Abstract
Vascular access is the Achilles’ heel of dialysis therapy among patient with end stage kidney disease. The development of neointimal hyperplasia and subsequent stenosis is common in vascular access and is associated with significant morbidity. Percutaneous transluminal angioplasty using balloon inflation was the standard therapy of these lesions. However, the balloon-based approaches were associated with poor vascular access patency rate necessitating new inventions. It is within this context that different types of stents were developed in order to improve the overall dialysis vascular access functionality. In this article, we review the available literature regarding the use of stents in treating dialysis vascular access stenotic lesions. Further, we review the major clinical trials of stent use in different anatomic locations and in different clinical scenarios.
Keywords: Stents, hemodialysis Vascular access, vascular stenosis, hemodialysis, arteriovenous access
Introduction
Hemodialysis vascular access dysfunction causes significant morbidity in patients with end stage kidney disease (ESKD). This dysfunction is usually associated with poor patency related to the development of vascular stenosis that is attributed to neointimal hyperplasia.1–3 Percutaneous transluminal balloon angioplasty (PTA) is considered the first-line treatment for the vascular access stenoses. Paradoxically, PTA itself can induce endothelial injury and may contribute to further neointimal hyperplasia and subsequent stenoses. Therefore, not surprisingly, PTA of the vascular access is associated with poor patency and high re-intervention rate. For instance, the primary patency rates for arteriovenous fistulas (AVFs) stenotic lesions following standard PTA are around 50% at 6 months and 20% at 12 months.4 Furthermore, these patency rate are even worse in patients with arteriovenous grafts (AVGs) as compared to those with AVFs.5,6 Importantly, the vascular access procedures are costly to our society. It is reported that, in 2013, the annual Medicare expenditure related to dialysis vascular access services in the United States was approximately $2.8 billion.7
Several novel therapies have been utilized to improve the dialysis vascular access function with some showing promising results.8 A recent study demonstrated significant improvement in the 6-month AVF patency following angioplasty using drug-coated balloons as compared to the standard balloon angioplasty.9 However, this study did not include AVGs or AVFs with thrombosis, in-stent stenosis or central venous stenotic lesions. Moreover, this study excluded patients with primary AVF failure. It is within this context that stents have been introduced during the last three decades for treating recurrent dialysis access stenotic lesions, recurrent access thrombosis, and occasionally for managing ruptured vessels encountered during angioplasty. The overarching goal of the present paper is to provide a comprehensive review of stent use in managing hemodialysis vascular access dysfunction.
Stent use in hemodialysis vascular access
The use of stents in managing hemodialysis vascular access dysfunction was first described in 1988.10 The classical indications for stent placement in a dialysis vascular access are recoil, rupture, and recurrent stenosis. A brief description of these terms is provided in the next few paragraphs.
Recoil lesion:
It is defined as residual stenosis of >30% following angioplasty with an appropriately sized balloon. This phenomenon occurs due to the elastic recoil of the vessel following balloon dilatation of the affected area. Importantly, the residual stenosis following angioplasty is associated with poor AV access survival outcomes.11
Vessel rupture:
This can occur during the balloon inflation of severely stenotic lesions of the vascular access. In most cases, the extravasation can be controlled with manual compression and balloon tamponade at the rupture site. However, if extravasation persists despite these measures, then stent placement at the site of rupture can be utilized in order to control the bleeding (Figure 1).12,13
Figure 1.
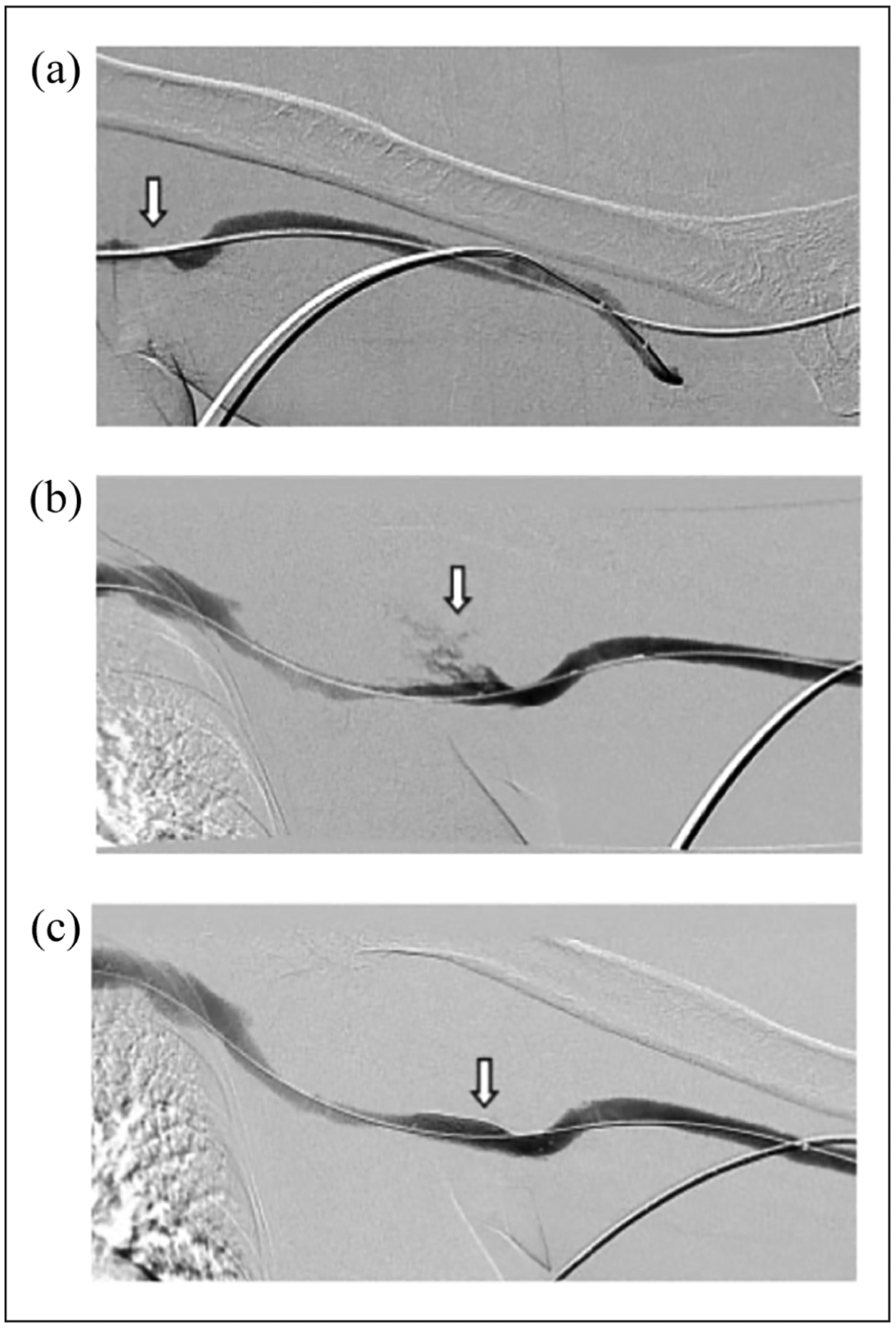
Stent placement to control extravasation following angioplasty: (a) stenosis (arrow) involving the venous anastomosis of a brachial-brachial arteriovenous graft, (b) an extravasation (arrow) into the axilla immediately after angioplasty to 8 mm is noted, and (c) there is complete cessation of extravasation after the placement of a bare metal stent (arrow).
Recurrent stenosis:
This phenomenon is commonly encountered in dialysis vascular access and is associated with access dysfunction and thrombosis. Stent deployment at the site of the affected segment can be used to manage these lesions. However, it is worth mentioning that the anatomic site of restenosis may not always be an ideal location for stent placement such as the cannulation zone of the vascular access. In these situations, surgical intervention such as patch angioplasty or jump graft placement may be preferred.
Regardless of the indication of stenting, the operator must always assess the impact of the stent deployment on the patient’s ESKD life-plan and on future AV access creation.14
Recently, however, the growing evidence suggests that stent-grafts are preferred over the conventional balloon angioplasty for managing the stenotic lesions developed at the graft-vein anastomosis seen in AVGs and for in-stent restenosis encountered in both AVFs and AVGs.
Taken together, the role of stents in managing dialysis vascular access malfunction has substantially evolved and expanded over the past decade. In the following paragraphs, we provide a brief description of the composition of stents, discuss the landmark clinical trials of the stent use in the dialysis vascular access, and review the complications associated with stent use. Further, the use of stents in special clinical scenarios will be discussed.
Composition of the stents
Endovascular stents are scaffolds or tube-like structures that provide mechanical endoluminal support to the vessel wall to maintain patency. These include bare metal stents, nitinol stents, and stent grafts.
The early generation of stents were made of stainless steel and, therefore, were called bare metal stents. One of the drawbacks of the bare metal stents is stent shortening upon deployment; additionally, these stents can develop kinks if they are deployed at sharp angles in the vascular access circuit. In order to address these pitfalls, the nitinol (nickel-titanium alloy) stent generation was developed. The term SMART (Shape Memory Alloy Recoverable Technology) was used to describe these nitinol stents. The unique structure of smart stents minimizes the foreshortening and allows the stent to be fully expanded to the pre-determined size.15
Despite the unique structure of the nitinol stents, they are not without complications. In fact, after deploying these stents, the tissue in-growth related to the migration of the smooth muscle cells and neovascularization, especially through the fenestrations of the bare metal stents, is proposed as a mechanism for the in-stent restenosis, which results in poor vascular access patency.
The aforementioned limitations of the bare metal and smart stents necessitated the development of the stent grafts (covered stents), which are metal stents by design and are covered with a fabric coat such as Dacron or expanded Polytetrafluorethylene (ePTFE) graft material. Theoretically, the design of the stent graft is intended to maintain the vessel patency through the endoluminal wall support while the biocompatible barrier is meant to prevent the tissue in-growth and restenosis.
Due to their common use in clinical practice, a brief description of the commonly available stent grafts is discussed below (See Table 1).
Table 1.
Properties of the commonly used stent-grafts in dialysis vascular access.
| Stent-graft | Stent skeleton | Stent-graft material | Stent diameter | Delivery system diameter |
|---|---|---|---|---|
| FLAIR ® | Nitinol | ePTFE lining on the luminal and the external surface. The luminal surface is carbon impregnated | 6–9 mm | 9 F |
| Viabahn ® | Nitinol | ePTFE lining on the luminal surface. ePTFE on the luminal surface is modified with a heparin bioactive surface | 5–13 mm | 7–10 F |
| Fluency Plus ® | Nitinol | ePTFE lining on the luminal and the external surfaces. The luminal surface is carbon impregnated | 6–13.5 mm | 8–10 F |
| Covera ® | Nitinol | ePTFE lining on the external surface | 6–10 | 8–9 F |
1. FLAIR® Endovascular Stent Graft (C. R. Bard, Tempe, Arizona):
The FLAIR® stent is a self-expandable nitinol stent with ePTFE material covering both the luminal and external surfaces. It comes in two configurations, flared and straight, with the flared configuration being 4 mm larger at the proximal end than the distal end of the stent.
2. Viabahn® Endovascular Stent Graft (W. L. Gore & Associates, Inc., Flagstaff, Arizona):
The Viabahn® stent is a self-expandable, flexible, nitinol stent that contains ePTFE covering the luminal surface of the stent. The ePTFE luminal surface of the Viabahn® stent is modified with a heparin bioactive surface.
3. Fluency Plus® Endovascular Stent Graft (C. R. Bard, Tempe, Arizona):
The Fluency Plus® stent is a self-expandable nitinol stent that is encapsulated between two layers of ePTFE along the entire length of the stent except at the flared ends of the stent graft.
4. Covera® Endovascular Stent Graft (C. R. Bard, Tempe, Arizona):
The Covera® stent graft is a self-expandable, flexible, nitinol stent that is covered with ePTFE and is available in flared and straight configurations.
Landmark trials of stent use in dialysis vascular access
Several earlier studies failed to show improvement in vascular access patency with the use of bare metal stents compared to standard balloon angioplasty.16–18 However, accumulative evidence from several recent randomized-control trials (RCTs) have shown that stent grafts are effective in improving the vascular access patency. The following clinical trials tested the efficacy of stents for treating the vascular access stenoses that are located at specific anatomic sites.
Graft-vein anastomosis stenosis in the AVG
In the United States, at least 20% of the prevalent hemodialysis patients use AVGs as the primary vascular access. Stenosis at the graft-vein anastomosis is the leading cause of AVG dysfunction and thrombosis. Several randomized controlled trials have compared the use of stent grafts to balloon angioplasty for the treatment of the graft-vein anastomosis stenosis.
FLAIR® Pivotal Trial.
The FLAIR® Pivotal Trial was a multicenter, prospective, RCT comparing the FLAIR® endovascular stent graft to standard PTA in functioning AVGs with stenosis at the graft-vein anastomosis.5 In this trial, a total of 190 patients were enrolled, of which 97 were randomly assigned to receive the FLAIR® stent graft and 93 were assigned to undergo PTA only. Follow-up angiography was performed at 6 months regardless of the AVG function. At 6-months, the primary patency of the target lesion was significantly better in the stent graft group (51% vs 23%, p ⩽ 0.001). Similarly, the primary patency rate of the access circuit was higher in the stent group as compared to the angioplasty counterparts (38% vs 20%, p = 0.008). The Flair trial was the first RCT to demonstrate better outcomes with stent graft use compared to PTA in patients with AVGs.
It is important to note that in the FLAIR trial, the 6-month patency of the graft-vein anastomosis stenosis following PTA alone was significantly lower compared to the patency rate described with in the previously conducted trials.16 In fact, most recent studies have reported 6-month primary patency rate in the range of 23%–35% with PTA alone, which is lower than the primary patency rate of 40%–60% reported in the older studies.16,19,20 The reason for this discrepancy is not entirely clear but, interestingly, it correlates with the time when ultra-pressure angioplasty balloon use became widely available.
RENOVA trial.
The RENOVA trial was a prospective, randomized, controlled post-approval study to assess the long-term safety and efficacy of the FLAIR® stent graft as compared to PTA for managing the graft-vein anastomosis stenosis in AVGs.21 Of the 270 patients enrolled in this study, 138 patients were randomized to the stent graft group and 132 patients were included in the PTA group. During the 2 year-follow-up, vascular access angiography and intervention were performed if clinically indicated. The study was completed by only 191 patients due to patient deaths (N = 74) and patients lost to follow up (N = 7). At 2-years, the primary patency of the target lesion was 26.9% in the stent graft group versus 13.5% in the PTA group (p = 0.001). Furthermore, the access circuit patency was 9.5% in the stent graft group and 5.5% in the PTA group (p = 0.01). Although the overall patency rates in the RENOVA trial were low in both the groups at 2 years, the trial demonstrated that the stent graft use was associated with a 1.7-fold better long-term primary patency than PTA.
REVISE trial.
The REVISE trial was a prospective, multicenter, RCT that compared the efficacy of the Viabahn stent grafts to PTA alone for the treatment of graft-vein anastomosis stenosis in both the failing AVGs as well as in the thrombosed AVGs.19 This was the first RCT to test the efficacy of stent grafts in thrombosed AVGs. Of the 293 patients, 145 patients were randomized to the stent graft group and 148 to the PTA only group for the treatment of stenosis at the graft-vein anastomosis: dysfunctional AVGs (n = 164) and thrombosed AVGs (N = 129).
At 6-months, the primary patency of the target lesion was better in the stent graft group compared to the PTA group (51.6% vs 34.2%, p = 0.006). Primary patency of the target lesion was much better in the dysfunctional AVGs compared to the thrombosed AVGs: 64.6% versus 36.1% in stent graft group, and 45.8% versus 23.5% in the PTA group. Despite the fact that the stent graft use in the thrombosed AVGs was less effective than its use in the dysfunctional AVGs, the thrombosed AVGs still performed better with stent grafts than with PTA (primary target lesion patency: 36.1% vs 23.5%). Overall, this trial demonstrated superior primary patency of the target lesion located at the graft-vein anastomosis in the AVGs with stent graft use compared to PTA.
A subsequent cost analysis of the REVISE trial was done to assess the cost of the index procedure, follow-up interventions, and total costs through 24 months. This analysis showed that at 24-months following the index procedure, the stent graft use reduced the number of rein-terventions in the access circuit by 27% (3.7 in the stent-graft group vs 5.1 in the PTA group, p = 0.005) but the total overall costs were similar in the two groups ($27,483 vs $28,664, p = 0.49).22 Stent graft placement initially costed more than PTA but at 24 months the total costs associated with these two interventions were not statistically different due to lower reintervention rate in the stent graft group. Moreover, the 24-month cost benefit with stent graft use was significant for the thrombosed AVGs ($30,329 in the stent graft group vs $37,206 in the PTA alone group, p = 0.027), and this difference was mainly driven by the lower reintervention rates in the stent graft group as compared to the PTA group (3.7 vs 6.2, p = 0.022).
In-stent restenosis
In-Stent restenosis is commonly encountered in dialysis vascular access and is associated with vascular access dysfunction.23 The RESCUE trial is the only trial to study the use of stent grafts for treating in-stent restenosis of the dialysis vascular access.
RESCUE trial.
The RESCUE trial was a prospective, multicenter, RCT that compared the Fluency® stent graft use with PTA alone for the treatment of in-stent restenosis in AVGs and AVFs.24 In this study, 275 patients with in-stent restenosis in the venous outflow circuit of a mature AVF or AVG were randomized to either the stent graft group (N = 132) or to the PTA alone group (N = 143). At 6-months, the access circuit primary patency was better in the stent graft group as compared to the angioplasty group: 18.6% versus 4.5% (p ⩽ 0.001). The 6-month target area primary patency was also superior in the stent graft group as compared to the angioplasty group (66.4% vs 12.3%, p ⩽ 0.001). Furthermore, patients who underwent stent graft placement maintained better access survival curves throughout the 2-year follow-up period as compared to their counterparts in the PTA group: (15.6% vs 4.3%; p ⩽ 0.001).
Cephalic arch stenosis
The cephalic arch is the perpendicular portion of the cephalic vein that lies in the region of the deltopectoral groove before it joins the axillary or subclavian vein. This area is particularly prone to stenosis, angioplasty-related rupture and is generally associated with poor patency. Two small studies have evaluated the role of stent grafts for the treatment of cephalic arch stenosis.
In a small study, Shemesh et al. compared the use of stent graft (Fluency Plus®) with the bare metal stent (Luminex®, C. R. Bard, Tempe, Arizona) for management of recurrent cephalic arch stenoses in 25 patients.25 The primary patency at 1-year was better in the stent graft group than the bare metal group (32% vs 0%, p = 0.0023), and the reintervention rate was also lower in the stent graft group as compared to their counterparts (0.9/pt.-year vs 1.9/pt.-year, p = 0.02).
In another study, Rajan et al., randomized 14 patients with de novo or in-stent cephalic arch stenosis to either the stent graft group or to the PTA group.26 The primary access circuit patency rates and primary target lesion patency rates at 3, 6, and 12 months were better in the stent graft group as compared to the PTA group; the target area patency was 100%, 100%, and 29% in the stent graft group versus 60%, 0%, 0% in the PTA group at 3, 6, and 12 months (p ⩽ 0.01). However, this study had several limitations including its small sample size.
Based on the available evidence, the first line of treatment for cephalic arch stenosis is PTA. However, stent graft placement or cephalic vein surgical turndown procedure are potential treatment options for recurrent cephalic arch stenosis or for lesions resistant to angioplasty.27,28 The operator has to be cautious when deploying a stent graft in the cephalic arch to avoid jailing of the ipsilateral axillary vein, which could jeopardize the creation of the future basilic vein or axillary vein based AV access.29
Central vein stenosis
Thoracic central vein stenosis is a common problem in dialysis vascular access. Moreover, it is difficult to ascertain the true incidence of central vein stenosis in dialysis patients as these lesions can be asymptomatic.30 Additionally, the treatment of the central vein stenotic lesions can be particularly challenging due to their resistance to angioplasty and their tendency to elastic recoil. There are no RCTs that have compared the efficacy of PTA to either bare metal stents or to stent grafts for the treatment of central vein stenosis in the dialysis vascular access. Basically, PTA is the first-line treatment for central vein stenosis.31 In clinical practice, the use of stents for the management of central vein stenosis is generally reserved for recurrent elastic symptomatic lesions. Interestingly, some recent reports suggest that the stent grafts may have better long term patency and lower rates of reintervention compared to PTA and bare metal stents.32,33
A small single center retrospective study compared the use of stent for central vein stenosis ipsilateral to the vascular access with converting the dialysis access into Hemodialysis Reliable Outflow (HeRO®, Jordan, UT) graft. The study concluded that the primary and secondary access patency rates were better in the HeRO® graft group compared to the stent group. The 6- and 12-month primary patency rates were 89% and 72% compared to 47% and 11%, respectively (p = <0.001). HeRO graft conversions also resulted in improvement of the 6- and 12-month secondary access patency rates (95% and 95% vs 79% and 58%; p = 0.006). It is noteworthy, however, that most stents used in this study were bare metal stents and all the AV accesses were functional before they were converted to HeRO® grafts, therefore, the outcomes of this report were likely skewed in favor of the HeRO® graft.34
Dialysis access related venous thoracic outlet syndrome (VTOS) is a rare but distinct clinical entity caused by subclavian vein stenosis due to its compression by the thoracic outlet, which is formed by the first rib, clavicle, and subclavius muscle. VTOS lesions can be resistant to PTA and stenting could be a treatment option. However, stent fracture is a potential complication due to the “nutcracker” effect of the first rib and the clavicle.35 Some studies suggest that surgical bone decompression followed by stenting may offer a better patency for VTOS lesions.36–38 Lastly, vein bypass surgery is rarely needed but has been performed for management of VTOS.39
Juxta-anastomosis stenosis
While juxta-anastomosis stenosis is a common cause of primary AVF failure, it may also occur in the mature and functioning AVFs. There are no RCTs comparing PTA to surgical revision for the treatment of juxta-anastomosis stenoses. Recent studies suggest that drug coated balloons may have a role in the management of juxta-anastomosis stenosis.9,40 Importantly, stent placement is generally not considered the first line treatment for juxta-anastomosis stenosis though its use has been described with good results in small studies.41–43 Larger studies are needed to further evaluate the role of stents in management of juxta-anastomosis stenosis.
Brachial-basilic angle of transposition stenosis or swing point stenosis
Brachio-basilic AVF creation usually requires the transposition of the basilic vein to a lateral and superficial position in order to make the fistula suitable for cannulation. This transposition usually creates a swing point within the fistula body that predisposes the basilic vein to stenosis. In general, PTA remains the first line of treatment for managing the stenotic lesions of the swing segment and the venovenous anastomosis of the transposed basilic vein. In a small study of 37 patients, where the patients served as their own controls, the use of stent graft improved the primary patency of the target lesion. The pre-stent primary patency rates of the target lesion were 29% and 3% at 6 and 12 months, respectively, whereas the post-stent primary patency rates of the target lesion were 57% and 40% at 6 and 12 months, respectively (p = 0.04). The stent placement improved the access primary assisted patency rates at 12 months (13% pre-stent vs 80% post-stent, p = <0.001) and also lowered the access reintervention rate (0.5/month pre-stent vs 0.15/month post-stent, p ⩽ 0.001).44
Special scenarios for stent use in a dialysis vascular access
Stent placement across the elbow joint
Stent placement at anatomic sites that expose the stent to repetitive bending and crushing forces, such as across the elbow joint, should be avoided if possible.45 If stent placement is absolutely indicated across the elbow joint, then a flexible stent graft is recommended. In the REVISE study, Viabahn® stent grafts were placed across the elbow joint in 22 patients and were found to be safe and effective.19 Importantly, the Viabahn® stent graft is FDA approved for use across the elbow joint.
Stent placement in the AV access cannulation zone
It is well acknowledged that stent placement in the cannulation zone of the vascular access is discouraged as the repeated cannulation through the stent can be associated with stent wall damage, stent infection, and pseudoaneurysm formation. The safety of cannulation through a stent graft has not been established. Therefore, stent graft use in the cannulation zone should only be used as the last resort for vascular access salvage.46
Stent placement in a pseudoaneurysm.
Pseudoaneurysms usually develop in the cannulation zone of an AV access and are believed to occur as a consequence of repeated cannulation at the same site causing injury to the vein or graft material. Additionally, pseudoaneurysm formation is further exacerbated by the high intra-access pressures seen with concurrent outflow vein stenosis. While surgical repair is considered the definite treatment for pseudoaneurysms, some interventionalists utilize stents to exclude these formations. The utilization of stents in excluding these pseudoaneurysms is associated with increased risk of infection.47 It is within this context that the use of stents graft to treat the dialysis access pseudoaneurysms should be reserved for patients in whom surgical repair is not feasible.
Stent placement in the setting of a cardiac implantable electronic devices
The use of cardiovascular implantable electronic devices (CIEDs) is common among dialysis patients.48 The presence of these devices within the central venous system can potentially cause endothelial injury, neointimal hyperplasia, and stenoses.49 Central vein stenosis is commonly seen in dialysis patients with CIEDs.50 Although PTA remains the first-line treatment for CIED-induced central venous stenoses, the post-PTA primary patency rates remain poor. In a multicenter study of 28 patients with CIED induced central venous stenosis, the primary patency rates following PTA were 18% and 9% at 6 and 12 months, respectively. In this study, the secondary patency rate was defined as the circuit patency until the access was surgically revised, clotted, abandoned, or lost to follow-up. The reported secondary patency rates following PTA were 95% at 6 months, 86% at 12 months, and 73% at 24 months.51 In a retrospective study of 14 patients, bare metal stents or stent grafts were used to treat CIED-induced central venous stenosis. The primary patency of the access circuit following the stent placement was 45.5% at 6 months and 9% at 12 months. Primary-assisted patency following the stent placement was 90.9% at 6 months and 80% at 12 months. Secondary patency was 100% at 6 months and 90% at 12 months. Furthermore, no device related adverse events were reported following stent placement, however, long-term follow-up was lacking.52
It is generally recommended to avoid stent use in managing central venous stenotic lesions in the setting of CIED as it can result in jailing of the device wires and potentially can cause CIED malfunction.53 If stent placement is considered absolutely necessary, it is recommended to extract the CIED leads prior to stent placement and to replace the leads after stent deployment when possible.54
KDOQI 2019 vascular access guidelines for stent use in dialysis vascular access
The recently published KDOQI 2019 Vascular Access Guidelines recommend stent graft placement in dialysis vascular access for the following indications (See Table 2).14
Table 2.
KDOQI 2019 vascular access guideline: indications for stent-graft use in AV access.
|
Complications associated with stent use in dialysis vascular access
Stent migration
Stent migration can occur immediately at the time of stent deployment or it can be a late complication (Figure 2(a) and (b)). The stent migration into the downstream veins can cause partial or complete venous occlusion.55 The loss of downstream venous segment following stent placement can limit future AV access options. Moreover, distal stent migration into the heart and pulmonary vessels have also been reported.56 Due to the morbidity associated with stent migration, displaced stents are usually retrieved using snare devices. In order to avoid this complication, it is critical to follow the stent device instructions and to accurately size the stent before it is deployed in the dialysis access.
Figure 2.
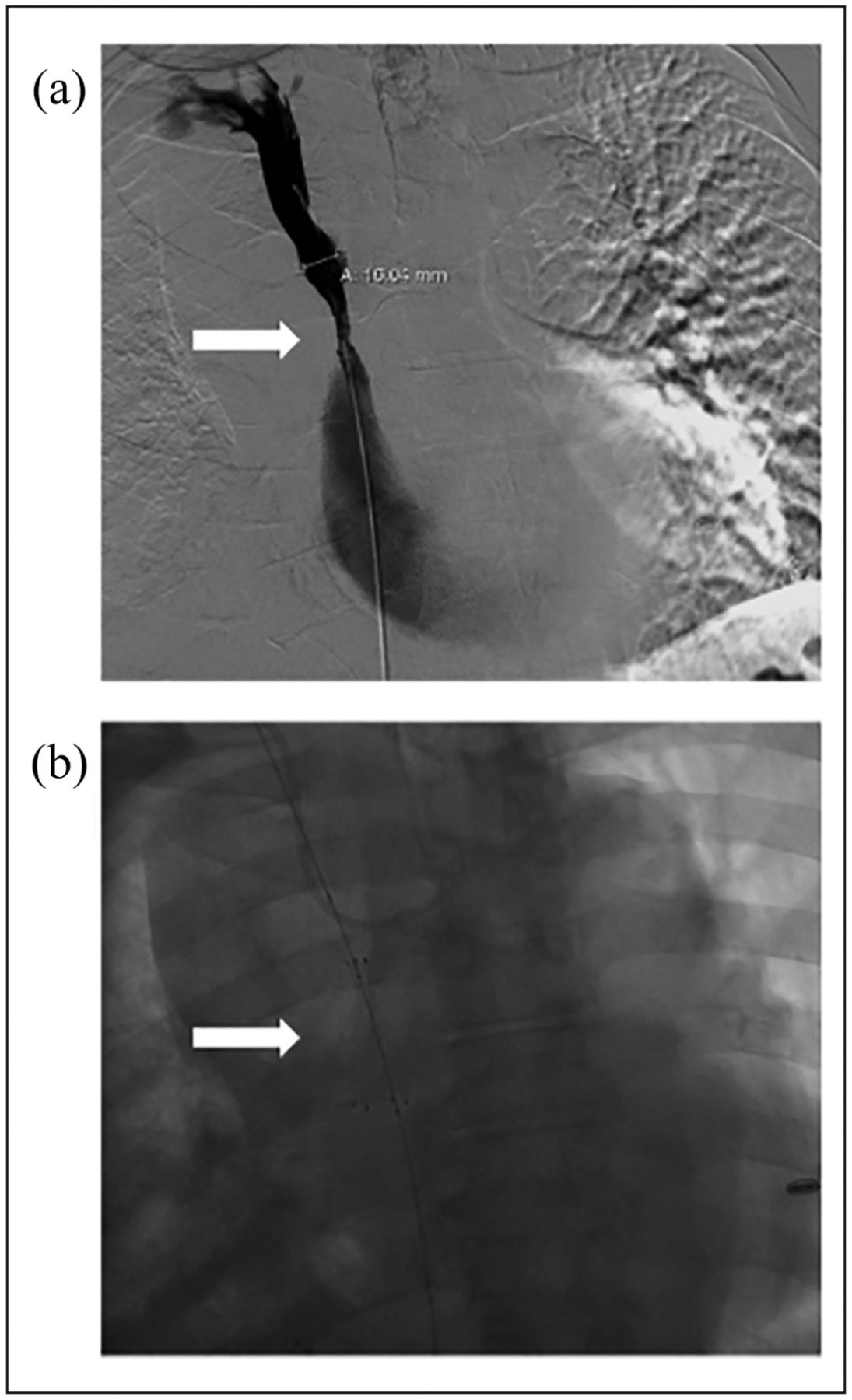
Stent migration: (a) venous stenosis (arrow) at the innominate vein and superior vena cava junction resistant to balloon angioplasty so decision was made to deploy a stent at the site of stenosis and (b) during stent deployment, the stent migrated into the inferior vena cava (arrow).
Stent fracture
Stent fracture can occur at sites that are prone to compressive mechanical forces, such as the subclavian vein stents and areas of repetitive bending and crushing, such as the stents across the elbow joint. Due to their flexibility properties, the stent grafts are better suited at the aforementioned locations.19
It is worth mentioning that stent fractures and stent (strut) protrusion have been described in the setting of repeated needle cannulation of the vascular access through the stent (Figures 3 and 4).57 These stent fractures can eventually lead to venous outflow obstruction and AV access thrombosis.55
Figure 3.
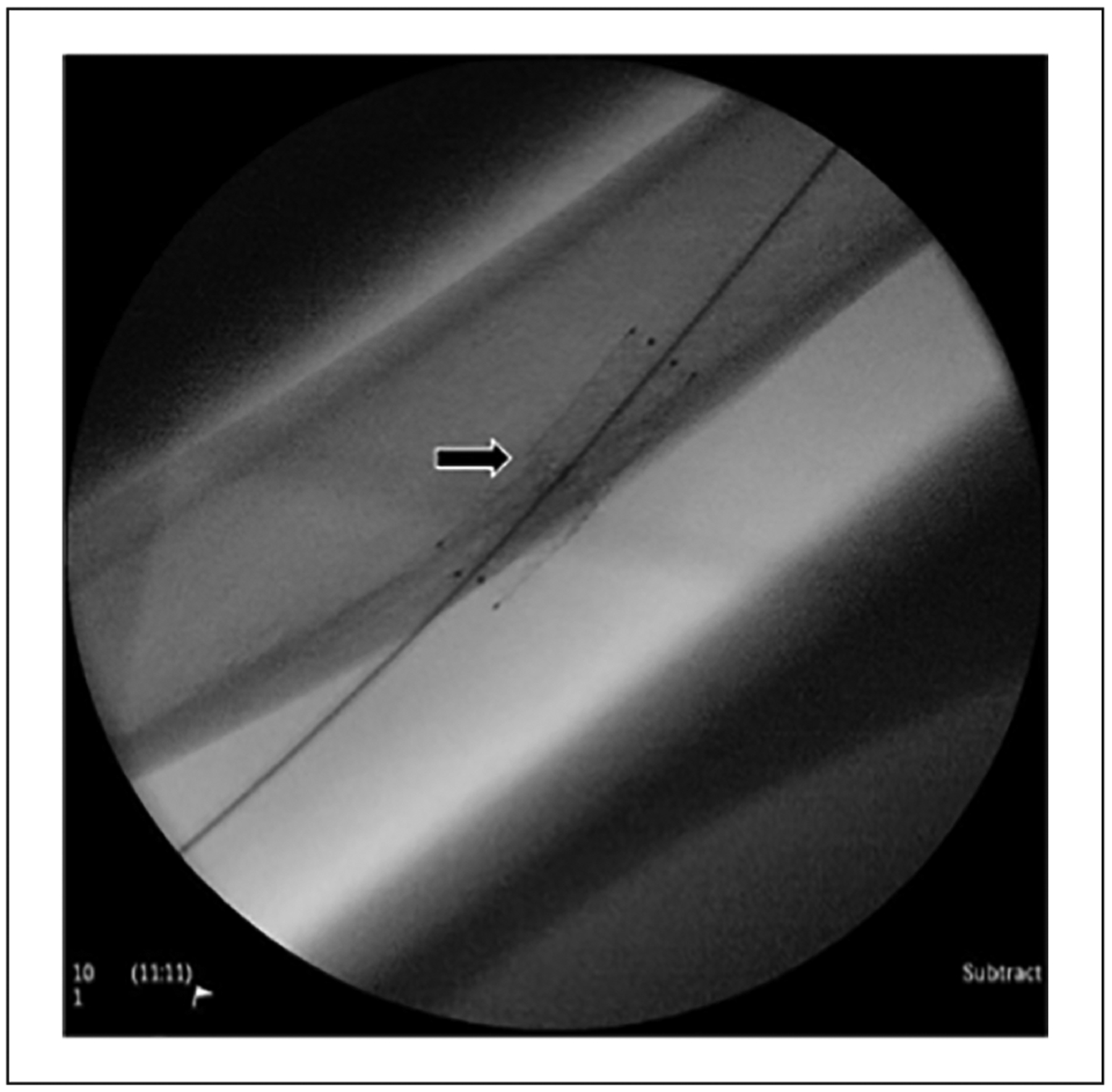
Stent fracture. A fractured stent in the outflow vein of a brachiocephalic arteriovenous fistula. The arrow points to the location of the fracture.
Figure 4.
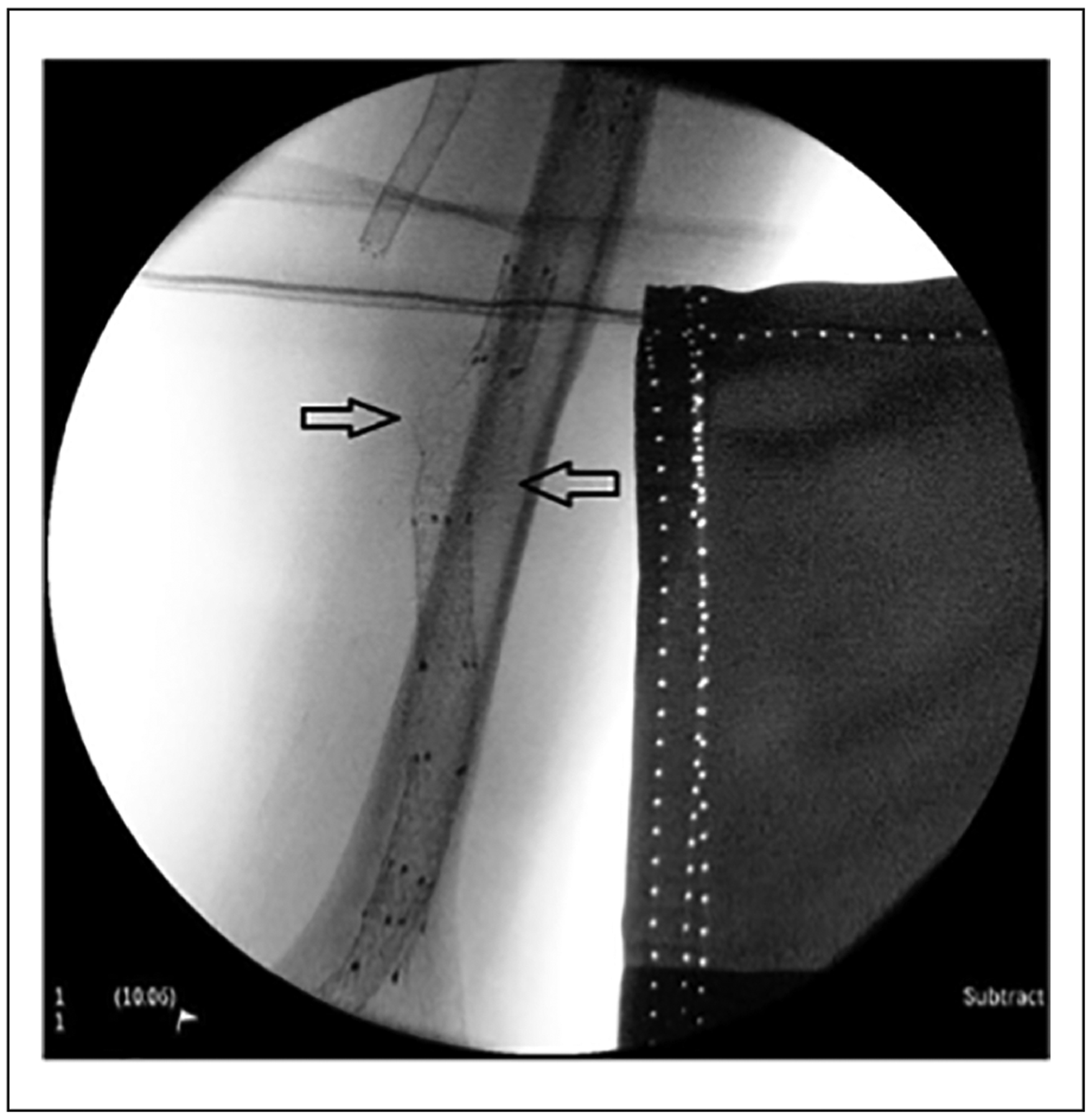
Stent protrusion. Stent material (struts) was cut by the repetitive cannulation from hemodialysis needle. The injured struts may protrude through the skin, which will carry risks to the dialysis patient’s healthcare providers. The arrow points at the damaged stent struts.
Infection
The risk factors for stent infection include repetitive needle cannulation through the stent wall and the use of stents for management of dialysis access pseudo-aneurysms. Stent associated infection is a serious complication and often requires surgical intervention.47 Of particular importance, stent placement must be avoided in the setting of any local or systemic infection. Seeding the stent with infection is associated with severe morbidity and potentially catastrophic outcomes.
Jailing of the veins
Stent placement in a venous outflow segment can potentially cause “jailing” of other veins, which can not only limit the option of surgical revision of the AV access, but it can also impact the option of creating a new AV access in the future.
Summary
Stents have emerged as a viable therapeutic option for dialysis vascular access dysfunction. The use of stents in vascular access must be guided by scientific evidence. In the past decade, clinical trials have demonstrated superiority of stent grafts over standard balloon angioplasty for the treatment of graft-vein anastomosis stenosis in the AVGs, and for in-stent stenosis in AVFs and AVGs. It is noteworthy that for the vast majority of vascular access stenoses, balloon angioplasty remains the first-line therapy. Prior to stent placement in a vascular access, it is imperative to consider alternative surgical options and assess the impact of the stent on the viability of future AV access options. As is often said in medicine, “First, do no harm.”
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Riella MC and Roy-Chaudhury P. Vascular access in haemodialysis: strengthening the Achilles’ heel. Nat Rev Nephrol 2013; 9: 348–357. [DOI] [PubMed] [Google Scholar]
- 2.Lee T and Roy-Chaudhury P. Advances and new frontiers in the pathophysiology of venous neointimal hyperplasia and dialysis access stenosis. Adv Chronic Kidney Dis 2009; 16: 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy-Chaudhury P, Sukhatme VP and Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol 2006; 17: 1112–1127. [DOI] [PubMed] [Google Scholar]
- 4.Yan Wee IJ, Yap HY, et al. A systematic review and meta-analysis of drug-coated balloon versus conventional balloon angioplasty for dialysis access stenosis. J Vasc Surg 2019; 70: 970–979 e973. [DOI] [PubMed] [Google Scholar]
- 5.Haskal ZJ, Trerotola S, Dolmatch B, et al. Stent graft versus balloon angioplasty for failing dialysis-access grafts. N Engl J Med 2010; 362: 494–503. [DOI] [PubMed] [Google Scholar]
- 6.Kim WS, Pyun WB and Kang BC. The primary patency of percutaneous transluminal angioplasty in hemodialysis patients with vascular access failure. Korean Circ J 2011; 41: 512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thamer M, Lee TC, Wasse H, et al. Medicare costs associated with arteriovenous fistulas among US hemodialysis patients. Am J Kidney Dis 2018; 72: 10–18. [DOI] [PubMed] [Google Scholar]
- 8.Lawson JH, Niklason LE and Roy-Chaudhury P. Challenges and novel therapies for vascular access in haemodialysis. Nat Rev Nephrol 2020; 16: 586–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lookstein RA, Haruguchi H, Ouriel K, et al. Drug-coated balloons for dysfunctional dialysis arteriovenous fistulas. N Engl J Med 2020; 383: 733–742. [DOI] [PubMed] [Google Scholar]
- 10.Zollikofer CL, Largiader I, Bruhlmann WF, et al. Endovascular stenting of veins and grafts: preliminary clinical experience. Radiology 1988; 167: 707–712. [DOI] [PubMed] [Google Scholar]
- 11.Lilly RZ, Carlton D, Barker J, et al. Predictors of arteriovenous graft patency after radiologic intervention in hemodialysis patients. Am J Kidney Dis 2001; 37: 945–953. [DOI] [PubMed] [Google Scholar]
- 12.Vesely TM. Role of stents and stent grafts in management of hemodialysis access complications. Semin Vasc Surg 2007; 20: 175–183. [DOI] [PubMed] [Google Scholar]
- 13.Dale JD, Dolmatch BL, Duch JM, et al. Expanded polytetrafluoroethylene-covered stent treatment of angioplasty-related extravasation during hemodialysis access intervention: technical and 180-day patency. J Vasc Interv Radiol 2010; 21: 322–326. [DOI] [PubMed] [Google Scholar]
- 14.Lok CE, Huber TS, Lee T, et al. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis 2020; 75: S1–S164. [DOI] [PubMed] [Google Scholar]
- 15.Sequeira A and Abreo K. The structure and function of endovascular stents: a primer for the interventional nephrolo-gist. Semin Dial 2014; 27: E10–E20. [DOI] [PubMed] [Google Scholar]
- 16.Beathard GA. Gianturco self-expanding stent in the treatment of stenosis in dialysis access grafts. Kidney Int 1993; 43: 872–877. [DOI] [PubMed] [Google Scholar]
- 17.Quinn SF, Schuman ES, Demlow TA, et al. Percutaneous transluminal angioplasty versus endovascular stent placement in the treatment of venous stenoses in patients undergoing hemodialysis: intermediate results. J Vasc Interv Radiol 1995; 6: 851–855. [DOI] [PubMed] [Google Scholar]
- 18.Sapoval MR, Turmel-Rodrigues LA, Raynaud AC, et al. Cragg covered stents in hemodialysis access: initial and midterm results. J Vasc Interv Radiol 1996; 7: 335–342. [DOI] [PubMed] [Google Scholar]
- 19.Vesely T, DaVanzo W, Behrend T, et al. Balloon angioplasty versus Viabahn stent graft for treatment of failing or thrombosed prosthetic hemodialysis grafts. J Vasc Surg 2016; 64: 1400–1410 e1401. [DOI] [PubMed] [Google Scholar]
- 20.Vesely TM and Siegel JB. Use of the peripheral cutting balloon to treat hemodialysis-related stenoses. J Vasc Interv Radiol 2005; 16: 1593–1603. [DOI] [PubMed] [Google Scholar]
- 21.Haskal ZJ, Saad TF, Hoggard JG, et al. Prospective, randomized, concurrently-controlled study of a stent graft versus balloon angioplasty for treatment of arteriovenous access graft stenosis: 2-year results of the RENOVA study. J Vasc Interv Radiol 2016; 27: 1105–1114 e1103. [DOI] [PubMed] [Google Scholar]
- 22.Mohr BA, Sheen AL, Roy-Chaudhury P, et al. Clinical and economic benefits of stent grafts in dysfunctional and thrombosed hemodialysis access graft circuits in the REVISE randomized trial. J Vasc Interv Radiol 2019; 30: 203–211 e204. [DOI] [PubMed] [Google Scholar]
- 23.Chan MR, Young HN and Yevzlin AS. The effect of in-stent restenosis on hemodialysis access patency. Hemodial Int 2009; 13: 250–256. [DOI] [PubMed] [Google Scholar]
- 24.Falk A, Maya ID, Yevzlin AS, et al. A prospective, randomized study of an expanded polytetrafluoroethylene stent graft versus balloon angioplasty for in-stent restenosis in arteriovenous grafts and fistulae: two-year results of the RESCUE study. J Vasc Interv Radiol 2016; 27: 1465–1476. [DOI] [PubMed] [Google Scholar]
- 25.Shemesh D, Goldin I, Zaghal I, et al. Angioplasty with stent graft versus bare stent for recurrent cephalic arch stenosis in autogenous arteriovenous access for hemodialysis: a prospective randomized clinical trial. J Vasc Surg 2008; 48: 1524–1531, 1531. [DOI] [PubMed] [Google Scholar]
- 26.Rajan DK and Falk A. A randomized prospective study comparing outcomes of angioplasty versus VIABAHN stent-graft placement for cephalic arch stenosis in dysfunctional hemodialysis accesses. J Vasc Interv Radiol 2015; 26: 1355–1361. [DOI] [PubMed] [Google Scholar]
- 27.D’Cruz RT, Leong SW, Syn N, et al. Endovascular treatment of cephalic arch stenosis in brachiocephalic arteriovenous fistulas: a systematic review and meta-analysis. J Vasc Access 2019; 20: 345–355. [DOI] [PubMed] [Google Scholar]
- 28.Vasanthamohan L, Gopee-Ramanan P and Athreya S. The management of cephalic arch stenosis in arteriovenous fistulas for hemodialysis: a systematic review. Cardiovasc Intervent Radiol 2015; 38: 1179–1185. [DOI] [PubMed] [Google Scholar]
- 29.Miller GA, Friedman A, Khariton A, et al. Access flow reduction and recurrent symptomatic cephalic arch stenosis in brachiocephalic hemodialysis arteriovenous fistulas. J Vasc Access 2010; 11: 281–287. [DOI] [PubMed] [Google Scholar]
- 30.MacRae JM, Ahmed A, Johnson N, et al. Central vein stenosis: a common problem in patients on hemodialysis. ASAIO J 2005; 51: 77–81. [DOI] [PubMed] [Google Scholar]
- 31.Bakken AM, Protack CD, Saad WE, et al. Long-term outcomes of primary angioplasty and primary stenting of central venous stenosis in hemodialysis patients. J Vasc Surg 2007; 45: 776–783. [DOI] [PubMed] [Google Scholar]
- 32.Quaretti P, Galli F, Moramarco LP, et al. Stent grafts provided superior primary patency for central venous stenosis treatment in comparison with angioplasty and bare metal stent: a retrospective single center study on 70 HEMODIALYSIS PATIENTS. Vasc Endovascular Surg 2016; 50: 221–230. [DOI] [PubMed] [Google Scholar]
- 33.Boutrous ML, Alvarez AC, Okoye OT, et al. Stent-graft length is associated with decreased patency in treatment of central venous stenosis in hemodialysis patients. Ann Vasc Surg 2019; 59: 225–230. [DOI] [PubMed] [Google Scholar]
- 34.Cline BC, Gage SM, Ronald J, et al. Treatment of arm swelling in hemodialysis patients with ipsilateral arteriovenous access and central vein stenosis: conversion to the hemodialysis reliable outflow graft versus stent deployment. J Vasc Interv Radiol 2020; 31: 243–250. [DOI] [PubMed] [Google Scholar]
- 35.Illig KA. Management of central vein stenoses and occlusions: the critical importance of the costoclavicular junction. Semin Vasc Surg 2011; 24: 113–118. [DOI] [PubMed] [Google Scholar]
- 36.Rajendran S, Cai TY, Loa J, et al. Early outcomes using dedicated venous stents in the upper limb of patients with venous thoracic outlet syndrome: a single centre experience. CVIR Endovasc 2019; 2: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wooster M, Fernandez B, Summers KL, et al. Surgical and endovascular central venous reconstruction combined with thoracic outlet decompression in highly symptomatic patients. J Vasc Surg Venous Lymphat Disord 2019; 7: 106– 112 e103. [DOI] [PubMed] [Google Scholar]
- 38.Auyang PL, Chauhan Y, Loh TM, et al. Medial claviculectomy for the treatment of recalcitrant central venous stenosis of hemodialysis patients. J Vasc Surg Venous Lymphat Disord 2019; 7: 420–427. [DOI] [PubMed] [Google Scholar]
- 39.Suliman A, Greenberg JI and Angle N. Surgical bypass of symptomatic central venous obstruction for arteriovenous fistula salvage in hemodialysis patients. Ann Vasc Surg 2008; 22: 203–209. [DOI] [PubMed] [Google Scholar]
- 40.Patane D, Giuffrida S, Morale W, et al. Drug-eluting balloon for the treatment of failing hemodialytic radiocephalic arteriovenous fistulas: our experience in the treatment of juxta-anastomotic stenoses. J Vasc Access 2014; 15: 338– 343. [DOI] [PubMed] [Google Scholar]
- 41.Swinnen J, Lean Tan K, Allen R, et al. Juxta-anastomotic stenting with aggressive angioplasty will salvage the native radiocephalic fistula for dialysis. J Vasc Surg 2015; 61: 436–442. [DOI] [PubMed] [Google Scholar]
- 42.Thomas SD, Peden S, Crowe P, et al. Interwoven nitinol stents to treat radiocephalic anastomotic arteriovenous fistula stenosis. J Endovasc Ther 2019; 26: 394–401. [DOI] [PubMed] [Google Scholar]
- 43.Quaretti P, Leati G, Moramarco LP, et al. Percutaneous transanastomotic stent graft deployment to salvage dysfunctional native forearm radiocephalic fistulae: feasibility and primary patency at 12 months. J Vasc Interv Radiol 2018; 29: 986–992. [DOI] [PubMed] [Google Scholar]
- 44.Nassar GM, Beathard G, Rhee E, et al. Management of transposed arteriovenous fistula swing point stenosis at the basilic vein angle of transposition by stent grafts. J Vasc Access 2017; 18: 482–487. [DOI] [PubMed] [Google Scholar]
- 45.Nikanorov A, Smouse HB, Osman K, et al. Fracture of self-expanding nitinol stents stressed in vitro under simulated intravascular conditions. J Vasc Surg 2008; 48: 435– 440. [DOI] [PubMed] [Google Scholar]
- 46.Shemesh D, Goldin I and Olsha O. Stent grafts for treatment of cannulation zone stenosis and arteriovenous graft venous anastomosis. J Vasc Access 2017; 18: 47–52. [DOI] [PubMed] [Google Scholar]
- 47.Kim CY, Guevara CJ, Engstrom BI, et al. Analysis of infection risk following covered stent exclusion of pseudoaneurysms in prosthetic arteriovenous hemodialysis access grafts. J Vasc Interv Radiol 2012; 23: 69–74. [DOI] [PubMed] [Google Scholar]
- 48.Saad TF, Hentschel DM, Koplan B, et al. Cardiovascular implantable electronic device leads in CKD and ESRD patients: review and recommendations for practice. Semin Dial 2013; 26: 114–123. [DOI] [PubMed] [Google Scholar]
- 49.Forauer AR and Theoharis C. Histologic changes in the human vein wall adjacent to indwelling central venous catheters. J Vasc Interv Radiol 2003; 14: 1163–1168. [DOI] [PubMed] [Google Scholar]
- 50.Da Costa SS, Scalabrini Neto A, Costa R, et al. Incidence and risk factors of upper extremity deep vein lesions after permanent transvenous pacemaker implant: a 6-month follow-up prospective study. Pacing Clin Electrophysiol 2002; 25: 1301–1306. [DOI] [PubMed] [Google Scholar]
- 51.Asif A, Salman L, Carrillo RG, et al. Patency rates for angioplasty in the treatment of pacemaker-induced central venous stenosis in hemodialysis patients: results of a multicenter study. Semin Dial 2009; 22: 671–676. [DOI] [PubMed] [Google Scholar]
- 52.Saad TF, Myers GR and Cicone J. Central vein stenosis or occlusion associated with cardiac rhythm management device leads in hemodialysis patients with ipsilateral arteriovenous access: a retrospective study of treatment using stents or stent-grafts. J Vasc Access 2010; 11: 293–302. [DOI] [PubMed] [Google Scholar]
- 53.Mehra S and Chelu MG. Implantable cardioverter-defibrillator shock after stenting across the device leads. Tex Heart Inst J 2016; 43: 88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkoff BL, Love CJ, Byrd CL, et al. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm 2009; 6: 1085–1104. [DOI] [PubMed] [Google Scholar]
- 55.El Kassem M, Alghamdi I, Vazquez-Padron RI, et al. The role of endovascular stents in dialysis access maintenance. Adv Chronic Kidney Dis 2015; 22: 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma AK, Sinha S and Bakran A. Migration of intravascular metallic stent into pulmonary artery. Nephrol Dial Transplant 2002; 17: 511. [DOI] [PubMed] [Google Scholar]
- 57.Zaleski GX, Funaki B, Rosenblum J, et al. Metallic stents deployed in synthetic arteriovenous hemodialysis grafts. AJR Am J Roentgenol 2001; 176: 1515–1519. [DOI] [PubMed] [Google Scholar]


