Summary
The phasic cardiovascular activity influences the central nervous system through the systolic baroreceptor inputs, inducing widespread inhibitory effects on behavior. Through transcranial magnetic stimulation (TMS) delivered during resting-state over the left primary motor cortex and across the different cardiac phases, we measured corticospinal excitability (CSE) and distinct indices of intracortical motor inhibition: short (SICI) and long (LICI) interval, corresponding to GABAA and GABAB neurotransmission, respectively. We found a significant effect of the cardiac phase on short-intracortical inhibition, without any influence on LICI. Specifically, SICI was stronger at systole compared to diastole. These results show a tight relationship between the cardiac cycle and the inhibitory neurotransmission within M1, and in particular with GABAA-ergic-mediated motor inhibition. We hypothesize that this process requires greater motor control via the gating mechanism and that this, in turn, needs to be recalibrated through the modulation of intracortical inhibition.
Subject areas: Biological sciences, Clinical neuroscience, Natural sciences, Neuroscience
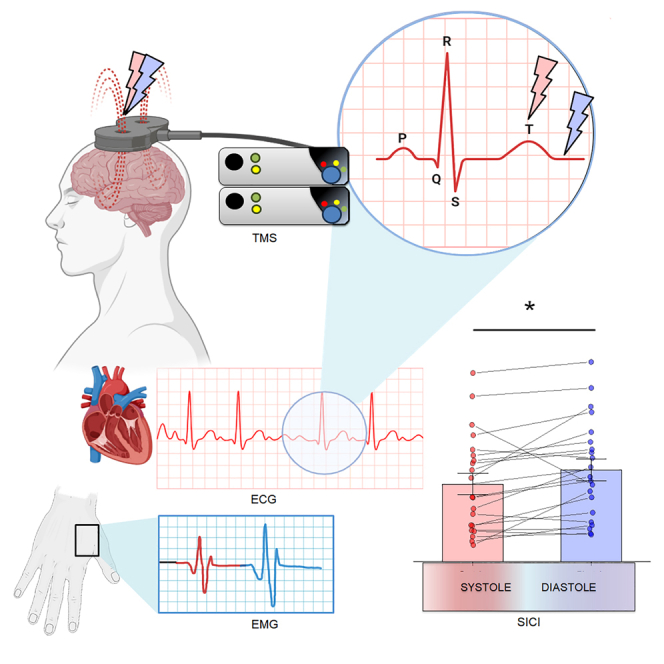
Graphical abstract
Highlights
-
•
TMS-ECG study to measure how the cardiac cycle affects the GABA of the motor cortex
-
•
Cardiovascular activity affects intracortical motor inhibition
-
•
Short-interval intracortical inhibition is stronger at systole than at diastole
-
•
GABAA-ergic-mediated motor inhibition is stronger at systole than at diastole
Introduction
The processing of incoming stimuli in the brain’s cortex is not isolated, but rather influenced by the ongoing activity within the same neural substrate.1,2 This phenomenon, known as state dependency, has been demonstrated in the visual stream, where inhibitory alpha oscillations play a role in limiting and prioritizing the neuronal processing of sensory representations in complex visual scenes.3,4,5,6 Furthermore, state dependency has also been observed in the motor system, where motor activity can influence sensory integration7 and action observation.8
This state dependency effect can be influenced, as previously pointed out, by both top-down factors9,10 such as brain oscillations or neurotransmitter modulation, and bottom-up factors11 such as interoception.12 In fact, fluctuations in the internal physiological state of the body can have a widespread impact on the central nervous system.13
One example of this is the influence of cardiovascular activity on cognition and behavior mediated by variations in baroreceptor inputs to brainstem nuclei.14 Sensory events occurring at different phases of the cardiac cycle, specifically systole (approximately 250/300 ms after the cardiac R-peak) and diastole (approximately 500 ms after the R-peak), have been found to be processed differently.15,16,17,18
Research has demonstrated that systolic baroreceptor activity particularly affects sensorimotor functions, exerting inhibitory effects on behavior.19 This can be attributed to the stronger and longer-lasting inhibitory effects produced by the phasic firing of arterial baroreceptors during systole compared to diastole.
These variations in phasic interoceptive signals driven by the cardiac cycle can even influence motor actions. For instance, reaction times have been observed to be slower during systole compared to diastole in early reports,20,21,22 but see the study by Salzman and Jaques.23 Consistently, linear decreases in RTs relative to the R-peak were reported in later studies24,25,26 and in more recent detailed investigations.27,28,29 However, the scenario is far complex as perception and action might be dynamically influenced by visceral drives according to recent perspectives,30 questioning the relevance of simple reaction times as indices of cardiac cycle effects on motor domain. On this matter, some inconsistencies have arisen studying the behavioral correlates of active and passive action tendencies.31,32 Interestingly, there is evidence to believe that the heart cycle might have an impact on motor control, as response inhibition efficiency seems to significantly improve when stop cues are presented at systole during the stop signal task (SST).33 These results support the hypothesis that the physiological fluctuations in the baroreceptor inputs conveyed to the brainstem nuclei and driven by phasic cardiovascular activity could have an impact on motor control and reactive inhibition. Specifically, the increase in the state of physiological arousal which occurs at systole seems to improve the performance on the SST. This task is used to estimate individuals’ ability to suppress a response already initiated, that is, the time required to inhibit a response, namely the stop signal reaction time (SSRT; shorter SSRT = better inhibition). Crucially, the inhibitory processes underlying the SST seem to be mainly modulated by the circuits of short-interval intracortical inhibition (SICI).34,35,36,37,38,39,40 SICI is elicited by pairing a subthreshold conditioning stimulus (CS) with a suprathreshold test stimulus (TS) delivered after 1–6 ms and it is thought to reflect fast-acting inhibitory postsynaptic potentials mainly mediated by GABAA neurotransmission. A significant increase in SICI has been measured online during stop trials compared to go trials, and it has been even hypothesized that the trait-like individual differences in the neurophysiological markers of intracortical inhibition (and in SICI in particular) might predict behavioral motor inhibition capacities,37,41 as supported by several negative correlations reported between SICI, measured at rest, and SSRT.35,38,42
However, despite the frequent aforementioned behavioral associations between the cardiac cycle and motor reactivity, no studies have yet investigated whether the heart cycle has an impact on the motor system by means of the GABAergic neurotransmitter modulation. To date, these cardiac-behavioral associations do not seem to have a neurophysiological counterpart, at least with regard to corticospinal excitability (CSE). In fact, converging studies reported no interactions between the cardiac cycle and CSE43,44,45,46 (see also the study by Al et al.47). Thus, we decided to focus not only on the excitability but also, and foremost on the neurophysiological markers of motor inhibition, measuring these across the different phases of cardiovascular activity. We here addressed the neurophysiological counterparts of this cardiac-behavioral association by investigating the relationship between the cardiac cycle and intracortical inhibition within the primary motor cortex (M1) by measuring SICI, long-interval intracortical inhibition (LICI), and motor evoked potential (MEP) elicited at systole and at diastole, respectively. While the amplitude of the MEPs provides an instantaneous readout of the state of the motor system by CSE,48,49 SICI and LICI consist in a reduction of CSE modulated by different GABAergic neurotransmission. LICI, differently from SICI that recruits faster GABAA receptors,50,51,52 is thought to reflect slower inhibitory postsynaptic potentials within M1 mainly mediated by the activity of GABAB receptors, and it is elicited by pairing a suprathreshold CS with a second suprathreshold TS delivered after 100 ms.51,53,54,55,56
Specifically, since SICI and LICI were both acquired through paired-pulses transcranial magnetic stimulation (TMS) procedures, we locked the respective CS either at systole or diastole, 250 and 500 ms after the R-peak of the electrocardiogram. MEP amplitudes were measured from the CS of LICI for each cardiac phase. The relative TS was delivered 3 (SICI) and 100 ms (LICI) after the CS. In this way, both SICI and LICI were evoked precisely at either systole or diastole, so as to test the hypothesis that GABAergic neurotransmitter modulation, and in particular fast-acting inhibitory postsynaptic potentials GABAA neurotransmission, might be affected by the physiological fluctuations of the baroreceptor inputs induced by phasic cardiovascular activity.
Results
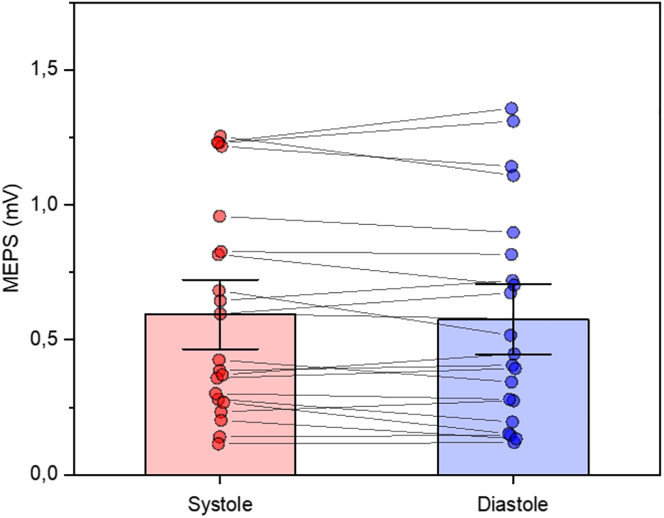
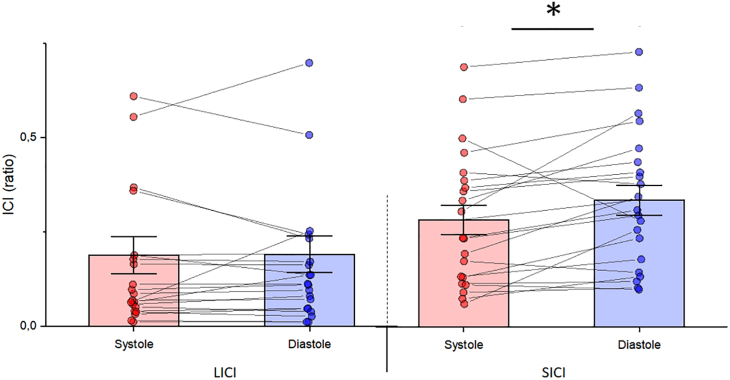
One participant (M) was excluded from the data analysis since heart rate (HR) was higher than 100 bpm.44 Overall, the average HR was 72 bpm, while the average resting motor threshold was 45% of the maximum stimulator output (ranging from 39% to 55%, SD = 5%). Mean MEP amplitude was 0.59 mV (SD = 0.39) at systole and 0.58 mV (SD = 0.40) at diastole. Paired sample t tests showed no difference in MEP amplitude between systole and diastole (t(20) = −1.04, p = 0.31 Cohen’s d = 0.22). The MEP data are shown in Figure 1. Mean SICI ratio was 0.28 (SD = 0.18) at systole and 0.34 (SD = 0.18) at diastole. Paired sample t tests showed a significant difference in SICI between systole and diastole (t(20) = −2.52, p = 0.02, Cohen’s d = 0.55). Mean LICI ratio was 0.19 (SD = 0.15) at systole and 0.19 (SD = 0.17) at diastole. Paired sample t tests showed no significant differences in LICI (t(20) = −0.15, p = 0.88 Cohen’s d = 0.03). SICI and LICI data are shown in Figure 2.
Figure 1.
Results of MEPs with t test analyses
Group MEPs as a function of cardiac phase with individual data points. Data are represented as mean ± SEM.
Figure 2.
Results of intracortical inhibition with t test analyses
Average LICI (left side) and SICI (right side) as a function of cardiac phase, with individual data points. Lower values indicate stronger LICI or SICI. Data are represented as mean ± SEM and asterisks show significant differences (p < 0.05).
Discussion
The present study was designed to study how an internal physiological body state could interact with the brain at rest.
Specifically, we were interested in determining whether the neurophysiological pattern of GABAergic-mediated intracortical inhibition within M1 varies across the different phases of the cardiac cycle. We here addressed this experimental question by measuring CSE, SICI, and LICI at systole and diastole, following the hypothesis that SICI could be stronger at systole rather than at diastole. This hypothesis is supported by the association between the strength of SICI and the efficiency of reactive response inhibition34,35,36,37,38 and the improvement of this behavioral inhibition during the systolic phase.33
In line with these converging pieces of evidence, our results showed a significant difference in SICI evoked across the two cardiac phases. Specifically, we observed stronger SICI during systole compared to diastole, which supports the hypothesis that the cardiac cycle might influence the neurophysiological pattern of intracortical inhibition within M1. These results imply that cardiac interoceptive signals might influence motor cortex inhibition and, crucially, the effect seems to be specific for SICI only, namely for fast-acting GABAA-ergic-mediated motor inhibition. Conversely, there was no significant difference in LICI measured at systole and diastole.
On a physiological level, two separate receptor types play a role in GABAergic inhibition, namely GABAA and GABAB. These receptors are responsible for enabling different form of inhibition57 since GABAA is acting through ionotropic mechanism and GABAB is a metabotropic receptor, leading respectively to a fast and powerful synaptic inhibition in contrast to a slow and mild modulation of neuronal function.58,59,60
It is widely accepted that SICI is primarily mediated by type A receptors52,61,62 and conversely, that LICI most likely occurs through neurotransmission via the GABAB receptor.63 Given this, our results suggest that cardiac-related signals interact selectively with a specific inhibitory mechanism (i.e., GABAA-mediated) which is associated with the phasic shaping of neuronal firing within a brief interval (e.g., systole). This could be plausible as it appears that SICI can be rapidly downregulated (activation phase) or upregulated (deactivation phase) depending on the movement phase, differently from LICI which does not exhibit significant modulation between rest and activation, nor between the activation and deactivation phases of the movement.64,65 Therefore, it could be supposed that heart-driven activity tunes motor cortical excitability by inhibition, carried by the most feasible substrate to achieve fast modulations (i.e., GABAA). We cannot, however, exclude that the influence of visceral signaling may be circumscribed solely on type-A receptors as they are more sensitive to GABA fluctuations relative to type-B receptors, which requires a higher concentration or a longer exposure to GABA.66
Moreover, our findings could be linked to motor-related beta oscillations67,68 as this rhythm in M1 has been associated with drives of GABAergic modulation.69,70,71 Crucially, it was found that movement-related beta desynchronizations, which are electroencephalogram premotor correlates, are coupled with GABAA modulation.72 Hence, it seems reasonable that we traced a specific effect on a GABAA readout measure (i.e., SICI), since the same receptor system may be functional for the modulation of the rhythms associated with motor control.
Our study also found the amplitude of MEPs to be consistent across different phases of the cardiac cycle. This observation aligns with previous literature43,44,45,46 and underscores the non-variability of MEP amplitude in relation to cardiac dynamics. Conversely, findings reported in another study47 diverge from our own. This variance could likely be ascribed to differences in the experimental methodology. Specifically, the timing of TMS in relation to the cardiac cycle is a critical variable that might complicate the direct comparison of outcomes. In our research, to preclude ambiguous interpretations, we intentionally administered TMS pulses outside the time window immediately adjacent to the R-peak. This approach was taken to ensure a clear delineation of the systolic phase. We based our definition on a time frame that correlates with the processing of central baroreceptors, a decision supported by a range of psychophysiological studies.73,74,75,76 Moreover, the potential for lateralization effects to contribute to the observed differences cannot be entirely dismissed.77 The pattern of corticospinal activation we documented, which did not differ between systole and diastole, indicates that the cardiac-related modulation of the SICI ratio is a specific effect, rather than a byproduct of altered CSE.
A multitude of converging results support the hypothesis that a stronger intracortical inhibition predicts higher motor control.78 Specifically, a lower SICI ratio is associated with a better inhibitory performance during the SST.36 Moreover, this heightened modulation of SICI during systole, as opposed to the diastole, aligns with the observed improvement in reactive motor inhibition performance when stop cues are presented during systole.33 With regard to behavior, it is worth mentioning that other authors reported how the inhibitory performance could not be impacted,79 or even impaired,80 depending on cardiac activity. The discrepancies among results may be related to the different types of tasks or stimuli used, possibly recruiting complementary mechanisms such as conflict resolution or deliberate inhibition. In fact, behavioral suppression is achieved by a series of subprocesses, encompassing either low-level perceptual processes (e.g., signal detection) or high-level cognitive control functions.81
In some circumstances, the actual effect of the cardiac cycle on response inhibition might be overshadowed by the presence of other subprocesses. This view aligns with the notion that the heart-dependent effects are moderate and susceptible to additional cognitive processes.80 It also highlights the necessity for further investigation on which inhibitory mechanisms are specifically influenced by cardiac contractions signals, since reactive33 and volitional79 motor control appear to be differently affected. Suitable attempts should assess distinct subprocesses related to motor control, matching as much as possible stimuli characteristics and task demands to avoid any bias of intervening processes.
In the present study, we can primarily interpret the results from a neurophysiological perspective that correspond with the aim of the work, proposing a GABA substrate accountable for the heart-driven SICI modulation. This in turn hints to a plausible behavioral scenario related to higher motor inhibition33,36,78 that should be carefully examined in future research.
Lastly, the neural modulation found here supports the principles of the gating-by-inhibition hypothesis.5,82 Originally observed in the visual system, this phenomenon is believed to improve spatial and temporal discrimination of various sensory inputs in the visual system.83 While initially proposed in relation to phase-coding and the organization of sequential sensory processing using alpha oscillations,3,4,5,6 the same working mechanism could be applicable to the motor system. According to this mechanism, inhibitory oscillations restrict and prioritize neuronal processing. At oscillatory peaks, inhibition effectively suppresses neuronal firing, preventing activation. During systole, the blood is ejected from the two ventricles, flowing into both the pulmonic and aortic circulation systems. Based on our findings, we hypothesize that this process requires greater motor control via the gating mechanism and that this, in turn, needs to be recalibrated through the modulation of intracortical inhibition. This recalibration serves two potential purposes: first, to prevent undesired muscle activations,84 and second, to enhance the differentiation between activity and rest, or more precisely, between engaged muscles and muscles at rest.61,85
Overall, these results show that cardiac interoceptive signals influence motor cortex inhibition and that this effect is specific for SICI, an index of GABAA-ergic-mediated motor inhibition, supporting the hypothesis that there might be a tight relationship between the phases of the cardiac cycle and the inhibitory neurotransmission within M1. This suggests that at systole, stronger physiological arousal signals prompt faster and more efficient responses to stop cues. This is in line not only with an increased likelihood of detecting and responding to salient events but also, at the neural level, with a stronger reactive SICI-mediated inhibition. To date, the present study has been the first one to show that the cardiac phasic interoceptive signals might influence not only behavior and cognition but also modulate intracortical inhibition within M1.
Limitations of the study
In this study, we investigated how the cardiovascular activity influences the central nervous system through intracortical-inhibition modulation. We delivered TMS pulse at fixed time points (250 and 500 ms) following the R-peak. Although these time windows correspond, respectively, to the time phases of systole and diastole, we cannot rule out any temporal variability that may have introduced noise into our results. Additionally, the scope of the present study could be extended by integrating an appropriate behavioral task into its design, to root this cardiac-driven neurophysiological effect into more functional and operative interpretations. Future studies will hopefully address these limitations.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Raw data | This paper | Reserved https://doi.org/10.17632/csb2w8f53m.1https://data.mendeley.com/drafts/csb2w8f53m |
| Software and algorithms | ||
| Psychtoolbox (version 3.0.14) | Psychophysics Toolbox | http://psychtoolbox.org/ RRID: SCR_002881 |
| Signal software | Cambridge Electronic Design Limited | http://ced.co.uk/products/sigovin RRID:SCR_017081 |
| MATLAB R2018a | MathWorks | https://www.mathworks.com/ RRID:SCR_001622 |
| Statistica | Statsoft | http://www.statsoft.com/Products/STATISTICA/Product-Index RRID:SCR_014213 |
| Other | ||
| Figure-of-eight TMS coil (7 cm diameter) | Magstim | https://www.magstim.com |
| Magstim 2002 stimulator | Magstim | https://www.magstim.com |
| CED power1401 | Cambridge Electronic Design Limited | http://ced.co.uk/ RRID:SCR_017282 |
| Biopac MP160 | Biopac Systems Inc | https://www.biopac.com RRID:SCR_014829 |
| DTU100 Digital Trigger Unit | Biopac Systems Inc | https://www.biopac.com RRID:SCR_014829 |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Mario Paci (mario.paci@unich.it).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Original data have been deposited at Mendeley Data and are publicly available as of the date of publication. The accession numbers or URL for the datasets are listed in the key resources table.
This paper does not report the original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Participants
We recruited 21 white/Caucasian right-handed participants (6 males, mean age 25.9 ± 3.2) with no history of cardiovascular or neuropsychiatric diseases, and no other contraindications to TMS,86 as self-reported. Handedness was assessed with the Edinburgh Handedness Inventory.87 Participants were informed about the experimental procedure and provided their written consent before taking part in the study. The study took place at ITAB (Institute for Advanced Biomedical Technologies) in Chieti. The number of participants was in line with previous works showing how individual differences in motor cortex inhibition measured at rest can predict motor inhibition performance.35,38 The study was approved by the Ethics Committee of the Department of Psychological Sciences, Health and Territory, University G. d’Annunzio, Chieti-Pescara and was conducted in accordance with the ethical standards of the last update of the Helsinki Declaration (2013).
Method details
ECG and EMG recordings
The electrocardiogram (ECG) was recorded using three disposable ECG surface electrodes set according to the standard lead II configuration and connected to a Biopac MP160 System (BIOPAC Systems, Inc., Goleta, CA, USA) supplied with a DTU100 Digital Trigger Unit (BIOPAC Systems, Inc., Goleta, CA, USA) module. Surface EMG activity from the right First Dorsal Interosseous (FDI) muscle was recorded using three disposable EMG electrodes placed in a belly-tendon montage and connected to a CED Micro 1401 (Cambridge Electronic Design, Cambridge, UK). ECG raw signals were amplified and band-pass filtered (0.5-40 Hz) while EMG raw signals were amplified (1000x), digitized at a sampling rate of 8 kHz, and filtered using an analogical online band-pass (20-250 Hz) and a 50 Hz notch filter. Both ECG and EMG recorded throughout the TMS procedure were stored for off-line analyses performed with AcqKnowledge software program (BIOPAC Systems, Inc., Goleta, CA, USA) and Signal 6.04 software (Cambridge Electronic Design, Cambridge, UK), respectively.
TMS protocol
Paired-pulse TMS was delivered over the left primary motor cortex using a 70 mm figure-of-eight coil with poster-anterior orientation connected to two 2002 monophasic Magstim stimulators (Magstim, Whitland, UK) integrated into a BiStim2 module. The coil was positioned tangentially to the scalp following the orthodox method,88,89 with the handle pointed backward, angled 45 degrees from the midline, perpendicular to the central sulcus. The hotspot of the right FDI muscle was identified by manoeuvring the coil around the left M1 hand area in steps of 0.5 cm until eliciting the maximum amplitude motor-evoked potentials (MEPs) using slightly suprathreshold stimuli. For each subject, FDI hotspot was marked on a head cap to maximize coil placement consistency through the testing sessions.
According to conventional criteria, individuals’ resting motor threshold (rMT) was identified by gradually adjusting the intensity of the stimulator until reaching the minimum percentage of the maximum stimulator output necessary to elicit consistent MEPs with a peak-to-peak amplitude > 50 μV during muscle relaxation in 5 out of 10 consecutive stimuli.90,91
For each participant, rMT was used to determine the specific intensity of TMS subthreshold and suprathreshold stimulations, which were set at 80% and 120% of this individual value, respectively. Specifically, the subthreshold conditioning stimuli of SICI was set at an intensity of 80% rMT, a percentage which has been confirmed not to induce MEPs.52,90 The intensity of the suprathreshold stimuli was set to 120% of rMT, a percentage that is considered appropriate for the SICI and LICI.55,92,93 The combinations of these different levels of stimulation intensity paired at the appropriate interstimulus interval (ISI) are considered appropriate to measure SICI (CS = 80% rMT, ISI = 3 ms, TS = 120% rMT) and LICI (CS = 120% rMT, ISI = 100 ms, TS = 120% rMT), respectively.94,95,96
TMS pulses were synchronized with the ECG signal using the cardiac R-peak as a digital trigger, to lock the CS at either systole or diastole (250 and 500 ms after the R-peak, respectively). This was implemented through a custom-made E-Prime script capable to integrate the signal received from the DTU100 module of the BIOPAC MP160 System with a Trigger Station™ (BrainTrends Ltd., Rome, Italy) used to trigger the TMS. SICI and LICI were measured across two distinct blocks of 50 stimulations each (100 total paired-pulses); CS were randomly locked at either systole or diastole, 250 or 500 ms after the cardiac R-peak, for a total of 25 SICI and 25 LICI for each cardiac phase. The resulting number of repetitions was considered optimal to obtain a reliable measure of ICI.97 Paired-pulses TMS stimulations were delivered with an interval jittered between 8 and 13 seconds in order to avoid any habituation or hysteresis effect. In each block of stimulation (i.e., SICI and LICI) the coil was controlled to adhere on the marked position of the cap. In order to reduce coil movements from the marked hotspot, coil position was fixed by a mechanical support and was continuously monitored by the experimenter. The whole procedure was performed while participants were resting, looking at a fixation cross. None of the participants reported any negative side effects during or after the TMS procedure.
Quantification and statistical analysis
Data pre-processing and analysis
MEPs were calculated as peak-to-peak amplitudes in a time window of 18 to 35 ms after the TMS pulse. Trials were rejected if during the acquisition stage any pronounced head movement occurred before or during the stimulation. Moreover, for each TMS-evoked parameter (MEP; SICI, LICI) trials were excluded from data analysis if any of the following conditions were not met: (1) root mean squared of the background EMG activity in the window of -50 to -5 ms prior the conditioning stimulus within mean ± 2 SD of all stimuli; (2) TMS-evoked activity not larger nor smaller than Mean ± 2.5 SD of all stimuli of the respective corresponding evoked activity.98 MEPs (in mV) were calculated as peak-to-peak amplitudes from the CS of LICI, within a time window from 20 to 35 ms after the TMS pulse, and averaged for each cardiac phase.55,93 SICI was calculated as the ratio between the mean TS of SICI (i.e., conditioned MEP) averaged for each cardiac phase and the respective averaged control MEP (i.e., CS of LICI) matching the cardiac phase, so as the lowest the value of this ratio, the higher the corresponding mediated inhibition in that condition. Conversely, LICI was calculated for each trial as the ratio between the TS and the respective CS and then averaged for each cardiac phase. Preliminary inspection, analysis, and offline extraction were all carried out using Signal 6.04 software (Cambridge Electronic Design, Cambridge, UK). Three independent paired two-tailed t-tests were performed to compare differences in MEP, SICI, and LICI between systole and diastole.
Acknowledgments
This work was supported by the “Departments of Excellence 2018–2022 and 2023–2027” initiative of the Italian Ministry of Education, University and Research for the Department of Neuroscience, Imaging and Clinical Sciences (DNISC) of the University of Chieti-Pescara, and by the “Search for Excellence” initiative of the University of Chieti-Pescara. This research was also supported by PNRR Project “Boost for Interdisciplinarity” (“NextGenerationEU”, “MIUR-Fondo Promozione e Sviluppo - DM 737/2021”, INTRIGUE).
Author contributions
M.P., F.F., and M.C. conceived the study. M.P., P.C., and M.G.P. developed and implemented the experimental setup. M.P. performed the experiment. P.C. and M.G.P. provided technical support during the experiment. M.P., P.C., and M.C. performed data analysis. M.P., P.C., P.D.L., and M.C. wrote the manuscript. P.C. and P.D.L. provided technical consultation for the revision of the manuscript. All authors drafted and approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: February 5, 2024
Contributor Information
Pasquale Cardellicchio, Email: pasquale.cardellicchio@gaslini.org.
Marcello Costantini, Email: marcello.costantini@unich.it.
References
- 1.Silvanto J., Pascual-Leone A. State-Dependency of Transcranial Magnetic Stimulation. Brain Topogr. 2008;21:1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silvanto J., Muggleton N., Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn. Sci. 2008;12:447–454. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Cecere R., Rees G., Romei V. Individual differences in alpha frequency drive crossmodal illusory perception. Curr. Biol. 2015;25:231–235. doi: 10.1016/j.cub.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coon W.G., Gunduz A., Brunner P., Ritaccio A.L., Pesaran B., Schalk G. Oscillatory phase modulates the timing of neuronal activations and resulting behavior. Neuroimage. 2016;133:294–301. doi: 10.1016/j.neuroimage.2016.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen O., Gips B., Bergmann T.O., Bonnefond M. Temporal coding organized by coupled alpha and gamma oscillations prioritize visual processing. Trends Neurosci. 2014;37:357–369. doi: 10.1016/j.tins.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Michalareas G., Vezoli J., van Pelt S., Schoffelen J.M., Kennedy H., Fries P. Alpha-Beta and Gamma Rhythms Subserve Feedback and Feedforward Influences among Human Visual Cortical Areas. Neuron. 2016;89:384–397. doi: 10.1016/J.NEURON.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardellicchio P., Hilt P.M., Dolfini E., Fadiga L., D’Ausilio A. Beta Rebound as an Index of Temporal Integration of Somatosensory and Motor Signals. Front. Syst. Neurosci. 2020;14:63. doi: 10.3389/fnsys.2020.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardellicchio P., Dolfini E., Hilt P.M., Fadiga L., D’Ausilio A. Motor cortical inhibition during concurrent action execution and action observation. Neuroimage. 2020;208 doi: 10.1016/j.neuroimage.2019.116445. [DOI] [PubMed] [Google Scholar]
- 9.Cattaneo L., Sandrini M., Schwarzbach J. State-Dependent TMS Reveals a Hierarchical Representation of Observed Acts in the Temporal, Parietal, and Premotor Cortices. Cereb. Cortex. 2010;20:2252–2258. doi: 10.1093/cercor/bhp291. [DOI] [PubMed] [Google Scholar]
- 10.Jacquet P.O., Avenanti A. Perturbing the Action Observation Network During Perception and Categorization of Actions’ Goals and Grips: State-Dependency and Virtual Lesion TMS Effects. Cereb. Cortex. 2015;25:598–608. doi: 10.1093/cercor/bht242. [DOI] [PubMed] [Google Scholar]
- 11.Schutter D.J.L.G., Smits F., Klaus J. Mind matters: A narrative review on affective state-dependency in non-invasive brain stimulation. Int. J. Clin. Health Psychol. 2023;23 doi: 10.1016/J.IJCHP.2023.100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelen T., Solcà M., Tallon-Baudry C. Interoceptive rhythms in the brain. Nat. Neurosci. 2023;26:1670–1684. doi: 10.1038/s41593-023-01425-1. [DOI] [PubMed] [Google Scholar]
- 13.Todd J., Cardellicchio P., Swami V., Cardini F., Aspell J.E. Weaker implicit interoception is associated with more negative body image: Evidence from gastric-alpha phase amplitude coupling and the heartbeat evoked potential. Cortex. 2021;143:254–266. doi: 10.1016/j.cortex.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 14.van der Veen F.M., van der Molen M.W., Jennings J.R. Selective inhibition is indexed by heart rate slowing. Psychophysiology. 2000;37:607–613. doi: 10.1017/S0048577200982301. [DOI] [PubMed] [Google Scholar]
- 15.Critchley H.D., Rotshtein P., Nagai Y., O’Doherty J., Mathias C.J., Dolan R.J. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. Neuroimage. 2005;24:751–762. doi: 10.1016/j.neuroimage.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Edwards L., Ring C., McIntyre D., Carroll D. Modulation of the human nociceptive flexion reflex across the cardiac cycle. Psychophysiology. 2001;38:712–718. doi: 10.1111/1469-8986.3840712. [DOI] [PubMed] [Google Scholar]
- 17.Garfinkel S.N., Barrett A.B., Minati L., Dolan R.J., Seth A.K., Critchley H.D. What the heart forgets: Cardiac timing influences memory for words and is modulated by metacognition and interoceptive sensitivity. Psychophysiology. 2013;50:505–512. doi: 10.1111/PSYP.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garfinkel S.N., Minati L., Gray M.A., Seth A.K., Dolan R.J., Critchley H.D. Fear from the Heart: Sensitivity to Fear Stimuli Depends on Individual Heartbeats. J. Neurosci. 2014;34:6573–6582. doi: 10.1523/JNEUROSCI.3507-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krypotos A.-M., Jahfari S., van Ast V.A., Kindt M., Forstmann B.U. Individual Differences in Heart Rate Variability Predict the Degree of Slowing during Response Inhibition and Initiation in the Presence of Emotional Stimuli. Front. Psychol. 2011;2 doi: 10.3389/fpsyg.2011.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birren J.E., Cardon P.V., Phillips S.L. Reaction time as a function of the cardiac cycle in young adults. Science. 1963;140:195–196. doi: 10.1126/science.140.3563.195-a. [DOI] [PubMed] [Google Scholar]
- 21.Saari M.J., Pappas B.A. Cardiac Cycle Phase and Movement and Reaction Times. Percept. Mot. Skills. 1976;42:767–770. doi: 10.2466/pms.1976.42.3.767. [DOI] [PubMed] [Google Scholar]
- 22.Weisz J., Ádám G. The influence of cardiac phase on reaction time depending on heart period length and on stimulus and response laterality. Psychobiology. 1996;24:169–175. [Google Scholar]
- 23.Salzman L.F., Jaques N. Heart rate and cardiac cycle effects on reaction time. Percept. Mot. Skills. 1976;42:1315–1321. doi: 10.2466/PMS.1976.42.3C.1315. [DOI] [Google Scholar]
- 24.Edwards L., Ring C., McIntyre D., Carroll D., Martin U. Psychomotor speed in hypertension: effects of reaction time components, stimulus modality, and phase of the cardiac cycle. Psychophysiology. 2007;44:459–468. doi: 10.1111/J.1469-8986.2007.00521.X. [DOI] [PubMed] [Google Scholar]
- 25.Stewart J.C., France C.R., Suhr J.A. The effect of cardiac cycle phase on reaction time among individuals at varying risk for hypertension. J. Psychophysiol. 2006;20:1–8. doi: 10.1027/0269-8803.20.1.1. [DOI] [Google Scholar]
- 26.McIntyre D., Ring C., Hamer M., Carroll D. Effects of arterial and cardiopulmonary baroreceptor activation on simple and choice reaction times. Psychophysiology. 2007;44:874–879. doi: 10.1111/J.1469-8986.2007.00547.X. [DOI] [PubMed] [Google Scholar]
- 27.Yang X., Jennings J.R., Friedman B.H. Exteroceptive stimuli override interoceptive state in reaction time control. Psychophysiology. 2017;54:1940–1950. doi: 10.1111/PSYP.12958. [DOI] [PubMed] [Google Scholar]
- 28.Saltafossi M., Zaccaro A., Perrucci M.G., Ferri F., Costantini M. The impact of cardiac phases on multisensory integration. Biol. Psychol. 2023;182 doi: 10.1016/J.BIOPSYCHO.2023.108642. [DOI] [PubMed] [Google Scholar]
- 29.Ren Q., Marshall A.C., Kaiser J., Schütz-Bosbach S. Multisensory integration of anticipated cardiac signals with visual targets affects their detection among multiple visual stimuli. Neuroimage. 2022;262 doi: 10.1016/J.NEUROIMAGE.2022.119549. [DOI] [PubMed] [Google Scholar]
- 30.Skora L.I., Livermore J.J.A., Roelofs K. The functional role of cardiac activity in perception and action. Neurosci. Biobehav. Rev. 2022;137 doi: 10.1016/J.NEUBIOREV.2022.104655. [DOI] [PubMed] [Google Scholar]
- 31.Galvez-Pol A., McConnell R., Kilner J.M. Active sampling in visual search is coupled to the cardiac cycle. Cognition. 2020;196 doi: 10.1016/J.COGNITION.2019.104149. [DOI] [PubMed] [Google Scholar]
- 32.Herman A.M., Tsakiris M. Feeling in Control: The Role of Cardiac Timing in the Sense of Agency. Affect. Sci. 2020;1:155–171. doi: 10.1007/S42761-020-00013-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rae C.L., Botan V.E., Gould Van Praag C.D., Herman A.M., Nyyssönen J.A.K., Watson D.R., Duka T., Garfinkel S.N., Critchley H.D. Response inhibition on the stop signal task improves during cardiac contraction. Sci. Rep. 2018;8:9136. doi: 10.1038/s41598-018-27513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chowdhury N.S., Livesey E.J., Harris J.A. Contralateral and Ipsilateral Relationships between Intracortical Inhibition and Stopping Efficiency. Neuroscience. 2019;415:10–17. doi: 10.1016/j.neuroscience.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 35.He J.L., Fuelscher I., Coxon J., Chowdhury N., Teo W.-P., Barhoun P., Enticott P., Hyde C. Individual differences in intracortical inhibition predict motor-inhibitory performance. Exp. Brain Res. 2019;237:2715–2727. doi: 10.1007/s00221-019-05622-y. [DOI] [PubMed] [Google Scholar]
- 36.Chowdhury N.S., Livesey E.J., Harris J.A. Individual differences in intracortical inhibition during behavioural inhibition. Neuropsychologia. 2019;124:55–65. doi: 10.1016/j.neuropsychologia.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Tran D.M.D., Chowdhury N.S., McNair N.A., Harris J.A., Livesey E.J. Linking cortical and behavioural inhibition: Testing the parameter specificity of a transcranial magnetic stimulation protocol. Brain Stimul. 2020;13:1381–1383. doi: 10.1016/j.brs.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Chowdhury N.S., Livesey E.J., Blaszczynski A., Harris J.A. Variations in response control within at-risk gamblers and non-gambling controls explained by GABAergic inhibition in the motor cortex. Cortex. 2018;103:153–163. doi: 10.1016/j.cortex.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Hermans L., Leunissen I., Pauwels L., Cuypers K., Peeters R., Puts N.A.J., Edden R.A.E., Swinnen S.P. Brain GABA levels are associated with inhibitory control deficits in older adults. J. Neurosci. 2018;38:7844–7851. doi: 10.1523/JNEUROSCI.0760-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hermans L., Maes C., Pauwels L., Cuypers K., Heise K.-F., Swinnen S.P., Leunissen I. Age-related alterations in the modulation of intracortical inhibition during stopping of actions. Aging (Albany. NY) 2019;11:371–385. doi: 10.18632/aging.101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paci M., Di Cosmo G., Perrucci M.G., Ferri F., Costantini M. Cortical silent period reflects individual differences in action stopping performance. Sci. Rep. 2021;11:15158. doi: 10.1038/s41598-021-94494-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardellicchio P., Koch G., Fadiga L., D’Ausilio A. Motor overload: GABAergic index of parallel buffer costs. Brain Stimul. 2021;14:1106–1108. doi: 10.1016/j.brs.2021.07.061. [DOI] [PubMed] [Google Scholar]
- 43.Amassian V.E., Cracco R.Q., Maccabee P.J., Cracco J.B., Rudell A., Eberle L. Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalogr. Clin. Neurophysiol. 1989;74:458–462. doi: 10.1016/0168-5597(89)90036-1. [DOI] [PubMed] [Google Scholar]
- 44.Bianchini E., Mancuso M., Zampogna A., Guerra A., Suppa A. Cardiac cycle does not affect motor evoked potential variability: A real-time EKG-EMG study. Brain Stimul. 2021;14:170–172. doi: 10.1016/J.BRS.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Ellaway P.H., Davey N.J., Maskill D.W., Rawlinson S.R., Lewis H.S., Anissimova N.P. Variability in the amplitude of skeletal muscle responses to magnetic stimulation of the motor cortex in man. Electroencephalogr. Clin. Neurophysiol. 1998;109:104–113. doi: 10.1016/S0924-980X(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 46.Filippi M.M., Oliveri M., Vernieri F., Pasqualetti P., Rossini P.M. Are autonomic signals influencing cortico-spinal motor excitability? A study with transcranial magnetic stimulation. Brain Res. 2000;881:159–164. doi: 10.1016/S0006-8993(00)02837-7. [DOI] [PubMed] [Google Scholar]
- 47.Al E., Stephani T., Engelhardt M., Villringer A. 2023. Cardiac Activity Impacts Cortical Motor Excitability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borgomaneri S., Vitale F., Avenanti A. Early changes in corticospinal excitability when seeing fearful body expressions. Sci. Rep. 2015;5 doi: 10.1038/srep14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cardellicchio P., Hilt P.M., Olivier E., Fadiga L., D’Ausilio A. Early modulation of intra-cortical inhibition during the observation of action mistakes. Sci. Rep. 2018;8:1784. doi: 10.1038/s41598-018-20245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cardellicchio P., Dolfini E., Fadiga L., D’Ausilio A. Parallel fast and slow motor inhibition processes in Joint Action coordination. Cortex. 2020;133:346–357. doi: 10.1016/j.cortex.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 51.Di Lazzaro V., Oliviero A., Meglio M., Cioni B., Tamburrini G., Tonali P., Rothwell J.C. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin. Neurophysiol. 2000;111:794–799. doi: 10.1016/S1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- 52.Kujirai T., Caramia M.D., Rothwell J.C., Day B.L., Thompson P.D., Ferbert A., Wroe S., Asselman P., Marsden C.D. Corticocortical inhibition in human motor cortex. J. Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coxon J.P., Stinear C.M., Byblow W.D. Intracortical inhibition during volitional inhibition of prepared action. J. Neurophysiol. 2006;95:3371–3383. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- 54.Greenhouse I., Oldenkamp C.L., Aron A.R. Stopping a response has global or nonglobal effects on the motor system depending on preparation. J. Neurophysiol. 2012;107:384–392. doi: 10.1152/jn.00704.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paulus W., Classen J., Cohen L.G., Large C.H., Di Lazzaro V., Nitsche M., Pascual-Leone A., Rosenow F., Rothwell J.C., Ziemann U. State of the art: Pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul. 2008;1:151–163. doi: 10.1016/j.brs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Ziemann U., Lönnecker S., Paulus W. Inhibition of human motor cortex by ethanol A transcranial magnetic stimulation study. Brain. 1995;118:1437–1446. doi: 10.1093/brain/118.6.1437. [DOI] [PubMed] [Google Scholar]
- 57.Nicoll R.A., Malenka R.C., Kauer J.A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990;70:513–565. doi: 10.1152/PHYSREV.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- 58.Bowery N. GABAB receptors and their significance in mammalian pharmacology. Trends Pharmacol. Sci. 1989;10:401–407. doi: 10.1016/0165-6147(89)90188-0. [DOI] [PubMed] [Google Scholar]
- 59.Ogata N. Pharmacology and physiology of GABAB receptors. Gen Pharmacol. 1990;21:395–402. doi: 10.1016/0306-3623(90)90687-H. [DOI] [PubMed] [Google Scholar]
- 60.Mott D.D., Lewis D.V. The Pharmacology and Function of Central GabaB Receptors. Int. Rev. Neurobiol. 1994;36:97–223. doi: 10.1016/S0074-7742(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 61.Ziemann U., Lönnecker S., Steinhoff B.J., Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp. Brain Res. 1996;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- 62.Ilić T.V., Meintzschel F., Cleff U., Ruge D., Kessler K.R., Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J. Physiol. 2002;545:153–167. doi: 10.1113/JPHYSIOL.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McDonnell M.N., Orekhov Y., Ziemann U. The role of GABAB receptors in intracortical inhibition in the human motor cortex. Exp. Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- 64.Lauber B., Taube W. Probing the link between cortical inhibitory and excitatory processes and muscle fascicle dynamics. Sci. Rep. 2023;13 doi: 10.1038/s41598-023-31825-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lauber B., Gollhofer A., Taube W. Differences inmotor cortical control of the soleus and tibialis anterior. J. Exp. Biol. 2018;221 doi: 10.1242/jeb.174680. [DOI] [PubMed] [Google Scholar]
- 66.Werhahn K.J., Kunesch E., Noachtar S., Benecke R., Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J. Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baker S.N., Olivier E., Lemon R.N. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J. Physiol. 1997;501:225–241. doi: 10.1111/j.1469-7793.1997.225bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murthy V.N., Fetz E.E. Oscillatory activity in sensorimotor cortex of awake monkeys: Synchronization of local field potentials and relation to behavior. J. Neurophysiol. 1996;76:3949–3967. doi: 10.1152/jn.1996.76.6.3949. [DOI] [PubMed] [Google Scholar]
- 69.Yamawaki N., Stanford I.M., Hall S.D., Woodhall G.L. Pharmacologically induced and stimulus evoked rhythmic neuronal oscillatory activity in the primary motor cortex in vitro. Neuroscience. 2008;151:386–395. doi: 10.1016/j.neuroscience.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 70.Hall S.D., Barnes G.R., Furlong P.L., Seri S., Hillebrand A. Neuronal network pharmacodynamics of GABAergic modulation in the human cortex determined using pharmaco-magnetoencephalography. Hum. Brain Mapp. 2010;31:581–594. doi: 10.1002/hbm.20889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jensen O., Goel P., Kopell N., Pohja M., Hari R., Ermentrout B. On the human sensorimotor-cortex beta rhythm: Sources and modeling. Neuroimage. 2005;26:347–355. doi: 10.1016/j.neuroimage.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 72.Hall S.D., Stanford I.M., Yamawaki N., McAllister C.J., Rönnqvist K.C., Woodhall G.L., Furlong P.L. The role of GABAergic modulation in motor function related neuronal network activity. Neuroimage. 2011;56:1506–1510. doi: 10.1016/J.NEUROIMAGE.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 73.Edwards L., Ring C., McIntyre D., Winer J.B., Martin U. Sensory detection thresholds are modulated across the cardiac cycle: Evidence that cutaneous sensibility is greatest for systolic stimulation. Psychophysiology. 2009;46:252–256. doi: 10.1111/J.1469-8986.2008.00769.X. [DOI] [PubMed] [Google Scholar]
- 74.Edwards L., Inui K., Ring C., Wang X., Kakigi R. Pain-related evoked potentials are modulated across the cardiac cycle. Pain. 2008;137:488–494. doi: 10.1016/J.PAIN.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Gray M.A., Beacher F.D., Minati L., Nagai Y., Kemp A.H., Harrison N.A., Critchley H.D. Emotional appraisal is influenced by cardiac afferent information. Emotion. 2012;12:180–191. doi: 10.1037/A0025083. [DOI] [PubMed] [Google Scholar]
- 76.Garfinkel S.N., Critchley H.D. Threat and the Body: How the Heart Supports Fear Processing. Trends Cogn. Sci. 2016;20:34–46. doi: 10.1016/J.TICS.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 77.Suarez-Roca H., Mamoun N., Sigurdson M.I., Maixner W. Baroreceptor Modulation of the Cardiovascular System, Pain, Consciousness, and Cognition. Compr. Physiol. 2021;11:1373–1423. doi: 10.1002/CPHY.C190038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duque J., Greenhouse I., Labruna L., Ivry R.B. Physiological markers of motor inhibition during human behavior. Trends Neurosci. 2017;40:219–236. doi: 10.1016/J.TINS.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rae C.L., Ahmad A., Larsson D.E.O., Silva M., Praag C.D.G.v., Garfinkel S.N., Critchley H.D. Impact of cardiac interoception cues and confidence on voluntary decisions to make or withhold action in an intentional inhibition task. Sci. Rep. 2020;10 doi: 10.1038/S41598-020-60405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Makowski D., Sperduti M., Blondé P., Nicolas S., Piolino P. The heart of cognitive control: Cardiac phase modulates processing speed and inhibition. Psychophysiology. 2020;57:e13490. doi: 10.1111/PSYP.13490. [DOI] [PubMed] [Google Scholar]
- 81.Raud L., Westerhausen R., Dooley N., Huster R.J. Differences in unity: The go/no-go and stop signal tasks rely on different mechanisms. Neuroimage. 2020;210 doi: 10.1016/J.NEUROIMAGE.2020.116582. [DOI] [PubMed] [Google Scholar]
- 82.VanRullen R. How to Evaluate Phase Differences between Trial Groups in Ongoing Electrophysiological Signals. Front. Neurosci. 2016;10 doi: 10.3389/fnins.2016.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blakemore C., Carpenter R.H., Georgeson M.A. Lateral Inhibition between Orientation Detectors in the Human Visual System. Nature. 1970;228:37–39. doi: 10.1038/228037a0. [DOI] [PubMed] [Google Scholar]
- 84.Levin O., Fujiyama H., Boisgontier M.P., Swinnen S.P., Summers J.J. Aging and motor inhibition: A converging perspective provided by brain stimulation and imaging approaches. Neurosci. Biobehav. Rev. 2014;43:100–117. doi: 10.1016/j.neubiorev.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 85.Mink J.W. The basal ganglia: focused selection and inhibition of competing motor programs. Prog. Neurobiol. 1996;50:381–425. doi: 10.1016/S0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 86.Rossi S., Hallett M., Rossini P.M., Pascual-Leone A., Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120:2008–2039. doi: 10.1016/J.CLINPH.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 88.Burden A. How should we normalize electromyograms obtained from healthy participants? What we have learned from over 25years of research. J. Electromyogr. Kinesiol. 2010;20:1023–1035. doi: 10.1016/j.jelekin.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 89.Farzan F., Barr M.S., Hoppenbrouwers S.S., Fitzgerald P.B., Chen R., Pascual-Leone A., Daskalakis Z.J. The EEG correlates of the TMS-induced EMG silent period in humans. Neuroimage. 2013;83:120–134. doi: 10.1016/j.neuroimage.2013.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Du X., Summerfelt A., Chiappelli J., Holcomb H.H., Hong L.E. Individualized Brain Inhibition and Excitation Profile in Response to Paired-Pulse TMS. J. Mot. Behav. 2014;46:39–48. doi: 10.1080/00222895.2013.850401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rossini P.M., Burke D., Chen R., Cohen L.G., Daskalakis Z., Di Iorio R., Di Lazzaro V., Ferreri F., Fitzgerald P.B., George M.S., et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015;126:1071–1107. doi: 10.1016/J.CLINPH.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garry M.I., Thomson R.H.S. The effect of test TMS intensity on short-interval intracortical inhibition in different excitability states. Exp. Brain Res. 2009;193:267–274. doi: 10.1007/s00221-008-1620-5. [DOI] [PubMed] [Google Scholar]
- 93.Hallett M. Transcranial magnetic stimulation: A primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 94.Fisher R.J., Nakamura Y., Bestmann S., Rothwell J.C., Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp. Brain Res. 2002;143:240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- 95.Roshan L., Paradiso G.O., Chen R. Two phases of short-interval intracortical inhibition. Exp. Brain Res. 2003;151:330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- 96.Vucic S., Cheah B.C., Krishnan A.V., Burke D., Kiernan M.C. The effects of alterations in conditioning stimulus intensity on short interval intracortical inhibition. Brain Res. 2009;1273:39–47. doi: 10.1016/j.brainres.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 97.Chang W.H., Fried P.J., Saxena S., Jannati A., Gomes-Osman J., Kim Y.-H., Pascual-Leone A. Optimal number of pulses as outcome measures of neuronavigated transcranial magnetic stimulation. Clin. Neurophysiol. 2016;127:2892–2897. doi: 10.1016/j.clinph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.İşcan Z., Schurger A., Vernet M., Sitt J.D., Valero-Cabré A. Pre-stimulus theta power is correlated with variation of motor evoked potential latency: a single-pulse TMS study. Exp. Brain Res. 2018;236:3003–3014. doi: 10.1007/s00221-018-5359-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data have been deposited at Mendeley Data and are publicly available as of the date of publication. The accession numbers or URL for the datasets are listed in the key resources table.
This paper does not report the original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.