Abstract
Apolipoprotein (APOE) ε4 allele is a strong risk factor for Alzheimer’s disease (AD) and cognitive decline. Epigenetic modifications such as DNA methylation (DNAm) play a central role in cognition. This study sought to identify DNAm sites in the APOE genomic region associated with cognitive performance in a racially diverse middle-aged cohort (n=411). Cognitive performance was measured by 11 standard neuropsychological tests. Two CpG sites were associated with the Card Rotation and Benton Visual Retention cognitive tests. The methylation level of the CpG site cg00397545 was associated with Card Rotation Test score (P=0.000177) and a novel CpG site cg10178308 was associated with Benton Visual Retention Test score (P=0.000084). Significant associations were observed among the dietary inflammatory index, which reflects the inflammatory potential of the diet, cognitive performance and the methylation level of several CpG sites. Our results indicate that DNAm in the APOE genomic area is correlated with cognitive performance and may presage cognitive
Keywords: cognition, APOE, biomarker, aging, DNA methylation, Dietary Inflammatory Index
1. Introduction
Apolipoprotein E (APOE) plays a central role in lipid and cholesterol metabolism in the central nervous system (CNS) (Mahley 2016). More specifically, APOE mediates the lipoprotein pathway that regulates cholesterol recycling and redistribution for cellular repair and maintenance (Boyles, Notterpek et al. 1990). In humans, the brain is the most cholesterol rich organ and synthesizes its own cholesterol. In the brain, APOE protein is mainly produced by astrocytes and is abundantly expressed in neurons and microglia under stress or injury conditions (Fernandez, Hamby et al. 2019).
The human APOE gene has three common alleles (ɛ2, ɛ3 and ɛ4), coding functionally distinct APOE isoforms. The ɛ4 allele is associated with increased risk of developing Alzheimer’s disease (AD) (Corder, Saunders et al. 1993, Okuizumi, Onodera et al. 1994). The APOE ε4 allele is the main genetic risk factor for sporadically occurring AD as well as the earlier stages of mild cognitive impairment (MCI) (Kryscio, Schmitt et al. 2006). While APOE ε4 allele carriers have an increased risk, ε2 allele carriers are protected from dementia (Farrer, Cupples et al. 1997). The APOE ε4 allele is also associated with greater cognitive decline in non-demented individuals (Bretsky, Guralnik et al. 2003). Additionally, the APOE ε4 allele moderates the effects of known risk factors of cognitive decline including sex, age, hypertension, diabetes, and smoking status (Farrer, Cupples et al. 1997, Carmelli, Swan et al. 1999, Dufouil, Tzourio et al. 2000, de Frias, Schaie et al. 2014, Andrews, Das et al. 2015, Riedel, Thompson et al. 2016). Furthermore, single nucleotide polymorphisms in the APOE linkage disequilibrium (LD) locus including TOMM40, NECTIN2 and APOC1 are associated with cognitive performance (Bartres-Faz, Clemente et al. 2001, Takei, Miyashita et al. 2009, Cervantes, Samaranch et al. 2011, Zhou, Peng et al. 2014).
Genomic variations, epigenetic modifications including histone modifications, DNA methylation (DNAm) and non-coding RNA (ncRNA) have been associated with many diseases. Epigenetic changes link altered gene expression with environmental factors such as exercise and diet. DNAm is the addition of a methyl group to the C-5 position of the cytosine pyrimidine ring. DNAm in the promotor region of genes can perturb transcription factor binding and suppress gene expression (Landgrave-Gomez, Mercado-Gomez et al. 2015). One study found that DNAm in the APOE gene had significant interindividual epigenetic variability in post-mortem brains, which may contribute to AD predisposition (Wang, Oelze et al. 2008). Several previous studies have examined the association between APOE DNAm and participants with diagnosed AD or mild cognitive impairment (Karlsson, Ploner et al. 2018, Shao, Shaw et al. 2018, Mancera-Paez, Estrada-Orozco et al. 2019). Two studies have evaluated the relationship of APOE DNAm and cognitive functions in cognitively healthy participants. One found DNAm in the APOE gene region was inversely associated with delayed recall during normal cognitive aging among 289 elderly African Americans with mean age 67 years (Liu, Zhao et al. 2018). The other study observed no association between general cognitive ability and APOE DNAm in a large European based cohort (Mur, McCartney et al. 2020). Most studies have focused on elderly AD patients and had relatively small sample sizes. The association between DNAm in the APOE genomic region and cognitive function in a middle-aged community-dwelling diverse cohort remains elusive.
Many studies have suggested that neuroinflammation plays an important role in AD pathogenesis (Morales, Guzman-Martinez et al. 2014, Heneka, Carson et al. 2015). Diet has been shown to be able to modulate systemic inflammation and neuroinflammation (Galland 2010, Tran, Tse et al. 2016). Accumulating evidence has suggested that dietary factors which can modulate inflammation may affect the progression of AD (Szczechowiak, Diniz et al. 2019). The Dietary inflammatory index (DII) was developed to be a sensitive measurement to identify the inflammatory potential of the diet with a higher score indicating greater proinflammatory potential (Shivappa, Steck et al. 2014). Recently, a study has shown higher DII scores were associated with an increase in the risk for dementia incidence (Charisis, Ntanasi et al. 2021). It is established that DNAm plays a pivotal role in the pathogenesis of cognitive decline, but little is known about the association between DII and DNAm. In this study, we aim to explore the association among DII, DNAm and cognitive performance in a community-dwelling middle-aged cohort.
Here, we characterized DNAm across four genes in the APOE genomic region (APOE, TOMM40, NECTIN2 and APOC1) in blood samples collected from 411 middle-aged, community dwelling, AAs and whites, cognitively intact participants in the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) cohort. Linear regression models were used to evaluate associations between DNAm and cognitive test scores adjusting for possible covariates. We tested for interactions among sex, age, race and APOE allele status and whether APOE gene region DNAm differences are associated with cognitive function. Our study on the DNAm of APOE region in a diverse middle-aged cohort may facilitate the development of new clinical biomarkers before the onset of dementia.
2. Material and methods
2.1. Study sample
The participants in this study were from the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) cohort of the National Institute on Aging Intramural Research Program, National Institutes of Health (NIH)(Evans, Lepkowski et al. 2010). HANDLS was initiated in 2004 and is an interdisciplinary, community-based prospective longitudinal study of AAs and whites based in Baltimore, Maryland. The HANDLS participants were drawn from 13 Baltimore neighborhoods (groups of contiguous census tracts) and were between the ages of 30–64 years at baseline. Poverty status was defined as above or below the 125% of the 2004 US federal poverty guidelines. Baseline data were collected through home visits and examination on the medical research vehicles (MRV). The participants were administered physical examination, medical history inquiries, cognitive testing, dietary recall interviews and other assessments. Details on the study design and protocols have been described elsewhere (Evans, Lepkowski et al. 2010). HANDLS was approved by the Institutional Review Board of National Institutes of Health. All participants provided written informed consent. The sample in this study consists of 411 participants with mean age 49 years and balanced by sex, race, and poverty status (Table 1, Figure S1).
Table 1.
Descriptive statistics of participants by APOE allele carrier status
| Variables | Total | APOE ε4+ | APOE ε4− |
|---|---|---|---|
| Number of Participants | 411 | 134 | 277 |
| Age (years) (mean [sd]) | 48.7 (8.67) | 48.6 (9.05) | 48.9 (8.16) |
| Sex (N [%]) | |||
| Male | 209 (50.9%) | 65 (48.5%) | 144 (52.0%) |
| Female | 202 (49.1%) | 69 (51.5%) | 133 (48.0%) |
| Race (N [%]) | |||
| White | 199 (48.4%) | 47 (35.1%) | 152 (54.9%) |
| AfrAm | 212 (51.6%) | 87 (64.9%) | 125 (45.1%) |
| Poverty Status (N [%]) | |||
| Above | 204 (49.6%) | 69 (51.5%) | 135 (48.7%) |
| Below | 207 (50.4%) | 65 (48.5%) | 142 (51.3%) |
| Smoking Status (N [%]) | |||
| Yes | 183 (47.3%) | 59 (44.7%) | 124 (44.8%) |
| NO | 204 (52.7%) | 65 (49.2%) | 139 (50.2%) |
| Diabetes (N [%]) | |||
| Yes | 48 (12.7%) | 17 (12.9%) | 31 (11.2%) |
| NO | 329 (87.3%) | 104 (78.8%) | 225 (81.2%) |
| CVD (N [%]) | |||
| Yes | 154 (43.9%) | 47 (35.6%) | 107 (38.6%) |
| NO | 197 (56.1%) | 59 (44.7%) | 138 (49.8%) |
| WRAT score (mean [sd]) | 42.40 (7.56) | 43.02 (7.72) | 41.49 (6.73) |
| BVRT score (mean [sd]) | 6.80 (4.31) | 6.24 (4.29) | 7.75 (4.27) |
| CRD score (mean [sd]) | 37.90 (17.32) | 38.82 (17.64) | 34.89 (16.98) |
411 of the 464 participants have APOE allele genotypes. Key: AfrAm, African American; CVD, cardiovascular disease; WRAT, Wide Range Achievement Test; BVRT, Benton Visual Retention Test; CRD, Card Rotation Test; sd, standard deviation.
2.2. Cognitive function measures
HANDLS participants were administered a battery of standard cognitive tests capturing different cognitive domains. The cognitive battery included: Mini-Mental State Examination total score (global mental status); California Verbal Learning Test, List A (verbal learning) and delayed free recall (verbal memory); Benton Visual Retention Test (visuospatial ability), total errors; Card Rotation Test (visuospatial ability); Digit Span Forward and Backward, total correct (attention, executive function/working memory); Animal Fluency Test (language/verbal fluency), total words minus intrusions/perseverations; Brief Test of Attention, total correct trials of 10; Trail-Making Test, part A (attention) and part B (executive function), number of seconds to completion; and the Clock-Drawing Test command total score (0–10) (visuo-constructional). For most of the cognitive tests, higher test scores indicate better cognitive performance, except for the Benton Visual Retention Test, which measures the total number of errors, and Trail-Making Test which record overall completion time.
2.3. DNA methylation measures
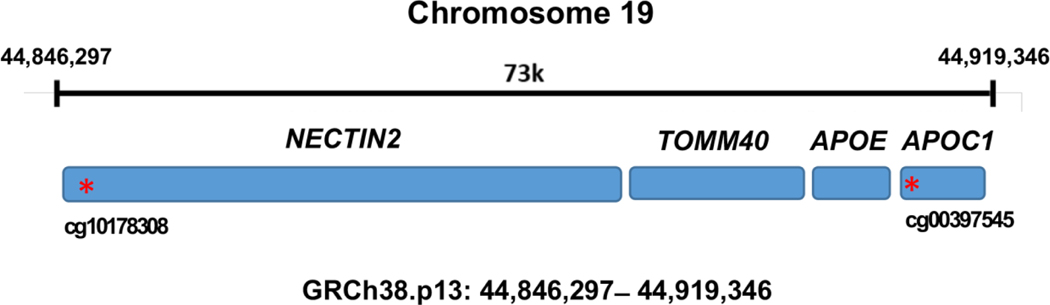
DNAm in peripheral white blood cell samples was profiled using the Illumina Human MethylationEPIC BeadChip as described previously (Tajuddin, Hernandez et al. 2019). Briefly, low quality samples and probes with low detection rates were excluded. Unrelated participants were selected to reduce the potential influence of shared genetics on the findings. We restricted the analysis to 69 CpGs located on chromosome 19 between 44,846,297 and 44,919,346 bp, which corresponded to the genomic region encompassing the PVRL2-TOMM40-APOE-APOC1 (UCSC GRCh38/hg19 genome build). For each CpG site, the methylation level used for analysis was defined as the β-value, adjusted for batch effects and white blood cell proportions. The proportion of each white blood cell type was estimated using Houseman’s method (Houseman, Accomando et al. 2012). β-value outliers were excluded. We used annotation from Illumina MethylationEPIC product files to determine whether each CpG site was in a promoter region or gene body. DNAm levels were measured from blood samples collected during the MRV examinations for 411 unrelated participants. None of the participants has been diagnosed with dementia by a physician.
2.4. APOE genotyping
To evaluate the potential confounding effect of APOE genotype, we assessed the APOE genotype of our participants (N=411) (Figure S1). Based on the identity of the nucleotides at SNP positions rs429358 (APOE-C112R) and rs7412 (APOE-R158C), participants with the ε3/ε4 and ε4/ε4 haplotypes were classified as ε4 carriers and the others as non-ε4 carriers. Taqman Assays (Applied Biosystems Assay-On-Demand part numbers C__3084793_20 and C__904973_10) were utilized to genotype these SNPs on a 7900HT Sequence Detection System (Applied Biosystems). Absolute quantification was performed on ThermoHybaidPCR cycler blocks using the following conditions across four stages. Stage 1: consisted of 1 cycle at 50 °C for a total of 2 min. Stage 2: consisted of 1 cycle at 95 °C for a total of 10 min. Stage 3: consisted of 40 cycles at 95 °C, each for a period of 15 s. Stage 4: consisted of 1 cycle at 60 °C for a total of 1 min. PCR plates were held at 4 °C until returning to the 7900HT Sequence Detection System to perform an allelic discrimination (Federoff, Jimenez-Rolando et al. 2012).
2.5. Covariates
Covariates were selected based on reported influence on cognitive performance from prior research. Specifically, sex, race, age, poverty status, body mass index, hypertension, smoking status, diabetes mellitus, cardiovascular disease status, APOE allele status and literacy level were chosen as the covariates (Deary, Whiteman et al. 2002, Scarmeas, Albert et al. 2006, Anstey, von Sanden et al. 2007, van den Berg, Kloppenborg et al. 2009, Carlsson 2010, Prickett, Brennan et al. 2015). Body mass index (BMI, kg/m2) was calculated from measured weight and height. Hypertension was defined as either an average of two measurements of systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg, current use prescribed antihypertensive medication, or self-reported diagnosis of hypertension. Smoking status was self-reported as part of the MRV exam. Diabetes mellitus was defined as fasting plasma glucose concentration ≥126 mg/dl (7.0 mmol/l), self-reported diabetes diagnosis, or current use of prescribed hypoglycemic agents. A “CVD” covariate was created for cardiovascular disease status of the participants, which was defined as having a self-reported history of hypertension, stroke, coronary artery disease, or blood clot. The Wide Range Achievement Test (WRAT-3) was administered as a measurement of literacy and is used here as an evaluation of an individual’s reading comprehension (Wilkinson 1993).
2.6. Dietary inflammatory index (DII) measures
The 24-hour dietary recall was used to collect food and beverage intakes of the HANDLS cohort. Data were collected through the computerized Automated Multiple-Pass Method (AMPM) established by the United States Department of Agriculture (USDA) (Moshfegh, Rhodes et al. 2008). The Dietary Inflammatory Index (DII) for HANDLS participants was calculated based on the 24-hour dietary recall data. The DII, developed by Shivappa and colleagues (Shivappa, Steck et al. 2014), consists of 45 dietary factors including a variety of macronutrients, micronutrients, vitamins, minerals, flavonoids, spices, and herbs. For this study, 35 out of 45 food parameters were available for the calculation of the DII. These dietary intakes included alcohol, vitamin B12, vitamin B6, carotene, caffeine, carbohydrates, cholesterol, energy, total fat, fiber, folate, iron, magnesium, mono-unsaturated fatty acid, niacin, omega_3 fatty acids, omega_6 fatty acids, protein, poly-unsaturated fatty acid, riboflavin, saturated fatty acids, selenium, thiamin, vitamin A, vitamin C, vitamin D, vitamin E, zinc, tea, flavan-3-ols, flavones, flavones, flavanones, anthocyanidins, isoflavones. DII was calculated for the subset of participants (N = 363) with dietary data.
2.7. Statistical analysis
All statistical analyses were performed in RStudio version 1.3.1093 (R version 4.0.3) (R Core Team 2020). For the analyses exploring effects of DNAm levels, linear regression models were used. The primary analysis was conducted in the full model with all covariates (N = 411). Linear regression was used to test for association between the methylation level of each CpG site and each cognitive measure, adjusting for all covariates stated above. For multiple comparisons, the threshold for statistical significance was Bonferroni‐corrected for each CpG (P<0.0007). Delta R2 was calculated comparing the model of interest with the same model excluding the predictor variable. Interaction terms among age and APOE status were first tested in the model but no significant terms were found (data not shown). Therefore, these interaction terms were omitted from the final model.
3. RESULTS
3.1. Description of study participant characteristics
Study participant characteristics are shown in Table 1 across APOE genotype groups. The age of the 411 participants ranged from 30.2 to 65.2 years with mean age of 48.7 years and standard deviation of 8.64. Other variables known to influence cognitive performance are listed in the table including smoking, diabetes, cardiovascular disease (CVD) status and the WRAT-3 which captures literacy level. Overall, 67.4% of the participants are non-APOE4 carriers and 33.6% of participants carry at least 1 APOE4 allele. No significant differences were found in sex, age, poverty, smoking, CVD status and WRAT-3 between the APOE4 carrier group and non-APOE4 carrier group. However, APOE4 carriers were significantly more prevalent among AAs (64.9%) compared to whites (35.1%) in our cohort. We examined the correlation among all the covariates. We found that smoking was more prevalent in men than women. However, the proportion of cardiovascular diseases were higher among women than men.
3.2. APOE methylation and cognitive function
We examined the association between cognitive tests scores and DNAm levels of CpGs in the APOE genomic area in 411 participants. Two significant associations were discovered in the full model after correcting 69 CpGs for multiple testing (Fig. 1, Table 2). One CpG cg00397545 located in the promotor region of APOC1 was significantly associated with the Card Rotation Test score (p = 0.000177), which captures visuospatial ability and has been reported be associated with cerebrospinal fluid amyloid β-peptide 42 (Aβ42) in AD patients (Shao, Shaw et al. 2018). Another CpG cg10178308 in the promotor region of NECTIN2 was significantly associated with Benton Visual Retention Test score (p = 0.000084), which captures visual recall ability and has not been reported before. The methylation level of the two significant CpGs were not correlated with each other. No significant interactions were found between CpGs and covariates.
Fig. 1.
Schematic for APOE genomic area Genomic region of NECTIN2-TOMM40-APOE-APOC2 locus on chromosome 19 Key: NECTIN2: Nectin Cell Adhesion Molecule 2; TOMM40: Translocase of Outer Mitochondrial Membrane 40; APOC1: Apolipoprotein C1 CpG sites marked with an asterisk (*) are in promoter regions (within 1.5 kb before the transcription start site of the gene)
Table 2.
Linear regression analyses between cognitive test scores and CpG methylation levels in APOE genomic region.
| Cognitive Test | Associated CpGs | Gene | P-value | β-value | ΔR2 | Previous Literature |
|---|---|---|---|---|---|---|
| Card Rotation Test | cg00397545 | APOC1 | 0.000177 | −0.69 | 0.04 | Associate with CSF Aβ42 in AD patients (Shao, Shaw et al. 2018) |
| Benton Visual Retention Test | cg10178308 | NECTIN2 | 0.000084 | −0.14 | 0.04 | Novel |
Shao, Y., M. Shaw, K. Todd, M. Khrestian, G. D’Aleo, P. J. Barnard, J. Zahratka, J. Pillai, C. E. Yu, C. D. Keene, J. B. Leverenz and L. M. Bekris (2018). “DNA methylation of TOMM40-APOE-APOC2 in Alzheimer’s disease.” J Hum Genet 63(4): 459–471.
3.3. Age and APOE genotypes on cognitive performance
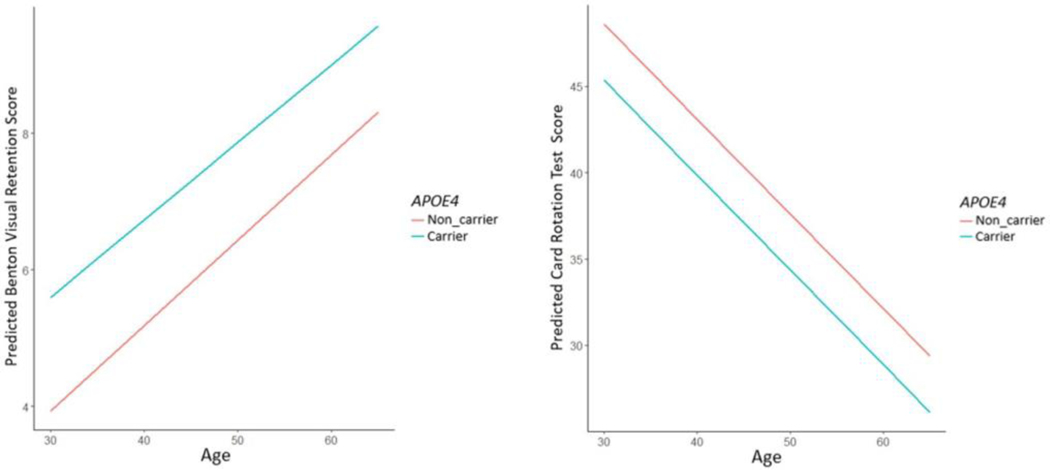
For middle-aged adults, when the development of dementia may be at its early stage, the APOE genotype effect on cognitive functioning remains unclear (Sager, Hermann et al. 2005, O’Dwyer, Lamberton et al. 2012, Patel, Stevens et al. 2013, Zimmermann, Alain et al. 2019). In this middle-aged cohort, age was strongly associated with performance on the Benton Visual Retention Test and Card Rotation Test (Table 3). We found the APOE ε4 allele was associated with worse performance on Benton Visual Retention Test (p = 0.009) (Fig. 2, Table 3). However, the effect of APOE genotype was reduced to a trend-level association (p = 0.08) after adjusting for covariates.
Table 3.
Association of cognitive test scores with age and APOE4 status.
| Age | APOE4 | |||
|---|---|---|---|---|
| Estimate | P-value | Estimate | P-value | |
| Basic Model (N=411) | ||||
| Benton Visual Retention Test | 0.1217 | <0.0001 | 1.4462 | 0.009 |
| Card Rotation Test | −0.5487 | <0.0001 | −3.2295 | 0.1 |
| Full Model (N=411) | ||||
| Benton Visual Retention Test | 0.1167 | 0.0004 | 0.9963 | 0.08 |
| Card Rotation Test | −0.4894 | <0.0001 | −0.8898 | 0.65 |
Full model is adjusted by sex, race, age, poverty status, body mass index, smoking status, diabetes mellitus, cardiovascular disease status, APOE4 allele status and WRAT score
Fig. 2.
Relationship between age and cognition test scores of (a) Benton Visual Retention Test and (b) Card Rotation Test based on APOE4 allele carrier status in an unadjusted model.
3.4. Association between DNA methylation and APOE genotype
DNA methylation, as one of the major epigenetic mechanisms, is regulated by DNA methyltransferases (Dnmts). Mutations in DNMT1 are associated with a form of neurodegenerative disease (Klein, Botuyan et al. 2011), suggesting that alterations in DNAm may play a crucial role in the pathogenesis of cognitive decline. Since little is known about the relationship between DNAm, APOE and cognition, we chose to examine DNAm in the genomic region of APOE. In our cohort, significant associations between APOE carrier status and DNAm level at 2 CpG sites were found after correcting for multiple testing in the full model with all available covariates. The 2 CpGs, cg07773593 (p = 0.0004) and cg13880303 (p = 3.2 × 10−8), were located closely to the promotor region of the APOC1 gene. Their methylation levels were strongly correlated with each other (r = 0.69) and the methylation level of both CpGs in APOE4 carriers were significantly reduced than non-APOE4 carriers.
3.5. Association among Dietary Inflammatory Index, cognitive performance and CpGs in APOE region
We discovered that the Benton Visual Retention Test score was significantly associated with cg10178308, which is located in the promotor region of the NECTIN2 gene. NECTIN2 functions in immune modulation and is expressed in neurons (Samanta, Ramagopal et al. 2012). Since inflammation plays a role in cognitive decline (Kravitz, Corrada et al. 2009, Bettcher, Wilheim et al. 2012), we wanted to examine the influence of inflammation on cognitive performance in our cohort. To test this, we investigated the inflammatory potential of the diet of our participants as there is evidence that diet component such as saturated fat promoted neuroinflammation (Milanski, Degasperi et al. 2009). We evaluated the association between DII score and cognitive test scores in our cohort using the full model (N = 363). We observed significant associations between DII and Benton Visual Retention Test (p < 0.001), Card Rotation Test (p =0.036), Mini-Mental State Examination (p < 0.001) and Animal Fluency Test (p = 0.015). We then examined the association between DII and the CpGs in the APOE genomic area using the full model and found that only one CpG cg10178308 passed multi-testing correction (p = 0.003).
4. DISCUSSION
We examined the relationship between the DNAm level, APOE carrier status and cognitive performance in a cross-sectional analysis of participants from age 30 to 65. Our study utilized a middle-aged cohort with both African Americans and Whites and identified a novel and a previously reported CpG site that were associated with either the Card Rotation Test (cg00397545) or the Benton Visual Retention Test (cg10178308) after Bonferroni correction for multiple testing (Table 2). However, we did not correct cognitive tests for multiple comparisons. Thus, the chance of Type I error remains a concern. We found significant associations between APOE carrier status and DNAm level at 2 APOE gene region CpGs (cg07773593 and cg13880303, located close to the promotor region of the APOC1 gene) after correcting 69 CpGs for multiple testing. We also observed a negative association between cognition and DII score, and another positive association between DII and the DNAm level of CpG cg10178308.
We identified a novel CpG, cg10178308 associated with the Benton Visual Retention Test score (P = 0.000084). Cg10178308 is located in the promotor region of Nectin Cell Adhesion Molecule 2 (NECTIN2) gene, which encodes a plasma membrane glycoprotein involved in adherens junctions as well as a modulator of T-cell signaling (Martinez and Spear 2001, Yu, Harden et al. 2009). NECTIN2 binding to CD226 and stimulates T-cell proliferation and cytokine production, including IL2, IL5, IL10, IL13, and Interferon γ. However, NECTIN2 binding with PVR Related Immunoglobulin Domain Containing Gene (PVRIG) inhibits T-cell proliferation (Tahara-Hanaoka, Shibuya et al. 2004, Zhu, Paniccia et al. 2016). SNPs in the NECTIN2 gene are associated with AD or cognitive impairment independent of APOE ε4 carrier status (Takei, Miyashita et al. 2009, Cruz-Sanabria, Bonilla-Vargas et al. 2018, Zhou, Chen et al. 2019). A recent study discovered that another CpG site, cg11670000, in NECTIN2 was associated with the Rey Auditory Verbal Learning test (RAVLT) delayed recall instrument, which evaluates verbal learning and memory (Liu, Zhao et al. 2018). The Benton Visual Retention Test is a widely used test of visual memory and visual construction (Sivan 1992), and the Benton Visual Retention Test of visual memory is highly sensitive to early dementia. One study discovered that more errors on the Benton Visual Retention Test was associated with an elevated risk of AD up to 15 years later compared to participants with fewer errors (Kawas, Corrada et al. 2003). The Benton Visual Retention Test score can be influenced by individual differences including age, socioeconomic status and educational attainment (Strauss, Sherman et al. 2006). In our study, we adjusted our model with participants’ age, literacy level and other covariates and found an inverse association between Benton Visual Retention Test score and DNAm of cg10178308 in a middle-aged cohort (average 49 years old).
Further, we identified that DNAm of the CpG site cg00397545 was associated with the Card Rotation Test score. This cg00397545 site has been reported to be associated with cerebrospinal fluid amyloid β-peptide 42 (Aβ42) in AD patients (Shao, Shaw et al. 2018). Cg00397545 is located in the promoter region of the APOC1 gene, which encodes apolipoprotein C1. APOC1 is mainly involved in lipoprotein metabolism and inhibits the APOE mediated uptake of very low density lipoprotein (Fuior and Gafencu 2019). Many studies have shown the APOC1 gene is a genetic risk factor for AD, in association with APOE allele status (Poduslo, Neal et al. 1998, Ki, Na et al. 2002, Tycko, Lee et al. 2004, Lucatelli, Barros et al. 2011). In mice, overexpression or knock out of human APOC1 resulted in impaired memory and learning functions (Berbée, Vanmierlo et al. 2011). Additionally, apolipoprotein C1 suppressed pro-inflammatory cytokine secretion from glial cells, which is dependent on APOE genotype in humans (Cudaback, Li et al. 2012). The Card Rotation Test is used to evaluate visuospatial ability, particularly spatial rotational ability. One study found that the Card Rotation Test showed the earliest change in rates of cognitive decline among 11 cognitive measurements in preclinical AD, suggesting its utility for early cognitive decline detection (Williams, An et al. 2020).
The neurodegenerative process can be caused by neuroinflammation due to the activation of immune cells that produce a large quantity of proinflammatory cytokines, which can cause damage to neurons (Fuhrmann, Bittner et al. 2010). An increased level of proinflammatory cytokines was found in the brain and CSF of people with dementia, suggesting the role of microglia in the pathology of dementia (Domingues, AB da Cruz e Silva et al. 2017). Furthermore, markers of neuroinflammation colocalize with amyloid plaque and neurofibrillary tangles, which are the hallmarks of AD and cognitive decline (Kuo, Yen et al. 2005). Another study suggested participants that have high inflammatory concentrations at baseline may have an increased risk of developing dementia after about two decades of follow up (Schmidt, Schmidt et al. 2002). The Dietary Inflammatory Index (DII) was developed to assess the inflammatory potential of nutrients and foods in the context of a dietary pattern (Shivappa, Steck et al. 2014). DII has been shown to have significant positive associations with blood inflammatory markers including Interleukin 6 (IL-6), IL-7, C-reactive protein (CRP), and tumor necrosis factor alpha (TNF-α) in diverse populations (Shivappa, Hébert et al. 2015, Tabung, Steck et al. 2015, Cervo, Scott et al. 2020). Two studies have found significant association or predictive value of DII score with modified Mini-Mental State Examination score in older women and older Korean populations (Hayden, Beavers et al. 2017, Shin, Kwon et al. 2018). Another study discovered a significant relationship between DII score and Animal Fluency Test score in older participants from the U.S. (Frith, Shivappa et al. 2018). In our study, we replicated the findings of association among DII score, Animal Fluency Test score and Mini-Mental State Examination score, while we found additional association including Benton Visual Retention Test, Card Rotation Test, and Digit Span Forward and Backward Tests.
Our study has several strengths. We are among the first to assess the relationship between cognitive performance and APOE methylation in a diverse, urban sample of middle-aged men and women. In addition, the DNAm profile of the APOE genomic area was characterized using high-density Methylation EPIC BeadChip, which has more CpG candidate sites compared to the previous Illumina HumanMethylation450 array. Moreover, a battery of 11 cognitive tests were performed to capture multiple domains of cognitive functions. To the best of our knowledge, this is the first study to characterize the relation between DII and DNAm. This study also has several limitations. First, only cross-sectional analysis of association between DNAm and cognitive performance has been conducted. Further longitudinal studies are necessary to infer causality and to characterize how DNAm and cognitive performance evolve with time. Moreover, we are not able to verify the correlation between blood DNAm with brain DNAm patterns as well as the relationship between DII with neuroinflammatory content. Blood DNAm in the APOE genomic area has modest correlation with brain DNAm (Hannon, Lunnon et al. 2015, Braun, Han et al. 2019). Consequently, our findings based on blood DNAm may not reflect possible pathological developments in the brain.
In conclusion, we identified 2 CpG sites in the APOE genomic area significantly associated with Benton Visual Retention Test and Card Rotation Test in a diverse middle-aged urban-dwelling cohort. Cross-sectional analysis discovered that the cognitive performance of participants differed by the DNAm level of the significant CpGs. We showed DII exhibits strong associations with cognitive performance and the DNAm of cg10178308. Future work may explore the relationship between DNAm and cognitive performance in a longitudinal cohort to further strengthen our understanding of its evolvement as aging progresses.
Supplementary Material
Highlights.
DNA methylation in the APOE genomic area correlates with cognitive performance.
The DNA methylation of two CpG sites was associated with performance on specific cognitive tests.
The Dietary Inflammatory Index associates with cognitive performance and DNA methylation level.
Footnotes
Botong Shen, Conceptualization, Writing - original draft, Writing - review & editing, Formal Analysis, Methodology, Dena Hernandez, Investigation, Data curation, Methodology, Kumaraswamynaidu Chitrala, Writing - review & editing, Formal Analysis, Data curation, Methodology, Marie T Fanelli-Kuczmarski, Writing - review & editing, Formal Analysis, Data curation, Nicole Noren. Hooten, Writing - review & editing, Natasha L. Pacheco, Writing - review & editing, Formal Analysis, Methodology, Nicolle A. Mode, Writing - review & editing, Formal Analysis, Data curation, Methodology, Alan B. Zonderman, Writing - review & editing, Formal Analysis, Funding Acquisition, Ngozi Ezike, Investigation, Resources, Michele K. Evans, Conceptualization, Writing - review & editing, Investigation, Resources, Funding Acquisition
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews S, Das D, Anstey KJ and Easteal S. (2015). “Interactive effect of APOE genotype and blood pressure on cognitive decline: the PATH through life study.” J Alzheimers Dis 44(4): 1087–1098. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, von Sanden C, Salim A. and O’Kearney R. (2007). “Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies.” American journal of epidemiology 166(4): 367–378. [DOI] [PubMed] [Google Scholar]
- Bartres-Faz D, Clemente IC, Junque C, Valveny N, Lopez-Alomar A, Sanchez-Aldeguer J, Lopez-Guillen A. and Moral P. (2001). “APOE and APOC1 genetic polymorphisms in age-associated memory impairment.” Neurogenetics 3(4): 215–219. [DOI] [PubMed] [Google Scholar]
- Berbée JF, Vanmierlo T, Abildayeva K, Blokland A, Jansen PJ, Lütjohann D, Gautier T, Sijbrands E, Prickaerts J. and Ramaekers F. (2011). “Apolipoprotein CI knock-out mice display impaired memory functions.” Journal of Alzheimer’s Disease 23(4): 737–747. [DOI] [PubMed] [Google Scholar]
- Bettcher BM, Wilheim R, Rigby T, Green R, Miller JW, Racine CA, Yaffe K, Miller BL and Kramer JH (2012). “C-reactive protein is related to memory and medial temporal brain volume in older adults.” Brain, behavior, and immunity 26(1): 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyles JK, Notterpek LM and Anderson LJ (1990). “Accumulation of apolipoproteins in the regenerating and remyelinating mammalian peripheral nerve. Identification of apolipoprotein D, apolipoprotein A-IV, apolipoprotein E, and apolipoprotein A-I.” J Biol Chem 265(29): 17805–17815. [PubMed] [Google Scholar]
- Braun PR, Han S, Hing B, Nagahama Y, Gaul LN, Heinzman JT, Grossbach AJ, Close L, Dlouhy BJ, Howard MA 3rd, Kawasaki H, Potash JB and Shinozaki G. (2019). “Genome-wide DNA methylation comparison between live human brain and peripheral tissues within individuals.” Transl Psychiatry 9(1): 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE and MacArthur A. Studies of Successful (2003). “The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging.” Neurology 60(7): 1077–1081. [DOI] [PubMed] [Google Scholar]
- Carlsson CM (2010). “Type 2 diabetes mellitus, dyslipidemia, and Alzheimer’s disease.” Journal of Alzheimer’s Disease 20(3): 711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Reed T, Schellenberg GD and Christian JC (1999). “The effect of apolipoprotein E epsilon4 in the relationships of smoking and drinking to cognitive function.” Neuroepidemiology 18(3): 125–133. [DOI] [PubMed] [Google Scholar]
- Cervantes S, Samaranch L, Vidal-Taboada JM, Lamet I, Bullido MJ, Frank-Garcia A, Coria F, Lleo A, Clarimon J, Lorenzo E, Alonso E, Sanchez-Juan P, Rodriguez-Rodriguez E, Combarros O, Rosich M, Vilella E. and Pastor P. (2011). “Genetic variation in APOE cluster region and Alzheimer’s disease risk.” Neurobiol Aging 32(11): 2107 e2107–2117. [DOI] [PubMed] [Google Scholar]
- Cervo MMC, Scott D, Seibel MJ, Cumming RG, Naganathan V, Blyth FM, Le Couteur DG, Handelsman DJ, Ribeiro RV and Waite LM (2020). “Proinflammatory diet increases circulating inflammatory biomarkers and falls risk in community-dwelling older men.” The Journal of nutrition 150(2): 373–381. [DOI] [PubMed] [Google Scholar]
- Charisis S, Ntanasi E, Yannakoulia M, Anastasiou CA, Kosmidis MH, Dardiotis E, Gargalionis AN, Patas K, Chatzipanagiotou S, Mourtzinos I, Tzima K, Hadjigeorgiou G, Sakka P, Kapogiannis D. and Scarmeas N. (2021). “Diet Inflammatory Index and Dementia Incidence: A Population-Based Study.” Neurology 97(24): e2381–e2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL and Pericak-Vance MA (1993). “Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families.” Science 261(5123): 921–923. [DOI] [PubMed] [Google Scholar]
- Cruz-Sanabria F, Bonilla-Vargas K, Estrada K, Mancera O, Vega E, Guerrero E, Ortega-Rojas J, Mahecha Maria F, Romero A, Montanes P, Celeita V, Arboleda H. and Pardo R. (2018). “Analysis of cognitive performance and polymorphisms of SORL1, PVRL2, CR1, TOMM40, APOE, PICALM, GWAS_14q, CLU, and BIN1 in patients with mild cognitive impairment and cognitively healthy controls.” Neurologia. [DOI] [PubMed]
- Cudaback E, Li X, Yang Y, Yoo T, Montine KS, Craft S, Montine TJ and Keene CD (2012). “Apolipoprotein CI is an APOE genotype-dependent suppressor of glial activation.” Journal of neuroinflammation 9(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias CM, Schaie KW and Willis SL (2014). “Hypertension moderates the effect of APOE on 21-year cognitive trajectories.” Psychol Aging 29(2): 431–439. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, Carothers A. and Whalley LJ (2002). “Cognitive change and the APOE ɛ4 allele.” Nature 418(6901): 932-932. [DOI] [PubMed] [Google Scholar]
- Domingues C, AB O. da Cruz e Silva and Henriques A. (2017). “Impact of cytokines and chemokines on Alzheimer’s disease neuropathological hallmarks.” Current Alzheimer Research 14(8): 870–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufouil C, Tzourio C, Brayne C, Berr C, Amouyel P. and Alperovitch A. (2000). “Influence of apolipoprotein E genotype on the risk of cognitive deterioration in moderate drinkers and smokers.” Epidemiology 11(3): 280–284. [DOI] [PubMed] [Google Scholar]
- Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF and Zonderman AB (2010). “Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status.” Ethnicity & disease 20(3): 267. [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N. and van Duijn CM (1997). “Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium.” JAMA 278(16): 1349–1356. [PubMed] [Google Scholar]
- Federoff M, Jimenez-Rolando B, Nalls MA and Singleton AB (2012). “A large study reveals no association between APOE and Parkinson’s disease.” Neurobiology of disease 46(2): 389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez CG, Hamby ME, McReynolds ML and Ray WJ (2019). “The Role of APOE4 in Disrupting the Homeostatic Functions of Astrocytes and Microglia in Aging and Alzheimer’s Disease.” Front Aging Neurosci 11: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith E, Shivappa N, Mann JR, Hébert JR, Wirth MD and Loprinzi PD (2018). “Dietary inflammatory index and memory function: population-based national sample of elderly Americans.” The British journal of nutrition 119(5): 552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, Haass C, LaFerla FM, Kretzschmar H. and Herms J. (2010). “Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease.” Nature neuroscience 13(4): 411–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuior EV and Gafencu AV (2019). “Apolipoprotein C1: Its Pleiotropic Effects in Lipid Metabolism and Beyond.” Int J Mol Sci 20(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland L. (2010). “Diet and inflammation.” Nutr Clin Pract 25(6): 634–640. [DOI] [PubMed] [Google Scholar]
- Hannon E, Lunnon K, Schalkwyk L. and Mill J. (2015). “Interindividual methylomic variation across blood, cortex, and cerebellum: implications for epigenetic studies of neurological and neuropsychiatric phenotypes.” Epigenetics 10(11): 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden KM, Beavers DP, Steck SE, Hebert JR, Tabung FK, Shivappa N, Casanova R, Manson JE, Padula CB and Salmoirago-Blotcher E. (2017). “The association between an inflammatory diet and global cognitive function and incident dementia in older women: The Women’s Health Initiative Memory Study.” Alzheimer’s & Dementia 13(11): 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT and Kummer MP (2015). “Neuroinflammation in Alzheimer’s disease.” Lancet Neurol 14(4): 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, Wiencke JK and Kelsey KT (2012). “DNA methylation arrays as surrogate measures of cell mixture distribution.” BMC bioinformatics 13(1): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson IK, Ploner A, Wang Y, Gatz M, Pedersen NL and Hagg S. (2018). “Apolipoprotein E DNA methylation and late-life disease.” Int J Epidemiol 47(3): 899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas CH, Corrada MM, Brookmeyer R, Morrison A, Resnick SM, Zonderman AB and Arenberg D. (2003). “Visual memory predicts Alzheimer’s disease more than a decade before diagnosis.” Neurology 60(7): 1089–1093. [DOI] [PubMed] [Google Scholar]
- Ki C-S, Na DL, Kim DK, Kim HJ and Kim J-W (2002). “Genetic association of an apolipoprotein CI (APOC1) gene polymorphism with late-onset Alzheimer’s disease.” Neuroscience letters 319(2): 75–78. [DOI] [PubMed] [Google Scholar]
- Klein CJ, Botuyan M-V, Wu Y, Ward CJ, Nicholson GA, Hammans S, Hojo K, Yamanishi H, Karpf AR and Wallace DC (2011). “Mutations in DNMT1 cause hereditary sensory neuropathy with dementia and hearing loss.” Nature genetics 43(6): 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz BA, Corrada MM and Kawas CH (2009). “Elevated C-reactive protein levels are associated with prevalent dementia in the oldest-old.” Alzheimer’s & Dementia 5(4): 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryscio RJ, Schmitt FA, Salazar JC, Mendiondo MS and Markesbery WR (2006). “Risk factors for transitions from normal to mild cognitive impairment and dementia.” Neurology 66(6): 828–832. [DOI] [PubMed] [Google Scholar]
- Kuo H-K, Yen C-J, Chang C-H, Kuo C-K, Chen J-H and Sorond F. (2005). “Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis.” The Lancet Neurology 4(6): 371–380. [DOI] [PubMed] [Google Scholar]
- Landgrave-Gomez J, Mercado-Gomez O. and Guevara-Guzman R. (2015). “Epigenetic mechanisms in neurological and neurodegenerative diseases.” Front Cell Neurosci 9: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhao W, Ware EB, Turner ST, Mosley TH and Smith JA (2018). “DNA methylation in the APOE genomic region is associated with cognitive function in African Americans.” BMC Med Genomics 11(1): 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucatelli JF, Barros AC, Da Silva VK, da Silva Machado F, Constantin PC, Dias AAC, Hutz MH and de Andrade FM (2011). “Genetic influences on Alzheimer’s disease: evidence of interactions between the genes APOE, APOC1 and ACE in a sample population from the South of Brazil.” Neurochemical research 36(8): 1533–1539. [DOI] [PubMed] [Google Scholar]
- Mahley RW (2016). “Central Nervous System Lipoproteins: ApoE and Regulation of Cholesterol Metabolism.” Arterioscler Thromb Vasc Biol 36(7): 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancera-Paez O, Estrada-Orozco K, Mahecha MF, Cruz F, Bonilla-Vargas K, Sandoval N, Guerrero E, Salcedo-Tacuma D, Melgarejo JD, Vega E, Ortega-Rojas J, Roman GC, Pardo-Turriago R. and Arboleda H. (2019). “Differential Methylation in APOE (Chr19; Exon Four; from 44,909,188 to 44,909,373/hg38) and Increased Apolipoprotein E Plasma Levels in Subjects with Mild Cognitive Impairment.” Int J Mol Sci 20(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez WM and Spear PG (2001). “Structural features of nectin-2 (HveB) required for herpes simplex virus entry.” J Virol 75(22): 11185–11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME and Takahashi HK (2009). “Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity.” Journal of Neuroscience 29(2): 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales I, Guzman-Martinez L, Cerda-Troncoso C, Farias GA and Maccioni RB (2014). “Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches.” Front Cell Neurosci 8: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ and Ingwersen LA (2008). “The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes.” The American journal of clinical nutrition 88(2): 324–332. [DOI] [PubMed] [Google Scholar]
- Mur J, McCartney DL, Walker RM, Campbell A, Bermingham ML, Morris SW, Porteous DJ, McIntosh AM, Deary IJ, Evans KL and Marioni RE (2020). “DNA methylation in APOE: The relationship with Alzheimer’s and with cardiovascular health.” Alzheimers Dement (N Y) 6(1): e12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dwyer L, Lamberton F, Matura S, Tanner C, Scheibe M, Miller J, Rujescu D, Prvulovic D. and Hampel H. (2012). “Reduced hippocampal volume in healthy young ApoE4 carriers: an MRI study.” PLoS One 7(11): e48895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuizumi K, Onodera O, Tanaka H, Kobayashi H, Tsuji S, Takahashi H, Oyanagi K, Seki K, Tanaka M, Naruse S. and et al. (1994). “ApoE-epsilon 4 and early-onset Alzheimer’s.” Nat Genet 7(1): 10–11. [DOI] [PubMed] [Google Scholar]
- Patel KT, Stevens MC, Pearlson GD, Winkler AM, Hawkins KA, Skudlarski P. and Bauer LO (2013). “Default mode network activity and white matter integrity in healthy middle-aged ApoE4 carriers.” Brain Imaging Behav 7(1): 60–67. [DOI] [PubMed] [Google Scholar]
- Poduslo S, Neal M, Herring K. and Shelly J. (1998). “The apolipoprotein CI A allele as a risk factor for Alzheimer’s disease.” Neurochemical research 23(3): 361–367. [DOI] [PubMed] [Google Scholar]
- Prickett C, Brennan L. and Stolwyk R. (2015). “Examining the relationship between obesity and cognitive function: a systematic literature review.” Obesity research & clinical practice 9(2): 93–113. [DOI] [PubMed] [Google Scholar]
- R Core Team (2020). R: A language and environment for statistical computing. Vienna, Austria, Foundation for Statistical Computing. [Google Scholar]
- Riedel BC, Thompson PM and Brinton RD (2016). “Age, APOE and sex: Triad of risk of Alzheimer’s disease.” J Steroid Biochem Mol Biol 160: 134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager MA, Hermann B. and La Rue A. (2005). “Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention.” J Geriatr Psychiatry Neurol 18(4): 245–249. [DOI] [PubMed] [Google Scholar]
- Samanta D, Ramagopal UA, Rubinstein R, Vigdorovich V, Nathenson SG and Almo SC (2012). “Structure of Nectin-2 reveals determinants of homophilic and heterophilic interactions that control cell-cell adhesion.” Proc Natl Acad Sci U S A 109(37): 14836–14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Albert S, Manly J. and Stern Y. (2006). “Education and rates of cognitive decline in incident Alzheimer’s disease.” Journal of Neurology, Neurosurgery & Psychiatry 77(3): 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Curb JD, Masaki K, White LR and Launer LJ (2002). “Early inflammation and dementia: a 25‐year follow‐up of the Honolulu‐Asia Aging Study.” Annals of neurology 52(2): 168–174. [DOI] [PubMed] [Google Scholar]
- Shao Y, Shaw M, Todd K, Khrestian M, D’Aleo G, Barnard PJ, Zahratka J, Pillai J, Yu CE, Keene CD, Leverenz JB and Bekris LM (2018). “DNA methylation of TOMM40-APOE-APOC2 in Alzheimer’s disease.” J Hum Genet 63(4): 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Kwon SC, Kim MH, Lee KW, Choi SY, Shivappa N, Hébert JR and Chung H-K (2018). “Inflammatory potential of diet is associated with cognitive function in an older adult Korean population.” Nutrition 55: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa N, Hébert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, Marcos A. and Huybrechts I. (2015). “Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study.” British Journal of Nutrition 113(4): 665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa N, Steck SE, Hurley TG, Hussey JR and Hebert JR (2014). “Designing and developing a literature-derived, population-based dietary inflammatory index.” Public Health Nutr 17(8): 1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa N, Steck SE, Hurley TG, Hussey JR and Hébert JR (2014). “Designing and developing a literature-derived, population-based dietary inflammatory index.” Public health nutrition 17(8): 1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan AB (1992). Benton visual retention test, Psychological Corporation San Antonio, TX. [Google Scholar]
- Strauss E, Sherman E. and Spreen O. (2006). A compendium of neuropsychological tests, New York: Oxford University Press. [Google Scholar]
- Szczechowiak K, Diniz BS and Leszek J. (2019). “Diet and Alzheimer’s dementia - Nutritional approach to modulate inflammation.” Pharmacol Biochem Behav 184: 172743. [DOI] [PubMed] [Google Scholar]
- Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, Hingle M, Hou L, Hurley TG and Jiao L. (2015). “Construct validation of the dietary inflammatory index among postmenopausal women.” Annals of epidemiology 25(6): 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara-Hanaoka S, Shibuya K, Onoda Y, Zhang H, Yamazaki S, Miyamoto A, Honda S, Lanier LL and Shibuya A. (2004). “Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112).” Int Immunol 16(4): 533–538. [DOI] [PubMed] [Google Scholar]
- Tajuddin SM, Hernandez DG, Chen BH, Noren Hooten N, Mode NA, Nalls MA, Singleton AB, Ejiogu N, Chitrala KN, Zonderman AB and Evans MK (2019). “Novel age-associated DNA methylation changes and epigenetic age acceleration in middle-aged African Americans and whites.” Clin Epigenetics 11(1): 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei N, Miyashita A, Tsukie T, Arai H, Asada T, Imagawa M, Shoji M, Higuchi S, Urakami K, Kimura H, Kakita A, Takahashi H, Tsuji S, Kanazawa I, Ihara Y, Odani S, Kuwano R. and Japanese D. Genetic Study Consortium for Alzheimer (2009). “Genetic association study on in and around the APOE in late-onset Alzheimer disease in Japanese.” Genomics 93(5): 441–448. [DOI] [PubMed] [Google Scholar]
- Tran DQ, Tse EK, Kim MH and Belsham DD (2016). “Diet-induced cellular neuroinflammation in the hypothalamus: Mechanistic insights from investigation of neurons and microglia.” Mol Cell Endocrinol 438: 18–26. [DOI] [PubMed] [Google Scholar]
- Tycko B, Lee JH, Ciappa A, Saxena A, Li C-M, Feng L, Arriaga A, Stern Y, Lantigua R. and Shachter N. (2004). “APOE and APOC1 promoter polymorphisms and the risk of Alzheimer disease in African American and Caribbean Hispanic individuals.” Archives of neurology 61(9): 1434–1439. [DOI] [PubMed] [Google Scholar]
- van den Berg E, Kloppenborg RP, Kessels RP, Kappelle LJ and Biessels GJ (2009). “Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: a systematic comparison of their impact on cognition.” Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1792(5): 470–481. [DOI] [PubMed] [Google Scholar]
- Wang SC, Oelze B. and Schumacher A. (2008). “Age-specific epigenetic drift in late-onset Alzheimer’s disease.” PLoS One 3(7): e2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS (1993). “Wide range achievement test–revision 3.” Wilmington, DE: Jastak Association 20. [Google Scholar]
- Williams OA, An Y, Armstrong NM, Kitner-Triolo M, Ferrucci L. and Resnick SM (2020). “Profiles of cognitive change in preclinical and prodromal Alzheimer’s disease using change-point analysis.” Journal of Alzheimer’s Disease(Preprint): 1–12. [DOI] [PMC free article] [PubMed]
- Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D. and Grogan JL (2009). “The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells.” Nat Immunol 10(1): 48–57. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Peng D, Yuan X, Lv Z, Pang S, Jiang W, Yang C, Shi X, Pang G, Yang Y, Xie H, Zhang W, Hu C. and Yang Z. (2014). “APOE and APOC1 gene polymorphisms are associated with cognitive impairment progression in Chinese patients with late-onset Alzheimer’s disease.” Neural Regen Res 9(6): 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Chen Y, Mok KY, Kwok TCY, Mok VCT, Guo Q, Ip FC, Chen Y, Mullapudi N, Alzheimer’s Disease Neuroimaging I, Giusti-Rodriguez P, Sullivan PF, Hardy J, Fu AKY, Li Y. and Ip NY (2019). “Non-coding variability at the APOE locus contributes to the Alzheimer’s risk.” Nat Commun 10(1): 3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Paniccia A, Schulick AC, Chen W, Koenig MR, Byers JT, Yao S, Bevers S. and Edil BH (2016). “Identification of CD112R as a novel checkpoint for human T cells.” J Exp Med 213(2): 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann J, Alain C. and Butler C. (2019). “Impaired memory-guided attention in asymptomatic APOE4 carriers.” Sci Rep 9(1): 8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.