Abstract
Background:
Previous work has indicated that differences in neurocognitive functioning may predict the development of adverse post-traumatic neuropsychiatric sequelae (APNS). Such differences may be vulnerability factors or simply correlates of APNS-related symptoms. Longitudinal studies that measure neurocognitive functioning at the time of trauma are needed to determine whether such differences precede the development of APNS.
Methods:
Here, we present findings from a subsample of 666 ambulatory patients from the AURORA (Advancing Understanding of RecOvery afteR trumA) study. All patients presented to EDs after a motor vehicle collision (MVC). We examined associations of neurocognitive test performance shortly after MVC with peritraumatic symptoms in the ED and APNS (depression, post-traumatic stress, post-concussive symptoms, and pain) 2 weeks and 8 weeks later. Neurocognitive tests assessed processing speed, attention, verbal reasoning, memory, and social perception.
Results:
Distress in the ED was associated with poorer processing speed and short-term memory. Poorer short-term memory was also associated with depression at 2 weeks post-MVC, even after controlling for peritraumatic distress. Finally, higher vocabulary scores were associated with pain 2 weeks post-MVC.
Limitations:
Self-selection biases among those who present to the ED and enroll in the study limit generalizability. Also, it is not clear whether observed neurocognitive differences predate MVC exposure or arise in the immediate aftermath of MVC exposure.
Conclusions:
Our results suggest that processing speed and short-term memory may be useful predictors of trauma-related characteristics and the development of some APNS, making such measures clinically-relevant for identifying at-risk individuals.
Keywords: Trauma, Longitudinal, Cognition, Neuropsychology, Digital neuropsychology, Digital cognitive assessment
1. Introduction
A substantial proportion of patients who present to the emergency department (ED) after a traumatic event go on to develop mild to severe APNS as a consequence of trauma exposure (Koenen et al., 2017; Santiago et al., 2013). Although one-third of all patients presenting to US EDs do so because of a trauma, only 10% are hospitalized (CDC, 2011). Yet, 90% of those not hospitalized go on to develop APNS (McLean et al., 2020). The most notable of these APNS are post-traumatic stress disorder (PTSD), depression, post-traumatic somatic symptoms (PTSS), and chronic/widespread pain (Boscarino, 2006; Kessler et al., 1995; McLean et al., 2020; Roberts et al., 2011). APNS contribute to substantial functional disability following trauma exposure and are a significant source of mortality and morbidity (Atwoli et al., 2015; McLean et al., 2020; Pacella et al., 2013). Trauma-exposed individuals who present to the ED are a large, high-risk population. Identifying and tailoring the right interventions for the right people in an ED context could have a major public health impact, reducing mortality, morbidity, and long-term disability. Such efforts are limited by critical knowledge gaps in our understanding of APNS and how to predict their development. Initiated by the National Institute of Mental Health in 2016, the AURORA (Advancing Understanding of RecOvery afteR traumA) study is designed to bridge these gaps through the collection and analysis of prospective genomic, neuroimaging, psychophysical, physiological, neurocognitive, digital phenotype, and self-report data from 5000 trauma survivors recruited from EDs, in the hours and days following trauma exposure and for one year thereafter (McLean et al., 2020). Critically, characterization of APNS and potential intermediate phenotypes draws from measures and biomarkers connected to the NIMH Research Domain Criteria (RDoC) (https://bit.ly/2pudCZH), allowing the AURORA data to be used to ultimately construct data-driven multidimensional phenotypes that are grounded in biology. AURORA study design and methods have been described in detail elsewhere (McLean et al., 2020).
As AURORA data collection is ongoing, initial analyses focus on peritraumatic symptoms and the development of traditional APNS in the first 8 weeks following trauma exposure. In this report, we focus specifically on the associations of neurocognitive test scores obtained shortly after motor vehicle collision (MVC) with peritraumatic symptoms assessed in the ED and the subsequent development of APNS 2 weeks and 8 weeks following MVC exposure. MVC is the most common life-threatening trauma experienced by people living in industrialized countries (Benjet et al., 2016). We limit our analyses to ambulatory individuals (i.e. excluding those with major somatic injuries) who presented to the ED after a MVC as these represent the vast majority of cases from initial AURORA data collection.
Associations of neurocognitive impairments with trauma exposure and APNS are well documented (Brandes et al., 2002; Golier et al., 2006; Hickling et al., 1998; Qureshi et al., 2011; Suliman et al., 2014; Vasterling and Brewin, 2005; Vasterling and Verfaellie, 2009). For PTSD, the most well studied APNS, effect sizes for neurocognitive differences tend to be small to moderate when comparing cases with healthy controls or other trauma-exposed individuals without PTSD (Scott et al., 2015). Longitudinal studies suggest that differences in neurocognitive function after trauma exposure are predictive of PTSD symptoms weeks to months later (Ben-Zion et al., 2018; Parslow and Jorm, 2007; Qureshi et al., 2011; Suliman et al., 2014). Not all studies find such differences, however (Crowell et al., 2002; Twamley et al., 2009; Zalewski et al., 1994). Of note, substantial evidence indicates that neurocognitive vulnerabilities for PTSD may not always arise in the aftermath of trauma, but may be attributable to pre-trauma risk (Vasterling and Verfaellie, 2009). Specifically, poorer neurocognitive functioning assessed before trauma exposure has been linked with the development of PTSD and other APNS after trauma in longitudinal analyses (Bomyea et al., 2012; Gale et al., 2008; Koenen et al., 2007; Macklin et al., 1998; Marx et al., 2009; Schäfer et al., 2018). Thus, neurocognitive dysfunction may be both a risk factor and an effect of APNS (Vasterling and Brewin, 2005).
MVC, in particular, has been linked with the development of APNS, including poorer neurocognitive function (Iverson et al., 2008). Several studies suggest that neurocognitive impairments after mild traumatic brain injury (TBI) are similar for those with brain injury vs injury to other parts of the body, suggesting that among those without loss of consciousness, mild TBI does not explain neurocognitive impairments (Babikian et al., 2011; Hanlon et al., 1999; Rieger et al., 2013). Differences in neurocognition are also associated with likelihood of MVC exposure, as pre-existing impairment in the elderly with mild cognitive impairment is associated with worse driving performance (Wadley et al., 2009) and increases the likelihood of future MVC (Ball et al., 2006). Thus, differences in neurocognitive function that are associated with the development of APNS might also be linked with pre-MVC vulnerability to APNS and MVC exposure.
Few studies have investigated the longitudinal associations of neurocognition with APNS in MVC. Given that APNS, like PTSD, are chronic (Kessler et al., 1995) and fluctuating (Shalev, 2003), lack of longitudinal data makes it difficult to disentangle differences in neurocognitive function that exist before or at the time of trauma exposure vs. those that develop concurrently with APNS or as a result of APNS. As MVC is one of the most frequently occurring types of trauma that presents to medical facilities (Benjet et al., 2016), identifying who is at risk of APNS and what interacting neurobiological, psychosocial, and neurocognitive factors lead to development of and recovery from APNS after MVC are critical clinical gaps for understanding APNS as well as improving care (Platts-Mills et al., 2012; Stein et al., 2016).
We present one of the initial papers from the AURORA study, based on neurocognitive data from 666 ambulatory participants who presented to EDs in the AURORA network across the United States after MVC. All participants completed objective, performance-based neurocognitive assessments in the ED and 48 h after discharge. We then assessed the development of four major APNS at 2 weeks and 8 weeks post-MVC, including PTSD, depression, pain, and somatic symptoms. Previous evidence indicates that most APNS are established within the 8 weeks following trauma exposure (Sterling et al., 2011). We hypothesized that neurocognition shortly after MVC would be associated with peritraumatic symptoms of distress and dissociation as measured in the ED, as well as APNS at 2 weeks and 8 weeks after trauma exposure.
2. Methods
2.1. Patients
Enrollment for AURORA began in September 2017. This analysis focuses on participants from the first data freeze, which includes participants who completed all assessments up to 8 weeks by March 2019, from 27 urban EDs in the US (McLean et al., 2020). The current analysis further focuses on ambulatory patients who were occupants of a vehicle involved in a MVC (within 72 h), who were the vast majority of potentially eligible participants (3981 / 5769; see Supplemental Fig. 1). Patients were age 18–65, able to speak and read English, able to follow the protocol at the time of enrollment, and able to use a smartphone, with access to a smartphone for at least 1 year following study enrollment. Patients were excluded if they had a solid organ injury Grade > 1 (based on American Association for the Surgery of Trauma; AAST), significant hemorrhage that required a chest tube or operation with anesthesia or were likely be admitted for > 72 h. Of 867 who met these criteria, provided informed consent, and completed baseline assessments, 666 also completed 2 week and 8-week assessments (see Supplementary Fig. 1). These inclusion/exclusion criteria were applied across the AURORA study and are not specific to the current analyses.
2.2. Measures
Patients completed interviewer-administered assessments with both self-report questions and biological sample collections (McLean et al., 2020). They also completed a battery of 10 neurocognitive assessments, including three tests in the ED and nine tests 48 h later. Self-report questions assessed peritraumatic symptoms of distress and dissociation. Web surveys were sent via text message at 2 weeks and 8 weeks, but could be completed with a telephone interviewer (if preferred). Details regarding consent and participant remuneration are described elsewhere (McLean et al., 2020). This protocol was approved on May 12, 2017 by the Biomedical IRB at UNC Chapel Hill through the Office of Human Research Ethics.
2.2.1. Sociodemographic and MVC information
Patient age, sex, race-ethnicity, education, marital status, family income before taxes, employment status, and MVC characteristics were collected (see Table 1). Patients were also assessed for injury severity (Abbreviated Injury Scale or AIS) (Loftis et al., 2018). Patient ratings of current pain and other somatic symptoms were collected and compared to the 30 days prior to the MVC. Further details of measures used to evaluate sociodemographic and MVC related information are included in Supplemental Materials.
Table 1.
Neurocognitive performance factors and participant demographic characteristics.
| % (SE) | F1 (Speeded Accuracy) | F2 (Cautious Accuracy) | |||
|---|---|---|---|---|---|
| b | (95% CI) | b | (95% CI) | ||
| Age | |||||
| 50+ | 18.3 (1.5) | −1.08** | (−1.31,−0.85) | 0.33** | (0.09,0.57)* |
| 35–49 | 28.7 (1.8) | −0.33** | (−0.54,−0.13) | 0.36** | (0.15,0.58)* |
| 25–34 | 30.5 (1.8) | −0.06 | (−0.25,0.14) | 0.19 | (−0.02,0.39) |
| 18–24 | 22.5 (1.6) | Ref | – | Ref | – |
| F3,623 | 36.8* | 4.1* | |||
| Sex (female) | 73 (1.7) | −0.09 | (−0.26,0.07) | 0.15 | (−0.02,0.31) |
| Race/ethnicity | |||||
| Non-Hispanic Black | 56.3 (1.9) | −0.44** | (−0.61,−0.27) | −0.57** | (−0.73,−0.4)* |
| Non-Hispanic White | 30 (1.8) | Ref | – | Ref | – |
| Hispanic | 10.5 (1.2) | 0.00 | (−0.26,0.26) | −0.22 | (−0.47,0.04) |
| Other | 3.2 (0.7) | −0.10 | (−0.54,0.34) | −0.11 | (−0.54,0.32) |
| F3,623 | 10.5* | 15.9* | |||
| Marital status | |||||
| Previously married2 | 14 (1.3) | −0.38** | (−0.60,−0.16) | −0.14 | (−0.36,0.08) |
| Never married | 43.5 (1.9) | 0.13 | (−0.03,0.29) | −0.18** | (−0.33,−0.02)* |
| Married/cohabitating | 42.5 (1.9) | Ref | – | Ref | – |
| F2,624 | 9.7* | 2.5 | |||
| Education | |||||
| Some college | 44 (1.9) | 0.03 | (−0.17,0.22) | −0.34** | (−0.52,−0.15)* |
| High school graduate | 24 (1.7) | −0.02 | (−0.25,0.21) | −0.76** | (−0.97,−0.55)* |
| Less than high school | 9.9 (1.2) | −0.35** | (−0.64,−0.06) | −0.91** | (−1.19,−0.64)* |
| College graduate | 22.1 (1.6) | Ref | – | Ref | – |
| F3,623 | 2.7* | 22.8* | |||
| Income 3 | |||||
| $19–35K | 31.5 (1.8) | −0.22** | (−0.41,−0.03) | −0.42** | (−0.60,−0.24)* |
| Less than $19K | 34.8 (1.8) | −0.24** | (−0.42,−0.05) | −0.71** | (−0.89,−0.53)* |
| More than $35K | 33.6 (1.8) | Ref | – | ||
| F2,624 | . | 3.7* | 31.1* | ||
| Employed (yes vs. no) | 77 (1.6) | 0.27** | (0.1,0.45) | 0.06 | (−0.11,0.24) |
Abbreviations: SE, standard error; CI, confidence interval; b, unstandardized linear regression coefficient. All models control for type of device used, pre-MVC PTSD, Depression, Pain, Sum of 20 Somatic Symptom Scores at ED.
Significant at the 0.05 level, two-sided test.
Significant at the 0.05 level, two-sided test, corrected for false discovery rate
Standardized to mean=0 and standard deviation=1.
Separated, divorced, or widowed.
Family income before taxes.
2.2.2. Peritraumatic distress and dissociation
Peritraumatic distress and dissociation were assessed with 8 items from the Peritraumatic Distress Inventory (PDI) (Brunet et al., 2001) and the 5-item revised Michigan Critical Events Perception Scale (MCEPS) (Michaels et al., 1999). Item were modified to ask about frequency of experiences “during and immediately after” the MVC (“none of the time”, “a little”, “some”, “most”, “all or almost all the time”). Cronbach’s α for new subscales was 0.80 for the PDI and 0.77 for the MCEPS. Each score was subsequently standardized to a mean of 0 and variance of 1.
2.2.3. Neurocognitive function
Peritraumatic neurocognitive function was assessed using the Test-MyBrain.org (TMB) digital research platform (Chaytor et al., 2020; Germine et al., 2012; Hartshorne and Germine, 2015; Passell et al., 2019). All tests were built in a combination of JavaScript and HTML, delivered through web applications that downloaded to the participant’s local device, ran in the browser, and then delivered data back to a central server. These measures were selected based on constructs of interest from the NIMH RDoC Matrix domains (Passell et al., 2019) and to provide a comprehensive neuropsychological assessment battery. Tests are described briefly below. For more information about test procedures, psychometric characteristics, and relationship with RDoC matrix domains of functioning, please see Supplemental Materials. All tests were developed and validated for self-administration in naturalistic environments (see Supplemental Materials for additional information about quality control measures), with good evidence for comparability between web versions of these tests and comparable lab/clinic or paper-and-pencil equivalents (Chaytor et al., 2020; Germine et al., 2012; Hartshorne and Germine, 2015). Basic quality control procedures were applied to ensure that data were excluded wherever there were clear indicators of lack of understanding or poor task compliance. These quality control rules are given in Supplemental Table 2.
In the ED, participants completed the TMB Simple Reaction Time test (basic psychomotor response speed) (Rutter et al., 2020), the TMB Choice Reaction Time test (processing speed, attention, and response selection/inhibition) (Rutter et al., 2020), and a Threat/Neutral Dot Probe test (attention biases to threat) (Bar-Haim et al., 2007). ED tests were completed on laptop or tablet computers in a quiet environment with minimal distractions.
48 h after discharge, participants completed additional measures on their own personal devices. These devices were classified based on operating system (Android 46–55%; iOS 35–42%; Mac OSX 3–4%; Windows 7–11%), screen size (smartphone size 83–88%; tablet size or larger 12–17%), and input type (mouse/keyboard 10–14%; touch 86–90%), based on known relationships between neurocognitive test scores and device variables (Passell et al., 2021). Variations in percentages reflect changes across time points. Device characteristics were controlled for in all analyses (Passell et al., 2021). Measures completed after discharge (48 h) were the TMB Multiracial Emotion Identification Test (emotion recognition) (Dodell-Feder et al., 2020; Passell et al., 2019), TMB Delay Discounting task (reward valuation) (Odum, 2011; Passell et al., 2019), an adaptation of the Probabilistic Reward Test (reward learning) (Passell et al., 2019; Pizzagalli et al., 2008, 2005), the TMB Gradual Onset Continuous Performance test (attention and response inhibition) (Fortenbaugh et al., 2018; Passell et al., 2019; Rosenberg et al., 2013; Vogel et al., 2020), the TMB Vocabulary test (general cognitive ability) (Chaytor et al., 2020; Cor et al., 2012; Hartshorne and Germine, 2015), the TMB Verbal Paired Associates test (verbal episodic memory) (Passell et al., 2019; Wilmer et al., 2012), the TMB Digit Symbol Matching test (processing speed) (Chaytor et al., 2020; Hartshorne and Germine, 2015; Joy et al., 2004), the TMB Forward Digit Span test (short-term memory) (Chaytor et al., 2020; Germine et al., 2012; Hartshorne and Germine, 2015), and the TMB Threat/Neutral Sternberg Memory test (working memory and memory biases for threat) (Passell et al., 2019; Sternberg, 1966). The Threat/Neutral Dot Probe test and Threat/Neutral Sternberg tests were ultimately dropped from the study and replaced with different tests (not included in Freeze 1) due to having no reliability (split-half and test-retest reliabilities indistinguishable from zero for threat-related difference score measures). Scores on these tests are excluded from the current analysis. The Probabilistic Reward Test was modified after freeze 1 data were collected due to low levels of reward related bias in this study (hypothetical rewards) based on the current forms. Since bias scores reliably varied between individuals, however, we included scores from the Probabilistic Reward test in our analyses. See Supplemental Materials for further information about neurocognitive task procedure. See Supplemental Table 1 for task psychometric information.
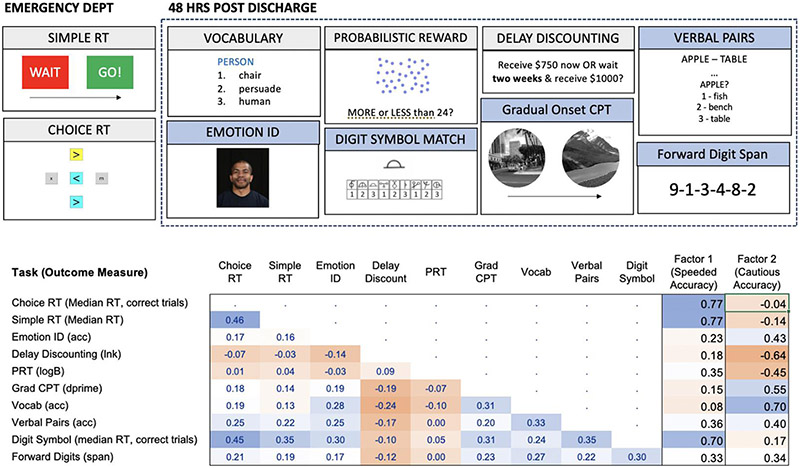
Task illustrations and primary outcome measures for each task are shown in Fig. 1.
Fig. 1. Measures of Neurocognitive Performance.
Performance-based neurocognitive assessments are administered in the Emergency Dept and 48 h after discharge. Tasks with blue shaded headings are administered three more times across the one-year duration of the AURORA study. The table gives polychoric correlations in the N = 666 person analytic sample, based on scores standardized to a mean of 0 and SD of 1. Factor loadings are given for two factors derived from exploratory factor analysis of neurocognitive data. Major outcomes measures from each task that are used in all analyses are indicated. For all measures, RT refers to reaction time and ACC refers to accuracy based on proportion correct. LnK (delay discounting) is the natural log of the hyperbolic discounting parameter, k, where higher scores reflect greater temporal discounting, or a preference for a smaller immediate reward. Dprime is a signal detection measure that reflects how well the participant was able to discriminate and accurately respond in the task. LogB is a signal detection measure of bias that reflects the tendency to select a rewarded response over a nonrewarded response, where higher scores indicate greater response bias to the rewarded response. Span is the number of digits the participant can accurately recall on at least one of two trials for each sequence length.
2.2.4. Acute stress disorder (ASD; 2 weeks) and post-traumatic stress disorder (PTSD)
PTSD-related symptoms were assessed at 2 and 8 weeks post-trauma using the PTSD Checklist for DSM-5 (PCL-5) (Bovin et al., 2016). The PCL-5 is a 20-item scale that assesses DSM-5 PTSD Criteria B-E on a 0–4 response scale based on how much the participant was “bothered by” a particular problem in the past 2 weeks (2 week survey) or 30 days (8 week survey). Summed raw scores (0–80) were calculated, with a liberal diagnostic threshold for ASD (2 weeks) or PTSD (8 weeks) of 31 or higher.
2.2.5. Self-reported depression
Self-reported depression symptoms were assessed at 2 and 8 weeks post-trauma using the 8 item PROMIS Depression Short-Form 8b (Cella et al., 2010; PROMIS Cooperative Group, 2021). Patients were asked how often they experienced each feeling in the preceding 2 weeks (2-week survey) or the past 30 days (8-week survey) on a 0–4 scale. Summed raw scores (0–32) were then converted to t-scores (mean = 50, standard deviation = 10), relative to the general US population. A score of 60 or greater was used as the threshold for moderate to severe depression.
2.2.6. Post-traumatic somatic symptoms (PTSS)
PTSS was assessed in the week 2 and week 8 surveys using the Rivermead Post-Concussion Symptoms Questionnaire (RPQ) (King et al., 1995), a 16-item scale used to assess post-concussion symptom severity after head injuries. We included 12 symptoms from the RPQ and asked patients to rate current symptom severity on a 10-point scale, where 0 = “no problem” and 10 = “a major problem.” This differs from the standard approach where symptoms are rated relative to symptom severity prior to head injury, as head injury was not experienced by all patients and similar symptoms can occur outside the context of head injury (Auvergne et al., 2016; McLean et al., 2009). Difference scores were calculated for each of the 12 symptoms, comparing 30 days before the MVC to the past 2 weeks (2-week survey) or past 30 days (8-week survey). Clinically significant new or worsening (CSNW) symptoms were those with a difference score of 2 or more. The number of CSNW post-concussion symptoms was summed to create a 0–12 continuous scale. Cronbach’s α was 0.90 for both the 2-week and 8-week scales. Based on previous research (Auvergne et al., 2016; Ulirsch et al., 2014), we defined PTSS as 3 or more clinically significant new or worsening (CSNW) post-concussion symptoms, where CSNW means a score increase of 2 or more from the 30 days before the MVC to the follow-up post-trauma time period.
2.2.7. Moderate/severe pain (MSP)
Pain outcomes were assessed using the Pain Intensity Numerical Rating Scale (PI-NRS), a single item measure of pain intensity (Farrar et al., 2001). Patients were asked to report the “usual intensity” of any and all physical pain in the past 2 weeks (2-week survey) or past 30 days (8-week survey) on a 0–10 scale, where 0 = “no pain or tenderness” and 10 = “severe pain or tenderness.” A score of 4 or more on this single item was used as the threshold to define moderate/severe pain (MSP). For our continuous scale, we used a count of the number of body regions with clinically significant new or worsening (CSNW) pain, which compared 18 body region pain severity scores at 30 days before the MVC to the past 2 weeks (2-week survey) or past 30 days (8-week survey). CSNW was defined as an increase in pain severity by 2 or more points (on a 0–10 response scale) from pre-trauma to post-trauma (Ulirsch et al., 2014). Cronbach’s α was 0.91 for 2-week CSNW and 0.95 for 8-week CSNW (Bortsov et al., 2013; McLean et al., 2014).
2.3. Analysis methods
Neurocognitive tests were selected to create a battery specifically for the AURORA study. To better characterize this test battery and understand its correlation structure, we conducted exploratory factor analysis across all 10 neurocognitive performance variables. We then looked at associations between neurocognitive factors and demographic / MVC variables to better characterize overall neurocognitive characteristics of the sample. All subsequent analyses treated neurocognitive variables individually, with appropriate correction for multiple comparisons.
For selection of covariates, we estimated bivariate associations between demographic / MVC variables and peritraumatic distress, peritraumatic dissociation, ASD / PTSD, Depression, PTSS, and MSP in our analytic sample. Candidate covariates were selected for inclusion across several AURORA analyses linking MVC with 2-week and 8-week outcomes, based on potential associations with ED symptoms or APNS (e.g. Joormann et al., 2020; Kessler et al., 2020). We also examined potential concussive factors through two variables – whether a participant reported hitting their head and the presence of mild TBI. Any variables significantly associated with these outcomes were included as covariates in all further models (see Supplemental Table 3). All analyses controlled for digital device characteristics (Passell et al., 2021). We also controlled for the frequency of four sets of symptoms (PTSD, Depression, PTSS, and MSP) in the 30 days prior to the ED based on retrospective self-report of such symptoms in the ED. Additional analyses (reported in Supplemental Materials) looked specifically at the relationship between neurocognitive performance, ED symptoms/APNS, and medication used prior to ED presentation, used in the ED, and prescribed in the ED.
For our primary analyses, we examined bivariate associations of neurocognitive performance and peritraumatic distress and dissociation. We then estimated logistic regression equations for the bivariate associations between each neurocognitive performance variable and APNS at 2 weeks and 8 weeks. For the AURORA study more broadly, APNS measures were dichotomized to permit inferences with respect to potential clinical decision thresholds. While this makes these analyses more practically useful, it does potentially reduce statistical power. The four APNS examined were threshold PTSD, Depression, PTSS, and Pain. These models were estimated with and without peritraumatic distress and dissociation as covariates, to examine the extent to which 2-week and 8-week outcomes were explained by variations in peritraumatic symptoms. For any significant neurocognition and APNS associations identified based on 8-week outcomes, we further controlled for 2-week APNS. Logits and logits ± 2 standard errors were exponentiated and are reported as odds-ratios (ORs) with 95% confidence intervals (CIs). Statistical significance was consistently evaluated using 0.05-level two-sided tests, with false discovery rate (FDR) correction based on the number of factors (two) or cognitive outcomes being considered (ten) for each analysis. All reported p values are FDR corrected, unless stated otherwise.
Procedures for handling missing data are described in Supplemental Materials.
In reporting of regression results, effect sizes were flipped where necessary (e.g. for reaction time-based scores) such that higher scores reflect better performance. Some scores (Delay Discounting and Probabilistic Reward Tests) were not interpreted in terms of better or worse performance, although steeper delay discounting and low response bias on the Probabilistic Reward Test have been linked with poorer mental health outcomes so may have functional significance (Lempert et al., 2019; Pizzagalli et al., 2008).
3. Results
3.1. Neurocognitive performance
Exploratory factor analysis of neurocognitive data yielded two latent factors (based on scree plot inspection), related to speeded accuracy and cautious accuracy, respectively. Tests with the highest loading on the speeded accuracy factor were those that measured processing speed (TMB Simple RT, TMB Choice RT, and TMB Digit Symbol Matching). The test with the highest loading on the cautious accuracy factor was TMB Vocabulary, with more modest loadings for tests requiring sustained attention where more cautious approaches might yield better scores (TMB Gradual Onset Continuous Performance Test, TMB Multiracial Emotion Identification Test, and TMB Verbal Paired Associates Test). TMB Delay Discounting scores (lnk) also loaded highly on the second factor, indicating that less temporal discounting (associated with lower impulsivity) was associated with higher scores on this factor. Together, these factors captured 42% of the variance in test scores. The two factors were positively correlated (r = 0.28). Fig. 1 shows correlations between tasks and factor loadings, expressed in terms of standardized regression coefficients.
3.2. Neurocognitive factors and demographic / motor vehicle collision variables
Speeded accuracy was associated with age (F(3623) = 36.8, p < 0.0001), race/ethnicity (F(3623) = 10.5, p < 0.0001), marital status (F (2624) = 9.7, p < 0.0001), education (F(3623) = 2.7, p < 0.05), income (F(2624) = 3.7, p < 0.05), and employment (F(1625) = 9.1,p < 0.01). Cautious accuracy was associated with age (F(3623) = 4.1,p < 0.01), race/ethnicity (F(3623) = 15.9,p < 0.0001), education (F(3623) = 22.8, p < 0.0001), and income (F(2624) = 31.1,p < 0.0001). Specifically, lower speeded accuracy factor scores were associated with middle and older age, non-Hispanic black race/ethnicity, being previously married, less than high school educational attainment, lack of employment, and income less than $35k per year. Lower cautious accuracy factor scores were associated with younger age, non-hispanic black race/ethnicity, never being married, lower levels of educational attainment, and lower income. Regression coefficients with 95% confidence intervals are given in Table 1.
Only cautious accuracy factor scores were associated with MVC characteristics, including the participants role in the collision (F(2624) = 5.6,p < 0.01), passenger injuries (F(1625) = 6.8, p < 0.05), and current severity of pain (F(1625) = 25.5,p < 0.0001). Specifically, lower cautious accuracy factor scores were associated with being a passenger in a motor vehicle collision, greater degree of passenger injuries, and greater pain severity. Regression coefficients with 95% confidence intervals are given in Table 2.
Table 2.
Neurocognitive performance factors and motor vehicle collision (MVC) characteristics.
| %/mean (SE) | F1 (Speeded Accuracy) | F2 (Cautious Accuracy) | |||
|---|---|---|---|---|---|
| b | (95% CI) | b | (95% CI) | ||
| Role in MVC | |||||
| Passenger | 23.3 (1.6) | −0.10 | (−0.29,0.08) | −0.31** | (−0.49,−0.13) |
| Driver with others | 19.2 (1.5) | 0.01 | (−0.19,0.2) | −0.09 | (−0.28,0.10) |
| Driver alone | 57.5 (1.9) | Ref | – | Ref | – |
| F2,624 | 0.7 | 5.6* | |||
| Your vehicle collided with | |||||
| Other moving vehicle | 68.2 (1.8) | −0.24* | (−0.46,−0.03) | 0.00 | (−0.21,0.22) |
| Stationary object | 17.9 (1.5) | −0.16 | (−0.43,0.10) | −0.02 | (−0.28,0.24) |
| Other1 | 14 (1.3) | Ref | – | Ref | – |
| F2,624 | . | 2.5 | 0.0 | ||
| Damage to your vehicle | |||||
| Severe | 58.3 (1.9) | −0.03 | (−0.34,0.27) | 0.02 | (−0.27,0.32) |
| Moderate | 26.4 (1.7) | 0.12 | (−0.20,0.44) | 0.11 | (−0.21,0.43) |
| Minor | 8.9 (1.1) | −0.02 | (−0.4,0.36) | 0.12 | (−0.26,0.49) |
| Other2 | 6.5 (1.0) | Ref | – | Ref | – |
| F3,623 | . | 1.1 | 0.5 | ||
| Passenger injuries (0–4) 3 | 0.4 (0.03) | −0.07 | (−0.15,0.00) | −0.10** | (−0.17,−0.02) |
| Others with injuries (any vs. none) 3 | 10.2 (1.2) | 0.05 | (−0.19,0.3) | −0.08 | (−0.32,0.16) |
| Transportation to ED | |||||
| Ambulance | 58.0 (1.9) | −0.16 | (−0.33,0.02) | 0.01 | (−0.16,0.18) |
| Other immediately | 14.7 (1.4) | 0.00 | (−0.24,0.24) | 0.12 | (−0.12,0.36) |
| Other delay | 27.3 (1.7) | Ref | – | Ref | – |
| F2,624 | . | 2.1 | 0.6 | ||
| Personal Injury | |||||
| Hit head (yes vs. no) | 57.4 (1.9) | 0.03 | (−0.12,0.18) | −0.14 | (−0.29,0.01) |
| MTBI (yes vs. no) | 27.5 (1.7) | −0.15 | (−0.33,0.02) | −0.06 | (−0.23,0.11) |
| AIS-Max4 (2+ vs 1) | 13.1 (1.3) | 0.16 | (−0.07,0.38) | 0.25* | (0.03,0.46) |
| Admitted (yes vs. no) | 4.1 (0.8) | −0.37 | (−0.74,0.00) | −0.16 | (−0.52,0.21) |
| Pain (severity) 5 | 6.6 (0.1) | −0.07 | (−0.14, 0.02) | −0.20** | (−0.28,−0.12) |
| Other somatic symptoms (severity) 6 | 1.7 (0.07) | −0.03 | (−0.14,0.08) | 0.02 | (−0.09,0.12) |
Abbreviations: SE, standard error; CI, confidence interval; MVC, motor vehicle collision; b, unstandardized linear regression coefficient. All models control for type of device used, pre-MVC PTSD, Depression, Pain, Sum of 20 Somatic Symptom Scores at ED.
Significant at the 0.05 level, two-sided test.
Significant at the 0.05 level, two-sided test, corrected for false discovery rate.
No collision (n = 81), “other” (n = 8), and “don’t know (n = 8).
None (n = 12) and “don’t know” (n = 31).
Moderate or severe injuries. Unstandardized values used for mean/SE reporting. Models use standardized values with mean=0 and standard deviation=1.
Max score of the nine AIS regions.
Self-reported 0–10 scale on pain intensity right now. Unstandardized values used for mean/SE reporting. Models use standardized values with mean=0 and standard deviation=1.
Sum of all differences in each somatic symptom between 30-day (self-reported 0–10 scale) and right now (self-reported 0–10 scale). Unstandardized values used for mean/SE reporting. Models use standardized values with mean=0 and standard deviation=1.
3.3. Neurocognition and peritraumatic symptoms
Peritraumatic distress (reported in the ED) was associated with TMB Choice RT scores (F(1644) = 7.5,p < 0.05) and TMB Forward Digit Span scores (F(1642) = 7.1,p < 0.05), after false discovery rate correction for ten comparisons (ten neurocognitive tests). The addition of Forward Digit Span to the model that included Choice RT resulted in significant model improvement (F(2639) = 6.6, p = 0.0014), indicating that each test explained unique variance in peritraumatic distress. Neurocognitive performance was not associated with peritraumatic symptoms of dissociation.
Standardized regression coefficients with 95% confidence intervals are given in Table 3.
Table 3. Neurocognitive performance and peritraumatic symptoms.
Bivariate models predicting distress/dissociation from neurocognitive performance, controlling for significant demographic and motor vehicle injury variables.
| Test | Peritraumatic Distress | Peritraumatic Dissociation | ||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | F statistic (DF) | Estimate | 95% CI | F statistic (DF) | |
| Choice RT | −0.09** | (−0.16,−0.03) | 7.5 (1644) | −0.07 | (−0.14,0.0) | 3.7 (1648) |
| Simple RT | −0.05 | (−0.12,0.02) | 1.8 (1644) | −0.01 | (−0.08,0.06) | 0.1 (1648) |
| Emotion ID | 0.06 | (−0.01,0.12) | 2.5 (1642) | 0.03 | (−0.04,0.1) | 0.9 (1646) |
| Delay Discounting | −0.02 | (−0.09,0.05) | 0.3 (1642) | −0.04 | (−0.11,0.03) | 1.2 (1646) |
| PRT | 0.01 | (−0.06,0.08) | 0.1 (1642) | −0.02 | (−0.09,0.05) | 0.3 (1646) |
| Grad CPT | 0.02 | (−0.05,0.09) | 0.3 (1641) | −0.02 | (−0.09,0.05) | 0.5 (1645) |
| Vocab | −0.04 | (−0.12,0.03) | 1.3 (1642) | 0.03 | (−0.04,0.11) | 0.9 (1646) |
| Verbal Pairs | −0.02 | (−0.09,0.05) | 0.2 (1642) | −0.02 | (−0.09,0.05) | 0.3 (1646) |
| Digit Symbol | −0.06 | (−0.12,0.02) | 2.4 (1642) | −0.03 | (−0.1,0.04) | 0.7 (1646) |
| Forward Digits | −0.09** | (−0.16,−0.02) | 7.1 (1642) | −0.02 | (−0.09,0.05) | 0.3 (1646) |
Significant at the 0.05 level, two-sided test.
Significant at the 0.05 level, two-sided test, corrected for false discovery rate.
3.4. Neurocognition and acute stress disorder (ASD) / post-traumatic stress disorder (PTSD)
The prevalence of ASD/PTSD in the aftermath of MVC was 41.0% (SE = 0.2) at 2 weeks and 42.0% (SE = 0.2) at 8 weeks. Neurocognitive performance was not associated with ASD at 2 weeks or PTSD at 8 weeks. Odds ratios with 95% confidence intervals for analyses controlling for peritraumatic symptoms are shown in Table 4.
Table 4.
Neurocognitive performance and APNS at 2 weeks and 8 weeks.
| Test | 2-week PTSD | 8-week PTSD | 2-week Depression | 8-week Depression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | χ2 | OR | 95% CI | χ2 | OR | 95% CI | χ2 | OR | 95% CI | χ2 | |
| Choice RT | 0.9 | (0.8,1.1) | 0.5 | 1.1 | (0.9,1.3) | 0.9 | 1.1 | (0.9,1.3) | 0.9 | 1.1 | (0.9,1.3) | 0.3 |
| Simple RT | 0.9 | (0.8,1.1) | 0.9 | 1 | (0.8,1.2) | 0.2 | 1.1 | (0.9,1.3) | 0.5 | 1.1 | (0.9,1.3) | 0.3 |
| Emotion ID | 1.1 | (0.9,1.3) | 0.4 | 1.2 | (1.0,1.4) | 2.4 | 1.1 | (0.9,1.4) | 0.8 | 1.2 | (1.0,1.5) | 3.4 |
| Delay Discounting | 1.1 | (1.0,1.4) | 2.1 | 0.8 | (0.7,1.0) | 3.3 | 1.1 | (0.9,1.4) | 1 | 1 | (0.8,1.2) | 0 |
| PRT | 1.1 | (0.9,1.3) | 0.7 | 1.1 | (0.9,1.3) | 1.4 | 1.2 | (1.0,1.4) | 2.4 | 1 | (0.9,1.3) | 0.2 |
| Grad CPT | 0.9 | (0.7,1.1) | 1.7 | 1 | (0.8,1.2) | 0 | 0.9 | (0.7,1.1) | 1.8 | 1 | (0.8,1.2) | 0 |
| Vocab | 1.1 | (0.9,1.3) | 0.4 | 1.3* | (1.0,1.6) | 5.6 | 1 | (0.8,1.2) | 0 | 1.2 | (1.0,1.5) | 3.3 |
| Verbal Pairs | 1 | (0.9,1.2) | 0.1 | 1.1 | (0.9,1.4) | 1.8 | 1.1 | (0.9,1.3) | 0.9 | 1.1 | (0.9,1.4) | 1.7 |
| Digit Symbol | 1 | (0.8,1.2) | 0.1 | 1.1 | (0.9,1.3) | 1.1 | 1.1 | (0.9,1.3) | 0.3 | 1.2 | (1.0,1.4) | 2.5 |
| Forward Digits | 0.9 | (0.7,1.0) | 2.2 | 0.9 | (0.7,1.0) | 2.6 | 0.7 ** | (0.6,0.9) | 10 | 0.9 | (0.8,1.1) | 0.5 |
| Test | 2-week PTSS | 8-week PTSS | 2-week Pain | 8-week Pain | ||||||||
| OR | 95% CI | χ2 | OR | 95% CI | χ2 | OR | 95% CI | χ2 | OR | 95% CI | χ2 | |
| Choice RT | 1 | (0.8,1.2) | 0.1 | 1.1 | (1.0,1.4) | 2 | 1.1 | (0.9,1.4) | 0.8 | 0.9 | (0.7,1.1) | 1 |
| Simple RT | 1 | (0.8,1.2) | 0 | 0.9 | (0.8,1.1) | 0.6 | 1 | (0.8,1.2) | 0.1 | 0.9 | (0.8,1.2) | 0.3 |
| Emotion ID | 1.1 | (0.9,1.3) | 0.9 | 1.2 | (1.0,1.4) | 2.7 | 1.1 | (0.9,1.4) | 1.2 | 1.1 | (0.9,1.3) | 1.4 |
| Delay Discounting | 0.8* | (0.7,1.0) | 4.6 | 0.9 | (0.7,1.0) | 2.8 | 0.9 | (0.7,1.1) | 1.2 | 0.9 | (0.7,1.1) | 1.5 |
| PRT | 0.9 | (0.8,1.1) | 0.4 | 0.9 | (0.8,1.1) | 0.5 | 1 | (0.8,1.2) | 0.1 | 1 | (0.8,1.2) | 0 |
| Grad CPT | 1 | (0.8,1.2) | 0 | 1.1 | (0.9,1.3) | 1.3 | 1.1 | (0.9,1.3) | 0.3 | 1.1 | (0.9,1.3) | 0.9 |
| Vocab | 1.2 | (1.0,1.5) | 3.8 | 1.3* | (1.1,1.6) | 6.3 | 1.5 ** | (1.2,1.8) | 12 | 1.2 | (1.0,1.4) | 2.9 |
| Verbal Pairs | 1.1 | (0.9,1.3) | 0.6 | 1.1 | (0.9,1.3) | 0.3 | 1 | (0.8,1.2) | 0.1 | 1.1 | (0.9,1.3) | 0.4 |
| Digit Symbol | 1 | (0.8,1.2) | 0 | 1.2* | (1.0,1.5) | 4.6 | 1.1 | (0.9,1.4) | 1.1 | 1.3* | (1.1,1.6) | 6.4 |
| Forward Digits | 1.2 | (1.0,1.4) | 2.3 | 1 | (0.9,1.2) | 0 | 1 | (0.8,1.2) | 0.1 | 0.9 | (0.8,1.1) | 1.1 |
Bivariate models predicting APNS from neurocognitive performance, controlling for significant demographic variables, motor vehicle injury variables, and peritraumatic distress and dissociation.
Significant at the 0.05 level, two-sided test.
Significant at the 0.05 level, two-sided test, corrected for false discovery rate.
3.5. Newocognition and threshold depression
The prevalence of Depression in the sample was 30.5% (SE = 1.8) at 2 weeks and 27.2% (SE = 1.7) at 8 weeks. Higher scores on Forward Digit Span (attention and short-term memory) were associated with lower rates of Depression at 2-weeks, even when controlling for peritraumatic distress and dissociation and FDR correction (X2(1) = 10.0, p < 0.05). This association was not significant for Depression at 8 weeks (X2(1) = 0.5, p = 0.82). No other associations between neurocognitive performance and Depression were significant. See Table 4.
3.6. Association of neurocognition with post-traumatic somatic syndrome (PTSS)
The prevalence of PTSS in the sample was 74.6% (SE = 1.7) at 2 weeks and 68.0% (SE = 1.8) at 8 weeks. Lower TMB Delay Discounting scores (less temporal discounting), higher TMB Vocabulary, and higher TMB Digit Symbol Matching scores were nominally associated with a greater likelihood of PTSS after MVC (Vocabulary X2(1) = 6.3, p < 0.05; Digit Symbol X2(1) = 4.6, p < 0.05), but these associations did not survive FDR correction. The pattern of associations was not affected by controlling for peritraumatic symptoms. See Table 4.
3.7. Association with moderate/severe pain (MSP) at 2-weeks and 8-weeks after mvc
The prevalence of MSP in the sample was 81.4% (SE = 1.5) at 2 weeks and 67.4% (SE = 1.8) at 8 weeks after MVC. Higher TMB Vocabulary scores were associated with greater likelihood of moderate to severe pain at 2-weeks (X2(1) = 12.4, p < 0.01). However, this relationship was not significant at 8 weeks (X2(1) = 2.9, p = 0.45). TMB Digit Symbol Matching scores were nominally associated with higher likelihood of moderate to severe pain at 8 weeks (X2(1) = 6.4, p < 0.05), but not after FDR correction. The patterns of associations was not affected by controlling for peritraumatic symptoms. See Table 4.
For all analyses reported above, results were unchanged when controlling for potential medication-related confounders (see Supplemental Materials).
4. Discussion
In the current study, we reported on associations of neurocognitive function, assessed in the ED and 48 h after exposure to motor vehicle collision trauma, and APNS in 666 freeze 1 participants from the AURORA longitudinal study. A major strength of the AURORA study is the prospective longitudinal design, which allows us to control for many baseline factors that could account for associations of neurocognition after trauma with the subsequent development of APNS, including sociodemographic characteristics, motor vehicle collision characteristics, personal injury, as well as PTSD, depression, pain and somatic symptoms 30 days prior to MVC. After controlling for these other variables, three main findings emerged. We found that slower processing speed and poorer short-term memory were associated with higher levels of peritraumatic distress, assessed in the ED. Poorer short-term memory (based on TMB Forward Digit Span scores) predicted threshold depression two weeks after MVC even after controlling for peritraumatic distress and dissociation. Higher vocabulary scores were also associated with greater likelihood of moderate/severe pain at two weeks, contrary to prediction. Vocabulary scores are often considered an indicator of premorbid general cognitive ability, as performance often better reflects learning over a lifetime rather than current psychological status (Lezak et al., 2004)}. The potential for self-selection bias driving this latter finding is discussed below.
The association between distress in the ED and cognitive processing speed replicates previous literature linking psychomotor speed with trauma-related symptoms (Gale et al., 2016). We did not, however, find any association between neurocognition and ED dissociation symptoms. As dissociation was only linked to severe distress in our sample, neurocognitive performance may be a better predictor of variations in distress across the full range rather than severe distress alone. Future analyses with larger sample sizes as part of the AURORA study will allow us to investigate this possibility.
The association between attention and short-term memory (forward digit span scores) and depression at 2 weeks has also been identified in previous studies, particularly for melancholic depression (see Bosaipo et al., 2017 for a review). This difference survived controlling for peritraumatic distress and dissociation, indicating that this relationship was not driven entirely by symptoms experienced at the time of trauma. Difficulties with attention and short-term memory may indicate underlying risk for developing depression after trauma.
In our analyses, neurocognitive performance was also related to the development of moderate to severe pain at follow-up – particularly vocabulary scores. However, this association was in the opposite direction of our prediction (Koenen et al., 2007; Vasterling and Verfaellie, 2009). That is, better vocabulary scores were associated with a higher rate of moderate to severe pain (at 2 weeks). Notably, the cautious accuracy neurocognitive factor – highly associated with vocabulary scores - was also associated with higher levels of personal injury after MVC (based on AIS injury scores). Together, these associations indicate self-selection factors that might drive this association. Patients choose emergency care for a wide range of reasons including perceived urgency, convenience, and alternative care options (Coster et al., 2017). Vocabulary is also related to education and income (Hoff, 2003). Higher income individuals may be less likely to come to the ED unless they have more severe personal injury. While these factors were controlled for in our analysis (education/income and personal injury), we may not have successfully accounted for all such potential confounding effects. We are currently developing a plan to contact individuals who chose not to come to the ED in the immediate aftermath of a MVC to determine whether such individuals differ in symptom and general cognitive ability (assessed by a brief vocabulary test) from those who came electively to the ED.
If self-selection biases were associated with neurocognitive function, this might also account for the relatively few significant associations between neurocognitive performance and APNS outcomes that we found in this study. For example, if pretrauma or peritraumatic neurocognition is positively associated with greater risk of APNS but negatively associated with likelihood of presenting to the ED (due to its association with socioeconomic status) given similar trauma severity, this would reduce our power to detect any significant associations.
Although it was our intention to include measures of threat-related biases in attention and working memory, our measures of these constructs were ultimately dropped from the study due to poor reliability. Currently, measures of threat-related biases in aspects of attention and executive functioning often have low (or no) psychometric reliability, leading to results that are not reproducible (Hedge et al., 2018; Parsons et al., 2019). Since freeze 1, these tasks have been replaced with measures of cognitive interpretation biases (Beard and Amir, 2009), social perception of threat (Rutter et al., 2019a, 2019b), and a Trauma Implicit Association Test (Lindgren et al., 2013) with confirmed psychometric reliability. This will allow us to probe the relationship between information processing of negative valence and the development of APNS in future analyses.
4.1. Limitations
Even though our sample was large relative to other prospective studies of APNS after trauma exposure, much larger samples are needed to carry out the powerful statistical analyses envisioned for the AURORA study (McLean et al., 2020). The AURORA study will ultimately enroll 5000 patients across sites, giving us adequate statistical power to address more complex research questions. Second, in addition to the potential ED self-selection biases described above, a substantial number of participants who were approached in the ED declined to enroll in the study. This is reasonable given the heavy burden of a 12-month study which includes deep phenotyping, but limits generalizability. Third, some of the variability in cognitive performance may be attributable to MVC-related concussion. While we attempted to control for concussive factors through inclusion of variables related to head injury as potential covariates, it is possible that the impact of concussion on cognitive performance was not fully accounted for in our analysis. Fourth, although every effort was made to ensure equivalence of testing across participants and occasions, neurocognitive testing in naturalistic settings necessarily involves a reduction of experimental control. While participants were instructed to complete testing in a quiet environment, for example, it was not possible to verify whether participants consistently followed these instructions. Finally, although we treated neurocognition here as a predictor of APNS, we do not know whether differences in neurocognition that predicted likelihood of APNS were pre-existing (pre-MVC differences) or whether they reflect differences that emerged in the immediate aftermath of MVC.
5. Conclusion
In this initial report, we sought to characterize the relationship between neurocognitive performance in the immediate aftermath of motor vehicle collision and the development of APNS up to 8 weeks following trauma. In general, we found that neurocognitive performance was linked with peritraumatic distress, with some initial associations with APNS 2 weeks and 8 weeks post-trauma. Our data also suggest the possibility of self-selection biases by neurocognitive function (e.g. presentation into the ED), suggesting that individuals with higher general cognitive ability may be less likely to present to the ED or consent to participate in the study unless they had greater injury severity and therefore higher rates of APNS development. Future analyses as the AURORA study continues, samples sizes increase, and as multidimensional APNS outcomes become available will help us tease apart these and other questions. The ultimate goal of these future analyses will be to identify new targets for intervention and better tools for risk stratification following exposure to trauma.
Supplementary Material
Acknowledgments
Data and/or research tools used in the preparation of this manuscript were obtained from the National Institute of Mental Health (NIMH) Data Archive (NDA). NDA is a collaborative informatics system created by the National Institutes of Health to provide a national resource to support and accelerate research in mental health. Dataset identifier: NIMH Data Archive 10.15154/1521156. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or of the Submitters submitting original data to NDA.
Disclosures
Over the past three years, LTG has served on the scientific advisory board of Sage Bionetworks, a nonprofit 501c3. RCK was a consultant for Datastat, Inc, Sage Pharmaceuticals, and Takeda. CWJ reports no direct conflicts related to this paper, and no ongoing conflicts. CWJ has been an investigator on studies funded by Roche Diagnostics, AstraZeneca, Janssen, and Hologic Inc, for which his department has received research funding. Over the past three years, DAP has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Posit Science, and Takeda Pharmaceuticals, as well as an honorarium from Alkermes for activities unrelated to the current project. JME reports support from the National Institutes of Health (NIH) through Grant Nos. R01HD079076 and R03HD094577: Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Center for Medical Rehabilitation Research. SS has received funding from the Florida Medical Malpractice Joint Underwriter’s Association Dr. Alvin E. Smith Safety of Healthcare Services Grant, the NIH/NIA-funded Jacksonville Aging Studies Center (JAX-ASCENT, R33AG05654), and the Florida Blue Foundation. There are no known direct conflicts related to this paper.
Nominclature
- ED
emergency department
- APNS
adverse post-traumatic neuropsychiatric sequelae
- AURORA
advancing understanding of recovery after trauma study
- MVC
motor vehicle collision
- PTSS
post-traumatic somatic symptoms
- TMB
test my brain (not-for-profit web research platform)
Footnotes
CRediT authorship contribution statement
Laura T. Germine: Conceptualization, Visualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Supervision, Writing – review & editing. Jutta Joormann: Methodology, Writing – original draft, Writing – review & editing. Eliza Passell: Methodology, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Lauren A. Rutter: Methodology, Formal analysis, Writing – review & editing. Luke Scheuer: Methodology, Resources, Writing – review & editing. Paolo Martini: Methodology, Resources, Writing – review & editing. Irving Hwang: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Sue Lee: Methodology, Formal analysis, Writing – review & editing. Nancy Sampson: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Deanna M. Barch: Methodology, Writing – review & editing. Stacey L. House: Methodology, Investigation, Data curation, Resources, Writing – review & editing. Francesca L. Beaudoin: Methodology, Investigation, Resources, Data curation, Writing – review & editing. Xinming An: Methodology, Investigation, Resources, Data curation, Writing – review & editing. Jennifer S. Stevens: Methodology, Investigation, Resources, Data curation, Writing – review & editing. Donglin Zeng: Methodology, Investigation, Resources, Data curation, Writing – review & editing. Sarah D. Linnstaedt: Methodology, Investigation, Resources, Data curation, Writing – review & editing. Tanja Jovanovic: Methodology, Investigation, Resources, Data curation, Writing – review & editing. Gari D. Clifford: Methodology, Investigation, Resources, Data curation, Writing – review & editing. Thomas C. Neylan: Methodology, Investigation, Resources, Data curation, Writing – review & editing. Scott L. Rauch: Methodology, Investigation, Resources, Data curation, Writing – review & editing. Christopher Lewandowski: Investigation, Resources, Writing – review & editing. Phyllis L. Hendry: Investigation, Resources, Writing – review & editing. Sophia Sheikh: Investigation, Resources, Writing – review & editing. Alan B. Storrow: Investigation, Resources, Writing – review & editing. Paul I. Musey: Investigation, Resources, Writing – review & editing. Christopher W. Jones: Resources, Writing – review & editing. Brittney E. Punches: Resources, Writing – review & editing. Meghan E. McGrath: Resources, Writing – review & editing. Jose L. Pascual: Resources, Writing – review & editing. Kamran Mohiuddin: Resources, Writing – review & editing. Claire Pearson: Resources, Writing – review & editing. David A. Peak: Resources, Writing – review & editing. Robert M. Domeier: Investigation, Resources, Writing – review & editing. Steven E. Bruce: Investigation, Writing – review & editing. Niels K. Rathlev: Investigation, Resources, Writing – review & editing. Leon D. Sanchez: Investigation, Resources, Writing – review & editing. Robert H. Pietrzak: Methodology, Writing – review & editing. Diego A. Pizzagalli: Methodology, Writing – review & editing. Steven E. Harte: Methodology, Resources, Writing – review & editing. James M. Elliott: Methodology, Writing – review & editing. Karesten C. Koenen: Conceptualization, Visualization, Methodology, Investigation, Resources, Data curation, Supervision, Writing – review & editing. Kerry J. Ressler: Conceptualization, Visualization, Data curation, Methodology, Resources, Supervision, Writing – review & editing. Samuel A. McLean: Conceptualization, Visualization, Methodology, Investigation, Resources, Data curation, Supervision, Writing – review & editing. Ronald C. Kessler: Conceptualization, Visualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Supervision, Writing – review & editing.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jad.2021.10.104.
References
- Atwoli L, Stein DJ, Koenen KC, McLaughlin KA, 2015. Epidemiology of posttraumatic stress disorder: prevalence, correlates and consequences. Curr. Opin. Psychiatry 28, 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auvergne L, Bortsov AV, Ulirsch JC, Peak DA, Jones JS, Swor RA, Domeier RM, Lee DC, Rathlev NK, Hendry PL, 2016. Association of epidemiologic factors and genetic variants influencing hypothalamic-pituitary-adrenocortical axis function with Postconcussive symptoms after minor motor vehicle collision. Psychosom. Med 78, 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babikian T, Satz P, Zaucha K, Light R, Lewis RS, Asarnow RF, 2011. The UCLA longitudinal study of neurocognitive outcomes following mild pediatric traumatic brain injury. J. Int. Neuropsychol. Soc. JINS 17, 886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KK, Roenker DL, Wadley VG, Edwards JD, Roth DL, McGwin G, Raleigh R, Joyce JJ, Cissell GM, Dube T, 2006. Can high-risk older drivers be identified through performance-based measures in a department of motor vehicles setting? J. Am. Geriatr. Soc 54, 77–84. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, Van Ijzendoorn MH, 2007. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol. Bull 133, 1. [DOI] [PubMed] [Google Scholar]
- Beard C, Amir N, 2009. Interpretation in social anxiety: when meaning precedes ambiguity. Cognit. Ther. Res 33, 406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zion Z, Fine NB, Keynan NJ, Admon R, Green N, Halevi M, Fonzo GA, Achituv M, Merin O, Sharon H, 2018. Cognitive flexibility predicts PTSD symptoms: observational and interventional studies. Front. Psychiatry 9, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjet C, Bromet E, Karam E, Kessler R, McLaughlin K, Ruscio A, Shahly V, Stein DJ, Petukhova M, Hill E, 2016. The epidemiology of traumatic event exposure worldwide: results from the world mental health survey consortium. Psychol. Med 46, 327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomyea J, Risbrough V, Lang AJ, 2012. A consideration of select pre-trauma factors as key vulnerabilities in PTSD. Clin. Psychol. Rev 32, 630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortsov AV, Platts-Mills TF, Peak DA, Jones JS, Swor RA, Domeier RM, Lee DC, Rathlev NK, Hendry PL, Fillingim RB, 2013. Pain distribution and predictors of widespread pain in the immediate aftermath of motor vehicle collision. Eur. J. Pain 17, 1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosaipo NB, Foss MP, Young AH, Juruena MF, 2017. Neuropsychological changes in melancholic and atypical depression: a systematic review. Neurosci. Biobehav. Rev 73, 309–325. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, 2006. Posttraumatic stress disorder and mortality among US Army veterans 30 years after military service. Ann. Epidemiol 16, 248–256. [DOI] [PubMed] [Google Scholar]
- Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, Keane TM, 2016. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders–fifth edition (PCL-5) in veterans. Psychol. Assess 28, 1379. [DOI] [PubMed] [Google Scholar]
- Brandes D, Ben-Schachar G, Gilboa A, Bonne O, Freedman S, Shalev AY, 2002. PTSD symptoms and cognitive performance in recent trauma survivors. Psychiatry Res. 110, 231–238. [DOI] [PubMed] [Google Scholar]
- Brunet A, Weiss DS, Metzler TJ, Best SR, Neylan TC, Rogers C, Fagan J, Marmar CR, 2001. The peritraumatic distress inventory: a proposed measure of PTSD criterion A2. Am. J. Psychiatry 158, 1480–1485. [DOI] [PubMed] [Google Scholar]
- CDC, 2011. National hospital ambulatory medical care survey: 2011 emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf (accessed 17 November 2021).
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, 2010. Initial adult health item banks and first wave testing of the patient-reported outcomes measurement information system (PROMIS™) network: 2005–2008. J. Clin. Epidemiol 63, 1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor NS, Barbosa-Leiker C, Germine LT, Fonseca LM, McPherson SM, Tuttle KR, 2020. Construct validity, ecological validity and acceptance of self-administered online neuropsychological assessment in adults. Clin. Neuropsychol 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cor MK, Haertel E, Krosnick JA, Malhotra N, 2012. Improving ability measurement in surveys by following the principles of IRT: the Wordsum vocabulary test in the. Gen. Soc. Survey Soc. Sci. Res 41, 1003–1016. [DOI] [PubMed] [Google Scholar]
- Coster JE, Turner JK, Bradbury D, Cantrell A, 2017. Why do people choose emergency and urgent care services? A rapid review utilizing a systematic literature search and narrative synthesis. Acad. Emerg. Med 24, 1137–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell TA, Kieffer KM, Siders CA, Vanderploeg RD, 2002. Neuropsychological findings in combat-related posttraumatic stress disorder. Clin. Neuropsychol 16, 310–321. [DOI] [PubMed] [Google Scholar]
- Dodell-Feder D, Ressler KJ, Germine LT, 2020. Social cognition or social class and culture? On the interpretation of differences in social cognitive performance. Psychol. Med 50, 133–145. [DOI] [PubMed] [Google Scholar]
- Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM, 2001. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94, 149–158. [DOI] [PubMed] [Google Scholar]
- Fortenbaugh FC, Rothlein D, McGlinchey R, DeGutis J, Esterman M, 2018. Tracking behavioral and neural fluctuations during sustained attention: a robust replication and extension. Neuroimage 171, 148–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale CR, Deary IJ, Boyle SH, Barefoot J, Mortensen LH, Batty GD, 2008. Cognitive ability in early adulthood and risk of 5 specific psychiatric disorders in middle age: the Vietnam experience study. Arch. Gen. Psychiatry 65, 1410–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale CR, Harris A, Deary IJ, 2016. Reaction time and onset of psychological distress: the UK health and lifestyle survey. J. Epidemiol. Community Health 70, 813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germine L, Nakayama K, Duchaine BC, Chabris CF, Chatterjee G, Wilmer JB, 2012. Is the web as good as the lab? Comparable performance from Web and lab in cognitive/perceptual experiments. Psychon. Bull. Rev 19, 847–857. [DOI] [PubMed] [Google Scholar]
- Golier JA, Harvey PD, Legge J, Yehuda R, 2006. Memory performance in older trauma survivors: implications for the longitudinal course of PTSD. Ann. N. Y. Acad. Sci 1071, 54–66. [DOI] [PubMed] [Google Scholar]
- Hanlon JAD, Martinovich Zoran, Kelly James P., Robert E, 1999. Effects of acute injury characteristics on neuropsychological status and vocational outcome following mild traumatic brain injury. Brain Inj. 13, 873–887. [DOI] [PubMed] [Google Scholar]
- Hartshorne JK, Germine LT, 2015. When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychol. Sci 26, 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedge C, Powell G, Sumner P, 2018. The reliability paradox: why robust cognitive tasks do not produce reliable individual differences. Behav. Res. Methods 50, 1166–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickling E, Gillen R, Blanchard E, Buckley T, Taylor A, 1998. Traumatic brain injury and posttraumatic stress disorder: a preliminary investigation of neuropsychological test results in PTSD secondary to motor vehicle accidents. Brain Inj. 12, 265–274. [DOI] [PubMed] [Google Scholar]
- Hoff E, 2003. The specificity of environmental influence: socioeconomic status affects early vocabulary development via maternal speech. Child Dev. 74, 1368–1378. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Brooks BL, Ashton VL, 2008. Cognitive impairment consequent to motor vehicle collisions. Foundations for Clinical and Forensic practice. Elsevier, pp. 243–309. Motor Vehicle Collisions. [Google Scholar]
- Joormann J, McLean SA, Beaudoin FL, An X, Stevens JS, Zeng D, Kessler RC, 2020. Socio-demographic and trauma-related predictors of depression within eight weeks of motor vehicle collision in the AURORA study. Psychol. Med 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy S, Kaplan E, Fein D, 2004. Speed and memory in the WAIS-III digit symbol—Coding subtest across the adult lifespan. Arch. Clin. Neuropsychol 19, 759–767. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB, 1995. Posttraumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatry 52, 1048–1060. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Ressler KJ, House SL, Beaudoin FL, An X, Stevens JS, McLean SA, 2020. Socio-demographic and trauma-related predictors of PTSD within 8 weeks of a motor vehicle collision in the AURORA study. Mol. Psychiatry 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N, Crawford S, Wenden F, Moss N, Wade D, 1995. The Rivermead Post concussion symptoms questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J. Neurol 242, 587–592. [DOI] [PubMed] [Google Scholar]
- Koenen K, Ratanatharathorn A, Ng L, McLaughlin K, Bromet E, Stein D, Karam E, Ruscio AM, Benjet C, Scott K, 2017. Posttraumatic stress disorder in the world mental health surveys. Psychol. Med 47, 2260–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Poulton R, Martin J, Caspi A, 2007. Early childhood factors associated with the development of post-traumatic stress disorder: results from a longitudinal birth cohort. Psychol. Med 37, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempert KM, Steinglass JE, Pinto A, Kable JW, Simpson HB, 2019. Can delay discounting deliver on the promise of RDoC? Psychol. Med 49, 190–199. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Fischer JS, 2004. Neuropsychological Assessment. Oxford University Press, USA. [Google Scholar]
- Lindgren KP, Kaysen D, Werntz AJ, Gasser ML, Teachman BA, 2013. Wounds that can’t be seen: implicit trauma associations predict posttraumatic stress disorder symptoms. J. Behav. Ther. Exp. Psychiatry 44, 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis KL, Price J, Gillich PJ, 2018. Evolution of the abbreviated injury scale: 1990–2015. Traffic Inj. Prev 19, S109–S113. [DOI] [PubMed] [Google Scholar]
- Macklin ML, Metzger LJ, Litz BT, McNally RJ, Lasko NB, Orr SP, Pitman RK, 1998. Lower precombat intelligence is a risk factor for posttraumatic stress disorder. J. Consult Clin. Psychol 66, 323. [DOI] [PubMed] [Google Scholar]
- Marx BP, Brailey K, Proctor SP, MacDonald HZ, Graefe AC, Amoroso P, Heeren T, Vasterling JJ, 2009. Association of time since deployment, combat intensity, and posttraumatic stress symptoms with neuropsychological outcomes following Iraq war deployment. Arch. Gen. Psychiatry 66, 996–1004. [DOI] [PubMed] [Google Scholar]
- McLean SA, Kirsch NL, Cheribeth UT, Sen A, Frederiksen S, Harris RE, Miao RF, 2009. Health status, not head injury, predicts concussion symptoms after minor injury. Am. J. Emerg. Med 27, 182–190. [DOI] [PubMed] [Google Scholar]
- McLean SA, Ressler K, Koenen KC, Neylan T, Germine L, Jovanovic T, Kessler R, 2020. The AURORA study: a longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Mol. Psychiatry 25, 283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean SA, Ulirsch JC, Slade GD, Soward AC, Swor RA, Peak DA, Jones JS, Rathlev NK, Lee DC, Domeier RM, 2014. Incidence and predictors of neck and widespread pain after motor vehicle collision among US litigants and nonlitigants. PAIN® 155, 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels AJ, Michaels CE, Moon CH, Smith JS, Zimmerman MA, Taheri PA, Peterson C, 1999. Posttraumatic stress disorder after injury: impact on general health outcome and early risk assessment. J. Trauma Acute Care Surg 47, 460–467. [DOI] [PubMed] [Google Scholar]
- Odum AL, 2011. Delay discounting: i am Ak, you are AK. J. Exp. Anal. Behav 96, 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacella ML, Hruska B, Delahanty DL, 2013. The physical health consequences of PTSD and PTSD symptoms: a meta-analytic review. J. Anxiety Disord 27, 33–46. [DOI] [PubMed] [Google Scholar]
- Parslow RA, Jorm AF, 2007. Pretrauma and posttrauma neurocognitive functioning and PTSD symptoms in a community sample of young adults. Am. J. Psychiatry 164, 509–515. [DOI] [PubMed] [Google Scholar]
- Parsons S, Kruijt AW, Fox E, 2019. Psychological science needs a standard practice of reporting the reliability of cognitive-behavioral measurements. Adv. Methods Pract. Psychol. Sci 2, 378–395. [Google Scholar]
- Passell E, Dillon DG, Baker JT, Vogel SC, Scheuer LS, Mirin NL, Rutter LA, Pizzagalli DA, Germine L, 2019. Digital cognitive assessment: results from the test my brain NIMH research domain criteria (RDoC) field test battery report. PsyArXiv. [Google Scholar]
- Passell E, Strong RW, Rutter LA, Kim H, Scheuer L, Martini P, Grinspoon L, Germine L, 2021. Cognitive test scores vary with choice of personal digital device. Behav. Res. Methods 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M, 2008. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J. Psychiatr. Res 43, 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP, 2005. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol. Psychiatry 57, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills TF, Hunold KM, Esserman DA, Sloane PD, McLean SA, 2012. Motor vehicle collision–related emergency department visits by older adults in the United States. Acad. Emerg. Med 19, 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROMIS Cooperative Group, 2021. Promis: Interpret Scores. https://www.healthmeasures.net/score-and-interpret/interpret-scores/promis (accessed 17 November 2021).
- Qureshi SU, Long ME, Bradshaw MR, Pyne JM, Magruder KM, Kimbrell T, Hudson TJ, Jawaid A, Schulz PE, Kunik ME, 2011. Does PTSD impair cognition beyond the effect of trauma? J. Neuropsychiatry Clin. Neurosci 23, 16–28. [DOI] [PubMed] [Google Scholar]
- Rieger BP, Lewandowski LJ, Callahan JM, Spenceley L, Truckenmiller A, Gathje R, Miller LA, 2013. A prospective study of symptoms and neurocognitive outcomes in youth with concussion vs orthopaedic injuries. Brain Inj. 27, 169–178. [DOI] [PubMed] [Google Scholar]
- Roberts AL, Gilman SE, Breslau J, Breslau N, Koenen KC, 2011. Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychol. Med 41, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M, Noonan S, DeGutis J, Esterman M, 2013. Sustaining visual attention in the face of distraction: a novel gradual-onset continuous performance task. Attent. Percept. Psychophys 75, 426–439. [DOI] [PubMed] [Google Scholar]
- Rutter LA, Dodell-Feder D, Vahia IV, Forester BP, Ressler KJ, Wilmer JB, Germine L, 2019a. Emotion sensitivity across the lifespan: mapping clinical risk periods to sensitivity to facial emotion intensity. J. Exp. Psychol. Gen 148, 1993. [DOI] [PubMed] [Google Scholar]
- Rutter LA, Scheuer L, Vahia IV, Forester BP, Smoller JW, Germine L, 2019b. Emotion sensitivity and self-reported symptoms of generalized anxiety disorder across the lifespan: a population-based sample approach. Brain Behav. 9, e01282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter LA, Vahia IV, Forester BP, Ressler KJ, Germine L, 2020. Heterogeneous indicators of cognitive performance and performance variability across the lifespan. Front. Aging Neurosci 12, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago PN, Ursano RJ, Gray CL, Pynoos RS, Spiegel D, Lewis-Fernandez R, Friedman MJ, Fullerton CS, 2013. A systematic review of PTSD prevalence and trajectories in DSM-5 defined trauma exposed populations: intentional and non-intentional traumatic events. PLoS ONE 8, e59236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer J, Zvielli A, Höfler M, Wittchen H-U, Bernstein A, 2018. Trauma, attentional dysregulation, and the development of posttraumatic stress: an investigation of risk pathways. Behav. Res. Ther 102, 60–66. [DOI] [PubMed] [Google Scholar]
- Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM, Krystal JH, Schweinsburg BC, 2015. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol. Bull 141, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev AY, 2003. Treating survivors in the acute aftermath of traumatic events. In: Proceedings of the Lecture at the 19th Annual Meeting of ISTSS. [Google Scholar]
- Stein DJ, Karam EG, Shahly V, Hill ED, King A, Petukhova M, Atwoli L, Bromet EJ, Florescu S, Haro JM, 2016. Post-traumatic stress disorder associated with life-threatening motor vehicle collisions in the WHO world mental health surveys. BMC Psychiatry 16, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling M, Hendrikz J, Kenardy J, 2011. Similar factors predict disability and posttraumatic stress disorder trajectories after whiplash injury. Pain 152, 1272–1278. [DOI] [PubMed] [Google Scholar]
- Sternberg S, 1966. High-speed scanning in human memory. Science 153, 652–654. [DOI] [PubMed] [Google Scholar]
- Suliman S, Stein DJ, Seedat S, 2014. Clinical and neuropsychological predictors of posttraumatic stress disorder. Medicine 93 (Baltimore). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Allard CB, Thorp SR, Norman SB, Cissell SH, Berardi KH, Grimes EM, Stein MB, 2009. Cognitive impairment and functioning in PTSD related to intimate partner violence. J. Int. Neuropsychol. Soc 15, 879–887. [DOI] [PubMed] [Google Scholar]
- Ulirsch J, Ballina L, Soward A, Rossi C, Hauda W, Holbrook D, Wheeler R, Foley KA, Batts J, Collette R, 2014. Pain and somatic symptoms are sequelae of sexual assault: results of a prospective longitudinal study. Eur. J. Pain 18, 559–566. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Brewin C, 2005. Neuropsychology of PTSD: Biological, cognitive, and Clinical Perspectives. Guilford Press. [Google Scholar]
- Vasterling JJ, Verfaellie M, 2009. Introduction-posttraumatic stress disorder: a neurocognitive perspective. J. Int. Neuropsychol. Soc. JINS 15, 826. [DOI] [PubMed] [Google Scholar]
- Vogel SC, Esterman M, DeGutis J, Wilmer JB, Ressler KJ, Germine LT, 2020. Childhood adversity and dimensional variations in adult sustained attention. Front. Psychol 11, 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadley VG, Okonkwo O, Crowe M, Vance DE, Elgin JM, Ball KK, Owsley C, 2009. Mild cognitive impairment and everyday function: an investigation of driving performance. J. Geriatr. Psychiatry Neurol 22, 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmer JB, Germine L, Chabris CF, Chatterjee G, Gerbasi M, Nakayama K, 2012. Capturing specific abilities as a window into human individuality: the example of face recognition. Cogn. Neuropsychol 29, 360–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski C, Thompson W, Gottesman I, 1994. Comparison of neuropsychological test performance in PTSD, generalized anxiety disorder, and control Vietnam veterans. Assessment 1, 133–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.