Abstract
Decades of research have characterized diverse immune cells surveilling the central nervous system (CNS). More recently, the discovery of osseous channels (so-called “skull channels”) connecting the meninges with the skull and vertebral bone marrow has revealed a new layer of complexity in our understanding of neuroimmune interactions. Here we discuss our current understanding of skull and vertebral bone marrow anatomy, its contribution of leukocytes to the meninges, and its surveillance of the CNS. We explore the role of this hematopoietic output on CNS health, focusing on the supply of immune cells during health and disease.
Introduction
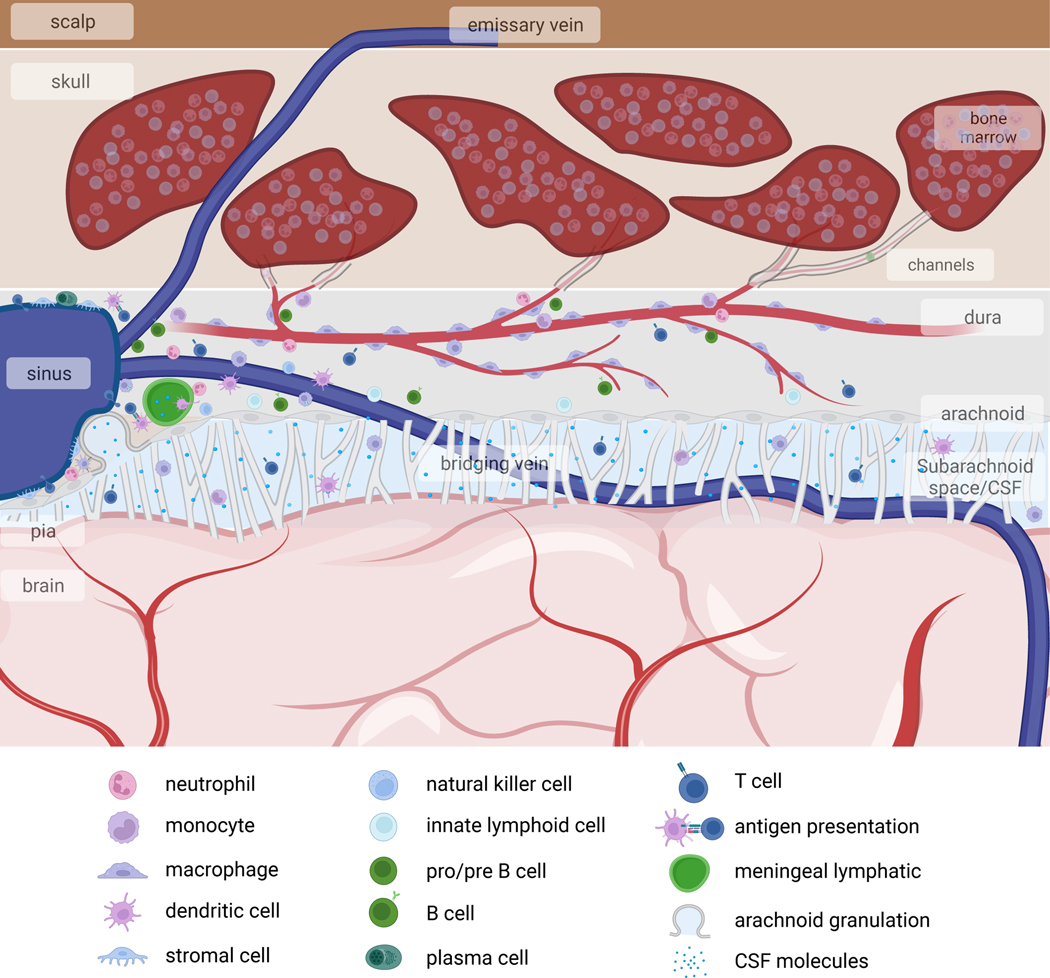
The central nervous system (CNS) was long thought to be isolated from the immune system, and the systems were believed to interact only in pathological conditions. Research over the past decades has challenged this model, revealing profound interactions between the immune system and CNS. Specifically, the CNS is not as isolated as once believed: leukocyte trafficking data dispute the concept of an impermeable CNS vasculature1,2, the brain’s meningeal layers and choroid plexus have a robust immune presence3–8, meningeal lymphatic vessels offer an exit for immune cells and molecules from the CNS9–11, and various meningeal compartments present brain autoantigens12–16. This collective evidence illustrates that CNS immune surveillance is concentrated at its borders (Fig. 1).
Fig. 1. Immune landscape at the outer borders of the CNS.
The CNS contains three membranous coverings, termed the meninges. The innermost layer, the pia mater, is in direct contact with the brain parenchyma. Above the pia is the arachnoid mater, and the subarachnoid space between these two membranes is filled with CSF. The subarachnoid space also contains arteries and veins that extend into the brain parenchyma. Above the arachnoid lies the dura mater, which contains the venous sinuses, draining cerebral veins, as well as meningeal lymphatic vessels, draining CSF. The dura mater is connected to the overlying skull bone marrow. These meningeal layers and the skull bone marrow harbor diverse immune cell subsets, many of which play important roles in brain development, social and cognitive behaviors, and CNS antigen presentation to the rest of the immune system.
Characterizing the immune compartment at CNS borders prompted investigations of these cells’ ontogeny. This led to the discovery that bone marrow within the skull and vertebrae supplies immune cells to the underlying meninges17–20. These immune cell reservoirs are anatomically connected to the CNS, in both mice and humans, and rapidly respond to perturbations in the CNS environment, suggesting specialized forms of neuroimmune communication exist between these two compartments. This discovery introduces a new layer of complexity in our understanding of neuroimmune communication, with considerable potential for uncovering new CNS disease mechanisms and therapeutic targets.
The purpose of this perspective is to summarize current knowledge regarding skull bone marrow and to discuss how hematopoietic output from this compartment may influence CNS health. We will particularly focus on the following questions: 1) What is the skull bone marrow? 2) How does this compartment communicate with and survey the underlying CNS? 3) What does the skull and vertebral bone marrow supply to the CNS during health and disease? To understand the potential role of this specialized immune cell supply, it is important to first review how leukocytes are generally produced in hematopoietic niches (Box 1).
Box 1. Overview of hematopoiesis.
Bone marrow is a widely dispersed organ that inhabits bones throughout the entire body. Its primary function is producing blood cells, including red blood cells, platelets, and most leukocyte subsets. Due to the relatively short life span of some leukocytes (e.g. neutrophils83 and monocytes84 live less than one week), the bone marrow is among the body’s most proliferative tissues, releasing billions of new cells each day. Given leukocytes’ vital role in responding to injury and infection, the marrow can be considered a key immune organ.
HSCs are the precursors for all circulating blood cells and are capable of long-term self-renewal75,85. They reside within specialized microenvironments, or niches, in bone marrow that promote their maintenance and differentiation into more committed progenitors and eventually mature cell types86. Collectively, HSCs and more committed progenitors are referred to as hematopoietic stem and progenitor cells (HSPC, Table 2). While this progressive differentiation of HSCs into committed progenitors and eventually mature cells was thought to occur in a hierarchical fashion with stepwise restriction of cell fate potential, recent evidence suggests that lineage bias may be predetermined at the level of individual HSCs through various priming mechanisms87–91. Discovery of these differentiation states has prompted characterization of surface markers that go beyond the classical hierarchical differentiation model and allow for the study of specific subpopulations92.
In addition to HSPCs and their progeny, stromal cells are integral components of the hematopoietic niche86. Bone marrow stroma, specifically mesenchymal stromal cells and vascular endothelial cells, are major producers of hematopoietic factors, such as stem cell factor (SCF), CXCL12, IL7, FLT3L, MCSF, IL34, IL15, GCSF, and others, which promote HSC maintenance as well as lymphoid and myeloid differentiation86,93–95. Sympathetic and nociceptive innervation are also major regulators of HSC mobilization. While sympathetic nerves indirectly regulate the circadian mobilization of HSCs via signaling to stromal cells34, nociceptive nerves directly induce mobilization via secretion of calcitonin gene-related peptide (CGRP), which activates HSC receptors36. In addition to locally produced factors, normal hematopoiesis can also be influenced by long-range signals originating outside the marrow, such as thrombopoietin produced in the liver, which promotes platelet formation and HSC maintenance96.
Skull and vertebral bone marrow channel anatomy
Skull and vertebral bone marrow is connected to the CNS borders through bone-encased vascular channels. These channels, the proposed function of which we will discuss in a later section, begin at the inner skull cortex—directly opposed to the dura—and extend into the marrow cavities17–24. X-ray microtomography (micro-CT, Table 1) of skull bone revealed that these structures are prevalent in both human and mouse skulls. The channels are distinct from emissary or diploic veins25 which are orders of magnitude larger, fewer in number, and traverse the entire skull thickness and connect to the central sinus26 (Fig 1).
Table 1.
Methods to Study Hematopoiesis
| Approach | Description/Application | Advantages | Disadvantages |
|---|---|---|---|
| Colony-forming assay | The CFU assay detects HSPCs that can generate uni- or multi-lineage mature cells. This assay also measures the proliferative capacity of primary human or murine HSPCs. Whole bone marrow or purified HSCs are plated with medium containing specific growth factors that promote the growth and differentiation of HSCs. Over days to weeks (depending on media and supplied factors), HSCs will grow and form visible colonies that can be morphologically classified based on the mature lineages they produce. | - Relatively rapid, low-cost method for testing the effects of molecules, genetic mutations, and drugs on HSC proliferative and differentiation capacity - Useful for the study of mouse and human HSPCs |
- In vitro system with added growth factors does not fully recapitulate the bone marrow microenvironment - Visual scoring of colony morphology/type can be inconsistent across scorers |
| Competitive transplantation assay | This assay allows for comparison of the ability of different HSCs to engraft, self-renew, and differentiate in vivo. It can be used to test the cell-intrinsic functional effects of specific gene knockouts. Two or more populations of HSCs are transplanted into the bone marrow of recipient animals in competition with each other. The relative proportion of labeled cells’ progeny in the blood and bone marrow can be measured over time, and used to determine the competitive advantage of each HSC population. | - Provides a reliable quantitative measure of a HSC’s ability to engraft, self-renew, and differentiate - Allows for long-term tracking of progeny from defined HSC populations |
- Very long timepoints (>16 weeks) generally required to properly assess multilineage reconstitution from transplanted HSCs - Typically requires myeloablative conditioning, which may alter normal bone marrow micro-environment |
| microCT | X-rays are focused onto a rotating specimen to generate 3D images of the skull. Acquired 3D reconstructions provide information about the native architecture of the channel network and surrounding marrow. Murine and human skull channels connecting the marrow to the underlying meninges were first visualized by microCT17,23. | - Does not destroy tissue being analyzed - High resolution (sub-millimeter) structural data - Useful for quantifying changes in skull channel frequency and morphology |
- Cannot be combined with immunostaining - Can be costly with large number of samples |
| Intravital microscopy of calvaria | Intravital imaging of the calvarial marrow has been used to study hematopoiesis because this niche is accessible for imaging17,22,23,72,73 and skull bone is thinner than long bone. Inflammatory stimuli like LPS induce changes across all hematopoietic organs, making the calvaria a useful representative. This approach requires the calvarial marrow to be exposed by removing the skin from the frontal/parietal bone regions. Mice can then be mounted on a stereotactic frame. | - Imaging cranial hematopoiesis in its native state - Allows serial imaging, i.e. changes over time - Applicable for analyses of both marrow anatomy and cellular dynamics |
- Labeling of specific cell subsets may require breeding of reporter mice - Certain HSPC populations are very rare and challenging to quantify - Photobleaching and long-term imaging may induce a local inflammatory response and alter the native biology |
| Tissue clearing | Tissue clearing permits deeper light penetration for improved imaging depth of whole tissue specimens. Specimens are processed in step-wise fashion to optically clear (delipidate) them in preparation for imaging. There are several in-depth reviews of optical clearing methods74 including RapiClear (Sunjin Labs) and vDISCO. | - Dramatically improves tissue imaging depth - Commercially available reagents - Can be coupled with immunostaining to visualize cell populations or anatomical structures |
- Longer, harsher clearing may damage native architecture - Certain fluorophores are not compatible with long-term clearing - Optimization of refractive index for imaging and clearing length require time |
| Organ bath | Herisson and colleagues describe an organ bath approach for ex vivo imaging of the skull marrow17. The skull bone is dissected, cut, and flipped upside down, then placed in a media bath that supports short-term cell survival. Factors in the organ bath may facilitate leukocyte migration out of the marrow for imaging analysis, which was used to first show cell trafficking through skull channels. | - Intermediate between fixed tissue and intravital imaging that can track cell dynamics in real time - Organ bath preparations can test specific cellular processes |
- Removal of the skull cap and subsequent imaging may not reflect in vivo conditions - Imaging can be challenging due to the irregular anatomy of the skull bone - Lifetime of ex vivo culture is short (<12 hours) |
| Calvarium bone-flap transplant | This method was employed to show that the skull bone marrow supplies myeloid cells to the underlying meninges19. A 4×6mm cranial window spanning the parietal and interparietal bones of the skull was made, with the dura left intact. An equivalent bone flap was harvested from a UBC-GFP mouse, which expresses GFP in all cells, and transplanted onto the non-fluorescent recipient. Successful engraftment of the marrow-containing bone flap allowed visualization and quantification of GFP+ monocytes and neutrophils in underlying meninges at 7 and 30 days post-transplant. | - Transplants from fluorescent reporter mice allow labeling and tracking of hematopoietic output from a large pocket of skull bone marrow - Transplants from genetically modified donors (Cre-lox, knockout, etc.) permit functional interrogation of genes within this CNS border compartment - Can be used as a model to assess the effects of decompressive craniectomy in various disease contexts. |
- Highly advanced procedure requires surgical proficiency - Invasive surgery results in tissue inflammation, the degree of which will vary by surgeon - Variable survival of the bone graft, depending on surgical proficiency - Transplanted area is only a small proportion of total skull bone marrow |
In mice, these channels are found throughout the frontal, parietal, and occipital bones with widths ranging from 20–25 μm23. Typically, a blood vessel transits skull channels, linking the dural vasculature to the bone marrow sinusoidal vasculature17,19,22,23. Electron microscopy shows a paravascular space between the bone and abluminal side of the channel vessel23. There are >1,000 channels across the entire mouse skull. In contrast, the human adult diploic vein network numbers 60–80 in total, illustrating the anatomical differences in these channel types27. The frontal and occipital bones have the highest channel density23, containing approximately 4-fold more connections than the parietal bone (~14 channels/mm2 vs. 3.9 channels/mm2). Though the frontal and occipital bones have similar channel density, channels are ~80 μm long in the frontal bone skull but ~110 μm long in the occipital bone. While the width, density, and length heterogeneity across bones is not well understood, we speculate that these anatomical differences may correlate with heterogeneity in channel function, i.e. their ability to facilitate cell migration or transport of soluble factors.
In humans, micro-CT of samples obtained during decompressive craniectomy revealed similar channels connecting the inner skull cortex with the marrow cavity17. These channels are 4- to 5-fold larger in diameter in humans than in mice17. As in mice, human skull channels connect the skull marrow cavities to the underlying meninges, though other channels also link the outer skull cortex to the periosteum. The function of these outer cortex-periosteum connections is not understood. Nevertheless, a first report demonstrated that human skull channels from either cortex are vascularized and similar in diameter17. Interestingly, these connections bear some similarity to recently described transcortical vessels (TCVs) of the long bones28. Long bone TCVs are capillaries that traverse bone cortex via ossified channels, connecting the bone marrow to the systemic circulation. Additionally, TCV channels have similar diameter, length, and morphologies compared to skull channels, suggesting these structures may serve similar functions across different bones.
Little is known about skull channels’ developmental origins or their status throughout the mammalian lifespan. Whether these channels are present from birth or develop and remodel postnatally and during aging remains an open question. Given the regional heterogeneity of skull channels observed in adult mice and human skull plasticity after birth, it would be surprising if channels were static throughout life. Furthermore, bone remodeling is a well-described process that occurs in other bones29 and may play a role in reshaping skull channels.
Skull and vertebral bone marrow channel function
The studies that initially identified skull bone marrow channels also proposed that these served as direct routes for leukocyte migration to the dural meninges17,18 (Fig. 2). Using Cx3cr1-GFP mice, which express green fluorescent protein in myeloid cells such as monocytes, dendritic cells, and macrophages, Herisson and colleagues observed monocytes, and antibody-stained neutrophils, within skull channels17. Real-time imaging of organ bath preparations (Table 1) revealed cells trafficking through a central CD31+ vessel of skull channels towards the underlying meninges17. Consistent with these findings, Cai and colleagues also detected cells within skull channels of optically cleared (Table 1) LysM-EGFP mice18, in which monocytes, macrophages, and neutrophils express green fluorescent protein. Spectrally resolved cell tracking after stroke induction indicated that skull-derived neutrophils are overrepresented in ischemic brain tissue compared to cells from remote long bones17. Given the limited time span covered by these ex vivo imaging and in vivo cell tracking experiments, the extent to which the skull contributes to steady-state immune populations in the meninges remained unclear.
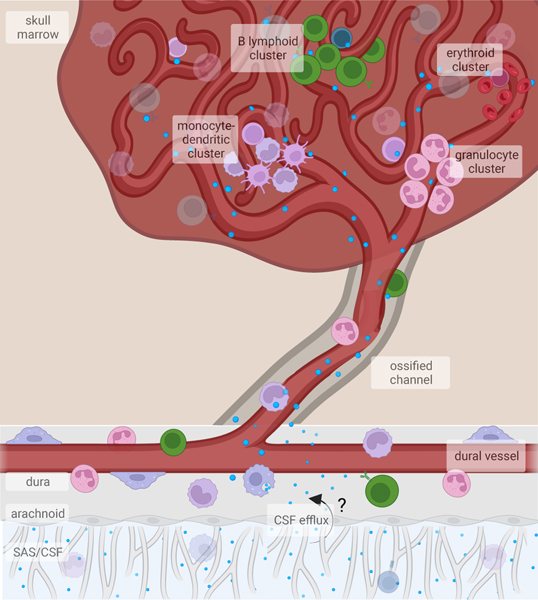
Fig. 2. CNS-associated bone marrow channels are bidirectional conduits.
CNS-associated bone marrow is anatomically connected to the underlying dural meninges through skull channels that cross the inner bone cortex into the marrow cavity. These channels contain a vessel that extends from the dura into the marrow and integrates into the sinusoidal vasculature. Within the marrow, HSPCs reside in perisinusoidal niches, where the processes of myelopoiesis, lymphopoiesis, and erythropoiesis are spatially segregated. Immune cells produced in the bone marrow traffic into the underlying meninges through channels. In addition to permitting cellular trafficking, the perivascular space allows CSF to flow into the bone marrow. Despite strong evidence supporting CSF efflux to the dura as well as the surrounding bone marrow, the precise route by which CSF is able to bypass the arachnoid mater remains unknown.
A few years after these initial reports, three groups showed that skull and vertebral bone marrow continuously supplies immune cell subsets to the meninges19,20,30. Using parabiosis, in which two mice are surgically joined to share a circulatory system, Cugurra and colleagues observed that the cranial and spinal dura had lower levels of immune cell chimerism than blood19. These findings raised the possibility that within the dura, myeloid cells — specifically neutrophils, monocytes, macrophages, and dendritic cells — were not all blood-derived. Skull transplantation (Table 1) experiments revealed migration of skull-derived myeloid cells into the underlying meninges19. Consistent with previous findings17,18,21, skull-derived cells appeared to egress along channels emerging from the interior bone cortex that is clad by the meninges.
Separate studies have also revealed that meningeal B cells may derive from skull bone marrow20,30. Parabiosis experiments demonstrated that meningeal B cells do not primarily derive from circulating B cells, and single-cell B cell receptor sequencing showed little clonal overlap between B cells from the meninges compared to blood, suggesting meningeal B cells have a local source20. While multiple studies report the presence of mature B cells and their progenitors within the dura20,30–32, there is disagreement regarding the source of immature B cells. While some studies claim that lymphocytes and their progenitors migrate from the surrounding skull bone marrow via skull channels20,30, others believe that lymphocyte progenitors are long-lived resident cells that locally maintain the meningeal lymphocyte pool31,32. Histological analysis of skull bone marrow shows immature B cells within the channels connecting to the underlying dura; however, it is still unclear whether sustained intrameningeal hematopoiesis separately contributes to the meningeal immune compartment (Box 2), as a typical hematopoietic microenvironment is only present in skull marrow.
Box 2. Can the meninges sustain hematopoiesis?
The question of whether the dural meninges serve as a hematopoietic niche outside of the skull bone marrow is currently a topic of debate. Evidence from multiple groups supports the presence of B-cell progenitors in the dural meninges20,30,31, but their origin remains unclear. Some argue that they derive from the overlying skull bone marrow20,30, while others claim that they are locally sustained through proliferation of resident HSPCs31,32. If the latter is true, it would be classified as extra-medullary hematopoiesis (EMH), a process in which hematopoiesis expands into facultative niches outside of the bone marrow. While the main site of hematopoiesis in adult mammals under normal circumstances is the bone marrow, hematopoiesis can transiently expand into such niches in response to severe hematopoietic stresses such as pregnancy, infection, or cancer97. Factors supporting EMH in the spleen, for example, have been well described and share many similarities with the bone marrow niche. In the spleen, HSCs are maintained around the red pulp sinusoidal vessels, and stromal cells and endothelial cells produce Cxcl12 and SCF97.
Despite the evidence supporting the presence of HSCs in the dura, whether or not sustained hematopoiesis occurs there is still unclear. While the dura shares some elements that are important for hematopoiesis, such as the expression of IL-7 and Cxcl1214,30 as well as sympathetic innervation98, there are several knowledge gaps that prevent us from definitively concluding that hematopoiesis is sustained within the meninges. Current evidence that HSCs can be isolated from the dura is strong, with colony-forming unit (CFU) assays and serial transplantation assays demonstrating that HSCs isolated from the dura are capable of multi-lineage reconstitution32. However, one major caveat with these findings stems from the structural adherence that normally exists between the dura and the skull bone marrow due to the high density of channels. Given that the dura needs to be peeled off the skullcap to isolate cells, current data describing intra-meningeal hematopoiesis cannot exclude the possibility that HSCs isolated from the meninges are skull-derived. Transcriptomic data show little difference between skull and dural HSCs, and histological evidence shows that HSCs can be found around dural sinuses in situ32. However, given the density of vascularized skull channels immediately overlying the dural sinuses, it is possible that these HSCs migrated through channels in vivo or were pulled out of overlying bone marrow during whole-mount preparation. The possibility of intra-meningeal hematopoiesis is intriguing and should be further explored with more definitive approaches. To prevent potential contamination during tissue separation, optically cleared whole-mount preparations of intact dura and skull22 can be used. Additionally, new genetic barcoding tools that allow quantification of clonal relationships in hematopoietic stem cells99 could decipher whether meningeal HSCs are derived from skull HSCs, or if they constitute a separate self-renewing population.
The exact means by which leukocytes from the skull traverse skull channels is only beginning to emerge. Our current understanding, based on in vivo imaging and tissue histology, is that immune cells likely migrate out of the skull bone marrow and into the meninges through either the channel’s perivascular space or along the channel’s vessel lumen before extravasating into the dura. In vivo imaging of young mice—which have thinner skulls, thus allowing better imaging of skull channels—showed neutrophils migrating intraluminally towards the underlying dura following stroke17. Histological assessment by Brioschi and colleagues demonstrated B cells along the outside of a lectin-labeled blood vessel within a skull channel20, suggestive of perivascular trafficking. Interestingly, time-lapse imaging of an ex vivo whole skull organ bath revealed Cx3cr1+ leukocytes crawling along the vessel wall towards the dural side of the channel opening17. Earlier work describing leukemic cell migration along the perivascular space into meninges21 also suggests perivascular migration to the dura.
While both intraluminal and abluminal modes of egress are possible—as has been shown for leukocytes in long bone TCVs28—whether certain skull-derived cell types are biased toward either form of migration, or whether heterogeneity exists in skull channels’ capacity to support either form of migration, remains to be seen. One challenge in observing skull channel migration in healthy conditions is the low frequency of ‘events’ per channel, paired with the high abundance of channels throughout the skull. While long-term parabiosis experiments suggest certain cell types in the meninges continuously derive from local bone marrow19,20,30, histological assessments show sparse (~1 LysM-GFP+ cell every other channel18) trafficking. It is clear that we need better in vivo imaging to directly observe the movement of immune cells within channels and determine their directionality and extent of migration. Such studies would provide more direct evidence supporting the hypothesis that channels serve as cell conduits from the skull marrow to the underlying meninges. Furthermore, investigating the mechanisms guiding this migration—such as chemokine gradients or extracellular matrix cues21—could provide insights into channels’ role in CNS health and disease.
Given this seemingly specialized hematopoietic source supplying the CNS border tissues and bone marrow’s ability to quickly respond to environmental triggers, the question arose whether the CNS could communicate with the surrounding bone marrow to instruct its immune supply. This led to the discovery of a neuroimmune signaling axis, in which cerebrospinal fluid can directly access the skull bone marrow through skull channels to provide context-dependent signals to this immune cell reservoir22,23 (Fig. 2). Fluorescent tracers injected into the cisterna magna of mice were found to accumulate within the skull bone marrow. The CSF tracer reached the skull bone marrow through skull channels via a paravascular route and was sampled by perivascular cells in both the channels and the marrow22,23. Interestingly, CSF flowing into skull marrow also interfaced with hematopoietic stem and progenitor cells (HSPC), suggesting this signaling axis could potentially instruct CNS-specific hematopoiesis22. Lastly, these studies demonstrated that CSF access to skull marrow occurs throughout the entire postnatal lifespan22, opening the possibility that changes in CSF composition can dynamically shape the hematopoietic output to the CNS. While these experiments revealed potential avenues of crosstalk between the CNS and the bone marrow, future studies using tools that probe native, unperturbed CSF flow will have to further validate this communication.
CSF outflow to skull marrow has been assessed in healthy humans as well as those with CSF circulation disorders such as idiopathic normal pressure hydrocephalus, communicating hydrocephalus and idiopathic intracranial hypertension33. MRI revealed extensions of the dural meninges into the skull marrow along parasagittal dura and more laterally. Intrathecal injection of the contrast agent gadobutrol into the CSF increased the signal in the skull marrow along parasagittal dura while the blood gadobutrol concentration remained much lower. Increased skull marrow signal after intrathecal gadobutrol injection was observed in patients with CSF disorders but less so in healthy controls. While these results suggest CSF pathology can increase the CSF efflux to skull marrow, the limited sensitivity of the method and the study’s focus on parietal marrow do not rule out that healthy subjects also experience CSF-to-bone marrow communication. Whether skull regions that have more channels, such as the occipital or frontal bones, or are more ventrally positioned, such as the sphenoid bone, feature higher CSF trafficking in humans is an important open question.
The discovery of CNS-to-skull marrow communication via soluble factors in CSF in mice raises the question if other mechanisms exist through which the CNS communicates with its surrounding bone marrow, such as innervation, which is known to promote immune responses in peripheral bone marrow34–36. Beyond revealing the homeostatic signaling mechanisms between these two compartments, CNS-to-bone marrow signaling may have implications for neurological disease, which we discuss below.
The skull and vertebrae are a major B cell source for the dural meninges in the healthy CNS20,30. During aging, B cell supply and maturation become disrupted. While meningeal B cells in young meninges have equally distributed clonality, aged meninges show dramatic clonal expansion of B cells20. These clonal populations significantly overlap with B cell clones in circulating blood, suggesting peripheral infiltration of antigen-experienced B cells into the aged dura. Mechanisms driving this shift in peripherally- versus skull and vertebral marrow-derived B cells in the meninges remain unclear but merit thorough investigation.
Skull and vertebral bone marrow in neurological disease
Stroke and CNS injury
Immune cell recruitment is fundamental to CNS injury progression37–40. Initial studies characterizing skull marrow-derived immune cell contributions to ischemic brain reported that migrating neutrophils and monocytes appear in the infarct within 6 hours after injury17, suggesting skull marrow is an early responder. Within skull marrow following stroke, Cxcl12, a hematopoietic niche retention factor, declines17, likely enabling egress of monocytes and neutrophils from the skull to the brain (Fig 3a). Consistent with this early response, a recent study demonstrated rapid transcriptional changes in skull marrow following ischemic stroke24.
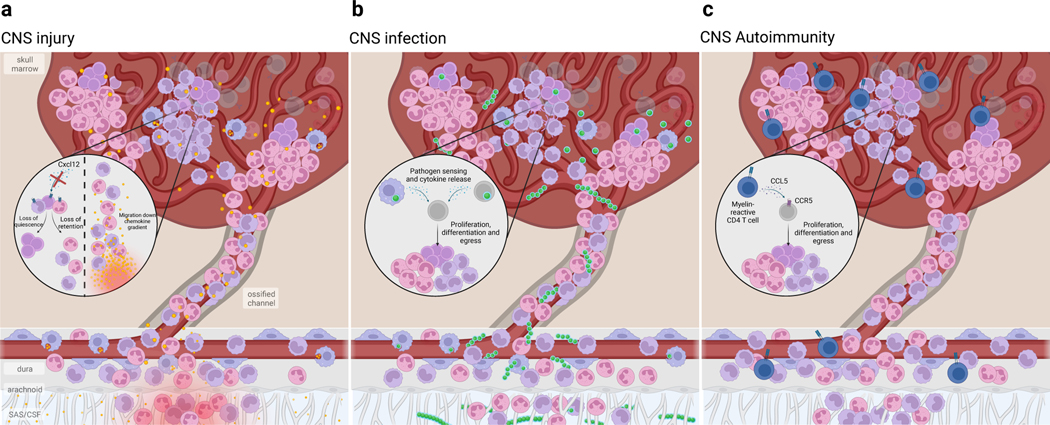
Fig. 3. Skull bone marrow responds to CNS perturbations.
Due to its proximity to the underlying CNS, the skull bone marrow can sense CNS impairment and mobilize immune supply to the underlying tissue. Alternatively, bone marrow niche perturbations can alter hematopoietic output to the CNS. a) Following CNS injury, such as spinal cord injury or stroke, HSPCs rapidly proliferate. This expands the monocyte and neutrophil supply into the underlying dura and injured CNS parenchyma. Such rapid response is due, in part, to CSF-contained cues entering the bone marrow and is likely a response to damage-associated molecular patterns (DAMP) resulting from tissue injury and cell death. Additionally, local retention cues such as Cxcl12 are downregulated, resulting in the mobilization of HSPCs and egress of monocytes and neutrophils. b) In bacterial meningitis, pathogens exploit the skull channel anatomy to invade the bone marrow. The rapid expansion of HSPCs as well as monocytes and neutrophils results from innate pathogen sensing, as well as local production of pro-inflammatory cytokines. c) In CNS autoimmune disease, autoreactive T cells home to the bone marrow and signal to hematopoietic stem cells to promote preferential over-production of monocytes and neutrophils. While this T cell homing and skewing of myelopoiesis seems to occur in both skull/vertebral bone marrow as well as femoral bone marrow, recent evidence suggests that skull/verterbal bone marrow-derived myeloid cells may be functionally distinct from their circulating counterparts during EAE. While there appears to be a conserved response in favor of myelopoiesis, whether these myeloid cells acquire phenotypes that are context-specific remains to be seen.
In humans, spatially distinct patterns of PET ligand uptake and binding to TSPO, an inflammation marker, occur following stroke24. In the skull marrow of 10 patients with acute stroke (<72 hours), tracer uptake followed ischemia patterns in underlying brain. Interestingly, longitudinal imaging in stroke patients 3 months post-stroke showed a global decrease in skull TSPO signal24. These human data suggest that skull bone marrow can respond to brain ischemia; however, analyzing larger cohorts would help uncover whether the skull marrow response varies with stroke location, size, or type. Additionally, while the signals evoking skull marrow responses after injury can be CSF-contained22, bone marrow innervation could also be playing a role, as has been shown in the femur41.
In addition to stroke, vertebral and skull bone marrow deliver monocytes to the spinal cord parenchyma and to the optic nerve, respectively, following injury19. Each of these CNS tissues has spatially associated bone marrow which connects to the meninges through bone channels, thus providing a local leukocyte source with a direct route for CNS infiltration. Beyond injury, tissue stress may trigger an adjacent bone marrow response, as a recent TSPO-PET study showed activated skull areas overlying occipital cortex in patients after migraine with visual aura42. Whether skull/vertebral-derived versus blood-derived myeloid cells differentially contribute to CNS injury and repair is still an open question worthy of further investigation.
CNS infection
Meningitis is caused by infection of the meningeal layers surrounding the CNS. Given its proximity, the skull marrow could serve as a sentinel for CNS pathogen invasion. Studies introducing bacteria into the CNS show that bacteria migrate along skull channels into the skull marrow23 (Fig. 3b). Within the bone marrow, macrophages engulf bacteria while HSPCs proliferate after engagement of their Toll-like receptors22,23. This proliferative response occurs within hours of infection, affects all studied myeloid progenitors of the mouse skull marrow, and is followed by mobilization of leukocytes to the underlying meninges22,23 (Fig. 3b).
While neurotropic viruses, such as Herpes simplex, West Nile and Zika virus cause structural and functional CNS damage resulting in cognitive decline43, whether or not these pathogens infect cells in the skull and vertebral marrow, thus potentially disrupting long-term immune supply to underlying CNS borders, remains unknown. Understanding how neurotropic viruses impact hematopoietic output to the CNS, and whether this reservoir can be therapeutically targeted to reverse neurological symptoms following infection, is of great interest.
Multiple sclerosis
Multiple sclerosis (MS) is a neurological disease resulting from autoimmune attack on CNS myelin, driven by waves of monocyte and lymphocyte entry into the brain and spinal cord44. In a mouse model of MS, autoreactive T cells were shown to migrate to bone marrow and amplify myelopoiesis via CCL5, increasing proliferation of CCR5+ myeloid-biased HSPCs45 (Fig. 3c). The elevated myeloid cell output then enhances CNS inflammation. Consistent with this enhanced marrow activation, TSPO-PET imaging of patients with primary progressive and relapsing remitting MS shows significant enhancement in the skull marrow24. In a separate study, parabiosis experiments revealed preferential migration of vertebral bone marrow-derived monocytes into the spinal dura and spinal cord parenchyma following EAE induction with spinal cord infiltrates regularly underlying vertebral bone channels 19. Single-cell RNA-sequencing of the vertebral bone marrow-enriched cell fractions versus those from circulating blood revealed distinct transcriptional phenotypes, with blood-derived cells displaying a Cxcl10hi signature that has previously been defined as pathogenic in EAE46. While transcriptional differences are present at baseline in monocytes from skull versus other bones22,24, it remains to be seen whether these phenotypes observed in diseased tissue are intrinsically primed or shaped by the tissue infiltration routes (e.g., channels versus blood).
Beyond functioning as a myeloid reservoir, skull and vertebral marrow and its cellular progeny may also be central to CNS tolerance in autoimmunity. The skull marrow is a lymphopoietic site and delivers both immature and mature B cells to the underlying dural tissue20,30. Because both of these tissues have access to CSF—and thus molecules arising from the CNS—they encounter a unique repertoire of CNS-associated antigens13,14,22,23,33, critical for extrathymic elimination of self-reactive B cells. Skull marrow-derived B cells harboring BCRs towards myelin oligodendrocyte glycoprotein (MOG), a critical component of CNS myelin, undergo negative selection as a result of endogenous MOG efflux to the dura30. While such MOG-reactive B cells are eliminated in the dura, not the skull bone marrow, continuous CSF access to skull and vertebral bone marrow opens the possibility that additional CNS-reactive B cells are shaped during their development in the skull to eliminate autoreactive lymphocytes.
CNS cancer
Leptomeningeal metastasis is a devastating complication of peripheral cancers and results in dissemination of malignant cells throughout the CNS47. Using an animal model of acute lymphoblastic leukemia (ALL), malignant cells were shown to accumulate within the skull bone marrow48 and subsequently migrate into the underlying meninges via integrin-mediated adhesion along laminin-coated vessels in skull channels21. Consistent with the recent discovery that CSF provides instructive cues to mobilize skull bone marrow, transwell experiments using either ALL, breast or lung cancer cells demonstrated that CSF contains chemoattractants that promote malignant cell migration21,49. These data suggest cancer cells may co-opt CSF-to-skull marrow cues as guideposts towards the CNS, and highlight the therapeutic potential of neutralizing CSF-contained molecules to curb CNS metastasis.
The tumor microenvironment—broadly composed of neural, immune, and stromal cells—is a critical regulator of tumor progression50. How does skull marrow cell production shape anti-tumor immunity? Do skull marrow-derived cells constitute a distinct immune population within the tumor microenvironment, as in mouse models of CNS autoimmunity19? Immunotherapies such as GD2-directed chimeric antigen receptor (CAR) T cells were recently developed to treat certain pediatric brain tumors51, many of which are located in close proximity to dense bone marrow regions51, suggesting skull and vertebral marrow-derived cells may be critical players within the tumor microenvironment. It is vital to decipher how CAR T cells impact hematopoietic output and whether this contributes to the therapeutic effects.
Alzheimer’s disease
The immune system plays a critical role in Alzheimer’s disease (AD) progression52. Because both neuroimmune interactions in AD and the skull marrow’s role in health have only recently been recognized, little is known about bone marrow changes in AD. In both a 5XFAD mouse model and human AD patients, TSPO-PET studies show the inflammatory response in the AD brain is paralleled by skull marrow activation in areas relevant to AD pathology, such as the skull covering the temporal and parietal cortices24. Interestingly, longitudinal imaging 18 months post-baseline scans showed increased TSPO signal in these same skull regions of AD patients24. Given the changes in CSF inflammatory mediators and soluble pathogenic proteins in AD53–56, chronic alterations in CSF composition may shape the recruitment and phenotype of cells produced in skull marrow.
The extent to which monocyte-derived macrophages contribute to AD is debated. While parabiosis studies have shown that circulating monocytes do not engraft into the brain in mouse models of AD57,58, recent single-cell RNA-seq data suggest that bone marrow-derived macrophages do engraft into the brain during aging and in mouse models of AD59. Thus, skull marrow may be a local source of monocytes in AD. Interestingly, one study found that in both aging and the APP/PS1 model of AD, in which both amyloid precursor protein and presenilin 1 are mutated, monocyte numbers in the leptomeninges were reduced, suggesting either their production in the skull or access to the leptomeninges might be impaired60. Additionally, anti-PD-1 antibodies that alleviate immune suppression improve cognitive performance in 5XFAD mice through the recruitment of bone marrow-derived macrophages61. It is possible that reactivation of T cells in the bone marrow via immune checkpoint inhibition enhances monocyte production and subsequent control of AD pathology.
Aging
Although aging is not a disease, both aging and neurodegenerative diseases are associated with CNS inflammation, leading to leukocyte accumulation and clonal expansion in the brain and its borders14,20,62–64. The extent to which these age-related shifts can be attributed to alterations in surrounding skull and vertebral marrow is unclear. For example, aging bone marrow shifts toward myelopoiesis and away from lymphopoiesis65,66, influenced by factors such as interleukin 1-beta (IL-1b), IL-6 and tumor necrosis factor-alpha (TNFa) from altered niche cells67–70. Additionally, skull bone marrow adiposity expands over time in a sex-specific manner, with females showing a dynamic increase between ages 45 to 70 that eventually matches baseline male adiposity levels71.
While the cellular makeup and hematopoietic output of the bone marrow niche shifts with age, it is unclear whether the skull and vertebral bone marrow’s ability to sense incoming CNS cues changes. Though CSF access to skull marrow remains consistent throughout the mouse lifespan22, aging’s effect on skull and vertebral bone marrow channel abundance or morphology, and its impact on immune cell egress to the dura or brain, are unknown. Lastly, given that fibrosis is a hallmark of aging meninges14, determining whether aberrant extracellular matrix deposition impacts skull channels is an important question.
Concluding remarks
Until recently, skull marrow was thought to be yet another blood cell production location. Instead, this hematopoietic compartment may be more important because it is uniquely connected to the CNS borders via skull channels, which enable a bidirectional “private communication” between marrow regions and neighboring meninges, neural tissues and the CSF. The resulting exchange of cells and signals is likely distinct from marrow interactions facilitated by the systemic circulation and may support specific functions in brain development, homeostasis and CNS defense. While a handful of studies have now documented the existence of skull channels and a few of their functions, many open questions regarding the influence of skull marrow on brain health remain (Box 3). Addressing these will improve our understanding of CNS inflammation, potentially adding a new topographical category of CNS immunity distinct from and positioned between brain-resident and blood-borne cells.
Box 3. Important Future Directions.
How does CSF travel across the arachnoid membrane to eventually reach the dura? Possible mechanisms include transcytosis across arachnoid or flow through gaps in the arachnoid mater.
What anatomical route gives skull- and vertebrae-derived cells access to the CNS parenchyma?
Hematopoiesis is supported by long-range signals produced in the kidney, for example. Does the CSF produce any CNS-specific cues that shape or support hematopoiesis in the skull and vertebrae?
Vascular fibrosis is a hallmark of aging. How does this process alter the perivascular space within the skull channels? Does this impact the ability of cells to migrate into underlying meninges?
Immune cell phenotypes are sensitive to vascular cues during migration. Does the vasculature within skull channels impact the phenotype of cells recruited to the meninges?
Do the meninges support in situ hematopoiesis separately from overlying skull and vertebral bone marrow?
CSF composition shifts throughout normal development and neurodegeneration. How do brain-derived molecules throughout life impact immune cell production in the adjacent bone marrow?
Are skull and vertebral bone marrow derived cells functionally distinct from those produced in other bone marrow? Or is this close anatomical organization beneficial only in terms of faster recruitment kinetics?
Surgical decompression via the removal of skull bone is performed in the setting of large strokes and other conditions with markedly elevated intracranial pressure100. Beyond the initial decompressive benefits, what are the long-term effects of removing portions of skull bone marrow? How does this impact immune composition of the dura, and could it impact CNS infection resolution or even cancer metastasis over time?
Is cell trafficking from the skull/vertebral bone marrow to underlying meninges circadian?
Table 2.
Mouse HSPC surface marker classification
| HSPC population | Surface markers | Ref. |
|---|---|---|
| Long-term HSC (LT-HSC) | Lin- Sca1+ CD117+ CD150+ CD48- | 75 |
| Short-term HSC (ST-HSC) | Lin- Sca1+ CD117+ CD150- CD48- | 75 |
| Multipotent progenitor (MPPMk/E) | Lin- Sca1+ CD117+ CD135- CD150+ CD48+ | 76 |
| Multipotent progenitor (MPPG/M) | Lin- Sca1+ CD117+ CD135- CD150- CD48+ | 76 |
| Multipotent progenitor (MPPLy) | Lin- Sca1+ CD117+ CD135+ CD150- CD48+ | 76 |
| Common lymphoid progenitor (CLP) | Lin- Sca1low CD117int/low CD135+ CD127+ | 77 |
| Common myeloid progenitor (CMP) | Lin- Sca1- CD117+ CD16/32lo CD135+ CD34+ CD115- | 78–80 |
| Granulocyte-monocyte progenitor (GMP) | Lin- Sca1- CD117+ CD16/32hi CD34+ CD115- Ly6C- | 78–80 |
| Monocyte-dendritic cell progenitor (MDP) | Lin- Sca1- CD117+ CD16/32lo CD135+ CD34+ CD115+ Ly6C- | 78,80 |
| Granulocyte progenitor (GP) | Lin- Sca1- CD117+ CD16/32hi CD34+ CD115- Ly6C+ | 78,80 |
| Common monocyte progenitor (cMoP) | Lin- Sca1- CD117+ CD16/32hi CD34+ CD115+ Ly6C+ | 78,80 |
| Common dendritic progenitor (CDP) | Lin- Sca1- CD117lo CD16/32lo CD135+ CD34+ CD115+ Ly6C- | 78,81,82 |
Acknowledgements
We acknowledge Kaley Joyes for editing the manuscript. This work was funded in part by U.S. federal funds from the National Institutes of Health (NS108419, NS127808, HL139598, HL142494, AT010416, NS096967), a Cure Alzheimer’s Fund grant (Berg Brain Entry and Exit Consortium), and the BJC HealthCare Investigators Program.
Footnotes
Competing interests
J.K. is a scientific advisor for Sana Biotechnology. M.N. has received funds or material research support from Alnylam, Biotronik, CSL Behring, GlycoMimetics, GSK, Medtronic, Novartis and Pfizer, as well as consulting fees from Biogen, Gimv, IFM Therapeutics, Molecular Imaging, Sigilon, Verseau Therapeutics and Bitterroot. The other authors declare no competing interests.
References
- 1.Vajkoczy P, Laschinger M. & Engelhardt B. α4-integrin-VCAM-1 binding mediates G protein–independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest 108, 557–565 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman L. Blocking adhesion molecules as therapy for multiple sclerosis: natalizumab. Nat Rev Drug Discov 4, 510–518 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Mrdjen D. et al. High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease. Immunity 48, 380–395.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Hove HV et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci 22, 1021–1035 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Fitzpatrick Z. et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature 587, 472–476 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dani N. et al. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell 184, 3056–3074.e21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanmarco LM et al. Gut-licensed IFNγ+ NK cells drive LAMP1+TRAIL+ anti-inflammatory astrocytes. Nature 1–7 (2021) doi: 10.1038/s41586-020-03116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croese T, Castellani G. & Schwartz M. Immune cell compartmentalization for brain surveillance and protection. Nat Immunol 1–10 (2021) doi: 10.1038/s41590-021-00994-2. [DOI] [PubMed] [Google Scholar]
- 9.Louveau A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aspelund A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212, 991–999 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Absinta M. et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 6, e29738 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schläger C. et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 530, 349–353 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Ringstad G. & Eide PK Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat Commun 11, 354 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rustenhoven J. et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell (2021) doi: 10.1016/j.cell.2020.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merlini A. et al. Distinct roles of the meningeal layers in CNS autoimmunity. Nat. Neurosci 25, 887–899 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Li Z. et al. Blockade of VEGFR3 signaling leads to functional impairment of dural lymphatic vessels without affecting autoimmune neuroinflammation. Sci. Immunol. 8, eabq0375 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Herisson F. et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nature Neuroscience 21, 1209–1217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai R. et al. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull–meninges connections. Nat Neurosci 1–11 (2018) doi: 10.1038/s41593-018-0301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cugurra A. et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science 373, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brioschi S. et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science 373, eabf9277 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao H. et al. Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature 560, 55–60 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzitelli JA et al. Cerebrospinal fluid regulates skull bone marrow niches via direct access through dural channels. Nat Neurosci 1–6 (2022) doi: 10.1038/s41593-022-01029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulous FE et al. Cerebrospinal fluid can exit into the skull bone marrow and instruct cranial hematopoiesis in mice with bacterial meningitis. Nat Neurosci 25, 567–576 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolabas ZI et al. Distinct molecular profiles of skull bone marrow in health and neurological disorders. Cell 186, 3706–3725.e29 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.S L, M D, B I, J I. & R T. The Diploic Veins: A Comprehensive Review with Clinical Applications. Cureus 11, e4422 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-González U. et al. The diploic venous system: surgical anatomy and neurosurgical implications. Neurosurg Focus 27, E2 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Alarfaj A. et al. Magnetic resonance imaging analysis of human skull diploic venous anatomy. Surg Neurology Int 12, 249 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grüneboom A. et al. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat Metabolism 1, 236–250 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raggatt LJ & Partridge NC Cellular and Molecular Mechanisms of Bone Remodeling*. J Biol Chem 285, 25103–25108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y. et al. Early developing B cells undergo negative selection by central nervous system-specific antigens in the meninges. Immunity (2021) doi: 10.1016/j.immuni.2021.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Schafflick D. et al. Single-cell profiling of CNS border compartment leukocytes reveals that B cells and their progenitors reside in non-diseased meninges. Nat Neurosci 24, 1225–1234 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Niu C. et al. Identification of hematopoietic stem cells residing in the meninges of adult mice at steady state. Cell Reports 41, 111592 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Ringstad G. & Eide PK Molecular trans-dural efflux to skull bone marrow in humans with cerebrospinal fluid disorders. Brain awab 388- (2021) doi: 10.1093/brain/awab388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katayama Y. et al. Signals from the Sympathetic Nervous System Regulate Hematopoietic Stem Cell Egress from Bone Marrow. Cell 124, 407–421 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Maryanovich M. et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat Med 24, 782–791 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao X. et al. Nociceptive nerves regulate haematopoietic stem cell mobilization. Nature 589, 591–596 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moalem G. et al. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med 5, 49–55 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Russo MV, Latour LL & McGavern DB Distinct myeloid cell subsets promote meningeal remodeling and vascular repair after mild traumatic brain injury. Nat Immunol 19, 442–452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mastorakos P. et al. Temporally distinct myeloid cell responses mediate damage and repair after cerebrovascular injury. Nat Neurosci 24, 245–258 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salvador AFM & Kipnis J. Immune response after central nervous system injury. Semin Immunol 101629 (2022) doi: 10.1016/j.smim.2022.101629. [DOI] [PubMed] [Google Scholar]
- 41.Courties G. et al. Ischemic Stroke Activates Hematopoietic Bone Marrow Stem Cells. Circ Res 116, 407–417 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadjikhani N. et al. Extra-Axial Inflammatory Signal in Parameninges in Migraine with Visual Aura. Ann Neurol 87, 939–949 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein RS et al. Neuroinflammation During RNA Viral Infections. Annu Rev Immunol 37, 73–95 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murúa SR, Farez MF & Quintana FJ The Immune Response in Multiple Sclerosis. Annu Rev Pathology Mech Dis 17, 121–139 (2022). [DOI] [PubMed] [Google Scholar]
- 45.Shi K. et al. Bone marrow hematopoiesis drives multiple sclerosis progression. Cell 185, 2234–2247.e17 (2022). [DOI] [PubMed] [Google Scholar]
- 46.Giladi A. et al. Cxcl10+ monocytes define a pathogenic subset in the central nervous system during autoimmune neuroinflammation. Nat Immunol 21, 525–534 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Wilcox JA, Li MJ & Boire AA Leptomeningeal Metastases: New Opportunities in the Modern Era. Neurotherapeutics 1–17 (2022) doi: 10.1007/s13311-022-01261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sipkins DA et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 435, 969–973 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boire A. et al. Complement Component 3 Adapts the Cerebrospinal Fluid for Leptomeningeal Metastasis. Cell 168, 1101–1113.e13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quail DF & Joyce JA The Microenvironmental Landscape of Brain Tumors. Cancer Cell 31, 326–341 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Majzner RG et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 603, 934–941 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen X. & Holtzman DM Emerging roles of innate and adaptive immunity in Alzheimer’s disease. Immunity 55, 2236–2254 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bateman RJ et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. New Engl J Medicine 367, 795–804 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barthélemy NR et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nat Med 26, 398–407 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nation DA et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med 25, 270–276 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taipa R. et al. Proinflammatory and anti-inflammatory cytokines in the CSF of patients with Alzheimer’s disease and their correlation with cognitive decline. Neurobiol Aging 76, 125–132 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Wang Y. et al. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J Exp Med 213, 667–675 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reed-Geaghan EG, Croxford AL, Becher B. & Landreth GE Plaque-associated myeloid cells derive from resident microglia in an Alzheimer’s disease model. J Exp Medicine 217, e20191374 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silvin A. et al. Dual ontogeny of disease-associated microglia and disease inflammatory macrophages in aging and neurodegeneration. Immunity 55, 1448–1465.e6 (2022). [DOI] [PubMed] [Google Scholar]
- 60.Wu X, Saito T, Saido TC, Barron AM & Ruedl C. Microglia and CD206+ border-associated mouse macrophages maintain their embryonic origin during Alzheimer’s disease. Elife 10, e71879 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dvir-Szternfeld R. et al. Alzheimer’s disease modification mediated by bone marrow-derived macrophages via a TREM2-independent pathway in mouse model of amyloidosis. Nat Aging 2, 60–73 (2022). [DOI] [PubMed] [Google Scholar]
- 62.Dulken BW et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 571, 205–210 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gate D. et al. CD4+ T cells contribute to neurodegeneration in Lewy body dementia. Science eabf 7266 (2021) doi: 10.1126/science.abf7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gate D. et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 577, 399–404 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sudo K, Ema H, Morita Y. & Nakauchi H. Age-Associated Characteristics of Murine Hematopoietic Stem Cells. J Exp Medicine 192, 1273–1280 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krishnarajah S. et al. Single-cell profiling of immune system alterations in lymphoid, barrier and solid tissues in aged mice. Nat Aging 1–16 (2021) doi: 10.1038/s43587-021-00148-x. [DOI] [PubMed] [Google Scholar]
- 67.Helbling PM et al. Global Transcriptomic Profiling of the Bone Marrow Stromal Microenvironment during Postnatal Development, Aging, and Inflammation. Cell Reports 29, 3313–3330.e4 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Schürch CM, Riether C. & Ochsenbein AF Cytotoxic CD8+ T Cells Stimulate Hematopoietic Progenitors by Promoting Cytokine Release from Bone Marrow Mesenchymal Stromal Cells. Cell Stem Cell 14, 460–472 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Yamashita M. & Passegué E. TNF-α Coordinates Hematopoietic Stem Cell Survival and Myeloid Regeneration. Cell Stem Cell 25, 357–372.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valletta S. et al. Micro-environmental sensing by bone marrow stroma identifies IL-6 and TGFβ1 as regulators of hematopoietic ageing. Nat Commun 11, 4075 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaufmann T. et al. Quantifying bone marrow adiposity and its genetic architecture from head MRI scans. Medrxiv 2022.08.19.22278950 (2022) doi: 10.1101/2022.08.19.22278950. [DOI] [Google Scholar]
- 72.Christodoulou C. et al. Live-animal imaging of native haematopoietic stem and progenitor cells. Nature 578, 278–283 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vandoorne K. et al. Imaging the Vascular Bone Marrow Niche During Inflammatory Stress. Circ Res 123, 415–427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ueda HR et al. Tissue clearing and its applications in neuroscience. Nat Rev Neurosci 21, 61–79 (2020). [DOI] [PubMed] [Google Scholar]
- 75.Oguro H, Ding L. & Morrison SJ SLAM Family Markers Resolve Functionally Distinct Subpopulations of Hematopoietic Stem Cells and Multipotent Progenitors. Cell Stem Cell 13, 102–116 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pietras EM et al. Functionally Distinct Subsets of Lineage-Biased Multipotent Progenitors Control Blood Production in Normal and Regenerative Conditions. Cell Stem Cell 17, 35–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kondo M, Weissman IL & Akashi K. Identification of Clonogenic Common Lymphoid Progenitors in Mouse Bone Marrow. Cell 91, 661–672 (1997). [DOI] [PubMed] [Google Scholar]
- 78.Liu Z. et al. Fate Mapping via Ms4a3-Expression History Traces Monocyte-Derived Cells. Cell 178, 1509–1525.e19 (2019). [DOI] [PubMed] [Google Scholar]
- 79.Akashi K, Traver D, Miyamoto T. & Weissman IL A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197 (2000). [DOI] [PubMed] [Google Scholar]
- 80.Yáñez A. et al. Granulocyte-Monocyte Progenitors and Monocyte-Dendritic Cell Progenitors Independently Produce Functionally Distinct Monocytes. Immunity 47, 890–902.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Onai N. et al. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol 8, 1207–1216 (2007). [DOI] [PubMed] [Google Scholar]
- 82.Naik SH et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol 8, 1217–1226 (2007). [DOI] [PubMed] [Google Scholar]
- 83.McCracken JM & Allen L-AH Regulation of Human Neutrophil Apoptosis and Lifespan in Health and Disease. J Cell Death 7, JCD.S11038 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patel AA et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Medicine 214, 1913–1923 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheshier SH, Morrison SJ, Liao X. & Weissman IL In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc National Acad Sci 96, 3120–3125 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Comazzetto S, Shen B. & Morrison SJ Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev Cell 56, 1848–1860 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang HH, Hemberg M, Barahona M, Ingber DE & Huang S. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature 453, 544–547 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Naik SH et al. Diverse and heritable lineage imprinting of early haematopoietic progenitors. Nature 496, 229–232 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Yu VWC et al. Epigenetic Memory Underlies Cell-Autonomous Heterogeneous Behavior of Hematopoietic Stem Cells. Cell 167, 1310–1322.e17 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Velten L. et al. Human haematopoietic stem cell lineage commitment is a continuous process. Nat Cell Biol 19, 271–281 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rodriguez-Fraticelli AE et al. Clonal analysis of lineage fate in native haematopoiesis. Nature 553, 212–216 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Triana S. et al. Single-cell proteo-genomic reference maps of the hematopoietic system enable the purification and massive profiling of precisely defined cell states. Nat Immunol 1–13 (2021) doi: 10.1038/s41590-021-01059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baryawno N. et al. A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell 177, 1915–1932.e16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tikhonova AN et al. The bone marrow microenvironment at single-cell resolution. Nature 569, 222–228 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baccin C. et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat Cell Biol 22, 38–48 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sauvage F. J. de et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature 369, 533–538 (1994). [DOI] [PubMed] [Google Scholar]
- 97.Inra CN et al. A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature 527, 466–471 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Keller JT, Marfurt CF, Dimlich RVW & Tierney BE Sympathetic innervation of the supratentorial dura mater of the rat. J Comp Neurol 290, 310–321 (1989). [DOI] [PubMed] [Google Scholar]
- 99.Li L. et al. A mouse model with high clonal barcode diversity for joint lineage, transcriptomic, and epigenomic profiling in single cells. (2023) doi: 10.1101/2023.01.29.526062. [DOI] [PubMed] [Google Scholar]
- 100.Hofmeijer J. et al. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurology 8, 326–333 (2009). [DOI] [PubMed] [Google Scholar]