Summary
Background
Infants born to women with HIV in settings with a high tuberculosis burden are at risk of tuberculosis infection and rapid progression to active disease. Maternal isoniazid preventive therapy might mitigate this risk, but optimal timing of therapy remains unclear. The TB APPRISE trial showed that initiation of isoniazid during pregnancy resulted in more frequent adverse pregnancy outcomes than when initiated postpartum. We aimed to determine the proportion of infants testing positive for tuberculosis infection born to mothers who initiated isoniazid therapy antepartum compared with postpartum using two commonly used tests, the test agreement, and predictors of test positivity.
Methods
TB APPRISE was a randomised, double-blind, placebo-controlled, non-inferiority trial done at 13 study sites across eight countries (Botswana, Haiti, India, South Africa, Tanzania, Thailand, Uganda, and Zimbabwe). Pregnant women with HIV on antiretroviral therapy were randomly assigned to receive immediate isoniazid preventive therapy (28 weeks isoniazid [300 mg daily], then placebo until week 40 after delivery) or deferred treatment (placebo until week 12 after delivery, then isoniazid [300 mg daily] for 28 weeks). Mother–infant pairs were followed up until 48 weeks after delivery. We included all liveborn infants with a tuberculin skin test or interferon-γ release assay (IGRA) at 44 weeks. The outcomes assessed in this secondary analysis were tuberculosis test positivity by study group, test agreement, and predictors of test positivity. This study was registered with ClinicalTrials.gov, NCT01494038.
Findings
Between Aug 19, 2014, and April 4, 2016, 956 mothers were randomly assigned, and 749 mother–child pairs were included in this secondary analysis. Of 749 infants, 694 (93%) received Bacille Calmette-Guérin (BCG) vaccination, 675 (90%) were born to mothers who had completed isoniazid treatment, 20 (3%) were exposed to tuberculosis, seven (1%) became HIV positive, and one (<1%) developed probable tuberculosis. 43 (6%; 95% CI 4–8]) of 732 infants had a positive IGRA test result and 55 (8%; 6–10) of 727 infants had a positive tuberculin skin test result. Test positivity did not differ by study group (p=0·88 for IGRA; p=0·44 for tuberculin skin test). Test agreement was poor (κ=0·107 [95% CI 0·002–0·212]). Infant tuberculin skin test positivity was associated with breastfeeding (adjusted odds ratio 6·63 [95% CI 1·57–27·9]), BCG vaccination (4·97 [1·50–16·43]), and maternal tuberculin skin test positivity at delivery (3·28 [1·70–6·33]); IGRA positivity was associated with female sex (2·09 [1·06–4·14]).
Interpretation
Deferral of maternal isoniazid preventive therapy to early postpartum had no effect on infant tuberculosis acquisition in our trial population, regardless of the diagnostic test used; however, tuberculosis test agreement is poor during infancy.
Funding
US National Institutes of Health.
Introduction
Tuberculosis disease affects an estimated 1·2 million children (aged <15 years) annually and is a leading cause of death for children under 5 years of age.1,2 Preventing tuberculosis during the first year of life is crucial since infants are at high risk for rapid progression to active disease after tuberculosis exposure and acquisition of infection.3,4 Women with HIV are at particularly high risk of developing tuberculosis during pregnancy and postpartum,5 and maternal tuberculosis is an important risk factor for infant tuberculosis. The incidence of tuberculosis infection among infants exposed to HIV ranges from 4% to 14% using interferon-γ release assay (IGRAs) and from 9% to 36% using tuberculin skin tests (TSTs).6,7 Routine tuberculosis screening and use of antiretroviral therapy (ART) and isoniazid preventive therapy among mothers could substantially reduce infant tuberculosis infection and active disease,8–10 but the optimal timing of maternal isoniazid preventive therapy remains unclear.
Globally, ART with isoniazid preventive therapy is recommended for all people living with HIV to reduce tuberculosis incidence.11–13 Both therapies have proven efficacy when used alone and stronger efficacy when combined.8,14 Prevention of maternal tuberculosis, including subclinical tuberculosis, reduces infant tuberculosis exposure and subsequent acquisition of infection and progression to active disease. However, it is only since 2018 that the isoniazid preventive therapy guidance has been implemented among an increasing number of pregnant women. There remains some concern that isoniazid exposure during pregnancy is associated with increased risk of adverse pregnancy outcomes. To more fully understand the risks and benefits of maternal isoniazid preventive therapy, studies are needed to determine whether immediately initiating isoniazid preventive therapy during pregnancy or delaying isoniazid preventive therapy until postpartum has a differential impact on the incidence of infant tuberculosis infection or disease.15
Here, we report the secondary outcomes of the TB Ante versus Postpartum Prevention with Isoniazid in HIV Seropositive mothers and their Exposed infants (TB APPRISE) trial. We aimed to assess the incidence of tuberculosis among infants by maternal study group and the concordance of the TST and IGRA for infant tuberculosis infection diagnosis; and to determine predictors of tuberculosis infection in infants exposed to HIV.
Methods
Study design and participants
TB APPRISE was a randomised, double-blind, placebo-controlled phase 4 trial done at 13 study sites across eight countries (Botswana, Haiti, India, South Africa, Tanzania, Thailand, Uganda, and Zimbabwe) with a tuberculosis prevalence of more than 60 cases per 100 000 population.15 The primary trial objective was to assess the safety of isoniazid preventive therapy among pregnant women with HIV, namely the incidence rate of treatment-related adverse events (Division of AIDS grade 3 or higher), and has been reported.15
Study sites enrolled pregnant women with HIV, aged 18 years or older, who were between 14 and 34 weeks of gestation and receiving ART. Exclusion criteria were suspected active tuberculosis, recent known tuberculosis exposure, tuberculosis disease treatment, or isoniazid for more than 30 days in the previous year and evidence of acute hepatitis within 90 days of study entry or peripheral neuropathy grade 1 or higher. All women provided written informed consent. The trial was approved by local and collaborating institutional review boards and was reviewed every 6 months by an independent data and safety monitoring board.
Full details of the trial design and conduct are provided in the protocol and the statistical analysis plan (appendix p 10).
Randomisation and masking
Participants were randomly assigned (1:1) to initiate isoniazid preventive therapy at study entry (antepartum; immediate isoniazid preventive therapy) or at 12-weeks postpartum (deferred isoniazid preventive therapy). Randomisation was stratified according to gestational age at entry (14 weeks to <24 weeks or 24 weeks to ≤34 weeks) and balanced by treatment group at each site. Randomisation was done using permuted blocks (block size 4) and was centrally coordinated by the patient registration system team at Frontier Science (Amherst, NY, USA). Study participants, investigators, and individuals assessing outcomes were masked to treatment assignment and IGRA result at entry; evidence of maternal tuberculosis infection was not required for entry.
Procedures
Women allocated to the immediate group received daily oral isoniazid 300 mg (Macleods Pharmaceuticals, Mumbai, India) for 28 weeks beginning at trial entry and then received placebo until week 40 after delivery. Women allocated to the deferred group received placebo beginning at entry until week 12 after delivery, followed by daily isoniazid 300 mg for 28 weeks. All mothers received locally supplied, open-label pyridoxine (vitamin B6) and prenatal multivitamins until 40 weeks postpartum. Maternal age, CD4 cell count, HIV viral load, and IGRA status were assessed at trial entry, and tuberculosis infection testing was repeated at delivery using TST. After delivery, mother–infant pairs were followed up for 48 weeks. Study visits were scheduled at week 4, 8, 12, 24, 36, 44, and 48 and for suspected active tuberculosis. At each visit, study staff assessed tuberculosis exposure history, WHO tuberculosis symptom screen (cough, weight loss, fever, and night sweats), breastfeeding history, and infant nutritional status (WHO weight-for-age Z score) and did a targeted physical exam. At each study visit, receipt of Bacille Calmette-Guérin (BCG) vaccination status was documented by recording if the infant had received a BCG vaccine at the current visit or since the last visit, including the date of receipt, and by assessing the infant for a visible BCG scar. Infants were tested for HIV at delivery, at week 48, and at the time of suspected tuberculosis. Mothers and infants received the local standard of care for prevention of mother-to-child transmission and HIV infection. In case of suspected tuberculosis, tuberculosis infection testing was performed (IGRA for mothers and TST for infants), and participants were referred for local treatment; all infants exposed to tuberculosis were referred for isoniazid preventive therapy, per local standard of care. All infants underwent tuberculosis infection testing at week 44 using IGRA or TST, or both.
Blood samples for IGRA were collected from infants before TST placement. IGRA was performed using QuantiFERON-Gold-in-tube (Qiagen, Venlo, the Netherlands). TST was performed using locally available purified protein derivative reagents (2 tuberculin-unit strength) administered intradermally via the mantoux method, and results were read within 3 days of purified protein derivative placement. Staff at all study sites were trained on how to perform IGRA and TST in a standardised manner and participated in a quality assurance programme for IGRA established by the IMPAACT Network.
Outcomes
In this prespecified secondary analysis, the main outcome was the proportion of infants with tuberculosis infection (positive IGRA or TST) at 44 weeks of life. A positive IGRA result was defined as an interferon-γ (IFN-γ) concentration of at least 0·35 IU/mL in response to Mycobacterium tuberculosis antigen stimulation after subtraction of an unstimulated control (as per manufacturer instructions); indeterminate results were considered negative. A positive TST result was defined as induration diameter of at least 5 mm in infants with HIV and at least 10 mm in infants without HIV.16 Additional pre-specified analyses were the assessment of test agreement and predictors of test positivity.
We also considered various alternative thresholds to define IGRA positivity, including IFN-γ concentrations of at least 0·2 IU/mL, which has been proposed for people with HIV,17 and IFN-γ of at least 0·7 IU/mL18,19 and at least 4·0 IU/mL, which have been reported in the literature as being more robust for diagnosing tuberculosis infection and predicting active disease, respectively, in children.17 For the TST, we also considered an induration diameter of at least 20 mm, which has been proposed for predicting active tuberculosis in children.17
Site investigators assessed and reported cases of suspected incident tuberculosis disease. All diagnoses were adjudicated by an independent endpoint review committee. We defined unconfirmed tuberculosis as probable or possible tuberculosis in accordance with the updated international consensus clinical case definitions for classification of intrathoracic tuberculosis for diagnostic studies in children.20 Possible tuberculosis was defined as compatible symptoms, positive acid-fast bacilli smear, or suggestive findings on chest radiography. Probable tuberculosis was defined as compatible symptoms and either positive acid-fast bacilli smear or suggestive findings on chest radiography.21
Statistical analysis
This analysis included all liveborn infants with an available tuberculosis infection test result. A small number of infants underwent tuberculosis infection testing outside of the week 44 window (plus or minus 2 weeks) and were included in the analysis if testing occurred at week 34 or later. Covariates and outcomes were summarised using descriptive statistics. IGRA test positivity and TST positivity were reported with associated 95% CIs, overall and by study group and study site. We used data from infants with results for both tests to construct a contingency table, and tuberculosis infection test agreement was evaluated using the κ statistic and conditional logistic regression.
Predictors of IGRA and TST positivity were evaluated using separate univariable and multivariable logistic regression models fitted to assess the association between tuberculosis infection test result and study group. Multivariable models including treatment group were fitted using a forward selection approach. Covariates with p values less than 0·15 in univariate analysis were considered for inclusion and were added to the model one at a time in the order of increasing univariable p value. A covariate remained in the model if the p value was less than 0·10. Adjusted odds ratios (aORs) with associated 95% CI are presented in forest plots. Potential maternal covariates were age, CD4 cell count, HIV-1 viral load, and IGRA status at entry; TST status at delivery; isoniazid preventive therapy completion, duration of isoniazid preventive therapy, and ART discontinuation for more than 4 weeks during the study. Potential infant covariates were sex, HIV status, tuberculosis exposure, isoniazid use, BCG vaccination, WHO weight-for-age Z score, duration of exclusive breastfeeding (time from delivery to the last visit where exclusive breastfeeding was reported), and timing of TST placement relative to IGRA blood draw (considered for IGRA analysis only).
All statistical analyses were performed using SAS (version 9.4). p values less than 0·05 were considered statistically significant. This trial was registered with ClinicalTrials.gov, NCT01494038.
Role of the funding source
The funder of the study was involved in the study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the paper for publication. The isoniazid and placebo were supplied by the Clinical Research Products Management Center of the National Institute of Allergy and Infectious Diseases. The isoniazid, placebo, and testing kits were purchased with trial funds; there was no commercial support for the trial.
Results
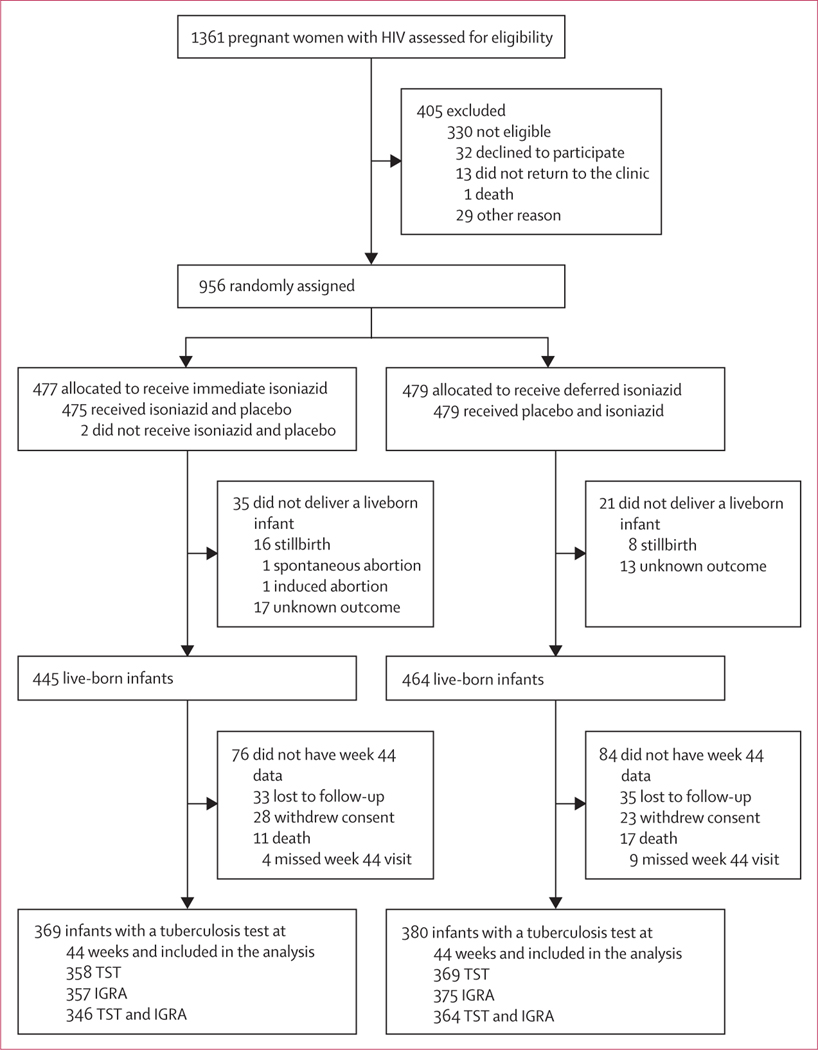
Between Aug 19, 2014, and April 4, 2016, 1361 pregnant women with HIV were assessed for eligibility, of whom 956 were randomly assigned (477 were allocated to the immediate group; 479 to the deferred group). 785 women completed the trial. Of the 909 liveborn infants, 749 (82%) had an IGRA or TST result available at week 44 and were included in this analysis. The analysis sample comprised 369 infants born to 366 women in the immediate isoniazid preventive therapy group and 380 infants born to 375 women in the deferred isoniazid preventive therapy group (figure 1).
Figure 1: Trial profile.
All liveborn infants with a tuberculosis infection test result at 44 weeks of life (at least one of IGRA or TST) were included in the analysis. IGRA=interferon-γ release assay. TST=tuberculin skin test.
Maternal and infant characteristics are summarised in table 1. The largest proportion of mother–infant pairs resided in sub-Saharan Africa (685 [91%] of 749 pairs), and the remaining 64 (9%) were from Thailand, India, and Haiti. At trial entry, the median maternal age was 29 years (IQR 24–34), the majority of infants were born to mothers who had an undetectable HIV-1 viral load (465 [62%] of 749), and the median maternal CD4 count was 493 cells per mm³ (349–679). The majority of infants were born to women who tested negative for tuberculosis infection at entry (466 [62%] of 749 infants) and delivery (610 [81%]), and who completed the entire isoniazid preventive therapy regimen (675 [90%]). After delivery, BCG vaccination was confirmed in 694 (93%) of 749 infants, and 600 (80%) infants were exclusively breastfed for any duration with a median duration of 30 weeks (17–30). 20 (3%) of 749 infants had documented tuberculosis exposure, including five (1%) with maternal tuberculosis exposure, and seven (1%) infants became HIV positive during follow-up.
Table 1:
Baseline characteristics of mother–infant pairs included in the analysis
| Immediate isoniazid preventive therapy (n=369) | Deferred isoniazid preventive therapy (n=380) | |
|---|---|---|
| Maternal characteristics | ||
| Country | ||
| Botswana (2 sites) | 53 (14%) | 54 (14%) |
| Haiti (1 site) | 6 (2%) | 9 (2%) |
| India (1 site) | 9 (2%) | 10 (3%) |
| South Africa (3 sites) | 59 (16%) | 53 (14%) |
| Tanzania (1 site) | 29 (8%) | 32 (8%) |
| Thailand (1 site) | 13 (4%) | 17 (4%) |
| Uganda (1 site) | 59 (16%) | 60 (16%) |
| Zimbabwe (3 sites) | 141 (38%) | 145 (38%) |
| Age at entry, years | 29 (25 to 34) | 29 (24 to 33) |
| CD4 count, cells per mm3 | 491 (348 to 670) | 499 (350 to 693) |
| Undetectable HIV-1 RNA | 229 (62%) | 236 (62%) |
| IGRA result | ||
| Positive* | 106 (29%) | 117 (31%) |
| Negative | 233 (63%) | 233 (61%) |
| Indeterminate | 23 (6%) | 25 (7%) |
| Unknown | 7 (2%) | 5 (1%) |
| TST result at delivery (or up to 6 weeks postpartum) | ||
| Positive† | 48 (13%) | 52 (14%) |
| Negative | 301 (82%) | 309 (81%) |
| Unknown | 20 (5%) | 19 (5%) |
| Completed entire isoniazid regimen | 336 (91%) | 339 (89%) |
| Duration of isoniazid therapy, weeks | 28 (27 to 29) | 28 (27 to 28) |
| Infant characteristics | ||
| Sex Female |
189 (51%) | 188 (49%) |
| Male | 180 (49%) | 192 (51%) |
| HIV positive‡ | 2 (1%) | 5 (1%) |
| Reported tuberculosis exposure§ | 10 (3%) | 10 (3%) |
| Timing of tuberculosis exposure, study week | 28·5 (11 to 41) | 30 (17 to 39) |
| BCG vaccination status (scar or record of receipt) | 344 (93%) | 350 (92%) |
| BCG scar present | 299 (81%) | 304 (80%) |
| Documented record of receipt | 242 (66%) | 260 (68%) |
| WHO weight-for-age Z score | ||
| Mean | −0·3 (2·8) | −0·5 (1·2) |
| Median | −0·5 (−1·3 to 0·3) | −0·5 (−1·4to 0·3) |
| Exclusive breastfeeding for any duration | 303 (82%) | 297 (78%) |
| Exclusive breastfeeding duration¶ | ||
| Mean (SD), weeks | 23·0 (12·9) | 21·9 (13·1) |
| Median (IQR), weeks | 30 (17 to 30) | 30 (10 to 30) |
| 0 week | 66 (18%) | 83 (22%) |
| >0–12 weeks | 20 (5%) | 17 (4%) |
| >12–24 weeks | 43 (12%) | 31 (8%) |
| >24–36 weeks | 212 (57%) | 235 (62%) |
| >36 weeks | 28 (8%) | 14 (4%) |
| Timing of tuberculosis tests | ||
| TST before IGRA blood draw (≥7 days) | 7 (2%) | 9 (2%) |
| TST after IGRA blood draw | 350 (95%) | 366 (96%) |
| IGRA not performed | 12 (3%) | 5 (1%) |
Data are n (%) median (IQR), unless otherwise indicated. Some percentages do not sum to 100 due to rounding. BCG=Bacille Calmette-Guérin. IGRA=interferon-γ release assay. TST=tuberculin skin test.
Defined as an interferon-γ concentration of ≥0·35 IU/mL.
Defined as an induration diameter ≥5 mm for mothers with HIV and ≥10 mm for mothers without HIV.
Assessed at birth, at 48 weeks after delivery, and at the time of suspected tuberculosis disease.
14 exposures were household contacts, including five maternal exposures, and six were non-household contacts.
Calculated as the time from birth to the last study visit where breastfeeding was reported.
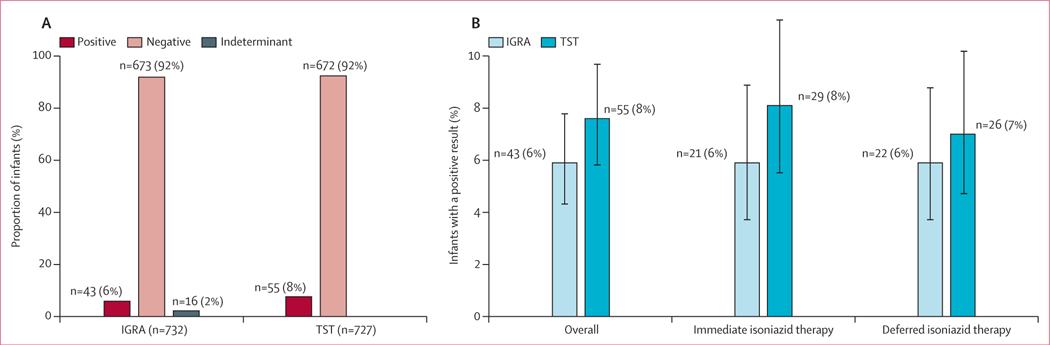
732 (98%) of 749 infants were tested by IGRA, and 727 (97%) were tested by TST. 43 (6%) of 732 infants had a positive IGRA test result, and 55 (8%) of 727 infants had a positive TST result (figure 2A). Tuberculosis infection test positivity did not differ by timing of maternal isoniazid preventive therapy using IGRA (6% [95% CI 4–9] for the immediate group vs 6% [4–9] for the deferred group; p=0·88) or TST (8% [5–11] vs 7% [5–10]; p=0·44; figure 2B). The findings remained unchanged when alternative thresholds for test positivity were considered. Overall, IGRA positivity was 8% (6–10) using an IFN-γ threshold of at least 0·2 IU/mL, 6% (5–8) using a threshold of at least 0·35 IU/mL, 3% (2–5) using a threshold of at least 0·7 IU/mL, and 2% (1–3) using a threshold of at least 4·0 IU/mL, and did not differ by study group (p=0·73; appendix p 3). Overall, TST positivity was 13% (11–16) for an induration diameter of at least 5 mm and 1% (0–2) for an induration diameter of at least 20 mm, and did not differ by study group (p=0·88; appendix p 3). IGRA and TST positivity varied by study site (appendix p 4).
Figure 2: Infant tuberculosis infection test positivity.
Tuberculosis infection test positivity in the overall analysis sample (A) and by study group (B). IGRA positivity was defined as an interferon-γ concentration of ≥0·35 IU/mL. TST positivity was defined as an induration diameter ≥5 mm for infants with HIV and ≥10 mm for infants without HIV infection. Test positivity did not differ by study group (p=0·88 for IGRA; p=0·44 for TST). IGRA=interferon-γ release assay. TST=tuberculin skin test.
Site investigators diagnosed five infants with unconfirmed tuberculosis disease during follow-up, including four with possible tuberculosis disease and one with probable tuberculosis (appendix p 5). Clinicians were adequately convinced of tuberculosis disease to start tuberculosis treatment in two of the five infants; one of whom was the only tuberculosis case confirmed by the endpoint review committee (appendix p 5). This infant had documented tuberculosis exposure, symptoms compatible with tuberculosis, perihilar infiltrates, and a positive TST with an induration diameter of 15 mm. After independent review of the medical records, the study endpoint review committee found no evidence of tuberculosis in the remaining infants, lowering the estimate for incident tuberculosis disease (one [<1%] of 749 infants).
710 (95%) of 749 infants had both an IGRA and TST result at week 44 (table 2). Positive test concordance was 1% (eight of 710), negative test concordance was 88% (622 of 710), and test discordance was 11% (80 of 710), yielding poor overall test agreement (κ=0·107; 95% CI 0·002–0·212; McNemar’s p value=0·1797). When tuberculosis infection test results were discordant, TST was more likely to be positive than IGRA, but the difference was not statistically significant (OR 1·4 [95% CI 0·9–2·1], p=0·22).
Table 2:
Tuberculosis infection test concordance and agreement among HIV-exposed infants who had both IGRA and TST at 44 weeks (n=710)
| TST positive | TST negative | Total | |
|---|---|---|---|
| IGRA positive | 8 | 34 | 42 |
| IGRA negative | 46 | 622 | 668 |
| Total | 54 | 656 | 710 |
IGRA positivity was defined as IFN-γ concentration ≥0·35 IU/mL. TST positivity was defined as induration diameter ≥5 mm for infants with HIV and ≥10 mm for infants without HIV infection. Negative IGRA included negative and indeterminate results; one infant who tested positive on IGRA did not have a TST. IGRA=interferon-γ release assay. TST=tuberculin skin test.
In univariable analysis, IGRA positivity was significantly associated with maternal TST positivity at delivery and infant female sex ( appendix p 6). Exposure to tuberculosis seemed to be associated with increased IGRA positivity, but there were few tuberculosis-exposed infants in our cohort (n=20), and the difference did not reach statistical significance (1·93 [0·43–8·63], p=0·39). Only female sex remained associated with IGRA positivity in multivariable models (aOR 2·09 [1·06–4·14], p=0·033; table 3).
Table 3:
Multivariable analysis of potential predictors of infant IGRA positivity at 44 weeks (n=732)
| IGRA result* |
Multivariable model |
|||
|---|---|---|---|---|
| Positive (n=43) | Negative (n=689) | Adjusted OR (95% CI) | p value | |
| Maternal study group | ||||
| Immediate isoniazid | 21 (49%) | 336 (49%) | 1·05 (0·55–2·00) | 0·88 |
| Deferred isoniazid | 22 (51%) | 353 (51%) | 1 (ref) | .. |
| Maternal TST positive at delivery (or up to 6 weeks postpartum)† | 10 (23%) | 87 (1%) | 2·09 (0·98–4·44) | 0·06 |
| Infant sex | ||||
| Female | 28 (8%) | 339 (92%) | 2·09 (1·06–4·14) | 0·033 |
| Male | 15 (4%) | 350 (96%) | 1 (ref) | .. |
Data are n (%) or median (IQR), unless stated otherwise. OR indicates likelihood of IGRA positivity. BCG=Bacille Calmette-Guérin. IGRA=interferon-γ release assay. OR=odds ratio. TST=tuberculin skin test.
Positive IGRA was defined as a IFN-γ concentration ≥0·35 IU/mL; negative IGRA included negative and indeterminate results.
Only includes mother–infant pairs for whom data were available.
Univariable and multivariable models identified several predictors of TST positivity, including infant BCG vaccination (presence of scar), increasing maternal CD4 count), maternal TST positivity at delivery, infant tuberculosis exposure, and exclusive breastfeeding (appendix p 7). Among these, the strongest predictors were exclusive breastfeeding for any duration (aOR 6·63 [95% CI 1·57–27·92], p=0·010), infant BCG scar (4·97 [1·50–16·43], p=0·009), and maternal TST positivity at delivery (3·28 [1·70–6·33], p=0·0004; table 4).
Table 4:
Multivariable analysis of potential predictors of infant TST positivity at 44 weeks (n=727)
| TST result* |
Multivariable model |
|||
|---|---|---|---|---|
| Positive (n=55) | Negative (n=672) | Adjusted OR (95% CI) | p value | |
| Maternal study group | ||||
| Immediate isoniazid | 29 (53%) | 329 (49%) | 1·26 (0·70–2·30) | 0·44 |
| Deferred isoniazid | 26 (47%) | 343 (51%) | 1 (ref) | .. |
| Maternal CD4 count at entry, cells per mm3 | 610·5 (276·5) | 511·5 (235·1) | 1·11 (0·99–1·25) | 0·08 |
| Maternal TST positive at delivery (up to 6 weeks postpartum)† | 17 (31%) | 80 (11·9%) | 3·28 (1·70–6·33) | 0·0004 |
| Infant BCG scar present† | 51 (93%) | 535 (80%) | 4·97 (1·50–16·43) | 0·009 |
| Exclusive breastfeeding for any duration | 53 (96%) | 528 (79%) | 6·63 (1·57–27·92) | 0·010 |
Data are n (%) or mean (SD). OR indicates likelihood of TST positivity. BCG=Bacille Calmette-Guérin. TST=tuberculin skin test. OR=odds ratio.
TST positivity was defined as induration diameter ≥5 mm for infants with HIV and ≥10 mm for infants without HIV.
Only includes mother–infant pairs for whom data were available.
Discussion
In the primary trial analysis, we previously reported a small but increased risk of composite adverse pregnancy outcomes and no difference in maternal tuberculosis when isoniazid preventive therapy was initiated during pregnancy versus postpartum.15 This secondary analysis builds on those findings and suggests that the timing of isoniazid preventive therapy initiation (antepartum or postpartum) had no effect on infant infection, as measured by either TST or IGRA. This is reassuring for real-world implementation. Although WHO currently recommends ART plus isoniazid preventive therapy for pregnant women with HIV, women who present late for antenatal care might require postpartum tuberculosis preventative therapy. National guidance might also differ; for example, the US Department of Health and Human Services offers the option to defer tuberculosis preventive therapy to postpartum in the absence of close contact with infectious tuberculosis.22 Overall, the goal is to prevent tuberculosis disease and infection, and our results suggest flexibility in the exact timing of maternal tuberculosis preventive therapy initiation. These specific data on incident tuberculosis infection among infants born to mothers on ART with or without isoniazid preventive therapy are also relevant for future tuberculosis burden estimates and for estimating sample size for prevention of infection vaccine trials involving infants exposed to HIV.
Compared with previous estimates of tuberculosis infection among infants exposed to HIV, we observed low incidence in our trial population, regardless of the diagnostic test used (6% with IGRA and 8% with TST).23–26 In contrast to our results, a 2008 study from South Africa reported overall tuberculosis infection incidence of 13% during the first year of life (IGRA or TST positive) and 15% IGRA positivity in infants without HIV infection:27 a study from Kenya also reported a tuberculosis infection incidence of 11% among infants in 2018. However, the same study found 5% IGRA positivity and 9% TST positivity among infants randomly assigned to receive isoniazid, which is more consistent with our findings.23 Furthermore, all mothers with HIV received ART, and most (70%) received isoniazid preventive therapy.23 We observed low incidence of infant tuberculosis infection and disease in the absence of infant isoniazid therapy. It is important to note that we excluded pregnant women with active tuberculosis or recent exposure to active tuberculosis. Additionally, mothers in our population had relatively good immune preservation or reconstitution, as shown by high CD4 T-cell counts at study entry, and all received isoniazid either during pregnancy or postpartum. These factors are likely to have reduced the risk of maternal tuberculosis disease during our study, and collectively these results support the combined benefit of maternal ART and isoniazid preventive therapy.
After independent review of five suspected tuberculosis cases, only one infant in our analysis sample was diagnosed with probable tuberculosis disease, but in two cases, health-care providers initiated anti-tuberculosis therapy. Although the diagnoses of possible tuberculosis disease were not fully substantiated, additional infants did receive post-exposure isoniazid or full therapy for possible tuberculosis disease, which could have averted cases of tuberculosis disease. Our estimated incidence of infant tuberculosis disease is more aligned with the proportion of infants demonstrating a plasma interferon-γ concentration of 4·0 IU/mL or higher in response to M tuberculosis antigen. This high quantitative threshold was previously associated with tuberculosis disease progression in children.17,28
There was substantial discordance between IGRA and TST results in our cohort of infants exposed to HIV, which highlights the current challenge of tuberculosis infection diagnosis during infancy. We previously reported poor test concordance among the mothers in our trial population.29 However, the infants were more likely to have the opposite discordance pattern, namely a positive TST and a negative IGRA test. Several mechanisms could explain the observation of higher TST positivity. The BCG vaccine was administered to 80% of our study participants. Receipt of BCG is associated with higher likelihood of TST positivity, but should have no impact on IGRA positivity.30–32 Similarly, other studies done in settings with high and low tuberculosis burden have shown high rates of discordance between IGRA and TST in young children and that there is higher concordance between the two tests in the absence of infant BCG vaccination. Additionally, in contrast to IGRA, non-specific TST conversion can occur in response to environmental exposure to non-tuberculosis mycobacteria.30 Furthermore, purified protein derivative administration technique and subjective interpretation of results might contribute to TST variability, whereas IGRA is subject to variation between laboratories33 and issues with reproducibility.34 Despite our attempt to reduce site variability by standardising the tuberculosis infection testing process and training all study personnel, the type of BCG vaccine and TST product could have contributed to variability in infant TST positivity by study site. However, our analysis also indicates a significant association between maternal and infant TST positivity at each site. This association could indicate an interpretation bias, environmental exposure to tuberculosis, or non-tuberculosis mycobacteria. The fact that maternal TST positivity was also associated with infant IGRA positivity in the univariable analysis supports increased, undocumented tuberculosis exposure as the underlying mechanism for the concordance of TST positivity among mothers and children.25
In addition to maternal TST positivity at delivery, our analysis identified several factors associated with either TST or IGRA positivity among infants. Increased odds of a positive tuberculin skin test result in infants was associated with breastfeeding and high maternal CD4 cell count, which might suggest an association of tuberculin skin test positivity with good nutritional and immunological status. However, it is unclear why nutritional and immunological advantages would not also affect IGRA results, since IGRAs measure the secretion of interferon-γ (an inflammatory cytokine) in response to tuberculosis-specific antigens. The performance characteristics of IGRAs and TSTs are often affected by the immune status of the host.35 In particular, some studies have noted the reduced sensitivity of IGRA compared to TST in high tuberculosis burden settings.36 In our analysis, infant female sex was the sole predictor of infant IGRA positivity. It is well established among adults that females are more likely than males to develop inflammatory reactions, including autoimmune disorders, and perhaps increased responses to vaccines.37 However, less is known about the impact of biological sex on infant inflammatory responses. A 2022 study among children with controlled perinatal HIV infection documented higher levels of inflammatory biomarkers in females at age 5–11 years than males, which suggests that factors other than sex hormones contribute to observed differences in inflammation.38 More studies are needed to fully understand sex-related differences in immune responses and how these might affect the interpretation of IGRA results. Additional studies are also needed to confirm and explain our observed associations between TST positivity and breastfeeding and maternal immunological status.
Our study had limitations. The use of TST and IGRA to detect tuberculosis infection in infants has several limitations, but they are the only tests available for use in clinics and tuberculosis programmes. Additionally, 160 mother–infant pairs did not complete 1 year of follow-up during the parent study, which was partially due to a letter that was distributed during the study to inform all participants about maternal deaths due to hepatotoxicity. The 160 infants who did not have IGRA or TST data and were excluded from this analysis were balanced between the two study groups. Thus, we would not expect the missing data to change the conclusions of our complete case analysis. Another potential limitation is that although 6 months of isoniazid is the current standard of care, progressively shorter regimens (eg, 3 months of isoniazid and rifapentine dosed weekly or 1 month of isoniazid and rifapentine dosed daily) are being considered for rollout in tuberculosis programmes, making incident pregnancy while on preventive therapy much less likely. However, the rollout will take time, and isoniazid preventive therapy is by far the most commonly used tuberculosis preventive therapy; thus, these data remain highly relevant. Overall, nearly 750 infants provided data for this analysis, and mothers were randomly assigned to receive antepartum or postpartum therapy, which enabled accurate assessment of the impact of the timing of maternal isoniazid preventive therapy on the primary outcome.
Overall, effective maternal ART and isoniazid preventive therapy, whether administered during pregnancy or postpartum, was associated with relatively low incidence of infant tuberculosis infection and possible or probable tuberculosis disease in the context of maternal and infant tuberculosis screening, as per WHO guidelines. Routine maternal tuberculosis screening remains important to prevent tuberculosis transmission to infants, and our study suggests no additional increased incidence of tuberculosis infection or tuberculosis disease in the case of postpartum-deferred maternal isoniazid preventive therapy. Our study also found poor concordance between TST and IGRA during infancy. Available tuberculosis infection tests have substantial limitations, underscoring the need for new diagnostics that can also predict tuberculosis progression. Preventing tuberculosis infection and disease in young children remains a global priority.
Supplementary Material
Research in context.
Evidence before this study
Before universal use of effective antiretroviral therapy (ART) during pregnancy, infants born to women with HIV were at high risk of exposure to Mycobacterium tuberculosis due to the high risk of maternal tuberculosis disease. To further mitigate the risk, isoniazid preventive therapy and ART are recommended for all pregnant women living with HIV in high tuberculosis burden settings. However, isoniazid preventive therapy during pregnancy has been associated with adverse pregnancy outcomes in some studies, indicating that the risks and benefits of using isoniazid preventive therapy during pregnancy must be carefully considered. The risk of infant tuberculosis infection when maternal isoniazid preventive therapy is deferred to the postpartum period in the context of maternal HIV is not known. We searched PubMed, Embase, ClinicalTrials.gov, and the EBSCO global health database for studies published in English between Jan 1, 2011 and Dec 31, 2022, using the terms “isoniazid”, “tuberculosis”, and “prophylaxis”. The search was restricted to clinical trials and observational studies. Our search found no randomised controlled studies of isoniazid preventive therapy during pregnancy before or since our study was completed.
Added value of this study
To our knowledge, this is the first randomised controlled trial to study the effect of antepartum versus postpartum initiation of maternal isoniazid preventive therapy on infant tuberculosis infection. We found that the timing of isoniazid therapy initiation among women receiving ART did not affect the incidence of infant tuberculosis infection. Additionally, compared with the published literature, we found low incidence of tuberculosis infection and tuberculosis disease among infants in our trial population as well as poor agreement between commonly used tuberculosis infection diagnostics.
Implications of all the available evidence
The results of this secondary analysis suggest that maternal isoniazid preventive therapy is associated with a low incidence of infant tuberculosis infection in the context of maternal and infant tuberculosis screening, and the timing of therapy initiation (antepartum or postpartum) can be flexible. These data can also inform estimates of tuberculosis infection burden and risk of tuberculosis disease progression in infants, which would guide scale up of public health policies for implementing tuberculosis preventive therapy as well as inform design trials of tuberculosis preventive therapies and vaccines.
Acknowledgments
This study was funded by the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) through grants from the US National Institutes of Health, National Institute of Allergy, and Infectious Diseases with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health (UM1AI068632, UM1AI068616, UM1AI106716, and HHSN275201800001). AG is supported by the US National Institutes of Health, National Institute of Allergy and Infectious Diseases (UM1AI069465). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank all the women and their infants who participated in the TB APPRISE trial. We also thank the TB APPRISE study team and the staff and investigators at all participating clinical research sites: Gaborone (Botswana), Molepolole (Botswana), Les Centres GHESKIO (Haiti), BJGMC-JHU (India), DTTC (South Africa), FAM-CRU (South Africa), Soweto (South Africa), KCMC (Tanzania), Chiang Mai University (Thailand), MU-JHU (Uganda), Harare Family Clinic (Zimbabwe), St Mary’s (Zimbabwe), and Seke North (Zimbabwe). We also acknowledge the Multinational Organization Network Sponsoring Translational and Epidemiological Research for assisting with figure formatting.
Footnotes
Data sharing
Data used in the analysis are available upon request via the IMPAACT Network data request form.
Declaration of interests
We declare no competing interests.
For the study protocol see https://www.impaactnetwork.org/studies/p1078
See Online for appendix
For the IMPAACT Network data request form see https://www.impaactnetwork.org/studies/submit-research-proposal
References
- 1.WHO. Global tuberculosis report Geneva: World Health Organization, 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed April 23, 2023). [Google Scholar]
- 2.Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Glob Health 2017; 5: e898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med 2012; 367: 348–61. [DOI] [PubMed] [Google Scholar]
- 4.Reid MJA, Arinaminpathy N, Bloom A, et al. Building a tuberculosis-free world: The Lancet Commission on tuberculosis. Lancet 2019; 393: 1331–84. [DOI] [PubMed] [Google Scholar]
- 5.Dhana A, Hamada Y, Kengne AP, et al. Tuberculosis screening among ambulatory people living with HIV: a systematic review and individual participant data meta-analysis. Lancet Infect Dis 2022; 22: 507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marquez C, Chamie G, Achan J, et al. Tuberculosis infection in early childhood and the association with HIV-exposure in HIV-uninfected children in rural Uganda. Pediatr Infect Dis J 2016; 35: 524–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cranmer LM, Kanyugo M, Jonnalagadda SR, et al. High prevalence of tuberculosis infection in HIV-1 exposed Kenyan infants. Pediatr Infect Dis J 2014; 33: 401–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rangaka MX, Wilkinson RJ, Boulle A, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet 2014; 384: 682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomes VF, Andersen A, Wejse C, et al. Impact of tuberculosis exposure at home on mortality in children under 5 years of age in Guinea-Bissau. Thorax 2011; 66: 163–67. [DOI] [PubMed] [Google Scholar]
- 10.Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis 2012; 55: 1532–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aemro A, Jember A, Anlay DZ. Incidence and predictors of tuberculosis occurrence among adults on antiretroviral therapy at Debre Markos referral hospital, Northwest Ethiopia: retrospective follow-up study. BMC Infect Dis 2020; 20: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalk E, Heekes A, Mehta U, et al. Safety and effectiveness of isoniazid preventive therapy in pregnant women living with human immunodeficiency virus on antiretroviral therapy: an observational study using linked population data. Clin Infect Dis 2020; 71: e351–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyathi S, Dlodlo RA, Satyanarayana S, et al. Isoniazid preventive therapy: uptake, incidence of tuberculosis and survival among people living with HIV in Bulawayo, Zimbabwe. PLoS One 2019; 14: e0223076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross JM, Badje A, Rangaka MX, et al. Isoniazid preventive therapy plus antiretroviral therapy for the prevention of tuberculosis: a systematic review and meta-analysis of individual participant data. Lancet HIV 2021; 8: e8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta A, Montepiedra G, Aaron L, et al. Isoniazid preventive therapy in HIV-infected pregnant and postpartum women. N Engl J Med 2019; 381: 1333–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Tuberculin skin testing fact sheet. https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.htm (accessed Aug 1, 2023). [Google Scholar]
- 17.Andrews JR, Nemes E, Tameris M, et al. Serial QuantiFERON testing and tuberculosis disease risk among young children: an observational cohort study. Lancet Respir Med 2017; 5: 282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nemes E, Abrahams D, Scriba TJ, et al. Diagnostic accuracy of early secretory antigenic target-6-free interferon-gamma release assay compared to QuantiFERON-TB gold in-tube. Clin Infect Dis 2019; 69: 1724–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazurek GH, Jereb J, Vernon A, et al. Updated guidelines for using interferon gamma release assays to detect mycobacterium tuberculosis infection-United States, 2010. MMWR Recomm Rep 2010; 59: 1–25. [PubMed] [Google Scholar]
- 20.Graham SM, Cuevas LE, Jean-Philippe P, et al. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis 2015; 61 (suppl 3): S179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S, Wu X, Hughes MD, et al. High prevalence of tuberculosis infection and disease in child household contacts of adults with rifampin-resistant tuberculosis. Pediatr Infect Dis J 2022; 41: e194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention, National Institutes of Health, HIV Medicine Association of the Infectious Diseases Society of America. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/mycobacterium-0 (accessed Aug 1, 2023). [Google Scholar]
- 23.LaCourse SM, Richardson BA, Kinuthia J, et al. A randomized controlled trial of isoniazid to prevent mycobacterium tuberculosis infection in kenyan human immunodeficiency virus-exposed uninfected infants. Clin Infect Dis 2021; 73: e337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez L, le Roux DM, Barnett W, Stadler A, Nicol MP, Zar HJ. Tuberculin skin test conversion and primary progressive tuberculosis disease in the first 5 years of life: a birth cohort study from Cape Town, South Africa. Lancet Child Adolesc Health 2018; 2: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez L, Cords O, Horsburgh CR, et al. The risk of tuberculosis in children after close exposure: a systematic review and individual-participant meta-analysis. Lancet 2020; 395: 973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaensbauer J, Young J, Harasaki C, Aiona K, Belknap R, Haas MK. Interferon-gamma release assay testing in children younger than 2 years in a us-based health system. Pediatr Infect Dis J 2020; 39: 803–07. [DOI] [PubMed] [Google Scholar]
- 27.Cranmer LM, Draper HR, Mandalakas AM, et al. High incidence of tuberculosis infection in hiv-exposed children exiting an isoniazid preventive therapy trial. Pediatr Infect Dis J 2018; 37: e254–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seddon JA, Shingadia D. Epidemiology and disease burden of tuberculosis in children: a global perspective. Infect Drug Resist 2014; 7: 153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinberg A, Aaron L, Montepiedra G, et al. Effects of pregnancy and isoniazid preventive therapy on mycobacterium tuberculosis interferon gamma response assays in women with HIV. Clin Infect Dis 2021; 73: e3555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pai M, Denkinger CM, Kik SV, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 2014; 27: 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garazzino S, Galli L, Chiappini E, et al. Performance of interferon-γ release assay for the diagnosis of active or latent tuberculosis in children in the first 2 years of age: a multicenter study of the Italian Society of Pediatric Infectious Diseases. Pediatr Infect Dis J 2014; 33: e226–31. [DOI] [PubMed] [Google Scholar]
- 32.Seddon JA, Paton J, Nademi Z, et al. The impact of BCG vaccination on tuberculin skin test responses in children is age dependent: evidence to be considered when screening children for tuberculosis infection. Thorax 2016; 71: 932–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa AG, Carvalho BKS, Araújo-Pereira M, et al. Lessons learned from implementation of an interferon gamma release assay to screen for latent tuberculosis infection in a large multicenter observational cohort study in Brazil. Microbiol Spectr 2021; 9: e0116321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tagmouti S, Slater M, Benedetti A, et al. Reproducibility of interferon gamma (IFN-γ) release assays. A systematic review. Ann Am Thorac Soc 2014; 11: 1267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiappini E, Bonsignori F, Mazzantini R, et al. Interferon-gamma release assay sensitivity in children younger than 5 years is insufficient to replace the use of tuberculin skin test in western countries. Pediatr Infect Dis J 2014; 33: 1291–93. [DOI] [PubMed] [Google Scholar]
- 36.Machingaidze S, Wiysonge CS, Gonzalez-Angulo Y, et al. The utility of an interferon gamma release assay for diagnosis of latent tuberculosis infection and disease in children: a systematic review and meta-analysis. Pediatr Infect Dis J 2011; 30: 694–700. [DOI] [PubMed] [Google Scholar]
- 37.Fischinger S, Boudreau CM, Butler AL, Streeck H, Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol 2019; 41: 239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinberg A, Giganti MJ, Sirois PA, et al. Coordination of inflammatory responses in children with perinatally acquired HIV infection. AIDS 2022; 36: 1117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.