enterocytes. Trophozites (Tr), macrogametocytes (Ma), and multinucleate parasites can be identified. (b) Low-power longitudinal section of a villus stained for ENR (green) and NTPase (red) showing the parasitophorous vacuoles positive for NTPase (red). Note the small ENR+ (green) apicoplasts associated with the small uninuclear parasites (arrowheads) but the larger structure and stronger signal from the multinucleate stage (arrow). (c) Section of a villus stained for ENR (green) and ENO2 (red) showing numerous strongly labeled nuclei for ENO2 of parasites with single and multiple nuclei. Note the strongly labeled large elongated structure centrally located in the multinucleate mid-stage schizonts (arrows) in comparison to the small structure associated with the uninuclear trophozoite (arrowhead). (d) Enlargement of a multinucleate mid-stage schizont. (e) Section stained for ENR (green) and ENO2 (red) showing an elongate apicoplast (arrow) stretching between the ENO+ (red) nuclei. Possible constriction in the apicoplast can be seen (arrowheads). (f) A mid-stage schizont single labeled for ENR (green) showing the complex branched structure of the apicoplast (arrow). (g) Control section stained with the preimmune serum showing the absence of any ENR labeling (green). The presence of parasites is confirmed by the DAPI staining of the nuclei. (h) Section stained for ENR (green) and ENO2 (red) showing a number of ENO2 positive nuclei and an apparently fragmented apicoplast (A). (i) Detail of a mature schizont showing the structure of the fully formed merozoites (Me). (j) Section through a mature schizont stained for ENR (green) and NTPase (red) showing the dense granules (DG) in the apical cytoplasm of the merozoites with the small apicoplast (arrowheads) located between them and the nucleus. (k) Extracellular merozoites stained for ENR (green) and ENO2 (red), which in the mature merozoites only stains the cytoplasm, showing the small spherical apicoplast (arrowhead) located adjacent to the nucleus. (l) Extracellular merozoites stained for ENR (green) and NTPase (red) showing the small spherical apicoplast located between the nucleus and the dense granules (DG). N, nucleus.
FIG. 5.
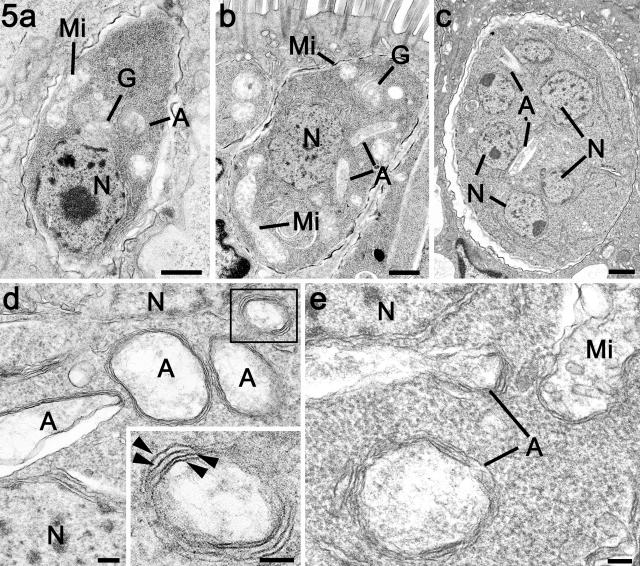
Electron micrographs illustrating the early stages of asexual development in the small intestine of the cat. (a) An early intracellular trophozoite showing the nucleus, Golgi body, mitochondrion, and apicoplast. Bar, 1 μm. (b) Later and larger trophozoite showing the increase in the size of the mitochondria and the apicoplast. Bar, 1 μm. (c) Mid-stage schizont with a number of nuclei and a centrally located elongated apicoplast. Bar, 1 μm. (d and e) Details from a multinucleated schizonts similar to that shown in panel c showing a number of profiles through the multimembraned apicoplast. Bar, 100 nm. (Inset) Enlargement of the enclosed area in panel d in which the presence of four membranes can be resolved (arrowheads). Bar, 100 nm. A, apicoplast; N, nucleus; G, Golgi body; Mi, mitochondria.
This finding was consistent with the ultrastructural observations where the early stages were characterized by an increase in the size of both the mitochondrion and the apicoplast prior to nuclear division (Fig. 5a and b). In the schizont, growth is accompanied by a number of nuclear divisions (Fig. 5c). In the larger, multinucleated stage prior to initiation of daughter formation, there were more numerous profiles of apicoplast-like structures (Fig. 5d and e) enclosed by possibly four unit membranes (Fig. 5d, inset). These observations would be consistent with the elongated branch structure seen by immunocytochemistry. During the later stages, daughter formation occurs within the cytoplasm in a manner similar to endodyogeny (Fig. 6a). At this stage, apicoplasts were observed to be associated with the nuclei and developing daughters (Fig. 6a, b, and c). In the developing and fully formed merozoites, the apicoplast was identified as a small structure adjacent to the nucleus (Fig. 6d).
FIG. 6.
(a) Low-power electron micrograph of a section through a late-stage schizont in which the early stages in the formation of a number of daughters (D) can be seen within the cytoplasm. Bar, 1 μm. (b) Detail of part of panel a showing the inner membrane complex of the developing daughters becoming associated with the nuclei. Note an apicoplast apparently entering a daughter. C, conoid; R, rhoptry; bar, 100 nm. (c) Detail of the cytoplasm of a late-stage schizont showing the formation of the apical end of the daughters within the cytoplasm. The daughters consist of the conoid (C), rhoptry precursor (R), and the membrane complex directed toward the nuclei. At this time apicoplasts can be seen adjacent to the nuclear pole (NP) and apparently entering the developing daughter. G, Golgi body; bar, 100 nm. (d) Part of a mature schizont showing the fully formed merozoite containing a nucleus, rhoptries (R), dense granules (DG), and small apicoplast adjacent to the nucleus. Bar, 500 nm. N, nucleus; A, apicoplast.
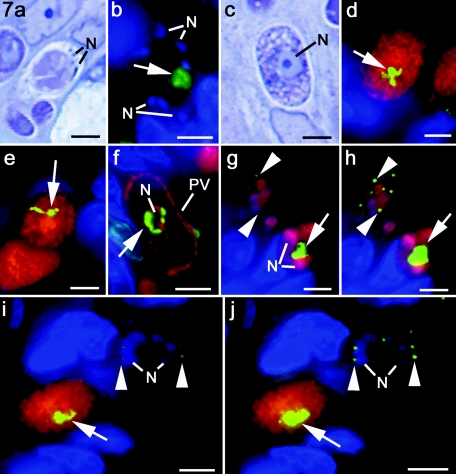
In summary, when asexual development is initiated, the apicoplast is a single spherical low-intensity structure. During growth and nuclear division, and prior to the initiation of daughter formation, the apicoplast enlarged into a centrally located, elongated branched structure with a marked increase in staining intensity. In the final stages during merozoite formation, the apicoplast divides and segregates, with each daughter receiving a single low-intensity staining structure. This disparity in staining intensity is best illustrated by examining the same field of parasites at two levels of exposure in the green (apicoplast-specific) channel. Note that when there is saturation of the signal from the apicoplast in the developing schizont (Fig. 7g) and macrogametocyte (Fig. 7i), the apicoplast of the merozoites in the mature schizont is difficult to discern. However, the presence of the apicoplast within the merozoites could be confirmed by amplifying the signal in the green channel (Fig. 7h and j).
FIG. 7.
Sections illustrating the sexual development of T. gondii in the villi of the small intestine of the cat. Panels a and c show 1-μm plastic sections stained with Azure A from material processed for electron microscopy. Panel b and panels c to j are sections that have been immunolabeled with anti-ENR in combination with other primary antibodies visualized with FITC (green) and Texas red (red), respectively. Bars, 5 μm. (a) Mature microgametocyte with a few microgametes, identified by their dense staining nucleus, located within the parasitophorous vacuole. (b) Similar stage to that shown in panel a immunostained for ENR (green) showing a single apicoplast (arrow) with a relatively low level of signal, while the microgamete nuclei are strongly stained with DAPI. (c) Section through a macrogametocyte showing the large nucleus with its nucleolus and the cytoplasm packed with granules. (d and e) Sections through macrogametes stained for ENR (green) and ENO2 (red) showing the cytoplasm labeled for ENO2 and a very strong ENR signal from a centrally located irregular shaped structure (arrow). (f) Macrogametes stained for ENR (green) and NTPase (red) showing NTPase labeling of the parasitophorous vacuole (PV) and ENR+ structure associated with the central nucleus. (g to j) Duplicate images of sections stained for ENR (green) and ENO2 (red) but in which one image shows saturation of the ENR signal within the apicoplast (arrow) of the multinucleate mid-stage schizont (g) or macrogametocyte (i), at which point it is difficult to identify the apicoplasts within the merozoites of the mature schizont (arrowheads). In the second pair of images (h and j) the presence of the apicoplasts within the merozoites was confirmed by increasing the level of signal in the green channel (arrowheads). N, nucleus.
It is more difficult to follow microgametogony because there are relatively few microgametocytes compared to the number of asexual stages and macrogametocytes (7). However, more mature microgametocytes can be identified by light microscopy due to the formation of the characteristic very dense comma-shaped nuclei of the microgametes around the periphery of the microgametocyte (Fig. 7a). Within the late microgametocytes, a single enlarged apicoplast was observed, but it displayed relatively low-level staining (Fig. 7b). There was no apicoplast division and the microgametes lacked an apicoplast. Again, this finding was consistent with details at the ultrastructural level, where it was possible to identify an apicoplast-like structure within the microgametocyte (data not shown). However, no such structure was ever observed within the microgametes, which consist of a nucleus, mitochondrion, and two flagella (12).
The early stages of macrogametogony can be identified by an increase in size but with no division of the nucleus. The mid to late stages can be identified due the granular cytoplasm (Fig. 7c). In the mid- and late-stage macrogametocytes, it was possible to identify lobulated or branched structures with a very strong ENR signal (Fig. 7d to f). They appeared in the central region of the cell, giving them a perinuclear location (Fig. 7f). An unexpected feature was that the single large nucleus of the macrogametocyte did not stain with DAPI. This may relate to the dispersal of the DNA within the large nucleus. A few mature macrogametes did not possess a strongly staining structure, but it is unclear whether this represents a down-regulation in the activity of the apicoplast in the mature macrogamete or early oocyst or a staining artifact due to the impenetrable nature of the developing oocyst wall.
By electron microscopy, the nucleus of the macrogametocyte is large with diffusely distributed chromatin and one large nucleolus (Fig. 8a). Electron microscopy also confirms the perinuclear location of multiple profiles of multimembraned vacuoles (Fig. 8a), which is consistent with the irregularly shaped structure observed by immunocytochemistry. During macrogametocyte development, wall-forming bodies of two types are synthesized, and numerous polysaccharide granules and a number of lipid droplets are deposited in the cytoplasm (Fig. 8a).
FIG. 8.
(a) Mature macrogametocyte showing the centrally located nucleus (N) with adjacent apicoplasts (A). The cytoplasm contains a number of wall-forming bodies type 1 (W1), a few wall-forming bodies type 2 (W2), and numerous polysaccharide granules (PG) and lipid droplets (L). Bar, 1 μm. (b) Enlargement of the apicoplast shown in panel a illustrating the complex shape and the formation of constriction rings (arrows). Bar, 100 nm. (c) Detail of an apicoplast within a developing schizont showing ring-like constriction (arrow). Bar, 100 nm.
Structure of the apicoplast.
In the present study it is possible to illustrate numerous examples of the apicoplast being limited by four unit membranes (Fig. 3d, 5d [inset], and 8b and c). However, in many sections, in certain areas of the periphery of the apicoplast the membranes appeared less distinct with fewer than four membranes being identified (Fig. 3d).
Division of the apicoplast.
The division of the apicoplast was examined during coccidian development. By immunofluorescence microscopy it appeared that during asexual multiplication, the large elongated apicoplast developed multiple constrictions that resulted in division of the apicoplast into a number of fragments (Fig. 4e and h). When viewed by electron microscopy, during both endopolygony and macrogametogony, the enlarged apicoplast exhibited multiple ring-like constrictions (Fig. 8b and c). There was fine dense material at these locations, which may be involved in apicoplast division (Fig. 8b and c). No other organelle appeared to be associated with the constrictions. However, there did appear to be a strong association between the apicoplast and the nucleus during the latter stages of daughter formation and throughout macrogametogony.
DISCUSSION
We previously reported the identification, cloning, and expression of biochemically active TgENR (25, 31). Herein, we immunized mice and produced polyclonal antibodies to this recombinant TgENR (Muench et al., unpublished observations). This antibody recognized a single band of the correct molecular weight in Western blots. We were then able to determine the presence of the enzyme and visualize apicoplast division and segregation in vivo in the coccidian life cycle stages in the cat intestine as well as the tachyzoite and bradyzoite in murine tissues.
We used electron micrographs of each of these stages to determine whether the localization of ENR coincides with the location and morphology of the apicoplast. Due to technical difficulties, it was not possible to confirm the localization of ENR by immunoelectron microscopy. We can confirm that in tachyzoites, ENR is located within the apicoplast in an identical manner to that described for in vitro studies of other apicoplast markers and green fluorescent protein (34).
In bradyzoites, the apicoplasts appeared elongated and, in certain cases, possibly two were present, and this was consistent with ultrastructural observations. However, in the early tissue cysts, it is known from electron microscopy that many of the bradyzoites are undergoing endodyogeny (11). Therefore, the increased size and duplicated apicoplasts within bradyzoites at 15 and 21 days postinfection could be related to continued parasite proliferation. However, the observation of strongly ENR-positive elongated apicoplasts within the bradyzoites in cysts at 1 to 24 months after infection cannot be associated with endodyogeny. In this situation, the increase in size may relate to a continuing physiological function within the “resting” bradyzoite. It has recently been reported for bradyzoites produced by in vitro cell culture that there is poor segregation of the apicoplast to the daughters, resulting in significant proportions of nonviable bradyzoites lacking an apicoplast (6). However, this missegregation was not observed in the present in vivo study. The in vitro study also reported that a proportion of parasites were undergoing “endopolygony” in addition to endodyogeny (5), which again contrasts with in vivo observations of only endodyogeny (11). It is possible that these differences may represent anomalies produced by the in vitro culture system.
Little is known about the molecular aspects of the coccidian stages of T. gondii, largely due to the difficulty in obtaining such stages for examination. Unfortunately, it has been impossible to culture any of the asexual or sexual stages that develop in the enterocytes of the cat. In the cat intestine, the unique form of asexual multiplication has been termed endopolygony and differs from endodyogeny in that a growth phase is associated with a number of nuclear divisions prior to the initiation of daughter formation, resulting in the simultaneous formation of a number (range, 16 to 30) of merozoites. In this aspect the process is similar to the classical schizogony undergone by many other apicomplexan parasites. The major difference between endopolygony and classical schizogony is that in schizogony, the nuclei move to the periphery and daughter formation is initiated directly beneath the plasmalemma, with the daughters budding into the parasitophorous vacuole. In endopolygony, the daughters form within the mother cell cytoplasm. When the apicoplast was examined during the initial growth and nuclear division phase, there was a marked increase in the size of the apicoplast and level of ENR signal. The apicoplast appeared as a single elongated or branched structure surrounded by a number of nuclei. This is similar to that reported for the apicoplast during schizogony in Plasmodium falciparum within erythrocytes (36). In the present semiquantitative study, the size and level of signal appear greater than required to provide a single small apicoplast to each daughter. It is possible that the increase in size and level of ENR signal is related to an increased physiological function of the apicoplast during the initial growth phase. It was noted that the increase in size of the apicoplast coincided with an increase in the size of the mitochondrion, representing evidence of increased metabolic activity. However, the growth phase during microgametogony does not appear to be associated with the marked increase in ENR signal. Since the microgametes lack an apicoplast, there is no need for the apicoplast to replicate, and this may explain the reduced size and signal. This also means that apicoplasts can only be inherited via the female (macrogamete) lineage, consistent with observations for Plasmodium sp. (3). The finding of an enlarged, strongly positive ENR apicoplast adjacent to the nucleus during the development of the macrogamete is consistent with the ultrastructural observations of multimembranous vacuoles. This is formal confirmation that these vacuoles are apicoplasts that contain ENR in this life cycle stage. The present study shows for the first time that the developing macrogametocyte has an enlarged apicoplast with what appears to be a highly elevated ENR signal. In this situation, the changes to the apicoplast are unrelated to nuclear division since none occurs within the macrogamete. However, macrogametogony is associated with the synthesis of the wall-forming bodies required to form the oocyst wall and storage material in the form of polysaccharide granules and lipid droplets. Indeed, the mature macrogamete must contain all the nutrients required for oocyst formation and extracellular sporulation. Sporulation is the only time T. gondii undergoes extracellular multiplication and results in the formation of eight daughters (sporozoites). In the absence of any access to additional nutrients while in the external environment, the macrogamete must have synthesized and/or stored all the material required to form eight haploid daughters. In addition to a metabolic role, it is possible that the increase in size of the apicoplast during macrogametogony may relate to its need to produce eight individual apicoplasts during sporulation.
The apicoplast is one of three genome-containing organelles in T. gondii, the others being the nucleus and mitochondrion. During asexual division these organelles are required to replicate their genomes, divide, and then segregate in such a manner that each daughter (whether two or more) receives one copy of each organelle and the accompanying organellar genome. Within the Apicomplexa, mitosis differs from that of other eukaryotes in that the nuclear membrane remains intact during the entire process. This involves an intranuclear spindle with the nuclear poles moving apart, but for the nucleus to finally divide in two, there is development of an inner and outer membrane to form a plane of nucleokinesis (5). However, the molecular mechanism involved in this process is still unknown. The process of apicoplast division and segregation has been elegantly studied in vitro during the formation of two daughters (endodyogeny) in the tachyzoite of T. gondii (23, 34). However, the studies reached different conclusions. Though both reports proposed that the apicoplast remains intimately associated with the centrioles or nuclear poles during nuclear division and segregation, one proposed that the apicoplast divides by a purely physical pull-push type of mechanism with the centrioles pulling the apicoplast into an elongated structure and the inner membrane complex of the daughters pushing into the midpoint (34). In contrast, the second study suggested that apicoplast division may occur before nuclear division and involve possible division or constriction rings (23). A purely external physical process would be unprecedented, as it contrasts with the way plastids divide in plants and algae, where there is formation of some form of division or constriction rings (reviewed in references 16, 21, 26, and 28). The physical hypothesis was partially based on the absence of FtsZ or its homologues from the T. gondii genome (reference 34 and F. L. Henriquez, unpublished observations). FtsZ is an important protein in bacterial division, and homologues are involved in chloroplast division in algae and plants, but other organelles, such as mitochondria, have evolved a mechanism that does not employ FtsZ (16). Indeed homologues to proteins thought to be involved in this alternative process, such as MinD and two isoforms of dynamin, were identified in the T. gondii genome database (Henriquez, unpublished observations). minD could not be amplified from cDNA generated from tachyzoites by using PCR, but the same primer set was able to amplify this gene from T. gondii genomic DNA (Henriquez, unpublished observations). This confirms that the minD gene is not a contaminant in the database. Identifying the genes encoding dynamin and MinD and demonstrating genomic DNA for minD does not necessarily indicate that they are required for division of the apicoplast in the different life cycle stages. Future mechanistic studies to characterize the roles, if any, of these and other proteins in organellar division will be of considerable interest. In addition, endodyogeny represents the most basic form of apicomplexan multiplication and is limited to a few cyst-forming coccidians. The situation is much more complex during endopolygony and schizogony, where multiple daughters are produced simultaneously. When the apicoplast was examined during endopolygony in T. gondii, it was observed that the initial nuclear divisions were dissociated from apicoplast division, with multinucleate stages possessing a single, enlarged apicoplast. This process was similar to that described during schizogony in P. falciparum (36). These authors proposed that apicoplast division was delayed until after nuclear division by an unknown feedback mechanism that determined how many portions the apicoplast would divide into (36). There are still many unanswered questions in relation to apicoplast division. Similarly, little is known about the process of mitochondrial division and segregation.
There has been much discussion about the exact structure of the apicoplast. In certain cases, four membranes have been identified as limiting the apicoplast. In the present study, it was possible to clearly identify four membranes, but in most organelles there were areas where only two or three membranes could be resolved. Three membranes have been suggested for Plasmodium (18) and it is possible that P. falciparum has lost a membrane during evolution, as has been reported for certain dinoflagellates (reviewed in reference 38). However, the multiple-membranous (whether three or four) nature of the structure clearly allows it to be differentiated from the mitochondrion and the nucleus, which are limited by two unit membranes.
In conclusion, we have identified apicoplasts containing ENR in all stages of parasite development, with the exception of the microgamete. The absence of an apicoplast and ENR in the microgamete indicates that there is maternal (macrogamete) mediated inheritance of this essential organelle.
Acknowledgments
We thank the following for kindly providing antibodies: S. Tomovo (anti-enolase 1 and 2), E. Petersen (anti-SAG1), U. Gross (anti-BAG1), K. Joiner (anti-NTPase), and K. Osteryoung and W. Margolin (anti-FtsZs). We thank L. Kelley for her assistance with preparation of the manuscript.
This work was supported by NIH grants R01 AI43228 (R.M. and C.W.R.) and AI27530 (R.M.), the BBSRC (D.W.R.), and an equipment grant from the Wellcome Trust Fund (D.J.P.F.). R. McLeod was the Jules and Doris Stein RPB Professor at the University of Chicago.
REFERENCES
- 1.Bermudes, D., K. R. Peck, M. A. Afifi, C. J. Beckers, and K. A. Joiner. 1994. Tandemly repeated genes encode nucleoside triphosphate hydrolase isoforms secreted into the parasitophorous vacuole of Toxoplasma gondii. J. Biol. Chem. 269:29252-29260. [PubMed] [Google Scholar]
- 2.Bohne, W., U. Gross, D. J. P. Ferguson, and J. Heesemann. 1995. Cloning and characterization of a bradyzoite-specifically expressed gene (hsp30/bag1) of Toxoplasma gondii, related to genes encoding small heat-shock proteins of plants. Mol. Microbiol. 16:1221-1230. [DOI] [PubMed] [Google Scholar]
- 3.Creasey, A., K. Mendis, J. Carlton, D. Williamson, I. Wilson, and R. Carter. 1994. Maternal inheritance of extrachromosomal DNA in malaria parasites. Mol. Biochem. Parasitol. 65:95-98. [DOI] [PubMed] [Google Scholar]
- 4.Dubey, J. P., I. T. Navarro, C. Sreekumar, E. Dahl, R. L. Freire, H. H. Kawabata, M. C. B. Vianna, O. C. H. Kwok, S. K. Shen, P. Thulliez, and T. Lehmann. 2004. Toxoplasma gondii infections in cats from Parana, Brazil: seroprevalence, tissue distribution, and biologic and genetic characterization of isolates. J. Parasitol. 90:721-726. [DOI] [PubMed] [Google Scholar]
- 5.Dubremetz, J. F. 1973. Etude ultrastructurale de la mitosis schizogonique chez la coccidie Eimeria necatrix. J. Ultrastruct. Res. 42:354-376. [PubMed] [Google Scholar]
- 6.Dzierszinski, F., M. Nishi, L. Ouko, and D. S. Roos. 2004. Dynamics of Toxoplasma gondii differentiation. Eukaryot. Cell 3:992-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson, D. J. P. 2002. Toxoplasma gondii and sex: essential or optional extra. Trends Parasitol. 18:355-359. [PubMed] [Google Scholar]
- 8.Ferguson, D. J. P., A. Birch-Andersen, W. M. Hutchison, and J. C. Siim. 1976. Ultrastructural studies on the endogenous development of Eimeria brunetti I. Schizogony. Acta Path. Microbiol. Scand. Sect. B 84:401-413. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson, D. J. P., A. Birch-Andersen, W. M. Hutchison, and J. C. Siim. 1977. Ultrastructural studies on the endogenous development of Eimeria brunetti III. Macrogametogony and the macrogamete. Acta. Path. Microbiol. Scand. Sect. B 85:78-88. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson, D. J. P., M.-F. Cesbron-Delauw, J.-F. Dubremetz, L. D. Sibley, K. A. Joiner, and S. E. Wright. 1999. The expression and distribution of dense granule proteins in the enteric (coccidian) forms of Toxoplasma gondii in the small intestine of the cat. Exp. Parasitol. 91:203-211. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson, D. J. P., and W. M. Hutchison. 1987. An ultrastructural study of early development and tissue cyst formation of Toxoplasma gondii in the brains of mice. Parasitol. Res. 73:483-491. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson, D. J. P., W. M. Hutchison, J. F. Dunachie, and J. C. Siim. 1974. Ultrastructural study of early stages of asexual multiplication and microgametogony of Toxoplasma gondii in the small intestine of the cat. Acta Path. Microbiol. Scand. Sect. B 82:167-181. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson, D. J. P., W. M. Hutchison, and J. C. Siim. 1975. The ultrastructural development of the macrogamete and formation of the oocyst wall of Toxoplasma gondii. Acta Path. Microbiol. Scand. Sect. B 83:491-505. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson, D. J. P., S. F. Parmley, and S. Tomavo. 2002. Evidence for nuclear location of two stage-specific isoenzymes of enolase in Toxoplasma gondii correlates with active parasite replication. Int. J. Parasitol. 32:1399-1410. [DOI] [PubMed] [Google Scholar]
- 15.Harning, D., J. Spenter, A. Metsis, J. Vuust, and E. Petersen. 1996. Recombinant Toxoplasma gondii surface antigen 1 (P30) expressed in Escherichia coli is recognized by human Toxoplasma-specific immunoglobulin M (IgM) and IgG antibodies. Clin. Diagn. Lab. Immunol. 3:355-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto, H. 2003. Plastid division: its origins and evolution. Int. Rev. Cytol. 222:63-98. [DOI] [PubMed] [Google Scholar]
- 17.He, C. Y., M. K. Shaw, C. H. Pletcher, B. Striepen, L. G. Tilney, and D. S. Roos. 2001. A plastid segregation defect in the protozoan parasite Toxoplasma gondii. EMBO J. 20:330-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins, J., R. Fowler, S. Krishna, I. Wilson, G. Mitchell, and L. Bannister. 1999. The plastid in Plasmodium falciparum asexual blood stages: a three-dimensional ultrastructural analysis. Protist 150:283-295. [DOI] [PubMed] [Google Scholar]
- 19.Jomaa, H., J. Wiesner, S. Sanderbrand, B. Altincicek, C. Weidemeyer, M. Hintz, I. Turbachova, M. Eberl, J. Zeidler, H. K. Lichtenthaler, D. Soldati, and E. Beck. 1999. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 285:1573-1576. [DOI] [PubMed] [Google Scholar]
- 20.Kohler, S., C. F. Delwiche, P. W. Denny, L. G. Tilney, P. Webster, R. J. Wilson, J. D. Palmer, and D. S. Roos. 1997. A plastid of probable green algal origin in Apicomplexan parasites. Science 275:1485-1489. [DOI] [PubMed] [Google Scholar]
- 21.Kuroiwa, T., H. Kuroiwa, A. Sakai, H. Takahashi, K. Toda, and R. Itoh. 1998. The division apparatus of plastids and mitochondria. Int. Rev. Cytol. 181:1-41. [DOI] [PubMed] [Google Scholar]
- 22.Ma, X., and W. Margolin. 1999. Genetic and function analyses of the conserved C-terminal core domain of Escherichia coli FtsZ. J. Bacteriol. 181:7531-7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuzaki, M., T. Kikuchi, K. Kita, S. Kojima, and T. Kuroiwa. 2001. Large amounts of apicoplast nucleoid DNA and its segregation in Toxoplasma gondii. Protoplasma 218:180-191. [DOI] [PubMed] [Google Scholar]
- 24.McFadden, G. I., M. E. Reith, J. Munholland, and N. Lang-Unnasch. 1996. Plastid in human parasites. Nature 381:482. [DOI] [PubMed] [Google Scholar]
- 25.McLeod, R., S. P. Muench, J. B. Rafferty, D. E. Kyle, E. J. Mui, M. J. Kirisits, D. G. Mack, C. W. Roberts, B. U. Samuel, R. E. Lyons, M. A. Dorris, W. K. Milhous, and D. W. Rice. 2001. Triclosan inhibits the growth of Plasmodium falciparum and Toxoplasma gondii by inhibition of Apicomplexan Fab I. Int. J. Parasitol. 31:109-113. [DOI] [PubMed] [Google Scholar]
- 26.Miyagishima, S. Y., K. Nishida, and T. Kuroiwa. 2003. An evolutionary puzzle: chloroplast and mitochondrial division rings. Trends Plant Sci. 8:432-438. [DOI] [PubMed] [Google Scholar]
- 27.Muench, S. P., J. B. Rafferty, R. McLeod, D. W. Rice, and S. T. Prigge. 2003. Expression, purification and crystallization of the Plasmodium falciparum enoyl reductase. Acta Crystallogr. D 59:1246-1248. [DOI] [PubMed] [Google Scholar]
- 28.Osteryoung, K. W., and J. Nunnari. 2003. The division of endosymbiotic organelles. Science 302:1698-1704. [DOI] [PubMed] [Google Scholar]
- 29.Rafferty, J. B., J. J. Simon, A. R. Stuitje, A. R. Slabas, T. Fawcett, D. W. Rice. 1994. Crystallization of the NADH-specific enoyl acyl carrier protein reductase from Brassica napus. J. Mol. Biol. 237:240-242. [DOI] [PubMed] [Google Scholar]
- 30.Roberts, C. W., R. McLeod, D. W. Rice, M. Ginger, M. L. Chance, and L. J. Goad. 2003. Fatty acid and sterol metabolism: potential antimicrobial targets in apicomplexan and trypanosomatic parasitic protozoa. Mol. Biochem. Parasitol. 126:129-142. [DOI] [PubMed] [Google Scholar]
- 31.Samuel, B. J., B. Hearn, D. G. Mack, P. Wender, J. Rothbard, M. J. Kirisits, E. Mui, C. W. Roberts, S. T. Prigge, D. W. Rice, S. P. Muench, A. B. Law, and R. McLeod. 2003. Delivery of antimicrobials into parasites. Proc. Natl. Acad. Sci. USA 100:14281-14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seeber, F. 2003. Biosynthetic pathways of plastid-derived organelles as potential drug targets against parasitic apicomplexa. Curr. Drug Targets Immune Endocr. Metabol. Disord. 3:99-109. [DOI] [PubMed] [Google Scholar]
- 33.Sheffield, H. G., and M. L. Melton. 1968. The fine structure and reproduction of Toxoplasma gondii. J. Parasitol. 54:209-226. [PubMed] [Google Scholar]
- 34.Striepen, B., M. J. Crawford, M. K. Shaw, L. G. Tilney, F. Seeber, and D. S. Roos. 2000. The plastid of Toxoplasma gondii is divided by association with the centrosomes. J. Cell Biol. 151:1423-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vivier, E., and A. Petitprez. 1972. Donnees ultrastructurales complementaires morphologiques et cytochemiques, sur Toxoplasma gondii. Protistologica 8:199-221. [Google Scholar]
- 36.Waller, R. F., M. B. Reed, A. F. Cowman, and G. I. McFadden. 2000. Protein trafficking to the plastid of Plasmodium falciparum is via the secretory pathway. EMBO J. 19:1794-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williamson, D. H., M. J. Gardener, P. Preiser, D. J. Moore, K. Rangachari, and R. J. M. Wilson. 1994. The evolutionary origin of the malaria parasite's 35kb circular DNA: new evidence supports a possible rhodaphyte ancestry. Mol. Gen. Genet. 243:249-252. [DOI] [PubMed] [Google Scholar]
- 38.Wilson, R. J. M. 2002. Progress with parasite plastids. J. Mol. Biol. 319:257-274. [DOI] [PubMed] [Google Scholar]
- 39.Zuther, E., J. J. Johnson, R. Haselkorn, R. McLeod, and P. Gornicki. 1999. Growth of Toxoplasma gondii is inhibited by aryloxyphenoxypropionate herbicides targeting acetyl-CoA carboxylase. Proc. Natl. Acad. Sci. USA 96:13387-13392. [DOI] [PMC free article] [PubMed] [Google Scholar]