Abstract
Firm conclusions regarding the differential effects of the maladaptive consequences of acute versus chronic stress on the etiology and symptomatology of stress disorders await a model that isolates chronicity as a variable for studying the differential effects of acute versus chronic stress. This is because most previous studies have confounded chronicity with the total amount of stress. Here, we have modified the stress-enhanced fear learning (SEFL) protocol, which models some aspects of posttraumatic stress disorder (PTSD) following an acute stressor, to create a chronic variant that does not have this confound. Comparing results from this new protocol to the acute protocol, we found that chronic stress further potentiates enhanced fear-learning beyond the nonassociative enhancement induced by acute stress. This additional component is not observed when the unconditional stimulus (US) used during subsequent fear learning is distinct from the US used as the stressor, and is enhanced when glucose is administered following stressor exposure, suggesting that it is associative in nature. Furthermore, extinction of stressor-context fear blocks this additional associative component of SEFL as well as reinstatement of generalized fear, suggesting reinstatement of generalized fear may underlie this additional SEFL component.
Keywords: Acute stress, Chronic stress, Stress-enhanced fear learning, Posttraumatic stress disorder, Generalization, Reinstatement
1. Introduction
Following exposure to traumatic stress, a percentage of individuals go on to develop a particularly debilitating psychiatric disorder referred to as posttraumatic stress disorder (PTSD). Chronic stress is thought to lead to more severe (Hoeboer et al., 2021), long-lasting forms of PTSD as compared to acute stress, but this remains to be conclusively validated. Many animal studies claim unique effects of chronic stress when compared to acute stress but virtually all such studies contain confounds that undermine this claim (e.g., Katz et al., 1981; Chappell et al., 1986; Bruinsma et al., 2016; Franco et al., 2016; Ghosal et al., 2017; Grissom and Bhatnagar, 2009). Every study that has attributed an effect to chronic stress contains one of two experimental confounds. The first is that the chronic stress condition is compared to an unstressed control. This comparison may allow conclusions about stress per se, but it says nothing about the factor of chronicity. In studies where chronic stress is compared to an acute stressor, there is also a very serious confound — the chronic stressor also provides considerably more total stress. This is because the chronic condition is compared to a more limited number of exposures to the same stressor. All current studies of chronic stress suffer from at least one of these two confounds (e.g., Katz et al., 1981; Chappell et al., 1986; Bruinsma et al., 2016; Franco et al., 2016; Ghosal et al., 2017; Grissom and Bhatnagar, 2009).
To develop a mechanistic understanding of why a single acute bout of intense stress can have such adverse consequences, we developed and characterized a rodent model, whereby a single bout of stress has a pronounced and prolonged impact on both behavior and physiology (Rau et al., 2005). Rats are exposed to a single, 90-min session containing 15 (1-mA, 1-s) footshocks in a ‘stressor context’. Following this stressor, mild Pavlovian fear conditioning becomes sensitized for at least 90 days without remission, so that a context or tone paired with a single shock bestows the conditional stimulus (CS) with a highly exaggerated, maladaptive level of fear (Rau et al., 2005). We have also provided compelling evidence that this stress-enhanced fear learning (SEFL) is a non-associative phenomenon that does not depend on learning about, or even fear of, the stressor context (Rau et al., 2005; Poulos et al., 2014; Rau and Fanselow, 2009; Long and Fanselow, 2012). This evidence includes, but is not limited to, the fact that neither complete extinction of fear of the stressor context (Rau et al., 2005; Long and Fanselow, 2012) nor infusion of the N-methyl-D-aspartate (NMDA) antagonist (2R)-amino-5-phosphonovaleric acid (APV) in a manner that completely blocks contextual fear conditioning during stress (Rau et al., 2005) have any effect on SEFL.
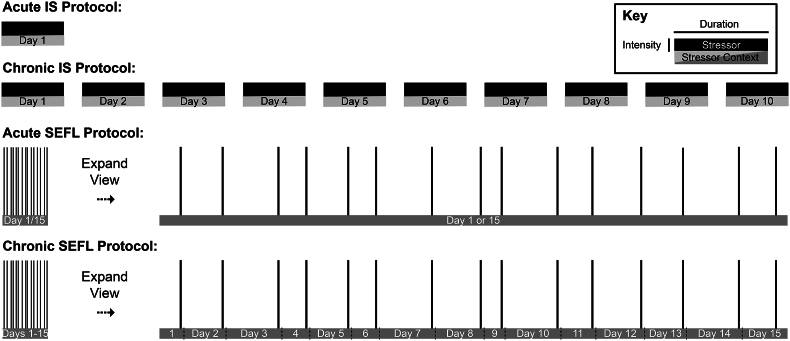
An advantage of the stressor we use in the SEFL model is that every aspect of it is quantifiable and titratable. The procedure itself is made of time (e.g., 90 min), number of shocks (e.g., 15), and their relationship (a variable rate of presentation averaging 1 shock every 6 min). This allows us to take our acute-stress condition and carve it into a chronic treatment of 15 daily epochs, where each epoch contains a pre-shock interval, a single shock, and a brief post-shock interval. The pre-shock interval for each epoch together with the post-shock interval from the prior epoch accounts for one of the inter-stressor intervals from the acute treatment (Fig. 1). Thus, time in the stressor context, time to next shock, and amount of shock are completely equated in the two conditions, with the only distinction being distribution of the 15 epochs over time.
Fig. 1.
The acute versus chronic SEFL protocol, in contrast to other common acute versus chronic stress (e.g., immobilization stress) protocols, isolates chronicity as a variable. Depicted: Chronic immobilization stress (IS) differs from acute IS not only in terms of chronicity of the stressor, but also in terms of total stress volume. Conversely, the chronic SEFL resembles acute SEFL in terms of the total amount of time exposed to the stressor context, the pattern of variable inter-stressor context exposure times, as well as total stress volume during stressor presentations, and only differs from acute SEFL in the chronicity of the stressor presentations.
There is clear theoretical and empirical precedent for the premise that spaced training, as opposed to massed training, favor the long-term retention of learning. This phenomenon has been observed for both nonassociative (i.e., habituation and sensitization) (Hinde, 1954; Carew et al., 1972; Davis, 1970; Masini et al., 2008; Menzel et al., 2001; Rose and Rankin, 2001; Sutton et al., 2002) as well as associative (e.g., spatial and declarative memory) (Commins et al., 2003; Goodrick, 1973) forms of learning across many species spanning several phyla. Therefore, in the present study, we test the hypothesis that chronic stress, unlike acute stress, enhances subsequent fear learning through the additional engagement of associative, rather than purely non-associative, processes.
There is also mounting evidence that post-stress glucose consumption has prophylactic effects on PTSD-like symptoms. Oral consumption of a concentrated glucose solution, within a 3-hr window following inescapable shock (Conoscenti et al., 2017), eliminates some symptoms in a PTSD model (Minor and Saade, 1997) and promotes hormetic resilience training (Plumb et al., 2015) in rats. Furthermore, oral, post-stress glucose consumption in humans improves hippocampus-dependent fear conditioning (Glenn et al., 2014). It is hypothesized that, by promoting more specific mental representations of stressor context, glucose treatment prevents maladaptive spillover of adaptive defense responses into inappropriate contexts. Therefore, in this study, we also test whether or not post-stress glucose consumption has a similar prophylactic effect within another model of PTSD, SEFL, and whether or not there is a differential effect of acute versus chronic versions of this model.
2. Material and methods
2.1. Subjects
Two-hundred and twenty male and female Long-Evans rats (250–400g) from Envigo were housed in individual cages with free access to food and water in a room maintained on a 12:12-h light/dark cycle for one week prior to experimental treatment. Experimentation occurred during the light portion of the cycle. The UCLA Institutional Animal Care and Use Committee approved all procedures involving animals, and all efforts were made to find and use alternatives to in vivo experimental approaches, if available, to reduce the number of animals used in the study and to minimize animal suffering. All groups in all experiments included an equal number of male and female rats, unless rats were excluded from analysis due to equipment malfunction or high baseline levels of freezing.
2.2. Apparatus
Rats were housed in metal hanging cages. Each cage was equipped with a standard glass (250 mL) water bottle with a rubber stopper and metal spout and a food hopper with standard laboratory rodent chow. All rats had constant access to food and water and could feed and drink ad libitum.
Stress pretreatment and testing occurred in Med Associates (St Alban, VT) behavioral testing chambers. Each chamber was equipped with an infrared camera, speaker for tone delivery, shock scrambler, and fluorescent and infrared light sources. The behavioral testing chambers in each testing room were controlled by a PC using Med Associates Video Freeze software that also automatically scores motion and freezing of the animal during the test session. A rat is considered freezing when image change is registered at less than 50 pixels for at least 1 s. Any baseline pixel change due to mechanical operation of the chamber or camera is measured before the animal's entry and subtracted from measurement during the trial. To create distinct contexts between stress pretreatment and subsequent fear conditioning and testing, the chamber's contextual features were modified using differential wall inserts, grid/non-grid floors, lighting, ambient noise, and odors. Transport, behavioral testing room location, and room lighting also differed among contexts. Unless otherwise stated, contexts differed on as many dimensions as possible to reduce generalization effects. More specifically, the behavioral testing chambers used for stress pretreament were configured with aluminum side walls, a clear acrylic (with blue dots) rear wall and ceiling, a flat stainless steel grid floor, the chamber white light on, the chamber infrared light on, the sound attenuation box fan on, and 1% acetic acid as odor. A white light was used to illuminate Room A housing the chambers, and rats were transported to this room in their home cages on a rack mounted to a cart and covered with a white sheet. The behavioral testing chambers used for generalization tests were configured with a black acrylic A-frame insert for side walls and ceiling, a white plastic rear wall, a white acrylic floor, the chamber white light on, the chamber infrared light on, the sound attenuation box fan on, and 70% isopropyl alcohol as odor. A white light was used to illuminate Room B, and rats were transported to this room in a covered black plastic tub filled with Sani-Chips (P.J. Murphy Forest Products; Montville, NJ) and carried by hand. The behavioral testing chambers used for the SEFL procedure were configured with curved white acrylic side and rear walls, a clear acrylic (with black stripes) ceiling, a staggered stainless steel grid floor, the chamber white light off, the chamber infrared light on, the sound attenuation box fan off, and 3% Simple Green (Sunshine Makers; Huntington Beach, CA) as odor. A red light was used to illuminate Room C, and rats were transported to this room in their home cages on a rack mounted to a cart and covered with a black sheet. The behavioral testing chambers used for tone tests were configured with aluminum side walls, a white plastic rear wall, a clear acrylic ceiling, a white acrylic floor, the chamber white light off, the chamber infrared light on, the sound attenuation box fan off, and 50% Windex (SC Johnson; Racine, WI) as odor. A red light was used to illuminate Room D, and rats were transported to this room in a covered black plastic tub filled with Bed-o’Cobs (The Andersons; Maumee, OH) and carried on a cart.
2.3. Procedure
In all experiments, stress pretreatment and subsequent testing occurred in Med Associates (St. Albans, VT) conditioning chambers. Chamber walls, ceiling, floor, lighting, ambient noise, and odor as well as behavioral testing room location and lighting and transport were adjusted to distinguish contexts. All rats were pre-handled in the vivarium by the experimenter for 1 min per day for 7 days prior to stress exposure and were habituated to transport to the room in which stress pretreatment later took place once per day for 3 days immediately prior to stress exposure. On Day 1 or 15, acutely-stressed groups received 15 (1-mA, 1-sec) unpredictable footshocks over a 90-min session. These rats remained in their home cages but were handled in the vivarium for a similar amount of time as chronically-stressed rats for the remaining 14 days of chronic-stress pretreatment. Chronically-stressed groups received 1 (1-mA, 1-sec), unpredictable footshock per day over 15 consecutive days (1 shock/day). Unstressed controls received identical context exposure without shock in either a single, 90-min session or across 15 days. Following stress pretreatment, all groups underwent subsequent testing. The generalization test involved an 8-min session within a context that differed from the stressor context only in terms of the shape of the walls/ceiling/floor and odor of the chamber as well as the method of transport. Extinction to the stressor context included 30-min sessions (each on subsequent days) of stressor context exposure without shock. Context preexposure also included 8-min or 30-min sessions (each on subsequent days across 3, 6, or 7 days) of exposure without shock to a novel context that differed more significantly from previously exposed contexts in terms of the shape of the walls/ceiling/floor, lighting, ambient noise, and odor of the chamber, lighting within the room, and method of transport. The single-shock/noise exposure occurred in the preexposed context following the extinction of fear generalization (3–6 days). All shocks were 1 mA in intensity and 1 s in length. All startle noise presentations consisted of a115-dB, 100-msec white noise. Single shock/startle noise followed a 3-min baseline period, to establish baseline freezing, and was followed by a 2-min post-shock/noise period, to assess fear acquisition. Rats were returned to the single-shock/noise context 24 h after exposure for an 8-min context test. In experiments involving fear testing to a shock associated tone, the single-shock was preceded by context preexposure, just as in the single-shock context. The tone consisted of a 65-dB, 2800-Hz tone — lasting 30 s and co-terminating with a 1-mA, 1-s shock. The tone was preceded by a 3-min baseline period, and followed by a 2-min post-tone/shock period. On tone test days, a 3-min baseline preceded the first tone and 1 min intervened between presentations. The last tone was followed by a 2-min post-tone period.
In experiments involving the glucose intervention, every group was pre-exposed to glucose in their drinking water over three consecutive days approximately ten days prior to the start of the experimental procedure (Minor and Saade, 1997). The cocktail consisted of 40% (w/v) glucose and 5% (w/v) sucrose dissolved in tap water. One group that received acute stress pretreatment and one group that received chronic stress pretreatment received one (AS) or fifteen (CS) day(s) of free access to the glucose cocktail for 6 h/day immediately following the end of each stress session (AS Glucose — ASG; CS Glucose — CSG). The other three groups (Chronic No Stress Water — CNW, AS Water — ASW, and CS Water — CSW) received only water during this time. We recorded total fluid consumption for all groups during this interval.
2.4. Statistical analysis
Software package SPSS (SAS Institute, Inc., Version 16.0; Cary, NC) was used for statistical analyses. One-way, two-way, and mixed-design ANOVAs were used when appropriate. Following significant interactions, Tukey post-hoc analyses on group means are reported. Statistical significance was noted when p values were less than .05. Data is presented as group means with error bars denoting group mean ± SEM. No statistical outliers were removed from the data; animals were excluded solely based on equipment malfunction or high baseline levels of freezing.
3. Results
3.1. Chronic stress produces greater generalization of stressor-context fear and greater enhancement of subsequent fear learning than acute stress
Previously, we showed that exposure to 15 1-mA shocks during a single, acute 90-min session enhances future fear learning (Rau et al., 2005; Rau and Fanselow, 2009), regardless of sex (Poulos et al., 2015). Given the emphasis on chronic stress in the literature, we tested whether a chronic version of the SEFL procedure, in which the 15 1-mA shocks and 90 min spent in the SEFL chamber are spaced out across fifteen days, would result in different effects. Importantly, we maintained an equivalent level of total stress in both the acute and chronic regimens. We also tested whether, like acute-stress-induced SEFL, the effects would be independent of sex.
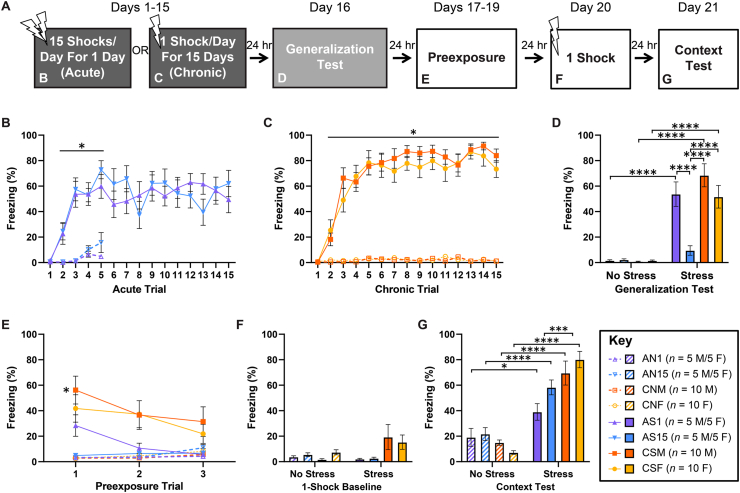
Rats within the chronic-stress group (CS) were placed into a stressor context for a variable amount of time on each of days 1–15 where they were exposed to 1 shock/day. Chronic-stress-exposure controls (CN) spent the same amount of time in the stressor context on each of fifteen days but were not exposed to any shocks. The acute-stress group (AS) received all fifteen shocks during a single 90-min session within the stressor context. At the same time, the acute-stress-exposure controls (AN) were placed in the same stressor context for the same amount of time without receiving any shocks. As the period from the first and the last shock stressor to the time of testing are both potentially important variables that cannot both be held constant between the acute- and chronic-stress groups, the acute-stress group was split into two subgroups: those whose stress onset was time-matched to chronic stress onset (acute day 1 — AS1) and those whose stress was time-matched to chronic stress termination (acute day 15 — AS15). One day after chronic and acute-day-15 stress exposure (15 days after acute-day-1 stress exposure), all rats were subjected to a fear generalization test in a new, distinct context. The following five days, all rats went through the SEFL test procedure, which consisted of 3 days of pre-exposure to a third, distinct context, exposure to a single shock in this new context on the fourth day, and a contextual fear memory test within this same context on the fifth day (Fig. 2A).
Fig. 2.
Impacts of stress chronicity and sex on subsequent fear learning. Depicted: Schematic illustration of the timeline of the experiment and the figure panels in which data from various components of the experiment are displayed (panel A). Percent freezing during stress pretreatment (panels B & C, days 1–15), generalization testing (panel D, day 16), context preexposure (panel E, days 17–19), single-shock baseline (panel F, day 20), and context test (panel G, day 21). Male (M) and female (F) rats received either acute stress exposure (15 footshocks) on day 1 or 15 (AS1 or AS15), chronic stress (CS) exposure (15 footshocks), or identical context exposure with no shock (No Stress; AN & CN). Following stress pretreatment, all rats were exposed to a novel context that shared some similar dimensions to the stress pretreatment context. All groups were then preexposed to a completely novel environment for 8 min/day for 3 consecutive days. Twenty-four hours after the termination of preexposure, all groups received a single footshock in the preexposed context. Then, all groups were tested for contextual fear learning 24 h later. All groups that received footshock during stress pretreatment readily reached asymptotic contextual fear conditioning (panels B & C). Groups that received chronic stress or that received acute stress on day 1 of the chronic stress procedure showed greater generalized fear compared to all other groups in the similar (panel D) or totally novel contexts (panel E). Increased baseline levels of generalized fear prior to the SEFL single-shock exposure were non-significant (panel F). AS and CS groups showed greater freezing behavior during the context test compared to unshocked controls, and CS showed higher freezing as compared to AS (panel G). Error bars denote mean ± SEM. *, ***, **** denotes significance (p ≤ .05, p ≤ .001, and p ≤ .0001, respectively) compared between indicated groups (horizontal square brackets), compared between each Stress group and its respective No Stress control (horizontal line), or compared between a specific Stress group and its respective No Stress control (free-standing).
During stress exposure, there was a rapid and significant increase in the percent of time both acute-stress (Fig. 2B) and chronic-stress (Fig. 2C) rats spent freezing as compared to their respective controls during a 2-min window prior to each shock across the 15 trials (Fig. 2B: mixed-design ANOVA (Acute groups) – Group (Stress-Day) x Trial: F(42,540) = 3.324, p ≤ .0001, Tukey post-hoc comparisons – ASD1,15-Trials 2–5 > AND1,15-Trials 2–5: p ≤ .05; Fig. 2C: mixed-design ANOVA (Chronic groups) – Group (Stress-Sex) x Trial: F(42,540) = 17.43, p ≤ .0001, Tukey post-hoc comparisons – CSM,F-Trials 2–15 > CNM,F-Trials 2–15: p ≤ .05), and there was no main effect of sex or chronicity on fear learning as measured by percent freezing following the fifteenth trial (data not shown). Beyond the fifth trial, comparisons in percent time freezing could not be made between acute-stress rats and their controls because many of the control rats started to engage in sleeping behavior, which Med Associates Video Freeze software cannot distinguish from freezing behavior, and, therefore, data from the control rats beyond the fifth trial was omitted from the acquisition curves in all relevant figures. During the generalization test, chronic-stress (both male — M, and female — F) and acute-day-1-stress rats displayed high freezing levels in this new context compared to their respective controls and acute-day-15-stress rats that displayed relatively low freezing levels (Fig. 2D; two-way ANOVA – Group (Chronicity-Day/Sex) x Stress: F(3,72) = 10.04, p ≤ .0001, Tukey post-hoc comparisons – AS1 > AN1: p ≤ .0001, AS1 > AS15: p ≤ .0001, CSM > CNM: p ≤ .0001, CSM > AS15: p ≤ .0001, CSF > CNF: p ≤ .0001, CSF > AS15: p ≤ .0001). In the case of chronic-stress male rats, this generalized fear was also observed at the beginning of pre-exposure to the SEFL context (Fig. 2E; mixed-design ANOVA Group (Chronicity-Stress-Day/Sex) x Trial: F(14,216) = 3.419, p ≤ .0001, Tukey post-hoc comparisons – CSM-Trial 1 > CNM-Trial 1: p = .012), but was extinguished by the end of the three trials. At baseline, prior to the SEFL single-shock exposure, neither the acute-stress groups nor the chronic-stress groups displayed significantly higher levels of freezing compared to their respective control groups (Fig. 2F; two-way ANOVA – Group (Chronicity-Day/Sex) x Stress: F(3,72) = 2.660, p = .055). The SEFL context test revealed that both acute-day-1 and acute-day-15 subgroups and male and female subgroups within the acute- and chronic-stress groups, respectively, showed an enhancement of contextual fear learning as compared to their respective non-stressed controls (Fig. 2G; two-way ANOVA – Group (Chronicity-Day/Sex) x Stress: F(3,72) = 7.256, p = .0003, Tukey post-hoc comparisons – AS1 > AN1: p = .022, AS15 > AN15: p ≤ .0001, CSM > CNM: p ≤ .0001, CSF > CNF: p ≤ .0001). Importantly, collapsing the data across day-1 and day-15 within the acute-stress group and across sex within the chronic-stress group revealed that chronic stress further enhances subsequent fear learning beyond the enhancement observed following acute stress (Fig. 2G; two-way ANOVA – Chronicity x Stress: F(1,76) = 7.256, p = .0087, Tukey post-hoc comparisons – CS > AS: p = .0003).
3.2. Extinction of stressor context fear attenuates SEFL following chronic but not acute stress
Reinstatement occurs when an extinguished response is renewed following re-exposure to the original US alone (Pavlov, 1927; Rescorla and Heth, 1975; Bouton and Bolles, 1979). Importantly, in addition to the original conditioned fear responses, generalized fear responses are also known to undergo reinstatement (Cameron et al., 2015). During the standard SEFL procedure, generalized contextual fear to the stressor context is extinguished during context preexposure to the context in which single-shock training occurs, and subsequent exposure to the single shock has the potential to cause reinstatement of this generalized fear. Therefore, one possible mechanism for the SEFL effect is reinstatement of generalized fear of the stressor context (previously extinguished within the SEFL test context during context preexposure sessions) having an additive effect on top of normal fear learning. Arguing against this account, we have previously shown that the SEFL effect following acute stress is unaffected by complete extinction of stressor context fear prior to the SEFL test (Rau et al., 2005; Long and Fanselow, 2012). However, the fact that substantial levels of generalized fear were observed in the present study following chronic stress as well as 15 days after acute stress, raises the question as to whether or not reinstatement of prior generalized fear might be contributing to the SEFL effect under these conditions. We, therefore, carried out an experiment in which we completely extinguished stressor context fear prior to performing the SEFL context test procedure. Although not statistically significant, the higher levels of baseline freezing observed in chronic-stress rats prior to SEFL single-shock exposure within the prior experiment (Fig. 2F) has the potential to confound interpretations regarding enhancement of SEFL. In all subsequent experiments, we, therefore, increased the duration of each context preexposure session from 8 min to 30 min and in some cases the number of days during which preexposure sessions took place from 3 days to 6 or 7 days.
If SEFL occurs through an associative mechanism, enhanced fear learning should only be observable when there is fear of the stressor context to generalize to similar contextual cues. While our use of distinct contexts in SEFL experiments attempts to minimize overlap in common cues across contexts, all conditioning contexts likely share some elemental features. By using auditory rather than contextual fear conditioning during the SEFL test procedure, we eliminate any commonality between stressor context and the conditional stimulus used in subsequent fear conditioning. Indeed, we have previously used this type of experiment to help validate that SEFL following acute stress arises from a non-associative, non-specific effect on all fear circuitry, including auditory fear learning circuitry. We, therefore, decided to similarly examine stress-enhanced auditory fear learning following chronic stress. A lack of enhanced auditory fear learning following chronic stress would help demonstrate an associative nature of this form of SEFL.
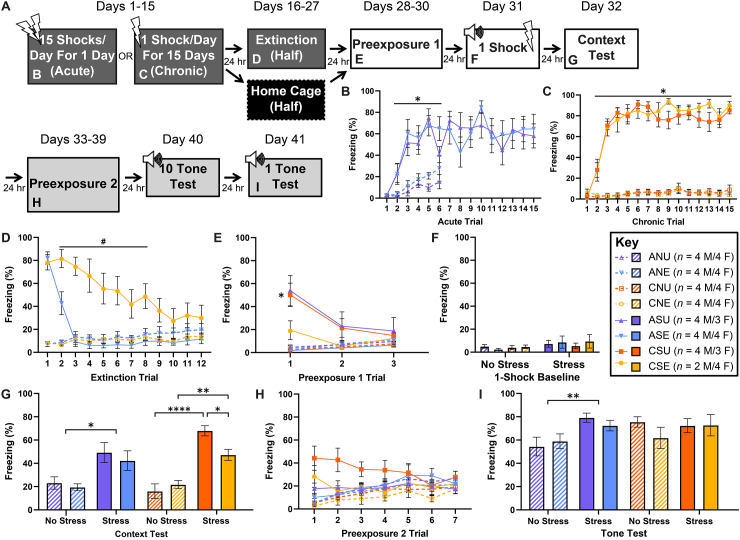
This experiment followed the same general timeline as the previous experiment with the following differences: 1) on days 16–27, half of the rats in each of the acute- and chronic-stressed and control groups went through 30 min/day of extinction training within the original stressor context while the other half of each group were left in their home cages and 2) the contextual-fear SEFL test was followed by an auditory-fear SEFL test procedure (Fig. 3A).
Fig. 3.
Effects of stressor context fear extinction on subsequent contextual and auditory fear learning. Depicted: Schematic illustration of the experiment timeline and the figure panels in which data from the experiment are displayed (panel A). Percent freezing during stress pretreatment (panels B & C, days 1–15), stressor context fear extinction (panel D, days 16–27), context preexposures (panels E & H, days 28–30 and days 33–39, respectively), single-shock baseline (panel F, day 31), context test (panel G, day 32), and tone test (panel I, day 41). Rats received either acute (AS) or chronic (CS) exposure to 15 footshocks, or identical context exposure with no shock (No Stress; AN/CN). Following stress pretreatment, rats received (E) or did not receive (U) extinction training to the stressor context. Following extinction training, all rats were preexposed to a novel context (30 min/day) for 3 consecutive days. Twenty-four hours after preexposure, all groups received a single footshock in the preexposed context. All groups were tested for contextual fear learning 24 h later, then preexposed to a third context (30 min/day) for 7 days, and, finally, tested for fear conditioning to the tone in this context. Rats that received chronic stress exhibited a slower rate of extinction (panel D). CSU rats exhibited higher levels of fear expression to the novel context (panel E). All groups displayed comparably low baseline levels of fear prior to the single-shock exposure (panel F). Stressed rats exhibited enhanced fear learning to the single-shock session. However, extinction training attenuated learning only in the chronic-stress group (panel G). Furthermore, only AS groups exhibited enhanced fear learning to the tone (panel I). Error bars denote mean ± SEM. *, **, and **** denotes significance (p ≤ .05, p ≤ .01, and p ≤ .0001, respectively) compared between indicated groups (horizontal square brackets), compared between each Stress group and its respective No Stress control (horizontal line), or compared between a specific Stress group and its respective No Stress control (free-standing). # denotes significance (p ≤ .05) compared between CSE and ASE (horizontal line).
As in the previous experiment, acute-stress (Fig. 3B) and chronic-stress rats (Fig. 3C) acquired fear of the stressor context across the 15 trials (Fig. 3B: mixed-design ANOVA (Acute groups) – Group (Stress-Extinction) x Trial: F(42,405) = 1.456, p = .0373, Tukey post-hoc comparisons – ASU,E-Trials 2–6 > ANU,E-Trials 2–6: p ≤ .05; Fig. 3C: mixed-design ANOVA (Chronic groups) – Group (Stress-Extinction) x Trial: F(42,375) = 12.56, p ≤ .0001, Tukey post-hoc comparisons – CSU,E-Trials 2–15 > CNU,E-Trials 2–15: p ≤ .05). Rats that went on to go through extinction showed similar fear learning within the stressor context as the rats that went on to be unextinguished, home-cage controls (Fig. 3B/C). Chronic-stress rats took significantly longer to extinguish fear of the stressor context than acute-stress rats (Fig. 3D; mixed-design ANOVA – Chronicity x Stress x Trial: F(11,312) = 6.042, p ≤ .0001, Tukey post-hoc comparisons – CSE-Trials 2–8 > ASE-Trials 2–8: p ≤ .05). After 12 days of extinction training, stressor-context fear was completely extinguished in acute-stress and largely extinguished in chronic-stress rats (Fig. 3D). More intensive preexposure (as compared to the last experiment) to the contextual-fear-SEFL context (30 min sessions/day for 3 days) again completely extinguished generalized fear of the stressor context in the unextinguished groups (Fig. 3E; mixed-design ANOVA – Extinction x Stress x Trial: F(2,168) = 11.388, p ≤ .0001, Tukey post-hoc comparisons – CSU-Trial 1 > CNU-Trial 1: p = .025). Importantly, following more intensive preexposure to the contextual-fear-SEFL context, no differences in baseline freezing were observed prior to the SEFL single-shock exposure (Fig. 3F; one-way ANOVA – Group (Chronicity-Stress-Extinction): F(7,52) = 0.4533, p = .863). Corroborating our previous findings, complete fear extinction of the stressor-context fear had no effect on contextual-fear SEFL in the acute-stress group, but, interestingly, reduced the level of contextual-fear SEFL in the chronic-stress group to a similar level as the acute-stress group (Fig. 3G; one-way ANOVA (Acute groups) – Stress: F(1,29) = 13.72, p = .0009; two-way ANOVA (Chronic groups) – Extinction x Stress: F(1,25) = 7.153, p = .013, Tukey post-hoc comparisons: CSU > CNU: p ≤ .0001, CSE < CSU: p = .043, CSE > CNE: p = .007). In unextinguished, but not extinguished, chronic-stress rats, despite generalized fear having been previously extinguished during preexposure to the second, distinct context, although just short of significant, there appeared to be some generalized fear at the beginning of preexposure to the third, distinct context (Fig. 3H). This is consistent with the interpretation that the extra component of SEFL for contextual-fear following chronic stress is reinstatement of extinguished generalized, but not extinguished non-generalized, fear. Importantly, the finding that extinction has no effect on contextual-fear SEFL in acute-stress rats held even when acute-day-1 rats were analyzed separately (data not shown). Further, in line with a non-associative mechanism for SEFL following acute stress, neither extinguished or unextinguished acute-stress rats showed any reinstatement of generalized fear in the third context (Fig. 3H). When data was collapsed across extinction subgroups, auditory fear learning was found to be enhanced by acute stress (Fig. 3I; one-way ANOVA (Acute groups) – Stress: F(1,29) = 10.01, p = .0036), confirming our previous findings (Rau et al., 2005). Surprisingly, enhancement of auditory fear learning was occluded by higher levels of fear learning in the chronic-stress control groups (Fig. 3I; two-way ANOVA (Chronic groups) – Extinction x Stress: F(1,25) = 0.910, p = .349; see discussion). Therefore, assessment of whether or not chronic stress engages associative mechanisms to produce SEFL requires another approach to probe pathways that are non-overlaping with stressor context fear conditioning.
3.3. SEFL occurs following both acute and chronic stress even when using a distinct unconditional stimulus to drive fear learning
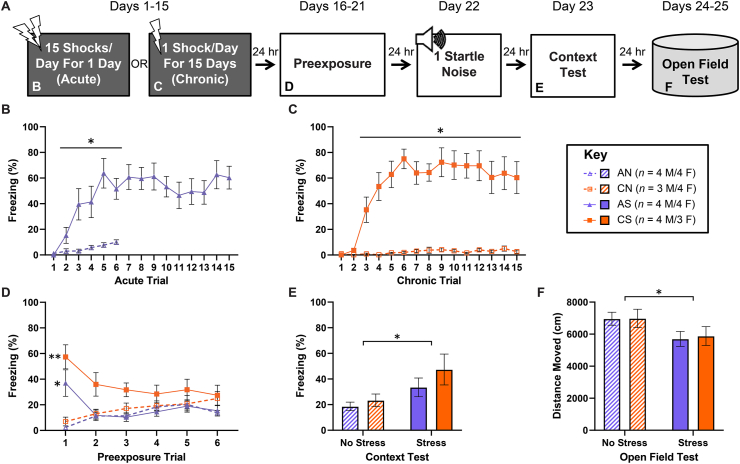
Complete extinction of stressor-context fear only partially blocked SEFL following chronic stress. This leaves three possibilities regarding the remaining component(s) of SEFL. They are: 1) one or more additional associative processes distinct from reinstatement of generalized fear, 2) one or more additional non-associative processes, and 3) a combination of additional distinct associative and non-associative processes. To help differentiate between these three scenarios, we sought another means of testing for non-associative components of SEFL following chronic stress. Using a CS that is not a component of the stressor context during subsequent fear learning is one way of assessing the non-specificity (non-associative nature) of SEFL; using a distinct US is another. We, therefore, carried out another experiment in which we substituted the shock normally used as the US during the SEFL test with a 115-dB, 100-ms burst of white noise, which has previously been demonstrated to have aversive valence (Pennington et al., 2020) and to reveal enhancement of fear learning following acute stress (Perusini et al., 2016) (Fig. 4A). In this experiment, we also took the opportunity to look for differences in stress-induced anxiety between the acute and chronic stress procedures by running two days of open field tests at the end of the experiment.
Fig. 4.
Impact of stress chronicity on subsequent fear learning reinforced by a novel aversive stimulus. Depicted: Schematic illustration of the timeline of the experiment and the figure panels in which data from various components of the experiment are displayed (panel A). Percent freezing during stress pretreatment (panels B & C, days 1–15), context preexposure (panel D, days 16–21), context test (panel E, day 23), and open field test (panel F, days 24–25). Rats received either acute (AS) or chronic (CS) exposure to 15 footshocks, or identical context exposure with no shock (No Stress; AN/CN). All groups were preexposed (30 min/day) to a novel environment for 6 consecutive days. Twenty-four hours after the termination of preexposure, all groups received a single startle stimulus in the preexposed context. All groups were tested for contextual fear learning 24 h later. Stressed rats exhibited enhanced contextual fear learning to the startle noise, but there was no difference between AS and CS (panel E). Additionally, stressed rats exhibited decreased exploratory behavior in the open field test as indicated by a decrease in distance travelled, but, again, there were no differences between AS and CS (panel F). Error bars denote mean ± SEM. *, ** denotes significance (p ≤ .05, p ≤ .01) compared between indicated groups (horizontal square brackets), compared between each Stress group and its respective No Stress control (horizontal line), or compared between a specific Stress group and its respective No Stress control (free-standing).
As in the previous experiments, acute-stress rats (Fig. 4B) and chronic-stress rats (Fig. 4C) showed fear learning within the stressor context (Fig. 4B: mixed-design ANOVA (Acute groups) – Stress x Trial: F(14,195) = 2.471, p = .0031, Tukey post-hoc comparisons – AS-Trials 2–6 > AN-Trials 2–6: p ≤ .05; Fig. 4C: mixed-design ANOVA (Chronic groups) – Stress x Trial: F(14,195) = 10.38, p ≤ .0001, Tukey post-hoc comparisons – CS-Trials 3–15 > CN-Trials 3–15: p ≤ .05). Generalized stressor-context fear was extinguished during SEFL-test context pre-exposure in both acute- and chronic-stress rats (Fig. 4D; mixed-design ANOVA – Group (Chronicity-Stress) x Trial: F(15,144) = 5.982, p ≤ .0001, Tukey post-hoc comparisons – CS-Trial 1 > CN-Trial 1: p = .003, AS-Trial 1 > AN-Trial 1: p = .047). Importantly, while stress had an overall effect of enhancing freezing during the context test (Fig. 4E; one-way ANOVA Stress: F(1,28) = 8.224, p = .0078), acute- and chronic-stress rats showed similar levels of SEFL, providing evidence for scenario 2, outlined above — chronic stress induces one or more non-associative processes in addition to associative generalization of stressor fear in order to produce SEFL within the standard SEFL procedure. Lastly, while there was an overall effect of stress in increasing anxiety, as measured by a decrease in movement within the open field test (Fig. 4F; one-way ANOVA – Stress: F(1,28) = 5.289, p = .0291), there was no observable difference in this measure of anxiety between acute- and chronic-stress rats.
3.4. Post-stress glucose selectively enhances SEFL in chronic-, but not acute-stressed, rats
Post-stress glucose consumption eliminates some behavioral impairments in the learned helplessness model of PTSD and comorbid depression (Conoscenti et al., 2017; Minor and Saade, 1997). In light of the fact that glucocorticoid signaling results in a delayed decrease in hippocampal glucose metabolism (Sapolsky, 1986) while hippocampal administration of glucose enhances memory formation in rodents (Dash et al., 2006) and oral ingestion of glucose enhances memory in humans (Manning et al., 1998; Korol and Gold, 1998), the prophylactic effect of post-stress glucose may lie in its ability to compensate for deficits in hippocampal glucose metabolism and memory formation mitigating aberrant fear generalization. If this is the case, post-stress glucose consumption would be expected to attenuate enhancement of subsequent fear learning following chronic, but not acute, stress. To test this hypothesis, we carried out a fourth SEFL experiment in which acute-day-1 or chronic-stress rats had access to a 40% (w/v) glucose/5% (w/v) sucrose (in drinking water) solution or a control water solution for 6 h/day immediately following each stress session (Fig. 5A).
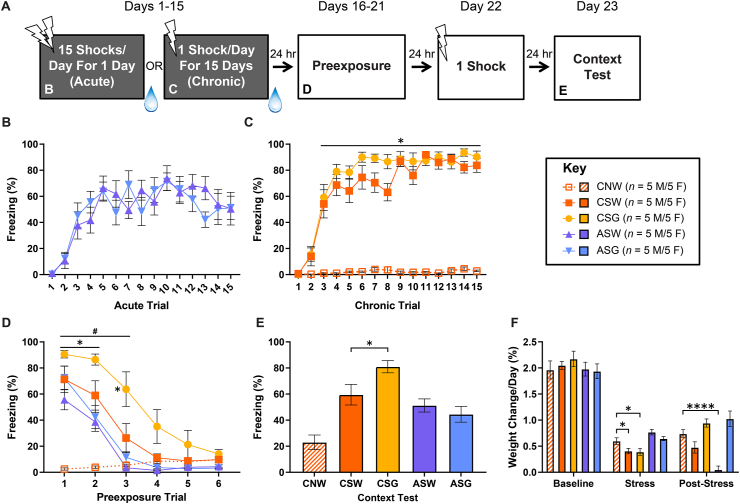
Fig. 5.
Post-stress glucose selectively enhances subsequent fear learning in chronically-stressed rats. Depicted: Schematic illustration of the experiment timeline and the figure panels in which data from the experiment are displayed (panel A). Percent freezing during stress pretreatment (panels B & C, days 1–15), context preexposure (panel D, days 16–21), and context test (panel E, day 23). Weights prior to, during, and following stress pretreatment are also reported (panel F). Rats received either acute (AS) or chronic (CS) exposure to 15 footshocks, or identical context exposure with no shock (No Stress; CN). Rats received daily access to a 40% glucose solution in drinking water (G) or drinking water alone (W) for 6 h immediately following the termination of each session of stress pretreatment. All groups were preexposed (30 min/day) to a novel environment for 6 consecutive days. Twenty-four hours after the termination of preexposure, all groups received a single footshock in the preexposed context. All groups were tested for contextual fear learning 24 h later. All groups that received footshock during stress pretreatment readily reached asymptotic contextual fear conditioning (panels B & C). Groups that received chronic or acute stress showed greater generalized fear compared to the unstressed group (panel D). However, chronically-stressed rats that received post-stress glucose (CSG) exhibited markedly higher levels of generalized fear when compared to those that received water (CSW; panel D). An identical trend was observed during the SELF context test (panel E). CS groups showed weight loss during stress pretreatment that was not blocked by glucose while AS groups showed weight loss following stress pretreatment that was blocked by glucose (panel F). Error bars denote mean ± SEM. *, **** denotes significance (p ≤ .05, p ≤ .0001) compared between indicated groups (horizontal square brackets), compared between each Stress group and CNW (horizontal line), or compared between a specific Stress group and CNW (free-standing). # denotes significance (p ≤ .05) between CSG and CSW (horizontal line).
Chronic-stress rats again showed normal fear acquisition during stress (Fig. 5C; mixed-design ANOVA – Group (Chronicity-Glucose) x Trial: F(28,405) = 18.225, p ≤ .0001, Tukey post-hoc comparisons – CSG-Trials 3–15 > CNW-Trials 3–15: p ≤ .05, CSW-Trials 3–15 > CNW-Trials 3–15: p ≤ .05). Notably, there was no observable difference in fear acquisition during stress between acute-stress rats that went on to receive glucose treatment and those that went on to receive water treatment (Fig. 5B). Also, glucose treatment in between stress pretreatment trials did not impact fear acquisition in the chronic-stress rats (Fig. 5C). Despite a lack of observable effect during fear learning, glucose treatment enhanced generalized stressor-context fear in chronic-stress, but not acute-stress, rats, and this generalized fear took longer to extinguish during the SEFL-test-context pre-exposure sessions (Fig. 5D; mixed-design ANOVA – Group (Chronicity-Glucose-Stress) x Trial: F(20,270) = 8.993, p ≤ .0001, Tukey post-hoc comparisons – ASG-Trials 1–2 > CNW-Trials 1–2: p ≤ .05, ASW-Trials 1–2 > CNW-Trials 1–2: p ≤ .05, CSW-Trials 1–2 > CNW-Trials 1–2: p ≤ .05, CSG-Trials 1–3 > CNW-Trials 1–3: p ≤ .05, CSG-Trials 1–3 > CSW-Trials 1–3: p ≤ .05). Glucose treatment was also found to enhance the SEFL effect in chronic-stress, but not acute-stress rats (Fig. 5E; one-way ANOVA – Group (Chronicity-Glucose-Stress): F(4,45) = 14.055, p ≤ .0001, Tukey post-hoc comparisons – CSG > CSW: p ≤ .05), and to block the weight loss following acute stress, but not during chronic stress (Fig. 5F; one-way ANOVA – Group (Chronicity-Glucose-Stress): F(4,45) = 15.61, p ≤ .0001, Tukey post-hoc comparisons – CSW-Stress < CNW-Stress: p ≤ .05, CSG-Stress < CNW-Stress: p ≤ .05, ASW-Post-Stress < CNW-Post-Stress: p ≤ .0001). The fact that glucose, a known enhancer of associative learning (Korol and Gold, 1998; Canal et al., 2005), enhances SEFL in chronic-stress, but not acute-stress, rats may support the conclusion that additional associative processes are engaged by the chronic form of SEFL.
4. Discussion
The experiments described above point to some similarities between the SEFL effect produced by acute stress and that produced by chronic stress. Firstly, like acute SEFL, chronic SEFL is similar for both sexes (Fig. 2G). Secondly, the fact that chronic stress produces a SEFL effect even when a different US (noise as opposed to shock) is used for subsequent fear learning, and that this effect is comparable in magnitude to the effect observed following acute stress (Fig. 4E) suggests acute and chronic stress both engage similar non-associative mechanisms. The experiments described above also provide evidence that differences in chronicity of stress exposure can lead to substantive differences in the consequent phenotype. Specifically, chronic stress produces deficits in stressor context fear extinction and further enhances subsequent fear learning above and beyond the enhancement caused by acute stress, and it does so by engaging an additional associative mechanism. The additional associative component of SEFL, engaged by chronic stress, is eliminated by extinction of stressor-context fear (Fig. 3G). Therefore, we suggest that the most parsimonious explanation for the additional component of SEFL evoked by chronic stress is that re-encountering the US, in the form of the single shock during contextual fear learning, reinstates generalized fear in the chronic-, but not acute-stress group. In favor of this explanation, following the SEFL context test, chronic-, but not acute-, stress rats (Fig. 3H) appeared to display generalized fear within a new context despite generalized fear having been previously extinguished. How exactly fear extinction within the original stressor context may block reinstatement of generalized fear remains to be further elucidated. In addition to exploring this question, future studies should also examine why fear extinction training is more effective than generalized fear extinction training at blocking reinstatement of generalized fear. While several questions remain to be addressed, it is noteworthy that, unlike the effects of fear extinction on fear itself, which tend to be specific (Vervliet et al., 2006; Pappens et al., 2015; Bouton and Todd, 2014; Bouton, 2002, 2004), the effects of fear extinction on fear reinstatement would appear to be more generalized. What is clear is that, comparable baseline levels of generalized fear prior to the SEFL single shock exposure between all groups in the extinction experiment (Fig. 3F) demonstrates that augmented SEFL following chronic stress is not simply the result of new fear compounding with preexisting augmented levels of unextinguished fear. Our findings mirror those of others describing an associative, extinction-sensitive component of SEFL acting on generalized stressor-context fear versus a non-associative, extinction-resistant component (Hassien et al., 2020). It is also noteworthy that complete extinction of stressor context fear had no effect on SEFL within the acute-day-1 stress group (data not shown), which, unlike the acute-day-15 stress group, displayed comparable levels of generalized stressor context fear as the chronic-stress group (Fig. 2D). This suggests that the additional component of SEFL following chronic, but not acute stress, arises from a mechanism that acts on levels of generalized stressor context fear following, but not prior to, reinstatement.
Splitting the acute-stress rats into two subgroups in our first experiment, those with a 15-day latency to the generalization test (acute-day-1) and those with a 1-day latency (acute-day-15), allowed us to conclude that the higher levels of generalized fear in chronic-stress as compared to acute-day-15-stress, but not as compared to acute-day-1-stress rats (Fig. 2D), are not due to differences in the chronicity of the stress but rather differences in the time between stress onset and the generalization test. This is consistent with the finding that systems memory consolidation not only causes contextual fear memories to become hippocampal independent, it also causes them to become more generalized (Wiltgen et al., 2010).
We were surprised to find glucose treatment enhanced generalized fear following chronic, but not acute, stress (Fig. 5D) because a previous study of ours failed to find any effect of glucose on contextual fear generalization in rats (Luyten et al., 2016), and, if anything, glucose is hypothesized to reduce fear memory generalization (Conoscenti et al., 2017; Minor and Saade, 1997; Glenn et al., 2014). However, it is important to note that, without a characterization of the generalization gradient (the curve describing the degree to which the elicitation of a conditioned response generalizes to a stimulus as a function of one or more dimensions of the CS) for the stressor context, one cannot distinguish between differences in generalized fear expression due to differences in the generality of the fear memory versus differences in the magnitude of the fear memory. It is possible that the latter mechanism is at play in this case as the fear acquisition curves of both glucose- and water-treated chronic-stress rats are both at ceiling. Based on the current findings, it is not possible to say whether this is indeed the case, but this interpretation could reconcile our present with previous findings (Luyten et al., 2016).
If our data proves translationally relevant, there are a couple additional points to note. Stressor context fear extinction deficits following chronic, as compared to acute stress (Fig. 3D), imply that more exposure therapy sessions may be required in order to successfully extinguish fear of the traumatic stressor context in PTSD patients following chronic traumatic stress as compared to those that experienced acute traumatic stress. Our finding that the chronic SEFL effect is larger than the acute SEFL effect supports clinical literature indicating that symptoms are more severe in patients with complex PTSD (CPTSD — PTSD following chronic traumatic stress) as compared to patients with PTSD (Hoeboer et al., 2021). Similarly, the fact that the extinction-resistant component of chronic SEFL is similar in magnitude to extinction-resistant acute SEFL (Fig. 3G) supports the clinical finding that traumatic stress chronicity is not predictive of prolonged exposure therapy outcomes (Hoeboer et al., 2021). Also, our data suggests that chronic, as compared to acute, stress may not lead to worse outcomes in terms of severity of anxiety (Fig. 4F) following a traumatic experience. Together, these findings highlight the importance of carrying out properly designed studies on stress chronicity as they will likely inform clinical treatment of PTSD.
One might argue that in the auditory fear SEFL experiment (Fig. 3H/I), potentiation of SEFL due to potentiation of the non-associative component of standard SEFL could be confounded with potentiation of SEFL due to reinstatement of generalized context fear, and, therefore, this experiment is not well suited towards teasing out the associative versus non-associative nature of the additional component of SEFL following chronic stress. However, auditory fear conditioning is well known to overshadow contextual fear conditioning (Tomie, 1976; Odling-Smee, 1975a, 1975b, 1978; Iberico et al., 2008; Esmoris-Arranz et al., 2008; Brasser and Spear, 2004) and should do so with minimal trials of learning in cases where the context but not the auditory cue has been previous explicitly unpaired with the US. Therefore, it would be quite surprising for reinstatement of generalized contextual fear to occur within the context of this experimental design. Having said this, the point is moot, considering SEFL was occluded in the case of auditory fear learning by high levels of fear learning in chronic-stress control rats (Fig. 3I). It is worth mention, though, that a likely explanation for this occlusion is that, unlike the acute-stress rats, who only experienced transportation and handling within the fear conditioning room on day 1 or day 15, the chronic-stress rats were transported to and handled within this room on each of days 1–15. Therefore, we hypothesize that chronic-stressed rats, including chronic-stress-control rats, were more habituated to the stimuli surrounding the stressor context, and, hence, the relative salience of the tone during subsequent auditory fear learning in the SEFL procedure was much higher, driving higher levels of fear learning that occluded the SEFL effect.
The apparent effects of our chronic stress procedure on associative learning are in line with the previously described hypothesis: spaced training will lead to greater associative learning when compared to massed training. However, it is important to recognize that the massed versus spaced rule is a descriptive law that belies multiple mechanisms that converge on a similar pattern. The enhanced impact of spaced training has been demonstrated for virtually every type of conditioning, skill learning, and cognitive phenomenon, and the effects range across a vast temporal space (Ebbinghaus, 1885; Kientzle, 1946; Underwood, 1952). In terms of biological mechanism, there is evidence that maximization of CREB activity (Tully et al., 1994; Silva et al., 1998; Liu et al., 2008), maximization of MAPK activity (Philips et al., 2007, 2013; Pagani et al., 2009; Seese et al., 2014), PKA/PKC crosstalk (Farah et al., 2009), and synaptic priming (Kramar et al., 2012) underlie the beneficial effects of spacing on long-term potentiation and learning, but these mechanisms act in very different time domains (minutes versus hours). Thus, while the spaced versus massed idea provided a rationale for our hypothesis, it does not provide a mechanistic explanation, which will require further direct investigation.
In conclusion, here we demonstrate that chronic stress engages an additional set of mechanisms, beyond those engaged by acute stress, that lead to further potentiation of subsequent fear learning. We provide evidence that these additional mechanisms are associative in nature and likely involve reinstatement of previously extinguished generalized fear of the stressor context.
Funding
This research was supported by National Institute of Mental Health R01-MH115678 (MSF) and the Staglin Center for Brain and Behavioral Health (MSF).
CRediT authorship contribution statement
Michael A. Conoscenti: Writing – review & editing, Writing – original draft, Investigation, Formal analysis. Daniel B. Weatherill: Writing – review & editing, Writing – original draft, Investigation, Formal analysis. Yuqing Huang: Writing – review & editing, Investigation. Raphael Tordjman: Writing – review & editing, Investigation. Michael S. Fanselow: Writing – review & editing, Supervision, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Nitish Patel for his contribution to this work.
Handling Editor: Rita Valentino
Contributor Information
Michael A. Conoscenti, Email: michael.conoscenti@neuro.utah.edu.
Daniel B. Weatherill, Email: dweatherill@mednet.ucla.edu.
Yuqing Huang, Email: fionahuangyq@g.ucla.edu.
Raphael Tordjman, Email: rtordjma@fiu.edu.
Michael S. Fanselow, Email: fanselow@psych.ucla.edu.
References
- Bouton M.E. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol. Psychiatr. 2002;52(10):976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton M.E. Context and behavioral processes in extinction. Learn. Mem. 2004;11(5):485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton M.E., Bolles R.C. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J. Exp. Psychol. Anim. Behav. Process. 1979;5(4):368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Bouton M.E., Todd T.P. A fundamental role for context in instrumental learning and extinction. Behav. Process. 2014;104:13–19. doi: 10.1016/j.beproc.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasser S.M., Spear N.E. Contextual conditioning in infants, but not older animals, is facilitated by CS conditioning. Neurobiology of learning and memory. Janus. 2004;81(1):46–59. doi: 10.1016/s1074-7427(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Bruinsma C.F., Savelberg S.M., Kool M.J., et al. An essential role for UBE2A/HR6A in learning and memory and mGLUR-dependent long-term depression. Hum. Mol. Genet. 2016;25(1):1–8. doi: 10.1093/hmg/ddv436. [DOI] [PubMed] [Google Scholar]
- Cameron G., Schlund M.W., Dymond S. Generalization of socially transmitted and instructed avoidance. Front. Behav. Neurosci. 2015;9:159. doi: 10.3389/fnbeh.2015.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal C.E., Stutz S.J., Gold P.E. Glucose injections into the dorsal hippocampus or dorsolateral striatum of rats prior to T-maze training: modulation of learning rates and strategy selection. Learn. Mem. 2005;12(4):367–374. doi: 10.1101/lm.88205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew T.J., Pinsker H.M., Kandel E.R. Long-term habituation of a defensive withdrawal reflex in aplysia. Science. 1972;175(4020):451–454. doi: 10.1126/science.175.4020.451. [DOI] [PubMed] [Google Scholar]
- Chappell P.B., Smith M.A., Kilts C.D., et al. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J. Neurosci. 1986;6(10):2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins S., Cunningham L., Harvey D., Walsh D. Massed but not spaced training impairs spatial memory. Behav. Brain Res. 2003;139(1–2):215–223. doi: 10.1016/s0166-4328(02)00270-x. [DOI] [PubMed] [Google Scholar]
- Conoscenti M.A., Hart E.E., Smith N.J., Minor T.R. Temporal parameters of post-stress prophylactic glucose treatment in rats. Stress. 2017;20(3):265–276. doi: 10.1080/10253890.2017.1334052. [DOI] [PubMed] [Google Scholar]
- Dash P.K., Orsi S.A., Moore A.N. Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-Mammalian target of rapamycin pathway. J. Neurosci. 2006;26(31):8048–8056. doi: 10.1523/JNEUROSCI.0671-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Effects of interstimulus interval length and variability on startle-response habituation in the rat. J. Comp. Physiol. Psychol. 1970;72(2):177–192. doi: 10.1037/h0029472. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus H. Dover; 1885. Memory: A Contribution to Experimental Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmoris-Arranz F.J., Mendez C., Spear N.E. Contextual fear conditioning differs for infant, adolescent, and adult rats. Behav. Process. 2008;78(3):340–350. doi: 10.1016/j.beproc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah C.A., Weatherill D., Dunn T.W., Sossin W.S. PKC differentially translocates during spaced and massed training in Aplysia. J. Neurosci. 2009;29(33):10281–10286. doi: 10.1523/JNEUROSCI.1533-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A.J., Chen C., Scullen T., et al. Sensitization of the hypothalamic-pituitary-adrenal Axis in a male rat chronic stress model. Endocrinology. 2016;157(6):2346–2355. doi: 10.1210/en.2015-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S., Packard A.E.B., Mahbod P., et al. Disruption of glucagon-like peptide 1 signaling in Sim1 neurons reduces physiological and behavioral reactivity to acute and chronic stress. J. Neurosci. 2017;37(1):184–193. doi: 10.1523/JNEUROSCI.1104-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn D.E., Minor T.R., Vervliet B., Craske M.G. The effect of glucose on hippocampal-dependent contextual fear conditioning. Biol. Psychiatr. 2014;75(11):847–854. doi: 10.1016/j.biopsych.2013.09.022. [DOI] [PubMed] [Google Scholar]
- Goodrick C.L. Maze learning of mature-young and aged rats as a function of distribution of practice. J. Exp. Psychol. 1973;98(2):344–349. doi: 10.1037/h0034421. [DOI] [PubMed] [Google Scholar]
- Grissom N., Bhatnagar S. Habituation to repeated stress: get used to it. Neurobiol. Learn. Mem. 2009;92(2):215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassien A.M., Shue F., Bernier B.E., Drew M.R. A mouse model of stress-enhanced fear learning demonstrates extinction-sensitive and extinction-resistant effects of footshock stress. Behav. Brain Res. 2020;379 doi: 10.1016/j.bbr.2019.112391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde R.A. Factors governing the changes in strength of a partially inborn response, as shown by the mobbing behaviour of the chaffinch (Fringilla coelebs). II. The waning of the response. Proc. R. Soc. Lond. B Biol. Sci. 1954;142(908):331–358. doi: 10.1098/rspb.1954.0029. [DOI] [PubMed] [Google Scholar]
- Hoeboer C.M., de Kleine R.A., Oprel D.A.C., Schoorl M., van der Does W., van Minnen A. Does complex PTSD predict or moderate treatment outcomes of three variants of exposure therapy? J. Anxiety Disord. 2021;80 doi: 10.1016/j.janxdis.2021.102388. [DOI] [PubMed] [Google Scholar]
- Iberico C., Vansteenwegen D., Vervliet B., Dirikx T., Marescau V., Hermans D. The development of cued versus contextual conditioning in a predictable and an unpredictable human fear conditioning preparation. Acta Psychol. 2008;127(3):593–600. doi: 10.1016/j.actpsy.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Katz R.J., Roth K.A., Carroll B.J. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev. Summer. 1981;5(2):247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- Kientzle M.J. Properties of learning curve under varied distributions of practice. J. Exp. Psychol. 1946;36:187–211. doi: 10.1037/h0061164. [DOI] [PubMed] [Google Scholar]
- Korol D.L., Gold P.E. Glucose, memory, and aging. Am. J. Clin. Nutr. 1998;67(4):764S–771S. doi: 10.1093/ajcn/67.4.764S. [DOI] [PubMed] [Google Scholar]
- Kramar E.A., Babayan A.H., Gavin C.F., et al. Synaptic evidence for the efficacy of spaced learning. Proc. Natl. Acad. Sci. U.S.A. 2012;109(13):5121–5126. doi: 10.1073/pnas.1120700109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.Y., Fioravante D., Shah S., Byrne J.H. cAMP response element-binding protein 1 feedback loop is necessary for consolidation of long-term synaptic facilitation in Aplysia. J. Neurosci. 2008;28(8):1970–1976. doi: 10.1523/JNEUROSCI.3848-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long V.A., Fanselow M.S. Stress-enhanced fear learning in rats is resistant to the effects of immediate massed extinction. Stress. 2012;15(6):627–636. doi: 10.3109/10253890.2011.650251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L., Schroyens N., Luyck K., Fanselow M.S., Beckers T. No effect of glucose administration in a novel contextual fear generalization protocol in rats. Transl. Psychiatry. 2016;6(9) doi: 10.1038/tp.2016.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning C.A., Stone W.S., Korol D.L., Gold P.E. Glucose enhancement of 24-h memory retrieval in healthy elderly humans. Behav. Brain Res. 1998;93(1–2):71–76. doi: 10.1016/s0166-4328(97)00136-8. [DOI] [PubMed] [Google Scholar]
- Masini C.V., Day H.E., Campeau S. Long-term habituation to repeated loud noise is impaired by relatively short interstressor intervals in rats. Behav. Neurosci. 2008;122(1):210–223. doi: 10.1037/0735-7044.122.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R., Manz G., Menzel R., Greggers U. Massed and spaced learning in honeybees: the role of CS, US, the intertrial interval, and the test interval. Learn. Mem. 2001;8(4):198–208. doi: 10.1101/lm.40001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor T.R., Saade S. Poststress glucose mitigates behavioral impairment in rats in the "learned helplessness" model of psychopathology. Biol. Psychiatr. 1997;42(5):324–334. doi: 10.1016/S0006-3223(96)00467-2. [DOI] [PubMed] [Google Scholar]
- Odling-Smee F.J. Background stimuli and the inter-stimulus interval during Pavlovian conditioning. Q. J. Exp. Psychol. 1975;27(3):387–392. doi: 10.1080/14640747508400498. [DOI] [PubMed] [Google Scholar]
- Odling-Smee F.J. The role of background stimuli during Pavlovian conditioning. Q. J. Exp. Psychol. 1975;27(2):201–209. doi: 10.1080/14640747508400480. [DOI] [PubMed] [Google Scholar]
- Odling-Smee F.J. The overshadowing of background stimuli: some effects of varying amounts of training and UCS intensity. Q. J. Exp. Psychol. 1978;30(4):737–746. doi: 10.1080/14640747808400698. [DOI] [PubMed] [Google Scholar]
- Pagani M.R., Oishi K., Gelb B.D., Zhong Y. The phosphatase SHP2 regulates the spacing effect for long-term memory induction. Cell. 2009;139(1):186–198. doi: 10.1016/j.cell.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappens M., Schroijen M., Van den Bergh O., Van Diest I. Retention of perceptual generalization of fear extinction. Int. J. Psychophysiol. 2015;98(3 Pt 2):520–528. doi: 10.1016/j.ijpsycho.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Pavlov I.P. Oxford University Press; 1927. Conditioned Reflexes: an Investigation of the Physiological Activity of the Cerebral Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington Z.T., Trott J.M., Rajbhandari A.K., et al. Chronic opioid pretreatment potentiates the sensitization of fear learning by trauma. Neuropsychopharmacology. 2020;45(3):482–490. doi: 10.1038/s41386-019-0559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perusini J.N., Meyer E.M., Long V.A., et al. Induction and expression of fear sensitization caused by acute traumatic stress. Neuropsychopharmacology. 2016;41(1):45–57. doi: 10.1038/npp.2015.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips G.T., Tzvetkova E.I., Carew T.J. Transient mitogen-activated protein kinase activation is confined to a narrow temporal window required for the induction of two-trial long-term memory in Aplysia. J. Neurosci. 2007;27(50):13701–13705. doi: 10.1523/JNEUROSCI.4262-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips G.T., Ye X., Kopec A.M., Carew T.J. MAPK establishes a molecular context that defines effective training patterns for long-term memory formation. J. Neurosci. 2013;33(17):7565–7573. doi: 10.1523/JNEUROSCI.5561-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb T.N., Cullen P.K., Minor T.R. Parameters of hormetic stress and resilience to trauma in rats. Stress. 2015;18(1):88–95. doi: 10.3109/10253890.2014.974154. [DOI] [PubMed] [Google Scholar]
- Poulos A.M., Reger M., Mehta N., et al. Amnesia for early life stress does not preclude the adult development of posttraumatic stress disorder symptoms in rats. Biol. Psychiatr. 2014;76(4):306–314. doi: 10.1016/j.biopsych.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos A.M., Zhuravka I., Long V., Gannam C., Fanselow M. Sensitization of fear learning to mild unconditional stimuli in male and female rats. Behav. Neurosci. 2015;129(1):62–67. doi: 10.1037/bne0000033. [DOI] [PubMed] [Google Scholar]
- Rau V., Fanselow M.S. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress. 2009;12(2):125–133. doi: 10.1080/10253890802137320. [DOI] [PubMed] [Google Scholar]
- Rau V., DeCola J.P., Fanselow M.S. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2005;29(8):1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Rescorla R.A., Heth C.D. Reinstatement of fear to an extinguished conditioned stimulus. J. Exp. Psychol. Anim. Behav. Process. 1975;1(1):88–96. [PubMed] [Google Scholar]
- Rose J.K., Rankin C.H. Analyses of habituation in Caenorhabditis elegans. Learn. Mem. 2001;8(2):63–69. doi: 10.1101/lm.37801. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. Glucocorticoid toxicity in the hippocampus: reversal by supplementation with brain fuels. J. Neurosci. 1986;6(8):2240–2244. doi: 10.1523/JNEUROSCI.06-08-02240.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seese R.R., Wang K., Yao Y.Q., Lynch G., Gall C.M. Spaced training rescues memory and ERK1/2 signaling in fragile X syndrome model mice. Proc. Natl. Acad. Sci. U.S.A. 2014;111(47):16907–16912. doi: 10.1073/pnas.1413335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A.J., Kogan J.H., Frankland P.W., Kida S. CREB and memory. Annu. Rev. Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Sutton M.A., Ide J., Masters S.E., Carew T.J. Interaction between amount and pattern of training in the induction of intermediate- and long-term memory for sensitization in aplysia. Learn. Mem. 2002;9(1):29–40. doi: 10.1101/lm.44802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A. Retardation of autoshaping: control by contextual stimuli. Science. 1976;192(4245):1244–1246. doi: 10.1126/science.192.4245.1244. [DOI] [PubMed] [Google Scholar]
- Tully T., Preat T., Boynton S.C., Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79(1):35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Underwood B.J. Studies of distributed practice. VI. The influence of rest-interval activity in serial learning. J. Exp. Psychol. 1952;43(5):329–340. doi: 10.1037/h0057936. [DOI] [PubMed] [Google Scholar]
- Vervliet B., Vansteenwegen D., Eelen P. Generalization gradients for acquisition and extinction in human contingency learning. Exp. Psychol. 2006;53(2):132–142. doi: 10.1027/1618-3169.53.2.132. [DOI] [PubMed] [Google Scholar]
- Wiltgen B.J., Zhou M., Cai Y., et al. The hippocampus plays a selective role in the retrieval of detailed contextual memories. Curr. Biol. 2010;20(15):1336–1344. doi: 10.1016/j.cub.2010.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]