Abstract
Objective: Recent studies have utilized innovative techniques to investigate the neural mechanisms underlying social and individual decision-making, aiming to understand how individuals respond to the world.
Method : In this review, we summarized current scientific evidence concerning the neural underpinnings of social decision-making and their impact on social behavior.
Results: Critical brain regions involved in social cognition and decision-making are integral to the process of social decision-making. Notably, the medial prefrontal cortex (mPFC) and temporoparietal junction (TPJ) contribute to the comprehension of others' mental states. Similarly, the posterior superior temporal sulcus (pSTS) shows heightened activity when individuals observe faces and movements. On the lateral surface of the brain, the inferior frontal gyrus (IFG) and inferior parietal sulcus (IPS) play a role in social cognition. Furthermore, the medial surface of the brain, including the amygdala, anterior cingulate cortex (ACC), and anterior insula (AI), also participates in social cognition processes. Regarding decision-making, functional magnetic resonance imaging (fMRI) studies have illuminated the involvement of a network of brain regions, encompassing the ventromedial prefrontal cortex (vmPFC), ventral striatum (VS), and nucleus accumbens (NAcc).
Conclusion: Dysfunction in specific subregions of the prefrontal cortex (PFC) has been linked to various psychiatric conditions. These subregions play pivotal roles in cognitive, emotional, and social processing, and their impairment can contribute to the development and manifestation of psychiatric symptoms. A comprehensive understanding of the unique contributions of these PFC subregions to psychiatric disorders has the potential to inform the development of targeted interventions and treatments for affected individuals.
Key Words: Brain, Decision-Making, Neurophysiology, Social Cognition, Social Structure
The phenomenon of decision-making encompasses an intricate mechanism by which an organism engages in the meticulous exploration of a myriad of potential avenues, carefully assessing their value and promptly discarding those that fail to meet its multifarious requirements. This cognitive endeavor necessitates a nuanced evaluation of diverse alternatives, involving both tangible and intangible parameters, to ascertain the optimal course of action that optimally satisfies the organism's multifaceted needs, ultimately culminating in the selection of the most valuable option (1). It is recognized that decision makers adjust their strategies based on their direct personal experiences and/or observing others' experiences in order to improve their performance (2). These behavior modification or problem solving strategies can be explained by the reinforcement learning theory (including model-free and model-based reinforcement learning) (3) (see Figure 1).
Figure 1.
Model-Based Versus Model-Free Reinforcement Learning
Accordingly, the probability of selecting each option is regulated by a set of value functions that are adjusted according to the decision makers’ experience. Based on the model-free reinforcement learning theory, the decision makers adjust their ineffective strategies using direct inputs from personal experiences (4, 5). In the realm of model-based reinforcement learning, an important aspect is the ability to concurrently and adeptly modify value functions for numerous actions. This can be achieved by integrating the individual's inner model of the environment, empowering them to make informed decisions without having to explicitly encounter the consequences of every single action. At the core of this process lies decision making, encompassing the utilization of environment representations, expectations, and prospective computations. This amalgamation allows the decision maker to cognitively forecast a future value, paving the way for more strategic and sophisticated actions. Ultimately, this framework unlocks the potential for adaptive and dynamic decision making, tailored to the individual's unique perspective and goals within their environment (4).
As a social species, many of our decisions take place in a social context; hence, they are called “social decisions” (6). A significant distinction between social decisions and individual decisions can be found in the fact that social decisions are a reciprocal process between two or more agents and are dependent on the interactions of multiple decision-makers (7). As a result of this discriminative feature, the social decision-making process faces two challenges. In the first place, it is important to understand that the mental states of social agents (humans) are dynamic and may change over the course of the process of making decisions (7). The second one is that human beings have altruistic tendencies and their decisions are influenced by both self-regarding and other-regarding considerations. In terms of reinforcement learning theory, model-based learning is more common than model-free learning in social decision making. The decision makers in a social context should have other decision makers' preferences in their minds as they make their subsequent decisions to reach a desired consequence.
Recently, a number of researchers have employed novel methods to identify the neural basis of social and individual decision making in order to discover how one responds to the world around them. In the present study, we review the current evidence regarding the neural underpinnings of social decision making and how these are influencing social behavior.
Brain Regions Involved in Social Decision Making
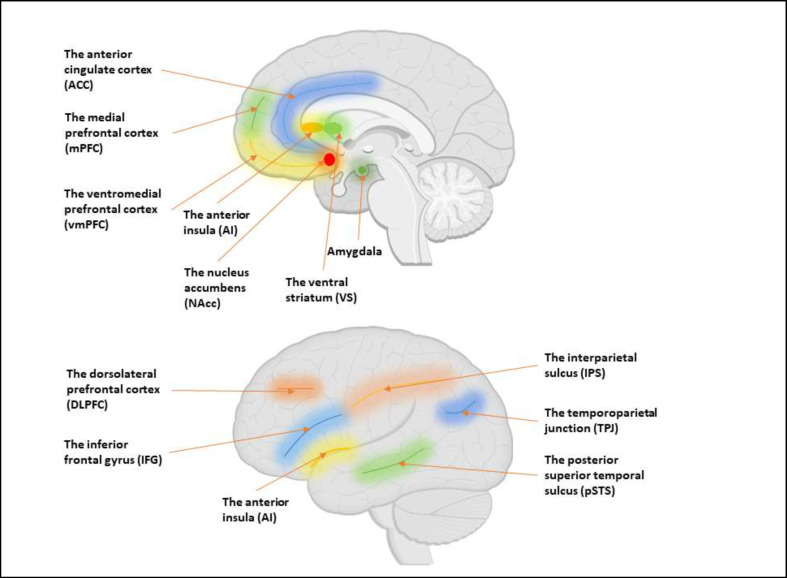
According to previous research, social decisions require the simultaneous involvement of both the social brain and the underlying structures involved in the decision-making process (8) (see Figure 2). The “social brain” network includes brain regions that are involved in social cognition. The social brain is commonly understood to encompass a network of brain regions that facilitate our ability to perceive and assess emotions, feelings, persistent traits, and actions of others. Moreover, it enables us to comprehend their intentions, desires, and beliefs (9-11).
Figure 2.
Brain Regions Involved in Social Decision-Making
Social Cognition
The primary and the most crucial elements of social interaction is the ability to express and interpret emotional cues from the face (12, 13). Thus, the first step in studying the social brain is to explore the brain regions involved in face recognition, which is an inherent ability of humans. It has been shown in a number of studies that the human brain responds differently to stimuli that are faces compared to those that are not (12, 14, 15). In the lateral occipitotemporal gyrus, there is a region called the “fusiform face area” (FFA), which is considerably more active in response to faces (16). Additionally, there are other regions that are involved in the processing of facial information, such as the occipital face area (OFA), superior temporal sulcus (STS), and the face-selective areas of the anterior temporal lobe (ATL) and prefrontal cortex (8, 17).
Other functions of the brain that are vital to social cognition include the ability to recognize facial expressions and emotions (18). A person's brain system for recognizing emotions varies depending on the type of stimuli, sensory modalities, as well as the characteristics of their face (18, 19). The lateral occipital cortex (LOC) and thalamus are especially involved when recognizing emotions from scenes, but when it comes to emotion recognition from faces, emotions are recognized by the fusiform face area (FFA) and middle temporal gyrus (MT); these two brain areas share many of the same structures, such as the amygdala, the medial prefrontal cortex (mPFC) and orbitofrontal cortex as well as the inferior temporal cortex and extrastriate occipital cortex (14).
Biological Motion Recognition
The biological motion entails recognizing the movements and actions of living beings. The pSTS is widely acknowledged as the principal brain region responsible for the recognition of biological motion. This region plays a crucial role in processing and interpreting visual information related to the movements of humans and animals (20). A recent study investigating the perception of emotional states through biological motion revealed an interconnected neural network composed of various brain regions. The identified regions can be categorized into two distinct networks: the action observation network (premotor cortex, inferior frontal gyrus, and inferior parietal lobule) and the mentalizing network (temporal-parietal junction, temporoparietal junction, dorsomedial prefrontal cortex, and lateral orbitofrontal cortex). Additionally, regions involved in processing body structure and movement, such as the extrastriate body area, fusiform body area, and posterior superior temporal sulcus, also contribute to this process. Notably, emotion-processing areas, such as the amygdala and hypothalamus, are activated by emotional body expressions, highlighting their important role in perceiving and interpreting emotional cues conveyed through biological motion (21).
Mentalizing
Mentalizing, which involves understanding the intentions, beliefs, and mental states of others, is a fundamental aspect of social cognition (22). Through multiple studies, researchers have identified key brain regions associated with mentalizing. The pSTS, temporal poles, and medial mPFC consistently exhibit increased activation during mentalizing tasks (23). The pSTS, responsible for processing biological motion, plays a crucial role in discerning the intricacies of others' mental realms (24). The temporal poles, involved in social perception and memory, contribute to understanding individuals' thoughts and emotions. Meanwhile, the mPFC, involved in self-referential thinking and perspective-taking, aids in comprehending the internal states of others in social interactions. Together, these brain regions form a coordinated network essential for navigating the complexities of social cognition and accurately interpreting the intentions and beliefs of others (25, 26).
Decision Making
The process of decision making, as mentioned earlier, is a goal-directed process that involves comparing various options as well as selecting the best option (from the point of view of the decision maker) based on previous personal experiences (direct learning) or acquired knowledge from others in order to reach a decision (observational learning) (1, 27).
Direct learning is characterized by reinforcement learning and the updating of reward prediction error as a teaching signal (28). Social learning, which replicates observed actions and values of others, has been primarily studied with single individuals, neglecting the collective influence of multiple social partners (29). There is a gap in research regarding the contribution of multiple social partners to social learning and the integration of their impact into existing models. Although direct learning is classified as a direct reward mechanism and social learning as a vicarious reward mechanism, neuroimaging studies have not provided a clear understanding of the brain networks involved. However, studies have demonstrated the involvement of brain areas like the vmPFC and ventral striatum in direct learning.
Social Decision-Making
Social decision-making involves various areas of the brain that are responsible for understanding and responding to social situations. The PFC is a crucial area involved in this process which helps evaluate social information and make decisions based on social norms, values, and expectations (30). The DLPFC is also essential in social decision-making. It is involved in higher-order cognitive processes, emotion regulation, and the integration of social and emotional information during decision-making (31). The DLPFC is responsible for planning and executing strategies, inhibiting impulsive responses, as well as assessing costs and benefits of social decisions. It helps differentiate between socially acceptable and unacceptable behaviors and understand others' thoughts and intentions. Disruptions in the DLPFC can lead to poor judgment and difficulties in considering social and emotional factors when making decisions in social situations (32).
The vmPFC is a critical brain region involved in social decision-making, emotional regulation, reward processing, and social cognition (33). It integrates emotional responses with cognitive processes and assesses the subjective value of social rewards and punishments. Damage or dysfunction in the vmPFC can impair decision-making in social situations and lead to deficits in empathy, moral reasoning, and social norm compliance (33). The vmPFC is also important in self-referential processing and self-related decision-making, helping us evaluate our own characteristics, values, and goals in relation to social interactions (34, 35).
The amygdala plays an essential role in social decision-making by processing emotions and determining the emotional significance of social cues (36). The ACC is involved in evaluating conflict, social pain, empathy, and moral judgments, allowing us to adjust our behavior based on social expectations (37). The insula integrates physical sensations with emotions and social experiences, facilitating empathy and decision-making based on others' feelings and perspectives (38). Other regions, including the TPJ and ventral striatum, contribute to understanding mental states and processing social rewards (39). Together, these interconnected brain regions enable us to evaluate social information, understand emotions and intentions, and make decisions that adhere to social norms. Dysfunction in or damage to these areas may impair empathy, judgment, and the ability to interpret social cues accurately.
Psychopathology
The PFC is a region in the frontal lobes of the brain that plays a crucial role in various cognitive and emotional processes. Several subregions of the PFC have been identified, each with distinct functions and connectivity patterns. In recent years, there has been growing interest in understanding how dysfunction in specific PFC subregions contribute to psychiatric disorders.
The mPFC is involved in self-referential processing and is disrupted in various psychiatric disorders such as major depressive disorder (MDD), bipolar disorder, and schizophrenia (40). Studies have shown reduced mPFC activity in individuals with depression and abnormalities in connectivity with other brain regions in anxiety disorders. The vmPFC plays a role in emotional regulation and decision-making, exhibiting dysfunction in disorders such as BPD and substance abuse (33). The DLPFC is important for executive functions and deficits in this area have been observed in ADHD (41) and schizophrenia (42). The TPJ is involved in social cognition and is dysfunctional in conditions such as ASD and schizophrenia, affecting understanding of others' mental states (43).
The pSTS is involved in processing social and emotional cues, and abnormal activity in this area is linked to autism and social anxiety disorder (44), while the IFG is responsible for language processing and is implicated in disorders like stuttering and aphasia (44). The IPS is involved in visuospatial processing and attentional control and displays dysfunction in disorders like neglect syndrome and developmental dyslexia. The ACC is important for emotion regulation and its abnormal functioning is observed in disorders like OCD and PTSD. The AI is involved in interoception and dysfunction is associated with somatization disorder and alexithymia. The VS and NAcc are part of the brain's reward circuitry and are implicated in addiction and depression due to dysregulation and reduced activation in these areas.
In conclusion, dysfunction in specific subregions of the PFC, including the mPFC, vmPFC, DLPFC, TPJ, pSTS, IFG, IPS, ACC, AI, VS, and NAcc, has been associated with various psychiatric disorders. These subregions play critical roles in cognitive, emotional, and social processing, and their dysfunction can contribute to the development and manifestation of psychiatric symptoms. Understanding the specific contributions of these PFC subregions to psychiatric disorders can potentially inform the development of targeted interventions and treatments for individuals affected by these conditions.
Limitation
The significant restriction of the study is that it does not follow a systematic review process, potentially resulting in the omission of pertinent information.
Conclusion
As mentioned earlier, the regions of the brain responsible for social cognition and decision-making processes play an essential role in social decision making. Certain brain areas, such as mPFC and TPJ, are involved in understanding others' mental states. Similarly, pSTS becomes active when observing faces and biological movements. On the lateral surface of the brain, the IFG and IPS contribute to social cognition. Additionally, the amygdala, ACC, and AI on the medial surface also play a part in social cognition processes (8). With respect to decision making, functional magnetic resonance imaging (fMRI) research has demonstrated the involvement of a network of brain regions, including the vmPFC as well as VS or NAcc (45). Dysfunction in specific sub-regions of the PFC has contributed to different psychiatric conditions. These subregions play critical roles in cognitive, emotional, and social processing, and their dysfunction can contribute to the development and manifestation of psychiatric symptoms. Understanding the specific contributions of these PFC subregions to psychiatric disorders has the potential to inform the development of targeted interventions and treatments for individuals affected by these conditions.
Acknowledgment
There is no financial support for this study.
Conflict of Interest
None.
References
- 1.Platt ML. Neural correlates of decisions. Curr Opin Neurobiol. 2002;12(2):141–8. doi: 10.1016/s0959-4388(02)00302-1. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Lengersdorff L, Mikus N, Gläscher J, Lamm C. Using reinforcement learning models in social neuroscience: frameworks, pitfalls and suggestions of best practices. Soc Cogn Affect Neurosci. 2020;15(6):695–707. doi: 10.1093/scan/nsaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chase HW, Kumar P, Eickhoff SB, Dombrovski AY. Reinforcement learning models and their neural correlates: An activation likelihood estimation meta-analysis. Cogn Affect Behav Neurosci. 2015;15(2):435–59. doi: 10.3758/s13415-015-0338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee D, Seo H, Jung MW. Neural basis of reinforcement learning and decision making. Annu Rev Neurosci. 2012;35:287–308. doi: 10.1146/annurev-neuro-062111-150512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins AGE, Shenhav A. Advances in modeling learning and decision-making in neuroscience. Neuropsychopharmacology. 2022;47(1):104–18. doi: 10.1038/s41386-021-01126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rilling JK, King-Casas B, Sanfey AG. The neurobiology of social decision-making. Curr Opin Neurobiol. 2008;18(2):159–65. doi: 10.1016/j.conb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Báez-Mendoza R, Vázquez Y, Mastrobattista EP, Williams ZM. Neuronal Circuits for Social Decision-Making and Their Clinical Implications. Front Neurosci. 2021;15:720294. doi: 10.3389/fnins.2021.720294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9(4):267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- 9.Gur RC, Gur RE. Social cognition as an RDoC domain. Am J Med Genet B Neuropsychiatr Genet. 2016;171b(1):132–41. doi: 10.1002/ajmg.b.32394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eslinger PJ, Anders S, Ballarini T, Boutros S, Krach S, Mayer AV, et al. The neuroscience of social feelings: mechanisms of adaptive social functioning. Neurosci Biobehav Rev. 2021;128:592–620. doi: 10.1016/j.neubiorev.2021.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry A, Raucher-Chéné D, Obert A, Gobin P, Vucurovic K, Barrière S, et al. Investigation of the neural correlates of mentalizing through the Dynamic Inference Task, a new naturalistic task of social cognition. Neuroimage. 2021;243:118499. doi: 10.1016/j.neuroimage.2021.118499. [DOI] [PubMed] [Google Scholar]
- 12.Xu P, Peng S, Luo YJ, Gong G. Facial expression recognition: A meta-analytic review of theoretical models and neuroimaging evidence. Neurosci Biobehav Rev. 2021;127:820–36. doi: 10.1016/j.neubiorev.2021.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Besag FMC, Vasey MJ. Social cognition and psychopathology in childhood and adolescence. Epilepsy Behav. 2019;100(Pt B):106210. doi: 10.1016/j.yebeh.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Grüter T, Grüter M, Carbon CC. Neural and genetic foundations of face recognition and prosopagnosia. J Neuropsychol. 2008;2(1):79–97. doi: 10.1348/174866407x231001. [DOI] [PubMed] [Google Scholar]
- 15.Scott LS, Arcaro MJ. A domain-relevant framework for the development of face processing. Nat Rev Psychol. 2023;2(3):183–95. [Google Scholar]
- 16.Burns EJ, Arnold T, Bukach CM. P-curving the fusiform face area: Meta-analyses support the expertise hypothesis. Neurosci Biobehav Rev. 2019;104:209–21. doi: 10.1016/j.neubiorev.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Freiwald WA. The neural mechanisms of face processing: cells, areas, networks, and models. Curr Opin Neurobiol. 2020;60:184–91. doi: 10.1016/j.conb.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anzellotti S, Caramazza A. The neural mechanisms for the recognition of face identity in humans. Front Psychol. 2014;5:672. doi: 10.3389/fpsyg.2014.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopatina OL, Komleva YK, Gorina YV, Higashida H, Salmina AB. Neurobiological Aspects of Face Recognition: The Role of Oxytocin. Front Behav Neurosci. 2018;12:195. doi: 10.3389/fnbeh.2018.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirai M, Senju A. The two-process theory of biological motion processing. Neurosci Biobehav Rev. 2020;111:114–24. doi: 10.1016/j.neubiorev.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Bachmann J, Munzert J, Krüger B. Neural Underpinnings of the Perception of Emotional States Derived From Biological Human Motion: A Review of Neuroimaging Research. Front Psychol. 2018;9:1763. doi: 10.3389/fpsyg.2018.01763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arioli M, Cattaneo Z, Ricciardi E, Canessa N. Overlapping and specific neural correlates for empathizing, affective mentalizing, and cognitive mentalizing: A coordinate-based meta-analytic study. Hum Brain Mapp. 2021;42(14):4777–804. doi: 10.1002/hbm.25570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luyten P, Fonagy P. The neurobiology of mentalizing. Personal Disord. 2015;6(4):366–79. doi: 10.1037/per0000117. [DOI] [PubMed] [Google Scholar]
- 24.Federici A, Parma V, Vicovaro M, Radassao L, Casartelli L, Ronconi L. Anomalous Perception of Biological Motion in Autism: A Conceptual Review and Meta-Analysis. Sci Rep. 2020;10(1):4576. doi: 10.1038/s41598-020-61252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schurz M, Radua J, Aichhorn M, Richlan F, Perner J. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci Biobehav Rev. 2014;42:9–34. doi: 10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Schurz M, Radua J, Tholen MG, Maliske L, Margulies DS, Mars RB, et al. Toward a hierarchical model of social cognition: A neuroimaging meta-analysis and integrative review of empathy and theory of mind. Psychol Bull. 2021;147(3):293–327. doi: 10.1037/bul0000303. [DOI] [PubMed] [Google Scholar]
- 27.Carcea I, Froemke RC. Biological mechanisms for observational learning. Curr Opin Neurobiol. 2019;54:178–85. doi: 10.1016/j.conb.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook TD, Goslin J. Principal components analysis of reward prediction errors in a reinforcement learning task. Neuroimage. 2016;124(Pt A):276–86. doi: 10.1016/j.neuroimage.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 29.Olsson A, Knapska E, Lindström B. The neural and computational systems of social learning. Nat Rev Neurosci. 2020;21(4):197–212. doi: 10.1038/s41583-020-0276-4. [DOI] [PubMed] [Google Scholar]
- 30.Tremblay S, Sharika KM, Platt ML. Social Decision-Making and the Brain: A Comparative Perspective. Trends Cogn Sci. 2017;21(4):265–76. doi: 10.1016/j.tics.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoder KJ, Decety J. The neuroscience of morality and social decision-making. Psychol Crime Law. 2018;24(3):279–95. doi: 10.1080/1068316X.2017.1414817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang D, Chen S, Wang S, Shi J, Ye H, Luo J, et al. Activation of the DLPFC Reveals an Asymmetric Effect in Risky Decision Making: Evidence from a tDCS Study. Front Psychol. 2017;8:38. doi: 10.3389/fpsyg.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiser J, Koenigs M. The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biol Psychiatry. 2018;83(8):638–47. doi: 10.1016/j.biopsych.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park HY, Park K, Seo E, Koo SJ, Bang M, Park JY, et al. Reduced activation of the ventromedial prefrontal cortex during self-referential processing in individuals at ultra-high risk for psychosis. Aust N Z J Psychiatry. 2020;54(5):528–38. doi: 10.1177/0004867419898529. [DOI] [PubMed] [Google Scholar]
- 35.Herold D, Spengler S, Sajonz B, Usnich T, Bermpohl F. Common and distinct networks for self-referential and social stimulus processing in the human brain. Brain Struct Funct. 2016;221(7):3475–85. doi: 10.1007/s00429-015-1113-9. [DOI] [PubMed] [Google Scholar]
- 36.Gothard KM. Multidimensional processing in the amygdala. Nat Rev Neurosci. 2020;21(10):565–75. doi: 10.1038/s41583-020-0350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalgleish T, Walsh ND, Mobbs D, Schweizer S, van Harmelen AL, Dunn B, et al. Social pain and social gain in the adolescent brain: A common neural circuitry underlying both positive and negative social evaluation. Sci Rep. 2017;7:42010. doi: 10.1038/srep42010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim A, Okuno HG. A recipe for empathy: Integrating the mirror system, insula, somatosensory cortex and motherese. Int J Soc Robot. 2015;7:35–49. [Google Scholar]
- 39.Flores LE Jr, Eckstrand KL, Silk JS, Allen NB, Ambrosia M, Healey KL, et al. Adolescents' neural response to social reward and real-world emotional closeness and positive affect. Cogn Affect Behav Neurosci. 2018;18(4):705–17. doi: 10.3758/s13415-018-0598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belzung C, Willner P, Philippot P. Depression: from psychopathology to pathophysiology. Curr Opin Neurobiol. 2015;30:24–30. doi: 10.1016/j.conb.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 41.Gu Y, Miao S, Han J, Zeng K, Ouyang G, Yang J, et al. Complexity analysis of fNIRS signals in ADHD children during working memory task. Sci Rep. 2017;7(1):829. doi: 10.1038/s41598-017-00965-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birnbaum R, Jaffe AE, Chen Q, Shin JH, Kleinman JE, Hyde TM, et al. Investigating the neuroimmunogenic architecture of schizophrenia. Mol Psychiatry. 2018;23(5):1251–60. doi: 10.1038/mp.2017.89. [DOI] [PubMed] [Google Scholar]
- 43.Quesque F, Brass M. The Role of the Temporoparietal Junction in Self-Other Distinction. Brain Topogr. 2019;32(6):943–55. doi: 10.1007/s10548-019-00737-5. [DOI] [PubMed] [Google Scholar]
- 44.Babinet MN, Cublier M, Demily C, Michael GA. Eye Direction Detection and Perception as Premises of a Social Brain: A Narrative Review of Behavioral and Neural Data. Cogn Affect Behav Neurosci. 2022;22(1):1–20. doi: 10.3758/s13415-021-00959-w. [DOI] [PubMed] [Google Scholar]
- 45.Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–74. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]