Abstract
E2F activity is regulated in part by the retinoblastoma family of tumor suppressor proteins. Viral oncoproteins, such as simian virus 40 (SV40) large-T antigen (TAg), adenovirus E1A, and human papillomavirus E7, can disrupt the regulation of cellular proliferation by binding to pRb family members and dissociating E2F-pRb family protein complexes. BK virus (BKV), which infects a large percentage of the human population and has been associated with a variety of human tumors, encodes a TAg homologous to SV40 TAg. It has been shown that BKV TAg, when expressed at low levels, does not detectably bind to pRb family members, yet it induces a serum-independent phenotype and causes a decrease in the overall levels of pRb family proteins. The experiments presented in this report show that, despite the lack of TAg-pRb interactions, BKV TAg can induce transcriptionally active E2F and that this induction does in fact require an intact pRb-binding domain as well as an intact J domain. In addition, E2F-pRb family member complexes can be detected in both BKV and SV40 TAg-expressing cells. These results suggest the presence of alternate cellular mechanisms for the release of E2F in addition to the well-established model for TAg-pRb interactions. These results also emphasize a role for BKV TAg in the deregulation of cellular proliferation, which may ultimately contribute to neoplasia.
Cell cycle progression is a tightly controlled process that involves the interactions of a complex network of proteins, including the members of the retinoblastoma family of tumor suppressor proteins and the E2F family of transcription factors. The retinoblastoma family includes the retinoblastoma susceptibility protein, pRb, and the related proteins p107 and p130. Overexpression of any of these three proteins can induce growth arrest, implicating this family of proteins as negative regulators of cell growth (32, 70, 92, 96, 99). Overexpression of E2F can induce quiescent cells to enter the S phase, implicating this family of proteins as positive regulators of cell growth (48). Further experiments have shown that ectopic expression of E2F in cells arrested by overexpression of pRb is sufficient to relieve the growth arrest phenotype (78, 99). In addition, E2F-pRb complexes have been shown to bind to and repress E2F-responsive promoters (see reference 94 for a review). The cell must therefore maintain a delicate balance between the active forms of these and other cell cycle regulators in order to maintain proliferative control. When pRb, E2F, and other key regulatory proteins are mutated or when their expression is altered, regulation is lost and cell proliferation can proceed unchecked. Evidence supporting the importance of these proteins in maintaining cell growth control comes from the discovery that many human cancers in addition to retinoblastoma have inactivating mutations in the retinoblastoma susceptibility gene, RB1, or in other genes whose products are involved in the pRb pathway (36, 43, 44, 56, 57, 59, 91).
The current model for pRb regulation of the G1-to-S-phase transition dictates that the hypophosphorylated form of pRb is the active, growth-suppressive form (10). Upon receiving a mitogenic or growth-stimulatory signal, active cyclin–cyclin-dependent kinase (CDK) complexes form within the cell and phosphorylate pRb in early to mid-G1 (see reference 93 for a review). This hyperphosphorylated form of pRb lacks growth-suppressive abilities, and the cell is set to progress through the cell cycle. This generalized model of regulation of pRb by cyclin-CDK complexes also applies to the other two members of the retinoblastoma family, p107 and p130 (5, 25, 28, 35, 45, 60, 64, 97).
The E2F transcription factors have been shown to be targets of pRb family member regulation (2, 3, 10, 39, 40, 81). These transcription factors are heterodimeric complexes of two families of proteins, E2F and DP. These complexes regulate the transcription of a variety of genes involved in cell cycle progression and DNA synthesis (18; see references 55, 71, and 84 for reviews). Five members of the E2F family have been identified (E2F1 to E2F5), and each member preferentially associates with a retinoblastoma family member in a cell cycle-dependent manner: pRb with E2F1 to E2F3, p107 with E2F4, and p130 with E2F4 and E2F5 (6, 22, 30, 41, 58, 93, 94). Additionally, it appears that specific E2F-pRb family complexes are active at various points of the cell cycle (12). The hypophosphorylated forms of pRb family members associate with E2F and block transcriptional activation. Upon phosphorylation of pRb, p107, and p130 by cyclin-CDK complexes, E2F is released and is free to activate cellular growth and proliferation (5, 13, 65, 89, 97, 99). It is through these multiple points of regulation that the cell is able to maintain very tight control over its own proliferation.
A great deal of our understanding of the role of the pRb pathway in cell cycle control has come from the use of DNA tumor viruses as molecular tools. Viruses such as polyomaviruses, human papillomaviruses (HPV), and adenoviruses require the cellular DNA replication machinery for replication of their own viral genomes (23). They have evolved mechanisms to subvert normal cellular growth control and induce the cell to enter S phase, during which the cellular replication machinery is readily available. It has been shown that one of the ways in which these viruses undermine cellular control of G1-to-S-phase progression is by encoding oncoproteins that bind to pRb family proteins. Simian virus 40 (SV40) large-T antigen (TAg), adenovirus E1A, and HPV E7 proteins all have been shown to bind to pRb family members. The pRb-binding domain is highly conserved among all three proteins and contains an LXCXE motif required for binding (9, 14, 17, 21, 26, 68, 96). This binding domain also has been shown to be required for viral transformation of the cell (4, 11, 24, 51, 60, 95). SV40 TAg binds to the hypophosphorylated, or active, form of pRb, causing the release of transcriptionally active E2F independent of the phase of the cell cycle or the presence of external mitogenic signals (9, 17, 62).
An additional domain of TAg that is highly conserved and required for transformation is the J domain, which includes the hexapeptide HPDKGG (amino acids 42 to 47) (74). This region of TAg shows extensive homology to the DnaJ family of molecular chaperone proteins (53, 83). In fact, recent evidence has shown that the large-T/small-t common region of SV40 TAg as well as the homologous family member BK virus (BKV) and JC virus TAgs can functionally substitute for the J domain of the Escherichia coli DnaJ protein (52). The J domain of TAg has been the focus of much interest recently and is now known to be required for multiple functions of TAg, including efficient viral replication, specific interaction with the hsp70 family member hsc70, and transformation (8, 79, 86). It has also been demonstrated that this domain of SV40 TAg is required for its ability to induce the turnover of p107 and p130 (87). Through its interactions with hsp family proteins and its function as a molecular chaperone, the J domain of TAg may affect the stability of pRb family proteins as well as the protein-protein complex formation required for efficient transformation by SV40.
We are interested in the human polyomavirus BKV. BKV infects at least 70 to 80% of the human population, establishing a persistent infection in the kidneys (19, 29, 72). The virus is thought to remain in a latent state, but reactivation can occur in immunocompromised patients, resulting in hemorrhagic cystitis (1). BKV transforms rodent cells both in vitro and in vivo (7, 16, 75, 85) and has been shown to transform human embryonic kidney cells in the presence of an activated ras oncogene (73, 76). Moreover, BKV DNA has been associated with a variety of human tumors, including brain, pancreatic islet, urinary tract, and Kaposi’s sarcoma (15, 66, 67, 90). Although a causative role in cancer has not been demonstrated, the widespread distribution of this virus in the human population, its association with human cancers, and its potent transforming ability in rodent cells have implicated a potential role for this virus as a cofactor in oncogenesis.
Given what is known about the interactions of SV40 TAg with tumor suppressor proteins and other known cell cycle regulators, we chose to examine the effects of BKV TAg on cell cycle regulatory proteins in order to further understand what effect this virus may have on the cell. In previous work, we demonstrated that BKV TAg has the ability to bind to members of the retinoblastoma family of tumor suppressor proteins both in vivo and in vitro (38). However, the levels of BKV TAg produced from viral promoter-enhancer elements were too low to bind to a significant amount of these tumor suppressor proteins in the cell. Of particular interest were the discoveries that these low levels of BKV TAg were sufficient to induce a reduction in the amounts of pRb, p107, and p130 and that the remaining proteins were predominantly in the hypophosphorylated forms. We have shown an equivalent effect of SV40 TAg on all three pRb family members, and others have also shown the same effect of SV40 TAg on p107 and p130 (87, 88) and HPV E7 on all three proteins (49). We also demonstrated that low levels of BKV TAg were sufficient to induce a serum- independent, semitransformed phenotype. In order to further understand the effects of BKV TAg on cell cycle regulation, we wished to examine the downstream targets of the pRb pathway, specifically E2F family members. In this report, we present data demonstrating that there is an increase in the levels of free, transcriptionally active E2F in cells despite the absence of detectable BKV TAg complexes with pRb, p107, or p130 and despite the presence of the hypophosphorylated forms of these proteins. Using BKV TAgs containing mutations in the pRb-binding or J domain, we show that this induction of E2F by BKV TAg requires both intact pRb-binding and intact J domains. This finding implies that BKV TAg can induce E2F activity via a mechanism that is dependent on TAg-pRb family interactions but independent of stable binding. In addition, we found that in the presence of both BKV TAg and SV40 TAg, E2F-pRb and E2F-p107 complexes can be readily detected. This result, along with the decrease in pRb family protein levels presumably mediated by the J domain, implies that even for SV40 TAg additional mechanisms may account for the increase in free E2F levels. Taken together, these results suggest the possibility of alternate roles for the pRb-binding domain of TAg.
MATERIALS AND METHODS
Cell cultures.
BSC-1 cells (American Type Culture Collection) are African green monkey kidney cells. BSC-BKT cells are BSC-1 cells stably transfected with the early region of BKV (Dun), encoding TAg and small-t antigen (38). COS-1 cells are African green monkey kidney cells expressing the early region of SV40 (31). 293 cells are human embryonic kidney cells expressing adenovirus early proteins E1A and E1B (34). PTP, BKT, E109K, and H42Q cells are BSC-1 cells stably transfected with an empty vector (PTP) or the BKV TAg expression constructs described below. BSC-tet cells are BSC-1 cells stably expressing the tetracycline transcriptional activator plasmid pUHD15-1 (33). All of these cells were maintained in Dulbecco’s modified Eagle’s medium (GIBCO) supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10% fetal bovine serum at 37°C in a 5% CO2 incubator. C33A cells (human cervical carcinoma; American Type Culture Collection) were grown in Eagle’s minimal essential medium (BioWhittaker) supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10% fetal bovine serum at 37°C in a 5% CO2 incubator.
Plasmids.
pBK-GEM contains the BKV early region in pGEM3zf− (38). PTP2000 is a retrovirus-based vector used to make the following constructs. pBKTPTP contains a BKV TAg cDNA which was cloned by PCR to delete the intron from the genomic clone. pE109KPTP contains a glutamic acid-to-lysine point mutation at amino acid 109, and pH42QPTP contains a histidine-to-glutamine point mutation at amino acid 42. Both mutants were cloned by PCR-directed mutagenesis and confirmed by DNA sequencing. Wild-type BKV TAg, the pRb-binding domain mutant, and the J domain mutant were cloned into pUHD10-3, which is the response plasmid for the tetracycline transactivator system, to generate pBKT-tet, pE109K-tet, and pH42Q-tet, respectively (33). pE2WTx4CAT, encoding the chloramphenicol acetyltransferase (CAT) reporter gene driven by an E2 core promoter and four copies of the E2F enhancer, was kindly provided by J. Nevins; we refer to this plasmid as pE2F-CAT. pΔE2F-CAT contains an XbaI-BglII deletion in plasmid pE2F-CAT which removes all four copies of the E2F enhancer. pE1AWT, which encodes the adenovirus E1A gene, was a kind gift from E. Moran (69). pAdCMVβ, which encodes the E. coli lacZ gene under the control of the cytomegalovirus promoter, was a kind gift from J. Chamberlain.
Antibodies.
The antibodies used were C-15 (anti-pRb polyclonal antibody) (Santa Cruz); C-18 (anti-p107 polyclonal antibody) (Santa Cruz); SD6, SD9, and SD15 (anti-p107 monoclonal antibodies) (gifts from N. Dyson); KH95 (anti-E2F1 monoclonal antibody) (Santa Cruz); C-20 (anti-E2F4 polyclonal antibody) (Santa Cruz); and PAb430 and PAb416 (anti-TAg monoclonal antibodies) (37).
E2F gel shift assay.
Whole-cell extracts were prepared as described previously (46) but with the following modifications. Subconfluent cells on 10-cm2 dishes were washed twice in phosphate-buffered saline, scraped into 1.5 ml of phosphate-buffered saline, microcentrifuged for 30 s at 4°C, and then resuspended in eight packed-cell volumes of lysis buffer (50 mM HEPES [pH 7.9]; 250 mM KCl; 0.1 mM EGTA; 0.1 mM EDTA; 0.1% Nonidet P-40 [NP-40]; 10% glycerol; 0.4 mM NaF; 0.4 mM Na3VO4; 1 μg each of aprotinin, leupeptin, and pepstatin per ml; 0.5 mM phenylmethylsulfonyl fluoride) on ice for 30 min. Lysates were then centrifuged at 100,000 × g for 20 min at 4°C. Supernatants were divided into aliquots and stored at −80°C. E2F DNA binding assays were performed as described previously (47) but with the following modifications. Reaction mixtures contained 3 μg of whole-cell extract in 15 μl of probe mix (10 mM HEPES, 20 mM KCl, 3 mM MgCl2, 0.5 mM EGTA, 30 μg of bovine serum albumin, 2 μg of sonicated salmon sperm DNA, 1.7% Ficoll, 1.3 mM dithiothreitol) and 0.6 ng of 32P-labeled probe. The probe was an EcoRI-HindIII fragment of the adenovirus E2 promoter missing the ATF binding site (61). Reaction mixtures were incubated at room temperature for 20 min and resolved in a 5% polyacrylamide (39:1 ratio of acrylamide to bisacrylamide) gel containing 2.5% glycerol in 0.25× Tris-borate-EDTA for 3 h at 250 V and 4°C. The oligonucleotides used as cold competitors in DNA binding reactions have been described elsewhere (47). For supershift assays, extracts were preincubated in the presence of antibodies for 15 min at room temperature. Labeled probe was then added, and the reaction mixtures were incubated for 15 min at room temperature. For immunoprecipitation-release reactions, 50 μg of whole-cell extract was incubated with 10 μl of antibody in a total volume of 150 μl of IP buffer (20 mM HEPES [pH 7.9]; 40 mM KCl; 6 mM MgCl2; 1 mM EGTA; 1 mM dithiothreitol; 0.1% NP-40; 0.4 mM NaF; 0.4 mM Na3VO4; 1 μg each of aprotinin, leupeptin, and pepstatin per ml; 0.5 mM phenylmethylsulfonyl fluoride) (46) on ice for 1 h. A total of 125 μl of 3% protein A–Sepharose beads in IP buffer was then added, and the samples were incubated at 4°C with rocking for 90 min. Immunocomplexes bound to the beads were washed three times with IP buffer and released by the addition of sodium deoxycholate to 0.8%. After incubation for 15 min on ice, NP-40 was added to a final concentration of 1.2% and the samples were incubated for 15 min on ice. Samples were pulsed in a microcentrifuge, and 4 μl of supernatant was used in a DNA binding reaction as described above. Supernatant (12 μl) was used for immunoblotting as described below.
Immunoblotting.
Whole-cell extracts prepared for gel shift assays were resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) with 8% polyacrylamide (54). Immunoblotting was performed as described previously (38).
Glutathione S-transferase (GST) fusion protein binding assays.
Lysates were prepared from BSC-tet cells transiently transfected with pBKT-tet, pE109K-tet, and pH42Q-tet, and binding assays were performed as previously described (38).
CAT assays.
BSC-1, BSC-BKT, and COS-1 cells were plated at 50,000 cells per well in a six-well plate. PTP, BKT, E109K, and H42Q cells were plated at 80,000 cells per well in a six-well plate. After 48 h, cells were transfected with 3 μg of pE2F-CAT or 3 μg of pΔE2F-CAT and 1 μg of pAdCMVβ. Lysates were harvested after 48 h, and 50 μl was used for CAT assays as described previously (63). β-Galactosidase assays were also performed as described previously (63), and transfection efficiencies were used to normalize CAT activity.
Growth curves.
The growth of cells in 0.1% serum-containing media was assayed as described previously (38).
RESULTS
In previous work, we demonstrated that BKV TAg, although unable to bind to detectable amounts of pRb, p107, or p130 due to the low levels of TAg protein present in the cell, was able to induce a serum-independent phenotype and to cause both a decrease in the amounts and a change in the steady-state phosphorylation status of the pRb family proteins. These results suggested that BKV TAg disrupted normal cell cycle regulation, possibly through an effect on the pRb family proteins. In order to determine if this was in fact the case, we chose to look at the effects of BKV TAg on the E2F family of transcription factors as downstream targets of the pRb pathway.
BKV TAg induces free E2F.
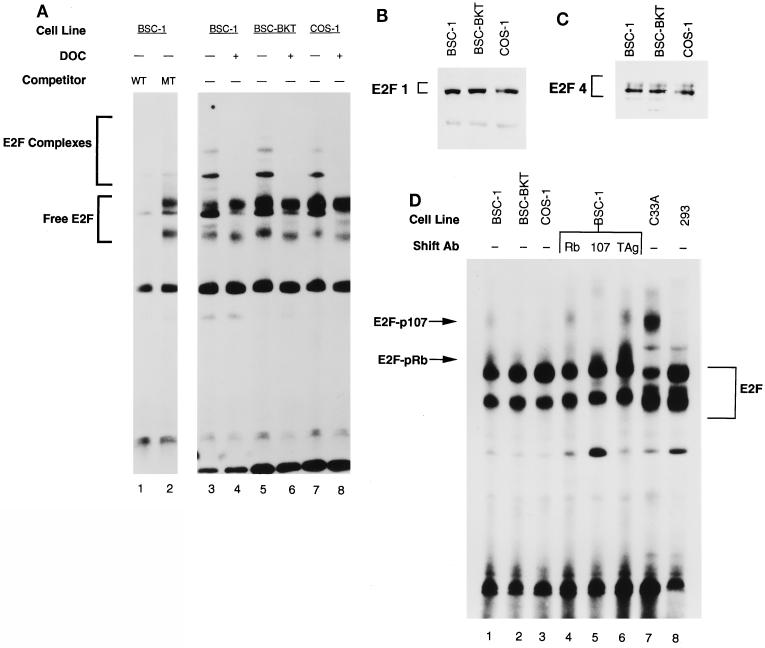
In order to examine the levels of free E2F and E2F-pRb family complexes in BKV TAg-expressing cells, DNA band shift assays were performed with whole-cell extracts from BSC-1 (no TAg), BSC-BKT (BKV TAg), and COS-1 (SV40 TAg) cells. We used cold wild-type or mutant oligonucleotide competitors in order to demonstrate the specificity of the DNA binding reactions (Fig. 1A, lanes 1 and 2). DNA binding reactions were performed in the presence and absence of deoxycholate in order to determine the mobility of the free E2F bound to the probe (Fig. 1A, lanes 4, 6, and 8). The results of this experiment demonstrated that there is more free E2F in the presence of BKV TAg or SV40 TAg than in the BSC-1 parental cell line (Fig. 1A, compare lanes 3, 5, and 7). Supershift experiments with antibodies against E2F indicated that E2F4 is the major family member whose levels were increased in the presence of TAg (data not shown). Quantitation of the free E2F in four separate experiments showed a reproducible 1.6-fold increase in free E2F levels in the presence of BKV TAg and a 1.8-fold increase in the presence of SV40 TAg. In previous work, we showed that there is 50 to 100 times as much SV40 TAg in COS-1 cells as there is BKV TAg in BSC-BKT cells (38). These results indicated that despite the difference in TAg levels, BKV TAg, like SV40 TAg, has the ability to induce free E2F.
FIG. 1.
Increased free E2F in the presence of BKV TAg. (A) Whole-cell extracts were prepared from subconfluent BSC-1, BSC-BKT, and COS-1 cells. Extracts were incubated with a labeled E2F probe for 30 min at room temperature and resolved by native 5% PAGE. Competition reactions were performed in the presence of a 150-fold excess of cold wild-type (WT) competitor (lane 1) or mutant (MT) competitor (lane 2). For lanes 4, 6, and 8, extracts were preincubated with 0.8% deoxycholate (DOC) for 15 min on ice, followed by 1.2% NP-40 for 15 min on ice, and then used for DNA binding reactions. Free E2F, E2F bound to the DNA probe. E2F Complexes, E2F-pRb family member complexes bound to the DNA probe. (B and C) Whole-cell extracts (25 μg) were separated by SDS–10% PAGE, transferred to nitrocellulose, and probed with KH95 (anti-E2F1; B) or C-20 (anti-E2F4; C). (D) Whole-cell extracts were prepared, and equivalent amounts of protein from each cell line were used in a DNA binding reaction as described for panel A. For supershift experiments (lanes 4 to 6), extracts from BSC-1 cells were preincubated for 15 min at room temperature with C-15 (anti-pRb), a mixture of SD6, SD9, and SD15 (anti-p107), or PAb430 (anti-TAg) as a negative control before the addition of the DNA probe. Ab, antibody.
The absence of BKV TAg-pRb family member complexes in the cell, combined with the ability of BKV TAg to induce free E2F, suggested that BKV TAg must affect the pRb pathway either by inactivating pRb, p107, and p130 through a mechanism other than stable binding or by directly inducing E2F synthesis. In order to determine if TAg has a direct effect on E2F levels, we used immunoblotting to assay the levels of E2F1 and E2F4 in BSC-1, BSC-BKT, and COS-1 cells. E2F1 and E2F4 were chosen as representative members of the E2F family because E2F4 was the predominant family member whose levels were increased in our DNA band shift assays. For these experiments, we used the same extracts as those used for the DNA band shift experiments in which we saw an increase in the overall levels of free E2F-DNA complexes. The results in Fig. 1B and C show that there was no detectable increase in the steady-state levels of E2F1 and E2F4 in the presence of BKV TAg or SV40 TAg. Therefore, BKV TAg must effectively release E2F from pRb family member complexes or complexes with other, as-yet-unknown proteins.
E2F-pRb and E2F-p107 complexes remain in BKV and SV40 TAg-expressing cells.
In order to look at the status of the remaining E2F complexes, we used DNA band shift assays to examine E2F complexes with pRb and p107 in TAg-expressing cell lines (Fig. 1D). To identify pRb-and p107-specific complexes in BSC-1 cells, which should contain both types of complexes, we used specific antibodies to supershift the complexes (Fig. 1D, lanes 4 to 6). The identities of the E2F-pRb, E2F-p107, and E2F-p130 complexes were confirmed with extracts prepared from serum-starved and serum-stimulated cells (data not shown). Extracts from C33A cells, which lack functional pRb (80), were also used to help verify the mobilities of the E2F-pRb complexes (Fig. 1D, lane 7). Having established the mobilities of the E2F complexes, we were then able to assay TAg-expressing cells for the presence of E2F-pRb and E2F-p107 complexes. Increased free E2F levels in the presence of BKV TAg were again detected (Fig. 1D, compare lanes 1 and 2). When we compared the band shift reactions for the BSC-1, BSC-BKT, and COS-1 cells, it appeared that both the pRb and the p107 complexes, but particularly the pRb complex, were still present in TAg-expressing cells. This was a surprising finding, given that SV40 TAg binding to pRb is thought to be mutually exclusive to E2F binding to pRb (9, 17, 21, 26). However, we found that not all of the hypophosphorylated pRb was bound to SV40 TAg in COS-1 cells (38). This finding is in agreement with the results of Ludlow et al., who found that 78% of the hypophosphorylated pRb was bound to SV40 TAg in stably transfected monkey cells (62). It is possible that the remaining hypophosphorylated pRb was seen in a complex with E2F in these band shift experiments. Interestingly, 293 cells, which express adenovirus E1A proteins, did not have significant amounts of E2F-pRb or E2F-p107 complexes (Fig. 1D, lane 8), suggesting that E1A is more effective at disrupting pRb family complexes with E2F.
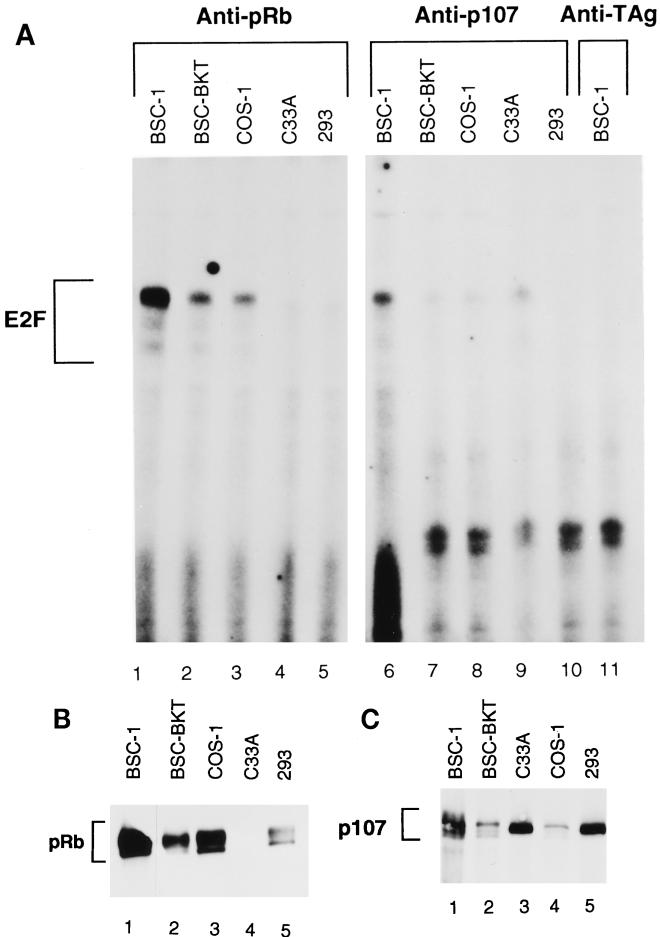
As an independent means of determining if E2F complexes with pRb or p107 were present in TAg-expressing cells, anti-pRb or anti-p107 immunoprecipitations were performed on the extracts, the immunoprecipitates were treated with deoxycholate, and the released proteins were tested in DNA binding reactions (Fig. 2A). An aliquot of each sample was also used for immunoblotting to confirm the effectiveness of the immunoprecipitations (Fig. 2B and C). Although more E2F was complexed with pRb in the BSC-1 cells, E2F complexes with pRb were clearly present in both the BSC-BKT and the COS-1 cells. In 293 cells, no E2F coimmunoprecipitated with pRb, again suggesting that E1A may be more effective at disrupting these complexes. C33A cells also showed no pRb-E2F complexes, as expected, due to the absence of functional pRb in these cells. There appeared to be much less E2F in a complex with p107 than with pRb in all of the cell extracts. Just as we found with pRb, some E2F-p107 complexes were found in BKV and SV40 TAg-expressing cell lines, whereas 293 cells lacked such complexes.
FIG. 2.
Presence of E2F-pRb and E2F-p107 complexes in TAg-expressing cells. (A) Total protein (50 μg) from whole-cell extracts was immunoprecipitated with C-15 (anti-pRb; lanes 1 to 5), C-18 (anti-p107; lanes 6 to 10), or PAb430 (anti-TAg; lane 11). Immunoprecipitates were treated with deoxycholate to disrupt protein-protein complexes, and 4 μl of supernatant was assayed for DNA binding activity under the same conditions as those described in the legend to Fig. 1. (B and C) Western blot analyses of immunoprecipitates used in panel A. Supernatant (12 μl) was separated by SDS–8% PAGE, transferred to nitrocellulose, and probed with C-15 (B) or C-18 (C). Note that the order of COS-1 and C33A cells is reversed in the two blots.
Figure 2B and C show the immunoblots for pRb and p107. From a comparison of lane 1 to lanes 2 and 3 of the anti-pRb blot in Fig. 2B and C, it is clear that less pRb was present in the BSC-BKT and COS-1 cells than in the control BSC-1 cells, confirming our previous results (38). The same was true for p107 when lanes 1, 2, and 4 of the anti-p107 blot in Fig. 2B and C were compared. 293 cells had significantly less pRb and p107, although the matching parental cell line would be required to make an absolute comparison. In both the pRb and the p107 immunoprecipitations, less than 1% remained in the supernatant, indicating that we were in fact immunoprecipitating all of the specific protein present in the cell and that E2F-containing complexes would not have been missed (data not shown).
Status of E2F in mutant BKV TAg-expressing cell lines.
The DNA band shift assays demonstrated that BKV TAg had the ability to induce free E2F and that some E2F-pRb and E2F-p107 complexes remained in both the BSC-BKT and the COS-1 cells despite the presence of either BKV or SV40 TAg. The increase in free E2F levels in the presence of SV40 TAg can be easily explained by virtue of the ability of SV40 TAg to bind to pRb, p107, and p130. However, our previous results showed that pRb family proteins in BSC-BKT cells are not bound to TAg and furthermore that BKV TAg causes an overall reduction in pRb, p107, and p130 levels, with only the hypophosphorylated, or growth-suppressive, forms of the proteins remaining. This puzzling contradiction suggested the possibility of alternate mechanisms for the increase in free E2F levels and led us to ask which domains of BKV TAg were involved.
Since our results indicated that the induction of free E2F by BKV TAg might be independent of interactions with pRb family members, we mutated the pRb-binding domain, expecting to confirm that these interactions were in fact not required. The E109K mutant in the pRb-binding domain of SV40 TAg has been shown to be defective for TAg-pRb family interactions (17). Based on recent data suggesting the importance of the J domain in SV40 TAg function, we chose to mutate the J domain of BKV TAg as well. Indeed, it has been suggested that the J domain of SV40 TAg is involved in the effect of SV40 TAg on p107 and p130 levels and phosphorylation states (8, 87). The H42Q mutation in SV40 TAg has been shown to functionally inactivate J domain function, as assayed by the ability of this domain of TAg to complement E. coli DnaJ activity, to interact with the hsp70 family member hsc70, and to aid in viral replication (8, 52).
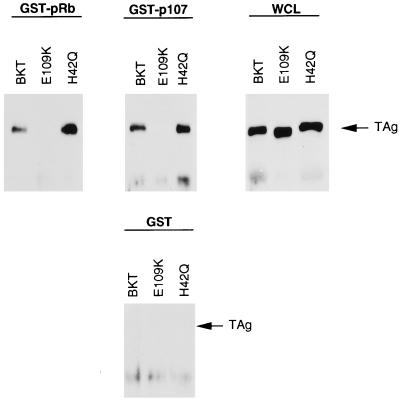
For these experiments, the wild-type BKV TAg gene was subcloned as a cDNA, and the mutations were then introduced into the cDNA construct by PCR-based mutagenesis. To assay first for the pRb- and p107-binding ability of the mutant TAgs, in vitro binding experiments were performed with purified GST-pRb and GST-p107 fusion proteins (Fig. 3) (25, 50, 77). Using equivalent amounts of lysates from BSC-tet cells transiently transfected with pBKT-tet, pE109K-tet, or pH42Q-tet, we were able to detect complex formation between BKV TAg and GST-pRb or GST-p107 in extracts from cells expressing both wild-type BKV TAg and the J domain mutant but not the pRb-binding domain mutant. None of the constructs interacted with GST alone, and TAg protein expression levels for all three TAgs were equivalent.
FIG. 3.
In vitro complex formation between mutant BKV TAgs and proteins pRb and p107. Whole cell-lysate (150 μg) from BSC-tet cells transiently transfected with pBKT-tet, pE109K-tet, and pH42Q-tet were incubated with equivalent amounts of each purified GST-Sepharose-bound fusion protein. Bound complexes were released and separated by SDS-PAGE, transferred to nitrocellulose, and probed with PAb416. For whole-cell lysate samples (WCL), 50 μg of lysate from each cell line was used.
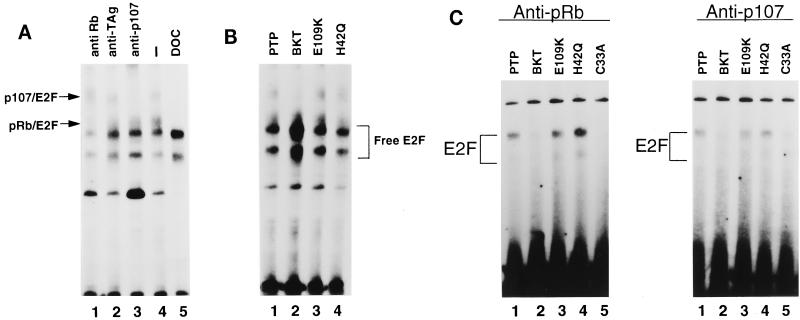
We also used cDNA constructs to stably transfect BSC-1 cells, and the resulting stable cell lines were named as follows: PTP (empty vector), BKT (wild-type BKV TAg), E109K (pRb-binding domain mutant), and H42Q (J domain mutant). Using whole-cell extracts from the cell lines, we performed DNA band shift analysis to determine the status of E2F in the cells. Specific antibodies were used to supershift the complexes in the PTP cells, which do not express any TAg, in order to confirm the identities of the E2F-pRb and E2F-p107 complexes (Fig. 4A). The mobilities of the E2F-pRb and E2F-p107 complexes were identical to those seen for the BSC-1 parental cells in Fig. 1D. A DNA binding reaction was also performed in the presence of deoxycholate in order to distinguish free E2F from the E2F-pRb complexes (Fig. 4A, lane 5).
FIG. 4.
E2F complexes in cell lines expressing mutant TAgs. (A) Supershift experiments were performed on PTP cells as described in the legend to Fig. 1D. For lane 5, PTP cell extract was preincubated with deoxycholate (DOC) and NP-40 as described in the legend to Fig. 1A. (B) Whole-cell extracts were prepared, and equivalent amounts of protein were used in DNA binding reactions as described in the legend to Fig. 1A. (C) Immunoprecipitations followed by deoxycholate reactions were performed as described in the legend to Fig. 2A, except that for anti-p107 immunoprecipitates, a cocktail of monoclonal antibodies SD6, SD9, and SD15 was used.
Having established the mobilities of the E2F complexes in PTP cells, we used whole-cell extracts from all four cell lines to analyze the status of free E2F and E2F-pRb and E2F-p107 complexes in the presence of wild-type and mutant BKV TAgs (Fig. 4B). BKT cells showed an increase in free E2F, as was seen previously with BSC-BKT cells, confirming that the BKV TAg cDNA construct behaves the same as genomic BKV TAg in this assay. Quantitation of free E2F in three separate experiments showed a reproducible 1.9-fold increase in free E2F levels in the presence of wild-type BKV TAg. In the presence of the pRb-binding domain or J domain mutant TAg, however, there was no detectable increase in free E2F levels (1.1-fold for E109K cells and 0.82-fold for H42Q cells). In addition, in both mutant cell extracts, the E2F-pRb and E2F-p107 complexes remained present at levels comparable to those present in PTP cells. BKT cells, on the other hand, retained some E2F-pRb and E2F-p107 complexes, but their levels were reduced in comparison to those in PTP cells.
For confirmation of the remaining E2F-pRb and E2F-p107 complexes in the mutant TAg-expressing cell lines, anti-pRb or anti-p107 immunoprecipitations were performed on the extracts. The immunoprecipitates were treated with deoxycholate to release the protein complexes, and the released proteins were then tested in DNA band shift assays (Fig. 4C). Again, the overall abundance of E2F-p107 complexes was lower than that of E2F-pRb complexes. BKT cells had lower levels of E2F-pRb and E2F-p107 complexes than PTP cells. However, in agreement with the band shift results shown in Fig. 4B, both E109K and H42Q cells contained amounts of E2F-pRb and E2F-p107 complexes equivalent to those in PTP cells. Again, wild-type BKV TAg seemed to be capable of inducing free E2F despite the continued presence of some E2F-pRb and E2F-p107 complexes. However, the mutant TAgs did not seem to induce free E2F, and the mutant cell lines seemed to have levels of E2F-pRb and E2F-p107 complexes equivalent to those in cells lacking any TAg. Taken together, these results suggest that both the pRb-binding domain and the J domain are required for the induction of free E2F.
E2F induced by BKV TAg is transcriptionally active.
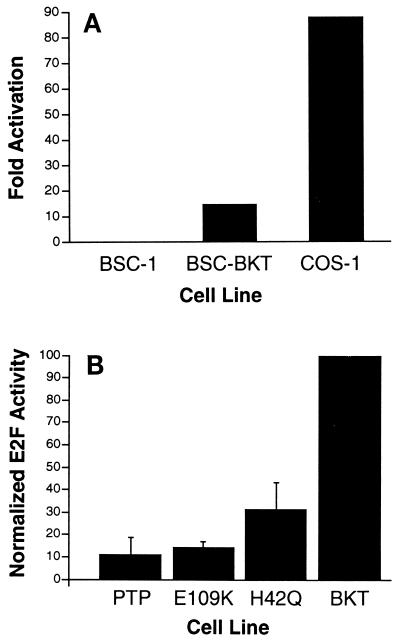
Having determined that BKV TAg is able to induce free E2F and that the pRb-binding and J domains are required for this function, we wanted to determine if this E2F is transcriptionally active. To assay for transcriptionally active E2F in the presence of TAg, we first transfected CAT reporter constructs into BSC-1, BSC-BKT, and COS-1 cells. We compared activity from a reporter construct containing four copies of the E2F enhancer linked to the CAT gene (pE2F-CAT) with activity from an isogenic construct lacking the E2F enhancer elements (pΔE2F-CAT). In BSC-BKT cells, CAT activity from the E2F binding site-linked CAT construct was 14-fold higher than activity from the construct lacking the E2F enhancer elements (Fig. 5A). No difference in CAT activity in the presence or absence of E2F binding sites was detected in BSC-1 cells. In COS-1 cells, there was an 87-fold activation of CAT activity from the pE2F-CAT construct as compared to the pΔE2F-CAT construct. These results demonstrated that despite the 100-fold difference in TAg levels, BKV TAg had only a sixfold reduced ability, as compared to SV40 TAg, to induce E2F activity in these cells. These results are in agreement with the DNA band shift assay results showing that BKV TAg could induce free E2F to a degree similar in magnitude to the induction of free E2F by SV40 TAg.
FIG. 5.
Increased transcriptionally active E2F in the presence of BKV TAg requires both the pRb-binding and the J domains. (A) Cells were transfected with 3 μg of pE2F-CAT or 3 μg of pΔE2F-CAT and 1 μg of pAdCMVβ. CAT assays were performed 48 h after transfection. Signals from the autoradiograms were quantitated with AMBIS Image Acquisition and Analysis software and normalized to the β-galactosidase activity for each extract. Fold activation, CAT activity from the pE2F-CAT construct divided by CAT activity from the pΔE2F-CAT construct. Data represent the results from three independent experiments. (B) CAT assays were performed as described in panel A. The fold activation of E2F in wild-type BKT cells after normalization to the background is 14.95. This level was set to 100%, and the activation from the mutant TAg-expressing cell lines is represented as a percentage of wild-type BKV TAg activity. Numbers represent averages from four separate experiments.
The pRb-binding domain and the J domain are both required for the induction of E2F activity.
CAT assays were also performed with wild-type and mutant TAg cDNA-expressing cell lines. Although we did not detect any increase in free E2F levels in the presence of pRb-binding domain or J domain mutant TAgs, it was possible that these TAgs induced an increase in transcriptionally active E2F levels which was not detectable by DNA band shift assays. Using the same constructs as those used above, we found that the levels of CAT activity with the pΔE2F-CAT construct were equivalent to background levels in all four cell lines (data not shown). With BKT cells, we found a 15-fold induction of E2F-dependent CAT activity over background activity, similar to the results for BSC-BKT cells. E2F activity induced by the pRb-binding domain mutant TAg was about 15% the activity induced by wild-type TAg (Fig. 5B). These results suggest that the pRb-binding domain is required for E2F induction by BKV TAg. This conclusion was surprising, given our previous data indicating that BKV TAg does not detectably bind to any of the pRb family members when present at low levels in the cell (38). The levels of BKV TAg in BKT cells were similar to those in BSC-BKT cells (data not shown).
We have shown that BKV TAg affects the levels and phosphorylation states of the pRb family proteins in the cell, leaving the remaining proteins predominantly in the hypophosphorylated forms (38). It is possible that this overall effect on the levels of pRb family proteins accounts for the increase in free E2F levels. H42Q cells, which express TAg lacking a functional J domain, retain approximately 30% wild-type E2F activity. This activity is similar to that of E109K cells and indicates the importance of the J domain in the induction of E2F. Interestingly, the J domain construct retains its ability to bind pRb family members and is significantly impaired in its induction of E2F, suggesting a role for other mechanisms in addition to direct pRb binding for the release of E2F.
The J domain is required for serum-independent growth.
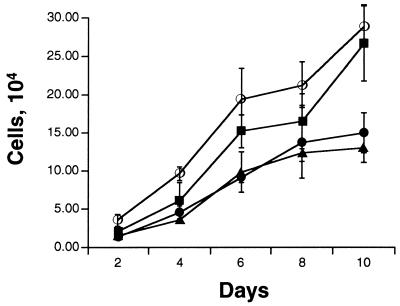
We previously showed that wild-type BKV TAg can induce serum-independent growth even at the low levels present in BSC-BKT cells (38). The mutant BKV TAg constructs were assayed for their ability to induce serum-independent growth in order to determine which domains are required for this phenotype. Equal numbers of PTP, BKT, E109K, and H42Q cells were seeded in medium containing 0.1% serum and counted every other day (Fig. 6). The wild-type BKV TAg-expressing cells grew in 0.1% serum, while PTP cells showed little growth. E109K cells grew significantly better in 0.1% serum than PTP cells, achieving almost the same level of growth as BKT cells. This result suggests that the pRb-binding domain is not absolutely required for serum-independent growth. Interestingly, H42Q cells were seriously impaired in their ability to grow in 0.1% serum and showed a growth curve similar to that of the empty vector PTP cells, suggesting that the J domain is involved in mediating serum-independent growth. All four cell lines grew equally well in 10% serum-containing media (data not shown). These results indicate that the induction of E2F can be genetically separated from growth in media with low serum concentrations, as E109K cells had very little E2F activity but grew in media with low serum concentrations.
FIG. 6.
The J domain but not the pRb-binding domain of BKV TAg is required for the induction of serum-independent growth. For each cell line, 2 × 104 cells were plated in medium containing 0.1% serum. Cells from duplicate wells were counted every other day. Values shown are averages from three independent experiments. Symbols: ○, BKT; ▪, E109K; •, PTP; ▴, H42Q.
DISCUSSION
This report addresses the mechanism of cell cycle deregulation by BKV TAg. In previous work, we demonstrated that at low levels of expression, BKV TAg did not bind to detectable amounts of pRb family proteins in the cell. However, BKV TAg induced a semitransformed phenotype, as has been shown (76), and caused a decrease in the overall levels of pRb, p107, and p130. In light of the effects of BKV TAg on these cell cycle regulatory proteins and the association of BKV DNA with a variety of human tumors, we thought it important to understand further the significance of this viral oncoprotein with respect to cellular proliferation.
Based on the serum independence phenotype, we wished to determine if BKV TAg was able to affect E2F activity. Our results showed that BKV TAg can induce transcriptionally active E2F. Since we obtained similar levels of induction with both genomic and cDNA clones, this activation of E2F is fully attributable to TAg. Furthermore, this induction is very similar in magnitude to that caused by SV40 TAg, despite 100-fold-lower levels of expression of BKV TAg. Although these results may indicate that the level of SV40 TAg is in vast excess with respect to its growth-stimulatory properties, these results also have unmasked a novel form of pRb protein family regulation. Specifically, these results suggest that BKV TAg, and probably SV40 TAg, may use a mechanism other than direct binding and dissociation of E2F-pRb complexes to cause an increase in free E2F levels. In an effort to characterize the domains of BKV TAg required for the induction of E2F, we used mutations in the pRb-binding and J domains to show that both of these domains are required for the induction of E2F activity.
Based on our previous results demonstrating that BKV TAg does not bind to a significant portion of pRb family proteins in the cell, we would have predicted that the induction of E2F activity occurs through a pRb-binding-independent mechanism. The data from the J domain mutant lend support to this prediction. This mutant TAg retains the ability to bind to pRb family proteins but is severely impaired in its induction of E2F activity. These data indicate that the stable binding of pRb family proteins alone is not sufficient for the induction of E2F or that stable binding may account for only a small portion of E2F induction. However, the data obtained here with the pRb-binding domain mutant suggest that the pRb-binding domain is required for E2F activation. These results pose an interesting puzzle as to how the pRb-binding domain is required to induce E2F, and several possibilities exist.
The first possibility is that BKV TAg is able to bind most of the pRb, p107, and p130 in the cell but that these interactions are transient in nature or outside of the limit of detection by immunoprecipitation and immunoblotting. Given our data that TAg is able to induce a decrease in the overall levels of pRb family proteins, it is possible that BKV TAg can bind to pRb, p107, and p130 just long enough to induce a rapid turnover of these proteins and that this transient interaction is not represented in the steady-state analysis. However, we have not detected interactions of newly synthesized pRb with BKV TAg using metabolically labeled cells (data not shown). Our experiments with GST fusion proteins also argue against a technical inability to detect complexes. In addition, we have shown SV40 TAg to have the same turnover effect on pRb family proteins, and yet interactions between SV40 TAg and pRb, p107, and p130 are readily detected (38). This apparent contradiction implies either that the TAgs behave very differently or that BKV TAg-pRb family interactions are not present in the cell even in a transient form.
An alternative possibility is that the pRb-binding domain has additional functions which have not yet been clearly defined. SV40 TAg has always been studied as the prototype for TAg-pRb family interactions, and since SV40 TAg does bind to most of the pRb, p107, and p130 in the cell, additional roles for the pRb-binding domain have not been sought. Although the levels of BKV TAg are too low to complex all the pRb family proteins, BKV TAg may cause amplification of a catalytic pathway, resulting in a similar outcome. In support of this idea, we have shown that BKV TAg and SV40 TAg have equivalent abilities to induce turnover and to affect the steady-state phosphorylation patterns of the pRb family. One possible function of the pRb-binding domain is modulation of cyclin-associated CDK activity. For BKV TAg, these interactions may result in a decrease or increase in cyclin-associated CDK activity toward the pRb family proteins or E2F in these cells.
Another role of the pRb-binding domain in the induction of E2F may involve the effect of BKV TAg on the overall levels of pRb, p107, and p130 (38). Both the pRb-binding and the J domains of SV40 TAg were recently shown to be required for an effect on the overall levels of p107 and p130, and the two domains may work together to promote the degradation of pRb family proteins (87, 88). It is possible that this reduction in the levels of pRb family proteins is sufficient to shift the equilibrium in the cell toward an excess of free E2F. However, a more detailed understanding of the exact roles of both the pRb-binding and the J domains in the modulation of pRb protein levels and E2F activity is required before the mechanisms can be fully understood.
Interestingly, the remaining pRb family proteins are primarily in the more hypophosphorylated forms, which would predictably bind to and prevent the transcriptional activation of E2F. In support of the notion of an effect on cell cycle regulation in the absence of pRb hyperphosphorylation are data demonstrating that the introduction of the RB1 gene into Saos-2 cells, which lack functional pRb, causes a flat cell morphology concurrent with G1 arrest. When cyclins A and E are overexpressed, pRb becomes phosphorylated and the growth arrest phenotype is relieved. However, coexpression of cyclin D1 causes a relief of the growth arrest phenotype in the absence of the phosphorylation of pRb. In addition, there is an apparent decrease in the overall levels of pRb in the presence of cyclin D1 (20, 42). Ewen et al. (27) also examined E2F-pRb complexes in the presence of cyclin D1. They showed that despite the lack of overt phosphorylation of pRb in the presence of cyclin D1 and the lack of significant interactions between cyclin D1 and pRb, the levels of the E2F-pRb complexes present in the cells were greatly reduced (27). It is possible then that BKV TAg acts on the retinoblastoma pathway in a manner similar to that of cyclin D1.
We have also shown here that neither BKV TAg nor SV40 TAg is able to completely disrupt all of the E2F-pRb or E2F-p107 complexes present in the cell. Although for SV40 TAg it has clearly been shown that the disruption of E2F-pRb family member complexes does occur, the nature of the disruption of these complexes appears to depend on the experimental system used. In TAg-expressing NIH 3T3 cells, the E2F-p107 complex but not the E2F-pRb complex is effectively disrupted by TAg (96). In contrast, Chellappan et al. have shown that purified GST-TAg completely disrupts the E2F-pRb complex in extracts from U937 human monocytic cells but not the E2F-p101 or E2F-p130 complex (9). In Rb +/+ and Rb −/− mouse embryo fibroblasts, a fraction of the E2F-p107 and E2F-p130 complexes remains resistant to SV40 TAg, and this resistance is not due to limiting amounts of TAg protein (98). These results, as well as those presented here, indicate that the disruption of pRb family member complexes by TAg may be cell type specific and that all pRb family member complexes with E2F need not be disrupted in order for TAg to establish a transformed phenotype. In support of this prediction, it has been shown that E2F-pRb complexes can be detected throughout the cell cycle and are therefore not mutually exclusive to cell cycle progression (81, 82). If both TAg proteins are able to disrupt the E2F-pRb–E2F equilibrium sufficiently, perhaps through alterations in pRb family protein levels, then complete sequestration of pRb family members is not necessary for the transformation of these cells.
Finally, using mutant BKV TAgs, we have shown that the induction of E2F and the induction of serum-independent growth are not directly related. The E109K mutant grew in 0.1% serum without the induction of E2F activity. The H42Q mutant was impaired in both the induction of E2F activity and serum-independent growth. These results suggest that BKV TAg must subvert some other cellular pathway to induce the serum independence phenotype. Although the data indicate that this pathway is affected by the J domain of BKV TAg, whether this is due to the effect of the J domain on pRb family proteins or other functions of the J domain has yet to be determined. In a very recent report, it was shown that both the pRb-binding domain and the J domain of SV40 TAg are required for growth in 1% serum (87). We suggest that there may be differences between SV40 TAg and BKV TAg, particularly with respect to the function of the pRb-binding domain.
We have sought to use BKV TAg not only as a tool to understand normal cellular regulation of proliferation but also to understand the potential effects of BKV in the normal host. BKV is found in a majority of the human population, and BKV DNA has been associated with a variety of tumors, including brain, pancreatic islet, urinary tract, and most recently Kaposi’s sarcoma (15, 66, 67, 90). This fact raises the possibility that BKV acts to potentiate carcinogenesis in these cells, perhaps through a cofactor function. Our results indicate that even at low levels, BKV TAg can have a significant effect on cell cycle regulation. A greater understanding of the specific mechanisms is required, however, before a role for BKV TAg in human cancers can be fully assessed.
ACKNOWLEDGMENTS
We thank the members of our laboratory for useful discussions and comments about this work, J. Nevins for the pE2F-CAT and GST constructs, E. Moran for the E1A construct, J. Chamberlain for the pAdCMVβ construct, N. Dyson for anti-p107 antibodies, and E. Harlow for various hybridomas.
This work was supported in part by American Cancer Society grant VM-11A, the Elsa U. Pardee Foundation, and a student development award from NIH Prostate SPORE grant P50 CA69568. K.F.H. was supported in part by the Nancy Newton-Loeb Foundation and a Rackham Predoctoral Fellowship from the University of Michigan.
ADDENDUM IN PROOF
Since the submission of this paper, Schaffhausen and colleagues have published data indicating that the J domain of mouse polyomavirus TAg also regulates pRb family function (J. Virol. 71:9410–9416, 1997).
REFERENCES
- 1.Arthur R R, Shah K V, Baust S J, Santos G W, Saral R. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N Engl J Med. 1986;315:230–234. doi: 10.1056/NEJM198607243150405. [DOI] [PubMed] [Google Scholar]
- 2.Bagchi S, Weinmann R, Raychaudhuri P. The retinoblastoma protein copurifies with E2F-I, an E1A-regulated inhibitor of the transcription factor E2F. Cell. 1991;65:1063–1072. doi: 10.1016/0092-8674(91)90558-g. [DOI] [PubMed] [Google Scholar]
- 3.Bandara L R, Adamczewski J P, Hunt T, La Thangue N B. Cyclin A and the retinoblastoma gene product complex with a common transcription factor. Nature. 1991;352:249–251. doi: 10.1038/352249a0. [DOI] [PubMed] [Google Scholar]
- 4.Barbosa M S, Edmonds C, Fisher C, Schiller J T, Lowy D R, Vousden K H. The region of the HPV E7 oncoprotein homologous to adenovirus E1a and SV40 large T antigen contains separate domains for Rb binding and casein kinase II phosphorylation. EMBO J. 1990;9:153–160. doi: 10.1002/j.1460-2075.1990.tb08091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beijersbergen R L, Carlee L, Kerkhoven R M, Bernards R. Regulation of the retinoblastoma protein-related p107 by G1 cyclin complexes. Genes Dev. 1995;9:1340–1353. doi: 10.1101/gad.9.11.1340. [DOI] [PubMed] [Google Scholar]
- 6.Beijersbergen R L, Kerkhoven R M, Zhu L, Carlee L, Voorhoeve P M, Bernards R. E2F-4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev. 1994;8:2680–2690. doi: 10.1101/gad.8.22.2680. [DOI] [PubMed] [Google Scholar]
- 7.Bollag B, Chuke W F, Frisque R J. Hybrid genomes of the polyomaviruses JC virus, BK virus, and simian virus 40: identification of sequences important for efficient transformation. J Virol. 1989;63:863–872. doi: 10.1128/jvi.63.2.863-872.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell K S, Mullane K P, Aksoy I A, Stubdal H, Zalvide J, Pipas J M, Silver P A, Roberts T M, Schaffhausen B S, DeCaprio J A. DnaJ/hsp40 chaperone domain of SV40 large T antigen promotes efficient viral DNA replication. Genes Dev. 1997;11:1098–1110. doi: 10.1101/gad.11.9.1098. [DOI] [PubMed] [Google Scholar]
- 9.Chellappan S, Kraus V B, Kroger B, Munger K, Howley P M, Phelps W C, Nevins J R. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci USA. 1992;89:4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chellappan S P, Hiebert S, Mudryj M, Horowitz J M, Nevins J R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- 11.Cherington V, Brown M, Paucha E, St. Louis J, Spiegelman B M, Roberts T M. Separation of simian virus 40 large-T-antigen-transforming and origin-binding functions from the ability to block differentiation. Mol Cell Biol. 1988;8:1380–1384. doi: 10.1128/mcb.8.3.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chittenden T, Livingston D M, DeCaprio J A. Cell cycle analysis of E2F in primary human T cells reveals novel E2F complexes and biochemically distinct forms of free E2F. Mol Cell Biol. 1993;13:3975–3983. doi: 10.1128/mcb.13.7.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cobrinik D. Regulatory interactions among E2Fs and cell cycle control proteins. Curr Top Microbiol Immunol. 1996;208:31–61. doi: 10.1007/978-3-642-79910-5_2. [DOI] [PubMed] [Google Scholar]
- 14.Cobrinik D, Whyte P, Peeper D S, Jacks T, Weinberg R A. Cell cycle-specific association of E2F with the p130 E1A-binding protein. Genes Dev. 1993;7:2392–2404. doi: 10.1101/gad.7.12a.2392. [DOI] [PubMed] [Google Scholar]
- 15.Corallini A, Pagnani M, Viadana P, Silini E, Mottes M, Milanesi G, Gerna G, Vettor R, Trapella G, Silvani V. Association of BK virus with human brain tumors and tumors of pancreatic islets. Int J Cancer. 1987;39:60–67. doi: 10.1002/ijc.2910390111. [DOI] [PubMed] [Google Scholar]
- 16.Dalrymple S A, Beemon K L. BK virus T antigens induce kidney carcinomas and thymoproliferative disorders in transgenic mice. J Virol. 1990;64:1182–1191. doi: 10.1128/jvi.64.3.1182-1191.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeCaprio J A, Ludlow J W, Figge J, Shew J Y, Huang C M, Lee W H, Marsilio E, Paucha E, Livingston D M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988;54:275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- 18.DeGregori J, Leone G, Ohtani K, Miron A, Nevins J R. E2F-1 accumulation bypasses a G1 arrest resulting from the inhibition of G1 cyclin-dependent kinase activity. Genes Dev. 1995;9:2873–2887. doi: 10.1101/gad.9.23.2873. [DOI] [PubMed] [Google Scholar]
- 19.Dorries K, Vogel E, Gunther S, Czub S. Infection of human polyomaviruses JC and BK in peripheral blood leukocytes from immunocompetent individuals. Virology. 1994;198:59–70. doi: 10.1006/viro.1994.1008. [DOI] [PubMed] [Google Scholar]
- 20.Dowdy S F, Hinds P W, Louie K, Reed S I, Arnold A, Weinberg R A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993;73:499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- 21.Dyson N, Buchkovich K, Whyte P, Harlow E. The cellular 107K protein that binds to adenovirus E1A also associates with the large T antigens of SV40 and JC virus. Cell. 1989;58:249–255. doi: 10.1016/0092-8674(89)90839-8. [DOI] [PubMed] [Google Scholar]
- 22.Dyson N, Dembski M, Fattaey A, Ngwu C, Ewen M, Helin K. Analysis of p107-associated proteins: p107 associates with a form of E2F that differs from pRB-associated E2F-1. J Virol. 1993;67:7641–7647. doi: 10.1128/jvi.67.12.7641-7647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckhart W. Polyomavirinae and their replication. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven Press Ltd.; 1990. pp. 1593–1607. [Google Scholar]
- 24.Egan C, Yee S P, Ferguson B, Rosenberg M, Branton P E. Binding of cellular polypeptides to human adenovirus type 5 E1A proteins produced in Escherichia coli. Virology. 1987;160:292–296. doi: 10.1016/0042-6822(87)90077-8. [DOI] [PubMed] [Google Scholar]
- 25.Ewen M E, Faha B, Harlow E, Livingston D M. Intereaction of p107 with cyclin A independent of complex formation with viral oncoproteins. Science. 1992;255:85–87. doi: 10.1126/science.1532457. [DOI] [PubMed] [Google Scholar]
- 26.Ewen M E, Ludlow J W, Marsilio E, DeCaprio J A, Millikan R C, Cheng S H, Paucha E, Livingston D M. An N-terminal transformation-governing sequence of SV40 large T antigen contributes to the binding of both p110Rb and a second cellular protein, p120. Cell. 1989;58:257–267. doi: 10.1016/0092-8674(89)90840-4. [DOI] [PubMed] [Google Scholar]
- 27.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 28.Frisque R J, Bream G L, Cannella M T. Human polyomavirus JC virus genome. J Virol. 1984;51:458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardner S D. Prevalence in England of antibody to human polyomavirus (B.K.) Br Med J. 1973;1:77–78. doi: 10.1136/bmj.1.5845.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ginsberg D, Vairo G, Chittenden T, Xiao Z X, Xu G, Wydner K L, DeCaprio J A, Lawrence J B, Livingston D M. E2F-4, a new member of the E2F transcription factor family, interacts with p107. Genes Dev. 1994;8:2665–2679. doi: 10.1101/gad.8.22.2665. [DOI] [PubMed] [Google Scholar]
- 31.Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- 32.Goodrich D W, Wang N P, Qian Y W, Lee E Y, Lee W H. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell. 1991;67:293–302. doi: 10.1016/0092-8674(91)90181-w. [DOI] [PubMed] [Google Scholar]
- 33.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 35.Hannon G J, Demetrick D, Beach D. Isolation of the Rb-related p130 through its interaction with CDK2 and cyclins. Genes Dev. 1993;7:2378–2391. doi: 10.1101/gad.7.12a.2378. [DOI] [PubMed] [Google Scholar]
- 36.Harbour J W, Lai S L, Whang-Peng J, Gazdar A F, Minna J D, Kaye F J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988;241:353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harlow E, Crawford L V, Pim D C, Williamson N M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981;39:861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris K F, Christensen J B, Imperiale M J. BK virus large T antigen: interactions with the retinoblastoma family of tumor suppressor proteins and effects on cellular growth control. J Virol. 1996;70:2378–2386. doi: 10.1128/jvi.70.4.2378-2386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helin K, Harlow E, Fattaey A. Inhibition of E2F-1 transactivation by direct binding of the retinoblastoma protein. Mol Cell Biol. 1993;13:6501–6508. doi: 10.1128/mcb.13.10.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiebert S W, Chellappan S P, Horowitz J M, Nevins J R. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992;6:177–185. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- 41.Hijmans E M, Voorhoeve P M, Beijersbergen R L, van ’t Veer L J, Bernards R. E2F-5, a new E2F family member that interacts with p130 in vivo. Mol Cell Biol. 1995;15:3082–3089. doi: 10.1128/mcb.15.6.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hinds P W, Mittnacht S, Dulic V, Arnold A, Reed S I, Weinberg R A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- 43.Horowitz J M, Park S H, Bogenmann E, Cheng J C, Yandell D W, Kaye F J, Minna J D, Dryja T P, Weinberg R A. Frequent inactivation of the retinoblastoma anti-oncogene is restricted to a subset of human tumor cells. Proc Natl Acad Sci USA. 1990;87:2775–2779. doi: 10.1073/pnas.87.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horowitz J M, Yandell D W, Park S H, Canning S, Whyte P, Buchkovich K, Harlow E, Weinberg R A, Dryja T P. Point mutational inactivation of the retinoblastoma antioncogene. Science. 1989;243:937–940. doi: 10.1126/science.2521957. [DOI] [PubMed] [Google Scholar]
- 45.Hunter T, Pines J. Cyclins and cancer. II. Cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 46.Ikeda M A, Jakoi L, Nevins J R. A unique role for the Rb protein in controlling E2F accumulation during cell growth and differentiation. Proc Natl Acad Sci USA. 1996;93:3215–3220. doi: 10.1073/pnas.93.8.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ikeda M A, Nevins J R. Identification of distinct roles for separate E1A domains in disruption of E2F complexes. Mol Cell Biol. 1993;13:7029–7035. doi: 10.1128/mcb.13.11.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson D G, Schwarz J K, Cress W D, Nevins J R. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 1993;365:349–352. doi: 10.1038/365349a0. [DOI] [PubMed] [Google Scholar]
- 49.Jones D L, Munger K. Analysis of the p53-mediated G1 growth arrest pathway in cells expressing the human papillomavirus type 16 E7 oncoprotein. J Virol. 1997;71:2905–2912. doi: 10.1128/jvi.71.4.2905-2912.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaelin W G, Jr, Pallas D C, DeCaprio J A, Kaye F J, Livingston D M. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell. 1991;64:521–532. doi: 10.1016/0092-8674(91)90236-r. [DOI] [PubMed] [Google Scholar]
- 51.Kalderon D, Smith A E. In vitro mutagenesis of a putative DNA binding domain of SV40 large-T. Virology. 1984;139:109–137. doi: 10.1016/0042-6822(84)90334-9. [DOI] [PubMed] [Google Scholar]
- 52.Kelley W L, Georgopoulos C. The T/t common exon of simian virus 40, JC, and BK polyomavirus T antigens can functionally replace the J-domain of the Escherichia coli DnaJ molecular chaperone. Proc Natl Acad Sci USA. 1997;94:3679–3684. doi: 10.1073/pnas.94.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelley W L, Landry S J. Chaperone power in a virus? Trends Biochem Sci. 1994;19:277–278. doi: 10.1016/0968-0004(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 54.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 55.La Thangue N B. DRTF1/E2F: an expanding family of heterodimeric transcription factors implicated in cell-cycle control. Trends Biochem Sci. 1994;19:108–114. doi: 10.1016/0968-0004(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 56.Lee E Y, To H, Shew J Y, Bookstein R, Scully P, Lee W H. Inactivation of the retinoblastoma susceptibility gene in human breast cancers. Science. 1988;241:218–221. doi: 10.1126/science.3388033. [DOI] [PubMed] [Google Scholar]
- 57.Lee W H, Bookstein R, Hong F, Young L J, Shew J Y, Lee E Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987;235:1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- 58.Lees J A, Saito M, Vidal M, Valentine M, Look T, Harlow E, Dyson N, Helin K. The retinoblastoma protein binds to a family of E2F transcription factors. Mol Cell Biol. 1993;13:7813–7825. doi: 10.1128/mcb.13.12.7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levine A J. The tumor suppressor genes. Annu Rev Biochem. 1993;62:623–651. doi: 10.1146/annurev.bi.62.070193.003203. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, Graham C, Lacy S, Duncan A M, Whyte P. The adenovirus E1A-associated 130-kD protein is encoded by a member of the retinoblastoma gene family and physically interacts with cyclins A and E. Genes Dev. 1993;7:2366–2377. doi: 10.1101/gad.7.12a.2366. [DOI] [PubMed] [Google Scholar]
- 61.Loeken M R, Brady J. The adenovirus EIIA enhancer. Analysis of regulatory sequences and changes in binding activity of ATF and EIIF following adenovirus infection. J Biol Chem. 1989;264:6572–6579. [PubMed] [Google Scholar]
- 62.Ludlow J W, DeCaprio J A, Huang C M, Lee W H, Paucha E, Livingston D M. SV40 large T antigen binds preferentially to an underphosphorylated member of the retinoblastoma susceptibility gene product family. Cell. 1989;56:57–65. doi: 10.1016/0092-8674(89)90983-5. [DOI] [PubMed] [Google Scholar]
- 63.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 64.Mayol X, Garriga J, Grana X. Cell cycle-dependent phosphorylation of the retinoblastoma-related protein p130. Oncogene. 1995;11:801–808. [PubMed] [Google Scholar]
- 65.Mayol X, Garriga J, Grana X. G1 cyclin/CDK-independent phosphorylation and accumulation of p130 during the transition from G1 to G0 lead to its association with E2F-4. Oncogene. 1996;13:236–246. [PubMed] [Google Scholar]
- 66.Monini P, Rotola A, De Lellis L, Corallini A, Secchiero P, Albini A, Benelli R, Parravicini C, Barbanti-Brodano G, Cassai E. Latent BK virus infection and Kaposi’s sarcoma pathogenesis. Int J Cancer. 1996;66:717–722. doi: 10.1002/(SICI)1097-0215(19960611)66:6<717::AID-IJC1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 67.Monini P, Rotola A, Di Luca D, De Lellis L, Chiari E, Corallini A, Cassai E. DNA rearrangements impairing BK virus productive infection in urinary tract tumors. Virology. 1995;214:273–279. doi: 10.1006/viro.1995.9928. [DOI] [PubMed] [Google Scholar]
- 68.Moran E. A region of SV40 large T antigen can substitute for a transforming domain of the adenovirus E1A products. Nature. 1988;334:168–170. doi: 10.1038/334168a0. [DOI] [PubMed] [Google Scholar]
- 69.Moran E, Zerler B, Harrison T M, Mathews M B. Identification of separate domains in the adenovirus E1A gene for immortalization activity and the activation of virus early genes. Mol Cell Biol. 1986;6:3470–3480. doi: 10.1128/mcb.6.10.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mudryj M, Devoto S H, Hiebert S W, Hunter T, Pines J, Nevins J R. Cell cycle regulation of the E2F transcription factor involves an interaction with cyclin A. Cell. 1991;65:1243–1253. doi: 10.1016/0092-8674(91)90019-u. [DOI] [PubMed] [Google Scholar]
- 71.Nevins J R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 72.Padgett B. Human papovaviruses. In: Tooze J, editor. DNA tumor viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1981. pp. 339–370. [Google Scholar]
- 73.Pater A, Pater M M. Transformation of primary human embryonic kidney cells to anchorage independence by a combination of BK virus DNA and the Harvey-ras oncogene. J Virol. 1986;58:680–683. doi: 10.1128/jvi.58.2.680-683.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peden K W, Pipas J M. Simian virus 40 mutants with amino-acid substitutions near the amino terminus of large T antigen. Virus Genes. 1992;6:107–118. doi: 10.1007/BF01703060. [DOI] [PubMed] [Google Scholar]
- 75.Portolani M, Barbanti-Brodano G, Placa M L. Malignant transformation of hamster kidney cells by BK virus. J Virol. 1975;15:420–422. doi: 10.1128/jvi.15.2.420-422.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Purchio A F, Fareed G C. Transformation of human embryonic kidney cells by human papovavirus BK. J Virol. 1979;29:763–769. doi: 10.1128/jvi.29.2.763-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qin X Q, Chittenden T, Livingston D M, Kaelin W G., Jr Identification of a growth suppression domain within the retinoblastoma gene product. Genes Dev. 1992;6:953–964. doi: 10.1101/gad.6.6.953. [DOI] [PubMed] [Google Scholar]
- 78.Qin X Q, Livingston D M, Ewen M, Sellers W R, Arany Z, Kaelin W G., Jr The transcription factor E2F-1 is a downstream target of RB action. Mol Cell Biol. 1995;15:742–755. doi: 10.1128/mcb.15.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sawai E T, Butel J S. Association of a cellular heat shock protein with simian virus 40 large T antigen in transformed cells. J Virol. 1989;63:3961–3973. doi: 10.1128/jvi.63.9.3961-3973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scheffner M, Munger K, Byrne J C, Howley P M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci USA. 1991;88:5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwarz J K, Devoto S H, Smith E J, Chellappan S P, Jakoi L, Nevins J R. Interactions of the p107 and Rb proteins with E2F during the cell proliferation response. EMBO J. 1993;12:1013–1020. doi: 10.1002/j.1460-2075.1993.tb05742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shirodkar S, Ewen M, DeCaprio J A, Morgan J, Livingston D M, Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle-regulated manner. Cell. 1992;68:157–166. doi: 10.1016/0092-8674(92)90214-w. [DOI] [PubMed] [Google Scholar]
- 83.Silver P A, Way J C. Eukaryotic DnaJ homologs and the specificity of Hsp70 activity. Cell. 1993;74:5–6. doi: 10.1016/0092-8674(93)90287-z. [DOI] [PubMed] [Google Scholar]
- 84.Slansky J E, Farnham P J. Introduction to the E2F family: protein structure and gene regulation. Curr Top Microbiol Immunol. 1996;208:1–30. doi: 10.1007/978-3-642-79910-5_1. [DOI] [PubMed] [Google Scholar]
- 85.Small J A, Khoury G, Jay G, Howley P M, Scangos G A. Early regions of JC virus and BK virus induce distinct and tissue-specific tumors in transgenic mice. Proc Natl Acad Sci USA. 1986;83:8288–8292. doi: 10.1073/pnas.83.21.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Srinivasan A, McClellan A J, Vartikar J, Marks I, Cantalupo P, Li Y, Whyte P, Rundell K, Brodsky J L, Pipas J M. The amino-terminal transforming region of SV40 large and small T antigens functions as a J-domain. Mol Cell Biol. 1997;17:4761–4773. doi: 10.1128/mcb.17.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stubdal H, Zalvide J, Campbell K S, Schweitzer C, Roberts T, DeCaprio J A. Inactivation of pRB-related proteins p130 and p107 mediated by the J domain of simian virus 40 large T antigen. Mol Cell Biol. 1997;17:4979–4990. doi: 10.1128/mcb.17.9.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stubdal H, Zalvide J, DeCaprio J A. Simian virus 40 large T antigen alters the phosphorylation state of the RB-related proteins p130 and p107. J Virol. 1996;70:2781–2788. doi: 10.1128/jvi.70.5.2781-2788.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suzuki-Takahashi I, Kitagawa M, Saijo M, Higashi H, Ogino H, Matsumoto H, Taya Y, Nishimura S, Okuyama A. The interactions of E2F with pRB and with p107 are regulated via the phosphorylation of pRB and p107 by a cyclin-dependent kinase. Oncogene. 1995;10:1691–1698. [PubMed] [Google Scholar]
- 90.Takemoto K K, Rabson A S, Mullarkey M F, Blaese R M, Garon C F, Nelson D. Isolation of papovavirus from brain tumor and urine of a patient with Wiskott-Aldrich syndrome. J Natl Cancer Inst. 1974;53:1205–1207. doi: 10.1093/jnci/53.5.1205. [DOI] [PubMed] [Google Scholar]
- 91.T’Ang A, Varley J M, Chakraborty S, Murphree A L, Fung Y K. Structural rearrangement of the retinoblastoma gene in human breast carcinoma. Science. 1988;242:263–266. doi: 10.1126/science.3175651. [DOI] [PubMed] [Google Scholar]
- 92.Vairo G, Livingston D M, Ginsberg D. Functional interaction between E2F-4 and p130: evidence for distinct mechanisms underlying growth suppression by different retinoblastoma protein family members. Genes Dev. 1995;9:869–881. doi: 10.1101/gad.9.7.869. [DOI] [PubMed] [Google Scholar]
- 93.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 94.Weinberg R A. E2F and cell proliferation: a world turned upside down. Cell. 1996;85:457–459. doi: 10.1016/s0092-8674(00)81244-1. [DOI] [PubMed] [Google Scholar]
- 95.Whyte P, Buchkovich K J, Horowitz J M, Friend S H, Raybuck M, Weinberg R A, Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988;334:124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- 96.Wolf D A, Hermeking H, Albert T, Herzinger T, Kind P, Eick D. A complex between E2F and the pRb-related protein p130 is specifically targeted by the simian virus 40 large T antigen during cell transformation. Oncogene. 1995;10:2067–2078. [PubMed] [Google Scholar]
- 97.Xiao Z X, Ginsberg D, Ewen M, Livingston D M. Regulation of the retinoblastoma protein-related protein p107 by G1 cyclin-associated kinases. Proc Natl Acad Sci USA. 1996;93:4633–4637. doi: 10.1073/pnas.93.10.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zalvide J, DeCaprio J A. Role of pRb-related proteins in simian virus 40 large-T-antigen-mediated transformation. Mol Cell Biol. 1995;15:5800–5810. doi: 10.1128/mcb.15.10.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu L, van den Heuvel S, Helin K, Fattaey A, Ewen M, Livingston D, Dyson N, Harlow E. Inhibition of cell proliferation by p107, a relative of the retinoblastoma protein. Genes Dev. 1993;7:1111–1125. doi: 10.1101/gad.7.7a.1111. [DOI] [PubMed] [Google Scholar]