Abstract
Evidence from large epidemiological studies has shown that obesity may predispose to increased Th2 inflammation and increase the odds of developing asthma. On the other hand, there is growing evidence suggesting that metabolic dysregulation that occurs with obesity, and more specifically hyperglycemia and insulin resistance, may modify immune cell function and in some degree systemic inflammation. Insulin resistance seldom occurs on its own, and in most cases constitutes a clinical component of metabolic syndrome, along with central obesity and dyslipidemia. Despite that, in some cases, hyperinsulinemia associated with insulin resistance has proven to be a stronger risk factor than body mass in developing asthma. This finding has been supported by recent experimental studies showing that insulin resistance may contribute to airway remodeling, promotion of airway smooth muscle (ASM) contractility and proliferation, increase of airway hyper-responsiveness and release of pro-inflammatory mediators from adipose tissue. All these effects indicate the potential impact of hyperinsulinemia on airway structure and function, suggesting the presence of a specific asthma phenotype with insulin resistance. Epidemiologic studies have found that individuals with severe and uncontrolled asthma have a higher prevalence of glycemic dysfunction, whereas longitudinal studies have linked glycemic dysfunction to an increased risk of asthma exacerbations. Since the components of metabolic syndrome interact with one another so much, it is challenging to identify each one’s specific role in asthma. This is why, over the last decade, additional studies have been conducted to determine whether treatment of type 2 diabetes mellitus affects comorbid asthma as shown by the incidence of asthma, asthma control and asthma-related exacerbations. The purpose of this review is to present the mechanism of action, and existing preclinical and clinical data, regarding the effect of insulin resistance in asthma.
Keywords: asthma, insulin resistance, antidiabetic drugs, metabolic dysfunction, obesity
1. Introduction
Asthma is one of the most common respiratory disorders worldwide [1], and in 2019, there were 262.4 million prevalent cases and 37.0 million new cases of asthma all over the world, contributing to 21.6 million disability-adjusted life years (DALYs) [2]. Chronic eosinophilic bronchitis with T helper 2 (Th2) inflammation, bronchial smooth muscle hyperplasia, easy epithelium shedding, mucus plug formation in the bronchi, and thickening of the subepithelial basement membrane are its hallmark pathologic features [1]. Due to its broad diagnostic standards, which include varying expiratory airflow limitation and respiratory symptoms, asthma is a heterogeneous disease with multiple pathogenic mechanisms (endotypes) and higher-order groupings sharing similar clinical characteristics (phenotypes) [3,4]. In the era of personalized medicine, significant progress has been achieved in disease phenotyping and endotyping with the evolution and utilization of dependable biomarkers precisely measured through reliable and repeatable techniques [5]. Even though several asthma phenotypes have been described, including allergic and non-allergic asthma, occupational, environmental (indoor/outdoor pollutants) [6] and aspirin-induced asthma, the pathologic characteristics of these phenotypes are very similar, while type 2 inflammation by Th2 and group 2 innate lymphoid (ILC2) cells is their crucial component [3]. Regarding severe uncontrolled asthma phenotypes, recently developed monoclonal antibody treatments have highlighted the importance of type 2 inflammation [7]. Despite the differences in the design of different studies, it is clear that a considerable minority of patients with severe uncontrolled asthma have a lack of atopy and type 2 inflammation, emphasizing the significance of non-allergic triggers to these patients [8,9].
Dysglycemia, glycemic dysfunction, and glucose dysmetabolism, (also referred to as disorders of glucose metabolism) have been identified as possible causes of severe asthma and as potential triggers of asthma exacerbation [10,11]. Asthma and insulin resistance appear to interact and interrelate through mechanisms and pathways that are not yet fully understood, thereby influencing each other’s natural course [12]. Compared to individuals with Th2 inflammation, patients with asthma and metabolic dysfunction have additional pathogenic and pathologic characteristics [11]. Often presented as metabolic syndrome, metabolic dysfunction has several significant clinical characteristics, such as insulin resistance/glucose intolerance, central obesity, dyslipidemia, and in certain cases, vitamin D (Vit D) deficiency [13,14].
It is commonly known that the treatment of asthma in obese patients can be challenging, partly because of the patients’ poor response to inhaled corticosteroids [15,16]. Treatment of insulin resistance has favorable effects on asthma management [17]. Additionally, metabolic dysfunction appears to be the primary cause of asthma exacerbation, according to a cohort analysis of asthmatic patients who were prone to exacerbations [18]. These characteristics highlight the association between asthma phenotype and metabolic dysfunction, and the significance of metabolic dysfunction in the pathogenesis of asthma. The impact of obesity and metabolic dysfunction on asthma is becoming increasingly apparent as these diseases’ prevalence rises worldwide.
In this context, the purpose of this review was to summarize current data on the deleterious role of insulin resistance in asthma, along with suggested mechanisms of asthma irritation.
2. Insulin Resistance
The most comprehensive definition of “insulin resistance” was formulated by Petersen and Shulman [19] as a “maladaptive in the setting of chronic overnutrition”. Even though its molecular causes are not entirely known, improper lipid accumulation in the liver is considered a major factor that contributes to decreased cellular sensitivity to insulin. To preserve glucose homeostasis, pancreatic B-cells produce more insulin, which results in a period of clinically silent hyperinsulinemia with normal blood glucose levels [10].
With time, blood glucose levels rise in tandem with reductions in insulin secretion as this compensatory hyperinsulinemia starts to falter [20]. This period can be identified by elevated glycated hemoglobin A1c (HbA1c), which is a measure of the average blood glucose levels over the previous 3 months, elevated fasting glucose (also named impaired fasting glucose, or IFG), or inadequate ability to completely offset hyperglycemic increases as a result of a glucose load (also named impaired glucose tolerance or IGT). Elevated IFG, IGT, or HbA1c are useful in diagnosing prediabetes [21]. Crucially, the human body still secretes more insulin during the prediabetic state, and eventually, there is a reduction in insulin secretion that coincides with overt hyperglycemia and pancreatic failure. Diabetes is diagnosed based on blood glucose or HbA1c levels [22].
Evidence from the Third National Health and Nutrition Examination Survey (NHANES III) has shown that one-third of nondiabetic adults in the United States (US) experience hyperinsulinemia associated with insulin resistance [23], whereas 12–43% of US adults have prediabetes. In the US, 11.3% of the population of all ages (more than 37 million people), had diabetes in 2019 [24]. Taken together, these data suggest that insulin resistance, prediabetes and diabetes are widespread and interrelated. Metabolic syndrome, which includes obesity, hypertension, dyslipidemia, and hyperglycemic tendencies, is closely linked to obesity (specifically abdominal obesity), while insulin resistance and hyperinsulinemia are the main molecular markers of this syndrome [25]. Indeed, large epidemiological studies [26,27] have shown that the main obesity-associated asthma risk was attributed to insulin resistance, and even maternal obesity raises the incidence of childhood asthma in susceptible children, pointing to systemic causes [28]. In the Korean Health and Genome Study (a population-based study of 10,038 Korean adults aged 40 to 69) [29], metabolic syndrome was related to asthma-like symptoms.
There is a strong connection between more severe and uncontrolled asthma and metabolic syndrome [30], which are often related to increased airway hyperresponsiveness, oxidative stress, regulation of systemic and pulmonary inflammation, and macrophage activation [31,32,33]. Modifications to nutrition and microbiome, immunity, inflammation and biophysical lung function associated with obesity may also likely have an impact on asthma, regardless of metabolic pathways [14,34]. Particularly, obesity-induced insulin resistance seems to precede macrophage accumulation and inflammation in adipose tissue [35] and has been linked to elevated inflammatory markers in serum and adipose tissue among patients with severe asthma [34]. On the other hand, sputum and serum expression of interleukin 6, which is recognized to participate in the development of insulin resistance, have been linked with more severe asthma and airway obstruction [36,37].
3. Insulin Resistance and Asthma Pathophysiology: A Missing Link
The degree of insulin resistance and the amount of circulating insulin are strongly correlated in most patients with prediabetes and insulin resistance. As a result, the lung and other peripheral organs that retain insulin sensitivity are susceptible to high levels of circulating insulin [30]. Studies have shown the presence of insulin receptors in the developing lung [38], but while the lungs’ extent of insulin sensitivity is unknown, it is assumed that (like most other tissues) hyperinsulinemia is accompanied by reduced insulin-like growth factor binding proteins (IGFBP-1 and -3) and increased free insulin-like growth factor (IGF-1) [39,40]. Both insulin and IGF-1 have significant impacts on cell proliferation and differentiation through several mechanisms of action, including increased fibrosis, increased epithelium to mucus transition, as well as increased contractility and mass of airway smooth muscle (ASM); some of these are associated with asthma phenotypes.
Insulin causes a hypercontractile phenotype of bovine ASM, similar to that seen in asthma, by increasing the production of b1-containing laminins through a phosphoinositide-3 kinase (PI-3K)/Akt-dependent signaling pathway [41]. Recent observations in humans [41] have shown that inhaled insulin can cause an abrupt loss in lung function due to airway smooth muscle contraction, suggesting that hyperinsulinemia may increase airway smooth muscle bulk or contraction. In mice, administration of intranasal insulin resulted in an enhancement in the deposition of collagen in the lungs, as well as increased airway hyper-responsiveness [42]. Furthermore, the lungs of mice treated with insulin showed activation of β-catenin by PI3K/Akt, which is a positive regulator of epithelial-mesenchymal transition and fibrosis, suggesting that hyperinsulinemia may have negative impacts on airway structure and function.
A key mechanism implicated in several biological processes, such as cell proliferation, morphogenesis, and development, is the Wnt/β-catenin-signaling pathway [43]. Multiple human abnormalities, such as malignancies and inflammatory, fibrotic, and metabolic disorders, have been linked to aberrant Wnt/β-catenin signaling [44]. Experimental studies in a murine model have illustrated that blocking Wnt/β-catenin signaling decreases pulmonary fibrosis [45]. The wnt/β-catenin-signaling pathway plays a crucial role in pulmonary arterial hypertension, in which vascular smooth muscle cell proliferation is a fundamental feature [46]. Considering that smooth muscle hyperplasia and subepithelial fibrosis are the hallmarks of airway remodeling, we can speculate that Wnt/β-catenin signaling plays a role in the process of airway remodeling in asthma. In the lung tissue of mice with chronic asthma, the use of a particular siRNA, which blocks β-catenin expression, resulted in airway remodeling and inflammation, reduction of subepithelial fibrosis and collagen accumulation, and downregulation of transforming growth factor-β production [47]. Furthermore, suppression of β-catenin in a model of chronic asthma prevented smooth muscle hyperplasia through downregulation of the tenascin C/platelet-derived growth factor receptor pathway, indicating that this pathway is abundantly expressed and controls the process of airway remodeling. Hence, insulin is thought to be involved in b-catenin-mediated unfavorable airway remodeling, since inhibiting this protein has been demonstrated to block the progression of subepithelial fibrosis, mucus metaplasia, and ASM hyperplasia in chronic asthma [48].
Remodeling of the airway wall is a feature of chronic airway inflammation and may play a significant role in airway hyperresponsiveness and lung function loss in patients with severe persistent asthma [49]. Both changes in extracellular matrix (ECM) [50] and increased mass of airway smooth muscle (ASM) [51] are characteristics of airway wall remodeling in these patients. ECM proteins known as laminins are frequently present in basement membranes with an increased expression being observed in the airways of patients with asthma compared with healthy controls [52]. Using bovine tracheal smooth muscle, Dekkers et al. showed a pivotal role for laminins in the establishment of an ASM phenotype that is hypercontractile and hypoproliferative after insulin exposure [41]. Thus, increased ASM contractility and contractile protein expression may be related to increased laminin synthesis by ASM in asthma. Moreover, the same study showed that eight days of administration of insulin causes an elevation in the expression of particular markers of the contractile phenotype in the smooth muscle cells and strips of the bovine trachea [53]. This was followed by a decrease in mitogenic responses and the installation of a phenotype functionally hypercontractile [54].
Additionally, it has been observed that high-fat-diet (HFD)-induced obesity enhanced the expression of TGF-β1 and insulin resistance in the lungs, which causes perivascular and peribronchial pulmonary fibrosis and aggravated airway hyper-responsiveness (AHR) to inhaled aerosolized methacholine (MCh) in mice [55].
Intranasal insulin increased the expression of TGF-β1 in the bronchial epithelium and caused lung fibrosis. HFD-induced AHR, lung fibrosis, and goblet cell hyperplasia were reduced by the anti-TGF-β1 antibody. Regarding airway hyper-responsiveness, muscarinic M2 receptors in parasympathetic nerves in the trachea of humans and rats are inhibited by insulin, which leads to an increase in acetylcholine release and airway contraction [56]. Loss of inhibitory M2 muscarinic receptor function on parasympathetic nerves and enhanced vagally mediated bronchoconstriction in obesity are strongly related [56]. Interestingly, Calco GN et al. demonstrated that cutting insulin receptors on sensory nerves blocked the increase in sensory nerve density and inhibited airway hyperreactivity in obese mice with hyperinsulinemia [57]. The same author [58], in a mouse model of maternal diet-induced obesity that recapitulates metabolic dysregulation, showed for the first time, that exposure to a maternal high-fat diet leads to hyperinnervation of airway sensory nerves and increased reflex bronchoconstriction in offspring fed a regular diet only [58]. These findings could clarify why obese people are more likely to experience asthma exacerbations since hyperinsulinemia is more common and predominant in obese people. They also imply that anticholinergic medications may be useful in treating this type of asthma. Furthermore, insulin-exposed open-ring guinea pig tracheal preparations induce ASM contraction via PI3-kinase and Rho kinase-dependent mechanisms mediated by contractile prostaglandin synthesis [59]. Xu et al. found [60] that insulin treatment decreased the β-agonist responsiveness of primary human ASM cells and obese mice via phosphorylating phosphodiesterase 4D and upregulating its downstream activity. In contrast, a recent study by Ferreira et al. [61] demonstrated that insulin deficiency correlated with decreased lung levels of STAT3, JNK, and ERK1/2. Additionally, in a mouse model of asthma, it blocked the onset of allergic inflammation, eosinophilic pulmonary infiltration, and airway hyperresponsiveness [61].
Delving even deeper into the pathophysiology of asthma, it is worth mentioning that mast cells are essential for the development and progression of inflammatory and acute-type allergic reactions. Lessman et al. [62], found that in mast cells generated from rabbit bone marrow, insulin and IGF-1 increased cell survival through the PI3-kinase pathway. These effects might be involved in the inverse link between atopic diseases, such as allergies and asthma, and type 1 diabetes mellitus, which is characterized by low insulin levels. Insulin-mediated stimulation of PI3-kinase and ERK pathways prevented human bronchial epithelial cell apoptosis, which may facilitate airway remodeling [63]. Accordingly, insulin is important in the pathogenesis of apoptosis-driven lung diseases (such as asthma and chronic obstructive pulmonary disease).
The lungs are not sterile, but they have a far lower bacterial load compared with other mucosal surfaces. This is partly because of strict control over the availability of nutrients, including glucose. Normally, the airway surface liquid has up to 12 times lower glucose levels compared with plasma [64]. Two mechanisms maintain this low concentration: glucose transporters that take up the glucose from the blood, and tight junctions that block it from entering the airway. Keeping glucose levels low may be a homeostatic strategy that prevents bacteria from growing by depriving them of an essential nutrient [65]. A higher risk of bacterial lung infection arises when these processes fail with resultant glucose increase, particularly in patients with underlying lung disease, like asthma [66]. In line with this, high glucose levels increase the likelihood of respiratory tract bacterial colonization in critically ill patients [66].
In the event of chronic hyperglycemia, advanced glycation end-products (AGEs) are actively generated and accumulate in the bloodstream and different organs [67]. By various mechanisms, AGEs also promote the expression of AGE receptors and play a crucial role in the onset of vascular complications in diabetes. Initially, the multiligand receptor known as the receptor for advanced glycation end products (RAGE) was suggested as a potential mediator in diabetes [68]. However, it was later discovered that membrane RAGE (mRAGE) signaling is pro-inflammatory, while soluble RAGE (sRAGE), a secreted form of RAGE, is mostly anti-inflammatory due to its ability to scavenge pro-inflammatory ligands [68]. Milutinovic et al. [69], revealed that RAGE is essential to the disease processes that lead to pulmonary eosinophilia, mucus hypersecretion, airway remodeling, and hyper-responsiveness of the airways in a model of dust mite-induced asthma/allergic airway disease. Airway hypersensitivity (resistance, tissue damping, and elastance), eosinophilic inflammation, and airway remodeling were all eliminated in the absence of RAGE. Additionally, the expression of IL-5 and IL-13 protein and mRNA in the lung was decreased [69].
Finally, insulin resistance, hyperglycemia, or both have been linked as a cause of accelerated decline in respiratory function, a condition that has been reported as “diabetic lung” [70]. The term “diabetic lung” includes several abnormalities of the respiratory function concerning control of ventilation, bronchomotor tone, lung volume, pulmonary diffusing capacity, and neuroadrenergic bronchial innervation. Although the precise pathogenetic processes through which insulin resistance, hyperglycemia, and diabetes mellitus may affect respiratory function are not yet fully understood, it is speculated that an increase in lung collagen and elastin [71], and the occurrence of subclinical nodular fibrosis, in addition to physiological impacts on the function of the respiratory muscles are responsible for this effect [72].
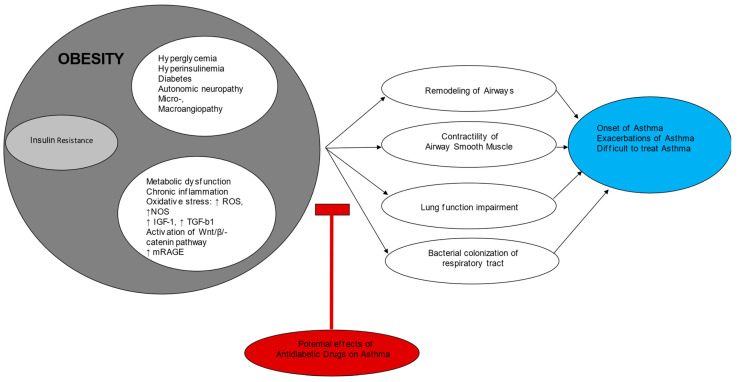
The suggested mechanisms of insulin resistance in asthma are shown in Table 1 and Figure 1.
Table 1.
Suggested mechanisms of insulin resistance in asthma.
| Enhanced Airway Remodeling |
| Increased collagen deposition in the lungs |
| Increased epithelium to mucus transition |
| Increased fibrosis |
| Increased airway smooth muscle mass |
| Increased expression of TGF-β1 |
| Increased Airway Smooth Muscle (ASM) contractility |
| Increased airway hyper-responsiveness |
| Loss of inhibitory M2 muscarinic receptor function |
| Enhanced vagally mediated bronchoconstriction |
| Lung function impairment |
| Reduced Forced Expiratory Volume in the 1st sec (FEV1) |
| Reduced Forced vital capacity (FVC) |
| Reduced Forced expiratory flow over the middle half of the FVC (FEF25–75%) |
| Increase the risk of respiratory tract bacterial colonization |
| Release of pro-inflammatory mediators from adipose tissue |
| Increased IL-6 |
| Increased TNF-α |
| Increased Th2-inflammation |
Figure 1.
Insulin resistance and asthma. IGF-1: insulin-like growth factor, mRAGE: membrane receptor advanced glycation end products, NOS: nitric oxide species, ROS: reactive oxygen species, TGF-b1: transforming growth factor b1.
4. Epidemiological-Observational Studies
Diabetes and disorders of glucose metabolism have been associated with reduced diffusing capacity and restrictive spirometry in subpopulations free from any pulmonary disease. This is based on 40 clinical trials evaluating lung function data of 3182 patients with diabetes [73]. However, no study has clarified whether hyperglycemia and consequently diabetes mellitus, increases the risk of asthma or vice versa [74]. It appears to be a bidirectional independent relationship between diabetes mellitus and asthma [74].
One of the first studies evaluating this issue was conducted in Denmark [26] and concluded that insulin resistance was related to a higher probability of manifesting asthma-like symptoms, thus promoting the theory that asthma and obesity may be associated with inflammatory processes also implicated in insulin resistance.
A few years afterward, Mueller et al. [75], using data from Singapore Chinese Health Study, also found a positive correlation between the likelihood of developing type 2 diabetes and self-reported, physician-diagnosed asthma. In agreement with the two aforementioned studies, the Nord-Trøndelag Health Study (HUNT) examining prospectively 23191 adults aged 19–55 years, without asthma at baseline, showed that metabolic syndrome was related to increased risk of incident asthma, indicating that physicians may take metabolic syndrome into account as a predictor of future risk of asthma [76]. The same was the result from another study that used multiple logistic regression analyses from the National Health and Nutrition Examination Survey (NHANES), 2001–2018, and found that an increase in the metabolism score for visceral fat (METS-VF) index was related to an increase in the incidence of asthma [77]. Recently, a cross-sectional analysis of 41,480 adults from the Korean National Health and Nutrition Examination Survey during the period 2007–2016, revealed that when both obesity and metabolic syndrome are present, the risk of developing asthma is highest [78]. The above observation is also seen in children: a study from Australia Illustrated signs of insulin resistance in 43% of children aged 6 to 17 who suffered from allergic asthma [79].
Together with the aforementioned epidemiological population-based studies, studies employing electronic and administrative health records similarly reported that the probability of developing asthma is higher in subjects with type 2 diabetes compared with non-diabetic individuals, suggesting that these 2 diseases are related [80,81]. Nevertheless, a major limitation in all these studies is that incident asthma was defined by self-report or through diagnostic codes and not objectively confirmed.
One step forward, Cardet et al. [82] hypothesized that insulin resistance is an effect modifier of the relationship between asthma and obesity. A history of physician-diagnosed current asthma and insulin resistance was obtained from 12,421 individuals, aged 18–85 years, utilizing the large National Health and Nutrition Examination Survey from 2003–2012. In logistic regression analysis, increased insulin resistance increases in obese individuals increased the likelihood of asthma [82]. This was not the case for other components of metabolic syndrome [82]. Therefore, it is possible that a subgroup of patients with asthma and obesity may be identified by their insulin resistance, and agents targeting insulin resistance may also improve asthma control in this subgroup [82]. This was confirmed recently, in another cross-sectional study of 1276 participants from the NHANES 2009–2012 database [83]. In this analysis, waist-to-hip ratio and insulin resistance independently predicted impaired pulmonary function in overweight/obese asthmatic adults [83]. Evaluations were based on forced expiratory volume in the 1st second (FEV1), forced vital capacity (FVC), and forced expiratory flow over the middle half of the FVC (FEF25–75%).
As indicated in a recent study by Peters and colleagues [84], in a SARP-3 (Severe Asthma Research Program–3) cohort, insulin resistance is independently linked to airflow limitation, diminished treatment responses, and an accelerated deterioration in lung function over time. In more detail, patients with insulin resistance had considerably lower FEV1 and FVC values and responded to β-adrenergic agonists and systemic corticosteroids with a lower FEV1 compared to individuals without insulin resistance. Moreover, individuals with moderate and severe insulin resistance had an annualized decline in FEV1 of −41 mL/year and −32 mL/year, respectively, compared to patients without insulin resistance who had an annualized decline of −13 mL/year [84]. What is noteworthy about the present study is the fact that after controlling for body mass index in regression models, the analysis showed that the effects of extra body fat on chest wall mechanics were unlikely to fully explain the association between worsening lung function and insulin resistance [84,85].
Accordingly, the association between insulin resistance, diabetes, and prevalent asthma is bidirectional. Other studies including patients with asthma have also reported a positive correlation between disorders of glucose metabolism and asthma morbidity. An increased risk of asthma exacerbation has been associated with a higher incidence of diabetes, hypertension, and obesity, according to a recent longitudinal analysis of the Severe Asthma Research Program cohort [18]. In a cross-sectional analysis using data from ERICA (Study of Cardiovascular Risk in Adolescents, Portuguese acronym ERICA), a multicenter, school-based countrywide study in a complex sample of adolescents aged 12–17 years, metabolic syndrome and insulin resistance were significantly associated with severe asthma in adolescents from Brazilia [86].
In an attempt to ascertain the relationship between pre-diabetes/diabetes and asthma exacerbations in an obese asthma cohort, Wu et al. [87], conducted a retrospective cohort of 5722 individuals with obese asthma in the United States, aged 18–64, from a claims-based health care database covering 2010–2015. It is worth underlining that in the current study, the investigators used HbA1c instead of a historical or self-reported diagnosis of diabetes, and pre-diabetes was defined as 5.7% ≤ HbA1c ≤ 6.4%, while diabetes was defined as HbA1c ≥ 6.5% [87]. Compared to patients with normal HbA1c, those in the pre-diabetes range experienced a 27% and those in the diabetes range experienced a 33% higher asthma exacerbation rate. Yang et al. [17] conducted a cross-sectional analysis of 47,606 adults with asthma who did not have diabetes mellitus from the UK Biobank to evaluate the association between lung function, HbA1c, and asthma-related hospitalizations. Both HbA1c per se and an HbA1c in the pre-diabetic or diabetic range were associated with ≥1 asthma hospitalization. Both HbA1c per se and HbA1c in the prediabetic/diabetic range were significantly and inversely associated with FEV1 and FVC [17].
Other studies have also shown that prediabetes or diabetes is linked with asthma exacerbations [88,89]. Although non-significant after adjustment for covariates, diabetes has been associated with asthma exacerbations [88]. A cross-sectional analysis of data from 709 participants in the US-based Severe Asthma Research Program (SARP)-3 cohort revealed that patients with exacerbation-prone asthma were more likely to self-report a diagnosis of diabetes mellitus than those without exacerbation-prone asthma [88]. Conversely, even after controlling for smoking status, overweight or obesity, and other potential confounders, a study of 130,547 patients (aged 12–80 years) in two UK databases found that type 1 or type 2 diabetes was strongly related to 1.53 times increased odds of hospitalizations due to asthma within the following year [89].
Finally, to evaluate the relationship between triglyceride-glucose index (TyG), a biomarker of metabolic syndrome and insulin resistance, with the risk of severe asthma exacerbation, Staggers KA et al. [90] designed a 5-year retrospective study. In it 108,219 patients with asthma from the US Veterans Health Administration were followed for a severe asthma exacerbation, from January 2015 to December 2019. The authors found that independent of obesity, eosinophils, smoking and asthma treatment, patients with an elevated TyG had a 6% greater risk of experiencing a severe asthma exacerbation [90].
Insulin resistance and glucose dysregulation have been further associated with impaired lung function in people with and without asthma. A multivariable analysis of 15,792 United States adults in the Atherosclerosis Risk in Communities (ARIC) Study showed that the % predicted of FEV1 and FVC were 2.4% and 3.6% lower, respectively, in adults with diabetes compared to those without diabetes after adjusting for smoking, body-mass index, waist circumference, and other covariates [91]. Conversely, in a U.S. nationwide survey study of 4257 adults without diabetes, there was a significant non-linear inverse association between elevated HbA1c and FEV1, FVC, and FEV1/FVC ratio, after adjusting for body-mass index and waist-to-hip ratio [92]. Unfortunately, the possible effects of respiratory comorbidities, including asthma, on glucose metabolism were not taken into account in either of those two trials. Moreover, according to a cross-sectional study of 1429 adolescents between the ages of 12 and 17 from the United States who participated in the 2007–2010 National Health and Nutrition Examination Survey [93], metabolic syndrome and insulin resistance were linked to impaired lung function in overweight/obese adolescents. In another cross-sectional study of diabetic and non-diabetic adults [94], a 1% absolute increase in HbA1c was associated with a −52 mL difference in FVC and a −25 mL difference in FEV1 in women, and a −128 mL difference in FVC and a −73 mL difference in FEV1 in men, showing an inverse association between glycemic measurements and pulmonary function. The findings of an additional cross-sectional observational cohort study of non-smoker African American adults from the Jackson Heart Study were also similar [95]. There was no significant difference in lung function between women and men with impaired glucose tolerance and those with normal glucose tolerance, while women and men with diabetes had lower FEV1 and FVC than those with normal glucose tolerance [95].
Ultimately, data from the Colombian Diabetes Association Center in Bogotá [96], showed that in comparison to patients with adequate control, patients with type 2 diabetes and inadequate glucose control had higher FEV1/FVC (0.013%) and lower mean residuals of FEV1 (75.4 mL) and FVC (121 mL), as well as higher levels of all inflammatory markers.
In light of all these data, we can assume that insulin resistance and metabolic syndrome are strongly related to asthma prevalence and may predict the impairment of lung function. However, the underlying mechanisms remain unclear. In an attempt to answer this query, 168 Hispanic and African American adolescents (13–18 years) from Children’s Hospital in Montefiore were divide.d into groups of 42 obese subjects with asthma, 42 normal-weight subjects with asthma, 40 obese subjects without asthma, and 44 healthy control subjects [33]. In children with obesity-related asthma, lung function deficiencies and non-atopic systemic inflammation have been linked to insulin resistance and dyslipidemia [33].
5. Current Antidiabetic Drugs and Asthma
If diabetes and insulin resistance aggravate asthma, it makes sense that treating these conditions could have a positive therapeutic impact, provided that the mechanism causing lung damage is reversible. In addition to their effects on glucose management, many antidiabetic drugs have additional actions that can also affect asthma [97].
According to in vivo and in vitro studies of the last decade, metformin exerts anti-inflammatory effects in airways [98,99]. In mice asthma models, metformin has been demonstrated to reverse eosinophilic infiltration of the lung tissue and reduce levels of pro-inflammatory cytokines, reactive oxygen species, and nitric oxide species [99]. Adenosine monophosphate-activated protein kinase (AMPK) is thought to be the likely key mechanism by which metformin has an anti-inflammatory effect on the airway [99]. Metformin has been observed to activate AMPK, and in a dose-dependent manner inhibit tumor necrosis factor (TNF)-α–induced NF-κB activation and TNF-α–induced IκB kinase activity [100]. Additionally, this agent appears to attenuate the TNF-α–induced gene expression of various proinflammatory and cell adhesion molecules (such as vascular cell adhesion molecule-1, E-selectin, intercellular adhesion molecule-1, and monocyte chemoattractant protein-1) in human umbilical vein endothelial cells (HUVECs) [100]. AMPK activity also reduces oxidative stress and contributes to the inhibition of tumor necrosis factor-α-induced inflammatory signaling and expression of inducible nitric oxide synthase mediated by nuclear factor-kB [99]. In male mice given a high-fat diet (HFD) for ten weeks to induce obesity, Calixto et al. showed that metformin reduced the exacerbation of allergic eosinophilic inflammation [98]. Given that metformin is proposed as a first-line therapeutic medication for diabetes [101], it may be a new therapeutic option for these patients. Indeed, metformin was associated with a reduced risk of asthma-related emergency department visits and hospitalizations in a US cohort of patients with diabetes and asthma [102].
An 11-year (2001–2011) retrospective cohort study that followed 1332 individuals with concurrent diabetes and asthma for three years to evaluate the asthma-related outcomes was conducted utilizing the Taiwan National Health Insurance Research Database [103]. The study concluded that metformin users experienced a lower risk of asthma exacerbation or asthma-related hospitalization compared to non-users [103]. Very recently, Wu et al. [104] identified 1749 patients with asthma and diabetes using the Johns Hopkins electronic health record from April 2013 to May 2018. The use of metformin, independently of glycemic control and obesity, was related to a lower hazard of asthma-related emergency department visits and hospitalizations [104]. Unfortunately, there are no data regarding the beneficial role of metformin from prospective controlled studies in asthmatic patients, with and without obesity.
Glucagon-like peptide 1 (GLP-1) is a hormone derived from the gut that enhances insulin sensitivity and increases pancreatic insulin production in response to oral food consumption [105]. The GLP-1Ra (GLP-1 receptor) exists in the lung and immune cells and may mediate several inflammatory pathways implicated in the pathogenesis of obesity-related asthma [106]. Preclinical studies have indicated that GLP-1 inhibited the production of pro-inflammatory cytokines, including TNF-a, by inactivating NF-κB in a protein kinase A-dependent manner [107,108]. Moreover, GLP-1 receptor agonists (GLP-1RAs) suppress RAGE expression, and thus reduce inflammation and bronchoconstriction [109]. Furthermore, the GLP-1RA liraglutide reduced mucus hypersecretion and airway inflammation in a mouse model of allergic asthma [110]. In addition, in mice models, GLP-1RAs reduced the release of T2 cytokines from type 2 innate lymphoid cells (ILC2), as well as mucus production after exposure to viral antigens and fungal allergens [111,112,113]. Interestingly, in isolated human airways, GLP-1R activation reduced contractile tone and reduced lipopolysaccharide-stimulated eosinophil activation [114,115].
GLP-1RAs have been linked with improvements in asthma outcomes in patients with asthma and diabetes mellitus. In the first uncontrolled preliminary study, 9 participants receiving a GLP-1RA for one-year improved asthma symptoms and decreased asthma exacerbations [116]. An electronic health records-based new user, active-comparator, retrospective cohort study of patients with type 2 diabetes and asthma showed that patients on GLP-1RAs for type 2 diabetes had fewer asthma exacerbations compared with other antidiabetic agents (sodium-glucose cotransporter-2 inhibitors [SGLT-2is], dipeptidyl peptidase inhibitors [DPP-4is], sulfonylureas, or basal insulin) [117].
Another new-user active-comparator analysis using a national claims database (2005–2017) also showed that patients with diabetes and chronic lower respiratory disease (CLRD) (a medical term that includes both COPD and asthma) who started GLP-1RA experienced fewer CLRD exacerbations compared to those starting DPP-4is [118]. In diabetes, a randomized clinical trial reported that liraglutide decreased serum surfactant protein D, which independently predicted improvements in FVC [119]. The addition of a GLP-1RA to metformin, as opposed to metformin alone or metformin plus insulin, improved lung function (FEV1 and FVC) in a prospective cohort of 32 patients with diabetes who did not have obstructive lung disease [120].
Sulfonylureas are frequently used as second-line antidiabetic therapy [121]. Sulfonylureas attach to receptors on the cell membranes of pancreatic beta cells and increase insulin secretion. Their main untoward effect is hypoglycemia [122,123]. The utilization of a representative UK primary care database in a retrospective cohort study revealed an association between sulfonylureas and a lower risk of incident asthma [124].
DPP-4is are preferable to sulfonylureas as second-line therapy after metformin initiation, due to their neutral effect on weight and the absence of hypoglycemia [125,126,127]. Very recently, studies utilizing in vitro models of human bronchial epithelial cells have demonstrated that DPP-4i inhibits pathways that lead to fibrosis [128] and oxidative stress [129]. Nonetheless, a retrospective observational matched cohort study found that treatment with DPP-4i did not improve asthma control, treatment stability or asthma exacerbations [130]. This has been confirmed in a network meta-analysis [131].
SGLT-2is promotes glycosuria [132]. An in vitro transcriptomics experiment in human proximal tubular cells revealed that the SGLT-2i canagliflozin decreased TNF receptor 1, matrix metalloproteinase 7, IL-6, and fibronectin 1 during two years of follow-up, as compared with the sulfonylurea glimepiride [133].
In 2021, 9 large randomized controlled trials (RCTs) were included in a fixed-effects meta-analysis to assess the relationship between SGLT2 inhibitors and the occurrence of 9 types of non-infectious respiratory disorders [134]. Compared to placebo, SGLT-2is decreased the incidence of severe adverse events related to asthma. Another meta-analysis with a similar design concluded that SGLT-2is reduced the risk of asthma in comparison with GLP-1RAs and DPP-4is [131]. Despite that, the extremely low incidence of asthma outcomes in both groups (treatment and placebo) limits the validity of both meta-analyses.
Thiazolidinediones (TZDs) are another class of antidiabetic agent. They bind to the gamma isoform of the peroxisome proliferator-activated receptor (PPARγ) and reduce insulin resistance and ectopic fat accumulation [135,136]. So far, the results of studies regarding the anti-inflammatory effect of TZDs on asthma are controversial. Τroglitazone reduced IL-6 in cultured human airway smooth muscle cells, in a dose-dependent manner [137], but a systematic review concluded that TZDs had no significant effect on IL-6 levels [138]. A large observational retrospective study of diabetic Veterans who had a diagnosis of asthma and were taking oral antidiabetic agents [139], showed that exposure to thiazolidinediones was linked with a significant reduction in the risk of asthma exacerbation and oral steroid prescription. Utilization of angiotensin-converting enzyme inhibitors (ACE-I) and/or TZD was also linked with a lower risk for incident asthma in obese/overweight patients with diabetes and/or hypertension, in another retrospective observational longitudinal data analysis, who included 77,278 Veterans with incident asthma [140].
Conversely, in a 12-week, randomized, placebo-controlled, double-blind trial, no difference was observed in asthma control, exhaled nitric oxide, or lung function between treatment groups [141]. Interestingly, patients receiving pioglitazone gained significantly more weight than those receiving placebo [141]. New safety concerns regarding the risk of bladder cancer with pioglitazone led to the early discontinuation of the study [141]. Finally, a RCT of pioglitazone in severe asthma found no beneficial effect on asthma quality of life, as well as many untoward effects [142]. Thus, TDZs do not appear to hold for asthma.
The potential effects of current classes of hypoglycemic therapies in the pathophysiology of asthma are shown in Table 2.
Table 2.
Potential effects of current classes of antidiabetics in pathophysiology of asthma.
| Antidiabetic Agent | Potential Beneficial Effects on Asthma | Putative Mechanisms |
|---|---|---|
| Metformin | Yes | Reverse lung tissue eosinophilic infiltrationProinflammatory cytokines
|
| GLP-1 | Yes |
|
| Dipeptidyl peptidase-4 inhibitors (DPP-4is) | Uncertain |
|
| Sodium-glucose cotransporter-2 inhibitors (SGLT-2) | Yes |
|
| Thiazolidinediones (TZD) | No |
|
| Sulfonylureas | Uncertain |
* A systematic review concluded that TZD does not significantly affect IL-6 levels. ↓: reduce.
6. Conclusions
Obesity, insulin resistance or glucose intolerance, dyslipidemia, and other key clinical features of metabolic dysfunction—typically manifested as metabolic syndrome—have been found to be possible risk factors for severe and uncontrolled asthma. More specifically, disorders of glucose metabolism, ranging from clinically silent insulin resistance through degrees of hyperglycemia defining prediabetes and diabetes, can precipitate changes in the lung consistent with asthma through pathways principally involving insulin excess. Recent experimental studies have shown that insulin resistance may contribute to an increase in systemic inflammation, modulation of immune function, effects on airway remodeling, promotion of airway smooth muscle (ASM) contractility and proliferation and increase of airway hyper-responsiveness. Evidence from observational studies also supports clinically relevant associations between glycemic dysregulation and asthma outcomes. In our opinion, there are four mechanisms of the effect of insulin resistance in asthma: (i) aging is associated with insulin resistance, which can lead to premature airway closure and airway damage, (ii) insulin directly contributes to airway dysfunction by causing airway inflammation through the activation of immunological and structural cells in the lungs, (iii) insulin can directly induce airway hyperresponsiveness by promoting the deposition of collagen fibroblasts in the airways, (iv) insulin is a pleiotropic hormone that affects endothelial cells in a variety of ways. When all the aforementioned are taken into consideration, a significant unmet therapeutic demand for drugs to ameliorate asthma management and control in patients with insulin resistance is revealed. A growing volume of epidemiologic evidence indicates that by focusing on the underlying glycemic dysregulation, specific types of hypoglycemic drugs may be able to treat both chronic diseases. To better understand the influence and the role of insulin resistance in asthma, more research is required, and novel antidiabetic drugs pertaining to asthma should be investigated. Expanding our understanding of the diverse range of biochemical mechanisms that result in an adult asthma diagnosis may also enable us to pinpoint important tactics to prevent insulin resistance, obesity, and asthma from emerging later in life.
Author Contributions
Conceptualization, P.S.; methodology, K.B.; data curation, K.B.; writing—original draft preparation, K.B. and A.I.P.; writing—review and editing, F.D., E.G. and N.P.; supervision, P.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
K.B., A.I.P., F.D., E.G. and P.S. declare no conflicts of interest. N.P. has been an advisory board member of AstraZeneca, Boehringer Ingelheim, MSD, Novo Nordisk, Pfizer, Takeda and TrigoCare International; has participated in sponsored studies by AstraZeneca, Eli Lilly, GSK, MSD, Novo Nordisk, Novartis and Sanofi-Aventis; has received honoraria as a speaker for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Elpen, MSD, Mylan, Novo Nordisk, Pfizer, Sanofi-Aventis and Vianex; and has attended conferences sponsored by TrigoCare International, Eli Lilly, Galenica, Novo Nordisk, Pfizer and Sanofi-Aventis.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.To T., Stanojevic S., Moores G., Gershon A.S., Bateman E.D., Cruz A.A., Boulet L.-P. Global Asthma Prevalence in Adults: Findings from the Cross-Sectional World Health Survey. BMC Public Health. 2012;12:204. doi: 10.1186/1471-2458-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2019 Chronic Respiratory Diseases Collaborators Global Burden of Chronic Respiratory Diseases and Risk Factors, 1990-2019: An Update from the Global Burden of Disease Study 2019. EClinicalMedicine. 2023;59:101936. doi: 10.1016/j.eclinm.2023.101936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.2023 GINA Main Report. [(accessed on 12 December 2023)]. Available online: https://ginasthma.org/2023-gina-main-report/
- 4.Tiotiu A., Novakova P., Baiardini I., Bikov A., Chong-Neto H., de-Sousa J.C., Emelyanov A., Heffler E., Fogelbach G.G., Kowal K., et al. Manifesto on United Airways Diseases (UAD): An Interasma (global Asthma Association—GAA) Document. J. Asthma. 2022;59:639–654. doi: 10.1080/02770903.2021.1879130. [DOI] [PubMed] [Google Scholar]
- 5.Fouka E., Domvri K., Gkakou F., Alevizaki M., Steiropoulos P., Papakosta D., Porpodis K. Recent Insights in the Role of Biomarkers in Severe Asthma Management. Front. Med. 2022;9:992565. doi: 10.3389/fmed.2022.992565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiotiu A.I., Novakova P., Nedeva D., Chong-Neto H.J., Novakova S., Steiropoulos P., Kowal K. Impact of Air Pollution on Asthma Outcomes. Int. J. Environ. Res. Public Health. 2020;17:6212. doi: 10.3390/ijerph17176212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papaioannou A.I., Fouka E., Bartziokas K., Kallieri M., Vontetsianos A., Porpodis K., Rovina N., Loukides S., Bakakos P. Defining Response to Therapy with Biologics in Severe Asthma: From Global Evaluation to Super Response and Remission. Expert Rev. Respir. Med. 2023;17:481–493. doi: 10.1080/17476348.2023.2226392. [DOI] [PubMed] [Google Scholar]
- 8.Moore W.C., Meyers D.A., Wenzel S.E., Teague W.G., Li H., Li X., D’Agostino R., Jr., Castro M., Curran-Everett D., Fitzpatrick A.M., et al. Identification of Asthma Phenotypes Using Cluster Analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loza M.J., Djukanovic R., Chung K.F., Horowitz D., Ma K., Branigan P., Barnathan E.S., Susulic V.S., Silkoff P.E., Sterk P.J., et al. Validated and Longitudinally Stable Asthma Phenotypes Based on Cluster Analysis of the ADEPT Study. Respir. Res. 2016;17:165. doi: 10.1186/s12931-016-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu T.D. Diabetes, Insulin Resistance, and Asthma: A Review of Potential Links. Curr. Opin. Pulm. Med. 2021;27:29–36. doi: 10.1097/MCP.0000000000000738. [DOI] [PubMed] [Google Scholar]
- 11.Park J.-W. Asthma Phenotype with Metabolic Dysfunction. Yonsei Med. J. 2022;63:1–7. doi: 10.3349/ymj.2022.63.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Škrgat S., Harlander M., Janić M. Obesity and Insulin Resistance in Asthma Pathogenesis and Clinical Outcomes. Biomedicines. 2024;12:173. doi: 10.3390/biomedicines12010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckel R.H., Grundy S.M., Zimmet P.Z. The Metabolic Syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 14.Peters U., Dixon A.E., Forno E. Obesity and Asthma. J. Allergy Clin. Immunol. 2018;141:1169–1179. doi: 10.1016/j.jaci.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S., Verma M., Michalec L., Liu W., Sripada A., Rollins D., Good J., Ito Y., Chu H., Gorska M.M., et al. Steroid Resistance of Airway Type 2 Innate Lymphoid Cells from Patients with Severe Asthma: The Role of Thymic Stromal Lymphopoietin. J. Allergy Clin. Immunol. 2018;141:257–268.e6. doi: 10.1016/j.jaci.2017.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambers E.S., Nanzer A.M., Pfeffer P.E., Richards D.F., Timms P.M., Martineau A.R., Griffiths C.J., Corrigan C.J., Hawrylowicz C.M. Distinct Endotypes of Steroid-Resistant Asthma Characterized by IL-17A(high) and IFN-γ(high) Immunophenotypes: Potential Benefits of Calcitriol. J. Allergy Clin. Immunol. 2015;136:628–637. doi: 10.1016/j.jaci.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang G., Han Y.-Y., Forno E., Yan Q., Rosser F., Chen W., Celedón J.C. Glycated Hemoglobin A, Lung Function, and Hospitalizations Among Adults with Asthma. J. Allergy Clin. Immunol. Pract. 2020;8:3409–3415.e1. doi: 10.1016/j.jaip.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters M.C., Mauger D., Ross K.R., Phillips B., Gaston B., Cardet J.C., Israel E., Levy B.D., Phipatanakul W., Jarjour N.N., et al. Evidence for Exacerbation-Prone Asthma and Predictive Biomarkers of Exacerbation Frequency. Am. J. Respir. Crit. Care Med. 2020;202:973–982. doi: 10.1164/rccm.201909-1813OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen M.C., Shulman G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018;98:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaccardi F., Webb D.R., Yates T., Davies M.J. Pathophysiology of Type 1 and Type 2 Diabetes Mellitus: A 90-Year Perspective. Postgrad. Med. J. 2016;92:63–69. doi: 10.1136/postgradmedj-2015-133281. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14–S31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010;33((Suppl. S1)):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C., Ford E.S., McGuire L.C., Mokdad A.H., Little R.R., Reaven G.M. Trends in Hyperinsulinemia among Nondiabetic Adults in the U.S. Diabetes Care. 2006;29:2396–2402. doi: 10.2337/dc06-0289. [DOI] [PubMed] [Google Scholar]
- 24.National Diabetes Statistics Report. 2022. [(accessed on 12 December 2023)]. Available online: https://repository.gheli.harvard.edu/repository/11854/
- 25.Terzano C., Morano S., Ceccarelli D., Conti V., Paone G., Petroianni A., Graziani E., Carnovale A., Fallarino M., Gatti A., et al. Effect of Insulin on Airway Responsiveness in Patients with Type 2 Diabetes Mellitus: A Cohort Study. J. Asthma. 2009;46:703–707. doi: 10.1080/02770900903056203. [DOI] [PubMed] [Google Scholar]
- 26.Thuesen B.H., Husemoen L.L.N., Hersoug L.-G., Pisinger C., Linneberg A. Insulin Resistance as a Predictor of Incident Asthma-like Symptoms in Adults. Clin. Exp. Allergy. 2009;39:700–707. doi: 10.1111/j.1365-2222.2008.03197.x. [DOI] [PubMed] [Google Scholar]
- 27.Appleton S.L., Adams R.J., Wilson D.H., Taylor A.W., Ruffin R.E. North West Adelaide Health Study Team Central Obesity Is Associated with Nonatopic but Not Atopic Asthma in a Representative Population Sample. J. Allergy Clin. Immunol. 2006;118:1284–1291. doi: 10.1016/j.jaci.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Scholtens S., Wijga A.H., Brunekreef B., Kerkhof M., Postma D.S., Oldenwening M., de Jongste J.C., Smit H.A. Maternal Overweight before Pregnancy and Asthma in Offspring Followed for 8 Years. Int. J. Obes. 2010;34:606–613. doi: 10.1038/ijo.2009.194. [DOI] [PubMed] [Google Scholar]
- 29.Lee E.J., In K.H., Ha E.S., Lee K.J., Hur G.Y., Kang E.H., Jung K.H., Lee S.Y., Kim J.H., Lee S.Y., et al. Asthma-like Symptoms Are Increased in the Metabolic Syndrome. J. Asthma. 2009;46:339–342. doi: 10.1080/02770900802660931. [DOI] [PubMed] [Google Scholar]
- 30.Agrawal A., Mabalirajan U., Ahmad T., Ghosh B. Emerging Interface between Metabolic Syndrome and Asthma. Am. J. Respir. Cell Mol. Biol. 2011;44:270–275. doi: 10.1165/rcmb.2010-0141TR. [DOI] [PubMed] [Google Scholar]
- 31.Scichilone N., Rizzo M., Benfante A., Catania R., Giglio R.V., Nikolic D., Montalto G., Bellia V. Serum Low Density Lipoprotein Subclasses in Asthma. Respir. Med. 2013;107:1866–1872. doi: 10.1016/j.rmed.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen F., Hancox R.J., Nair P., Hansen H.S., Siersted H.C., Nybo M. Associations between Airway Hyperresponsiveness, Obesity and Lipoproteins in a Longitudinal Cohort. Clin. Respir. J. 2013;7:268–275. doi: 10.1111/crj.12000. [DOI] [PubMed] [Google Scholar]
- 33.Rastogi D., Fraser S., Oh J., Huber A.M., Schulman Y., Bhagtani R.H., Khan Z.S., Tesfa L., Hall C.B., Macian F. Inflammation, Metabolic Dysregulation, and Pulmonary Function among Obese Urban Adolescents with Asthma. Am. J. Respir. Crit. Care Med. 2015;191:149–160. doi: 10.1164/rccm.201409-1587OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sideleva O., Suratt B.T., Black K.E., Tharp W.G., Pratley R.E., Forgione P., Dienz O., Irvin C.G., Dixon A.E. Obesity and Asthma: An Inflammatory Disease of Adipose Tissue Not the Airway. Am. J. Respir. Crit. Care Med. 2012;186:598–605. doi: 10.1164/rccm.201203-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimobayashi M., Albert V., Woelnerhanssen B., Frei I.C., Weissenberger D., Meyer-Gerspach A.C., Clement N., Moes S., Colombi M., Meier J.A., et al. Insulin Resistance Causes Inflammation in Adipose Tissue. J. Clin. Investig. 2018;128:1538–1550. doi: 10.1172/JCI96139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters M.C., McGrath K.W., Hawkins G.A., Hastie A.T., Levy B.D., Israel E., Phillips B.R., Mauger D.T., Comhair S.A., Erzurum S.C., et al. Plasma Interleukin-6 Concentrations, Metabolic Dysfunction, and Asthma Severity: A Cross-Sectional Analysis of Two Cohorts. Lancet Respir. Med. 2016;4:574–584. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neveu W.A., Allard J.L., Raymond D.M., Bourassa L.M., Burns S.M., Bunn J.Y., Irvin C.G., Kaminsky D.A., Rincon M. Elevation of IL-6 in the Allergic Asthmatic Airway Is Independent of Inflammation but Associates with Loss of Central Airway Function. Respir. Res. 2010;11:28. doi: 10.1186/1465-9921-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baffi C.W., Wood L., Winnica D., Strollo P.J., Jr., Gladwin M.T., Que L.G., Holguin F. Metabolic Syndrome and the Lung. Chest. 2016;149:1525–1534. doi: 10.1016/j.chest.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attia N., Tamborlane W.V., Heptulla R., Maggs D., Grozman A., Sherwin R.S., Caprio S. The Metabolic Syndrome and Insulin-like Growth Factor I Regulation in Adolescent Obesity. J. Clin. Endocrinol. Metab. 1998;83:1467–1471. doi: 10.1210/jc.83.5.1467. [DOI] [PubMed] [Google Scholar]
- 40.Singh S., Prakash Y.S., Linneberg A., Agrawal A. Insulin and the Lung: Connecting Asthma and Metabolic Syndrome. J. Allergy. 2013;2013:627384. doi: 10.1155/2013/627384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dekkers B.G.J., Schaafsma D., Tran T., Zaagsma J., Meurs H. Insulin-Induced Laminin Expression Promotes a Hypercontractile Airway Smooth Muscle Phenotype. Am. J. Respir. Cell Mol. Biol. 2009;41:494–504. doi: 10.1165/rcmb.2008-0251OC. [DOI] [PubMed] [Google Scholar]
- 42.Singh S., Bodas M., Bhatraju N.K., Pattnaik B., Gheware A., Parameswaran P.K., Thompson M., Freeman M., Mabalirajan U., Gosens R., et al. Hyperinsulinemia Adversely Affects Lung Structure and Function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016;310:L837–L845. doi: 10.1152/ajplung.00091.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacDonald B.T., Tamai K., He X. Wnt/beta-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.George S.J. Wnt Pathway: A New Role in Regulation of Inflammation. Arterioscler. Thromb. Vasc. Biol. 2008;28:400–402. doi: 10.1161/ATVBAHA.107.160952. [DOI] [PubMed] [Google Scholar]
- 45.Henderson W.R., Jr., Chi E.Y., Ye X., Nguyen C., Tien Y.-T., Zhou B., Borok Z., Knight D.A., Kahn M. Inhibition of Wnt/beta-catenin/CREB Binding Protein (CBP) Signaling Reverses Pulmonary Fibrosis. Proc. Natl. Acad. Sci. USA. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Jesus Perez V., Yuan K., Alastalo T.-P., Spiekerkoetter E., Rabinovitch M. Targeting the Wnt Signaling Pathways in Pulmonary Arterial Hypertension. Drug Discov. Today. 2014;19:1270–1276. doi: 10.1016/j.drudis.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwak H.J., Park D.W., Seo J.-Y., Moon J.-Y., Kim T.H., Sohn J.W., Shin D.H., Yoon H.J., Park S.S., Kim S.-H. The Wnt/β-Catenin Signaling Pathway Regulates the Development of Airway Remodeling in Patients with Asthma. Exp. Mol. Med. 2015;47:e198. doi: 10.1038/emm.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumawat K., Koopmans T., Gosens R. β-Catenin as a Regulator and Therapeutic Target for Asthmatic Airway Remodeling. Expert Opin. Ther. Targets. 2014;18:1023–1034. doi: 10.1517/14728222.2014.934813. [DOI] [PubMed] [Google Scholar]
- 49.Bousquet J., Jeffery P.K., Busse W.W., Johnson M., Vignola A.M. Asthma. From Bronchoconstriction to Airways Inflammation and Remodeling. Am. J. Respir. Crit. Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 50.Fernandes D.J., Bonacci J.V., Stewart A.G. Extracellular Matrix, Integrins, and Mesenchymal Cell Function in the Airways. Curr. Drug Targets. 2006;7:567–577. doi: 10.2174/138945006776818700. [DOI] [PubMed] [Google Scholar]
- 51.Ebina M., Takahashi T., Chiba T., Motomiya M. Cellular Hypertrophy and Hyperplasia of Airway Smooth Muscles Underlying Bronchial Asthma. A 3-D Morphometric Study. Am. Rev. Respir. Dis. 1993;148:720–726. doi: 10.1164/ajrccm/148.3.720. [DOI] [PubMed] [Google Scholar]
- 52.Altraja A., Laitinen A., Virtanen I., Kämpe M., Simonsson B.G., Karlsson S.E., Håkansson L., Venge P., Sillastu H., Laitinen L.A. Expression of Laminins in the Airways in Various Types of Asthmatic Patients: A Morphometric Study. Am. J. Respir. Cell Mol. Biol. 1996;15:482–488. doi: 10.1165/ajrcmb.15.4.8879182. [DOI] [PubMed] [Google Scholar]
- 53.Schaafsma D., McNeill K.D., Stelmack G.L., Gosens R., Baarsma H.A., Dekkers B.G.J., Frohwerk E., Penninks J.-M., Sharma P., Ens K.M., et al. Insulin Increases the Expression of Contractile Phenotypic Markers in Airway Smooth Muscle. Am. J. Physiol. Cell Physiol. 2007;293:C429–C439. doi: 10.1152/ajpcell.00502.2006. [DOI] [PubMed] [Google Scholar]
- 54.Gosens R., Nelemans S.A., Hiemstra M., Grootte Bromhaar M.M., Meurs H., Zaagsma J. Insulin Induces a Hypercontractile Airway Smooth Muscle Phenotype. Eur. J. Pharmacol. 2003;481:125–131. doi: 10.1016/j.ejphar.2003.08.081. [DOI] [PubMed] [Google Scholar]
- 55.Park Y.H., Oh E.Y., Han H., Yang M., Park H.J., Park K.H., Lee J.-H., Park J.-W. Insulin Resistance Mediates High-Fat Diet-Induced Pulmonary Fibrosis and Airway Hyperresponsiveness through the TGF-β1 Pathway. Exp. Mol. Med. 2019;51:1–12. doi: 10.1038/s12276-019-0258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nie Z., Jacoby D.B., Fryer A.D. Hyperinsulinemia Potentiates Airway Responsiveness to Parasympathetic Nerve Stimulation in Obese Rats. Am. J. Respir. Cell Mol. Biol. 2014;51:251–261. doi: 10.1165/rcmb.2013-0452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calco G.N., Maung J.N., Jacoby D.B., Fryer A.D., Nie Z. Insulin Increases Sensory Nerve Density and Reflex Bronchoconstriction in Obese Mice. JCI Insight. 2022;7:e161898. doi: 10.1172/jci.insight.161898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calco G.N., Alharithi Y.J., Williams K.R., Jacoby D.B., Fryer A.D., Maloyan A., Nie Z. Maternal High-Fat Diet Increases Airway Sensory Innervation and Reflex Bronchoconstriction in Adult Offspring. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023;325:L66–L73. doi: 10.1152/ajplung.00115.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schaafsma D., Gosens R., Ris J.M., Zaagsma J., Meurs H., Nelemans S.A. Insulin Induces Airway Smooth Muscle Contraction. Br. J. Pharmacol. 2007;150:136–142. doi: 10.1038/sj.bjp.0706985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu R., Gopireddy R.R., Wu Y., Wu L., Tao X., Shao J., Wang W., Li L., Jovanovic A., Xu B., et al. Hyperinsulinemia Promotes Heterologous Desensitization of β Adrenergic Receptor in Airway Smooth Muscle in Obesity. FASEB J. 2020;34:3996–4008. doi: 10.1096/fj.201800688RR. [DOI] [PubMed] [Google Scholar]
- 61.Ferreira S.S., Oliveira M.A., Tsujita M., Nunes F.P.B., Casagrande F.B., Gomes E., Russo M., Tavares de Lima W., Martins J.O. Insulin Modulates the Immune Cell Phenotype in Pulmonary Allergic Inflammation and Increases Pulmonary Resistance in Diabetic Mice. Front. Immunol. 2020;11:84. doi: 10.3389/fimmu.2020.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lessmann E., Grochowy G., Weingarten L., Giesemann T., Aktories K., Leitges M., Krystal G., Huber M. Insulin and Insulin-like Growth Factor-1 Promote Mast Cell Survival via Activation of the Phosphatidylinositol-3-Kinase Pathway. Exp. Hematol. 2006;34:1532–1541. doi: 10.1016/j.exphem.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 63.Numata T., Araya J., Fujii S., Hara H., Takasaka N., Kojima J., Minagawa S., Yumino Y., Kawaishi M., Hirano J., et al. Insulin-Dependent Phosphatidylinositol 3-kinase/Akt and ERK Signaling Pathways Inhibit TLR3-Mediated Human Bronchial Epithelial Cell Apoptosis. J. Immunol. 2011;187:510–519. doi: 10.4049/jimmunol.1004218. [DOI] [PubMed] [Google Scholar]
- 64.Tregoning J.S., Mallia P. Modulating Airway Glucose to Reduce Respiratory Infections. Expert Rev. Respir. Med. 2019;13:121–124. doi: 10.1080/17476348.2019.1563487. [DOI] [PubMed] [Google Scholar]
- 65.Baker E.H., Baines D.L. Airway Glucose Homeostasis: A New Target in the Prevention and Treatment of Pulmonary Infection. Chest. 2018;153:507–514. doi: 10.1016/j.chest.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 66.Gill S.K., Hui K., Farne H., Garnett J.P., Baines D.L., Moore L.S.P., Holmes A.H., Filloux A., Tregoning J.S. Increased Airway Glucose Increases Airway Bacterial Load in Hyperglycaemia. Sci. Rep. 2016;6:27636. doi: 10.1038/srep27636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rhee S.Y., Kim Y.S. The Role of Advanced Glycation End Products in Diabetic Vascular Complications. Diabetes Metab. J. 2018;42:188–195. doi: 10.4093/dmj.2017.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramasamy R., Yan S.F., Schmidt A.M. RAGE: Therapeutic Target and Biomarker of the Inflammatory Response--the Evidence Mounts. J. Leukoc. Biol. 2009;86:505–512. doi: 10.1189/jlb.0409230. [DOI] [PubMed] [Google Scholar]
- 69.Milutinovic P.S., Alcorn J.F., Englert J.M., Crum L.T., Oury T.D. The Receptor for Advanced Glycation End Products Is a Central Mediator of Asthma Pathogenesis. Am. J. Pathol. 2012;181:1215–1225. doi: 10.1016/j.ajpath.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pitocco D., Fuso L., Conte E.G., Zaccardi F., Condoluci C., Scavone G., Incalzi R.A., Ghirlanda G. The Diabetic Lung--a New Target Organ? Rev. Diabet. Stud. 2012;9:23–35. doi: 10.1900/RDS.2012.9.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ofulue A.F., Thurlbeck W.M. Experimental Diabetes and the Lung. II. In Vivo Connective Tissue Metabolism. Am. Rev. Respir. Dis. 1988;138:284–289. doi: 10.1164/ajrccm/138.2.284. [DOI] [PubMed] [Google Scholar]
- 72.Kida K., Utsuyama M., Takizawa T., Thurlbeck W.M. Changes in Lung Morphologic Features and Elasticity Caused by Streptozotocin-Induced Diabetes Mellitus in Growing Rats. Am. Rev. Respir. Dis. 1983;128:125–131. doi: 10.1164/arrd.1983.128.1.125. [DOI] [PubMed] [Google Scholar]
- 73.Van den Borst B., Gosker H.R., Zeegers M.P., Schols A.M.W.J. Pulmonary Function in Diabetes: A Metaanalysis. Chest. 2010;138:393–406. doi: 10.1378/chest.09-2622. [DOI] [PubMed] [Google Scholar]
- 74.Rayner L., McGovern A., Creagh-Brown B., Woodmansey C., de Lusignan S. Type 2 Diabetes and Asthma: Systematic Review of the Bidirectional Relationship. Curr. Diabetes Rev. 2019;15:118–126. doi: 10.2174/1573399814666180711114859. [DOI] [PubMed] [Google Scholar]
- 75.Mueller N.T., Koh W.-P., Odegaard A.O., Gross M.D., Yuan J.-M., Pereira M.A. Asthma and the Risk of Type 2 Diabetes in the Singapore Chinese Health Study. Diabetes Res. Clin. Pract. 2013;99:192–199. doi: 10.1016/j.diabres.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brumpton B.M., Camargo C.A., Jr., Romundstad P.R., Langhammer A., Chen Y., Mai X.-M. Metabolic Syndrome and Incidence of Asthma in Adults: The HUNT Study. Eur. Respir. J. 2013;42:1495–1502. doi: 10.1183/09031936.00046013. [DOI] [PubMed] [Google Scholar]
- 77.Liu Q., Han X., Chen Y., Gao Y., Yang W., Huang L. Asthma Prevalence Is Increased in Patients with High Metabolism Scores for Visceral Fat: Study Reports from the US. Front. Endocrinol. 2023;14:1162158. doi: 10.3389/fendo.2023.1162158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shim J.-S., Kim M.-H., Cho Y.-J. Risk of Asthma And/or Wheezing in Obese Individuals with or without Metabolic Syndrome: From the Korea National Health and Nutrition Examination Survey Data. Allergy Asthma Proc. 2024;45:e1–e8. doi: 10.2500/aap.2024.45.230070. [DOI] [PubMed] [Google Scholar]
- 79.Arshi M., Cardinal J., Hill R.J., Davies P.S.W., Wainwright C. Asthma and Insulin Resistance in Children. Respirology. 2010;15:779–784. doi: 10.1111/j.1440-1843.2010.01767.x. [DOI] [PubMed] [Google Scholar]
- 80.Thomsen S.F., Duffy D.L., Kyvik K.O., Skytthe A., Backer V. Risk of Asthma in Adult Twins with Type 2 Diabetes and Increased Body Mass Index. Allergy. 2011;66:562–568. doi: 10.1111/j.1398-9995.2010.02504.x. [DOI] [PubMed] [Google Scholar]
- 81.Ehrlich S.F., Quesenberry C.P., Jr., Van Den Eeden S.K., Shan J., Ferrara A. Patients Diagnosed with Diabetes Are at Increased Risk for Asthma, Chronic Obstructive Pulmonary Disease, Pulmonary Fibrosis, and Pneumonia but Not Lung Cancer. Diabetes Care. 2010;33:55–60. doi: 10.2337/dc09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cardet J.C., Ash S., Kusa T., Camargo C.A., Jr., Israel E. Insulin Resistance Modifies the Association between Obesity and Current Asthma in Adults. Eur. Respir. J. 2016;48:403–410. doi: 10.1183/13993003.00246-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sadeghimakki R., McCarthy H.D. Interactive Effects of Adiposity and Insulin Resistance on the Impaired Lung Function in Asthmatic Adults: Cross-Sectional Analysis of NHANES Data. Ann. Hum. Biol. 2019;46:56–62. doi: 10.1080/03014460.2019.1572223. [DOI] [PubMed] [Google Scholar]
- 84.Peters M.C., Schiebler M.L., Cardet J.C., Johansson M.W., Sorkness R., DeBoer M.D., Bleecker E.R., Meyers D.A., Castro M., Sumino K., et al. The Impact of Insulin Resistance on Loss of Lung Function and Response to Treatment in Asthma. Am. J. Respir. Crit. Care Med. 2022;206:1096–1106. doi: 10.1164/rccm.202112-2745OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nie Z., Fryer A.D., Jacoby D.B., Drake M.G. Mechanisms of Obesity-Related Asthma: Is Insulin Getting on Your Nerves? Am. J. Respir. Crit. Care Med. 2023;207:109–110. doi: 10.1164/rccm.202207-1419LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuschnir F.C., Felix M.M.R., Caetano Kuschnir M.C., Bloch K.V., Azevedo de Oliveira Costa Jordão E., Solé D., Ledo Alves da Cunha A.J., Szklo M. Severe Asthma Is Associated with Metabolic Syndrome in Brazilian Adolescents. J. Allergy Clin. Immunol. 2018;141:1947–1949.e4. doi: 10.1016/j.jaci.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 87.Wu T.D., Brigham E.P., Keet C.A., Brown T.T., Hansel N.N., McCormack M.C. Association Between Prediabetes/Diabetes and Asthma Exacerbations in a Claims-Based Obese Asthma Cohort. J. Allergy Clin. Immunol. Pract. 2019;7:1868–1873.e5. doi: 10.1016/j.jaip.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Denlinger L.C., Phillips B.R., Ramratnam S., Ross K., Bhakta N.R., Cardet J.C., Castro M., Peters S.P., Phipatanakul W., Aujla S., et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am. J. Respir. Crit. Care Med. 2017;195:302–313. doi: 10.1164/rccm.201602-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Price D., Wilson A.M., Chisholm A., Rigazio A., Burden A., Thomas M., King C. Predicting Frequent Asthma Exacerbations Using Blood Eosinophil Count and Other Patient Data Routinely Available in Clinical Practice. J. Asthma Allergy. 2016;9:1–12. doi: 10.2147/JAA.S97973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Staggers K.A., Minard C., Byers M., Helmer D.A., Wu T.D. Metabolic Dysfunction, Triglyceride-Glucose Index, and Risk of Severe Asthma Exacerbation. J. Allergy Clin. Immunol. Pract. 2023;11:3700–3705.e2. doi: 10.1016/j.jaip.2023.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yeh H.-C., Punjabi N.M., Wang N.-Y., Pankow J.S., Duncan B.B., Cox C.E., Selvin E., Brancati F.L. Cross-Sectional and Prospective Study of Lung Function in Adults with Type 2 Diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care. 2008;31:741–746. doi: 10.2337/dc07-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McKeever T.M., Weston P.J., Hubbard R., Fogarty A. Lung Function and Glucose Metabolism: An Analysis of Data from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2005;161:546–556. doi: 10.1093/aje/kwi076. [DOI] [PubMed] [Google Scholar]
- 93.Forno E., Han Y.-Y., Muzumdar R.H., Celedón J.C. Insulin Resistance, Metabolic Syndrome, and Lung Function in US Adolescents with and without Asthma. J. Allergy Clin. Immunol. 2015;136:304–311.e8. doi: 10.1016/j.jaci.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kabeya Y., Kato K., Tomita M., Katsuki T., Oikawa Y., Shimada A. Association of Glycemic Status with Impaired Lung Function among Recipients of a Health Screening Program: A Cross-Sectional Study in Japanese Adults. J. Epidemiol. 2014;24:410–416. doi: 10.2188/jea.JE20140016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hickson D.A., Burchfiel C.M., Liu J., Petrini M.F., Harrison K., White W.B., Sarpong D.F. Diabetes, Impaired Glucose Tolerance, and Metabolic Biomarkers in Individuals with Normal Glucose Tolerance Are Inversely Associated with Lung Function: The Jackson Heart Study. Lung. 2011;189:311–321. doi: 10.1007/s00408-011-9296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dennis R.J., Maldonado D., Rojas M.X., Aschner P., Rondón M., Charry L., Casas A. Inadequate Glucose Control in Type 2 Diabetes Is Associated with Impaired Lung Function and Systemic Inflammation: A Cross-Sectional Study. BMC Pulm. Med. 2010;10:38. doi: 10.1186/1471-2466-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ge D., Foer D., Cahill K.N. Utility of Hypoglycemic Agents to Treat Asthma with Comorbid Obesity. Pulm. Ther. 2023;9:71–89. doi: 10.1007/s41030-022-00211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calixto M.C., Lintomen L., André D.M., Leiria L.O., Ferreira D., Lellis-Santos C., Anhê G.F., Bordin S., Landgraf R.G., Antunes E. Metformin Attenuates the Exacerbation of the Allergic Eosinophilic Inflammation in High Fat-Diet-Induced Obesity in Mice. PLoS ONE. 2013;8:e76786. doi: 10.1371/journal.pone.0076786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park C.S., Bang B.-R., Kwon H.-S., Moon K.-A., Kim T.-B., Lee K.-Y., Moon H.-B., Cho Y.S. Metformin Reduces Airway Inflammation and Remodeling via Activation of AMP-Activated Protein Kinase. Biochem. Pharmacol. 2012;84:1660–1670. doi: 10.1016/j.bcp.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 100.Hattori Y., Suzuki K., Hattori S., Kasai K. Metformin Inhibits Cytokine-Induced Nuclear Factor kappaB Activation via AMP-Activated Protein Kinase Activation in Vascular Endothelial Cells. Hypertension. 2006;47:1183–1188. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- 101.Executive Summary: Standards of Medical Care in Diabetes—2013. Diabetes Care. 2012;36:S4–S10. doi: 10.2337/dc13-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu T.D., Keet C.A., Fawzy A., Segal J.B., Brigham E.P., McCormack M.C. Association of Metformin Initiation and Risk of Asthma Exacerbation. A Claims-Based Cohort Study. Ann. Am. Thorac. Soc. 2019;16:1527–1533. doi: 10.1513/AnnalsATS.201812-897OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li C.-Y., Erickson S.R., Wu C.-H. Metformin Use and Asthma Outcomes among Patients with Concurrent Asthma and Diabetes. Respirology. 2016;21:1210–1218. doi: 10.1111/resp.12818. [DOI] [PubMed] [Google Scholar]
- 104.Wu T.D., Fawzy A., Akenroye A., Keet C., Hansel N.N., McCormack M.C. Metformin Use and Risk of Asthma Exacerbation Among Asthma Patients with Glycemic Dysfunction. J. Allergy Clin. Immunol. Pract. 2021;9:4014–4020.e4. doi: 10.1016/j.jaip.2021.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pabreja K., Mohd M.A., Koole C., Wootten D., Furness S.G.B. Molecular Mechanisms Underlying Physiological and Receptor Pleiotropic Effects Mediated by GLP-1R Activation. Br. J. Pharmacol. 2014;171:1114–1128. doi: 10.1111/bph.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bendotti G., Montefusco L., Lunati M.E., Usuelli V., Pastore I., Lazzaroni E., Assi E., Seelam A.J., El Essawy B., Jang J., et al. The Anti-Inflammatory and Immunological Properties of GLP-1 Receptor Agonists. Pharmacol. Res. 2022;182:106320. doi: 10.1016/j.phrs.2022.106320. [DOI] [PubMed] [Google Scholar]
- 107.Li Z., Li S., Wang N., Xue P., Li Y. Liraglutide, a Glucagon-like Peptide-1 Receptor Agonist, Suppresses Osteoclastogenesis through the Inhibition of NF-κB and MAPK Pathways via GLP-1R. Biomed. Pharmacother. 2020;130:110523. doi: 10.1016/j.biopha.2020.110523. [DOI] [PubMed] [Google Scholar]
- 108.Baker R.G., Hayden M.S., Ghosh S. NF-κB, Inflammation, and Metabolic Disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nguyen D.-V., Linderholm A., Haczku A., Kenyon N. Glucagon-like Peptide 1: A Potential Anti-Inflammatory Pathway in Obesity-Related Asthma. Pharmacol. Ther. 2017;180:139–143. doi: 10.1016/j.pharmthera.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu T., Wu X.-L., Zhang W., Xiao M. Glucagon Like Peptide-1 (GLP-1) Modulates OVA-Induced Airway Inflammation and Mucus Secretion Involving a Protein Kinase A (PKA)-Dependent Nuclear Factor-κB (NF-κB) Signaling Pathway in Mice. Int. J. Mol. Sci. 2015;16:20195–20211. doi: 10.3390/ijms160920195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Toki S., Goleniewska K., Reiss S., Zhang J., Bloodworth M.H., Stier M.T., Zhou W., Newcomb D.C., Ware L.B., Stanwood G.D., et al. Glucagon-like Peptide 1 Signaling Inhibits Allergen-Induced Lung IL-33 Release and Reduces Group 2 Innate Lymphoid Cell Cytokine Production in Vivo. J. Allergy Clin. Immunol. 2018;142:1515–1528.e8. doi: 10.1016/j.jaci.2017.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bloodworth M.H., Rusznak M., Pfister C.C., Zhang J., Bastarache L., Calvillo S.A., Chappell J.D., Boyd K.L., Toki S., Newcomb D.C., et al. Glucagon-like Peptide 1 Receptor Signaling Attenuates Respiratory Syncytial Virus-Induced Type 2 Responses and Immunopathology. J. Allergy Clin. Immunol. 2018;142:683–687.e12. doi: 10.1016/j.jaci.2018.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Toki S., Newcomb D.C., Printz R.L., Cahill K.N., Boyd K.L., Niswender K.D., Peebles R.S., Jr. Glucagon-like Peptide-1 Receptor Agonist Inhibits Aeroallergen-Induced Activation of ILC2 and Neutrophilic Airway Inflammation in Obese Mice. Allergy. 2021;76:3433–3445. doi: 10.1111/all.14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rogliani P., Calzetta L., Capuani B., Facciolo F., Cazzola M., Lauro D., Matera M.G. Glucagon-Like Peptide 1 Receptor: A Novel Pharmacological Target for Treating Human Bronchial Hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 2016;55:804–814. doi: 10.1165/rcmb.2015-0311OC. [DOI] [PubMed] [Google Scholar]
- 115.Mitchell P.D., Salter B.M., Oliveria J.P., El-Gammal A., Tworek D., Smith S.G., Sehmi R., Gauvreau G.M., Butler M., O’Byrne P.M. Glucagon-like Peptide-1 Receptor Expression on Human Eosinophils and Its Regulation of Eosinophil Activation. Clin. Exp. Allergy. 2017;47:331–338. doi: 10.1111/cea.12860. [DOI] [PubMed] [Google Scholar]
- 116.Khan F., Mat A., Hogan A.E., Kent B.D., Eigenheer S., Corrigan M.A., O’Shea D., Butler M.W. Preliminary Asthma-Related Outcomes Following Glucagon-like Peptide 1 Agonist Therapy. QJM Int. J. Med. 2017;110:853–854. doi: 10.1093/qjmed/hcx125. [DOI] [PubMed] [Google Scholar]
- 117.Foer D., Beeler P.E., Cui J., Karlson E.W., Bates D.W., Cahill K.N. Asthma Exacerbations in Patients with Type 2 Diabetes and Asthma on Glucagon-like Peptide-1 Receptor Agonists. Am. J. Respir. Crit. Care Med. 2021;203:831–840. doi: 10.1164/rccm.202004-0993OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Albogami Y., Cusi K., Daniels M.J., Wei Y.-J.J., Winterstein A.G. Glucagon-Like Peptide 1 Receptor Agonists and Chronic Lower Respiratory Disease Exacerbations Among Patients With Type 2 Diabetes. Diabetes Care. 2021;44:1344–1352. doi: 10.2337/dc20-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.López-Cano C., Ciudin A., Sánchez E., Tinahones F.J., Barbé F., Dalmases M., García-Ramírez M., Soto A., Gaeta A.M., Pellitero S., et al. Liraglutide Improves Forced Vital Capacity in Individuals with Type 2 Diabetes: Data From the Randomized Crossover LIRALUNG Study. Diabetes. 2022;71:315–320. doi: 10.2337/db21-0688. [DOI] [PubMed] [Google Scholar]
- 120.Rogliani P., Matera M.G., Calzetta L., Hanania N.A., Page C., Rossi I., Andreadi A., Galli A., Coppola A., Cazzola M., et al. Long-Term Observational Study on the Impact of GLP-1R Agonists on Lung Function in Diabetic Patients. Respir. Med. 2019;154:86–92. doi: 10.1016/j.rmed.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 121.Kudaravalli J., Vijayalakshmi G., Kiran Kishore K. Safety and Efficacy of Sulfonylurea Drugs in Type 2 Diabetes Mellitus. Apollo Med. 2013;10:165–168. doi: 10.1016/j.apme.2013.05.002. [DOI] [Google Scholar]
- 122.Hougen I., Whitlock R.H., Komenda P., Rigatto C., Clemens K.K., Tangri N. Safety of Add-on Sulfonylurea Therapy in Patients with Type 2 Diabetes Using Metformin: A Population-Based Real-World Study. BMJ Open Diabetes Res. Care. 2021;9:e002352. doi: 10.1136/bmjdrc-2021-002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Douros A., Dell’Aniello S., Yu O.H.Y., Filion K.B., Azoulay L., Suissa S. Sulfonylureas as Second Line Drugs in Type 2 Diabetes and the Risk of Cardiovascular and Hypoglycaemic Events: Population Based Cohort Study. BMJ. 2018;362:k2693. doi: 10.1136/bmj.k2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rayner L.H., Mcgovern A., Sherlock J., Gatenby P., Correa A., Creagh-Brown B., deLusignan S. The Impact of Therapy on the Risk of Asthma in Type 2 Diabetes. Clin. Respir. J. 2019;13:299–305. doi: 10.1111/crj.13011. [DOI] [PubMed] [Google Scholar]
- 125.GRADE Study Research Group. Nathan D.M., Lachin J.M., Balasubramanyam A., Burch H.B., Buse J.B., Butera N.M., Cohen R.M., Crandall J.P., Kahn S.E., et al. Glycemia Reduction in Type 2 Diabetes—Glycemic Outcomes. N. Engl. J. Med. 2022;387:1063–1074. doi: 10.1056/NEJMoa2200433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee Y.-S., Jun H.-S. Anti-Diabetic Actions of Glucagon-like Peptide-1 on Pancreatic Beta-Cells. Metabolism. 2014;63:9–19. doi: 10.1016/j.metabol.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 127.Lyu X., Zhu X., Zhao B., Du L., Chen D., Wang C., Liu G., Ran X. Effects of Dipeptidyl Peptidase-4 Inhibitors on Beta-Cell Function and Insulin Resistance in Type 2 Diabetes: Meta-Analysis of Randomized Controlled Trials. Sci. Rep. 2017;7:44865. doi: 10.1038/srep44865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun J., Chu S., Lu M., Pan Q., Li D., Zheng S., Ma L. The Roles of Dipeptidyl Peptidase-4 and Its Inhibitors in the Regulation of Airway Epithelial-Mesenchymal Transition. Exp. Lung Res. 2020;46:163–173. doi: 10.1080/01902148.2020.1753853. [DOI] [PubMed] [Google Scholar]
- 129.Ma L., Chang E., Ruan X., Zhang B., Tang F., Zhang J. The Protective Effects of Omarigliptin against Lipopolysaccharide (LPS)- Induced Inflammatory Response and Expression of Mucin 5AC (MUC5AC) in Human Bronchial Epithelial Cells. Mol. Immunol. 2022;141:108–115. doi: 10.1016/j.molimm.2021.11.013. [DOI] [PubMed] [Google Scholar]
- 130.Colice G., Price D., Gerhardsson de Verdier M., Rabon-Stith K., Ambrose C., Cappell K., Irwin D.E., Juneau P., Vlahiotis A. The Effect of DPP-4 Inhibitors on Asthma Control: An Administrative Database Study to Evaluate a Potential Pathophysiological Relationship. Pragmat. Obs. Res. 2017;8:231–240. doi: 10.2147/POR.S144018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang A., Tang H., Zhang N., Feng X. Association between Novel Glucose-Lowering Drugs and Risk of Asthma: A Network Meta-Analysis of Cardiorenal Outcome Trials. Diabetes Res. Clin. Pract. 2022;183:109080. doi: 10.1016/j.diabres.2021.109080. [DOI] [PubMed] [Google Scholar]
- 132.Story of Discovery: SGLT2 Inhibitors: Harnessing the Kidneys to Help Treat Diabetes. [(accessed on 20 December 2023)]; Available online: https://www.niddk.nih.gov/news/archive/2016/story-discovery-sglt2-inhibitors-harnessing-kidneys-help-treat-diabetes.
- 133.Heerspink H.J.L., Perco P., Mulder S., Leierer J., Hansen M.K., Heinzel A., Mayer G. Canagliflozin Reduces Inflammation and Fibrosis Biomarkers: A Potential Mechanism of Action for Beneficial Effects of SGLT2 Inhibitors in Diabetic Kidney Disease. Diabetologia. 2019;62:1154–1166. doi: 10.1007/s00125-019-4859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Qiu M., Ding L.-L., Zhan Z.-L., Liu S.-Y. Use of SGLT2 Inhibitors and Occurrence of Noninfectious Respiratory Disorders: A Meta-Analysis of Large Randomized Trials of SGLT2 Inhibitors. Endocrine. 2021;73:31–36. doi: 10.1007/s12020-021-02644-x. [DOI] [PubMed] [Google Scholar]
- 135.Boden G., Cheung P., Mozzoli M., Fried S.K. Effect of Thiazolidinediones on Glucose and Fatty Acid Metabolism in Patients with Type 2 Diabetes. Metabolism. 2003;52:753–759. doi: 10.1016/S0026-0495(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 136.Soccio R.E., Chen E.R., Lazar M.A. Thiazolidinediones and the Promise of Insulin Sensitization in Type 2 Diabetes. Cell Metab. 2014;20:573–591. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhu M., Flynt L., Ghosh S., Mellema M., Banerjee A., Williams E., Panettieri R.A., Jr., Shore S.A. Anti-Inflammatory Effects of Thiazolidinediones in Human Airway Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2011;45:111–119. doi: 10.1165/rcmb.2009-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen R., Yan J., Liu P., Wang Z. Effects of Thiazolidinedione Therapy on Inflammatory Markers of Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE. 2015;10:e0123703. doi: 10.1371/journal.pone.0123703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rinne S.T., Feemster L.C., Collins B.F., Au D.H., Perkins M., Bryson C.L., O’Riordan T.G., Liu C.-F. Thiazolidinediones and the Risk of Asthma Exacerbation among Patients with Diabetes: A Cohort Study. Allergy Asthma Clin. Immunol. 2014;10:34. doi: 10.1186/1710-1492-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sood A., Qualls C., Murata A., Kroth P.J., Mao J., Schade D.S., Murata G. Potential for Repurposing Oral Hypertension/diabetes Drugs to Decrease Asthma Risk in Obesity. J. Asthma. 2023;60:802–810. doi: 10.1080/02770903.2022.2097919. [DOI] [PubMed] [Google Scholar]
- 141.Dixon A.E., Subramanian M., DeSarno M., Black K., Lane L., Holguin F. A Pilot Randomized Controlled Trial of Pioglitazone for the Treatment of Poorly Controlled Asthma in Obesity. Respir. Res. 2015;16:143. doi: 10.1186/s12931-015-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kaler M., Barochia A.V., Weir N.A., Cuento R.A., Stylianou M., Roth M.J., Filie A.C., Vaughey E.C., Nathan S.D., Levine S.J. A Randomized, Placebo-Controlled, Double-Blinded, Crossover Trial of Pioglitazone for Severe Asthma. J. Allergy Clin. Immunol. 2017;140:1716–1718. doi: 10.1016/j.jaci.2017.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request.