SUMMARY
Neurons of the mammalian central nervous system fail to regenerate. Substantial progress has been made towards identifying the cellular and molecular mechanisms that underlie regenerative failure, and how altering those pathways can promote cell survival and/or axon regeneration. Here we summarize those findings while comparing the regenerative process in the central versus the peripheral nervous system. We also highlight studies that advance our understanding of the mechanisms underlying neural degeneration in response to injury, as many of these mechanisms represent primary targets for restoring functional neural circuits.
Huberman & Kolodkin eTOC blurb
Successful clinical regeneration of central nervous system neurons following injury is still out of reach, but recent mechanistic discoveries illuminate a roadmap to achieve this long-held dream of neuroscience research.
INTRODUCTION
Physical injuries and neurodegenerative diseases often bring about irreversible damage and loss of function to the central nervous system (CNS). In mammals, such loss of function is due to the inability of adult mammalian CNS neurons to regenerate. A limited degree of CNS self-repair exists early in development; however, the ability to spontaneously regenerate is dramatically reduced after parturition.
Tremendous effort has been devoted to characterizing the cellular and molecular origins of so-called CNS ‘regenerative failure’, yet a complete understanding of these factors is still lacking. Efforts to promote CNS regeneration, particularly by removing factors known to restrict CNS regeneration such as myelin-associated proteins, have been met with mixed success. In some instances, increased neuronal survival and a small amount of axon re-extension was observed, but in every case these approaches have failed to restore normal, or even near-normal, circuit function. To date, only a handful of experimental approaches have yielded CNS regeneration to a degree that inspired human clinical trials. No treatments yet exist for successfully stimulating regeneration of CNS neurons in humans. Nevertheless, a number of key mechanistic discoveries have been made that point to new directions for the field. We posit that to achieve this goal of re-establishing functional connectivity following CNS damage in humans, multi-faceted strategies will be required that promote: i) neuronal survival, ii) axon re-extension, iii) synapse re-formation, iv) myelination, and v) experience-dependent refinement of newly formed circuits.
Here, we summarize our current understanding of mammalian neuronal responses to injury and highlight key advances directed toward overcoming CNS regenerative failure. We provide a brief overview of the model systems most commonly used to study CNS injuries in mammals to highlight their relative advantages. We consider both the extrinsic and intrinsic mechanisms activated by injury, with a focus on events that trigger axon degeneration because they constrain the viability of pro-regeneration approaches.
Model species and pathways for exploring CNS regeneration
Many key discoveries that advanced our understanding of the molecular and cellular mechanisms involved in axon regeneration and degeneration, including the identification of signaling pathways that regulate regeneration in mammals, were originally made in lower vertebrates (frogs, fish, salamanders) and invertebrates (worms and flies).
Two valuable models for studying mammalian CNS regeneration are the spinal cord system and the visual system. Both carry out vital sensory-motor functions and have well defined anatomical elements whose physiologies and relation to behavior are known. The spinal system includes descending projections from the brain to the spinal cord that make up the corticospinal tract (CST), ascending projections from peripheral sensory neurons that compose the spinothalamic tract, and intra-spinal connections (Figure 1A and 1B). Together, these pathways offer the opportunity to explore diverse features of neural circuits. Further, sensory neurons allow for unique insight into spinal circuit regeneration since dorsal root ganglia (DRG) neurons have both a centrally extending axon that cannot regenerate and a peripherally extending branch that readily regenerates following injury (Figure 1B). This highlights that the differential ability of CNS and peripheral nervous system (PNS) axons to regenerate depends on interactions with the environment (CNS versus PNS) and their subsequent intracellular responses.
Figure 1: Model systems for studying CNS regeneration: spinal system and visual system.
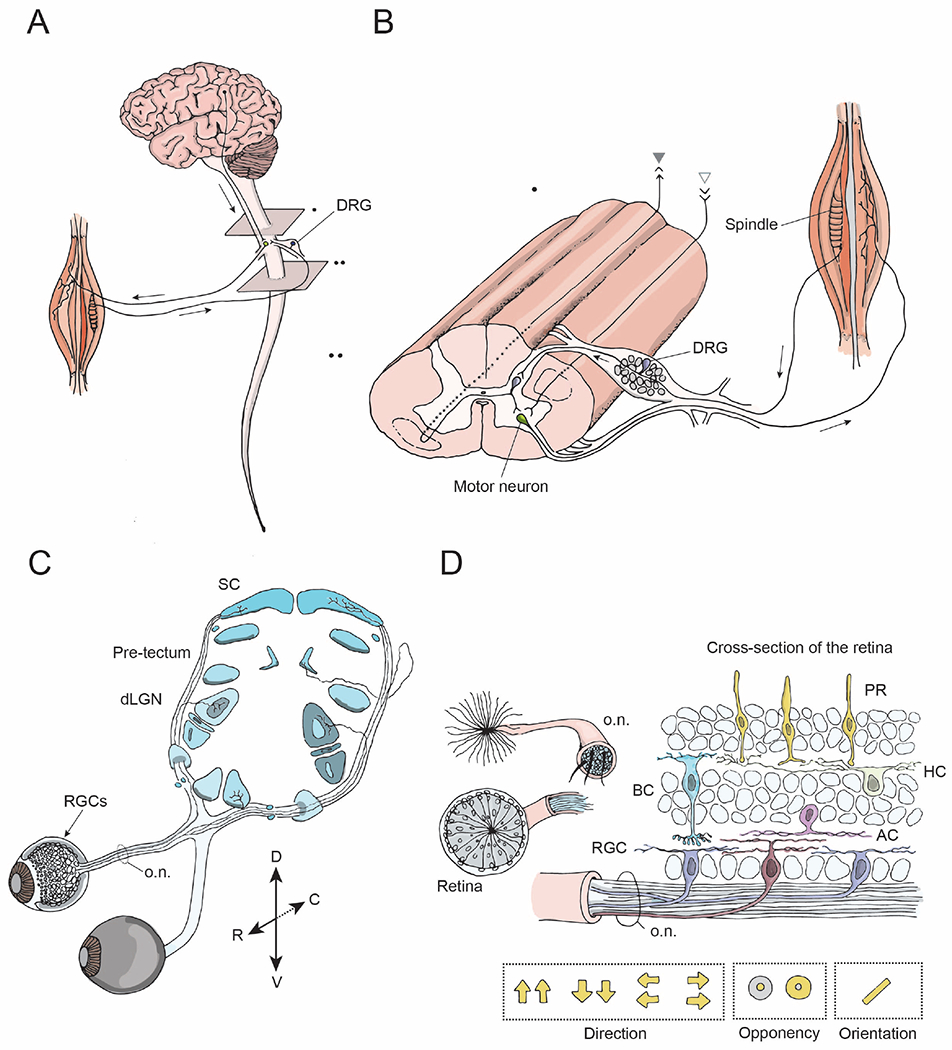
(A) The brain and the spinal cord exchange information via descending projections from the brain to the spinal cord (arrow), and ascending projections. The spinal cord receives sensory input from external stimuli via the dorsal root ganglia (DRG) neurons. Output signals from the brain and the spinal cord are relayed to the muscles via motor neurons and their peripheral projections.
(B) A longitudinal view of the spinal cord showing sensory inputs, interneurons, and motor outputs. The spinothalamic tract is comprised of ascending projections from interneurons (gray, closed arrowhead), whereas the corticospinal tract consists of descending projections from the brain to the spinal cord (gray, open arrowhead). The DRG neurons have central and peripheral axonal branches.
(C) The visual system includes retinal ganglion cells (RGCs, arrow), the neurons that relay sensory input from the eye, their axons that form the optic nerve (o.n.) and their projections to target regions within the brain including the dorsal lateral geniculate nucleus (dLGN) and the superior colliculus (SC) (blue).
(D) Cross-section of the retina and the optic nerve. The retina consists of five types of neural cells: photoreceptors, i.e. rods and cones (PR, yellow), bipolar cells (BC, blue), horizontal cells (HC, green), amacrine cells (AC, pink) and ~40 different types of retinal ganglion cells (RGC, brown, purple). RGCs are the only neurons that send projections from the retina into the optic nerve and have various receptive field properties as indicated by the yellow arrows and circles.
For somewhat distinct reasons, the visual system is also an attractive model system to study regeneration. Eye-to-brain circuits, collectively referred to as the retinofugal system, consist of retinal ganglion cells (RGCs), the output neurons of the eye, and their connections with central target areas (Figure 1C). In many species, RGCs are easily accessible for drug treatments, viral infection or anatomical labeling via injections into the eye. Moreover, RGCs and many of their central targets have well defined physiological receptive field properties (Figure 1D), allowing for precise assessments of functional recovery. Despite being located outside the cranial vault, RGCs are bona fide CNS neurons.
These two model systems, the spinal and visual systems, together offer comprehensive platforms for investigating neural regeneration. Indeed, many pro-regenerative treatments that were effective in the spinal system have proven effective in the visual system and vice versa (Liu et al., 2010; Park et al., 2008).
NEURONAL SURVIVAL
Dead neurons cannot regenerate. Thus, the goal of re-establishing functional neural circuits following CNS injury is first contingent upon maintaining the survival of damaged neurons. Some CNS neuronal subtypes appear less susceptible to damage-induced death than others. Identifying the molecular programs that render select neuron subtypes more resilient or particularly susceptible to the effects of neuronal insults has revealed both pro-survival and pro-death signaling proteins expressed to varying degrees following injury. These represent attractive entry points for the overall goal of enhancing CNS regeneration.
Subtype specific susceptibility
In the mammalian retina, very few RGCs die within the first three days after damage to the optic nerve (the dense axon bundle that exits each eye to innervate the brain) (Tran et al., 2019). Eight days after injury to the optic nerve, however, up to 70% of RGCs are dead and cleared away by glial phagocytosis (Tran et al., 2019). This provides a very narrow therapeutic window for interventions aimed at sparing and/or regenerating injured RGCs. Nonetheless, some RGCs die faster and others resist injury-induced-death until the second half of the eight day window. Still others resist injury-induced death entirely. Interestingly, these differences relate to RGC subtype identity in a systematic way.
RGCs are divided into ~40 subtypes, each responding best to particular qualities of visual information. To date, studies of mammalian optic nerve repair have generally addressed regrowth (or lack thereof) following either: i) optic nerve transection or ii) optic nerve crush. Exploration of varying degrees of pressure applied to the optic nerve crush are rare due to lack of instrumentation to accurately assess crush pressures. Alpha-RGCs (αRGCs), among the largest RGC subtypes, are motion-selective (but not direction-selective) and survive for several months following optic nerve transection (Duan et al., 2015b; Holländer et al., 1985). Intrinsically photosensitive retinal ganglion cells (ipRGCs), which respond directly to light due to the presence of melanopsin protein and which set the central circadian clock (Hattar et al., 2002), are also resilient to optic nerve crush (ONC, Figure 2A) (Pérez de Sevilla Müller et al., 2014). Moreover, they resist the onset of death following other types of insults, including elevated intraocular pressure (a model of induced glaucoma, Figure 2B) and N-methyl-D-aspartate (NMDA)-receptor-mediated excitotoxicity (Cui et al., 2015). Recent work also suggests that a population of direction-selective (DS) retinal ganglion cells (On-DSGCs) can survive up to one month following ONC, although anatomical changes suggest these cells are actively undergoing apoptosis (Lilley et al., 2019). Other unidentified subtypes are resilient to cell death following ONC, but these make up a very small fraction of surviving RGCs (Tran et al., 2019).
Figure 2: CNS injury models.
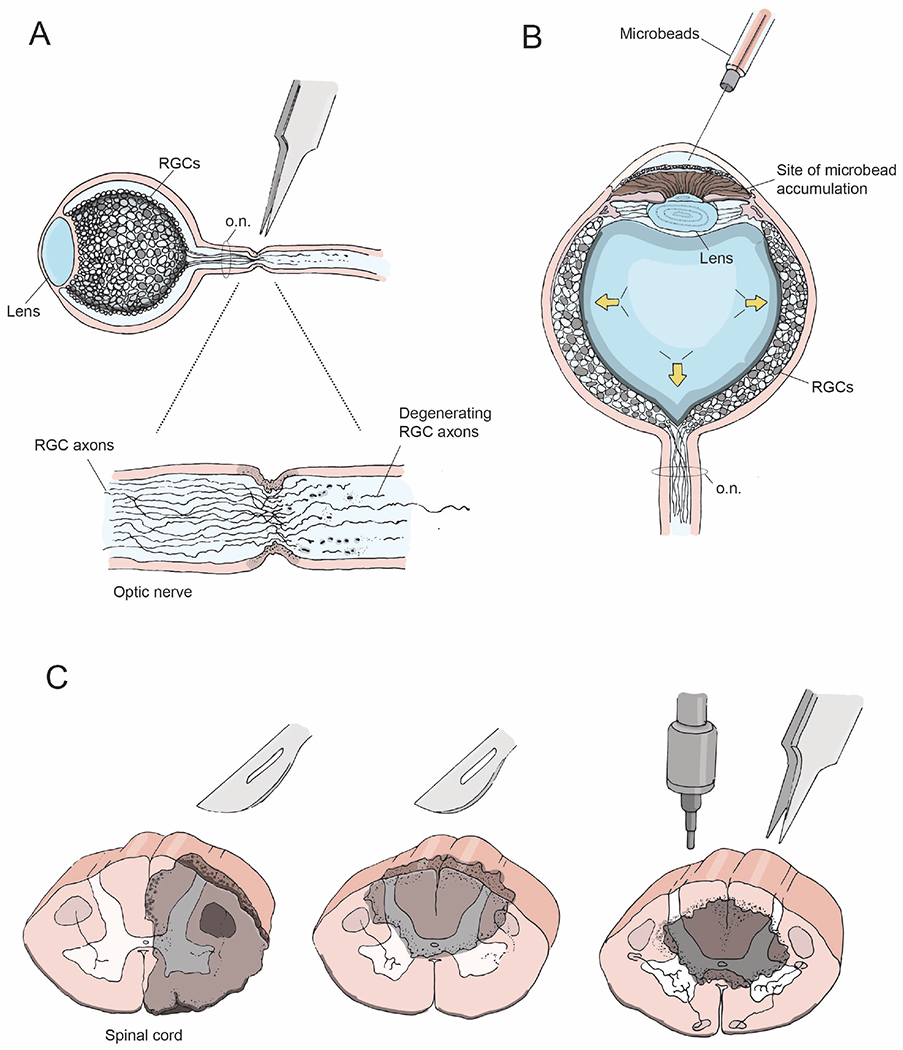
(A) Optic nerve crush injury showing axon degeneration distal to the injury site
(B) Bead-induced mouse model of glaucoma, wherein microbeads injected into the eye increase intraocular pressure (arrows) and mimic the degenerative effects of glaucoma
(C) Coronal views of the spinal cord depicting lesion sites (gray) following unilateral transection (left), dorsal bilateral hemisection (middle), and a contusion or crush injury (right).
Why are some RGC subtypes less susceptible to injury than others? One possibility is that endogenous levels of mammalian target of rapamycin (mTOR), a factor that promotes RGC axonal outgrowth during development, are higher in αRGCs as compared to other RGC subtypes and thereby endow these cells with increased damage resilience (Duan et al., 2015b). This can be attributed to select expression of osteopontin, an mTOR activator, in these cells (Duan et al., 2015b). In addition, phosphoinositide 3-kinase (PI3K)-AKT activation that accompanies melanopsin signaling in ipRGCs underlies the resilience of these cells following injury (Li et al., 2008; Pérez de Sevilla Müller et al., 2014). Furthermore, transcriptional activation of signal transducers and activators of transcription-3 (STAT3) in RGCs following injury bestows neuroprotective effects via stimulation of inflammatory signaling events, thereby increasing RGC survival (Leibinger et al., 2013), though the extent to which individual subtypes of RGCs are affected by STAT3 activation requires further investigation.
While it is likely that subtype-specific neuronal survival following spinal cord injury is regulated by similar mechanisms as RGCs, the degree to which neurons survive following injury is also highly dependent on the type and location of the insult (Figure 2C). The most commonly used methods to produce a spinal cord injury either lesion a large number of axons in a specific spinal segment, such as the contusion model and spinal cord crush, or lesion specific ascending or descending tracts, for example using unilateral or bilateral spinal hemisections (Figure 2C, and reviewed in Steward and Willenberg, 2017). Upper motor neurons (UMNs), whose axons comprise the CST, are highly susceptible to axonal transection, with nearly 40% of these neurons undergoing apoptosis within 1 week following lesion (Hains et al., 2003). By contrast, neurons that elaborate descending projections of the rubrospinal and vestibulospinal tracts appear less vulnerable than UMNs to contusion injury, but this is highly dependent on the severity of the injury (Hassannejad et al., 2018).
Within the spinal cord, short thoracic propriospinal (STP) neurons are more susceptible to cell death in both contusion and transection injuries than are long descending propriospinal tract (LDPT) neurons (Hassannejad et al., 2018). Interestingly, approximately 5% of LDPT projections remain intact two weeks post-contusion injury, whereas STP axons are completely lost (Conta and Stelzner, 2004). Despite the loss of projections, numerous STP somas are still present for at least two weeks following severe contusion, offering a therapeutic window to promote regeneration of this cell type.
A complete understanding of differential neuronal survival in both the retina and the SC following injury remains to be unveiled. Meanwhile, insights into the transcriptional programs of resilient neurons is starting to reveal not only the kinetics of apoptosis, but also the specific pro-survival genes and signaling pathways that serve to stabilize all cell types following injury (Duan et al., 2015a; Tran et al., 2019).
Reprogramming
Across development, multiple pro-growth signaling pathways that regulate cell size and neurite elaboration become gradually suppressed, preventing aberrant growth in adulthood. It was once thought that only PNS neurons re-activate developmental pro-growth signaling pathways to support axon regeneration following injury. However, recent work suggests that corticospinal neurons transiently reprogram to an embryonic, pro-growth state following CST transection both in the presence and absence of neural progenitor cell grafts (Poplawski et al., 2020). However, this regenerative transcriptome is quickly downregulated in the absence of grafted neural progenitor cells at the injury site, suggesting that a pro-regenerative extrinsic environment can retrogradely influence sustained gene expression. Transcriptional profiling of RGCs following ONC suggests that RGCs do not share this reprogramming property with lesioned corticospinal neurons (Tran et al., 2019). Nevertheless, the possibility that other injured CNS neurons spontaneously reprogram, albeit transiently, has therapeutic implications.
To sustain pro-growth signaling in injured CNS neurons, many groups have sought to exogenously reprogram these cells to a development-like state. One way to achieve this is to manipulate the balance of oncogenes and tumor suppressors, including Lin28 and Let7, respectively, to favor activation of growth-promoting pathways. Initially described as a key regulator of developmental timing in C. elegans (Ambros and Horvitz, 1984), Lin28 can also reprogram differentiated cells to a stem cell-like state in multiple contexts (Li et al., 2017a). In mammals, Lin28 expression increases in injured DRGs and regulates the activity of several growth-promoting pathways, including PI3K-Akt-mTOR and GSK3β, to sustain axon regeneration (Wang et al., 2018; Zhang et al., 2019). Further, increased expression of the Let-7 target genes c-myc and Tet3 following injury suggests that Lin28 acts as a reprogramming factor in injured DRGs.
In the CNS, where Lin28 levels remain low following injury, Lin28 overexpression mediated by adeno-associated viral delivery either before or after lesion in both RGCs and CST neurons promotes long-distance axon regeneration (Nathan et al., 2020; Wang et al., 2018). Manipulating Lin28 activity in neurons following injury to promote regeneration suggests potential benefits in treating human CNS injuries. However, like mTOR, increasing Lin28 in humans may carry additional concerns related to oncogenesis.
An alternative approach is to initiate a global reprogramming of neurons to a development-like state by altering their epigenetic landscape following injury. Ten-eleven translocation methylcytosine dioxygenases (Tets) are a family of enzymes that promote the demethylation of CpG sites throughout the genome. Interestingly, sciatic nerve injury (SNI) leads to increased expression of Tet3 in DRG neurons, subsequently activating the expression of known regeneration-associated genes such as STAT3, c-myc, and ATF3 (Weng et al., 2017). Tet3 knockdown and a related Tet family member, Tet1, are required to promote spontaneous axon regeneration and for the pro-regenerative effects of mTOR upregulation in lesioned RGCs. Further evidence supporting a role for Tet family members in initiating a global regenerative program comes from the finding that the pro-regenerative effects garnered by reprogramming injured RGCs using Oct4, Sox2, and Klf4 overexpression are Tet1- and Tet2-dependent (Lu et al., 2020). This treatment can recover some visual behaviors in a glaucoma mouse model and can also stimulate robust RGC axon regeneration in aged mice, supporting the idea that Tet-dependent epigenetic reprogramming is a master switch to allow long-distance axon regrowth following injury. Although Lin et al., 2020, observed no tumor growth in Oct4, Sox2, Klf4 expressing eyes, it remains important to determine whether such broad genomic manipulations are deleterious to proper cellular function. For instance, how do these manipulations impact neuronal properties such as subtype identity and presynaptic connectivity? Answers to these questions will help determine the clinical potential for exogenous reprogramming of neurons to restore function following injury.
AXON REGENERATION
Axon regeneration is defined as axon regrowth and the subsequent innervation of target regions following injury, resulting in recovery of neuronal function and behavior. While many studies document robust CNS axon regrowth following modulation of pro-growth signaling pathways in injured neurons, or alteration of extrinsic factors that inhibit axon re- extension, none have achieved complete regeneration of function. This is due to the long trajectories that mammalian projection neurons often must navigate to reach their developmental target regions, and the presence of inhibitory factors encountered by these re- extending axons. This is especially true in the visual system since the optic nerve consists of RGC axons and glial cells and no classical synapses, thereby necessitating complete re-growth of injured axons into the brain to restore electrical connectivity. Although the optic nerve serves as a conduit for RGC axons, as is the case in development, regenerating axons must traverse the optic chiasm before ascending into the brain. To navigate the optic chiasm, an important choice point, developing RGC axons interact with a combination of attractive and repulsive guidance cues that ultimately mediate axon decussation (crossing) or non-decussation at the CNS midline (Petros et al., 2008). Although a few regenerating axons have been shown to cross the chiasm following growth stimulating treatments (Lim et al., 2016; de Lima et al., 2012), whether the midline glial cells and the guidance cues they express are present post-injury in adults remains unknown. Transcriptomic analyses of midline glial cells in uninjured and injured conditions may reveal molecular targets that can be manipulated to encourage extension of regenerating axons into and beyond the chiasm to achieve robust long-distance regeneration.
The spinal system includes neuronal cell bodies throughout the entire length of the spinal cord, allowing relay circuits to bypass the injury site and achieve connectivity with distal targets. Despite these limitations there are now several promising strategies for promoting long-distance regrowth of severed axons, even if we are far from a complete understanding of the events required to re-establish functional neuronal circuits. Since PNS neurons can regenerate, there is much to learn about key features that bestow PNS neurons with this property. Therefore, we discuss next current findings on regeneration and the re-establishment of connectivity following injury by describing factors that promote PNS regeneration and then intrinsic and extrinsic hurdles that constrain CNS regeneration.
PNS neurons regenerate following injury
The PNS has a remarkable capacity to regenerate following injury. A striking example can be found in reptiles and amphibia such as lizards and salamanders, where a damaged or even completely amputated tail or limb fully regenerates to restore normal movement. In mammals, PNS neurons regenerate less quickly and extensively than in reptiles or amphibians, but more readily than CNS neurons. For example, spinal motor neurons that project in peripheral nerves regenerate axons and reinnervate muscle targets following injury even in adulthood (Kang and Lichtman, 2013). A primary distinction between the CNS and PNS lies in each system’s response to injury. Following injury in the PNS, myelinating glia called Schwann cells undergo dedifferentiation and subsequent trans-differentiation, allowing them to carry out various functions that facilitate debris clearance (Jessen and Mirsky, 2016). Further, PNS neurons have cell-intrinsic responses to injury that greatly increase their capacity to regenerate compared to CNS neurons (Hoffman, 2010).
What is known about the molecular properties that bestow PNS neurons with the capacity to regenerate? Recent evidence implicates injury-induced reprogramming within peripheral sensory neurons that aids in regeneration following injury (Chandran et al., 2016; Renthal et al., 2020). This feature is perhaps best illustrated by DRG neurons, which are bipolar in shape; their cell body resides adjacent to the spinal cord and their axons have two branches—one that projects into the CNS and another that extends into the PNS. Following damage to a DRG neuron, the peripheral branch readily regenerates at a rate of ~1mm per day, whereas the central branch fails to regenerate (Schwab and Bartholdi, 1996). In the PNS, transcriptional activation of Atf3 in injured DRG neurons within 3 days suppresses cell identity and boosts regeneration (Renthal et al., 2020). Additionally, local translation of mTOR in axons following sciatic nerve injury upregulates not only mTOR, but also other proteins important for retrograde injury signaling, such as STAT3, that promote regeneration of the peripheral branch (Terenzio et al., 2018). In the CNS, the absence of such a robust injury-induced increase in growth-promoting proteins and the absence of protein trafficking in axons, particularly alpha9 integrin, a class of receptors that promote neurite outgrowth during development, considerably limit regeneration of the central branch (Andrews et al., 2009).
These findings indicate that both the environment encountered by regrowing axons following injury and the internal state of PNS neurons are key features for supporting PNS axon regeneration. Classic studies of Aguayo and colleagues showed that peripheral nerve grafts used as physical bridges promote regeneration of central axons in the spinal cord and from the retina to the brain (David and Aguayo, 1981). Similarly, a conditioning PNS lesion, or introduction of a cAMP analog, alters the intrinsic state of DRG neurons, supporting central axon regrowth past the injury site through an otherwise inhibitory environment; this suggests that CNS neurons may retain the potential to regenerate axons (Neumann and Woolf, 1999; Neumann et al., 2002). Thus, the two main categories of factors that influence mammalian CNS regeneration following injury are: i) cell-intrinsic factors including transcriptional programs and growth-promoting signaling pathways, and ii) extrinsic factors such as the molecules and cells in the environment encountered by damaged axons (reviewed in Crair and Mason, 2016).
Intrinsic factors that regulate CNS regeneration
Neuron-intrinsic factors play a critical role in determining CNS regenerative capability. Though our knowledge of the genes expressed in mature CNS neurons that limit regeneration has increased in recent years (Bray et al., 2019; Tran et al., 2019), we are far from a complete understanding of the intrinsic responses that impact CNS axon regeneration.
Initiating axon growth from lesioned CNS neurons
An important measure of whether a neuron can successfully regenerate is its ability to form an axonal growth cone (Figure 3A). Similar to development, growth cones of regenerating axons sample their environment as they pathfind toward their targets (Figure 3B). What are the key influences that determine whether an injured axon forms an active growth cone or a retraction bulb–the characteristic post-injury axon tip that resembles a dystrophic growth cone? Selective transection of the CNS-projecting branch of DRG axons revealed injured axons re-growing either from the tip of the axon or sprouting from the proximal node of Ranvier within 24 hours following a lesion (Kerschensteiner et al., 2005). A third of the injured axons began regenerating within two days of the injury, at approximately 4 microns/hour, considerably slower than PNS axons (Kerschensteiner et al., 2005). These rapid, dynamic changes in the injured axon, proximal to the lesion site, suggest the presence of an initial, albeit short-lived, regenerative response in CNS axons. The majority of injured CNS axons, however, form retraction bulbs as they encounter proteoglycan substrates. They then protrude, undulate and endocytose vesicles, ultimately remaining dystrophic without advancing further (Tom et al., 2004). Recent work demonstrates that destabilized and disorganized microtubules cause active growth cones to become dystrophic, an effect that can be overcome by administering epothilone B, an FDA approved drug, or by specifically deleting a small GTPase ras homolog gene family member A (RhoA) in neurons (Ruschel et al., 2015; Stern et al., 2021). In the visual system, other mechanisms have been shown to be effective in initiating a growth cone; for example, following ONC, overexpression of doublecortin-like kinases (DCLK2) promotes growth cone initiation resulting in more axons regenerating past the lesion site in the optic nerve (Nawabi et al., 2015). Ultimately, growth cone formation is an important aspect of axon regeneration involving a cascade of events, some of which overlap with mechanisms activated by axonal responses to injury (Bradke et al., 2012).
Figure 3: Axon growth following injury.
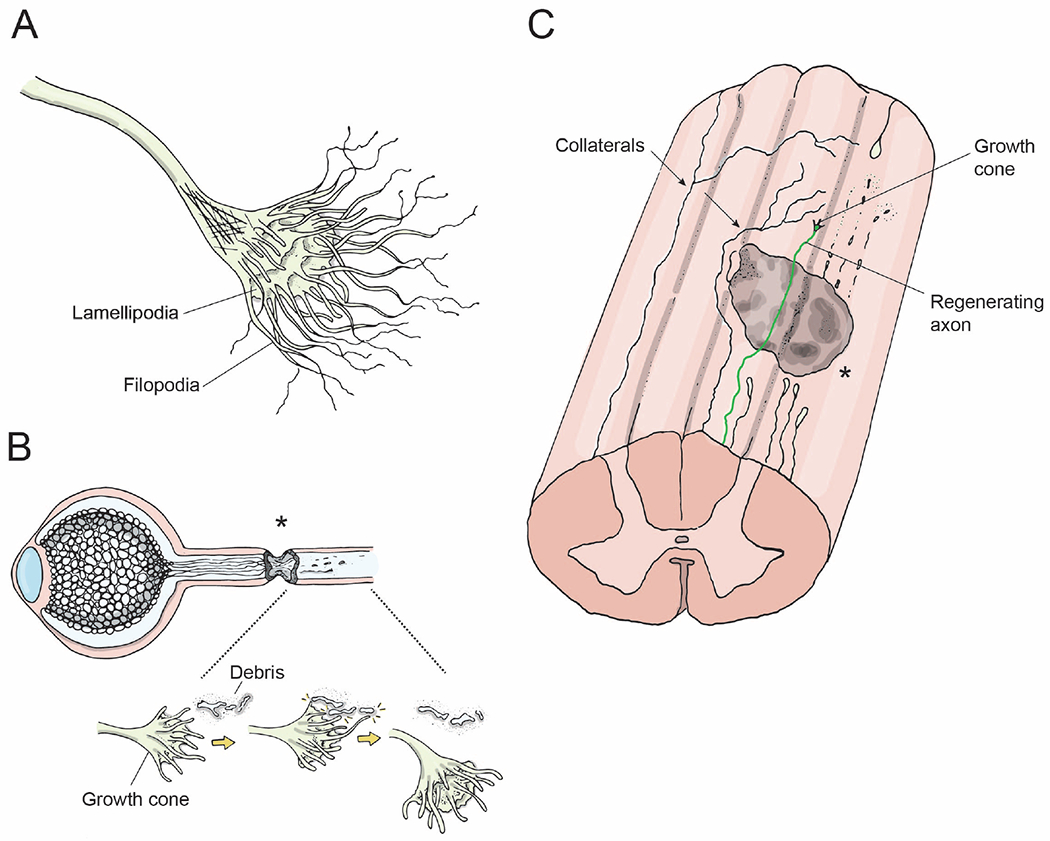
(A) Growth cone, i.e. the leading edge of an axon. The fingerlike protrusions are filiopodia and lamellipodia that are composed of actin filaments and are crucial for the growth cone’s ability to grow towards or away from environmental cues.
(B) Axons regenerating following an optic nerve crush injury. Growth cones at the leading edge of regenerating axons grow past the lesion site (asterisk), interact with microglia/axonal-debris/guidance cues, and accordingly alter their direction of growth.
(C) Longitudinal view of the spinal cord showing a lesion site (gray), distal processes of injured axons degenerating (dotted lines), a regenerating axon extending a growth cone distal to the lesion site (color), and two spared axons extending collaterals circumventing the lesion site and extending to target neurons distal to the lesion (arrows).
Transcriptional regulation of axon growth
Several transcriptional programs active in developing neurons are downregulated postnatally and have the capacity to influence the regenerative potential of mature CNS neurons. For example, the cell-intrinsic signaling pathway that regulates mTOR to modulate cell survival, growth, metabolism, and protein synthesis is elevated in newborn CNS neurons; however, inhibition by phosphatase/tensin homologue (PTEN) causes mTOR signaling levels to rapidly taper down, becoming low-to-absent in most neurons by adulthood. Genetic deletion of PTEN, a negative regulator of mTOR, leads to robust regenerative responses by RGC axons following ONC (Park et al., 2008). Manipulating the mTOR pathway has proved to be a robust approach for promoting axon extension and cell survival after acute injury in both the visual and spinal systems (Duan et al., 2015b; Jin et al., 2015; de Lima et al., 2012; Sun et al., 2011). Further, suppression of the PTEN pathway along with other neuron-intrinsic transcriptional programs that suppress cytokine signaling through SOCS3 synergistically induces robust regeneration of RGC axons and also improves sprouting of corticospinal axons following injury (Jin et al., 2015; Leibinger et al., 2013; Luo et al., 2013; Sun et al., 2011).
Transcriptional programs can also be modulated to activate growth-promoting elements, as is the case with the transcription factor SRY-Box 11 (Sox11). Overexpression of Sox11 in RGCs increases axon growth following ONC (Norsworthy et al., 2017). Interestingly, Sox11 overexpression is deleterious to α RGC cell survival and only promotes regeneration of non-αRGC axons, thus demonstrating neuronal subtype-specific effects (Chang et al., 2021; Norsworthy et al., 2017; Welsbie et al., 2017). Similarly, another family of transcription factors, the Kruppel-like proteins (KLFs), have distinct effects on the regulation of axon growth after injury in the visual and spinal systems; KLF-4 and -9 are up-regulated postnatally during development and suppress axon regeneration (Trakhtenberg et al., 2018), while KLF-6 and -7 are normally down-regulated postnatally but upon activation support axon re-growth (Moore et al., 2009). Interestingly, Klf-6, but not Klf-7, expression is higher in ipRGCs following ONC (Bray et al., 2019), potentially increasing the resilience of these neurons after injury. Similarly, several transcriptional programs involving epigenetic control of injury induced transcription, and also signaling pathways that have pro-regenerative effects, have been described (reviewed in Mahar and Cavalli, 2018).
Extrinsic factors regulating CNS regeneration
Major extrinsic factors that regulate regeneration include myelin debris from injured axons and the astrocytes, fibroblasts, extracellular matrix components, microglia and blood-borne immune cells that are present or infiltrate the lesion site to form a glial scar. In the PNS, Schwann cells facilitate axon regrowth by clearing axonal debris following peripheral injury. Upon physical injury or degeneration, Schwann cells downregulate their production of myelin and transdifferentiate into repair cells. With the assistance of resident macrophages and blood-borne leukocytes, Schwann cells initiate a rapid response that facilitates clearing of debris, up-regulation of trophic factors, and regeneration of axonal components (Brosius Lutz et al., 2017; Kang et al., 2014). Additionally, Schwann cells also form bridges called ‘regeneration tracks,’ or ‘Bungner’s bands’: strings of Schwann Cells along which injured axons navigate back to their targets (Jessen and Mirsky, 2016; Son and Thompson, 1995).
Injury to the CNS, by contrast, is met with significantly delayed clearance of CNS myelin. The myelin sheaths that encase neurons in the CNS are made up of oligodendrocytes. Oligodendrocytes, unlike Schwann cells, do not undergo injury-induced reprogramming and do not phagocytose myelin debris. Further, in the CNS microglia themselves do not phagocytose myelin debris as efficiently as macrophages. As a result, myelin sheaths are not cleared away from injury sites, thereby leaving behind a “ghost” of the former axon trajectory. In some instances, myelin ghost sheaths remain years after the injury and are never cleared away (Vargas and Barres, 2007). This lack of myelin clearance is a barrier to axon regeneration through damaged territory, impeding circuit restoration (Filbin, 2003).
Another impediment to CNS regeneration was thought to be the glial scar formed around the lesion site. In the CNS, the glial scar is composed of fibroblasts, extracellular matrix (ECM) components and inflammatory immune cells at the lesion core, all surrounded by reactive astrocytes (Adams and Gallo, 2018). Though research into the glial scar has mainly centered on the inhibitory effects of cells surrounding the lesion and how they negatively affect axon regeneration, recent evidence questions this inhibitory role of the glial scar after injury.
Glial scar in axon regeneration
Astrocyte activation at the site of CNS lesions results in the deposition of ECM molecules such as chondroitin sulfate proteoglycans (CSPGs) and the formation of the glial scar, which for many years has been considered exclusively refractory to axon extension (Silver and Miller, 2004). In addition to CSPGs, inhibitory cues such as semaphorin 3A and ephrin B within the scar also contribute to regenerative failure (Bundesen et al., 2003; Pasterkamp et al., 2001). Other cues including semaphorin 5A and semaphorin 6D are also elevated at the injury site following SCI, resulting in increased axonal dieback of CST neurons (Ueno et al., 2020). This axonal retraction can be suppressed by removal of these semaphorins or conditional deletion of their Neuropilin 1 or Plexin A1 holoreceptor complex components, respectively, from CST neurons prior to injury (Ueno et al., 2020).
However, not all molecules found in the glial scar are inhibitory to axon growth since some glycosaminoclycan side chains actually promote axon extension (Miller and Hsieh-Wilson, 2015). Since the CSPG side chain governs the response of interacting axon growth cones, a potential therapeutic strategy investigated by many groups is the enzymatic degradation of inhibitory CSPG glycosaminoclycans using chondroitinase ABC (chABC), resulting in increased axon growth and some recovery of motor function in rodents following lesion (Barritt et al., 2006; Moon et al., 2001). In addition, chABC combined with cell transplants or optimization of the mode of chABC delivery may substantially promote regeneration and improve functional recovery (Muir et al., 2019; Rosenzweig et al., 2019). Encouragingly, intraparenchymal chABC injections in rhesus monkeys following spinal cord hemisection also results in increased synapse formation in gray matter caudal to the injury site and also to partial restoration of motor function (Rosenzweig et al., 2019). Administering chABC to treat human patients afflicted with traumatic nerve injury is promising, but additional studies will determine whether the beneficial effects of this treatment occur in other CNS injury models.
In addition to inhibitory factors, transcriptomic profiling analyses reveal that cells occupying the glial scar also express a host of cues that are permissive for axon regrowth (Anderson et al., 2016). Among these, reactive astrocytes and non-astrocytic cells such as fibroblasts upregulate CSPG subtypes known to support axon growth (Anderson et al., 2016). Interestingly, axons fail to regenerate within the CNS when the astrocytic scar is ablated following a conditioning peripheral lesion, suggesting that components of the glial scar can, in fact, promote axon regrowth. Further, axon guidance signaling components such as Plexin-B2 are upregulated in injury-activated microglia and macrophages and play a role in organizing the glial scar (Zhou et al., 2020). Investigation of the role of microglia in neonatal mice following SCI shows that microglia form transient ECM bridges and express peptidase inhibitors that promote axon re-growth and wound healing (Li et al., 2020). Therefore, a conundrum of using chABC and other approaches to ablate the glial scar is the degradation of attractive and repulsive cues expressed by its constituents and that are essential for directing axon growth. One approach is to identify key elements within the scar that are refractory to axon growth and specifically manipulate those components, as shown by the role of astrocyte-specific activation of RhoA in regulating injury-induced scarring and CSPG production, which ultimately promotes axonal regeneration (Stern et al., 2021). In the visual system, glial scars may not be as refractory to axon regeneration as they are in the spinal cord, since manipulations of intrinsic growth mechanisms such as mTOR can trigger substantial regeneration without any need to suppress glial scarring (Park et al., 2008).
Taken together, the glial scar should not be viewed as a homogeneous inhibitory environment but, rather, as a complex milieu of both permissive and inhibitory signals through which extending axons must traverse to reach their targets (reviewed in Adams and Gallo, 2018; Tran et al., 2021).
Axon guidance molecules in regeneration
Axon guidance molecules play a critical role in neural circuit formation during development by directing the growth of axons and influencing target selection (Chédotal, 2019). These molecules can elicit attractive or repulsive responses from growth cones (Bashaw and Klein, 2010; Tessier-Lavigne and Goodman, 1996). Expression of many axon guidance molecules persists into adulthood; however, the role of guidance cues in adulthood and whether they provide the same attractive or repulsive forces as they do during neural development, varies from one cue to another (Giger et al., 2010). Consequently, the role of guidance cues in re-forming neural circuits after injury, though promising, is complex and requires thorough investigation in order to understand how guidance cues might be successfully applied to regenerating axons (Giger et al., 2010; Harel and Strittmatter, 2006).
Repulsive guidance molecule A (RGMa) is a glycosylphosphatidylinositol (GPI)-linked membrane-associated protein that binds to its receptor, Neogenin (Neo1), to specify repulsive axon guidance. Following spinal cord injury, RGMa is upregulated in neurons and oligodendrocytes and is also expressed by astrocytes, activated microglia and macrophages. Monoclonal antibodies that neutralize the inhibitory effects of RGMa by specifically binding to this ligand promote axon regeneration and also improve functional recovery 6 weeks after injury (Mothe et al., 2017). RGMa-Neo1 signaling is also known to promote cell survival, but not axon re-growth following optic nerve transection in the adult rat retina (Koeberle et al., 2010). These observations provide another promising therapeutic avenue involving receptor disinhibition to neutralize anti-regenerative factors and thereby promote axon re-extension following injury.
The Wnt signaling pathway is also implicated in providing repulsive signaling via its Ryk receptor during CNS regeneration. Following cervical spinal cord injury, inhibition of Wnt-Ryk signaling either using conditional knockout of Ryk or function blocking monoclonal antibodies resulted in enhanced sprouting of lesioned CST axons (Hollis et al., 2016). These studies also demonstrated that when mice received task-specific forelimb training, their cortical maps underwent significant reorganization, resulting in novel recruitment of cortical areas that normally only control the hindlimb. Importantly, this work suggests that the greatest axon collateral branch formation and behavioral recovery following injury can be attributed to the joint contributions of task-oriented learning and blockade of Ryk receptor function. Recent evidence in the visual system shows that Wnt5a, acting at the optic chiasm, promotes decussation of contralateral RGC axons and repels axons of ipsilateral RGCs via EphB1 receptor (Morenilla-Palao et al., 2020). Whether such mechanisms will be automatically reinstated or whether they need to be exogenously introduced after injury remains unclear. In the mammalian retinocollicular system, RGC axon injury triggers upregulation of select guidance cues in the target (Symonds et al., 2007), but whether this also occurs in the mammalian CNS remains unknown.
Injury models that spare some axons, though not ideal for understanding the total regenerative potential of a particular treatment, are meritorious since they are clinically relevant. Most injuries in humans caused by accidents or trauma likely result in spared axons. Axon regeneration and sprouting refer to differences in the origin of a regenerating axon; a newly regenerating axon arises from the injured axon itself, whereas sprouting refers to collaterals extending from spared axons that were not severed by the injury (Figure 3C) (reviewed in: Fischer et al., 2017; Steward and Willenberg, 2017). The formation of axon collaterals allows mostly uninjured axons (and a few injured ones) to elaborate new processes that bypass the lesion site and promote connection and subsequent synaptogenesis with neuronal processes distal to the lesion site. Thus, there is a pressing need to identify strategies that can preserve the remaining neurons and their processes and to maximize their potential to restore function.
A more typical approach for enhancing functional recovery after CNS injury is to use guidance cues to promote the formation of collateral branches from the axon shaft (Figure 3C). Ephrins, a family of canonical guidance cues, play major inhibitory roles in the post-injury environment by blocking axonal sprouting following both ONC and SCI (Duffy et al., 2012; Joly et al., 2014; Overman et al., 2012). These studies demonstrate that ephrin/Eph expression is increased post-injury, mainly by reactive astrocytes. However, the use of ephrin/Eph blockers to neutralize injury-induced increases in expression was not successful in improving regeneration by permitting axonal sprouting. Remarkably, a combination of behavioral activity, fostered by forced-use of the affected limb, along with Ephrin-A5 blockade following stroke, resulted in a small but significant increase in axonal sprouting compared to controls with no forced-use. Several additional studies show that axon collateral sprouting improves functional recovery of motor circuits following spinal cord injury (Fouad et al., 2001; Lee et al., 2010; Massey et al., 2006). A caveat to consider is that aberrant, or constitutive upregulation, of growth-signaling factors and guidance cue signaling pathways can lead to profuse, but abnormal, growth that may not be conducive for the functional re-connection of neural circuits (Bray et al., 2017; Pernet and Schwab, 2014; Pernet et al., 2013). Identifying and combining key guidance molecules and growth factors necessary for encouraging growth post-injury may promote synaptogenesis of regenerating axons.
RESTORING SYNAPTIC CONNECTIVITY IN DISTAL TARGET REGIONS
A central goal of CNS regeneration studies is to understand how to achieve long distance axon regrowth and connectivity to distal targets to restore CNS function. However, can regenerating axons properly reinnervate their developmental targets without exogenous application of guidance cues? In the mammalian spinal cord, injured corticospinal motor axons can successfully navigate through a neural progenitor cell graft and re-innervate appropriate pre-motor interneurons distal to the lesion (Ceto et al., 2020; Kumamaru et al., 2019). Trans-synaptic labeling in these studies demonstrates that premotor, but not presensory, interneurons become synaptically connected to regenerating corticospinal motor axons, suggesting that successful re-innervation of distal targets in the mammalian spinal cord is indeed possible independent of exogenous guidance cues.
Likewise, in the mammalian visual system regenerating RGC axons can re-innervate retinorecipient targets following ONC, resulting in partial recovery of visual function. Intraocular injections of zymosan and a cAMP analog elicits an inflammatory response in RGCs that, when combined with mTOR upregulation, leads to long distance axon regeneration into retinorecipient centers (de Lima et al., 2012). Though it is unclear whether target-specific regeneration occurs following this treatment, the partial recovery of visual behaviors, including the optomotor response and circadian photoentrainment, suggests this is the case.
Another strategy to promote long-distance RGC axon regeneration involves combining mTOR activation with biased visual stimulation to stimulate retinal activity only in the injured eye. A small subset of regenerating RGC axons navigate through the optic chiasm and re-innervate retinorecipient centers following this approach (Lim et al., 2016). The mechanistic basis at play here is unknown, yet during development neural activity is known to promote RGC survival and axon outgrowth (Goldberg et al., 2002)—processes that may involve activity-dependent delivery of TrkB and other trophic receptors to the RGC cell surface (Meyer-Franke et al., 1998). To address the issue of target specific re-innervation, the Cochlin-GFP (CoCH-GFP) mouse line, in which many labeled RGCs are α-RGCs, was used (Lim et al., 2016). Regenerating CoCH-GFP–positive RGC axons re-extend into some of their normal developmental targets, including the ventral lateral geniculate nucleus and superior colliculus but avoided others, including the hypothalamic suprachiasmatic nucleus and brainstem medial terminal nucleus.
However, aside from a few rare instances of long-distance axon regeneration, most studies have failed to achieve the degree of axon regrowth following ONC required to assess whether regenerated axons can indeed form functional synapses in retinorecipient targets. In order to address this issue, implementation of a distal injury model in which the optic tract is severed immediately proximal to the superior colliculus has been employed (Bei et al., 2016). Co-deletion of PTEN and SOCS3 leads to target reinnervation and the formation of functional synapses following optic nerve lesion, but not restoration of visual behavior. The regenerated axons in this injury model are demyelinated, however voltage-gated potassium channel blockers can enhance conductivity, ultimately leading to recovery of visual function. These experiments provide hope that, given the proper stimulation paradigm, regrowing axons can properly re-innervate their developmental targets and restore function following CNS injury.
AXON DEGENERATION
The sequelae of events leading to axon degeneration are well known. Axon degeneration is a multi-stage process that occurs following a traumatic injury to the nervous system. An injury can present in the form of a contusion and does not have to breach the skull or spinal cord to cause degeneration. Degeneration can also originate from neurons themselves without any overt physical insult, as in amyotrophic lateral sclerosis (ALS), Alzheimer’s or Parkinson’s disease, wherein genetic predispositions, age-related triggers, or environmental stressors can affect neuronal survival and cause progressive degeneration and dysfunction of neuronal circuits (Hou et al., 2019). Regardless of the initial cause, a key issue is whether the degeneration occurs in the CNS or in the PNS, since these two divisions of the nervous system diverge sharply in their capacity to support regeneration and replenish damaged neurons.
When axons are crushed or severed the portion of the axon that is distal to the injury, and therefore disconnected from the soma, disintegrates in a fragmented manner by a process called Wallerian degeneration (Figure 4A). Disconnection from target cells and the inability of the parent neuron to receive target-derived trophic support may cause the proximal stump of the axon to degenerate further, although not as dramatically as the distal axon. Axon degeneration, therefore, proceeds in both directions from the injury site, with distal axons completely disappearing over time while the proximal axons “die back” and may then begin to regenerate. Despite recent progress in achieving long-distance axon regrowth following CNS injury, no pro-regenerative strategies have completely restored neural circuit function. One possible impediment to functional regeneration that remains to be investigated is whether the delayed clearing of degenerating neuronal elements further curbs the growth of regenerating axons.
Figure 4: Axon degeneration and immune response to injury.
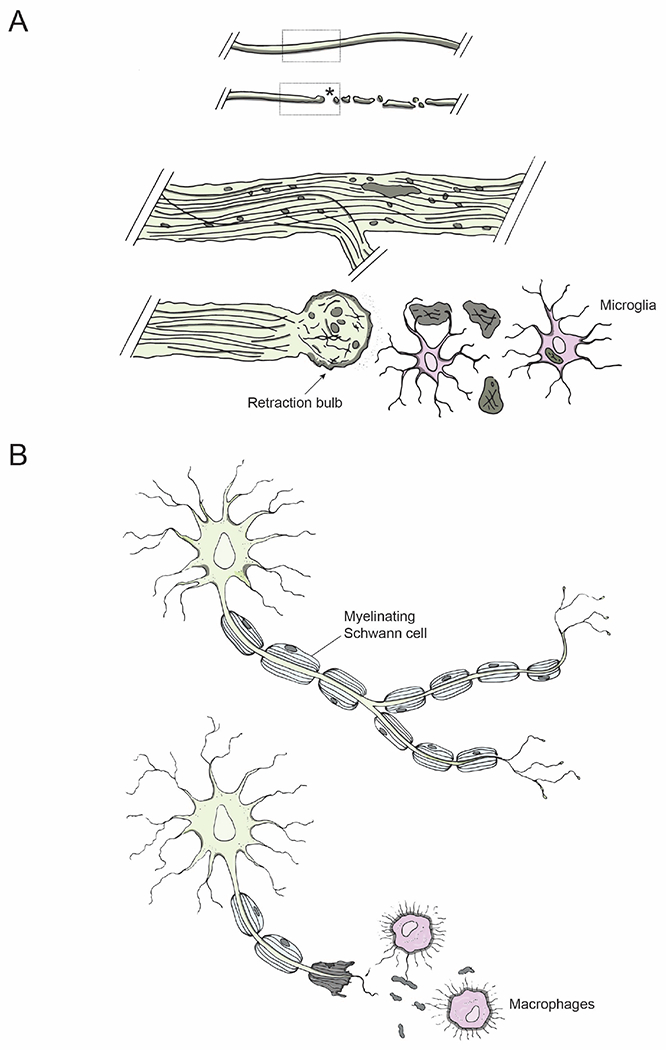
(A) Degenerative mechanisms following injury: An intact axon before injury; an injured axon undergoing Wallerian degeneration distal to the lesion site (asterisk). Dotted regions of the intact and injured axons are shown magnified below: retraction bulb (arrow) sealing the axolemma at the proximal end of an injured axon and microglia (pink) clearing axonal debris.
(B) Injury in the PNS: Schwann cells in the PNS myelinate regenerating axons (top); macrophages phagocytose axonal debris (bottom, pink).
The degeneration process has a deleterious component, the damage and loss of neurons, but also an adaptive component, the clearance of debris from the site of injury. Indeed, the latter is a distinguishing factor that enhances the regenerative capacity of PNS neurons and circuits (Vargas and Barres, 2007). Conversely, lack of clearance of injury-induced debris, in particular the persistence of myelin, is thought to be a major contributor to the lack of regeneration observed in the mature CNS.
Underlying molecular pathways
To achieve robust CNS regenerative outcomes, it is imperative to not only understand the events that unfold after an injury but to also take into account how degeneration impacts the regeneration of surviving neurons. We consider here observations that provide insight into the underlying signaling pathways that mediate degeneration.
A genome-wide screen using small interference RNAs (siRNAs) identified DLK as an important regulator of cell death (Watkins et al., 2013; Welsbie et al., 2013, 2017). DLK, a mitogen-activated protein kinase present in both the CNS and PNS, regulates apoptosis, axon growth and degeneration during development with a conserved function in many organisms including C. elegans and Drosophila (He and Jin, 2016). DLK activates c-Jun N-terminal kinase (JNK) signaling, and it induces both apoptotic and regeneration-associated gene expression (Watkins et al., 2013; Welsbie et al., 2013). In the PNS, mTOR and DLK are required to transport phosphorylated-STAT3 mediated injury signals from peripheral axons to the cell body, to initiate pro-regenerative pathways and they are robustly upregulated in peripheral axons following conditioning lesions (Shin et al., 2012; Terenzio et al., 2018). Similarly, in the CNS DLK protein levels first increase in the axons and later in their cell bodies following ONC (Watkins et al., 2013; Welsbie et al., 2013). Remarkably, DLK loss-of-function in an ONC model promotes increases RGC survival but results in very little axon regeneration. These findings suggest DLK is a signal conveyed by the injured axon to its soma that can ultimately mediate cell death in injured neurons.
Calcium
One of the main events leading to degeneration is the influx of Ca2+ to the axon from the extracellular space through injury-induced plasma membrane holes called mechanopores (Williams et al., 2014). Elevated intra-axonal Ca2+ initiates degenerative mechanisms, including calpain activation and autophagy, that break down cytoskeletal components in the injured axon (Knöferle et al., 2010; Ma, 2013). However, not all injured axons undergo these events since the severity of axonal injury likely influences the degree to which axons are able to evade Ca2+-dependent degeneration. For example, a small percentage of axons recover from a spinal cord contusion through spontaneous closure of mechanopores and restoration of pre-injury intra-axonal calcium levels (Williams et al., 2014).
To overcome the deleterious effects of increased intracellular calcium following severe axonal injury, various strategies have been employed that block autophagy or inhibit calcium-signaling using calcium-channel blockers or chelating agents (Knöferle et al., 2010; Ribas et al., 2017). Calpain inhibitors can also limit axon degeneration, but they appear to preferentially spare neurofilaments over microtubules (Park et al., 2013). Intriguingly, a recent investigation of the role of Ca2+ signaling in RGC survival showed that reactivation of Calmodulin-dependent protein kinase II (CaMKII), a key regulator of Ca2+ homeostasis, provides neuroprotective effects on RGC survival and RGC axon regeneration following injury (Guo et al., 2021). Further, CaMKII reactivation acts as a positive regulator of RGC survival and axon regeneration in models of excitotoxicity and glaucoma, slowing disease progression. Therefore, increased Ca2+ levels, and possibly a dysregulation of calcium homeostasis following injury, negatively affects cell survival. Regardless of the intervention, efforts to restore intracellular calcium levels to basal conditions after injury may preserve distal axon integrity and increase the likelihood of functional recovery.
Acute axon degeneration
There are at least two distinct phases during which axon degeneration occurs: an initial acute axon degeneration (AAD) and subsequent Wallerian Degeneration (WD) phase. As the name suggests, AAD is the response that takes place within the first few minutes following an injury. Though the time kinetics may vary among different systems, resulting in distinct phases of degeneration, AAD in general results in a rapid degeneration of both the proximal and distal stumps of the axon over a few hundred microns. For example, following a spinal cord lesion in which labeled central projections of DRG axons are selectively transected and then imaged in vivo, AAD starts within the first 24 hours and lasts less than five minutes, causing both proximal and distal stumps to retract ~300-400 microns (Kerschensteiner et al., 2005).
In an optic nerve crush model AAD initiates a similar retraction of the proximal stump by ~400 microns, however in this scenario AAD does not occur as a distinct phase and is instead interspersed with the formation of retraction bulbs, perhaps due to the structural simplicity of the optic nerve (Knöferle et al., 2010). Regardless, considering the range over which AAD affects injured axons, the therapeutic potential of addressing the molecular mechanisms underlying AAD may prove to be limited in promoting long distance regeneration. What, then, might be the purpose of an immediate rapid degenerative response as compared to the slower WD response? The proximal axon degeneration that is characteristic of AAD is thought to be a necessary step for subsequent initiation of glia-mediated events surrounding the injury. However, experiments that specifically inhibit this initial response while preserving WD are necessary to fully understand the functional implications of AAD.
Following AAD, retraction bulbs form on the axon stumps (Figure 4A). Both the proximal and distal stumps of injured axons close their axolemma to contain the anterograde and retrograde transport of proteins and other molecules. During this phase retraction bulbs form at the ends of CNS axons within the first 30 minutes after ONC, or within 30 hours after SCI. Retraction bulbs remain more or less stable unless the axon establishes contact with a macrophage, which triggers phagocytosis within an hour (Evans et al., 2014).
Wallerian degeneration
The Wallerian degeneration phase involves the well-studied, evolutionarily conserved cell-autonomous program that neurons undergo following an injury or insult and has been best investigated in the context of the sciatic nerve and DRG neurons in the PNS (Vargas and Barres, 2007). It is generally thought that all CNS neurons undergo Wallerian-type degeneration after injury, although that has not been systematically explored. During WD, distal segments of injured axons form connected beadlike swellings that gradually become separated and then more rapidly fragment and disappear. Notably, classical work showed that a spontaneous autosomal dominant mutation in the WldS gene conferred neuroprotective effects and a delay in the degradation of the injured PNS axon (Perry et al., 1990). This mutation results in the mobilization of the enzyme nicotinamide mononucleotide adenylytransferase (NMNAT1) from the nucleus to the axoplasm, leading to an increase in nicotinamide adenine dinucleotide (NAD+) levels (Sasaki et al., 2016; Wang et al., 2015). Importantly, a local increase of NAD+ in cultured embryonic DRG neurons within 4 hours after injury is sufficient to protect injured axons, similar to what is provided by the WldS mutation (Wang et al., 2015). These results point towards a promising therapeutic window during which axonal degeneration might be prevented (Wang et al., 2015).
Similar to WldS, the NAD+-cleaving enzyme sterile alpha and TIR motif containing 1 (Sarm1) regulates axon degeneration (Gerdts et al., 2015; Osterloh et al., 2012). Axon injury causes NMNAT2 degradation and an increase in axonal nicotinamide mononucleotide (NMN) levels (Gilley et al., 2017). Normally, both NMN and NAD+ compete for binding to the ARM domain of Sarm1. However, increased NMN levels and subsequent binding to Sarm1 induces a conformational change that activates the NADase activity of Sarm1, initiating an axon destruction program; therefore, Sarm1 is a major regulator of axonal degeneration and is an attractive candidate for strategies designed to promote axon regeneration (Figley et al., 2021; Gilley et al., 2017; Tian et al., 2020).
Although WD was initially described as a self-destruction program that axons undergo upon trophic deprivation or injury, recent in vitro studies in embryonic sensory neurons support the idea that WD is an active process regulated by the soma (Vargas and Barres, 2007). Normally, neurotrophic factor (NGF) binds to its receptor Tropomyosin receptor kinase A (TrkA) at the distal end of the axon and initiates several signaling events, including AkT signaling. However, trophic deprivation results in the phosphorylation of Akt, subsequently activating JnK/DLK and leading to a cascade of signaling events that ultimately engages Puma, a pro-apoptotic protein and a key regulator of degeneration. Upon trophic deprivation, transcriptional regulation of Puma in the cell body sends pro-apoptotic caspase-dependent signals back to the distal axon (Simon et al., 2016). Interestingly, further investigation shows that the anterograde signaling that activates axonal-caspase is also mediated by the tumor suppressor p53 and is likely distinct from the somatic and axonal caspase activation induced by Puma (Simon et al., 2021). A screen for cleavage targets enriched by trophic deprivation identified the neuronally-enriched protein RUN- and –FYVE-domain-containing protein 3 (Rufy3), that is cleaved downstream of caspase3 and is required for WD, independent of Puma (Hertz et al., 2019). These findings highlight the presence of a quiescent apoptotic machinery that is already present in the axon; one that is readily activated by the cell body upon trophic deprivation. Future work examining these signaling pathways in the CNS of adults may prove valuable in understanding the mechanistic differences that determine how cells in the CNS versus PNS respond to degeneration in their respective environments.
Trophic factor deprivation-induced cell death can also occur at the dendrites of a neuron. Reduced responsiveness of RGCs to insulin-like growth factor-1 (IGF-1) leads to activation of the AkT pathway, followed by a trophic deprivation response (Ambacher et al., 2012). IGF-1 receptors normally accumulate in RGC primary cilia, however they are dramatically reduced within a week of ONC as a result of strong inhibition from amacrine cell hyperactivity (Zhang et al., 2019). Remarkably, overexpression of Lin28 in amacrine cells robustly promotes RGC cell survival and axon regeneration, which is further potentiated by overexpression of IGF-1 (Zhang et al., 2019). Similarly, exogenously supplied IGF-1 promotes survival of corticospinal neurons in a proximal injury model (Hollis et al., 2009). Thus, trophic factor responsiveness by the cell body and axon may produce diverse regenerative responses in different neuronal subpopulations.
In light of the crosstalk between axonal and somal signaling pathways in response to an injury, it is understandable that there is overlap among apoptotic signaling pathways and those responsible for axonal degeneration. Various kinases, including DLK, JNK and MAPK, have been implicated in the regulation of cell death and axon degeneration, albeit to varying degrees in each context (Shin et al., 2012; Watkins et al., 2013). It is critical to identify downstream effectors of these signaling pathways and to determine how they differentially trigger degeneration in the soma and the axon. For example, deletion of Sarm1 does not impede activation of DLK/JNK in RGC somas after ONC and hence fails to prevent RGC death, even though it reduces axon degeneration (Fernandes et al., 2018). With a better understanding of the downstream signaling, these overlapping pathways may offer opportunities to simultaneously alleviate cell death and slow axon degeneration, thus providing attractive therapeutic strategies.
Immune mechanisms of axon degeneration
In the weeks following injury, the immune system is tasked with the removal of damaged axons (Figure 4). In the PNS, severed axons and dying cells are phagocytosed, myelin debris is cleared, and macrophages are swiftly recruited for further containment of the lesion following injury (Figure 4B) (Vargas and Barres, 2007). However, in the CNS this relatively simple process becomes maladaptive, leading to an exaggerated immune response that inhibits axon re-extension. The earliest response to an injury is initiated by glial subtypes, including microglia and reactive astrocytes in the CNS, and by Schwann cells in the PNS (Beck et al., 2010; Greenhalgh et al., 2020). Interactions between immune cells and glia can exert a significant influence on the regenerative capacity of the nervous system and must be considered carefully when identifying new therapeutic approaches (reviewed in Greenhalgh et al., 2020).
Studies on microglia offer further insight into the role of glia and the immune system in the injured environment since microglia can be classified as both immune cells and as a glial cell type (Greenhalgh et al., 2020). Microglia are an important component of the glial scar that forms after an injury, where they proliferate and populate the overall lesion site. This component of the glial scar forms within 7 days of an injury and is sandwiched between the fibrotic and astrocytic scars (Beck et al., 2010; Bellver-Landete et al., 2019). Depleting microglia after SCI reduces IGF-1 expression, leading to a disorganized astrocytic scar and impaired locomotor recovery; this demonstrates an important role for microglia in inducing the astrocytic response via IGF-1 (Bellver-Landete et al., 2019). Microglia also exert an anti-regenerative influence by inducing the production of reactive astrocytes via cytokine secretion, including interleukin 1α (IL-1α), tumor necrosis factor (TNF) and the complement component 1 (C1q). These three cytokines secreted by microglia together induce one of two types of cytotoxic reactive astrocytes that are responsible for killing RGCs in an ONC or glaucoma model. Blocking all three cytokines prevents the formation of cytotoxic astrocytes and increases RGC survival, highlighting axotomy-induced cytokine secretion as the cause of RGC death (Liddelow et al., 2017). Interestingly, injury or cytokine-toxins alone are insufficient to cause RGC death. A triple knockout mouse line that prevents astrocytes from becoming activated preserves RGCs after injury, while the toxins injected into a healthy adult mouse do not affect RGC viability (Guttenplan et al., 2020).
One of the major tasks for the immune system after a nerve injury is clearance of injury-induced debris, including degenerating myelin sheaths and fragmented axons (Figure 4B). Unlike the CNS, in which axonal and myelin debris are cleared over a prolonged period of ~90 days or longer, WD in the PNS adopts a faster time frame with debris clearance completed by ~30 days, partly due to rapid recruitment of macrophages (George and Griffin, 1994). Further, within the first few days post-injury in the PNS, Schwann cells reprogram into repair cells that clear myelin debris (Vargas et al., 2010). A compression injury or nerve transection to the PNS triggers a rapid and well-orchestrated immune response, comprised of macrophages and blood-borne leukocytes in the injured region. Neutrophils also enter the injured nerve within hours, followed by monocytes that subsequently differentiate into macrophages. In addition to fiber debris removal, macrophages phagocytose apoptotic cell bodies in the injured nerve and thereby contribute to inflammation resolution (Kalinski et al., 2020). Repair SC (rSC) and macrophages employ different mechanisms for myelin phagocytosis. The engulfment receptors MerTK and Axl are key players in rSC (Brosius Lutz et al., 2017), while antibody- dependent opsonization of myelin debris is a primary mechanism for Fc receptor- mediated phagocytosis by macrophages (Kuhlmann et al., 2002).
What are the consequences of preventing degeneration?
While we do not have a clear answer to this question, it is important to consider the negative consequences of delaying, or preventing, degeneration following injury. The pathological changes that take place following spinal cord injury in WldS mice reveal that, apart from a slower degenerative response, scar formation is also considerably slower in these mutant mice (Zhang et al., 1996). Delayed scar formation in the Wlds mice also results in delayed disappearance of the lesion site and leads to incomplete healing. More recently, it was shown that delayed degeneration in WldS mice results in a subsequent delay in collateral sprouting from spared axons, leading to delayed functional recovery (Collyer et al., 2014). Therefore, degeneration and the inflammatory responses to injury may be as important as the regenerative mechanisms critical for mediating functional nervous system repair.
In the PNS, delayed WD significantly affects the recruitment of macrophages, regeneration of sensory and motor axons as assessed by reinnervation of muscle targets and the restoration of action potentials (Bisby and Chen, 1990; Brown et al., 1991). Interestingly, the regenerative ability of motor axons in WldS mice was comparable to that observed in control mice, albeit with smaller action potentials, while sensory axons suffered in their ability to reinnervate muscles, an effect attributed to the intact injury environment. Degeneration, therefore, is necessary to clear the distal stumps that can otherwise prevent regenerating axons from navigating past the lesion site and reinnervating targets. This highlights the need for further study to assess whether clearance of debris accelerates overall regeneration repair mechanisms, and whether promoting rapid clearing of degenerating axons should be included in strategies designed to enhance axon regeneration.
CONCLUSION
Cell death and clearance of neuronal debris, decreased intrinsic growth ability, and the presence of inhibitory factors all contribute to CNS regenerative failure following trauma. We have considered recent progress in efforts to stimulate axon regeneration which, if leveraged with approaches that accelerate distal axon regeneration, represent rational strategies for restoring CNS function following injury. Though some studies have demonstrated long-distance axon regeneration following ONC (Lim et al., 2016; de Lima et al., 2012), the manipulations required were multifaceted, and only a very small number of axons reached central targets. Nevertheless, these results suggest that, given the proper treatment, regrowing axons can navigate long distances and re-innervate target regions.
Although many of the strategies for promoting neuronal regeneration described thus far facilitate some degree of regeneration, and even functional recovery, the prophylactic nature of many approaches makes it difficult to extrapolate to the clinic. Nevertheless, a few promising studies demonstrate that provision of extrinsic and intrinsic factors after SCI promotes axon regeneration (Kadoya et al., 2009; Nathan et al., 2020). A combinatorial treatment administered up to 15 months post-spinal cord injury consisting of a conditioning lesion, a syngenic bone marrow stromal cell graft, and delivery of an NT-3 gradient is able to sustain axon regrowth through and past the lesion site (Kadoya et al., 2009). Additionally, overexpression of Lin28a within 5 days of either spinal cord injury or ONC supports robust axon regeneration of lesioned axons (Nathan et al., 2020). These positive outcomes serve as models for future work directed toward determining tractable approaches for stimulating robust axon regeneration in humans. Overall, combinatorial approaches such as increasing mTOR and neural activity (Lim et al., 2016), increasing growth factor signaling and improving conduction with potassium channel blockers (Bei et al., 2016), transplanting neurons or reprogramming cells to differentiate into neurons (Lu et al., 2020; Venugopalan et al., 2016), and many other approaches have led to significant advances in addressing neuronal survival, axon re-extension, and myelination following injury. Future work investigating mechanisms to promote dendritic regeneration, synapse formation and experience-dependent plasticity of re-formed neural circuits will greatly increase our understanding of how to achieve meaningful functional regeneration. Additionally, as each approach uncovers new means to promote survival and regeneration, identifying the best, yet most tractable, combination of approaches to fully repair neural circuits is crucial, as is consideration of expanding model organisms and anatomical pathways for testing regeneration-stimulating candidates.
When considering approaches that might hold the most therapeutic potential for human treatment, we must remember that many of the strategies described in this review are limited by the possibility of oncogenic effects brought on by upregulating pro-growth pathways active early in development. For this reason, many of these studies serve as models for understanding mechanisms of axon regrowth and target re-innervation following injury, with the goal of transitioning these observations into safe regeneration-promoting strategies. For example, it is possible that combining a non-oncogenic, axon growth stimulating, approach such as zinc chelation with a strategy to promote rapid clearance of degenerating axons might yield a desirable clinical outcome while decreasing the risk of deleterious side effects (Li et al., 2017b).
Other strategies aimed at restoring function following CNS injury also deserve consideration. Grafts of neural stem cells and neural progenitor cells have shown promising results, supporting regeneration and promoting functional recovery after SCI (Figure 5) (Cummings et al., 2005; Kadoya et al., 2016; Kumamaru et al., 2019). Transplanted RGCs that integrate and extend axons to visual targets in the brains of uninjured mice also provide a promising direction for replacing degenerating cells, thereby promoting RGC axon regeneration following injury (Venugopalan et al., 2016). These advances, along with the extensive work that demonstrates robust axon regeneration in a wide range of model systems, provide hope that restoration of CNS function following injury will be a reality for human patients in the near future.
Figure 5: Clinically relevant therapeutic strategies.
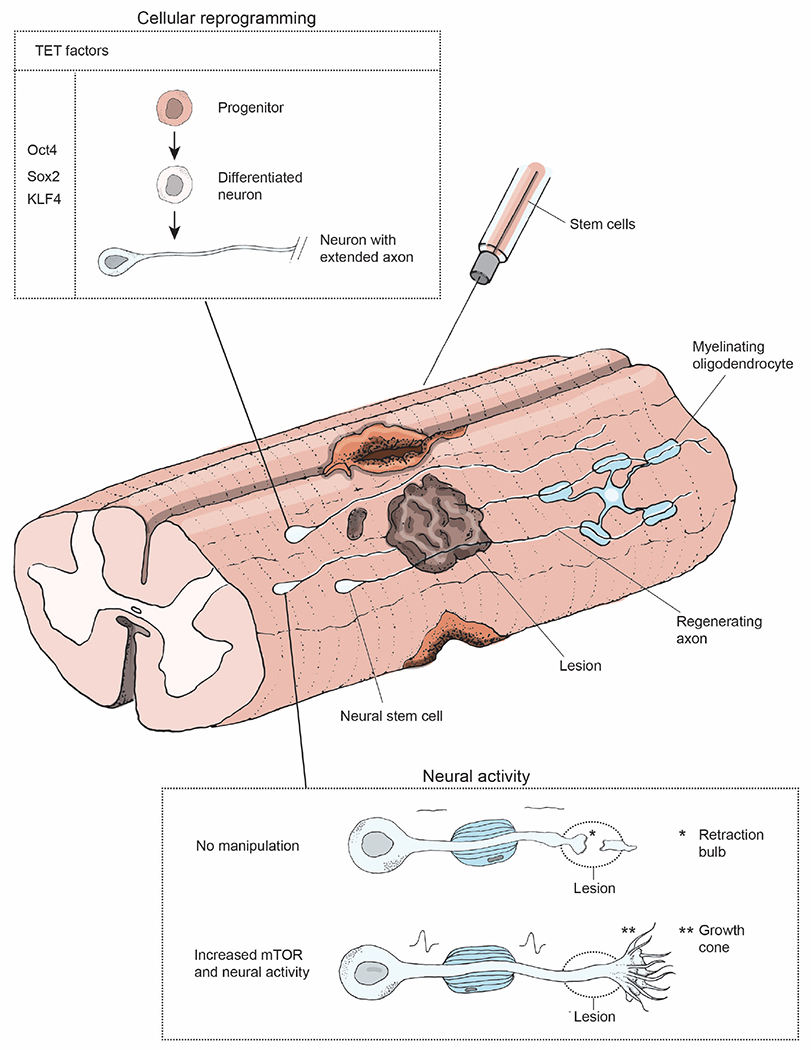
The most promising therapeutic strategies to promote re-connectivity of neural circuits are illustrated. Stem cells can be utilized to promote functional recovery following injury by neural stem cell grafts or by overexpressing TET factors (Oct4, Sox2, and KLF4)(Kumamaru et al., 2019; Lu et al., 2020) to reprogram cells to a development-like state that encourages axon regrowth. Upregulation of mTOR signaling and neuronal activity can promote axon regeneration past the lesion site(Lim et al., 2016), while voltage-gated potassium channel blocker can be utilized to promote myelin reformation in regenerating axons (Bei et al., 2016).
ACKNOWLEDGMENTS
We thank Roman Giger, Jeffrey Goldberg and Zhigang He for helpful comments on this manuscript. All illustrations were drawn by Natalie Hamilton. Work in the authors’ laboratories is supported by EY027713 to ALK and ADH; The Gilbert Family Foundation Vision Restoration Initiative to ADH; National Science Foundation Graduate Research Fellowship Program (NSF GRFP) to NRH; Knights Templar Eye Foundation Career Starter Grant to SGV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
Authors have no competing interests.
REFERENCES
- 1.Adams KL, and Gallo V (2018). The diversity and disparity of the glial scar. Nat. Neurosci 21, 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambacher KK, Pitzul KB, Karajgikar M, Hamilton A, Ferguson SS, and Cregan SP (2012). The JNK- and AKT/GSK3β- signaling pathways converge to regulate Puma induction and neuronal apoptosis induced by trophic factor deprivation. PLoS One 7, e46885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambros V, and Horvitz HR (1984). Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226, 409–416. [DOI] [PubMed] [Google Scholar]
- 4.Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, and Sofroniew MV (2016). Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews MR, Czvitkovich S, Dassie E, Vogelaar CF, Faissner A, Blits B, Gage FH, ffrench-Constant C, and Fawcett JW (2009). Alpha9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J. Neurosci 29, 5546–5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, McMahon SB, and Bradbury EJ (2006). Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J. Neurosci 26, 10856–10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bashaw GJ, and Klein R (2010). Signaling from axon guidance receptors. Cold Spring Harb. Perspect. Biol 2, a001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, and Anderson AJ (2010). Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 133, 433–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bei F, Lee HHC, Liu X, Gunner G, Jin H, Ma L, Wang C, Hou L, Hensch TK, Frank E, et al. (2016). Restoration of Visual Function by Enhancing Conduction in Regenerated Axons. Cell 164, 219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellver-Landete V, Bretheau F, Mailhot B, Vallières N, Lessard M, Janelle M-E, Vernoux N, Tremblay M-À, Fuehrmann T, Shoichet MS, et al. (2019). Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun 10, 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bisby MA, and Chen S (1990). Delayed wallerian degeneration in sciatic nerves of C57BL/Ola mice is associated with impaired regeneration of sensory axons. Brain Res. 530, 117–120. [DOI] [PubMed] [Google Scholar]
- 12.Bradke F, Fawcett JW, and Spira ME (2012). Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat. Rev. Neurosci 13, 183–193. [DOI] [PubMed] [Google Scholar]
- 13.Bray ER, Noga M, Thakor K, Wang Y, Lemmon VP, Park KK, and Tsoulfas P (2017). 3D Visualization of Individual Regenerating Retinal Ganglion Cell Axons Reveals Surprisingly Complex Growth Paths. ENeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bray ER, Yungher BJ, Levay K, Ribeiro M, Dvoryanchikov G, Ayupe AC, Thakor K, Marks V, Randolph M, Danzi MC, et al. (2019). Thrombospondin-1 Mediates Axon Regeneration in Retinal Ganglion Cells. Neuron 103, 642–657.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brosius Lutz A, Chung W-S, Sloan SA, Carson GA, Zhou L, Lovelett E, Posada S, Zuchero JB, and Barres BA (2017). Schwann cells use TAM receptor-mediated phagocytosis in addition to autophagy to clear myelin in a mouse model of nerve injury. Proc. Natl. Acad. Sci. U. S. A 114, E8072–E8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown MC, Perry VH, Lunn ER, Gordon S, and Heumann R (1991). Macrophage dependence of peripheral sensory nerve regeneration: possible involvement of nerve growth factor. Neuron 6, 359–370. [DOI] [PubMed] [Google Scholar]
- 17.Bundesen LQ, Scheel TA, Bregman BS, and Kromer LF (2003). Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J. Neurosci 23, 7789–7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ceto S, Sekiguchi KJ, Takashima Y, Nimmerjahn A, and Tuszynski MH (2020). Neural Stem Cell Grafts Form Extensive Synaptic Networks that Integrate with Host Circuits after Spinal Cord Injury. Cell Stem Cell 27, 430–440.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandran V, Coppola G, Nawabi H, Omura T, Versano R, Huebner EA, Zhang A, Costigan M, Yekkirala A, Barrett L, et al. (2016). A Systems-Level Analysis of the Peripheral Nerve Intrinsic Axonal Growth Program. Neuron 89, 956–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang K-C, Bian M, Xia X, Madaan A, Sun C, Wang Q, Li L, Nahmou M, Noro T, Yokota S, et al. (2021). Posttranslational modification of Sox11 regulates RGC survival and axon regeneration. ENeuro 8, ENEURO.0358-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chédotal A. (2019). Roles of axon guidance molecules in neuronal wiring in the developing spinal cord. Nat. Rev. Neurosci 20, 380–396. [DOI] [PubMed] [Google Scholar]
- 22.Collyer E, Catenaccio A, Lemaitre D, Diaz P, Valenzuela V, Bronfman F, and Court FA (2014). Sprouting of axonal collaterals after spinal cord injury is prevented by delayed axonal degeneration. Exp. Neurol 261, 451–461. [DOI] [PubMed] [Google Scholar]
- 23.Conta AC, and Stelzner DJ (2004). Differential vulnerability of propriospinal tract neurons to spinal cord contusion injury. J. Comp. Neurol 479, 347–359. [DOI] [PubMed] [Google Scholar]
- 24.Crair MC, and Mason CA (2016). Reconnecting Eye to Brain. J. Neurosci 36, 10707–10722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui Q, Ren C, Sollars PJ, Pickard GE, and So K-F (2015). The injury resistant ability of melanopsin-expressing intrinsically photosensitive retinal ganglion cells. Neuroscience 284, 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, and Anderson AJ (2005). Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. U. S. A 102, 14069–14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.David S, and Aguayo AJ (1981). Axonal Elongation into Peripheral Nervous System “Bridges” after Central Nervous System Injury in Adult Rats. New Series 214, 931. [DOI] [PubMed] [Google Scholar]
- 28.Duan H, Ge W, Zhang A, Xi Y, Chen Z, Luo D, Cheng Y, Fan KS, Horvath S, Sofroniew MV, et al. (2015a). Transcriptome analyses reveal molecular mechanisms underlying functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. U. S. A 112, 13360–13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan X, Qiao M, Bei F, Kim I-J, He Z, and Sanes JR (2015b). Subtype-specific regeneration of retinal ganglion cells following axotomy: effects of osteopontin and mTOR signaling. Neuron 85, 1244–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duffy P, Wang X, Siegel CS, Tu N, Henkemeyer M, Cafferty WBJ, and Strittmatter SM (2012). Myelin-derived ephrinB3 restricts axonal regeneration and recovery after adult CNS injury. Proc. Natl. Acad. Sci. U. S. A 109, 5063–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans TA, Barkauskas DS, Myers JT, Hare EG, You JQ, Ransohoff RM, Huang AY, and Silver J (2014). High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp. Neurol 254, 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandes KA, Mitchell KL, Patel A, Marola OJ, Shrager P, Zack DJ, Libby RT, and Welsbie DS (2018). Role of SARM1 and DR6 in retinal ganglion cell axonal and somal degeneration following axonal injury. Exp. Eye Res 171, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Figley MD, Gu W, Nanson JD, Shi Y, Sasaki Y, Cunnea K, Malde AK, Jia X, Luo Z, Saikot FK, et al. (2021). SARM1 is a metabolic sensor activated by an increased NMN/NAD+ ratio to trigger axon degeneration. Neuron 109, 1118–1136.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Filbin MT (2003). Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci 4, 703–713. [DOI] [PubMed] [Google Scholar]
- 35.Fischer D, Harvey AR, Pernet V, Lemmon VP, and Park KK (2017). Optic nerve regeneration in mammals: Regenerated or spared axons? Exp. Neurol 296, 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fouad K, Pedersen V, Schwab ME, and Brösamle C (2001). Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr. Biol 11, 1766–1770. [DOI] [PubMed] [Google Scholar]
- 37.George R, and Griffin JW (1994). Delayed macrophage responses and myelin clearance during Wallerian degeneration in the central nervous system: the dorsal radiculotomy model. Exp. Neurol 129, 225–236. [DOI] [PubMed] [Google Scholar]
- 38.Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, and Milbrandt J (2015). SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science 348, 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giger RJ, Hollis ER 2nd, and Tuszynski MH (2010). Guidance molecules in axon regeneration. Cold Spring Harb. Perspect. Biol 2, a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilley J, Ribchester RR, and Coleman MP (2017). Sarm1 Deletion, but Not WldS, Confers Lifelong Rescue in a Mouse Model of Severe Axonopathy. Cell Rep. 21, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GTA, and Barres BA (2002). Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron 33, 689–702. [DOI] [PubMed] [Google Scholar]
- 42.Greenhalgh AD, David S, and Bennett FC (2020). Immune cell regulation of glia during CNS injury and disease. Nat. Rev. Neurosci 21, 139–152. [DOI] [PubMed] [Google Scholar]
- 43.Guo X, Zhou J, Starr C, Mohns EJ, Li Y, Chen EP, Yoon Y, Kellner CP, Tanaka K, Wang H, et al. (2021). Preservation of vision after CaMKII-mediated protection of retinal ganglion cells. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guttenplan KA, Stafford BK, El-Danaf RN, Adler DI, Münch AE, Weigel MK, Huberman AD, and Liddelow SA (2020). Neurotoxic Reactive Astrocytes Drive Neuronal Death after Retinal Injury. Cell Rep. 31, 107776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hains BC, Black JA, and Waxman SG (2003). Primary cortical motor neurons undergo apoptosis after axotomizing spinal cord injury. J. Comp. Neurol 462, 328–341. [DOI] [PubMed] [Google Scholar]
- 46.Harel NY, and Strittmatter SM (2006). Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat. Rev. Neurosci 7, 603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassannejad Z, Zadegan SA, Shakouri-Motlagh A, Mokhatab M, Rezvan M, Sharif-Alhoseini M, Shokraneh F, Moshayedi P, and Rahimi-Movaghar V (2018). The fate of neurons after traumatic spinal cord injury in rats: A systematic review. Iran. J. Basic Med. Sci 21, 546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hattar S, Liao HW, Takao M, Berson DM, and Yau KW (2002). Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295, 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He Z, and Jin Y (2016). Intrinsic Control of Axon Regeneration. Neuron 90, 437–451. [DOI] [PubMed] [Google Scholar]
- 50.Hertz NT, Adams EL, Weber RA, Shen RJ, O’Rourke MK, Simon DJ, Zebroski H, Olsen O, Morgan CW, Mileur TR, et al. (2019). Neuronally Enriched RUFY3 Is Required for Caspase-Mediated Axon Degeneration. Neuron 103, 412–422.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffman PN (2010). A conditioning lesion induces changes in gene expression and axonal transport that enhance regeneration by increasing the intrinsic growth state of axons. Exp. Neurol 223, 11–18. [DOI] [PubMed] [Google Scholar]
- 52.Holländer H, Bisti S, and Maffei L (1985). Long term survival of cat retinal ganglion cells after intracranial optic nerve transection. Exp. Brain Res 59, 633–635. [DOI] [PubMed] [Google Scholar]
- 53.Hollis ER 2nd, Lu P, Blesch A, and Tuszynski MH (2009). IGF-I gene delivery promotes corticospinal neuronal survival but not regeneration after adult CNS injury. Exp. Neurol 215, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hollis ER 2nd, Ishiko N, Yu T, Lu C-C, Haimovich A, Tolentino K, Richman A, Tury A, Wang S-H, Pessian M, et al. (2016). Ryk controls remapping of motor cortex during functional recovery after spinal cord injury. Nat. Neurosci 19, 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, and Bohr VA (2019). Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol 15, 565–581. [DOI] [PubMed] [Google Scholar]
- 56.Jessen KR, and Mirsky R (2016). The repair Schwann cell and its function in regenerating nerves. J. Physiol 594, 3521–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin D, Liu Y, Sun F, Wang X, Liu X, and He Z (2015). Restoration of skilled locomotion by sprouting corticospinal axons induced by co-deletion of PTEN and SOCS3. Nat. Commun 6, 8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joly S, Jordi N, Schwab ME, and Pernet V (2014). The Ephrin receptor EphA4 restricts axonal sprouting and enhances branching in the injured mouse optic nerve. Eur. J. Neurosci 40, 3021–3031. [DOI] [PubMed] [Google Scholar]
- 59.Kadoya K, Tsukada S, Lu P, Coppola G, Geschwind D, Filbin MT, Blesch A, and Tuszynski MH (2009). Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron 64, 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kadoya K, Lu P, Nguyen K, Lee-Kubli C, Kumamaru H, Yao L, Knackert J, Poplawski G, Dulin JN, Strobl H, et al. (2016). Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat. Med 22, 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalinski AL, Yoon C, Huffman LD, Duncker PC, Kohen R, Passino R, Hafner H, Johnson C, Kawaguchi R, Carbajal KS, et al. (2020). Analysis of the immune response to sciatic nerve injury identifies efferocytosis as a key mechanism of nerve debridement. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kang H, and Lichtman JW (2013). Motor axon regeneration and muscle reinnervation in young adult and aged animals. J. Neurosci 33, 19480–19491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang H, Tian L, Mikesh M, Lichtman JW, and Thompson WJ (2014). Terminal Schwann cells participate in neuromuscular synapse remodeling during reinnervation following nerve injury. J. Neurosci 34, 6323–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kerschensteiner M, Schwab ME, Lichtman JW, and Misgeld T (2005). In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat. Med 11, 572–577. [DOI] [PubMed] [Google Scholar]
- 65.Knöferle J, Koch JC, Ostendorf T, Michel U, Planchamp V, Vutova P, Tönges L, Stadelmann C, Brück W, Bähr M, et al. (2010). Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc. Natl. Acad. Sci. U. S. A 107, 6064–6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koeberle PD, Tura A, Tassew NG, Schlichter LC, and Monnier PP (2010). The repulsive guidance molecule, RGMa, promotes retinal ganglion cell survival in vitro and in vivo. Neuroscience 169, 495–504. [DOI] [PubMed] [Google Scholar]
- 67.Kuhlmann T, Wendling U, Nolte C, Zipp F, Maruschak B, Stadelmann C, Siebert H, and Brück W (2002). Differential regulation of myelin phagocytosis by macrophages/microglia, involvement of target myelin, Fc receptors and activation by intravenous immunoglobulins. J. Neurosci. Res 67, 185–190. [DOI] [PubMed] [Google Scholar]
- 68.Kumamaru H, Lu P, Rosenzweig ES, Kadoya K, and Tuszynski MH (2019). Regenerating corticospinal axons innervate phenotypically appropriate neurons within neural stem cell grafts. Cell Rep. 26, 2329–2339.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee H, McKeon RJ, and Bellamkonda RV (2010). Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. U. S. A 107, 3340–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leibinger M, Andreadaki A, Diekmann H, and Fischer D (2013). Neuronal STAT3 activation is essential for CNTF- and inflammatory stimulation-induced CNS axon regeneration. Cell Death Dis. 4, e805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li C, Sako Y, Imai A, Nishiyama T, Thompson K, Kubo M, Hiwatashi Y, Kabeya Y, Karlson D, Wu S-H, et al. (2017a). A Lin28 homologue reprograms differentiated cells to stem cells in the moss Physcomitrella patens. Nat. Commun 8, 14242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li S-Y, Yau S-Y, Chen B-Y, Tay DK, Lee VWH, Pu M-L, Chan HHL, and So K-F (2008). Enhanced survival of melanopsin-expressing retinal ganglion cells after injury is associated with the PI3 K/Akt pathway. Cell. Mol. Neurobiol 28, 1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y, Andereggen L, Yuki K, Omura K, Yin Y, Gilbert H-Y, Erdogan B, Asdourian MS, Shrock C, de Lima S, et al. (2017b). Mobile zinc increases rapidly in the retina after optic nerve injury and regulates ganglion cell survival and optic nerve regeneration. Proc. Natl. Acad. Sci. U. S. A 114, E209–E218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y, He X, Kawaguchi R, Zhang Y, Wang Q, Monavarfeshani A, Yang Z, Chen B, Shi Z, Meng H, et al. (2020). Microglia-organized scar-free spinal cord repair in neonatal mice. Nature 587, 613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung W-S, Peterson TC, et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lilley BN, Sabbah S, Hunyara JL, Gribble KD, Al-Khindi T, Xiong J, Wu Z, Berson DM, and Kolodkin AL (2019). Genetic access to neurons in the accessory optic system reveals a role for Sema6A in midbrain circuitry mediating motion perception. J. Comp. Neurol 527, 282–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lim J-HA, Stafford BK, Nguyen PL, Lien BV, Wang C, Zukor K, He Z, and Huberman AD (2016). Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat. Neurosci 19, 1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Lima S, Koriyama Y, Kurimoto T, Oliveira JT, Yin Y, Li Y, Gilbert H-Y, Fagiolini M, Martinez AMB, and Benowitz L (2012). Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc. Natl. Acad. Sci. U. S. A 109, 9149–9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, et al. (2010). PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci 13, 1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu Y, Brommer B, Tian X, Krishnan A, Meer M, Wang C, Vera DL, Zeng Q, Yu D, Bonkowski MS, et al. (2020). Reprogramming to recover youthful epigenetic information and restore vision. Nature 588, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo X, Salgueiro Y, Beckerman SR, Lemmon VP, Tsoulfas P, and Park KK (2013). Three-dimensional evaluation of retinal ganglion cell axon regeneration and pathfinding in whole mouse tissue after injury. Exp. Neurol 247, 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma M. (2013). Role of calpains in the injury-induced dysfunction and degeneration of the mammalian axon. Neurobiol. Dis 60, 61–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mahar M, and Cavalli V (2018). Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci 19, 323–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Massey JM, Hubscher CH, Wagoner MR, Decker JA, Amps J, Silver J, and Onifer SM (2006). Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J. Neurosci 26, 4406–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG Jr, Reichardt LF, and Barres BA (1998). Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron 21, 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miller GM, and Hsieh-Wilson LC (2015). Sugar-dependent modulation of neuronal development, regeneration, and plasticity by chondroitin sulfate proteoglycans. Exp. Neurol 274, 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moon LD, Asher RA, Rhodes KE, and Fawcett JW (2001). Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat. Neurosci 4, 465–466. [DOI] [PubMed] [Google Scholar]
- 88.Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, and Goldberg JL (2009). KLF family members regulate intrinsic axon regeneration ability. Science 326, 298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morenilla-Palao C, López-Cascales MT, López-Atalaya JP, Baeza D, Calvo-Díaz L, Barco A, and Herrera E (2020). A Zic2-regulated switch in a noncanonical Wnt/βcatenin pathway is essential for the formation of bilateral circuits. Sci. Adv 6, eaaz8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mothe AJ, Tassew NG, Shabanzadeh AP, Penheiro R, Vigouroux RJ, Huang L, Grinnell C, Cui Y-F, Fung E, Monnier PP, et al. (2017). RGMa inhibition with human monoclonal antibodies promotes regeneration, plasticity and repair, and attenuates neuropathic pain after spinal cord injury. Sci. Rep 7, 10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Muir E, De Winter F, Verhaagen J, and Fawcett J (2019). Recent advances in the therapeutic uses of chondroitinase ABC. Exp. Neurol 321, 113032. [DOI] [PubMed] [Google Scholar]
- 92.Nathan FM, Ohtake Y, Wang S, Jiang X, Sami A, Guo H, Zhou F-Q, and Li S (2020). Upregulating Lin28a promotes axon regeneration in adult mice with optic nerve and spinal cord injury. Mol. Ther 28, 1902–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nawabi H, Belin S, Cartoni R, Williams PR, Wang C, Latremolière A, Wang X, Zhu J, Taub DG, Fu X, et al. (2015). Doublecortin-like kinases promote neuronal survival and induce growth cone reformation via distinct mechanisms. Neuron 88, 704–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neumann S, and Woolf CJ (1999). Regeneration of Dorsal Column Fibers into and beyond the Lesion Site following Adult Spinal Cord Injury. Neuron 23, 83–91. [DOI] [PubMed] [Google Scholar]
- 95.Neumann S, Bradke F, Tessier-Lavigne M, and Basbaum AI (2002). Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron 34, 885–893. [DOI] [PubMed] [Google Scholar]
- 96.Norsworthy MW, Bei F, Kawaguchi R, Wang Q, Tran NM, Li Y, Brommer B, Zhang Y, Wang C, Sanes JR, et al. (2017). Sox11 Expression Promotes Regeneration of Some Retinal Ganglion Cell Types but Kills Others. Neuron 94, 1112–1120.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, et al. (2012). dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science 337, 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Overman JJ, Clarkson AN, Wanner IB, Overman WT, Eckstein I, Maguire JL, Dinov ID, Toga AW, and Carmichael ST (2012). A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proc. Natl. Acad. Sci. U. S. A 109, E2230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park JY, Jang SY, Shin YK, Suh DJ, and Park HT (2013). Calcium-dependent proteasome activation is required for axonal neurofilament degradation. Neural Regeneration Res. 8, 3401–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, et al. (2008). Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322, 963–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pasterkamp RJ, Anderson PN, and Verhaagen J (2001). Peripheral nerve injury fails to induce growth of lesioned ascending dorsal column axons into spinal cord scar tissue expressing the axon repellent Semaphorin3A. Eur. J. Neurosci 13, 457–471. [DOI] [PubMed] [Google Scholar]
- 102.Pérez de Sevilla Müller L, Sargoy A, Rodriguez AR, and Brecha NC (2014). Melanopsin ganglion cells are the most resistant retinal ganglion cell type to axonal injury in the rat retina. PLoS One 9, e93274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pernet V, and Schwab ME (2014). Lost in the jungle: new hurdles for optic nerve axon regeneration. Trends Neurosci. 37, 381–387. [DOI] [PubMed] [Google Scholar]
- 104.Pernet V, Joly S, Dalkara D, Jordi N, Schwarz O, Christ F, Schaffer DV, Flannery JG, and Schwab ME (2013). Long-distance axonal regeneration induced by CNTF gene transfer is impaired by axonal misguidance in the injured adult optic nerve. Neurobiol. Dis 51, 202–213. [DOI] [PubMed] [Google Scholar]
- 105.Perry VH, Lunn ER, Brown MC, Cahusac S, and Gordon S (1990). Evidence that the Rate of Wallerian Degeneration is Controlled by a Single Autosomal Dominant Gene. Eur. J. Neurosci 2, 408–413. [DOI] [PubMed] [Google Scholar]
- 106.Petros TJ, Rebsam A, and Mason CA (2008). Retinal Axon Growth at the Optic Chiasm: To Cross or Not to Cross. Annu. Rev. Neurosci 31, 295–315. [DOI] [PubMed] [Google Scholar]
- 107.Poplawski GHD, Kawaguchi R, Van Niekerk E, Lu P, Mehta N, Canete P, Lie R, Dragatsis I, Meves JM, Zheng B, et al. (2020). Injured adult neurons regress to an embryonic transcriptional growth state. Nature 581, 77–82. [DOI] [PubMed] [Google Scholar]
- 108.Renthal W, Tochitsky I, Yang L, Cheng Y-C, Li E, Kawaguchi R, Geschwind DH, and Woolf CJ (2020). Transcriptional Reprogramming of Distinct Peripheral Sensory Neuron Subtypes after Axonal Injury. Neuron 108, 128–144.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ribas VT, Koch JC, Michel U, Bähr M, and Lingor P (2017). Attenuation of Axonal Degeneration by Calcium Channel Inhibitors Improves Retinal Ganglion Cell Survival and Regeneration After Optic Nerve Crush. Mol. Neurobiol 54, 72–86. [DOI] [PubMed] [Google Scholar]
- 110.Rosenzweig ES, Salegio EA, Liang JJ, Weber JL, Weinholtz CA, Brock JH, Moseanko R, Hawbecker S, Pender R, Cruzen CL, et al. (2019). Chondroitinase improves anatomical and functional outcomes after primate spinal cord injury. Nat. Neurosci 22, 1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ruschel J, Hellal F, Flynn KC, Dupraz S, Elliott DA, Tedeschi A, Bates M, Sliwinski C, Brook G, Dobrindt K, et al. (2015). Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science 348, 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sasaki Y, Nakagawa T, Mao X, DiAntonio A, and Milbrandt J (2016). NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD+ depletion. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schwab ME, and Bartholdi D (1996). Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev 76, 319–370. [DOI] [PubMed] [Google Scholar]
- 114.Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, and DiAntonio A (2012). Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron 74, 1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Silver J, and Miller JH (2004). Regeneration beyond the glial scar. Nat. Rev. Neurosci 5, 146–156. [DOI] [PubMed] [Google Scholar]
- 116.Simon DJ, Pitts J, Hertz NT, Yang J, Yamagishi Y, Olsen O, Teţćc Mark M, Molina H, and Tessier-Lavigne M (2016). Axon Degeneration Gated by Retrograde Activation of Somatic Pro-apoptotic Signaling. Cell 164, 1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Simon DJ, Belsky DM, Bowen ME, Ohn CYJ, O’Rourke MK, Shen R, Kim G, Pitts J, Attardi LD, and Tessier-Lavigne M (2021). An anterograde pathway for sensory axon degeneration gated by a cytoplasmic action of the transcriptional regulator P53. Dev. Cell 56, 976–984.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Son YJ, and Thompson WJ (1995). Schwann cell processes guide regeneration of peripheral axons. Neuron 14, 125–132. [DOI] [PubMed] [Google Scholar]
- 119.Stern S, Hilton BJ, Burnside ER, Dupraz S, Handley EE, Gonyer JM, Brakebusch C, and Bradke F (2021). RhoA drives actin compaction to restrict axon regeneration and astrocyte reactivity after CNS injury. Neuron. [DOI] [PubMed] [Google Scholar]
- 120.Steward 0, and Willenberg R (2017). Rodent spinal cord injury models for studies of axon regeneration. Exp. Neurol 287, 374–383. [DOI] [PubMed] [Google Scholar]
- 121.Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner BA, et al. (2011). Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 480, 372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Symonds ACE, King CE, Bartlett CA, Sauvé Y, Lund RD, Beazley LD, Dunlop SA, and Rodger J (2007). EphA5 and ephrin-A2 expression during optic nerve regeneration: a “two-edged sword.” Eur. J. Neurosci 25, 744–752. [DOI] [PubMed] [Google Scholar]
- 123.Terenzio M, Koley S, Samra N, Rishal I, Zhao Q, Sahoo PK, Urisman A, Marvaldi L, Oses-Prieto JA, Forester C, et al. (2018). Locally translated mTOR controls axonal local translation in nerve injury. Science 359, 1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tessier-Lavigne M, and Goodman CS (1996). The molecular biology of axon guidance. Science 274, 1123–1133. [DOI] [PubMed] [Google Scholar]
- 125.Tian W, Czopka T, and López-Schier H (2020). Systemic loss of Sarm1 protects Schwann cells from chemotoxicity by delaying axon degeneration. Commun. Biol 3, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tom VJ, Steinmetz MP, Miller JH, Doller CM, and Silver J (2004). Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci 24, 6531–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Trakhtenberg EF, Li Y, Feng Q, Tso J, Rosenberg PA, Goldberg JL, and Benowitz LI (2018). Zinc chelation and Klf9 knockdown cooperatively promote axon regeneration after optic nerve injury. Exp. Neurol 300, 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tran AP, Warren PM, and Silver J (2021). New insights into glial scar formation after spinal cord injury. Cell Tissue Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tran NM, Shekhar K, Whitney IE, Jacobi A, Benhar I, Hong G, Yan W, Adiconis X, Arnold ME, Lee JM, et al. (2019). Single-Cell Profiles of Retinal Ganglion Cells Differing in Resilience to Injury Reveal Neuroprotective Genes. Neuron 104, 1039–1055.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ueno M, Nakamura Y, Nakagawa H, Niehaus JK, Maezawa M, Gu Z, Kumanogoh A, Takebayashi H, Lu QR, Takada M, et al. (2020). Olig2-Induced Semaphorin Expression Drives Corticospinal Axon Retraction After Spinal Cord Injury. Cereb. Cortex 30, 5702–5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vargas ME, and Barres BA (2007). Why is Wallerian degeneration in the CNS so slow? Annu. Rev. Neurosci 30, 153–179. [DOI] [PubMed] [Google Scholar]
- 132.Vargas ME, Watanabe J, Singh SJ, Robinson WH, and Barres BA (2010). Endogenous antibodies promote rapid myelin clearance and effective axon regeneration after nerve injury. Proc. Natl. Acad. Sci. U. S. A 107, 11993–11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Venugopalan P, Wang Y, Nguyen T, Huang A, Muller KJ, and Goldberg JL (2016). Transplanted neurons integrate into adult retinas and respond to light. Nat. Commun 7, 10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang JT, Medress ZA, Vargas ME, and Barres BA (2015). Local axonal protection by WldS as revealed by conditional regulation of protein stability. Proc. Natl. Acad. Sci. U. S. A 112, 10093–10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang X-W, Li Q, Liu C-M, Hall PA, Jiang J-J, Katchis CD, Kang S, Dong BC, Li S, and Zhou F-Q (2018). Lin28 signaling supports mammalian PNS and CNS axon regeneration. Cell Rep. 24, 2540–2552.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Watkins TA, Wang B, Huntwork-Rodriguez S, Yang J, Jiang Z, Eastham-Anderson J, Modrusan Z, Kaminker JS, Tessier-Lavigne M, and Lewcock JW (2013). DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc. Natl. Acad. Sci. U. S. A 110, 4039–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Welsbie DS, Yang Z, Ge Y, Mitchell KL, Zhou X, Martin SE, Berlinicke CA, Hackler L Jr, Fuller J, Fu J, et al. (2013). Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc. Natl. Acad. Sci. U. S. A 110, 4045–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Welsbie DS, Mitchell KL, Jaskula-Ranga V, Sluch VM, Yang Z, Kim J, Buehler E, Patel A, Martin SE, Zhang P-W, et al. (2017). Enhanced functional genomic screening identifies novel mediators of dual leucine zipper kinase-dependent injury signaling in neurons. Neuron 94, 1142–1154.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weng Y-L, An R, Cassin J, Joseph J, Mi R, Wang C, Zhong C, Jin S-G, Pfeifer GP, Bellacosa A, et al. (2017). An intrinsic epigenetic barrier for functional axon regeneration. Neuron 94, 337–346.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Williams PR, Marincu B-N, Sorbara CD, Mahler CF, Schumacher A-M, Griesbeck O, Kerschensteiner M, and Misgeld T (2014). A recoverable state of axon injury persists for hours after spinal cord contusion in vivo. Nat. Commun 5, 5683. [DOI] [PubMed] [Google Scholar]
- 141.Zhang Y, Williams PR, Jacobi A, Wang C, Goel A, Hirano AA, Brecha NC, Kerschensteiner D, and He Z (2019). Elevating Growth Factor Responsiveness and Axon Regeneration by Modulating Presynaptic Inputs. Neuron 103, 39–51.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang Z, Fujiki M, Guth L, and Steward O (1996). Genetic influences on cellular reactions to spinal cord injury: a wound-healing response present in normal mice is impaired in mice carrying a mutation (WldS) that causes delayed Wallerian degeneration. J. Comp. Neurol 371, 485–495. [DOI] [PubMed] [Google Scholar]
- 143.Zhou X, Wahane S, Friedl M-S, Kluge M, Friedel CC, Avrampou K, Zachariou V, Guo L, Zhang B, He X, et al. (2020). Microglia and macrophages promote corralling, wound compaction and recovery after spinal cord injury via Plexin-B2. Nat. Neurosci 23, 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]


