Abstract
Introduction
Despite new therapies for relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL), treatments with chemotherapy, single-agent rituximab/obinutuzumab, single-agent lenalidomide, or combinations of these agents continue to be commonly used.
Methods
This retrospective study utilized longitudinal data from 4226 real-world electronic health records to characterize outcomes in patients with R/R DLBCL. Eligible patients were diagnosed with DLBCL between January 2010 and March 2022 and had R/R disease treated with ≥ 1 prior systemic line of therapy (LOT), including ≥ 1 anti-CD20-containing regimen.
Results
A total of 573 patients treated with ≥ 1 prior LOT were included (31.2% and 13.4% with ≥ 2 and ≥ 3 prior LOTs, respectively). Median duration of follow-up was 7.7 months. Most patients (57.1%) were male; mean standard deviation (SD) age was 63 (14.7) years. Overall and complete response rates (95% confidence interval (CI) were 52% (48–56) and 23% (19–27). Median duration of response and duration of complete response were 3.5 and 18.4 months. Median progression-free and overall survival (95% CI) was 3.0 (2.8–3.3) and 12.9 (10.1–16.9) months, respectively. Patients with a higher number of prior LOTs, primary refractoriness, refractoriness to last LOT, refractoriness to last anti-CD20-containing regimen, and prior CAR T exposure had worse outcomes (i.e., challenging-to-treat R/R DLBCL) compared with those without these characteristics.
Conclusions
Outcomes in patients with R/R DLBCL treated with chemotherapy, single-agent rituximab/obinutuzumab, single-agent lenalidomide, or combinations of these agents remain poor, especially for those with challenging-to-treat R/R DLBCL. These findings underscore the unmet need for new, safe, and effective therapies, especially for challenging-to-treat R/R DLBCL populations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02775-9.
Keywords: Bispecific antibody, Chimeric antigen receptor, Immunotherapy, Observational, Real-world evidence, Retrospective, Salvage therapy, Standard of care
Key Summary Points
| Why carry out this study? |
| Diffuse large B-cell lymphoma (DLBCL) is an aggressive and common form of non-Hodgkin lymphoma. Typically, first-line treatment consists of chemo-immunotherapy (CIT); however, this treatment ultimately fails for up to half of patients |
| For these patients, clinical outcomes (e.g., survival, disease progression) are poor. As the treatment landscape evolves, real-world data provide valuable historical benchmarks |
| What did this study ask? |
| We conducted this study to characterize outcomes in a real-world cohort of patients with relapsed or refractory (R/R) DLBCL treated with standard-of-care CIT over the last 10 years and to examine the impact of demographic, clinical, and treatment characteristics on clinical outcomes |
| What was learned from this study? |
| We found that patients with R/R DLBCL treated with standard-of-care CIT have suboptimal outcome, and have declining response rates, progression-free survival, and overall survival as they progress through additional lines of therapy |
| In particular, the most challenging-to-treat patients with the worst outcomes were characterized by the following: refractory disease (i.e., primary refractory, refractory to their most recent line of therapy, and/or refractory to their last anti-CD20-containing regimen), higher number of prior lines of therapy, and prior treatment with chimeric antigen receptor T-cell therapy. These findings highlight the unmet need for new, safe, and effective life-prolonging therapies for R/R DLBCL, especially for challenging-to-treat patients |
Introduction
Diffuse large B-cell lymphoma (DLBCL), an aggressive form of non-Hodgkin lymphoma (NHL), is the most common type of lymphoma, accounting for ~ 30% of all NHL cases [1], with the annual number of new US cases estimated at 5.6 per 100,000 persons [2] and an estimated national prevalence of up to 142,889 cases [3]. For patients with DLBCL, the most commonly recommended first-line (1L) treatment is multi-agent chemo-immunotherapy (CIT) with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) [4]. However, up to 50% of patients with DLBCL relapse after or are refractory to 1L CIT [5, 6], and their clinical outcomes are poor, especially in high-risk subgroups (double-hit or double-expressor DLBCL) [7–10]. In a 2021 study of the Veteran Affairs Cancer Registry System (N = 270), patients with relapsed or refractory (R/R) DLBCL had a median progression-free survival (PFS) ranging from 5 to 6 months [11, 12] and median overall survival (OS) of < 1 year [11–14].
The recommended treatment for patients with R/R DLBCL who are not frail and lacking major comorbidities is salvage chemotherapy followed by consolidation with high-dose chemotherapy and autologous stem cell transplantation (HDT-ASCT) [4, 15]. It is estimated that at least half of patients with R/R DLBCL are ineligible for HDT-ASCT because of advanced age, comorbidities, or other access issues [16]. Among those who are eligible, only 30% to 40% respond to salvage chemotherapy, allowing them to subsequently undergo HDT-ASCT [5]. Even among patients with R/R DLBCL who respond to salvage therapy and undergo HDT-ASCT consolidation, about 50% ultimately relapse following transplantation [5]. As such, for most patients with R/R DLBCL, there are no curative treatment options [5]. Instead, chemotherapy, single-agent rituximab/obinutuzumab, single-agent lenalidomide, or combinations of these agents remain common components of standard of care (SOC) in patients with R/R DLBCL [17, 18].
Recently approved therapeutic options, such as chimeric antigen receptor T cell (CAR T) therapy, polatuzumab vedotin, tafasitamab + lenalidomide, loncastuximab tesirine, or selinexor, may be considered treatment options for patients with R/R DLBCL, including those who have received ≥ 2 lines of therapy (LOTs) and/or who are not eligible for ASCT [19–27]. In phase 1/2 clinical trials, CAR T was associated with overall response rates (ORR) ranging from 52 to 82% (of 93–256 treated patients in the non-intention-to-treat population) [22, 28, 29]. More recently, CAR T therapies have demonstrated significant benefit in phase 3 randomized trials against salvage therapy with the intent to consolidate with HDT-ASCT among patients with primary refractory DLBCL or with relapse within 12 months of 1L therapy [30], representing a major advance for patients with R/R DLBCL. However, the use of CAR T therapy has been underscored by high rates of disease progression within 12 months of treatment, serious toxicities, limited eligibility for treatment (47.8% ineligible in a real-world Dutch study), and logistical challenges related to manufacturing, distribution, and workforce training [22, 31–35]. In clinical trials of polatuzumab vedotin (n = 40 treated), selinexor (N = 127), and loncastuximab tesirine (N = 145), ORR ranged from 28 to 48% [24, 36, 37]. ORR was higher in a clinical trial (N = 80) of tafasitamab + lenalidomide (60%), although 50% of study patients had only one prior LOT, and patients with primary refractory disease were excluded from participation [38]. Real-world outcomes of polatuzumab-based and tafasitamab-based regimens have also been suboptimal and worse than in clinical trials [39–41]. These outcomes highlight an unmet medical need for innovative therapies with better efficacy and tolerability.
With the therapeutic landscape evolving rapidly for R/R DLBCL, real-world data are needed to provide historical benchmarks regarding treatment response and survival, and also to contextualize outcomes from uncontrolled clinical trials [42]. Several US Food and Drug Administration (FDA) approvals of novel therapies for serious or life-threatening cancers, including R/R DLBCL, have been based on results from uncontrolled studies across heterogenous patient populations [19, 22, 25–29, 42].
This study characterizes a real-world cohort of patients with R/R DLBCL treated with SOC therapy over the last 10 years. The study objective was to describe clinical outcomes in a real-world cohort of patients treated with chemotherapy, single-agent rituximab/obinutuzumab, single-agent lenalidomide, or combinations of these agents in the R/R DLBCL setting and to examine the impact of demographic, clinical, and treatment characteristics on clinical outcomes.
Materials and Methods
Study Design
This observational, retrospective cohort study utilized longitudinal data from the COTA electronic health records (EHR) database (COTA, New York, NY, USA). The COTA EHR database contains de-identified patient demographic and clinical information, including diagnostic, treatment, and outcomes data, for an estimated 4226 patients (at the time of the analysis) with DLBCL from US academic medical centers and community practice sites. Patients eligible for this study had a recorded diagnosis of DLBCL and DLBCL treatment at any time between January 2010 and March 2022.
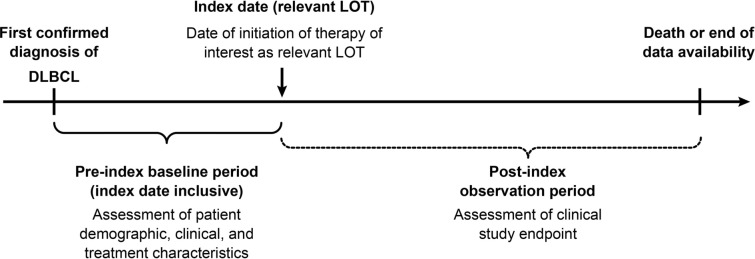
Figure 1 summarizes the study design. COTA’s proprietary algorithm was used to define what constituted a LOT. The index date for a given LOT was defined as the initiation date of the first eligible treatment with the relevant LOT. The pre-index baseline period was defined as the time between the first confirmed DLBCL diagnosis and the index date (inclusive). The post-index observation period was defined as the duration from the index date until death or end of data availability (i.e., end of the study period), whichever occurred first. To be considered as the index LOT, the LOT must have comprised a regimen of interest administered in 2L or later (≥ 2L) treatment setting (i.e., R/R setting). Regimens of interest were defined as chemotherapy, single-agent rituximab/obinutuzumab, single-agent lenalidomide, or combinations of these agents (see the Supplementary Material for a list of qualifying regimens). Each patient contributed data to the analysis of outcomes for just one LOT. If the patient had records indicating more than one qualifying LOT, one LOT was randomly selected as the index LOT.
Fig. 1.
Study design. DLBCL diffuse large B-cell lymphoma, LOT line of therapy
Given that the COTA database houses de-identified, secondary data, this analysis did not require Institutional Review Board approval.
Patient Population
To be included in the study, patients had to be age ≥ 18 years with documented CD20+ mature B-cell neoplasm with de novo DLBCL (including “double-hit” or “triple-hit” DLBCL) or DLBCL histologically transformed from all indolent subtypes. Additionally, patients were required to have R/R disease previously treated with ≥ 1 systemic antineoplastic therapy that included ≥ 1 anti-CD20 monoclonal antibody-containing regimen. Relapsed disease was defined as disease that recurred ≥ 6 months after completion of therapy. Refractory disease was defined as disease that either progressed or relapsed within 6 months following completion of therapy. Patients had to have been treated with a regimen of interest (detailed above and in the Supplementary Material) in the ≥ 2L setting. If patients had a history of exposure to ASCT or novel therapies such as anti-CD19 CAR T, they must have progressed to a regimen of interest for inclusion in this analysis. Other inclusion criteria were an Eastern Cooperative Oncology Group (ECOG) performance status 0–2 and ≥ 1 post-index evaluable response assessment, or a date of death, if the death occurred before the post-index evaluable response assessment.
Patients meeting any of the following criteria prior to or on the index date were excluded: central nervous system lymphoma or known history of prior malignancies other than NHL, chronic heart failure or acute myocardial infarction within 6 months of the index date, or human immunodeficiency virus infection.
Outcomes
Outcomes included ORR, complete response (CR), duration of response (DOR), duration of complete response (DOCR), PFS, and OS. ORR was defined as the proportion of patients with CR or partial response (PR) of any duration as the best documented response for the given LOT. CR was the proportion of patients with CR of any duration as the best documented response for the given LOT. DOR was the time from the first documentation of CR or PR to the earliest of first documented progressive disease (PD) or death. DOCR was the time from the first documentation of CR to the earliest of first-documented PD or death. DOR and DOCR were only calculated for patients who achieved ORR and CR, respectively, for a given LOT. PFS was defined as the time from initiation of the given LOT to the earliest of first-documented PD or death. OS was the time from initiation of an index LOT to all-cause death.
For DOR, DOCR, PFS, and OS, death due to any cause was considered. For DOR, DOCR, and PFS, patients without an assessment of PD or death date prior to initiating the subsequent LOT were censored at the date of their last clinical assessment. For OS, patients without a death date were censored at the date of last recorded follow-up. All study outcomes were evaluated both overall and stratified by LOT. ORR was also stratified by number of prior LOTs, primary refractoriness, refractoriness to last LOT, prior exposure status to CAR T, and refractoriness to most recent anti-CD20-containing regimen. In addition, subgroup analyses were conducted among patients treated with ≥ 2 and ≥ 3 prior LOTs.
Finally, the associations among patient demographic, clinical, and treatment characteristics and clinical outcomes were assessed. When multiple observations of patient demographic or clinical characteristics were available, the observation closest to the index date was used.
Statistical Analysis
Continuous variables are presented as mean, standard deviation (SD), and median values. Categorical variables are presented as frequencies and percentages. Time-to-event parameters are described using Kaplan-Meier estimates (median time and corresponding 95% confidence intervals [CIs]). Chi-square and Fisher’s exact tests were used to determine the significance in the difference between ORRs for patients by primary refractoriness, refractoriness status to last LOT, refractoriness status to most recent anti-CD20-containing regimen, and (prior) exposure status to CAR T. The number and percentage of missing data were recorded, and observations with missing endpoint information were excluded from analysis.
Bivariate and multivariate logistic regression analyses were performed to assess the association between binary response outcomes and patient demographic, clinical, and treatment characteristics, with odds ratios (ORs) and corresponding 95% CIs computed. Bivariate and multivariate Cox proportional-hazards regression analyses were performed to assess the association between time-to-event outcomes and patient demographic, clinical, and treatment characteristics, with hazard ratios (HRs) and corresponding 95% CIs computed. If variables were highly correlated in multivariate analysis (e.g., primary refractoriness, refractoriness to last LOT, and refractoriness to most recent anti-CD20-containing regimen), models were run one at a time with each variable. The Akaike information criterion was used to select the final model and reported associations.
All statistical analyses and data tabulations were calculated using Statistical Analysis Software (SAS®) for Windows release 9.4 (64 bit) or later (SAS Institute Inc., Cary, NC, USA) or RStudio (Boston, MA, USA; version 3.6.2).
Results
Baseline Demographic and Clinical Characteristics
In total, 573 patients who had been treated with ≥ 1 prior LOT were included in the study (Table 1). Of these, 179 (31.2%) were treated with ≥ 2 prior LOTs and 77 (13.4%) were treated with ≥ 3 prior LOTs. Median duration of follow-up for patients treated with ≥ 1, ≥ 2, and ≥ 3 prior LOTs, respectively, was 7.7 months, 5.4 months, and 4.4 months. Table 2 shows patient baseline demographic and clinical characteristics. Over half (57.1%) were male and 81.9% were white. Mean (SD) patient age was 63 (14.7) years, and 50.1% were diagnosed after age 65. Most patients (68.8%) had only one prior LOT, and the percentage of patients with prior (pre-index) CAR T, ASCT, or polatuzumab-based therapies was low (≤ 7.0% for all). Most patients had received only one previous anti-CD20-containing regimen (73.7%), while the remainder (26.3%) received ≥ 2 previous anti-CD20-containing regimens. A total of 21 patients (3.7%) had single-agent rituximab, single-agent obinutuzumab, or single-agent lenalidomide as the qualifying LOT for this analysis. ECOG status was 0 or 1 for 82.0% of patients, 68.6% had Ann Arbor stage III/IV disease, 44.7% had bulky disease, 30.7% had B symptoms, 32.5% had International Prognostic Index (IPI) scores of 3–5 at diagnosis, and 8.7% had double-/triple-hit DLBCL as determined by fluorescence in situ hybridization (FISH). Regarding refractory status, 62.0% were primary refractory, 68.6% were refractory to their last LOT, and 67.7% were refractory to their most recent anti-CD20-containing regimen. Of those with previous CAR T (n = 16), 87.5% were refractory to CAR T.
Table 1.
Patient attrition
| Criteria | N |
|---|---|
| Adult ≥ 18 years old and diagnosed with DLBCL on or after January 1, 2010 | 4226 |
| Previously treated with > 1 line of systemic therapy, including ≥ 1 anti-CD20-containing regimen | 1188 |
| Treated with regimen of interest | 920 |
| ECOG performance status 0–2 | 764 |
| ≥ 1 post-index evaluable response assessment, or date of death | 756 |
| Treated with ≥ 1 prior LOT, after applying exclusion criteria | 573 |
| Treated with ≥ 2 prior LOTs, after applying exclusion criteria | 179 |
| Treated with ≥ 3 prior LOTs, after applying exclusion criteria | 77 |
DLBCL diffuse large B-cell lymphoma, ECOG Eastern Cooperative Oncology Group, LOT line of therapy
Table 2.
Patient demographic and clinical characteristics
| Characteristic | N = 573 |
|---|---|
| Male | 327 (57) |
| Race (white) | 469 (81.9) |
| Missing | 16 (2.8) |
| Age, mean (SD) [range], years | 63 (14.7) [20–89] |
| Age at diagnosis | |
| ≤ 65 | 286 (49.9) |
| 66–75 | 168 (29.3) |
| > 75 | 119 (20.8) |
| Year of diagnosis | |
| 2010–2015 | 301 (52.5) |
| 2016–2021 | 272 (47.5) |
| Number of prior LOTs | |
| 1 | 394 (68.8) |
| 2 | 102 (17.8) |
| 3 | 42 (7.3) |
| ≥ 4 | 35 (6.1) |
| Prior novel treatments (pre-index) | |
| CAR T | 16 (2.8) |
| ASCT | 40 (7.0) |
| Polatuzumab based | 7 (1.2) |
| Selinexor | 1 (0.2) |
| Number of previous anti-CD20-containing regimens | |
| 1 | 422 (73.7) |
| 2 | 107 (18.7) |
| 3 | 35 (6.1) |
| ≥ 4 | 9 (1.6) |
| ECOG PS | |
| 0 | 161 (28.1) |
| 1 | 309 (53.9) |
| 2 | 103 (18.0) |
| Ann Arbor stage III/IV | 393 (68.6) |
| Missing | 50 (8.7) |
| Bulky diseasea | 256 (44.7) |
| Missing | 133 (23.2) |
| B symptoms | 176 (30.7) |
| Missing | 29 (5.1) |
| DLBCL molecular classification | |
| GCB | 212 (37.0) |
| Non-GCB | 176 (30.7) |
| Unknown/missing | 185 (32.3) |
| IPI scores 3–5 at diagnosis | 186 (32.5) |
| Missing | 177 (30.9) |
| Extranodal involvement (any number) | 458 (79.9) |
| Missing | 82 (14.3) |
| Primary refractory | 355 (62.0) |
| Refractory to last LOT | 393 (68.6) |
| Refractory to most recent anti-CD20-containing regimen | 388 (67.7) |
| Double-/triple hitb | 50 (8.7) |
| Missing | 254 (44.3) |
| CAR T refractory | 14/16 (87.5) |
| Biomarker | |
| BCL2 | 97 (16.9) |
| Missing | 276 (48.2) |
| BCL6 | 83 (14.5) |
| Missing | 292 (51.0) |
| CMYC | 66 (11.5) |
| Missing | 243 (42.4) |
| Creatinine | |
| < Lower limit | 36 (6.3) |
| > Upper limit | 69 (12.0) |
| Missing | 21 (3.7) |
| LDH | |
| < Lower limit | 9 (1.6) |
| > Upper limit | 295 (51.5) |
| Missing | 31 (5.4) |
| Albumin (< lower limit) | 182 (31.8) |
| Missing | 37 (6.5) |
| Hematocrit (< lower limit) | 383 (66.8) |
| Missing | 38 (6.6) |
| Comorbidities | |
| Diabetes without chronic complication | 126 (22.0) |
| Diabetes with chronic complication | 7 (1.2) |
| Renal disease | 82 (14.3) |
| Obesity | 76 (13.3) |
| Chronic pulmonary disease | 60 (10.5) |
| Rheumatic disease | 28 (4.9) |
| Cerebrovascular disease | 26 (4.5) |
| Myocardial infarction | 23 (4.0) |
| Unspecified viral hepatitis B without hepatic coma | 13 (2.3) |
| Peptic ulcer disease | 13 (2.3) |
| Peripheral vascular disease | 13 (2.3) |
| Mild liver disease | 11 (1.9) |
| Dementia | 11 (1.9) |
| Moderate to severe liver disease | 10 (1.8) |
| Unspecified viral hepatitis C without hepatic coma | 10 (1.8) |
Data are n (%) unless otherwise specified
ASCT autologous stem cell transplant, CAR T chimeric antigen receptor T cell, DLBCL diffuse large B-cell lymphoma, ECOG PS Eastern Cooperative Oncology Group performance status, FISH fluorescence in situ hybridization, GCB germinal center B-cell, IPI International Prognostic Index, LDH lactate dehydrogenase, LOT line of therapy
aBulky disease is defined as tumor measurements ≥ 10 cm and/or mediastinal disease > 1/3 of the transthoracic diameter, within 30 days of initial diagnosis
bBased on FISH
Response Rates
Response rates and DOR overall and by number of prior LOTs are shown in Table 3. Overall, in this R/R patient population, ORR (95% CI) was 52% (48–56), CR (95% CI) was 23% (19–27), and PR (95% CI) was 29% (25–33). ORR (95% CI) for patients with 1 (n = 394 evaluable patients), 2 (n = 102), 3 (n = 42), and ≥ 4 (n = 35) prior LOTs, respectively, was 57% (52–62), 45% (35–55), 36% (22–52), and 31% (17–49). CRs (95% CI) were 27% (23–32), 17% (10–25), 7% (1–19), and 9% (2–23) for patients with 1, 2, 3, and ≥ 4 prior LOTs, respectively. Overall, median DOR and median DOCR were 3.5 and 18.4 months, respectively. Median DOR (95% CI) for patients with 1 (n = 226 evaluable patients), 2 (n = 46), 3 (n = 15), and ≥ 4 (n = 11) prior LOTs, respectively, was 4.5 (3.2–5.7), 2.9 (1.7–5.2), 2.1 (1.1–4.1), and 2.5 (1.3–3.2) months. Median DOCR (95% CI) for patients with 1 (n = 108 evaluable patients), 2 (n = 17), 3 (n = 3), and four (n = 3) prior LOTs, respectively, was 23.5 (13.0–44.0), 9.6 (4.9–not reached [NR]), 18.2 (2.2–NR), and 4.6 (2.1–NR) months.
Table 3.
Response rates and duration of response, overall and by number of prior LOTs
| Response rate (% [95% CI]), mDOR, mDOCR, mPFS, and mOS (months [95% CI]) | |||||||
|---|---|---|---|---|---|---|---|
| Number of prior LOTs | |||||||
| ≥ 1 | ≥ 2 | ≥ 3 | 1 | 2 | 3 | ≥ 4 | |
| N | 573 | 179 | 77 | 394 | 102 | 42 | 35 |
| ORR | 52 (48–56) | 40 (33–48) | 34 (23–45) | 57 (52–62) | 45 (35–55) | 36 (22–52) | 31 (17–49) |
| CR | 23 (19–27) | 13 (8–19) | 8 (3–16) | 27 (23–32) | 17 (10–25) | 7 (1–19) | 9 (2–23) |
| PR | 29 (25–33) | 27 (21–35) | 26 (17–37) | 30 (25–35) | 28 (20–38) | 29 (16–45) | 23 (10–40) |
| N with ORR | 298 | 72 | 26 | 226 | 46 | 15 | 11 |
| mDOR | 3.5 (3.0–4.7) | 2.7 (1.9–3.4) | 2.3 (1.3–3.3) | 4.5 (3.2–5.7) | 2.9 (1.7–5.2) | 2.1 (1.1–4.1) | 2.5 (1.3–3.2) |
| N with CR | 131 | 23 | 6 | 108 | 17 | 3 | 3 |
| mDOCR | 18.4 (11.8–32.7) | 9.6 (4.9–28) | 8.5 (2.1–NR) | 23.5 (13–44) | 9.6 (4.9–NR) | 18.2 (2.2–NR) | 4.6 (2.1–NR) |
| N | 573 | 179 | 77 | 394 | 102 | 42 | 35 |
| mPFS | 3.0 (2.8–3.3) | 2.4 (2.0–2.7) | 1.9 (1.5–2.5) | 3.6 (3.0–4.2) | 2.6 (2.1–3.2) | 1.9 (1.5–2.5) | 1.7 (1.2–2.9) |
| mOS | 12.9 (10.1–16.9) | 6.2 (5.2–10.9) | 5.1 (3.2–7.3) | 17.4 (13.0–26.7) | 10.6 (5.9–16.4) | 6.1 (3.1–12.6) | 4.1 (2.9–11.2) |
CR complete response; DOCR duration of complete response; DOR duration of response; LOT line of therapy; m median; NR not reached; ORR overall response rate; OS overall survival; PFS progression-free survival; PR partial response
For patients treated with ≥ 2 prior LOTs, ORR (95% CI) was 40% (33–48), CR (95% CI) was 13% (8–19), and PR (95% CI) was 27% (21–35). For patients treated with ≥ 3 prior LOTs, ORR (95% CI) was 34% (23–45), CR (95% CI) was 8% (3–16), and PR (95% CI) was 26% (17–37) (Table 3).
Progression-free Survival and Overall Survival
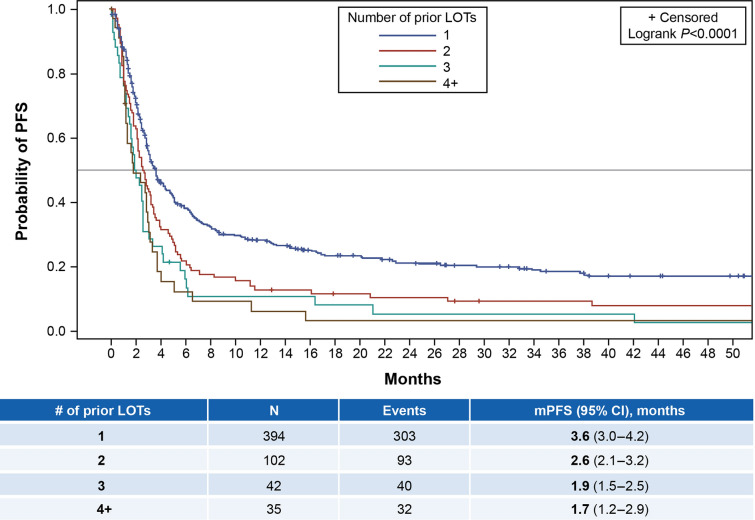
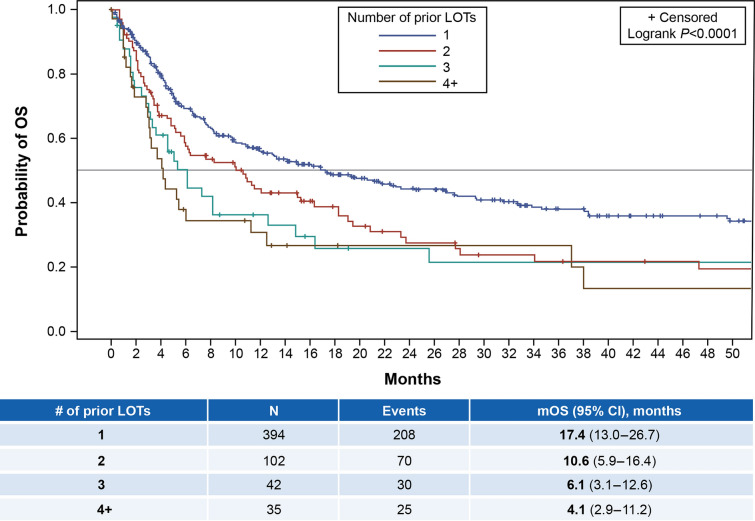
Overall, median PFS (95% CI) was 3.0 (2.8–3.3) months and median OS (95% CI) was 12.9 (10.1–16.9) months (Table 3). Figure 2 shows the Kaplan-Meier plot for PFS by the exact number of prior LOTs, while Fig. 3 shows similar data for OS. Median PFS (95% CI) for patients with 1 (n = 394 evaluable patients), 2 (n = 102), 3 (n = 42), and ≥ 4 (n = 35) prior LOTs, respectively, was 3.6 (3.0–4.2), 2.6 (2.1–3.2), 1.9 (1.5–2.5), and 1.7 (1.2–2.9) months; median OS (95% CI) for these patient groups was 17.4 (13.0–26.7), 10.6 (5.9–16.4), 6.1 (3.1–12.6), and 4.1 (2.9–11.2) months, respectively. See Supplemental Figs. 1–4 for Kaplan-Meier plots of OS by subgroup (e.g., primary refractory, refractory to last LOT, refractory to last anti-CD20-containing regimen, and CAR T failure). Median OS ranged from 5.2 to 10.0 months for these subgroups.
Fig. 2.
Kaplan-Meier plot for PFS by number of prior LOTs. LOTs lines of therapy, m median, PFS progression-free survival
Fig. 3.
Kaplan-Meier plot for OS by number of prior LOTs. LOTs lines of therapy, m median, OS overall survival
For the subgroup of patients with ≥ 2 prior LOTs, median PFS (95% CI) was 2.4 (2.0–2.7) months and median OS (95% CI) was 6.2 (5.2–10.9) months. For patients treated with ≥ 3 prior LOTs, median PFS (95% CI) was 1.9 (1.5–2.5) months and median OS (95% CI) was 5.1 (3.2–7.3) months (Table 3).
Associations Between Patient Characteristics and Clinical Endpoints
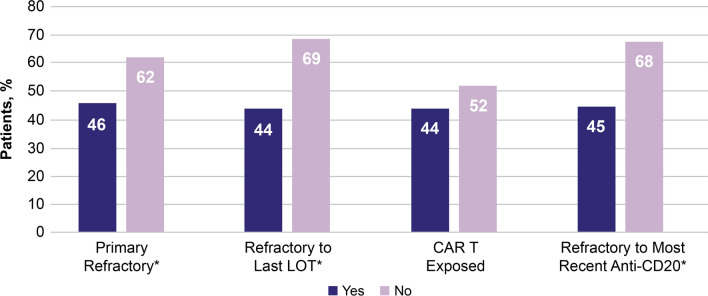
Figure 4 shows ORR by treatment history in the overall cohort, including patients who were primary refractory, refractory to last LOT, CAR T exposed, and refractory to most recent anti-CD20-containing regimen. ORRs were significantly lower for patients who had primary refractory disease (46% vs 62% not primary refractory; P = 0.0001), were refractory to their last LOT (44% vs 69% not refractory to last LOT; P < 0.0001), and/or refractory to their most recent anti-CD20-containing regimen (45% vs 68% not refractory to last anti-CD20-containing regimen; P < 0.0001). There was no significant difference in ORR between the very small number of patients (n = 16) who were CAR T-exposed (44%) vs those who were not (52%).
Fig. 4.
ORR (95% CI) by treatment history. *Statistical significance. Note the small sample size for patients with previous CAR T exposure (n = 16). CAR T chimeric antigen receptor T cell, LOT line of therapy, ORR overall response rate
Tables 4 and 5 show regression analysis results. In bivariate analysis, the following factors were significantly associated with worse outcomes (reduced likelihood of ORR and CR; increased risk of progression [PFS] and mortality [OS]) across all models: increasing prior LOTs (2, 3, or ≥ 4 vs 1 prior LOTs), IPI risk score 3–5 (vs 0–2); primary refractoriness (vs not primary refractory), refractoriness to last LOT (vs relapsed), and refractoriness to last anti-CD20-containing regimen (vs relapsed). In addition, CAR T failure was significantly associated with a 92% increased risk of mortality. Note that the sample size for patients with previous CAR T exposure was small (n = 16).
Table 4.
Odds ratios for likelihood of achieving overall response and complete response
| Effect | ORR (95% CI) | CR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| Bivariate | Multivariate | Bivariate | Multivariate | |||||
| Odds ratio | P value | Odds ratio | P value | Odds ratio | P value | Odds ratio | P value | |
| Year of diagnosis, 2016–2021 vs 2010–2015 | 0.58 (0.41, 0.80) | 0.001 | 0.58 (0.40, 0.83) | 0.003 | 0.72 (0.49, 1.07) | 0.104 | 0.68 (0.45, 1.05) | 0.079 |
| Number of prior LOTs, 2 vs 1 | 0.61 (0.39, 0.95) | 0.027 | 0.68 (0.42, 1.12) | 0.129 | 0.53 (0.30, 0.93) | 0.028 | 0.48 (0.25, 0.93) | 0.030 |
| Number of prior LOTs, 3 vs 1 | 0.41 (0.21, 0.80) | 0.009 | 0.50 (0.24, 1.03) | 0.061 | 0.20 (0.06, 0.67) | 0.009 | 0.18 (0.05, 0.68) | 0.011 |
| Number of prior LOTs, ≥ 4 vs 1 | 0.34 (0.16, 0.72) | 0.004 | 0.46 (0.21, 1.04) | 0.062 | 0.25 (0.07, 0.83) | 0.023 | 0.26 (0.07, 0.94) | 0.040 |
| ECOG performance status, 1 vs 0 | 0.80 (0.54, 1.17) | 0.241 | 1.01 (0.67, 1.54) | 0.955 | 0.72 (0.47, 1.11) | 0.136 | 0.94 (0.58, 1.50) | 0.790 |
| ECOG performance status, 2 vs 0 | 0.63 (0.38, 1.04) | 0.068 | 0.90 (0.51, 1.60) | 0.722 | 0.46 (0.24, 0.87) | 0.016 | 0.67 (0.33, 1.37) | 0.273 |
| B symptoms (yes/no) | 0.60 (0.42, 0.87) | 0.006 | 0.72 (0.48, 1.09) | 0.120 | 0.65 (0.41, 1.02) | 0.058 | 0.82 (0.50, 1.34) | 0.428 |
| IPI, 3–5 vs 0–2 | 0.56 (0.37, 0.83) | 0.004 | 0.63 (0.38, 1.03) | 0.067 | 0.52 (0.32, 0.86) | 0.010 | 0.72 (0.39, 1.33) | 0.292 |
| Primary refractory (yes/no) | 0.52 (0.37, 0.74) | < 0.001 | 0.59 (0.40, 0.86) | 0.007 | 0.56 (0.38, 0.83) | 0.004 | 0.68 (0.44, 1.07) | 0.094 |
| Refractory to last LOT (vs relapsed) | 0.36 (0.25, 0.52) | < 0.0001 | 0.45 (0.30, 0.69) | < 0.001 | 0.41 (0.27, 0.61) | < 0.0001 | 0.59 (0.37, 0.93) | 0.025 |
| Refractory to last anti-CD20-containing regimen (vs relapsed) | 0.39 (0.27, 0.56) | < 0.0001 | 0.49 (0.33, 0.74) | < 0.001 | 0.43 (0.29, 0.64) | < 0.0001 | 0.61 (0.39, 0.96) | 0.033 |
In bivariate analysis, the following variables were significant but not included in the table because they were highly correlated with other variables: month from diagnosis to index date, time since discontinuation of last LOT, and time from first LOT to disease progression. Nonsignificant variables in both analyses were: age at diagnosis; Ann Arbor stage (III or IV vs I or II); bulky disease (yes vs no), cell of origin (non-GCB vs GCB), double-/triple-hit DLBCL (yes vs no), number of extranodal sites, previous ASCT (yes vs no), and previous CAR T (yes vs no; analyzed only in bivariate regression). Note the small sample size for patients with previous CAR T exposure (n = 16)
ASCT autologous stem cell transplant, CAR T chimeric antigen receptor T cell, CI confidence interval, CR complete response, DLBCL diffuse large B-cell lymphoma, ECOG Eastern Cooperative Oncology Group, IPI International Prognostic Index, LOT line of therapy, ORR overall response rate
Table 5.
Hazard ratios for risk of PFS and OS
| Effect | PFS (95% CI) | OS (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| Bivariate | Multivariate | Bivariate | Multivariate | |||||
| Hazard ratio | P value | Hazard ratio | P value | Hazard ratio | P value | Hazard ratio | P value | |
| Age at diagnosis (1-year increase) | 1.00 (0.99, 1.01) | 0.826 | 1.00 (0.99, 1.01) | 0.532 | 1.02 (1.01, 1.03) | < 0.0001 | 1.00 (1.00, 1.01) | 0.395 |
| Number of extranodal sites | 1.14 (1.04, 1.26) | 0.005 | 1.06 (0.88, 1.28) | 0.536 | 1.19 (1.08, 1.32) | 0.001 | 1.07 (0.96, 1.20) | 0.242 |
| Number of prior LOTs, 2 vs 1 | 1.46 (1.16, 1.84) | 0.002 | 1.40 (0.94, 2.08) | 0.098 | 1.48 (1.13, 1.94) | 0.005 | 1.45 (1.12, 1.87) | 0.005 |
| Number of prior LOTs, 3 vs 1 | 1.89 (1.35, 2.63) | 0.000 | 1.98 (1.06, 3.67) | 0.032 | 1.78 (1.21, 2.62) | 0.003 | 1.73 (1.19, 2.52) | 0.005 |
| Number of prior LOTs, ≥ 4 vs 1 | 2.01 (1.39, 2.90) | 0.000 | 2.35 (1.20, 4.60) | 0.013 | 2.11 (1.39, 3.20) | 0.000 | 1.70 (1.14, 2.55) | 0.010 |
| ECOG performance status, 1 vs 0 | 1.32 (1.06, 1.64) | 0.012 | 0.94 (0.68, 1.29) | 0.686 | 1.41 (1.08, 1.84) | 0.012 | 1.07 (0.85, 1.35) | 0.551 |
| ECOG performance status, 2 vs 0 | 1.44 (1.10, 1.90) | 0.009 | 1.05 (0.68, 1.63) | 0.818 | 2.17 (1.57, 2.98) | < 0.0001 | 1.17 (0.85, 1.60) | 0.344 |
| Ann Arbor stage, III or IV vs I or II | 1.43 (1.14, 1.79) | 0.002 | 1.07 (0.73, 1.55) | 0.731 | 1.50 (1.14, 1.98) | 0.004 | 1.35 (1.02, 1.78) | 0.043 |
| B symptoms (yes/no) | 1.31 (1.08, 1.59) | 0.007 | 1.08 (0.77, 1.51) | 0.661 | 1.13 (0.89, 1.43) | 0.310 | 1.27 (1.03, 1.57) | 0.027 |
| IPI, 3–5 vs 0–2 | 1.41 (1.13, 1.75) | 0.002 | 1.05 (0.68, 1.63) | 0.827 | 2.24 (1.73, 2.92) | < 0.0001 | 1.09 (0.76, 1.57) | 0.629 |
| Previous CAR T treatment (yes/no) | 1.57 (0.92, 2.67) | 0.097 | – | – | 1.92 (1.05, 3.51) | 0.035 | – | – |
| Primary refractory (yes/no) | 1.45 (1.20, 1.75) | 0.000 | 1.31 (0.97, 1.77) | 0.082 | 1.39 (1.11, 1.74) | 0.004 | 1.32 (1.07, 1.63) | 0.011 |
| Refractory to last LOT (yes vs relapsed) | 1.67 (1.37, 2.05) | < 0.0001 | 1.32 (0.96, 1.81) | 0.091 | 1.75 (1.37, 2.23) | < 0.0001 | 1.39 (1.11, 1.75) | 0.005 |
| Refractory to last anti-CD20-containing regimen (yes vs relapsed) | 1.66 (1.36, 2.03) | < 0.0001 | 1.35 (0.99, 1.85) | 0.061 | 1.64 (1.29, 2.08) | < 0.0001 | 1.39 (1.11, 1.74) | 0.005 |
In bivariate analysis, the following variables were significant but not included in the table because they were highly correlated with other variables: month from diagnosis to index date, time since discontinuation of last LOT, and time from first LOT to disease progression. Nonsignificant variables in both analyses were: bulky disease (yes vs no), previous ASCT (yes vs no), double-/triple-hit DLBCL (yes vs no), and year of diagnosis (2016–2021). Note the small sample size for patients with previous CAR T exposure (n = 16)
ASCT autologous stem cell transplant, CI confidence interval, DLBCL diffuse large B-cell lymphoma, ECOG Eastern Cooperative Oncology Group, IPI International Prognostic Index, LOT line of therapy, OS overall survival, PFS progression-free survival
In multivariate regression analysis, the following factors were significantly associated with a reduced likelihood of achieving an overall response (Table 4): primary refractoriness (OR 0.59 [95% CI 0.40–0.86]; P = 0.007 vs not primary refractory), refractoriness to last LOT (0.45 [0.30–0.69]; P < 0.001 vs relapsed), refractoriness to last anti-CD20-containing regimen (0.49 [0.33–0.74]; P < 0.001 vs relapsed), and year of diagnosis 2016–2021 (0.58 [0.40–0.83]; P = 0.003 vs diagnosed 2010–2015). In addition, the following factors were associated with a reduced likelihood of achieving CR in multivariate analysis (Table 4): increasing prior LOTs (2 vs 1 prior LOTs, OR 0.48 [95% CI 0.25–0.93]; P = 0.030; 3 vs 1 prior LOTs, 0.18 [0.05–0.68]; P = 0.011; ≥ 4 vs 1 prior LOTs, 0.26 [0.07–0.94]; P = 0.040), refractoriness to last LOT (0.59 [0.37–0.93]; P = 0.025 vs relapsed), and refractoriness to last anti-CD20-containing regimen (0.61 [0.39–0.96]; P = 0.033 vs relapsed).
In multivariate regression analysis, the following factors were significantly associated with an increased risk of disease progression (Table 5): increasing prior LOTs (3 vs 1 prior LOTs, OR 1.98 [95% CI 1.06–3.67]; P = 0.032; ≥ 4 vs 1 prior LOTs, 2.35 [1.20–4.60]; P = 0.013). In addition, the following factors were significantly associated with increased mortality risk (Table 5): Ann Arbor stage III–IV (OR 1.35 [95% CI 1.02–1.78]; P = 0.043 vs I–II); B symptoms (1.27 [1.03–1.57]; P = 0.027 vs none), increasing prior LOTs (2 vs 1 prior LOTs, 1.45 [1.12–1.87]; P = 0.005; 3 vs 1 prior LOTs, 1.73 [1.19–2.52]; P = 0.005; ≥ 4 vs 1 prior LOTs, 1.70 [1.14–2.55]; P = 0.010), primary refractoriness (1.32 [1.07–1.63]; P = 0.011 vs not primary refractory), refractoriness to last LOT (1.39 [1.11–1.75]; P = 0.005 vs relapsed), and refractoriness to last anti-CD20-containing regimen (1.39 [1.11–1.74]; P = 0.005 vs relapsed).
Double-/triple-hit DLBCL was not significantly associated with worse outcomes in any of the models, although data were missing for this variable in 44.3% of patients.
Discussion
This observational, retrospective claims database study of real-world patients with R/R DLBCL treated with chemotherapy, single-agent rituximab/obinutuzumab, single-agent lenalidomide, or combinations of these agents showed poor overall response and survival in patients treated with ≥ 1 prior LOT, with worse outcomes in patients treated with ≥ 2 and ≥ 3 prior LOTs. As patients progressed through LOTs, they had progressively poorer outcomes, with a disproportionately larger attenuation of CR vs ORR. Attenuation was also observed for PFS, OS, DOR, and DOCR as patients progressed through LOTs. Additionally, this study identified some factors that characterize challenging-to-treat R/R DLBCL patients: treatment with multiple prior LOTs, primary refractoriness, refractoriness to last LOT and/or last anti-CD20-containing regimen, and prior CAR T exposure.
Previous studies have characterized ORR and survival in DLBCL. SCHOLAR-1 was an international multicohort analysis (N = 636) that pooled patients from two real-world observational cohorts and two phase 3 clinical trials [5]. The study retrospectively evaluated outcomes in patients with refractory DLBCL (28% primary refractory) in the rituximab era [5], which followed the drug’s 2006 FDA approval for 1L treatment of DLBCL in combination with chemotherapy [43]. In SCHOLAR-1, enrolled patients had refractory disease, defined as PD after ≥ 4 cycles of 1L chemotherapy, stable disease after two cycles of later-line chemotherapy as best response, or relapse ≤ 12 months following ASCT [5]. In these patients, ORR and CR were 26% and 7%, respectively, slightly lower than current study results for patients with ≥ 2 (40%) and ≥ 3 (34%) prior LOTs, and markedly lower than patients with ≥ 1 prior LOT (52%), although patient selection criteria differed from the current study. Median OS in SCHOLAR-1 was 6.3 months [5], consistent with the median OS identified for patients treated with ≥ 2 prior LOTs in the current study. Also consistent with the current results is a prior real-world database analysis of CIT-treated patients with R/R DLBCL (N = 212) who received ≥ 3L treatment between 2014 and 2020. These patients had an ORR and CR of 35.9% and 9.4% in the 3L setting and 40.7% and 6.3% in the 4L setting, respectively. Median OS was 7.7 months and 4.4 months, respectively [18]. These slightly different findings from the present study may be due to differing definitions of a LOT and different selection criteria, as the CIT cohort excluded patients treated with lenalidomide and obinutuzumab. Lastly, a recent, retrospective, observational cohort study (RE-MIND2) analyzed outcomes in R/R DLBCL patients treated with systemic therapies in the ≥ 2L setting (N = 76). RE-MIND2 reported an ORR and CR of 48.7% and 21.1%, with a median PFS of 5.8 months and a median OS of 11.6 months, respectively. Notably, the RE-MIND2 cohort excluded double-/triple-hit patients and had a smaller proportion of primary refractory patients (15.8%) and patients who were refractory to last LOT (46.1%) than the current study [44]. Taken together, the evidence suggests poor real-world outcomes for patients with R/R DLBCL, despite the availability of multiple rituximab-based regimens and other novel therapies.
In the current study, several treatment-related factors were significantly associated with poorer outcomes, effectively characterizing a very challenging-to-treat R/R DLBCL patient subgroup. Characteristics consistently associated with worse ORR, PFS, OS, and DOR were an increasing number of prior LOTs, primary refractoriness, refractoriness to last LOT, and refractoriness to last anti-CD20-containing regimen. In multivariate analysis, patients with ≥ 4 vs 1 prior LOT had a 74% reduction in the likelihood of CR, a 135% increased risk of PD, and a 70% increased risk of mortality. Patients who were primary refractory vs not had a 41% reduction in the likelihood of overall response and a 32% increased mortality risk. These patterns continued for patients who were refractory to their last LOT vs those who were relapsed; these patients had a 55% reduction in likelihood of overall response, a 41% reduction in likelihood of CR, and a 39% increased mortality risk. Lastly, patients who were refractory to their last anti-CD20-containing regimen vs those who were relapsed had a 51% reduced likelihood of overall response, a 39% reduced likelihood of CR, and a 39% increased mortality risk. It was notable that, in bivariate analysis, despite the small number of CAR T failures, there was a 92% increased mortality risk in patients who failed prior CAR T therapy.
The strengths of this real-world study are its large sample size and its capture of data spanning an entire decade. This study also included patients who are typically underrepresented in clinical trials. For example, nearly two-thirds of study patients were primary refractory, nearly half had bulky disease, and one in ten was heavily pretreated with ≥ 3 prior LOTs. These features have led to patients being excluded from participating in clinical trials of novel DLBCL treatments [36, 38].
This analysis also had several limitations. In retrospective real-world observational studies, outcomes monitoring may be inconsistent relative to clinical trial protocols. As such, outcomes may be subject to surveillance bias depending on the frequency of clinical assessments. Due to the retrospective and observational nature of the data, this study may be subject to incomplete records and/or data documentation, variability in the quality of information recorded, and differences in clinical practices across study sites [45, 46]. While the overall study sample size was reasonable, some subgroups were smaller (e.g., prior CAR T exposure and later LOTs), limiting the ability to conduct robust analyses that adjust for all factors, including potential confounders. COTA’s proprietary algorithm was used to define what constituted a LOT; this definition may have differed from what the treating clinicians had intended. Finally, if patients in the COTA database have systematically different demographic or clinical characteristics than the overall population of patients with DLBCL, the outcomes of this study may not be fully representative or generalizable to the general patient population with DLBCL in the US.
Conclusions
This study demonstrates that treatment outcomes remain poor in patients with R/R DLBCL treated with SOC. Factors consistently associated with worse outcomes were primary refractory disease, refractory to last LOT, refractory to last anti-CD20-containing regimen, number of prior therapies, and prior CAR T exposure. These factors characterize challenging-to-treat R/R patients and highlight the unmet need for new, safe, and effective therapies to improve outcomes in patients with R/R DLBCL.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical Writing, Editorial, and Other Assistance.
Medical writing and editing were provided by Naseem Bazargan and Jim Wood of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and funded by Genmab. We thank the participants of the study.
Author Contributions
Andrew Ip: Study design and concept, review and interpretation of the results, author review. Alex Mutebi: Study design and concept, review and interpretation of the results, author review. Tongsheng Wang: Study design and concept, analysis of results, review and interpretation of the results, author review. Monika Jun: Study design and concept, review and interpretation of the results, author review. Anupama Kalsekar: Study design and concept, review and interpretation of the results, author review. Fernando Rivas Navarro: Study design and concept, review and interpretation of the results, author review. Anthony Wang: Study design and concept, review and interpretation of the results, author review. Rajesh Kamalakar: Study design and concept, review and interpretation of the results, author review. Mariana Sacchi: Study design and concept, review and interpretation of the results, author review. Brian Elliott: Study design and concept, review and interpretation of the results, author review. All authors have made substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data or the creation of new software used in the work; drafted the work or revised it critically for important intellectual content; approved the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy of integrity of any part of the work are appropriately investigated and resolved.
Funding
Genmab A/S and AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the manuscript. Genmab A/S provided funding for the Open Access fee.
Data Availability
The data sets generated during and/or analyzed during the current study are not publicly available due to the E7438-G000-101 trial IPD being commercial-in-confidence.
Declarations
Conflict of Interest
Andrew Ip: Honoraria: Pfizer; Speakers Bureau: Seagen; Advisory Board: Secura Bio, AstraZeneca, TG Therapeutics; Equity Interest: COTA. Alex Mutebi, Tongsheng Wang, Monika Jun, Anupama Kalsekar, Fernando Rivas Navarro, Mariana Sacchi, and Brian Elliott: Employees of Genmab and own stock. Anthony Wang and Rajesh Kamalakar: Employees of AbbVie and own stock.
Ethical Approval
Given that the COTA database houses de-identified, secondary data, this analysis did not require Institutional Review Board approval. All authors had full permission to access and use the COTA EHR database for this study.
Footnotes
Prior Presentation: Presented at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition; December 10–13, 2022; New Orleans, LA, and virtual.
References
- 1.Lymphoma - non-Hodgkin: Subtypes. 2022 28 Jun 2023]. https://www.cancer.net/cancer-types/lymphoma-non-hodgkin/subtypes. Accessed 28 Jun 2023.
- 2.Cancer stat facts: NHL — diffuse large b-cell lymphoma (DLBCL). 2023 28 Jun 2023]. https://seer.cancer.gov/statfacts/html/dlbcl.html. Accessed 28 Jun 2023.
- 3.Chihara D, Johnston K, Bolatova T, et al. An epidemiological model to estimate the prevalence of diffuse large B-cell lymphoma in the United States. Clin Lymphoma Myeloma Leuk. 2022;22:e1092–e1099. doi: 10.1016/j.clml.2022.08.008. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: B-cell lymphomas v5. 2023 [cited 2023 August 10, 2023]. https://www.nccn.org/professionals/physician_gls/default_nojava.aspx. Accessed August 10, 2023.
- 5.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–1808. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–1861. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 7.Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117:2319–2331. doi: 10.1182/blood-2010-09-297879. [DOI] [PubMed] [Google Scholar]
- 8.Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121:4021–31; quiz 250. [DOI] [PMC free article] [PubMed]
- 9.Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood. 2014;124:2354–2361. doi: 10.1182/blood-2014-05-578963. [DOI] [PubMed] [Google Scholar]
- 10.Herrera AF, Mei M, Low L, et al. Relapsed or refractory double-expressor and double-hit lymphomas have inferior progression-free survival after autologous stem-cell transplantation. J Clin Oncol. 2017;35:24–31. doi: 10.1200/JCO.2016.68.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chien HC, Morreall D, Patil V, et al. Real-world practice patterns and outcomes in veterans with relapsed/refractory diffuse large B-cell lymphoma. Future Oncol. 2021;17:411–422. doi: 10.2217/fon-2020-0522. [DOI] [PubMed] [Google Scholar]
- 12.Halwani AS, Chien H-C, Morreall DK, et al. Survival patterns in patients with relapsed or refractory diffuse large b cell lymphoma: treatment trajectories and responses after the first relapse [abstract] Blood. 2019;134(suppl 1):1622. doi: 10.1182/blood-2019-127719. [DOI] [Google Scholar]
- 13.Rovira J, Valera A, Colomo L, et al. Prognosis of patients with diffuse large B cell lymphoma not reaching complete response or relapsing after frontline chemotherapy or immunochemotherapy. Ann Hematol. 2015;94:803–812. doi: 10.1007/s00277-014-2271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farooq U, Maurer MJ, Thompson CA, et al. Clinical heterogeneity of diffuse large B cell lymphoma following failure of front-line immunochemotherapy. Br J Haematol. 2017;179:50–60. doi: 10.1111/bjh.14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkozy C, Sehn LH. Management of relapsed/refractory DLBCL. Best Pract Res Clin Haematol. 2018;31:209–216. doi: 10.1016/j.beha.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011:498–505. doi: 10.1182/asheducation-2011.1.498. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Laliberté F, Germain G, et al. Real-world characteristics, treatment patterns, health care resource use, and costs of patients with diffuse large B-cell lymphoma in the U.S. oncologist. 2021;26:e817-e26. [DOI] [PMC free article] [PubMed]
- 18.Hamadani M, Liao L, Yang T, Chen L, Moskowitz C. Characteristics and clinical outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma who received at least 3 lines of therapies. Clin Lymphoma Myeloma Leuk. 2022;22:373–381. doi: 10.1016/j.clml.2021.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Chavez JC, Bachmeier C, Kharfan-Dabaja MA. CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Ther Adv Hematol. 2019;10:2040620719841581. doi: 10.1177/2040620719841581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sermer D, Batlevi C, Palomba ML, et al. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv. 2020;4:4669–4678. doi: 10.1182/bloodadvances.2020002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochenderfer JN, Somerville RPT, Lu T, et al. Long-duration complete remissions of diffuse large b cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther. 2017;25:2245–2253. doi: 10.1016/j.ymthe.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 23.Polivy [package insert]. South San Francisco, CA: Genentech, Inc.; 2020.
- 24.Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38:155–165. doi: 10.1200/JCO.19.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monjuvi [package insert]. Boston, MA, USA: MorphoSys US Inc.; 2021.
- 26.Zynlonta [package insert]. Murray Hill, NJ, USA: ADC Therapeutics SA; 2021.
- 27.Xpovio [package insert]. Newton, MA: Karyopharm Therapeutics Inc.; 2022.
- 28.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 29.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westin J, Sehn LH. CAR T cells as a second-line therapy for large B-cell lymphoma: a paradigm shift? Blood. 2022;139:2737–2746. doi: 10.1182/blood.2022015789. [DOI] [PubMed] [Google Scholar]
- 31.Di Rocco A, Di Rocco A, Farcomeni A, et al. Relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL) patients: a retrospective analysis of eligibility criteria for CAR-T cell therapy [abstract 2888]. Blood. 2019;134(Supplement_1):2888.
- 32.Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thornton Snider J, Brauer M, Kee R, et al. The potential impact of CAR T-cell treatment delays on society. Am J Manag Care. 2019;25:379–386. [PubMed] [Google Scholar]
- 34.Kuhnl A, Roddie C, Kirkwood AA, et al. A national service for delivering CD19 CAR-Tin large B-cell lymphoma - the UK real-world experience. Br J Haematol. 2022;198:492–502. doi: 10.1111/bjh.18209. [DOI] [PubMed] [Google Scholar]
- 35.Spanjaart AM, Pennings E, Mutsaers P, et al. Population-based real world results of CD19-directed CAR T-cell therapy for patients with relapsed or refractory large B-CELL LYMPHOMA: a Dutch CAR T-cell tumorboard experience [abstract P1462] HemaSphere. 2022;6(suppl 3):1344–1345. doi: 10.1097/01.HS9.0000848704.84354.44. [DOI] [Google Scholar]
- 36.Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22:790–800. doi: 10.1016/S1470-2045(21)00139-X. [DOI] [PubMed] [Google Scholar]
- 37.Kalakonda N, Maerevoet M, Cavallo F, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020;7:e511–e522. doi: 10.1016/S2352-3026(20)30120-4. [DOI] [PubMed] [Google Scholar]
- 38.Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21:978–988. doi: 10.1016/S1470-2045(20)30225-4. [DOI] [PubMed] [Google Scholar]
- 39.Hamadani M, Liao L, Wilson L, Howarth A, Flores C, Chen L. Real-world outcomes in relapsed/refractory DLBCL patients who received polatuzumab vedotin PLUS bendamustine and rituximab or tafasitamab plus lenalidomide by line of therapy [abstract] Blood. 2022;140(suppl 1):8058–8060. doi: 10.1182/blood-2022-167753. [DOI] [Google Scholar]
- 40.Qualls D, Buege MJ, Dao P, et al. Tafasitamab and lenalidomide in relapsed/refractory large B cell lymphoma (R/R LBCL): real world outcomes in a multicenter retrospective study [abstract] Blood. 2022;140(supplement 1):787–789. doi: 10.1182/blood-2022-167620. [DOI] [Google Scholar]
- 41.Liebers N, Duell J, Fitzgerald D, et al. Polatuzumab vedotin as a salvage and bridging treatment in relapsed or refractory large B-cell lymphomas. Blood Adv. 2021;5:2707–2716. doi: 10.1182/bloodadvances.2020004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feinberg BA, Gajra A, Zettler ME, Phillips TD, Phillips EG, Jr, Kish JK. Use of real-world evidence to support FDA approval of oncology drugs. Value Health. 2020;23:1358–1365. doi: 10.1016/j.jval.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Mohammed R, Milne A, Kayani K, Ojha U. How the discovery of rituximab impacted the treatment of B-cell non-Hodgkin's lymphomas. J Blood Med. 2019;10:71–84. doi: 10.2147/JBM.S190784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nowakowski GS, Yoon DH, Peters A, et al. Improved efficacy of tafasitamab plus lenalidomide versus systemic therapies for relapsed/refractory DLBCL: RE-MIND2, an observational retrospective matched cohort study. Clin Cancer Res. 2022;28:4003–4017. doi: 10.1158/1078-0432.CCR-21-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandis N. Cohort studies: part 2. Am J Orthod Dentofacial Orthop. 2014;146:686–687. doi: 10.1016/j.ajodo.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Sedgwick P. Retrospective cohort studies: advantages and disadvantages. BMJ. 2014;348:g1072. doi: 10.1136/bmj.g1072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analyzed during the current study are not publicly available due to the E7438-G000-101 trial IPD being commercial-in-confidence.