Abstract
The purified Rel/NF-κB (p50/p65) complex and Sp1 markedly activate transcription from the human immunodeficiency virus type 1 (HIV-1) promoter in a highly purified HeLa reconstituted transcription system. Transcriptional activation by NF-κB and Sp1 requires both TFIID and the USA fraction. The USA-derived coactivators PC2 and PC4 fully reconstitute the USA coactivator activity, both by repressing the basal level of transcription and by potentiating activator function to yield large increases in the levels of transcription induction. Under limiting concentrations, PC2 and PC4 also show synergistic effects. The C-terminal portion (amino acids 416 to 550) of the p65 subunit of NF-κB is a potent activator when assayed as a Gal fusion in the reconstituted transcription system and interacts both with TATA-binding protein (TBP) and with several human TBP-associated factors (TAFs) that include TAFII250. The p65 activation domain mediates transcription activation in the presence of partially reconstituted TFIID species that include a minimal complex containing only TBP and TAFII250. These studies also show that, like USA components, TAFs can serve both to repress TBP-mediated transcription and, following activator interactions, to reverse the repression and effect a net increase in activity. Taken together, these data underscore the importance of both TAFs and specific USA-derived coactivators for optimal activation of the HIV-1 promoter, as well as certain parallels in their overall mechanisms of action.
The general inducible NF-κB activity consists of a heterodimer of two subunits, p50 (NF-κB) and p65 (RelA), that both bind DNA (28, 68). p50 and p65, along with other members of the Rel family, share a 300-residue N-terminal Rel homology domain that contains sequences required for DNA binding, protein dimerization, and nuclear localization. In addition to the Rel homology domain, the p65, c-Rel, and RelB proteins contain flanking regions involved in transactivation (for reviews, see references 2 and 69). Transient expression assays have revealed that the p65 subunit is responsible for most of the activation potential of Rel/NF-κB (52, 59), and a strong activation domain, which is lacking in the p50 subunit, has been mapped to its C-terminal domain (48, 58, 60). p50 does not usually activate transcription in transient transfection experiments, although it has been reported to do so in vitro (17, 34).
A well-studied target promoter for NF-κB activity is the proximal enhancer of human immunodeficiency virus type 1 (HIV-1). In addition to three Sp1 sites, this enhancer contains two NF-κB sites to which several combinations of NF-κB subunits can bind and activate transcription both in vivo and in nuclear extracts (15, 28, 34). In addition, it has been shown that these two activators cooperate during transcription of the HIV-1 promoter in vivo (50, 51). While specific DNA-binding factors like constitutive Sp1 and induced NF-κB modulate the expression level of the HIV-1 promoter, RNA polymerase II (pol II), and general initiation factors (TFIIA, TFIIB, TFIID, TFIIE, TFIIF, and TFIIH) are required for both activator-independent (basal) and activator-dependent transcription. Furthermore, and in contrast to basal transcription, activator-dependent transcription in mammalian cell-free systems generally requires cofactor activities that include both the USA fraction (upstream factor stimulatory activity) and TATA-binding protein (TBP)-associated factors (TAFs) within TFIID (for reviews, see references 5, 27, 48, 54, and 70).
The USA fraction contains both positive and negative cofactors (PCs and NCs), and the four human PCs (PC1, PC2, PC3, and PC4) characterized so far are distinct from components within TFIID (for a review, see reference 27). Importantly, these USA-derived cofactors effect a large increase in promoter activity in the presence of activators and are essential even in systems reconstituted with homogeneous TFIID (10). Human TFIID itself is composed of at least 13 TAFs and TBP (for a review, see reference 5). Consistent with its key role in recruiting other basal factors to the promoter, TFIID was implicated in transcriptional activation by the demonstration of physical and functional interactions between TFIID and various activators, and in several cases, activator-induced changes in TFIID binding were correlated with increased recruitment of downstream factors (for reviews, see references 5, 54 and 70). Although in vivo and in vitro studies have shown that the NF-κB complex can bind NF-κB sites and activate transcription, very little is known about the cofactors required for its transcriptional activity in vitro. Only two studies have analyzed NF-κB activity in systems reconstituted with isolated factors, and both showed a requirement for a crude USA fraction and for TFIID (34, 60). It therefore becomes important to determine whether individual components of the complex USA fraction (reviewed in reference 27) can function alone or in combination to restore the full coactivator activity of USA. Also of key importance is an understanding of the role of individual human TAFs (in reconstituted TFIID species) in both basal and NF-κB-mediated transcription, especially in light of core promoter-specific effects of TAFs in both metazoans (reviewed in reference 54) and yeast (61).
Here, we report a dissection of specific coactivator requirements for HIV-1 promoter-driven transcription by NF-κB (p50/p65) and Sp1 in a transcription system reconstituted with highly purified factors. We show that the synergistic action of USA-derived coactivators PC2 and PC4 fully reconstitutes the USA coactivator function, including both basal repression and activator-enhanced transcription. In addition, we have used highly purified native TFIID and partially reconstituted TFIID complexes to show that TAFs, like USA components, serve both to repress the TBP-mediated basal transcription level and, following activator interactions, to reverse the repression and effect a net increase in overall activity. Thus, our data show more diverse roles for TAFs in transcription regulation that go beyond their simple involvement in recruiting TFIID to the promoter upon interactions with upstream activators.
MATERIALS AND METHODS
Expression and purification of activators and TAFs.
cDNAs corresponding to the coding regions of p50 and p65 (20, 46) were used to generate, by PCR, their respective PCR fragments with appropriate flanking restriction sites (XbaI and EcoRI or KpnI). PCR products were cloned into the baculovirus expression vector pVLMH6 generated by insertion of the hexahistidine (6His) oligonucleotide linker 5′-GATCCATATGAGAGGATCGCATCACCATCACCATCACT-3′ into the BamHI-XbaI sites of pVL1393 (62). Each construct was verified by sequencing. The hexahistidine tag allows purification through a nickel affinity column. The original initiation methionine of each protein was mutated to valine to avoid any internal initiation of translation. To produce the mature form of p50 without processing of its precursor, a translational terminator was introduced at codon position 452. For p65, a full-length cDNA was used. The p50/p65 heterodimer was made in vivo by coinfecting Sf9 cells with the corresponding viruses for each subunit. To avoid contamination of the heterodimer with p50 homodimers, only p65 carried the hexahistidine tag in the coinfection. Recombinant baculoviruses were then isolated and characterized for protein expression by Western blot analysis. For each protein, one recombinant baculovirus was selected to infect an Sf9 suspension culture. For the coinfection experiments, the ratio between the two viruses was 10 to 1 in favor of the virus that carries the subunit without the hexahistidine tag (p50). The p50/p65 complex was then purified by affinity chromatography on a nickel column, followed by ion-exchange chromatography.
The pVL derivatives used for the expression of hemagglutinin (HA)-hTAFII250, FLAG-hTAFII100, and FLAG-hTAFII55 have already been described (11, 21, 66). Expression pVL plasmids for FLAG-hTAFII80, FLAG-hTAFII80, FLAG-hTBP, His-TBP, FLAG-hTAFII80, FLAG-hTAFII31, nontagged hTAFII31, and FLAG-Sp1 were constructed by PCR. In each case, an NdeI site at the N-terminal end and an appropriate restriction enzyme site at the C-terminal end following the natural stop codon were created. The large number of primers used in the PCRs has precluded description of their exact sequences, but the information is available upon request. The PCR-generated fragments were then inserted into adaptor plasmids pFLAG(S)-7 and pFLAG(AS)-7 carrying the appropriate epitope tag (10) and subsequently subcloned into either pVL-1392 or pVL-1393. For each TFIID subunit or for Sp1, an individual recombinant baculovirus was generated by cotransfecting corresponding cDNA and BaculoGold linearized baculovirus DNA (Pharmingen) into Sf9 cells. Each recombinant baculovirus was further amplified by repeated infection of Sf9 cells. For production of recombinant proteins, Sf9 cells were infected by the corresponding recombinant viruses and harvested 48 h postinfection. Recombinant proteins were purified from infected cells. Nuclear extracts were prepared in buffer C (20 mM Tris [pH 7.9], 20% glycerol, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml, 1 μg of pepstatin per ml) containing 400 mM KCl (BC400) and 0.1% Nonidet P-40 (NP-40) (13). Clarified extracts were subjected to the appropriate method of purification: affinity purification on an anti-FLAG antibody (M2 agarose; Kodak) or anti-HA antibody (12CA5 monoclonal antibody) column or chromatography on Ni-nitrilotriacetic acid (NTA) agarose (Qiagen, Chatsworth, Calif.). After extensive washing, bound proteins were eluted, respectively, in BC100 buffer containing FLAG- or HA-peptides (10, 75) or 250 mM imidazole (for Ni-NTA) and further purified by one or two steps of ion-exchange chromatography. The recombinant proteins were more than 90% pure as judged by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and Coomassie blue or silver staining.
A GST (glutathione S-transferase)-p65ct construct was created by inserting a cDNA fragment corresponding to the C-terminal 135 amino acids of p65, flanked by NdeI and EcoRI sites, into a pGEX-2T derivative (23). The GST fusion protein was expressed in Escherichia coli, solubilized by sonication of cells in lysis buffer (11) and removal of insoluble debris by centrifugation, and purified on glutathione-Sepharose (Pharmacia). A 1-μg sample of the purified protein was used for each binding assay.
Bacterially expressed FLAG-Gal4 fusion proteins were purified as described previously (11, 38) and consist of the Gal4 DNA-binding domain fused either to a duplicated copy of the transcriptional activation domain of p53 (amino acids 1 to 57) or to a single copy of a C-terminal portion (amino acids 416 to 550) of p65 (4, 42, 55). cDNA fragments that express the corresponding activation domains were amplified by PCR, using primers which introduced unique XbaI and EcoRI restriction sites, and inserted into a pGAL4 adaptor plasmid 3′ of, and in frame with, the encoded Gal4 DNA-binding domain (amino acids 1 to 94). The corresponding recombinant pGAL4 plasmids were then digested with XhoI and EcoRI, and Gal-p65ct and Gal-p53nt fragments were subcloned into pFLAG-GAL4-11d (11). Constructs were verified by sequencing.
In vitro RNA pol II transcription assays.
Nuclear extracts were prepared as previously described (13). General transcription factors and the USA cofactor fraction were purified from HeLa and 3–10 (FLAG-TBP cell line) nuclear extracts as previously described (10, 41). For TFIIA, the P11 fraction in buffer C containing 0.1 M KCl (BC100) was applied to a DEAE-cellulose (DE52) column, eluted with BC300, dialyzed to BC100, and loaded onto a Q-Sepharose column. The column was then washed with BC300 and eluted with BC500. The TFIIA (BC500) fraction was further purified on an Ni-NTA column (12). For TFIIE/F/H, the P11 0.5 M KCl fraction was applied to a DEAE-cellulose (DE52) column, eluted with BC300, dialyzed to BC100, and passed through a double-stranded DNA-cellulose column to remove contaminating TFIID activity. The flowthrough fraction from the DNA affinity column was applied to a Mono S fast protein liquid chromatography column and eluted with BC300 after washing of the column with BC150. For TFIID, the P11 0.85 M KCl fraction (from 3–10 cells) was dialyzed against BC100, applied to a DEAE-cellulose column, and eluted with BC300. After dialysis against BC100, fTFIID was immunopurified by using an anti-FLAG antibody column (M2 agarose; Kodak). For TFIIB, recombinant FLAG-TFIIB was expressed in, and purified from, E. coli. To prepare the USA fraction, the P11 0.85 M KCl fraction was dialyzed against BC100, applied twice to a DE52 column to limit TFIID contamination, and loaded onto a heparin-Sepharose column. The column was washed with BC300, and the USA fraction was eluted with BC500. Finally, the TFIIE/F/H and USA fractions were depleted of any residual TFIID by using M2 agarose and antigen-purified antibodies against TAFII100 and TAFII31. RNA pol II was purified essentially as previously described (41).
By using the purified transcription factors described above, in vitro transcription assays were carried out in 25-μl reaction mixtures containing 40 ng of a pMLΔ53 template and either 100 ng of pG5HMC2AT or 50 ng of pMHIVWT or pMHIVMT. pMHIVMT contains mutated HIV-1 NFκB binding sites (34). All transcription factors were added simultaneously to the reaction mixtures if not indicated otherwise in the figure legends. 32P-labeled RNA was phenol-chloroform extracted, ethanol precipitated, analyzed directly by urea–4% PAGE, and visualized by autoradiography. Quantitation was done by PhosphorImager.
Purification of PC2 and PC4.
Recombinant PC4 was purified as previously described (18). PC2 was purified essentially as previously described (36). Briefly, the P11 0.85 M KCl fraction was dialyzed to BC100 and applied to a DE52 column. The flowthrough fraction and the BC150 step eluate were combined, dialyzed to BC050, and applied to a second DE52 column. The column was developed with a linear gradient of BC050 to BC250. Fractions containing the PC2 activity (peak at 90 mM KCl) were applied to a Mono S fast protein liquid chromatography column that was developed with a linear gradient of 0.1 to 0.5 M KCl in BC buffer. PC2 activity was eluted between 200 and 300 mM KCl and concentrated on S-Sepharose.
Partial TFIID reconstitution.
By the method of Chen et al. (8), human hTAFII250 containing a fused N-terminal HA epitope tag was immobilized on protein A-Sepharose containing covalently linked monoclonal antibodies directed against the HA epitope. After extensive washing (BC1000 with 0.1% NP-40), the beads were incubated sequentially (at 4°C for 4 h) with molar excesses of additional TFIID subunits. After each incubation, unbound materials were removed by several washes with 100 volumes of BC150 (with 0.1% NP-40). Finally, the resulting complex was eluted with HA peptide (1 mg/ml) in BC100 (with 0.1% NP-40).
In vitro protein-protein interaction assays.
The p65 activation domain interactions were carried out by using TAFs and TBP expressed in, and purified from, Sf9 cells. In each reaction, 1 μg of purified GST, FLAG-GAL4(1–94), GST-p65ct, or FLAG-GAL4p65ct was immobilized on either glutathione beads or anti-FLAG M2 agarose. After washing of the beads, purified TBP and different TAFs (100 ng of each) were added to the appropriate binding reaction mixtures in BC150 with 100 μg of bovine serum albumin–0.1% NP-40 in a 500-μl total volume. After incubation at 30°C for 1 h, the beads were washed four times with 500 μl of incubation buffer. Bound proteins were eluted with SDS loading buffer and analyzed with the inputs by SDS-PAGE and Western blotting.
RESULTS
Transcription activation of the HIV-1 promoter by NF-κB and Sp1.
Full-length FLAG-tagged Sp1 (fSp1) and a hexahistidine-tagged p65/p50 heterodimer were produced in Sf9 cells by coinfection with corresponding baculovirus vectors and purified to over 90% homogeneity (Fig. 1A and B) by affinity chromatography. The function of the recombinant activators was analyzed in a well-defined in vitro transcription system that was reconstituted with recombinant and highly purified general transcription factors from HeLa cells and dependent on the addition of FLAG epitope-tagged and affinity-purified TFIID (f-TFIID) (10; see also below). The ability of this system to support both basal and activator-dependent transcription was tested simultaneously by using two templates whose correctly initiated products could be differentiated by the sizes of their transcribed G-less cassettes. Basal transcription was assayed on a template containing only the adenovirus (Ad) major late (AdML) core promoter sequence, whereas activator-dependent transcription was assayed by using an earlier-described (41) HIV-1 template that contains natural NF-κB- and Sp1-binding sites and the adjacent HIV TATA element. In all cases, levels of transcription were quantitated by PhosphorImager and the levels of induction cited throughout Results are based on these values.
FIG. 1.
Analysis of purified recombinant proteins. (A) NF-κB heterodimer 6his-p65-p50 heterodimer purified from Sf9 cells. (B) Purified FLAG-Sp1 (f-Sp1) purified from Sf9 cells. (C) Purified FLAG-Gal-p53 (fGal-p53nt) and FLAG-Gal-p65ct (fGal-p65ct) expressed in and purified from bacteria. (D) GST-p65ct purified from bacteria. Proteins were resolved by SDS-PAGE and visualized by Coomassie blue staining. M, markers with protein molecular sizes indicated on the left in kilodaltons.
Both recombinant NF-κB (p50/p65 heterodimer) and Sp1 activate transcription of the HIV-1 promoter in this system (Fig. 2). The levels of induction by NF-κB (50 ng of the p50 subunit in the p50/p65 complex) and Sp1 (10 ng of f-Sp1) were 14-fold and 8-fold, respectively. NF-κB activation was dependent on functional NF-κB sites, since no activation was obtained when the mutated template (pMHIVMT) was used (Fig. 2, lane 3 versus lane 4).
FIG. 2.
Site-specific transcriptional activation of the HIV-1 promoter by NFκB and Sp1. Transcription activation in the highly purified transcription system by NFκB and Sp1. A p50/p65 heterodimer containing 50 ng of the p50 subunit or 10 ng of purified f-Sp1 was incubated with DNA templates with f-TFIID (4-ng TBP content) and 1 μl of USA (heparin-Sepharose) at 25°C for 10 min before addition of the other general factors and RNA pol II. The test template consisted of 50 ng of either wild-type HIV-1 pMHIVWT (WT; lanes 1, 2, 5, and 6) or mutated HIV-1 pMHIVMT (MT; lanes 3 and 4). The control template consisted of 40 ng of pMLΔ53, which contains the major late core promoter sequence.
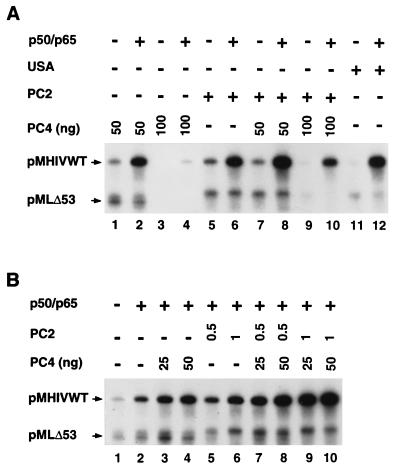
Identification of USA-derived components required for natural NF-κB activity.
Since we have shown previously that the USA fraction is required for full activation by natural NF-κB, we wished to determine which USA-derived PCs are responsible for the activity and to further investigate their mechanism(s) of action. In this regard, it was also important to determine whether there is functional redundancy in the individual factors or whether they can act synergistically. We focused on two USA-derived PCs, PC2, and PC4, since they are the most potent of the USA-derived coactivators and since they together recapitulate the function of the essential USA activity (see below). To test for an independent effect of the well-characterized coactivator PC4 (18, 33) on NF-κB (p50/p65) activity, we replaced the USA fraction with various amounts of purified recombinant PC4. First, without USA or purified PCs, NF-κB activated transcription by less than 2.5-fold (Fig. 3B, lane 1 versus lane 2). In agreement with previous observations on other promoters, basal (activator-independent) transcription activities on both test HIV-1 and control AdML templates were mildly increased at low PC4 concentrations (Fig. 3A, lane 1, versus Fig. 3B, lane 1) but markedly decreased at a high concentration (Fig. 3A, lane 3). PC4 enhanced the ability of p50/p65 to activate transcription of the HIV-1 promoter, but the level of induction was greater at the higher level of PC4 (sixfold) than at the lower level (fourfold) (Fig. 3A, lane 3 versus lane 4 and lane 1 versus lane 2). The increased induction by NF-κB at higher PC4 levels reflects a net decrease of basal HIV transcription that is specifically (but only partially) reversed by NF-κB.
FIG. 3.
Effect of purified USA-derived positive cofactors PC2 and PC4 on NF-κB activity. (A) Effects of various concentrations of PC2 and PC4 on p50/p65 activity. Reaction mixtures contained the indicated amounts of purified PC4 and/or PC2 (2 μl of the Mono S fraction). (B) Effects of low concentrations of PC2 and PC4 in combination on NF-κB activity. Reaction mixtures contained the indicated amounts of PC4 and/or PC2 (0.5 or 1 μl of the Mono S fraction). Other components and reaction conditions were as described in the legend to Fig. 2. The assays in panels A and B are directly comparable since they were performed simultaneously with the same reagents under identical incubation, processing, and autoradiographic exposure conditions.
The coactivator PC2 is less well characterized than PC4 but appears to consist of a circa 500-kDa complex (36). As observed for PC4, a purified PC2 fraction mildly increased the basal activities on both the HIV-1 and AdML templates (Fig. 3A, lane 5, versus Fig. 3B, lane 1) and also showed a significant coactivator function with NF-κB (Fig. 3A, lane 5 versus lane 6). However, the level of induction was lower for PC2 (threefold) than for the higher level of PC4 (sixfold) (Fig. 3A, lanes 5 and 6 and lanes 3 and 4), due primarily to more efficient reduction of basal transcription by PC4. This also reflects, in PC2, both the absence of an activator-reversible NC activity (as observed for PC4) and the presence of a stronger basal stimulatory activity that may be repressed by other NC functions in the unfractionated USA fraction. Interestingly, the addition of high concentrations of PC4 to reaction mixtures containing PC2 increased the overall level of induction by NF-κB from 3-fold (Fig. 3A, lanes 5 and 6) to 7.8-fold (Fig. 3A, lanes 9 and 10). However, and consistent with the NC potential of PC4, this resulted primarily from a selective reduction in basal activity rather than a large net increase in total activity. In this regard, lowering the basal activity enhances substantially the overall level of induction by an activator and more closely mimics the in vivo situation. Taken together, these results indicate that the combination of PC2 and PC4, with their intrinsic positive and negative activities, can have a dual effect on transcription—both decreasing the basal level of transcription and potentiating higher levels of activation by natural NF-κB. However, the higher level of induction (12-fold) evident with unfractionated USA (Fig. 3A, lane 11 versus 12) suggests that optimal induction may still require other cofactors present in the crude USA fraction.
We next investigated whether a possible synergism between PC2 and PC4 on NF-κB function could be observed at limiting concentrations of these two coactivators. As already mentioned, NF-κB activated transcription only by 2.5-fold in the absence of USA or purified PCs (Fig. 3B, lane 1 versus lane 2). In the presence of purified PC4 (50 ng) or PC2 (1 μl) alone, NF-κB activated transcription by 5.6-fold and 5.7-fold, respectively (Fig. 3B, lane 1 versus lanes 4 and 6). A combination of PC4 and PC2, at the same concentrations, led to an 11-fold induction of transcription by NF-κB (Fig. 3B, lane 1 versus lane 10) but had only moderate effects on basal activity in the absence of NF-κB (Fig. 3A, lane 7, versus Fig. 3B, lane 1). This indicates that PC2 and PC4 coactivator functions can be additive for NF-κB activity.
PC4 and PC2 together can reconstitute USA functions for activation by Sp1 and show synergistic effects.
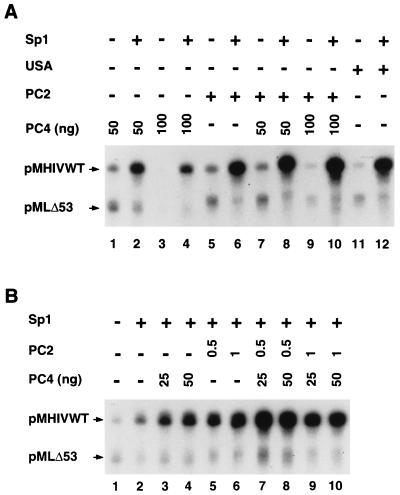
Similar to the above observations for activation by NF-κB, Sp1 activated the HIV promoter only about twofold in the absence of USA (Fig. 4B, lane 1 versus lane 2) and either PC2 or PC4 alone could substitute, to different degrees, for the effect of USA on Sp1 function on the HIV promoter (Fig. 4A, lanes 1 to 6 and lanes 11 and 12). A titration of PC4 showed variable effects on the levels of activation by Sp1 (from threefold with 50 ng of PC4 to eightfold with 100 ng of PC4) (Fig. 4A, lane 1 versus lane 2 and lane 3 versus lane 4). The increased induction by Sp1 at higher PC4 levels reflects a net decrease in basal HIV transcription, which is efficiently reversed by Sp1 activity. This effect of Sp1 in reversing the inhibitory effect of PC4 on basal transcription is specific to the HIV promoter relative to the control AdML promoter (Fig. 4A, lanes 1 to 4).
FIG. 4.
Combination of PC2 and PC4 can fully reconstitute USA cofactor activity for Sp1 function. (A) Effects of various concentrations of PC2 and PC4 on Sp1 activity. Reaction mixtures contained the indicated amounts of purified PC4 and/or PC2 (2 μl of the Mono S fraction). (B) Synergistic effects of low concentrations of PC2 and PC4 on Sp1 function. Reaction mixtures contained the indicated amounts of PC4 and/or PC2 (0.5 and 1 μl of the Mono S fraction). Other components and reaction conditions were as described in the legend to Fig. 2. The assays in panels A and B were performed simultaneously with the same reagents under identical incubation, processing, and autoradiographic exposure conditions.
The purified PC2 fraction also showed a significant coactivator function with Sp1 (Fig. 4A, lane 5 versus lane 6). In this case, the overall transcription activity was higher with PC2 than with PC4 (Fig. 4A, lane 6 versus lanes 2 and 4). However, the level of induction was lower with PC2 than with PC4 (fourfold versus eightfold) (Fig. 4A, lanes 3 and 4 and lanes 5 and 6), similar to what was observed in the NF-κB analysis. This is due primarily to a more efficient reduction of basal transcription by PC4 that can be reversed by Sp1. Significantly, a combination of appropriate concentrations of PC2 and PC4 fully reconstituted the USA effect on Sp1 function (Fig. 4A, lanes 9 and 10 versus lanes 11 and 12). The levels of induction by Sp1 were 14-fold with a combination of PC2 and PC4 and 11-fold with USA. This combined effect of PC2 and PC4, relative to their independent effects, is due mainly to a reduction of basal transcription to the same level observed with the USA fraction and to an effective reversal of this inhibition in the presence of Sp1, thus leading to a higher level of induction. These results demonstrate that while several PCs may coexist in the USA fraction, the maintenance of a low (more physiological) level of basal activity and the function of at least some activators may depend, necessarily, only on the activities of very few PCs.
We next examined whether a possible synergistic effect of PC2 and PC4 on Sp1 function could be observed at otherwise limiting concentrations of PC2 and PC4. As mentioned above, without USA or purified PCs, Sp1 activated transcription by only twofold (Fig. 4B, lane 1 versus lane 2). With lower levels of purified PC2 (0.5 μl) or PC4 (25 ng) alone, Sp1 activated transcription by 4.5-fold and 4-fold, respectively (Fig. 4B, lane 1 versus lanes 3 and 5). A combination of the same amounts of PC2 and PC4 led to a 13-fold induction of transcription by Sp1 (Fig. 4B, lane 1 versus lane 7), whereas basal transcription was relatively unaffected by PC2 and PC4 alone (Fig. 4A, lane 7, versus Fig. 4B, lane 1). The greater-than-additive effect of both PC2 and PC4 indicates a synergistic effect of these two positive coactivators on Sp1 function. It is possible that this moderate synergism would be amplified in the context of a more natural chromatin template, where other constraints and cofactors may be involved.
The p65 C-terminal region is a potent activation domain in the highly purified system.
Previous studies performed in vivo (see the introduction) have shown that the p65 subunit has a strong activation domain in its C-terminal region. We next examined whether this C-terminal region (amino acids 416 to 550) can activate transcription in a purified transcription system when fused to the Gal4 DNA-binding domain. In vitro transcription assays were performed in the presence or absence of purified, bacterially expressed activators consisting of the Gal4 DNA-binding domain fused either to the C-terminal portion of the NF-κB p65 subunit (fGal-p65ct) or to the transcription activation domain from tumor suppressor protein p53 (fGal-p53nt, used as a control) (Fig. 5). Basal transcription was assayed on the AdML template, whereas the reporter for activator-dependent transcription for Gal fusion proteins contains five copies of the Gal4 DNA-binding site upstream of the HIV TATA box and the AdML initiator (Inr) element linked to a G-less cassette of 380 bp (10). Results in Fig. 5 show that the p53 (lanes 2 and 3 versus lane 1) and p65 (lanes 6 and 7 versus lane 1) activation domains are both potent transcription activators in the highly purified system, whereas the Gal4 DNA-binding domain alone showed no activation (lanes 4 and 5 versus lane 1). The strong activation (over 20-fold) by multiply bound p65 activation domains suggests that they most likely interact with several targets within TFIID, general factors or cofactors to facilitate formation of the preinitiation complex (PIC).
FIG. 5.
C-terminal region of p65 strongly activates transcription in vitro. The C-terminal region of p65 (the last 135 amino acids) and the N-terminal activation domain of p53 (amino acids 1 to 57, duplicated) were fused to the minimal Gal4 DNA-binding domain (amino acids 1 to 94), expressed in bacteria as FLAG-tagged proteins, and purified. These Gal fusion proteins were then tested for their activation potential in transcription assays. As a control, Gal4 (amino acids 1 to 94) alone was used. Transcription assays were performed with no Gal proteins (lane 1) or 10 and 20 ng of either fGal-p53 (lanes 2 and 3), fGal4(1–94) (lanes 3 and 4), or fGal-p65ct (lanes 6 and 7). The templates were pMLΔ53, as a control, and Gal4-responsive pG5HMC2AT containing five Gal4-binding sites upstream of the HIV TATA box core promoter.
The p65 activation domain interacts with both TBP and several TAFs.
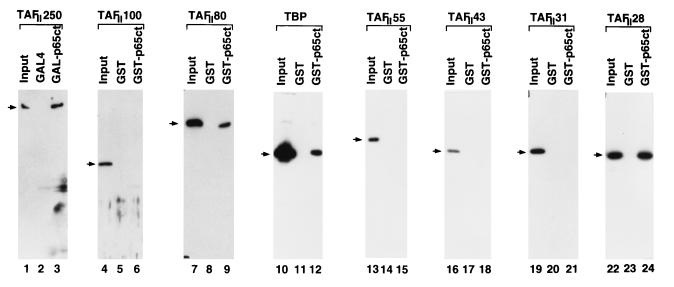
TFIID is indispensable for activator-dependent transcription (see below) under our standard in vitro assay conditions with purified factors, and earlier studies (see Discussion) have indicated that activator-dependent transcription can be achieved through specific interactions between activation domains of transcription factors and specific TFIID subunits. Therefore, we next examined potential protein-protein interactions between the potent C-terminal activation domain of p65 and different human TFIID subunits. A GST-p65 activation domain fusion protein (GST-p65ct) and fGalp65ct were expressed in bacteria, purified, and immobilized on glutathione-Sepharose or on M2 agarose. TBP and the human TAFs were overexpressed from baculovirus vectors in Sf9 cells, purified, and used for solution interactions with p65ct. After extensive washing, specific complexes were analyzed by SDS-PAGE and immunoblotting. Interactions were observed with TBP, TAFII250, TAFII80, and TAFII28 but not with TAFII100, TAFII55, or TAFII31 (Fig. 6). Although Schmitz et al. (60) reported an interaction between TBP and p65ct, this is the first report of p65-TAF interactions. Interactions with TAFs may be more relevant for activation than the interaction with TBP, since TBP alone does not support the high level of transcription activation by NF-κB that we have observed with TFIID (see below). These results are consistent with activation domains of transcription factors interacting with basal factor (TBP) or coactivator (TAF) components of TFIID. The redundancy of interactions may be required for synergistic activation of transcription (6).
FIG. 6.
In vitro interactions between the activation domain of NF-κB p65 and the human TFIID subunits. Purified HA-TAFII250 was incubated with equivalent amounts of M2 agarose-bound FLAG-Gal4(1–94) or FLAG-Gal-p65ct that was bound on M2 agarose, in BC150, 0.1% NP-40, and 100 μg of bovine serum albumin. After extensive washing with BC500, bound protein was resolved by SDS-PAGE and assayed by Western blotting. Input samples contained 10% of the amounts used for binding. Similarly, purified hTBP, hTAFII100, hTAFII80, hTAFII55, hTAFII43, hTAFII31, and hTAFII28 were incubated with either GST alone or GST-p65ct immobilized on glutathione beads. Reactions were then processed as for the TAFII250 analysis, except that the washing was performed with BC150. The arrow indicates the band corresponding to the appropriate TFIID subunit.
The p65 activation domain supports transcriptional activation with partial TFIID complexes.
We first compared the abilities of TBP and TFIID, at equimolar amounts based on quantitative Western blot analysis, to mediate activation by fGal-p65ct on a template containing five Gal4 sites upstream of the HIV TATA element. Our complementation system is TAF/TFIID-free, as shown both by functional analysis (Fig. 7C, lanes 7 and 8) and by Western blot analysis of different fractions (data not shown). Therefore, any transcription in this system is absolutely dependent on addition of exogenous TBP or TFIID. While transcription activation reached 17-fold in the presence of f-TFIID, it was only 1.8-fold in the presence of an equimolar amount TBP (Fig. 7B, lane 1 versus lane 2 and lane 3 versus lane 4). This result shows a clear requirement for TAFs for significant transcription activation by the NF-κB activation domain. Interestingly, whereas basal transcription was comparable for TBP and TFIID on the control AdML template, it was slightly higher for TBP than for TFIID on the test promoter (Fig. 7B, lane 1 versus lane 3). This may reflect inhibitory effects of TAFs on TBP binding and function on some promoters in the absence of activators or specific TAF-DNA interactions (54).
FIG. 7.
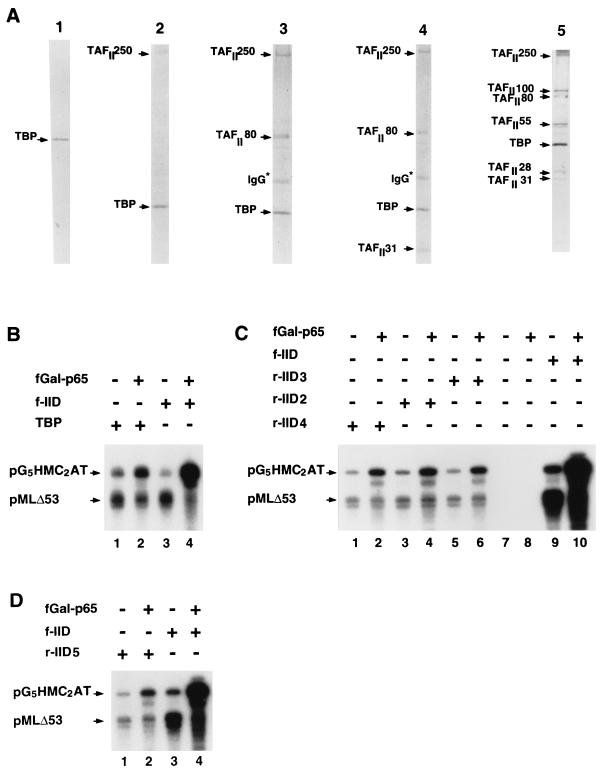
In vitro reconstitution and functional analysis of partial TFIID species. (A) Partially reconstituted TFIID species were resolved by SDS-PAGE and visualized by silver staining. The complexes were assembled with purified subunits that were individually expressed in Sf9 cells via baculovirus vectors (see Materials and Methods). Lane 1 contained TBP alone. Lanes 2, 3, 4, and 5 contained, respectively, hTBP-TAFII250 (r-IID2), hTBP-TAFII250-TAFII80 (r-IID3), hTBP-TAFII250-TAFII80-TAFII31 (r-IID4), and hTBP-TAFII250-TAFII100-TAFII80-TAFII55-TAFII31-TAFII28 (r-IID5) complexes. (B) Comparison of hTBP and f-TFIID transcriptional activities. Reaction mixtures contained hTBP alone (4 ng) (lanes 1 and 2) or highly purified f-TFIID (f-IID) (lanes 3 and 4) containing the same amount (4 ng) of hTBP. Purified recombinant fGal-p65ct (20 ng) was added to the samples in the even-numbered lanes. Transcription was assayed as described in the legend to Fig. 5. (C) Comparison of transcriptional activities of intact TFIID and partial TFIID complexes. The TFIID-dependent transcription system was supplemented with no protein (lanes 7 and 8), hTBP-TAFII250 (r-IID2, lanes 3 and 4), hTBP-TAFII250-TAFII80 (r-IID3, lanes 5 and 6), hTBP-TAFII250-TAFII80-TAFII31 (r-IID4, lanes 1 and 2), or f-TFIID (lanes, 9 and 10). Added TFIID and partial TFIID complexes contained the same amount of TBP (4 ng). Transcription was tested in the absence (lanes 1, 3, 5, and 7) or presence (lanes 2, 4, 6, and 8) of 20 ng of purified fGal-p65ct. (D) Comparison of transcription activities of intact TFIID and a seven-component partial TFIID complex. The TFIID-dependent transcription system was supplemented with either the partial complex hTBP-TAFII250-TAFII100-TAFII80-TAFII55-TAFII31-TAFII28 (r-IID5, lanes 1 and 2) or highly purified f-TFIID (lanes 3 and 4). Added TFIID and r-IID5 complexes contained the same amount (4 ng) of TBP. Reaction mixtures in lanes 2 and 4 contained 20 ng of fGal-p65-ct.
The human TFIID complex contains at least 13 TAFs in addition to TBP (for a review, see reference 5), and studies with corresponding cDNA-encoded proteins have documented a number of TBP-TAF and TAF-TAF interactions that may also occur within the natural TFIID complex (5, 11, 14, 21–23, 64, 65). Based on these studies, and others whose results are not shown here, we began the in vitro assembly of human TFIID to study the direct requirement of specific TAFs for both basal and activated transcription.
By use of a method similar to the one employed by Chen et al. (8) for the assembly of Drosophila TFIID, several combinations of human TAFs and TBP were assembled on an HA epitope-tagged TAFII250 column, eluted with HA peptides, and analyzed by SDS-PAGE and silver staining (Fig. 7A). Taking into account the differential staining of different TAFs, the purified complexes appear to contain roughly stoichiometric amounts of the recombinant TFIID subunits.
To study the TAF requirement for activation, we utilized complexes that contained, in addition to TBP-TAFII250, several different TAFs (TAFII80, TAFII80-TAFII31, or TAFII100-TAFII80-TAFII55-TAFII31-TAFII28) (Fig. 7A). Complexes with either TAFII80 or TAFII80-TAFII31 were designed to test whether these two complexes can mediate transcription activation by the p65ct acidic activator, as was shown for the corresponding Drosophila TAFs in the case of the TAF-interacting p53 acidic activation domain (67). The larger complex (containing seven subunits) was assembled to test possible effects of other currently available human TAFs on both basal and activated transcription.
We then compared equimolar amounts (based on TBP content) of f-TFIID and partial, in vitro-assembled TFIID species for both basal and fGal-p65ct-activated transcription. First, our data show that the partial complexes containing TAFII250 have a markedly reduced capacity for basal transcription compared to natural TFIID (Fig. 7C, lanes 1, 3, and 5 versus lane 9) or to TBP (Fig. 7B, lane 3 versus lane 1). DNA footprinting experiments show that this is due to the failure of TBP, when complexed to TAFII250 in the absence of a complete set of TAFs, to efficiently bind DNA (data not shown). This effect is specific for TAFII250, since a complex containing only TBP, TAFII80, and TAFII31 showed DNA binding quantitatively similar to that observed with TFIID, but restricted to the TATA box on the AdML template, and basal transcription activities comparable to those observed with f-TFIID (47a). The inhibitory effect of TAFII250 on TBP function is consistent with the results of studies with Drosophila TAFII250 and TBP (32, 45).
All of the TAFII250-containing partial TFIID species tested were capable of mediating a moderate level (3.5-fold) of transcription activation by fGal-p65ct that was specific for the test template containing the Gal4 sites (Fig. 7C and D). Significantly, for the partial complexes, the overall level of transcription in the presence of the activator only reached a level comparable to the level of basal transcription observed with complete f-TFIID alone (Fig. 7C, lanes 2, 4, and 6 versus lane 9, and Fig. 7D, lane 2 versus lane 3), which in turn is slightly lower than the level of basal transcription obtained with TBP (Fig. 7B, lane 3 versus lane 1). Thus, the “activation” observed in this analysis appears to reflect primarily a reversal of TAF-mediated repression rather than the large net increase in activity (above the TBP level) observed with intact TFIID. Nonetheless, it is possible that this activation represents part of the natural activation mechanism and that it may be further enhanced by the presence of missing human TAFs that, in this study, include TAFII135 and the putative human homolog of Drosophila TAFII150 (8, 71). The missing TAFs also could effect the basal transcription of the partial complexes.
The activated transcription that is observed with various TAFII250-containing partial complexes appears to be directly related to the interaction of the activation domain of p65 with TAFII250, rather than other TAFs. This is suggested by the finding of comparable levels of activation with the complex that contains only TBP and TAFII250 and with those complexes that contain additional TAFs (Fig. 7C and D) and by the observation that a stable TBP-TAFII80-TAFII31 complex shows the same basal activity as TBP alone and fails to mediate any activation by p65 (data not shown). Human TAFII250 has been reported to interact physically with two activators (7, 19), although functional data linking these TAFII250 interactions to transcription activation are lacking. Our study is the first to suggest such a role for hTAFII250. It is still possible, however, that interaction of p65ct with TBP reverses the inhibitory effect of TAFII250-TBP interaction (45).
Different core promoters have variable effects on both basal and activated transcription in the presence of TFIID and partial TFIID complexes.
In the assays described above, the core promoter of the Gal4-p65-responsive template contained the HIV-1 TATA element and a downstream Inr element. We next examined whether a different core promoter within the Gal4-responsive template would alter the effect of TAFs on either basal or fGal-p65-activated transcription when assayed with TBP, complete f-TFIID, and partial TFIID complexes. In this case, the template contained five Gal4 sites upstream of the adenovirus E1b TATA and natural E1b Inr regions, and the results are as follows. First, while TFIID and TBP supported comparable levels of basal transcription on the AdML template (Fig. 8, lanes 9 and 11), basal activity on the E1b template was clearly lower (circa fivefold) with TFIID than with TBP (Fig. 8, lanes 9 and 11). This underscores a selective negative effect of TAFs on TBP activity that is dependent on the type of core promoter used and is consistent with the AdML versus HIV core promoter comparisons in Fig. 7B. Second, and significantly, fGal4-p65ct activated transcription 42-fold in the presence of f-TFIID but only 2-fold in the presence of TBP alone (Fig. 8, lane 9 versus lane 10 and lane 11 versus lane 12), showing again the coactivator function of TAFs but in the context of a different core promoter. It is important to note that this high level of induction in the presence of TFIID reflects the dual role of TAFs: an inhibitory effect on TBP-mediated basal activity that is reversed by the activator and a coactivator function that leads to a net increase in activity (above the TBP level) in response to the activator (Fig. 8, lane 10 versus lane 12). This demonstrates clearly that high levels of induction with a net increase above the intrinsic TBP-mediated basal activity can be achieved by combined antirepression and true (net) activation mechanisms.
FIG. 8.
Different core promoters have various effects on both basal and activated transcription in the presence of TFIID and partial TFIID complexes. fGal-p65 transcriptional activation was assayed with TBP, f-TFIID, or partial TFIID complexes on a Gal4-responsive template containing the Ad E1b TATA and Inr regions. The TFIID-dependent transcription system was supplemented either with the partial complexes r-IID2 (lanes 1 and 2), r-IID3 (lanes 3 and 4), r-IID4 (lanes 5 and 6), and r-IID5 (lanes 7 and 8) or with complete f-TFIID (f-IID, lanes 9 and 10). Lanes 11 and 12 contained hTBP alone. All additions were adjusted to the same amount of TBP (4 ng). Purified recombinant fGal-p65ct (20 ng) was added to the reaction mixtures in the even-numbered lanes. Transcription reactions were performed as described in the legend to Fig. 7.
Similar to what was observed in the analysis of Fig. 7 for the HIV-1-based template, all partial TFIID complexes showed a basal activity lower than that observed with TBP alone and all mediated activation by Gal4-p65ct on the E1b-based template (Fig. 8, lanes 1, 3, 5, and 7 versus lanes 2, 4, 6, and 8). However, on the E1b promoter, the absolute levels of activated transcription observed with the partial complexes were significantly higher than the level of basal transcription observed with complete f-TFIID alone (Fig. 8, lanes 2, 4, 6, and 8 versus lane 9). This indicates that on the E1b core promoter-containing template, the induction of transcription in the presence of partial TFIID complexes represents, at least in part, a net increase in activity rather than simply a reversal of the TAF-mediated repression (see also below). However, consistent with the observations on the HIV template, the failure to achieve the high absolute level of activity observed with natural TFIID argues for additional functions of other TAFs in achieving maximal levels of activation.
A minimal TFIID complex, TBP-TAFII250, effects a net level of activation by fGalp65ct in the presence of PC2 and PC4.
In the assays described in Fig. 7 and 8, we used the unfractionated USA fraction together with partial TFIID complexes to investigate the TAF requirement for activated transcription and showed that all of the TAFII250-containing partial TFIID species tested were capable of mediating transcriptional activation by fGalp65ct. We next wished to determine the effect of a minimal TBP-TAFII250 complex on activated transcription in the presence of purified USA components PC2 and PC4 by using the Gal4-responsive template with the E1b core promoter. First, we found that without USA or purified PCs, Galp65ct specifically repressed transcription (by 2.5-fold) from the test template (Fig. 9, lane 1 versus lane 2). Second, and consistent with its general inhibitory role, the addition of unfractionated USA had a repressive effect on basal transcription from both the test and the control AdML templates (Fig. 9, lane 3 versus lane 1). Consistent with the results of Fig. 8 (lanes 1 and 2), addition of Galp65ct to a reaction mixture containing the USA fraction resulted in 5.5-fold activation (Fig. 9, lane 4 versus lane 3). This level of induction is due mainly to specific (from the test template only) reversal of the inhibitory effect of USA and further enhancement above the basal level of transcription. Third, purified PC2 alone mildly increased basal activity on both test and control templates (Fig. 9, lane 5 versus lane 1) and showed a coactivator function with fGalp65 (Fig. 9, lane 5 versus lane 6). The level of induction for PC2 (2.5-fold) reflects a modest net increase in the overall level of transcription. Addition of PC4 alone had no effect on basal transcription from either template (Fig. 9, lane 7 versus lane 1), and somewhat surprisingly, the further addition of the activator still effected a mild repression (1.5-fold) of transcription (Fig. 9, lane 7 versus lane 8). Importantly, however, the addition of PC4 to reaction mixtures containing PC2 increased the overall level of induction by fGalp65ct from 2.5-fold (Fig. 9, lane 5 versus lane 6) to 7-fold (Fig. 9, lane 9 versus lane 10). The sevenfold induction represents a clear net activation of transcription and not just an antirepression mechanism, since the basal transcription level with a combination of PC2 and PC4 is comparable to the basal transcription level without any USA components (Fig. 9, lane 9 versus lane 1). Taken together, these data show that a strong net activation (increase in transcription above the basal level obtained without any USA components) by the NF-κB p65 activation domain can be achieved with only a TBP-TAFII250 complex in the presence of two USA components: PC2 and PC4. This, plus the failure to see activation with TBP alone, provides further support for the relevance of the p65-TAFII250 interaction.
FIG. 9.
A minimal TFIID complex, TBP-TAFII250, effects a net level of activation by fGalp65ct in the presence of PC2 and PC4. fGalp65 transcriptional activation was assayed with the TBP-TAFII250 complex on a Gal-responsive template containing the Ad E1b core promoter. All reaction mixtures contained the TBP-TAFII250 partial complex. As indicated, the reaction mixtures were also supplemented with either unfractionated USA (lanes 3 and 4), 2 μl of the PC2 Mono S fraction (lanes 5, 6, 9, and 10), or purified PC4 (50 ng) (lanes 7 to 10). Purified recombinant fGalp65ct (20 ng) was added to the reaction mixtures in the even-numbered lanes.
DISCUSSION
HIV-1 and Ad promoters have been used to study cofactor requirements and mechanisms for activation by NF-κB and Sp1 through cognate binding sites. These studies have shown that recombinant NF-κB and Sp1 proteins strongly activate transcription in a purified system, that activation is mediated by TAFs and USA-derived components (PC2 and PC4), that high levels of induction from a low (more physiological) basal activity result from both negative and positive functions of these two distinct classes of coactivators, and that some of the effects are core promoter specific. These results both support and extend previous observations regarding TAF function in metazoans (reviewed in references 54 and 70) and are also relevant to the recent finding of a core promoter-specific TAF function in yeast (61).
USA-derived cofactors: independent and cooperative functions in repression of basal transcription and in activator-mediated transcription.
Early studies have shown that USA is required for the activity of several Gal4 fusion activators, NF-κB p50 homodimers, Sp1, and USF (18, 33–35, 41, 60). Our data for the HIV-1 promoter confirm these early reports and further show a persistent requirement for the USA fraction for NF-κB and Sp1 functions, even in the presence of essentially homogeneous (affinity-purified) TFIID. This argues against a conditional requirement for USA to eliminate (for example) nonspecific inhibitory effects of other contaminating proteins. Importantly, USA effected both a large net increase in activator-dependent transcription and a significant repression of basal (activator-independent) transcription, leading to a very high level of transcriptional induction that more closely mimics the in vivo situation.
We next investigated the ability of two components (PC2 and PC4) of the USA fraction to support transcription activation by both NF-κB and Sp1. Our results show that in both cases, either highly purified PC2, which appears to be physically distinct from other PCs and TAFs on the basis of molecular size (circa 500 kDa) and chromatographic properties, or recombinant PC4 (a 15-kDa protein) is sufficient for mediation of activator-dependent transcription. However, the basal transcription levels observed with these positive cofactors alone are generally higher than the basal level observed with the USA fraction. This indicates that low levels of basal activity and the consequent high levels of induction by activators require either the function of negative cofactors like NC1 (40) that are usually present in the crude USA fraction or a balance of PCs that, in some cases (18), may also inhibit basal transcription at high concentrations. In this regard, we have shown that a combination of PC2 and PC4, at the appropriate concentrations, can restore the total function of the USA fraction (low basal plus high activator-dependent transcription) when assayed with Sp1. This suggests that single components of the USA fraction may act either alone or in combination with other USA components to simultaneously repress the basal activity and enhance the activity of specific activators in an optimal fashion. Consistent with this, and at low cofactor concentrations, we have also observed some synergism between PC2 and PC4 in mediating activation by Sp1. Similarly, in the case of NF-κB (p50/p65), either PC2 or PC4 can support the activator function, although a combination of both PC2 and PC4 results in a high absolute level of activation and a higher level of induction than that observed with either alone.
In summary, our data demonstrate that single components of the USA fraction can function in combination to restore all or part of the coactivator activity of USA when naked DNA is used as the template. Other components from the USA fraction, or from other subnuclear fractions, also may be utilized in more physiological situations, where constraints of chromatin structure may require other types of coactivators or chromatin-remodeling factors (reviewed in reference 30) for activator functions. In this regard, Pazin et al. (49) recently reported an in vitro synergism between Sp1 and NF-κB activities that was dependent upon the use of chromatin (rather than DNA) templates. This could reflect a requirement for additional cofactors such as those described here, although the observed activation appeared to reflect mainly antirepression effects and no specific (co)factor requirements were determined. Thus, in our system, it will be important to determine whether there is a more stringent requirement for other USA components or whether the weak cooperativity that we have observed between PC2 and PC4 may be amplified when activator functions are tested on chromatin templates.
Role of TAFs in activator-dependent transcription.
As we have demonstrated, efficient activation by Sp1 and NF-κB requires not only the USA-derived cofactors, but also the TAFs that are tightly associated with TBP in the TFIID complex. In general, the efficiency of PIC assembly or function is controlled by the presence of transcription factors usually bound to specific upstream sequences. Some models of how transcription factors influence PIC assembly involve interactions with TFIID that, through qualitative and/or quantitative effects on TFIID binding, enhance the recruitment of downstream factors (1, 5, 9, 25, 26, 37, 73). Whereas TFIID was found to mediate both basal and activator-dependent transcription in cell-free systems reconstituted with purified components, TBP elicited only basal transcription (10, 16, 24, 53, 57, 63, 75). Following the demonstration of a number of specific activator-TAF interactions (reviewed in reference 5), the direct involvement of specific TAFs in the function of specific activators was shown in studies using Drosophila TFIID complexes (or subcomplexes) reconstituted with the recombinant TAFs in vitro (8, 67). In addition, the synergistic function of two activators that interact with two different TAFs was demonstrated with reconstituted Drosophila TFIID complexes, and the mechanism was shown to involve enhanced TFIID binding (recruitment) to the promoter (56).
Since the C-terminal region of p65 is a very strong activator when fused to a Gal4 DNA-binding domain and tested in the purified reconstituted transcription system, and since TFIID is a target for many activators, we have analyzed the interactions of the p65 activation domain with different human TAFs and TBP. The activator p65 interacts directly both with TBP and with several TAFs that include TAFII80 and TAFII250. The interaction with TAFII80 is consistent with data published for acidic activation domains, which seem to interact preferentially with TAFII80 and TAFII31 and their Drosophila homologs TAFII60 and TAFII40 (31, 39, 67). However, as shown here, p65ct also interacts strongly with TAFII250, and this interaction appears to be directly relevant to transcription activation since a complex that contains only TBP and TAFII250, but not TBP alone, supports p65 activity in the absence of TAFII80. These data support the conclusions of earlier studies (see above) implicating specific TAFs in the function of other activators (reviewed in reference 70). Although several studies have shown that the activation domains of c-rel and p65 can both interact with TBP and TFIID (29, 60, 74), these interactions were not shown to be directly relevant to activation by the NF-κB proteins. Because we have shown directly that TFIID, rather than TBP alone, is required for activated transcription, TAF interactions with p65 appear to be more relevant for transcriptional activation by p65. While other studies have shown that two viral activators, E1A and ICP4, can interact with human TAFII250 (7, 19), our study is the first to suggest a direct role of TAFII250 in activated transcription. The failure of other p65-interacting TAFs (e.g., TAFII80) to enhance the function, in activation, of TAFII250-TBP complexes suggests either that these interactions do not occur in the context of the partial TFIID complex or that they are redundant with the p65-TAFII250 interaction. The functional consequence of such interactions could also be that additional TAFs are required.
Dual roles of TAFs: TAF-mediated repression of TBP function and TAF-mediated net increase in transcriptional activation.
Another important property of TAFs that we have observed is their ability, on certain core promoters, to inhibit TBP-mediated basal activity within native TFIID compared to TBP alone (Fig. 7 and 8). In the presence of an activator, there is both a reversal of TAF-mediated repression of TBP function and a TAF-mediated net increase in overall transcription activity. This leads to a high level of transcription induction that more closely mimics the physiological situation. However, with partially reconstituted TFIID complexes, and depending on the core promoter used, the absolute levels of transcription in the presence of the fGal4-p65 activator were only comparable to (HIV-1 promoter) or moderately greater than (Ad5 E1b promoter) the level of basal activity observed with natural (complete) TFIID alone and 20-fold (HIV-1 promoter) to 25-fold (E1b promoter) lower than the levels observed with TFIID in the presence of the activator. Our results on the E1b core promoter, especially with PC2 and PC4 in place of USA, are more consistent with previous indications from Tjian and colleagues (8, 56) that specific TAFs can serve as coactivators in partial complexes and result in levels of activator-dependent transcription greater than basal levels. Our failure to fully recapitulate the expected large net increases of activity with partial complexes could be due to the absence of a specific subset of the TAFs in the reconstituted system. Alternatively (see also below), human TFIID may function optimally only as a complete entity, which may allow other DNA interactions and conformational changes important for the efficient recruitment and function of other general transcription factors (references above; 9, 47). Nonetheless, by using partial TFIID complexes, we have dissociated two functions of TAFs in transcription regulation: intrinsic repressive effects on TBP binding and function that may be core promoter-specific and coactivator functions, leading to reversal of the repressive effects and large net increases in activation, that may be activator specific.
Our demonstration that TAFII250 represses the basal transcription activity of TBP on the HIV promoter is in agreement with what was reported earlier for Drosophila TAFII250 and TBP (32, 45). However, no apparent repression of TBP by Drosophila TAFII250 was observed when the latter was reconstituted in vitro by Chen et al. (8). This may reflect differences in the functional TBP content of various complexes (or preparations of complexes), the specific promoters used, or the purity of the reconstituted systems. We also have observed greater repression of basal activity with all of the partial TAFII250-containing complexes tested compared to native TFIID, suggesting that additional TAF-TAF and/or TAF-DNA interactions partially relieve the repression within native TFIID. In this regard, an early study by Nakatani et al. (44) showed that downstream promoter interactions are necessary to reverse TAF-mediated repression of natural TFIID binding and function (relative to TBP) in the gfa core promoter, and activator-TAF interactions could serve similar functions in other situations.
Finally, recent studies with yeast have shown that most TAFs are dispensable for activated transcription from many genes in vivo but necessary for cell cycle progression (43, 72). These results led the investigators to propose that TAFs are only required for transcription of a subset of genes, and Shen and Green (61) have recently identified several yeast TAFII145-dependent genes whose TAF requirements are dictated by their (as yet undefined) core promoters rather than by specific activators. Thus, the TAF properties discussed above—effects on TBP binding and function that may be core promoter specific—and the presence of additional coactivators (3) for specific activators could explain, at least in part, the observations in yeast. Moreover, while the transcription of some genes in metazoans could prove to be TAF independent, it is also possible that metazoans have broader TAF requirements than yeast (65).
ACKNOWLEDGMENTS
We thank C.-M. Chiang for the FLAG-Gal4 expression plasmid, D. K. Lee for the E1b construct, and T. Oelgeschläger for critical comments on the manuscript.
This work was supported by a grant from the Tebil Foundation to The Rockefeller University and by NIH grants AI32737 and CA 42567 to R.G.R.
REFERENCES
- 1.Abmayr S M, Workman J L, Roeder R G. The pseudorabies immediate early protein stimulates in vitro transcription by facilitating TFIID:promoter interactions. Genes Dev. 1988;2:542–553. doi: 10.1101/gad.2.5.542. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin A S. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1995;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Björklund S, Kim Y-J. Mediator of transcriptional regulation. Trends Biochem Sci. 1996;21:335–337. doi: 10.1016/s0968-0004(96)10051-7. [DOI] [PubMed] [Google Scholar]
- 4.Blair W S, Bogerd H P, Madore S J, Cullen B R. Mutational analysis of the transcriptional analysis of RelA: identification of a highly synergistic minimal acidic activation module. Mol Cell Biol. 1994;14:7226–7234. doi: 10.1128/mcb.14.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burley S K, Roeder R G. Biochemistry and structural biology of transcription factor IID (TFIID) Annu Rev Biochem. 1996;65:769–799. doi: 10.1146/annurev.bi.65.070196.004005. [DOI] [PubMed] [Google Scholar]
- 6.Carey M, Lin Y-Y, Green M R, Ptashne M. A mechanism for synergistic activation of a mammalian gene by GAL4 derivatives. Nature. 1990;345:361–364. doi: 10.1038/345361a0. [DOI] [PubMed] [Google Scholar]
- 7.Carrozza M J, Deluca N A. Interaction of the viral protein ICP4 with TFIID through TAF250. Mol Cell Biol. 1996;16:3085–3093. doi: 10.1128/mcb.16.6.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J-L, Attardi L D, Verrijzer C P, Yokomori K, Tjian R. Assembly of recombinant TFIID reveals differential coactivator requirements for distinct transcriptional activators. Cell. 1994;79:93–105. doi: 10.1016/0092-8674(94)90403-0. [DOI] [PubMed] [Google Scholar]
- 9.Chi T, Carey M. Assembly of the isomerized TFIIA-TFIID-TATA ternary complex is necessary and sufficient for gene activation. Genes Dev. 1996;10:2540–2550. doi: 10.1101/gad.10.20.2540. [DOI] [PubMed] [Google Scholar]
- 10.Chiang C-M, Ge H, Wang Z, Hoffmann A, Roeder R G. Unique TATA-binding protein-containing complexes and cofactors involved in transcription by RNA polymerase II and III. EMBO J. 1993;12:2749–2762. doi: 10.1002/j.1460-2075.1993.tb05936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang C-M, Roeder R G. Cloning of an intrinsic human TFIID subunit that interacts with multiple transcriptional activators. Science. 1995;267:531–536. doi: 10.1126/science.7824954. [DOI] [PubMed] [Google Scholar]
- 12.Dejong J, Roeder R G. A single cDNA, hTFIIA/α, encodes both the p35 and p19 subunits of human TFIIA. Genes Dev. 1993;7:2220–2234. doi: 10.1101/gad.7.11.2220. [DOI] [PubMed] [Google Scholar]
- 13.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubrovskaya V, Lavigne A-C, Davidson I, Acker J, Staub A, Tora L. Distinct domains of hTAFII100 are required for functional interaction with transcription factor TFIIFβ (RAP30) and incorporation into the TFIID complex. EMBO J. 1996;15:3702–3712. [PMC free article] [PubMed] [Google Scholar]
- 15.Duckett C S, Perkins N D, Kowalil T F, Schmid R M, Huang E S, Baldwin A S, Jr, Nabel G J. Dimerization of NF-κB2 with RelA (p65) regulates DNA binding, transcription activation, and inhibition by IκBα (MAD-3) Mol Cell Biol. 1993;13:1315–1322. doi: 10.1128/mcb.13.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dynlacht B D, Hoey T, Tjian R. Isolation of coactivators associated with the TATA-binding protein that mediates transcriptional activation. Cell. 1991;66:563–576. doi: 10.1016/0092-8674(81)90019-2. [DOI] [PubMed] [Google Scholar]
- 17.Fujita T, Nolan G P, Ghosh S, Baltimore D. Independent modes of transcriptional activation by the p50 and p65 subunits of NF-κB. Genes Dev. 1992;6:775–787. doi: 10.1101/gad.6.5.775. [DOI] [PubMed] [Google Scholar]
- 18.Ge H, Roeder R G. Purification, cloning, and characterization of a human coactivator, PC4, that mediates transcriptional activation of class II genes. Cell. 1994;78:513–523. doi: 10.1016/0092-8674(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 19.Geisberg J V, Chen J-L, Ricciardi R P. Subregions of the adenovirus E1A transactivation domain target components of the TFIID complex. Mol Cell Biol. 1995;15:6283–6290. doi: 10.1128/mcb.15.11.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh S, Gifford A M, Riviere L R, Tempst P, Nolan G P, Baltimore D. Cloning of the p50 DNA binding subunit of NF-κB—homology to Rel and Dorsal. Cell. 1990;62:1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- 21.Hisatake K, Ohta T, Takada R, Guermah M, Horikoshi M, Nakatani Y, Roeder R G. Evolutionary conservation of human TATA-binding-polypeptide-associated factors TAFII31 and TAFII80 and interactions of TAFII80 with other TAFs and with general transcription factors. Proc Natl Acad Sci USA. 1995;92:8195–8199. doi: 10.1073/pnas.92.18.8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann A, Chiang C-M, Oelgeschläger T, Xie X, Burley S K, Nakatani Y, Roeder R G. A histone octamer-like structure within TFIID. Nature. 1996;380:356–359. doi: 10.1038/380356a0. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann A, Roeder R G. Cloning and characterization of human TAF20/15. J Biol Chem. 1996;271:18194–18202. doi: 10.1074/jbc.271.30.18194. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann A, Sinn E, Yamamoto T, Wang J, Roy A, Horikoshi M, Roeder R G. Highly conserved core domain and unique N terminus with presumptive regulatory motifs in a human TATA factor TFIID. Nature. 1990;346:387–390. doi: 10.1038/346387a0. [DOI] [PubMed] [Google Scholar]
- 25.Horikoshi M, Carey M F, Kakidani H, Roeder R G. Mechanism of action of a yeast activator: direct effect of GAL4 derivatives on mammalian TFIID-promoter interactions. Cell. 1988;54:665–669. doi: 10.1016/s0092-8674(88)80011-4. [DOI] [PubMed] [Google Scholar]
- 26.Horikoshi M, Hai T, Lin Y-S, Green M R, Roeder R G. Transcription factor ATF interacts with TATA factor to facilitate establishment of a preinitiation complex. Cell. 1988;54:1033–1042. doi: 10.1016/0092-8674(88)90118-3. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser K, Meisterernst M. The human general cofactors. Trends Biochem Sci. 1996;21:342–345. [PubMed] [Google Scholar]
- 28.Kawakami K, Scheidereit C, Roeder R G. Identification and purification of a human immunoglobulin-enhancer-binding protein (NF-κB) that activates transcription from a human immunodeficiency virus type 1 promoter in vitro. Proc Natl Acad Sci USA. 1988;85:4700–4704. doi: 10.1073/pnas.85.13.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerr L D, Ransone L J, Wamsley P, Schmitt M J, Boyer T G, Zhou Q, Berk A J, Verma I M. Association between proto-oncoprotein Rel and TATA-binding protein mediates transcription activation by NF-κB. Nature. 1993;338:39–44. doi: 10.1038/365412a0. [DOI] [PubMed] [Google Scholar]
- 30.Kingston R E, Bunker C A, Imbalzano A N. Repression and activation by multiprotein complexes that alter chromatin structure. Genes Dev. 1996;10:905–920. doi: 10.1101/gad.10.8.905. [DOI] [PubMed] [Google Scholar]
- 31.Klemm R D, Goodrich J A, Zhou S, Tjian R. Molecular cloning and expression of the 32-kDa subunit of human TFIID reveals interactions with VP16 and TFIIB that mediate transcriptional activation. Proc Natl Acad Sci USA. 1996;92:5788–5792. doi: 10.1073/pnas.92.13.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kokubo T, Gong D-W, Wooton J C, Horikoshi M, Roeder R G, Nakatani Y. Drosophila 230-KD TFIID subunit, a functional homolog of the human cell cycle gene product, negatively regulates DNA binding of the TATA box-binding subunit of TFIID. Genes Dev. 1993;7:1033–1046. doi: 10.1101/gad.7.6.1033. [DOI] [PubMed] [Google Scholar]
- 33.Kretzschmar M, Kaiser K, Lottspeich F, Meisterernst M. A novel mediator of class II gene transcription with homology to viral immediate-early transcription regulators. Cell. 1994;78:525–534. doi: 10.1016/0092-8674(94)90429-4. [DOI] [PubMed] [Google Scholar]
- 34.Kretzschmar M, Meisterernst M, Scheidereit C, Li G, Roeder R G. Transcriptional regulation of the HIV-1 promoter by NF-κB in vitro. Genes Dev. 1992;6:761–774. doi: 10.1101/gad.6.5.761. [DOI] [PubMed] [Google Scholar]
- 35.Kretzschmar M, Meisternst M, Roeder R G. Identification of human DNA topoisomerase I as a cofactor for activator-dependent transcription by RNA polymerase II. Proc Natl Acad Sci USA. 1993;90:11508–11512. doi: 10.1073/pnas.90.24.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kretzschmar M, Stelzer G, Roeder R G, Meisterernst M. RNA polymerase II cofactor PC2 facilitates activation of transcription by GAL4-AH in vitro. Mol Cell Biol. 1994;14:3927–3937. doi: 10.1128/mcb.14.6.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lieberman P M, Berk A J. A mechanism for TAFs in transcriptional activation: activation domain enhancement of TFIID-TFIIA promoter DNA complex formation. Genes Dev. 1994;8:995–1006. doi: 10.1101/gad.8.9.995. [DOI] [PubMed] [Google Scholar]
- 38.Lin Y S, Carey M F, Ptashne M, Green M R. GAL4 derivatives function alone and synergistically with mammalian activators in vitro. Cell. 1988;54:659–664. doi: 10.1016/s0092-8674(88)80010-2. [DOI] [PubMed] [Google Scholar]
- 39.Lu H, Levine A J. Human TAFII31 protein is a transcriptional coactivator of the p53 protein. Proc Natl Acad Sci USA. 1995;92:5154–5158. doi: 10.1073/pnas.92.11.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meisterernst M, Roeder R G. Family of proteins that interact with TFIID and regulate promoter activity. Cell. 1991;67:557–567. doi: 10.1016/0092-8674(91)90530-c. [DOI] [PubMed] [Google Scholar]
- 41.Meisterernst M, Roy A L, Lieu H M, Roeder R G. Activation of class II transcription by regulatory factors is potentiated by a novel activity. Cell. 1991;66:981–993. doi: 10.1016/0092-8674(91)90443-3. [DOI] [PubMed] [Google Scholar]
- 42.Moore P A, Ruben S M, Rosen C A. Conservation of transcriptional activation functions of the NF-κB p50 and p65 subunits in mammalian cells and Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:1666–1674. doi: 10.1128/mcb.13.3.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moqtaderi Z, Bai Y, Poon D, Weil A P, Struhl K. TBP-associated factors are not generally required for transcription activation in yeast. Nature. 1996;383:188–191. doi: 10.1038/383188a0. [DOI] [PubMed] [Google Scholar]
- 44.Nakatani Y, Horikoshi M, Brenner M, Yamamoto T, Besnard F, Roeder R G, Freese E. A downstream initiation element required for efficient TATA box binding and in vitro function of TFIID. Nature. 1990;348:86–88. doi: 10.1038/348086a0. [DOI] [PubMed] [Google Scholar]
- 45.Nishizawa J-I, Kokubo T, Horikoshi M, Roeder R G, Nakatani Y. Drosophila TAFII230 and the transcriptional activator VP16 bind competitively to the TATA box-binding domain of the TATA box-binding protein. Proc Natl Acad Sci USA. 1997;94:85–90. doi: 10.1073/pnas.94.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nolan G P, Ghosh S, Liou H, Tempst P, Baltimore D. DNA binding and IκB inhibition of the cloned p65 subunit of NF-κB, a Rel-related polypeptide. Cell. 1991;64:961–969. doi: 10.1016/0092-8674(91)90320-x. [DOI] [PubMed] [Google Scholar]
- 47.Oelgeschläger T, Chiang C-M, Roeder R G. Topology and reorganization of a human TFIID-promoter complex. Nature. 1996;382:735–738. doi: 10.1038/382735a0. [DOI] [PubMed] [Google Scholar]
- 47a.Oelgeschläger, T., M. Guermah, and R. G. Roeder. Unpublished data.
- 48.Orphanides G, Lagrange T, Reinberg D. The general transcription factors of RNA polymerase II. Genes Dev. 1996;10:2657–2683. doi: 10.1101/gad.10.21.2657. [DOI] [PubMed] [Google Scholar]
- 49.Pazin M J, Sheridan P L, Cannon K, Cao Z, Keck J G, Kadonaga J T, Jones K A. NF-κB-mediated chromatin reconfiguration and transcriptional activation of the HIV-1 enhancer in vitro. Genes Dev. 1996;10:37–49. doi: 10.1101/gad.10.1.37. [DOI] [PubMed] [Google Scholar]
- 50.Perkins N D, Agranoff A B, Pascal E, Nabel G J. An interaction between the DNA-binding domains of RelA(p65) and Sp1 mediates human immunodeficiency virus gene activation. Mol Cell Biol. 1994;14:6570–6583. doi: 10.1128/mcb.14.10.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perkins N D, Edwards N L, Duckett C S, Agranoff A, Shmid R M, Nabel G. A cooperative interaction between NFκB and Sp1 is required for HIV-1 enhancer activation. EMBO J. 1993;12:3551–3558. doi: 10.1002/j.1460-2075.1993.tb06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perkins N D, Shmid R M, Duckett C S, Leung K, Rice N R, Nabel G J. Distinct combinations of NF-κB subunits determine the specificity of transcriptional activation. Proc Natl Acad Sci USA. 1992;89:1529–1533. doi: 10.1073/pnas.89.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pugh B F, Tjian R. Mechanism of transcriptional activation by Sp1: evidence for coactivators. Cell. 1990;61:1187–1197. doi: 10.1016/0092-8674(90)90683-6. [DOI] [PubMed] [Google Scholar]
- 54.Roeder R G. The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem Sci. 1996;21:327–335. [PubMed] [Google Scholar]
- 55.Ruben S M, Dillon P J, Schreck R, Henkel T, Chen C H, Maher M, Baeuerle P A, Rosen C A. Isolation of a rel-related human cDNA that potentially encodes the 65-kD subunit of NF-κB. Science. 1991;251:1490–1493. doi: 10.1126/science.2006423. [DOI] [PubMed] [Google Scholar]
- 56.Sauer F, Hansen S K, Tjian R. Multiple TAFIIs directing synergetic activation of transcription. Science. 1995;270:1783–1788. doi: 10.1126/science.270.5243.1783. [DOI] [PubMed] [Google Scholar]
- 57.Sawadogo M, Roeder R G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985;43:165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- 58.Schmid R M, Liptay S, Betts J, Nabel G. Structural and functional analysis of NF-κB. J Biol Chem. 1994;269:32162–32167. [PubMed] [Google Scholar]
- 59.Schmitz M L, Baeuerle P A. The p65 subunit is responsible for the strong transcription activating potential of NF-κB. EMBO J. 1991;10:3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmitz M L, Stelzer G, Altmann H, Meisterernst M, Baeuerle P A. Interaction of the C-terminal transactivation domain of p65NF-κB with TATA-binding protein, transcription factor IIB, and coactivators. J Biol Chem. 1995;270:7219–7226. doi: 10.1074/jbc.270.13.7219. [DOI] [PubMed] [Google Scholar]
- 61.Shen W-C, Green M R. Yeast TAFII145 functions as a core promoter selectivity factor, not a general coactivator. Cell. 1997;90:615–624. doi: 10.1016/s0092-8674(00)80523-1. [DOI] [PubMed] [Google Scholar]
- 62.Summers M, Smith G E. A manual of methods for baculovirus vectors and insect cell culture procedures. Tex. Agric. Exp. Stn. Bull. No. 1555. 1987. [Google Scholar]
- 63.Tanese N, Pugh B F, Tjian R. Coactivators for a proline-rich activator purified from the multisubunit human TFIID complex. Genes Dev. 1991;5:2212–2224. doi: 10.1101/gad.5.12a.2212. [DOI] [PubMed] [Google Scholar]
- 64.Tanese N, Saluja D, Vassalo M F, Chen J-L, Admon A. Molecular cloning and analysis of two subunits of the human TFIID complex: hTAFII130 and hTAFII100. Proc Natl Acad Sci USA. 1996;93:13611–13616. doi: 10.1073/pnas.93.24.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tansey W P, Herr W. TAFs: guilt by association? Cell. 1997;88:729–732. doi: 10.1016/s0092-8674(00)81916-9. [DOI] [PubMed] [Google Scholar]
- 66.Tao Y, Guermah M, Martinez E, Oelgeschläger T, Hasegawa S, Takada R, Yamamoto T, Horikoshi M, Roeder R G. Specific interactions and potential functions of human TAFII100. J Biol Chem. 1997;272:6714–6721. doi: 10.1074/jbc.272.10.6714. [DOI] [PubMed] [Google Scholar]
- 67.Thut C J, Chen J-L, Klemm R, Tjian R. p53 transcriptional activation mediated by coactivators TAFII40 and TAFII60. Science. 1995;367:100–104. doi: 10.1126/science.7809597. [DOI] [PubMed] [Google Scholar]
- 68.Urban M B, Schreck R, Baeuerle P A. NF-κB contacts DNA by a heterodimer of the p50 and p65 subunits. EMBO J. 1991;10:1817–1825. doi: 10.1002/j.1460-2075.1991.tb07707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 70.Verrijzer C P, Tjian R. TAFs mediate transcriptional activation and promoter selectivity. Trends Biochem Sci. 1996;21:338–342. [PubMed] [Google Scholar]
- 71.Verrijzer C P, Yokomori K, Chen J-L, Tjian R. Drosophila TAFII150: similarity to yeast gene TSM-1 and specific binding to core promoter DNA. Science. 1994;264:933–941. doi: 10.1126/science.8178153. [DOI] [PubMed] [Google Scholar]
- 72.Walker W W, Reese J C, Apone L A, Green M R. Transcription activation in cells lacking TAFIIs. Nature. 1996;383:185–188. doi: 10.1038/383185a0. [DOI] [PubMed] [Google Scholar]
- 73.Workman J L, Abmayr S M, Cromlish W A, Roeder R G. Binding of transcription factor TFIID to the major late promoter during in vitro nucleosome assembly potentiates subsequent initiation by RNA polymerase II. Cell. 1988;51:613–622. doi: 10.1016/0092-8674(87)90130-9. [DOI] [PubMed] [Google Scholar]
- 74.Xu X, Prorock C, Ishikawa H, Maldonaldo E, Ito Y, Gelinas C. Functional interaction of the v-Rel and c-Rel oncoproteins with TATA-binding protein and association with transcription factor IIB. Mol Cell Biol. 1993;13:6733–6741. doi: 10.1128/mcb.13.11.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Q, Lieberman P M, Boyer T G, Berk A J. Holo-TFIID supports transcriptional stimulation by diverse activators and from a TATA-less promoter. Genes Dev. 1992;6:1964–1974. doi: 10.1101/gad.6.10.1964. [DOI] [PubMed] [Google Scholar]