Abstract
Background
Intermittent urethral self‐dilatation is sometimes recommended to reduce the risk of recurrent urethral stricture. There is no consensus as to whether it is a clinically effective or cost‐effective intervention in the management of this disease.
Objectives
The purpose of this review is to evaluate the clinical effectiveness and cost‐effectiveness of intermittent self‐dilatation after urethral stricture surgery in males compared to no intervention. We also compared different programmes of, and devices for, intermittent self‐dilatation. .
Search methods
We searched the Cochrane Incontinence Group Specialised Register (searched 7 May 2014), CENTRAL (2014, Issue 4), MEDLINE (1 January 1946 to Week 3April 2014), PREMEDLINE (covering 29 April 2014), EMBASE (1 January 1947 to Week 17 2014), CINAHL (31 December 1981 to 30 April 2014) OpenGrey (searched 6 May 2014), ClinicalTrials.gov (6 May 2014), WHO International Clinical Trials Registry Platform (6 May 2014), Current Controlled Trials (6 May 2014) and the reference lists of relevant articles.
Selection criteria
Randomised and quasi‐randomised trials where one arm was a programme of intermittent self‐dilatation for urethral stricture were identified. Studies were excluded if they were not randomised or quasi‐randomised trials, or if they pertained to clean intermittent self‐catheterisation for bladder emptying.
Data collection and analysis
Two authors screened the records for relevance and methodological quality. Data extraction was performed according to predetermined criteria using data extraction forms. Analyses were carried out in Cochrane Review Manager (RevMan 5). The primary outcomes were patient‐reported symptoms and health‐related quality of life, and risk of recurrence; secondary outcomes were adverse events, acceptability of the intervention to patients and cost‐effectiveness. Quality of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results
Eleven trials were selected for inclusion in the review, including a total of 776 men. They were generally small; all were of poor quality and all were deemed to have high risk of bias.
Performing intermittent self‐dilatationversus not performing intermittent self‐dilatation
The data from six trials were heterogeneous, imprecise and had a high risk of bias, but indicated that recurrent urethral stricture was less likely in men who performed intermittent self‐dilatation than men who did not perform intermittent self‐dilatation (RR 0.70, 95% CI 0.48 to 1.00; very low quality evidence). Adverse events were generally poorly reported: two trials did not report adverse events and two trials reported adverse events only for the intervention group. Meta‐analysis of the remaining two trials found no evidence of a difference between performing intermittent self‐dilatation and not performing it (RR 0.60, 95% CI 0.11 to 3.26). No trials formally assessed acceptability, and no trials reported on patient‐reported lower urinary tract symptoms, patient‐reported health‐related quality of life, or cost‐effectiveness.
One programme of intermittent self‐dilatationversus another
We identified two trials that compared different durations of intermittent self‐dilatation, but data were not combined. One study could not draw robust conclusions owing to cross‐over, protocol deviation, administrative error, post‐hoc analysis and incomplete outcome reporting. The other study found no evidence of a difference between intermittent self‐dilatation for six months versus for 12 months after optical urethrotomy (RR 0.67, 95% CI 0.12 to 3.64), although again the evidence is limited by the small sample size and risk of bias in the included study. Adverse events were reported narratively and were not stratified by group. No trials formally assessed acceptability, and no trials reported on patient‐reported lower urinary tract symptoms, patient‐reported health‐related quality of life, or cost‐effectiveness.
One device for performing intermittent self‐dilatationversus another
Three trials compared one device for performing intermittent self‐dilatation with another. Results from one trial at a high risk of bias were too uncertain to determine the effects of a low friction hydrophilic catheter and a standard polyvinyl chloride catheter on the risk of recurrent urethral stricture (RR 0.32, 95% CI 0.07 to 1.40). Similarly one study did not find evidence of a difference between one percent triamcinolone gel for lubricating the intermittent self‐dilatation catheter versus water‐based gel on risk of recurrent urethral stricture (RR 0.68, 95% CI 0.35 to 1.32). Two trials reported adverse events, but one did not provide sufficient detail for analysis. The other small study reported fewer instances of prostatitis, urethral bleeding or bacteriuria with a low friction hydrophilic catheter compared with a standard polyvinyl chloride catheter (RR 0.13, 95% CI 0.02 to 0.98). ‘Happiness with the intervention’ was assessed using a non‐validated scale in one study, but no trials formally assessed patient‐reported health‐related quality of life or acceptability. No trials reported on patient‐reported lower urinary tract symptoms or cost‐effectiveness.
GRADE quality assessment
The evidence that intermittent self‐dilatation reduces the risk of recurrent urethral stricture after surgical intervention was downgraded to 'very low' on the basis that the studies comprising the meta‐analysis were deemed to have high risk of bias, and the data was imprecise and inconsistent.
Insufficient evidence
No trials provided cost‐effectiveness data or used a validated patient‐reported outcome measure, and adverse events were not reported rigorously. Acceptability of the intervention to patients has not been assessed quantitatively or qualitatively.
Authors' conclusions
Performing intermittent self‐dilatation may confer a reduced risk of recurrent urethral stricture after endoscopic treatment. We have very little confidence in the estimate of the effect owing to the very low quality of the evidence. Evidence for other comparisons and outcomes is limited. Further research is required to determine whether the apparent benefit is sufficient to make the intervention worthwhile, and in whom.
Keywords: Humans, Male, Dilatation, Dilatation/adverse effects, Dilatation/instrumentation, Dilatation/methods, Patient Satisfaction, Postoperative Complications, Postoperative Complications/prevention & control, Randomized Controlled Trials as Topic, Recurrence, Self Care, Self Care/adverse effects, Self Care/instrumentation, Self Care/methods, Urethral Stricture, Urethral Stricture/prevention & control, Urethral Stricture/therapy
Plain language summary
Asking men to pass a catheter into their own urethra to try to stop a urethral stricture from coming back
BACKGROUND
About one in 300 men are affected by a condition called urethral stricture where part of the urethra scars causing it to become narrow. Most urethral strictures are caused by injury or infection. The main symptom is difficulty passing urine. In at least half of patients, urethral strictures come back within two years after they have a surgical operation called optical urethrotomy to stretch their urethral stricture. For this reason there is considerable interest in finding ways to reduce the chance of a urethral stricture coming back.
Intermittent self‐dilatation is a treatment designed to stop urethral strictures returning. The man passes a thin, usually disposable, catheter tube into the urethra himself at regular intervals to try to keep the scarred area from narrowing down again. It is thought to work by preventing the cut edges of a stricture from sticking together, but there are some risks including infection and injury to the urethra.
We do not know whether intermittent self‐dilatation is a good treatment for urethral stricture.
STUDY CHARACTERISTICS
We found 11 trials involving a total of 776 men across eight countries for this review.
KEY RESULTS
A combination of results from six trials involving a total of 404 participants indicated that men with urethral stricture who perform intermittent self‐dilatation may have less chance of their urethral stricture coming back than men with a urethral stricture who do not perform intermittent self‐dilatation. We can not be confident about this finding, however, because the quality of the evidence was very low.
There were no trials that looked at whether intermittent self‐dilatation is a cost‐effective health care intervention and there were no trials that used reliable health questionnaires to find out whether intermittent self‐dilatation reduces men's urinary symptoms or improves their overall well being.
On the whole the trials did not report side effects in a way that was useful for estimating the risks of performing intermittent self‐dilatation.
We do not know yet whether certain types of catheter are better than others for performing intermittent self‐dilatation. It is also unclear how often or for how long men should perform intermittent self‐dilatation to give themselves the best chance of staying free from urethral strictures.
QUALITY OF THE EVIDENCE
The trials in this review were generally small and poorly designed or poorly explained. All of the trials were conducted in a way which meant they had a high chance of generating an answer that does not represent the truth.
Summary of findings
Summary of findings for the main comparison. Intermittent self‐dilatation compared to no treatment for males after urethral stricture surgery.
| Intermittent self‐dilatation compared to no treatment for males after urethral stricture surgery | ||||||
| Population: males after urethral stricture surgery Intervention: intermittent self‐dilatation Comparison: no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Intermittent self‐dilatation | |||||
| Recurrent urethral stricture Follow‐up: 8‐24 months | 618 per 1000 | 433 per 1000 (297 to 618) | RR 0.7 (0.48 to 1) | 404 (6 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by two for risk of bias: all six trials comprising the quantitative synthesis were judged high risk of bias in two or more domains. 2 Downgraded by two for inconsistency: the point estimates of the effect size vary widely; the statistical test for heterogeneity is highly significant (P = 0.003), and the I2 is large (72%). 3 Downgraded by two for imprecision: the total number of events was less than 300 and the 95% confidence interval of the effect size is 0.48 to 1.00 (> 50% and includes the line of no effect).
Background
Urethral stricture is the most common cause of difficulty passing urine in young and middle aged men. The prevalence varies worldwide but indicative numbers from North America are 200 per 100,000 for men in their 20s rising to 900 per 100,000 for men in their 70s (Santucci 2007). In the National Health Service in the United Kingdom, urethral strictures account for approximately 16,000 hospital admissions and 12,000 operations annually, at a cost of GBP 10M (Mundy 2010). Strictures have a tendency to recur after treatment, and the concept of intermittent self‐dilatation was popularised in the 1980s as a means of reducing the risk of recurrence (Lawrence 1988).
Description of the condition
A urethral stricture is a scar of the spongy erectile tissue that surrounds the anterior urethra. Gradual contraction of this scar constricts the urethral lumen leading to progressive lower urinary tract symptoms (LUTS), the hallmark of this condition. Any process that injures the urethra can cause a urethral stricture. In developed countries one in two strictures are iatrogenic (following catheterisation or endoscopic prostate surgery, for example) and in one in three cases no cause can be identified (Lumen 2009). The pattern of aetiology is different in developing countries where sexually transmitted infection and pelvic trauma are more likely to be responsible (Ahmed 1998).
For men who present for the first time with a urethral stricture the standard treatment is an operation called endoscopic urethrotomy. A cold blade mounted on an endoscope is passed into the urethra and the stricture is incised longitudinally through to the healthy tissue underneath. This incision allows the narrow section to expand, returning the urethra to an adequate diameter. The alternatives to endoscopic urethrotomy are simple blind dilatation, where the stricture is stretched with a set of lubricated dilators or sounds; and urethroplasty, where the diseased part of the urethra is exposed through a cut in the skin behind the scrotum and then reconstructed.
Depending on the site and length of the stricture, men undergoing their first endoscopic urethrotomy have somewhere between a 25% and 89% chance of their stricture recurring (Lauritzen 2009). Some men perform intermittent self‐dilatation after an operation with the aim of delaying the onset of symptoms and recurrence.
Description of the intervention
Intermittent self‐dilatation is a treatment for urethral stricture where the patient passes a catheter tube or rod‐shaped device into their urethra at regular intervals to prevent the stricture from coming back.
The intermittent self‐dilatation device can be the same type of sterile disposable catheter used by people who perform intermittent self‐catheterisation to empty their bladder. There are cultural variations and a stainless steel chopstick was found to be safe and cost‐effective in Taiwan (Yu‐Hung Lin 2006). In principle, the device should be clean to minimise the risk of introducing infection, and it should have a low co‐efficient of friction to facilitate atraumatic passage.
It is usual for a healthcare professional to first teach the patient how to pass the intermittent self‐dilatation device safely. Once comfortable with the technique, men are given a programme of dilatation to follow at home. Men are usually advised to dilate more frequently to begin with (daily for example) and to lengthen the interval in a stepwise fashion thereafter. Intermittent self‐dilatation can continue for a fixed period or indefinitely depending on the stricture, the patient and the doctor recommending it as a treatment. There is no general consensus as to which device or programme of intermittent self‐dilatation works best.
How the intervention might work
Performing intermittent self‐dilatation regularly splints the urethra open. It might prevent the cut edges of a stricture from sticking together and contracting after an operation (Lawrence 1988).
Why it is important to do this review
Intermittent self‐dilatation is an invasive procedure with associated cost and morbidity (urinary tract infection for example). We do not know whether performing intermittent self‐dilatation after a urethral stricture operation is better than having a urethral stricture operation and then doing nothing afterwards.
This review focuses on intermittent self‐dilatation; there is another Cochrane review which focuses on dilatation, urethrotomy and urethroplasty (Wong 2012).
Objectives
The purpose of this review is to evaluate the clinical effectiveness and cost‐effectiveness of intermittent self‐dilatation after urethral stricture surgery in the management of urethral stricture disease in males.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled trials in which at least one arm is a programme of intermittent self‐dilatation for urethral stricture. We did not consider studies that evaluated devices for intermittent self‐catheterisation for bladder emptying.
Types of participants
Male patients of all ages with a urethral stricture. The urethral stricture may be at any site, of any length or aetiology. Intermittent self‐dilatation is a treatment that is generally only instituted after an operation to widen a urethral stricture, therefore it was expected that participants would have had at least one surgical intervention for the condition.
Types of interventions
Intermittent self‐dilatation is a programme of repeated urethral self‐dilatation using a rod‐shaped device. All permutations of device, dilation frequency and programme duration were eligible for inclusion.
One arm of any eligible trial had to involve allocation to a programme of intermittent self‐dilatation. The programme of intermittent self‐dilatation normally follows, and could be coupled to, an endoscopic intervention for urethral stricture. Where that was the case, intermittent self‐dilatation was assessed independent of the operation preceding it. Comparison interventions were therefore: no treatment; and the programmes of intermittent self‐dilatation themselves.
The following comparisons were made:
1. intermittent self‐dilatation versus no intervention;
2. one regimen of intermittent self‐dilatation (e.g. catheterisation frequency or duration of programme) versus another;
3. one device to perform intermittent self‐dilatation versus another.
Types of outcome measures
Primary outcomes
Risk of recurrent urethral stricture (measured as length of time to reintervention, or number of men requiring reintervention)
Patient‐reported lower urinary tract symptoms (validated patient‐reported outcome measures (PROMs), symptom and bother scores)
Patient‐reported health‐related quality of life (validated condition‐specific and generic utility measures)
Secondary outcomes
Adverse events
Rates of:
urinary tract infection;
urethral trauma;
hospitalisation.
Acceptability
Rate of withdrawal from the programme of intermittent self‐dilatation.
Cost‐effectiveness
Additional treatment cost;
Incremental cost per quality‐adjusted life year (QALY);
Other health economic outcomes.
Other outcomes
Those not specified but reported in eligible trials were considered.
Quality assessment
Two independent review authors used the Grading of Recommendations Assessment, Development and Education (GRADE) system to rate the quality of evidence (Guyatt 2008). GRADE is a systematic approach to making judgements about quality of evidence and accordingly the strength of recommendations that can be made based on meta‐analyses. It assesses methodological flaws, consistency of results, generalisability of results and how effective the treatment has been shown to be at addressing outcomes that are judged to be of utmost importance to patients. GRADE profiler 3.6.1 was used to create the Summary of Findings table. The outcome we retrospectively selected for GRADE quality assessment was "risk of risk of recurrent urethral stricture".
Search methods for identification of studies
No language or other limitations were imposed on any of the searches described below.
Electronic searches
This review drew on the search strategy developed for the Incontinence Review Group as a whole. Relevant trials were identified from the Group's Specialised Register of controlled trials, which is described under the Incontinence Group's module in The Cochrane Library. The Register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in Process and handsearching of journals and conference proceedings. Date of last search: 7 May 2014.
The terms used to search the Incontinence Group Specialised Register were:
({DESIGN.CCT*} OR {DESIGN.RCT*}) AND ({topic.urine.urethralStricture*})
(Searches of the keyword field of Reference Manager 2012).
Urethral stricture disease lies outside the stated remit of the Cochrane Incontinence Review Group. To ensure inclusion of all relevant trials, the electronic databases MEDLINE, MEDLINE in Process, CENTRAL, EMBASE, CINAHL, OpenGrey, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (ICTRP) and Current Controlled Trials (including the UK National Research Register) were separately interrogated with terms pertaining to urethral stricture disease. MEDLINE and MEDLINE in Process were searched using the Cochrane highly sensitive search strategy (Lefebvre 2011). For details of the search terms used in MEDLINE and MEDLINE in Process please see Appendix 1 and for all other search strategies used in the databases detailed below please see Appendix 2.
The following databases were searched:
CENTRAL (2014, Issue 4), searched on 6 May 2014.
MEDLINE (January 1946 to Week 3 April 2014), searched on 30 April 2014.
MEDLINE In Process (29 April 2014), searched on 30 April 2014.
EMBASE and EMBASE Classic (January 1947 to Week 17 2014), searched on 1 May 2014.
CINAHL (on EBSCOhost) (31 December 1981 to 30 April 2014), searched on 1 May 2014.
OpenGrey, searched on 6 May 2014.
Also the following clinical trials registries/platforms were searched on 6 May 2014:
WHO International Clinical Trials Registry Platform (ICTRP)
Current Controlled Trials (including the UK National Research Register)
ClinicalTrials.gov
Citations and abstracts were examined by two independent review authors and reports of potentially relevant trials were retrieved in full.
Searching other resources
Reference lists of identified trials and review articles were searched to find further relevant trials not identified elsewhere.
Data collection and analysis
Selection of studies
Randomised and quasi‐randomised trials identified from the Specialised Register and electronic searches were screened for eligibility and selected for inclusion by two independent review authors.
Data extraction and management
Data relevant to the pre‐stated outcomes, characteristics of the study, interventions and participants were extracted to data collection forms by two independent review authors.
Studies were excluded from the analyses if they were non‐randomised or quasi‐randomised trials or did not meet other inclusion criteria. We did not consider studies that evaluated devices for intermittent self‐catheterisation for bladder emptying. Reasons for exclusion are stated in Characteristics of excluded studies table.
Assessment of risk of bias in included studies
Two review authors independently assessed methodological quality using the Cochrane Collaboration tool for assessing risk of bias. The quality of the trials is documented under the headings:
Adequate sequence generation
Allocation concealment
Blinding
Incomplete outcome data addressed
Free of selective reporting
Funding/conflict of interest
Studies were not necessarily excluded from the analyses on the basis of methodological quality.
The nature of intermittent self‐dilatation means that study participants cannot be blinded to the intervention.
Measures of treatment effect
Dichotomous data were presented as risk ratios (RR) with 95% confidence intervals (CI).
Dealing with missing data
We intended to seek clarification from the trialists where trial data were collected but were not fully reported, or the reported form was unsuitable for analysis in this review.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar to carry out a clinically meaningful meta‐analysis. The presence of statistical heterogeneity was assessed through visual inspection of forest plots, the χ2 test for heterogeneity (< 10%) and the I2 statistic (> 50%) (Higgins 2003). Reasons for heterogeneity were explored.
Assessment of reporting biases
There were insufficient studies per outcome to identify reporting bias by funnel plot.
Data synthesis
Trial data were handled according to the processes described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Where possible, data for each outcome was aggregated from included studies in a formal meta‐analysis of treatment effect. Analyses were carried out using the RevMan analyses software in Review Manager (RevMan 5). Data that could not be combined quantitatively were assessed qualitatively.
Subgroup analysis and investigation of heterogeneity
The data did not permit the intended subgroup analysis by type of urethral stricture (that is site, length or aetiology) or preceding operation (that is endoscopic urethrotomy or blind dilatation).
A random‐effects model was used because there was evidence of clinical and statistical heterogeneity,
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Eleven trials were selected for inclusion in this review. The studies were conducted in Pakistan, Denmark, UK, Iran, USA, Kenya, Tunisia and Finland. Collectively they included 776 male participants with urethral stricture disease randomised to a programme of intermittent self‐dilatation or no treatment, or a type of device for performing intermittent self‐dilatation, after optical urethrotomy. The number of participants in each study ranged from 49 to 146, aged between 10 and 87 years where stated. No trials evaluated intermittent self‐dilatation after urethral reconstruction.
Results of the search
The systematic literature search yielded 295 records. One further record (Khalid 2007) was identified by searching the reference lists of included studies. Twenty duplicate records were eliminated. Two hundred and sixty three records were excluded based on their title or abstract because they were not relevant to the review or were not randomised trials, including two records that were almost certainly not randomised trials and the full‐text could not be retrieved from the authors or the publishers (Khalid 2007; Suhail 2011). Therefore 13 studies were considered for inclusion in the review (Figure 1).
1.
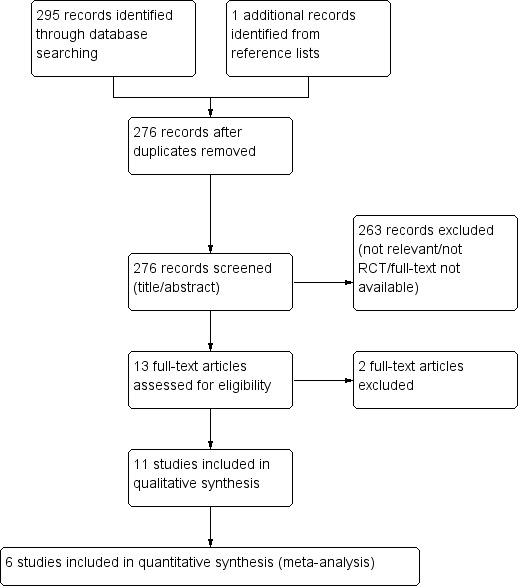
PRISMA study flow diagram
Included studies
The following interventions were included:
Six trials evaluated intermittent self‐dilatation versus no treatment after optical urethrotomy (Afridi 2010; Bodker 1992; Husmann 2006; Khan 2011; Kjaergaard 1994; Matanhelia 1995).
Two trials evaluated one programme of intermittent self‐dilatation versus another (Harriss 1994; Tammela 1993).
One trial evaluated intermittent self‐dilatation versus a programme of outpatient urethral dilatation by a surgeon using sounds (Ngugi 2007).
Two trials evaluated one type of device versus another for intermittent self‐dilatation (Hosseini 2008; Sallami 2011).
Excluded studies
Two potentially eligible trials were excluded:
One trial evaluated intermittent hydraulic self‐dilatation which is per se a form of urethral self‐dilatation but is not intermittent self‐dilatation using a device (Kaisary 1985);
One trial evaluated intermittent outpatient urethral dilatation by a surgeon, which is not intermittent self‐dilatation (Tunc 2002).
Risk of bias in included studies
We classified all 11 included studies as high risk of bias in two or more domains. A graphical display of the risk of bias assessment is presented in Figure 2 and Figure 3. None of the reports included a funding or conflict of interest statement.
2.
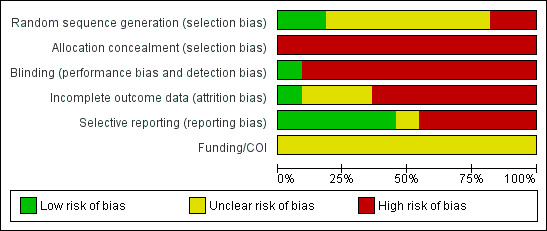
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
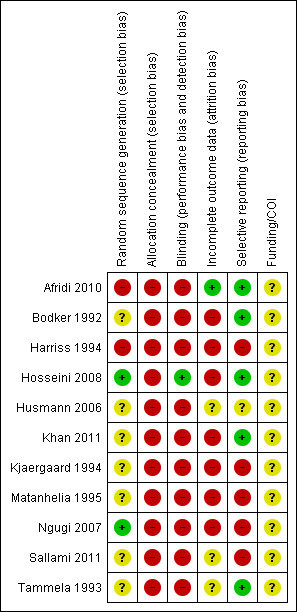
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Adequate sequence generation
Two trials were overtly quasi‐randomised because they used alternation or another predicable method of generating the randomisation sequence: (Afridi 2010; Harriss 1994). Only two trials used an adequate method (Hosseini 2008; Ngugi 2007). The remainder were judged as 'unclear' mostly due to lack of information.
Allocation concealment
Allocation concealment was globally inadequate. We judged there to be high risk of bias in this domain in all 11 trials.
Blinding
Blinding of participants is probably not possible in trials evaluating intermittent self‐dilatation versus not performing intermittent self‐dilatation owing to the self‐performed and invasive nature of the intervention. We judged blinding as high risk of bias in 10 of the 11 included trials. One trial that evaluated lubrication of the intermittent self‐dilatation catheter with triamcinolone ointment versus a water‐based gel (Hosseini 2008) packaged both medications in identical tubes, was double‐blind and deemed low risk of bias in this domain.
Incomplete outcome data
Reported outcome data was inadequate in seven (Bodker 1992; Harriss 1994; Hosseini 2008; Khan 2011; Kjaergaard 1994; Matanhelia 1995; Ngugi 2007) of the 11 included studies which were therefore judged to have high risk of attrition bias. In general, participants who did not attend follow‐up, died during the study period or discontinued intermittent self‐dilatation owing to an adverse event or outcome were excluded from the final analysis. We did not require data clarification from the study authors to undertake our pre‐stated analyses.
Selective reporting
Five (Afridi 2010; Bodker 1992; Hosseini 2008; Khan 2011; Tammela 1993) of the 11 included trials appeared free of selective reporting and were judged low risk of bias in this domain.
Other potential sources of bias
No other potential sources of bias were identified.
Conflict of interest statement
None of the reports included a conflict of interest statement.
Subgroup analysis
The data did not permit any of the pre‐stated subgroup analyses.
Effects of interventions
See: Table 1
Comparison 1: intermittent self‐dilatation versus no treatment
Six trials addressed this comparison (Afridi 2010; Bodker 1992; Husmann 2006; Khan 2011; Kjaergaard 1994; Matanhelia 1995).
Primary outcomes
Patient‐reported lower urinary tract symptoms
No trials reported this outcome.
Patient‐reported health‐related quality of life
No trials reported this outcome.
Risk of recurrent urethral stricture
There was significant heterogeneity (I2 statistic = 72%) therefore data from all six trials and 404 recruited participants (Afridi 2010; Bodker 1992; Husmann 2006; Khan 2011; Kjaergaard 1994; Matanhelia 1995) were combined for pooled analysis using a random‐effects model. Recurrent urethral stricture was less common in men who performed intermittent self‐dilatation (85/197, 43%) than men who did not perform intermittent self‐dilatation (128/207, 62%) (RR 0.70, 95% CI 0.48 to 1.00, Analysis 1.1). Estimates using a fixed‐effect model were similar but the CI became narrower (RR 0.71, 95% CI 0.59 to 0.85). The number needed to treat to prevent one urethral stricture recurrence is 5.4. Heterogeneity was expected given that no two trials defined recurrent urethral stricture in the same way; nor employed the same programme of intermittent self‐dilatation uniformly; nor followed‐up participants for the same length of time. The six trials in this comparison defined the presence of recurrent urethral stricture variably based on urethrographic appearance, cystoscopy or maximum urinary flow rate. Only Husmann 2006 defined recurrence as the need for re‐intervention.
1.1. Analysis.
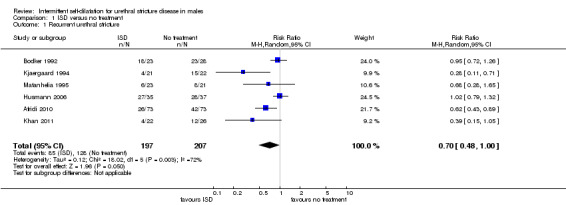
Comparison 1 ISD versus no treatment, Outcome 1 Recurrent urethral stricture.
The GRADE approach was used to assess the quality of the evidence indicating that intermittent‐self dilatation may reduce the risk of recurrent urethral stricture (Table 1). The recommendation was downgraded two levels on the basis that the studies comprising the meta‐analysis were deemed to have high risk of bias, and evidence of imprecision and inconsistency. Thus, our judgement informed by the GRADE approach is that the quality of the evidence is 'very low' and accordingly we have very little confidence in the estimate of the effect.
Bodker 1992 reported that the median time to recurrence was greater in men who performed intermittent self‐dilatation than men who did not perform intermittent self‐dilatation after optical urethrotomy (7 months versus 4 months respectively).
Secondary outcomes
Adverse events
No trials reported adverse events rigorously or completely. Four trials gave a narrative account of adverse events associated with performing intermittent self‐dilatation (Bodker 1992, Khan 2011, Kjaergaard 1994; Matanhelia 1995) including pain, haematuria, symptomatic and asymptomatic bacteruria and epididymitis.
Bodker 1992 reported that two of 28 (7.1%) patients in the intermittent self‐dilatation arm of the trial experienced urethral haemorrhage and that the remaining 26 (92.9%) patients did not experience haematuria, pain on catheter insertion or infection. Two of 28 (7.1%) patients in the treatment arm died during the study; the causes of death are not stated. The rate of adverse events in the control arm is not given for comparison.
Khan 2011 reported rates of urinary tract infection, defined as one or more positive urine cultures or epididymitis, of 18.1% (4/22) and 15.3% (4/26) in the intervention and control arms respectively.
Kjaergaard 1994 reported a lower rate of bacteruria or epididymitis in men who performed intermittent self‐dilatation compared with men who did not perform intermittent self‐dilatation [1/21 (4.7%) versus 5/22 (22.7%) respectively, P = 0.4)].
Matanhelia 1995 reported that no patient in the intermittent self‐dilatation arm of this trial developed urinary tract infection.
Combining the data using a random‐effects model from the two small studies that reported sufficient data (Khan 2011 and Kjaergaard 1994) gave a risk ratio of 0.60 favouring intermittent self‐dilatation but the 95% CI crossed the line of no effect (0.11 to 3.26) and the result was not statistically significant (Analysis 1.2).
Afridi 2010 and Husmann 2006 did not report adverse events.
Acceptability
No trials formally evaluated the concept of acceptability of intermittent self‐dilatation to patients.
Two studies (Khan 2011 and Kjaergaard 1994) made the identical claim that 'all of the patients who completed the prescribed CISC (intermittent self‐dilatation) program considered the method fully acceptable and all were able to perform CISC at home with no problems.' Matanhelia 1995 commented that 'patients generally found the procedure acceptable.' No trials described the means of assessment of acceptability to participants.
Cost‐effectiveness
No trials reported this outcome.
Comparison 2: One programme of intermittent self‐dilatation versus another
Two trials addressed this comparison (Harriss 1994; Tammela 1993). Both trials investigated the effect of the duration of the programme of intermittent self‐dilatation as opposed to the frequency with which intermittent self‐dilatation was performed by participants.
Patient‐reported lower urinary tract symptoms
Neither trial reported this outcome.
Patient‐reported health‐related quality of life
Neither trial reported this outcome.
Risk of recurrent urethral stricture
The stated objective of Harriss 1994 was to determine the duration of intermittent self‐dilatation required to 'stabilise' a urethral stricture. One hundred and one men were allocated by odd or even hospital number to intermittent self‐dilatation for a period of six months or a period of three years after optical urethrotomy. Participants in both arms performed intermittent self‐dilatation twice weekly for one month and then weekly. Robust conclusions cannot be drawn from the presented data owing to cross‐over, protocol deviation, administrative error, post‐hoc analysis and incomplete outcome reporting. It is notable that none of the 10 patients who performed intermittent self‐dilatation to the end of the study developed a recurrence.
Tammela 1993 randomised 49 men with recurrent urethral stricture to intermittent self‐dilatation for six months or intermittent self‐dilatation for 12 months after optical urethrotomy. The authors defined recurrence as the need for surgical re‐intervention. No patient developed a recurrence within the first six months of follow‐up. Three men who performed intermittent self‐dilatation for 12 months and two men who performed intermittent self‐dilatation for the first six months, developed recurrent urethral stricture thereafter (RR 0.67, 95% CI 0.12 to 3.64, Analysis 2.1): the numbers were too small to be reliable.
2.1. Analysis.
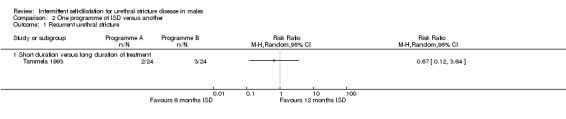
Comparison 2 One programme of ISD versus another, Outcome 1 Recurrent urethral stricture.
Adverse events
Both reports gave a narrative account of the adverse events encountered in the trials but did not stratify those adverse events by study arm. Tammela 1993 stated that 10 and two of 48 patients who completed the study developed asymptomatic and symptomatic bacteruria respectively. Harriss 1994 reported that 21 of 101 (21%) men enrolled in the trial died of unrelated diseases during follow‐up .
Acceptability
Neither trial made a formal assessment of acceptability. Harriss 1994 stated that 'most patients, even the frail and elderly, took to the procedure very easily.'
Cost‐effectiveness
Neither trial reported this outcome.
Comparison 3: One device for intermittent self‐dilatation versus another
Three trials were relevant to this comparison (Ngugi 2007; Sallami 2011; Hosseini 2008).
One trial evaluated triamcinolone gel (a synthetic corticosteroid) for lubricating the intermittent self‐dilatation catheter compared to a water‐based gel (Hosseini 2008).
One trial evaluated a low friction hydrophilic catheter (LoFric™) for performing intermittent self‐dilatation compared to a standard Nelaton polyvinyl chloride (PVC) catheter (Sallami 2011).
One trial evaluated intermittent self‐dilatation versus regular outpatient urethral dilatation by a surgeon with Clutton sounds (Ngugi 2007).
Patient‐reported lower urinary tract symptoms
No trials assessed this outcome.
Patient‐reported health‐related quality of life
No trials used a psychometrically robust patient‐reported outcome measure to assess health‐related quality of life.
Ngugi 2007 employed a novel, non‐validated questionnaire to compare regular outpatient urethral dilatation with sounds by a surgeon with intermittent self‐dilatation. Participants were asked to say how happy they were with the intervention one, three and six months after enrolment in the trial using a Likert‐type happiness scale. At each time point a greater proportion of men who were randomised to intermittent self‐dilatation reported being happier with their intervention than those men who were randomised to outpatient urethral dilatation with sounds (100% versus 73% at one month, 88% versus 12% at three months and 85% versus 20% six months post‐enrolment).
Risk of recurrent urethral stricture
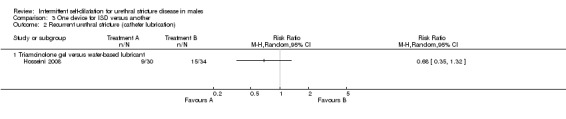
Sallami 2011 reported a lower rate of recurrent urethral stricture in men randomised to a low‐friction hydrophilic catheter (LoFric™) versus a standard Nelaton PVC catheter for performing intermittent self‐dilatation (RR 0.32, 95% CI 0.07 to 1.40, Analysis 3.1.1). Hosseini 2008 reported a lower rate of recurrent urethral stricture in men randomised to 1% triamcinolone gel versus water‐based gel for lubrication of the intermittent self‐dilatation catheter (RR 0.68, 95% CI 0.35 to 1.32, Analysis 3.1.2,). In both cases the 95% confidence interval of the effect size crosses the line of no effect and the trials were too small for significance.
3.1. Analysis.
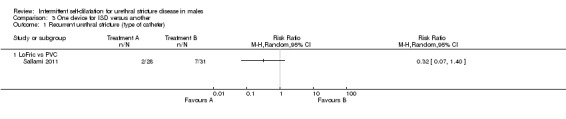
Comparison 3 One device for ISD versus another, Outcome 1 Recurrent urethral stricture (type of catheter).
Adverse events
Using a LoFric™ catheter conferred a lower rate of adverse events than using a standard Nelaton PVC catheter in one study (Sallami 2011). In a cohort of 31 men performing intermittent self‐dilatation with a LoFric™ catheter there were no instances of prostatitis or urethral bleeding and one instance of bacteriuria versus one, two and four instances respectively in a cohort of 28 men performing intermittent self‐dilatation with a PVC catheter (RR 0.13, 95% CI 0.02 to 0.98, Analysis 3.3) . Ngugi 2007 commented that there was a higher rate of urinary tract infection in the group receiving regular outpatient urethral dilatation with sounds versus the group who performed intermittent self‐dilatation. No further details are given.
3.3. Analysis.
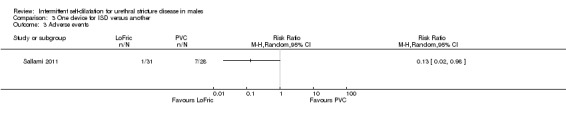
Comparison 3 One device for ISD versus another, Outcome 3 Adverse events.
Acceptability
No trials made a formal assessment of acceptability.
Sallami 2011 commented that 30 of 31 men performing intermittent self‐dilatation using a LoFric™ catheter considered the device fully acceptable versus seven of 28 men using a standard PVC catheter, with the caveat that only 10 of 31 men who performed intermittent self‐dilatation with the LoFric™ catheter were able to do so at home without any difficulty.
Cost‐effectiveness
No trials assessed the cost‐effectiveness of one device for performing intermittent self‐dilatation compared with another.
Discussion
Summary of main results
Repeated transurethral intervention for urethral stricture can lead to a chronic disease state necessitating regular treatment throughout the course of a man's life. In the assessment of therapeutic interventions for urethral stricture the outcome measure of single greatest importance is arguably therefore the rate of, or interval to, recurrence, as a proxy for the length of the time a man will spend with symptomatic disease and thus the number of quality‐adjusted life years they will lose as a result. Unfortunately there is no consensus as to the definition of recurrent urethral stricture across trials, case series or clinical guidelines. The trials included in this review variably employed cystoscopic and urethrographic appearance, maximum urinary flow rate and re‐intervention.
In practice it is usually a patient's symptoms or level of bother that dictate the need for intervention but the use of psychometrically validated patient‐reported outcome measures in the management of this disease is a relatively recent development (Jackson 2011). Pooled analysis of six trials indicated that men who perform intermittent self‐dilatation may have a lower chance of developing recurrent urethral stricture than men who do not perform intermittent self‐dilatation. This finding is tempered by the absence of reliable evidence regarding the intervention in the following areas:
a quantitative or qualitative evaluation of acceptability of intermittent self‐dilatation to patients;
the comparative rate and nature of adverse events versus no treatment;
cost‐effectiveness;
effect on feasibility of future urethral reconstruction.
The programme of intermittent self‐dilatation varied across the studies in this review and the optimum frequency and timing of this technique, or whether indeed there is a generalisable optimum programme or protocol, cannot be determined from the available body of evidence. The effect of the duration of intermittent self‐dilatation on risk of recurrence was compared in two trials (Harriss 1994, Tammela 1993). These data suggest that increasing the duration for which intermittent self‐dilatation is performed delays the onset of recurrence. This is in some respects axiomatic and there is no evidence that performing intermittent self‐dilatation has any enduring preventative benefit once the treatment has been discontinued.
Most of the available devices for performing intermittent self‐dilatation have not been robustly compared. Sallami 2011 reported a reduction in recurrence rate associated with the use of a low‐friction hydrophilic catheter versus a standard PVC catheter. Hosseini 2008 found that one percent triamcinolone gel is superior to conventional water‐based gel in terms of prevention of urethral stricture recurrence.
Overall completeness and applicability of evidence
Most of the trials in this review were undertaken before the proliferation of urethral reconstruction which many urethral surgeons, accepting the absence of high level evidence, would regard as the standard of care for recurrent urethral stricture in men who are fit enough to have the procedure.
Quality of the evidence
This systematic review has highlighted a paucity of reliable data on the subject of intermittent self‐dilatation for urethral stricture disease in men. Relevant randomised trials were small, few in number and were generally of low methodological quality or poorly reported, with a high risk of bias. The evidence that performing intermittent self‐dilatation reduces the risk of recurrent urethral stricture is 'very low' quality on the basis of the GRADE approach such that we have very little confidence in the effect estimate.
Potential biases in the review process
The method for assessing the quality of evidence was not specified at the time of protocol writing and was selected while conducting the review. Selection of GRADE at this stage could be a potential source of bias.
Agreements and disagreements with other studies or reviews
There are no other systematic reviews of intermittent self‐dilatation for the management of urethral stricture disease in males.
Authors' conclusions
Implications for practice.
Performing intermittent self‐dilatation may confer a reduced risk of recurrent urethral stricture, although our confidence in the evidence is very low. This benefit has to be counter‐balanced against the burden of performing the procedure. It is not yet known whether performing intermittent self‐dilatation is generally acceptable to patients, alleviates symptoms, improves health‐related quality of life or is cost‐effective versus doing nothing at all after an operation for urethral stricture. It is also unknown whether intermittent self‐dilatation is effective at reducing the risk of recurrent urethral stricture after it has been discontinued, whether it is required to be performed indefinitely to be effective, or whether performing intermittent self‐dilatation has a detrimental effect on the feasibility and outcome of future reconstructive urethral surgery.
Implications for research.
A trial incorporating robust patient‐reported and health economic outcome measures and adhering to the CONSORT recommendations is required to determine the benefits and harms of intermittent self‐dilatation in the management of urethral stricture disease in males (Schulz 2010).
Acknowledgements
We thank Sheila Wallace for undertaking the systematic literature search.
Appendices
Appendix 1. MEDLINE and PREMEDLINE search strategies
The search strategy used in MEDLINE (1946 to April Week 3 2014) and PREMEDLINE (29 April 2014) (both on OVID SP) (both last searched on 30 April 2014) is given below:
| 1. | controlled clinical trial.pt. |
| 2. | randomized controlled trial.pt. |
| 3. | randomized controlled trials/ |
| 4. | random allocation/ |
| 5. | double blind method/ |
| 6. | single blind method/ |
| 7. | clinical trial.pt. |
| 8. | exp clinical trials/ |
| 9. | placebos/ |
| 10. | placebo$.tw. |
| 11. | random$.tw. |
| 12. | research design/ |
| 13. | volunteer$.tw. |
| 14. | (clin$ adj25 trial$).tw. |
| 15. | ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw. |
| 16. | factorial.tw. |
| 17. | cross‐over studies/ |
| 18. | crossover.tw. |
| 19. | latin square.tw. |
| 20. | (balance$ adj2 block$).tw. |
| 21. | (animals not humans).sh. |
| 22. | or/1‐20 |
| 23. | 22 not 21 |
| 24. | exp clinical trial/ |
| 25. | clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/ or controlled clinical trials as topic/ or randomized controlled trials as topic/ or multicenter studies as topic/ |
| 26. | or/1‐20,24‐25 |
| 27. | 26 not 21 |
| 28. | urethrotom$.tw. |
| 29. | (dilatat$ adj25 urethra$).tw. |
| 30. | self dilatat$.tw. |
| 31. | urethroplast$.tw. |
| 32. | urethral stricture/ |
| 33. | (urethra$ adj5 (stricture$ or stenos$)).tw. |
| 34. | or/28‐33 |
| 35. | 34 and 27 |
Appendix 2. Other search strategies
CENTRAL (on OVID SP), 2014 Issue 4 was searched on 6 May 2014 using the following strategy:
1. urethrotom$.tw. 2. (dilatat$ adj25 urethra$).tw. 3. (self adj3 calibrat$).tw. 4. self dilatat$.tw. 5. urethroplast$.tw. 6. urethral stricture/ 7. (urethra$ adj5 (stricture$ or stenos$)).tw. 8. (urethra$ adj3 narrow$).tw. 9. or/1‐8 10. cochrane incontinence group.gc. 11. 9 not 10 12. ((urin$ or urethra$) adj5 catheter$).tw. 13. ((intermittent or bladder) adj5 catheter$).tw. 14. dilatat$.tw. 15. calibrat$.tw. 16. or/12‐15 17. or/6‐8 18. 16 and 17 19. 18 not 10 20. 11 or 19
Embase and Embase Classic (on OVID SP) were searched (1947 to 2014 Week 17) on 1 May 2014:
| 1. | Randomized Controlled Trial/ |
| 2. | controlled study/ |
| 3. | clinical study/ |
| 4. | major clinical study/ |
| 5. | prospective study/ |
| 6. | meta analysis/ |
| 7. | exp clinical trial/ |
| 8. | randomization/ |
| 9. | crossover procedure/ or double blind procedure/ or parallel design/ or single blind procedure/ |
| 10. | Placebo/ |
| 11. | latin square design/ |
| 12. | exp comparative study/ |
| 13. | follow up/ |
| 14. | pilot study/ |
| 15. | family study/ or feasibility study/ or pilot study/ or study/ |
| 16. | placebo$.tw. |
| 17. | random$.tw. |
| 18. | (clin$ adj25 trial$).tw. |
| 19. | ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw. |
| 20. | factorial.tw. |
| 21. | crossover.tw. |
| 22. | latin square.tw. |
| 23. | (balance$ adj2 block$).tw. |
| 24. | or/1‐23 |
| 25. | (nonhuman not human).sh. |
| 26. | 24 not 25 |
| 27. | factorial design/ |
| 28. | parallel design/ |
| 29. | triple blind procedure/ |
| 30. | community trial/ |
| 31. | intervention study/ |
| 32. | experimental study/ |
| 33. | prevention study/ |
| 34. | quasi experimental study/ |
| 35. | or/27‐34 |
| 36. | 24 or 35 |
| 37. | 36 not 25 |
| 38. | urethra stricture/ |
| 39. | urethra stenosis/ |
| 40. | (urethra$ adj5 (stricture$ or stenos$)).tw. |
| 41. | (urethra$ adj5 narrow$).tw. |
| 42. | 41 or 38 or 39 or 40 |
| 43. | urethroplasty/ or urethrotomy/ |
| 44. | urethroplast$.tw. |
| 45. | urethrotom$.tw. |
| 46. | dilatat$.tw. |
| 47. | intermittent catheterization/ |
| 48. | catheter$.tw. |
| 49. | bladder catheterization/ or urethral catheterization/ |
| 50. | 49 or 46 or 45 or 43 or 44 or 48 or 47 |
| 51. | 42 and 50 and 37 |
CINAHL (on EBSCOhost) covering 31 December 1981 to 30 April 2014 was last searched on 1 May 2014 using the following strategy:
| Search Terms | Search Options |
| S36 | S23 AND S35 |
| S35 | S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 |
| S34 | TI (urethroplast*) or AB (urethroplast*) |
| S33 | TI (urethrotom*) or AB (urethrotom*) |
| S32 | TI (urethra* N25 dilat*) or AB (urethra* N25 dilat*) |
| S31 | TI (urethra* N25 narrow*) or AB (urethra* N25 narrow*) |
| S30 | TI (urethra* N25 stenos*) or AB (urethra* N25 stenos*) |
| S29 | TI (urethra* N25 stricture*) or AB (urethra* N25 stricture*) |
| S28 | "URETHRA" AND "DILATATION" |
| S27 | "URETHRAL DILATATION" |
| S26 | "URETHRA DILATATION" |
| S25 | "SELF DILATATION" |
| S24 | (MH "Urethral Stricture") |
| S23 | S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 |
| S22 | TI ( singl* N25 blind* OR singl* N25 mask* OR doubl* N25 blind* or doubl* N25 mask* OR trebl* N25 blind* OR trebl* N25 mask*OR tripl* N25 blind* OR tripl* N25 mask* ) or AB ( singl* N25 blind* OR singl* N25 mask* OR doubl* N25 blind* or doubl* N25 mask* OR trebl* N25 blind* OR trebl* N25 mask*OR tripl* N25 blind* OR tripl* N25 mask* ) |
| S21 | (MH "Comparative Studies") |
| S20 | (MH "Clinical Research+") |
| S19 | (MH "Static Group Comparison") |
| S18 | (MH "Quantitative Studies") |
| S17 | (MH "Crossover Design") or (MH "Solomon Four‐Group Design") |
| S16 | (MH "Factorial Design") |
| S15 | (MH "Community Trials") |
| S14 | (MH "Random Sample") |
| S13 | TI balance* N2 block* or AB balance* N2 block* |
| S12 | TI "latin square" or AB "latin square" |
| S11 | TI factorial or AB factorial |
| S10 | TI clin* N25 trial* or AB clin* N25 trial* |
| S9 | (MH "Study Design") |
| S8 | (AB random*) OR (TI random*) |
| S7 | (AB placebo*) OR (TI placebo*) |
| S6 | (MH "Placebos") |
| S5 | PT Clinical Trial |
| S4 | (MH "Clinical Trials+") |
| S3 | MH (random assignment) OR (crossover design) |
| S2 | cross‐over |
| S1 | crossover |
Open Grey was last searched on 6 May 2014 using the following terms:
urethra* stricture*
self dilatation
ClinicalTrials.gov (via CRS), WHO ICTRP and Current Controlled Trials (all registers) were last searched on 6 May 2014 using the following terms:
self dilatation OR urethra* stricture* OR urethra* stenos* OR urethra* narrow*
Data and analyses
Comparison 1. ISD versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrent urethral stricture | 6 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.48, 1.00] |
| 2 Adverse events | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.26] |
| 2.1 Urinary tract infection/bacteriuria | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.26] |
1.2. Analysis.
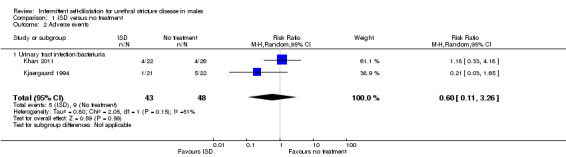
Comparison 1 ISD versus no treatment, Outcome 2 Adverse events.
Comparison 2. One programme of ISD versus another.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrent urethral stricture | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Short duration versus long duration of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. One device for ISD versus another.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrent urethral stricture (type of catheter) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 LoFric vs PVC | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Recurrent urethral stricture (catheter lubrication) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Triamcinolone gel versus water‐based lubricant | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
3.2. Analysis.
Comparison 3 One device for ISD versus another, Outcome 2 Recurrent urethral stricture (catheter lubrication).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Afridi 2010.
| Methods | Full text. July 2004 ‐ June 2008. Objective: find out the role of intermittent self‐dilatation for the prevention of recurrent urethral stricture. Quasi‐randomisation: alternate allocation. Statistical methods: not described. |
|
| Participants | Pakistan. 146 men with anterior urethral stricture. Age: not stated. Exclusions: posterior urethral stricture; post‐urethroplasty; stricture; unable to perform intermittent self‐dilatation; >3 strictures; stricture >4cm; obliterative urethral stricture; para‐urethral abscess; fistula. |
|
| Interventions | Group A (n = 73): optical urethrotomy Group B (n = 73): optical urethrotomy then intermittent self‐dilatation for 5 months |
|
| Outcomes |
PROs: no Health economic: no Adverse events: no Acceptability: no Recurrence rate: Number of men with urethral stricture 8 months after optical urethrotomy. Definition of recurrence: urethrogram. Group A : 42/73 Group B: 26/73 |
|
| Notes | intermittent self‐dilatation programme: daily for 4 weeks; alternate days for 4 weeks; every 3 days for 4 weeks; weekly for 8 weeks. Withdrawals: nil. Subgroups: first versus recurrent stricture; stricture length; aetiology |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: 'divided into two groups on alternate basis.' |
| Allocation concealment (selection bias) | High risk | Quote: 'divided into two groups on alternate basis.' |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated. Impossible to blind participants. Outcome assessors probably not blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported. |
| Funding/COI | Unclear risk | No statement. |
Bodker 1992.
| Methods | Full text. Objective: find the effect of treatment of recurrent urethral stricture by optical urethrotomy followed by intermittent self‐dilatation for 3 months. States randomised; no details. Statistical methods: Chi2. |
|
| Participants | Denmark. 61 men with recurrent anterior urethral stricture. Age: range 18‐87; Median: Observation 76; Intervention 70 Exclusions: prostatic urethral stricture, bladder cancer. |
|
| Interventions | Observation (n = 33): optical urethrotomy Intervention (n = 28): optical urethrotomy then intermittent self‐dilatation for 3 months |
|
| Outcomes |
PROs: no Health economic: no Adverse events: Intervention: 2/28 urethral haemorrhage; Observation: nil reported. Acceptability: no Recurrence rate: Number of men with recurrent urethral stricture 1 year after optical urethrotomy. Definition of recurrence: flow rate < 10ml/s. Observation: 23/28 Intervention: 18/23 Time to recurrence: Median time after optical urethrotomy: Observation: 4 (range 2‐12) months Intervention: 7 (range 5‐15) months |
|
| Notes | intermittent self‐dilatation programme: twice weekly for 1 month then weekly for 2 months Withdrawals: Observation: 3 death, 1 DNA Intervention: 2 bleeding, 2 death, 1 DNA Subgroups: no. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract states 'randomized to undergo internal urethrotomy'; thereafter described as allocated to groups. |
| Allocation concealment (selection bias) | High risk | Probably not done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated. Impossible to blind participants. Outcome assessors probably not blind. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 men in intervention group withdrawn owing to haemorrhage should have been evaluated for recurrence. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported. |
| Funding/COI | Unclear risk | No statement. |
Harriss 1994.
| Methods | Full text. 1985‐89. Objective: ascertain the duration of intermittent self‐dilatation required to allow stabilization of urethral strictures following urethrotomy. Randomisation: odd/even hospital number. Statistical methods: Chi2 with Yates' correction; Wilcoxon signed rank test. |
|
| Participants | UK. 101 men with recurrent urethral stricture. Age: range 24‐78, median 67. Exclusions: nil (states all men with the disease attending the department) |
|
| Interventions | Group 1: optical urethrotomy then intermittent self‐dilatation for 6 months Group 2: optical urethrotomy then intermittent self‐dilatation for 3 years (intended). |
|
| Outcomes |
PROs: no Health economic: no Adverse events: Not stratified by group: overall: 21 death, 6 UTI, 'several' haematuria. Acceptability: No objective assessment. Quote 'most patients ... took to the procedure very easily.' Recurrence rate: Number of men with recurrent urethral stricture. Census time point unclear; follow‐up range 24‐78 months. Definition of recurrence: cystoscopy. Group 1 (6 months): 19/48 Group 2A (12‐36 months): 4/28 Group 2B (> 36 months): 0/10 Time to recurrence: no |
|
| Notes | intermittent self‐dilatation programme: twice weekly for 1 month then weekly. Withdrawals: 8/21 men who died of 'unrelated disease' in the study period with insufficient follow‐up, 7 DNA (Group 1 = 3; Group 2 = 4) Protocol not followed: significant deviation from intended 36‐month programme of intermittent self‐dilatation in Group 2 owing to death, administrative error and patient preference. Subgroups: no. 10 men on permanent intermittent self‐dilatation after optical urethrotomy = zero recurrence. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Odd/even hospital number. |
| Allocation concealment (selection bias) | High risk | Odd/even hospital number. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated. Probably not done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of patients withdrawn sufficient to introduce clinically relevant bias. |
| Selective reporting (reporting bias) | High risk | Narrative presentation of results; post‐hoc analysis; variable follow‐up and time point of recurrence not stated. |
| Funding/COI | Unclear risk | No statement. |
Hosseini 2008.
| Methods | Full text. Objective: evaluate intermittent self‐dilatation in combination with triamcinolone gel for lubrication of the catheter after optical urethrotomy. Double‐blind, placebo‐controlled trial. Randomisation: random numbers table. Statistical methods: Chi2, Student's t test. |
|
| Participants | Iran. 70 male participants with urethral stricture. Age: range 10‐80; Mean: intervention 37.7; Control 34.5 Exclusions: complete urethral obstruction; stricture > 1.5 cm. |
|
| Interventions | Control (n = 35): optical urethrotomy then intermittent self‐dilatation with water‐based lubricant for 6 months. Intervention (n=35): optical urethrotomy then intermittent self‐dilatation with 1 ml 1% triamcinolone gel for 6 months. |
|
| Outcomes |
PROs: no Health economic: no Adverse events: Not objectively reported. Metaquote: 'no febrile UTIs or complications specific to Triamcinolone.' Acceptability: No objective assessment. Quote 'all of the patients who completed the prescribed CISC program considered the method fully acceptable.' Recurrence rate: Number of men with recurrent urethral stricture 12 months after optical urethrotomy. Definition of recurrence: cystoscopy. Control: 15/34 Intervention: 9/30 P = 0.24 Time to recurrence: no |
|
| Notes | intermittent self‐dilatation: daily week 1, alternate days week 2, twice weekly week 3, weekly week 4, every 2 weeks for 1 month, monthly for 3 months. Withdrawals: Control: 1 DNA Intervention: 5 DNA Subgroups: no. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Refers to random table. Probably done. |
| Allocation concealment (selection bias) | High risk | Not described. Probably not done. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quotes: 'the patient and the physicians involved in the research project were blind to the type of the lubricants' which were 'packed in similar tubes.' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalance in numbers across intervention groups possibly related to outcome and sufficient to introduce clinically relevant bias in intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported. |
| Funding/COI | Unclear risk | No statement. |
Husmann 2006.
| Methods | Full text. 1986‐2005. Objective: to answer the question 'does post‐op intermittent self‐dilatation influence long term results of optical urethrotomy?' States randomised; no details. Statistical methods: Chi2 |
|
| Participants | USA. 72 men with < 1cm pendulous penile urethral stricture following hypospadias repair. Age: not stated, presumed adults, conclusion refers to follow‐up through adulthood. Exclusions: meatal or bulbar stricture. |
|
| Interventions | Control: optical urethrotomy (n = 37) Intervention: optical urethrotomy then intermittent self‐dilatation for 3 months (n = 35) |
|
| Outcomes |
PROs: no Health economic: no Adverse events: no Acceptability: no Recurrence rate: Number of men with recurrent urethral stricture 2 years after optical urethrotomy. Definition of recurrence: re‐intervention. Control: 28/37 Intervention: 27/35 Time to recurrence: no |
|
| Notes | intermittent self‐dilatation programme: daily. Withdrawals: not reported. Subgroups: type of hypospadias repair: tubularised graft, tubularised flap, onlay flap, urethral plate. Authors' conclusion: addition of intermittent self‐dilatation following optical urethrotomy has no benefit for preventing stricture recurrence [in men who have had hypospadias surgery]. Note: patients recruited over 19‐year period. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: 'randomized to treatment based on 1 of 4 types of initial hypospadias repair'; 'arbitrarily assigned to treatment.' Probably not done. |
| Allocation concealment (selection bias) | High risk | Randomised according to type of hypospadias repair. Probably not done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated. Impossible to blind participants. Outcome assessors probably not blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomised not stated. Reports only patients that completed the study. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. Presents analysis that was not pre‐specified. |
| Funding/COI | Unclear risk | No statement. |
Khan 2011.
| Methods | Full text. June 2007 ‐ June 2010. Objective: determine the role of intermittent self‐dilatation in the prevention of recurrence of urethral stricture after optical urethrotomy; study the frequency of postoperative complications and tolerability of intermittent self‐dilatation. States randomised; no details. |
|
| Participants | Pakistan. 60 men with anterior urethral stricture. Age: range 20‐38; mean Control 37.3, Treatment 42.5 Exclusions: prostate or bladder cancer, inability to learn intermittent self‐dilatation. |
|
| Interventions | Control: optical urethrotomy (n = 30) Treatment: optical urethrotomy then intermittent self‐dilatation for 1 year (n = 30) |
|
| Outcomes |
PROs: no. Health economic: no. Adverse events: Control: 3 UTI; 1 epididymitis Treatment: 4 UTI, zero epididymitis. Acceptability: no objective assessment. Quote 'All of the patients who completed the prescribed CISC program considered the method fully acceptable.' Recurrence rate: Number of men with recurrent urethral stricture 12 months after optical urethrotomy. Definition of recurrence: cystoscopy. Control: 12/26 Treatment: 4/22 P < 0.01 Time to recurrence: no |
|
| Notes | intermittent self‐dilatation: twice a day for 1 week, once a day for 4 weeks, then weekly for one year. Withdrawals: Control: 4 (2 DNA, 1 emigration, 2 symptomatic declined cystoscopy) Treatment: 8 (4 DNA, 1 death, 3 unable to perform intermittent self‐dilatation) Subgroups: no. Large sections of this paper including the results are described verbatim in Kjaergaard 1994; numbers similar. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote 'randomly divided into treatment group and control group.' Insuffucient information to make judgement. |
| Allocation concealment (selection bias) | High risk | Not stated. Probably not done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated. Impossible to blind participants. Outcome assessors probably not blind. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of participants withdrawn from treatment arm enough to impact effect estimate. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported. |
| Funding/COI | Unclear risk | Not stated. |
Kjaergaard 1994.
| Methods | Full text. August 1987 ‐ August 1991. Objective: investigate the effect of intermittent self‐dilatation on the prevention of urethral stricture after optical urethrotomy. States randomised. Statistical methods: Life‐table, logrank, Fisher's exact test. |
|
| Participants | Denmark. 55 men with anterior urethral stricture. Age: range 28‐85 (median 68) Exclusions: prostate or bladder cancer, inability to learn intermittent self‐dilatation. |
|
| Interventions | Control: optical urethrotomy (n = 24) Treatment: optical urethrotomy then intermittent self‐dilatation for 1 year (n = 31). |
|
| Outcomes |
PROs: no Health economic: no Adverse events: Number of men with positive urine culture or epididymitis Control: 5/22 Treatment: 1/21 P = 0.4 Acceptability: Not objectively assessed. Quote 'All of the patients who completed the prescribed CIC programme considered the method fully acceptable.' Recurrence rate: Number of men with recurrent urethral stricture 12 months after optical urethrotomy. Definition of recurrence: cystoscopy. Control: 15/22 Treatment: 4/21 P < 0.01 |
|
| Notes | intermittent self‐dilatation programme: weekly. Withdrawals: Control: 2 (2 protocol violation) Treatment: 10 (4 DNA, 1 death, 1 rUTI, 4 unable to perform intermittent self‐dilatation) Subgroups: no. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is unclear. Insufficient detail to make a judgement. |
| Allocation concealment (selection bias) | High risk | Not stated. Probably not done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated. Probably not done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of participants withdrawn from treatment arm enough to impact effect estimate. |
| Selective reporting (reporting bias) | High risk | Pre‐stated outcomes not reported: time to recurrence; second year data. |
| Funding/COI | Unclear risk | Astra Meditec Limited, Denmark supplied LoFric catheters for this study. |
Matanhelia 1995.
| Methods | Full text. 1989‐1991. No stated objective. States randomly allocated; no details. Statistical methods: Log rank x2 |
|
| Participants | UK. 51 men with anterior urethral stricture. Age: not stated. Exlcusions: not stated. |
|
| Interventions | Control: optical urethrotomy (n = 21) Treatment: optical urethrotomy then intermittent self‐dilatation for 3 months (n = 23) |
|
| Outcomes |
PROs: no Health economic: no Adverse events: Zero UTI intermittent self‐dilatation arm. Quote 'none developed urinary tract infections.' Acceptability: No objective assessment. Quote 'patients generally found the procedure acceptable.' Recurrence rate: Number of men with recurrent urethral stricture 12 months after optical urethrotomy. Definition of recurrence: flow rate < 12 ml/s. Control: 8/21 Treatment: 6/23 Time to recurrence: no |
|
| Notes | intermittent self‐dilatation programme: twice daily for 2 weeks, daily for 3 weeks, twice weekly for 3 weeks, weekly for 4 weeks. Withdrawals: 7 (6 DNA, 1 death); not stratified by arm. Subgroups: First or recurrent stricture. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: 'Patients were randomly allocated.' No further details. |
| Allocation concealment (selection bias) | High risk | Not described. Probably not done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated. Probably not done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/51 patients entered into the trial did not attend follow‐up, allocation unspecified. Sufficient to impact on intervention effect. |
| Selective reporting (reporting bias) | High risk | No pre‐stated objective. UTI data incompletely reported. |
| Funding/COI | Unclear risk | Not stated. |
Ngugi 2007.
| Methods | Full text. October 1998 ‐ June 1999. Objective: compare clean intermittent self catheterisation and urethral dilation with sounds in the management of recurrent urethral strictures. Randomised study; random number sequence generated by computer. Statistical methods: Chi2, Fisher's exact test. |
|
| Participants | Kenya. 49 male patients with recurrent urethral stricture. Age: range 15‐75; mean Control 40.0, Treatment 40.7 Exclusions: none stated. |
|
| Interventions | Control (Group B): dilatation with sounds at 1, 3 and 6 months (n = 22) Treatment (Group A): intermittent self‐dilatation for 6 months (n = 27) |
|
| Outcomes |
PROs: quality of life; non‐validated questionnaire; at 1, 3 and 6 months. Respectively: Control: 6/22, 15/17, 12/15 unhappy Treatment: 0/25, 3/25, 3/20 unhappy P = 0.01, 0.01, 0.12 Health economic: no Adverse events: No objective assessment. Quote 'higher rate of infection in the dilatation group at 3 and 6 months.' Acceptability: no. Recurrence rate: no. Flowrate: At baseline (preoperative), 1, 3 and 6 months. Respectively: Control mean: 9.9 ± 10.7, 8.2 ± 3.9, 5.4 ± 3.4, 7.7 ± 2.7 ml/s Treatment mean: 11.7 ± 10.9, 18.9 ± 9.9, 18.9 ± 9.8, 18.6 ± 11.5 ml/s P = 0.80, 0.0002, 0.0002, 0.002 |
|
| Notes | intermittent self‐dilatation programme: twice daily. Withdrawals: unclear: states 13 and 10 lost to follow‐up at 3 and 6 months respectively, does not tally with results table. Subgroups: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number sequence generated by computer. Probably done. |
| Allocation concealment (selection bias) | High risk | Not described. Probably not done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No details. Probably not done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Flowrate means and unspecified measure of dispersion given. Quality of Life (QoL) data missing for 14/49 patients at 6 months. Report states n = 10 and 13 lost to follow‐up at 3 and 6 months respectively. |
| Selective reporting (reporting bias) | High risk | Non‐specific pre‐stated objective. QoL Likert‐type scale stated in methods but not presented in results. |
| Funding/COI | Unclear risk | Not stated. |
Sallami 2011.
| Methods | Full text. August 2005 ‐ February 2008. Objective: compare intermittent self‐dilatation after optical urethrotomy for urethral stricture using a low‐friction hydrophilic catheter (LoFric) or standard Nelaton polyvinyl chloride (PVC) catheter. States block randomisation. Statistical methods: Life table, log rank, Chi2. |
|
| Participants | Tunisia. 62 men with anterior or posterior urethral stricture < 2cm. Age: range 21‐86; mean Control 60.9, Treatment 62 Exclusions: prostate or bladder cancer, patients requiring antibiotic prophylaxis, need for CISC for bladder drainage, incapable of following study protocol. |
|
| Interventions | Control: optical urethrotomy then intermittent self‐dilatation with Nelaton catheter (n = 31). Treatment: optical urethrotomy then intermittent self‐dilatation with LoFric catheter (n = 31). |
|
| Outcomes |
PROs: Ease of use after 6 catheter insertions: 5‐item VAS non‐validated questionnaire (trouble, convenience, pain, comfort, general opinion). Mean pain, comfort and opinion scores favoured LoFric; trouble and convenience NS. Health economic: no Adverse events: Control: 7 (1 prostatitis, 2 bleeding, 4 positive urine culture) Treatment: 1 positive urine culture Acceptability: Number of men who considered the treatment acceptable. Means of assessment of acceptability to patients not described. Control: 7/28 Treatment 30/31 Recurrence rate: Definition of recurrence: flow rate < 14 ml/s Number of men with recurrent urethral stricture 2 years after optical urethrotomy. Control: 7/28 Treatment: 2/31 P = 0.15 Time to recurrence: no. |
|
| Notes | intermittent self‐dilatation programme: twice monthly for 3 months then monthly for 1 year Witdrawals: n = 3 Nelaton arm (1 DNA, 2 rUTI). Authors conclude: LoFric catheter significantly increased the degree of comfort and satisfaction and decreased the feeling of pain when the catheter was removed or inserted, when compared with a conventional PVC catheter. Note: Results state only 10/31 in treatment arm able to perform intermittent self‐dilatation at home without problems. Subgroups: no. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States block randomisation. No further details. |
| Allocation concealment (selection bias) | High risk | Not stated. Probably not done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated. Probably not done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 control group patients withdrawn (2 UTI, 1 DNA); none from the treatment group. |
| Selective reporting (reporting bias) | High risk | Time to first recurrence analysis stated in Methods but not reported in Results. Results conflict with statement that only 10/31 in treatment arm able to perform intermittent self‐dilatation at home without problems. |
| Funding/COI | Unclear risk | Not stated. |
Tammela 1993.
| Methods | Full text. Objective: compare the effect of treatment of urethral stricture by optical urethrotomy followed by intermittent self‐dilatation for 6 versus 12 months. Randomised study; method of randomisation not described. Statistical methods: Student's t test, Chi2. |
|
| Participants | Finland. 49 men with recurrent urethral stricture. Age: mean Group A 58, Group B 62 Exlcusions: prostate or bladder cancer, scarred prostatic urethra; inadequate vision, dexterity, mental capacity. |
|
| Interventions | Group A: optical urethrotomy and intermittent self‐dilatation for 12 months (n = 24). Group B: optical urethrotomy and intermittent self‐dilatation for 6 months (n = 25). |
|
| Outcomes |
PROs: no. Health economic: no. Adverse events: Asymptomatic bacteruria n = 10/48 Symptomatic bacteruria n = 2/48 Acceptability: 1/48 unable to perform intermittent self‐dilatation. Recurrence rate: Number of men with recurrent urethral stricture 1 year after optical urethrotomy Definition of recurrence: need for further treatment. Group A: 3/24 Group B: 2/24 All recurrences occurred > 6 months therefore number of men with recurrent urethral stricture 6 months ‐ 1 year after optical urethrotomy: Group A (intermittent self‐dilatation): 3/24 Group B (no intermittent self‐dilatation): 2/24 Flowrate: 3, 6, 9 and 12 months after optical urethrotomy. Group A mean: 18 ± 7, 17 ± 9, 17 ± 3, 18 ± 6 ml/s Group B mean: 17 ± 6, 17 ± 7, 14 ± 7, 12.5 ± 3 ml/s At 12 months p < 0.05 |
|
| Notes | intermittent self‐dilatation: twice weekly for 1 month then weekly. Withdrawals: n = 1 (Group B) unable to perform intermittent self‐dilatation at home. Subgroups: no. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised into 2 groups. No further details. |
| Allocation concealment (selection bias) | High risk | Not stated. Probably not done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated. Probably not done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Means and SD presented only. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported. |
| Funding/COI | Unclear risk | Not stated. |
Abbreviations
CISC clean intermittent self‐catherisation
CONSORT Consolidated Standards of Reporting Trials
DNA did not attend
ISD intermittent self‐dilatation
LUTS lower urinary tract symptoms
NS Not statistically‐significant
PRO patient‐reported outcome
PROM patient‐reported outcome measure
PVC polyvinyl chloride
QALY quality‐adjusted life year
QoL quality of life
rUTI recurrent urinary tract infection
VAS visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Kaisary 1985 | Full text. Evaluates technique called 'hydraulic self‐dilatation' (manual occlusion of the meatus during voiding) following optical urethrotomy. Not intermittent self‐dilatation. |
| Khalid 2007 | Abstract only. Full text sought from author and publisher and not available. Probably observational case series of optical urethrotomy plus intermittent self‐dilatation. Probably non‐randomised study. |
| Suhail 2011 | Abstract only. Full text sought from author and publisher and not available. Evaluates optical urethrotomy versus optical urethrotomy then intermittent self‐dilatation. Described as comparative cross‐sectional study. Participants 'divided into groups.' Probably non‐randomised study. |
| Tunc 2002 | Full text. Optical urethrotomy alone versus serial urethral dilatation performed by a surgeon. Not intermittent self‐dilatation. |
Differences between protocol and review
Use of the GRADE methodology was not specified in the protocol but was adopted for the review to assess the quality of the evidence pertaining to the effect of intermittent self‐dilatation on risk of recurrent urethral stricture.
Contributions of authors
Matthew Jackson (MJ), Rajan Veeratterapillay (RV), Chris Harding (CH) and Trevor Dorkin (TD) were responsible for the conception of the protocol. MJ and CH were responsible for screening search results, screening retrieved papers against the inclusion criteria and appraising the quality of papers. MJ, RV and TD were responsible for extracting data. MJ and TD were responsible for checking the data for inter‐observer agreement. MJ was responsible for entering data into Review Manager. MJ and RV were responsible for assessing risk of bias. MJ was responsible for carrying out the analysis. MJ and RV were responsible for interpreting the analysis. MJ, RV and CH were responsible for drafting the review. MJ, RV, CH and TD were responsible for reviewing the final manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR, UK.
The Cochrane Incontinence Group is supported by NIHR funding
Declarations of interest
Matthew J Jackson: nil
Rajan Veeraterapillay: nil
Christopher K Harding: speaker at meetings for Astellas, GSK, Lilly, Allergan and B Braun Advisory Board for AMS
Trevor J Dorkin: speaker at meetings for Lilly, Pfizer and Astellas
New
References
References to studies included in this review
Afridi 2010 {published data only}
- Afridi NG, Khan M, Nazeem S, Hussain A, Ahmad S, Aman Z. Intermittent urethral self dilatation for prevention of recurrent stricture. Journal of Postgraduate Medical Institute 2010;24(3):239‐43. [Google Scholar]
Bodker 1992 {published data only}
- Bodker A, Ostri P, Rye‐Andersen J, Edvardsen L, Struckmann J. Treatment of recurrent urethral stricture by internal urethrotomy and intermittent self‐catheterization: a controlled study of a new therapy. Journal of Urology 1992;148(2 Pt 1):308‐10. [DOI] [PubMed] [Google Scholar]
Harriss 1994 {published data only}
- Harriss DR, Beckingham IJ, Lemberger RJ, Lawrence WT. Long‐term results of intermittent low‐friction self‐catheterization in patients with recurrent urethral strictures. British Journal of Urology 1994;74(6):790‐2. [DOI] [PubMed] [Google Scholar]
Hosseini 2008 {published data only}
- Hosseini J, Kaviani A, Golshan AR. Clean intermittent catheterization with triamcinolone ointment following internal urethrotomy. Urology Journal 2008;5(4):265‐8. [PubMed] [Google Scholar]
Husmann 2006 {published data only}
- Husmann DA, Rathbun SR. Long‐term followup of visual internal urethrotomy for management of short (less than 1 cm) penile urethral strictures following hypospadias repair. Journal of Urology 2006;176(4 Pt 2):1738‐41. [DOI] [PubMed] [Google Scholar]
Khan 2011 {published data only}
- Khan S, Khan RA, Ullah A, ul Haq F, ur Rahman A, Durrani SN, et al. Role of clean intermittent self catheterisation (CISC) in the prevention of recurrent urethral strictures after internal optical urethrotomy. Journal of Ayub Medical College, Abbottabad : JAMC 2011;23(2):22‐5. [PubMed] [Google Scholar]
Kjaergaard 1994 {published data only}
- Kjaergaard B, Walter S, Bartholin J, Andersen JT, Nohr S, Beck H, et al. Prevention of urethral stricture recurrence using clean intermittent self‐catheterization. British Journal of Urology 1994;73(6):692‐5. [DOI] [PubMed] [Google Scholar]
Matanhelia 1995 {published data only}
- Matanhelia SS, Salaman R, John A, Matthews PN. A prospective randomized study of self‐dilatation in the management of urethral strictures. Journal of the Royal College of Surgeons of Edinburgh 1995;40(5):295‐7. [PubMed] [Google Scholar]
Ngugi 2007 {published data only}
- Ngugi PM, Kassim A. Clean intermitent catheterisation in the management of urethral strictures. East African Medical Journal 2007;84(11):522‐4. [DOI] [PubMed] [Google Scholar]
Sallami 2011 {published data only}
- Sallami S, Mouine Y, Rhouma SB, Cherif K, Dahmani A, Horchani A. Clean intermittent catheterization following urethral stricture surgery using a low friction catheter versus conventional plastic catheter: a prospective, randomized trial [online]. UroToday International Journal 2011; Vol. 4, issue 1.
Tammela 1993 {published data only}
- Tammela TL, Permi J, Ruutu M, Talja M. Clean intermittent self‐catheterization after urethrotomy for recurrent urethral strictures. Annales Chirurgiae et Gynaecologiae ‐ Supplementum 1993;206:80‐3. [PubMed] [Google Scholar]
References to studies excluded from this review
Kaisary 1985 {published data only}
- Kaisary AV. Postoperative care following internal urethrotomy. Urology 1985;26(4):333‐6. [DOI] [PubMed] [Google Scholar]
Khalid 2007 {published data only}
- Khalid M, Sanaullah, Ahmad M, Hussain K, Husain S. Clean intermittent self dilatation as an adjunct to optical urethrotomy for rehabilitation of anterior urethral stricture. Pakistan Postgraduate Medical Journal 2007;18(1):11‐3. [Google Scholar]
Suhail 2011 {published data only}
- Suhail MA, Memon S‐U, Shaikh U. The comparative role of optical urethrotomy with and without clean intermittent self catheterization (CISC) in urethral stricture. Medical Forum Monthly 2011;22(11):13. [Google Scholar]
Tunc 2002 {published data only}
- Tunc M, Tefekli A, Kadioglu A, Esen T, Uluocak N, Aras N. A prospective, randomized protocol to examine the efficacy of postinternal urethrotomy dilations for recurrent bulbomembranous urethral strictures. Urology 2002;60(2):239‐44. [DOI] [PubMed] [Google Scholar]
Additional references
Ahmed 1998
- Ahmed A, Kalayi GD. Urethral stricture at Ahmadu Bello University Teaching Hospital, Zaria. East African Medical Journal 1998;75(10):582‐5. [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, Schünemann HJ, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(78650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jackson 2011
- Jackson MJ, Sciberras J, Mangera A, Brett A, Watkin N, N'Dow J, et al. Defining a patient‐reported outcome measure for urethral stricture surgery. European Urology 2011;60(1):60‐8. [DOI] [PubMed] [Google Scholar]
Lauritzen 2009
- Lauritzen M, Greis G, Sandberg A, Wedren H, Ojdeby G, Henningsohn L. Intermittent self‐dilatation after internal urethrotomy for primary urethral strictures: a case‐control study. Scandanavian Journal of Urology and Nephrology 2009;42:220‐5. [DOI] [PubMed] [Google Scholar]
Lawrence 1988
- Lawrence WT, MacDonagh RP. Treatment of urethral stricture disease by internal urethrotomy followed by intermittent 'low‐friction' self‐catheterization: preliminary communication. Journal of the Royal Society of Medicine 1988;81(3):136‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lumen 2009
- Lumen N, Hoebeke P, Willemsen P, Troyer B, Pieters B, Oosterlinck W. Etiology of urethral stricture disease in the 21st century. Journal of Urology 2009;182(3):983‐7. [DOI] [PubMed] [Google Scholar]
Mundy 2010
- Mundy AR, Andrich DE. Urethral strictures. BJU International 2010;107(1):6‐26. [DOI] [PubMed] [Google Scholar]
Reference Manager 2012
- Reference Manager Professional Edition Version 12. New York: Thomson Reuters 2012.
Santucci 2007
- Santucci RA, Joyce GF, Wise M. Male urethral stricture disease. Journal of Urology 2007;177(5):1667‐74. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine 2010;8:18. [PUBMED: 20334633] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2012
- Wong SS W, Aboumarzouk OM, Narahari R, O'Riordan A, Pickard R. Simple urethral dilatation, endoscopic urethrotomy, and urethroplasty for urethral stricture disease in adult men. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD006934.pub3] [DOI] [PubMed] [Google Scholar]
Yu‐Hung Lin 2006
- Yu‐Hung Lin, William Ji‐Sien Huang, Kuang‐Kuo Chen. Using stainless steel chopstick for self‐performing urethral sounding in preventing recurrence of anterior urethral stricture. Journal of the Chinese Medical Association 2006;69(4):189‐92. [DOI] [PubMed] [Google Scholar]


