Abstract
Background
Protein intake plays an important role in maintaining the health status of older adults. However, few epidemiologic studies examined midlife protein intake in relation to healthy aging.
Objectives
The objective of this study was to evaluate the long-term role of dietary protein intake in healthy aging among female participants in the prospective Nurses’ Health Study (NHS) cohort.
Methods
We included 48,762 NHS participants aged <60 y in 1984. Total protein, animal protein, dairy protein (a subset of animal protein), and plant protein were derived from validated food frequency questionnaires. Healthy aging was defined as being free from 11 major chronic diseases, having good mental health, and not having impairments in either cognitive or physical function, as assessed in the 2014 or 2016 NHS participant questionnaires. We used multivariate logistic regression adjusted for lifestyle, demographics, and health status to estimate the odds ratios (ORs) and 95% confidence intervals for protein intake in relation to healthy aging.
Results
A total of 3721 (7.6%) NHS participants met our healthy aging definition. Protein intake was significantly associated with higher odds of healthy aging. The ORs (95% confidence intervals) per 3%-energy increment with healthy aging were 1.05 (1.01, 1.10) for total protein, 1.07 (1.02, 1.11) for animal protein, 1.14 (1.06, 1.23) for dairy protein, and 1.38 (1.24, 1.54) for plant protein. Plant protein was also associated with higher odds of absence of physical function limitations and good mental status. In substitution analyses, we observed significant positive associations for the isocaloric replacement of animal or dairy protein, carbohydrate, or fat with plant protein (ORs for healthy aging: 1.22–1.58 for 3% energy replacement with plant protein).
Conclusions
Dietary protein intake, especially plant protein, in midlife, is associated with higher odds of healthy aging and with several domains of positive health status in a large cohort of female nurses.
Keywords: healthy aging, diet, protein, animal protein, plant protein, lifestyle
Introduction
The number of United States adults ≥60 y is expected to nearly double by 2060[1]. However, increased longevity has not resulted in extended health span because of the occurrence of chronic diseases and impairments in physical and cognitive function among older adults [2]. Diet is an important modifiable factor of several chronic diseases [3,4], frailty [5], premature death [6], and successful or healthy aging [7,8], which are relevant to this vulnerable population.
In particular, protein intake plays an important role in maintaining good health status in older adults, especially in promoting physical function [9,10]. Data from clinical trials and observational studies suggest that higher protein intake is associated with decreased rate of muscle loss and improved physical performance in older adults [11,12]. Dietary protein also helps maintain physical mobility [9,13,14] and is associated with decreased risk of hip fractures and bone mass density loss [[15], [16], [17]]. Furthermore, dietary protein has been associated with maintenance of cognitive function [18,19]. However, most of these studies had short follow-up times, and the associations of specific protein sources (animal or plant) were inconclusive for most outcomes [9,20,21].
With respect to protein sources, animal protein intake in middle adulthood has been associated with an increased risk of premature death from chronic diseases driven by cardiovascular disease (CVD) mortality [22]. Plant protein intake in older adulthood was also associated with a lower risk of frailty in a previous study in the Nurses’ Health Study (NHS) [23]. Furthermore, higher plant protein intake was associated with a better probability of achieving healthy aging defined by changes in functional impairments, self-reported health/vitality, mental health, and use of health services in the Seniors-Estudio Sobre Nutrición y Riesgo Cardiovascular in Spain [24]. Therefore, more detailed studies are needed to assess the role of dietary protein—and protein sources—in healthy aging, taking into account physical function, cognitive health, and mental status in conjunction with chronic disease incidence in older adults [25].
The objective of our longitudinal study was to evaluate the association of protein intake in middle-aged female nurses in the United States with the likelihood of healthy aging, defined as longevity with the absence of major chronic diseases, good mental health, and no impairment in either cognitive or physical function, using data from the NHS [25]. We hypothesized that higher intakes of protein intake would be associated with greater odds of healthy aging.
Methods
Study population
The NHS was established in 1976 with 121,700 registered female nurses aged 30–55 y at enrollment [26]. Follow-up questionnaires were administered at baseline and every 2 y thereafter to collect information on lifestyle practices and medical history. The follow-up rate of NHS participants was >90% in most cycles.
From the 81,702 participants who returned the 1984 questionnaire, we excluded participants who had a history of any of the 11 chronic diseases that make up the healthy aging phenotype (see below) at baseline (1984) as well as participants aged ≥60 y in 1984 (Supplementary Figure 1). We excluded participants if they left >70 food items blank on the baseline food frequency questionnaire (FFQ) and those who reported unusual total energy intake levels (i.e., daily energy intake <500 or >3500 kcal/d). We also excluded participants without baseline information on protein intake, participants who did not return the 2016 questionnaire, those who skipped >5 items on the physical function scale of the Medical Outcomes Study Short-Form Health Survey (SF-36), and those who skipped any item on the subjective memory or 15-item Geriatric Depression Scale (GDS-15), leaving a sample of 48,762 NHS participants for the present analysis. The study protocol was approved by the Institutional Review Boards of Brigham and Women’s Hospital and Tufts University. The return of a completed questionnaire was considered as informed consent.
Assessment of diet
To collect information about habitual diet, NHS participants responded to an FFQ with 61 items in 1980. The FFQ was subsequently expanded to 131 items in 1984 and 1986 and every 4 y thereafter to obtain updated dietary information. Consumption frequency for each food in the FFQ was captured in 9 categories ranging from “never or less than once per month” to “6 or more times per day.” The intakes of total protein and protein from animal, dairy, and plant sources were calculated for each participant by multiplying the consumption frequency of each food item by its protein content and then adding the protein intake across all food items. The intake of other nutrients was estimated using the same procedure. The nutrient contents of food items were obtained from the Harvard University Food Composition Database. We expressed the intakes of each protein variable as a percent of total energy intake by multiplying their respective intake in grams per day by the energy contribution of each gram of protein (4 kcal/g) and then dividing by total energy intake. The primary animal protein sources in the 1984 and 1986 FFQs were beef, chicken, milk, fish/seafood, and cheese (Supplementary Figure 2). The main contributors to dairy protein (a subset of animal protein) were milk, cheese, pizza, yogurt, and ice cream. The main plant protein sources were bread, vegetables, fruits, pizza, cereal, baked items, mashed potatoes, nuts, beans, peanut butter, and pasta (Supplementary Figure 2). FFQs have demonstrated good validity and reproducibility in assessing overall diet and protein intake against 7-d diet records [27,28]. The deattenuated correlations between protein intake assessed by diet records and FFQs in prior validation studies in the NHS were 0.53 [27] and 0.64 [28]. For the main analysis, we used the 1984 and 1986 FFQs to ascertain dietary intake. We calculated intakes of total protein, animal protein, dairy protein, and plant protein by averaging the self-reported dietary intakes from the 1984 and 1986 FFQs and expressed them as percent of total energy intake.
Assessment of healthy aging
Healthy aging was defined as a composite end point: being free from 11 major chronic diseases, having no impairment in memory or physical function, and being in good mental health, as defined previously for the NHS participants [29]. All remaining participants were considered to be usual agers, which included those who did not meet our healthy aging definition and those who died before 2016 (Supplementary Figure 1). The assessment of healthy aging was conducted based on 4 domains listed below.
Assessment of chronic diseases.
We determined the clinical diagnoses of 11 major chronic diseases from the biennial follow-up questionnaires, which were subsequently confirmed by a review of medical records or pathology reports, telephone interviews, and supplementary questionnaires. These conditions were selected because they are primary causes of mortality in the United States or are considered to be highly debilitating [30]. Previous studies have reported high validity of self-reported health information in the NHS [31,32]. The list of 11 chronic diseases included cancer (except for nonmelanoma skin cancer), type 2 diabetes, myocardial infarction, coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty, congestive heart failure, stroke, kidney failure, chronic obstructive pulmonary disease, Parkinson disease, multiple sclerosis, and amyotrophic lateral sclerosis from the biennial follow-up questionnaires. Participants who did not report a history of any of these 11 diseases by the end of follow-up (2016) were considered to be free from chronic diseases.
Subjective memory.
Subjective memory was assessed on the basis of 7 questions included in the 2014 follow-up questionnaire regarding self-reported memory complaints about the change in ability to remember things and trouble in remembering recent events, short lists, 1 s to the next, spoken instructions, following conversations or plot, and finding the way on familiar streets [29,33,34]. No impairment in memory was defined as having 1 memory complaint at most.
Physical function.
Physical function was assessed on the basis of 10 questions from the Medical Outcomes Study SF-36, which is a 36-item questionnaire that evaluates physical function and mental health which was administered in 2016 [35]. The absence of impairment in physical function was defined as having no limitations in moderate activities (e.g., walking a few blocks and bathing) and no more than moderate limitations in vigorous activities (e.g., running, lifting heavy objects, and strenuous sports).
Assessment of mental health status.
Study participants’ mental health status was assessed in 2016 by using the 15-item GDS-15, in which lower scores indicate better mental health [36]. Good mental health status was defined as a GDS-15 score of ≤1, which corresponds to the median value in this cohort.
Assessment of covariates
We obtained information from the biennial follow-up questionnaires about participants’ education level, marital status, body height and weight, lifestyle practices, such as physical activity and cigarette smoking, medication use, including use of aspirin and postmenopausal hormones, and health status including history of hypertension and hypercholesterolemia. Race was self-reported in the biennial questionnaires (White, Black, American Indian, Asian, or other) and was categorized as White or Other. We quantified dietary quality using the 2010 Alternative Healthy Eating Index (AHEI) score, which is based on foods and nutrients predictive of chronic disease risk [37]. The AHEI consists of 10 dietary components, which are scored based on adherence to optimal intake from 0 (poorest adherence) to 10 (best adherence) [37].
Statistical analysis
We used the averaged protein intakes derived from the 1984 and 1986 FFQs for the primary analysis. We modeled each dietary protein variable continuously, expressed as a percentage of total energy, and also categorized in dietary quintiles based on the present data distributions. We did not observe evidence of nonlinearity in the associations between each protein exposure and odds of healthy aging evaluated by fitting cubic splines to the logistic regression models (Supplementary Figure 3) [38]. We used multivariable-adjusted logistic regression analysis to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for the association of each dietary protein exposure with the odds of healthy aging as well as with the odds of each of the 4 individual domains: absence of the 11 chronic diseases examined, no memory complaints, no physical function limitations, and good mental health status. In our basic model, we adjusted for the age of the participants in 1984. In multivariable-adjusted models, we further adjusted for race, education, marital status, postmenopausal hormone use, smoking status, alcohol intake, physical activity, baseline history of hypertension or hypercholesterolemia, aspirin, and multivitamin use, sugar-sweetened beverage (SSB) intake, and total energy intake. We also adjusted for BMI (in kg/m2) in a separate model. These are potential confounding variables with known associations with healthy aging. In our final regression models, we further adjusted the associations of animal intake for plant protein intake and the associations of plant protein intake for animal protein. We adjusted the associations for dairy protein for intakes of nondairy animal protein and plant protein. For the tests of trend, we assigned median values of each quintile and modeled this variable in the logistic regressions. We used a missing indicator for missing categorical covariate data. No values were missing for continuous covariates.
We performed analyses to estimate the effect of substituting 3% of energy from each dietary protein variable for the equivalent energy contribution from total carbohydrates, refined carbohydrates, carbohydrates from whole grains, total fat, SFA, MUFA, and PUFA using multivariate logistic models. For each comparison, we simultaneously included each protein variable and the corresponding replacement macronutrient, both modeled continuously in a multivariate logistic model. The ORs and 95% CIs for the isocaloric substitution association were derived from the difference between the regression coefficients for each variable [39]. To assess the substitution associations for total, whole grain, or refined-grain carbohydrates, we used the same list of covariates listed in multivariate model 2 except for SSB intake. We used the same multivariate logistic model to assess the substitution associations for the fat variables. In these models, we simultaneously included SFA, PUFA, MUFA, and trans fatty acids variables.
We conducted multiple sensitivity analyses to evaluate the robustness of our observed associations. In 1 analysis, we examined long-term protein intake with healthy aging by using the cumulatively averaged protein intakes derived from the FFQs from 1984 to either 2002 (12–14 y lag) to 2006 (8–10 y lag). In these models, we stopped updating dietary intakes after diagnosing any 1 of the 11 chronic diseases that are part of our healthy aging definition. We modeled protein intake in increments of 10 g of calorie-adjusted protein per day and expressed in (g/kg) of body weight per day. We evaluated the associations of each protein variable with odds of healthy aging, including adjustment for fruit and vegetable intake, which contribute to plant protein intake. We conducted an analysis excluding participants who died before the healthy aging assessment and performed a separate analysis including the participants who did not respond to the 2016 questionnaire. We used the responses for the domains of healthy aging assessed in 2012 to evaluate the consistency of our results. We conducted a sensitivity analysis evaluating cognitive function using the Telephone Interview for Cognitive Status (TICS) [33]. We also measured physical function using the FRAIL scale, which consists of 5 components: Fatigue, Resistance, Ambulation, Illness, and Loss of Weight. The FRAIL scale has been operationalized in the NHS cohort by using the first 3 criteria from the SF-36, the report of ≥5 chronic diseases [23,40], and ≥5% decrease in body weight reported in a 2 y period. Frailty was defined as having ≥3 criteria in the FRAIL scale [23,40]. Lastly, we repeated our analyses after including participants who scored ≤5 points on the GDS-15 scale, and in a separate sensitivity analysis, we excluded participants who skipped ≥4 items on the GDS-15 scale and repeated the analyses.
We conducted subset analyses by fitting logistic models stratified by the median values of participant age in 1984 (≤49 y and >49 y), BMI averaged in 1984 and 1986 (≤25, >25), baseline physical activity [≤5.5 metabolic equivalent (MET)-h/wk, >5.5 MET-h/wk) and averaged 1984 and 1986 AHEI (≤44.3, 44.3). We tested for effect modification by including a cross-product term between these variables and the protein intake variables, modeled continuously.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Results were considered to be statistically significant when P value was <0.05 (2-tailed).
Results
The present analysis included 48,762 participants, of whom 3721 (7.6%) achieved healthy aging. 15,131 (31.0%) participants remained free from diagnoses of any of the 11 chronic diseases examined, 23,215 (47.6%) did not report memory complaints, 7303 (15.0%) did not develop any physical function limitations, and 18,211 (37.3%) maintained good mental health status.
Baseline characteristics
The mean (SD) age of participants at baseline was 48.6 (6.3) y, 38.6% of participants had BMI levels >25.0, 22.9% were current smokers, and 88.2% were married. Mean total protein consumption as percent energy (SD) was 18.3% (3.0%); this included 13.3% (3.1%) animal protein, 3.6% (1.7%) dairy protein, and 4.9% (1.0%) plant protein. The mean intakes were relatively consistent for each variable across the follow-up cycles with examples for the intakes derived from the 1984, 1994, and 2006 FFQs presented in Supplementary Table 1. Table 1 presents age-standardized summary statistics by quintiles of baseline protein intake expressed in percent of total energy intake. Total protein consumption was positively associated with intakes of animal protein, dairy protein, chicken, fish, legumes, eggs, dairy products, fruits and vegetables, and the AHEI (Table 1). Total protein intake was also positively associated with higher education levels, being physically active, higher BMI, and a higher proportion of baseline history of hypertension and hypercholesterolemia (Table 1). Conversely, total protein intake was inversely associated with intakes of total carbohydrates, nuts, alcohol, and SSBs.
TABLE 1.
Baseline age-adjusted characteristics of participants in the Nurses’ Health Study according to quintiles of total protein intake (percent of total energy)
| Quintiles of total protein intake |
|||||
|---|---|---|---|---|---|
| Q1 (n = 9752) | Q2 (n = 9753) | Q3 (n = 9752) | Q4 (n = 9753) | Q5 (n = 9752) | |
| Age (y)1 | 48.3 ± 6.4 | 48.3 ± 6.2 | 48.5 ± 6.2 | 48.8 ± 6.2 | 49.2 ± 6.1 |
| Total protein intake (% of total energy)2 | 14.2 ± 1.3 | 16.5 ± 0.4 | 18.0 ± 0.4 | 19.5 ± 0.5 | 22.5 ± 1.9 |
| Total protein intake (g/d)2 | 57.7 ± 5.7 | 66.9 ± 2.9 | 72.3 ± 2.8 | 78.1 ± 3.2 | 89.2 ± 7.7 |
| Animal protein intake (% of total energy)2 | 9.5 ± 1.5 | 11.6 ± 1.0 | 13.1 ± 1.0 | 14.6 ± 1.0 | 17.7 ± 2.3 |
| Animal protein intake (g/d)2 | 38.6 ± 6.5 | 47.2 ± 4.6 | 52.7 ± 4.5 | 58.5 ± 4.8 | 70.0 ± 8.9 |
| Dairy protein intake (% of total energy)2 | 2.8 ± 1.2 | 3.3 ± 1.4 | 3.6 ± 1.5 | 4.0 ± 1.7 | 4.4 ± 2.1 |
| Dairy protein intake (g/d)2 | 10.9 ± 4.8 | 13.1 ± 5.3 | 14.4 ± 6.0 | 15.8 ± 6.8 | 17.8 ± 8.3 |
| Plant protein intake (% of total energy)2 | 4.7 ± 1.1 | 4.9 ± 0.9 | 4.9 ± 0.9 | 4.9 ± 0.9 | 4.8 ± 1.0 |
| Plant protein intake (g/d)2 | 19.1 ± 4.2 | 19.6 ± 3.6 | 19.6 ± 3.5 | 19.6 ± 3.6 | 19.1 ± 4.0 |
| Race, White (%) | 94.9 | 95.1 | 95.8 | 95.0 | 94.9 |
| Married (%) | 87.4 | 90.1 | 90.6 | 90.1 | 89.1 |
| Education | |||||
| Registered nurse (%) | 76.0 | 72.7 | 70.6 | 68.8 | 67.8 |
| Bachelors (%) | 16.6 | 18.5 | 19.6 | 20.5 | 19.8 |
| Master’s or higher (%) | 7.4 | 8.8 | 9.8 | 10.7 | 12.4 |
| Physical activity (MET-h/wk)2 | 10.6 ± 22.0 | 12.3 ± 19.5 | 12.1 ± 17.7 | 12.8 ± 17.5 | 14.1 ± 21.3 |
| BMI (kg/m2) | |||||
| BMI <25 (%) | 68.7 | 65.9 | 62.0 | 58.8 | 51.9 |
| BMI 25 to <30 (%) | 21.5 | 23.3 | 25.2 | 27 | 29.9 |
| BMI ≥30 (%) | 9.8 | 10.8 | 12.8 | 14.2 | 18.2 |
| Alcohol intake (g/d)2 | 9.6 ± 15.0 | 7.8 ± 11.2 | 6.5 ± 9.4 | 5.6 ± 8.1 | 4.1 ± 6.4 |
| Smoking status | |||||
| Never smoker (%) | 41.8 | 43.2 | 45.1 | 42.8 | 41.2 |
| Past smoker (%) | 29.3 | 33.6 | 33.5 | 36.0 | 39.0 |
| Current smoker (%) | 28.9 | 23.2 | 21.4 | 21.2 | 19.8 |
| Postmenopausal hormone use | |||||
| Premenopausal | 49.3 | 49.5 | 49.2 | 49.4 | 47.7 |
| Never used (%) | 30.3 | 29.8 | 29.7 | 28.7 | 29.4 |
| Former user (%) | 11.5 | 11.8 | 12.3 | 12.7 | 12.7 |
| Current user (%) | 8.9 | 8.9 | 8.8 | 9.2 | 10.2 |
| Current aspirin use (%) | 70.2 | 73.0 | 71.8 | 71.4 | 70.4 |
| Multivitamin use (%) | 32.7 | 34.6 | 36.3 | 38.3 | 39.2 |
| Hypertension (%) | 15.8 | 15.1 | 16.8 | 17.8 | 20.5 |
| High cholesterol (%) | 4.9 | 5.4 | 5.1 | 6.0 | 7.3 |
| Total energy (kcal/d)2 | 1902 ± 531 | 1837 ± 491 | 1769 ± 469 | 1705 ± 451 | 1568 ± 434 |
| Total fat intake (% of total energy)2 | 33.0 ± 5.5 | 34.2 ± 4.9 | 34.3 ± 4.9 | 34.4 ± 5.0 | 34.2 ± 5.6 |
| Total carbohydrate intake (% of total energy)2 | 50.9 ± 7.5 | 48.1 ± 6.4 | 46.9 ± 6.1 | 45.6 ± 6.2 | 43.1 ± 6.8 |
| AHEI2 | 40.8 ± 9.2 | 42.9 ± 8.9 | 44.5 ± 8.9 | 46.3 ± 9.0 | 49.8 ± 9.4 |
| Carbohydrates from whole grains (% of total energy)2 | 2.9 ± 2.9 | 3.3 ± 2.7 | 3.5 ± 2.8 | 3.7 ± 2.9 | 3.7 ± 3.0 |
| Red and processed red meat intake (servings/wk)2 | 7.3 ± 4.1 | 8.0 ± 4.2 | 8.2 ± 4.2 | 8.1 ± 4.4 | 7.6 ± 4.8 |
| Chicken intake (servings/wk)2 | 2.4 ± 1.6 | 3.1 ± 1.8 | 3.5 ± 2.0 | 4.1 ± 2.2 | 5.3 ± 3.5 |
| Fish intake (servings/wk)2 | 1.2 ± 0.8 | 1.6 ± 1.0 | 1.8 ± 1.2 | 2.2 ± 1.4 | 3.1 ± 2.1 |
| Legume intake (servings/wk)2 | 2.5 ± 1.7 | 2.7 ± 1.6 | 2.8 ± 1.7 | 2.8 ± 1.8 | 2.9 ± 2.0 |
| Nut intake (servings/wk)2 | 1.7 ± 2.3 | 1.7 ± 2.2 | 1.6 ± 2.0 | 1.5 ± 2.0 | 1.3 ± 1.8 |
| Egg intake (servings/wk)2 | 2.1 ± 1.7 | 2.3 ± 1.8 | 2.3 ± 1.9 | 2.4 ± 2.0 | 2.5 ± 2.2 |
| Dairy intake (servings/wk)2 | 13.0 ± 8.9 | 14.2 ± 8.4 | 14.6 ± 8.5 | 14.9 ± 8.4 | 14.8 ± 8.7 |
| SSB intake (servings/wk)2 | 4.5 ± 6.1 | 2.2 ± 3.1 | 1.5 ± 2.4 | 1.0 ± 1.8 | 0.6 ± 1.2 |
| Fruit intake (servings/wk) | 9.6 ± 7.5 | 10.2 ± 7.2 | 10.4 ± 6.7 | 10.6 ± 6.9 | 10.7 ± 6.8 |
| Vegetable intake (servings/wk)2 | 17.5 ± 8.3 | 19.4 ± 8.6 | 20.1 ± 8.9 | 21.2 ± 9.2 | 22.4 ± 10.0 |
AHEI, alternative healthy eating index; BMI, body mass index; MET, metabolic equivalent; Q, quintile; SD, standard deviation; SSB, sugar-sweetened beverage.
Summary statistics include age-standardized means and SDs for continuous covariates (except for age) and proportions for categorical variables by quintiles of protein intake expressed in percent of total energy intake.
Mean, SD (all such values)
Protein intake and healthy aging
In our multivariable-adjusted regression model 1, we did not observe statistically significant associations of total protein or dairy protein intakes with the odds of healthy aging (Table 2). However, consumption of animal protein was associated with 6% (95% CI: 2%, 9%) lower odds of healthy aging, and plant protein was significantly associated with 46% (30%, 65%) higher odds of healthy aging for every 3%-energy increment (Table 2). After further adjustment for BMI (multivariate model 2), consumption of total and plant protein was associated with 5% (1%, 10%) and 31% (16%, 48%) higher odds of healthy aging for every 3%-energy increment, whereas animal and dairy protein intakes were not significantly associated with healthy aging, respectively (Table 2). After mutual adjustment for plant and animal protein sources (multivariate model 3), the associations were strengthened. Each 3%-energy increment of animal or dairy protein intake was associated with 7% (2%, 11%) and 14% (6%, 23%) higher odds of healthy aging, whereas the association for each 3%-energy increment of plant protein associated with 38% (24%, 54%) higher odds of healthy aging, respectively (Table 2). The associations of the cumulatively averaged intakes of total, animal, or dairy protein through either 2002 or 2006 with the odds of healthy aging were similar to the associations observed using the baseline protein variables, whereas the associations for plant protein were stronger across all statistical models (Supplementary Table 2).
TABLE 2.
Odds ratios (ORs) (95% confidence intervals) of healthy aging (n = 3721) assessed in 2014/2016 according to intake of protein in 1984/1986 among 48,762 participants in the Nurses’ Health Study (ORs >1 denote greater odds of healthy aging)
| Quintile of protein intake |
P-trend5 | ORs (95% CI) for 3%-energy increment | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Total protein | |||||||
| Healthy ager/participants (n) | 723/9752 | 781/9753 | 796/9752 | 748/9753 | 673/9752 | ||
| Median intake (IQR) % energy | 14.6 (13.6, 15.2) | 16.5 (16.2, 16.9) | 17.3 (17.6, 18.3) | 19.4 (19.0, 19.9) | 21.9 (21.1, 23.3) | ||
| Age-adjusted model1 | 1.00 | 1.12 (1.00, 1.25) | 1.18 (1.06, 1.31) | 1.16 (1.03, 1.29) | 1.11 (0.99, 1.25) | 0.06 | 1.05 (1.01, 1.09) |
| Multivariate model 12 | 1.00 | 0.96 (0.85, 1.08) | 0.99 (0.88, 1.11) | 0.95 (0.84, 1.07) | 0.89 (0.78, 1.01) | 0.07 | 0.97 (0.93, 1.01) |
| Multivariate model 23 | 1.00 | 0.99 (0.88, 1.11) | 1.06 (0.94, 1.20) | 1.08 (0.95, 1.21) | 1.08 (0.95, 1.23) | 0.11 | 1.05 (1.01, 1.10) |
| Animal protein | |||||||
| Healthy ager/participants (n) | 775/9752 | 758/9753 | 783/9752 | 754/9753 | 651/9752 | ||
| Median intake (IQR) % energy | 9.6 (8.6, 10.2) | 11.6 (11.2, 12.0) | 13.1 (12.7, 13.4) | 14.6 (14.2, 15.1) | 17.3 (16.3, 18.7) | ||
| Age-adjusted model1 | 1.00 | 0.99 (0.89, 1.10) | 1.03 (0.92, 1.14) | 1.01 (0.91, 1.13) | 0.94 (0.84, 1.05) | 0.38 | 0.99 (0.94, 1.05) |
| Multivariate model 12 | 1.00 | 0.91 (0.81, 1.01) | 0.92 (0.82, 1.03) | 0.90 (0.80, 1.00) | 0.81 (0.72, 0.91) | 0.001 | 0.94 (0.91, 0.98) |
| Multivariate model 23 | 1.00 | 0.93 (0.83, 1.04) | 0.99 (0.89, 1.11) | 1.02 (0.91, 1.15) | 0.99 (0.88, 1.12) | 0.64 | 1.01 (0.98, 1.05) |
| Multivariate model 34 | 1.00 | 0.98 (0.87, 1.10) | 1.07 (0.95, 1.20) | 1.12 (0.995, 1.27) | 1.13 (0.99, 1.29) | 0.01 | 1.07 (1.02, 1.11) |
| Dairy protein | |||||||
| Healthy ager/participants (n) | 693/9752 | 729/9753 | 799/9752 | 750/9753 | 750/9752 | ||
| Median intake (IQR) % energy | 1.7 (1.4, 2.0) | 2.6 (2.4, 2.8) | 3.4 (3.2, 3.6) | 4.3 (4.0, 4.6) | 5.9 (5.4, 6.8) | ||
| Age-adjusted model1 | 1.00 | 0.97 (0.87, 1.09) | 1.11 (0.99, 1.24) | 1.05 (0.94, 1.17) | 1.17 (1.05, 1.31) | 0.002 | 1.12 (1.06, 1.20) |
| Multivariate model 12 | 1.00 | 0.93 (0.83, 1.04) | 0.99 (0.89, 1.11) | 0.93 (0.83, 1.04) | 1.04 (0.92, 1.17) | 0.42 | 1.05 (0.98, 1.12) |
| Multivariate model 23 | 1.00 | 0.95 (0.85, 1.07) | 1.03 (0.91, 1.15) | 0.97 (0.86, 1.09) | 1.06 (0.94, 1.20) | 0.26 | 1.06 (0.99, 1.13) |
| Multivariate model 34 | 1.00 | 0.97 (0.87, 1.09) | 1.06 (0.95, 1.20) | 1.02 (0.91, 1.15) | 1.17 (1.03, 1.33) | 0.009 | 1.14 (1.06, 1.23) |
| Plant protein | |||||||
| Healthy ager/participants (n) | 611/9752 | 750/9753 | 726/9752 | 796/9753 | 838/9752 | ||
| Median intake (IQR) % energy | 3.7 (3.4, 4.0) | 4.4 (4.2, 4.5) | 4.8 (4.7, 4.9) | 5.2 (5.1, 5.4) | 6.0 (5.7, 6.4) | ||
| Age-adjusted model1 | 1.00 | 1.26 (1.13, 1.42) | 1.26 (1.12, 1.41) | 1.47 (1.31, 1.65) | 1.77 (1.58, 1.99) | <0.0001 | 1.82 (1.64, 2.03) |
| Multivariate model 12 | 1.00 | 1.12 (0.996, 1.26) | 1.10 (0.98, 1.24) | 1.22 (1.08, 1.37) | 1.42 (1.26, 1.60) | <0.0001 | 1.46 (1.30, 1.65) |
| Multivariate model 23 | 1.00 | 1.12 (0.99, 1.26) | 1.08 (0.96, 1.22) | 1.17 (1.03, 1.32) | 1.32 (1.16, 1.49) | <0.0001 | 1.31 (1.16, 1.48) |
| Multivariate model 34 | 1.00 | 1.15 (1.02, 1.29) | 1.12 (0.99, 1.27) | 1.23 (1.08, 1.40) | 1.41 (1.24, 1.62) | <0.0001 | 1.38 (1.24, 1.54) |
BMI, body mass index; CI, confidence interval; IQR, interquartile range; MET, metabolic equivalent; OR, odds ratio; SSB, sugar-sweetened beverage.
Logistic model adjusted for baseline age.
Multivariate logistic model 1 was adjusted for baseline age (continuous), race (White, other), education (registered nurse, bachelor, or graduate), marital status (married, other), postmenopausal hormone use (premenopausal; never, past user, current user), smoking status (never smoked; former smoker, 0.1–14.9, 15.0–29.9, >30 pack-y), alcohol intake (0, 0.1–4.9, 5.0–14.9, >15.0 g/d), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, >27 MET/wk), baseline history of hypertension or hypercholesterolemia (yes, no), aspirin use (never, past, current), multivitamin use (yes, no), SSB intake, and total energy intake (kcal/d, quintiles).
Multivariate model 2 included the covariates in multivariate model 1 and was additionally adjusted for BMI (averaged 1984 and 1986; <22.5, 22.5–24.9, 25.0–27.5, 27.5–30.0, 30.0–34.9, >35.0).
Multivariate model 3 included covariates in multivariate model 2 with additional mutual adjustment of animal and plant protein. The models for dairy protein were adjusted for plant protein and other animal protein (nondairy protein).
P-trend was calculated by assigning median values to each quintile and was treated as a continuous variable.
Table 3 presents the associations of dietary protein intake modeled continuously per 3%-energy increment with each domain of healthy aging. Total and animal protein intake were significantly associated with higher chronic disease risk in all models. However, dairy protein and plant protein intake were associated with higher odds of absence of chronic diseases. None of the protein intake exposures were associated with the absence of memory complaints after mutual adjustment for plant and animal protein. Animal protein and plant protein were associated with 5% (95% CI: 2%, 9%) and 41% (27%, 57%) higher odds of being free of physical limitations in fully adjusted models, respectively (Table 3). However, only plant protein was significantly associated with higher odds of having good mental status. The results for the cumulatively averaged intakes of each dietary protein variable through either 2002 or 2006 with each domain of aging were stronger (Supplementary Table 3).
TABLE 3.
Odds ratios (ORs) (95% confidence intervals) of individual domains of healthy aging assessed in 2014/2016 according to intake of protein in 1984/1986 among 48,762 participants in the Nurses’ Health Study (ORs >1 denote greater odds of healthy aging)
| ORs (95% CI) for 3%-energy increment |
||||
|---|---|---|---|---|
| Absence of chronic diseases (n = 15,131)5 | No impairment in memory (n = 23,215)6 | No physical function limitations (n = 7303)7 | Good mental status (n = 18,211)8 | |
| Total protein | ||||
| Age-adjusted model1 | 0.97 (0.95, 0.99) | 1.02 (1.00, 1.04) | 1.04 (1.01, 1.07) | 1.03 (1.01, 1.05) |
| Multivariate model 12 | 0.91 (0.89, 0.93) | 0.97 (0.95, 0.99) | 0.95 (0.92, 0.98) | 0.97 (0.95, 0.996) |
| Multivariate model 23 | 0.95 (0.93, 0.97) | 0.99 (0.96, 1.01) | 1.03 (0.999, 1.07) | 1.01 (0.98, 1.03) |
| Animal protein | ||||
| Age-adjusted model1 | 0.94 (0.92, 0.96) | 0.99 (0.97, 1.01) | 0.98 (0.95, 1.01) | 0.99 (0.97, 1.01) |
| Multivariate model 12 | 0.91 (0.89, 0.93) | 0.97 (0.94, 0.99) | 0.93 (0.90, 0.95) | 0.96 (0.94, 0.98) |
| Multivariate model 23 | 0.95 (0.93, 0.97) | 0.98 (0.96, 1.00) | 1.00 (0.97, 1.03) | 0.99 (0.97, 1.02) |
| Multivariate model 34 | 0.95 (0.93, 0.98) | 0.99 (0.97, 1.01) | 1.05 (1.02, 1.09) | 1.01 (0.99, 1.04) |
| Dairy protein | ||||
| Age-adjusted model1 | 1.11 (1.07, 1.15) | 1.06 (1.02, 1.09) | 1.09 (1.04, 1.14) | 1.08 (1.04, 1.12) |
| Multivariate model 12 | 1.03 (0.99, 1.07) | 0.97 (0.93, 1.00) | 1.00 (0.95, 1.06) | 0.98 (0.94, 1.02) |
| Multivariate model 23 | 1.04 (1.00, 1.08) | 0.97 (0.93, 1.01) | 1.02 (0.96, 1.07) | 0.98 (0.95, 1.03) |
| Multivariate model 34 | 1.02 (0.98, 1.06) | 0.97 (0.94, 1.02) | 1.08 (1.02, 1.15) | 1.01 (0.97, 1.06) |
| Plant protein | ||||
| Age-adjusted model1 | 1.46 (1.37, 1.56) | 1.42 (1.34, 1.51) | 1.85 (1.70, 2.01) | 1.54 (1.44, 1.64) |
| Multivariate model 12 | 1.17 (1.09, 1.26) | 1.12 (1.05, 1.20) | 1.47 (1.37, 1.61) | 1.20 (1.11, 1.28) |
| Multivariate model 23 | 1.09 (1.01, 1.17) | 1.09 (1.02, 1.17) | 1.32 (1.20, 1.45) | 1.14 (1.06, 1.22) |
| Multivariate model 34 | 1.02 (0.94, 1.10) | 1.08 (0.998, 1.16) | 1.41 (1.27, 1.57) | 1.16 (1.07, 1.26) |
BMI, body mass index; CI, confidence interval; MET, metabolic equivalent; OR, odds ratio; SSB, sugar-sweetened beverage.
Logistic model adjusted for baseline age.
Multivariate logistic model 1 was adjusted for baseline age (continuous), race (White, other), education (registered nurse, bachelor, or graduate), marital status (married, other), postmenopausal hormone use (premenopausal; never, past user, current user), smoking status (never smoked; former smoker, 0.1–14.9, 15.0–29.9, >30 pack-y), alcohol intake (0, 0.1–4.9, 5.0–14.9, >15.0 g/d), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, >27 MET/wk), baseline history of hypertension or hypercholesterolemia (yes, no), aspirin use (never, past, current), multivitamin use (yes, no), SSB intake, and total energy intake (kcal/d, quintiles).
Multivariate model 2 included the covariates in multivariate model 1 and was additionally adjusted for BMI (averaged 1984 and 1986; <22.5, 22.5–24.9, 25.0–27.5, 27.5–30.0, 30.0–34.9, >35.0).
Multivariate model 3 included covariates in multivariate model 2 with additional mutual adjustment of animal and plant protein. The models for dairy protein were adjusted for plant protein and other animal protein (nondairy protein).
Absence of chronic disease was defined as not being diagnosed with a history of 11 chronic diseases (cancer, type-2 diabetes, myocardial infarction, coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty, congestive heart failure, stroke, kidney failure, chronic obstructive pulmonary disease, Parkinson disease, multiple sclerosis, and amyotrophic lateral sclerosis) by the end of follow-up (2016).
No impairment in memory was defined as having 1 memory complaint at most on the basis of 7 regarding self-reported memory complaints about the change in the ability to remember things and trouble in remembering recent events, short lists, 1 s to the next, spoken instructions, following conversations or plot, and finding the way on familiar streets.
No physical function limitations were defined as having no limitations in moderate activities (e.g., walking a few blocks, bathing) and no more than moderate limitations on vigorous activities (e.g., running, lifting heavy objects, strenuous sports from the Medical Outcomes Study Short-Form Health Survey.
Good mental status was defined as a Geriatric Depression Scale-15 score ≤1.
Substitution analyses
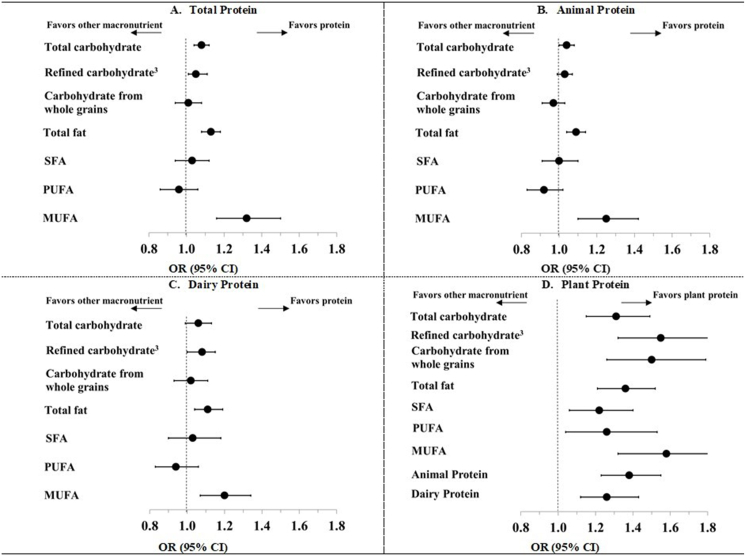
In isocaloric substitution analyses, substituting each dietary protein variable for total fat, total carbohydrates, or refined carbohydrate were largely associated with better odds of achieving healthy aging: the ORs (95% CIs) for every 3%-energy increment ranged from 1.03 (0.99, 1.07) for the replacement of refined carbohydrate with dairy protein to 1.08 (1.00, 1.15) for the replacement with plant protein (Figure 1 and Supplementary Table 4). Substituting the intakes of total, animal, and dairy protein variables for other macronutrients, such as carbohydrates from whole grains, PUFA, or SFA, was not significantly associated with the odds of healthy aging. In contrast, substituting 3% of plant protein energy was significantly associated with 22%–58% higher odds of healthy aging when replacing the equivalent calories from SFA, PUFA, MUFA, or animal or dairy protein (Figure 1 and Supplementary Table 4). Furthermore, isocaloric substitutions of plant protein for total carbohydrate, total fat, animal, or dairy protein were associated with higher odds of being free of chronic diseases. Replacing calories from total fat and total or total carbohydrate with dairy protein was associated with higher odds of being free from the 11 chronic diseases examined (Supplementary Table 4). Lastly, isocaloric substitutions of total and animal protein most macronutrients were associated with 3–14% lower (less favorable) odds of being free of chronic diseases (Supplementary Table 4). Replacing calories from total carbohydrates or fat for the equivalent calories from protein was associated with higher odds of being free of physical function limitations. Furthermore, replacing calories from all macronutrient variables with the equivalent calories from plant protein was associated with 20%–60% higher odds of having no physical function limitations (Supplementary Table 4).
FIGURE 1.
Odds ratios (ORs) (95% confidence intervals) of healthy aging (n = 3721) associated with isocaloric substitution of protein (total, animal, dairy, and plant) for dietary carbohydrate (total, refined, and from whole grains) and dietary fatty acids (total, saturated, polyunsaturated, and trans) modeled in 3%-energy increments in 48,762 participants in the Nurses’ Health Study.1,2 BMI, body mass index; CI, confidence interval; MET, metabolic equivalent; MUFA, monounsaturated fatty acid; OR, odds ratio; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid; TFA, trans fatty acid.
1The isocaloric substitutions can be interpreted as the effect of substituting 3% of calories contributed by a given dietary protein variable for the corresponding calories contributed by either dietary carbohydrate or dietary fat variables on the odds of healthy aging. For each comparison, we simultaneously included each protein variable and the corresponding replacement macronutrient in a multivariate logistic, both modeled continuously. The ORs and 95% CIs for the isocaloric substitution association were derived from the difference between the regression coefficients for each variable.
2To assess the substitution associations for total, whole grain, or refined-grain carbohydrates, we used multivariate logistic models adjusted for baseline age (continuous), race (White, other), education (registered nurse, bachelor, or graduate), marital status (married, other), postmenopausal hormone use (premenopausal; never, past user, the current user), smoking status (never smoked; former smoker, 0.1–14.9, 15.0–29.9, >30 pack-y), alcohol intake (0, 0.1–4.9, 5.0–14.9, >15.0 g/d), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, >27 MET/wk); BMI (averaged 1984 and 1986; <22.5, 22.5–24.9, 25.0–27.5, 27.5–30.0, 30.0–34.9, >35.0), baseline history of hypertension or hypercholesterolemia (yes, no); aspirin use (never, past, current); multivitamin use (yes, no), and total energy intake (kcal/d, continuous) We used the same multivariate logistic model to assess the substitution associations for the fat variables. Of note, we simultaneously included SFA, PUFA, MUFA, and TFA variables in the same model.
3Refined carbohydrate was defined as the sum of carbohydrates from refined grains, potatoes, and added sugar
In our stratified analyses, we did not observe statistically significant heterogeneity by age in 1984 (≤49 y and >49 y), baseline BMI (≤25 and >25), physical activity (≤5.3 MET-h/wk and >5.3 MET-h/wk) or AHEI (≤44.3 and >44.3) on the associations of each protein intake with odds of healthy aging (P-interaction > 0.05) (Supplementary Table 5).
Sensitivity analyses
In our sensitivity analyses, we observed similar associations in the analysis that modeled protein intake in increments of 10 g of calorie-adjusted protein per day (Supplementary Table 6) or in grams per kilogram of body weight per day (Supplementary Table 7). Our observed associations were attenuated after adjustment by intakes of fruits and vegetables, but the associations for plant protein remained statistically significant (Supplementary Table 8). Additionally, we observed similar associations in the analysis that excluded participants who died before assessing healthy aging (Supplementary Table 9) and those with missing 2016 questionnaire responses (data not shown). Of note, the baseline protein intake and other baseline characteristics were similar for the participants in the entire study sample (n = 48,762) or those with missing 2016 questionnaire responses (n = 17,716) (Supplementary Table 10). Additionally, we observed similar associations in the analysis that used the healthy aging domains assessed in 2012 (data not shown). Lastly, the pattern of associations remained virtually unchanged in our analysis evaluating cognitive function using TICS (Supplementary Table 11) or by expanding the inclusion of participants using the GDS-15 scale to those who skipped ≥4 items (Supplementary Table 12). We observed similar associations for plant protein but attenuated associations for total, dairy, and animal protein for those who scored ≤5 points on the GDS_15 scale (data not shown). The associations of healthy aging using the FRAIL scale were attenuated for total protein but otherwise were consistent for animal, dairy, and plant protein with those in our main analyses (Supplementary Table 13).
Discussion
Our prospective cohort study assessed the role of protein intake in a population of middle-aged female nurses in the development of healthy aging at ages 70–93 y. In general, we observed positive (favorable) associations between dietary protein intake and the odds of healthy aging in later life. However, we observed heterogeneous associations for individual domains of healthy aging by various sources of dietary proteins. Consumption of total and animal protein was inversely (unfavorably) associated with the development of several chronic diseases, whereas the consumption of dairy and plant protein was favorably associated with this domain. Furthermore, we observed a modest favorable association between animal protein intake with the absence of physical function limitations, whereas the respective associations for plant protein were stronger and consistent across all statistical models. Additionally, plant protein intake was favorably associated with mental health status later in life. In our substitution analyses, we observed that animal protein intake was unfavorably associated with the incidence of chronic diseases when compared with calories primarily from carbohydrates (total, refined, or whole grain) or from total fat, SFA, MUFA, or PUFA. In contrast, isocaloric substitution of plant protein for animal or dairy protein, total or refined carbohydrate, total fat, or SFA were favorably associated with the odds of healthy aging, absence of chronic disease, good physical function, and good mental status. Lastly, the results of our sensitivity analyses were similar to our main results, which supported the robustness of our findings.
Comparison with other studies
Our results are consistent with a study of older adults in the Seniors-Estudio Sobre Nutrición y Riesgo Cardiovascular study in Spain, which found that higher plant protein consumption was associated with higher odds of healthy aging [24]. Additionally, replacing animal protein, total carbohydrates, or total fat with plant protein was significantly associated with higher odds of healthy aging in that population, which is in line with our findings [24]. One key difference was that our study evaluated these associations for protein intake during midlife; however, the associations were consistent with those observed for intakes in older adulthood. The unfavorable association of animal protein intake with chronic disease risk observed in our study is consistent with observational studies linking animal protein intake with increased risk of death from various chronic diseases, particularly from CVD [22]. Moreover, replacing animal protein with plant protein has been associated with a reduced risk of all-cause mortality [22,41] and CVD-related mortality [41]. Furthermore, plant protein intake has been associated with a reduced risk of several chronic diseases, including coronary artery disease [42,43], type-2 diabetes [44,45], and stroke [46].
With respect to physical function, our results are consistent with 2 observational studies that reported favorable associations of protein intake from midlife to older adulthood with physical function in older age [10,13]. Moreover, higher protein intake in older adulthood was associated with reduced risk of frailty [14] or slower progression from healthy physical function to frailty [47]. Our findings are also in agreement with previous observational evidence [20,21,23] supporting a role for plant protein in the maintenance of muscle mass, improved physical function, and reduced risk of frailty in older adults.
Our largely null results for the associations of protein intake with the absence of memory complaints are consistent with prospective studies of cognitive function in older adults, which have observed null associations for total protein intake and heterogeneous associations for animal and plant protein sources [19]. Of note, we observed favorable associations for replacing dietary carbohydrates with plant protein in the memory domain, which is consistent with recent findings from a study in the Health Professionals Follow-up Study and the NHS [18]. Furthermore, plant protein was previously associated with maintenance of mental scores in older adults [48,49]. Lastly, the role of protein intake in mental health has not been explored among older adult populations and has been inconclusive in other studies [50].
Potential mechanisms
The mechanisms explaining the associations between protein intake and healthy aging are complex and not fully understood. Regarding physical function, some lines of evidence suggest that the activation of the mammalian target of the rapamycin complex 1 signaling pathway decreases with age [11]. Dietary protein and exercise activate the mammalian target of rapamycin complex 1 signaling, thereby stimulating muscle protein synthesis, which is associated with improved physical function in older adults [11,51]. There are several potential mechanisms that may explain the differential associations between plant and animal protein intake on the chronic disease domain of the healthy aging phenotype. Plant protein has been associated with favorable levels of important risk factors of cardiometabolic diseases, such as reduced LDL cholesterol, lower blood pressure, and insulin sensitivity [[52], [53], [54], [55], [56], [57]], and decreased levels of proinflammatory markers [58,59]. Conversely, total and animal protein intakes were positively associated with concentrations of insulin-like growth factor 1, which has been implicated in the growth of malignant cells in breast and prostate tissue [[60], [61], [62]]. In our study, dietary protein was favorably associated with physical function in older age, and this relationship was stronger for plant protein. Studies of older adult populations have found that protein intake has been associated with decreased lean mass loss in older age [9,63]. Animal protein supplementation studies in older adults have been implicated in lean mass gains [64], which were potentially related to its amino acid composition. However, lean mass gains related to short-term supplementation [9] have been inconsistent with long-term prospective studies, which have observed favorable associations between plant protein intake and frailty risk but no significant associations for animal protein intake [23,65].
One potential explanation is that plant protein is associated with a reduced risk of chronic diseases over a long follow-up period; in turn, chronic diseases are associated with reduced physical function and frailty in older adults [66,67]. Furthermore, these studies have included the incidence of chronic diseases in their definition of frailty, which highlights the role of plant protein, given its beneficial associations with the risk of chronic conditions [23]. A second explanation is that short-term studies in older adult populations may observe a stronger role of dietary protein, which is largely comprised of animal protein, as it may mitigate the loss of muscle mass in older adults [9]. We also note that although all protein sources were associated with better odds of healthy aging, dietary components related to plant protein sources, including dietary fiber, micronutrients, and polyphenols, may have contributed to the stronger associations observed for plant protein. Our observed associations were attenuated when adjusted for intakes of fruits and vegetables; thus, we cannot discount the contributions of other components of those foods that contributed to plant protein intake. Lastly, the associations we observed for substituting refined carbohydrates for dietary protein are also likely because of the potential adverse effects of refined carbohydrate intake on cardiometabolic health [68].
Strengths and limitations
Our study has several strengths. To our knowledge, this is the first study that evaluated the role of protein intake in midlife in relation to healthy aging in later life. Dietary risk factors assessed in midlife likely represent the relevant etiologic development window for most chronic conditions as they tend to develop over many years. The substantial lag between the assessment of diet and the evaluation of the domains of healthy aging minimized the likelihood of reverse causation, biasing our results. Furthermore, the sensitivity analysis of diets followed up through 2002 or 2006 demonstrated that there is a low likelihood of misclassification of dietary exposures over the follow-up period.
However, our study may be subject to several limitations as well. Our study population included mostly White females, which limits the generalizability of our findings to other populations. Although we excluded participants with several chronic diseases at baseline to minimize reverse causation, we were unable to exclude those with physical function limitations or impairments in mental status or memory function at baseline because those data were unavailable for the baseline period. We also acknowledge the possibility that participants with impairments in physical or cognitive function may be less likely to return the biennial questionnaires. However, the baseline diet was similar between participants who returned the questionnaires for physical and cognitive functions and those who did not, suggesting that the loss to follow-up was probably nondifferential in terms of exposure assessments. In addition, the long lag time between dietary assessments and memory loss assessments may bias the associations toward the null when the more recent diet is more relevant to cognitive function decline at older ages [69]. Because of the observational nature of our study, we cannot exclude the possibility of residual or unmeasured confounding, particularly from the food sources of each protein variable, particularly those from plant sources. Our self-reported assessment of physical function using SF-36 may add imprecision to the assessment of that domain. However, SF-36 measurements have been validated for use in populations of older adults [70,71]. Similarly, assessing subjective memory complaints using a self-reported questionnaire may introduce misclassification of the outcome. Of note, subjective memory complaints have been associated with performance on objective cognitive tests in the NHS [33,34]. Moreover, our observed associations were consistent in sensitivity analyses using the FRAIL scale to measure physical function and TICS as an objective method to evaluate memory complaints. In addition, we conducted multiple substitution analyses simultaneously and did not adjust for multiple comparisons, which may lead to the possibility of false positive findings. Lastly, there is a possibility of measurement error in assessing dietary exposures; however, the FFQ has been previously validated against dietary records. Using averaged measurements across FFQ cycles helped decrease the likelihood of random measurement error.
In conclusion, the findings from this large prospective study suggest that dietary protein intake, and especially the consumption of plant protein, in middle-aged female nurses, may be related to higher odds of healthy aging. Plant protein intake was favorably associated with several domains of health status of older adults, including good physical function and good mental health status. Our study contributes evidence to the role of protein in the etiology of healthy aging and adds specific insights to the importance of protein sources and relevant etiologic windows in midlife, which may contribute to providing recommendations regarding the amount of protein intake to promote healthy aging. Future research is warranted to verify these findings in other populations and to elucidate the mechanisms underlying the associations between protein intake and healthy aging.
Author contributions
The authors’ responsibilities were as follows – AVAK, QS: were responsible for project conception and study design; AVAK: conducted the data analysis; AVAK, QS: wrote the paper; MKS, PFJ, PS, MW, AHE, WCW: participated in study design and provided critical feedback on revisions. AVAK, QS: had primary responsibility for the final content; and all authors: read, provided critical revisions, and approved the final manuscript.
Funding
This study was supported by the USDA Agricultural Research Service under Cooperative Agreement No. 58-8050-9-004 and by the NIH grants CA186107, P01 CA87969, DK120870, U2C DK129670, DK127601, HL060712, HL034594, HL035464, and HL088521. AVAK was supported by training grant 1KL2TR002545. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA or the official views of the NIH. The funders had no role in study design, data collection and analysis, publication decisions, or manuscript preparation.
Data availability
Data described in the manuscript, code book, and analytic code will not be made available because of participant confidentiality and privacy concerns. Further information, including the procedures to obtain and access data from the Nurses’ Health Study, is described at https://www.nurseshealthstudy.org/researchers (contact e-mail: nhsaccess@channing.harvard.edu).
Conflict of interest
The authors report no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.11.010.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Vespa J., Medina L., Armstrong D. Demographic turning points for the United States: population projections for 2020 to 2060. Curr. Popul. Rep. P. 2020;1144:25. [Google Scholar]
- 2.Beard J.R., Officer A., de Carvalho I.A., Sadana R., Pot A.M., Michel J.P., et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387(10033):2145–2154. doi: 10.1016/S0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarmeas N., Anastasiou C.A., Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet. Neurol. 2018;17(11):1006–1015. doi: 10.1016/S1474-4422(18)30338-7. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2019 Diseases and Injuries Collaborators, Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Struijk E.A., Hagan K.A., Fung T.T., Hu F.B., Rodríguez-Artalejo F., Lopez-Garcia E. Diet quality and risk of frailty among older women in the Nurses’ Health Study. Am. J. Clin. Nutr. 2020;111(4):877–883. doi: 10.1093/ajcn/nqaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sotos-Prieto M., Bhupathiraju S.N., Mattei J., Fung T.T., Li Y., Pan A., et al. Association of changes in diet quality with total and cause-specific mortality. N. Engl. J. Med. 2017;377(2):143–153. doi: 10.1056/NEJMoa1613502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samieri C., Sun Q., Townsend M.K., Chiuve S.E., Okereke O.I., Willett W.C., et al. The association between dietary patterns at midlife and health in aging: an observational study. Ann. Intern. Med. 2013;159(9):584–591. doi: 10.7326/0003-4819-159-9-201311050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts S.B., Silver R.E., Das S.K., Fielding R.A., Gilhooly C.H., Jacques P.F., et al. Healthy aging-nutrition matters: start early and screen often. Adv. Nutr. 2021;12(4):1438–1448. doi: 10.1093/advances/nmab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houston D.K., Nicklas B.J., Ding J., Harris T.B., Tylavsky F.A., Newman A.B., et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008;87(1):150–155. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- 10.Hruby A., Sahni S., Bolster D., Jacques P.F. Protein intake and functional integrity in aging: the Framingham heart study offspring. J. Gerontol. A. Biol. Sci. Med. Sci. 2020;75(1):123–130. doi: 10.1093/gerona/gly201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naseeb M.A., Volpe S.L. Protein and exercise in the prevention of sarcopenia and aging. Nutr. Res. 2017;40:1–20. doi: 10.1016/j.nutres.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Tessier A.J., Chevalier S. An update on protein, leucine, omega-3 fatty acids, and vitamin D in the prevention and treatment of sarcopenia and functional decline. Nutrients. 2018;10(8) doi: 10.3390/nu10081099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mustafa J., Ellison R.C., Singer M.R., Bradlee M.L., Kalesan B., Holick M.F., et al. Dietary protein and preservation of physical functioning among middle-aged and older adults in the Framingham offspring study. Am. J. Epidemiol. 2018;187(7):1411–1419. doi: 10.1093/aje/kwy014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coelho-Júnior H.J., Milano-Teixeira L., Rodrigues B., Bacurau R., Marzetti E., Uchida M. Relative protein intake and physical function in older adults: A systematic review and meta-analysis of observational studies. Nutrients. 2018;10(9) doi: 10.3390/nu10091330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace T.C., Frankenfeld C.L. Dietary protein intake above the current RDA and bone health: A systematic review and meta-analysis. J. Am. Coll. Nutr. 2017;36(6):481–496. doi: 10.1080/07315724.2017.1322924. [DOI] [PubMed] [Google Scholar]
- 16.Rizzoli R., Biver E., Bonjour J.P., Coxam V., Goltzman D., Kanis J.A., et al. Benefits and safety of dietary protein for bone health-an expert consensus paper endorsed by the European Society for Clinical and Economical Aspects of Osteopororosis, Osteoarthritis, and Musculoskeletal Diseases and by the International Osteoporosis Foundation. Osteoporos. Int. 2018;29(9):1933–1948. doi: 10.1007/s00198-018-4534-5. [DOI] [PubMed] [Google Scholar]
- 17.Fung T.T., Meyer H.E., Willett W.C., Feskanich D. Protein intake and risk of hip fractures in postmenopausal women and men age 50 and older. Osteoporos. Int. 2017;28(4):1401–1411. doi: 10.1007/s00198-016-3898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeh T.S., Yuan C., Ascherio A., Rosner B.A., Blacker D., Willett W.C. Long-term dietary protein intake and subjective cognitive decline in US men and women. Am. J. Clin. Nutr. 2022;115(1):199–210. doi: 10.1093/ajcn/nqab236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coelho-Júnior H.J., Calvani R., Landi F., Picca A., Marzetti E. Protein intake and cognitive function in older adults: A systematic review and meta-analysis. Nutr. Metab. Insights. 2021;14 doi: 10.1177/11786388211022373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan R., Leung J., Woo J., Kwok T. Associations of dietary protein intake on subsequent decline in muscle mass and physical functions over four years in ambulant older Chinese people. J. Nutr. Health Aging. 2014;18(2):171–177. doi: 10.1007/s12603-013-0379-y. [DOI] [PubMed] [Google Scholar]
- 21.Houston D.K., Tooze J.A., Garcia K., Visser M., Rubin S., Harris T.B., et al. Protein intake and mobility limitation in community-dwelling older adults: the health ABC study. J. Am. Geriatr. Soc. 2017;65(8):1705–1711. doi: 10.1111/jgs.14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z., Glisic M., Song M., Aliahmad H.A., Zhang X., Moumdjian A.C., et al. Dietary protein intake and all-cause and cause-specific mortality: results from the Rotterdam Study and a meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2020;35(5):411–429. doi: 10.1007/s10654-020-00607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Struijk E.A., Fung T.T., Rodríguez-Artalejo F., Bischoff-Ferrari H.A., Hu F.B., Willett W.C., et al. Protein intake and risk of frailty among older women in the Nurses’ Health Study. J. Cachexia. Sarcopenia. Muscle. 2022;13(3):1752–1761. doi: 10.1002/jcsm.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortolá R., Struijk E.A., García-Esquinas E., Rodríguez-Artalejo F., Lopez-Garcia E. Changes in dietary intake of animal and vegetable protein and unhealthy aging. Am. J. Med. 2020;133(2):231–239.e7. doi: 10.1016/j.amjmed.2019.06.051. [DOI] [PubMed] [Google Scholar]
- 25.Rowe J.W., Kahn R.L. Successful aging, Gerontologist. 1997;37(4):433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- 26.Colditz G.A., Hankinson S.E. The Nurses’ Health Study: lifestyle and health among women. Nat. Rev. Cancer. 2005;5(5):388–396. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 27.Willett W.C., Sampson L., Browne M.L., Stampfer M.J., Rosner B., Hennekens C.H., et al. The use of a self-administered questionnaire to assess diet four years in the past. Am. J. Epidemiol. 1988;127(1):188–199. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 28.Yuan C., Spiegelman D., Rimm E.B., Rosner B.A., Stampfer M.J., Barnett J.B., et al. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am. J. Epidemiol. 2018;187(5):1051–1063. doi: 10.1093/aje/kwx328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma W., Hagan K.A., Heianza Y., Sun Q., Rimm E.B., Qi L. Adult height, dietary patterns, and healthy aging. Am. J. Clin. Nutr. 2017;106(2):589–596. doi: 10.3945/ajcn.116.147256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J., Murphy S.L., Kochanek K.D., Arias E. Mortality in the United States, 2021. NCHS Data Brief. 2022;(456):1–8. [PubMed] [Google Scholar]
- 31.Colditz G.A., Martin P., Stampfer M.J., Willett W.C., Sampson L., Rosner B., et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am. J. Epidemiol. 1986;123(5):894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 32.Barr R.G., Herbstman J., Speizer F.E., Camargo C.A. Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am. J. Epidemiol. 2002;155(10):965–971. doi: 10.1093/aje/155.10.965. [DOI] [PubMed] [Google Scholar]
- 33.Amariglio R.E., Townsend M.K., Grodstein F., Sperling R.A., Rentz D.M. Specific subjective memory complaints in older persons may indicate poor cognitive function. J. Am. Geriatr. Soc. 2011;59(9):1612–1617. doi: 10.1111/j.1532-5415.2011.03543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samieri C., Proust-Lima C., Glymour M.M., Okereke O.I., Amariglio R.E., Sperling R.A., et al. Subjective cognitive concerns, episodic memory, and the APOE ε4 allele. Alzheimers Dement. 2014;10(6):752–759 e1. doi: 10.1016/j.jalz.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McHorney C.A., Ware J.E., Raczek A.E. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 36.de Craen A.J., Heeren T.J., Gussekloo J. Accuracy of the 15-item geriatric depression scale (GDS-15) in a community sample of the oldest old. Int. J. Geriatr. Psychiatry. 2003;18(1):63–66. doi: 10.1002/gps.773. [DOI] [PubMed] [Google Scholar]
- 37.Chiuve S.E., Fung T.T., Rimm E.B., Hu F.B., McCullough M.L., Wang M., et al. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012;142(6):1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durrleman S., Simon R. Flexible regression models with cubic splines. Stat. Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 39.Kipnis V., Freedman L.S., Brown C.C., Hartman A., Schatzkin A., Wacholder S. Interpretation of energy adjustment models for nutritional epidemiology. Am. J. Epidemiol. 1993;137(12):1376–1380. doi: 10.1093/oxfordjournals.aje.a116647. [DOI] [PubMed] [Google Scholar]
- 40.Morley J.E., Malmstrom T.K., Miller D.K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J. Nutr. Health Aging. 2012;16(7):601–608. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang J., Liao L.M., Weinstein S.J., Sinha R., Graubard B.I., Albanes D. Association between plant and animal protein intake and overall and cause-specific mortality. JAMA Intern. Med. 2020;180(9):1173–1184. doi: 10.1001/jamainternmed.2020.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernstein A.M., Sun Q., Hu F.B., Stampfer M.J., Manson J.E., Willett W.C. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122(9):876–883. doi: 10.1161/CIRCULATIONAHA.109.915165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Preis S.R., Stampfer M.J., Spiegelman D., Willett W.C., Rimm E.B. Dietary protein and risk of ischemic heart disease in middle-aged men. Am. J. Clin. Nutr. 2010;92(5):1265–1272. doi: 10.3945/ajcn.2010.29626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shang X., Scott D., Hodge A.M., English D.R., Giles G.G., Ebeling P.R., et al. Dietary protein intake and risk of type 2 diabetes: results from the Melbourne Collaborative Cohort Study and a meta-analysis of prospective studies. Am. J. Clin. Nutr. 2016;104(5):1352–1365. doi: 10.3945/ajcn.116.140954. [DOI] [PubMed] [Google Scholar]
- 45.Malik V.S., Li Y., Tobias D.K., Pan A., Hu F.B. Dietary protein intake and risk of Type 2 diabetes in US men and women. Am. J. Epidemiol. 2016;183(8):715–728. doi: 10.1093/aje/kwv268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X.W., Yang Z., Li M., Li K., Deng Y.Q., Tang Z.Y. Association between dietary protein intake and risk of stroke: A meta-analysis of prospective studies. Int. J. Cardiol. 2016;223:548–551. doi: 10.1016/j.ijcard.2016.08.106. [DOI] [PubMed] [Google Scholar]
- 47.Teh R., Mendonça N., Muru-Lanning M., MacDonell S., Robinson L., Kerse N. Dietary protein intake and transition between frailty states in octogenarians living in New Zealand. Nutrients. 2021;13(8) doi: 10.3390/nu13082843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matison A.P., Milte C.M., Shaw J.E., Magliano D.J., Daly R.M., Torres S.J. Association between dietary protein intake and changes in health-related quality of life in older adults: findings from the AusDiab 12-year prospective study. BMC Geriatr. 2022;22(1):211. doi: 10.1186/s12877-022-02894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts R.O., Roberts L.A., Geda Y.E., Cha R.H., Pankratz V.S., O’Connor H.M., et al. Relative intake of macronutrients impacts risk of mild cognitive impairment or dementia. J. Alzheimers. Dis. 2012;32(2):329–339. doi: 10.3233/JAD-2012-120862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watson N.A., Dyer K.A., Buckley J.D., Brinkworth G.D., Coates A.M., Parfitt G., et al. Comparison of two low-fat diets, differing in protein and carbohydrate, on psychological wellbeing in adults with obesity and type 2 diabetes: a randomised clinical trial. Nutr. J. 2018;17(1):62. doi: 10.1186/s12937-018-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Makanae Y., Fujita S. Role of exercise and nutrition in the prevention of sarcopenia. J. Nutr. Sci. Vitaminol. (Tokyo) 2015:61. doi: 10.3177/jnsv.61.S125. Suppl S125–S127. [DOI] [PubMed] [Google Scholar]
- 52.Anderson J.W., Johnstone B.M., Cook-Newell M.E. Meta-analysis of the effects of soy protein intake on serum lipids. N. Engl. J. Med. 1995;333(5):276–282. doi: 10.1056/NEJM199508033330502. [DOI] [PubMed] [Google Scholar]
- 53.Lamarche B., Desroches S., Jenkins D.J., Kendall C.W., Marchie A., Faulkner D., et al. Combined effects of a dietary portfolio of plant sterols, vegetable protein, viscous fibre and almonds on LDL particle size. Br. J. Nutr. 2004;92(4):657–663. doi: 10.1079/bjn20041241. [DOI] [PubMed] [Google Scholar]
- 54.He J., Gu D., Wu X., Chen J., Duan X., Chen J., et al. Effect of soybean protein on blood pressure: a randomized, controlled trial. Ann. Intern. Med. 2005;143(1):1–9. doi: 10.7326/0003-4819-143-1-200507050-00004. [DOI] [PubMed] [Google Scholar]
- 55.Tremblay F., Lavigne C., Jacques H., Marette A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu. Rev. Nutr. 2007;27:293–310. doi: 10.1146/annurev.nutr.25.050304.092545. [DOI] [PubMed] [Google Scholar]
- 56.Elliott P., Stamler J., Dyer A.R., Appel L., Dennis B., Kesteloot H., et al. Association between protein intake and blood pressure: the INTERMAP Study. Arch. Intern. Med. 2006;166(1):79–87. doi: 10.1001/archinte.166.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vasei M.H., Hosseinpour-Niazi S., Ainy E., Mirmiran P. Effect of dietary approaches to stop hypertension (DASH) diet, high in animal or plant protein on cardiometabolic risk factors in obese metabolic syndrome patients: A randomized clinical trial. Prim. Care Diabetes. 2022;16(5):634–639. doi: 10.1016/j.pcd.2022.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Azadbakht L., Kimiagar M., Mehrabi Y., Esmaillzadeh A., Hu F.B., Willett W.C. Soy consumption, markers of inflammation, and endothelial function: a cross-over study in postmenopausal women with the metabolic syndrome. Diabetes Care. 2007;30(4):967–973. doi: 10.2337/dc06-2126. [DOI] [PubMed] [Google Scholar]
- 59.Li J., Glenn A.J., Yang Q., Ding D., Zheng L., Bao W., et al. Dietary protein sources, mediating biomarkers, and incidence of Type 2 diabetes: findings from the Women’s Health Initiative and the UK Biobank. Diabetes Care. 2022;45(8):1742–1753. doi: 10.2337/dc22-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen N.E., Appleby P.N., Davey G.K., Kaaks R., Rinaldi S., Key T.J. The associations of diet with serum insulin-like growth factor I and its main binding proteins in 292 women meat-eaters, vegetarians, and vegans. Cancer Epidemiol. Biomarkers Prev. 2002;11(11):1441–1448. [PubMed] [Google Scholar]
- 61.Holmes M.D., Pollak M.N., Willett W.C., Hankinson S.E. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol. Biomarkers Prev. 2002;11(9):852–861. [PubMed] [Google Scholar]
- 62.Watts E.L., Perez-Cornago A., Fensom G.K., Smith-Byrne K., Noor U., Andrews C.D., et al. Circulating insulin-like growth factors and risks of overall, aggressive and early-onset prostate cancer: a collaborative analysis of 20 prospective studies and Mendelian randomization analysis. Int. J. Epidemiol. 2023;52(1):71–86. doi: 10.1093/ije/dyac124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mangano K.M., Sahni S., Kiel D.P., Tucker K.L., Dufour A.B., Hannan M.T. Dietary protein is associated with musculoskeletal health independently of dietary pattern: the Framingham Third Generation Study. Am. J. Clin. Nutr. 2017;105(3):714–722. doi: 10.3945/ajcn.116.136762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Komar B., Schwingshackl L., Hoffmann G. Effects of leucine-rich protein supplements on anthropometric parameter and muscle strength in the elderly: a systematic review and meta-analysis. J. Nutr. Health Aging. 2015;19(4):437–446. doi: 10.1007/s12603-014-0559-4. [DOI] [PubMed] [Google Scholar]
- 65.Sandoval-Insausti H., Pérez-Tasigchana R.F., López-García E., García-Esquinas E., Rodríguez-Artalejo F., Guallar-Castillón P. Macronutrients intake and incident frailty in older adults: A prospective cohort study. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71(10):1329–1334. doi: 10.1093/gerona/glw033. [DOI] [PubMed] [Google Scholar]
- 66.Afilalo J., Alexander K.P., Mack M.J., Maurer M.S., Green P., Allen L.A., et al. Frailty assessment in the cardiovascular care of older adults. J Am. Coll. Cardiol. 2014;63(8):747–762. doi: 10.1016/j.jacc.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh M., Stewart R., White H. Importance of frailty in patients with cardiovascular disease. Eur. Heart J. 2014;35(26):1726–1731. doi: 10.1093/eurheartj/ehu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blaak E.E., Riccardi G., Cho L. Carbohydrates: separating fact from fiction. Atherosclerosis. 2021;328:114–123. doi: 10.1016/j.atherosclerosis.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 69.Shishtar E., Rogers G.T., Blumberg J.B., Au R., Jacques P.F. Long-term dietary flavonoid intake and risk of Alzheimer disease and related dementias in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 2020;112(2):343–353. doi: 10.1093/ajcn/nqaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McHorney C.A., Ware J.E., Jr., Lu J.F., Sherbourne C.D. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med. Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Schumaier A.P., Matar R.N., Ramalingam W.G., Archdeacon M.T. Patient-reported outcomes for fractures of the acetabulum: A comparison between patient-reported outcomes information system and traditional instruments. J. Am. Acad. Orthop. Surg. 2022;30(2):71–78. doi: 10.5435/JAAOS-D-20-01324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will not be made available because of participant confidentiality and privacy concerns. Further information, including the procedures to obtain and access data from the Nurses’ Health Study, is described at https://www.nurseshealthstudy.org/researchers (contact e-mail: nhsaccess@channing.harvard.edu).