Abstract
The analysis of the single-cell transcriptome has emerged as a powerful tool to gain insights on the basic mechanisms of health and disease. It is widely used to reveal the cellular diversity and complexity of tissues at cellular resolution by RNA sequencing of the whole transcriptome from a single cell. Equally, it is applied to discover an unknown, rare population of cells in the tissue. The prime advantage of single-cell transcriptome analysis is the detection of stochastic nature of gene expression of the cell in tissue. Moreover, the availability of multiple platforms for the single-cell transcriptome has broadened its approaches to using cells of different sizes and shapes, including the capture of short or full-length transcripts, which is helpful in the analysis of challenging biological samples. And with the development of numerous packages in R and Python, new directions in the computational analysis of single-cell transcriptomes can be taken to characterize healthy versus diseased tissues to obtain novel pathological insights. Downstream analysis such as differential gene expression analysis, gene ontology term analysis, Kyoto Encyclopedia of Genes and Genomes pathway analysis, cell-cell interaction analysis, and trajectory analysis has become standard practice in the workflow of single-cell transcriptome analysis to further examine the biology of different cell types. Here, we provide a broad overview of single-cell transcriptome analysis in health and disease conditions currently applied in various studies.
Keywords: Single-cell transcriptome, cell types, R, python, RNA sequencing
INTRODUCTION
The discovery of cells (1) led to the understanding of life at the microscopic level. The cell is the smallest structural and functional unit of the human body, including all living organisms. Its proper function is important in health. Once it is altered, which occurs in a disease condition, the detection of these altered cells is important for the diagnosis, prognosis, and treatment of this pathological condition. In the human body, there are multiple types of cells (Fig. 1(A)), and these cells are heterogeneous but have similar characteristics to form a complex functional tissue. And these complex tissues contribute to proper organ functions in the system, and all systems in the human body coordinate to maintain normal physiology. Therefore, the single cell is essential to maintaining the integrity of all levels of cellular organizations from tissues to organs to systems in normal physiology. Furthermore, in the cell, the genes are efficiently translated into proteins after their expression (2), and these proteins are the molecular machine that keeps the cell alive by carrying out innumerable functions as well as building cell structures. And in the human genome, there are approximately 20,000 genes, and all these genes are not expressed in a particular single cell. Rather, some gene expression is specific to certain kind of cell type, and this pattern of gene expression leads to different types of cells in the tissue. Hence, the expression pattern of genes in a cell is critical in health and disease.
Fig. 1. Different types of human cells and depiction of the process of single-cell RNA sequencing.
A, Schematic diagram illustrating human cells. Various shapes and sizes of human cells are shown, such as adipocyte, ciliated columnar cell, Langerhans cell, goblet cell, myofibroblast cell, neuron, sperm, and columnar cell. B, Overview of the single-cell RNA sequencing process. Initially, the single-cell suspension is prepared from the tissue sample by dissociation methods. Then, the cells are separated into single cells with lysis buffer, reverse transcriptase, beads, and oil to create a chamber. Inside the oil chamber, the single cell is lysed and cDNA is synthesized. Subsequently, the library is prepared, and sequencing is performed on different platforms according to the manufacturer's instructions. Images of human cells are used from biorender.com.
Frequently, the study of gene expression at messenger RNA (mRNA) level is performed by popularmethods such as Polymerase chain reaction, quantitative PCR, northern blot, microarray, bulk RNA sequencing (RNA-seq), etc., which are carried out at tissue level and give only an average global gene expression value (3). However, the tissue is made up of diverse range of cells, and measuring the gene expression of thousands of individual single cells simultaneously from a tissue was challenging technically. But recently, a single-cell transcriptome technology was developed to address this technical problem (4), and it has revolutionized the study of gene expression of single cells across health and disease, and has become a tool that is used to understand healthy tissue at cellular resolution and to elucidate the underlying mechanisms of disease in humans. It has also become a useful tool to reveal the unbiased cellular heterogeneity in the tissue, to understand cell to cell variations, to find defective signaling pathways, to detect changes during disease condition, to screen potential drugs, and to evaluate drug response in treatment (5).
The first individual single-cell transcriptome sequencing was performed from the blastomere and oocyte after isolating single cells manually under a microscope (4). Generally, in this process, the population of total RNA from a single cell is faithfully converted into complementary DNA (cDNA) fragments by reverse transcription, and then all the cDNAs are sequenced in parallel, and it is referred to as single-cell RNA-seq (scRNA-seq). After sequencing, the raw sequences are analyzed by widely available computational tools to obtain a tissue atlas. On the other hand, single nucleus can also be used after isolation from the cell for RNA-seq, and it is known as single-nuclei RNA-seq (snRNA-seq) (6). Especially, the snRNA-seq tool is useful in case of difficult tissue to isolate cells from, such as adipocytes, cardiomyocytes, and neurons, or when the sample is stored frozen. In addition to the single-cell or nuclei transcriptome method, there has been rapid development in the single-cell spatial transcriptome method too (7), which gives not only gene expression value but also the location of the cells in the tissue section under study. At present, there are established automated methods to process and sequence individual single cells and generate cellular maps of tissues in unprecedented details. After completing the single-cell transcriptome experiment, in general, the sequencing or processed datasets are deposited and made available freely in the public databases for further reanalysis. The most common databases used for the deposition of scRNA-seq datasets are NCBI (GEO) and ArrayExpress (EBI). And other single-cell–dedicated databases are Single Cell Portal—Broad Institute, Single Cell Expression Atlas-EBI (8), SCPortalen (9), Panglaodb (10), CancerSEA (11), etc.
This review provides a concise overview of the single-cell transcriptome as applied in healthy individuals and in various disease conditions. We briefly go over multiple platforms and workflows that are important for single-cell transcriptome analysis. The results obtained from earlier studies are outlined as an example of applications that will be useful to design single-cell transcriptome experiments to understand normal as well as disease processes in projects relevant to biomedical and clinical research.
PLATFORMS OF SINGLE-CELL TRANSCRIPTOME
The plate-based or microwell-based, microfluidic-based, and droplet-based methods are commonly selected procedures for single-cell capture or isolation in the scRNA-seq technique (12). In the plate-based method, a large number of microwells containing beads coated with oligonucleotide are used for scRNA-seq experiment (13,14). It is a useful technique for any size and shape of the cell, for example, adipocytes, cardiomyocytes, neurons, astrocytes, etc. However, in the microfluidic methods (15), a chip is used where multiple narrow microfluidic channels are present to isolate single cells, and then these isolated cells are lysed and reverse transcribed into cDNA, whereas the droplet-based method also uses a chip but generates oil droplet containing single cells, oligonucleotides to capture mRNA, lysis buffer, and reverse transcriptase (RT) enzymes for cDNA synthesis (16,17). At present, microfluidic-based and droplet-based methods are combined together in a single device, and this device is a popular choice for single-cell capture or isolation and RT reaction. After using either of these methods, the library is prepared for the next-generation sequencing (NGS) technology.
Based on the current methods, a multitude of platforms are available commercially, such as STRT-Seq (18), CEL-Seq (19), SMART-Seq (20), FluidigmC1 (21), Smart-Seq2 (22), MARS-Seq (23), CytoSeq (24), Drop-Seq (17), inDrop (16), 10x Chromium (25), Seq-well (13), etc., to capture individual cells with reagents (26). Some of these devices produce relatively long reads and are called long-read scRNA-seq technology, and it is useful to obtain information regarding the diversity of proteins by calculating splice variants, whereas other devices produce short reads and are called short read scRNA-seq technology. And other attributes typically included or not for the scRNA-seq experiments are use of unique molecular identifier (UMI) and the coverage of transcript among different platforms (27) (Table 1). Furthermore, depending on the total number of cells they can process, some platforms have low throughput, whereas others have high throughput, and the protocol to be used can be chosen based on the needs of the experiment (26).
Table 1.
Some examples of scRNA-seq approaches
| Protocols | Long reads | Short reads | UMI | Platforms | Reference |
|---|---|---|---|---|---|
| STRT-Seq | – | 5′ end | Yes | Microfluidics | [18] |
| CEL-Seq | – | 3′ end | Yes | Plate based | [19] |
| SMART-Seq | Full length | – | No | Plate based | [20] |
| FluidigmC1 | – | 3′ end | Yes | Microfluidics | [21] |
| Smart-Seq2 | Full length | – | No | Plate based | [22] |
| MARS-Seq | – | 3′ end | Yes | Plate based | [23] |
| CytoSeq | – | 3′ end | Yes | Microwell | [24] |
| DropSeq | – | 3′ end | Yes | Droplet | [17] |
| inDrop | – | 3′ end | Yes | Droplet | [16] |
| 10x Chromium | – | 3′ end | Yes | Droplet | [25] |
| Seq-well | – | 3′ end | Yes | Nanowell | [13] |
Certain populations of cells from the tissue sample can be selectively used for scRNA-seq experiment after separating them from other different cell types. This isolation of a specific population of cells is achieved by cell enrichment methods. Typically used cell enrichment instruments are fluorescence-activated cell sorters (FACS) (28) and magnetic-activated cell sorters (MACS) (29). The FACS uses fluorescence from expressed protein or fluorochrome-conjugated antibodies against cell surface markers to enrich cells, whereas the MACS uses magnetic bead–conjugated antibodies against cell surface markers. Although we can study certain populations of cells in isolation, it is always important to include all cell types to get a tissue atlas. However, in the tissue atlas, the most pivotal hurdle present during analysis of scRNA-seq data is the lack of correct detection of some cell populations (5), which is mostly due to the elimination of lowly expressed genes during feature selection. In addition, there is a limitation of capturing low abundant transcripts of the cell while sequencing in some of the current methods, and to solve this limitation, a deep sequencing can be performed.
WORKFLOW OF SINGLE-CELL TRANSCRIPTOME ANALYSIS
The preparation of a single-cell suspension from the tissue sample is the first step in the process of scRNA-seq experiment. The single-cell suspension is prepared by either physical methods or enzymatic digestion of the tissue sample (30). At this point, we need to ensure that the single cells are viable and not stressed too much to maintain their native gene expression profile. Then, single cells are handled by various platforms to acquire transcriptomes from individual cells. Subsequently, a library of individual single cells is sequenced by NGS technologies to get raw sequence information (31) (Fig. 1(B)). And over the years, there has been rapid development of many bioinformatics tools and pipelines for the robust analysis of scRNA-seq data. Mostly, the scRNA-seq data are handled by the data analysis software such as R and Python. Both R and Python have a large collection of packages contributed by researchers around the world for the analysis of scRNA-seq data. For example, Seurat (32) in R and SCANPY (33) in Python are the two most popular packages for the scRNA-seq data analysis, which are not only actively maintained but also timely updated.
After sequencing the library, the preprocessing of the transcriptome is required based on the different methods used, and the workflow is depicted in Figure 2(A). In this step, the cells are identified using the cell barcode and demultiplexed UMI, and the reads from the cells are aligned to the reference genome to obtain the mRNA count matrix. The common software used for the alignment to the genome is STAR (34), Kallisto (35), Bustools (36), Salmon (37), Alevin (38), and 10x Genomics (25). Next, the quality control procedure of the count matrix is performed to remove dead, doublet, and empty cells from the scRNA-seq data based on the number of gene per cell and mitochondrial gene content, and afterward, normalization and batch correction are performed if multiple samples are used (39). During the sequencing of the sample, uneven sequencing depths of the cells will likely occur because of both biological and technical variations. This noise is corrected by data normalization methods, which is important for the downstream analysis. Generally, the data normalization is done by dividing gene expression by total gene expression of the cells and multiplying by scale factor 10,000 with natural log transformation at the end, with the assumption that the scRNA-seq data have normal distribution (40). Other popular packages routinely applied for scRNA-seq data normalization are scran, scNorm, sctransform, etc. In addition, the processing of multiple sample sets for scRNA-seq experiment can introduce variation among samples, which may be contributed by different people carrying out experiments, different time of experiments, or use of different reagents; therefore, the batch correction is important to correctly interpret the results of scRNA-seq data analysis to get true biological signals. There are multiple algorithms available for batch correction, such as canonical correlation analysis (41), mutual nearest neighbors (42), LIGER (43), harmony (44), etc. Of note, we get scRNA-seq data in high-dimensional format, which needs to be reduced to low-dimensional format to effectively visualize and interpret by cell clustering and cell type identification. However, in the scRNA data, there are only a few genes (500–2000) that are highly variable and informative enough to actually draw conclusion on the expression profile of the cells (45). These highly variable genes are chosen by feature selection function and then are used by other algorithms for the dimensional reduction purposes. The most common method for the unsupervised linear dimensional reduction is principal component analysis (PCA) (46).
Fig. 2. Workflow of single-cell RNA sequencing data analysis and downstream functional analysis.
A, Sequential steps of single-cell RNA sequencing data analysis. First, the raw data are preprocessed to get the count matrix. The count matrix data is then subjected to quality control and normalization. In the normalized count matrix data, a few highly variable genes are selected and used for the dimensional reduction method. Then, the cells are clustered using selected algorithms. B, Downstream functional analysis of single-cell RNA sequencing data. Differentially expressed gene analysis, trajectory analysis, and cell-cell interaction analysis are a few examples of downstream functional analysis to extract biological insights from the single-cell RNA sequencing experiment. C, Three examples of plots generated from single-cell RNA sequencing data analysis: the violin plot, the UMAP plot, and the heat map. UMAP, uniform manifold approximation and projection.
A number of PCA is used further downstream in the nonlinear dimensional reduction method for cells clustering and projection in 2D or 3D plots. These implement multidimensional scaling algorithm (47), and the most commonly used models are t-distributed stochastic neighbor embedding (48) and uniform manifold approximation and projection (UMAP) (49). After clustering the cells, the identification of cell types and cell states is accomplished by marker gene analysis by downstream analysis (Fig. 2(B)). The differentially expressed marker gene analysis among clusters is the key for characterizing the cell types and biological attributes in the scRNA data (5). Moreover, the downstream analysis such as pseudotime or trajectory analysis (50) can be performed to get an idea about the order of cells based on dynamic gene expression pattern in single cells. This allows us to detect the progenitor cells from the population of mature cells while undergoing differentiation and the direction of the cell progression. Besides trajectory analysis, we can further study co-expression of genes, gene regulatory network, cell-cell interaction, GO/KEGG enrichment analysis, etc. in the scRNA-seq data. All the scRNA-seq data analysis steps generate various plots that can be analyzed and displayed as results (Fig. 2(C)).
SINGLE-CELL TRANSCRIPTOME IN HEALTH
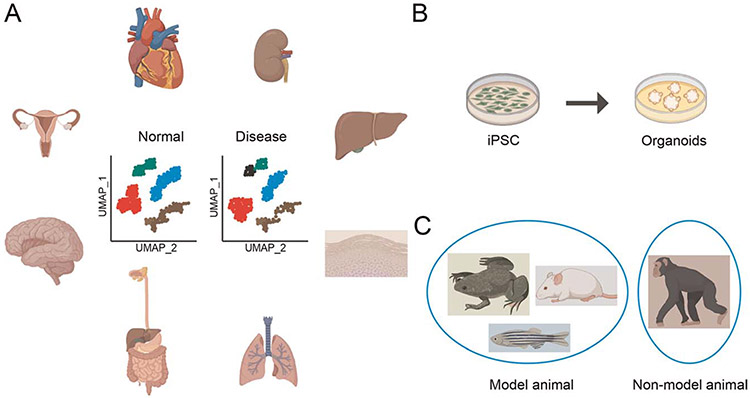
A healthy cell is important for tissue homeostasis and normal physiology. The cells are exclusively produced by cell division at the beginning of embryonic development after fertilization between egg and sperm. The zygote divides into two cells, and subsequent cell divisions give rise to billions of cells in the adult human body, and most of these cells are constituents of various organs. A recent study using a scRNA-seq tool has shed light in the early human embryonic development of tissues during gastrulation at single-cell resolution (51). This study identified pluripotent epiblast, primordial germ cells, red blood cells, and mesodermal and endodermal cell types in early human embryos and made comparative observations with other animal embryos. Moreover, the scRNA-seq experiment of pre-implantation embryos and embryonic stem cells by Yan et al. (52) provided deeper insights into the transcriptomic landscapes of the early human embryonic development, including its stem cells. They discovered that human epiblast cells and in vitro human embryonic stem cells had different transcriptome signatures. Understanding cells in the early embryonic development is essential because any abnormalities present in these cells can result in a birth defect. This technology has been successfully applied to normal cell, induced pluripotent stem cell (iPSC), and organoids research (53,54) (Fig. 3(B)), but most of the time, scRNA-seq data are obtained from the model animals, such as Drosophila (55), planaria (56), Caenorhabditis elegans (57), Ciona (58), Zebrafish (59), Xenopus (60), mouse (61), etc., and nonmodel animals (62) (Fig. 3(C)). The scRNA-seq experiments in model animals have provided deeper understanding of the biology at single-cell resolution. Importantly, the scRNA-seq tool has been able to reveal the cellular heterogeneity of the tissues as well as aided in the discovery of a rare population of cells (63), which is important for the understanding of healthy tissues. Of note, a healthy tissue single-cell transcriptomic atlas is always required as a reference to compare and predict when the changes occur during disease conditions (Fig. 3(A)).
Fig. 3. Applications of the single-cell RNA sequencing method.
A, A single-cell RNA sequencing tool is applied in the study of human organs like the heart, kidney, liver, skin, lung, digestive tract, brain, uterus, etc. in both healthy and diseased conditions. Inset depicts different clusters present in the UMAP of normal and disease conditions after single-cell RNA sequencing data analysis. B, The disease model of iPSC cells can generate organoids to study the mechanism of disease and investigate the drug response by single-cell RNA sequencing. C, The single-cell RNA sequencing method is applied in both model and nonmodel animal research. All the images are created from biorender.com, except UMAP plots. UMAP, uniform manifold approximation and projection.
One of the landmark experiments recently carried out in science is building the Human Cell Atlas (HCA) by implementing scRNA-seq technology from The Tabula Sapiens Consortium and others (64-66). This project has targeted to capture cells of major tissues from the adult human body. Previously, a single organ or tissues were used for single-cell transcriptome analysis and lacked a systemic comparison of cells among different organs of the body, which is important to understand the transcriptional profiles of similar cell types in the normal physiology of the body. In the HCA dataset, a little more than a million cells are present, with 500 different cell types as identified from more than 30 different adult human tissues, using 68 donors (67). This pan-tissue single-cell atlas is extremely important for understanding tissue microenvironment and cellular transcriptional signature required to maintain tissue homeostasis. And to build human pan-tissue atlas, the HCA project included both solid and liquid tissues such as lung, skin, kidney, heart, muscle, pancreases, brain, etc. and hematologic cells. Yet, another important study profiled all the immune cells across several organs during development of the human by scRNA-seq and the spatial transcriptomic method (68). For this project, the immune cells were captured from prenatal hematopoietic organs (yolk sac, liver, and bone marrow), lymphoid organs (thymus, spleen, and lymph node), and nonlymphoid peripheral organs (skin, kidney, and gut) to obtain insights into tissue-specific immune cells properties during human development. A detailed single-cell profile of adult human heart cells was also reported by Litviňuková et al. (69). They analyzed single cells from six different anatomical regions of the adult human heart and found cell type heterogeneity of cardiomyocytes, pericytes, and fibroblast with difference between atrial and ventricular regions. Another essential human organ is adipose tissue, which acts as an endocrine organ to maintain metabolic homeostasis. The single-cell atlas of human and mouse white adipose tissue was reported by Emont et al. (70). In this project, both subcutaneous and visceral adipose tissues depot was characterized at single-cell resolution. Besides humans, mostly the scRNA-seq data are collected from a mouse model using various tissues and organs, and it is very important resource for the biomedical research.
It is logical to carry out scRNA-seq study in healthy tissues because the stochastic nature of gene expression, distinct normal cell types, cellular behavior, cell-cell interaction, and cellular ecosystem within and between tissues are still unknown. Another reason to perform scRNA-seq experiment in a healthy tissue is mostly due to the presence of different cell types in the tissue, and when we want to compare gene expression or cell-cell interaction between two cell populations, we need scRNA-seq technology because bulk RNA-seq technology cannot provide cellular resolution (3). For instance, the heart is composed of cardiomyocytes, fibroblast, endocardial cells, mesenchymal cells, epicardial cells, etc., but if we need to examine the gene expression pattern between cardiomyocytes and endocardial cells populations, we need the scRNA-seq method. However, we can purify certain populations of cells to study in isolation, but it is not an ideal method for studying at single-cell resolution because it will lose tissue ecosystem. In addition, there is continuous differentiation of progenitor cells into mature cells in a healthy tissue, and this process can be uncovered by analyzing the direction of differentiation between two cell populations using trajectory methods such as RNA velocity (71), monocle (72), slingshot (73), PAGA (74), etc. We can also use scRNA-seq technology to study adult stem cells in a healthy tissue (75), which is critical for the replenishment of adult cells in a tissue. Hence, the application of the scRNA-seq tool is wide in the biomedical research, and its potential use in the clinical settings is feasible in the near future.
SINGLE-CELL TRANSCRIPTOME ANALYSIS IN DISEASE
Human disease is a complex process caused by pathological changes in the normal cell types. These pathological phenotypes of cells are currently investigated by the scRNA-seq method in various research laboratories. Most diseases typically studied by this method include cancer, aging, cardiovascular disease, lung disease, kidney disease, Alzheimer, infectious disease, diabetes mellitus, liver disease, etc., and it has covered a vast number of other conditions that are medically relevant. In the database search alone, the articles regarding application of the scRNA-seq method in different disease conditions revealed that mostly cancer researcher implemented this method in their studies (Fig. 4). Preferably, the single-cell transcriptome tools are useful for identifying gene markers to detect diseases as well as to understand the mechanisms of disease and its microenvironment and potentially find therapeutic targets (76). And to understand the mechanism of disease by using the scRNA-seq method, various animal models as well as humanized animal models are commonly used (77). Recently, the iPSC model and organoid model of disease have become popular choices for the elucidation of the pathology of disease based on the scRNA-seq method (53,78). In case of the mouse model, the gene of interests related to a particular disease condition is perturbed by genetics, and the phenotype is examined by the scRNA-seq method to understand the underlying molecular and cellular mechanisms (79) to get deeper insights into the disease, and additional attempts are made to identify the disease target to find cure.
Fig. 4. Statistics of articles in the database about single-cell RNA sequencing applications.
The data for published articles were retrieved from PubMed using the query as single-cell RNA-seq AND disease and setting the publication date from 2013 to 2023. The number of articles was then recorded for each disease in Excel, and the percentage was calculated. The pie graph was then generated in Excel using the same dataset.
Cancer is a disease of different organs and is often described as heterogeneous in the context of cell types (80). Extensive applications of single-cell transcriptome in the cancer research mean there is incredible potential to understand disease and hopefully find druggable targets. Major contributions of scRNA-seq experiments in cancer research are identification of cancer stem cell (81) and circulating tumor cell (82), understanding cancer metastasis (83), recognizing heterogeneity of cancer cells (84), changes in cancer microenvironment (85), investigating cancer drug resistance (86), evaluating cancer immunotherapy (87), discovering cancer targets (88), etc. There is also interpersonal variation in cancer pathogenesis at the population level as well as ethnic variation due to genetic and environmental impacts (89). Commonly, aging is one of the risk factors for cancer (90). Although aging in itself is a natural process, it is also a disease. Aging accompanies many distinct cellular changes, albeit naturally, resulting in weak tissue functions (91). However, healthy aging (92) is now an active area of research to understand chronic diseases in humans. Mostly transcriptional alterations in adult stem cells are observed as a consequence of aging, which was revealed by scRNA-seq experiments in humans (93). Similar study of aging in human skin demonstrated cell type changes that included downregulation of HES1 in fibroblast and KLF6 in basal cells by scRNA-seq analysis (94). Interestingly, the opposite process of aging is regeneration, and different organs or tissues have regenerative ability, but it is found that in humans, regeneration is very limited compared with lower animals (95). Notably, the single-cell transcriptome has enabled to identify new populations of cells responsible for regeneration and to understand the regeneration process using different animal models (96). In the future, harnessing the regeneration process to repair damaged tissues to cure diseases will potentially be applicable in the clinics. And with increase in the quality of life and life expectancy, the cardiovascular disease has become the most prevalent diseases that occur in humans (97). It accounts for most of the clinically related death in the world. Indeed, to understand congenital heart defect, the single-cell analysis from iPSC-derived cardiomyocytes with Tbx5+/− haploinsufficiency revealed disruption in the heart cell lineages (98). Furthermore, the single cell from the cardiogenic heart region of the Hand2−/− mouse embryo provided evidence of a cellular mechanism of outflow tract anomaly (99). The outflow tract anomaly is the prime cause of congenital heart defect in newborns. Yet in another study, the activation of fibroblasts was observed by single-cell transcriptome analysis in adult heart disease and identified MEOX1 as a responsible gene during activation of fibroblasts leading to fibrosis (100). The fibrosis in the heart causes permanent damage and lead to poor heart condition and even death.
In humans, the lung is one of the critical organs for life. The lung suffers from many diseases that ultimately cause death because of respiratory failure. Sikkema et al. (101) recently built a lung atlas after integration of scRNA-seq data of lung samples from diverse demographic individuals to understand disease-associated changes in humans. This project accumulated 2.2 million cells from 444 individuals across healthy and diseased lungs, and this huge dataset can serve as a resource for the scientific community. Also, in the fibrotic lung disease analysis by the single-cell transcriptome, it was revealed that changes occurred in epithelial phenotype and identified multiple fibroblast cells producing large extracellular matrix content as a mechanism of pulmonary fibrotic pathology (102). Similarly, the kidney disease model of mouse was analyzed by single-cell transcriptome. This study by Kirita et al. (103) reported that an acute kidney injury in a mouse model showed the failure of certain types of kidney cells to repair as the prime cause of developing later chronic kidney disease. This finding provides new strategy to target therapeutically to improve health of the kidney after injury. Notably, using both single-nucleus Assay for Transposase Accessible Chromatin sequencing and snRNA-seq analyses of the late-stage Alzheimer disease in humans, Morabito et al. (104) pinpointed distinct epigenomic and transcriptomic changes in the glial cell population in the brain and identified SREBF1 as a causative transcription factor. Another group demonstrated that APOE3 lipoprotein with TREM2 affects microglial cell populations in response to amyloid β in the Alzheimer disease model by the scRNA-seq method (105).
Remarkably, our understanding of infectious disease by the scRNA-seq method has spurred the development of new therapeutic agents in a short time. A very infectious disease called COVID-19 caused a recent pandemic in human history (106). The COVID-19 patient's bronchoalveolar lavage fluid sample analysis by the scRNA-seq method revealed diverse epithelial cells and immune cells affected by this virus (107). And there was markedly increased expression of ACE2 and TMPRSS2 in the epithelial cells of the COVID-19 patient's sample. Another most common disease present in a large population of humans is diabetes mellitus, which affects multiple organs. Specific analysis of diabetic nephropathy in humans by scRNA-seq experiment showed excessive secretion of potassium and observed transcriptomic changes in glomerular cell-type, proximal convoluted tubule, distal convoluted tubule, and principal cells as early signs of kidney impairment (108). Equally, the application of scRNA-seq analysis to traumatic injury and sepsis or shock has proven fruitful in understanding its etiology. In case of a severe burn injury, the human patients experience a complex systemic health problem manifesting as a chronic hypermetabolic state (109). Knuth et al. (110) used snRNA-seq of subcutaneous adipose tissue from severely burned patients to demonstrate that there is an abnormal regulation of adipokine and inflammation. Of note, the cell composition of adipose tissue and particularly immune cells was altered between the early and late stages of severe burn patients, which may have driven the hypermetabolic state. Cho et al. (111) used scRNA-seq analysis of the bone marrow immune microenvironment to understand the immune response after septic shock and found significant changes in the immune cell composition due to systemic infection. Mainly, the myeloid cell population decreased at acute phase (1 day) but increased postsepsis (1 month) in the murine model. In another study, Sun et al. (112) used the scRNA-seq tool and identified a GSDMD-expressing subpopulation of macrophages in sepsis patients, which was responsible for pyroptosis-related pathway regulation and was also associated with a poor prognosis. In adult sepsis patients, Liu et al.(113) screened potential core genes to understand transcriptional regulation and used the scRNA-seq method to study its expression pattern. FOXO3, PPARA, SP1, and STAT3 were expressed in monocytes, NK-T cells, and B-cells, whereas SPI1 was exclusively expressed in monocytes. Furthermore, FOXO3, SP1, SPI1, STAT3, and USF1 were upregulated, whereas PPARA was downregulated in sepsis patients. Also, in human liver cirrhosis, a scar-associated TRM2+CD9+ subpopulation of cells was identified by scRNA-seq experiment for playing a role in its pathogenesis. In addition, cell-cell interaction of some cell populations from the same datasets indicated TNFSF12A, PDGFR, and NOTCH signaling activity led to profibrogenic pathway dysregulation in human liver cirrhosis (114). In summary, we can solely apply the single-cell transcriptome method in collected samples from human patients to understand the disease mechanism and eventually find cures.
CONCLUSIONS
The detailed workflow of scRNA-seq, its application in the individual diseases, and exploring mechanisms of disease have been extensively discussed in the review articles published elsewhere over the past decades. We have witnessed steady progress in the single-cell technology field in a very short period. Particularly, the application and development of scRNA-seq technology have hugely impacted the biomedical research field. It has become the most frequently used tool in understanding and uncovering the basic process in health and disease, yet it faces many inherent technical challenges such as dropout effect, low coverage of transcripts, influence of enzyme or mechanical force during single-cell preparation, etc. (30), which are being mitigated by gradual improvement in the development of scRNA-seq technology. Besides single-cell transcriptome, other single-cell technologies available are single-cell genomics (115), single-cell ATAC sequencing (116), single-cell chip sequencing (117), single-cell Hi-C sequencing (118), single-cell proteomics (119), etc., which are equally applied across health and disease conditions to understand their underlying mechanisms of molecular pathologies. Furthermore, a single-cell multi-omics approach enables researchers to capture two or more single-cell data from the same sample, and this approach comprehensively characterizes the single-cell state by multiple molecular information (120). Here, we summarized and broadly highlighted single-cell transcriptome, some of its different platforms, experimental and computational workflow, and its importance in the normal healthy tissues and in disease conditions.
Funding:
R01AG080040-01A1, 1R01GM133961-01A1.
Footnotes
Conflict of interest: The authors report no conflicts of interest.
REFERENCES
- 1.Hooke R: Micrographia. London: The Royal Society, 1665. [Google Scholar]
- 2.Das S, Vera M, Gandin V, et al. Intracellular mRNA transport and localized translation. Nat Rev Mol Cell Biol. 2021;22(7):483–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers DC, Carew AM, Lukowski SW, et al. : Transcriptomics and single-cell RNA-sequencing. Respirology. 2019:29–36. [DOI] [PubMed] [Google Scholar]
- 4.Tang F, Barbacioru C, Wang Y, et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat Methods. 2009. May;6(5):377–382. [DOI] [PubMed] [Google Scholar]
- 5.Andrews TS, Kiselev VY, McCarthy D, et al. Tutorial: guidelines for the computational analysis of single-cell RNA sequencing data. Nat Protoc. 2021;16:1–9. [DOI] [PubMed] [Google Scholar]
- 6.Grindberg RV, Yee-Greenbaum JL, McConnell MJ, et al. RNA-sequencing from single nuclei. Proc Natl Acad Sci U S A. 2013;110(49):19802–19807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ståhl PL, Salmén F, Vickovic S, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science. 2016;353(6294):78–82. [DOI] [PubMed] [Google Scholar]
- 8.Moreno P, Huang N, Manning JR, et al. User-friendly, scalable tools and workflows for single-cell RNA-seq analysis. Nat Methods. 2021;18(4):327–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abugessaisa I, Noguchi S, Böttcher M, et al. SCPortalen: human and mouse single-cell centric database. Nucleic Acids Res. 2018;46(D1):D781–D787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franzén O, Gan L-M, Björkegren JLM. PanglaoDB: a web server for exploration of mouse and human single-cell RNA sequencing data. Database (Oxford). 2019; 2019:baz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan H, Yan M, Zhang G, et al. CancerSEA: a cancer single-cell state atlas. Nucleic Acids Res. 2019. Jan 8;47(D1):D900–D908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paik DT, Cho S, Tian L, et al. Single-cell RNA sequencing in cardiovascular development, disease and medicine. Nat Rev Cardiol. 2020;17:457–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gierahn TM, Wadsworth MH 2nd, Hughes TK, et al. Seq-Well: portable, low-cost rna sequencing of single cells at high throughput. Nat Methods. 2017;14(4):395–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picelli S, Björklund ÅK, Faridani OR, et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat Methods. 2013;10(11):1096–1098. [DOI] [PubMed] [Google Scholar]
- 15.Wyatt Shields CW 4th, Reyes CD, López GP. Microfluidic cell sorting: a review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip. 2015;15(5):1230–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein AM, Mazutis L, Akartuna I, et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell. 2015;161(5):1187–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macosko EZ, Basu A, Satija R, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161(5):1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Islam S, Zeisel A, Joost S, et al. Quantitative single-cell RNA-seq with unique molecular identifiers. Nat Methods. 2014;11(2):163–166. [DOI] [PubMed] [Google Scholar]
- 19.Hashimshony T, Wagner F, Sher N, et al. CEL-Seq: single-cell RNA-Seq by multiplexed linear amplification. Cell Rep. 2012;2(3):666–673. [DOI] [PubMed] [Google Scholar]
- 20.Ramsköld D, Luo S, Wang YC, et al. Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nat Biotechnol. 2012; 30(8):777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLaughter DM. The use of the Fluidigm C1 for RNA expression analyses of single cells. Curr Protoc Mol Biol. 2018;122(1):e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picelli S, Faridani OR, Björklund AK, et al. Full-length RNA-seq from single cells using Smart-seq2. Nat Protoc. 2014;9(1):171–181. [DOI] [PubMed] [Google Scholar]
- 23.Jaitin DA, Kenigsberg E, Keren-Shaul H, et al. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science. 2014;343(6172):776–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan HC, Fu GK, SPA F. Expression profiling. Combinatorial labeling of single cells for gene expression cytometry. Science. 2015;347(6222):1258367. [DOI] [PubMed] [Google Scholar]
- 25.Zheng GXY, Terry JM, Belgrader P, et al. Massively parallel digital transcriptional profiling of single cells. Nat Commun. 2017;8:14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valihrach L, Androvic P, Kubista M. Platforms for single-cell collection and analysis. Int J Mol Sci. 2018;19(3):807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adil A, Kumar V, Jan AT, et al. Single-cell transcriptomics: current methods and challenges in data acquisition and analysis. Front Neurosci. 2021;15:591122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hulett HR, Bonner WA, Barrett J, Herzenberg LA. Cell sorting: automated separation of mammalian cells as a function of intracellular fluorescence. Science. 1969;166(3906):747–749. [DOI] [PubMed] [Google Scholar]
- 29.Miltenyi S, Müller W, Weichel W, et al. High gradient magnetic cell separation with MACS. Cytometry. 1990;11(2):231–238. [DOI] [PubMed] [Google Scholar]
- 30.Nayak R, Hasija Y. A hitchhiker's guide to single-cell transcriptomics and data analysis pipelines. Genomics. 2021;113(2):606–619. [DOI] [PubMed] [Google Scholar]
- 31.Levy SE, Boone BE. Next-generation sequencing strategies. Cold Spring Harb Perspect Med. 2019. Jul;9(7):a025791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satija R, Farrell JA, Gennert D, et al. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol. 2015;33(5):495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf FA, Angerer P, Theis FJ. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 2018;19(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bray NL, Pimentel H, Melsted P, et al. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34(5):525–527. [DOI] [PubMed] [Google Scholar]
- 36.Melsted P, Booeshaghi AS, Gao F, Beltrame E, Lu L, Eldjárn K, et al. Modular and efficient pre-processing of single-cell RNA-seq. 2019;. bioRxiv [Internet]. Available at 10.1101/673285. Accessed April 24, 2023. [DOI] [Google Scholar]
- 37.Patro R, Duggal G, Love MI, et al. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14(4):417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srivastava A, Malik L, Sarkar H, et al. A Bayesian framework for inter-cellular information sharing improves dscRNA-seq quantification. Bioinformatics. 2020;36:I292–I299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luecken MD, Theis FJ. Current best practices in single-cell RNA-seq analysis: a tutorial. Mol Syst Biol. 2019;15(6):e8746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stuart T, Butler A, Hoffman P, et al. Comprehensive integration of single-cell data. Cell. 2019;177(7):1888–1902.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodosthenous T, Shahrezaei V, Evangelou M. Integrating multi-OMICS data through sparse canonical correlation analysis for the prediction of complex traits: a comparison study. Bioinformatics. 2020;36(17):4616–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haghverdi L, Lun ATL, Morgan MD, et al. Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat Biotechnol. 2018;36(5):421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welch JD, Kozareva V, Ferreira A, et al. Single-cell multi-omic Integration compares and contrasts features of brain cell identity. Cell. 2019;177(7):1873–1887.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korsunsky I, Millard N, Fan J, et al. Fast, sensitive and accurate integration of single-cell data with harmony. Nat Methods. 2019;16(12):1289–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider I, Cepela J, Shetty M, et al. Use of “default” parameter settings when analyzing single cell RNA sequencing data using Seurat: a biologist's perspective. J Transl Genet Genom. 2020. [Google Scholar]
- 46.Hotelling H. Analysis of a complex of statistical variables into principal components. J Educ Psychol. 1933;24(6):417–441. [Google Scholar]
- 47.Kruskal JB. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika. 1964;29(1):1–27. [Google Scholar]
- 48.Van DerMaaten L, Hinton G. Visualizing data using t-distributed stochastic neighbor embedding (tSNE). J Mach Learn Res. 2008;9(86):2579–2605. [Google Scholar]
- 49.McInnes L, Healy J, Melville J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. 2018. Available at http://arxiv.org/abs/1802.03426. Accessed February 9, 2023. [Google Scholar]
- 50.Saelens W, Cannoodt R, Todorov H, et al. A comparison of single-cell trajectory inference methods. Nat Biotechnol. 2019;37(5):547–554. [DOI] [PubMed] [Google Scholar]
- 51.Tyser RCV, Mahammadov E, Nakanoh S, et al. Single-cell transcriptomic characterization of a gastrulating human embryo. Nature. 2021;600(7888):285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan L, Yang M, Guo H, et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat Struct Mol Biol. 2013;20(9):1131–1139. [DOI] [PubMed] [Google Scholar]
- 53.Wesely J, Kotini AG, Izzo F, et al. Acute myeloid leukemia iPSCs reveal a role for RUNX1 in the maintenance of human leukemia stem cells. Cell Rep. 2020;31(9):107688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Combes AN, Zappia L, Er PX, et al. Single-cell analysis reveals congruence between kidney organoids and human fetal kidney. Genome Med. 2019;11(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H, Janssens J, de Waegeneer M, et al. Fly Cell Atlas: a single-nucleus transcriptomic atlas of the adult fruit fly. Science. 2022;375(6584):eabk2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fincher CT, Wurtzel O, de Hoog T, et al. Cell type transcriptome atlas for the planarian Schmidtea mediterranea. Science. 2018;360(6391):eaaq1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao J, Packer JS, Ramani V, et al. Comprehensive single-cell transcriptional profiling of a multicellular organism. Science. 2017;357(6352):661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao C, Lemaire LA, Wang W, et al. Comprehensive single-cell transcriptome lineages of a proto-vertebrate. Nature. 2019;571(7765):349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Metikala S, Casie Chetty S, Sumanas S. Single-cell transcriptome analysis of the zebrafish embryonic trunk. PLoS One. 2021;16(7):e0254024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee J, Møller AF, Chae S, et al. A single-cell, time-resolved profiling of Xenopus mucociliary epithelium reveals nonhierarchical model of development. Sci Adv. 2023;9(14):eadd5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao J, Spielmann M, Qiu X, et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature. 2019;566(7745):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen D, Sun J, Zhu J, et al. Single cell atlas for 11 non-model mammals, reptiles and birds. Nat Commun. 2021;12(1):7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hwang B, Lee JH, Bang D. Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med. 2018;50(8):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tabula Sapiens Consortium*, Jones RC, Karkanias J, Krasnow MA, et al. The Tabula Sapiens: a multiple-organ, single-cell transcriptomic atlas of humans. Science. 2022;376(6594):eabl4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eraslan G, Drokhlyansky E, Anand S, et al. Single-nucleus cross-tissue molecular reference maps toward understanding disease gene function. Science. 2022;376(6594):eabl4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Domínguez Conde C, Xu C, Jarvis LB, et al. Cross-tissue immune cell analysis reveals tissue-specific features in humans. Science. 2022;376(6594):eabl5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Z, Zhang Z. Mapping cell types across human tissues. Science. 2022;376(6594):695–696. [DOI] [PubMed] [Google Scholar]
- 68.Suo C, Dann E, Goh I, et al. Mapping the developing human immune system across organs. Science. 2022;376(6597):eabo0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Litviňuková M, Talavera-Loópez C, Maatz H, et al. Cells of the adult human heart. Nature. 2020;588(7838):466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Emont MP, Jacobs C, Essene AL, et al. A single-cell atlas of human and mouse white adipose tissue. Nature. 2022;603(7903):926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.La Manno G, Soldatov R, Zeisel A, et al. RNA velocity of single cells. Nature. 2018;560(7719):494–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trapnell C, Cacchiarelli D, Grimsby J, et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol. 2014;32(4):381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Street K, Risso D, Fletcher RB, et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics. 2018;19(1):477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolf FA, Hamey FK, Plass M, et al. PAGA: graph abstraction reconciles clustering with trajectory inference through a topology preserving map of single cells. Genome Biol. 2019;20(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dell'Orso S, Juan AH, Ko KD, et al. Single cell analysis of adult mouse skeletal muscle stem cells in homeostatic and regenerative conditions. Development. 2019;146(12):dev174177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van de Sande B, Lee JS, Mutasa-Gottgens E, et al. Applications of single-cell RNA sequencing in drug discovery and development. Nat Rev Drug Discov. 2023;22(6):496–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng L, Yu H, Wrobel JA, et al. Identification of pathogenic TRAIL-expressing innate immune cells during HIV-1 infection in humanized mice by scRNA-Seq. JCI Insight. 2020;5(11):e135344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cochrane DR, Campbell KR, Greening K, et al. Single cell transcriptomes of normal endometrial derived organoids uncover novel cell type markers and cryptic differentiation of primary tumours. J Pathol. 2020;252(2):201–214. [DOI] [PubMed] [Google Scholar]
- 79.Chen WS, Liang Y, Zong M, et al. Single-cell transcriptomics reveals opposing roles of Shp2 in Myc-driven liver tumor cells and microenvironment. Cell Rep. 2021;37(6):109974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanahan D. Hallmarks of Cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. [DOI] [PubMed] [Google Scholar]
- 81.Zheng H, Pomyen Y, Hernandez MO, et al. Single-cell analysis reveals cancer stem cell heterogeneity in hepatocellular carcinoma. Hepatology. 2018;68(1):127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kojima M, Harada T, Fukazawa T, et al. Single-cell DNA and RNA sequencing of circulating tumor cells. Sci Rep. 2021;11(1):22864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olalekan S, Xie B, Back R, et al. Characterizing the tumor microenvironment of metastatic ovarian cancer by single-cell transcriptomics. Cell Rep. 2021;35(8):109165. [DOI] [PubMed] [Google Scholar]
- 84.Pandiani C, Strub T, Nottet N, et al. Single-cell RNA sequencing reveals intratumoral heterogeneity in primary uveal melanomas and identifies HES6 as a driver of the metastatic disease. Cell Death Differ. 2021;28(6):1990–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen K, Wang Q, Li M, et al. Single-cell RNA-seq reveals dynamic change in tumor microenvironment during pancreatic ductal adenocarcinoma malignant progression. EBioMedicine. 2021;66:103315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stewart CA, Gay CM, Xi Y, et al. Single-cell analyses reveal increased intratumoral heterogeneity after the onset of therapy resistance in small-cell lung cancer. Nat Cancer. 2020;1:423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Z, Wang ZX, Chen YX, et al. Integrated analysis of single-cell and bulk RNA sequencing data reveals a pan-cancer stemness signature predicting immunotherapy response. Genome Med. 2022;14(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang H, Yu D, Yang P, et al. Revealing the transcriptional heterogeneity of organspecific metastasis in human gastric cancer using single-cell RNA sequencing. Clin Transl Med. 2022;12(2):e730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Özdemir BC, Dotto GP. Racial differences in Cancer susceptibility and survival: more than the color of the skin? Trends Cancer. 2017;3(3):181–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22(17):R741–R752. [DOI] [PubMed] [Google Scholar]
- 91.López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153(6):1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aronson L. Healthy aging across the stages of old age. Clin Geriatr Med. 2020;36(4):549–558. [DOI] [PubMed] [Google Scholar]
- 93.Oliva AD, Gupta R, Issa K, et al. Aging-related olfactory loss is associated with olfactory stem cell transcriptional alterations in humans. J Clin Invest. 2022;132(4):e155506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zou Z, Long X, Zhao Q, et al. A single-cell transcriptomic atlas of human skin aging. Dev Cell. 2021;56(3):383–397.e8. [DOI] [PubMed] [Google Scholar]
- 95.Goldman JA, Poss KD. Gene regulatory programmes of tissue regeneration. Nat Rev Genet. 2020;21(9):511–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aztekin C, Hiscock TW, Marioni JC, et al. Identification of a regeneration—organizing cell in the Xenopus tail. Science. 2019;364(6441):653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Costantino S, Paneni F, Cosentino F. Ageing, metabolism and cardiovascular disease. J Physiol. 2016;594(8):2061–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kathiriya IS, Rao KS, Iacono G, et al. Modeling human TBX5 haploinsufficiency predicts regulatory networks for congenital heart disease. Dev Cell. 2021;56(3):292–309.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Soysa TY, Ranade SS, Okawa S, et al. Single-cell analysis of cardiogenesis reveals basis for organ-level developmental defects. Nature. 2019;572(7767):120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alexanian M, Przytycki PF, Micheletti R, et al. A transcriptional switch governs fibroblast activation in heart disease. Nature. 2021;595(7867):438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sikkema L, Strobl D, Zappia L, Madissoon E, Markov N, Zaragosi L, et al. An integrated cell atlas of the human lung in health and disease. 2022. bioRxiv [Internet]. Available at 10.1101/2022.03.10.483747. Accessed May 14, 2023. [DOI] [Google Scholar]
- 102.Habermann AC, Gutierrez AJ, Bui LT, et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci Adv. 2020;6(28):eaba1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kirita Y, Wu H, Uchimura K, et al. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc Natl Acad Sci U S A. 2020;117(27):15874–15883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morabito S, Miyoshi E, Michael N, et al. Single-nucleus chromatin accessibility and transcriptomic characterization of Alzheimer's disease. Nat Genet. 2021;53(8):1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fitz NF, Nam KN, Wolfe CM, et al. Phospholipids of APOE lipoproteins activate microglia in an isoform-specific manner in preclinical models of Alzheimer's disease. Nat Commun. 2021;12(1):3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Crook H, Raza S, Nowell J, et al. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. [DOI] [PubMed] [Google Scholar]
- 107.Chen H, Liu W, Wang Y, et al. SARS-CoV-2 activates lung epithelial cell proinflammatory signaling and leads to immune dysregulation in COVID-19 patients. EBioMedicine. 2021;70:103500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilson PC, Wu H, Kirita Y, et al. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc Natl Acad Sci U S A. 2019;116(39):19619–19625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jeschke MG, van Baar ME, Choudhry MA, et al. Burn injury. Nat Rev Dis Primers. 2020;6(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Knuth CM, Ricciuti Z, Barayan D, et al. Single-nuclei RNA profiling reveals disruption of adipokine and inflammatory signaling in adipose tissue of burn patients. Ann Surg. 2023;278(6):e1267–e1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cho DS, Schmitt RE, Dasgupta A, et al. Acute and sustained alterations to the bone marrow immune microenvironment following polymicrobial infection. Shock. 2022;58(1):45–55. [DOI] [PubMed] [Google Scholar]
- 112.Sun Z, Zhang T, Ning C, et al. Construction of sepsis diagnostic models and identification of macrophage subpopulations based on pyroptosis-related genes. Shock. 2023;60(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu J, Li S, Xiong D, et al. Screening of potential core genes in peripheral blood of adult patients with sepsis based on transcription regulation function. Shock. 2023;59(3):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramachandran P, Dobie R, Wilson-Kanamori JR, et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575(7783):512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gawad C, Koh W, Quake SR. Single-cell genome sequencing: current state of the science. Nat Rev Genet. 2016;17(3):175–188. [DOI] [PubMed] [Google Scholar]
- 116.Buenrostro JD, Wu B, Litzenburger UM, et al. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature. 2015;523(7561):486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rotem A, Ram O, Shoresh N, et al. Single-cell ChIP-seq reveals cell subpopulations defined by chromatin state. Nat Biotechnol. 2015;33(11):1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nagano T, Lubling Y, Stevens TJ, et al. Single-cell Hi-C reveals cell-to-cell variability in chromosome structure. Nature. 2013;502(7469):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kelly RT. Single-cell proteomics: progress and prospects. Mol Cell Proteomics. 2020;19(11):1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee J, Hyeon DY, Hwang D. Single-cell multiomics: technologies and data analysis methods. Exp Mol Med. 2020;52(9):1428–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]