Abstract
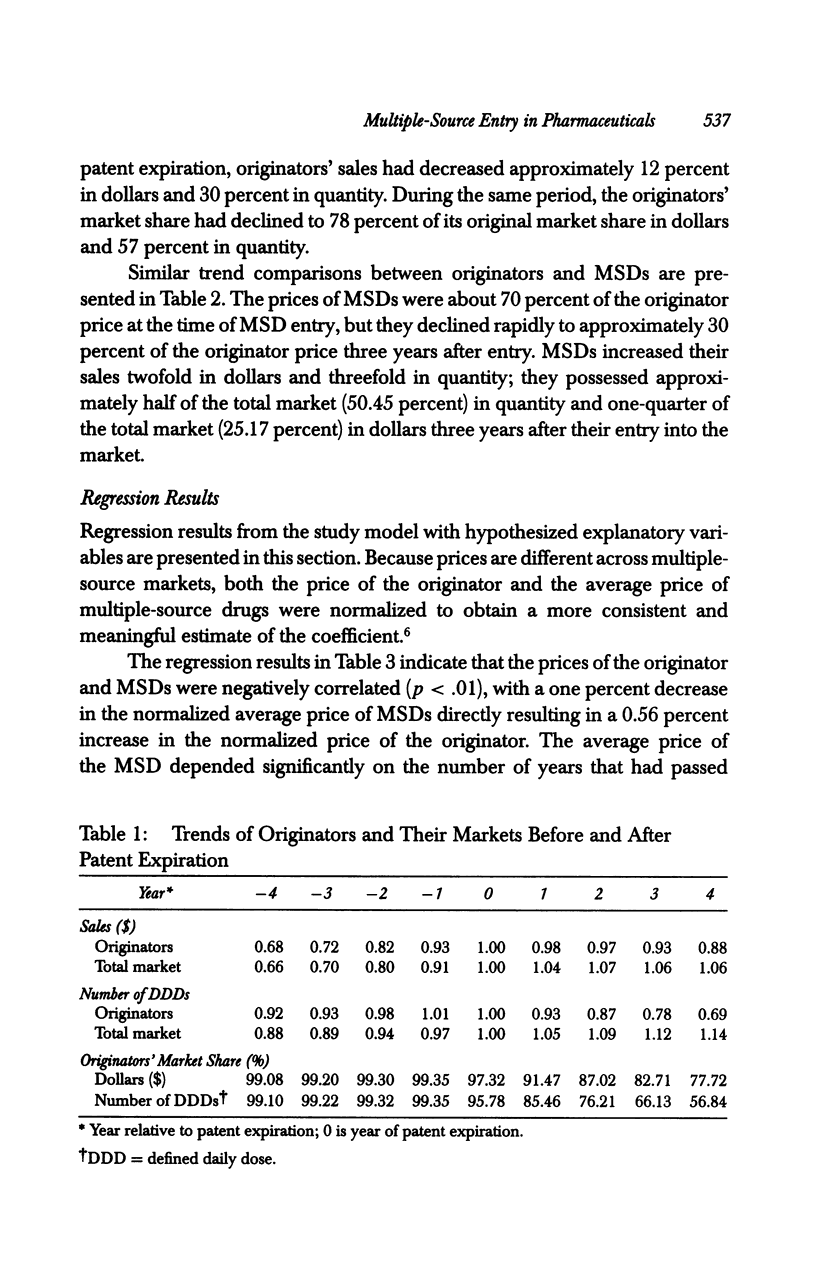
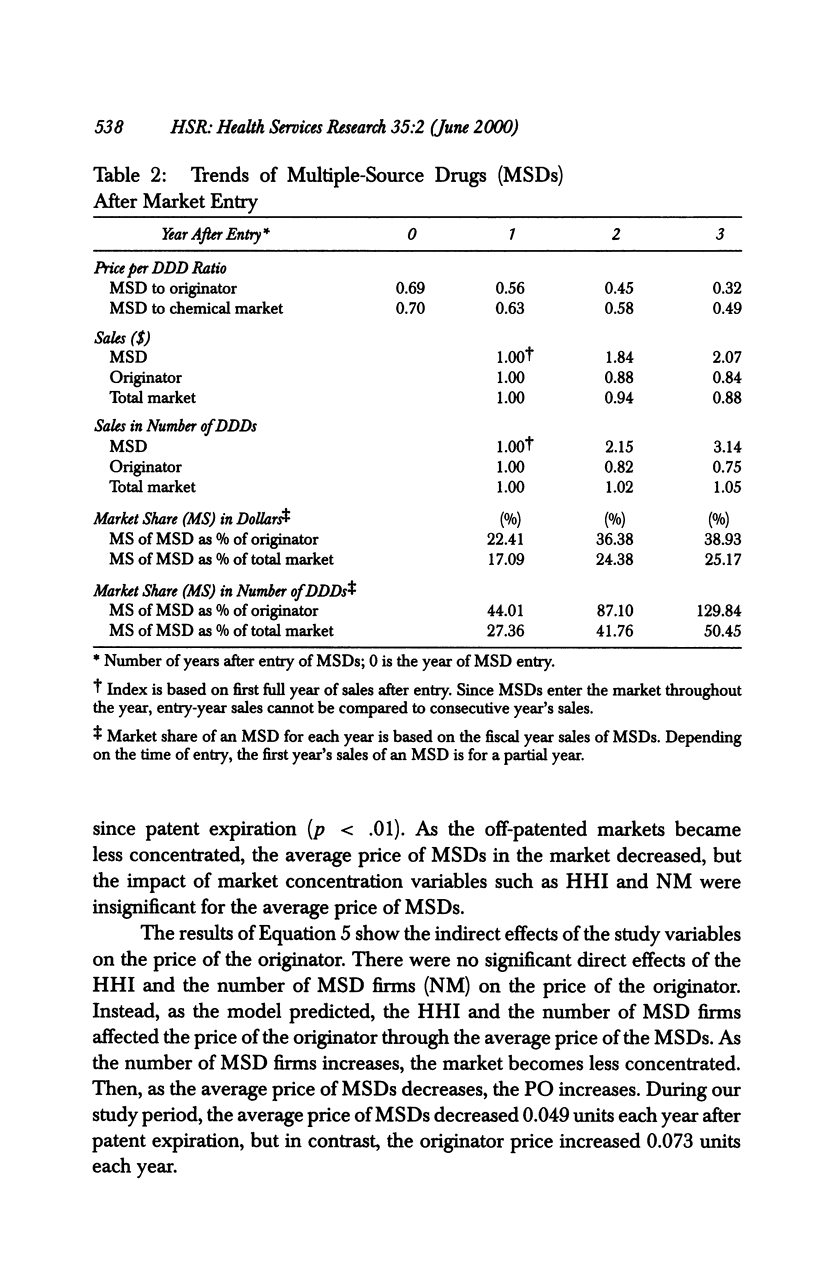
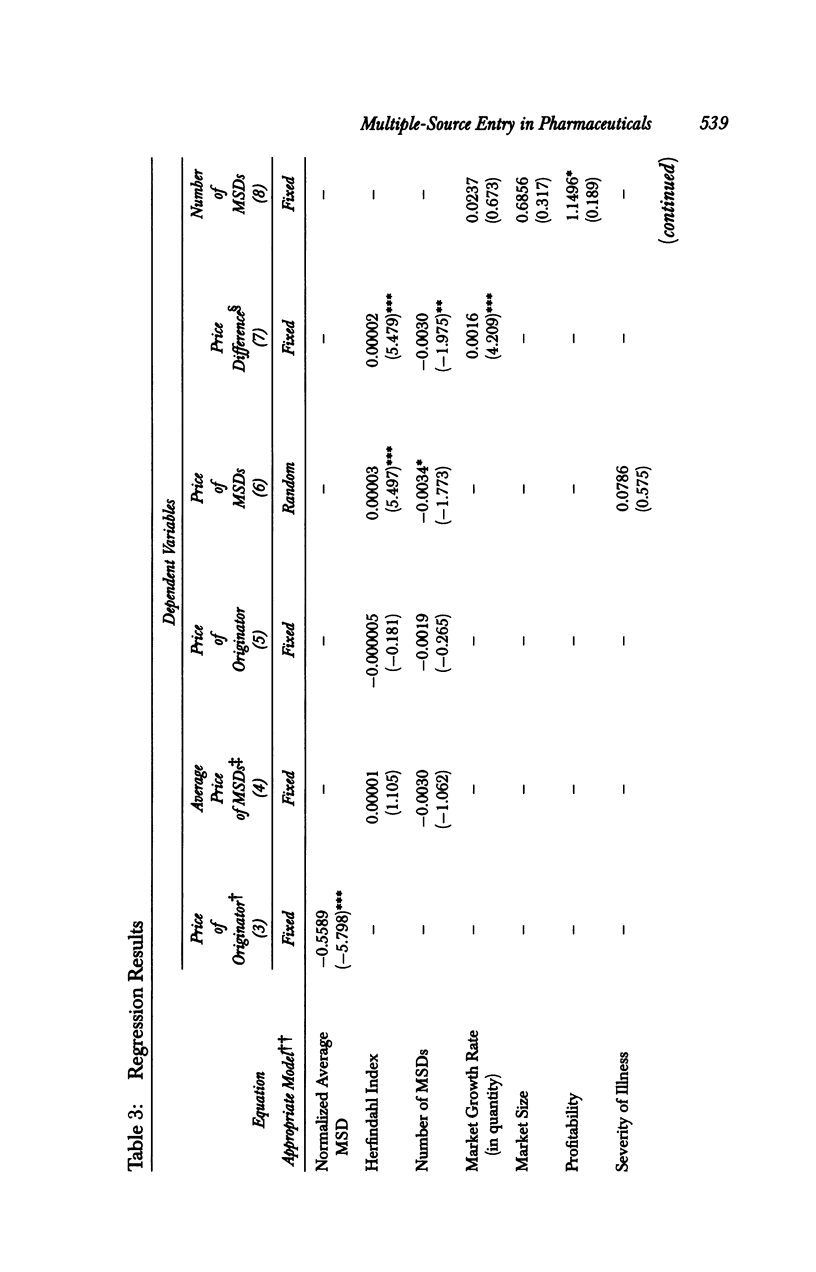
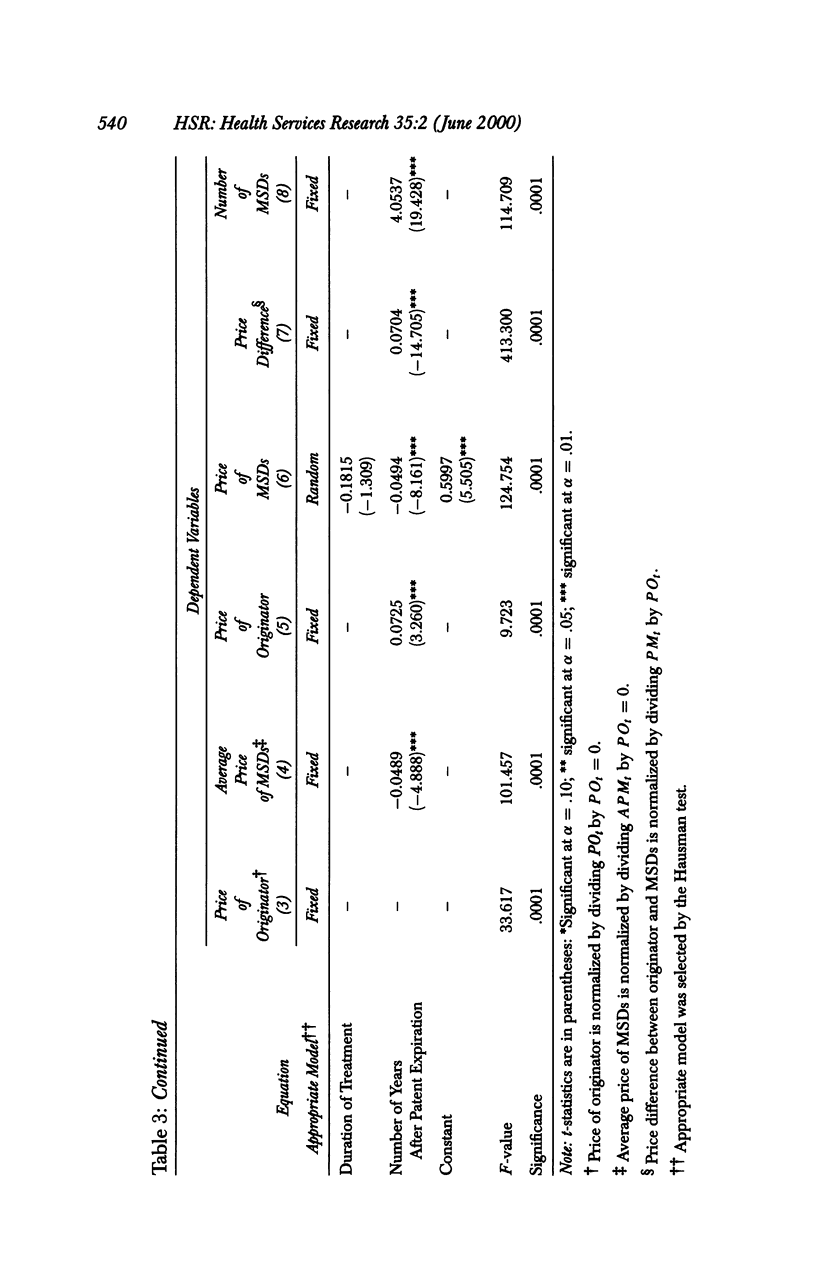
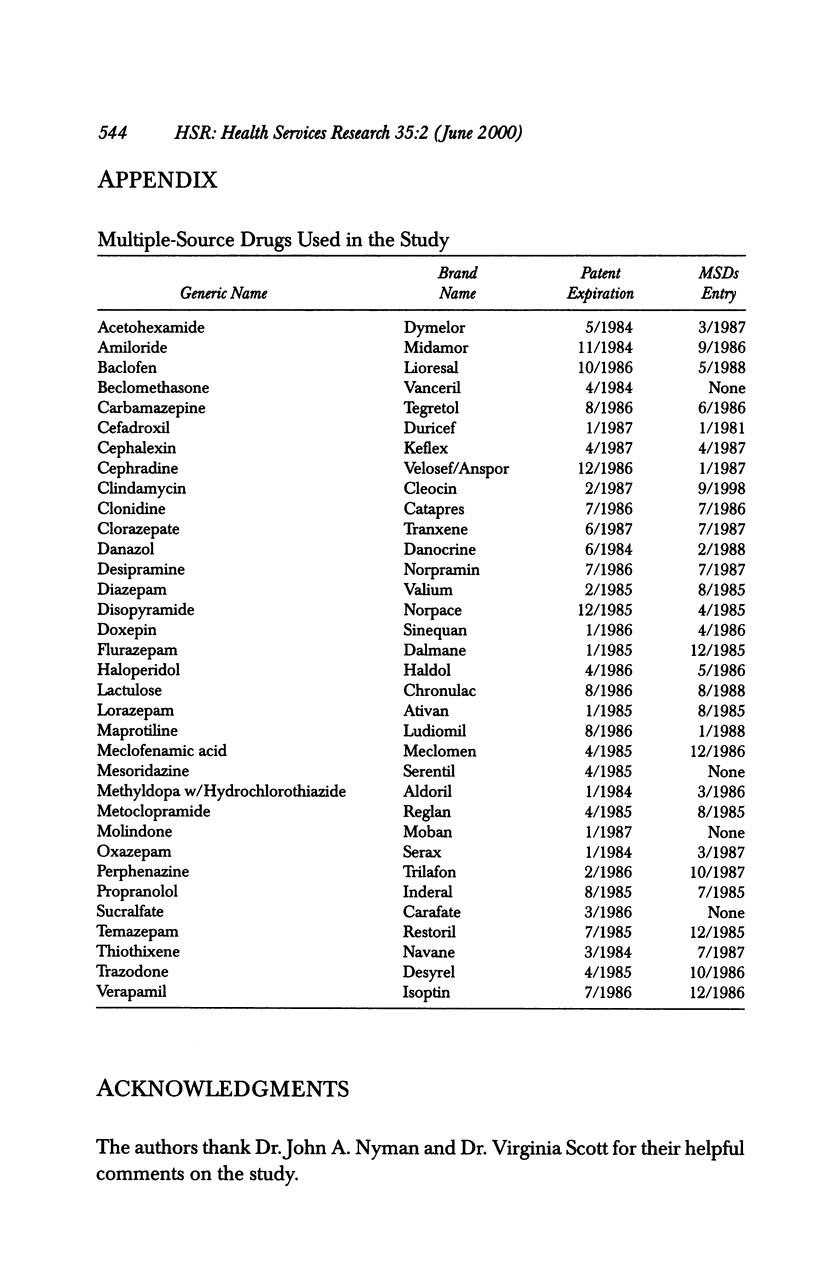
OBJECTIVE: To analyze the effect of multiple-source drug entry on price competition after patent expiration in the pharmaceutical industry. DATA SOURCES: Originators and their multiple-source drugs selected from the 35 chemical entities whose patents expired from 1984 through 1987. Data were obtained from various primary and secondary sources for the patents' expiration dates, sales volume and units sold, and characteristics of drugs in the sample markets. STUDY DESIGN: The study was designed to determine significant factors using the study model developed under the assumption that the off-patented market is an imperfectly segmented market. PRINCIPAL FINDINGS: After patent expiration, the originators' prices continued to increase, while the price of multiple-source drugs decreased significantly over time. By the fourth year after patent expiration, originators' sales had decreased 12 percent in dollars and 30 percent in quantity. Multiple-source drugs increased their sales twofold in dollars and threefold in quantity, and possessed about one-fourth (in dollars) and half (in quantity) of the total market three years after entry. CONCLUSION: After patent expiration, multiple-source drugs compete largely with other multiple-source drugs in the price-sensitive sector, but indirectly with the originator in the price-insensitive sector. Originators have first-mover advantages, and therefore have a market that is less price sensitive after multiple-source drugs enter. On the other hand, multiple-source drugs target the price-sensitive sector, using their lower-priced drugs. This trend may indicate that the off-patented market is imperfectly segmented between the price-sensitive and insensitive sector. Consumers as a whole can gain from the entry of multiple-source drugs because the average price of the market continually declines after patent expiration.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Grabowski H., Vernon J. Longer patents for increased generic competition in the US. The Waxman-Hatch Act after one decade. Pharmacoeconomics. 1996;10 (Suppl 2):110–123. doi: 10.2165/00019053-199600102-00017. [DOI] [PubMed] [Google Scholar]
- Scherer F. M. Post-patent barriers to entry in the pharmaceutical industry. J Health Econ. 1985 Mar;4(1):83–87. doi: 10.1016/0167-6296(85)90026-8. [DOI] [PubMed] [Google Scholar]


