Abstract
G protein-coupled receptors (GPCRs) are the largest family of cell surface receptors and a critical class of regulators of mammalian physiology. Also known as seven transmembrane receptors (7TMs), GPCRs are ubiquitously expressed and versatile, detecting a diverse set of endogenous stimuli, including odorants, neurotransmitters, hormones, peptides, and lipids. Accordingly, GPCRs have emerged as the largest class of drug targets, accounting for upward of 30% of all prescription drugs. The view that ligand-induced GPCR responses originate exclusively from the cell surface has evolved to reflect accumulating evidence that receptors can elicit additional waves of signaling from intracellular compartments. These events in turn shape unique cellular and physiological outcomes. Here, we discuss our current understanding of the roles and regulation of compartmentalized GPCR signaling.
Keywords: G protein-coupled receptor, spatial encoding, endosomal signaling, cAMP, PKA, adenylyl cyclase, PDE, retromer, arrestin
From a cell surface-centric to a spatially encoded model of GPCR action
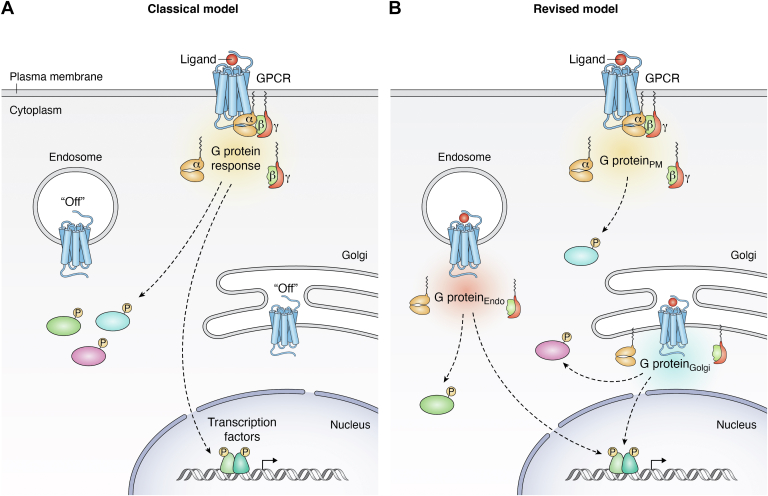
The classical view had been that GPCR signaling takes place at the cell surface (1). The agonist-bound receptor undergoes a conformational change that promotes the activation of the heterotrimeric protein complex, Gαβγ. G proteins are classified into four main families according to their Gα subunit. Gαs and Gαi/o interact with adenylyl cyclase enzymes to stimulate or inhibit the production of cyclic AMP (cAMP), respectively. Gαq activates the mobilization of calcium via phospholipase C (PLC), while Gα12/13 signals vias Rho GTPase stimulation. These second messengers initiate signaling cascades that lead to the regulation of key kinases and transcription factors that coordinate a cellular response. For instance, cAMP activates protein kinase A (PKA). Following ligand binding, the GPCR undergoes post-translational modifications, including phosphorylation of its intracellular loops and/or the C-terminal tail by GPCR kinases (GRKs) (2). This in turn facilitates the recruitment of β-arrestins one and 2. Classically, the association of arrestins with the receptor was presumed to terminate its signaling in two ways: by sterically hindering interactions with the G protein and by promoting GPCR internalization through the scaffolding of the AP2 adaptor complex and clathrin (2, 3, 4). Subsequently, the internalized receptor is sorted and degraded in lysosome or recycled back to the plasma membrane for additional cycles of activation (Fig. 1A).
Figure 1.
Spatial encoding of GPCR signaling.A, classical model: GPCRs initiate all G protein-dependent responses from the cell surface, and are turned “off” following internalization. B, revised model: Active GPCRs on intracellular membranes (endosomes, Golgi) stimulate a “second wave” of G protein-dependent signaling that gives rise to unique cellular responses, including protein phosphorylation and transcriptional reprogramming. endo, endosome; PM, plasma membrane; TF, transcription factor.
This traditional model has undergone significant revision in the past 2 decades that highlights the immense complexity of the cascades and their resulting signaling outputs. For example, it is now well-appreciated that the functions of arrestins are multifaceted and extend beyond receptor desensitization. Arrestin-coordinated signaling scaffolds downstream of GPCR activation give rise to responses that are distinct from the ones driven by the G protein. While not the subject of the current review, the phenomenon of “arrestin-biased signaling” is of high physiological and pharmacological significance (5, 6).
A more recent shift in the classical model involves the discovery that receptors can activate G proteins from intracellular compartments. Early evidence supporting the activation of mammalian G proteins inside the cell emerged from studies of the metabotropic glutamate receptor, mGluR5, and the hormone receptors, TSHR (thyroid stimulating hormone receptor) and PTHR (parathyroid hormone receptor). There, biochemical and pharmacological manipulations were utilized to demonstrate that G protein-dependent production of the second messengers Ca2+ and cAMP is blunted in the absence of activation of the intracellular receptor fractions (7, 8, 9). However, these studies relied on indirect and on in vitro methods to examine intracellular GPCR signaling (global endocytic blockade, cell fractionation, temporal correlation between receptor endocytosis and second messenger kinetics). The first direct demonstration of endosomal GPCR and G protein activity emerged from experiments utilizing nanobody-based conformational biosensors in live cells. A study employed two biosensors: Nanobody 80 (Nb80) raised against purified active beta2-adrenergic receptor (β2AR), and Nb37 recognizing a catalytic intermediate of the G protein activation cycle (10). The nanobodies co-localized with over-expressed β2AR at the cell surface and on early endosomes following ligand-induced receptor activation and internalization (10). The same strategy has since been applied to directly demonstrate the intracellular GPCR/G protein activity for several other receptors, most notably also for GPCRs expressed at native levels (11, 12, 13, 14, 15).
What are the roles of intracellular G protein signaling? This question is only beginning to be addressed, but emerging evidence supports that intracellular receptor activity drives discrete cellular functions. In parallel genome- and proteome-wide investigations carried out on native adrenergic receptors in HEK293 cells it was demonstrated that endosomal β2AR/cAMP signaling gives rise to unique transcriptional and phospho-signaling (16, 17). Two orthogonal approaches were utilized to pinpoint the functional contributions of endosomal signaling: acute pharmacological inhibition of endocytosis and local cAMP production via a photoactivatable adenylyl cyclase (“opto-cyclase”) tethered to different subcellular sites. With these, it was found that the entire gamut of transcriptional changes driven by adrenergic receptor activation is selectively induced from the endosome (17). Likewise, endosomal GPCR/cAMP was reported as the primary driver of protein phosphorylation via PKA (16). Notably, by carefully matching the light stimulation conditions to yield comparable cAMP production in their opto-cyclase experiments, the authors demonstrated that the location of signaling alone, rather than the total amount of second messenger, dictates these unique outcomes (16, 17). Similar to the β2AR, intracellular activity from endogenous TSHR/Gαs is required for the regulation of discrete PKA substrates and for the induction of several known downstream transcriptional target genes (9, 11). Hence, this paradigm extends beyond a single GPCR, and it remains to be determined if the same principle applies to all receptors that signal via cAMP production.
Therefore, rather than being strictly plasma membrane-delimited, receptor signaling is compartmentalized, as the cell can functionally differentiate the subcellular location of GPCR/G protein activation when initiating various responses (Fig. 1B). Although also referred to as “location bias” or “spatial bias,” this review uses the term “spatial encoding” of GPCR signaling to refer to this phenomenon.
Diversity of intracellular GPCR signaling
To date, reports have linked virtually all sub-cellular compartments to GPCR activity transduced via different Gα subunits (18). Examples of organelles that harbor signaling receptors include the early endosome (7, 10), very early endosome (19), Golgi (12, 13, 14), endoplasmic reticulum (20), mitochondrion (21), and nucleus (22, 23). The ever-growing list of active intracellular GPCRs is comprised of receptors that couple to Gαs, Gαq, and Gαi. As we discuss below, there are ongoing efforts to shed light on the consequences and regulation of intracellular GPCRs, but based on the information at hand, the functions and mechanisms of spatial encoding are likely to be diverse. Examples of mechanisms that may promote diverse outcomes from intracellular GPCRs include the following.
-
•
Intracellular compartments can harbor receptors that couple to different G proteins: A straightforward scenario for how intracellular receptor activation can lead to discrete outcomes involves GPCR signaling via distinct G proteins. The early endosome, one of the best characterized signaling compartments to date, has been linked to the acute activation of both Gαs- (e.g., β2AR (10, 16)) and Gαi-coupled receptors (e.g., delta- and mu-opioid receptors (12)).
-
•
Receptors that signal from the same compartment and couple to the same G protein exhibit different signaling kinetics: In the case of certain receptors (e.g., Vasopressin receptor 2 (V2R) and PTHR (24, 25)), the endosomal population gives rise to persistent cAMP production. In contrast, other GPCRs (10, 16) undergo acute and reversible G protein/cAMP activation on endosomes. Whether the distinct temporal dynamics of the endosomal cAMP signal encode additional functional information remains to be determined.
-
•
Receptors that signal from the same compartment via the same G protein take different trafficking routes to get to their destination: Although the TSHR and beta1-adrenergic receptor (β1AR) both activate Gαs at the Golgi (11, 14), these receptors traffic via distinct mechanisms. The TSHR undergoes ligand-dependent internalization into early endosomes followed by retrograde trafficking to the Golgi. On the other hand, the β1AR is delivered to the Golgi through the biosynthetic pathway. Therefore, it is likely that the Golgi activity and residence of each receptor is subject to unique regulation.
-
•
The same receptor signals from multiple intracellular compartments: Lastly, some receptors stimulate G protein signaling from multiple organelles. The Gαq-coupled GPCR mGluR5 elicits calcium responses from the ER and nuclear membranes (20, 26). Two opioid receptors, delta (DOR) and mu (MOR), activate Gαi signaling from early endosomes and the Golgi (12). The gamut of unique downstream responses that can be generated by the same signal originating from each of these organelles is unexplored.
Intracellular receptors: (patho)physiology and pharmacology
Recent studies have highlighted the role of endomembrane signaling in numerous physiological and pathophysiological processes and have suggested that spatial encoding of GPCR signaling can be exploited to improve therapeutic strategies that target these pathways. We highlight several of these reports below and reference additional studies that further support this model (27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38).
Work on GPCRs coupled to each of the main Gα proteins supports that compartmentalized receptor activity critically shapes physiology across tissues. In the brain, key neuronal processes are mediated by mGluR5/Gαq signaling. It was found that the metabotropic glutamate receptor is enriched in the perinuclear region of hippocampal neurons, and this intracellular population is required to elicit sustained Gαq/Ca2+ signaling and long-term depression (LTD) after chemical and low-frequency stimulation (39). Similarly, intracellular signaling is the driver of essential physiology for another neuronal GPCR, the melatonin type 1 receptor (MT1R), which couples to Gαi. Stimulation of MT1R on the mitochondrial membrane of cerebrocortical neurons has neuroprotective effects via blockade of cytochrome c release and associated caspase activation (21). Intracellular GPCR/G protein activation is also important outside of the central nervous system. In kidney cells, this persistent activity of endosomal V2R leads to the translocation of aquaporin water channels and epithelial ion channels to the apical membrane, resulting in increased water and sodium permeability (40). While physiologically advantageous in the contexts outlined above, intracellular GPCRs have also been implicated as drivers of disease. For example, selective stimulation of Golgi-localized β1AR in cardiac myocytes results in cardiac hypertrophy through Gαs-dependent induction of phosphoinositide hydrolysis (15). Likewise, nerve injury in spinal dorsal horn neurons leads to an increase in the population of perinuclear mGluR5 and neuropathic pain (41).
The demonstration that intracellular GPCR/G protein signaling has physiological and pathophysiological implications has raised the intriguing possibility that this phenomenon can be exploited therapeutically. One strategy would rely on the distinct pharmacodynamic properties of existing compounds in order to selectively target the receptor at a given compartment. Of specific interest are the affinity, hydrophobicity, and endocytic efficacy of the ligand. Indeed, several studies have provided proof-of-principle for the utility of such approaches. Long-acting agonists that remain bound to the PTHR following internalization into endosomes stimulate sustained Gαs/cAMP signaling and elicit elevated serum calcium and serum vitamin D (7, 42). This contrasts with an agonist that dissociates rapidly from the receptor and does not induce endosomal signaling (7, 43, 44). Further, a pharmacologic approach relying on agonists and antagonists with distinct hydrophobicity has been applied to selectively inactivate the pathological functions of several intracellular GPCRs. As one example, the increase in neuropathic pain following perinuclear mGluR5 activation in the model of nerve injury that we discuss above can be attenuated by the application of a membrane-permeant mGluR5 antagonist (41). Similarly, beta-adrenergic receptor antagonists that can access the Golgi-localized population of β1ARs are effective at preventing cardiac hypertrophy compared to antagonists that cannot permeate the plasma membrane (15). The range of endocytic efficacies of GPCR agonists presents yet another important tool to manipulate compartmentalized receptor activity. For the β2AR, it was reported that dopamine, a ligand that does not induce robust internalization, leads to weaker transcriptional signaling compared to epinephrine, a ligand that drives receptor endocytosis (17). In addition to targeting the receptor itself, the transporters responsible for ligand entry into the cells and into the lumen of organelles could also be manipulated for therapeutic purposes. Catecholamines are transported into the cell via organic cation transporters (OCT proteins). Inhibition of OCT3 with corticosterone in cultured cardiac myocytes abolishes norepinephrine-induced β1AR activation at the Golgi and subsequent cardiac hypertrophy (15). Likewise, decreasing intracellular glutamate uptake via the excitatory amino acid transporter, EAAT-3, reduces perinuclear mGluR5 activity and neuropathic pain (41). Finally, the machinery mediating site-selective GPCR activation could be targeted therapeutically to manipulate physiological outcomes. As we discuss below, only a handful of intracellular GPCR regulators have been identified to date, and most of these are components of the trafficking machinery. Emerging evidence supports the benefit of targeting the endocytic machinery in certain disease contexts. Tissue-selective knockdown of dynamin, the GTPase required for membrane scission of endocytic vesicles, prevents intracellular PAR2-induced inflammation and nociception (38). Further, pharmacological inhibition of dynamin reduces CXCR3-induced inflammatory response in vivo by preventing the sustained endosomal signal from the receptor (45). Therefore, elucidation of the mechanistic basis of compartmentalized receptor signaling is a key area of active investigation with translational potential. We next summarize the current understanding of how spatial encoding is established.
What makes the endosome a functionally specialized GPCR signaling compartment?
In this section, we focus on the early endosome, as it is currently one of the most extensively characterized sites of intracellular GPCR signaling. We outline what is presently known about the regulation of spatially-encoded receptor activity, and we speculate about additional characteristics of the endosomal compartment that are likely of regulatory significance and should be interrogated in future studies (Fig. 2).
Figure 2.
Mechanisms regulating intracellular GPCR activity. The assembly of unique biochemical complexes (PDEs, ACs, arrestins, retromer) at the cell surface compared to endosomes underlies spatially biased GPCR signaling. Endosomes are also biophysically distinct from the plasma membrane. Their discrete size, three-dimensional intracellular positioning, ionic and lipid compositions impact receptor activity and the resulting cascade outputs. AC, adenylyl cyclase; PDE, phosphodiesterase.
Unique molecular factors shape diverse downstream signaling dynamics and outcomes
Adenylyl cyclases (ACs) are a relevant class of G protein effectors that mediate the production of cAMP in response to G protein binding and calcium. Immunocytochemical staining analyses have detected colocalization of distinct AC isoforms with GPCRs on endosomes. The dopamine receptor D1 (D1R) is present together with Gαs and AC5 on endosomes of striatal neurons (46). In primary thyroid cells, AC3 is the main isoform colocalizing with the TSHR/Gαs complex on endosomes and at the Golgi (9). In addition to colocalization, studies have also explored the functional role of AC isoforms in intracellular GPCR activity. It was demonstrated that AC9 is required for the selective transduction of endosomal β2AR/Gαs signaling (47). The authors found that β2AR and Gαs activation promotes the endocytosis of AC9 from the plasma membrane into early endosomes, and this internal pool of AC9 mediates cAMP production (47). While transmembrane ACs represent one facet of this regulation of signaling, the soluble AC enzyme, sAC, also plays a mechanistic role in spatial encoding. An initial demonstration was provided for the corticotropin-releasing hormone receptor 1 (CRHR1), which elicits sustained Gαs/cAMP signaling specifically via sAC stimulation at endosomes (48). Lastly, there could be crosstalk between transmembrane and soluble AC signaling downstream of intracellular GPCRs. According to a recent report, endosomal TSH receptors simulate local transmembrane AC activity, which in turn leads to calcium accumulation and the subsequent activation of nuclear sAC signaling (49).
GPCR signal compartmentalization is further shaped by local phosphodiesterase (PDE) activity. A report showed that cAMP generated at the cell surface undergoes more efficient PDE-dependent hydrolysis compared to cAMP coming from the endosome (16). Similarly, another study described that activation of receptors with low doses of ligand leads to the generation of highly localized, nanometer-sized cAMP signaling domains, which are abolished upon PDE inhibition (50). Therefore, there likely exists selective enrichment of PDEs around the plasma membrane compared to the cell core. Indeed, at least one PDE isoform, PDE4B, shows prevalent plasma membrane localization at steady state across different cell types (51, 52). In addition, PDEs can be recruited to the cell surface in a receptor-dependent manner. It was demonstrated that activation of the β2AR leads to arrestin-induced translocation of PDE4D3 and PDE4D5 to the receptor (53). This relocalization takes place within minutes, a timeframe that is consistent with enzyme enrichment at the plasma membrane (53). Ultimately, these PDE gradients impact the cellular response. A study found that inhibition of PDE activity renders β2AR signals originating from the plasma membrane capable of generating endosome-selective outcomes, such as transcriptional signaling (17). Therefore, local PDE hydrolysis is an important contributor to spatially-encoded GPCR signaling.
In addition to the two key classes of established cAMP effectors, arrestins also play a vital role in determining the duration of the endosomal signal. Receptors that elicit persistent cAMP (e.g., V2R, PTHR) form an intracellular super-complex, which consists of the GPCR, G protein, and arrestin (24, 25). Because arrestin binding was classically presumed to terminate G protein signaling, these initial observations raised a conundrum regarding the ability of arrestins to stimulate sustained G protein activation when part of that complex. It was subsequently revealed that the endosomal GPCR/arrestin complex adopts a distinct conformation with arrestin binding to the receptor tail alone (without binding to the core), therefore stabilizing a unique signaling complex (24). Further, β-arrestins are not the only arrestin family proteins with roles in mediating intracellular GPCR signaling dynamics. Arrestin domain-containing protein 3 (ARRDC3), an α-arrestin, interacts with β2AR on endosomes to delay receptor recycling (54). As a result, ARRDC3 modulates β2AR endosomal residence and shapes the duration of intracellular cAMP (54).
The retromer complex is another protein assembly with essential functions in the regulation of endosomal GPCR residence and signaling. The complex consists of vacuolar protein sorting subunits (VPS) and their associated sorting nexin proteins (SNX) and plays critical roles in GPCR retrograde trafficking and recycling (55, 56). A series of elegant studies on the PTHR have yielded a detailed characterization of the role of the retromer in terminating endosomal signaling. It was discovered that PTHR signaling leads to PKA-dependent activation of vacuolar ATPase (v-ATPase), followed by endosome acidification and PTHR/retromer association (57). This interaction serves to terminate the endosomal signal. Specifically, it was shown that depletion of VPS35 retains the receptor in early endosomes and triggers more sustained cAMP signaling, while VPS29/VPS26 overexpression has the opposite effect (55). Ultimately, the precise timing of this inactivation of intracellular PTHR by the retromer is critical for physiology, as SNX27 knockout mice exhibit skeletal growth defects and reduced bone mass (58). Furthermore, it is likely that the regulatory roles of the retromer complex in shaping compartmentalized GPCR signaling are of broad significance across receptors. Even in the case of the β2AR, a GPCR that signals via acute cAMP production at endosomes, uncoupling of the receptor/retromer complex impacts the duration of intracellular signaling. Specifically, it was shown that mutating a motif within the C terminal β2AR tail, which is required for retromer association, leads to β2AR redistribution from retromer-positive tubules into the endosome body (59). This in turn gives rise to increased endosomal Gαs activation (59).
Therefore, the selective enrichment of AC and PDE enzymes across subcellular compartments together with the formation of complexes between the endosomal GPCR, arrestins and the retromer modulates the duration of intracellular signaling. However, our knowledge of the biochemical mechanisms at play is incomplete and many additional mediators remain to be uncovered.
Unique biophysical properties of endosomes underlie spatially encoded signaling
While the mechanistic efforts to understand spatially-encoded GPCR signaling have focused largely on the distinct biochemical makeup of different signaling compartments, several biophysical characteristics of endosomes have also been explored and shown to be of likely regulatory significance in these processes.
Early endosomes maintain a unique three-dimensional distribution within the cell. Compared to the plasma membrane, endosomes are perinuclear, and thus their localization has been interrogated as one property that could mediate the propagation of distinct GPCR responses. A recent study redistributed β2AR-containing endosomes toward the cell periphery with the aid of a heterodimerization approach that exploits rapamycin-induced complex formation between a kinesin motor and an early endosome-resident protein (60). Notably, this relocalization strategy did not impact the endosomal biochemical complexes required for β2AR trafficking and signaling (60). Yet, disrupting the intracellular distribution of endosomes was found to inhibit β2AR-induced transcriptional signaling, a spatially-encoded response for the receptor (17, 60). Evidence was put forth demonstrating that the propagation of the GPCR signal originating from a natively positioned endosome relies on the optimal subcellular geometry of this organelle with respect to the cell-peripheral enrichment of PDEs and the perinuclear localization of PKA (60).
Another distinguishing biophysical characteristic of the endosome is its intraluminal pH. Early endosomes have a slightly acidic pH of ∼6.2 to 6.5, while the plasma membrane has a neutral pH of ∼7.4 (61, 62). The impact of protonation on GPCR activity is therefore directly relevant to the phenomenon of receptor compartmentalization. A handful of studies are beginning to address this question, and the data support a complex relationship between pH and GPCR activity. An early in vitro investigation employed biophysical and biochemical assays to assess the role of protonation in β2AR activation, and found that acidic pH of 6.5 favors the transition from inactive to active receptor state (63). Specifically, the authors reported an increase in β2AR basal activity and a higher rate of agonist-induced conformational change with decreasing pH (63). While it remains to be seen whether these findings translate to intact cells, it appears that in the case of the β2AR, the acidic environment of the endosome may be favorable for signaling even in the absence of agonist. In contrast, as discussed in the preceding section, endosome acidification serves as a negative feedback loop to terminate the activity of a different GPCR, the PTHR, by enhancing ligand dissociation (57). Recent work representing the most comprehensive interrogation to date of the effects of proton concentrations on GPCR/ligand signaling helps reconcile these findings. The study demonstrates that pH-dependent regulation is widespread among GPCRs, but the impact is receptor- and ligand-specific (64). Through a high-throughput platform for expressing and studying human GPCRs in yeast, the authors assessed the activity of 280 GPCR/G protein combinations at pH 5.0 and 7.0. They discovered that for some receptors, ligand-dependent activation is exclusive for either low or high pH, while other receptors display graded pH sensitivity (64). Interestingly, protonation can also impact which G proteins the receptor couples to depending on the ligand. When stimulated with full agonist niacin, hydroxycarboxylic acid receptor 2 (HCAR2) couples stronger to Gαi/o at low pH and Gα12/13 at high pH. In contrast, the same receptor couples to both Gαi/o and Gα13 at low pH when activated with full agonist butyrate (64). Additionally, for some GPCRs, pH alters ligand efficacy and/or potency (64). Therefore, protonation is an essential regulator of GPCR signaling, and it is imperative to comprehensively evaluate its impact in the context of the endosomal receptor/ligand/G protein interplay.
Further, distinct organelles are enriched in unique phospholipids, and there is evidence that the lipid environment can mediate G protein coupling, GTPase activity and the recruitment of receptor effectors. Systematic characterization of the impact of lipids found in mammalian cell membranes revealed that negatively charged lipids are important for β2AR activation. Specifically, phosphatidylglycerol (PG) increased agonist binding and provided the best lipid environment for the recruitment of Nb80, which was used as a Gα protein surrogate (65). The significance of phospholipids on GPCR activity was further underscored by an in vitro mass spectrometry study that evaluated the impact of phospholipids on three Class A GPCR/G protein complexes (66). It was demonstrated that all three receptors exhibit preferential binding to phosphatidylinositol-4,5-bisphosphate (PtdIns (4, 5)P2 or PIP2), a phospholipid enriched at the plasma membrane (67). This association in turn stabilizes the active receptor state and enhances coupling to the G protein and GTPase activity (66). Phospholipids may impact the association of the G proteins as well. Molecular dynamics (MD) simulations pinpointed distinct interactions between phospholipids enriched in either the plasma membrane or the Golgi and residues on an engineered truncated version of the Gαi protein, mini-Gsi (68). These interactions included a selective association between mini-Gsi and PIP2. The MD simulation results were collaborated by experimental evidence demonstrating preferential recruitment of mini-Gsi to receptors at the plasma membrane, but not at the Golgi (69). In addition, the lipid composition of membranes regulates preferential G protein coupling. Fluorescence spectroscopy analysis of G protein coupling to the β2AR revealed that negatively charged phospholipids facilitate association with Gαs by impairing coupling to Gαi (70). Finally, phospholipids can play active roles in the recruitment and modulation of GPCR effectors. For example, studies have reported that PIP2 regulates the relocalization, orientation, and activity of GRK2 at the plasma membrane (71, 72, 73, 74), and positively coordinates the interactions between certain GPCRs and β-arrestin (75). Thus, distinct lipids may impact signaling by orchestrating unique GPCR/effector associations. Ultimately, it would be important to reconcile the in vitro work with in-cell studies, and the latter would necessitate strategies for compartment-specific assessment of how lipids shape GPCR activity. One approach could be to deplete phospholipid species in select compartments as demonstrated by a recent study, which successfully reduced endosomal phosphatidylinositol-3-phosphate (PI3P) content with acute rapamycin-dependent recruitment of inositol 3-phosphatase MTM1 to early endosomes (76).
Lastly, it is worth noting that the size of endosomes may also impact GPCR signaling. While the significance of this biophysical property has not been assessed experimentally, based on known parameters we predict that the intraluminal concentration of agonists is likely saturating in the case of most receptor/agonist pairs. The diameter of an early endosome is ∼100 to 500 nm (77). A single molecule of ligand present within the lumen of a vesicle in this size range would present in the mid-nanomolar to low-micromolar range of concentration (∼30 nM–3 μM). Therefore, the endosome may present an optimal environment for prolonged activation. This may be especially relevant in the case of membrane-impermeant ligands that co-internalize with the GPCR, as such ligands would remain “trapped” in the endosome lumen. Further experiments are required to determine the precise concentrations of ligands in endosomes and other intracellular compartments.
Thus, the unique biophysical characteristics of each signaling compartment are of high regulatory significance, and the contributions of each property to the range of spatially encoded GPCR-induced outcomes warrant additional investigation.
Intracellular GPCR activation and the fidelity of signal processing
Why has the cell evolved mechanisms for cell surface and intracellular activation of GPCRs? We speculate that compartmentalization of receptor signaling could serve to regulate the fidelity and robustness of stimulus detection (Fig. 3).
Figure 3.
Speculative model for the role of intracellular GPCR signaling in ligand recognition.A, compartmentalized GPCR activation as a cellular mechanism to distinguish between endogenous agonists with distinct chemical properties or between endogenous and synthetic agonists. B, intracellular signaling as a cellular mechanism to interpret stimulus strength. Low dose of stimulation would activate the plasma membrane-delimited fraction of receptors, leading to localized and transient responses (e.g., ion channel phosphorylation). Only high concentrations of ligand would drive efficient endocytosis or transport into the cell via transporters, and consequently would trigger extensive cellular reprogramming.
One scenario where intracellular signaling may mediate pathway specificity involves the detection of ligands with different pharmacodynamic properties at the receptor (Fig. 3A). Epinephrine and dopamine are structurally similar endogenous catecholamines produced from the same precursor, yet epinephrine is a full agonist that binds to and activates beta-adrenergic receptors with high affinity, while dopamine is a very weak partial agonist (17, 78). Interestingly, it was reported that when the doses of epinephrine and dopamine are matched to yield comparable total cAMP production, only epinephrine induces β2AR internalization and subsequent activation of transcriptional signaling (17). Further, although not evolutionarily intended, compartmentalization of GPCR signaling could regulate the recognition of native from synthetic ligands. Using conformation-selective nanobodies and mini-G probes, it was uncovered that the DOR is active at the cell surface and at the Golgi following accumulation through the biosynthetic pathway (12, 69). Unlike intracellular β2ARs, which are responsible for initiating the majority of phospho- and transcriptional signaling (16, 17), Golgi-localized DORs are uncoupled from the transcriptional response and generate a very weak phosphoresponse (69). Instead, both downstream outcomes are mediated through activation of DOR at the plasma membrane (69). Notably, native neuropeptide agonists are impermeant and would access DORs only at the cell exterior, while synthetic opioid receptor agonists (e.g., fentanyl) can activate both subcellular fractions of receptors. It is tempting to speculate that Golgi-resident DORs have not evolved as the major drivers of cellular responses, because they are inaccessible to endogenous ligands. With these two examples, we propose a model of co-evolution between functional encoding of the receptor signaling site and the chemical properties of the ligand to ultimately direct the GPCR response in favor of a select stimulus (Fig. 3A).
Intracellular GPCR activation may further serve as a cellular mechanism to “interpret” stimulus strength. In this model, weak receptor stimulation would activate plasma membrane-delimited responses to trigger quick cellular adaptation, while activation with high doses of ligand would initiate a second wave via intracellular signaling to give rise to more persistent changes (e.g., transcriptional reprogramming, translation of new proteins, etc.) (Fig. 3B). Indeed, it has been reported that weak activation of GPCRs results in highly localized responses, typically involving protein post-translational modifications. For example, while multiple PKA substrates can be phosphorylated by high-dose stimulation of β1ARs in cardiac myocytes, activation of the plasma membrane fraction of adrenergic receptors with an autoantibody, a de facto weak partial agonist, results in very local modulation of the proximal ryanodine two receptors (RyR2) (79). Is strong stimulation of the pathways more likely to lead to intracellular signaling? For GPCRs, which require ligand-induced internalization for endosomal signaling, stimulus strength would be expected to correlate with intracellular activity, as the fraction of endocytosed receptors is generally proportional to agonist concentrations (80). It would be straightforward to test this model through careful mapping of the magnitude of the induction of a spatially-encoded response across a range of stimulus doses. In fact, a study found that transcriptional upregulation of a β2AR target gene, which requires endosomal receptor activation, increased from ∼1.2- to 24-fold (∼16-fold range) when cells were stimulated with concentrations of isoproterenol in the 100 pM-10 nM range (81). β2AR endocytosis similarly increased in a dose-dependent manner (81). Therefore, more internal ligand-bound receptors presumably result in more robust transcriptional reprogramming. The relationship is less straightforward when it comes to intracellular activation of GPCRs delivered to internal membranes through the biosynthetic pathway. There, stimulation of the receptor in the cell interior necessitates either a membrane-permeant agonist or a ligand transporter. Curiously, some transporters implicated in ligand uptake in the context of intracellular GPCR signaling are in fact low-affinity high-capacity transporters (82). Both β1AR and D1R are present on Golgi membranes, and in a native system, activation of intracellular β1ARs or D1Rs requires transport of norepinephrine/epinephrine or dopamine via organic cation transporters OCT3 and OCT2, respectively (13, 14). The reported Km values for OCT2 and dopamine, and OCT3 and norepinephrine/epinephrine are in the high micromolar-low millimolar range (83, 84, 85, 86). Therefore, uptake into the cell and subsequent activation of intracellular receptors would necessitate high concentrations of ligand, effectively imposing a stimulus strength filter over the initiation of the second wave of signaling.
Outlook
Spatial encoding of GPCR activity has emerged as a novel paradigm for functional diversification of signaling with important physiological and therapeutic implications. Yet, much still remains to be learned about this phenomenon. A caveat of many studies is their reliance on GPCR over-expression and/or the use of tractable rather than physiologically relevant cell models. In addition, even for the best-characterized receptors with endosomal activity, we lack a definitive link between the molecular, cellular, and physiological relevance of intracellular GPCRs. A systematic and comprehensive examination of the molecular and cellular consequences in appropriate model systems on the one hand, and in vivo physiology on the other, is therefore warranted. Global genomic and proteomic approaches to illuminate the vast spectrum of signaling outcomes at the cellular level would be of particular value in such analyses. In addition, the advent of single-cell approaches provides another opportunity to delve into the universality of spatially encoded GPCR signaling across multiple cell types, tissues, and pathologies. The implications of compartmentalized GPCR activity for drug action are also poorly understood. Extensive profiling of ligands with a range of pharmacodynamic properties at any given receptor would serve as an informative approach and allow insights into how ligand hydrophobicity and endocytic efficacy impact the gamut of GPCR responses. Lastly, to be able to harness the therapeutic potential of compartmentalized GPCR activity, there is also an urgent need to pinpoint the detailed regulatory mechanisms. As discussed, the propagation of site-selective responses is shaped by biochemical and biophysical regulatory mechanisms, and our understanding of these mechanisms is very poor. Therefore, the elucidation of novel effectors and unique organelle properties that give rise to spatially-encoded outcomes across GPCRs, compartments and cell types would inform the development of more selective interventions to target these pathways.
Data availability
Data can be shared upon request
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank members of the Tsvetanova lab, Dr Laura Wingler and Dr Roshanak Irannejad for helpful discussions and feedback. We regret that we were unable to cite the many additional studies that have contributed to this field due to space constraints. This work was supported by the National Institutes of Health (R01-NS127847 to N. G. T.).
Author contributions
M. J. K., B. K. A. W., and N. G. T. conceptualization; N. G. T. funding acquisition; M. J. K. and N. G .T. writing; original draft; M. J. K., B. K. A. W., and N. G. T. writing; review and editing.
Reviewed by members of the JBC Editorial Board. Edited by Kirill Martemyanov
References
- 1.Sriram K., Insel P.A. G protein-coupled receptors as targets for approved drugs: how many targets and how many drugs? Mol. Pharmacol. 2018;93:251–258. doi: 10.1124/mol.117.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurevich V.V., Gurevich E.V. GPCR signaling regulation: the role of GRKs and arrestins. Front. Pharmacol. 2019;10:125. doi: 10.3389/fphar.2019.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohse M.J., Benovic J.L., Codina J., Caron M.G., Lefkowitz R.J. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 4.Cahill T.J., 3rd, Thomsen A.R., Tarrasch J.T., Plouffe B., Nguyen A.H., Yang F., et al. Distinct conformations of GPCR-β-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc. Natl. Acad. Sci. U. S. A. 2017;114:2562–2567. doi: 10.1073/pnas.1701529114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefkowitz R.J., Shenoy S.K. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 6.Shenoy S.K., Lefkowitz R.J. β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol. Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrandon S., Feinstein T.N., Castro M., Wang B., Bouley R., Potts J.T., et al. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat. Chem. Biol. 2009;5:734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jong Y.J., Kumar V., Kingston A.E., Romano C., O'Malley K.L. Functional metabotropic glutamate receptors on nuclei from brain and primary cultured striatal neurons. Role of transporters in delivering ligand. J. Biol. Chem. 2005;280:30469–30480. doi: 10.1074/jbc.M501775200. [DOI] [PubMed] [Google Scholar]
- 9.Calebiro D., Nikolaev V.O., Gagliani M.C., de Filippis T., Dees C., Tacchetti C., et al. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irannejad R., Tomshine J.C., Tomshine J.R., Chevalier M., Mahoney J.P., Steyaert J., et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godbole A., Lyga S., Lohse M.J., Calebiro D. Internalized TSH receptors en route to the TGN induce local G(s)-protein signaling and gene transcription. Nat. Commun. 2017;8:443. doi: 10.1038/s41467-017-00357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoeber M., Jullié D., Lobingier B.T., Laeremans T., Steyaert J., Schiller P.W., et al. A genetically encoded biosensor reveals location bias of opioid drug action. Neuron. 2018;98:963–976.e965. doi: 10.1016/j.neuron.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puri N.M., Romano G.R., Lin T.Y., Mai Q.N., Irannejad R. The organic cation transporter 2 regulates dopamine D1 receptor signaling at the Golgi apparatus. Elife. 2022;11 doi: 10.7554/eLife.75468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irannejad R., Pessino V., Mika D., Huang B., Wedegaertner P.B., Conti M., et al. Functional selectivity of GPCR-directed drug action through location bias. Nat. Chem. Biol. 2017;13:799–806. doi: 10.1038/nchembio.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nash C.A., Wei W., Irannejad R., Smrcka A.V. Golgi localized β1-adrenergic receptors stimulate Golgi PI4P hydrolysis by PLCε to regulate cardiac hypertrophy. Elife. 2019;8 doi: 10.7554/eLife.48167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsvetanova N.G., Trester-Zedlitz M., Newton B.W., Peng G.E., Johnson J.R., Jimenez-Morales D., et al. Endosomal cAMP production broadly impacts the cellular phosphoproteome. J. Biol. Chem. 2021;297 doi: 10.1016/j.jbc.2021.100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsvetanova N.G., Von Zastrow M. Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat. Chem. Biol. 2014;10:1061–1065. doi: 10.1038/nchembio.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jong Y.I., Harmon S.K., O'Malley K.L. GPCR signalling from within the cell. Br. J. Pharmacol. 2018;175:4026–4035. doi: 10.1111/bph.14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sposini S., Jean-Alphonse F.G., Ayoub M.A., Oqua A., West C., Lavery S., et al. Integration of GPCR signaling and sorting from very early endosomes via opposing APPL1 mechanisms. Cell Rep. 2017;21:2855–2867. doi: 10.1016/j.celrep.2017.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jong Y.J., Kumar V., O'Malley K.L. Intracellular metabotropic glutamate receptor 5 (mGluR5) activates signaling cascades distinct from cell surface counterparts. J. Biol. Chem. 2009;284:35827–35838. doi: 10.1074/jbc.M109.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suofu Y., Li W., Jean-Alphonse F.G., Jia J., Khattar N.K., Li J., et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E7997–E8006. doi: 10.1073/pnas.1705768114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boivin B., Lavoie C., Vaniotis G., Baragli A., Villeneuve L.R., Ethier N., et al. Functional beta-adrenergic receptor signalling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc. Res. 2006;71:69–78. doi: 10.1016/j.cardiores.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Kumar V., Jong Y.J., O'Malley K.L. Activated nuclear metabotropic glutamate receptor mGlu5 couples to nuclear Gq/11 proteins to generate inositol 1,4,5-trisphosphate-mediated nuclear Ca2+ release. J. Biol. Chem. 2008;283:14072–14083. doi: 10.1074/jbc.M708551200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomsen A.R.B., Plouffe B., Cahill T.J., 3rd, Shukla A.K., Tarrasch J.T., Dosey A.M., et al. GPCR-G protein-β-arrestin super-complex mediates sustained G protein signaling. Cell. 2016;166:907–919. doi: 10.1016/j.cell.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wehbi V.L., Stevenson H.P., Feinstein T.N., Calero G., Romero G., Vilardaga J.P. Noncanonical GPCR signaling arising from a PTH receptor-arrestin-Gβγ complex. Proc. Natl. Acad. Sci. U. S. A. 2013;110:1530–1535. doi: 10.1073/pnas.1205756110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sergin I., Jong Y.I., Harmon S.K., Kumar V., O'Malley K.L. Sequences within the C terminus of the metabotropic glutamate receptor 5 (mGluR5) are responsible for inner nuclear membrane localization. J. Biol. Chem. 2017;292:3637–3655. doi: 10.1074/jbc.M116.757724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jimenez-Vargas N.N., Pattison L.A., Zhao P., Lieu T., Latorre R., Jensen D.D., et al. Protease-activated receptor-2 in endosomes signals persistent pain of irritable bowel syndrome. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E7438–e7447. doi: 10.1073/pnas.1721891115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen D.D., Lieu T., Halls M.L., Veldhuis N.A., Imlach W.L., Mai Q.N., et al. Neurokinin 1 receptor signaling in endosomes mediates sustained nociception and is a viable therapeutic target for prolonged pain relief. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Don-Salu-Hewage A.S., Chan S.Y., McAndrews K.M., Chetram M.A., Dawson M.R., Bethea D.A., et al. Cysteine (C)-x-C receptor 4 undergoes transportin 1-dependent nuclear localization and remains functional at the nucleus of metastatic prostate cancer cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorvin C.M., Rogers A., Hastoy B., Tarasov A.I., Frost M., Sposini S., et al. AP2σ mutations impair calcium-sensing receptor trafficking and signaling, and show an endosomal pathway to spatially direct G-protein selectivity. Cell Rep. 2018;22:1054–1066. doi: 10.1016/j.celrep.2017.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bénard G., Massa F., Puente N., Lourenço J., Bellocchio L., Soria-Gómez E., et al. Mitochondrial CB₁ receptors regulate neuronal energy metabolism. Nat. Neurosci. 2012;15:558–564. doi: 10.1038/nn.3053. [DOI] [PubMed] [Google Scholar]
- 32.Yarwood R.E., Imlach W.L., Lieu T., Veldhuis N.A., Jensen D.D., Klein Herenbrink C., et al. Endosomal signaling of the receptor for calcitonin gene-related peptide mediates pain transmission. Proc. Natl. Acad. Sci. U. S. A. 2017;114:12309–12314. doi: 10.1073/pnas.1706656114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q., Zhang H., Xu H., Guo D., Shi H., Li Y., et al. 5-HTR3 and 5-HTR4 located on the mitochondrial membrane and functionally regulated mitochondrial functions. Sci. Rep. 2016;6 doi: 10.1038/srep37336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Revankar C.M., Cimino D.F., Sklar L.A., Arterburn J.B., andProssnitz E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 35.Jimenez-Vargas N.N., Gong J., Wisdom M.J., Jensen D.D., Latorre R., Hegron A., et al. Endosomal signaling of delta opioid receptors is an endogenous mechanism and therapeutic target for relief from inflammatory pain. Proc. Natl. Acad. Sci. U. S. A. 2020;117:15281–15292. doi: 10.1073/pnas.2000500117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tadevosyan A., Vaniotis G., Allen B.G., Hébert T.E., Nattel S. G protein-coupled receptor signalling in the cardiac nuclear membrane: evidence and possible roles in physiological and pathophysiological function. J. Physiol. 2012;590:1313–1330. doi: 10.1113/jphysiol.2011.222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joyal J.S., Nim S., Zhu T., Sitaras N., Rivera J.C., Shao Z., et al. Subcellular localization of coagulation factor II receptor-like 1 in neurons governs angiogenesis. Nat. Med. 2014;20:1165–1173. doi: 10.1038/nm.3669. [DOI] [PubMed] [Google Scholar]
- 38.Latorre R., Hegron A., Peach C.J., Teng S., Tonello R., Retamal J.S., et al. Mice expressing fluorescent PAR(2) reveal that endocytosis mediates colonic inflammation and pain. Proc. Natl. Acad. Sci. U. S. A. 2022;119 doi: 10.1073/pnas.2112059119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purgert C.A., Izumi Y., Jong Y.J., Kumar V., Zorumski C.F., O'Malley K.L. Intracellular mGluR5 can mediate synaptic plasticity in the hippocampus. J. Neurosci. 2014;34:4589–4598. doi: 10.1523/JNEUROSCI.3451-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feinstein T.N., Yui N., Webber M.J., Wehbi V.L., Stevenson H.P., King J.D., Jr., et al. Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J. Biol. Chem. 2013;288:27849–27860. doi: 10.1074/jbc.M112.445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vincent K., Cornea V.M., Jong Y.I., Laferriere A., Kumar N., Mickeviciute A., et al. Intracellular mGluR5 plays a critical role in neuropathic pain. Nat. Commun. 2016;7 doi: 10.1038/ncomms10604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White A.D., Pena K.A., Clark L.J., Maria C.S., Liu S., Jean-Alphonse F.G., et al. Spatial bias in cAMP generation determines biological responses to PTH type 1 receptor activation. Sci. Signal. 2021;14 doi: 10.1126/scisignal.abc5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horwitz M.J., Tedesco M.B., Sereika S.M., Syed M.A., Garcia-Ocaña A., Bisello A., et al. Continuous PTH and PTHrP infusion causes suppression of bone formation and discordant effects on 1,25(OH)2 vitamin D. J. Bone Miner Res. 2005;20:1792–1803. doi: 10.1359/JBMR.050602. [DOI] [PubMed] [Google Scholar]
- 44.Horwitz M.J., Tedesco M.B., Sereika S.M., Hollis B.W., Garcia-Ocaña A., Stewart A.F. Direct comparison of sustained infusion of human parathyroid hormone-related protein-(1-36) [hPTHrP-(1-36)] versus hPTH-(1-34) on serum calcium, plasma 1,25-dihydroxyvitamin D concentrations, and fractional calcium excretion in healthy human volunteers. J. Clin. Endocrinol. Metab. 2003;88:1603–1609. doi: 10.1210/jc.2002-020773. [DOI] [PubMed] [Google Scholar]
- 45.Eiger D.S., Boldizsar N., Honeycutt C.C., Gardner J., Kirchner S., Hicks C., et al. Location bias contributes to functionally selective responses of biased CXCR3 agonists. Nat. Commun. 2022;13:5846. doi: 10.1038/s41467-022-33569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotowski S.J., Hopf F.W., Seif T., Bonci A., von Zastrow M. Endocytosis promotes rapid dopaminergic signaling. Neuron. 2011;71:278–290. doi: 10.1016/j.neuron.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lazar A.M., Irannejad R., Baldwin T.A., Sundaram A.B., Gutkind J.S., Inoue A., et al. G Protein-regulated endocytic trafficking of adenylyl cyclase type 9. Elife. 2020;9 doi: 10.7554/eLife.58039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inda C., Dos Santos Claro P.A., Bonfiglio J.J., Senin S.A., Maccarrone G., Turck C.W., et al. Different cAMP sources are critically involved in G protein-coupled receptor CRHR1 signaling. J. Cell Biol. 2016;214:181–195. doi: 10.1083/jcb.201512075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pizzoni A., Zhang X., Naim N., Altschuler D.L. Soluble cyclase-mediated nuclear cAMP synthesis is sufficient for cell proliferation. Proc. Natl. Acad. Sci. U. S. A. 2023;120 doi: 10.1073/pnas.2208749120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anton S.E., Kayser C., Maiellaro I., Nemec K., Möller J., Koschinski A., et al. Receptor-associated independent cAMP nanodomains mediate spatiotemporal specificity of GPCR signaling. Cell. 2022;185:1130–1142.e11. doi: 10.1016/j.cell.2022.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Blackman B.E., Horner K., Heidmann J., Wang D., Richter W., Rich T.C., et al. PDE4D and PDE4B function in distinct subcellular compartments in mouse embryonic fibroblasts. J. Biol. Chem. 2011;286:12590–12601. doi: 10.1074/jbc.M110.203604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terrin A., Di Benedetto G., Pertegato V., Cheung Y.F., Baillie G., Lynch M.J., et al. PGE(1) stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: role of compartmentalized phosphodiesterases. J. Cell Biol. 2006;175:441–451. doi: 10.1083/jcb.200605050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perry S.J., Baillie G.S., Kohout T.A., McPhee I., Magiera M.M., Ang K.L., et al. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- 54.Tian X., Irannejad R., Bowman S.L., Du Y., Puthenveedu M.A., von Zastrow M., et al. The α-arrestin ARRDC3 regulates the endosomal residence time and intracellular signaling of the β2-adrenergic receptor. J. Biol. Chem. 2016;291:14510–14525. doi: 10.1074/jbc.M116.716589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feinstein T.N., Wehbi V.L., Ardura J.A., Wheeler D.S., Ferrandon S., Gardella T.J., et al. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat. Chem. Biol. 2011;7:278–284. doi: 10.1038/nchembio.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Temkin P., Lauffer B., Jager S., Cimermancic P., Krogan N.J., von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat. Cell Biol. 2011;13:715–721. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gidon A., Al-Bataineh M.M., Jean-Alphonse F.G., Stevenson H.P., Watanabe T., Louet C., et al. Endosomal GPCR signaling turned off by negative feedback actions of PKA and v-ATPase. Nat. Chem. Biol. 2014;10:707–709. doi: 10.1038/nchembio.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan A.S., Clairfeuille T., Landao-Bassonga E., Kinna G., Ng P.Y., Loo L.S., et al. Sorting nexin 27 couples PTHR trafficking to retromer for signal regulation in osteoblasts during bone growth. Mol. Biol. Cell. 2016;27:1367–1382. doi: 10.1091/mbc.E15-12-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varandas K.C., Irannejad R., von Zastrow M. Retromer endosome exit domains serve multiple trafficking destinations and regulate local G protein activation by GPCRs. Curr. Biol. 2016;26:3129–3142. doi: 10.1016/j.cub.2016.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willette B.K.A., Zhang J.F., Zhang J., Tsvetanova N.G. Endosome positioning coordinates spatially selective GPCR signaling. Nat. Chem. Biol. 2023;10 doi: 10.1038/s41589-023-01390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murphy R.F., Powers S., Cantor C.R. Endosome pH measured in single cells by dual fluorescence flow cytometry: rapid acidification of insulin to pH 6. J. Cell Biol. 1984;98:1757–1762. doi: 10.1083/jcb.98.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scott C.C., Gruenberg J. Ion flux and the function of endosomes and lysosomes: pH is just the start: the flux of ions across endosomal membranes influences endosome function not only through regulation of the luminal pH. Bioessays. 2011;33:103–110. doi: 10.1002/bies.201000108. [DOI] [PubMed] [Google Scholar]
- 63.Ghanouni P., Schambye H., Seifert R., Lee T.W., Rasmussen S.G., Gether U., et al. The effect of pH on beta(2) adrenoceptor function. Evidence for protonation-dependent activation. J. Biol. Chem. 2000;275:3121–3127. doi: 10.1074/jbc.275.5.3121. [DOI] [PubMed] [Google Scholar]
- 64.Kapolka N.J., Rowe J.B., Taghon G.J., Morgan W.M., O'Shea C.R., Isom D.G. Proton-gated coincidence detection is a common feature of GPCR signaling. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2100171118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dawaliby R., Trubbia C., Delporte C., Masureel M., Van Antwerpen P., Kobilka B.K., et al. Allosteric regulation of G protein-coupled receptor activity by phospholipids. Nat. Chem. Biol. 2016;12:35–39. doi: 10.1038/nchembio.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yen H.Y., Hoi K.K., Liko I., Hedger G., Horrell M.R., Song W., et al. PtdIns(4,5)P(2) stabilizes active states of GPCRs and enhances selectivity of G-protein coupling. Nature. 2018;559:423–427. doi: 10.1038/s41586-018-0325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Posor Y., Jang W., Haucke V. Phosphoinositides as membrane organizers. Nat. Rev. Mol. Cell Biol. 2022;23:797–816. doi: 10.1038/s41580-022-00490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wan Q., Okashah N., Inoue A., Nehmé R., Carpenter B., Tate C.G., et al. Mini G protein probes for active G protein-coupled receptors (GPCRs) in live cells. J. Biol. Chem. 2018;293:7466–7473. doi: 10.1074/jbc.RA118.001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Radoux-Mergault A., Oberhauser L., Aureli S., Gervasio F.L., Stoeber M. Subcellular location defines GPCR signal transduction. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adf6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strohman M.J., Maeda S., Hilger D., Masureel M., Du Y., Kobilka B.K. Local membrane charge regulates β(2) adrenergic receptor coupling to G(i3) Nat. Commun. 2019;10:2234. doi: 10.1038/s41467-019-10108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pitcher J.A., Touhara K., Payne E.S., Lefkowitz R.J. Pleckstrin homology domain-mediated membrane association and activation of the beta-adrenergic receptor kinase requires coordinate interaction with G beta gamma subunits and lipid. J. Biol. Chem. 1995;270:11707–11710. doi: 10.1074/jbc.270.20.11707. [DOI] [PubMed] [Google Scholar]
- 72.DebBurman S.K., Ptasienski J., Benovic J.L., Hosey M.M. G protein-coupled receptor kinase GRK2 is a phospholipid-dependent enzyme that can be conditionally activated by G protein betagamma subunits. J. Biol. Chem. 1996;271:22552–22562. doi: 10.1074/jbc.271.37.22552. [DOI] [PubMed] [Google Scholar]
- 73.DebBurman S.K., Ptasienski J., Boetticher E., Lomasney J.W., Benovic J.L., Hosey M.M. Lipid-mediated regulation of G protein-coupled receptor kinases 2 and 3. J. Biol. Chem. 1995;270:5742–5747. doi: 10.1074/jbc.270.11.5742. [DOI] [PubMed] [Google Scholar]
- 74.Yang P., Homan K.T., Li Y., Cruz-Rodríguez O., Tesmer J.J., Chen Z. Effect of lipid composition on the membrane orientation of the G protein-coupled receptor kinase 2-gβ1γ2 complex. Biochemistry. 2016;55:2841–2848. doi: 10.1021/acs.biochem.6b00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang W., Masureel M., Qu Q., Janetzko J., Inoue A., Kato H.E., et al. Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature. 2020;579:303–308. doi: 10.1038/s41586-020-1953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uchida Y., Rutaganira F.U., Jullié D., Shokat K.M., von Zastrow M. Endosomal phosphatidylinositol 3-kinase is essential for canonical GPCR signaling. Mol. Pharmacol. 2017;91:65–73. doi: 10.1124/mol.116.106252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klumperman J., andRaposo G. The complex ultrastructure of the endolysosomal system. Cold Spring Harb. Perspect. Biol. 2014;6:a016857. doi: 10.1101/cshperspect.a016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swaminath G., Xiang Y., Lee T.W., Steenhuis J., Parnot C., Kobilka B.K. Sequential binding of agonists to the beta2 adrenoceptor. Kinetic evidence for intermediate conformational states. J. Biol. Chem. 2004;279:686–691. doi: 10.1074/jbc.M310888200. [DOI] [PubMed] [Google Scholar]
- 79.Lin T.Y., Mai Q.N., Zhang H., Wilson E., Chien H.C., Yee S.W., et al. Cardiac contraction and relaxation are regulated by distinct subcellular cAMP pools. Nat. Chem. Biol. 2023;10:62–73. doi: 10.1038/s41589-023-01381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hammer M.M., Wehrman T.S., Blau H.M. A novel enzyme complementation-based assay for monitoring G-protein-coupled receptor internalization. FASEB J. 2007;21:3827–3834. doi: 10.1096/fj.07-8777com. [DOI] [PubMed] [Google Scholar]
- 81.Hurley T.R., Luo K., Sefton B.M. Activators of protein kinase C induce dissociation of CD4, but not CD8, from p56lck. Science. 1989;245:407–409. doi: 10.1126/science.2787934. [DOI] [PubMed] [Google Scholar]
- 82.Koepsell H. General overview of organic cation transporters in. Brain Handb. Exp. Pharmacol. 2021;266:1–39. doi: 10.1007/164_2021_449. [DOI] [PubMed] [Google Scholar]
- 83.Duan H., Wang J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J. Pharmacol. Exp. Ther. 2010;335:743–753. doi: 10.1124/jpet.110.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amphoux A., Vialou V., Drescher E., Brüss M., Mannoury La Cour C., Rochat C., et al. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50:941–952. doi: 10.1016/j.neuropharm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 85.Gründemann D., Köster S., Kiefer N., Breidert T., Engelhardt M., Spitzenberger F., et al. Transport of monoamine transmitters by the organic cation transporter type 2, Oct2. J. Biol. Chem. 1998;273:30915–30920. doi: 10.1074/jbc.273.47.30915. [DOI] [PubMed] [Google Scholar]
- 86.Busch A.E., Karbach U., Miska D., Gorboulev V., Akhoundova A., Volk C., et al. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol. Pharmacol. 1998;54:342–352. doi: 10.1124/mol.54.2.342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be shared upon request