Abstract
Background
Androgenetic alopecia (AGA) is the most common form of hair loss. Studies have suggested a potential link to metabolic disorders, but with conflicting results. To elucidate the lipidomics profile and sex-specific variations in AGA, while exploring correlation between AGA and metabolic syndrome (MetS).
Methods
The AGA patients (n = 83) and healthy controls (n = 84) were collected in the study. The lipid profiles were analyzed using ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS). Serum levels of important factors associated with AGA, namely dihydrotestosterone (DHT), prostaglandin D2 (PGD2) and transforming growth factor-β1 (TGF-β1) were quantified using ELISA.
Results
Compared with controls, AGA patients had a higher probability of MetS (26.51% vs 11.9%, P < 0.05). Fifty-one differentially expressed lipids were identified in AGA. The kind of triglyceride (TG) were significantly increased, while phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), and phosphatidylserine (PS) exhibited remarkable decrease. PC (16:2/21:6), PC (34:4p), PE (41:7), PE (44:12), PG (40:9), PI (32:2) and TG (15:0/18:1/18:1) were identified as potential biomarkers of AGA with the highest specificity. The levels of DHT, PGD2 and TGF-β1 were significantly elevated in AGA. All seven lipids showed significant correlations with DHT, PC (34:4p) and TG (15:0/18:1/18:1) were significantly associated with PGD2, TGF-β1 displayed exclusively correlation with TG (15:0/18:1/18:1) (all P < 0.05). Furthermore, these lipids were also significantly linked to systolic blood pressure and BMI, while some of them also showed significant associations with total cholesterol and HDL-C. In subgroups, forty-two differentially expressed lipids were identified in male AGA vs male control and eighty-one in female AGA vs female control. PC (16:2/21:6) was the only specific lipids common to both sexes.
Conclusions
Aberrant lipid metabolism was observed in AGA, with distinct lipidomic profiles between male and female AGA. The potential biomarkers were closely related to DHT, PGD2, TGF-β1 and MetS-related indicators. It provides the foundation for revealing the mechanisms of AGA.
Keywords: Androgenetic alopecia, Lipidomics, Lipid metabolism, Biomarkers, Metabolic syndrome
1. Introduction
Androgenetic alopecia (AGA) is the most common type of hair loss, characterized by progressive reduction of hair density. The prevalence of AGA is on the rise globally, with males accounting for 45.72% and females accounting for 5.05% [1]. Compared with the previous 10 years, the incidence of AGA in males has more than doubled in China, with no significant change in female [2]. The pathogenesis in males and females is different and has not been fully clarified, it is related mainly to the increased sensitivity of hair follicles to androgens caused by the change of 5α-reductase activity, the increase in dihydrotestosterone (DHT) level, or androgen receptor overexpression [3]. This condition has become a major cause of anxiety and depression in patients. Many studies have shown that AGA is potentially associated with hyperlipidemia and insulin resistance, appearing abnormal lipid metabolism in patients [4]. Lipids are small signal and energy-providing molecules, which are also the main components of the membranes of living organisms. Lipids are not only involved in growth and development, nerve signal transduction, and other physiological processes, but are also closely related to the occurrence and development of various pathologies, such as metabolic syndrome (MetS), tumors, and coronary heart disease [5]. Studies have shown that lipids affect hair follicle (HF) biology through the HF association with the lipid-rich sebaceous gland [6]. Understanding metabolite differences is helpful to reveal disease characteristics and mechanisms. Therefore, lipid metabolites in AGA patients and healthy controls were detected by ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS), which was used to analyze the lipid profiles of AGA patients and the alterations in lipid profiles between different sexes of AGA patients. The impact of MetS-related indicators on lipid metabolites were explored, and the correlation between AGA and MetS was analyzed based on clinical characteristics.
2. Materials and methods
2.1. Sample collection
Eighty-three AGA patients were recruited from the department of dermatology of the First Affiliated Hospital of Anhui Medical University. They were diagnosed and evaluated simultaneously by two deputy chief dermatologists. The patients with AGA meet the following criteria: Nordwood-Hamilton classification Ⅲ-Ⅵ in males and Ludwig classification Ⅱ-Ⅲ in females [7,8]. Eighty-four healthy controls were enrolled from the physical examination center of the hospital who had no hair loss. Exclusion criteria for all participants: (1) Having autoimmune, mental, infectious or major metabolic diseases; (2) Received antitumor, immunosuppressive, radiation therapy or systemic glucocorticoid therapy in the last three months; (3) Using antibiotics within the past 30 days; (4) Pregnancy or lactation period. In addition, the subjects were further divided by sex into two subgroups: males (M)-AGA vs M-control and females (F)-AGA vs F-control. This study was approved by the hospital ethics committee (Approval ID: PJ2022-04-31). All participants signed an informed consent form.
Fasting blood samples were collected from all participants and placed in coagulation-promoting tubes. Then, the serum was separated by centrifugation and stored at −80 °C for further analysis. The levels of DHT, prostaglandin D2 (PGD2) and transforming growth factor-β1 (TGF-β1) were detected in serum by ELISA (Preferred Biotechnology, Shanghai, China) according to manufacturer's instructions, which were calculated according to the calibration curve. The levels of serum total cholesterol (CHOL), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), triglyceride (TG) and fasting blood glucose (FBG) were quantitatively detected by ADVIA Chemistry XPT system (Siemens, Akishima, Tokyo, Japan). The enzymatic method was used for CHOL, direct method for HDL-C and LDL-C, GPO-PAP method for TG and glucose oxidase method for FBG. The height, weight, waist circumference (WC), systolic blood pressure (SBP), diastolic blood pressure (DBP), and body mass index (BMI) of all participants were measured. The diagnosis of MetS was based on the International Diabetes Federation's diagnostic criteria [9].
2.2. Sample preparation
The serum samples were defrosted, and 100 μL of each sample was transferred into a new EP tube. The extracting reagents (methanol and chloroform = 1:2, V/V) (Sigma, Qingpu, Shanghai, China) were added into this tube, followed by vortexing and centrifugation. Subsequently, the underlying liquid was collected and separated into a new EP tube. The liquid was further dried with liquid nitrogen, and the residue was redissolved with complex solution (isopropyl alcohol: acetonitrile 4:6 V/V and 0.2% formic acid) (Macklin, Zhangjiang, Shanghai, China). The samples were subjected to a vortex–ultrasound–vortex procedure, then centrifuge for 10min at 12,000 rpm at 4 °C and filtered into sample vials for analysis. Quality control (QC) samples were prepared by mixing 10 μL of each sample into a pooled samples.
2.3. Non-targeted lipidomics analysis
The Dionex Ultimate 3000 UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA) coupled to a Q Exactive Hybrid Quadrupole-Orbitrap Mass Spectrometer system (Thermo Fisher, San Jose, CA, USA) was used to analyze the metabolic profiles in both the ESI-positive and the ESI-negative ion modes.
The parameters of the HPLC instrument were as follows. An Acclaim C30 chromatographic column (3.0 μm, 2.1 mm × 150 mm) (Thermo Fisher Scientific, Waltham, MA, USA) was utilized with the following mobile phases: (A) acetonitrile/water (60:40 V/V), 0.1% formic acid, 0.1% ammonium formate and (B) isopropanol/acetonitrile (90:10 V/V, containing 0.1% formic acid, 0.1% ammonium formate). Gradient elution was performed at a flow rate of 260 μL/min for 30 min (Table S1).
2.4. Data processing and statistical analysis
The original UHPLC-MS/MS data were processed by LipidSearch 4.1 Software (Thermo Fisher Scientific, San Jose, CA, USA), including those of the peak extraction, identification, and peak alignment. The extracted data were then further processed by deleting the values with relative standard deviation (RSD) > 30%, filtering the missing value of more than 50% peak area, and filling the zero value with half of the minimum value. The positive and negative ion data were combined. Lipid metabolites with the same name and smaller peak area were deleted, the total data of peak area was normalized by using the online MetaboAnalyst platform (https://www.metaboanalyst.ca).
The Simca-P 14.1 software (Umetrics, Umea, Vasterbotten, Sweden) was used to perform principle component analysis (PCA) for dimensionality reduction of multivariate original data. The orthogonal partial least-squares discriminant analysis (OPLS-DA) was utilized to visualize the metabolic alterations among groups, and permutation testing was applied to further evaluate the quality of the model. The differentially expressed lipids were screened on the basis of the combination of a statistically significant threshold of variable importance of projection (VIP) values obtained from the OPLS- DA mode, fold-change (FC) between two groups from univariate analysis and P-values from a two-tailed Student's t-test, the P-value was corrected using the false discovery rate (FDR) method by Benjamini-Hochberg. With the following criteria: VIP >1, FC > 2 or <0.5, and Padj < 0.05. The potential biomarkers were selected by time-dependent receiver operating characteristic (ROC) analysis with the area under ROC curve (AUC) value > 0.8.
Clinical data were presented as mean ± standard error. Independent-sample t-test and nonparametric test were used for analysis of the statistical significance using SPSS 17.0. Spearman correlation tests were used to assess the relationship of metabolites with DHT, PGD2, TGF-β1 and MetS-related indicators. P < 0.05 was considered to indicate a statistically significant difference.
3. Results
3.1. 1Characteristics of the lipid metabolites
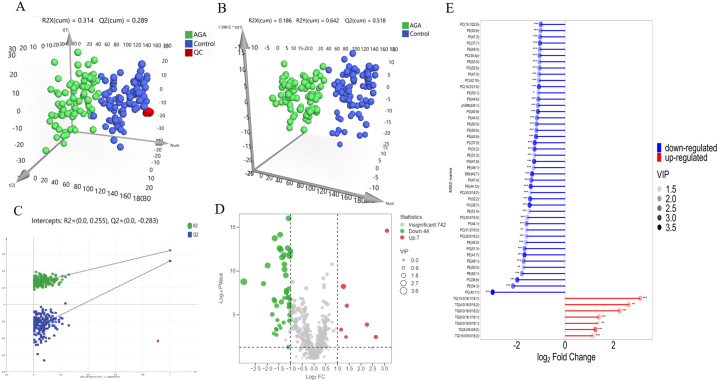
UHPLC-MS was used for analysis of serum samples from 83 AGA patients and 84 healthy controls. The AGA group and the healthy control group were matched for age and sex (Table 1). Total 192 and 1944 lipid metabolites were detected in the positive and negative ion modes, respectively. In the PCA score plots, the samples were observed basically in the 95% confidence intervals with the clustered QC samples (Fig. 1A), indicating that the test model was successfully created. A clear group differentiation was achieved by a supervised OPLS-DA model (Fig. 1B), and the 200-permutation test showed that the experimental results were stable and reproducible (Fig. 1C).
Table 1.
The comparison of clinical features and DHT, PGD2, TGF-β1 between AGA and control.
| Variable | AGA (n = 83) | Control (n = 84) | P-value |
|---|---|---|---|
| Sex | 0.565 | ||
| Male, n (%) | 53 (63.86%) | 50 (59.52%) | |
| Female, n (%) | 30 (36.14%) | 34 (40.48%) | |
| Age | 28.14 ± 7.25 | 27.71 ± 5.13 | 0.968 |
| Male, year | 30.15 ± 7.01 | 29.04 ± 5.19 | 0.361 |
| Female, year | 24.6 ± 6.34 | 25.76 ± 4.45 | 0.089 |
| WC, cm | 86.25 ± 12.81 | 80.99 ± 8.77 | 0.002 |
| BMI, kg/m2 | 24.31 ± 4.07 | 22.09 ± 2.52 | 0.000 |
| SBP, mmHg | 123.81 ± 15.75 | 117.82 ± 12.64 | 0.007 |
| DBP, mmHg | 78.80 ± 13.80 | 77.39 ± 8.66 | 0.434 |
| FBG, mmol/L | 4.87 ± 0.47 | 4.85 ± 0.62 | 0.419 |
| CHOL, mmol/L | 4.32 ± 0.88 | 4.50 ± 0.76 | 0.055 |
| HDL-C, mmol/L | 1.12 ± 0.28 | 1.24 ± 0.31 | 0.007 |
| LDL-C, mmol/L | 2.48 ± 0.80 | 2.41 ± 0.63 | 0.820 |
| TG, mmol/L | 1.32 ± 0.90 | 1.21 ± 1.03 | 0.214 |
| Incidence of MetS, n (%) | 22 (26.51%) | 10 (11.90%) | 0.017 |
| DHT, pg/ml | 1159.49 ± 250.69 | 1033.29 ± 204.14 | 0.000 |
| PGD2, pg/ml | 714.48 ± 302.19 | 607.30 ± 281.68 | 0.044 |
| TGF-β1, ng/ml | 19.32 ± 6.04 | 15.77 ± 3.72 | 0.000 |
AGA: Androgenetic alopecia, WC: Waist circumference, BMI: Body mass index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, FBG: Fasting blood glucose, CHOL: Cholesterol, TG: Triglyceride, LDL-C: Low density lipoprotein cholesterol, HDL-C: High density lipoprotein cholesterol, MetS: Metabolic syndrome, DHT: Dihydrotestosterone, PGD2: Prostaglandin D2, TGF-β1: Transforming growth factor-β1.
Fig. 1.
The different metabolic profile between AGA patients and healthy controls by multivariate statistical analysis. (A) PCA and (B) OPLS-DA plot observed differences in samples between AGA patients (n = 83) and controls (n = 84). (C) Validation of model by permutation test, R2Y (cum) = 0.642, Q2Y (cum) = 0.518, P < 0.05. (D–E) Fifty-one lipids with significant differences were screened in AGA with the threshold of VIP > 1, Padj < 0.05, and FC > 2 or < 0.5. Seven upregulated lipids were all belonged to TGs shown in red, forty-four downregulated lipids in green(D) or blue(E). PC: Phosphatidylcholines, PE: Phosphatidylethanolamines, PG: Phosphatidylglycerol, PI: Phosphatidylinositols, PS: Phosphatidylserine, phSM: Phytosphingosine, SM: Sphingomyelins, TG: Triglycerides. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
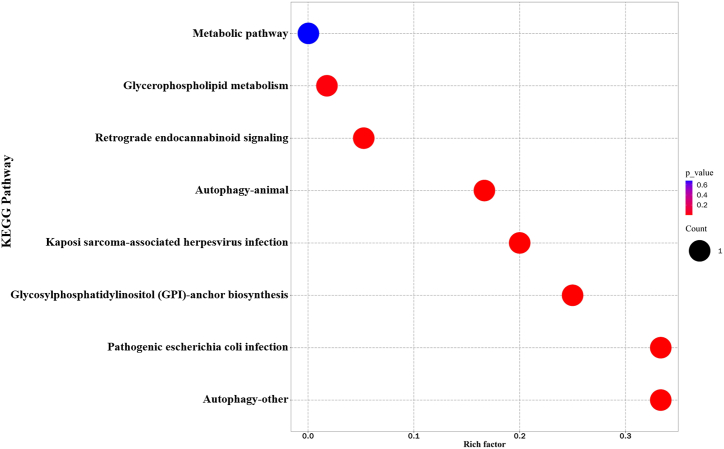
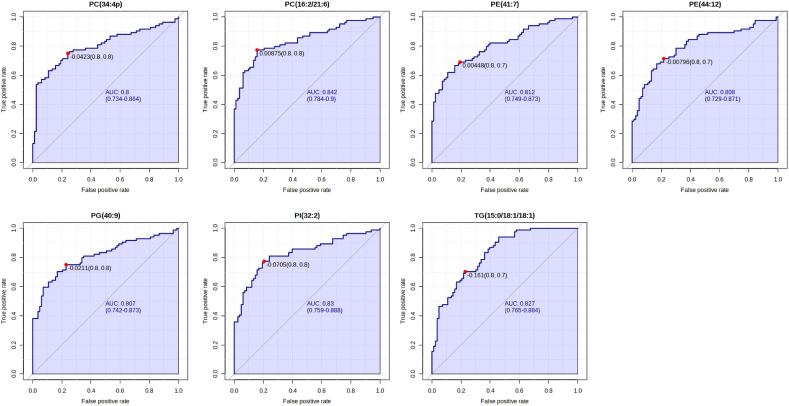
Among the detected metabolites, the lipid species included phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), phosphatidylserine (PS), lysophosphatidylcholine (LPC), ceramide (Cer), sphingomyelin (SM), diglyceride (DG) and TG. Compared with control group, fifty-one lipids with significant differences were screened out from AGA group (Table S2). Seven lipids were upregulated and all belonged to TGs, namely TG (15:0/18:1/18:1), TG (16:0/9:0/18:2), TG (6:0/16:0/18:2), TG (6:0/18:1/18:1), TG (6:0/18:1/18:2), TG (4:0/16:0/18:2) and TG (8:0/8:0/8:0). The remaining 44 lipids were downregulated, including phospholipids and sphingolipids (Fig. 1D–E). KEGG pathway analysis showed that the differentially expressed lipids were enriched in several pathways, including autophagy, pathogenic escherichia coli infection, glycosylphosphatidylinositol (GPI)-anchor biosynthesis, kaposi sarcoma-associated herpesvirus infection, retrograde endocannabinoid signaling, glycerophospholipid metabolism and metabolic pathways (Fig. 2). PC (16:2/21:6), PC (34:4p), PE (41:7), PE (44:12), PG (40:9), PI (32:2) and TG (15:0/18:1/18:1) were identified as potential biomarkers of AGA which has highly specificity with a threshold of AUC >0.8 (Fig. 3).
Fig. 2.
The enrichment analysis of important metabolic pathways. The abscissa represents the rich factor, the ordinate represents the KEGG pathway. Node size indicates the number of lipids enriched in the pathway, node color indicates P-value. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Seven lipids with the highest specificity of AGA by receiver operating characteristic curve analysis. The specific lipids which considered as the potential biomarkers were differentially expressed lipids with AUC >0.8. PC: Phosphatidylcholines, PE: Phosphatidylethanolamines, PG: Phosphatidylglycerol, PI: Phosphatidylinositols, TG: Triglycerides.
3.2. The characteristics of lipid metabolites in male and female AGA
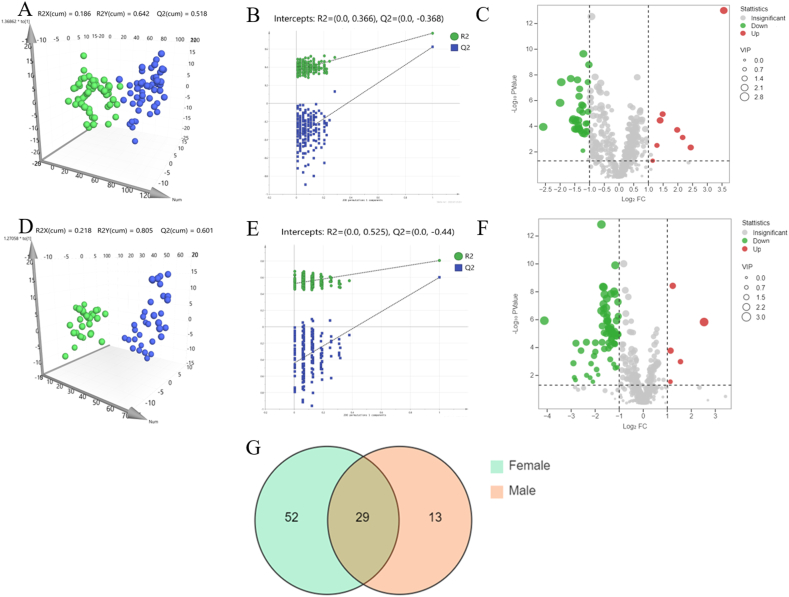
The effects of sex on AGA lipid metabolites were explored by OPLS-DA (Fig. 4A and D) and permutation test (Fig. 4B and E). The OPLS-DA results revealed significant differences between M-AGA vs M-control and F-AGA vs F-control. In male AGA, forty-two differential lipid metabolites were identified, while in female AGA, eighty-one differential lipid metabolites were detected, with the criteria of VIP >1, P-value <0.05, and FC > 2 or <0.5. Venn plot was utilized to identify the shared and distinct lipid metabolites between AGA patients of different sexes (Fig. 4G), 13 lipid metabolites were unique to male AGA patients, 52 lipid metabolites were unique to female AGA patients, and 29 lipid metabolites shared by both sexes (Tables S3 and S4).
Fig. 4.
The comparison of lipidomics characteristics between male and female AGA. (A–C) OPLS-DA plot showed metabolic differences between M-AGA (n = 53) and M-control (n = 50), the model was validated by permutation tests, R2Y (cum) = 0.773, Q2Y (cum) = 0.622. The volcanic plot showed the overall distribution of metabolites in M-AGA. (D–F) OPLS-DA plot showed metabolic differences between F-AGA (n = 30) and F-control (n = 34), the model was validated by permutation tests, R2Y (cum) = 0.805, Q2Y (cum) = 0.601. The volcanic plot show the overall distribution of metabolites in F-AGA. Red represents up-regulated metabolites, green represents down-regulated metabolites. (G) The Venn diagram showed the intersection of differential lipids between M-AGA and F-AGA. A total of 29 lipids were identified as common to both sexes, while 52 lipids were exclusively detected in female AGA and 13 lipids were exclusive to male AGA. M-AGA: Male androgenetic alopecia, M-control: Male control, F-AGA: Female androgenetic alopecia, F-control: Female control. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Compared with male controls, TG (4:0/16:0/18:2), TG (6:0/16:0/18:1), TG (6:0/16:0/18:2), TG (6:0/18:1/18:1), TG (15:0/18:1/18:1), TG (16:0/9:0/18:2), TG (22:1/18:2/22:1) and PS (37:4) were significantly upregulated in male AGA, PC, PE, PG, PI, PS (50:5), PS (50:6)), SM(d23:0), SM(d42:7) and TG (18:0/16:0/22:6) were downregulated (Fig. 4C). Among these differential lipid metabolites, TG (15:0/18:1/18:1), PC (16:2/21:6) and PC (43:6e) were the possible potential biomarkers of male AGA with the threshold of AUC >0.8 (Table 2). However, Compared with female controls, LPC (21:3), PE (18:0/18:2), TG (58:9), TG (15:0/18:1/18:1) and TG (8:0/8:0/8:0) were significantly upregulated in female AGA, whereas the other seventy-six lipid metabolites, including PE, PC, PG, PS, Cer, SM, TG (27:5/16:0/18:3), TG (18:0/18:0/20:4) and TG (12:0/12:0/14:0) were significantly downregulated (Fig. 4F). Forty-four highly specific lipid metabolites with AUC >0.8 were screened out, including PCs, PEs, PGs, PIs, TGs, SM(d42:7) and Cer(d30:0). Among them, LPC (21:3), phSM (d34:1), phSM (d32:1), PI (32:2) and TG (8:0/8:0/8:0) had the highest specificity with AUC >0.9 (Table 2). It was speculated that they may be the potential biomarker of female AGA. PC (16:2/21:6) was the only lipid metabolites with high specificity shared by both sexes.
Table 2.
The lipids with the highest specificity in males and female AGA.
| Group | Metabolites | Padj | FC | VIP | AUC |
|---|---|---|---|---|---|
| M-AGA vs M-control | PC (16:2/21:6) | 1.62E−09 | 0.49 | 2.30 | 0.81 |
| PC (43:6e) | 2.27E−10 | 0.43 | 2.52 | 0.84 | |
| TG (15:0/18:1/18:1) | 9.59E−14 | 11.80 | 2.34 | 0.88 | |
| F-AGA vs F-control | Cer (d30:0) | 1.16E−04 | 0.37 | 1.96 | 0.85 |
| LPC (21:3) | 3.74E−09 | 2.34 | 1.95 | 0.90 | |
| PC (15:1/22:6) | 5.69E−07 | 0.49 | 2.23 | 0.87 | |
| PC (37:6) | 5.38E−08 | 0.48 | 2.42 | 0.87 | |
| PC (37:7) | 9.32E−09 | 0.43 | 2.63 | 0.89 | |
| PC (16:2/21:6) | 1.45E−08 | 0.44 | 2.61 | 0.89 | |
| PC (34:4p) | 1.05E−05 | 0.48 | 1.99 | 0.82 | |
| PC (37:9) | 6.46E−06 | 0.40 | 2.39 | 0.81 | |
| PC (38:7) | 7.63E−08 | 0.32 | 2.68 | 0.86 | |
| PE (33:1/16:0) | 2.87E−06 | 0.31 | 2.20 | 0.82 | |
| PE (41:7) | 2.30E−07 | 0.30 | 2.64 | 0.84 | |
| PE (44:12) | 4.50E−06 | 0.37 | 2.41 | 0.83 | |
| PE (51:1) | 1.29E−06 | 0.35 | 2.13 | 0.82 | |
| PE (51:5) | 4.49E−06 | 0.38 | 2.05 | 0.81 | |
| PE (52:6) | 1.39E−06 | 0.36 | 2.19 | 0.83 | |
| PE (54:1) | 1.09E−05 | 0.39 | 2.25 | 0.80 | |
| PG (40:9) | 1.40E−08 | 0.39 | 2.62 | 0.89 | |
| PG (41:8) | 1.54E−08 | 0.33 | 2.73 | 0.88 | |
| PG (51:6) | 3.89E−06 | 0.37 | 2.12 | 0.81 | |
| PG (52:6) | 3.64E−06 | 0.36 | 2.14 | 0.81 | |
| PG (53:5) | 8.73E−06 | 0.32 | 2.07 | 0.81 | |
| PG (56:5) | 3.29E−06 | 0.40 | 2.08 | 0.82 | |
| phSM (d32:1) | 1.26E−10 | 0.45 | 2.68 | 0.91 | |
| phSM (d34:1) | 1.48E−13 | 0.30 | 2.92 | 0.94 | |
| phSM (d42:1) | 1.18E−06 | 0.46 | 2.45 | 0.88 | |
| PI (32:2) | 4.30E−09 | 0.31 | 2.68 | 0.90 | |
| PI (33:2) | 4.76E−09 | 0.32 | 2.80 | 0.88 | |
| PI (44:0) | 4.03E−07 | 0.35 | 2.22 | 0.84 | |
| PI (46:5) | 7.62E−06 | 0.42 | 1.94 | 0.80 | |
| PI (47:0) | 1.65E−07 | 0.32 | 2.32 | 0.85 | |
| PI (47:3) | 1.35E−06 | 0.36 | 2.25 | 0.82 | |
| PI (48:0) | 1.10E−06 | 0.36 | 2.13 | 0.83 | |
| PI (48:4) | 6.43E−06 | 0.44 | 1.90 | 0.80 | |
| PS (42:6) | 3.08E−08 | 0.35 | 2.67 | 0.87 | |
| PS (49:0) | 3.67E−07 | 0.32 | 2.32 | 0.84 | |
| PS (50:0) | 1.01E−05 | 0.40 | 2.01 | 0.80 | |
| PS (50:1) | 8.41E−06 | 0.40 | 1.83 | 0.81 | |
| PS (50:5) | 6.53E−07 | 0.35 | 2.23 | 0.83 | |
| PS (50:6) | 4.88E−06 | 0.36 | 2.05 | 0.81 | |
| PS (51:6) | 6.90E−06 | 0.40 | 2.04 | 0.81 | |
| PS (52:9) | 4.53E−06 | 0.39 | 2.08 | 0.82 | |
| SM (d42:7) | 1.37E−07 | 0.34 | 2.64 | 0.85 | |
| TG (8:0/8:0/8:0) | 1.48E−06 | 5.79 | 2.98 | 0.92 | |
| TG (27:5/16:0/18:3) | 6.49E−06 | 0.40 | 2.05 | 0.80 |
M-AGA: Male androgenetic alopecia, M-control: Male control, F-AGA: Female androgenetic alopecia, F-control: Female control, Padj: Adjusted P-value, FC: fold change, VIP: variable importance of projection, AUC: the area under ROC curve, Cer-Ceramides, LPC-Lysophosphatidylcholines, PC-Phosphatidylcholines, PE-Phosphatidylethanolamines, PG-Phosphatidylglycerol, phSM-Phytosphingosine, PI-Phosphatidylinositols, PS-Phosphatidylserine, SM-Sphingomyelins, TG-Triglycerides.
3.3. Association of DHT, GPD2 and TGF-β1 with lipid metabolites
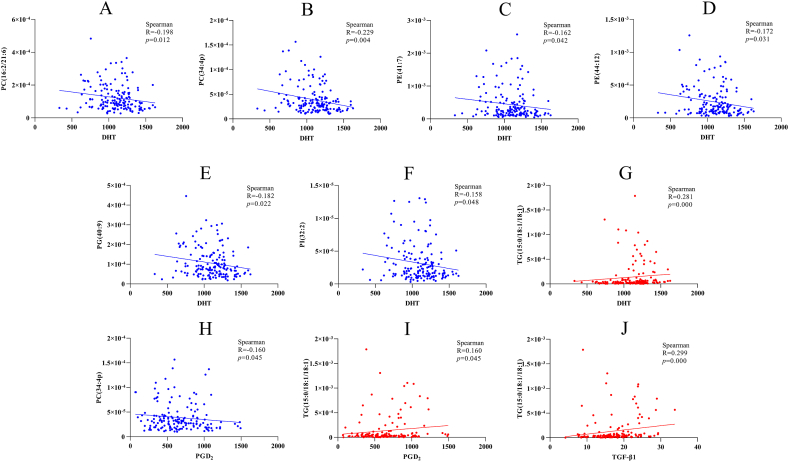
Compared to healthy controls, the levels of DHT, GPD2 and TGF-β1 were significantly higher in AGA patients (all P < 0.05) (Table 1). The correlation analysis revealed significant associations between DHT, GPD2, TGF-β1 and some of the specific lipids screened out above. DHT exhibited negative correlation with PC (16:2/21:6), PC (34:4p), PE (41:7), PE (44:12), PG (40:9) and PI (32:2) (all P < 0.05) (Fig. 5A–F). PGD2 displayed negative correlation with PC (34:4p) (R = −0.16, P = 0.045) (Fig. 5H). Notably, DHT, PGD2 and TGF-β1 was demonstrated positive correlations with TG (15:0/18:1/18:1) (P < 0.05) (Fig. 5G and I–J).
Fig. 5.
The positive results between DHT, PGD2, TGF-β1 and lipids with highest specificity by spearman correlation analysis. (A–G) The significant correlation between PC (16:2/21:6), PC (34:4p), PE (41:7), PE (44:12), PG (40:9), PI (32:2) and TG (15:0/18:1/18:1) with DHT. (H–I) PGD2 was significant correlation with PC (34:4p) and TG (15:0/18:1/18:1). (J) TGF-β1 only showed a significant correlation with TG (15:0/18:1/18:1). All P < 0.05. Blue represents negative correlation and red represents positive correlation. PC: Phosphatidylcholines, PE: Phosphatidylethanolamines, PG: Phosphatidylglycerol, PI: Phosphatidylinositols, TG: Triglycerides, DHT: Dihydrotestosterone, PGD2: Prostaglandin D2, TGF-β1: Transforming growth factor-β1. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. Characteristics of the MetS-related indicators and their correlation with lipid metabolites
The levels of SBP, WC, and BMI were higher, but HDL-C was lower in AGA group than control group (P < 0.05). Nevertheless, the levels of CHOL, LDL-C, TG, FBG, and DBP showed no significantly differences between two groups, as visible in Table 1. In addition, the probability of MetS in AGA (26.51%) was significantly higher than that in control (11.9%) (P < 0.05).
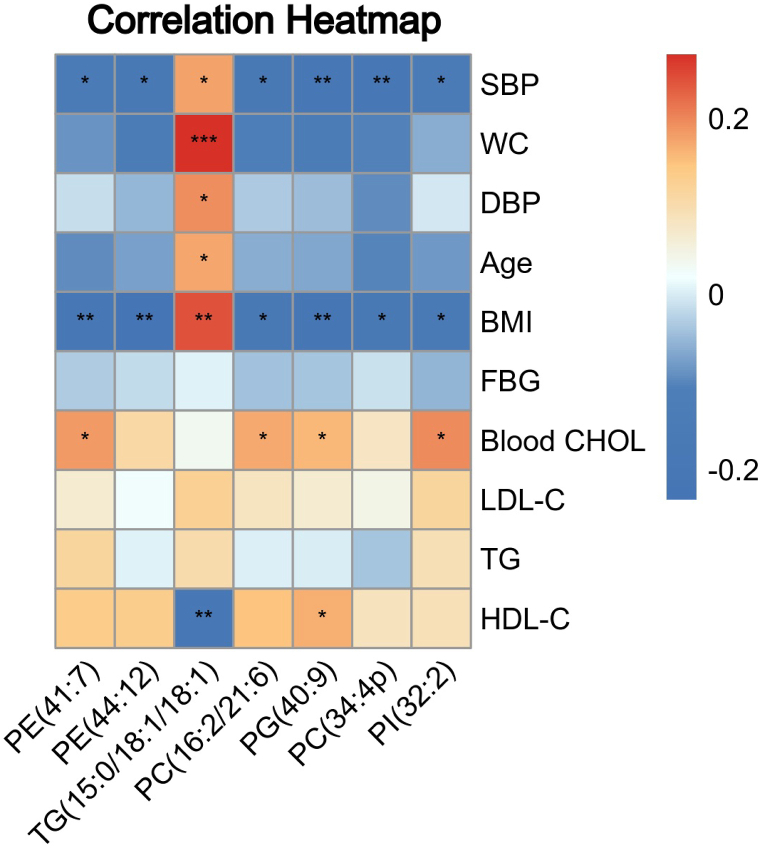
The effect of MetS-related indicators on the specific lipids of AGA was demonstrated by correlation heatmap analysis (Fig. 6). In AGA patients, phospholipids were significant negatively correlated with the levels of SBP and BMI, partial phospholipids were positively with the levels of HDL-C and CHOL. While the correlation between TG (15:0/18:1/18:1) and these clinical indications showed opposite results, TG (15:0/18:1/18:1) were positively related to the levels of WC, BMI, SBP, DBP and age, negatively correlated with the level of HDL-C. But all of them were not significantly associated with levels of FBG, LDL-C and TG.
Fig. 6.
The correlation between lipids with highest specificity in AGA and MetS-related indications. The correlation between specific lipids and MetS-related indications in AGA. Red represents positive correlation, blue represents negative correlation, * represents P < 0.05, ** represents P < 0.01, *** represents P < 0.001. PE: Phosphatidylethanolamines, TG: Triglycerides, PC: Phosphatidylcholines, PG: Phosphatidylglycerol, PI: Phosphatidylinositols, SBP: Systolic blood pressure, WC: Waist circumference, DBP: Diastolic blood pressure, BMI: Body mass index, FBG: Fasting blood glucose, CHOL: Cholesterol, LDL-C: Low density lipoprotein cholesterol, TG: Triglyceride, HDL-C: High density lipoprotein cholesterol. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Lipidomics has opened up a new and important field for research on the relationships between bioactive lipids and diseases. Glycerophospholipids are a large class of lipids composed of a glycerol backbone, a polar head, and two fatty acyl chains at the positions sn-1 and sn-2. Based on the polar head subunits, they are divided into five subclasses: PC, PE, PG, PI, and PS [10]. It has been found that the dysfunction of glycerophospholipid metabolism is related to lipid regulation, lipoprotein, systemic energy metabolism and atherosclerosis [11]. The study indicated that there were significant changes in lipid metabolism of AGA compared with healthy controls. The relative abundances of PC, PE, PG, PI, and SM were lower in AGA, while that of TG were higher. Seven specific lipid metabolites were identified in AGA, namely PC (16:2/21:6), PC (34:4p), PE (41:7), PE (44:12), PG (40:9), PI (32:2) and TG (15:0/18:1/18:1). These lipids are speculated to serve as potential biomarkers for AGA, indicating their possible involvement in the pathogenesis of AGA.
DHT, TGF-β1 and PGD2 are important factors in AGA. DHT can cause dermal papilla cells senescence in AGA through mitochondrial dysfunction, and stimulate dermal cells to inhibit hair growth and hair follicle differentiation, resulting in premature termination of the hair growth phase in the hair follicle [12,13]. TGF-β1 is a hair growth inhibitory factor, which is an androgen mediated signaling protein [14]. PGD2 inhibits hair growth in mouse and human hair follicles, and can promote the occurrence of degenerative period, leading to the increase of hair follicles and hair follicle miniaturization in resting period [15]. The levels of DHT, TGF-β1, and PGD2 were found to be significantly elevated in AGA. Notably, PC (16:2/21:6), PC (34:4p), PE (41:7), PE (44:12), PG (40:9), PI (32:2) and TG (15:0/18:1/18:1) exhibited associations with DHT, while some of these specific lipids were also related to PGD2 and TGF-β1. These findings provide further evidence supporting the involvement of these lipids in the pathogenesis of AGA.
PC is considered to be the most biologically active phospholipid with regenerative and antioxidant effects. It is a major source of many second messengers such as arachidonic acid and DG. PC produces linoleic acid under the action of secreted phospholipase A, and linoleic acid and its derivatives play an important role in inflammation and metabolic diseases [16], which may affect AGA process by affecting scalp barrier and scalp inflammation. In our study, PC (16:2/21:6) and PC (34:4p) were significantly decreased in AGA, and PC (16:2/21:6) was a highly specific lipid metabolite for AGA in both sexes.
PE is the second most abundant phospholipid, the ethanolamine group of PE has been reported to be widely involved in various life processes, such as promoting oxidative phosphorylation, initiating autophagy and endoplasmic reticulum stress [17,18]. KEGG pathway analysis showed that the differentially expressed lipids were enriched in various pathways such as autophagy. The key differentially expressed lipid in autophagy we identified was PE. Relevant studies have found that autophagy plays a key role in maintaining hair growth, and activation of autophagy can induce hair follicle activation and promote hair growth [19]. In this study, PE (41:7) and PE (44:12) were declined significantly in AGA.
As members of the phospholipid family, PG (40:9) and PI (32:2) were also significantly reduced in AGA in this study. PG is responsible for vital biological processes within cells [20], which are involved in electron transport in photosynthesis, inflammatory response reduction, lipoprotein maturation, and extracellular stress sensing [21]. PI generates phosphoinositides using phospholipases, which are vital mediators of several membrane signaling pathways [22]. Studies have shown that the PI3K-Akt signaling pathway plays a key role in hair follicle regeneration. PI3K inhibition significantly inhibits the hair follicle regeneration mediated by epidermal stem cells and skin-derived precursors [23,24].
TG is the most important energy-storage molecule in the human body, which is essential for normal cell growth, metabolism, and function. The composition and content of TG molecules are directly related to atherosclerosis, diabetes, obesity, and other diseases [25]. Suzuki et al. found a significant increase of TG content in the scalp of male patients with AGA [26]. This study showed that TG levels were elevated and TG (15:0/18:1/18:1) was a highly specific lipid metabolite in AGA. Not only that, we found that male AGA had more lipid metabolites of TG compared with female AGA, but the differential lipid metabolites of male AGA were obviously less than those of female AGA, indicating sex has an impact on lipid metabolism, which was consistent with the results of Wolrab [27]. Notably, three metabolites, TG (15:0/18:1/18:1), PC (16:2/21:6), and PC (43:6E) were presumed to be potential biomarkers of male AGA. While in female AGA, forty-four possible potential biomarkers were identified, but LPC (21:3), phSM (d34:1), phSM (d32:1), PI (32:2) and TG (8:0/8:0/8:0) had the highest specificity.
Among the differential lipid metabolites unique to different sexes of AGA, PS (37:4) was significantly increased in male AGA. Studies have shown that PS exposure may be an important event in acne skin inflammation and subsequent apoptosis [28]. The synthetic pathway of PS may affect AGA process by stimulating apoptosis of hair follicle cells. While in female AGA, LPC (21:3) was significantly rised, Cer(d30:0), phSM (d34:1) and phSM (d32:1) were significantly droped. LPC was a phospholipid messenger between cells, it can activate G protein-coupled receptors, cause growth hormone-like effects, promote the proliferation and contraction of fibroblasts and smooth muscle cells, and promote platelet aggregation. Studies have revealed that LPC is closely related to hypertension and coronary atherosclerosis [29]. SM and Cer were belonged to sphingolipids. SM can serve as a stable membrane building block, and as a generator and regulator of potent metabolic signals [30]. Cer is an essential component of the physical barrier of skin. It has been reported that synthetic Cer may induce the proliferation of human dermal papilla cells by regulating the Wnt/β-catenin and BMP2/4 signaling pathways, thereby stimulating hair growth [31].
Since AGA has been recognized as a risk factor for cardiovascular disease, many studies have shown that AGA patients have significantly worse metabolic profiles than healthy controls [32,33]. However, the associations between the pathologic mechanism of AGA and metabolic disorders have not been elucidated. Our findings revealed that AGA patients had higher probability of MetS than healthy participants, which was consistent with the results of an earlier study [34]. The prevalence of MetS in AGA patients was 26.51%, which was 2.23 times that of healthy controls, but slightly lower than the results obtained by Zhu et al. in 2022 [1], which may be related to the different ages of AGA patients participating in these studies, our subjects were much younger. Research has shown that the prevalence of MetS increases with age [34].
Small changes in phospholipid levels appeared to have a large effect on parameters associated with MetS [11]. In specific lipid metabolites of AGA, phospholipids were negatively correlated with SBP and BMI, partially positively correlated with HDL-C and blood CHOL. While TG (15:0/18:1/18:1) was positively correlated with WC, BMI, SBP, DBP and age, negatively correlated with HDL-C. In our study, AGA patients had lower HDL-C levels, indicating that AGA have higher levels of atherogenic lipids, which may partially explain the association with increased cardiovascular disease risk in AGA. Besides, WC and BMI were significantly higher in AGA. Obesity has been shown to inhibit the sonic hedgehog signal transduction in hair follicle stem cells, leading to hair follicle miniaturization and eventually hair loss [35], which further demonstrated the positive link between AGA and obesity. Furthermore, SBP was significantly higher in AGA. This finding was consistent with some studies [36,37]. This effect may be due to raised levels of salt-corticosteroids such as aldosterone in AGA. It is speculated that the relevant management of BP can provide certain benefits for prevention and progression of AGA.
This was a non-target study, without quantitative and functional research, so future investigations with more samples are needed to confirm our findings.
This is the first study to investigate lipid metabolites by lipidomics in the serum of male and female AGA patients. It was revealed that AGA patients exhibit elevated level of TG and decreased levels of PC, PE, PG, PI, PS, LPC, and SM. The lipid profiles in AGA differed between males and females. AGA patients are more prone to developing MetS. The specific lipids in AGA have shown correlation with DHT, PGD2 and TGF-β1, while being largely influenced by MetS-related indicators, especially SBP, BMI, HDL-C and blood CHOL. This study provides a foundation for revealing the pathogenesis and discovering biomarkers of AGA.
Ethics approval and consent to participate
This study was approved by the hospital ethics committee (Approval ID: PJ2022-04-31). All participants signed an informed consent form.
Funding
This study was funded by the National Natural Science Foundation of China (No. 82373481, 82203920), the Key Project of Natural Science Research in Colleges and Universities in Anhui Province (No. 2023AH053302) and research fund of Anhui Medical University (No. 2022xkj202).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
CRediT authorship contribution statement
Shuqin Wang: Formal analysis. Mei Li: Conceptualization. Shichun Qin: Investigation. Rui Wang: Methodology. Liping Dong: Methodology. Sheng Wang: Project administration. Fengli Xiao: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to express our gratitude to the individuals and their families who participated in this project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e26204.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Zhu H., et al. A community-oriented survey on the association between androgenetic alopecia and metabolic syndrome in Chinese people. Front. Med. 2022;9 doi: 10.3389/fmed.2022.1009578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiu Y., et al. Systematic review and meta-analysis of the association between metabolic syndrome and androgenetic alopecia. Acta Derm. Venereol. 2022;102 doi: 10.2340/actadv.v101.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devjani S., et al. Androgenetic alopecia: therapy update. Drugs. 2023;83(8):701–715. doi: 10.1007/s40265-023-01880-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawoud N.M., et al. Circulating and/or cutaneous irisin resistance: a novel link among androgenetic alopecia, comorbid metabolic syndrome and cardiovascular risks. J. Cosmet. Dermatol. 2023;22(9):2584–2597. doi: 10.1111/jocd.15760. [DOI] [PubMed] [Google Scholar]
- 5.Dalhaimer P. Lipid droplets in disease. Cells. 2019;8(9) doi: 10.3390/cells8090974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lattouf C., et al. Treatment of alopecia areata with simvastatin/ezetimibe. J. Am. Acad. Dermatol. 2015;72(2):359–361. doi: 10.1016/j.jaad.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Norwood O.T. Male pattern baldness: classification and incidence. South. Med. J. 1975;68(11):1359–1365. doi: 10.1097/00007611-197511000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br. J. Dermatol. 1977;97(3):247–254. doi: 10.1111/j.1365-2133.1977.tb15179.x. [DOI] [PubMed] [Google Scholar]
- 9.Alberti K.G., Zimmet P., Shaw J. The metabolic syndrome–a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 10.Tyurina Y.Y., et al. Redox (phospho)lipidomics of signaling in inflammation and programmed cell death. J. Leukoc. Biol. 2019;106(1):57–81. doi: 10.1002/JLB.3MIR0119-004RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Veen J.N., et al. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017;1859(9 Pt B):1558–1572. doi: 10.1016/j.bbamem.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Jung Y.H., et al. Cyanidin 3-O-arabinoside suppresses DHT-induced dermal papilla cell senescence by modulating p38-dependent ER-mitochondria contacts. J. Biomed. Sci. 2022;29(1):17. doi: 10.1186/s12929-022-00800-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu D., et al. Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomed. Pharmacother. 2021;137 doi: 10.1016/j.biopha.2021.111247. [DOI] [PubMed] [Google Scholar]
- 14.Liang Y., et al. Adipose mesenchymal stromal cell-derived exosomes carrying MiR-122-5p antagonize the inhibitory effect of dihydrotestosterone on hair follicles by targeting the TGF-β1/SMAD3 signaling pathway. Int. J. Mol. Sci. 2023;24(6) doi: 10.3390/ijms24065703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu Y.C., et al. CXXC5 mediates DHT-induced androgenetic alopecia via PGD(2) Cells. 2023;12(4) doi: 10.3390/cells12040555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choque B., et al. Linoleic acid: between doubts and certainties. Biochimie. 2014;96:14–21. doi: 10.1016/j.biochi.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Shinzawa-Itoh K., et al. Structures and physiological roles of 13 integral lipids of bovine heart cytochrome c oxidase. EMBO J. 2007;26(6):1713–1725. doi: 10.1038/sj.emboj.7601618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichimura Y., et al. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408(6811):488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- 19.Chai M., et al. Stimulation of hair growth by small molecules that activate autophagy. Cell Rep. 2019;27(12):3413–3421.e3. doi: 10.1016/j.celrep.2019.05.070. [DOI] [PubMed] [Google Scholar]
- 20.Struzik Z.J., et al. Stereospecific synthesis of phosphatidylglycerol using a cyanoethyl phosphoramidite precursor. Chem. Phys. Lipids. 2020;231 doi: 10.1016/j.chemphyslip.2020.104933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang G., et al. Concise synthesis of ether analogues of lysobisphosphatidic acid. Org. Lett. 2005;7(18):3837–3840. doi: 10.1021/ol051194w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goncalves M.D., Hopkins B.D., Cantley L.C. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N. Engl. J. Med. 2018;379(21):2052–2062. doi: 10.1056/NEJMra1704560. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y., et al. PI3K/Akt signaling pathway is essential for de novo hair follicle regeneration. Stem Cell Res. Ther. 2020;11(1):144. doi: 10.1186/s13287-020-01650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kageyama T., et al. Impacts of manipulating cell sorting on in vitro hair follicle regeneration. J. Biosci. Bioeng. 2022;134(6):534–540. doi: 10.1016/j.jbiosc.2022.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Han X., Ye H. Overview of lipidomic analysis of triglyceride molecular species in biological lipid extracts. J. Agric. Food Chem. 2021;69(32):8895–8909. doi: 10.1021/acs.jafc.0c07175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki K., et al. Scalp microbiome and sebum composition in Japanese male individuals with and without androgenetic alopecia. Microorganisms. 2021;9(10) doi: 10.3390/microorganisms9102132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolrab D., et al. Lipidomic profiling of human serum enables detection of pancreatic cancer. Nat. Commun. 2022;13(1):124. doi: 10.1038/s41467-021-27765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou M., et al. Lipidomics reveals skin surface lipid abnormity in acne in young men. Br. J. Dermatol. 2018;179(3):732–740. doi: 10.1111/bjd.16655. [DOI] [PubMed] [Google Scholar]
- 29.Kraemer M.P., et al. Effects of diet and hyperlipidemia on levels and distribution of circulating lysophosphatidic acid. J. Lipid Res. 2019;60(11):1818–1828. doi: 10.1194/jlr.M093096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goñi F.M. Sphingomyelin: what is it good for? Biochem. Biophys. Res. Commun. 2022;633:23–25. doi: 10.1016/j.bbrc.2022.08.074. [DOI] [PubMed] [Google Scholar]
- 31.Oh J.H., et al. Synthesized ceramide induces growth of dermal papilla cells with potential contribution to hair growth. Ann. Dermatol. 2019;31(2):164–174. doi: 10.5021/ad.2019.31.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lie C., Liew C.F., Oon H.H. Alopecia and the metabolic syndrome. Clin. Dermatol. 2018;36(1):54–61. doi: 10.1016/j.clindermatol.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Hamed A.M., Fatah M.A., Shams G.M. Androgenetic alopecia and metabolic syndrome: is Alarin a missing link? J. Clin. Aesthet. Dermatol. 2022;15(7):32–37. [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S., et al. Sex and age differences in the impact of metabolic syndrome and its components including A body shape index on arterial stiffness in the general population. J. Atherosclerosis Thromb. 2022;29(12):1774–1790. doi: 10.5551/jat.63371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morinaga H., et al. Obesity accelerates hair thinning by stem cell-centric converging mechanisms. Nature. 2021;595(7866):266–271. doi: 10.1038/s41586-021-03624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozbas Gok S., Akin Belli A., Dervis E. Is there really relationship between androgenetic alopecia and metabolic syndrome? Dermatol. Res. Pract. 2015;2015 doi: 10.1155/2015/980310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danesh-Shakiba M., Poorolajal J., Alirezaei P. Androgenetic alopecia: relationship to anthropometric indices, blood pressure and life-style habits. Clin. Cosmet. Invest. Dermatol. 2020;13:137–143. doi: 10.2147/CCID.S231940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.