Abstract
Normally, skeletal muscle accounts for 70–80% of insulin-stimulated glucose uptake in the postprandial hyperglycemia state. Consequently, abnormalities in glucose uptake by skeletal muscle or insulin resistance (IR) are deemed as initial metabolic defects in the pathogenesis of type 2 diabetes mellitus (T2DM). Globally, T2DM is growing in exponential proportion. The majority of T2DM patients are treated with sulfonylureas in combination with other drugs to improve insulin sensitivity. Glycosylated sulfonylureas (sulfonylurea–glucosamine analogues) are modified analogues of sulfonylurea that have been previously reported to possess antidiabetic activity. The aim of this study was to evaluate the impact of glycosylated sulfonylureas on the insulin signalling pathway at the molecular level using L6 skeletal muscle cell (in vitro) and extracted soleus muscle (ex vivo) models. To create an in vitro model, insulin resistance was established utilizing a high insulin–glucose approach in differentiated L6 muscle cells from Rattus norvegicus. Additionally, for the ex vivo model, extracted soleus muscles, adult Sprague-Dawley rats were subjected to a solution containing 25 mmol L−1 glucose and 100 mmol L−1 insulin for 24 hours to induce insulin resistance. After insulin resistance, compounds under investigation and standard medicines (metformin and glimepiride) were tested. The differential expression of PI3K, IRS-1, PKC, AKT2, and GLUT4 genes involved in the insulin signaling pathway was evaluated using qPCR. The evaluated glycosylated sulfonylurea analogues exhibited a significant increase in the gene expression of insulin-dependent pathways both in vitro and ex vivo, confirming the rejuvenation of the impaired insulin signaling pathway genes. Altogether, glycosylated sulfonylurea analogues described in this study represent potential therapeutic anti-diabetic drugs.
Normally, skeletal muscle accounts for 70–80% of insulin-stimulated glucose uptake in the postprandial hyperglycemia state.
Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by insufficient control of the blood glucose level leading to persistent hyperglycemia, which could be attributed to impaired insulin secretion, insulin action, or both.1 Type 2 diabetes mellitus (T2DM) is a heterogeneous metabolic subtype of DM, which is characterized by impaired insulin secretion from pancreatic β-cells, hyperinsulinemia, glucose intolerance and insulin resistance.2–4 The International Diabetes Federation anticipated the number of people with diabetes to increase steeply and exceed 592 million in 2025.1
Insulin is a hormone that regulates plasma glucose homeostasis and promotes glucose consumption, glycogenesis and protein synthesis in adipose tissue, skeletal muscle, and the liver.5–7 Insulin resistance is a key factor in the etiology and development of T2DM. Normally, skeletal muscle accounts for 70–80% of insulin-stimulated glucose uptake in the postprandial hyperglycemia state. Consequently, abnormalities in glucose uptake by skeletal muscle are deemed as initial metabolic defects in the development and pathogenesis of T2DM.7,8 AKT and Rac1 signaling pathways are jointly required for insulin-stimulated glucose uptake in skeletal muscle and downregulated in insulin resistance.5,7,9
Insulin activates the cellular uptake of glucose using a glucose transporter (GLUT4), which will be translocated from an intracellular pool to the plasma membrane via the downstream PI3K/AKT signaling pathway.3,8,10,11 Upon binding to the insulin receptor, insulin induces receptor conformational changes, which lead to the autophosphorylation of tyrosine residues, which activates another kinase, phosphatidylinositol-3 kinase (PI3K), and hence, increases the level of intracellular phosphatidylinositol 3,4,5-trisphosphate (PIP3). Later, PIP3 activates AKT2 at the plasma membrane, which is necessary for subsequent glucose uptake into the skeletal muscle tissue.9,12 Consequently, glucose is either converted to glycogen for storage, enters the glycolytic pathway, or oxidized for energy production. PIP3 phosphatases hydrolyse PIP3 to phosphatidylinositol 3,4,5-disphosphate (PIP2) in order to stop the PIP3-kinase signalling pathway.13 Severe complications, including hyperglycemia and microvascular and macrovascular complications, are associated with impaired glucose uptake in the skeletal muscle of patients with T2DM.14
At the cellular level, it has been reported that sulfonylureas and metformin enhance insulin-mediated glucose utilization in muscle tissue by a distal mechanism to the insulin receptor.15,16 Sulfonylurea drugs are recommended as a second-line therapy for T2DM management.17 Suaifan et al. reported the potential antidiabetic activity of a series of glycosylated sulfonylurea analogues in a streptozotocin-induced diabetic rat model.18,19 These candidates were designed to retain the pharmacophoric features of glimepiride sulfonylurea with the addition of the glucosamine moiety, an analogue to N-acetylglucosamine, one of the cell wall peptidoglycans with clinical and medicinal importance.19–22
Contrary to other skeletal muscle cell lines, L6 skeletal muscle cells (a rat cell line) and myotubes (a mouse cell line) have exhibited insulin-dependent glucose uptake in animal muscle cells in culture.23,24 Hence, they could be adopted to successfully develop an in vitro model to investigate glucose uptake in muscle cells. Moreover, previous studies reported the successful induction of diabetes using high glucose–insulin levels in L6 myotubes, C2C12 cells and isolated skeletal muscle cells.25,26 A marked decrease in insulin-induced AKT2 phosphorylation was observed in the skeletal muscle of normal-glucose-tolerance offspring of T2DM parents compared to healthy subjects.25–27 Therefore, in this work, L6 myotubes will be used as an in vitro diabetic model to investigate the mechanism of action of glycosylated sulfonylureas.
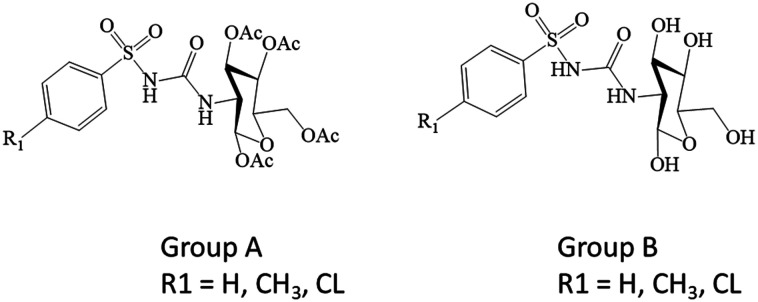
In the current study, we have explored the effect of 1,3,4,6-tetra-O-acetyl-2-deoxy-2-(benzenesulfonylurea)-d-glucopyranose analogues (group A, acetylated) and 2-deoxy-2-(benzene sulfonylurea)-d-glucopyranose analogues (group B, deacetylated), presented in Scheme 1, on the underlying insulin signaling pathway with a particular focus on the PI3K/AKT cascade using in vitro and ex vivo experimental models.
Scheme 1. Chemical structures of glycosylated sulfonylurea analogues. Group A (acetylated); 1,3,4,6-tetra-O-acetyl-2-deoxy-2-(benzenesulfonylurea)-d-glucopyranose analogues. Group B (deacetylated); 2-deoxy-2-(benzene sulfonylurea)-d-glucopyranose analogues.
To assess the impact of the glucosamine moiety conjugated to the sulfonylurea drug, two positive controls were employed, one of which was glimepiride, a sulfonylurea antidiabetic medication and the other was metformin which is currently the most commonly prescribed medication for the treatment of T2DM. The rationale and design of the research was to evaluate the efficacy of glycosylated sulfonylurea analogues in rejuvenating the impaired insulin dependent signalling pathway compared to sulfonylurea glimepiride.
Materials and methods
Reagents, kits and instruments
Dulbecco's modified Eagle's medium (DMEM), penicillin–streptomycin antibiotics (Nacalai Tesque, Inc. Japan), fetal bovine serum (FBS) (Tico Europe, South America), trypsin-EDTA (Nacalai Tesque, Inc. Japan), trypan blue solution (Sigma-Aldrich, USA), horse serum (Gibco, New Zealand), insulin (EMD Millipore Chemicals, France), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) powder (Sigma-Aldrich, USA), glucose (EMD Millipore, Chemicals, France), magnesium sulphate anhydrous (Systerm, Malaysia), potassium phosphate monobasic (J. Koern Chemicals, Malaysia), sodium chloride (Gene Chemical, France), potassium chloride (J. Koern Chemicals), calcium chloride dihydrate (Merck, Germany), sodium bicarbonate (R &M, UK), chloroform (Friendemann Schmidt's Chemicals, Germany), isopropanol (Merck, Germany), D-NASE free water (Bioline, Germany), liquid nitrogen (Merck, Germany), TRIsure solution (Bioline, Germany), absolute alcohol (AnApur, Malaysia), bovine serum albumin (BSA) (Sinar Scientific, Malaysia), glimepiride (Amaryl), metformin (Glucophage), 3,4,6-tetra-O-acetyl-2-deoxy-2-(benzenesulfonylurea)-d-glucopyranose analogues (group A1–3) and 2-deoxy-2-(benzenesulfonylurea)-d-glucopyranose (group B1–3) previously synthesized by Prof. Ghadeer Suaifan, InnuPREP RNA mini kit (Analytik Jena, Germany), Sensifast cDNA synthesis kit (Bioline, USA), My Taq HS mix kit (Bioline, USA) and SensiFAST SYBR HI-ROX kit (Bioline, USA). Water bath WB29 (Memmert, Germany), analytical balance AB204-S (Mettler Toledo, Switzerland), biosafety cabinet class II (Gelmar, Singapore), CO2 incubator (Esco, Singapore), inverted microscope (Nikon, Japan), hemocytometer chamber (Neubauer, Germany) and FLUOstar Omega plate reader (Thermo Fisher, USA).
Cell culture
Rat L6 myoblast cells (ATCC-CRL-1458) procured from Bio-Focus Scientific, Malaysia were maintained in DMEM containing 10% (v/v) FBS and 1% penicillin (100 U mL−1) cultured in a humidified atmosphere of 95% air and 5% CO2 at 37 °C until cells reached 70–80% confluency. The cells were subcultured in a solution of 0.25% (w/v) trypsin-EDTA and 0.05% glucose in PBS.28
Cytotoxicity
The cytotoxicity study was carried out according to the Mosmann protocol29 using L6 rat muscle cells with a final density of 2 × 104 cells per mL. The cell suspension (20 μL per well) was seeded into a 96-well plate and was incubated at 37 °C, 5% CO2, 95% air and 100% relative humidity to allow cell attachment. Glycosylated sulphonylurea analogues (group A (1, 2 and 3) and group B (1, 2 and 3)) and standard drugs (glimepiride and metformin) were solubilized in dimethyl sulfoxide and stored frozen prior to use.
After 24 h, the cells were treated with five concentrations (50–250 μM) of the glycosylated sulphonylurea analogues (groups A and B), standard drugs (glimepiride and metformin) and negative control (0.1% DMSO). Aliquots of 100 μL of the above concentrations of group A (1, 2 and 3) and group B (1, 2 and 3) were added to appropriate wells, which already contained 100 μL of medium. After the addition of tested compounds, the plate was incubated for 24 h. The cell medium was replaced in the dark with 100 μL fresh medium per well containing 0.5 mg mL−1 MTT (filtered with a 0.22 μm syringe filter) and incubated for another 4 h in the incubator. The supernatant was removed and 100 μL DMSO was added per well. The control wells contained the medium with tested compounds in complete media. All concentrations of tested compounds were performed in triplicate. Absorbance was read at 570 nm using a FLUOstar Omega microplate reader. All experiments were repeated three times in triplicate. Using the absorbance measurement, the cytotoxic effect of the analogues was expressed as cell viability using the following equation:10,30
Differentiation of skeletal muscle cells to myotubes and induction of insulin resistance
L6 rat skeletal muscle cells were differentiated into myotubes via DMEM containing 2% horse serum with 1% penicillin for 7 days. All the cultures were grown in a T-25 flask under an atmosphere of 95% air and 5% CO2 at 37 °C. Insulin resistance induction was performed as previously reported by Zhou et al.28 In brief, differentiated myotubes were grown at a density of 2 × 104 cells per well on 96 well microplates and were pre-treated with DMEM containing 25 mM glucose and 100 mM insulin for 24 h. The high glucose–insulin medium was discarded after 24 h incubation and was replaced with a medium containing 5 mM glucose in the absence of insulin for 5 h. The glucose enriched medium was discarded after 5 h and replaced with a 100 mM insulin medium for another 30 min. This subsequent incubation with glucose and insulin aimed to induce insulin resistance.31 Glimepiride, metformin, group A (acetylated A1, A2 and A3) and group B (deacetylated B1, B2 and B3) were prepared in DMEM with 2% inactivated FBS32 as summarized in Scheme 2.
Scheme 2. Experimental grouping and treatment.
Induction of insulin resistance in the isolated soleus muscle
Healthy female adult Sprague-Dawley (SD) rats weighing 150–200 g and 8 weeks old were used in this study. The rats had a fasting blood glucose level below 200 mg dL−1 and no physical defect. After two weeks of acclimatization in the laboratory facility, the rats were dissected, and soleus muscles (2 cm long) were extracted, snap-frozen in a polypropylene vial and stored at −80 °C for the ex vivo study. To induce insulin resistance in the soleus muscle tissues, a four-step incubation procedure with buffer was adopted.15,33 In the first step: isolated soleus muscle tissues were incubated in gassed glass vials (95% O2–5% CO2) containing 50 mL of Krebs–Henseleit buffer (KHB) supplemented with 0.1% BSA and 1 mM pyruvate for 24 h at 37 °C. In the second step: the KHB–BSA buffer was replaced with KHB containing 125 mM glucose and 500 mM insulin (high glucose/insulin) for 24 h. In the third step: the buffer was removed and replaced with KHB containing 25 mM glucose for 5 h at 37 °C. And in the last step, the buffer was replaced with KHB–glucose buffer containing 1000 mM insulin for 30 min. Insulin resistance-induced soleus muscle tissues were incubated with KHB containing the tested compound for 24 h. Then a gene expression analysis experiment was conducted. Generally, using high glucose intake to induce T2DM and insulin resistance would mimic the primary pathophysiology underlying this disorder in humans.34 Therefore, this method was adopted due to its sustainability and reliability. In addition, this approach has the ability to target the insulin-dependent signalling pathway of IRS–PI3K–PKC–AKT–GLUT4 genes.
RNA isolation and real-time polymerase chain reaction (PCR)
After incubation of L6 rat skeletal myotubes and insulin-resistant soleus muscle with glimepiride, metformin, group A (1, 2 and 3) and group B (1, 2 and 3) compounds for 24 h, total RNA was isolated using an InnuPREP RNA mini kit (Analytik Jena, Germany) and TRIsure extraction buffer (Bioline, Germany). The absorbance of each extracted RNA was measured using a NanoDrop 2000 spectrometer (Eppendorf, Germany) at 260/280 nm. Complementary DNA (cDNA) synthesis was done according to the manufacturer protocol of the SensiFast cDNA synthesis kit (Bioline, USA). cDNA and primers (Table 1) were prepared with a SensiFast SYBR HI-ROX kit (Bioline, USA) for real-time PCR. Table 1 shows the sequence of the primers and the product length (bp) of the primers used for the experiment. The amplification conditions were 95 °C (2 min) for DNA polymerase activation, followed by 95 °C (5 s), 60 °C (10 s) and 72 °C (20 s). The melting curve was constructed at the end of the reaction to confirm that amplification is properly performed. β-Actin and γ-actin were used as housekeeping genes.23 The data were analysed using Applied Biosystems Step One Software Version 2.3.
Primer sequence used in the gene expression study.
| S. no. | Name of gene | Primer sequence | Product length (bp) |
|---|---|---|---|
| 1. | IRS-1 | 5′-CGCTACATCCAGGTGCTAC-3′(F) 5′-GAAACCACTGAGGACTGCGA-3′(R) | 164 |
| 2. | PI3KCA | 5′-CAGATCATCCGAATCATGGAGAAC-3′(F) 5′-AGGCCTCCTTTGCACTGAAT-3′(R) | 155 |
| 3. | PKC-theta | 5′-CGAGGCAAGGTCTCAAGTGT-3′(F) 5′-TAAGGTGCGAGCCTGTTGAG-3′(R) | 146 |
| 4. | AKT 2 | 5′-CAGGCACCCCTTCCTTACAG-3′(F) 5′-GGTACACCACATCCGTCGAG-3′(R) | 200 |
| 5 | GLUT4 | 5′-TCATCAACGCCCCACAGAAA-3′(F) 5′-GGAGGAAATCATGCCACCCA-3′(R) | 158 |
| 6. | β-Actin | 5′-CCCTAAGGCCAACCGTGAAAA-3′(F) 5′-TACGTACATGGCTGGGGTGT-3′(R) | 150 |
| 7. | γ-Actin | 5′-GGATCTCTGTCGAGCACCATGTAG-3′(F) 5′-GGACTTTCCCACCCTGTTAGAC-3′(R) | 145 |
Statistical analysis
All data were presented as mean ± standard deviation using XL STAT version 14.0. The data were statistically analysed by one-way ANOVA followed by Duncan's multiple range post hoc test. The statistically different values (p < 0.05) were denoted with different case letters (b–e) as follows: b compared vs. diabetic control group; c compared vs. glimepiride; d compared vs. metformin; e for 1 μM groups A and B vs. 2 μM groups A and B. When relevant, f was used as an indication for significant A1 (1 μM) vs. 1 μM group A (A2, A3) and g for A1 (2 μM) vs. 2 μM group A (A2, A3), “a” diabetic control group.
Results
The MTT assay was performed to assess the cytotoxicity of the developed glycosylated sulfonylurea analogues. Results for both group A (acetylated analogues A1, A2 and A3) and group B (deacetylated analogues B1, B2 and B3) at various concentrations (50–250 μM) showed no toxic effect on L6 skeletal muscle cells as the percentage of cell viability ranged from 50–90%, except for B1 which was lower than 50% (Fig. 1).
Fig. 1. Cytotoxicity assay (MTT) results using L6 skeletal muscle cells. Cells were incubated with increasing concentrations ranging from 50–250 μM of each treatment.
Effect of glycosylated sulfonylurea analogues on the expression of insulin receptor substrate (IRS-1)
Insulin-resistant L6 skeletal muscle cells treated with the antidiabetic drugs (glimepiride and metformin) and sulfonylurea analogues (group A and group B) illustrated a significant increase in the expression of IRS-1 when compared with untreated insulin-resistant cells (Fig. 2). Moreover, both sulfonylurea analogues (groups A and B) at 1 μM and 2 μM exhibited comparable IRS-1 expression levels to glimepiride and metformin (Fig. 2). Meanwhile, IRS-1 in the insulin resistant isolated soleus muscle (ex vivo) treated with the sulfonylurea analogues (groups A and B) showed higher expression compared to that with the metformin and glimepiride treated groups (Fig. 2).
Fig. 2. Relative quantification of IRS-1 expression in glimepiride, metformin, group A (acetylated) and group B (deacetylated) (at 1 μM and 2 μM) treated diabetic groups. (I): In vitro. (II): Ex vivo. The gene was normalized against a geometric mean of two housekeeping genes (β-actin and γ-actin). All the data are presented as mean ± standard error mean where one-way ANOVA with Duncan's multiple range post hoc test was conducted using StatPlus. The statistically different values (p < 0.05) are denoted with different case letters (b–d) as follows: b compared vs. diabetic control group (a); c compared vs. glimepiride; d compared vs. metformin.
Effect of glycosylated sulfonylurea analogues on the expression of phosphatidylinositol-3 kinase (PI3K)
As illustrated in Fig. 3, the PI3K gene expression was found to be significantly higher in insulin-resistant L6 skeletal muscle cells treated with sulfonylurea analogues (groups A and B) compared to untreated and treated (glimepiride and metformin) diabetic groups. On the other hand, the PI3K expression was higher in all treated groups ex vivo.
Fig. 3. Relative quantification of PI3K expression in glimepiride, metformin, group A (acetylated) and group B (deacetylated) (at 1 μM and 2 μM) treated diabetic groups. (I): In vitro. (II): Ex vivo. The gene was normalized against a geometric mean of two housekeeping genes (β-actin and γ-actin). All the data are presented as mean ± standard error mean where one-way ANOVA with Duncan's multiple range post hoc test was conducted using StatPlus. The statistically different values (p < 0.05) are denoted with different case letters (b–d) as follows: b compared vs. diabetic control group (a); c compared vs. glimepiride; d compared vs. metformin.
Effect of glycosylated sulfonylurea analogues on the expression of protein kinase C (PKC-α)
As shown in Fig. 4, the PKC-α gene expression was upregulated in the insulin-resistant L6 skeletal muscle cell (in vitro) and insulin-resistant soleus tissue (ex vivo) models, but was downregulated in the groups treated with glimepiride, metformin, and groups A and B. All the treatment groups exhibited similar behaviour both in vitro and ex vivo.
Fig. 4. Relative quantification of PKC expression in glimepiride, metformin, group A (acetylated) and group B (deacetylated) (at 1 μM and 2 μM) treated diabetic groups. (I): In vitro. (II): Ex vivo. The gene was normalized against a geometric mean of two housekeeping genes (β-actin and γ-actin). All the data are presented as mean ± standard error mean where one-way ANOVA with Duncan's multiple range post hoc test was conducted using StatPlus. The statistically different values (p < 0.05) are denoted with different case letters (b–d) as follows: b compared vs. diabetic control group (a); c compared vs. glimepiride; d compared vs. metformin.
Effect of glycosylated sulfonylurea analogues on the expression of protein kinase B (PKB/AKT 2)
The protein kinase B (PKB/AKT 2) in vitro gene expression was significantly highest in the metformin-treated groups. However, it was almost the same for the glimepiride and 2 μM group A and B treated cells compared to the 1 μM treated cells, as shown in Fig. 5. A similar pattern was observed in the ex vivo test, except that the expression of PKB/AKT2 was significantly higher in the 2 μM group A and B treated groups compared to glimepiride (Fig. 5).
Fig. 5. Relative quantification of AKT2 expression in glimepiride, metformin, group A (acetylated) and group B (deacetylated) at (1 μM and 2 μM) treated diabetic groups. (I): In vitro. (II): Ex vivo. The gene was normalized against a geometric mean of two housekeeping genes (β-actin and γ-actin). All the data are presented as mean ± standard error mean where one-way ANOVA with Duncan's multiple range post hoc test was conducted using StatPlus. The statistically different values (p < 0.05) are denoted with different case letters (b–d) as follows: b compared vs. diabetic control group (a); c compared vs. glimepiride; d compared vs. metformin.
Effect of glycosylated sulfonylurea analogues on the expression of glucose transporter 4 (GLUT4) vesicles
As shown in Fig. 6, the GLUT4 gene significantly upregulated the cells treated with metformin, and very closely the same expression was observed in the cells treated with both 1 μM and 2 μM group A and B compounds compared to the glimepiride and diabetic groups in vitro. The GLUT4 gene expression was almost the same across the board except for the diabetic group ex vivo as illustrated in Fig. 6.
Fig. 6. Relative quantification of GLUT4 expression in glimepiride, metformin, group A (acetylated) and group B (deacetylated) (at 1 μM and 2 μM) treated diabetic groups. (I): In vitro. (II): Ex vivo. The gene was normalized against a geometric mean of two housekeeping genes (β-actin and γ-actin). All the data are presented as mean ± standard error mean using StatPlus. The data were statistically analyzed by one-way ANOVA followed by Duncan's multiple range post hoc test. The statistically different values (p < 0.05) are denoted with different case letters (b–d) as follows: b compared vs. diabetic control group (a); c compared vs. glimepiride; d compared vs. metformin.
Discussion
Owing to the adverse effects of the commonly used anti-diabetic drugs, T2DM patients require the use of sulfonylureas in combination with other drugs to improve insulin sensitivity. Therefore, the current research aims to test glycosylated sulfonylureas (groups A and B) designed to be glimepiride analogues by merging the pharmacophoric features of glimepiride with the glucosamine moiety to identify and develop newer and safer alternatives for managing diabetes and insulin resistance.18
Proper functioning of the insulin signalling pathway is necessary for glucose uptake in skeletal muscle, which, along with adipose tissue, is the primary storage site for glucose in the human body, accounting for 60–80% of insulin-stimulated glucose uptake.35 The insulin signaling pathway recruits a series of genes that culminate in the activation and translocation of GLUT4 (glucose transporter) vesicles to the cell membrane. This pathway could be insulin-dependent or insulin-independent.36–38 Impairment or malfunction of the insulin signaling pathway will lead to insulin resistance (IR), with a consequent reduction in insulin transmission, to persistent hyperglycemia and eventually to the development of T2DM. Malfunctions of insulin signaling and IR development could be attributed to the downregulation of gene expression or impairment in the phosphorylation of downstream proteins involved in the signaling, such as IRS–PI3K–PKC–AKT–GLUT4–AMPK. T2DM may also develop because of the high expression of PKC-α. Naturally, mounting evidence has shown that T2DM patients have decreased expression of the IRS gene and increased expression of the PKC-α gene.39–41
Starting with IRS-proteins, which are the chief player receptors in the insulin-dependent and insulin-independent pathway, IRS proteins play a vital role by phosphorylating the downstream proteins associated with the glucose uptake events upon binding with insulin.42–44 Downregulation of the IRS gene or non-phosphorylation of IRS proteins will lead to a reduction in the phosphorylation of downstream signaling genes/proteins in the pathway.
As is observed in the current experiment, there is a decrease in the expression of the IRS-1–PI3K–AKT–GLUT4 genes in the in vitro and ex vivo diabetic models. Downregulation of these genes may be because of an increase in oxidative stress generated from the developed hyperglycemia. Many reports have shown that oxidative stress affects the expression of the IRS gene and the other downstream genes in the insulin signaling pathway.37,45 PI3K is an intracellular lipid kinase and an element of cell membranes which is phosphorylated into phosphoinositides phosphatidylinositol-4-phosphate (PIP2) and phosphatidylinositol-4,5-bisphosphate (PIP3). These phosphoinositides are responsible for the activation of AKT/PKB via PDK-1 in skeletal muscle tissues for glucose uptake. The observed decreased expression of the PI3K gene could be explained by the decreased expression of IRS-1; our results aligned with the observation of Chunyu Tian et al.46 Similar to IRS-1, the PI3K expression has been linked to insulin resistance and reduction in insulin secretion.37 AKT was also downregulated in the insulin-resistant control group, which could be attributed to the decreased expression of both IRS and PI3K genes. AKT is a serine/threonine kinase, also known as PKB. AKT is the only isoform of the AKT, which is highly expressed in the skeletal muscle tissues, adipose tissue, and liver tissue.41,47 Understandably, the expression of the GLUT4 gene was reduced in the insulin-resistant control group, which is mainly associated with the lower expression of the IRS–PI3K–AKT genes. Inactivation of IRS-1, PI3K, and AKT2 in the insulin-resistant control group will directly affect the translocation of glucose in the skeletal muscle tissues or adipose tissue.
Glucose is a hydrophilic molecule and, hence, cannot diffuse through the lipid bilayer of the cell surface membrane. Consequently, glucose entry into cells needs to be facilitated by membrane transporters. The GLUT (glucose transporter) family facilitates glucose transportation in cells and tissues. GLUT4 is a member of the GLUT family, responsible for glucose transportation in the skeletal muscle tissues.37,48,49 Therefore, downregulation of the genes mediating glucose uptake in skeletal muscle leads to the disruption of insulin signal transduction and hence, hyperglycemia development.46
PKC is a group of serine/threonine protein kinases. It has been shown that PKCs can be activated by oxidants, such as H2O2 and mitochondrial superoxide, induced by the elevated glucose level.50 PKCα and β isoforms are responsible for the inhibition of the PI3K/AKT insulin signaling pathway, while the PKCδ isoform upregulates GLUT4 vesicles via the PI3K/AKT and MAPK pathways.33,37 It was found that the PKC-α gene is usually upregulated in T2DM,39,41 owing to the increased oxidative stress caused by hyperglycemia. PKC-α is responsible for the inactivation of insulin-dependent and insulin-independent pathways.41,51 Herein, PKC-α expression was high in the insulin-resistant control group treated with saline.
Glimepiride, a third generation of sulfonylureas, is known to lower plasma blood glucose levels in T2DM. According to Haupt et al.,52 glimepiride stimulated insulin-induced glycogen synthesis by causing the phosphorylation of IRS-1. Sulfonylureas translocate glucose into the cell membrane through activation of IRS–PI3K–PKC–AKT–GLUT4 signalling proteins.34,52 However, metformin has been reported to be able to activate both insulin-dependent and independent signalling pathways.53,54
Similarly, treatment with group A (acetylated) and B (deacetylated) sulfonylurea analogues showed upregulation of the IRS–PI3K–AKT–GLUT4 genes and downregulated expression of PKC-α. Several reports have shown that oxidative stress, increased levels of lipids and fatty acids and destruction of beta-cells of islets of Langerhans play an important role in the reduced expression of IRS–PI3K–AKT–GLUT4.37,45 PKC-α is activated by oxidants such as H2O2 and mitochondrial superoxide induced by elevated glucose levels.55 The increase in expression of these genes: IRS–PI3K–AKT–GLUT4 and reduction in expression of PKC-α may be related to the in vitro and in vivo antioxidant properties of tested compounds (unpublished results). Since oxidative stress plays a role in the impairment of the IRS–PI3K–AKT–GLUT4 pathway and destruction of beta-cells, which is critical in the development of IR and insulin secretion, quenching the oxidative stress will reverse the effect as was seen in the experiment. Hyperglycemia (elevated plasma glucose) generates oxidants such as H2O2 and mitochondrial superoxide, which contribute to the impairment of the insulin pathway through hypo-expression of the IRS–PI3K–AKT–GLUT4 genes and elevate the expression of the PKC-α gene.55 The high expression of the PKC-α gene inactivates IRS–PI3K–AKT–GLUT4 leading to IR.
Ma et al.56 reported that treatment with glimepiride increases the expression of the IRS-1 gene in an insulin resistance model. The expression of the IRS–PI3K–AKT–GLUT4–PKC-α genes in the tested compounds was close to that in glimepiride (in vitro and ex vivo), with minor differences amongst the acetylated and deacetylated groups (group A and group B). Thus, these results suggest that tested compounds share the same mechanism of IRS-1 phosphorylation, stimulate the insulin-induced glycogen synthesis, and translocate glucose into the cell membrane through activation of the PI3K/AKT pathway.52 The tested glycosylated sulfonylurea analogues rejuvenate the impaired IRS–PI3K–AKT–GLUT4 insulin signaling pathway, which could be attributed to their antioxidant, antihyperglycemic and antiglycation properties. Further research is underway to investigate the mechanism of action of the developed glycosylated sulfonylurea analogues and whether they possess any effect on insulin-independent signaling pathways.
Conclusion
The present study reports the mechanism of action of the antidiabetic sulfonylurea-glucosamine analogues. Interestingly, the tested analogues rejuvenated insulin resistance via activating the insulin-dependent (IRS–PI3K/AKT) pathway in both insulin-resistant skeletal muscle cells (in vitro) and soleus muscles (ex vivo). Results revealed that the tested analogs restored insulin sensitivity by stimulating the insulin-dependent (IRS–PI3K/AKT) pathway genes, in both in vitro and ex vivo models. These findings could be related to the compounds' hypoglycemic and antiglycation properties. The sulfonylurea–glucosamine analogues exhibited a similar pattern when compared to prescription drugs. In summary, compounds under investigation enhanced glucose uptake and reactivated the insulin-dependent pathway genes. Therefore, these compounds hold promise as medications targeting diabetes. Further research should be carried out to verify the mechanism of action using different experimental models.
Ethics approval
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of University Kebangsaan Malaysia and approved by the Animal Ethics Committee of University Kebangsaan Malaysia.
Conflicts of interest
Disclosures related to phenylsulfonylurea derivatives of 2-amino-2-deoxy-d-glucopyranose, their method of preparation, and the use thereof have been registered for patent (Reg. No. PCT/JO2022/050010).
Supplementary Material
Acknowledgments
The authors acknowledge the Deanship of Scientific Research at the University of Jordan (Grant numbers 2213, 2460) and UCSI University for providing the fund and facility for this research work.
References
- Shi G.-J. Li Y. Cao Q.-H. Wu H.-X. Tang X.-Y. Gao X.-H. Yu J.-Q. Chen Z. Yang Y. Biomed. Pharmacother. 2019;109:1085–1099. doi: 10.1016/j.biopha.2018.10.130. [DOI] [PubMed] [Google Scholar]
- Galicia-Garcia U. Benito-Vicente A. Jebari S. Larrea-Sebal A. Siddiqi H. Uribe K. B. Ostolaza H. Martín C. Int. J. Mol. Sci. 2020;21:6275. doi: 10.3390/ijms21176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman E. C. Front. Nutr. 2021;8:707371. doi: 10.3389/fnut.2021.707371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanshu D. Ali W. Wamique M. J. Diabetes Metab. Disord. 2020;19:1959–1966. doi: 10.1007/s40200-020-00641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. S. Hossain K. S. Das S. Kundu S. Adegoke E. O. Rahman M. A. Hannan M. A. Uddin M. J. Pang M.-G. Int. J. Mol. Sci. 2021;22:6403. doi: 10.3390/ijms22126403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M. C. Shulman G. I. Physiol. Rev. 2018:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton L. Shannon C. Gastaldelli A. DeFronzo R. A. Metabolism. 2022;129:155142. doi: 10.1016/j.metabol.2022.155142. [DOI] [PubMed] [Google Scholar]
- Honka M.-J. Latva-Rasku A. Bucci M. Virtanen K. A. Hannukainen J. C. Kalliokoski K. K. Nuutila P. Eur. J. Endocrinol. 2018;178:523–531. doi: 10.1530/EJE-17-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka N. Araki N. Satoh T. PLoS One. 2019;14:e0212219. doi: 10.1371/journal.pone.0212219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagbo I. J. van de Venter M. Koekemoer T. Bradley G. J. Evidence-Based Complementary Altern. Med. 2018;2018:4170372. doi: 10.1155/2018/4170372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. Liu G. Guo J. Su Z. Int. J. Biol. Sci. 2018;14:1483. doi: 10.7150/ijbs.27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira R. M. Moura L. P. D. Muñoz V. R. Silva A. S. R. D. Gaspar R. S. Ropelle E. R. Pauli J. R. Mot. Rev. Educ. Fis. 2017;23:e101609. [Google Scholar]
- Zhang X. Yang S. Chen J. Su Z. Front. Endocrinol. 2018;9:802. doi: 10.3389/fendo.2018.00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A. Mousa M. Abdelmannan D. Tay G. Hassoun A. Alsafar H. Front. Endocrinol. 2023;14:1143067. doi: 10.3389/fendo.2023.1143067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwabueze O. P. Sharma M. Balachandran A. Gaurav A. Abdul Rani A. N. Małgorzata J. Beata M.-M. Lavilla C. A. Billacura M. P. Pharmaceuticals. 2022;15:1317. doi: 10.3390/ph15111317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulett N. A. Scalzo R. L. Reusch J. E. Nutrients. 2022;14:647. doi: 10.3390/nu14030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douros A. Dell'Aniello S. Yu O. H. Y. Filion K. B. Azoulay L. Suissa S. BMJ. 2018;362:k2693. doi: 10.1136/bmj.k2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaifan G. A. R. Y. Shehadeh M. B. Darwish R. M. Al-Ijel H. Abbate V. Molecules. 2015;20:20063–20078. doi: 10.3390/molecules201119676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaifan G. A., Shehadeh M. B. and Okechukwu P., PCT/JO2022/050010, 2022, https://patents.google.com/patent/WO2022269652A1/en
- Suaifan G. A., Mohammed A. A. and Shehadeh M. B., PCT/JO2019/050011, 2020, https://patents.google.com/patent/WO2020202239A1/en
- Mohammed A. A. Suaifan G. A. Shehadeh M. B. Okechukwu P. N. Eur. J. Med. Chem. 2020;202:112513. doi: 10.1016/j.ejmech.2020.112513. [DOI] [PubMed] [Google Scholar]
- Mohammed A. A. Suaifan G. A. Shehadeh M. B. Okechukwu P. N. Drug Dev. Res. 2019;80:179–186. doi: 10.1002/ddr.21508. [DOI] [PubMed] [Google Scholar]
- Abdelmoez A. M. Sardón Puig L. Smith J. A. Gabriel B. M. Savikj M. Dollet L. Chibalin A. V. Krook A. Zierath J. R. Pillon N. J. Am. J. Physiol. 2020;318:C615–C626. doi: 10.1152/ajpcell.00540.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz K. E. Thurmond D. C. Compr. Physiol. 2020;10:785–809. doi: 10.1002/cphy.c190029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S. Adachi S.-i. Yoshizawa F. Yagasaki K. Curr. Issues Mol. Biol. 2021;43:1293–1306. doi: 10.3390/cimb43030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwoudt S. Mulya A. Fealy C. E. Martelli E. Dasarathy S. Naga Prasad S. V. Kirwan J. P. Am. J. Physiol. 2017;313:C575–C583. doi: 10.1152/ajpcell.00123.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijuin T. Hosooka T. Takenawa T. Mol. Cell. Biol. 2016;36:108–118. doi: 10.1128/MCB.00921-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q. Yang X. Xiong M. Xu X. Zhen L. Chen W. Wang Y. Shen J. Zhao P. Liu Q.-H. Cell. Physiol. Biochem. 2016;38:2030–2040. doi: 10.1159/000445562. [DOI] [PubMed] [Google Scholar]
- Mosmann T. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Edirs S. Jiang L. Xin X. Aisa H. A. J. Pharmacol. Sci. 2018;137:212–219. doi: 10.1016/j.jphs.2018.06.011. [DOI] [PubMed] [Google Scholar]
- Huang C. Somwar R. Patel N. Niu W. Torok D. Klip A. Diabetes. 2002;51:2090–2098. doi: 10.2337/diabetes.51.7.2090. [DOI] [PubMed] [Google Scholar]
- Jiang Y. Singh P. Yin H. Zhou Y. X. Gui Y. Wang D. Z. Zheng X. L. S. M. R. Group L. C. I. O. Alberta J. Cell. Physiol. 2013;228:1989–1995. doi: 10.1002/jcp.24365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsoulieris E. N. Drossopoulou G. I. Kotsopoulou E. S. Vlahakos D. V. Lianos E. A. Tsilibary E. C. PLoS One. 2016;11:e0158873. doi: 10.1371/journal.pone.0158873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X. Wang L. Wang Z. Chai S. Zhu X. Ren W. Chang X. J. Clin. Biochem. Nutr. 2019;64:194–200. doi: 10.3164/jcbn.18-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormazabal V. Nair S. Elfeky O. Aguayo C. Salomon C. Zuñiga F. A. Cardiovasc. Diabetol. 2018;17:1–14. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L. Wang Q. Jin J. Xiang Z. Chen T. Shen S. Wang H. Gao Q. Wang Y. PLoS One. 2017;12:e0188029. doi: 10.1371/journal.pone.0188029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Świderska E. Strycharz J. Wróblewski A. Szemraj J. Drzewoski J. Śliwińska A. Blood Glucose Levels. 2018;1:1–18. [Google Scholar]
- Zhang Z.-Y. Miao L.-F. Qian L.-L. Wang N. Qi M.-M. Zhang Y.-M. Dang S.-P. Wu Y. Wang R.-X. Front. Endocrinol. 2019;10:640. doi: 10.3389/fendo.2019.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassaway B. M. Petersen M. C. Surovtseva Y. V. Barber K. W. Sheetz J. B. Aerni H. R. Merkel J. S. Samuel V. T. Shulman G. I. Rinehart J. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E8996–E9005. doi: 10.1073/pnas.1804379115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin D. P. White M. F. Brazil D. P. Diabetologia. 2016;59:2280–2291. doi: 10.1007/s00125-016-4072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher J. Kleinridders A. Kahn C. R. Cold Spring Harbor Perspect. Biol. 2014;6:a009191. doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C. Chang H. La X. Li J.-a. Ma L. Evid.-Based Complementary Altern. Med. 2018;2018:4084259. doi: 10.1155/2018/4084259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamran M. Kharazmi F. Malekzadeh K. Talebi A. Khosravi F. Soltani N. Biol. Trace Elem. Res. 2019;190:396–404. doi: 10.1007/s12011-018-1555-z. [DOI] [PubMed] [Google Scholar]
- Yousef A. A. Behiry E. G. Allah W. M. A. Hussien A. M. Abdelmoneam A. A. Imam M. H. Hikal D. M. Appl. Clin. Genet. 2018:99–106. doi: 10.2147/TACG.S171096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ishaq R. K. Abotaleb M. Kubatka P. Kajo K. Büsselberg D. Biomolecules. 2019;9:430. doi: 10.3390/biom9090430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C. Chang H. La X. Li J.-a. Evid.-Based Complementary Altern. Med. 2017;2017:4393529. doi: 10.1155/2017/4393529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie R. W. Elliott B. T. Diabetes, Metab. Syndr. Obes.: Targets Ther. 2014:55–64. doi: 10.2147/DMSO.S48260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadt A. Al-Hasani H. Pfluegers Arch. 2020;472:1273–1298. doi: 10.1007/s00424-020-02417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R. Fang X. Wei J. Wu H. Wang X. Tian J. Front. Physiol. 2022;13:822333. doi: 10.3389/fphys.2022.822333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna R., McNeill T. H., Elhiani A. A. and Gundimeda U., in Methods in Enzymology, Elsevier, 2013, vol. 528, pp. 79–98 [DOI] [PubMed] [Google Scholar]
- Santo-Domingo J. Galindo A. N. Cominetti O. De Marchi U. Cutillas P. Dayon L. Wiederkehr A. Cell Commun. Signaling. 2019;17:1–19. doi: 10.1186/s12964-018-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt A. Kausch C. Dahl D. Bachmann O. Stumvoll M. Haring H.-U. Matthaei S. Diabetes Care. 2002;25:2129–2132. doi: 10.2337/diacare.25.12.2129. [DOI] [PubMed] [Google Scholar]
- Ma R. Yi B. Riker A. I. Xi Y. Acta Pharmacol. Sin. 2020;41:1403–1409. doi: 10.1038/s41401-020-00508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmadhun N. Y. Lassaletta A. D. Chu L. M. Sellke F. W. J. Thorac. Cardiovasc. Surg. 2013;145:258–266. doi: 10.1016/j.jtcvs.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldes P. King G. L. Circ. Res. 2010;106:1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma P. Gu B. Xiong W. Tan B. Geng W. Li J. Liu H. PLoS One. 2014;9:e112243. doi: 10.1371/journal.pone.0112243. [DOI] [PMC free article] [PubMed] [Google Scholar]